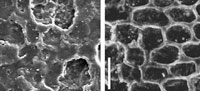
 The study of cuticular and epidermal features in fossil plant impressions using silicone replicas for scanning electron microscopy
The study of cuticular and epidermal features in fossil plant impressions using silicone replicas for scanning electron microscopy
Article number: 15.2.23A
https://doi.org/10.26879/309
Copyright Palaeontological Association, August 2012
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 7 November 2011. Acceptance: 11 March 2012
{flike id=279}
ABSTRACT
The study of epidermal and cuticular features is crucial in palaeobotanical investigations. In fossil plant impressions organic material is not preserved and cuticular data is commonly believed to be missing. This work describes a redefined version of the silicone cast technique for SEM examination known since almost 40 years, but unfortunately rarely used in routine palaeobotanical studies. The use of silicone (vinylpolysiloxane) instead latex casts offers significant advantages such as easier handling, higher reproduction of surface details, and elimination of electrostatic charge accumulation. The results indicate that silicone represents an improvement over latex. With this technique excellent results can be achieved, possibly making visible several plant surface structures, including epidermal cells, stomata, papillae, trichomes and striations on the rachis. Moreover, this technique demonstrates that impression fossils can provide similar useful data like those seen in compressed fossils. The effectiveness of this technique is demonstrated with several examples of fossil plants from the Triassic Madygen Lagerstätte. The application of this simple, non-destructive and extremely effective technique provides significant biological information on the cuticular and epidermal features in fossil plant impressions despite the absence of cuticles, to resolve taxonomic problems as well as to infer diverse ecological adaptations.
Philippe Moisan. Forschungsstelle für Paläobotanik am Institut für Geologie und Paläontologie, Westfälische Wilhelms-Universität Münster, Hindenburgplatz 57, 48143 Münster, Germany. f_mois01@uni-muenster.de
Keywords: silicone replicas; fossil plant impressions; SEM; epidermal and cuticular features; palaeobotany
Final citation: Moisan, Philippe. 2012. The study of cuticular and epidermal features in fossil plant impressions using silicone replicas for scanning
electron microscopy. Palaeontologia Electronica Vol. 15, Issue 2;23A,9p;
palaeo-electronica.org/content/2012-issue-2-articles/279-replicas-for-sem-examination
INTRODUCTION
The use of scanning electron microscopy (SEM) is a valuable tool in palaeontology for providing three-dimensional images of macro- and microfossils (Hill, 1990; Collinson, 1999). In palaeobotany the first studies using the SEM were carried out in palynology (e.g., Hibbert, 1967; Chaloner, 1968; Taylor, 1968; Drew and Tschudy, 1968; Heywood, 1969; Muir, 1970), in turn the first SEM studies of plant macrofossils focused on tracheids, lignites and cuticles (e.g., Taylor, 1968; Alvin and Muir, 1969; Taylor and Millay, 1969; Alvin, 1970). Since then, the SEM has become a standard method for studying fossil plant cuticles. Fossil plants are preserved in a variety of forms, compression and impression fossils being the most common types of preservation. Fossil plant compressions occur in sediments that were not exposed to high temperatures, thermal alteration or oxidation, and as a consequence a highly compressed coalified layer with cuticle can be retained. Cuticles are of special interest in palaeobotanical studies, providing valuable information for identifying plant remains as well as for plant taxonomy, correlation of dispersed organs, whole plant reconstructions, and ecological and palaeoclimatic interpretations (see Kerp, 1990; McElwain and Chaloner, 1996; Kerp and Krings, 1999; Barclay et al., 2007). The cuticular morphology and anatomy of fossil plants has been commonly studied with the complementary use of light and electron microscopes (SEM/TEM), thus providing the most detailed information on the structure of fossil cuticles (e.g., Niklas et al., 1978; Archangelsky and Taylor, 1986; Archangelsky et al., 1986; Archangelsky, 1991; Guignard et al., 1998). Standard procedures for extracting cuticles from compression fossils include chemical methods known as bulk acid maceration, and transfer methods coupled with cellulose acetate films, nail polish films, collodion films and polyester resins (see Dilcher, 1974; Kouwenberg et al., 2007; Escapa et al., 2010).
