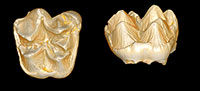
 Deciduous dentition and dental eruption sequence of Bothriogenys fraasi (Anthracotheriidae, Artiodactyla) from the Fayum Depression, Egypt
Deciduous dentition and dental eruption sequence of Bothriogenys fraasi (Anthracotheriidae, Artiodactyla) from the Fayum Depression, Egypt
Article number: 19.3.38A
https://doi.org/10.26879/646
Copyright Palaeontological Association, October 2016
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 13 February 2016. Acceptance: 16 August 2016
{flike id=1595}
ABSTRACT
Paleogene anthracotheres are poorly documented from Afro-Arabian localities. This is due, in large part, to the fragmentary nature of the specimens that have been described. However, sediments in the Jebel Qatrani Formation, Fayum Depression, Egypt, preserve the richest anthracothere assemblage in all of Afro-Arabia. Unlike other samples, the Fayum collection includes many complete dentitions, skulls, and partial skeletons. Based on these extensive collections, this study provides the first description of the complete deciduous dentition and dental eruption sequence for the early Oligocene anthracothere Bothriogenys fraasi. A detailed discussion concerning the pattern and timing of dental growth in B. fraasi is provided, and the ontogenetic sequence documented for B. fraasi is compared with those available for suoids and hippos, the two extant groups currently considered as possible sister taxa to anthracotheres. Results show that anthracotheres and suoids share a more similar dental emergence pattern, and one that may be close to the primitive condition for artiodactyls, while hippos have a very different dental eruption sequence as a consequence of their highly divergent life history pattern. As a growing body of life history research indicates that taxa in close phylogenetic proximity may be expected to share features of their dental developmental pattern, this finding suggests a useful test of competing hypotheses of a relationship between Anthracotheriidae and either Hippopotamidae or Suiformes can potentially be developed based on eruption patterns.
Hesham M. Sallam, Mansoura University Vertebrate Paleontology Center, Department of Geology, Mansoura University, Mansoura, 35516, Egypt, and Department of Evolutionary Anthropology, Duke University, Durham, NC 27708, USA sallam@mans.edu.eg
Afifi H. Sileem, Vertebrate Paleontology Section, Cairo Geological Museum, Cairo, Egypt, afifi.sileem@yahoo.com
Ellen R. Miller, Department of Anthropology, Wake Forest University, Winston-Salem, NC 27106, USA, millerer@wfu.edu
Gregg F. Gunnell, Division of Fossil Primates, Duke Lemur Center, Durham, NC 27705, USA, gregg.gunnell@duke.edu
Keywords: Fayum; Egypt; Oligocene; juvenile; dentition; Artiodactyla
Final citation: Sallam, Hesham M., Sileem, Afifi H., Miller, Ellen R., and Gunnell, Gregg F. 2016. Deciduous dentition and dental eruption sequence of Bothriogenys fraasi (Anthracotheriidae, Artiodactyla) from the Fayum Depression, Egypt. Palaeontologia Electronica 19.3.38A: 1-17. https://doi.org/10.26879/667
palaeo-electronica.org/content/2016/1595-anthracotheriidae-from-egypt
INTRODUCTION
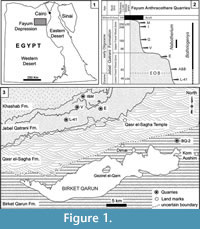 The terrestrial mammal-bearing localities of the Jebel Qatrani Formation, Fayum Depression, Egypt, including the well-known quarries L-41, A, B, E, V, I, and M, are among the most productive Paleogene fossil localities known anywhere in Africa (Figure 1). The Jebel Qatrani deposits range in age from late Eocene through early Oligocene (~34 to ~30 Ma) (Kappelman et al., 1992; Seiffert, 2006; Seiffert et al., 2008), and fossils recovered from the Jebel Qatrani have profoundly influenced our understanding of primate (e.g., Seiffert et al., 2010; Seiffert, 2012), and mammalian evolution (e.g., Simons and Rasmussen, 1990; Simons, 2008). However, relatively few of these mammalian studies have focused on anthracotheres (Andrews, 1906; Schmidt, 1913; Black, 1978; Holroyd et al., 1996; Ducrocq, 1997; Sileem et al., 2015; Sileem et al., 2016), despite the fact that anthracotheres are among the most abundant fossils recovered from Fayum deposits.
The terrestrial mammal-bearing localities of the Jebel Qatrani Formation, Fayum Depression, Egypt, including the well-known quarries L-41, A, B, E, V, I, and M, are among the most productive Paleogene fossil localities known anywhere in Africa (Figure 1). The Jebel Qatrani deposits range in age from late Eocene through early Oligocene (~34 to ~30 Ma) (Kappelman et al., 1992; Seiffert, 2006; Seiffert et al., 2008), and fossils recovered from the Jebel Qatrani have profoundly influenced our understanding of primate (e.g., Seiffert et al., 2010; Seiffert, 2012), and mammalian evolution (e.g., Simons and Rasmussen, 1990; Simons, 2008). However, relatively few of these mammalian studies have focused on anthracotheres (Andrews, 1906; Schmidt, 1913; Black, 1978; Holroyd et al., 1996; Ducrocq, 1997; Sileem et al., 2015; Sileem et al., 2016), despite the fact that anthracotheres are among the most abundant fossils recovered from Fayum deposits.
Anthracotheriidae is an extinct family of artiodactyls known from Eocene - Miocene deposits across Laurasia and into Africa (but not elsewhere in Gondwana). Behaviorally, they are thought to have occupied a range of browsing niches over both their long temporal and broad geographic ranges, although whatever their specific feeding ecologies were they are widely acknowledged to have been semi-aquatic based on skeletal proportions and inferred habitats where fossils are found (e.g., Black, 1978; Pickford, 1991; Holroyd et al., 2010; Miller et al., 2014). As a group, identification of the closest living anthracothere relative within Artiodactyla is contested, with some researchers favoring a suiform affiliation (e.g., Pickford, 2007), and others supporting a closer relationship with extant Hippopotamidae (e.g., Black, 1978; Boisserie and Lihoreau, 2006; O’Leary et al., 2012).
