|
|
|
SYSTEMATICS
Order PIPERALES
Berchtold and Presl, 1820 Genus PIPERITES Goeppert, 1854
Piperites sp.
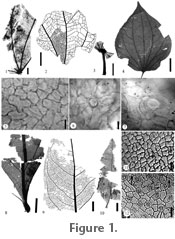
Description: Leaves petiolate. Lamina incomplete, ovate, unlobed, > 4.8 cm long by 2.5 cm wide. Apex missing, base and margin fragmented. Petiole marginal, 1 cm long with scattered trichomes (Figure 1.3). Venation basal actinodromous, consisting of 5–7 primary veins, and two secondary veins. Interior secondaries chevron, diverging from primaries at 60–80║ (Figure 1.1–1.2). Simple agrophic veins, minor secondaries simple brochidodromous. Tertiary and quaternary veins irregular reticulate (Figure 1.2). Adaxial surface glabrous; epidermal cells mostly pentagonal with some tetragonal (average size ca. 23 x 32.9 Ám), surface is smooth, and cell walls mostly arcuate, some sinuolate to sinuous, or rarely sinuate (Figure 1.5). Abaxial surface with numerous basal cells (average size ca. 15.8 x 22.3 Ám), and occasional minute glandular cells; epidermal cells smooth (average size ca. 24.9 x 33.6 Ám) (Figure 1.6–1.7). Stomata sunken, barley visible, and tetracytic (average size ca. 31.4 x 31.6 Ám) (Figure 1.7). Comments: Characters such as palmate venation and interconnecting secondary veins suggest the families Piperaceae, Malvaceae, Cecropiaceae, Urticaceae, and Menispermaceae. Within Urticaceae, genera such as Cecropia (Judd and Olmstead 2004; Systma et al. 2002), and Cecropiaceae, genera such as Pourouma, venation is similar, but the petiolar attachment is peltate in Cecropia (Burger 1977; Hammel 1986), and the deeply lobed lamina of Pourouma (Burger 1977; Berg et al. 1990) do not match the fossil specimen. Within the Malvaceae, genera such as Sterculia and Theobroma are basal plinerved, with glandular cells and epidermal cell features that are similar to the fossils, but the predominance of stellate trichomes are lacking in the fossil specimens (Robyns 1964; Hussin and Sani 1998). The numerous basal cells and some glandular cells, and stomatal shapes appear most similar to those found in the Piperaceae (Pant and Banerji 1965; Bornstein 1989) and Malvaceae, but Malvaceae contain stomatal ledges. In Menispermaceae, the presence of basal and glandular cells is similar to the fossil but Menispermaceae have stomatal ledges and stomatal shape is not tetracytic (Krukoff and Moldenke 1938, 1941, 1942, 1943; Krukoff and Barneby 1970; Barneby and Krukoff 1971; Wilkinson 1989; Hong et al. 2001). Characters such as venation (Figure 1.1–1.3), basal cells (Figure 1.6), epidermal and stomatal shape (Figure 1.5, 1.7) suggest a relationship to Piperaceae, near Piper rosei C.DC. and P. novogalicianum A.J. Bornstein (Figure 1.4) (Burger 1971; Bornstein 1989). The family Piperaceae are tropical trees, shrubs, lianas, herbs, and sometimes epiphytic, with five genera and ca. 3000 species, with Piper having around 1000 species. Due to the lack of an apex, fragmented base, and poorly defined stomata, assignment of the fossil to any one species of Piperaceae is not advisable and is referred to the fossil genus Piperites. Piperaceae pollen has been found in the Pleistocene and Holocene of Costa Rica (Kesel 1974; Horn 1985, 1992; Hooghiemstra et al. 1992; Northrop and Horn 1996; Arford 2001; Clement and Horn 2001; Anchukaitis and Horn 2005; Kennedy and Horn 2008). Leaves of Piperites have been reported from the Miocene of Costa Rica (Berry 1921a), and the Tertiary of Venezuela (Berry 1936) and Indonesia (van Konijnenburg-van Cittert et al. 2004).