However, none of these techniques can be applied to fossil plant impressions, because organic matter (i.e., the proper cuticle) is not preserved, and therefore valuable information that cuticles can offer is commonly believed to be missing. Although no organic material is preserved in impression fossils, features of the cuticles and epidermis may be preserved as imprints when the plant remains are embedded in a very fine-grained sediment matrix. In this case, replicas of the plant surfaces can be made and successfully examined with the SEM. Despite the technique having a great potential in palaeobotany, surprisingly little attention has been given to the application of silicone and latex casts for detailed studies in fossil plant impressions. The use of silicone replicas instead of latex has proven to be extremely helpful for the study of the Middle to Late Triassic flora from the Madygen Formation of Kyrgyzstan, considered one of the most spectacular early Mesozoic Lagerstätte worldwide because of the abundance and diversity of animal and plant fossils (see Dobruskina, 1995; Voigt et al., 2006, 2009; Shcherbakov, 2008; Sues and Fraser, 2010; Moisan et al., 2011).
In this paper, a redefined version of the silicone cast technique for SEM analysis is presented, and illustrated with examples from the Madygen fossil flora. SEM images of latex were also obtained and compared with silicone replicas in order to identify differences between both materials. The technique using silicone is simple, not time-consuming, offering excellent results and high quality SEM images to study the cuticular and epidermal features, despite the absence of cuticle in fossil plant impressions. A main goal of this paper is to encourage a more widely use of this easy but very effective technique to palaeontologists working with impression fossils.
PREVIOUS INVESTIGATIONS USING REPLICAS AND SEM
The SEM generates surface images by detecting secondary electrons emitted from electrically conductive specimens, offering several advantages such as a large depth of field, high resolution and magnification. The SEM has been widely used for studying cuticular features in fossil plant compressions in which cuticles have been preserved. Although, impression fossils lack of the proper cuticle, the use of replicas coupled with SEM have a considerable potential for revealing cuticular and epidermal features as was earlier pointed out by Dilcher (1974). Chaloner and Gay (1973) were the first who published a method for the examination of the plant impression surfaces under high magnification. They used latex replicas for SEM studies of Palaeozoic lycopod stems showing leaf scars and cushions as well as stomata and cells. Chaloner and Collinson (1975) first removed the organic remains of compression specimens by burning before making latex casts of the clean sediment matrix surface. Removing the coaly material in a stove for making fine details visible was already practised by Stur (1883). Using this method was possible to observe leaf trace cicatricules, parichnos scars, striated cortical surfaces and stomata that could not be seen in the coaly compressions. Chaloner et al. (1979) prepared latex replicas of a Permian lycopsid for SEM examination revealing stomatal bands and cells. Rigby (1978) used latex replicas to obtain SEM images of the micropyle and cells of a glossopterid fructification. Kerp (1984) also obtained SEM images by preparing latex replicas of a Permian sphenopsid, recognizing cell patterns and stomata. Edwards et al. (1989) made latex replicas of a zosterophyll plant showing bases of spines, cells, papillae and probable stomata.
The use of silicone replicas for SEM studies in palaeobotany was introduced by Watson and Alvin (1976). They obtained images of tracheids, stomata, hairs and papillae in silicified conifer remains. Reihman and Schabilion (1976) used silicone in Pennsylvanian fern-like foliage allowing the examination of papillate surfaces and stomata surrounded by papillae. Tavares and Rohn (2009) made silicone replicas of a petrified Permian pecopterid showing cells and probable trichome scars on the pinnules. Moisan et al. (2011) prepared silicone replicas of cycadophytes for SEM examination, obtaining detailed images of epidermal cells and papillae of the plant surfaces. These papers are the only attempts made so far to study cuticular and epidermal features by SEM examination using silicone replicas. Additionally, silicone replicas of complete specimens can be photographed and have been used to discern details not seen in the original material (e.g., Manchester, 1992; Van der Ham and Van Konijnenburg-van Cittert, 2003, 2004; Van der Ham et al., 2001, 2003, 2004; Van der Ham and Dortangs, 2005). A useful overview on the use of casting and moulding in palaeontology is summarized by Rigby and Clark (1965).