Much of the recent work on anthracotheres has been concerned with establishing their alpha taxonomy, as reflected in observable differences among adult dental morphologies (e.g., Lihoreau and Ducrocq, 2007, but for exceptions see Pickford, 2006; 2008). However, many of these investigations have been conducted without adequate material. The mandibular and maxillary material available in many museum collections preserves jaws with only molars and premolars, while many of the most striking differences among anthracothere taxa are manifest in their anterior dentition (e.g., presence/absence of diastemata, reduced v. caniniform canines, relative size, and orientation of incisors), even when the morphology of the cheek teeth remains more conservative.
In contrast, the collection of anthracothere material from the Jebel Qatrani Formation is comprised of a large number of anthracothere specimens (N= ~2270, including isolated teeth and postcranial elements), that are currently recognized as representing six species in three genera. A fairly large proportion of these preserve at least part of the anterior dentition and 12% (N=33/270) of the specimens attributed to B. fraasi are juveniles.
Here we provide the first documentation of the deciduous dentition and dental eruption sequence of the anthracothere, Bothriogenys fraasi, a taxon known from the early Oligocene upper sequence in the Jebel Qatrani Formation. This work represents the first detailed account of an ontogenetic sequence for any anthracothere taxon and is made possible by the decades of fieldwork in the Fayum that recovered such a large sample of remarkably well-preserved juvenile specimens. Results from this investigation into the pattern and timing of dental growth in B. fraasi will serve as a reference for comparison with other anthracothere species, and provides empirical information from growth and development towards resolving the phylogenetic relationships within anthracotheres, and between anthracotheres and other artiodactyl groups.
MATERIAL AND METHODS
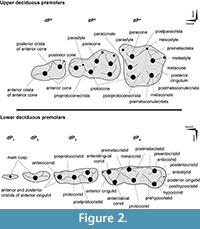
Tooth position is referenced as dI, dC, dP, P, and M (for deciduous incisors, canine, premolars, and permanent premolars and molars, respectively), with upper and lower teeth designated by superscript and subscript numbers (respectively), such that, for example, dP4 is the fourth upper deciduous premolar. We follow the nomenclature of Bärmann and Rössner (2011) for dental features (cusps and crests) of upper and lower deciduous premolars (Figure 2).
 In the following we refer to the first premolar of Bothriogenys as dP1/1. Based on the available material, there is no evidence for P1/1 replacement suggesting that Bothriogenys fraasi retained a dP1/1 into adulthood. Morphology is not especially helpful to determine whether or not the retained tooth is deciduous or permanent because, unlike dP3/3 and dP4/4, dP 2/2 is neither molariform or of an exaggerated form but more like permanent P2/2 suggesting that the first deciduous premolar would be simple as well.
In the following we refer to the first premolar of Bothriogenys as dP1/1. Based on the available material, there is no evidence for P1/1 replacement suggesting that Bothriogenys fraasi retained a dP1/1 into adulthood. Morphology is not especially helpful to determine whether or not the retained tooth is deciduous or permanent because, unlike dP3/3 and dP4/4, dP 2/2 is neither molariform or of an exaggerated form but more like permanent P2/2 suggesting that the first deciduous premolar would be simple as well.
It is not uncommon for mammals (some pigs, hippopotamus, horses, hyraxes) to retain deciduous first premolars that are not replaced (Zeigler, 1971; Luckett, 1993; Smith, 2000). Only Tapirus and maybe some archaeocete whales show evidence of replacement of the first premolar among mammals and in the case of the latter the evidence is not compelling (Luckett, 1993; Uhen, 2000). Therefore, the first upper and lower premolars of Bothriogenys fraasi are here considered to be retained deciduous teeth.
All specimens are cataloged in the Duke University Lemur Center, Division of Fossil Primates and designated with the prefix DPC. The studied materials were scanned using a Nikon XT H 225 ST micro-CT scanner housed at Duke University’s Shared Materials Instrumentation Facility, and three-dimensional reconstructions were rendered in Avizo v.8. Dental measurements were taken from digital surface models (in Stanford “ply” format). All 3D digital materials are available for viewing and direct download at www.morphosource.org, (morphosource.org/index.php/Detail/ProjectDetail/Show/project_id/224). See also Table 1 for digital object identifiers, DOIs that directly link to 3D digital media associated with each specimen. More details yet are provided in Supplemental Material. Reuse of these data should cite MorphoSource, the DOI and this paper.
DESCRIPTION
Upper Deciduous Teeth
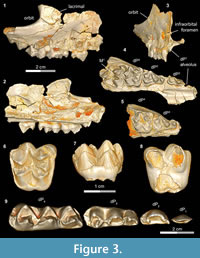 A complete upper and lower deciduous premolar dentition for Bothriogenys fraasi is available as a composite from specimens recovered from Fayum Quarries I, M, and O. DPC 5167 (Figure 3.1-4, Table 1) is a right maxillary fragment with light post-mortem distortion that has led to minor surface cracks and displacements. However, the specimen clearly preserves the alveolus for dP 1, and crowns of dP2 -M1 , with M1 half exposed. The upper deciduous second premolar in DPC 5167 is triangular, has two roots, and is separated from the mesial aspect of dP3 by a very short diastema. The crown of the tooth is convex labially and concave lingually, is longer than it is wide, and has an oval occlusal outline, owing to a slightly wider posterior region. The crown bears two main cusps, the anterior of which is larger and occupies the mesial part of the tooth, and the posterior cusp is smaller and occupies the distolabial portion of the crown. The anterior cusp has a pyramid shape with three crests, the mesial of which is curved and joins a very short and low anterocingulum. The distolabial crest of the anterior cusp is longer than the mesial crest, and slopes down to meet the mesial crest of the posterior cusp, creating a small inverted V-shape on the labial border of the tooth. The distolingual crest of the anterior cusp is straight and short and runs distolingually. The distal crest of the posterior cusp has the same length as the mesial crest, and runs distally to end as a small projection in the distolabial corner of the crown. There is a low and weakly developed but broad cingulum extending around the lingual margin of the posterior cusp that connects distally with the distal crest of the posterior cusp, and joins the distolingual crest of the anterior cusp mesially. The dP2 is the least worn of the upper deciduous dentition.
