 Eocene/Oligocene deep-water agglutinated foraminifers (DWAF) assemblages from the Madonie Mountains (Sicily, Southern Italy)
Eocene/Oligocene deep-water agglutinated foraminifers (DWAF) assemblages from the Madonie Mountains (Sicily, Southern Italy)
Andrea Benedetti
Article number: 20.1.4A
https://doi.org/10.26879/660
Copyright Palaeontological Association, February 2017
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 14 March 2016. Acceptance: 4 February 2017
{flike id=1746}
ABSTRACT
Quantitative and qualitative analysis of deep-water agglutinated foraminifer (DWAF) assemblages from Portella Colla (Madonie Mts.) reveal a variation of the trophic continuum in late Eocene and early Oligocene times. Twenty-nine samples were processed obtaining 138 agglutinated species, 59 of which are in open nomenclature, belonging to 46 genera attributed to four functional morphogroups according to their life position and feeding strategies. Faunal density increases upsection, whereas specific diversity fluctuates and reaches its minimum within the E/O transition. In the uppermost Eocene, cyclamminids, Haplophragmoides and Repmanina charoides, epifaunal and lower infaunal forms which prefer well-oxigenated bottom water and a normal food supply, prevail. Within the E/O transition the DWAF abundance broadly decreases and the assemblages are dominated by opportunistic taxa such as Repmanina charoides. In the lower Rupelian, suspension-feeders and assemblages rich in Paratrochamminoides are present, and hyaline taxa disappear. At the end of the lower Rupelian, Nothia, ammodiscids and hyaline foraminifers reappear, and deep infaunal morphogroup becomes dominant marking low-oxygen bottom water conditions. In the upper Rupelian, deep infaunal communities (rheophacids and Caudammina) dominate the assemblages, and epifaunal surface-dwelling foraminifers decrease. The LO of Caudammina gutta coincides with an increase in sand deposition (transition from Caltavuturo Fm. to Portella Colla Clays), a decrease in suspension feeders and surface-dwellers, and the dominance of oligotypic assemblages by Reticulophragmium rotundidorsatum. The oxygenation of bottom waters return to levels similar to those of the upper Eocene, with a medium to high nutrient supply as inferred from the abundance of cyclamminids.
Andrea Benedetti. Dipartimento di Scienze della Terra, “Sapienza” Università di Roma, P.le A. Moro 5, 00185, Italy; GIRMM -- Informal Group of Micropaleontological and Malacological Researches. andrea.benedetti@uniroma1.it
Keywords: agglutinated foraminifer; Eocene; Oligocene; paleobathymetry; ecology; climatic change; Ocean Drilling Program
Final citation: Benedetti, Andrea. 2017. Eocene/Oligocene deep-water agglutinated foraminifers (DWAF) assemblages from the Madonie Mountains (Sicily, Southern Italy). Palaeontologia Electronica 20.1.4A: 1-66. https://doi.org/10.26879/660
palaeo-electronica.org/content/2017/1746-e-o-dwaf-from-sicily
INTRODUCTION
Deep water agglutinated foraminifer (DWAF) assemblages are well known in the Carpathians flysch (e.g., Grzybowski, 1896, 1898, 1901; Geroch, 1960, 1966), from deep water core exploration (e.g., Gradstein and Berggren, 1981; Kuhnt and Collins, 1996; Kaminski et al., 2006; Kender et al., 2008), and are well documented from the Apennine (Montanaro Gallitelli, 1943, 1955, 1958; Petters and Gandolfi, 1948; Lipparini, 1951; Nicosia, 1952; Emiliani, 1954; Accordi, 1958; Dallan, 1962; Coltro, 1963; Wezel, 1966; Dallan Nardi, 1968; Morlotti and Kuhnt, 1992; Morlotti, 1998a, 1998b). Such faunal assemblages were described by Brouwer (1965) under the name “ Rhabdammina faunas” for the Cretaceous-Paleogene flysches. The record of late Eocene and early Oligocene DWAF assemblages in Italy are very scarce (Lipparini, 1951; Bellagamba and Coccioni, 1990; Morlotti and Kuhnt, 1992), whereas a more complete record is available for the Atlantic Ocean Drilling Program (ODP) exploration (e.g., Osterman and Spiegler, 1996; Kaminski et al., 2006, 2009; Kender et al., 2008, 2009; Kaminski and Ortiz, 2014).
The Eocene-Oligocene transition (EOT) was characterized by a glacial climatic and oceanographic change (e.g., Coxall and Pearson, 2007) from the shallow water seas to the deep ocean. An extended period of global cooling is widely documented (e.g., Zachos et al., 2001; Katz et al., 2008) and extinctions are recognized in different phyletic lineages (Wade and Pearson, 2008; Ortiz and Kaminski, 2012; Prothero et al., 2013; Kaminski and Ortiz, 2014).
With respect to shallow-water environments, where an abrupt change in benthic communities is well documented (Benedetti, 2010; Cotton and Pearson, 2011), deep-sea benthic foraminifers underwent a gradual extinction from the middle-late Eocene (Kaminski, 2005; Ortiz and Kaminski, 2012). The EOT was characterized by a faunal turnover among DWAF (Kaminski and Gradstein, 2005; Kaminski, 2005; Kaminski and Ortiz, 2014), possibly reflecting an abrupt drop in the calcite compensation depth (CCD) (Coxall et al., 2005); this results in a poorly documented record of DWAF from the early Oligocene (Kaminski, 2005; Kaminski and Gradstein, 2005; Cetean and Kaminski, 2011).
Some poorly investigated assemblages have been identified and preliminarly described (Benedetti and Pignatti, 2008; 2009; Benedetti, 2010) from the uppermost Eocene and lower Oligocene clays of the Caltavuturo Formation cropping out at Portella Colla (Madonie Mts., Sicily). These previous works provided new evidence of diverse Oligocene DWAF assemblages containing 74 species and defined three faunal assemblages: 1, a Cyclammina assemblage, characterized by the dominance of cyclamminids, ammodiscids, Paratrochamminoides, with some Cibicidoides, but few elongated and cylindric forms; 2, a Rhabdammina assemblage, characterizing the lower Rupelian sediments, and dominated by tubular suspension feeding forms; 3, a Caudammina assemblages dominated by elongated and seriate infaunal forms (Benedetti and Pignatti, 2008). In the sediments of Caltavuturo Fm., Benedetti and Pignatti (2008, 2009) described some taxa that are usually known from the Cretaceous to the Eocene. In particular the new species Caudammina gutta Benedetti and Pignatti, 2009 represents the first documentation of this genus after the middle Eocene. These studies excluded the possibility of mixture among different-age taxa, because none of the recovered foraminifers shows signs of reworking (except isolated tests of Late Cretaceous orbitoids within few interbedded calcarenites). All the foraminifers indicate an Eocene-Oligocene age, because the DWAF assemblages show a coherent vertical distribution, and according to the biometric study, this reveals the occurrence of a complex evolutionary trend (Benedetti and Pignatti, 2009). In addition, agglutinated foraminifers considered extinct from the Eocene, were recently recovered and recognized in Miocene sediments by Kaminski et al. (2006, 2009) and Kender et al. (2009), thus suggesting strict environmental and ecological controls on the disappearance of such taxa from different areas. The aim of this work is to provide a taxonomic identification of the investigated taxa, and to give a paleoenvironmental interpretation of the foraminiferal assemblages, with particular regards to the faunal turnover at the EOT.
MATERIALS AND METHODS
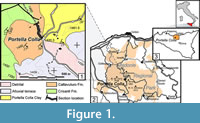 The Caltavuturo Formation (Schmidt di Friedberg et al., 1960) belongs to the Imerese Domain and is generally described as red or green calcilutites and marls with intercalated resedimented larger foraminiferal biocalcarenites (Basilone, 2012). This sedimentary succession crops out discontinuously in northeastern Sicily, and spans from the Cretaceous to the lower Oligocene (Basilone, 2012). The Caltavuturo Fm. at Portella Colla (Figure 1) is about 30 m thick, and consists of red to dark greenish clays and marly-clays, with low calcium carbonate content, with some interbedded turbiditic levels dominated by numulitids and orbitoidiforms (Figure 2). The investigated succession unconformably overlies the light-grey limestone of the Crisanti Formation, and it is covered without any hiatuses by the silty-clay of the Portella Colla member of the Numidian Flysch (Wezel, 1966).
The Caltavuturo Formation (Schmidt di Friedberg et al., 1960) belongs to the Imerese Domain and is generally described as red or green calcilutites and marls with intercalated resedimented larger foraminiferal biocalcarenites (Basilone, 2012). This sedimentary succession crops out discontinuously in northeastern Sicily, and spans from the Cretaceous to the lower Oligocene (Basilone, 2012). The Caltavuturo Fm. at Portella Colla (Figure 1) is about 30 m thick, and consists of red to dark greenish clays and marly-clays, with low calcium carbonate content, with some interbedded turbiditic levels dominated by numulitids and orbitoidiforms (Figure 2). The investigated succession unconformably overlies the light-grey limestone of the Crisanti Formation, and it is covered without any hiatuses by the silty-clay of the Portella Colla member of the Numidian Flysch (Wezel, 1966).
 A total of 29 samples were collected at variable intervals, from 10 cm to 2 m. The sampling intervals were spaced closer near the suspected Eocene/Oligocene boundary, following the preliminary results in Benedetti (2010) and Benedetti and Pignatti (2008). The age control of Portella Colla section was previously based on the distribution of larger foraminifers in the resedimented layers (Benedetti, 2010), because the planktonic foraminifers are scarce and not useful to biostratigraphic characterization. Upsection the larger foraminifer assemblages appear to be diacronous and stratigraphically continuous, but some reworked Cretaceous orbitoids occur. The biometrical analysis of megalospheric specimens of Nephrolepidina reveals a direct evolutionary progression of the measured populations (Benedetti and Pignatti, 2013). The foraminifers in the clayey levels can be instead considered autochtonous in comparison to those of the calcareous levels. The macrofossils are extremely rare and essentially represented by small teeth of fish; radiolarians are absent. In the clayey samples of the Caltavuturo Fm., the assemblages are primarily composed of DWAF (Benedetti and Pignatti, 2008, 2009); hyaline foraminifers, both benthic and planktic, are rare and often absent. The scarce planktic foraminifers are poorly preserved and dominated by taxa with low stratigraphic resolution, so they are not useful for a detailed biostratigrafic reconstruction of the investigated succession. Only two samples contain recognizable taxa: single specimens of Turborotalia ampliapertura ¸ Subbotina corpulenta, S. eocaena, and Dentoglobigerina cf. galavisi occur in PC060601 (suggesting a late Eocene-early Oligocene age), whereas Subbotina gortanii , Dentoglobigerina tripartita, D. tapuriensis and Globigerina venezuelana were extracted from PC8 (Oligocene s.l.).
A total of 29 samples were collected at variable intervals, from 10 cm to 2 m. The sampling intervals were spaced closer near the suspected Eocene/Oligocene boundary, following the preliminary results in Benedetti (2010) and Benedetti and Pignatti (2008). The age control of Portella Colla section was previously based on the distribution of larger foraminifers in the resedimented layers (Benedetti, 2010), because the planktonic foraminifers are scarce and not useful to biostratigraphic characterization. Upsection the larger foraminifer assemblages appear to be diacronous and stratigraphically continuous, but some reworked Cretaceous orbitoids occur. The biometrical analysis of megalospheric specimens of Nephrolepidina reveals a direct evolutionary progression of the measured populations (Benedetti and Pignatti, 2013). The foraminifers in the clayey levels can be instead considered autochtonous in comparison to those of the calcareous levels. The macrofossils are extremely rare and essentially represented by small teeth of fish; radiolarians are absent. In the clayey samples of the Caltavuturo Fm., the assemblages are primarily composed of DWAF (Benedetti and Pignatti, 2008, 2009); hyaline foraminifers, both benthic and planktic, are rare and often absent. The scarce planktic foraminifers are poorly preserved and dominated by taxa with low stratigraphic resolution, so they are not useful for a detailed biostratigrafic reconstruction of the investigated succession. Only two samples contain recognizable taxa: single specimens of Turborotalia ampliapertura ¸ Subbotina corpulenta, S. eocaena, and Dentoglobigerina cf. galavisi occur in PC060601 (suggesting a late Eocene-early Oligocene age), whereas Subbotina gortanii , Dentoglobigerina tripartita, D. tapuriensis and Globigerina venezuelana were extracted from PC8 (Oligocene s.l.).
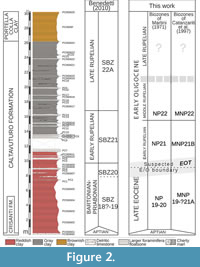
In this work new biostratigraphic data are added from poorly preserved calcareous nannofossils, although several samples were barren of recognizable taxa (Figure 2). The oldest sediments recovered from the investigated succession are referred to the late Eocene (biozones NP19-20 of Martini, 1971) based on the occurrence of Dictyoccites bisectus, Coccolithus formosus, Reticulofenestra umbilicus, Discoaster tanii, D. nodifer, D. deflandrei (about 80%), and rosette-shaped discoasters (about 20%). Latest Eocene sediments occcur at least up to samples PC0606067 and PC3, which contains nannofossil assemblage of Dictyoccites bisectus, Coccolithus formosus, Reticulofenestra umbilicus, Isthmolithus recurvus, Sphenolithus radians, and common Discoaster saipanensis. In the absence of more precise data, these levels are referred to the biozone NP19-NP20 of Martini (1971). A more accurate attribution according to the biozonal scheme of Agnini et al. (2014) is not possible because of the preservation of the material. Based on the occurrence of Dictyoccites bisectus, D. scrippsae, Isthmolithus recurvus, Reticulofenestra umbilicus, Clausicoccus obrutus, Discoaster tanii, D. nodifer , D. deflandrei, and the absence of rosette-shaped Discoaster, the sample PC060609 is dated as early Oligocene, zone MP21B of Catanzariti et al. (1997) corresponding to the upper part of NP21 of Martini (1971). A middle Rupelian age is assigned from the sample PC13 by the occurrence of Dictyoccites bisectus, Reticulofenestra umbilicus, Ismolithus recurvus, Sphenolithus predistentus, Cyclicargolithus floridanus, Discoaster tanii, D. nodifer, D. deflandrei, and by the absence of Coccolithus formosus marking the MNP22 of Catanzariti et al. (1997), or more simply NP22 of Martini (1971). The upper part of the section is barren in recognizable calcareous nannofossils and is assigned to the late Rupelian according to the study of resedimented larger foraminifers (Benedetti, 2010; Benedetti and Pignatti, 2013).
Samples were weighed, disaggregated by peroxide hydrogen solution, sieved over a 63 µm mesh, and all foraminifers were picked from each dry residue to determine the total abundance per gram. Foraminifers were mounted in standard micropaleontological slides. Selected specimens were photographed at a FEI QUANTA 400 MK2 scanning electron microscope (SEM), and the plates were assembled by the vectorial imaging software Canvas 11. The number of species per sample, the percentages of epifaunal and infaunal forms, the specific diversity, richness, and the index of eveness were calculated using the software PAST (Hammer et al., 2001; Hammer and Harper, 2006). The content in calcium carbonate in the investigated samples was determined with a Dietrich-Fruhling calcimeter in the Laboratory of Sedimentology of the University of Rome “La Sapienza”.
FACTORS CONTROLLING THE DWAF ASSEMBLAGES
According to Stainforth (1952), water turbidity is the main factor controlling the distribution of the arenaceous agglutinated foraminifers, reducing the activity of symbionts typical of calcareous organisms. The living agglutinated foraminifers occur in a great variety of environments: in shallow waters, lagoons, transitional environments, in proximity of river deltas, and in deep waters. A progressive increase in agglutinating taxa diversity from marginal marine to deep sea has been recorded (Nagy et al., 2000; Murray and Alve, 2011). In particular, modern taxa with organo-agglutinated walls are exclusively found in environments where the water is under-saturated in carbonate, such as in high intertidal marshes, and in the deep sea below the CCD (Murray and Alve, 2011), but also in fjords (Murray et al., 2003), and on deep continental shelves (Murray and Pudsey, 2004). The increase in arenaceous foraminifers with depth usually corresponds to a decrease in calcareous-walled foraminifers (Nagy et al., 2000).
Living DWAF assemblages have been reported from depths of 11 km (Akimoto et al., 2001), therefore, the temperature should be another dominant factor in the distribution of the DWAF. Comparing the “ Rhabdammina faunas” with recent agglutinated foraminifer-dominated assemblages described by Brady (1884) and Saidova (1961), Brouwer (1965) held this kind of assemblage typical of abyssal depth below the CCD. Consequently Gradstein and Berggren (1981) found that the availability of calcium carbonate is the main factor controlling the benthic foraminifer distribution. A lot of agglutinants have organic cement resistant to the corrosion of the deep seawater enriched in carbon dioxide. This would explain why DWAF are predominant in waters unsaturated in calcium carbonate, as well as in low salinity and low temperature conditions, or in oxygen depleted or pH fluctuating waters, such as in brackish environments. The organic cement typical of DWAF, however, tends to degrade after death and burial, so a limited number of specimens are preserved in the fossil record (Corliss, 1985). More likely, therefore, is that a single dominant factor does not exist, and DWAF distribution is influenced by bathymetry, oxygenation of the bottom- and interstitial waters, sedimentary input, nutrients, availability of calcium carbonate, type of substrate, hydrodynamic effects, and other factors that rule the equilibrium of the communities (Kuhnt et al., 1989).
