MARS, PANSPERMIA, AND THE ORIGIN OF
LIFE:
WHERE DID IT ALL BEGIN?
During the nineteenth century, when steady-state cosmological theories were in vogue, Lord Kelvin, Svante Arrhenius, and other eminent scientists believed that the transfer of life from one planet to another was a process made inevitable by the infinite extent and duration of the universe. This hypothesis, known as panspermia, subsequently fell out of favor, partly as a result of the acceptance of the Big Bang theory. Most efforts to understand the origin of life have since been framed by the assumption that life began on Earth.
 However,
in the last decade data have begun to accumulate suggesting that panspermia may
in fact be a natural and frequently occurring process. Recent paleomagnetic
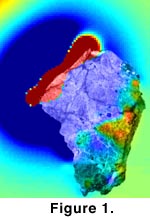
studies on Martian meteorite ALH84001 have shown that this rock traveled from
Mars to Earth without its interior becoming warmer than 40ºC (Weiss
et al. 2000) (Fig. 1). Experiments
aboard the European Space Agency’s Long Duration Exposure Facility indicate
that bacterial spores can survive in deep space for more than five years (Horneck
et al. 1994; Horneck
1999), and laboratory experiments demonstrate that bacteria can survive the
shocks and jerks expected for a rock ejected from Mars (Mastrapa
et al. 2001). Finally, dynamical studies indicate that the transfer of rocks
from Mars to Earth (and to a limited extent, vice versa) can proceed on a
biologically short time scale, making it likely that organic hitchhikers have
traveled between these planets many times during the history of the Solar System
(Mileikowsky et al. 2000; Weiss
and Kirschvink 2000). These studies demand a re-evaluation of the long-held
assumption that terrestrial life evolved in isolation on Earth.
However,
in the last decade data have begun to accumulate suggesting that panspermia may
in fact be a natural and frequently occurring process. Recent paleomagnetic
studies on Martian meteorite ALH84001 have shown that this rock traveled from
Mars to Earth without its interior becoming warmer than 40ºC (Weiss
et al. 2000) (Fig. 1). Experiments
aboard the European Space Agency’s Long Duration Exposure Facility indicate
that bacterial spores can survive in deep space for more than five years (Horneck
et al. 1994; Horneck
1999), and laboratory experiments demonstrate that bacteria can survive the
shocks and jerks expected for a rock ejected from Mars (Mastrapa
et al. 2001). Finally, dynamical studies indicate that the transfer of rocks
from Mars to Earth (and to a limited extent, vice versa) can proceed on a
biologically short time scale, making it likely that organic hitchhikers have
traveled between these planets many times during the history of the Solar System
(Mileikowsky et al. 2000; Weiss
and Kirschvink 2000). These studies demand a re-evaluation of the long-held
assumption that terrestrial life evolved in isolation on Earth.
Three lines of evidence have been proposed to support the idea that by 4.0 billion years ago (Ga), life somewhere had evolved to a fairly high level of complexity. These challenge the assumption that the late heavy bombardment (Cohen et al. 2000) was inimical to the origin and continuity of life. First, the presence of isotopically depleted graphite inclusions in apatite crystals in Archean rocks from Greenland (Mojzsis et al. 1996) were interpreted as the action of biological carbon fractionation, perhaps through photosynthesis. However, this has been cast in doubt because the apatite has U/Pb and Pb/Pb ages of ~1.5 Ga, indicating either that they formed much later than the BIFs and/or their isotopic ratios were reset by a high-temperature metamorphic event (Sano et al. 1999). The geological context of the sampling area has also been questioned (Myers and Crowley 2000).
The second line of evidence is the possible presence of 3.9-4.1 Ga magnetofossils (indistinguishable from those made by modern Earthly bacteria) in the ALH84001 carbonates (Friedmann et al. 2001; Thomas-Keprta et al. 2000, 2001). Although this hypothesis remains highly controversial, no one has yet documented a plausible, inorganic mechanism for producing similar particles. The ferrite industry (which makes small magnetic particles for disk drives and magnetic recording tapes, and is worth $35 billion per year) has tried to make similar particles of magnetite inorganically for the past 60 years and has failed. The inorganic synthesis of magnetosome-like magnetite particles is clearly not a simple feat. Although Golden et al. (2001) claim to have produced magnetofossil-like magnetites from heating of siderite, they have not yet documented the shape and chemistry of those inorganic magnetites in detail (see Thomas-Keprta et al. 2001). Nevertheless, their mechanism should be studied further given that shocks have probably affected ALH84001 carbonates.
 The
third line of evidence is recent molecular-clock analyses which exploit the
large number of completely sequenced genomes of Bacteria, Archaea, and Eukarya,
and indicate that the Last Common Ancestor (LCA) of all living organisms dates
back to about 4 Ga (Hedges et al.