MATERIAL AND METHODS
The fossil plant impressions used in this study were collected from the Middle to Late Triassic Madygen Formation situated in northern foothills of the Turkestan Mountains of Central Asia in southwestern Kyrgyzstan. Plant fossils from the Madygen fossil localities lack of cuticles, however, they occur in very fine-grained sediment matrix, resulting in an exceptional preservation and perfect natural casts of the plant surfaces, showing cell pattern, stomata, striations, papillae and trichome bases. In order to compare directly the differences between silicone and latex replicas, both materials were tested in the same specimens. See Chaloner and Gay (1973) for technical details of the use of latex to fossil plants.
 Casts of plant surfaces were made using vinylpolysiloxane (VPS) (Provil® novo light, ISO 4823, Heraeus Kulzer, Wehrheim, Germany), a high-quality impression material based on low-viscosity silicone, conventionally used in dental laboratories. The VPS is supplied in double cartridges (base paste and matching catalyst paste). A dispensing gun and a mixing tip attached to the outlet of the cartridge are required for an automatic and homogenous mixing. The base/catalyst mixing ratio of VPS is 1:1. Prior to the preparation of VPS casts, an accurate examination of the specimens under stereomicroscope is recommended to select the most appropriate areas of the plant surface to replicate and examine with the SEM. Trapezium-shaped pieces of VPS casts (Figure 1) were cut out using a single-edged razor blade or scalpel and mounted with conductive thermoplastic adhesive (Tempfix®, Wetzlar, Germany) on geological microscope slides (48 x 28 mm) using a hot plate at about 40°C. Subsequently, the VPS casts were sputter-coated with gold for 3–4 minutes with a sputter current of 20 mA using a Balzers Union SCD 040. The VPS casts were examined and photographed with a JEOL 840 scanning electron microscope at the Interdisciplinary Centre for Electron Microscopy and Analysis (ICEM), Westfälische Wilhelms-Universität Münster, using the image-analysis software analySIS 3.2 (Soft Imaging System, Münster, Germany).
Casts of plant surfaces were made using vinylpolysiloxane (VPS) (Provil® novo light, ISO 4823, Heraeus Kulzer, Wehrheim, Germany), a high-quality impression material based on low-viscosity silicone, conventionally used in dental laboratories. The VPS is supplied in double cartridges (base paste and matching catalyst paste). A dispensing gun and a mixing tip attached to the outlet of the cartridge are required for an automatic and homogenous mixing. The base/catalyst mixing ratio of VPS is 1:1. Prior to the preparation of VPS casts, an accurate examination of the specimens under stereomicroscope is recommended to select the most appropriate areas of the plant surface to replicate and examine with the SEM. Trapezium-shaped pieces of VPS casts (Figure 1) were cut out using a single-edged razor blade or scalpel and mounted with conductive thermoplastic adhesive (Tempfix®, Wetzlar, Germany) on geological microscope slides (48 x 28 mm) using a hot plate at about 40°C. Subsequently, the VPS casts were sputter-coated with gold for 3–4 minutes with a sputter current of 20 mA using a Balzers Union SCD 040. The VPS casts were examined and photographed with a JEOL 840 scanning electron microscope at the Interdisciplinary Centre for Electron Microscopy and Analysis (ICEM), Westfälische Wilhelms-Universität Münster, using the image-analysis software analySIS 3.2 (Soft Imaging System, Münster, Germany).
RESULTS AND DISCUSSION
In the course of a systematic revision of the diverse Madygen fossil flora this effective and simple technique described above was successfully applied to several groups of plants. Several features of the plant surfaces were made visible using the VPS such as epidermal cells, papillae, stomata, venation pattern and striations. About 400 VPS replicas of the plant surfaces were made and studied.