FUNCTIONAL MORPHOGROUPS
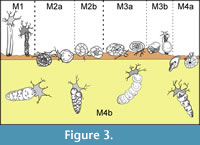 The analysis of functional morphogroups of agglutinated foraminifers is a fundamental tool for paleoenvironmental interpretations; the relative frequence of different trophic groups in a sample is dependent on environmental factors such as the organic productivity and the bottom-water oxygenation. The different agglutinated foraminifer taxa can be divided in morphotypes on the basis of test morphology that reflects the life position and the feeding strategies (Jones and Charnock, 1985; Nagy et al ., 1995, 1997; Bąk et al ., 1997; van den Akker et al ., 2000; Kaminski et al., 2006) (Figure 3-Figure 4). The morphotypes can be combined into morphogroups according to the preferred habitat, and the life position respect to the sediment/water interface (infaunal or epifaunal).
The analysis of functional morphogroups of agglutinated foraminifers is a fundamental tool for paleoenvironmental interpretations; the relative frequence of different trophic groups in a sample is dependent on environmental factors such as the organic productivity and the bottom-water oxygenation. The different agglutinated foraminifer taxa can be divided in morphotypes on the basis of test morphology that reflects the life position and the feeding strategies (Jones and Charnock, 1985; Nagy et al ., 1995, 1997; Bąk et al ., 1997; van den Akker et al ., 2000; Kaminski et al., 2006) (Figure 3-Figure 4). The morphotypes can be combined into morphogroups according to the preferred habitat, and the life position respect to the sediment/water interface (infaunal or epifaunal).
In this work four principal morphogroups, subdivided into seven morphotypes, are distinguished (Figure 4). Morphogroup M1 includes tubular and branched forms living in erect position, perpendicular to the substrate. These foraminifers are suspension feeders that filter bottom waters with extroflexed pseudopoda at the end of the tubular chambers. They are abundant in bathyal and abyssal environments without strong currents under stable conditions (Kaminski and Schröder, 1987; Kuhnt and Kaminski, 1989; Nagy et al., 2000). This morphogroup is composed of mostly astrorhizids and bathysiphonids. Kaminski et al. (2006) also inserted Arthrodendron subnodosiformis, but not A. grandis, in M1 in association with tubular branched forms; however, Kaminski et al. (2006) did not provide support for this placement, and so this hypothesis is herein rejected.
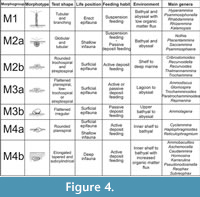 The morphogroup M2, predominantly characterized by deposit feeders, globular taxa living on or just below the substrate, is subdivided in two subgroups. The morphotype M2a includes spherical or subspherical forms and the saccamminids, among which is Psammosphaera, a globular form without evident aperture that lives just under the water/sediment boundary (Kaminski et al ., 1995). The thick-walled tubular Nothia is here considered a deposit feeder as suggested by Geroch and Kaminski (1993), even if it is classified as suspension feeder by Jones and Charnock (1985) and Kaminski et al. (2006). Nothia is placed in M2a rather than in M1 on the basis of data collected in this work, that show that Nothia distribution is directly proportional to deposit feeders rather than to tubular suspension feeders . Van den Akker et al. (2000) also included the genera Arthrodendron and Caudammina in M2a. In this study, however, these taxa are instead referred to the morphogroup M4b (Figure 4). The morphotype M2b comprises both rounded trochospiral and streptospiral forms (Recurvoides and Thalmannammina), in addition to some planoconvex trchospiral forms (Trochammina). Following Bąk et al . (1997), Galeotti et al. (2004), and Kaminski et al. (2006), Cribrostomoides is also included in M2b.
The morphogroup M2, predominantly characterized by deposit feeders, globular taxa living on or just below the substrate, is subdivided in two subgroups. The morphotype M2a includes spherical or subspherical forms and the saccamminids, among which is Psammosphaera, a globular form without evident aperture that lives just under the water/sediment boundary (Kaminski et al ., 1995). The thick-walled tubular Nothia is here considered a deposit feeder as suggested by Geroch and Kaminski (1993), even if it is classified as suspension feeder by Jones and Charnock (1985) and Kaminski et al. (2006). Nothia is placed in M2a rather than in M1 on the basis of data collected in this work, that show that Nothia distribution is directly proportional to deposit feeders rather than to tubular suspension feeders . Van den Akker et al. (2000) also included the genera Arthrodendron and Caudammina in M2a. In this study, however, these taxa are instead referred to the morphogroup M4b (Figure 4). The morphotype M2b comprises both rounded trochospiral and streptospiral forms (Recurvoides and Thalmannammina), in addition to some planoconvex trchospiral forms (Trochammina). Following Bąk et al . (1997), Galeotti et al. (2004), and Kaminski et al. (2006), Cribrostomoides is also included in M2b.
The morphogroup M3 consists of two morphotypes. M3a includes flattened forms with planispiral (Ammodiscus) trochospiral (Trochamminoides) or streptospiral coiling (Paratrochamminoides, Repmanina). Morphotype M3b includes all the sessile, agglutinated foraminifers referred to Tolypammininae, passive herbivores and deposit feeders that live fixed to the substrate or other organisms, such as tests of others foraminifers. Ammolagena clavata is the unique representative of M3b recovered in this work. Ammolagena is a cosmopolitan epibiont species common in various deep water assemblages from upper bathyal to abyssal environments, but it has also been found in shallow environments and in tropical seas (Cushman, 1928). Ammolagena clavata is, however, usually common in sediments deposited above the CCD, selecting the tests of other agglutinated foraminifers as a substrate for settlement (Waśkowska, 2014).
The morphogroup M4 comprises all the shallow or deep infaunal taxa, which are split into two different morphotypes. These taxa tolerate high organic matter flux (Kaminski et al ., 1995). The planispirally coiled Cyclammina, Haplophragmoides, and Reticulophragmium belong to the morphotype M4a; these taxa live in the sediment near the water/sediment boundary. They are herbivores or active deposit feeders able to resort to more omnivorous feeding in answer to environmental modifications (Jones and Charnock, 1985). Haplophragmoides lives up to 4 cm deep in the sediment (Kaminski et al., 1995), and is a generalist form adapted to a wide variety of environments (Nagy et al., 2000) indicative of low oxygenation in the bottom waters (Green et al., 2004). The M4a abundance, therefore, is dependent on changes in organic productivity rather than bathymetry (Kaminski et al., 2006). All the plurilocular, lengthened, seriate, cylindrical, or flattened forms belong to the morphotype M4b; the lengthened tests are particularly adapted to a deep infaunal life position and these foraminifers are passive deposit feeders. As an example, Karrerulina lives 10 to 20 cm deep in the sediment, and its abundance indicates a low rate of sedimentation and low oxygenation of the bottom water under oligotrophic conditions (Kender et al., 2005). Intermediate and deep infaunal dominated assemblages are usually directly proportional to the organic matter content of the sea-floor sediments and to the oxygenation of the bottom waters (Van der Zwaan et al ., 1999) and, even in case of parity in the oxygen content of the waters, infaunal-dominated assemblages are common in samples with higher organic carbon content (Kaminski et al ., 1995). On the contrary, epifaunal- and shallow infaunal-dominated assemblages are typical of environments with normal oxygenation, although in this condition all the morphogroups are usually represented (Kaminski et al., 1995).
In unstable environments, benthic communities are “physically controlled” and consist of infaunal r-strategists that live at the limit of the environmental tolerance (Kaminski et al., 1995; Preece et al., 1999). Under these conditions the specific diversity is controlled by the frequency of the reduction of the stressed populations (such as after periodic events of anoxia). In the most extreme environments, the specific diversity and the number of foraminifers decrease to those occurring under abiotic conditions (Kuhnt and Kaminski, 1993; Kaminski et al ., 1995). Under oligotrophic conditions all the morphogroups are represented, and accordingly the DWAF diversity is high and the organisms are typically K-strategists. Under eutrophic conditions the specific diversity generally decreases, and hyaline benthic foraminifers dominate the assemblages. Moreover, the whole fauna is primarily concentrated in the upper few centimeters and on the surface of the sediment (Kuhnt et al., 1996). Bottom waters depleted in oxygen are usually the result of epipelagic, eutrophic conditions. A high epipelagic productivity contributes to a fall of nutrients and induces deficiency of oxygen in the sediment/water interface. An elevated organic matter flux favors two groups of benthic foraminifers: 1) specialized forms, such as infaunal taxa tolerating low oxygenation; 2) opportunist forms that quickly (or seasonally) respond to elevated organic matter supply, which may tolerate oxygen content below the normal concentration (Kuhnt et al ., 1996). The position of the “redox boundary”, representing the limit of the benthic activity in the sediment, is fundamental, and it is due to the consumption of the oxygen in the interstitial waters by the activity of aerobic bacteria. In absence of burrowing macrofauna, which may rework the sediments, the position of the redox boundary depends, therefore, on the rate of organic flux. Under oligotrophic conditions the redox boundary can be several meters deep or even absent. Under very elevated rates of organic flux, the boundary can be close to the water/sediment interface, and tends to devastate the niches occupied by infaunal foraminifers (Kuhnt et al ., 1996). Deep infaunal taxa and Haplophragmoides fully exploit the whole infaunal area between the sediment surface and the redox boundary (Gooday, 1996). Under extremely eutrophic conditions, epifaunal morphogroups dominate, with especially Ammodiscus and Glomospira (Kaminski et al., 1996), the infaunal taxa survive and live within the sediment.
RESULTS
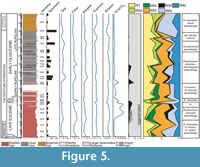 A total of 138 species, of which 59 are in open nomenclature, have been isolated from the marly-clays of the Portella Colla section; additional taxa have been recognized only at generic rank (see Appendix for complete list). Many of the recognized species, particularly Ammolagena clavata, Psammosiphonella cylindrica, P. linearis, Repmanina charoides, and Rhabdamminadiscreta, are cosmopolitan and have a wide stratigraphic range. A total of eight assemblages have been identified according to the functional morphogroup analysis, the specific diversity, and the faunal density (Figure 5, Figure 6, Figure 7, Figure 8).
A total of 138 species, of which 59 are in open nomenclature, have been isolated from the marly-clays of the Portella Colla section; additional taxa have been recognized only at generic rank (see Appendix for complete list). Many of the recognized species, particularly Ammolagena clavata, Psammosiphonella cylindrica, P. linearis, Repmanina charoides, and Rhabdamminadiscreta, are cosmopolitan and have a wide stratigraphic range. A total of eight assemblages have been identified according to the functional morphogroup analysis, the specific diversity, and the faunal density (Figure 5, Figure 6, Figure 7, Figure 8).
Repmanina charoides-Psammosiphonella linearis-Haplophragmoides walteri Assemblage (Upper Eocene)
The red clays at the base of the investigated succession have a low calcium carbonate content, and low faunal density; whereas the specific diversity reaches discrete values and all the morphogroups are represented, although M2b and M3b are subordinate. The assemblages are dominated by Haplophragmoides walteri, Repmanina charoides, and Psammosiphonella linearis. With the exception of the latter species, the tubular morphotypes belonging to M1 are rare. M4b is represented above all by Karrerulina horrida. This assemblage suggests good oxygenation of the seafloor, according to the rarity or absence of deep infaunal morphotypes and the occurrence of oligotrophic conditions.
Reticulophragmium acutidorsatum-Paratrochamminoides spp. Assemblage (uppermost Eocene)
 In the upper Eocene red clay, the specific diversity and density tend to decrease, the CaCO3 content increases up to 20%, and hyaline taxa typical of deep environments are present, including Cibicidoides havanensis and C. grimsdalei (van Morkhoven et al., 1986). Among the DWAF, cyclamminids, Haplophragmoides, Ammodiscus latus, Ammolagena clavata, Paratrochamminoides, and Pseudonodosinella elongata dominate . Parisi and Coccioni (1988) and Molina et al. (2006) described peaks in R. amplectens (or cyclamminids in general) in assemblage with C. havanensis and C. grimsdalei near the E/O boundary. These assemblages resemble the “Paratrochamminoides assemblages” of Kuhnt and Kaminski (1989), typical of red clays with a low number of tubular taxa (Kender et al., 2005), and characterizing oligotrophic environments (Kaminski et al., 1996).
In the upper Eocene red clay, the specific diversity and density tend to decrease, the CaCO3 content increases up to 20%, and hyaline taxa typical of deep environments are present, including Cibicidoides havanensis and C. grimsdalei (van Morkhoven et al., 1986). Among the DWAF, cyclamminids, Haplophragmoides, Ammodiscus latus, Ammolagena clavata, Paratrochamminoides, and Pseudonodosinella elongata dominate . Parisi and Coccioni (1988) and Molina et al. (2006) described peaks in R. amplectens (or cyclamminids in general) in assemblage with C. havanensis and C. grimsdalei near the E/O boundary. These assemblages resemble the “Paratrochamminoides assemblages” of Kuhnt and Kaminski (1989), typical of red clays with a low number of tubular taxa (Kender et al., 2005), and characterizing oligotrophic environments (Kaminski et al., 1996).
Repmanina charoides-Ammodiscus tenuissimus Assemblage (Eocene/Oligocene transition)
 Near the hypothesized E/O boundary, the number of taxa abruptly decreases; the assemblages are dominated by opportunist taxa such as Repmanina charoides. The reduced carbon matter flux is reflected by the disappearance of the M1 and M3b morphogroups. Infaunals are instead rare, and M4b is not represented in this interval. The specific diversity registers a peak because most of the recovered taxa, although very few (n=7-9), are represented only by one or two individuals. The CaCO3 content reaches 12%, but no calcareous foraminifers have been recovered in the autochtonous assemblages. The calcium carbonate is probably linked to the occurrence of reworked late Eocene larger foraminifers, such as Nummulites incrassatus, Discocyclina dispansa dispansa and Orbitoclypeus varians , within the sample PC060608 described in Benedetti (2010).
Near the hypothesized E/O boundary, the number of taxa abruptly decreases; the assemblages are dominated by opportunist taxa such as Repmanina charoides. The reduced carbon matter flux is reflected by the disappearance of the M1 and M3b morphogroups. Infaunals are instead rare, and M4b is not represented in this interval. The specific diversity registers a peak because most of the recovered taxa, although very few (n=7-9), are represented only by one or two individuals. The CaCO3 content reaches 12%, but no calcareous foraminifers have been recovered in the autochtonous assemblages. The calcium carbonate is probably linked to the occurrence of reworked late Eocene larger foraminifers, such as Nummulites incrassatus, Discocyclina dispansa dispansa and Orbitoclypeus varians , within the sample PC060608 described in Benedetti (2010).
Opportunists survive, such as Ammodiscus tenuissimus, Reticulophragmium acutidorsatum, and especially Repmanina charoides, an epifaunal taxon that quickly responds to elevated flux of organic matter and to rapid changes of seafloor conditions (Arreguin-Rodriguez et al., 2014). The morphogroups M2, M3a, and M4a dominate within the interval, and robust taxa, such as Nothia robusta, disappear. Haplophragmoides disappears before the E/O boundary, and the infaunal taxa are absent, suggesting a sudden fall of the organic productivity . The causes of this change in the faunal content may be related to some alteration in the water mass characteristics, or more likely to the global cooling recognized at the base of the Oligocene (Miller et al., 1987).
Psammosiphonella linearis-Rhabdammina discreta Assemblage (lowermost Rupelian)
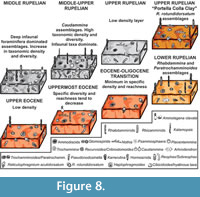 In the lowermost Rupelian, suspension-feeding tubular taxa (Psammosiphonella and Rhabdammina) become abundant, but the specific density and diversity are low. Paratrochamminoides and Repmanina charoides dominate, whereas Cibicidoides, Haplophragmoides, and Reticulophragmium disappear. Among the infaunal forms Arthrodendron grandis appears in association with Karrerulina horrida and Pseudonodosinella elongata. The presence of these taxa, especially Arthrodendron, could reflect elevated rate of organic matter input to the seafloor (Kaminski et al., 1996). The clay color is greenish gray recording decrease in the oxygenation of the bottom-waters. This assemblage is similar to the “ Rhabdammina faunas” of Kuhnt and Kaminski (1989), typical of flysch-type sedimentation. At the base of the Oligocene, the turbiditic supply becomes more significant, and the larger foraminifer-bearing layers increase in frequence and thickness.
In the lowermost Rupelian, suspension-feeding tubular taxa (Psammosiphonella and Rhabdammina) become abundant, but the specific density and diversity are low. Paratrochamminoides and Repmanina charoides dominate, whereas Cibicidoides, Haplophragmoides, and Reticulophragmium disappear. Among the infaunal forms Arthrodendron grandis appears in association with Karrerulina horrida and Pseudonodosinella elongata. The presence of these taxa, especially Arthrodendron, could reflect elevated rate of organic matter input to the seafloor (Kaminski et al., 1996). The clay color is greenish gray recording decrease in the oxygenation of the bottom-waters. This assemblage is similar to the “ Rhabdammina faunas” of Kuhnt and Kaminski (1989), typical of flysch-type sedimentation. At the base of the Oligocene, the turbiditic supply becomes more significant, and the larger foraminifer-bearing layers increase in frequence and thickness.
Paratrochamminoides-Ammolagena clavata Assemblage (lower-middle Rupelian)
At the transition from the lower to the upper Rupelian, the faunal density increases considerably, whereas the CaCO3 content decreases. The assemblages are composed of suspension-feeding taxa, and deep infaunal, elongate forms indicating continuous flows enriched in organic matter and nutrients. Hyaline taxa are present (Cibicidoides and stilostomellids). Arthrodendron grandis, Karrerulina horrida, and Karrerulina conversa suggest an elevated supply of organic matter. Haplophragmoides walteri, Reticulophragmium acutidorsatum, Ammolagena clavata, Paratrochamminoides, Ammodiscus latus, Psammosphaera irregularis, and rhabdamminids are abundant. In addition, the FO of Caudammina in the Oligocene is recorded. The specific diversity is high, and the assemblages are similar to the “Rhabdammina assemblages” described by Kender et al. (2005).
