|
|
|
SYSTEMATIC PALAEONTOLOGY
Phylum MOLLUSCA Cuvier, 1797 Diagnosis. Laterally compressed univalves with slight to extensive curvature of the ventral aperture margin. Remarks. Includes Stenotheca Salter in Hicks, 1872, Mellopegma Runnegar and Jell, 1980, Eurekapegma MacKinnon, 1985, and Acanthotheca gen. nov. Parkhaev (in Gravestock et al. 2001) placed Watsonella within the Family Stenothecidae. However, the split shell of Watsonella (Dzik 1994) excludes it from the stenothecids, which are univalved, although Watsonella is surely closely related to stenothecids. Anabarella Vostokova, 1962 has been considered a member of the Stenothecidae (Runnegar and Jell 1980; Parkhaev in Gravestock et al. 2001), but Anabarella plana, the type species of Anabarella, is more similar to Watsonella in overall form, shell microstructure, and age (Kouchinsky 1999) than it is to other stenothecids. Therefore, we tentatively exclude Anabarella from the Stenothecidae. To complicate matters, the form named Anabarella australis Runnegar in Bengtson et al., 1990 is clearly a stenothecid, bearing striking similarities in form to Stenotheca and Mellopegma. Further taxonomic revision is needed. The type species of Anabarella, A. plana Vostokova, 1962, has a greater degree of coiling than other stenothecids (Gubanov and Peel 2003), and we view this trait as the distinguishing characteristic of Anabarella. Mellopegma differs from other stenothecids in being the most elongate form (typical l/w = 4) without a zygion; Eurekapegma is similar to Mellopegma but possesses a zygion; Stenotheca is the only stenothecid that is both tall and narrow; and Acanthotheca is the widest and has the greatest ratio of height to length. The life orientation of the shell in many Cambrian 'monoplacophorans' has been debated, with Peel (1991a, 1991b) having suggested that a large group of these fossils (placed in his Class Helcionelloida) had a shell coiled toward the posterior (endogastric), different from modern monoplacophorans where the shell coils toward the anterior (exogastric). Runnegar and Jell (1980) had previously defined the Family Stenothecidae as exogastric. Runnegar (1996) questioned the basis of Peel's interpretation, in particular inferring an evolutionary link between stenothecids and bivalves that would indicate the long dorsal margin of stenothecids like Mellopegma is homologous to the bivalve ligament. If true, stenothecids would have been exogastric. However, without distinct soft part data for most of these forms it is difficult to know with high certainty which are endogastric and which are exogastric.
Stenothecids may include the ancestors of bivalves and rostroconchs. Genus MELLOPEGMA Runnegar and Jell, 1976 Type species. Mellopegma georginense Runnegar and Jell, 1976. Other species. Mellopegma indecorum (Missarzhevsky in Rozanov et al., 1969), Mellopegma schizocheras sp. nov., Mellopegma simesi (MacKinnon, 1985), and Mellopegma uslonicum (Parkhaev, 2004). Diagnosis. Stenothecid molluscs with elongate shells (typical l/w >4) that lack a zygion and possess a strongly curved ventral margin. Description. Laterally compressed, elongate univalves with strongly curved ventral (aperture) margin. Slight coiling (less than half a whorl; cladistic character 4, state 0; Appendix 1), with gently convex supra-apical dorsal margin and concave sub-apical margin that ends in a flat to downward-sloping shelf. Shell microstructure of outer layer prismatic and inner layer calcitic semi-nacre (character 17, state 1). Tubercles (granules) occur sporadically on surface of internal moulds, revealing shell pores that extend through most or all of the thickness of the shell (character 14, state 1). Inner sculpture of gentle to prominent comarginal ridges (character 6, state 1). Remarks. The genus name Mellopegma is neuter gender because the ending word, pegma, Greek for fastened or thick, is neuter. The gender of all adjective-based species names should be modified to be in agreement with the gender of the genus name (International Code of Zoological