 Cannibalism in Holocene muricid snails in the Beagle Channel, at the extreme southern tip of South America: an opportunistic response?
Cannibalism in Holocene muricid snails in the Beagle Channel, at the extreme southern tip of South America: an opportunistic response?
Article number: 16.1.4A
https://doi.org/10.26879/344
Copyright Palaeontological Association, January 2013
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 14 August 2012. Acceptance: 6 January 2013
{flike id=373}
ABSTRACT
This work documents the occurrence of drillholes on muricid Trophon geversianus shells from a Holocene raised marine deposit in the Beagle Channel, located on the extreme southern tip of South America (~ 55ºS). Based on drillhole morphology and previous data under laboratory conditions these predatory holes are attributed to conspecifics, thus suggesting cannibalism. It appears that when food is scarce and the alternative prey (Tawera gayi and other clams) is not available, T. geversianus may increase the frequency of cannibalism in order to compensate for the loss of bivalve prey. Cannibalism therefore developed at ca. 4000 yr. BP as a response to the lack of clams, which would have disappeared during a sudden hydrological local event that affected the filter feeders.
Sandra Gordillo. Centro de Investigaciones en Ciencias de la Tierra, Consejo Nacional de Investigaciones Científicas y Técnicas – Universidad Nacional de Córdoba (CICTERRA, CONICET-UNC). Centro de Investigaciones Paleobiológicas (CIPAL), Facultad de Ciencias Exactas, Físicas y Naturales, Universidad Nacional de Córdoba. Av. Vélez Sársfield 1611 X5016GCA Córdoba, Argentina
Key words: cannibalism; muricid gastropods; drillholes; Holocene; Tierra del Fuego
Final citation: Gordillo, Sandra. 2013. Cannibalism in Holocene muricid snails in the Beagle Channel, at the extreme southern tip of South America: an opportunistic response?, Palaeontologia Electronica Vol. 16, Issue 1; 4A, 13p. https://doi.org/10.26879/344
palaeo-electronica.org/content/2013/373-cannibalism-in-gastropods
INTRODUCTION
Cannibalism is not only widespread in the animal kingdom, it is actually important in the ecology of many species of aquatic and terrestrial communities (Fox, 1975; Polis, 1981). This intraspecific interaction has the potential to alter the functional relationship of predator-prey interactions (Rudolf, 2008).
In the fossil record cannibalism has been identified during the Cambrian, as seen in priapulids from the Burgess Shale; and, as modern priapulids are also known to be cannibals, this feeding behavior has remained remarkably similar for 530 million years (Brett and Walker, 2002).
Cannibalism in fossil marine gastropods has been often considered in naticids. For example, Kelley (1991), and then Dietl and Alexander (2000) examined cannibalism by Miocene-Pleistocene naticid gastropods from Maryland. More recently, Martinell et al. (2010) investigated confamiliar predation in Pliocene naticids from southern France, and Serralta (2010) described cannibalism in Polinices marambioensis from the La Meseta Formation (Eocene) in Antarctica.
Although research on cannibalism in fossil muricid gastropods is extremely scarce, Spanier (1987) attributed drillholes in the Holocene muricid snail Chicoreus ramosus from the Red Sea to conspecifics, and Dávid (1997) found signs of cannibalism in the Oligocene of Hungary through muricid boring observed on the shells of muricid gastropods.
The ecological significance of cannibalism has been discussed in previous research on naticids (see Kelley, 1991). On the one hand, cannibalism is seen as evidence of predator ineptitude, or an absence of bivalve prey (Stanton and Nelson, 1980; Polis, 1981). On the other hand, cannibalism is considered the result of selective predation in order to maximize energy gain per unit foraging time, as shown by cost-benefit ratios (Kitchell et al., 1986; Kelley, 1991; Kelley and Hansen, 2007), thus suggesting that it is sometimes more beneficial for naticids to prey upon themselves rather than bivalves. In relation to muricids, Spanier (1986, 1987) associated cannibalism of snails from the Red Sea with the absence of an alternative food.
The objective of this work is to document the occurrence of intraspecific predation in specimens of the muricid Trophon geversianus of ca. 4000 years BP from the Beagle Channel, and to discuss the ecological reasons behind this occasional phenomenon.
Muricid Gastropods and Cannibalism
Muricid gastropods are a diverse family of predator snails, comprising around 1,600 living and 1,200 fossil Cenozoic species (Barco et al., 2010; Merle et al., 2011). They are common members of shallow marine benthic communities in most areas of the world, mainly preying on bivalves and barnacles, leaving a recognizable drillhole on the skeleton of their victims. Thus drilling is preserved in the fossil record and can be used to determine patterns of predation in the past (Vermeij, 1980; Kelley and Hansen, 2003).
In southern South America, the most common living drilling muricid along the sea and channel shores is the whelk T. geversianus, and predation by this species appears to be an important cause of mortality in epifaunal mussels and semi-infaunal venerid clams (Gordillo, 1994; Gordillo and Archuby, 2012). In the Beagle Channel, T. geversianus inhabits the rocky and gravelly shores, which are mainly covered by mussels (Ingólfsson, 2005), but this snail is also associated with Tawera gayi clams, which live in soft substrates (Lomovasky et al. 2005).