Caudammina Assemblage (middle-upper Rupelian)
Beginning at the upper Rupelian, the assemblages are dominated by M4, whereas deposit-feeding epifaunal taxa tend to decrease. The assemblages are dominated by reophacids and, above all, by Caudammina gutta, a species described from the Portella Colla outcrop (Benedetti and Pignatti, 2009). Rhabdamminids are common and Nothia robusta becomes common. The occurrence of Reticulophragmium projectum, a species described for the Oligocene of the Beaufort-Mackenzie basin from Schröder-Adams and McNeil (1994), characterizes the first occurrence of this taxon in the Meditterranean area. The high organic carbon flux rate is proved by an increase in the faunal density, and by the abundance of deep and intermediate infaunal taxa. In particular deep infaunal taxa dominate in low-oxygen environments and are adapted to live in areas with a high level of organic flux (Kaminski and Gradstein, 2005).
Low-density Interval (upper Rupelian)
In the uppermost clay layers of the Caltavuturo Fm., a low-diversity interval occurs. The assemblages are characterized by very rare DWAF, and are mainly composed of rhabdamminids and cyclamminids. This interval marks a return to oligotrophic conditions, preceding the deposition of the Portella Colla Clay where Reticulophragmium rotundidorsatum -dominated assemblages occur.
Reticulophragmium rotundidorsatum Assemblage (upper Rupelian, Portella Colla Clay)
Passing into the Portella Colla Clays, the faunas suffer a further change. Caudammina disappears, suspension-feeding and epifaunal deposit-feeding taxa decrease, and the assemblages become oligotipic, dominated by Reticulophragmium rotundidorsatum and R.projectum. Hyaline foraminifers are absent. Arthrodendron and Karrerulina dominate among the infaunals; Haplophragmoideswalteri is replaced by H. carinatum , the suspension feeders decrease considerably responding to the increase of siliciclastic sedimentation rate flux. The fragmented specimens of Nothia robusta, occurring within this assemblage, show a very thick test characterized by coarse agglutination. In the Portella Colla Clay, there is a marked increase in the siliciclastic contribution; the quartz grains become more frequent in the clayey and especially in the turbiditic layers, the tubular forms have a coarser agglutination, and, among these, dominate large specimens of N. robusta. The occurrence of M4 marks high productivity without increase in sedimentation flux, since deep infaunal forms, such as Karrerulina, numerous in the uppermost samples, cannot tolerate high rates of sedimentation.
DISCUSSION
The Investigated Assemblages
 The DWAF assemblages studied in the Portella Colla section represent a unicum in the Mediterranean Oligocene, and they are here compared to ODP projects. The ODP sites 643 (Norwegian Sea, North Atlantic; Kaminski et al., 1990) and 647 (Southern Labrador Sea, North Atlantic; Kaminski et al., 1989; Kaminski, 2005; Kaminski and Ortiz, 2014) constitute the most complete Paleogene successions from high latitude available for DWAF analysis, and their zonations are compared with the assemblages recovered at Portella Colla (Figure 9). Late Eocene sediments are characterized by the occurrence of Reticulophragmium and Haplophragmoides- dominated assemblages. The species R. amplectens, poorly documented in the upper Eocene Portella Colla sediments, dominates the middle to late Eocene North Atlantic and is also common in the Artic Ocean (Schröder-Adams and McNeil, 1994; McNeil, 1996). Reticulophragmium acutidorsatum, commonly recorded from the Eocene to Miocene (e.g., Kender et al., 2005, 2009), at Portella Colla occurs in the whole the Caltavuturo Fm., with a peak in the latest Eocene (sample PC3). Reticulophragmium rotundidorsatum, a species common throughout the Oligocene to the Miocene (e.g., Kaminski, 2005; Kaminski et al., 2006), and dominating the uppermost samples in the investigated section, marks the latest Eocene in the Carpathians (Geroch and Nowak, 1984). Haplophragmoides carinatum, which spans from the late Eocene to the Oligocene in the Artic Ocean, at Portella Colla appears to be restricted to the Oligocene. The lower Oligocene generally contains impoverished DWAF assemblages, especially in the Tethys realm, whereas the Spirosigmoilinella assemblage appears to be restricted to the North Atlantic area (Kaminski and Gradstein, 2005).
The DWAF assemblages studied in the Portella Colla section represent a unicum in the Mediterranean Oligocene, and they are here compared to ODP projects. The ODP sites 643 (Norwegian Sea, North Atlantic; Kaminski et al., 1990) and 647 (Southern Labrador Sea, North Atlantic; Kaminski et al., 1989; Kaminski, 2005; Kaminski and Ortiz, 2014) constitute the most complete Paleogene successions from high latitude available for DWAF analysis, and their zonations are compared with the assemblages recovered at Portella Colla (Figure 9). Late Eocene sediments are characterized by the occurrence of Reticulophragmium and Haplophragmoides- dominated assemblages. The species R. amplectens, poorly documented in the upper Eocene Portella Colla sediments, dominates the middle to late Eocene North Atlantic and is also common in the Artic Ocean (Schröder-Adams and McNeil, 1994; McNeil, 1996). Reticulophragmium acutidorsatum, commonly recorded from the Eocene to Miocene (e.g., Kender et al., 2005, 2009), at Portella Colla occurs in the whole the Caltavuturo Fm., with a peak in the latest Eocene (sample PC3). Reticulophragmium rotundidorsatum, a species common throughout the Oligocene to the Miocene (e.g., Kaminski, 2005; Kaminski et al., 2006), and dominating the uppermost samples in the investigated section, marks the latest Eocene in the Carpathians (Geroch and Nowak, 1984). Haplophragmoides carinatum, which spans from the late Eocene to the Oligocene in the Artic Ocean, at Portella Colla appears to be restricted to the Oligocene. The lower Oligocene generally contains impoverished DWAF assemblages, especially in the Tethys realm, whereas the Spirosigmoilinella assemblage appears to be restricted to the North Atlantic area (Kaminski and Gradstein, 2005).
A faunal turnover of DWAF taxa has been observed at the E/O boundary (Kaminski, 2005; Ortiz and Kaminski, 2012; Kaminski and Ortiz, 2014), and it generally linked to a drop in the CCD (Coxall et al., 2005; Ortiz and Kaminski, 2012). In particular, the late Eocene assemblages are usually dominated by Pseudonodosinella elongata (e.g., Kaminski and Huang, 1991), Reticulophragmium amplectens , Ammodiscus latus and Spiroplectammina trinitatensis (Kaminski and Ortiz, 2014), whereas the earliest Oligocene assemblages consist mainly of Ammodiscids and glomospirids (Kaminski et al., 1989). In the investigated section, red clays from the upper Eocene to the lower Oligocene levels suggest that the seafloor and deep-waters were well-oxygenated. The relatively high specific diversity of the first recognized assemblage also reflects oxygenation of bottom water. In the uppermost Eocene, the specific diversity and richness tend to decrease and cyclamminids dominate the assemblages in association with Ammodiscus latus , Pseudonodosinella elongata, Ammolagena clavata, and Paratrochamminoides. Near the E/O boundary, the DWAF assemblages usually record an abrupt faunal turnover and a reduction of abundance and diversity (Kaminski et al . , 1989; Kaminski and Huang, 1991; Kaminski, 2005; Kaminski and Ortiz, 2014). The DWAF assemblages are extremely impoverished, epifaunal opportunistic taxa survive, such as ammodiscids and glomospirids with smooth and well cemented tests (Kaminski et al ., 1989).
The acme of Ammodiscus latus in the late Eocene and early Oligocene, with a hiatus in the lowermost Oligocene, is a global event with a high potential for correlation (Kaminski and Ortiz, 2014). Also at Portella Colla, A. latus disappears before the EOT up to the middle Rupelian sediments. Pseudonodosinella elongata commonly occurs along the entire section, but it disappears before the suspected EO boundary. Reticulophragmium amplectens is usually recorded as abundant in the late Eocene, and its last occurrence coincides with the Eocene/Oligocene boundary (e.g., Kaminski, 2005). The last occurrence of at least 10 DWAF species is well-documented at the end of the Eocene (Ortiz and Kaminski, 2012; Kaminski and Ortiz, 2014), some of which were not recovered along the Portella Colla section (see below). Ammolagena clavata disappears at the EOT, and reappears in the lower-middle Rupelian; its occurrence is indicative of favourable environmental conditions (i.e., a high supply of organic matter, well oxygenated bottom sediment, and low-energy water) (Waśkowska, 2014). Overall, the faunal assemblage recovered in the upper Eocene clays reflects an oligotrophic environment in well-oxygenated seafloor waters.
The DWAF assemblages suffer a decrease in abundance in the EOT where some ammodiscid and glomospirids such as Ammodiscus tenuissimus and Repamina charoides survive . Suspension feeders belonging to the morphogroup M1 and deep-infaunal taxa of M4 disappear within this interval. The glomospirid Repmanina charoides builds its wall by agglutinating particles with organic cement (Arreguin-Rodriguez et al., 2014), and their abundance is traditionally interpreted as controlled by the dissolution of CaCO3, and by the changes in the CCD and carbonate availability (e.g., Kuhnt and Urqhart, 2001). Arreguin-Rodriguez et al. (2014) argued that the CaCO3 dissolution is not the only cause of the dominance of glomospirids, which instead act as opportunists under stressed conditions due to their ability to feed on refractory organic matter. During the EOT at ODP site 647 an increase in the deep infaunal taxa of morphogroup M4b has been observed (Kaminski and Ortiz, 2014), suggesting an intensified productivity (Ortiz and Kaminski, 2012). The assemblages are diverse and indicative of well-oxygenated bottom waters. This datum contrasts with the assemblages described here, which document a decrease in deep infaunal taxa and the number of taxa. The R. charoides-A. tenuissimus assemblage is the result of a short-term event reflecting eutrophic conditions in which opportunistic forms with an epiphaunal mode of life, such as Ammodiscus and Glomospira, survive during an abrupt change of the organic matter supply to the seafloor (e.g., Kaminski et al., 1996). The absence of deep infaunal taxa and the rarity of shallow-infaunal taxa could reflect a shift in the redox boundary, thus limiting the benthic activity in the sediments (Kuhnt et al., 1996). The calcareous taxa disappear within this interval confirming a change in the trophic conditions.
The dominance of morphogroups M1, M2b, and M4b in the lowermost Oligocene (Psammosiphonella linearis - Rhabdammina discreta assemblage) suggests an intensified organic supply at the seafloor and an increase in productivity. The diversity increases in the Paratrochamminoides-Ammolagena clavata assemblage, in which tubular forms, M1, M3a, and M4 morphogroups dominate. In this assemblage peaks in abundance of Haplophragmoides walteri, Reticulophragmium acutidorsatum, Pseudonodosinella elongata, Ammodiscus latus, Arthrodendron grandis, A. clavata, and Paratrochamminoides are observed. Peaks in R. acutidorsatum and P. elongata are known from the Oligocene of the Celebes Sea (Kaminski and Huang, 1991), but are rare in scaglia-type formations (Kaminski and Gradstein, 2005). The acme of A. latus is generally recorded in the lower Oligocene sediments of the Carpathians, where it defines a partial range zone from the FO of the nominate taxon to the FO of Reticulophragmium rotundidorsatum (Kaminski, 2005). At Portella Colla, A. latus disappears before the first common occurrence of R. rotundidorsatum and reappears in the uppermost samples. Ammolagena clavata has been described from high diversity flysh-type assemblages indicating favourable environmental conditions such as high supply of organic matter, low energy water conditions, and well-oxygenated bottom sediments (Waśkowa, 2014).
The Caudammina assemblage represents a unicum for the Oligocene DWAF assemblages, being this taxon unknown for this time interval since the erection of the species C. gutta Benedetti and Pignatti, 2009. The high occurrence of M4b marks a high organic carbon flux rate and low-oxygen, whereas the robuste tubular forms of M1 could indicate currents strong enough to resuspend the surficial sediments. Caudammina is typical of bathyal to abyssal environments (Kaminski and Gradstein, 2005), and its high occurrence in the upper part of the investigated section suggests a deepening of the basin. The uppermost sediments of the Caltavuturo Fm. are characterized by a low density interval in which the number of taxa, their diversity, and the morphogroups M4a and M4b decrease, marking oligotrophic conditions. A peak in abundance of R. charoides, P. linearis, and Paratrochamminoides spp. is noteworthy, and indicates a new short-time phase of cooling (Pälike et al., 2006). Unfortunately, as stated above, no accurate biostratigraphic data are available for the upper part of the investigated section.
The assemblages in the Portella Colla Clay are dominated by Haplophragmoides and Reticulophragmium belonging to morphotype M4a, and suggesting low oxygenation (Green et al., 2004), with subordinate deep infaunal of M4b and tubular forms of M1. In the investigated samples, Haplophragmoides carinatus replaces the typical H. walteri forms, whereas Reticulophragmium rotundidorsatum seems to substitute for R. acutidorsatum. Although R. rotundidorsatum is known from the late Eocene (Kaminski and Gradstein, 2005), its highest occurrence usually marks middle late Miocene of the Norwegian-Greenland Sea (Osterman and Spiegler, 1996; Kaminski et al., 2006) and the Oligocene-Miocene of the Beaufort-MacKenzie Basin (Schröder-Adams and McNeil, 1994). The species R. projectum, described from the Oligocene of the Beaufort-MacKenzie Basin (Schröder-Adams and McNeil, 1994) and previously dubitatively recognized (Benedetti and Pignatti, 2008), is herein described from the late Rupelian of the Mediterranean area.
Bathymetry
The sedimentary successions of the Caltavuturo Formation have been described as having been deposited along the slope, between the platform margin and the basin, where turbidity currents reworked the carbonates from the Panormid carbonate platform (Abate et al., 1988; Pescatore et al., 1987). Calderone et al. (1980) and Dongarrà and Ferla (1982) provided a different environmental interpretation of the deposits cropping out at Portella Colla according to the analysis of the clay minerals. They suggested that the clays were deposited in a restricted depositional environment enriched in iron and organic matter. The premature diagenesis caused a modification in the sediments chemical composition with the dissolution of calcium carbonate, due to hyperaline bottom waters (Calderone et al., 1980). Such scenario is implausible, however, according to the micropaleontological interpretation of the assemblages occurring in the Caltavuturo Fm. and in the Portella Colla Clay. In addition, the analysis of resedimented larger foraminfers excludes high salinity values or other stress conditions in the basin (Benedetti, 2015). The bathymetric interpretation of the investigated faunas is challenging because of some common glauconitic layers (glauconite does not exceed 800 meters in depth according to Odin and Matter, 1981), the abundance of the displaced larger foraminiferal tests, and the absence of significant biosiliceous component testifying the occurrence of taxa living under the CCD.
DWAF assemblages are usually described as typical of epibathyal to abyssal environments (e.g., Kaminski and Gradstein, 2005). Their abundance is linked to the water depth, but is also dependent on factors such as water circulation and acidification (e.g., Gradstein and Berggren, 1981; Kuhnt et al., 1989). At Portella Colla, hyaline tests are uncommon and planktonic foraminifers are rare or absent, thus reflecting dissolution of calcium carbonate at depth below the CCD. In particular, the planktonic foraminiferal tests tend to be greatly affected by dissolution (Zachos et al., 1996). Kuhnt and Kaminski (1989) and Kuhnt et al . (1989) proposed a paleobathymetric distribution of DWAF assemblages: 1) the middle-slope assemblages (500-1500 m) are constituted by flysch-type agglutinated foraminifers with common ataxophragmiids, and variable number of planktonic foraminifers (0-99%) and of calcareous-walled benthic taxa; 2) the lower slope assemblages (from 1500 m to more than 2500 m) are dominated by Rhabdammina and other tubular taxa, whereas hyaline taxa are rare or often absent, fragmented, and corroded; 3) the abyssal assemblages are characterized by high diversity, are dominated by Recurvoides and Paratrochamminoides, and are deprived of autochthonous calcareous foraminifers.
 The bathymetric range of selected and frequent taxa from the investigated deposits is schematized in Figure 10. The percentage abundance of Recurvoides in the Caltavuturo Fm. is low in each sample and, although the Caudammina and Reticulophragmium rotundidorsatum assemblages could reflect an abyssal environment, it is likely that the true depositional environment was the middle slope. We must take into account that recent foraminiferal assemblages show a bathymetric distribution strictly dependent on the water temperature with a shallower limit for some species at the highest latitudes (Milam and Anderson, 1981), and bathymetric migrations (recorded even stagionally) are linked to the cooling of the ocean waters. Near the E/O boundary a global cooling is well documented on the strength of isotopic analyses on benthic foraminifers (Miller et al., 1987, Zachos et al., 1996) linked to an abrupt > 1 km drop of the CCD (Haq, 1981; Zachos et al., 1996; Rea and Lyle, 2005; Katz et al., 2008). Local rises of the CCD are possible and DWAF dominated assemblages barren in calcareous taxa are described from the Congo’s delta in a bathyal environment (Kender et al., 2008). The sea level drop is also registered in the Caltavuturo Fm. by the shallow-water taxa occurring in the turbiditic layers. The deposition of these calcarenites could represent a sea-level drop that activated erosion at shallow marine settings and transport of shallower elements towards deeper environment in the basin (e.g., Alegret et al., 2008).