Order LAURALES
Jussieu ex Berchtold and Presl, 1820
Laurophyllum sp. Material: Puerto Viejo – UF18882–32687, 32688, 32689, 32690, 32692, 32693. Description: Leaves petiolate. Lamina incomplete, elliptic, unlobed, 6.0 cm long and 2–4.7 cm wide at the middle. Apex angle acute (Figure 1.10), with straight flanks, base angle acute, with cuneate flanks (Figure 1.8). Margin entire. The petiole marginal, 4.0 mm long by 2.0 mm wide. Primary venation pinnate, secondary venation eucamptodromous (Figure 1.9), secondary vein angle of divergence near the base 30–40° (Figure 1.8), middle 35–60°, and apex 30–90°. Basal veins are subopposite, remaining veins alternate; basal secondaries arch laterally and upward extending out to the margin, middle and upper secondaries arching slightly or straight from the midvein outward to near margin, then an abrupt upward curve to the superadjacent vein. Intersecondary veins 1–3 per intercosta, traversing more than Ż distance to the margin before forking into two branches, one joining the superadjacent secondary, and one joining the subadjacent secondary (Figure 1.9). Tertiary venation reticulate and tertiary vein angle obtuse. Marginal vein present. Adaxial surface glabrous; epidermal cells tetragonal to pentagonal with some hexagonal (average size ca. 8.7 x 15.5 Ám), surface smooth, cell walls straight, slightly curved to slightly sinuate with thin sections or knobby projections along anticlinal walls (Figure 1.11, arrow). Abaxial surface glabrous but with a few trichome bases; epidermal cells pentagonal to tetragonal (average size ca. 7.5 x 15.1 Ám), surface smooth, cell walls straight, slightly curved or slightly sinuate with thin sections or knobby projections along the anticlinal walls (Figure 1.12, arrow). Stomatal complex is random, brachyparacytic, and hemiparacytic (stomata average size ca. 9.1 x 13.6 Ám) with an inner rim scale on the guard cell, trapezoid in shape, and cutinized inner walls of the subsidiary cells adjacent to the guard cell scales. Stomatal pore is flat to round topped to slightly flared, opening slit-like, stomatal index ca. 13.5–16.3, and subsidiary cell size ca. 4.6–6.3 x 9.2–16.3 Ám. Comments: Extant species of Lauraceae have predominately simple, entire-margined leaves with acrodromous, brochidodromous, and camptodromous venation (Wolfe 1977; Christophel and Rowett 1996; Carpenter et al. 2007). Thus, the character of fossil leaf venation alone is not reliable for placement within Lauraceae. A cuticular character unique to Lauraceae is paracytic stomata with cuticular scales between the small embedded guard cells and overaching subsidiary cells (Bandulksa 1926; Dilcher 1963; Hill 1986). The presence of entire leaves with the above mention stomatal character provides a stable placement of the fossils with Lauraceae. The fossils provide numerous characters that are similar to extant species of Ocotea, Nectandra, and Beilschmiedia such as overall leaf shape, size, venation (Figure 1.8–1.10), epidermal shape, size, and stomatal type. The trapezoid-shaped inner rim scale of the guard cells (Figure 1.12) is characteristic of Ocotea (Dilcher 1963), Nectandra, and pan-tropical species Beilschmiedia (Nishida and Christophel 1999) but in Beilschmiedia the epidermal cells are highly sinuate or knobby (buttressed) (Christophel and Rowett 1996). Due to the difficulty of distinguishing between Ocotea and Nectandra based on leaf morphology, placement within either genus is not advisable. Here, we follow Hill's (1986) advice and place our specimens in the organ genus Laurophyllum based on characters of entire-margined leaves, cuticular scales mentioned above, and slit like stomatal openings on the outer abaxial surface. Cuticular characters of our specimens are similar to the dispersed fossil cuticle organ species Piliparicutis hradekensis except the stomatal size is twice the size in P. hradekensis (Schneider 2005). Venation and cuticular specimen UF18882-32689 is unique with trichome bases limited to the abaxial surface and the stomatal index is higher. Thus, there is the possibility of more than one species of Laurophyllum. Lauraceae are a subtropical to tropical family of woody plants, with over 50 genera and 2500–3000 species (Rohwer 1993). Ocotea, with over 300 species, are found in subtropical to tropical Central America to South America, Madagascar, Africa, and the Canary Islands. Nectandra, with about 120 species, are found in subtropical to tropical Central to South America (Rohwer 1993). Fossil leaves and reproductive structures of Lauraceae are known from mid- and Late Cretaceous localities in the Northern Hemisphere, and well-preserved leaves of Lauraceae are commonly found in Cenozoic floras of Europe, North America, and Australia (Hill 1986; Carpenter et al. 2007). Within the neotropics, fossil leaves of Laurophyllum are found in the Miocene of Argentina (Anzˇtegui and Ace˝olaza 2008), and Nectandra leaves in the Cenozoic of Central America, and South America (Engelhardt 1895; Berry 1921a, 1923, 1929, 1936; Anzˇtegui and Ace˝olaza 2008). The presence of Ocotea leaves in the Pleistocene of Costa Rica (Horn et al. 2003) is now in question due to leaf similarities of Ocotea and Nectandra.