This species has also been recovered from Holocene and Pleistocene marine deposits located in southern Argentina and Chile (Gordillo, 1999; Gordillo and Isla, 2011; Gordillo et al., 2011). Previous research showed the ability of this predator to capture and subdue mobile prey under laboratory conditions. In this region, T. geversianus drills holes primarily in the clam T. gayi, or the mussel M. chilensis, depending on which one is dominant in the substrate, while other species would only be occasional food items (Gordillo and Amuchástegui, 1998; Andrade and Ríos, 2007; Gordillo et al., 2011; Gordillo and Archuby, 2012).
In muricids, intraspecific predation (cannibalism) has been shown to take place in living gastropods (e.g., Paine, 1966; Spanier, 1986; Rilov et al., 2004; Vasconcelos et al., 2012), although very little information is available for the snail T. geversianus. Osorio (2002) first reported cannibalism in T. geversianus under aquarium conditions, and more recently Cumplido et al. (2011), studying the embryological development of this species, observed drilling attempts in a prehatching embryo of T. geversianus, thus also suggesting cannibalism.
Muricid Drillholes
To excavate a drillhole, muricids penetrate the shell of their prey by a mechanical scraping action of the radula, together with chemical activities of carbonic anhydrase, chelating agents and enzymes secreted by the ABO (accessory boring organ) situated in the foot (Carriker, 1981). More recent studies also showed that muricids can produce different secretions to paralyze their prey (Andrews et al., 1991; West et al., 1994; Roseghini et al., 1996). After extracellular digestion, muricids take their food in fluid form; and given that they suction the nutrient-rich fluids of the prey´s body tissues, they really are liquid feeders (Crothers, 1985) and also feed on suspended and soluble organic nutrients (Lau and Leung, 2004).
The drilling mechanism in T. geversianus, including the drilling and the feeding phase, is a very slow process. Under laboratory conditions, it mostly takes between 6-10 days (Gordillo and Amuchástegui, 1998; Andrade and Ríos, 2007; Gordillo and Archuby, 2012).
Predation patterns and bore holes by T. geversianus on different prey have been described in previous papers (Gordillo, 1994, 1998; Gordillo and Amuchástegui, 1998; Gordillo and Archuby, 2012, in press). Other drilling predators from the Beagle Channel are the muricids Xymenopsis muriciformis and Acanthina monodon. Drillholes produced by X. muriciformis resemble a cylinder and are relatively smaller than those produced by T. geversianus (Gordillo, 1998). A. monodon only lives on rocky substrates where it feeds on mussels and barnacles, leaving different types of predation damage (Gordillo, 2001; Gordillo and Archuby, 2012). In any case A. monodon seems to bore less frequently since it normally uses a labral spine to open the mussels Mytilus chilensis and Aulacomya atra and barnacles, but also this species was able to drill cylindrical holes on Brachidontes purpuratus under laboratory conditions.
Muricid holes can usually be distinguished from those made by naticids by their size and shape; in general, muricid holes tend to be smaller and more cylindrical (Kelley and Hansen, 2003), although holes drilled by T. geversianus may vary from conical to cylindrical depending on the prey species drilled (Gordillo and Amuchástegui, 1998).
Finally, and given that T. geversianus drillholes look more like the classical description of naticid drillholes, it is important to point out that naticids (although present in the Beagle Channel) are practically absent in the studied area. Furthermore, there is also no evidence indicating that naticids from the Beagle Channel produce holes in the shells of their prey; and there is even a possibility that they ingest their prey without drilling.
MATERIALS AND METHODS
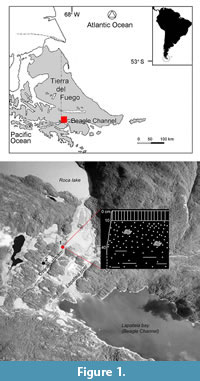 The material considered in this study comes from a raised marine outcrop located in southern Tierra del Fuego, on the northern coast of the Beagle Channel (Figure 1). T. geversianus specimens with drillholes were collected from the upper part of Site 1 (Figure 1.2). A second site (Site 2; Figure 1.2) previously studied is mentioned here because it will be a reference for comparisons.
The material considered in this study comes from a raised marine outcrop located in southern Tierra del Fuego, on the northern coast of the Beagle Channel (Figure 1). T. geversianus specimens with drillholes were collected from the upper part of Site 1 (Figure 1.2). A second site (Site 2; Figure 1.2) previously studied is mentioned here because it will be a reference for comparisons.
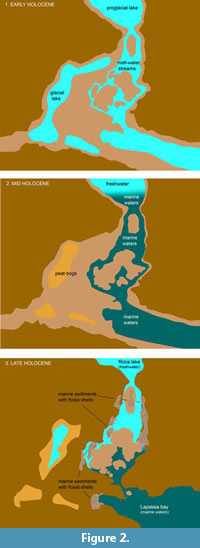
As indicated by previous geomorphological and paleontological studies (Rabassa et al., 1986; Gordillo et al., 1993; Gordillo et al., 2005; Rabassa et al., 2009), it is an environmentally complex area (Figure 2). During the last Pleistocene glaciations this area was covered by ice, but around 12 kyr. BP the ice disappeared from the Beagle Channel. After that, during the early Holocene this area was a low energy freshwater environment (Figure 2.1), but during mid-Holocene times (ca. 7500-4000 yr. BP), this area became a marine archipelago (Figure 2.2), and today it is a freshwater environment flanked by the rivers Ovando and Lapataia (Figure 2.3).