The bathymetric range of selected and frequent taxa from the investigated deposits is schematized in Figure 10. The percentage abundance of Recurvoides in the Caltavuturo Fm. is low in each sample and, although the Caudammina and Reticulophragmium rotundidorsatum assemblages could reflect an abyssal environment, it is likely that the true depositional environment was the middle slope. We must take into account that recent foraminiferal assemblages show a bathymetric distribution strictly dependent on the water temperature with a shallower limit for some species at the highest latitudes (Milam and Anderson, 1981), and bathymetric migrations (recorded even stagionally) are linked to the cooling of the ocean waters. Near the E/O boundary a global cooling is well documented on the strength of isotopic analyses on benthic foraminifers (Miller et al., 1987, Zachos et al., 1996) linked to an abrupt > 1 km drop of the CCD (Haq, 1981; Zachos et al., 1996; Rea and Lyle, 2005; Katz et al., 2008). Local rises of the CCD are possible and DWAF dominated assemblages barren in calcareous taxa are described from the Congo’s delta in a bathyal environment (Kender et al., 2008). The sea level drop is also registered in the Caltavuturo Fm. by the shallow-water taxa occurring in the turbiditic layers. The deposition of these calcarenites could represent a sea-level drop that activated erosion at shallow marine settings and transport of shallower elements towards deeper environment in the basin (e.g., Alegret et al., 2008).
 In an outcrop 700 m north of Portella Colla (section FO), in the red clays of the Caltavuturo Fm., three volcanoclastic layers enriched in quartz were found (Figure 11). The clays above and below these levels have no carbonatic content. Volcanic activity can explain the acidification of the bottom water, and the absence of calcareous taxa in response to a local rise of the CCD, thus resulting in a migration of some taxa, such as Caudammina, from abyssal environments to shallower ones. Therefore, the Portella Colla assemblages were most likely deposited under the CCD from the middle to lower bathyal zone in a middle-lower slope setting, as evidenced by the shallower known distribution of selected taxa (Figure 10).
In an outcrop 700 m north of Portella Colla (section FO), in the red clays of the Caltavuturo Fm., three volcanoclastic layers enriched in quartz were found (Figure 11). The clays above and below these levels have no carbonatic content. Volcanic activity can explain the acidification of the bottom water, and the absence of calcareous taxa in response to a local rise of the CCD, thus resulting in a migration of some taxa, such as Caudammina, from abyssal environments to shallower ones. Therefore, the Portella Colla assemblages were most likely deposited under the CCD from the middle to lower bathyal zone in a middle-lower slope setting, as evidenced by the shallower known distribution of selected taxa (Figure 10).
Remarks on the Taxonomic Composition of the Assemblages
Deep-sea Eocene to Miocene assemblages usually contain varying proportions of calcareous and agglutinated taxa (Kaminski and Gradstein, 2005). From the EOT, an increase in calcium-cemented taxa and low DWAF density is usually described (Kaminski and Ortiz, 2014). In the early Oligocene organic-cemented agglutinated foraminifers dominate and diversity decreases (Kaminski and Gradstein, 2005). Although some poorly preserved bathyal hyaline foraminifers, such as Cibicidoides, occur along the investigated section (Benedetti and Pignatti, 2008, plate 2, figures 14-15), the faunal assemblages recovered in the samples of the Portella Colla section contain very peculiar deep-water faunas. In all the investigated samples, taxa with calcareous cement and with agglutinated calcitic grains are absent, such as Dorothia, Karreriella, Tritaxia and Vulvulina which are instead common in the coeval sediments of the Gratteri Formation cropping near Isnello (Benedetti and D’Amico, 2012), about 9 km north of the Portella Colla outcrop. In addition, in the sediments of Portella Colla, typical Eocene-Oligocene taxa, such as Duquepsammina cubensis , Spiroplectammina trinitatensis, S. spectabilis, and Turrilina alsatica, are absent. The Scaglia-type formations also contain calcareous-cemented forms such as Remesella, Karreriella , and Spiroplectammina (e.g., Kaminski and Gradstein, 2005). In the Numidian flysch of Morocco, S. spectabilis is very rare or absent because of the lack of organic matter or other nutrients (Kaminski et al., 1996). At Portella Colla, the frequency of species belonging to the morphogroup M4, except for the sample near the EOT, seems to be a good indicator of the abundance of organic matter reaching the seafloor. So, the absence of calcareous agglutinated taxa must be explained not as much on the trophic resources, but rather on the post-mortem dissolution of the tests, linked to the acidification of the water possibly due to the volcanic activity documented in the section FO (Figure 11).
SUMMARY
The taxonomic and stratigraphic importance of the uppermost Eocene and Oligocene DWAF assemblages, recovered at Portella Colla, is noteworthy; this work provides the most complete record of foraminifers in the clay of the Caltavuturo Fm., and several as yet unrecorded species are reported in open nomenclature. The cosmopolitan species described are well known from the Paleogene to Miocene of the North Atlantic region (Schroeder-Adams and McNeil, 1994; Osterman and Spiegler, 1996; Kaminski et al., 2006; Kaminski et al., 2009), but they are poorly described from the Mediterranean area, especially for the Oligocene. This study provides new data about the distribution of the poorly-known species Caudammina gutta and Reticulophragmium projectum in lower Oligocene sediments of Mediterranean area.
Nine assemblages were recognized according to the faunal content, faunal density, and species diversity. The DWAF assemblages suggest good oxygenation in the uppermost Eocene samples, whereas during the EOT a minimum in the specific diversity has been recorded. The occurrence of opportunistic taxa, such as Ammodiscids and glomospirids (especially Repmanina charoides), and the absence of infaunal taxa indicate an upward migration of the redox boundary. At the base of the Rupelian, deep-infaunal foraminiferal assemblages suggest a decrease in bottom water oxygenation, and an increase in nutrients supply. Repmanina charoides is known as opportunistic species with an ecological advantage in the post-extinction benthic ecosystem (Arreguin-Rodriguez et al., 2014), since it is able to feed on less labile organic matter. In particular, glomospirids may reproduce rapidly and colonize the post-extinction empty niches. This study confirms that the EOT was a time of significant faunal turnover among DWAF, which responded to variations in the nutrient's availability, sea level fall, and climatic changes.
SYSTEMATIC PALAEONTOLOGY
The suprageneric classification of Loeblich and Tappan (1987, 1992) is followed and integrated with those of Kaminski (2004, 2014), Mikhalevich (2013), and Pawlowski et al. (2013). The specimens were investigated under an optic microscope and drawn using a camera lucida to detect details. Most taxa are illustrated with SEM photographs. For some specimens the generic or suprageneric classification is undetermined. The material is stored in the collection Benedetti in the micropaleontological laboratory of the University of Rome "La Sapienza".
Phylum FORAMINIFERA d'Orbigny, 1826
Class ASTRORHIZATA Saidova, 1981
Order ASTRORHIZIDA Lankester, 1885
Superfamily ASTRORHIZOIDEA Brady, 1881
Family RHABDAMMINIDAE Brady, 1884
Subfamily RHABDAMMININAE Brady, 1884
Genus RHABDAMMINA M. Sars in Carpenter, 1869
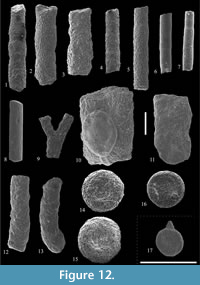
Rhabdammina discreta Brady, 1884
Figure 12.1-2
1884 Rhabdamminadiscreta; Brady, p. 268, pl. 22, figs. 7, 8.
 1896 Rhabdammina subdiscreta; Grzybowski, p. 275, pl. 8, figs. 5, 6.
1896 Rhabdammina subdiscreta; Grzybowski, p. 275, pl. 8, figs. 5, 6.
1954 Psammosiphonella discreta (Brady); Avnimelech, p. 65.
2005 Psammosiphonella discreta (Brady); Kaminski and Gradstein, p. 117, pl. 5/6, figs. 1-8.
Material. 134 specimens from 22 samples.
Description. Tubular and rectilinear test, oval in outline. Thick wall with coarse agglutinated grains. The test presents irregularly distanced constrictions, aperture a simple terminal opening.
Distribution. From Cretaceous to recent, cosmopolitan.
Rhabdammina eocenica Cushman and Hanna, 1927
Figure 12.3
1927 Rhabdamminaeocenica; Cushman and Hanna, p. 209, pl. 13, fig. 1.
1990 Bathysiphon eocenicus; Cushman and Hanna; Bellagamba and Coccioni, pl. 1, fig. 1.
Material. 21 specimens from 12 samples.
Description. Thick and weakly flattened test with an elliptical trasversal section.
Remarks. Differs from R. rhabdammina for the lack of constrictions and for the flattening of the test.
Rhabdammina spp.
Material. 17 specimens from 10 samples.
Description. Included in this group are all fragmentary and tubular specimens not assigned to any known species.
Subfamily BATHYSIPHONINAE Avnimelech, 1952
Genus BATHYSIPHON Sars, 1872
Bathysiphon spp.
Figure 12.8
Material. Three specimens from two samples.
Description. Tubular test with selective agglutination of sponge spicules.
Genus NOTHIA Pflaumann, 1964
Nothia excelsa (Grzybowski, 1898)
Figure 12.9
1898 Dendrophrya excelsa; Grzybowski, p. 272, pl. 10. figs. 1-4.
1960 Dendrophrya excelsa Grzybowski; Geroch, p. 121, pl. 1, figs. 1-11.
1993 Nothia excelsa (Grzybowski) emend. Geroch and Kaminski, 1993; Kaminski and Geroch, p. 245, pl. 1, figs. 2-6, 15a, b.
2009 Nothia excelsa (Grzybowski); Kender, Kaminski and Jones, p. 493, pl. 1, fig. 5.
Material. 57 specimens from 21 samples.
Description. Tubular and flattened test, rarely branched, usually straight or curved. Wall thick, moderately coarse agglutinated, composed of quartz grains.
Distribution. Well-known species from Cretaceous to the late Eocene of Carpathians; described from the Miocene by Osterman and Spiegler (1996) and Kender et al. (2009).
Paleoecology. Nothia excelsa was regarded as an epifaunal deposit-feeders form colonizing turbiditic deposit surfaces (Geroch and Kaminski, 1993); recently Kaminski et al. (2006) and Kender et al. (2008) set this species into the erect epifauna morphogroup M1.
Remarks. In the investigated samples this species usually occurs broken and fragmentated; only one branched specimen was found in the sample PC060603.
Nothia cf. latissima (Grzybowski, 1898)
Figure 12.11
cf. 1898 Dendrophrya latissima; Grzybowski, p. 273, pl. 10, fig. 8.
Material. Three specimens from the samples PC060624.
Description. Test tubular, flattened, compressed with wall thin composed of medium sized grains.
Distribution. Nothia latissima is a cosmopolitan taxon common in flysch-type assemblages from the Cretaceous to the Eocene (Kaminski and Gradstein, 2005); Kaminski et al. (2006) and Kender et al. (2009) signal this species in the Miocene of Greenland Sea and Angola.
Nothia robusta (Grzybowski, 1898)
Figure 12.10
1898 Dendrophrya robusta; Grzybowski, p. 273, pl. 10, fig. 7.
1993 Rhabdammina robusta (Grzybowski); Kaminski and Geroch, p. 247, pl. 1, figs. 7-9b, 16a, b.
1995 Nothia robusta (Grzybowski); Holbourn and Kaminski, p. 438, pl. 1, figs. 12, 13.
1996 Rhabdammina robusta (Grzybowski); Kaminski, Kuhnt, and Radley, p. 16, pl. 1, figs. 2-18.
2005 Nothiarobusta (Grzybowski); Kaminski and Gradstein, p. 114, pl. 4.
2009 Nothia robusta (Grzybowski); Kender, Kaminski, and Jones, p. 493, pl. 1, fig. 7.
Material. 93 specimens from 16 samples.
Description. Test large, tubular, straight, compressed with a longitudinal furrow. Wall thick, coarse agglutinated with medium sized grains.
Remarks. Typical branched forms were absent, we have found only fragmented specimens. Nothia robusta differs from N. excelsa in having larger size and thicker wall.
Distribution. Cosmopolitan taxon known from the Late Cretaceous to the early Miocene (Kaminski and Gradstein, 2005).
Nothia spp.
Material. Eight specimens from three samples.
Description. Specimens with large tubular and flattened test not belonging to the listed species.
Genus PSAMMOSIPHONELLA Avnimelech, 1952
Psammosiphonella cylindrica (Glaessner, 1937)
Figure 12.4-5
1937 Rhabdammina cylindrica; Glaessner, p. 354, pl. 1, fig. 1.
1952 Psammosiphonella cylindrica (Glaessner); Avnimelech, p. 65.
1992 Rhabdammina cilindrica; Glaessner; Morlotti and Kuhnt, p. 223, pl. 2, fig. 1.
2005 Psammosiphonella cylindrica (Glaessner); Kaminski and Gradstein, p. 119, pl. 5/6, fig. 9-13.
2009 Rhabdammina cylindrica (Glaessner); Kender, Kaminski and Jones, p. 492, pl. 1, fig. 1.
Material. 238 specimens from 21 samples.
Description. Test tubular with circular section. Wall thick, composed of quartz grains dispersed in siliceous cement. Surface rough, aperture a simple terminal opening. Against the light is clearly visible the central siphon.
Distribution. Known in flysh-type deposits from the Cretaceous.
Remarks. Psammosiphonella cylindrica differs from Rhabdammina discreta Brady, 1884 in the absence of constrictions, and in having smoother surface. Psammosiphonella linearis is smaller and thinner, and has a more evident siphon.
Psammosiphonella linearis (Brady, 1879)
Figure 12.6-7
1879 Rhabdammina linearis; Brady, p. 37, pl. 3, figs. 10, 11.
1952 Oculosiphon linearis (Brady); Avnimelech, p. 65, fig. 9.
1987 Oculosiphon linearis (Brady); Loeblich and Tappan, p. 23, pl. 15, fig. 1.
2005 Rhabdammina linearis (Brady); Kaminski and Gradstein, p. 122, pl. 7, figs. 1-8.
2009 Rhabdammina linearis (Brady); Kender, Kaminski, and Jones, p. 493, pl. 1, fig. 2.
Material. 80 specimens from 19 samples.
Description. Test small, tubular, wall thin and finely agglutinated. Aperture a single terminal opening.
Distribution. From the Cretaceous to Recent (Kaminski and Gradstein, 2005).
Superfamily KOMOKIOIDEA Tendal and Hessler, 1977
Family RHIZAMMINIDAE Wiesner, 1931
Genus RHIZAMMINA Brady, 1879
Rhizammina indivisa Brady, 1884
Figure 12.12-13
1884 Rhizammina indivisa; Brady, p. 277, pl. 29, figs. 5-7.
1966 Rhizammina indivisa Brady; Geroch, p. 434, fig. 6 (1-7) (cum syn.).
1981 Rhizammina indyvisa Brady; Morgiel and Olszewska, p. 7, pl. 1, fig. 2.
1990 Rhizammina indivisa Brady; Bellagamba and Coccioni, pl. 1, fig. 5.
Material. 56 specimens from 19 samples.
Description. Test tubular, commonly flattened and curved.
Distribution. Jurassic-Recent.
Rhizammina spp.
Material. Seven specimens from four samples.
Description. Included in this group are all the fragmented forms with small size, test tubular and curved, with a wall thin, and irregular in outline.
Order SACCAMMININA Lankester, 1885
Superfamily SACCAMMINOIDEA Brady, 1884
Family SACCAMMINIDAE Brady, 1884
Subfamily SACCAMMININAE Brady, 1884
Genus PLACENTAMMINA Thalmann, 1947
Placentammina placenta (Grzybowski, 1898)
Figure 12.16
1898 Reophax placenta; Grzybowski, p. 276, pl. 10, figs. 9, 10.
1943 Placentammina placenta; Majzon, p. 152, pl. 3, fig. 7a-c.
1954 Saccammina placenta (Grzybowski); Geroch and Gradzinski, p. 36.
1960 Saccammina placenta (Grzybowski); Geroch, p. 121, pl. 2, figs. 1-6.
1987 Placentammina placenta (Grzybowski); Loeblich and Tappan, p. 31, 32, pl. 21, figs. 12-19.
2005 Placentammina placenta (Grzybowski) emend. Geroch, 1960; Kaminski and Gradstein, p. 136, pl. 11, figs. 1-6.
Material. 11 specimens from eight samples
Description. Test medium size, single chamber circular in outline and compressed in both the sides. Wall composed of quartz grains of different size. Aperture small and circular on a raised neck in more or less eccentric position.
Distribution. Common in flysch-type deposits from the Late Cretaceous to the Eocene of Carpathians; signaled in the Oligo-Miocene flysches of Mediterranean areas.
Genus SACCAMMINA Carpenter, 1869
Saccammina grzybowskii (Schubert, 1902)
Figure 12.17
1902 Reophax grzybowskii; Schubert, p. 20, pl. 1, fig. 13a, b.
2005 Saccammina grzybowskii (Schubert); Kaminski and Gradstein, p. 132, pl. 10, figs. 1-9.
Material. Two specimens from the sample PC060621.
Description. Unilocular test, circular in outline, and compressed. Peripheral aperture on a raised neck.
Distribution. Cretaceous-Neogene (Kaminski and Gradstein, 2005).
Superfamily PSAMMOSPHAEROIDEA Haeckel, 1894
Family PSAMMOSPHAERIDAE Haeckel, 1894
Subfamily PSAMMOSPHAERINAE Haeckel, 1894
Genus PSAMMOSPHAERA Schulze, 1875
Psammosphaera irregularis (Grzybowski, 1896)
Figure 12.14-15
1896 Keramosphaera irregularis; Grzybowski, p. 273, pl. 8, fig. 12, no fig. 13.
1966 Psammosphaera levigata White; Geroch, p. 436, pl. 7, figs. 18-20.
cf. 1981 Psammosphaera sp. var. B; Gradstein and Berggren, p. 241, pl. I, fig. 16.