Order MAGNOLIALES
Jussieu ex Berchtold and Presl, 1820
Oxandra sp.
Description: Dispersed carpels of a berry, ellipsoid, 2.4 cm long, 1.3 cm wide, and 0.8 cm high. Surface finely verrucose and glabrous, apex rounded and base narrows to 0.5 cm wide. Carpel wall is woody, 1–1.3 mm thick, black, and the pulp is spongy (Figure 2.1–2.3). Comments: Carpel characters (Figure 2.1–2.3) of the fossil are very similar to Annonaceae, and in particular Oxandra xylopioides Diels (Figure 2.4) (Diels 1927; van Setten and Koek-Noorman, 1992). Annonaceae are a pantropical family of trees, shrubs, rarely subshrubs, or climbers, with 128 genera and ca. 2300 species (Kessler 1993). Oxandra are trees to shrubs, with around 22 species, and are found in the West Indies and Panama to South Brazil (Kessler 1993). Fossil seeds of Annonaceae are known from the Maastrichtian of Nigeria, and then diversify in the Eocene London Clay flora (Doyle and Thomas 1997). Based on the phylogenetic analyses of Annonaceae using rbcL and trnL-F plastid DNA sequences, Oxandra may have originated within the Miocene (Richardson et al. 2004). Leaves of Annona have been reported in the Tertiary of Venezuela (Berry 1921b, 1936), Panama (Berry 1918), and in the Miocene of Mexico (Berry 1923) and Costa Rica (Berry 1921a). Leaves of Guatteria are published from the Tertiary of Panama (Berry 1918). Annonaceous pollen has been reported in the Pleistocene of Costa Rica (Kesel 1974). In this report, we correct the previous identification of Xylopia (Horn et al. 2003) to Oxandra.
Order MALPIGHIALES
Jussieu ex Berchtold and Presl, 1820
Parinari sp. 1 Material: Puerto Viejo – UF18882–32684, 32685, 32686, 32694, 32695, 32696, 32697, 33596, 33597, 53698. Description: Leaves petiolate. Lamina incomplete, elliptic to oblong, unlobed, symmetrical, 3.3–7.4 cm long by 1.2–3.5 cm wide, laminar ratio 2.1:1–2.8:1 (Figure 2.7–2.8). Apex missing, base angle acute to obtuse, with cuneate to rounded flanks; 1–2 basilaminar sessile glands present (Figure 2.7, 2.10). Margin entire. Petiole marginal, 6 mm long and 1–2 mm wide, bearing 1–3 sessile glands and simple single-celled trichomes (Figure 2.9). Primary venation pinnate; secondary venation eucamptodromous. More than 21 secondary veins per side, alternate to opposite, spacing regular, angle of divergence 50–60°, arching slightly from midvein outward to near margin, then arching abruptly towards apex, unbranched (Figure 2.7–2.8). Tertiary venation alternate percurrent, tertiary vein angle obtuse (Figure 2.8). Adaxial surface glabrous; epidermal cells mostly pentagonal, some tetragonal (average size ca. 6.6 x 9.6 Ám), surface smooth, cell walls straight to slightly curved (Figure 2.5). Abaxial surface with simple single celled trichomes around sunken stomata; epidermal cells mostly pentagonal with some tetragonal (average size ca. 5.1 x 6.7 Ám), surface smooth, cell walls straight to slightly curved (Figure 2.6). Stomatal complex with cells crowded against each other and stomata brachyparacytic (stomata average size ca. 9.5 x 11.6 Ám). Subsidiary cells form a circular shape with each guard cell distinctly arching outward into the center of its adjacent subsidiary cell (sometimes referred to as "butterfly-shape" Prance 1972); subsidiary cell