Site 1
All complete, unbroken T. geversianus shells (N=20) lying on a layer of sand over an exposed area of 20 x 20 m were collected. In this area, a radiocarbon date by Coronato et al. (1999) gave an age of 4160+/-45 yr. BP (Site 1; Figure 1).
The low number of T. geversianus shells collected per unit area in Site 1 is consistent with the proportion of the species in living communities (0.52 ind. m2; Andrade et al., 2009). Ten T. geversianus shells (50% of the total shells) showed signs of incomplete or complete drilling attempts.
 Site 2
Site 2
Another level from this area, with clams in life position, gave a radiocarbon age of 4425+/-55 yr. BP (Rabassa et al., 1986; Site 2; Figure 1). The fauna of site 2 was previously studied (Gordillo, 1999) and is dominated by filter feeding bivalves T. gayi (63%) and Hiatella sp. (19%) among other taxa which contribute most to the biomass. Bivalves normally occur as whole joined valves, oriented in life position, or horizontally, randomly oriented within the bed. Gastropods represent a minor proportion, although exhibit the highest richness. The most common gastropods are Pareuthria plumbea, which feed on carrion and the drilling gastropods T. geversianus and X. muriciformis which prey upon bivalves.
After examination and taking into account previous studies centered on laboratory experiments, which include the analysis of the morphology of drillholes (see above Muricid drillholes section), predatory drillings on T. geversianus shells were attributed to conspecifics. Each specimen of T. geversianus was measured with a digital caliper (precision of 0.01 mm) to determine height, width and thickness in millimeters. In specimens exhibiting borings, for each drilling attempt, outer and inner drillhole diameters were measured. In addition, each shell with borings was divided into five uneven sectors as an approach for evaluating site selectivity. These sectors were: the spire (sector 1); the last whorl in ventral position (sector 2) and the last whorl in dorsal position, subdivided into upper (sector 3), central (sector 4) and lower (sector 5) zones. The last subdivision into three zones (sectors 3, 4 and 5) was made to better discriminate site selectivity in dorsal position, taking into account the life position of the species with the dorsal side upwards.
To determine prey effectiveness, the number of complete holes was compared to the total number of drilling attempts. To analyze size selectivity and frequency of cannibalism, the proportion of bored versus unbored shells in relation to prey size was determined. Three size classes were considered: between 20.00 and 29.99 mm (class I); between 30.00 and 39.99 mm (class II) and between 40.00 and 49.99 mm (class III). To infer the relative amount of prey biomass as energetic value, the biovolume was calculated. As biomass in bivalves and gastropods is correlated with the volume of the body cavity within the shell we determined the relationships of size to biovolume. Powell and Stanton (1985) proposed an operational measurement of biovolume based on the conversion of measured shell parameters (i.e., length, height, width) into cubic form. However, in this paper we measured true biovolume as the paleontological analog of biomass. For this purpose, the biovolume consumed by the predator was constructed for T. geversianus and for T. gayi, its main prey in Holocene deposits in the area (see: Muricid gastropods and cannibalism section), as follows. From hundreds of modern specimens of these two species collected from the Beagle Channel we selected a subsample of 10 specimens for each species, covering all size ranges. In the case of T. geversianus, it was within the range of 13-75 mm in height and was between 7-50 mm in width. To calculate the biovolume, a graduated glass cylinder was used, and measurements were taken of the fluid displaced when introducing each specimen. With these data, an equation for each species was obtained. In T. geversianus, the cavity of the last whorl was filled with plasticine. In this way species-specific equations were used to generate biomass derived from data sets. These data were used in conjunction with shell thickness as a function of drilling time, to be interpreted in association with a cost-benefit analysis (Kitchell et al., 1981; Kidwell, 1991). To infer predator-prey size and cost-benefit relationships, equations were based on unpublished data obtained previously under laboratory conditions. To test the predator-prey size relationship, the predator size for each borehole diameter was calculated based on nine observations of three different T. geversianus specimens eating T.gayi clams under laboratory conditions. Predator size for each bored shell of T. geversianus was then estimated using this equation. To test whether patterns of prey selection by T. geversianus is related to biomass and energetic profitability, two bored shells from each prey species (i.e., T. gayi versus T. geversianus) of the same thickness were compared in biomass.
As drilling time includes drilling action and ingestion time, the calculations discriminated between these two phases. In this respect, we considered data on predation time (in days) obtained under laboratory conditions with T. geversianus preying on T. gayi. For each bored specimen of T. gayi we took into account (1) the number of days taken by T. geversianus from when it was placed on the prey until it left, (2) the shell thickness (as a measure of borehole depth) and (3) biovolume measurements based on empty shells. We correlated predation time (a) with borehole depth in order to evaluate whether predation time would increase in direct proportion to the drilling action required to excavate the shell and (b) with biovolume to evaluate whether predation time would increase in direct proportion to the volume of shell material eaten.