2001) (Fig. 2). This phylogeny (Hedges
et al. 2001) is more pleasing than earlier attempts using fewer gene systems
because it intrinsically agrees with two deep-time aspects of the direct
geological record: it agrees with the presence of the first biomarkers
(2-methylhopanoids) possibly indicative of the cyanobacteria at around 2.5 Ga (Summons
et al. 1999), and with the direct fossil record of organelle-bearing
eukaryotes at about 2.1 Ga (Han and
Runnegar 1992). Hence, it is probably the Hadean of Earth, or perhaps even
the Noachian of Mars, in which we must look for Darwin’s "warm little
pond".
The
third line of evidence is recent molecular-clock analyses which exploit the
large number of completely sequenced genomes of Bacteria, Archaea, and Eukarya,
and indicate that the Last Common Ancestor (LCA) of all living organisms dates
back to about 4 Ga (Hedges et al.
2001) (Fig. 2). This phylogeny (Hedges
et al. 2001) is more pleasing than earlier attempts using fewer gene systems
because it intrinsically agrees with two deep-time aspects of the direct
geological record: it agrees with the presence of the first biomarkers
(2-methylhopanoids) possibly indicative of the cyanobacteria at around 2.5 Ga (Summons
et al. 1999), and with the direct fossil record of organelle-bearing
eukaryotes at about 2.1 Ga (Han and
Runnegar 1992). Hence, it is probably the Hadean of Earth, or perhaps even
the Noachian of Mars, in which we must look for Darwin’s "warm little
pond".
Because life on Earth depends on a variety of
biochemical respiratory chains involving electron transport, we argue that the
presence of electrochemical gradients is one of the most critical prerequisites
for life’s initiation and would form intense selection pressures during its
initial evolution. A recent genetic analysis of the respiratory chains in the
Archaea, Eukarya and Bacteria (Castresana
and Moreira 1999) indicates that terminal oxidases linked to oxygen,
nitrate, sulfate, and sulfur were all present in the last common ancestor of
living organisms. An alternative interpretation, massive lateral gene transfer (Doolittle
2000), can be ruled out at least for O2 because most of the
cytochrome oxidase genes (with the exception of a small domain in one organism)
track the phylogeny of rRNA (Schutz et al. 2000). Figure
3 shows the typical electrochemical cascades that operate in a modern,
neutral oceanic system, with the basal oxidase of the LCA indicated in red. Our
LCA must have evolved on a world in which these redox gradients were present in
large enough quantities to evolve and to be maintained, and in which metastable,
energetic compounds could diffuse across redox boundaries. "Smothered"
environments (like Europa’s ocean; Gaidos
et al. 1999) that lack significant electrochemical gradients are difficult
for existing life to survive in, and would have been even more difficult for it
to arise in. 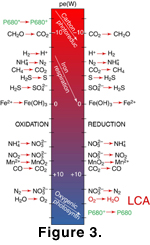 Since the efficiency of biological systems tends to improve over time as a
result of the processes of random mutation and natural selection (Darwin
1859), primitive metabolic electron transport systems likely could not
extract energy as efficiently from the redox couples shown in Fig.
3 as do those of modern organisms. In fact, many of the intermediate steps
in these electron transport chains arose through gene duplication events from
ancestral proteins (Schutz et al.
2000). Hence, the ancestral forms could not have been as efficient at
recovering metabolic energy as are the modern ones. For this reason, we argue
that life is more likely to have evolved on the planet (either Earth or Mars)
that had the broadest dynamical range of electrochemical species early in its
history.
Since the efficiency of biological systems tends to improve over time as a
result of the processes of random mutation and natural selection (Darwin
1859), primitive metabolic electron transport systems likely could not
extract energy as efficiently from the redox couples shown in Fig.
3 as do those of modern organisms. In fact, many of the intermediate steps
in these electron transport chains arose through gene duplication events from
ancestral proteins (Schutz et al.
2000). Hence, the ancestral forms could not have been as efficient at
recovering metabolic energy as are the modern ones. For this reason, we argue
that life is more likely to have evolved on the planet (either Earth or Mars)
that had the broadest dynamical range of electrochemical species early in its
history.
So now it is important to compare the probable environments of the early Earth with that of early Mars in order to evaluate which of these two bodies, during the first half-billion years of the solar system, might have produced an environment most suitable for the origin of life based on redox chemistry. This involves deducing the early environments of Mars and of Earth during a time interval when Earth lacks an extensive sedimentary rock record and when the Martian record must be accessed indirectly.