size ca. 6.7 x 10.7 Ám (Figure 2.6). Comments: Morphological characters of the fossil such as leaf entire, elliptic to oblong shape, basilaminar and petiolar glands, simple trichomes, and pinnate venation are similar to Euphorbiaceae (Tetrorchiduim), Malpighiaceae (Bunchosia, Byrsonima [elliptic form], Heteropterys, Lophopterys, and Mascagnia), and Chrysobalanaceae (Parinari and Licania). In Tetrorchiduim (Burger and Huft 1995), the placement of the laminar glands, and low number of secondary vein pairs do not match the fossil leaf. In Bunchosia, Byrsonima [elliptic form], Heteropteris, Lophopterys, and Mascagnia (Anderson 1982, 1995, 1999, 2001; Amorim 2001; Anderson and Davis 2001), the low number of secondary vein pairs does not match the fossil. The venation and anatomy of the fossil leaves do suggest a close affiliation with Parinari or Licania (Prance 1972). The basilaminar and petiolar glands are characteristic for both Parinari (Figure 2.13) and Licania, along with single celled trichomes on the petiole. Both genera have characteristic sunken stomatal regions (Prance 1972). Whole leaf sizes tend to be smaller than Licania but in the range of Parinari (Figure 2.7–2.8, 2.13). The secondary vein angle of divergence resembles Licania, but the number and spacing of secondary veins per side resemble Parinari (Figure 2.7–2.8). Adaxial and abaxial epidermal characteristics are similar between the fossil, Parinari and Licania but abaxial cell surfaces in Licania have some striations while Parinari tend to be smooth. Abaxial trichomes are similar to Parinari. The stomata for both Parinari and Licania are brachyparacytic and the lateral subsidiary cells extend out in a butterfly wing shape, but stomatal and subsidiary size tends to be larger than observed for Licania and is in the size range observed for Parinari. When all the characters are considered, the fossils are best placed in the genus Parinari. Chrysobalanaceae are a lowland pantropical family of trees and shrubs, with 17 genera and 494 species (Prance and White 1988). Parinari is also pantropical with 49 species (Prance and White 1988). The fossil history of Chrysobalanaceae is limited, with pollen found in the Oligocene (Muller 1981) and phytoliths in the Pleistocene of Panama (Piperno and Jones 2003).
Parinari sp. 2 Material: Puerto Viejo – UF18882–33066, 33067. Description: An elliptic drupe, 3.7–5.3 cm long by 1.3–2.0 cm wide; tapered apically, base rounded; bilocular, each single seeded (Figure 2.11–2.12). Exocarp smooth, 1 mm thick; mesocarp spongy/fibrous, 1 mm thick; endocarp thick and bony, 3–5 mm thick. Locules 9 mm long by 6 mm wide, smooth interior. Fibrous strands near attenuate end. Comments: Characters of the fossil (Figure 2.11–2.12) fit well with Parinari (Prance 1972; van Roosmalen 1985; Roth 1987) except extant Parinari has densely pubescent locules while the locules of the fossil lacks pubescence. The subgenus Licania, section Pulverulenta, has similar endocarp characters but the exocarp is wrinkled to ridged, and is densely pubescent in the locules. Parinari fruits have been reported in the Miocene of Ethiopia (Tiffney et al. 1994), Pliocene of Colombia (Wijninga and Kuhry, 1990; Wijninga, 1996) and the Holocene of Zambia (Phillipson 1976).