As a comparison, the relative abundance of the predator T. geversianus with respect to its potential prey, in different fossil and modern sites located on the Beagle Channel was compiled. Data analyses were done in Microsoft Excel and with the software PAST 2.02 (Hammer et al., 2001).
RESULTS
 In relation to the age of fossil T. geversianus shells with drillholes, and since the exposed layer in which they were recovered is located at a relatively higher position with respect to both layers dated in ca. 4400-4100 yr. BP, it is highly likely to be younger (i.e., ca. 4000 years because after this age the sea retreated).
In relation to the age of fossil T. geversianus shells with drillholes, and since the exposed layer in which they were recovered is located at a relatively higher position with respect to both layers dated in ca. 4400-4100 yr. BP, it is highly likely to be younger (i.e., ca. 4000 years because after this age the sea retreated).
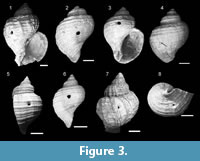
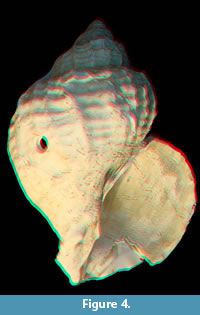 Figure 3 shows T. geversianus shells with predatory drillholes. In addition, Figure 4 provides a 3-D effect on the T. geversianus specimen illustrated as Figure 3.1.
Figure 3 shows T. geversianus shells with predatory drillholes. In addition, Figure 4 provides a 3-D effect on the T. geversianus specimen illustrated as Figure 3.1.
These holes are circular to oval when seen from above, oriented to the shell surface and conical in cross section. All of them (100%) are located on the last whorl. Of these, 25% are in the ventral region (sector 2) and 75% in the dorsal region, distributed as follows: 25% in the upper zone (sector 3), 62.5% in the central area (sector 4) and 12.5% in the lower part (sector 5). The highest percentage in the dorsal area would relate to the life position of the snails, and not to a preference for a particular area.
T. geversianus completed drilling in eight out of 10 attempts (Prey effectiveness gave a value of 0.8), but as the incomplete borings are located on shells which also exhibit a second complete borehole (e.g., Figure 3.3-3.4), 100% of the specimens were eaten by the same or by another predator. Thus, drilling was 100% successful. However, 20% of failed attempts still cost the predator time and energy without immediate gain.
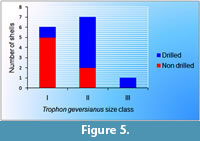 Figure 5 shows that there is no complete overlapping in size between drilled and non drilled specimens of T. geversianus from the same assemblage. Specimens with drillholes tend to have larger size.
Figure 5 shows that there is no complete overlapping in size between drilled and non drilled specimens of T. geversianus from the same assemblage. Specimens with drillholes tend to have larger size.
To estimate predator size, the height of T. geversianus was inferred by the inner diameter of boreholes excavated on T. gayi shells under laboratory conditions: Ln Y = 1.29 Ln X + 0.13; (R2=0.72)
This equation was applied to each prey specimen with a complete borehole, and it was observed that predators appear to be smaller (specimens 2, 5, 6 and 7) or larger (specimens 1, 3 and 4) than their prey (Figure 6).
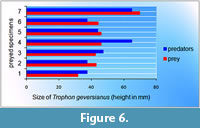 Taking into account that this is a mobile prey, it is more plausible that a small predator will find it easier to eat for several days on the dorsal side of a larger victim than a smaller one. On the contrary, in the case of specimens 1, 3 and 4, the predators were apparently larger than their prey, and they probably manipulated their prey prior to making the hole in their victims. In the case of specimen 4, it appears to be considerably larger than its prey, which showed a borehole located on the ventral side, indicating that this specimen was perhaps manipulated and turned before being drilled.
Taking into account that this is a mobile prey, it is more plausible that a small predator will find it easier to eat for several days on the dorsal side of a larger victim than a smaller one. On the contrary, in the case of specimens 1, 3 and 4, the predators were apparently larger than their prey, and they probably manipulated their prey prior to making the hole in their victims. In the case of specimen 4, it appears to be considerably larger than its prey, which showed a borehole located on the ventral side, indicating that this specimen was perhaps manipulated and turned before being drilled.
To analyze the cost-benefit relationship an equation for each species was calculated based on modern specimens collected (Table 1).
 These equations were applied in order to compare two boreholes of the same inner diameter (depth of 2.16 mm) excavated on valves of each of the two species: i.e., one T. gayi specimen of 33 mm long and one T. geversianus of 43.1 mm high. Similar values of biovolume were obtained for the two taxa, although slightly higher in T. geversianus: i.e., 7.27 for T. gayi and 8.56 for T. geversianus. These values indicate that for the same cost (to pass through the shell wall of T.geversianus or T. gayi), there is no benefit in one of these two food items, since the biomass of the two species is similar.