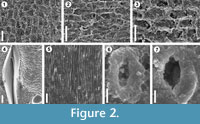 The results obtained demonstrate that silicone replicas (VPS) for SEM examination are very useful for the study of epidermal and cuticular features in fossil plant impressions despite the absence of cuticles, providing data about the morphology of plant surface structures that cannot be readily recognized with the stereomicroscope, which is usually the only way to examine impression fossils. This study also confirms that the use of VPS offers important advantages and improvements over the conventionally used latex replicas. A good example of this situation is the occurrence in the pteridosperm Ptilozamites of longitudinal striations (Figure 2.5) and stomata with subsidiary cells forming a ring-like structure (Figures 2.6-7) considered as a diagnostic character of this genus (Popa and McElwain, 2009). These structures were only recognized using the VPS replicas, and attempts with latex to observe such structures were unsuccessful. The identification of stomata from VPS replicas is important due to their effective application to identify and classify fossil plants. Chaloner and Collinson (1975) pointed out the difficulty to identify stomata from latex replicas; however this example in Ptilozamites shows that using VPS replicas, detailed information can be obtained from the plant surfaces, while with latex some microscopic details cannot be replicated adequately. In this study, in contrast to previous works in which this technique was applied to a limited number of taxa, it was used successfully in several different groups of plants including bryophytes (Figure 2.2), lycopsids (Figure 2.1, Figure 3.2, 3.4), sphenopsids (Figure 2.3) and gymnosperms (Figure 2.5-7, Figure 3.6, 3.8) making visible epidermal and cuticular structures, even of organisms of very delicate nature like bryophyte that normally have a very poor and low preservation potential and its fossil record is at best very fragmentary. Therefore, applying this technique to impression fossils preserved in fine grained sediments can offers similar results to those reported for cuticles in compressed floras.
The results obtained demonstrate that silicone replicas (VPS) for SEM examination are very useful for the study of epidermal and cuticular features in fossil plant impressions despite the absence of cuticles, providing data about the morphology of plant surface structures that cannot be readily recognized with the stereomicroscope, which is usually the only way to examine impression fossils. This study also confirms that the use of VPS offers important advantages and improvements over the conventionally used latex replicas. A good example of this situation is the occurrence in the pteridosperm Ptilozamites of longitudinal striations (Figure 2.5) and stomata with subsidiary cells forming a ring-like structure (Figures 2.6-7) considered as a diagnostic character of this genus (Popa and McElwain, 2009). These structures were only recognized using the VPS replicas, and attempts with latex to observe such structures were unsuccessful. The identification of stomata from VPS replicas is important due to their effective application to identify and classify fossil plants. Chaloner and Collinson (1975) pointed out the difficulty to identify stomata from latex replicas; however this example in Ptilozamites shows that using VPS replicas, detailed information can be obtained from the plant surfaces, while with latex some microscopic details cannot be replicated adequately. In this study, in contrast to previous works in which this technique was applied to a limited number of taxa, it was used successfully in several different groups of plants including bryophytes (Figure 2.2), lycopsids (Figure 2.1, Figure 3.2, 3.4), sphenopsids (Figure 2.3) and gymnosperms (Figure 2.5-7, Figure 3.6, 3.8) making visible epidermal and cuticular structures, even of organisms of very delicate nature like bryophyte that normally have a very poor and low preservation potential and its fossil record is at best very fragmentary. Therefore, applying this technique to impression fossils preserved in fine grained sediments can offers similar results to those reported for cuticles in compressed floras.