Family HUMIRIACEAE
Jussieu ex St. Hilaire et al., 1829
Sacoglottis tertiaria Berry Material: Puerto Viejo – UF18882–33581, 33582, 33583, 33584, 33600, 33601.
Comments: Characters of the fossil such as shape, size, woody endocarp with irregular warts and collapsed lacunae, and carpel arrangement are similar to Humiriaceae and in particular Sacoglottis and Schistostemon (Cuatrecasas 1961; Burger and Zamora 1991). A recent review of Humiriaceae has determined that Schistostemon should be a subgenus of Sacoglottis (Herrera et al. 2010). Humiriaceae are trees and shrubs with eight genera and 49 species, found in the tropics of Central America, South America, and Western Africa. Sacoglottis, with eight species, are found in Central America, South America, and Western Africa while Schistostemon, with seven species, are found in South America (Cuatrecasas 1961). Fossil fruits of Sacoglottis have been reported from the Oligocene and Pliocene of Colombia (Reid 1933; Cuatrecasas 1961; Wijninga and Kuhry 1990; Wijninga 1996), and the Pliocene of Bolivia (Berry 1922). Our specimens are very similar to Sacoglottis tertiaria Berry (1922) of Bolivia.
Family MALPIGHIACEAE
Jussieu, 1789
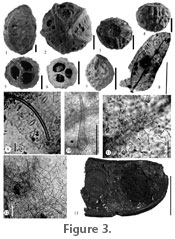
Byrsonima sp. Material: Puerto Viejo – UF18882–33602, 33603, 33604, 33605, 33606, 33607. Description: Fruit with endocarp preserved, non-dehiscent. Shape is globose, apex rounded, and base with a shallow indentation (Figure 3.3). Average length 7.6 mm, average equatorial diameter 7.0 mm. Endocarp woody with rugose surface (Figure 3.4), trilocular with a single seed per locule, and vascular bundle down center of the fruit. Locules are dorsally depressed ovate and symmetrically arranged about the long axis of the fruit (Figure 3.5). Comments: Fossil fruit characters of shape, three woody carpels, and each carpel with a single locule are similar to the families Euphorbiaceae, Violaceae, and Malpighiaceae. Violaceae fruits are predominately capsular, dehiscent into three valves (van Roosmalen 1985), while in the fossil specimen there is no trace of longitudinal grooves indicative of capsules. In Euphorbiaceae, drupaceous fruits that are non-dehiscent, and trilocular with a single seed per locule is found in Drypetes, although the endocarp is hard but brittle, not evident in the fossil specimen that includes a thick endocarp (Berry et al. 1999). Within Malpighiaceae, morphological characters of an endocarp with a rugose surface, a shallow basal indentation, and locule shape and arrangement place the fossil within Byrsonima (Figure 3.6–3.7) (Robertson 1972, figure 1h, i; Anderson 1982, 1999, 2001; van Roosmalen 1985). Malpighiaceae are pantropical to subtropical trees, shrubs, subshrubs, or lianas, with 66 genera and 1200 species (Anderson 1990). Byrsonima are trees, shrubs, or subshrubs with 135 species, found in S.E. Florida, the Caribbean, and from Mexico to South America. Fossil pollen of Malpighiaceae are found in the Miocene of Costa Rica (Graham 1987a, 1987b) and leaves in the Tertiary of Panama (Berry 1918). Fossil pollen of Byrsonima occurs in the Quaternary of Costa Rica (Kesel 1974; Horn 1985; Graham and Dilcher 1995).