Nomenclature, ICZN, 34.2). Therefore, we herein carry out justified emendations mandated by the ICZN in modifying the following names of species assigned to Mellopegma: M. georginensis Runnegar and Jell, 1976 becomes M. georginense; M. indecora (Missarzhevsky, 1989) becomes M. indecorum; and M. uslonica Parkhaev, 2004 becomes M. uslonicum. The type species of Mellopegma, M. georginense Runnegar and Jell, 1976, was defined from numerous internal moulds of laterally-compressed shells from the middle Cambrian Gowers Formation of the Georgina Basin, Australia. Zhou and Xiao (1984) described Mellopegma nana from the early Cambrian of northern China and South Australia, although Parkhaev (in Gravestock et al. 2001) reassigned this species to the genus Figurina. Zhou and Xiao (1984) also named Mellopegma rostratum, which was reassigned to Mackinnonia by Parkhaev (in Gravestock et al. 2001), an assignment supported by Skovsted (2004), Wotte (2006), and Wagner (2008). The photographs in Zhou and Xiao (1984, figures 3.7-11) support both reassignments. MacKinnon (1985) described Eurekapegma, a form that he argued was very similar to Mellopegma except that the former had an internal plate between the lateral walls that he termed a zygion. Based on the great similarity between these two genera, and the fact that Mellopegma is slightly older than Eurekapegma, MacKinnon (1985) suggested that the former may have given rise to the latter. Whether the presence of the zygion warrants classification in a separate genus is subjective, but it is clear that Eurekapegma cooperi MacKinnon, 1985 is closely related to the species of Mellopegma and may have been a descendent of M. schizocheras sp. nov. Mellopegma differs from Anabarella Vostokova, 1962 in that it is less coiled, has a pronounced sub-apical shelf, and has greater curvature of the aperture margin. Mellopegma differs from Stenotheca Hicks, 1872 in that it has a greater ratio of length to height, and typically has a more pronounced sub-apical shelf. Mellopegma differs from Watsonella in having an undivided shell with a lower ratio of height to length. A summary of the taxonomic history of Mellopegma is provided in Table 1. Range. Early to middle Cambrian. Occurrence. Australia, New Zealand, Russia, and Canada.
Mellopegma georginense
Runnegar and Jell, 1976
Diagnosis. Laterally compressed, elongate shell with prominent comarginal ridges that continue around the anterior and posterior margins. Supra-apical dorsal margin gently to strongly convex. Sub-apical margin strongly concave, terminating in a flat to downward sloped shelf. Internal moulds covered with regularly spaced pores and ropy comarginal ridges.
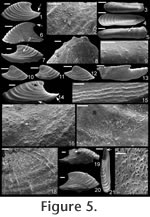
Description. Shell elongate, laterally compressed, 2-3 times longer than tall (character 10, state 1); 5-7 times longer than wide. Aperture narrow at mid-point (character 20, state 1); widened at either end; upturned at both sub-apical and supra-apical ends. Apex located close to most distal point of sub-apical margin (~20% of shell length); short part of dorsum sharply concave; long part of dorsum mildly convex. Inner shell texture of coarse comarginal rugae; often with fine, sinuous striations. Periostracum with curved ridges that extend from the apex to the aperture.
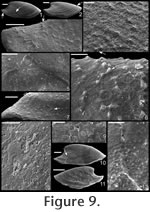
Remarks. The specimens of Mellopegma georginense shown herein are from a locality in the Georgina Basin about 300 km from the type locality (Gowers Formation in both cases). This species is dominant in many of the beds of the Gowers Formation. The granules shown in Runnegar and Jell (1976, figure 8.b.6) are regularly spaced, but are most likely a line of pore fillings that commonly parallels growth lines in internal moulds of Mellopegma georginense and Mellopegma schizocheras sp. nov.
See remarks in descriptions of other species for distinction from M. georginense. Range. Early to middle Cambrian. Occurrence. Middle Cambrian Gowers Formation, Georgina Basin, Australia and early Cambrian "Anse Maranda Formation," Québec, Canada.