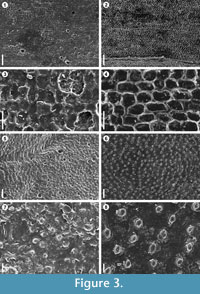 In this study was observed that latex replicas produce significant electrostatic charge of the surfaces making it difficult to distinguish biological structures of the fossil plants (Figures 3.5, 3.7), while using the VPS, electrostatic charge was completely eliminated (Figures 3.6, 3.8). In this sense, the shape of VPS casts and, moreover, sputter-coating with gold instead of carbon are important to prevent accumulation of electrostatic charge and to enhance electron emission from the surface. The VPS also offers markedly better resolution, making it possible to capture sharp and clear SEM images with minute details. An example of the resolution made possible by this method was observed in the herbaceous lycopsid Isoetites in which epidermal cells using latex replicas were not distinguishable (Figures 3.1, 3.3), but with VPS the pattern, size and shape of the cells were clearly visible (Figures 3.2, 3.4). Reihman and Schabilion (1976) pointed out that silicone replicas were superior to latex in reproducing lower epidermal features of Alethopteris sullivanti, and they found that silicone replicas, at higher magnifications above 250x, show textural artifacts resulting in unsatisfactory images. This problem has not been observed with VPS, where observations of the replicas with magnifications up to 1000x are possible. Other favourable properties of VPS, include easy handling, cures quickly within 3–4 minutes at room temperature, and it is completely free of air bubbles. A critical point of the use of latex is the presence of air bubbles, occurring in almost all of the latex replicas (see Figures 3.1, 3.3, 3.5). Deformation and fissures of the latex replica surfaces were also found in this study (Figure 2.4, Figure 3.5, 3.7). Watson and Alvin (1976) mentioned problems with replicas having finely wrinkled surfaces that could not be eliminated. The VPS replicas do not shrink, and wrinkles are not evident at high magnifications, even after examining the replicas one year later.
In this study was observed that latex replicas produce significant electrostatic charge of the surfaces making it difficult to distinguish biological structures of the fossil plants (Figures 3.5, 3.7), while using the VPS, electrostatic charge was completely eliminated (Figures 3.6, 3.8). In this sense, the shape of VPS casts and, moreover, sputter-coating with gold instead of carbon are important to prevent accumulation of electrostatic charge and to enhance electron emission from the surface. The VPS also offers markedly better resolution, making it possible to capture sharp and clear SEM images with minute details. An example of the resolution made possible by this method was observed in the herbaceous lycopsid Isoetites in which epidermal cells using latex replicas were not distinguishable (Figures 3.1, 3.3), but with VPS the pattern, size and shape of the cells were clearly visible (Figures 3.2, 3.4). Reihman and Schabilion (1976) pointed out that silicone replicas were superior to latex in reproducing lower epidermal features of Alethopteris sullivanti, and they found that silicone replicas, at higher magnifications above 250x, show textural artifacts resulting in unsatisfactory images. This problem has not been observed with VPS, where observations of the replicas with magnifications up to 1000x are possible. Other favourable properties of VPS, include easy handling, cures quickly within 3–4 minutes at room temperature, and it is completely free of air bubbles. A critical point of the use of latex is the presence of air bubbles, occurring in almost all of the latex replicas (see Figures 3.1, 3.3, 3.5). Deformation and fissures of the latex replica surfaces were also found in this study (Figure 2.4, Figure 3.5, 3.7). Watson and Alvin (1976) mentioned problems with replicas having finely wrinkled surfaces that could not be eliminated. The VPS replicas do not shrink, and wrinkles are not evident at high magnifications, even after examining the replicas one year later.
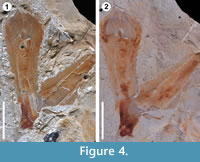 Another important difference found between these two compounds is shown in Figure 4. Latex replicas leave a considerable quantity of residues on the fossils, and even more in some cases, partially destroying the original material (Figure 4.1). In contrast to silicone replicas that are non-destructive, they do not leave residues on the original material (Figure 4.2). To conclude, the use of VPS for the replication of plant surfaces in fossil impressions is a non-destructive, quick and easy technique that can be applied without risk of destruction or damage to museum material and unique type specimens.
Another important difference found between these two compounds is shown in Figure 4. Latex replicas leave a considerable quantity of residues on the fossils, and even more in some cases, partially destroying the original material (Figure 4.1). In contrast to silicone replicas that are non-destructive, they do not leave residues on the original material (Figure 4.2). To conclude, the use of VPS for the replication of plant surfaces in fossil impressions is a non-destructive, quick and easy technique that can be applied without risk of destruction or damage to museum material and unique type specimens.