Likewise, the principal gaseous components produced by volcanic emissions likely had equilibrated with the reducing components then present in the outer mantles of these planetary bodies. A recent study of Martian meteorites (Wadhwa, 2001) has shown that the mantle of Mars is more reduced than that of the Earth, while the crust of Mars is still quite oxidized. The difference between the two planets is probably a result of the fact that Mars lacks plate tectonics, which on Earth recycles oxidized crust back into the mantle. However, on Earth, plate tectonics did not necessarily oxidize the mantle quickly. Kump et al. (2001) argue that during Archean time subducted oceanic crust may well have penetrated to the lower mantle, implying that the bulk of volcanic gasses equilibrated with the more primitive and more reducing upper mantle, until a turn-over event near the Archean/Proterozoic boundary. Both planets, then, possessed at least local environments sufficiently reducing to allow the accumulation of pre-biotic compounds. The question then focuses on determining which planet would have contained a better source of oxidizing atmospheric compounds capable of diffusing into this primordial soup to promote organic evolution.
Figure 2 (adapted from Kasting 1993) shows the redox history of Earth’s surface, as inferred through the geological record. Although today both Earth and Mars have extremely oxidizing surficial environments, there has been a long debate about the redox potential of the Earth's surface prior to the Phanerozoic (summarized in Schopf and Klein 1992). Sedimentary rocks of Archean and early Proterozoic age typically contain banded iron formations (BIFs) and detrital, stream-rounded pebbles of the minerals pyrite and uraninite. Based on modern analogs, these are usually taken as indicative of more reducing conditions. In contrast, the strongest line of evidence for oxygen production in the Archean was the presence of filaments with strong similarities to cyanobacteria from black cherts in the 3.5 Ga Warawoona group of Australia (Schopf 1993; Schopf and Packer 1987). These claims supported an environmental model of small oxygenic ‘islands’ of photosynthetic life separated by sharp redox gradients from an otherwise anaerobic environment (e.g., Cloud 1988). However, Buick (1988) has maintained that the actual black cherts sampled for these studies were secondary hydrothermal silica deposits of much younger age which cross-cut the primary bedding (an observation with which we concur after having visited these localities with him this past July). These putative fossils are probably not a constraint on oxygen in the Archean environment. Furthermore, it is unclear why the cyanobacteria, if equipped with the incredible power of the oxygen-releasing Photosystem-II, did not follow an explosive exponential growth pattern to take over the world as soon as they evolved. Even the presence of Archean stromatolites does not help, given their possible abiogenicity (Grotzinger and Knoll 1999) and the possibility that their photosystems were non-oxygenic. In fact, the oldest direct evidence for oxygenic photosynthesis (Photosystem-II) is the Kalahari Manganese Field, which precipitated as oxides in the aftermath of the Paleoproterozoic Snowball glaciation event at about 2.3 Ga (Kirschvink et al. 2000). These deposits could well be the result of run-away oxygenation of the surface environment as a result of the newly evolved Photosystem-II. As shown on Fig. 2, the Kalahari Manganese Field falls well within the error range for the evolution of cyanobacteria given by the Hedges et al. (2001) molecular-clock study, but it is clearly out of range for the LCA.
In recent years, several new developments have bolstered the case for a strongly reducing surficial environment on Earth during Archean and earliest Proterozoic time. First, shallow-water carbonates of this age typically contain a trace of Mn+2, which is only soluble under anoxic conditions (Kirschvink et al. 2000; Veizer 1994), requiring oxidants of either nitrate or O2 to be oxidized to the insoluble Mn+4 form (see Fig. 3). But nitrate formation in the ocean requires O2 (Kirschvink et al. 2000), so this is de facto a constraint on O2. The Mn+2 presumably was incorporated in the carbonate during initial precipitation, but it disappears after the first Snowball event at 2.3 Ga. This implies that post-Snowball oxygen levels were high enough to keep Mn+2 out of solution in the surficial waters. Second, Sumner (1997) has noted that Fe+2 has a strong inhibitory effect on the nucleation of carbonate minerals, and that many of the shallow-water early Archean carbonates display textures compatible with the presence of ferrous iron in the shallow, mixed surface waters.
Third, recent work on terrestrial mass-independent sulfur isotope fractionation (Farquhar et al. 2000) suggests that units older than the Snowball were derived from an environment reducing enough to allow gaseous sulfur compounds to be cycled within the atmosphere. The implications of sulfur fractionations for the early Martian redox environment are less clear. Although ~4 Ga ALH84001 pyrites also show enormous mass-independent sulfur fractionations, these do not require a reducing atmosphere but instead can be explained by the lack of crustal recycling on Mars, in combination with an early influx of isotopically heterogeneous materials to the planet (Greenwood et al. 2000). Furthermore, mass-independent fractionation of oxygen isotopes (17O with reference to 16O and 18O) in ALH84001 carbonates suggest passage of this element through an atmospheric ozone phase (Farquhar et al. 1998).