1995 Psammosphaera irregularis (Grzybowski); Bubík, p. 84, pl. 1, figs. 15, 16.
Material. 27 specimens from 12 samples.
Description. Test small to medium in size, single chamber, lenticular, circular in outline with raised peripheral margin. Wall thick composed of medium to coarse quartz grains, aperture not clearly visible. In some specimens a small aperture appears in between the grains.
Distribution. Known in flysh-type deposits from the Late Cretaceous to Eocene (Kaminski and Gradstein, 2005).
Psammosphaera cf. laevigata White, 1928
Figure 13.1
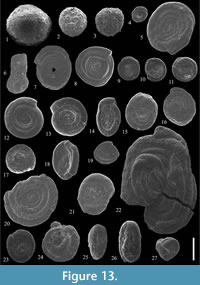 cf. 1977 Psammosphaera laevigata White; Samuel, p. 24, pl. 2, fig. 11.
cf. 1977 Psammosphaera laevigata White; Samuel, p. 24, pl. 2, fig. 11.
Material. Four specimens from three samples.
Description. Test globular to lenticular, with circular and rounded periphery. Wall thick composed of quartz grains. Aperture not visible.
Psammosphaera sp. 1
Figure 13.2
cf. 1966 Psammosphaera laevigata White; Geroch, p. 436, fig. 7 (18, 20).
? 1972 Psammosphaera laevigata White; Hanzlíková, p. 33, pl. I, figs. 7, 8.
1995 Psammosphaera sp.1; Bubík, 84, pl. 1, figs. 11a-13b.
1995 Psammosphaera fusca Schulze; Rögl, p. 252, pl. 1, fig. 11.
Material. Five specimens from three samples.
Description. Test small, flattened, single chamber, circular in outline with a raised and rounded periphery.
Remarks. Differs from P. irregularis in having a smaller size.
Psammosphaera sp. 2
Figure 13.3
Material. One specimen from the sample PC060615.
Description. Test small to medium in size, single chamber, inflated, globular, subspherical. Wall thick, composed of quartz grains. Aperture is a small opening between grains.
Psammosphaera sp. 3
Figure 13.4
Material. One specimen from the sample PC060615.
Description. Test free, small, circular in outline with a central depression in both the sides. Wall thin, finely agglutinated.
Remarks. Differs from Psammosphaera sp. 1 in having smaller size and sparser agglutination.
Order AMMODISCIDA Mikhalevich, 1980
Suborder HIPPOCREPININA Saidova, 1981
Superfamily HIPPOCREPINOIDEA Rhumbler, 1895
Family HYPERAMMINIDAE Eimer and Fickert, 1899
Subfamily HYPERAMMININAE Eimer and Fickert, 1899
Genus HYPERAMMINA Brady, 1878
Hyperammina spp.
Figure 13.6
Material. Eight specimens from seven samples.
Description. This group includes all the tubular, straight or weakly curved flattened fragmented forms, with a large piriform or suboval proloculus.
Superfamily HORMOSINELLOIDEA Rauser and Reitlinger, 1986
Family AMMOLAGENIDAE Kaminski, Henderson, Cetean, and Waśkowska, 2009
Genus AMMOLAGENA Eimer and Fickert, 1899
Ammolagena clavata (Jones and Parker, 1860)
Figure 14.1-2
 1860 Trochammina irregularis (d'Orbigny) var. clavata; Jones and Parker, p. 304.
1860 Trochammina irregularis (d'Orbigny) var. clavata; Jones and Parker, p. 304.
1884 Webbina clavata Jones and Parker; Brady, p. 349-350, pl. 41I, figs. 12-16 (cum syn.).
2004 Ammolagena clavata (Jones and Parker); Nigam, Mazumder, and Saraswat, p. 74, pl. 1, fig. a-d.
Material. 96 specimens from 20 samples.
Description. Test attached, large inflated and ovoid proloculus, followed by an undivided elongate irregular tube-like chamber, not increasing in size. Wall smooth, thin, finely agglutinated. Two apertures, one at the base of the proloculus as a simple opening surrounded by a lip, one at the end of the tube-like chamber.
Distribution. Cosmopolitan, known from the Cretaceous to Recent, recently signaled in the Indian Ocean (Nigam et al., 2004).
Remarks. Attached form, sometimes encrusting Rhabdamminids, Ammodiscus or lituolids.
Family HORMOSINELLIDAE Rauser and Reitlinger, 1986
Genus CAUDAMMINA Montanaro-Gallitelli, 1955
Caudammina gutta Benedetti and Pignatti, 2009
Figure 14.3-7
2009 Caudammina gutta; Benedetti and Pignatti, p. 344, pl. 1, figs. 1-18.
Material. 126 specimens from nine samples.
Description. Test free, large, flask-like, originally plurilocular comprised of rounded or pyriform pseudochambers without internal partitions. Wall thick, imperforate, and finely agglutinated with a smooth surface. Aperture at the open end of the short neck.
Distribution. Late Eocene?-Oligocene.
Remarks. Mikhalevich (2013) erroneously includes the subfamily Caudammininae within the family Saccamminidae, since Caudammina is clearly not monothalamous.
Genus SUBREOPHAX Saidova, 1975
Subreophax cf. guttifer (Brady, 1881)
Figure 14.8
?1881 Reophax guttifera; Brady, p. 49.
?1977 Reophax pseudoscalaria; Samuel, p. 36, pl. 3, fig. 4a, b.
1992 Subreophax cf. guttifer (Brady); Morlotti and Kuhnt, p. 223, pl. 3, fig. 5.
Material. Two specimens from two samples.
Description. Test free, subcylindic, three flattened and subcircular chambers, interconnected and oblique respect the axis of growth. Aperture at the end of the last chamber.
Subreophax cf. pseudoscalaris (Samuel, 1977)
Figure 14.9
cf. 1977 Reophax pseudoscalaria; Samuel, p. 35, 36, pl. 3, fig. 4a, b.
cf. 2005 Subreophax pseudoscalaris (Samuel); Kaminski and Gradstein, p. 281, pl. 56, figs. 1-6 (partim).
Material. Five specimens from five samples.
Description. Test free, uniseriate, composed of discoidal, compressed chamber increasing gradually in size.
Distribution. Subreophax pseudoscalaris is described from the Senonian to Lower Eocene (Kaminski and Gradstein, 2005).
Subreophax scalaris (Grzybowski, 1896)
Figure 14.10
1896 Reophax guttifera Brady var. scalaria; Grzybowski, p. 277, pl. 8, fig. 26a, b.
1977 Reophax scalaria Grzybowski; Samuel, p. 35, 36, pl. 3, fig. 6a, b, pl. 19, fig. 4.
1992 Subreophax scalaris (Grzybowski); Morlotti and Kuhnt, p. 223, pl. 3, fig. 5.
2005 Subreophax scalaris (Grzybowski); Kaminski and Gradstein, p. 278, pl. 55, figs. 1-7.
2009 Subreophax scalaris (Grzybowski); Kender, Kaminski, and Jones, p. 497, pl. 3, figs. 3, 4.
Material. Six specimens from six samples.
Description. Test free, uniseriate, elongated, curved axis. Several discoidal and compressed chambers partially embraced increasing very slowly in size.
Distribution. Lowermost Cretaceous to Oligocene (Kaminski and Gradstein, 2005), more recently reported also from the Miocene by Kender et al. (2009).
Subreophax splendidus (Grzybowski, 1898)
Figure 14.11-12
1898 Reophax splendidus; Grzybowski, p. 278, pl. 10, fig. 16.
1993 Subreophax splendidus (Grzybowski); Kaminski and Geroch, p. 251, pl. 3, figs. 11a-12b.
2005 Subreophax splendidus (Grzybowski); Kender, Kaminski, and Cieszkowski, p. 262, fig. 11C.
Material. Eight specimens from five specimens.
Description. Test free, large, weakly curved. Chambers irregular flattened and elliptic, suture incise. Aperture wide and terminal. Wall coarsely agglutinated.
Suborder AMMODISCINA Mikhalevich, 1980
Superfamily AMMODISCOIDEA Reuss, 1862
Family AMMODISCIDAE Reuss, 1862
Subfamily AMMODISCINAE Reuss, 1862
Genus AMMODISCUS Reuss, 1862
Ammodiscus cretaceus (Reuss, 1845)
Figure 13.5
1845 Operculina cretacea; Reuss, p. 35, pl. 13, figs. 64, 65.
1860 Cornuspira cretacea Reuss; Reuss, p. 177 , pl. 1, fig. 1.
1978 Ammodiscus cretaceus (Reuss); Krasheninnikov and Pflaumann, p. 569, pl. 2, fig. 7.
2005 Ammodiscus cretaceus (Reuss); Kaminski and Gradstein, p. 145, pl. 14, figs. 1-10.
2009 Ammodiscus cretaceus (Reuss); Kender, Kaminski, and Jones, p. 495, pl. 1, fig. 16, pl. 2, fig. 5.
Material. 10 specimens from seven samples.
Description. Test large, circular, biconcave, composed of a spherical proloculus followed by a single tubular chamber planispirally coiled. The whorls (up to 11) increase gradually in size. Wall thin and finely agglutinated. Aperture at the end of the tubular chamber.
Distribution. Cosmopolitan taxon; known from the Cretaceous to the late Eocene (Kaminski and Gradstein, 2005). Signaled from the Oligocene of the Beaufort-Mackenzie Basin (Schröder-Adams and McNeil, 1994), and from the Miocene of the Congo Fan (Kender et al., 2008, 2009).
Ammodiscus incertus (d’Orbigny, 1839)
Figure 13.7-8
1839 Operculina incerta; d’Orbigny, p. 49, pl. 6, figs. 16, 17.
?1884 Ammodiscus incertus d’Orbigny; Brady, p. 370, pl. 38, fig. 1-3.
2004 Ammodiscus incertus d’Orbigny; Govindan, p. 225, pl. 4, figs. 17, 19.
Material. Four specimens from four samples.
Description. Test discoidal commonly biconcave, composed of a single tubular chamber.
Remarks. Ammodiscus incertus differs from A. cretaceus (Reuss, 1845) in having a constant height of the whorls, smaller size, and a typical biconvex test.
Ammodiscus latus Grzybowski, 1898
Figure 13.12-13
1898 Ammodiscus latus; Grzybowski, p. 282, 283, pl. 10, figs. 27, 28.
1898 Ammodiscus umbonatus; Grzybowski, p. 283, pl. 10, figs. 29, 30.
1954 Lituotuba lata (Grzybowski); Geroch and Gradzinski, p. 39, tabl. 4, figs. 1, 2.
1960 Lituotuba lata (Grzybowski); Geroch, p. 126, pl. 4, fig. 8.
1992 Ammodiscus latus Grzybowski; Morlotti and Kuhnt, p. 221, pl. 1, fig. 3.
2005 Ammodiscus latus Grzybowski; Kaminski and Gradstein, p. 150, pl. 16a, figs. 1-8, pl. 16b, figs. 1-6.
2009 Ammodiscus latus Grzybowski; Kender, Kaminski, and Jones, p. 495, pl. 1, fig. 17.
Material. 42 specimens from nine samples.
Description. Spherical proloculus followed by a single tubular chamber planispirally coiled for 4.5 whorl increasing in size gradually. Wall thick and finely agglutinated, sutures depressed. The last portion of the tubular chamber is typically uncoiled. Aperture a simple terminal opening.
Distribution. Cosmopolitan taxon described from the Eocene to Miocene (Kender et al., 2009).
Remarks. The uncoiled stage is not visible in each specimen.
Ammodiscus cf. latus Grzybowski, 1898
Figure 13.15-16
cf. 1898 Ammodiscus latus; Grzybowski, p. 282, 283, pl. 10, figs. 27, 28.
Material. Four specimens from the sample PC11.
Remarks. Differs from A. latus in having a more flattened test, and for the lack of the uncoiled stage.
Ammodiscus tenuissimus (Gümbel, 1862)
Figure 13.9-11
1862 S pirillina tenuissima; Gümbel, p. 214, pl. 13, fig. 2.
1898 Ammodiscus tenuissimus; Grzybowski, p. 282, pl. 10, fig. 35.
1966 Ammodiscus tenuissimus Guembel; Geroch, p. 437, fig. 8 (1, 4).
1993 Ammodiscus tenuissimus Grzybowski; Kaminski and Geroch, p. 253, figs. 1-3b.
1995 Ammodiscus tenuissimus Gümbel; Holbourn and Kaminski, p. 442, pl. III, fig. 1.
1996 Ammodiscus tenuissimus Grzybowski; Kaminski, Kuhnt, and Radley, p. 10, pl. 1, fig. 4.
Material. 25 specimens from eight samples.
Description. Test free, small size (less than 0.5 mm), very thin and flattened, circular or weakly elliptical, composed of a single chambers planispirally arranged in several closest whorls. Wall thin, finely agglutinated. Aperture terminal.
Distribution. Cosmopolitan species common from the Late Cretaceous to the Eocene (Kaminski and Gradstein, 2005). Recently signaled from the Miocene of the Greenland Sea (Kaminski et al., 2006).
Ammodiscus peruvianus Berry, 1928
Figure 13.14
1928 Ammodiscus peruvianus; Berry, p. 342, pl. 27.
1954 Ammodiscus grzybowski; Emiliani, p. 106.
2006 Ammodiscus peruvianus (Berry); Kaminski, Silye, and Kender, p. 382, pl. 1, fig. 20.
Material. Five specimens in the sample PC060617.
Description. Test free, elliptical in outline, composed of a single chambers planispirally coiled. Wall thin, finely agglutinated. Aperture terminal.
Distribution. Cosmopolitan species common from the Cretaceous to the Eocene, and signaled from the Oligocene of the northern Appennines (Emiliani, 1954), and from the Miocene of Greenland Sea (Kaminski et al ., 2006).
Remarks. Ammodiscus peruvianus differs from A. cretaceus for the elliptical outline.
Ammodiscus sp. 1
Figure 13.17
Material. One specimen from the sample PC060607.
Description. Test free, small in size, incomplete, composed of a large sized proloculus followed by a single tubular chamber for about two whorls. Wall thick, medium to coarse agglutinated.
Remarks. Differs from A. latus and A. cf. latus in having higher chamber and for the coarser agglutination of the wall.
Ammodiscus sp. 2
Figure 13.18
Material. Two specimens from two samples.
Description. Test free, planispirally coiled, biconcave, evoluted with weakly embraced whorls. The single tubular chamber is arranged in seven whorls increasing gradually in size. Wall thin, finely agglutinated.
Ammodiscus sp. 3
Figure 13.19
Material. One single specimen from the sample PC060603.
Description. Test free, planispirally coiled, strictly biconcave, evolute with partially embraced whorls. The tubular chamber completes 11 whorls around the axis. Wall thin, finely agglutinated. Aperture single at the end of the chamber.
Remarks. Differs from Ammodiscus sp. 2 in having a higher number of whorls, and smaller size.
Ammodiscus spp.
Material. Six specimens from two samples.
Description. In this group are included all fragmented and compressed test unrecognizable at specific level.
Subfamily AMMOVERTELLININAE Saidova, 1981
Genus ANNECTINA Suleymanov, 1963
Annectina biedai Gradstein and Kaminski, 1997
Figure 13.20
1997 Annectina biedai; Gradstein and Kaminski, p. 218. figs. 2, 3, 1a-2c.
2005 Annectina biedai Gradstein and Kaminski; Kaminski and Gradstein, p. 195, pl. 29, figs. 1-5 (cum syn.).
Material. Two specimens from the sample PC060618.
Description. Test free, discoidal and flattened, circular or elliptic in outline. The short initial stage triloculina-like coiled is followed by several planispirally coiled whorls.
Distribution.Middle Eocene-late Oligocene (Kaminski and Gradstein, 2005).
Annectina cf. grzybowskii (Jurkiewicz, 1960)
Figure 13.21
cf. 1960 Glomospira grzybowskii; Jurkievicz, p. 339, pl. 38, figs. 7, 10, 11.
cf. 1992 Glomospirella biedai Samuel; Morlotti and Kuhnt, p. 222, pl. 1, figs. 10, 11.
Material. Three specimens from the sample PC060617.
Description. Test elliptic or subcircular composed of a single chamber with an initial milioline-like stage followed by few planispirally coiled whorls. Wall thin, medium sized quartz grains immerse into abundant cement. Aperture a single opening at the end of the tubular chamber.
Remarks. Differs from A. grzybowskii, species descrive from the Campanian to the Paleocene, in having smaller size.
Subfamily USBEKISTANIINAE Vyalov, 1968
Genus GLOMOSPIRA Rzehak, 1885 Glomospira extendens Emiliani, 1954
Figure 15.1
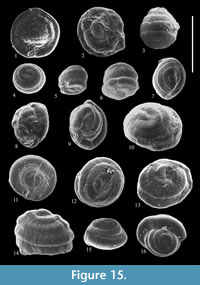 1954 Glomospira charoides (Jones and Parker), var. extendens; Emiliani, p. 133, pl. 22 (3), fig. 14a-c.
1954 Glomospira charoides (Jones and Parker), var. extendens; Emiliani, p. 133, pl. 22 (3), fig. 14a-c.
Material. One single specimen from the sample PC11.
Description. Test free, small, composed of a proloculus followed by a tubular undivided chamber initially arranged in 3-4 glomospirine whorls, whereas the last two whorls are coiled around an axis at 90°. Wall smooth and finely agglutinated. Aperture terminal at the end of the tube-like chamber.