ACKNOWLEDGEMENTS
This paper constitutes a part of the author's Ph.D. thesis. This research was supported in part by the Deutscher Akademischer Austausch Dienst (DAAD grant A/06/27956). I wish to thank Hans Kerp (Münster) for critically reading the manuscript and for valuable comments and suggestions. I am very indebted to all the members of the expeditions to Madygen, Kyrgyzstan (2008-2009), especially Sebastian Voigt, Jörg W. Schneider, Andreas Brosig, Marvin Preuße, Jan Fischer and Michael Buchwitz (all of Freiberg) for their great help in the field. I am grateful to Benni Bomfleur (Lawrence) for support and helpful discussion throughout the course of this work and for comments on an early draft of this manuscript. Thanks to Thorsten Grund (Münster) for technical help and advice with the SEM. Thomas Wotte (Münster) kindly transported the fossil plant material collected in 2009 from Freiberg to Münster. The latex was kindly provided by Gerd Schreiber (Münster). Thanks to Christian Pott and Steve McLoughlin (Stockholm) organizers of the symposium "Old techniques, improved methods and new approaches to study fossil plants a symposium on modern methods in Palaeobotany" at the 8th EPPC held in Budapest where the author presented preliminary results using this technique.
REFERENCES
Alvin, K.L. 1970. The study of fossil leaves by SEM. Proceedings of the Third Annual Scanning Electron Microscope Symposium, IIT Research Institute, Chicago, 121–128.
Alvin, K.L. and Muir, M.D. 1969. Scanning electron microscopy?a new method of studying lignite. Review of Palaeobotany and Palynology, 9:115–118.
Archangelsky, S. 1991. Ultrastructural studies in fossil plant cuticles. Current Science, 61:676–677.
Archangelsky, S. and Taylor, T.N. 1986. Ultrastructural studies of fossil plant cuticles. II. Tarphyderma gen. n., a Cretaceous conifer from Argentina. American Journal of Botany, 73:1577–1587.
Archangelsky, S., Taylor, T.N., and Kurmann, M.H. 1986. Ultrastructural studies of fossil plant cuticles: Ticoa harrisii from the early Cretaceous of Argentina. Botanical Journal of the Linnean Society, 92:101–106.
Barclay, R., McElwain, J., Dilcher, D., and Sageman, B. 2007. The Cuticle Database: Developing an interactive tool for taxonomic and paleoenvironmental study of the fossil cuticle record. Courier Forschungsinstitut Senckenberg, 258:39–55. In Jarzen, D.M., Manchester, S.R., Retallack, G.J., and Jarzen, S.A. (eds.), Advances in Angiosperm Paleobotany and Paleoclimatic Reconstruction - Contributions Honoring David L. Dilcher and Jack A. Wolfe. E. Schweizerbart'sche Verlagsbuchhandlung, Stuttgart.
Chaloner, W.G. 1968. British pre-quaternary palynology: A historical review. Review of Palaeobotany and Palynology, 6:21–40.
Chaloner, W.G. and Gay, M.M. 1973. Scanning electron microscopy of latex casts of fossil plant impressions. Palaeontology, 16:645–649.
Chaloner, W.G. and Collinson, M.E. 1975. Application of SEM to a sigillarian impression fossil. Review of Palaeobotany and Palynology, 20:85–101.
Chaloner, W.G., Leistikow, K.U., and Hill, A. 1979. Brasilodendron gen. nov. and B. pedroanum (Carruthers) comb. nov., a Permian lycopod from Brazil. Review of Palaeobotany and Palynology, 28:117–136.
Collinson, M.E. 1999. Scanning electron microscopy of megafossils and mesofossils, p. 57–64. In Jones, T.P. and Rowe, N.P. (eds.), Fossil Plants and Spores: Modern Techniques. Geological Society, London.
Dilcher, D.L. 1974. Approaches to the identification of angiosperm leaf remains. The Botanical Review, 40:1–157.
Dobruskina, I.A. 1995. Keuper (Triassic) flora from Middle Asia (Madygen, South Fergana). Bulletin of the New Mexico Museum of Natural History and Science, 5:1–49.