These equations were applied in order to compare two boreholes of the same inner diameter (depth of 2.16 mm) excavated on valves of each of the two species: i.e., one T. gayi specimen of 33 mm long and one T. geversianus of 43.1 mm high. Similar values of biovolume were obtained for the two taxa, although slightly higher in T. geversianus: i.e., 7.27 for T. gayi and 8.56 for T. geversianus. These values indicate that for the same cost (to pass through the shell wall of T.geversianus or T. gayi), there is no benefit in one of these two food items, since the biomass of the two species is similar.
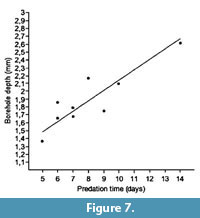
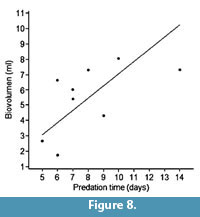 The relationships between predation time and shell thickness (borehole depth), and between predation time and biovolume (biomass) are plotted in Figure 7 and Figure 8, respectively, showing a correlation coefficient of 0.88 in the first case and a lower value of 0.59 in the second graph. Thus there is a better relationship between the energy invested in excavating the drillhole and the borehole depth than the biomass obtained.
The relationships between predation time and shell thickness (borehole depth), and between predation time and biovolume (biomass) are plotted in Figure 7 and Figure 8, respectively, showing a correlation coefficient of 0.88 in the first case and a lower value of 0.59 in the second graph. Thus there is a better relationship between the energy invested in excavating the drillhole and the borehole depth than the biomass obtained.
Finally, Table 2 shows great differences in the proportion of drilling predators and their potential prey in this study compared to other sites of different ages also located along the Beagle Channel. In addition to the different ratio, in the studied site this trophic interaction occurs within the same trophic level (intraguild), while in the other cases it involves a different trophic level (carnivorous and filter feeders) and organisms with a different mode of life (i.e., epifauna and semi-infauna), thus showing interguild predation. In this situation we have a competitive intraspecific interaction, since a cannibal can be cannibalized.
DISCUSSION
This study, while providing an ecological explanation of intraspecific predation in Holocene muricids from the Beagle Channel, also represents the first mention of cannibalism in Cenozoic fossil mollusks from South America.
Size frequency analysis in conjunction with the analysis of boreholes confirms that eaten specimens have a larger size, and that small specimens eat the larger ones. However, large specimens may also eat small prey if the predator initially manipulates the prey, or if the predator eats a dying or decaying specimen that offers no resistance. Although the number of T. geversianus recovered is relatively low, it is consistent with their relative abundance in modern and fossil communities in the region (Gordillo, 1999; Andrade et al., 2009; Gordillo and Archuby, 2012).
Several studies focused on naticid gastropods conclude that cannibalism results from selective predation in order to maximize energy gain, rather than from ineptitude of the predator or absence of bivalve prey (Kitchell et al., 1981; Kelley, 1991). More recently, Gould (2010) suggested that naticid cannibalism is more likely in low competition environments in the presence of bivalve prey.
In contrast, few studies on muricids (Spanier, 1986) have shown that cannibalism only occurred in the absence of bivalve prey. The present study supports the view of the absence of bivalve prey, as described below.
Polis (1981) in a classic study of intraspecific predation attributed cannibalism to the unavailability of alternative prey. As predicted by foraging theory, an animal may expand its diet beyond the normal limits of acceptable prey during periods of hunger or limited food supply (Polis, 1981), and cannibalism may enhance the survival of an individual during the transition time needed to discover common prey (Morton, 1987).
Taking into account previous studies in the region (Gordillo, 1993, 1999; Gordillo et al., 2011), it appears that prior to the onset of cannibal snails, the study area was inhabited by shallow benthic communities dominated by suspension feeder clams (site 2). Gordillo (1999) described two local benthic paleocommunities preserved in life position and composed of suspension feeders (80-90%), grazers (5-10%) and carnivores (1-5%). The main suspension feeder is the venerid T. gayi and the muricid T. geversianus appeared as an important predator of the dominant clams (Gordillo et al., 2011). These assemblages were preserved in life position due to a phenomenon of mass mortality (Gordillo, 1993), attributed to abrupt changes in salinity conditions caused by the invasion of continental waters in areas hitherto occupied by sea water.
In this respect, it is believed that a sudden event, such as the increase in brackish water in this area, produced a time-lag between the proportion of filter feeders and carnivores. In other words, these changes produced the mortality of filter feeders that did not adapt to sudden changes. This situation made the main prey (T. gayi) less available, which modified the relationship between filter feeders and predators, thus leading to cannibalism. It appears that when the main prey is not easily available, T. geversianus, as an opportunistic predator, may increase the frequency of cannibalism. It therefore follows that if prey abundance decreases then there are relatively more predators, and cannibalism increases.
Under this abrupt change in situation, the behavior of T. geversianus shifted from non-cannibal to cannibal, thus showing it to have flexible feeding behavior. This predator is therefore a generalistic, selective and opportunistic predator, and is occasionally cannibalistic.
Furthermore, given that small specimens of T. geversianus normally inhabit the intertidal zone, it is also plausible that when the sea level dropped (before this area was definitively disconnected from the sea), T. geversianus specimens living in the intertidal zone moved to the subtidal waters inhabited by larger specimens. Thus, a higher number of predators could also cause intraspecific predation. Too many predators and too little prey therefore results in cannibalism.