Mellopegma schizocheras sp. nov.
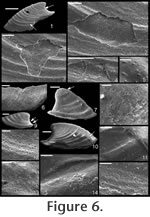
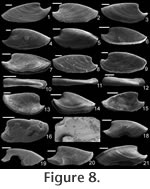
Etymology. From the Greek schizo, to split or cleave, and cheras, silt. The small, blade-like shape of this species would have allowed it to easily navigate (or slice) through interstitial sediment. Material. Holotype (Figure 8.1; CPC 40456), three paratypes (Figure 8.8,10, 15; CPC 40464, 40465, 40470), and numerous other specimens from near the type locality. Type locality. Just above the Bronco Stromatolith Bed of the Gowers Formation, c. 200 m East-Northeast along strike from section 415 (see Shergold and Southgate 1986, Southgate 1986, and Vendrasco et al. 2010).
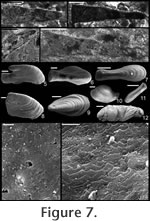
Description. Elongate, laterally compressed univalve with faint internal ridges on the internal mould. The internal ridges roughly parallel the growth lines and fade out near the anterior and posterior margins. These ridges vary in this species in depth, spacing, and number. Dorsal margin broadly convex, sub-apical margin concave with extended shelf. Tubercles present over much of surface of some internal moulds, but typically most common along the dorsal margin, including the apex, and along the internal ridges. Polygonal texture on sub-apical lip of many specimens, probably representing prismatic shell microstructure of outer shell layer. Angular texture with imprints of parallelogram-shaped tablets occur on all other regions of internal moulds, representing highly organized version of calcitic semi-nacre. The angular imprints are visible in various states of preservation on all specimens examined. Many specimens had an internal thickening parallel to, and just below, dorsum (Figure 8.5, 8.7, 8.21). Remarks. This species differs from Mellopegma georginense, with which it co-occurs, in having a more dorso-ventrally compressed, elongate shell with less distinct internal rugae. These rugae are present along the dorsal ridge of the internal moulds of M. georginense, whereas they thin out at the dorsal ridge of Mellopegma schizocheras sp. nov. Specimens of M. schizocheras tend to be more elongate than those of M. georginense (compare Figure 5.3-5 with Figure 8.10-12), although some specimens of M. georginense are also elongate (Figure 5.21). The polygonal texture differs dramatically between these two species: in M. georginense the polygons are thick walled, with a small diameter, and occur over much of the surface of the internal mould (Figure 5.2, 5.16, 5.22, Figure 6.12, 6.15); in M. schizocheras sp. nov. the polygons are thin walled, with a larger diameter, and occur near the aperture margin at and near the anterior or posterior edge of the internal mould (Figure 9.3). The size overlap of these two species, as well as the form of juveniles deduced from small specimens (Figure 5.10-12, Figure 6.7, 6.9, Figure 8.13-15) and growth lines in adults reveal that these two morphotypes are not different ontogenetic stages of the same animal. Moreover, this does not appear to be a case of sexual dimorphism, as juveniles of both morphs look different. See remarks in descriptions of following Mellopegma species for distinction from M. schizocheras. Range. Middle Cambrian (Floran). Occurrence. Only from Gowers Formation in the Thorntonia region of the Georgina Basin, Australia.
Mellopegma simesi (MacKinnon, 1985) comb. nov.
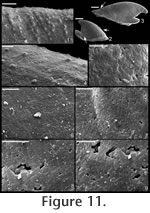
. Description. Laterally compressed, elongate shell with less than half a coil. Margin below apex strongly concave; dorsal margin weakly to strongly convex. Faint internal ridges on some specimens. Raised sub-apical margin, forming a shelf ranging from nearly horizontal to angling downward at 45º. Supra-apical margin tends to be wider than sub-apical margin (Figure 10.17, 10.19-20; cladistic character 21, state 1; Appendix 1). Granules apparent in some internal moulds, paralleling growth lines (Figure 10.17, Figure 11.1). Prominent protoconch (Figure 10.12, 10.17) bulbous. Pegma or pegma-like structure developed to varying degrees (Figure 10, Figure 11.2-3; character 1, state 1).