A complete upper and lower deciduous premolar dentition for Bothriogenys fraasi is available as a composite from specimens recovered from Fayum Quarries I, M, and O. DPC 5167 (Figure 3.1-4, Table 1) is a right maxillary fragment with light post-mortem distortion that has led to minor surface cracks and displacements. However, the specimen clearly preserves the alveolus for dP 1, and crowns of dP2 -M1 , with M1 half exposed. The upper deciduous second premolar in DPC 5167 is triangular, has two roots, and is separated from the mesial aspect of dP3 by a very short diastema. The crown of the tooth is convex labially and concave lingually, is longer than it is wide, and has an oval occlusal outline, owing to a slightly wider posterior region. The crown bears two main cusps, the anterior of which is larger and occupies the mesial part of the tooth, and the posterior cusp is smaller and occupies the distolabial portion of the crown. The anterior cusp has a pyramid shape with three crests, the mesial of which is curved and joins a very short and low anterocingulum. The distolabial crest of the anterior cusp is longer than the mesial crest, and slopes down to meet the mesial crest of the posterior cusp, creating a small inverted V-shape on the labial border of the tooth. The distolingual crest of the anterior cusp is straight and short and runs distolingually. The distal crest of the posterior cusp has the same length as the mesial crest, and runs distally to end as a small projection in the distolabial corner of the crown. There is a low and weakly developed but broad cingulum extending around the lingual margin of the posterior cusp that connects distally with the distal crest of the posterior cusp, and joins the distolingual crest of the anterior cusp mesially. The dP2 is the least worn of the upper deciduous dentition.
The dP3 is well-preserved in DPC 5167 and DPC 20439 (Figure 3.1-5). The dP3 is longer than wide, and has a roughly triangular outline with a narrower anterior portion and a broader posterior one. The occlusal surface has five major cusps (anterior cone, protocone, paracone, metacone, and metaconule), all of which are more or less equal in size, and all of which are about the same height, except for the paracone, which is somewhat smaller. The anterior cone occupies the most mesial portion of the crown and bears three crests. The lingual crest originates at the apex and runs lingually, curving distally to end at the base of the protocone and so enclosing a lingual anterior valley. There are two labial crests running from the anterior cone, which together delimit a small fovea on the labial border of the anterior cone. The protocone is the highest cusp in the crown and is situated distal to the anterior cone. The protocone has a conical shape with a mesial crest that joins the distal crest of the anterior cone at a level lower than the apex, creating a V-shaped crest that helps close the anterior valley labially. The postparacrista slopes down distolabially to merge with the mesostyle, and the premetacrista slopes down mesolabially to connect with the mesostyle. Together these crests form an inverted V- shape that protrudes as a mesostyle and weakly closes the posterior valley labially. The mesostyle is crestiform, weakly developed, and has a worn surface on both specimens. The metacone is pyramid shaped and bears three crests, the distal of which runs distally and interrupts the posterior cingulum. The premetacrista is the mostly heavily worn crest on the crown. The metaconule is transverse to the metacone and connected to it via a short crest. The postmetaconulecrista runs distolabially and ends at the midpoint of the posterior cingulum. The premetaconulecrista runs mesially and terminates at the base of the protocone, closing the posterior valley lingually. The posterior valley of dP3 is broader and longer than the anterior valley. The posterior cingulum is relatively well developed, in particular the labial aspect and starts from the base of the mesostyle, courses around the posterior margin of the tooth, and terminates at the base of the protocone.
The dP4 occlusal surface is nearly identical to that of the first upper molar, differing only in being relatively smaller and having less robust cusps and crests. DPC 3224 (Figure 3.6-8) is the most well-preserved dP 4 in the available material. The dP4 has a semi-quadrate outline and four main cusps (paracone, protocone, metacone, and metaconule), all of which are about equal in size. The mesostyle is well developed, placed slightly distal of center on the labial wall of the tooth, and extends farther labially than the parastyle. The lingual side of the mesostyle is invaded by the postparacrista and premetacrista, extending the protofossa labially in a V-shape between the paracone and metacone. There is no cingulum that runs distally from the mesostyle to meet the postmetacrista as is seen in M1. The parastyle is distinct and crestiform and forms the mesiolabial corner of the crown. The paraconule is distinct, triangular in shape, and situated at the midpoint between the paracone and the protocone, from which it is separated by deep, narrow notches. The preparacristule runs down mesiolabially toward the base of the paracone, whereas the postparacristule runs distally and fades before reaching the main valley of the crown. The preprotocrista is short and runs mesially to connect with the lingual base of the paraconule, and there are two postprotocristae that run distolabially. The metaconule bears three cristae; the premetacristule runs mesiolabially toward the main valley and connects with the most lingual postprotocrista, interrupting the course of the main valley. The postmetacristule is well developed, runs distolabially, and fuses with the distal cingulum. The lingual metacristule becomes a continuation of the mesial portion of the lingual cingulum. The distal cingulum is moderately developed, extends lingually, and courses around the lingual base of the metaconule, forming the distal portion of the lingual cingulum. The mesial cingulum is well developed but low, and runs lingually from the parastyle. In DPC 5167, the cingulum courses around the lingual base of the protocone and merges with the lingual metacristule, while in DPC 3224 and 20439, the cingulum terminates at the mesial base of the protocone. The main valley is closed lingually either via a low connection between the base of the protocone and the metaconule, or by a continuous lingual cingulum.
Lower Deciduous Teeth
DPC 11416 (Figure 3.9, Figure 4) preserves the complete lower deciduous premolars series (dP1-4) and several other juvenile mandibular specimens of Bothriogenys fraasi preserve parts of the deciduous series (Table 1).
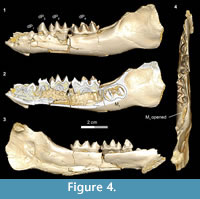 The dP1 is visible in DPC 11416 but the tooth is not completely erupted. The dP1 is a small, peg-like tooth, with a labiolingually compressed crown and an oval base. The dP1 crown has one main cusp, from which run mesial and distal cristids, the distal one being longer than the mesial one. The tooth is single-rooted, convex laterally, concave medially, and curves distally.