Cannibalism can be dangerous for organisms because the roles of predator and prey can easily switch, and the original cannibal can become cannibalized (Dietl and Alexander, 2000). However, occasional cannibalistic behavior may be beneficial when alternative prey is not available in order to compensate for the loss of food.
As T. geversianus specimens with drillholes only appeared as fossils in a layer of sediment, it is believed that cannibalism is a circumstantial situation which naturally would not have been much more common. Hence, it appears that this community was subject to a highly competitive regime only during a brief lapse of time within the Holocene (i.e., ca. 4000 yr. BP). It is also likely that after this event, the whole population died as this region was disconnected from the sea.
Interestingly, this particular situation of a highly competitive regime was apparently reversed in benthic communities living in the Beagle Channel, since cannibalism has not been mentioned, except for isolated (only two shells) modern and reworked shells of T. geversianus collected along the beach.
When ecologically comparing T. geversianus and T. gayi as prey, some differences can be seen. Although in both cases they are mobile prey, they differ because T. gayi is a semi-infaunal burrower, and can buried completely below the sediment, while T. geversianus is an epifaunal mobile prey. In a comparison with the present, it was noted that bored T. geversianus shells are rare and modern T. gayi exhibit high levels of predation by T. geversianus.
Taking into account that T. geversianus exhibits a preference for dominant bivalves such as the mussel M. chilensis and the clam T. gayi it is considered that in this case cannibalism is opportunistic or occasional and is stimulated by other factors associated with nutritional stress and high conspecific density. This interpretation is reinforced with data on modern and other Holocene paleocommunities dominated by clams, in which no bored T. geversianus shell was found. Thus, T. geversianus did not prey on congeners when alternative prey is present.
Differences in size frequency distribution and predator size are explained as follows. Cannibalism as an event could be explained by variation in the predator/prey ratio as see in Table 2. This new situation, unfavorable for predators, would cause high intraspecific competition. In this competition, larger individuals would have benefited, leaving the smaller ones the only option to feed on its own congeners. The latter is reinforced by the interpretation based on borehole size (to infer predator size), which showed that larger individuals were eaten by the smaller ones.
Thus, the existence of occasional cannibalism at ca. 4000 yr. BP suggests a change in the ecological interaction between T. geversianus and alternative prey. This intraspecific interaction arose due to abrupt changes in the density of interacting functional groups (a reduction of filter feeders in this case), which had local consequences (cannibalism), but it is unknown if this event had long-term consequences for the T. geversianus population. Perhaps this predator increased its range of food items.
In summary, occasional cannibalism in the muricid T. geversianus appears to be caused when the predator/prey ratio is disturbed for any reason which causes a decrease in their usual food, resulting in high intraspecific competition that causes intraspecific predation. More work in muricids under laboratory conditions is needed to reinforce and to extend these interpretations to other members of this family and to assess evolutionary implications of this occasional interaction.
ACKNOWLEDGMENTS
The author is grateful to C.E. Gómez who helped during fieldwork in November of 2007 and to S.N. Amuchástegui who collaborated in laboratory experiments at the Centro Austral de Investigaciones Científicas (CADIC). To S. Gerber (University of Bath, United Kingdom) and P. Kelley (University of North Carolina, Wilmington, USA) for their comments and constructive suggestions. Funding was provided by the CONICET (PIP 09-260). To Aaron Swartz, a strong defender of open access academic journals, who died in the fight for make them freely available to the public.
REFERENCES
Andrade, C. and Ríos, C. 2007. Estudio experimental de los hábitos tróficos de Trophon geversianus (Pallas, 1774) (Gastropoda: Muricidae): Selección y manipulación de presas. Anales Instituto Patagonia (Chile), 35:45-54.
Andrade, C., Montiel, A., and Quiroga, E. 2009. Estimación de producción secundaria y productividad para una población intermareal de Trophon geversianus (Bahía Laredo, Estrecho de Magallanes). Anales Instituto Patagonia (Chile), 37:73-84.
Andrews, E.B., Elphick, M.R., and Thorndyke, M.C. 1991. Pharmacologically active constituents of the accessory salivary and hypobranchial glands of Nucella lapillus. Journal of Molluscan Studies, 57:136-138.
Barco, A., Claremont, M., Reid, D.G., Houart, R., Bouchet, P., Williams, S.T., Cruaud, C., Couloux, A., and Oliverio, M. 2010. A molecular phylogenetic framework for the Muricidae, a diverse family of carnivorous gastropods. Molecular and Phylogenetic Evolution, 56:1025-1039.
Brett, C.E. and Walker, S.E. 2002. Predators and predation in Paleozoic marine environments. The Paleontological Society Papers, 8:93-118.
Carriker, M.R. 1981. Shell penetration and feeding by naticacean and muricacean predatory gastropods: A synthesis. Malacologia, 20:403-422.
Coronato, A., Rabassa, J., Borromei, A., Quattrocchio, M., and Bujalesky, G. 1999. Nuevos datos sobre el nivel relativo del mar durante el Holoceno en el Canal Beagle, Tierra del Fuego, Argentina. I Congreso Argentino de Cuaternario y Geomorfología Actas, 1:27-28.