Remarks. This species was originally considered to be a member of Anabarella, but it shares more distinguishing characters with the type species of Mellopegma (M. georginense
Runnegar and Jell, 1976) than the type species of Anabarella (A. plana
Vostokova, 1962).
Mellopegma simesi can be distinguished from all other species of Mellopegma in having a more pronounced pegma or pegma-like structure. It also differs from M. georginense in having much less prominent internal ridges; from M. indecorum and M. schizocheras sp. nov. in having a less smoothly rounded sub-apical margin; and from M. uslonicum in having a more inset sub-apical region. There is some uncertainty in assigning this species to Mellopegma, primarily due to the presence of two characters of this species not seen in any other species of Mellopegma: elongate apical neck (i.e., sharply in-curved sub-apical margin) and abrupt transition between sub-apical surface and shelf. These two characters are seen in specimens of Anabarella, but this species differs from Anabarella in the ways listed above. Stenotheca drepanoida shares with M. simesi a pegma-like structure just above the sub-apical shelf (Parkhaev in Gravestock et al. 2001, pl. xliii, figure 1) and an elongate sub-apical neck. However, M. simesi differs from Stenotheca drepanoida—and in fact other species of Stenotheca—in having: (1) a more elongate shell; (2) less lateral compression; and (3) greater curvature of the ventral margin. While M. simesi has striking similarities with species of Stenotheca, Anabarella, and Mellopegma, current data indicate it shares the greatest similarity with Mellopegma, and consequently we classify it as such; this classification is supported by our cladistic analysis (Figure 4). Some of the character states of M. simesi not seen in M. georginense—including an incurved sub-apical slope and lack of prominent internal ridges—are seen in M. schizocheras. Range. Middle Cambrian. Occurrence. Gowers Formation in the Thorntonia region of the Georgina Basin, Australia and Tasman Formation, New Zealand.
Mellopegma uslonicum
Parkhaev, 2004
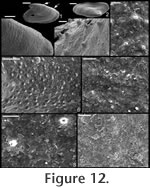
Description. Elongate, laterally compressed shell with gently convex dorsal margin and gently concave sub-apical margin with slight lateral flaring of aperture. Internal mould typically covered with tubercles or granules (Figure 13.1, 13.3, 13.4), revealing nearly isotropically spaced cavities or pores along the inner shell surface. External ornament of ridges. Remarks. Parkhaev (2004) named Mellopegma uslonica (now M. uslonicum) for specimens from the early Cambrian (Botoman) of Transbaikalia. These early Cambrian forms differ from the type species in being slightly less compressed laterally and typically less elongate, but otherwise are similar in form to M. georginense.
M. uslonicum differs from all other known species of Mellopegma in having equally spaced pores over the entire inner surface of the shell, and in having a sub-apical margin that is less recessed. Range. Early Cambrian (Botoman). Occurrence. Emyaksin Formation from the eastern flanks of the Anabar Uplift of the Siberian Platform and the Bystraya Formation of the Chita Region, Russia.