The dP1 is visible in DPC 11416 but the tooth is not completely erupted. The dP1 is a small, peg-like tooth, with a labiolingually compressed crown and an oval base. The dP1 crown has one main cusp, from which run mesial and distal cristids, the distal one being longer than the mesial one. The tooth is single-rooted, convex laterally, concave medially, and curves distally.
The dP2 is a double-rooted tooth with one main cusp that has its apex in the middle of the crown. The tooth has a triangular shape in lateral view. The labial surface of the crown is convex, and the lingual surface is concave. The crown bears two main cristids. The distal cristid in some cases branches into two cristids, leading to a relatively narrow groove. On the distal border, there is a weakly developed cingulid that extends mesially onto the lingual border of the crown.
The dP3 is double-rooted molariform with a crown length greater than width and has a generally rhomboidal outline. The occlusal surface is occupied by four major cusps (anteroconid, protoconid, entoconid, and hypoconid). The anteroconid is concave lingually and convex labially. In some cases, it bears three cristids, the two mesial ones of which delimit a small depression similar to the corresponding fovea on the dP3. The mesial cristid of the anteroconid slopes down toward the mesiolingual corner of the crown and forms the very mesial tip of the tooth. The distal cristid of the anteroconid is relatively weakly developed when compared with the mesial cristid. The labial cingulid is low and well developed, and runs from the labial base of the protoconid coursing around the anteroconid (in some specimens), and meets with the distal cristid of the anteroconid. The protoconid is the largest and tallest cusp and is situated distal to the anteroconid, from which it is separated by a narrow and deep notch. The labial side of the protoconid is concave, and the lingual side is somewhat convex, with low and well-developed lingual cingulid . The hypoconid is relatively large, occupies the distolabial corner of the crown, and has relatively well-developed prehypocristid and posthypocristid, which run mesiolingually and distolingually, respectively, from its tip. The entoconid is the smallest cusp and it is positioned transversely relative to the hypoconid, from which it is separated by a deep and narrow valley. The preentocristid meets the prehypocristid mesially, both of which join the postprotocristid at the posterior valley. The postentocristid runs distolabially from the entoconid to connect with the posthypocristid distally, and both continue as a single cristid that connects to the distal cingulid, forming the distal tip of the crown. In some specimens, the entoconid lacks a postentocristid. The distal cingulid is relatively broad and limited to the posterior border of the tooth.
The dP4 is larger than dP3, it is longer than it is wide, and the tooth is rhomboidal in occlusal outline, with a wide talonid and a narrow trigonid. The tooth bears six major cusps (anterolingual conid, anterolabial conid, metaconid, entoconid, protoconid, and hypoconid). On the occlusal surface, the lingual cusps align mesodistally, and are slightly higher than the labial cusps. The mesial sides of the latter cusps show some wear in specimens of more advanced aged. On the lingual border of the tooth, there are two deep V-shaped notches separating the lingual cusps, while on the labial border, two wide and deep sinuses separate the labial cusps. The entostylid is distinct and the smallest cusp of the crown. The anterolingual conid is placed mesial to the anterolabial conid, from which it is separated via a narrow and deep notch. The cusp has three cristids, the most mesial of which runs mesiolingually, forming the mesial tip of the crown. The labial cristid of the anterolingual conid runs mesiolabially to join the mesial cristid of the anterolabial conid, forming a V-shape at the mesiolabial corner of the crown. The distal cristid is directed toward the base of the metaconid. The anterolabial conid has two cristids, the distal cristid of which slopes down distolingually to attach to the junction of the preprotocristid, the premetacristid, and the distal cristid of mesiolingual conid. The metaconid has three cristids (mesial, distal, labial), is situated transverse to the protoconid, and is separated from the protoconid by a deep, narrow valley. The postprotocristid meets with the labial cristid of the metaconid at a high level on the crown. The postmetacristid is distally oriented and slopes down from the tip of the cusp to reach the distal basin of the crown. The entoconid has three main cristids; the preentocristid slopes down from the tip of the tooth to end at the base of the postmetacristid, leaving the distal basin open lingually; the postentocristid is directed distally and curves to connect with the posthypocristid, forming a V-shape and closing the basin between hypoconid and entoconid distally; the labial cristid of the entoconid runs mesiolabially and ends at the base of the hypoconid. The prehypocristid is well developed and slopes down from the hypoconid toward the junction between the postprotocristid and the postmetacristid. The distal cingulid is well developed but short, and bears a distinct small entostylid, which is connected mesially with the tip of the distal V-shape pattern of the tooth via a short crestid.
TOOTH ERUPTION SEQUENCE AND COMPARISONS
A nearly complete lower tooth eruption sequence can be documented for Bothriogenysfraasi (Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Table 2). This eruption sequence can be compared with that of the extant Hippopotamus (Laws, 1968), and the European wild boar Sus scrofa (Matschke, 1967). Information about the dental eruption sequence for both taxa is available, and both taxa represent higher level groups that have been frequently cited as being sister taxa to or derived from anthracotheres (Geisler and Uhen, 2003; Pickford, 2008; Boisserie et al., 2010; Lihoreau et al., 2015).
In the developmentally earliest stage (Figure 4.1-4), Bothriogenys fraasi exhibits an exposed dP1 crown tip, a nearly completely erupted dP2, fully erupted dP3-4, and an M1 visible in the crypt but not yet having begun to erupt. There is no evidence of a C1 -dP1 diastema at this point, which must represent a very early post-partum stage. This stage roughly corresponds to Laws’ (1968) Hippopotamus groups I and II, which he interprets to represent ages between birth and six months, respectively. Laws (1968) noted that in hippos only the second through fourth deciduous premolars were replaced but that there was no evidence that the first deciduous premolar was replaced, only lost through ontogeny.
There are some differences between Bothriogenys and Hippopotamus during these early stages of development. The first deciduous premolar in Hippopotamus erupts before dP2, while it has clearly just begun to erupt when dP2 is nearly fully erupted in Bothriogenys. As in Hippopotamus, dP1 is not replaced in Bothriogenys (or in any other Fayum anthracothere for which there is data) but unlike Hippopotamus, Bothriogenys does not lose dP1 later in ontogeny, typically retaining it throughout life.