Crothers, J.H. 1985. Dog-whelks: an introduction to the biology of Nucella lapillus. Field Studies, 6:291-360.
Cumplido, M., Pappalardo, P., Fernández, M., Averbuj, A., and Bigatti, G. 2011. Embryogenic development, feeding and intracapsular oxygen availability in Trophon geversianus (Gastropoda: Muricidae). Journal of Molluscan Studies, 77:429-436
Dávid, Á. 1997. Predation by muricid gastropods on Late-Oligocene (Egerian). Molluscs collected from Wind Brickyard, Eger, Hungary. Malacological Newsletter 16:5-12.
Dietl, G.P. and Alexander R.R. 2000. Post-Miocene shift in stereotypic naticid predation on confamilial prey from the Mid-Atlantic Shelf: Coevolution with dangerous prey. Palaios, 15:414-429.
Fox, L.R. 1975. Cannibalism in natural populations. Annual Review of Ecology and Systematics, 6:87-106.
Gordillo, S. 1993. Mortalidad en masa de moluscos subfósiles en Tierra del Fuego. In Resúmenes Jornadas Nacionales de Ciencias del Mar, 76. Puerto Madryn.
Gordillo, S. 1994. Perforaciones en bivalvos subfósiles y actuales del Canal Beagle, Tierra del Fuego. Ameghiniana, 31:177-185.
Gordillo, S. 1998. Trophonid gastropod predation on recent bivalves from the Magellanic Region, p. 251-254. In Johnston, P.A. and Haggart, J.W. (eds.), Bivalves: An Eon of Evolution. Paleobiological Studies Honoring Norman N. Newell. University of Calgary Press, Canada.
Gordillo, S. 1999. Holocene molluscan assemblages in the Magellan Region. Scientia Marina, 63 (Supl. 1):15-22.
Gordillo, S. 2001. Marcas de la depredación de Acanthina Fisher von Waldheim, 1807 (Gasteropoda: Muricidae) sobre Bivalvia. Ameghiniana, 38:55-60.
Gordillo, S. and Amuchástegui, S. 1998. Estrategias de depredación del gastrópodo perforador Trophon geversianus (Pallas) (Muricoidea: Trophonidae). Malacologia, 39:83-91.
Gordillo, S. and Archuby, F. 2012. Predation by drilling gastropods and asteroids upon mussels in rocky shallow shores of southernmost South America: paleontological implications. Acta Palaeontologica Polonica, 57:633-646.
Gordillo, S. and Archuby, F. in press. Live-live and live-dead interactions in marine death assemblages: the case of the Patagonian clam Venus antiqua. Acta Palaeontologica Polonica. http://dx.doi.org/10.4202/app.2011.0176
Gordillo, S. and Isla, F. 2011. Faunistic changes between the Middle/Late Pleistocene and the Holocene on the Atlantic coast of Tierra del Fuego: molluscan evidence. Quaternary International, 233:101-112.
Gordillo S., Coronato A.M.J., and Rabassa, J.O. 1993. Late Quaternary evolution of a subantarctic paleofjord, Tierra del Fuego. Quaternary Science Reviews, 2:889-897.
Gordillo S., Coronato, A., and Rabassa, J. 2005. Quaternary molluscan faunas from the island of Tierra del Fuego after the Last Glacial Maximum. Scientia Marina, 69 (Suppl. 2):337-348.
Gordillo, S., Martinelli, J., Cárdenas, J., and Bayer, S. 2011. Testing ecological and environmental changes during the last 6000 years: a multiproxy approach based on the bivalve Tawera gayi from southern South America. Journal of the Marine Biological Association of the United Kingdom, 91:1413-1427.
Gould, E.S. 2010. Unexpected rates of cannibalism under competitive conditions by the naticid gastropod Neverita duplicata (Say). Unpublished MS thesis, University of North Carolina, Wilmington, USA.
Hammer, Ø., Harper, D.A.T., and Ryan, P.D. 2001. Past: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4.1.23A:9pp., 178kb; http://palaeo-electronica.org/2001_1/past/issue1_01.htm.
Ingólfsson, A. 2005. Community structure and zonation patterns of rocky shores at high latitudes: an interoceanic comparison. Journal of Biogeography, 32:169-182.
Kelley, P.H. 1991. Apparent cannibalism by Chesapeake Group naticid gastropods: A predictable result of selective predation. Journal of Paleontology, 65:75-79.
Kelley, P.H. and Hansen, T.A. 2003. The fossil record of drilling predation on bivalves and gastropods, 113-133. In Kelley, P.H., Kowalewski, M., and Hansen, T.A. (eds.), Predator–prey interactions in the fossil record. Plenum Press/Kluwer, New York.
Kelley, P.H. and Hansen, T.A. 2007. A case for cannibalism: Confamilial and specific predation by naticid gastropods, Cretaceous through Pleistocene of the United States Coastal Plain, p. 151-170. In Elewa, A.M.T. (ed.), Predation in Organisms: A Distinct Phenomenon. Springer Verlag, Berlin.