Glomospira irregularis (Grzybowski, 1898)
Figure 13.27
1898 Ammodiscus irregularis; Grzybowski, p. 285, pl. 11, figs. 2, 3.
1966 Glomospira irregularis (Grzybowski); Geroch, p. 460, pl. 8, figs. 11, 12.
2005 Glomospira irregularis (Grzybowski); Kender, Kaminski, and Cieszkowski, p. 261, fig. 10Q.
2009 Glomospira irregularis (Grzybowski); Kender, Kaminski, and Jones, p. 496, pl. 2, fig. 12.
Material. 10 specimens from six samples.
Description. Test free composed of a tube-like undivided chamber streptospirally coiled. Wall thick, finely agglutinated, smooth surface. Aperture at the end of the tubular chamber.
Distribution. Cosmopolitan species known from the Cretaceous to Recent (Kender et al., 2009).
Glomospira gordialis (Jones and Parker, 1860)
Figure 15.2
1860 Trochammina squamata Jones and Parker var. charoides; Jones and Parker, p. 304.
1884 Ammodiscus gordialis Jones and Parker; Brady, p. 333, pl. 38, figs. 7-9.
1995 Glomospira gordialis (Jones and Parker); Holbourn and Kaminski, p. 444, pl. 3, fig. 9.
2005 Glomospira gordialis (Jones and Parker); Kender, Kaminski, and Cieszkowski, p. 261, fig. 10P.
2009 Glomospira gordialis (Jones and Parker); Kender, Kaminski, and Jones, p. 496, pl. 2, fig. 11.
Material. 16 specimens from eight samples.
Description. Test free, small, circular in outline, comprised of a small proloculus followed by a single undivided chamber trochospirally enrolled in the early whorls, then glomospirally coiled along a general plane; last whorl deviates from the general coiling, embracing previous stage. Wall smooth, finely agglutinated; aperture simple opening at the end of the tube.
Distribution. Cosmopolitan species known from the Cretaceous to Recent (Kender et al., 2009).
Glomospira serpens (Grzybowski, 1898)
Figure 13.25-26
1898 Ammodiscus serpens; Grzybowski, p. 285, pl. 10, figs. 31-33.
1977 Glomospirella serpens (Grzybowski); Samuel, p. 30, pl. 4, figs. 2, 4.
1982 Glomospira serpens (Grzybowski); Miller, Gradstein, and Berggren, p. 20, pl. 1, fig. 13.
2005 Glomospira serpens (Grzybowski); Kender, Kaminski, and Cieszkowski, p. 261, fig. 10R.
Material. 21 specimens from six samples.
Description. Test flattened, ovate in outline, a single undivided chamber milioline-like coiled. Wall smooth, finely agglutinated; aperture is a simple opening at the end of the tube.
Distribution. Cosmopolitan species known from the Upper Cretaceous to the Eocene of Carpathians and Alps.
Glomospira sp. 1
Figure 13.23
Material. One single specimen from the sample PC11.
Description. Initial stage streptospirally coiled, followed by two planispirally enrolled whorls increasing rapidly in size.
Glomospira sp. 2
Figure 13.22
Material. One single specimen from the sample PC15.
Description. Test large, elliptic in outline, short initial stage glomospirine-like coiled followed by five planispirally coiled whorls weakly irregular. Wall thick with sparse agglutinated grains. Aperture at the end of the tubular chamber.
Glomospira sp. 3
Figure 13.24
Material. One single specimen from the sample PC13.
Description. Proloculus followed by an undivided tubular chamber with an initial stage streptospirally coiled; the diameter of the tube is constant and does not increase substantially in size. Last whorls are arranged in a subplanar coil perpendicular to the main direction of coiling of the glomospirine stage which results decentralized. Wall thick and finely agglutinated.
Genus REPMANINA Suleymanov in Arapova and Suleymanov, 1966
Repmanina charoides (Jones and Parker, 1860)
Figure 15.3-16
1860 Trochammina squamata Jones and Parker var. charoides; Jones and Parker, p. 304.
1884 Ammodiscus charoides (Jones and Parker); Brady, p.334, 335, pl. 38, figs. 10-16.
1928 Glomospira charoides (Jones and Parker), var corona; Cushman and Jarvis, p. 89, pl. 12, figs. 9-11.
1989 Repmanina charoides (Jones and Parker); Coccioni, p. 95, pl. 3, fig. 22.
2005 Glomospira charoides (Jones and Parker); Kender, Kaminski, and Cieszkowski, p. 261, fig. 10M.
Material. 131 specimens from 24 samples.
Description. Test small, subspheric, circular in outline or flattened, composed of a long tube-like chamber streptospirally enrolled around the proloculus (never visible) about up to three different axes of coiling. Wall finely agglutinated, surface smooth.
Distribution. Cosmopolitan taxon known from the Jurassic to Recent (Kaminski and Gradstein, 2005).
Remarks. The outern morphology of R. charoides is widely variable, Berggren and Kaminski (1990) recognize a dozen of synonim subdivided into four groups.
Family LITUOTUBIDAE Loeblich and Tappan, 1984
Genus LITUOTUBA Rhumbler, 1895
Lituotuba lituiformis (Brady, 1879)
Figure 16.16
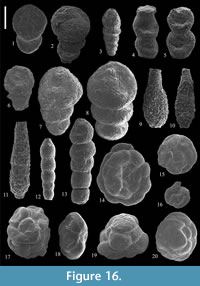 1879 Trochammina lituiformis; Brady, p. 59, pl. 5, Zfi. 16.
1879 Trochammina lituiformis; Brady, p. 59, pl. 5, Zfi. 16.
2005 Lituotuba lituiformis (Brady); Kaminski and Gradstein, p. 287, pl. 58, figs. 1-8.
Material. One single specimen from the sample PC060615.
Description. Test free, composed of a subspheric proloculus followed by a tube glomospirine or streptospirally coiled, which tends to uncoil and become rectilinear carrying on with the ontogeny. The tube is initially undivided, after some constrictions at irregular distance define the chambers.
Distribution. Cretaceous to Recent (Kaminski and Gradstein, 2005).
Genus PARATROCHAMMINOIDES Soliman, 1972
Paratrochamminoides acervulatus (Grzybowski, 1896)
Figure 16.14
1896 Trochammina acervulata; Grzybowski, p. 274, pl. 9, fig. 4.
2005 Paratrochamminoides acervulatus (Grzybowski); Kaminski and Gradstein, p. 290, pl. 59, figs. 1-7.
Material. Nine specimens from six samples.
Description. Test free, ovoid, the sack-shaped chambers are arranged in a conic and irregular trocospire. The ventral side shows a depressed umbelicus. Wall thick, finely agglutinated, aperture at the base of the last chamber.
Distribution. Late Cretaceous-Eocene (Kaminski and Gradstein, 2005).
Paratrochamminoides deflexiformis (Noth, 1912)
Figure 16.15
1912 Trochammina deflexiformis; Noth, p. 14, pl. 1, fig. 10.
2005 Paratrochamminoides deflexiformis (Noth); Kaminski and Gradstein, p. 293, pl. 60, figs. 1-4.
Material. Four specimens from four samples.
Description. Test free, subelliptic in outline, three glomospirine-like coiled whorls and open umbelicus. Subspheric chambers increasing gradually in size, sutures incise. Wall finely agglutinated, thick, smooth surface.
Distribution. Maastrichtian-Eocene.
Paratrochamminoides draco (Grzybowski, 1901)
Figure 16.18
1901 Trochammina draco; Grzybowski, p. 280, pl. 8, fig. 10.
1995 Paratrochamminoides draco (Grzybowski); Bubík, p. 84, pl. 3, fig. 2a, b.
1996 Paratrochamminoides draco (Grzybowski); Kaminski, Kuhnt, and Radley, p. 16, pl. 3, fig. 8.
Material. One specimen from the sample PC11.
Description. Test free, large, elliptic in outline. Initially 2-3 glomospirine-like coiled whorls, followed by a reversed whorl of about 180°. Chambers elongated, sack-shaped, sutures very incise. Wall thick, medium to finely agglutinated. Aperture simple at the end of the last chamber.
Paratrochamminoides aff. gorayskii (Grzybowski, 1898)
Figure 17.12
aff. 1898 Ammodiscus gorayskii; Grzybowski, p. 286, pl. 11, fig. 5.
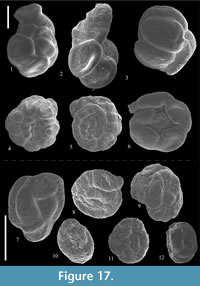 Material. Two specimens from the sample PC060615.
Material. Two specimens from the sample PC060615.
Description. Test small, elliptic in outline, triloculine-like coiled. The chambers are subdivided by septa few evident. Wall finely agglutinated.
Remarks. The collected specimens resemble P. gorayskii (Grzybowski) differing in having a shorter spire.
Paratrochamminoides heteromorphus (Grzybowski, 1898)
Figure 17.1-2
1898 Trochammina heteromorpha; Grzybowski, p. 286, pl. 11, fig. 16.
1943 Trochamminoides koeroesmezooensis; Majzon, p. 156, pl. 2, fig. 16a-c.
2005 Paratrochamminoides heteromorphus (Grzybowski); Kaminski and Gradstein, p. 298, pl. 62, figs. 1-10.
Material. 18 specimens from nine samples.
Description. Test free, large, initiallly trocospirally or streptospirally coiled, last chambers uncoiled. Chambers globose increasing in size in the uncoiled portions, where they become tubular and more or less compressed. Sutures incise. Wall finely agglutinated, smooth surface. Aperture simple at the end of the last chamber.
Distribution. Campanian-Eocene (Kaminski and Gradstein, 2005).
Paratrochamminoides mitratus (Grzybowski, 1901)
Figure 16.19
1901 Trochammina mitrata; Grzybowski, p. 280, pl. 8, fig. 3.
2005 Paratrochamminoides mitratus (Grzybowski); Kaminski and Gradstein, p. 302, pl. 63, figs. 1-7.
Material. Four specimens from four samples.
Description. Test large, streptospirally coiled, composed of several chambers increasing slowly in size. The coiling plane abruptly changes. Wall finely agglutinated.
Distribution. Turonian-Oligocene (Kaminski and Gradstein, 2005).
Paratrochamminoides olszewskii (Grzybowski, 1898)
Figure 17.9
1898 Trochammina olszewskii; Grzybowski, p. 298, pl. 11, fig. 6.
2005 Paratrochamminoides olszewskii (Grzybowski); Kaminski and Gradstein, p. 305, pl. 64, figs. 1-7.
Material. Five specimens from five samples.
Description. Test free, oval in outline, glomospirine-like coiled, 2-3 whorls are externally visible. The initial stage is undivided, going on with the ontogeny some constrictions define the chambers. The last whorl tends to become planispiral or weakly irregular. Wall thin, finely agglutinated.
Distribution. Cenomanian-Eocene (Kaminski and Gradstein, 2005).
Paratrochamminoides cf. olszewskii (Grzybowski, 1898)
Figure 17.7
cf. 1898 Trochammina olszewskii; Grzybowski, p. 298, pl. 11, fig. 6.
Material. Three specimens from three samples.
Remarks. Differs from P. olszewskii in the lack of the planispiral final stage.
Paratrochamminoides aff. olszewskii (Grzybowski, 1898)
Figure 17.11
aff. 1898 Trochammina olszewskii; Grzybowski, p. 298, pl. 11, fig. 6.
Material. One specimen from the sample PC060615.
Description. Test free, small, glomospirine-like coiled. Initially a tubular undivided chambers, later constrictions subdivided the chambers with septa. Wall thin, finely agglutinated.
Remarks. Differs from P. olszewskii in having a shorter initial tube-like chamber and in the lack of the planispiral whorl.
Paratrochamminoides sp. 1
Figure 16.17
Material. One specimen from the sample PC060601.
Description. Test free, large, elliptic in outline, streptospirally coiled. Chambers large and compressed, sack-shaped, sutures incise. Wall finely agglutinated.
Paratrochamminoides spp.
Material. 139 specimens from 22 samples.
Description. In this group are included all the specimens not referred at any known species.
Genus CONGLOPHRAGMIUM Bermúdez and Rivero, 1963
Conglophragmium deforme (Grzybowski, 1898)
Figure 16.20
1898 Trochammina deformis; Grzybowski, p. 288, pl. 11, figs. 20-22.
1995 Paratrochamminoides deformis (Grzybowski); Rögl, p. 256, pl. 2, figs. 15-19.
2004 Conglophragmium deformis (Grzybowski); Kaminski and Kuhnt, p. 279.
Material. One specimen from the sample PC060623.
Description. Test free, large, oval in outline. Initially streptospirally coiled, later an involute planispire with 5-6 globose, elongated and subrectangular chambers componing the last whorl. Aperture interiomarginal.
Conglophragmium irregulare (White, 1928)
Figure 17.3
1928 Trochammina irregularis; White, p. 307, pl. 42, fig. 1.
1937 Trochamminoides irregularis (White); Glaessner, p. 360, pl. 1, fig. 9a, b.
1990 Paratrochamminoides irregularis (White); Kuhnt, p. 320, pl. 5, fig. 10.
2004 Conglophragmium irregularis (White); Kaminski and Kuhnt, p. 279.
Material. 21 specimens from 10 samples.
Description. Test free, large, elliptic in outline. Chambers large, globose or sack-shaped, irregularly coiled. Coiling direction continuously alternating.
Distribution. From Cretaceous to Oligocene (Kaminski and Gradstein, 2005).
Family TROCHAMMINOIDAE Haynes and Nwabufo-Ene, 1998
Genus TROCHAMMINOIDES Cushman, 1910
Trochamminoides coronatus (Brady, 1879)
Figure 17.4
1879 Trochammina coronata; Brady, p. 39, pl. 5, fig. 15.
1977 Trochamminoides coronatus Brady; Krasheninnikov and Pflaumann, p. 570, pl. 4, fig. 4a, b.
Material. 14 specimens from seven samples.
Description. Test free, large, biconcave, composed of few whorls arranged in a low trochospire. Chambers globose and elongated, sutures distinct, periphery lobate. Aperture simple at the end of the last chamber.
Trochamminoides dubius (Grzybowski, 1901)
Figure 17.8, 10
1901 Ammodiscus dubius; Grzybowski, p. 274, pl. 8, figs. 12, 14.
1993 Trochamminoides dubius (Grzybowski); Kaminski and Geroch, p. 275, pl. 15, figs. 9-12.
2005 Trochamminoides dubius (Grzybowski); Kaminski and Gradstein, p. 308, pl. 65, figs. 1-8.
Material. 10 specimens in six samples.
Description. Test free, small, compressed. Initial coil glomospirine-like, becoming planispiral through the ontogeny. The test is composed of four whorls, in the last whorl 4 ½ tubular chambers occur. Wall finely agglutinated.
Distribution. Maastrichtian-Oligocene (Kaminski and Gradstein, 2005).
Trochamminoides grzybowskii Kaminski and Geroch, 1992
Figure 17.5
1898 Trochammina elegans Rzehak; Grzybowski, p. 287, pl. 11, fig. 10.
1977 Trochamminoides elegans (Grzybowski); Samuel, p. 45, pl. 4, fig. 17.
1992 Trochamminoides grzybowskii; Kaminski and Geroch, p. 64, fig. 1a, b.
2005 Trochamminoides grzybowskii Kaminski and Geroch; Kaminski and Gradstein, p. 311, pl. 66, figs. 1-4.
Material. Four specimend from four samples.
Description. Test free, large, elliptic or subcircular in outline, 2-3 whorls planispirally evolute coiled. Chambers numerous, subspheric, sutures depressed, well distinct at regular distance. Wall finely agglutinated, smooth surface. Aperture small at the base of the last chamber.
Distribution. Coniacian-Eocene (Kaminski and Gradstein, 2005).
Trochamminoides intermedius (Grzybowski, 1896)
Figure 18.5
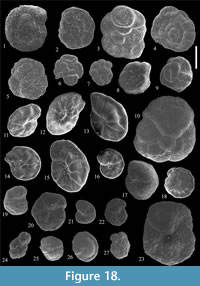 1896 Trochammina intermedia; Grzybowski, p. 282, pl. 8, fig. 53a-c.
1896 Trochammina intermedia; Grzybowski, p. 282, pl. 8, fig. 53a-c.
Material. Three specimens from two samples.
Description. Test free, oval, compressed, trocospirally coiled, with chambers flattened and square-shaped. Sutures incise.
Trochamminoides proteus (Karrer, 1866)
Figure 18.2
1866 Trochammina proteus; Karrer, pl. 1, fig. 8.
1977 Trochamminoides proteus (Karrer); Samuel, p. 46-47, pl. 5, fig. 5a, b.
2005 Trochamminoides proteus (Karrer); Kender, Kaminski and Cieszkowski, p. 264, fig. 12E, F.
Material. Six specimens from five samples.
Description. Test free, large, oval in outline. Initially glomospirine-like coiled becoming planispiral going on with the ontogeny. Chambers globose increasing gradually in size. In the last whorl 6-9 chambers occur. Wall finely agglutinated.
Distribution. Cosmopolitan species known from the Late Cretaceous to the late Eocene (Kender et al ., 2005).
Trochamminoides cf. proteus (Karrer, 1866)
Figure 18.3
cf. 1866 Trochammina proteus; Karrer, pl. 1, fig. 8.
Material. One specimen from the sample PC060624.
Description. A species referred to the genus Trochamminoides with six subcircular and compressed chambers in the last whorl. Periphery lobate. Wall finely agglutinated with some medium to large sized quartz grains.