When compared with Sus, Bothriogenys stage I roughly corresponds to the documented sequence of eruption for the extant taxon but there are differences. Bothriogenys stage I documents the beginning of eruption of dP1, full eruption of the other three deciduous premolars, and M1 has yet to appear. In Sus, following the full eruption of dP2-4 (which occurs by 100 days), M1 is in place at ~172 days followed by P1 (or dP1) at ~204 days. Thus, the first permanent molar erupts before P1 (or dP1) in Sus while the opposite is true in Bothriogenys. Bothriogenys stage I also shows no wear on the deciduous premolars.
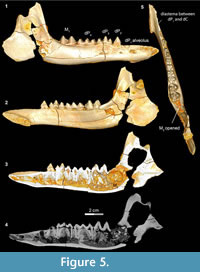 Stage II in Bothriogenys (Figure 5) documents the beginning of the development of the C1 -dP1 diastema and roughly corresponds to Laws’ (1968) Hippopotamus group III, which occurs at approximately one year of age. In Bothriogenys, dP1-4 are fully erupted as is M1, while M 2 is just appearing in the crypt but has not yet begun to erupt, and there is no opening on the surface of the mandible to indicate its presence. As in Hippopotamus, dP4 shows relatively heavy wear at this stage but unlike in Hippopotamus dP3 remains unworn in Bothriogenys. In Sus, the equivalent of Bothriogenys stage II is reached by an age of about 33 weeks (roughly six months of age).
Stage II in Bothriogenys (Figure 5) documents the beginning of the development of the C1 -dP1 diastema and roughly corresponds to Laws’ (1968) Hippopotamus group III, which occurs at approximately one year of age. In Bothriogenys, dP1-4 are fully erupted as is M1, while M 2 is just appearing in the crypt but has not yet begun to erupt, and there is no opening on the surface of the mandible to indicate its presence. As in Hippopotamus, dP4 shows relatively heavy wear at this stage but unlike in Hippopotamus dP3 remains unworn in Bothriogenys. In Sus, the equivalent of Bothriogenys stage II is reached by an age of about 33 weeks (roughly six months of age).
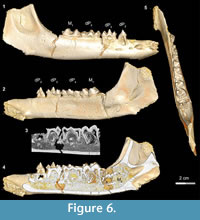 Stage III in Bothriogenys (Figure 6) shows the continuation of mandibular growth as the C1 -dP1 diastema approaches adult length. The early beginning of crown formation for permanent P2-4 can be seen in scanned images and their apices point horizontally. The M2 crypt is open on the mandibular surface so that M2 is visible. This stage corresponds roughly to Laws’ (1968) Hippopotamus groups IV and V (ages three to four years, respectively). Like in Hippopotamus group IV, dP3, and dP4 both show relatively heavy wear, and M1 has also begun to wear but M2 is not visible on the mandibular surface in Hippopotamus group IV. By Hippopotamus group V, the M2 is visible in its crypt but this group differs from Bothriogenys stage III in having both permanent P2 and P3 visible on the surface whereas they have barely begun to form in Bothriogenys. This stage corresponds to roughly one year of age in Sus (Matschke, 1967).
Stage III in Bothriogenys (Figure 6) shows the continuation of mandibular growth as the C1 -dP1 diastema approaches adult length. The early beginning of crown formation for permanent P2-4 can be seen in scanned images and their apices point horizontally. The M2 crypt is open on the mandibular surface so that M2 is visible. This stage corresponds roughly to Laws’ (1968) Hippopotamus groups IV and V (ages three to four years, respectively). Like in Hippopotamus group IV, dP3, and dP4 both show relatively heavy wear, and M1 has also begun to wear but M2 is not visible on the mandibular surface in Hippopotamus group IV. By Hippopotamus group V, the M2 is visible in its crypt but this group differs from Bothriogenys stage III in having both permanent P2 and P3 visible on the surface whereas they have barely begun to form in Bothriogenys. This stage corresponds to roughly one year of age in Sus (Matschke, 1967).
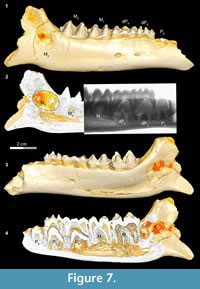 In Bothriogenys stage IV (Figure 7) permanent P2 is now exposed on the surface, P3 and P4 have not yet begun to erupt but their crowns are fully formed beneath dP3-4, M1-2 are fully erupted, and M3 is visible in its crypt. There is moderate to heavy wear present on dP3-4 and M1, but M2 remains unworn or only slightly worn at this stage. This stage corresponds to Hippopotamus groups VII and VIII (ages 8 to 11 years, respectively) as documented by Laws (1968). The main differences with Hippopotamus are that Bothriogenys has not erupted permanent P2-4 yet but does have a fully erupted M2, which does not occur in Hippopotamus until group VIII. By Hippopotamus group VIII, only dP4 remains from the deciduous dentition, dP1 is lost (may happen as early as group VI), and M3 is still not visible on the surface of the mandible.
In Bothriogenys stage IV (Figure 7) permanent P2 is now exposed on the surface, P3 and P4 have not yet begun to erupt but their crowns are fully formed beneath dP3-4, M1-2 are fully erupted, and M3 is visible in its crypt. There is moderate to heavy wear present on dP3-4 and M1, but M2 remains unworn or only slightly worn at this stage. This stage corresponds to Hippopotamus groups VII and VIII (ages 8 to 11 years, respectively) as documented by Laws (1968). The main differences with Hippopotamus are that Bothriogenys has not erupted permanent P2-4 yet but does have a fully erupted M2, which does not occur in Hippopotamus until group VIII. By Hippopotamus group VIII, only dP4 remains from the deciduous dentition, dP1 is lost (may happen as early as group VI), and M3 is still not visible on the surface of the mandible.