Kidwell, S.M. 1991. Taphonomic feedback (live/dead interactions) in the genesis of bioclastic beds: keys to reconstructing sedimentary dynamics, p. 268-282. In Einsele, G., Ricken, W., and Seilacher, A. (eds.), Cycles and Events in Stratigraphy. Springer Verlag, Berlin.
Kitchell, J.A., Boggs, C.H., Kitchell, J.F., and Rice, J.A. 1981. Prey selection by naticid gastropods: Experimental tests and applications to the fossil record: Paleobiology, 7:533-552.
Kitchell, J.A., Boggs, C.H., Rice, J.A., Kitchell, J.F., Hoffman, A., and Martinell, J. 1986. Anomalies in naticid predatory behavior: A critique and experimental observations. Malacologia, 27:291-298.
Lau, D.C.P. and Leung, K.M.Y. 2004. Feeding physiology of the carnivorous gastropod Thais clavigera (Kuster): do they eat ''soup''? Journal of Experimental Marine Biology and Ecology, 312:43-66.
Lomovasky, B., Brey, T., and Morriconi, E. 2005. Population dynamics of the venerid bivalve Tawera gayi (Hupé, 1854) in the Ushuaia Bay, Beagle Channel. Journal of Applied Ichthyology, 21:64-69.
Martinell, J., Domenech, R., Aymar, J., and Kowalewski, M. 2010. Confamiliar predation in Pliocene naticid gastropods from southern France: Utility of preexisting collections in Quantitative Paleoecology. Palaios, 25:221-228.
Merle, D., Garrigues, B., and Pointier, J.-P. 2011. Fossil and Recent Muricidae of the World, Part Muricinae. ConchBooks, Hackenheim.
Morton, B. 1987, Juvenile growth of the South China Sea whelk Hemifusus tuba (Gmelin) (Prosobranchia: Melongenidae) and the importance of sibling cannibalism in estimates of consumption. Journal of Experimental Marine Biology and Ecology, 109:1-14.
Osorio, C. 2002. Moluscos marinos en Chile, especies de importancia económica. Guía para su identificación. Facultad de Ciencias, Universidad de Chile. p. 1-110.
Paine, R.T. 1966. Food web complexity and species diversity. The American Naturalist, 100:65-75.
Polis, G.A. 1981. The evolution and dynamics of intraspecific predation. Annual Review of Ecology and Systematics, 12:225-251.
Powell, E.N. and Stanton, R.J., Jr. 1985. Estimating biomass and energy flow of molluscs in palaeo-communities. Paleontology, 28:1-34.
Rabassa, J., Heusser, C., and Stuckenrath, R. 1986. New Data on Holocene Sea Transgression in the Beagle Channel: Tierra del Fuego, Argentina. Quaternary of South America and Antarctic Peninsula, 4:291:309.
Rabassa, J., Coronato, A., Gordillo, S., Candel, M., and Martinez, M. 2009. Paleoambientes litorales durante la trasgresión marina Holocena en Bahía Lapataia, Canal Beagle, Parque Nacional Tierra del Fuego, Argentina. Revista de la Asociación Geológica Argentina, 65:648-659.
Rilov, G., Benayahu, Y., and Gasith, A. 2004. Life on the edge: do biomechanical and behavioral adaptations to wave-exposure correlate with habitat partitioning in predatory whelks? Marine Ecology Progress Series, 282:193-204.
Roseghini, M., Severini, C., Erspamer, G.F., and Erapamer, V. 1996. Choline esters and biogenic amines in hypobranchial gland of 55 molluscan species of the neogastropod Muricoidea superfamily. Toxicon, 34:33-55.
Rudolf, V.H.W. 2008. The impact of cannibalism in the prey on predator-prey systems. Ecology, 89:3116-3127.
Serralta, D. 2010. Predación sobre Polinices marambioensis (Gastropoda, Naticidae) en el alomiembro Cucullaea (Formación La Meseta), Eoceno de la Península Antártica. Unpublished MS thesis, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de La Pampa, Argentina.
Spanier, E. 1986. Cannibalism in muricid snails as a possible explanation for archaeological findings. Journal of Archaeological Science, 13:463-468.
Spanier, E. 1987. A fossil record of shell boring: possible evidence for sea level changes in the Red Sea. Estuarine, Coastal and Shelf science, 24:873-879.
Stanton, R.J. and Nelson, P.C. 1980. Reconstruction of the Trophic Web in Paleontology: Community Structure in the Stone City Formation (Middle Eocene, Texas). Journal of Paleontology, 54:118-135.
Vasconcelos, P., Pereira, M., Constantino, R., Barroso, C.M., and Gaspar, M.B. 2012. Growth of the purple dye murex, Bolinus brandaris (Gastropoda: Muricidae), marked and released in a semi-intensive fish culture earthen pond. Scientia Marina, 76:67-78.
Vermeij, G.J. 1980. Drilling predation of bivalves in Guam: Some paleoecological considerations. Malacologia, 19:329- 334
West, D.J., Andrews, E.B., Mcvean, A.R., Osborne, D.J., and Thorndyke, M.C. 1994. Isolation of serotonin from the accessory salivary glands of the marine snail Nucella lapillus. Toxicon, 32:1261-1264.