Fourth, the rarity of glacial features during Archean time, coupled with solar evolution models arguing for a faint-young-sun with 30% less luminosity than today, has long presented a climatic paradox in its own right. Suggestions of a massive, 10-bar CO2 greenhouse atmosphere (Kasting 1993) conflict with lower estimates of atmospheric CO2 from Archean paleosols (Rye et al. 1995). However, Pavlov et al. (2000) note that an Archean atmosphere rich in methane would solve this paradox, and would provide an elegant trigger for the Paleoproterozoic Snowball event as soon as Photosystem-II were to evolve. If these indicators are extrapolated blindly back to the Hadean Earth, environments capable of producing sharp redox gradients needed for the evolution of primitive life would be rare to nonexistent.
The temporal evolution of oxygen in the Martian atmosphere is much less understood. In the present-day atmosphere, H and O atoms are thought to be lost from the atmosphere to space in a self-regulating ratio of 2:1, which maintains a constant oxidation state for the planet. This loss ratio is maintained today because the rates of formation and photolysis of H2O adjust to maintain the appropriate supplies of free H and O available for loss (Yung and DeMore 1999). The constant 2:1 loss ratio is maintained despite the fact that the principal loss mechanisms for these two elements are vastly different. Hydrogen is lost mainly via thermal escape (Nair et al. 1994). On the other hand, O, a much heavier element, is lost chiefly from the impingement of the solar wind plasma and magnetic field (e.g., atmospheric sputtering, dissociative recombination, and direct ion pick-up; Fox 1997; Hutchins et al. 1997). Both hydrogen and oxygen loss have had a much less significant effect on Earth’s atmosphere both because of Earth’s higher gravity and strong magnetic field (the latter reduces solar-wind-induced ionization as well as loss of ionized species due to interaction with the solar-wind magnetic field).
Although Mars currently lacks a global magnetic field, we have recently learned that it had a strong field at 4 Ga or earlier (Acuna et al. 1999, Weiss, B. P., Vali, H., Baudenbacher, F. J., Stewart, S. T., and Kirschvink, J. L., unpublished manuscript). The ancient field would have protected against the loss of O (Jakosky and Phillips 2001). If today’s self-regulating loss mechanisms for H and O also operated in this early period in Mars history, this would imply that H loss would have also have been much less in this period. However, there are good reasons to believe that this was not the case. The D/H ratio of the present-day atmosphere is ~5 times that of the Earth, indicating that a large amount of H (and, by implication, H2O) has been lost to space. However, the 18O/16O ratio is not much different than the terrestrial value, indicating that O and H loss were decoupled in the past (Owen 1992). This means that the protection afforded by the early magnetic field might have meant dramatically more loss of H than O, and so provided a cascade of oxidants to drive organic evolution. Given that Mars's mantle was likely more reduced than that of Earth (Wadhwa, 2001), such oxidizing conditions at the surface would imply that Mars had much larger redox gradients than Earth at the same time.
Isotopic studies of the Martian meteorite ALH84001 are consistent with a neutral to oxidizing surface environment in which the carbonates formed about 4 billion years ago, including contributions from photo-irradiated ozone (Farquhar et al. 1998). This ozone would have shielded an early Martian biosphere from UV radiation, a protection presumably lacking on Earth at the same time (Pavlov et al. 2001). Also, the possible presence of magnetofossils in this meteorite argues for the presence of vertical redox gradients, which magnetotactic bacteria need for their survival (Chang and Kirschvink 1989). The oldest magnetofossils yet identified on Earth are from the post-Snowball Gunflint Chert at about 2.1 Ga (Chang and Kirschvink 1989), roughly coincident with the appearance of the first eukaryotes (Han and Runnegar 1992).
At face value, all of these lines of evidence suggest that, compared to early Earth, early Mars might have had a greater supply of biologically useable energy and was perhaps, by implication, a better place for the origin of life. And so we salute you, all you descendants of space-traveling microbes from the Red Planet!
We thank F. Macdonald and F. Baudenbacher for assembling the image of Figure 1, and the NASA Astrobiology Institute and the NASA Exobiology program for supporting this research.
This text is a modified version of the Carl Sagan Lecture given by Joseph Kirschvink at the American Geophysical Union Meeting in December, 2001 (webcast of lecture here).
Joseph L. Kirschvink. Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena CA 91125, USA
and the Department of Earth and Planetary Sciences, Tokyo University, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan
Benjamin P. Weiss. Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena CA 91125, USA
Copyright: Coquina Press
31 January 2002
http://palaeo-electronica.org