 Bothriogenys stage IV documents an eruption pattern similar to that seen in Sus. At this stage, Bothriogenys has P2 erupting but retains dP 3-4 and has a fully erupted M1-2. In Sus, at the point where M 2 is fully erupted (at ~385 days) dP2-4 are still retained, and there are no signs of permanent premolars (except for P13. In Sus the permanent premolars are fully erupted by ~490 days.
Bothriogenys stage IV documents an eruption pattern similar to that seen in Sus. At this stage, Bothriogenys has P2 erupting but retains dP 3-4 and has a fully erupted M1-2. In Sus, at the point where M 2 is fully erupted (at ~385 days) dP2-4 are still retained, and there are no signs of permanent premolars (except for P13. In Sus the permanent premolars are fully erupted by ~490 days.
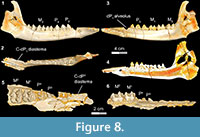
Stage V in Bothriogenys (Figure 8) documents the appearance of the full adult dentition and the completion of mandibular growth wherein the C1 -dP1 diastema reaches its maximum length. DPC 10677, right partial maxilla with P2 -M 1-2, shows that P3 and P4 are exposed simultaneously, which represents the early phase of stage V. At this stage only M1 exhibits moderate wear, while all other cheek teeth essentially have no evidence of wear. This stage is reached by group XII in Hippopotamus, which occurs at 22 years. At this point in Hippopotamus, M1 and M2 are exhibiting heavy wear and cusp tips are beginning to wear on P2-4 and M3. In Sus, a fully adult dentition is acquired by ~755 days with the eruption of M3 .
DISCUSSION AND CONCLUSIONS
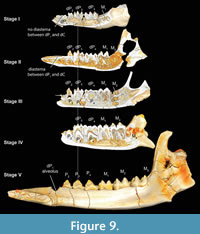 From the previous description, it is clear that the Bothriogenys tooth eruption sequence (Figure 9) differs from both those of Hippopotamus and wild boars (Sus). The main difference with Hippopotamus is that the anthracothere retains all deciduous premolars through the period when the third molar becomes visible in its crypt on the mandibular surface. Only the crown of permanent P2 becomes visible at the end of this sequence (Figure 9, Stage IV). In Hippopotamus, no deciduous premolars remain by the time M3 has appeared in its crypt on the mandibular surface, and all three permanent premolars (and dP1) are fully erupted. With regard to the overall sequence of premolar versus molar eruption, Bothriogenys is more similar to Sus than it is to Hippopotamus, except for the sequence of erupting the first deciduous premolar before M1 in Bothriogenys, and Sus doing the opposite.
From the previous description, it is clear that the Bothriogenys tooth eruption sequence (Figure 9) differs from both those of Hippopotamus and wild boars (Sus). The main difference with Hippopotamus is that the anthracothere retains all deciduous premolars through the period when the third molar becomes visible in its crypt on the mandibular surface. Only the crown of permanent P2 becomes visible at the end of this sequence (Figure 9, Stage IV). In Hippopotamus, no deciduous premolars remain by the time M3 has appeared in its crypt on the mandibular surface, and all three permanent premolars (and dP1) are fully erupted. With regard to the overall sequence of premolar versus molar eruption, Bothriogenys is more similar to Sus than it is to Hippopotamus, except for the sequence of erupting the first deciduous premolar before M1 in Bothriogenys, and Sus doing the opposite.
It is unclear why Hippopotamus exhibits an accelerated eruption of its permanent premolars relative to that seen in Bothriogenysand Sus, however, this is a somewhat common phenomenon in longer lived species Smith (2000), referred to the pattern of permanent teeth erupting earlier in more slow growing, longer-lived species as Schultz’s Rule. She found evidence to support Schultz’s Rule across a broad array of mammalian taxa including many species of ungulates, hippopotamus among them. Based on Smith’s 2000 study, it is reasonable to postulate that artiodactyl groups that show this shift in eruption patterns (such as that seen in Hippopotamus) may represent a specialization within the order. Slower loss of the deciduous cheek tooth dentition (relative to permanent tooth eruption) therefore may be primitive for artiodactyls, given that both Bothriogenys and Sus exhibit this pattern.
Part of the explanation for these differences in eruption pattern probably has to do with differences in longevity as suggested by Schultz’s Rule (Smith, 2000). Based on differences in body mass (Eisenberg, 1981), members of Hippopotamus almost certainly have longer life spans than did members of Bothriogenys, and because of this it may benefit members of Hippopotamus to have permanent premolars in place to function in conjunction with the molars in a manner different from that of anthracotheres, especially later in their lives. Given the similarity in body size between Bothriogenys fraasi and Sus scrofa, it is a reasonable assumption that Bothriogenys probably reached reproductive age by the middle of the second year of life and had a life span of about 12 years (Myers et al., 2016), quite different from that seen in Hippopotamus (reproductive age at three and half years and life span of ~55 years).
Examining Laws (1968) Hippopotamus groups XII through XX, it is clear that premolars, based on increasing wear, begin to participate in food processing by age 22 and become important alternative agents for processing as molars become heavily worn. Given that anthracotheres did not live as long as hippos, the permanent premolars were probably less important as alternative food processing agents and may have been more important in food acquisition and initial preparation.
Further evidence that Hippopotamus and anthracotheres use their permanent premolars differently can be seen in the wear patterns of premolars versus molars in the two groups. In Hippopotamus of advanced age (past the age of 35) all cheek teeth show signs of heavy apical wear such that all teeth become flattened and dentine becomes exposed on the surface. In anthracotheres (at least in all documented Fayum forms), similar wear can be found on the molars but the permanent premolars never exhibit apical wear and, in fact, seldom exhibit any wear at all except along crest surfaces. Premolars remain lightly worn, even in individuals of advanced age. This suggests that anthracothere premolars were used for initial acquisition and slicing of vegetation but were not employed for crushing and grinding functions.