Drew, C.M. and Tschudy, B.D. 1968. Aquilapollenites: fossil pollen as seen under the scanning electron microscope. Geological Society of America Bulletin, 79:1829–1832.
Edwards, D., Kenrick, P., and Carluccio, L.M. 1989. A reconsideration of cf. Psilophyton princeps (Croft & Lang, 1942), a zosterophyll widespread in the Lower Old Red Sandstone of South Wales. Botanical Journal of the Linnean Society, 100:293–318.
Escapa, I.H., Axsmith, B.J., Taylor, T.N., and Taylor, E.L. 2010. Modifications of the transfer technique for studying complex plant structures. Review of Palaeobotany and Palynology, 159:62–68.
Guignard, G., Thévenard, F., and Van Konijnenburg-van Cittert, J.H.A. 1998. Cuticle ultrastructure of the cheirolepidiaceous conifer Hirmeriella muensteri (Schenk) Jung. Review of Palaeobotany and Palynology, 104:115–141.
Heywood, V.H. 1969. Scanning electron microscopy in the study of plant materials. Micron, 1:1–14.
Hibbert, F.A. 1967. The use of scanning electron microscopy in the study of Carboniferous miospores. New Phytologist, 66:825–826.
Hill, C.R. 1990. Scanning electron microscopy in palaeobotany, p. 193–234. In Claugher, D. (ed.), Scanning electron microscopy in taxonomy and functional morphology. Clarendon Press, Oxford.
Kerp, J.H.F. 1984. Aspects of Permian palaeobotany and palynology. III. A new reconstruction of Lilpopia raciborskii (Lilpop) Conert et Schaarschmidt (Sphenopsida). Review of Palaeobotany and Palynology, 40:237–261.
Kerp, H. 1990. The study of fossil gymnosperms by means of cuticular analysis. Palaios, 5:548–569.
Kerp, H. and Krings, M. 1999. Light microscopy of fossil cuticles, p. 52–56. In Jones, T.P. and Rowe, N.P. (eds.), Fossil plants and spores: modern techniques. The Geological Society, London.
Kouwenberg, L.L.R., Hines, R.R., and McElwain, J.C. 2007. A new transfer technique to extract and process thin and fragmented fossil cuticle using polyester overlays. Review of Palaeobotany and Palynology, 145:243–248.
Manchester, S.R. 1992. Flowers, fruits, and pollen of Florissantia, an extinct Malvalean genus from the Eocene and Oligocene of western North America. American Journal of Botany, 79:996–1008.
McElwain, J.C. and Chaloner, W.G. 1996. The fossil cuticle as a skeletal record of environmental change. Palaios, 11:376–388.
Moisan, P., Voigt, S., Pott, C., Buchwitz, M., Schneider, J.W., and Kerp, H., 2011. Cycadalean and bennettitalean foliage from the Triassic Madygen Lagerstätte (SW Kyrgyzstan, Central Asia). Review of Palaeobotany and Palynology, 164:93–108.
Muir, M.D. 1970. Scanning electron microscopy in palynology. Review of Palaeobotany and Palynology, 10:85–97.
Niklas, K.J., Brown Jr., R.M, Santos, R., and Vian, B. 1978. Ultrastructure and cytochemistry of Miocene angiosperm leaf tissues. Proceedings of the National Academy of Sciences, 75:3263–3267.
Popa, M.E. and McElwain, J.C. 2009. Bipinnate Ptilozamites nilssonii from Jameson Land and new considerations on the genera Ptilozamites Nathorst 1878 and Ctenozamites Nathorst 1886. Review of Palaeobotany and Palynology, 153:386–393.
Reihman, M.A. and Schabilion, J.T. 1976. Cuticles of two species of Alethopteris. American Journal of Botany, 63:1039–1046.
Rigby, J.F. 1978. Permian glossopterid and other cycadopsid fructifications from Queensland. Geological Survey of Queensland, 367:3–21.