Order FABALES Bromhead, 1838
Morphotype PV1-28 Material: Puerto Viejo – UF18882–33578. Description: Leaflet complete, oblong, asymmetrical, unlobed, 7–8 mm long by 2.5–3.0 mm wide, lamina ratio 2.6:1–2.8:1 (Figure 3.8). Apex angle acute and mucronate, with convex flanks, base angle obtuse, with concavo-convex flanks. Margin entire. Petiole missing. Primary venation pinnate; secondary venation brochidodromous, basal secondary veins present and arching upward to midsection of leaflet. Secondary vein spacing increasing toward base. Adaxial surface with short unicellular trichomes (average size ca. 2 x 25 Ám) with an acute apex (usually broken off) and thickened base; epidermal cells mostly tetragonal, some pentagonal (average size ca. 7.4 x 13.6 Ám), surface smooth, cell walls straight to slightly curved (Figure 3.11). Abaxial surface with short trichomes (average size ca. 3 x 40 Ám) with an acute apex (usually broken off) and slightly thickened base (Figure 3.9); epidermal cells mostly tetragonal, some pentagonal (average size ca. 8 x 19.2 Ám), surface smooth, cell walls straight to arcuate (Figure 3.9). Marginal trichomes present, unicellular, and conical (average size ca. 15 x 90 Ám) (Figure 3.10). Stomatal complex random and hypostomatic, paracytic to anisocytic (stomata average size ca. 7.5 x 17 Ám) with thin inner rim, stomatal index 9.7; in paracytic stomata, subsidiary cells equal to unequal in size, (average size ca. 4.7 x 26.1 Ám) (Figure 3.12). Comments: Overall shape and size of the fossil leaflet is similar to Zygophyllaceae except in Zygophyllaceae there are more basal secondary veins, and the leaflets are amphistomatic. The leaflet architecture, prominent basal secondary veins, and trichomes of the fossil (Figure 3.8–3.9) are similar to the tribes Mimoseae (Acacia) and Ingeae (Albizia, Pithecellobium, Calliandra, and Zapoteca) (Woodson and Schery 1950; Leelavathi and Ramayya 1982; Ghosh and Roy 1986; Grosso et al. 1994; Ogundipe and Akinrinlade 1998). Acacia and Pithecellobium tend to be amphistomatic while Calliandra and Zapoteca are hypostomatic. Albizia shows both amphistomatic and hypostomatic condition, although when amphistomatic, the adaxial stomata are very sparse. Acacia, Albizia, Pithecellobium, and Calliandra tend to have both paracytic and anisocytic stomata with equal to unequal subsidiary cells and unicellular hairs. Calliandra and Zapoteca epidermal cell walls are sinuate. Although no one genus contains all the characters noted in the fossil, Ingeae seems closer in character traits to the fossil. More than one specimen is needed for character variability, allowing for a more precise placement within a recognized taxa, so a temporary Morphotype category (Johnson 1989) will be used. The tribe Ingeae are trees, shrubs, or rarely climbers, found in the pantropical to subtropical regions, with 17 genera and ~1000 species (Nielsen 1981; Herendeen et al. 1992). Ingeae pollen is known from the Eocene of Egypt (Herendeen et al. 1992), and possible leaflets from the Eocene of Kentucky (Herendeen and Dilcher 1987) and the Tertiary of Costa Rica (Berry 1921a, Graham 1992). Genus LEGUMINOCARPON Goeppert, 1855
Leguminocarpon sp. 1
Description: Pod fragmented, > 5.5 cm long by 1–1.9 cm wide, linear to slightly curved, straight; rounded to short tapered apex and oblique with longitudinal axis of fruit; curved tapered base aligned with longitudinal axis of the fruit (Figure 3.13, Figure 4.1–4.2). Marginal sutures 1 mm thick, medial along entire length of fruit, possibly dehiscence along one suture. Fruit non-winged, and surface with small papilla. Seeds 9–10 mm long by 5–6 mm wide, ovate, perpendicular to fruit length; funiculus 3 mm long by 0.5 mm wide. Comments: The fragmented nature of the pods makes it difficult to place this fossil within a subfamily status, although the papillose nature of the fruit wall is not usually found in the subfamily Caesalpinioideae (Cowan 1981). Although a fruit that is dehiscent along one suture can be found in all three subfamilies, the general shape of the pod, base, and apex of the fossil fruits (Figure 3.13, Figure 4.1–4.2) are close to Albizia and Havardia (Mimosodieae: Nielsen 1981; Kirkbridge et al. 1999). The fossil fruits share characters with Albizia such as apex oblique to longitudinal axis and margin with thickened sutural area, but again the fragmentary nature of the fossils preclude placement within Albizia. Mimosites has been used for fossil legume fruits and leaves but this name should be restricted to the tribe Mimoseae (Herendeen and Dilcher 1990). Calliandra pollen has been found in the Quaternary of Panama (Barlett and Barghoorn 1973) and the Pliocene of Colombia (Caccavari 1996) while Calliandra and Acacia pollen are found in the Pleistocene of Costa Rica (Kesel 1974).