In sum, new information presented here about the dental eruption sequence of Bothriogenys fraasi, and comparisons among B. fraasi, Sus, and Hippopotamus, shows that, at least anthracotheres and hippos, have very different dental emergence sequences as a consequence of having highly divergent life history patterns. This finding suggests that a useful test of the hypothesis that Hippopotamidae and bothriodontine anthracotheres may be closely related (Orliac et al., 2010; Lihoreau et al., 2015) can be executed when more complete juvenile dentitions of relevant anthracotheres and early hippos are found. A growing body of life history studies indicate that taxa in close phylogenetic proximity can be expected to share features of their dental developmental pattern (Tattersall and Schwartz, 1974; Byrd, 1981; Greenwald, 1988; Schwartz et al., 2005; Asher and Lehmann, 2008; Asher and Olbricht, 2009; Bastl et al., 2011; Cianco et al., 2012; Asher, 2013; Bastl and Nagel, 2014; Veitschegger and Sanchez-Villagra, 2015). Therefore, the lack of a shared pattern between B. fraasi, the only bothriodont anthracothere for which this information is currently available, and Hippopotamus , suggests additional evidence is required to test hypotheses of relatedness between these two groups.
ACKNOWLEDGEMENTS
We are thankful to V. Yarborough for preparing the fossils described here, C. Riddle for providing access to the fossil collection at the Division of Fossil Primates, Duke Lemur Center and J. Thostenson and C. Crawford (Duke University) for providing access to micro-CT scanning facilities. This research was funded by U.S. National Science Foundation grants BCS-0416164 to E.R. Seiffert (ERS) and E.L. Simons, BCS-0819186 (ERS) and BCS-1231288 to ERS, J.G. Fleagle, G.F. Gunnell, and D.M. Boyer. Funding was also provided by grants from The Leakey Foundation to E.R.S. This is Duke Lemur Center publication #1327 and NSF BCS 1552848 to D.M. Boyer. This paper is a contribution to project BR/121/A3/PALEURAFRICA of the Belgian Science Policy Office.
REFERENCES
Andrews, C.W. 1906. A descriptive catalogue of the tertiary vertebrata of the Fayum, Egypt. Based on the collection of the Egyptian government in the Geological Museum, Cairo, and on the collection in the British Museum (Natural History).
Asher, R. J. 2013. Dental eruption in ruminants and other mammals. Zitteliana, 31:18.
Asher, R.J. and Lehmann, T. 2008. Dental eruption in afrotherian mammals. BMC Biology, 6:14.
Asher, R.J. and Olbricht, G. 2009. Dental ontogeny in Macroscelides proboscideus (Afrotheria) and Erinaceus europaeus (Lipotyphla). Journal of Mammalian Evolution, 16:99-115.
Bärmann, E.V. and Rössner, G.E. 2011. Dental nomenclature in Ruminantia: Towards a standard terminological framework. Mammalian Biology, Zeitschriftfür Säugetierkunde, 76:762-768.
Bastl, K.A. and Nagel, D. 2014. First evidence of the tooth eruption sequence of the upper jaw in Hyaenodon (Hyaenodontidae, Mammalia) and new information on the ontogenetic development of its dentition. Paläontologische Zeitschrift, 88:481-494.
Bastl, K.A., Morlo, M., and Nagel, D. 2011. Differences in the tooth eruption sequence in Hyaenodon (‘Creodonta’: Mammalia) and implications for the systematics of the genus, Journal of Vertebrate Paleontology, 31:181-192.
Black, C. C. 1978. Anthracotheriida, p. 423-434. In Maglio V.J. and Cooke H.B.S., (eds.), Evolution of African Mammals. Harvard University Press, Cambridge.
Boisserie, J.R. and Lihoreau, F. 2006. Emergence of Hippopotamidae: new scenarios. Comptes Rendus Palevol, 5:749-756.
Boisserie, J.R., Lihoreau, F., Orliac, M., Fisher, R.E., Weston, E.M., and Ducrocq, S. 2010. Morphology and phylogenetic relationships of the earliest known hippopotamids (Cetartiodactyla, Hippopotamidae, Kenyapotaminae). Zoological Journal of the Linnean Society, 158:325-366.
Byrd, K.E. 1981. Sequences of dental ontogeny and callitrichid taxonomy. Primates, 22:103-118.
Ciancio, M.R., Castro, M.C., Galliari, F.C., Carlini, A.A., and Asher, R.J. 2012. Evolutionary implications of dental eruption in Dasypus (Xenarthra). Journal of Mammalian Evolution, 19:1-8. doi:10.1007/s10914-011-9177-7.
Ducrocq, S. 1997. The anthracotheriid genus Bothriogenys (Mammalia, Artiodactyla) in Africa and Asia during the paleogene: phylogenetical and paleobiogeographical relationships. Stuttgarter Beitrage zur Naturkunde, B (Geologie und Palaontologie), 250:1-44.
Eisenberg, J.F. 1981. The Mammalian Radiations. University of Chicago Press, Chicago.
Geisler, J.H. and Uhen, M.D. 2003. Morphological support for a close relationship between hippos and whales. Journal of Vertebrate Paleontology, 23:991-996.
Greenwald, N.S.1988. Patterns of tooth eruption and replacement in multituberculate mammals. Journal of Vertebrate Paleontology, 8:265-277.
Holroyd, P.A., Simons E.L., Bown, T.M., Polly, P.D., and Kraus M.J. 1996. New records of terrestrial mammals from the upper Eocene Qasr el Sagha Formation, Fayum Depression, Egypt. Palaeovertebrata, 23:175-192.
Holroyd, P.A., Lihoreau P., Gunnell G.F., and Miller E.R. 2010. Anthracotheriidae, p. 843-85. In Werdelin L. and Sanders W.J. (eds.), Cenozoic Mammals of Africa, University of California Press, Berkeley.
Kappleman, J. 1992. The age of the Fayum primates as determined by paleomagnetic reversal stratigraphy. Journal of Human Evolution, 22:495-503.
Laws, R.M. 1968. Dentition and ageing of the hippopotamus. East African Wildlife Journal, 6:19-52.
Lihoreau, F., Boisserie, J.R., Manthi, F.K., and Ducrocq, S. 2015. Hippos stem from the longest sequence of terrestrial cetartiodactyl evolution in Africa. Nature Communications, 6:6264. doi:10.1038/ncomms7264.
Lihoreau, F. and Ducrocq, S. 2007. Family Anthracotheriidae, p. 89-105. In Prothero D.R. and Foss S.E. (eds.), The Evolution of Artiodactyls. The Johns Hopkins University Press, Baltimore.