Rigby, J.K. and Clark, D.L. 1965. Casting and moulding, p. 389–413. In Kummel, B. and Raup, D. (eds.), Handbook of paleontological techniques. W.H. Freeman and Company, San Francisco.
Shcherbakov, D.E. 2008. Madygen, Triassic Lagerstätte number one, before and after Sharov. Alavesia, 2:113–124.
Stur, D. 1883. Zur Morphologie und Systematik der Culm- und Carbonfarne. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften Wien, 88:633–846.
Sues, H.-D. and Fraser, N.C. 2010. Triassic Life on Land. The Great Transition. Columbia University Press, New York.
Tavares, T.M.V. and Rohn, R. 2009. First record of petrified Permian pecopterids from the Paraná Basin, Brazil (Corumbataí Formation, Passa Dois Group, northeastern State of São Paulo): Morphology, anatomy and paleoecological implications. Journal of South American Earth Sciences, 27:60–73.
Taylor, T.N. 1968. Application of the scanning electron microscope in paleobotany. Transactions of the American Microscopical Society, 87:510–515.
Taylor, T.N. and Millay, M.A. 1969. Application of the scanning electron microscope in paleobotany. Proceedings of the Second Annual Scanning Electron Microscope Symposium, IIT Research Institute, Chicago. 197–205.
Van der Ham, R.W.J.M. and Dortangs, R.W. 2005. Structurally preserved ascomycetous fungi from the Maastrichtian type area (NE Belgium). Review of Palaeobotany and Palynology, 136:48–62.
Van der Ham, R.W.J.M. and Van Konijnenburg-van Cittert, J.H.A. 2003. Rare conifers from the type area of the Maastrichtian (Upper Cretaceous, southeast Netherlands). Scripta Geologica, 126:111–119.
Van der Ham, R.W.J.M. and Van Konijnenburg-van Cittert, J.H.A. 2004. Coniferen uit het Krijt van Zuid-Limburg en omgeving (Conifers from the Cretaceous of southern Limburg and adjacent areas). Natuurhistorisch Maandblad, 93:26–32.
Van der Ham, R.W.J.M., Van Konijnenburg-van Cittert, J.H.A., and Van der Burgh, J. 2001. Taxodiaceous conifers from the Maastrichtian type area (Late Cretaceous, NE Belgium, SE Netherlands). Review of Palaeobotany and Palynology, 116:233–250.
Van der Ham, R.W.J.M., Van Konijnenburg-Van Cittert, J.H.A., and Nieuwenhuis, E.A.P.M. 2004. Cunninghamites ubaghsii (Taxodiaceae?) from the Maastrichtian type area (Late Cretaceous, SE Netherlands) rediscovered. Bulletin van het Koninklijk Belgisch Instituut voor Natuurwetenschappen–Aardwetenschappen, 74:89–96.
Van der Ham, R.W.J.M., Van Konijnenburg-van Cittert, J.H.A., Dortangs, R.W., Herngreen, G.F.W., and Van der Burgh, J. 2003. Brachyphyllum patens (Miquel) comb. nov. (Cheirolepidiaceae?): remarkable conifer foliage from the Maastrichtian type area (Late Cretaceous, NE Belgium, SE Netherlands). Review of Palaeobotany and Palynology, 127:77–97.
Voigt, S., Haubold, H., Meng, S., Krause, D., Buchantschenko, J., Ruckwied, K., and Götz, A.E. 2006. Die Fossil-Lagerstätte Madygen: Ein Beitrag zur Geologie und Paläontologie der Madygen-Formation (Mittel- bis Ober- Trias, SW-Kirgisistan, Zentralasien). Hallesches Jahrbuch für Geowissenschaften, 22:85–119.
Voigt, S., Buchwitz, M., Fischer, J., Moisan, P., and Kogan, I. 2009. Lagerstätte Madygen – outstanding window to a continental Triassic ecosystem. Journal of Vertebrate Paleontology, 29 (supplement to number 3):196A.
Watson, J. and Alvin, K.L. 1976. Silicone rubber casts of silicified plants from the Cretaceous of Sudan. Palaeontology, 19:641–650.