Order ERICALES
Berchtold and Presl, 1820
Morphotype PV1-12 Material: Puerto Viejo – UF18882–32803, 32804, 33598, 33599, 45285, 53697. Description: Lamina incomplete, elliptic, unlobed. Apex short attenuate, rounded end (Figure 4.3) and micro aristate, base fragmented but tending convex. Margin entire. Primary venation pinnate, secondary venation festooned brochidodromous with two-three series of exmedial loops, last series close against the margin (Figure 4.4). Secondary veins alternate, spacing irregular, angle of divergence 50–60║. Secondaries gently curving upward and out toward the margin. Secondary attachment decurrent. Intersecondary veins 1–3 per intercosta, traversing more or less one half the distance to the margin then forking into two branches and joining adjacent intersecondaries or secondaries. Tertiary and quaternary veins irregular reticulate, marginal vein lacking, midrib flat. Adaxial surface glabrous; epidermal cells mostly tetragonal (average size ca. 12.9 x 17.6 Ám), surface smooth, cell walls sinulate to sinuate (Figure 4.5). Abaxial surface glabrous; epidermal cells mostly tetragonal (average size ca. 12.1 x 17.0 Ám), surface smooth, cell walls sinuous to sinuate (Figure 4.6). Stomata paracytic to anomocytic (average size ca. 8.5 x 11.5 Ám), stomatal index 10.8. Comments: The form and venation of the fossil leaves are very similar to Pouteria (Sapotaceae) (Figure 4.9) and Macrolobium (Leguminosae). The adaxial and abaxial sinuate cell walls of the fossil are very similar to Pouteria while in Macrolobium, abaxial cell walls are straight to slightly undulating (UF5127, 5131, 5212; Watson and Dallwitz 1983, 1993). Although characters such as attenuate apex, festooned brochidodromous venation, lack of a marginal vein, and a flat midrib (Pennington 1990) place the fossil leaf close to Pouteria, the fragmentary nature of the leaves prevent a precise affiliation to any one species, so we place the fossil into the morphotype designation. Sapotaceae are pantropical trees, shrubs, or lianas, with 53 genera and 1100 species. Pouteria are pantropical with 325 species and are confined to lowland rainforest below 1000 m altitude (Pennington 2004). Fossil pollen of Sapotaceae are found worldwide through the Cenozoic (Harley 1990) and more specifically of Pouteria in the Pliocene and Pleistocene of Costa Rica (Kesel 1974; Graham and Dilcher 1998). Fossil Sapotaceous leaves are found in the Eocene/Miocene of Argentina (Berry 1938).
Morphotype PV1-27 Material: Puerto Viejo – UF18882–32825. Description: Seed ellipsoid, 3.3 cm long by 1.4 cm wide, hilum adaxial covering full length of seed, scar 2 mm wide at base, 8.0 mm wide at apex (Figure 4.7). Comments: Fossil seed characters of shape, and hilum position and extent fits well in Sapotaceae, and in particular Pouteria (Figure 4.8). INCERTAE SEDIS
Morphotype PV1-16 Material: Puerto Viejo – UF18882–53700. Description: Lamina incomplete, elliptic, unlobed, > 2 cm long by 1.7 cm wide. Apex missing, base slightly cordate with rounded flanks (Figure 4.10). Margin entire. Primary venation pinnate, secondary venation festooned brochidodromous with two series of unorganized exmedial loops. Secondary veins alternate, spacing irregular, angle of divergence 52–86║. Ascendence of secondaries unequal, one side with two secondary loops reaching middle of lamina, other side with three secondary loops reaching middle of lamina. Intersecondaries 1–2 per intercosta, traversing less than or more than one half the distance to margin then joining tertiary veins. Tertiary and quaternary veins irregular reticulate, marginal vein present. |
|