Mellopegma indecorum (Missarzhevsky in
Rozanov et al., 1969)
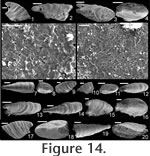
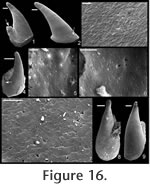
Description. Shell elongate, laterally compressed in sub-apical region of aperture, laterally expanded to a great degree along rest of aperture. Internal ornament of comarginal rugae. Dorsal margin smoothly convex; sub-apical margin sharply concave (forming an emargination), ending in a prominent sub-apical shelf. Remarks. Missarzhevsky (1989) assigned the name Mellopegma indecora (now M. indecorum) to specimens from the early Cambrian (Tommotian) of Russia. He had previously assigned this species to the genus Anabarella (Missarzhevsky in Rozanov et al. 1969). Parkhaev (2004) recognized M. indecorum as a valid species of Mellopegma. Although Wagner (2008) classified M. indecorum as a species of Anabarella, he more recently stated his agreement with Missarzhevsky's and Parkhaev's decision (Wagner, personal commun., 2010). The specimens of Mellopegma indecorum photographed by Missarzhevsky (1989, pl. 6, figs. 10-11) show significant lateral compression and strongly pronounced internal ridges. Although his photographs lack detail, new photos of the specimen in his figure 11 (Figure 14.1-6 here) suggest these are in fact members of Mellopegma. In addition, the specimens shown in Missarzhevksy in Rozanov et al. (1969, pl. 4, figs. 7-8) are laterally compressed and show the internal ridges, curved ventral margin, and upturned sub-apical shelf (character 5, state 1) typical of Mellopegma. These features are clearly seen on the holotype of this species (Missarzhevksy in Rozanov et al. 1969, pl. 4, fig. 8), and the prominent sub-apical shelf and lesser coiling it shows are distinct from what characterizes Anabarella. We therefore consider M. indecorum to be a valid species of Mellopegma. This species appears to belong to Mellopegma, as it shares with other species of this genus the same lateral profile, lateral compression in the sub-apical half of the shell, internal ornament of comarginal rugae, and laminar inner shell microstructure. However, this species differs from all other known species of Mellopegma in having a much more expanded supra-apical region of the shell (Figure 14.3-4, 14.20). This lateral expansion is similar to what occurs in a few equivocal forms of Mellopegma from the middle Cambrian (Figure 7.7, 7.9), although these two unusual specimens differ from M. indecorum in having a more pronounced sub-apical shelf in one case (Figure 7.7), and in having a greater amount of expansion and more convex sub-apical margin in the other (Figure 7.9). The occurrence of M. indecorum reveals that Mellopegma existed during the Tommotian, and that it co-occurred with Watsonella. M. indecorum is intermediate in extent of lateral compression between non-stenothecid helcionellids and typical stenothecids. The holotype of M. indecorum is listed from sample 183e on p. 144 of Rozanov et al. (1969), but in the caption to pl. IV, fig. 8, on p. 303 the same specimen is attributed to sample M302/1-2 (Rozanov et al. 1969), which is the same age but from the middle Lena River. The holotype and another specimen illustrated by Rozanov et al. (1969, pl. IV, figs. 7-8) appear to be missing from Missarzhevsky's collection at present, but sample 183e yielded two incomplete moulds of M. indecorum figured herein (Figure 14.7-12) along with other fossils of Tommotian Age (AVK, personal observation). We conclude that the holotype of M. indecorum should be attributed to sample 183e, and that the reference to sample M302/1-2 is a mistake. However, according to Rozanov et al. (1969, p. 145), M. indecorum is indeed present in M302/1-2 and also in sample M303/2, the latter collected by Missarzhevsky from a nearby locality of the same age. Range. Early Cambrian (Tommotian). Occurrence. Kyndyn and Pestrotsvet Formations, Siberian Platform.
Mellopegma sp. Remarks. An unusual specimen of Mellopegma from the middle Cambrian Gowers Formation of Australia looks to be intermediate in lateral view between M. schizocheras and M. simesi, but has a lateral expansion at the supra-apical end of the aperture that is seen in neither. However, a number of characters suggest this is a member of Mellopegma, including: lateral compression at sub-apical end, prominent sub-apical shelf, sharply concave sub-apical margin (forming an embayment), gently convex dorsal ridge, and internal ridges.