Remarks. Differs from T. proteus in having less chambers and a larger size.
Trochamminoides septatus (Grzybowski, 1898)
Figure 18.1
1898 Ammodiscus septatus; Grzybowski, p. 283, pl. 11, fig. 1.
1993 Trochamminoides septatus (Grzybowski); Kaminski and Geroch, p. 255, pl. 5, fig. 9a-c.
2005 Trochamminoides septatus (Grzybowski); Kender, Kaminski, and Cieszkowski, p. 264, fig. 12G.
Material. One specimen from the sample PC060604.
Description. Test free, large, planispirally ammodiscine-type coiled, with constrictions forming chambers. Wall thin, but coarsely agglutinated.
Trochamminoides subcoronatus (Grzybowski, 1896)
Figure 17.6
1896 Trochammina subcoronata; Grzybowski, p. 283, 284, pl. 9, fig. 3a-c.
1996 Trochamminoides subcoronatus (Grzybowski); Kuhnt and Collins, p. 214.
2001 Trochamminoides subcoronatus (Grzybowski); Kuhnt and Urquhart, p 56, pl. 3, fig. 9 (cum syn.).
Material. 16 specimens from nine samples.
Description. Test free, very large, subelliptic or circular in outline, planispirally coiled with 6-8 elongated sack-shaped chambers in the last whorl.
Remarks. Trochamminoides coronatus has a higher number of chambers.
Trochamminoides variolarius (Grzybowski, 1898)
Figure 18.6
1898 Trochammina variolaria; Grzybowski, p. 288, pl. 11, fig. 15.
1993 Trochamminoides variolarius (Grzybowski); Kaminski and Geroch, p. 261, pl. 9, figs. 5, 6.
2005 Trochamminoides variolarius (Grzybowski); Kender, Kaminski, and Cieszkowski, p. 264, fig. 12J.
Material. Six specimens from four samples.
Description. Test free, small, subsquare in outline, composed of two whorls, with 4-5 chambers in the last whorl. Coiling planispiral or weakly trochospiral. Chambers triangular increasing rapidly in size. Wall finely agglutinated, smooth surface.
Trochamminoides velascoensis Cushman, 1926
Figure 18.4
1926 Trochamminoides velascoensis; Cushman, p. 583, pl. 15, fig. 2a, b.
2005 Trochamminoides velascoensis (Cushman); Kender, Kaminski, and Cieszkowski, p. 264, fig. 12K.
Material. Three specimens from three samples.
Description. Test free, subcircular in outline, planispirally coiled. Numerous subrectangular and flattened chambers (10 in the last whorl). Suture few evident.
Distribution. Trochamminoides velascoensis was firstly described from the Velasco Formation of Mexico (Cushman, 1926), and recently signaled from the Eocene of Carpathians (Kender et al ., 2005).
Trochamminoides sp. 1
Figure 18.7
Material. One single specimen from the sample PC060603.
Description. Test free, small, initially glomospirine-like coiled, becoming planispiral in the latest whorls. Chambers globose, sutures incise. Ten chambers in the last whorl.
Trochamminoides sp. 2
Figure 18.8
Material. One specimen from the sample PC060604.
Description. Very similar in shape to T. velascoensis , but the subdivision of the chambers is arranged as in T. septatus.
Trochamminoides sp. 3
Figure 18.9
Material. Two specimens from the sample PC060618.
Description. Test free, glomospirine-like coiled. Chambers flattened and subsquared. Periphery quadrangular and lobate.
Trochamminoides sp. 4
Figure 18.10
Material. Two specimens from two samples.
Distribution. Test free, very large, quadrangular in outline, low trocospirally coiled. Chambers subrectangular and very large increasing rapidly in size. In the dorsal side a low spire is visible, whereas three large chambers occur in the last whorl.
Trochamminoides spp.
Material. 22 specimens from 12 samples.
Description. In this group are included all the broken or incomplete specimens with planispiral or low trocospiral mode of coiling, and chambers globose or flattened.
Order LITUOLIDA Lankester, 1885
Suborder HORMOSININA Mikhalevich, 1980
Superfamily HORMOSINOIDEA Haeckel, 1894
Family ASCHEMOCELLIDAE Vyalov, 1966
Genus ARTHRODENDRON Ulrich, 1904
Arthrodendron grandis (Grzybowski, 1898)
Figure 14.14
1898 Reophax grandis; Grzybowski, p. 277, pl. 10, figs. 13-15.
1993 Aschemocella grandis (Grzybowski); Kaminski and Geroch, p. 249, pl. 2, figs. 8-10.
2005 Aschemocella grandis (Grzybowski); Kender, Kaminski, and Cieszkowski, p. 262, fig. 11D, E.
2008 Arthrodendron grandis (Grzybowski); Kaminski, Uchman, Neagu, and Cetean, p. 108.
2009 Aschemocella grandis (Grzybowski); Kender, Kaminski, and Jones, p. 497, pl.3, fig. 5.
Material. 56 specimens from 14 samples.
Description. Test free, large, uniseriate and curved, composed of discoidal and compressed chambers with raised periphery. Aperture single, peripheric, supported by a raised neck. Wall coarsely agglutinated.
Distribution. Species cosmopolitan known from the Campanian to the early Miocene (Kaminski and Gradstein, 2005).
Remarks. Only isolated chambers have been recovered.
Arthrodendron subnodosiformis (Grzybowski, 1898)
Figure 14.13
1898 Hyperammina subnodosiformis; Grzybowski, p. 274, pl. 10, figs. 5, 6.
1993 Aschemocella subnodosiformis (Grzybowski); Kaminski and Geroch, p. 248, pl. 1, figs. 10a-13.
2006 Aschemocella subnodosiformis (Grzybowski); Kaminski, Silye, and Kender, p. 384.
2008 Arthrodendron subnodosiformis (Grzybowski); Kaminski, Uchman, Neagu, and Cetean, p. 108.
Material. Eight specimens from four samples.
Description. Test free, large, uniseriate, flattened, consisting of single elongated, piriform, and compressed chambers increasing rapidly in size. Wall thin and finely agglutinated.
Distribution. Grzybowski (1898) described A. subnodosiformis from the Paleogene of the Polish Carpathians; Kaminski et al. (2006) signaled it from the Miocene of Greenland Sea.
Remarks. The chambers are usually isolated and fragmentated.
Genus KALAMOPSIS de Folin, 1883
Kalamopsis grzybowskii (Dylążanka, 1923)
Figure 14.15-19
1923 Hyperammina grzybowskii; Dylążanka, p. 65.
1966 Kalamopsis grzybowskii (Dylążanka); Geroch, p. 438, fig. 6.
1987 Silicotuba grzybowskii (Dylążanka); Loeblich and Tappan, p. 26, pl. 16, figs. 10-13.
2002 Kalamopsis grzybowskii (Dylążanka); Holbourn and Henderson, p. 11, figs. 2, 3.
Material. 47 specimens from 12 samples.
Description. Test free, bottle-like, elongated with constrictions, straight or curved, often tubular with longitudinal groove. Aperture terminal at the end of the open part of the chamber. Wall finely agglutinated, smooth surface.
Distribution. Flysh-type cosmopolitan taxon known from the lower Jurassic (?Oxfordian); Kuhnt et al. (2002) signaled K. grzybowskii from the Oligocene of the Southern China Sea.
Family REOPHACIDAE Cushman, 1927
Genus REOPHAX de Montfort, 1808
Reophax duplex Grzybowski, 1896
Figure 16.1
1896 Reophax duplex; Grzybowski, p. 276, pl. 8, figs. 23-25.
2002 Reophax duplex Grzybowski; Kuhnt, Holbourn, and Zhao, p. 132, pl. 3, figs. 6, 7.
Material. 20 specimens from 11 samples.
Description. Test uniseriate composed of two subspheric chambers, sometimes more or less compressed. The first chamber is smaller and more globular than the second one. Aperture is a single opening on a raised neck. Wall thick and coarsely agglutinated.
Reophax pilulifer Brady, 1884
Figure 16.2
1884 Reophax pilulifera; Brady, p. 292, pl. 30, figs. 18-20.
1992 Reophax pilulifer Brady; Morlotti and Kuhnt, p. 223, pl. 2, figs. 10, 12.
1994 Hormosina pilulifer Brady; Schröder-Adams and McNeil, p. 37, pl. 4, fig. 3.
non 1995 Reophax pilulifer Brady; Bubík, p. 86, pl. 9, fig. 7.
2005 Reophax pilulifer Brady; Kender, Kaminski and Cieszkowski, p. 262, fig. 11F, G.
2009 Reophax pilulifer (Brady 1884); Kender, Kaminski, and Jones, p. 498, pl. 3, fig. 9.
Material. 14 specimens from 10 samples.
Description. Test free, uniseriate, straight, 2-4 circular and compressed chambers increasing in size gradually. Wall thick, coarsely agglutinated.
Distribution. Cosmopolitan taxon originally described from the Recent.
Reophax sp. 1
Figure 16.3
Material. One single specimen from the sample PC060615.
Description. Test free, uniseriate, straight axis, six chambers increasing gradually in size. Wall coarsely agglutinated. Aperture circular at the end of the last chamber.
Family HORMOSINIDAE Haeckel, 1894
Subfamily HORMOSININAE Haeckel, 1894
Genus HORMOSINA Brady, 1879
Hormosina trinitatensis Cushman and Renz, 1946
Figure 16.6
1946 Hormosina globulifera Brady var. trinitatensis; Cushman and Renz, p. 14, pl. 1, figs. 15-19.
1995 Hormosina trinitatensis Cushman and Renz; Bubík, p. 82, 83, pl. 9, fig. 3 (cum syn.).
2005 Hormosina trinitatensis Cushman and Renz; Kaminski and Gradstein, p. 241, pl. 43, figs. 1-11.
Material. Nine specimens from five samples.
Description. Test free, seriate, composed of 2-6 weakly embracing globular chambers increasing slowly in size. Wall medium to finely agglutinated.
Distribution. Late Campanian-Eocene (Kaminski and Gradstein, 2005).
Hormosina velascoensis (Cushman, 1926)
Figure 16.4-5
1926 Nodosinella velascoensis; Cushman, p. 583, pl. 20, fig. 9.
1981 Nodellum velascoense (Cushman); Morgiel and Olszewska, p. 7, pl. 1, fig. 1.
2005 Hormosina velascoensis (Cushman); Kaminski and Gradstein, p. 243, pl. 44, figs. 1-8.
Material. 15 specimens from nine samples.
Description. Test free, robust, uniseriate; five or more subcylindric or fuse-like chambers sideways compressed. Wall medium to finely agglutinated, aperture is a single circular opening at the apex of the last chamber.
Distribution. Campanian-Oligocene (Kaminski and Gradstein, 2005).
Hormosina sp. 1
Figure 16.7
Material. Two specimens from the sample PC060624.
Description. Test free, seriate, composed of three flattened weakly embracing chambers increasing rapidly in size. Wall thick, coarsely agglutinated; aperture is a circular opening in the peripherical zone of the last chamber.
Hormosina sp. 2
Figure 16.8
Material. One specimen from the sample PC060615.
Description. Test free, uniseriate; composed of three flattened weakly embracing chambers increasing gradually in size. Wall thick, coarsely agglutinated; aperture is a circular opening in the peripherical zone of the last chamber.
Hormosina spp.
Material. Eight specimens from seven samples.
Description. In this group are included all the incomplete or bad preserved specimens, with uniseriate and straight test with partially embracing chambers, not referred to the species above mentioned.
Genus PSEUDONODOSINELLA Saidova, 1970
Pseudonodosinella elongata Grzybowski, 1898
Figure 16.9-11
1898 Reophax elongatus; Grzybowski, p. 279, pl. 10, figs. 19, 20.
1960 Reophax elongata Grzybowski; Geroch, p. 123, pl. 3, figs. 8, 9.
2005 Pseudonodosinella elongata Grzybowski; Kaminski and Gradstein, p. 256, pl. 48, figs. 1-9.
Material. 153 specimens from 21 samples.
Description. Test free, uniseriate, composed of elongated piriform chambers never found connected in this work. Wall thick, coarsely agglutinated.
Distribution. Geroch and Nowak (1984) restricted the range of P. elongata to the middle-late Eocene of Polish; Kaminski et al. (2006) signaled P. elongata from the Miocene of Greenland Sea.
Pseudonodosinella nodulosa Brady, 1879
Figure 16.12-13
1879 Reophax nodulosa; Brady, p. 52, pl. 4, figs. 7, 8.
1898 Reophax subnodulosa Grzybowski; p. 279, pl. 10, figs. 17, 18.
1987 Pseudonodosinella nodulosa Brady; Loeblich and Tappan, p. 61, pl. 46, figs. 5, 6.
2005 Pseudonodosinella nodulosa (Brady); Kaminski and Gradstein, p. 259, pl. 49, figs. 1-9.
2009 Pseudonodosinella nodulosa (Brady); Kender, Kaminski, and Jones, p. 498, pl. 3, fig. 12.
Material. 23 specimens from 12 samples.
Description. Test free, elongated, uniseriate, straight or slightly curved; ovoid or piriform embracing chambers. Wall finely agglutinated with medium sized quartz grains. Sutures moderately incise, aperture terminal.
Distribution. Cosmopolitan taxon known from the Eocene to Recent (Kaminski and Gradstein, 2005).
Suborder LITUOLINA Lankester, 1885
Superfamily LITUOLOIDEA de Blainville, 1827
Family HAPLOPHRAGMOIDIDAE Maync, 1952
Genus HAPLOPHRAGMOIDES Cushman, 1910
Haplophragmoides carinatus Cushman and Renz, 1941
Figure 18.11-13
1941 Haplophragmoides carinatum; Cushman and Renz, p. 2, pl. 1, fig. 1.
2004 Haplophragmoides carinatus (Cushman and Renz); Green, Kaminski, and Sikora, p. 124, pl. 1, fig. 8.
Material. 21 specimens from seven samples.
Description. Test free, planispirally involute coiled, depressed in the umbelical zone, periphery lobate and carinate. Chambers triangular in shape increasing gradually in size, sutures incise and slightly curved. Wall finely agglutinated.
Distribution. At Portella Colla occurs only in the upper Rupelian.
Remarks. Haplophragmoides carinatus differs from H. walteri in having a tranversal section more lenticular, and the last chamber elongated and strictly triangular.
Haplophragmoides eggeri Cushman, 1926
Figure 18.17
1926 Haplophragmoides eggeri; Cushman, p. 583, pl. 15, fig. 1a, b.
2005 Haplophragmoides eggeri Cushman; Kaminski and Gradstein, p. 342, pl. 75, figs. 1-6.
Material. Five specimens from four samples.
Description. Test free, oval in outline, planispirally coiled with depressed umbelicus. The chambers (6-7 in the last whorl) increase slowly in size. Sutures usually indistinct. Wall medium to coarsely agglutinated.
Distribution. Late Cretaceous-Eocene (Kaminski and Gradstein, 2005).
Haplophragmoides excavatus Cushman and Waters, 1927
Figure 18.18
1927 Haplophragmoides excavatus; Cushman and Waters, p. 82, pl. 10, fig. 3a-b.
2005 Haplophragmoides excavatus Cushman and Waters; Kaminski and Gradstein, p. 360, pl. 82, figs. 1-7.
Material. Four specimens from three samples.
Description. Test free, subcircular in outline, planispirally involute coiled with depressed umbilicus, lobate and carinate periphery. Chambers (12-13 in the last whorl) increase very slowly in size. Sutures few incise. Wall finely agglutinated, surface smooth.
Distribution. Late Cretaceous-Oligocene (Kaminski and Gradstein, 2005).
Remarks. Haplophragmoides excavatus differs from H. carinatus and H. walteri in having a more circular periphery, and a greater number of chambers in the last whorl.
Haplophragmoides cf. kirki Wickenden, 1932
Figure 18.19
cf. 1932 Haplophragmoides kirki; Wickenden, p. 85, pl. 1, fig. 1a-c.
Material. Three specimens from three samples.
Description. Test free, medium sized, planispirally coiled, rounded periphery, chambers weakly lobate. 3.5-4 chambers in the last whorl increasing slowly in size. Sutures distinct and depressed.
Remarks. Haplophragmoides kirki is smaller, and has 4-4.5 chambers in the last whorl.
Haplophragmoides horridus (Grzybowski, 1901)
Figure 18.22
1901 Haplophragmium horridum; Grzybowski, p. 270, pl. 7, fig. 12.
2005 Haplophragmoides horridus (Grzybowski); Kaminski and Gradstein, p. 347, pl. 77, figs. 1-6.
2009 Haplophragmoides horridus (Grzybowski); Kender, Kaminski, and Jones, p. 500, pl. 5, fig. 8.
Material. Eight specimens from seven samples.
Description. Test free, planispirally involute coiled, laterally compressed with 4½ triangular chambers in the last whorl. The last chamber is much larger than the previous ones. Sutures incise, rounded periphery. Wall coarsely agglutinated. Aperture interiomarginal.
Distribution. Originally described from the Paleogene of the Polish Carpathians, and ascribed to Maastrichtian-Eocene by Kaminski and Gradstein (2005), H. horridus has been recently reported from the Miocene of Congo Fan (Kender et al., 2009).
Haplophragmoides cf. horridus (Grzybowski, 1901)
Figure 18.23
cf. 1901 Haplophragmiums horridum; Grzybowski, p. 270, pl. 7, fig. 12.
Material. One single specimen from the sample PC060604.
Description. Similar to H. horridus, but very larger and with only 4 chambers in the last whorl.
Haplophragmoides cf. latissimisuturalis Smith, 1971
Figure 19.1-2
cf. 1901 Haplophragmoides latissimisuturalis; Smith, p. 25, pl. 1, fig. 6a-c, pl. 2, fig. 3a-b.