Luckett, W.P. 1993. An ontogenetic assessment of dental homologies in therian mammals, p. 182-204. In Szalay, F.S., Novacek, M.J., and McKenna, M.C. (eds.), Mammal Phylogeny, Volume 1. Springer, Berlin.
Matschke, G.H. 1967. Aging European wild hogs by dentition. Journal of Wildlife Management, 31:109-113.
Miller, E.R., Gunnell, G.F., Abdel Gawad, M., Hamdan, M., El-Barkooky, A.N., and Clementz, M.T. 2014. Anthracotheres from Wadi Moghra, early Miocene, Egypt. Journal of Paleontology, 88:967-981.
Myers, P., Espinosa, R., Parr, C.S., Jones, T., Hammond, G.S., and Dewey. T.A. 2016. The Animal Diversity Web (online). Accessed at animaldiversity.org.
O’Leary M.A., Patel B.A., and Coleman M.N. 2012. Endocranial petrosal anatomy of Bothriogenys (Mammalia, Artiodactyla, Anthracotheriidae), and petrosal volume and density comparison among aquatic and terrestrial artiodactyls and outgroups. Journal of Paleontology, 86:44-50.
Orliac, M., Boisserie, J.R., Lihoreau, F., and MacLatchy, L. 2010. Early Miocene hippopotamids (Cetartiodactyla) constrain the phylogenetic and spatiotemporal settings of hippopotamid origin. Proceedings of the National Academy of Sciences, USA, 107:11871-11876.
Pickford, M. 1991. Revision of the Neogene Anthracotheriidae of Africa. p. 1491-1525. In Salem M.J. (ed.), The Geology of Libya. Elsevier, Amsterdam.
Pickford, M. 2006. Sexual and individual morphometric variation in Libycosaurus (Mammalia, Anthracotheriidae) from the Maghreb and Libya. Geobios, 39:267-310.
Pickford, M. 2007. A new suiform (Artiodactyla, Mammalia) from the Early Miocene of East Africa. Comptes Rendus Palevol, 6:221-229.
Pickford, M. 2008. Libycosaurus petrocchii Bonarelli, 1947, and Libycosaurus anisae, Black, 1972 (Anthracotheriidae, Mammalia): Nomenclatural and geochronological implications. Annales de Paléontologie, 94:39-55.
Schmidt, M. 1913. Ueber Paarhufer der fluviomarinen Schichted des Fajum, odontographisches und osteologisches Material. Geologische und Paläontologische Abhandlungen, 15:153-264.
Schwartz, G.T., Mahoney, P., Godfrey, L.R., Cuozzo, F.B., Jungers, W.L., and Randria, G.F.N. 2005. Dental development in Megaladapis edwardsi (Primates, Lemuriformes): Implications for understanding life history variation in subfossil lemurs, Journal of Human Evolution, 49:702-721.
Seiffert, E.R. 2006. Revised age estimates for the later Paleogene mammal faunas of Egypt and Oman. Proceedings of the National Academy of Sciences, USA, 103:5000-5005.
Seiffert, E.R. 2012. Early primate evolution in Afro-Arabia. Evolutionary Anthropology, 21: 239-253.
Seiffert, E.R., Bown, T.M., Clyde, W.C., and Simons, E.L. 2008. Geology, paleoenvironment, and age of Birket Qarun Locality 2 (BQ-2), Fayum Depression, Egypt. p. 71-86. In Fleagle J.G. and Gilbert C.C. (eds.), Elwyn L. Simons:A Search for Origins, Springer, New York.
Seiffert, E.R., Simons, E.L., Boyer, D.M., Perry, J.M.G., Ryan, T.M., and Sallam, H.M. 2010. A fossil primate of uncertain affinities from the earliest late Eocene of Egypt. Proceedings of the National Academy of Sciences, 107:9712-9717
Sileem, A.H., Sallam, H.M., Hewaidy, A.A., Gunnell, G.F., and Miller, E.R. 2015. Anthracotheres (Mammalia, Artiodactyla) from the upper-most horizon of the Jebel Qatrani Formation, latest early Oligocene, Fayum Depression, Egypt. Egyptian Journal of Paleontology, 15:1-11.
Sileem, A.H., Sallam, H.M., Hewaidy, A.A., Miller, E.R., and Gunnell, G.F. 2016. A new anthracothere (Artiodactyla) from the early Oligocene, Fayum, Egypt, and the mystery of African ‘Rhagatherium’ solved. Journal of Paleontology , 90.1:170-181.
Simons, E.L. 2008. Eocene and Oligocene mammals of the Fayum, Egypt. p. 87-105. In Fleagle, J.G. and Gilbert, C.C. (eds.), Elwyn Simons: A Search for Origins. Springer, New York.
Simons, E.L. and Rasmussen, D.T. 1990. Vertebrate paleontology of Fayum: history of research, faunal review and future prospects, p. 627-638. In Said, R. (ed.), The Geology of Egypt. A.A. Balkema, Rotterdam.
Smith, B.H. 2000. ‘Schultz’s Rule’ and the evolution of tooth emergence and replacement patterns in primates and ungulates, p. 212-227. In Teaford, M.F., Smith, M.M., and Ferguson, M.W.J. (eds.), Development, Function and Evolution of Teeth. Cambridge University Press, Cambridge.
Tattersall, I. and Schwartz, J.H. 1974. Craniodental morphology and the systematics of the Malagasy lemurs (Primates, Prosimii). Anthropological Papers of the American Museum of Natural History, 52:139-192.
Uhen, M.D. 2000. Replacement of deciduous first premolars and dental eruption in archaeocete whales. Journal of Mammalogy, 81:123-133.
Veitschegger, K. and Sánchez-Villagra, M.R. 2015. Tooth eruption sequences in cervids and the effect of morphology, life history, and phylogeny. Journal of Mammalian Evolution. doi: 10.1007/s10914-015-9315-8.
Zeigler, A.C. 1971. A theory of the evolution of therian dental formulas and replacement patterns. Quarterly Review of Biology, 46:226-249.