Mellopegma? Remarks. One unusual specimen from the middle Cambrian Gowers Formation of Australia has internal ridges and bulbous apex similar to Mellopegma spp., and remarkably similar shell microstructure (Figure 7.13) to other members of this species. However, it differs from other species of Mellopegma in having a convex, nearly vertical sub-apical margin. Moreover, this specimen shows a high degree of lateral expansion at the aperture, unusual for most species of Mellopegma but seen to a lesser extent in Mellopegma indecorum (Figure 14.4, 14.16, 14.18). Genus STENOTHECA Salter in Hicks, 1872 Type species. Stenotheca cornucopia Salter in Hicks, 1872. Other species. Stenotheca acutacosta Walcott, 1890, Stenotheca clotho Walcott, 1912, Stenotheca drepanoida (He and Pei in He et al., 1984), Stenotheca manchurica Kobayashi, 1933, Stenotheca pojetai Runnegar and Jell, 1976, Stenotheca tepee Runnegar and Jell, 1976, and Stenotheca transbaikalica Parkhaev, 2004. Description. Highly laterally compressed, tall univalve with a sub-triangular lateral profile, slight coiling (about 1/8 to just over 1/4 one whorl), concave sub-apical margin (embayment) that borders an angular to flat sub-apical shelf. Remarks. The type species is poorly known. One specimen was drawn by Salter (in Hicks, 1872), and then three specimens were drawn—with more depicted detail—by Cobbold (1934). Cobbold (1934) considered one specimen (possibly the one drawn by Salter, in Hicks 1872) to be the holotype. He also drew two additional specimens, revealing some variation in the sub-apical slope from slightly convex to moderately concave. Both Salter (in Hicks 1872) and Cobbold (1934) depict strong growth lines in these specimens, but Cobbold's drawing of the apparent holotype was more elongate than Salter's and had a curvature of the apical tip not seen in Salter's. However, because the type specimens appear to have been lost, Runnegar (in Bengtson et al. 1990) designated the clearly similar species Stenotheca acutacosta Walcott, 1890 as the secondary standard for the genus. This species has the same degree of lateral compression, slight coiling, and prominent internal ridges as depicted in the drawings of S. cornucopia. Stenotheca? from Landing and Bartowski (1996) has the lateral compression typical of stenothecids, but is fragmentary and lacks distinguishing features. Range. Early Cambrian (Botoman) to middle Cambrian. Occurrence. United Kingdom (Wales), Canada (Newfoundland), China (Henan), and Australia (South Australia).
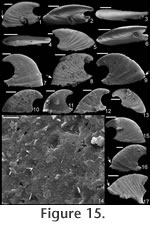
Stenotheca drepanoida (He and Pei in
He et al., 1984)
Material. Twelve new specimens from the early Cambrian of Australia, UNEL localities L1852, 1874, and 1876 (Bengtson et al. 1990). One specimen (Figure 15.17; SMNH Mo167632) from the early Cambrian of Siberia (sample 96-7/70, from the lower Botoman Stage of Siberia—Kouchinsky 2000a). Diagnosis. Shell elongate, highly laterally compressed. Sharply concave sub-apical margin that terminates in a short sub-apical shelf. Internal and external ornament of comarginal rugae, less prominent on inner surface of shell. Typically a constriction on internal mould just above supra-apical margin. Apex curved a little more than 1/4 of a complete whorl, although some specimens show less coiling. High degree of variation in lateral profile. Description. Shell elongate (c. 1.75 times longer than tall), laterally compressed (c. 5 times longer than wide); coiled less than half a whorl; dorsal margin strongly convex; margin under apex strongly concave; raised, narrow sub-apical shelf, curving slightly dorsally; tapered towards the sub-apical margin (Figure 15.3-4). Faint internal ridges become less prominent at anterior and posterior margins. Protoconch smooth, somewhat bulbous (Figure 15.2-3). Remarks. In the original description of Stenotheca drepanoida (He and Pei in He et al., 1984) eight photographs were provided of multiple specimens, although a holotype was not designated. These specimens reveal significant variation in lateral profile in terms of degree of coiling, nearly grading in lateral profile into some specimens of Anabarella australis Runnegar (in Bengtson et al. 1990, fig. 163d-e). Only one of the specimens shown by He and Pei in He et al. (1984, pl. ii, fig. 3) is nearly as triangular in lateral profile as is the type species (based on illustrations) or the secondary standard species (based on photographs). Additional specimens that are somewhat intermediate between A. australis and S. cornucopia/ S. acuticostata were referred to this species by Feng et al. (1994, pl. iii, figs. 3, 6, 7, 14, 15). Runnegar (in Bengtson et al. 1990) named a number of specimens from the early Cambrian of South Australia as Stenotheca cf. drepanoida. Parkhaev (in Gravestock et al. 2001) illustrated additional specimens from the same region and time period, classifying them as Stenotheca drepanoida. Clearly Runnegar's and Parkhaev's specimens and those herein described as Stenotheca drepanoida are members of the same species. Whether they are the same species as was shown in the original description of Stenotheca drepanoida is more speculative due to the wide variation in the latter, but some specimens between these different sets are remarkably similar (e.g., compare He and Pei in He et al., 1984, pl. ii, fig. 3 with Figure 15.7 herein). Thus, it seems reasonable that all these specimens belong to S. drepanoida. Runnegar (in Bengtson et al. 1990) noted that species such as Mellopegma simesi and Stenotheca drepanoida are difficult to classify. Much of the difficulty in classification of stenothecids, especially for Stenotheca drepanoida, is due to the remarkable intraspecific variation found in each bed. This variation makes classification difficult, but also reveals a striking degree of fuel for evolution in these stenothecid species. Stenotheca drepanoida has less coiling and a more flared sub-apical margin compared with Anabarella. It has a greater degree of elongation and greater coiling than in S. acuticostata or S. cornucopia. Stenotheca drepanoida differs from Mellopegma georginense and Mellopegma schizocheras sp. nov. in having a more protruding, tubular apex and a less extensive sub-apical shelf; it differs from Mellopegma simesi comb. nov. and Mellopegma uslonicum in having a more smoothly rounded sub-apical margin; and from Mellopegma indecorum in being more laterally compressed. Range. Early Cambrian. Occurrence. Xinji Formation, Fangcheng County, Henan Province, China; and Mernmerna Formation and Parara and Ajax Limestones, South Australia. Acanthotheca gen. nov. Type species. Acanthotheca junior (Runnegar, 1996), by monotypy. Etymology. From the Greek acanthos, meaning thorn, and the Greek theca, meaning cup or container, with reference to the overall thorn shape of the shell ('cup'). Diagnosis. Shell small (c. 1 mm long), tall, subconical, slightly laterally compressed; ventral margin curved; sharp boundary in sub-apical slope at shelf. Remarks. Acanthotheca shares key characters with other stenothecids, including a curved ventral margin, a gently curved dorsal margin, some lateral compression, and a laminar—possibly calcitic semi-nacre—inner shell layer. Although it lacks the same degree of lateral compression as most other stenothecids, its striking similarity in form to species such as Mellopegma simesi indicate it should be classified in the Stenothecidae. Acanthotheca differs from Mellopegma in having less lateral compression and a strongly developed pegma-like structure; it differs from Stenotheca in having less lateral compression, a greater degree of curvature of the ventral margin, and strongly developed pegma-like structure; it differs from Anabarella in coiling less than 1/2 a whorl; it differs from Ribeiria in coiling at all and in having a much more elongate apex, a less developed pegma, and greatest width in the supra-apical rather than sub-apical region. Range. Middle Cambrian (Floran). Occurrence. Only from Gowers Formation in the Thorntonia region of the Georgina Basin, Australia.
Acanthotheca junior (Runnegar, 1996)
Diagnosis. As for genus. Remarks. Runnegar (1996) classified this species as a member of Ribeiria based on the overall shape and apparent pegma in the one known specimen at the time. Vendrasco et al. (2010) illustrated many more specimens that reveal the pegma-like structure is variously developed in different individuals. In addition, this species differs from members of Ribeiria Sharpe, 1853 in being taller/less elongate, having a shell with at least some coiling (~1/4 whorl), and having the greatest width in the supra-apical rather than sub-apical region of the shell. Acanthotheca junior may be ancestral to Ribeiria. Range and Occurrence. As for genus. |
|