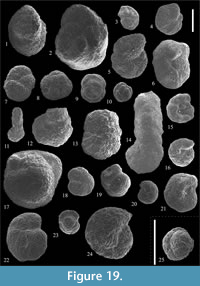 cf. 2004 Haplophragmoides latissimisuturalis Smith; Green, Kaminski, and Sikora, p. 124, pl. 2, fig. 1.
cf. 2004 Haplophragmoides latissimisuturalis Smith; Green, Kaminski, and Sikora, p. 124, pl. 2, fig. 1.
Material. 11 specimens from six samples.
Description. Test free, large, planispirally involute coiled, often compressed, subcircular and rounded periphery. Chambers large not well distinct, increasing gradually in size. Sutures limbate in the latest chambers. Wall medium agglutinated. Aperture interiomarginal.
Distribution. Haplophragmoides latissimisuturalis was described from the Oligocene of California (Smith, 1971), and from the Miocene of the Gulf of Mexico (Green et al ., 2004).
Haplophragmoides porrectus Maslakova, 1955
Figure 18.20
1955 Haplophragmoides porrectus; Maslakova, p. 47, pl. 3, figs. 5, 6.
2005 Haplophragmoides porrectus Maslakova; Kaminski and Gradstein, p. 353, pl. 354, figs. 1-6.
Material. Four specimens from three samples.
Description. Test free, rounded, planispirally involute coiled, composed of two whorls. The last one consists of 5-6 triangular chambers. Sutures depressed. Wall finely agglutinated.
Distribution. Paleocene-Eocene (Kaminski and Gradstein, 2005).
Haplophragmoides cf. porrectus Maslakova, 1955
Figure 18.24
cf. 1955 Haplophragmoides porrectus; Maslakova, p. 47, pl. 3, figs. 5, 6.
Material. One single specimen from the sample PC060615.
Description. Test free, small, compressed. Five chambers in the last whorl, sutures incise.
Haplophragmoides walteri (Grzybowski, 1898)
Figure 18.14-16
1898 Trochammina walteri; Grzybowski, pl. 11, fig. 31.
1960 Haplophragmoides walteri (Grzybowski); Geroch, p. 49, pl. 5, fig. 5.
2005 Haplophragmoides walteri (Grzybowski); Kender, Kaminski, and Cieszkowski, p. 266, fig. 13A.
2009 Haplophragmoides walteri (Grzybowski); Kender, Kaminski, and Jones, p. 500, pl. 5, fig. 9.
Material. 36 specimens from 16 samples.
Description. Test free, subcircular in outline, planispirally involute coiled, sutures incise. Chambers subtriangular, compressed near the periphery. Wall thin, finely agglutinated, surface smooth.
Distribution. Cosmopolitan species recorded from the Late Cretaceous to middle Miocene (Kender et al., 2009).
Remarks. In this taxon we include typical H. walteri morphotype, and the H. walteri - H. carinatus transitional forms common at the base of the Oligocene. At Portella Colla H. walteri is totally substituted by H. carinatus in the late Rupelian.
Haplophragmoides cf. walteri (Grzybowski, 1898)
Figure 18.21
1988 Haplophragmoides sp . cf. walteri (Grzybowski); Moullade, Kuhnt, and Thuro, p. 364, pl. 8, fig. 7.
1990 Haplophragmoides cf. walteri (Grzybowski); Kuhnt, p. 314, pl. 4, figs. 10-12.
Material. Four specimens from three samples.
Description. A small flattened Haplophragmoides with 4-5 chambers in the last whorl.
Remarks. Differs from H. walteri in having a smaller size, and a lower number of chambers in the last whorl.
Haplophragmoides sp. 1
Figure 18.25-26
Material. Six specimens from two samples.
Description. Test free, small, planispirally involute coiled with depressed umbilicus. Periphhery circular and acute. 10-11 not well distinct chambers in the last whorl. Wall finely agglutinated.
Remarks. Differs from H. excavatus in having a smaller size, sutures less evident, and lower number of chambers.
Haplophragmoides sp. 2
Figure 18.27
Material. One specimen from the sample PC060603.
Description. Test free, small, planispirally involute coiled, with 6 globose chambers in the last whorl. Sutures incise, wall thick, coarsely agglutinated.
Haplophragmoides sp. 3
Figure 19.3
Material. One specimen from the sample PC060615.
Description. Test free, subcircular in outline, planispiral involute coil with 7 chambers in the ultimate whorl. Sutures few incise, and acute periphery. In umbilical position, one chamber of the penultimate whorl is visible.
Remarks. Differs from H. walteri by the smaller size, sutures not sigmoidal, and in having less chambers.
Haplophragmoides sp. 4
Figure 19.4
Material. One specimen from the sample PC060601.
Description. Planispiral involute coil with 4.5 triangular chambers increasing gradually in size in the last whorl. Sutures depressed.
Haplophragmoides sp. 5
Figure 19.5
Material. Two specimens from the sample PC060615.
Description. Planispiral involute coil, with 5-6 globose chambers increasing very rapidly in size in the last whorl. Sutures straight not incise.
Haplophragmoides sp. 6
Figure 19.6
Material. Two specimens from the sample PC060621.
Description. Planispiral involute coil, with five triangular chambers in the last whorl. Sutures few depressed, rounded periphery.
Haplophragmoides spp.
Material. Seven specimens from six samples.
Description. In this group are included all the compressed and incomplete specimens not determined at a specific rank.
Family SPHAERAMMINIDAE Cushman, 1933
Subfamily PRAESPHAERAMMININAE Kaminski and Filipescu, 2000
Genus PRAESPHAERAMMINA Kaminski and Filipescu, 2000
Praesphaerammina subgaleata (Vašiček, 1947)
Figure 19.7-8
1947 Cystammina subgaleata; Vašiček, p. 247, pl. 1, fig. 15, text fig. 3.
1981 Sphaerammina subgaleata Vašiček; Morgiel and Olszewska, p. 12, pl. 9, fig. 2.
2004 Praesphaerammina subgaleata (Vašiček); Green, Kaminski, and Sikora, p. 125, pl. 3, fig. 1.
2005 Praesphaerammina subgaleata (Vašiček); Kaminski and Gradstein, p. 369, pl. 85, figs. 1-6.
Material. Nine specimens from eight samples.
Description. Test free, flattened and subelliptic, the last chamber covers over 60% the previous chambers.
Distribution. Middle Eocene-late Miocene (Kaminski and Gradstein, 2005).
Family LITUOLIDAE de Blainville, 1827
Subfamily AMMOMARGINULINAE Podobina, 1978
Genus AMMOBACULITES Cushman, 1910
Ammobaculites agglutinans (d’Orbigny, 1846)
Figure 19.11
1846 Spirolina agglutinans; d’Orbigny, p. 137, pl. 7, figs. 10-12.
2005 Ammobaculites agglutinans (d’Orbigny); Kaminski and Gradstein, p. 324, pl. 70, figs. 1-8.
Material. Two specimens from two samples.
Description. Test free, robust, initially planispiral involute coil, becoming uniseriate in the last chambers. The coiled portion consists of 4-5 chambers in the last whorl, separated by straight and depressed sutures. The uniseriate stage is rectilinear, composed of low, circular in section, chambers separated by indistinct sutures. Aperture terminal at the end of the ultimate chamber. Wall medium to coarse agglutinated.
Distribution. Late Cretaceous-Recent (Kaminski and Gradstein, 2005).
Ammobaculites sp. 1
Figure 19.12
Material. One incomplete specimen from the sample PC060607.
Description. Seven chambers visible in the last whorl of the planispiral coiled stage, separated by indistinct sutures. Umbilical region strongly depressed. The unique complete chamber of the uniseriate stage is very short and larger compared to previous chambers. Wall thick, coarsely agglutinated.
Remarks. Differs from A. agglutinans (d’Orbigny, 1846) in having more chambers in the planispiral portion increasing faster in size.
Ammobaculites sp. 2
Figure 19.14
Material. One specimen from the sample PC060625.
Description. Test free, flattened, with tapered periphery. Five chambers, triangular in section, in the planispiral involute portion, followed by 5 chambers uniserially arranged with acute periphery. Sutures indistinct, wall finely agglutinated with sparse quartz grains.
Ammobaculites sp. 3
Figure 19.13
Material. One specimen from the sample PC15.
Description. Test free, large, initially planispirally involute coiled with 4 chambers divided by depressed sutures in the last whorl. The unique preserved chamber of the uniseriate stage is very large, and has a rectangular section. Wall thick, coarsely agglutinated.
Remarks. Differs from A. agglutinans (d’Orbigny, 1846) by the larger size, and by the shape of the ultimate preserved chamber. Ammobaculites sp. 1 has more chambers and sutures less depressed.
Superfamily RECURVOIDOIDEA Alekseychik-Mitskevich, 1973
Family AMMOSPHAEROIDINIDAE Cushman, 1927
Subfamily AMMOSPHAEROIDININAE Cushman, 1927
Genus AMMOSPHAEROIDINA Cushman, 1910
Ammosphaeroidina pseudopauciloculata (Mjatliuk, 1966)
Figure 19.9-10
1896 Trochammina pauciloculata Brady; Grzybowski, p. 23, pl. 8, figs. 51, 52.
1960 Cystamminapauciloculata (Brady); Geroch, p. 66, pl. 6, fig. 8.
1966 Cystamminella pseudopauciloculata Mjatliuk; p. 264, pl. 1, figs. 5-7, pl. 2, fig. 6, pl. 3, fig. 3.
1990 Ammosphaeroidina pseudopauciloculata (Mjatliuk); Kuhnt, p. 311, pl. 5, fig. 1.
2004 Ammosphaeroidina pseudopauciloculata (Mjatliuk): Green, Kaminski, and Sikora, p. 125, pl. 5, fig. 3.
Material. 25 specimens from 10 samples.
Description. Test free, compressed, trochospirally coiled, trilobate in outline, elliptic and elongated in axial view or quadrilobate in ventral view, subelliptic in lateral view; 3-4 chambers in the last whorl increasing regularly in size covering the previous whorls. Chambers circular in outline, lenticular or subspheric with rounded periphery; sutures distinct, depressed. Aperture not distinguishable; wall smooth, finely agglutinated.
Distribution. Cosmopolitan species common from the Cretaceous to the Eocene (Kaminski and Gradstein, 2005), less frequent in the Oligocene, and signaled in the Miocene (Green et al., 2004; Kender et al., 2009).
Subfamily RECURVOIDINAE Alekseychik-Mitskevich, 1973
Genus BUDASHEVAELLA Loeblich and Tappan, 1964
Budashevaella multicamerata (Voloshinova and Budasheva, 1961)
Figure 19.15-16
1961 Circus multicameratus; Voloshinova and Budasheva, p. 201, pl. 7, fig. 6a-c, pl. 8, figs. 1a-c, 6a-c.
2005 Budashevaella multicamerata (Voloshinova); Kaminski and Gradstein, p. 386, pl. 90, figs. 1-6.
2006 Budashevaella multicamerata Voloshinova and Budasheva; Kaminski, Silye, and Kender, p. 384, pl. 4, fig. 6a-b.
2009 Budashevaella multicamerata Voloshinova and Budasheva; Kender, Kaminski, and Jones, p. 502, pl. 6, fig. 8.
Material. Two specimens from two samples.
Description. Test initially streptospirally coiled, later planispirally coiled, with 2 or 2½ whorls exteriorly visible. Chambers inflated, sutures depressed in the planispiral portion. The streptospiral coiled portion is observable in the umbilical region. Wall thick with sparse agglutination.
Distribution. Middle Eocene-Miocene (Kaminski and Gradstein, 2005).
Genus CRIBROSTOMOIDES Cushman, 1910
Cribrostomoides subglobosus (Cushman, 1910)
Figure 19.17
1910 Haplophragmoides subglobosum; Cushman, p. 105, figs. 162-164.
2005 Cribrostomoides subglobosus (Cushman); Kaminski and Gradstein, p. 391, pl. 92, figs. 1-3.
2009 Cribrostomoides subglobosus (Cushman); Kender, Kaminski and Jones, p. 502, pl. 6, fig. 9.
Material. Six specimens from four samples.
Description. Test free, globose. Initially planispirally coiled, later planispiral involute, with 5-6 chambers in the last whorl increasing rapidly in size. Rounded periphery. Sutures depressed excepted for the last 2 chambers. Wall coarsely agglutinated.
Distribution. Late Cretaceous-Recent (Kaminski and Gradstein, 2005).
Cribrostomoides spp.
Material. 29 specimens from 14 samples.
Description. Broken and incomplete specimens with wall coarsely agglutinated, and initial streptospiral coil.
Genus RECURVOIDELLA Uchio, 1960
Recurvoidella lamella (Grzybowski, 1898)
Figure 19.18
1898 Trochammina lamella; Grzybowski, p. 290, pl. 11, fig. 25.
2005 Recurvoidella lamella Grzybowski; Kaminski and Gradstein, p. 399, pl. 94, figs. 1-3.
Material. Five specimens from five samples.
Description. Test free, initial streptospiral coil, later planispiral involute with 4-5 subglobose chambers in the last whorl, increasing rapidly in size. Periphery lobate, sutures depressed.
Distribution. Paleocene-Eocene (Kaminski and Gradstein, 2005).
Genus RECURVOIDES Earland, 1934
Recurvoides anormis Mjatliuk, 1970
Figure 19.19
1970 Recurvoides anormis; Mjatliuk, p. 84, pl. 18, fig. 4, pl. 19, figs. 1-4.
2005 Recurvoides anormis Mjatliuk; Kaminski and Gradstein, p. 402, pl. 95, figs. 1-7.
Material. One specimen from the sample PC5.
Description. Test globose with large and rounded periphery. Involute streptospiral coil, the ultimate five chambers are planispirally enrolled. 6-7 chambers in the last whorl. Aperture at the base of the last chamber; wall thick with sparse agglutination.
Distribution. Campanian-Eocene (Kaminski and Gradstein, 2005).
Recurvoides nucleolus (Grzybowski, 1898)
Figure 19.22
1898 Trochammina nucleolus; Grzybowski, p. 291, pl. 11, figs. 28, 29.
1993 Recurvoides nucleolus (Grzybowski) emend Samuel, 1977; Kaminski and Geroch, p. 265, pl. 11, fig. 4a-d.
2005 Recurvoides nucleolus (Grzybowski) emend Samuel, 1977; Kaminski and Gradstein, p. 408, figs. 97-1, 97-2.
Material. Three specimens from three samples.
Description. Test free, rounded, and planoconvex. Initial streptospiral coil without changes in direction, followed by a short plano- or slowly trochospiral coil.
Distribution. Maastrichtian-Eocene (Kaminski and Gradstein, 2005).
Recurvoides walteri (Grzybowski, 1898)
Figure 19.23
1898 Haplophragmium walteri; Grzybowski, p. 280, pl. 10, fig. 24.
2005 Recurvoides walteri Grzybowski; Kaminski and Gradstein, p. 415, pl. 100, figs. 1-3.
Material. One specimen from the sample PC5.
Description. Test free, rounded, involute streptosporal coil with direction of coil changing during the ontogeny. Chambers increase slowly in size. Aperture at the base of the ultimate chamber. Test thick with sparse agglutination.
Distribution. Paleocene-Eocene (Kaminski and Gradstein, 2005).
Recurvoides sp. 1
Figure 19.20
Material. One specimen from the sample PC060601.
Description. Test subglobose, initial streptospirally coiled, followed by 3-4 chambers arranged planispirally. Chambers inflated increasing gradually in size, sutures depressed. Wall finely to medium agglutinated.
Recurvoides sp. 2
Figure 19.21
Material. One specimen from the sample PC060623.
Description. Test free, initial streptospiral coil, the ultimate whorl is planispirally involute coiled. The last chambers increase abruptly in size. Sutures are depressed and evident in the umbilical region.
Recurvoides spp.
Material. 29 specimens from 14 samples.
Description. Broken or incomplete specimens not recognizable at specific rank.
Genus THALMANNAMMINA Pokorny, 1951
Thalmannammina subturbinata (Grzybowski, 1898)
Figure 19.25
1898 Haplophragmium subturbinatum; Grzybowski, p. 280, pl. 10, fig. 23.
1993 Thalmannammina subturbinata (Grzybowski) emend. Pokorny, 1951; Kaminki and Geroch, p. 252, pl. 4, fig. 5a-d.
2005 Thalmannammina subturbinata (Grzybowski) emend. Pokorny, 1951; Kaminki and Gradstein, p. 419, pl. 101a-b.
Material. One specimen from the sample PC060604.
Description. Test globular with 10-12 chambers visible in the outer whorl. Streptospiral thalmannina-type coil with axis of coil changing abruptly of 90°, followed by a second opposite refolding. Wall finely agglutinated, a little aperture at the base of the last chamber.
Distribution. Latest Cretaceous-Eocene (Kaminski and Gradstein, 2005).
Suborder TROCHAMMININA Saidova, 1981
Superfamily TROCHAMMINOIDEA Schwager, 1877
Family TROCHAMMINIDAE Schwager, 1877
Subfamily TROCHAMMININAE Schwager, 1877
Genus TROCHAMMINA Parker and Jones, 1859
Trochamminabifaciata Friedberg, 1901
Figure 19.24
1901 Trochammina bifaciata; Friedberg, p. 460, pl. 2, fig. 1a-b.
Material. One specimen from the sample PC060604.
Description. Test free, compressed, discoidal. Chambers not distinct on the dorsal side, whereas in the ventral side a well developed spire occur. Aperture interiomarginal, wall finely agglutinated with rough surface.
Trochammina sp. 1
Figure 20.1
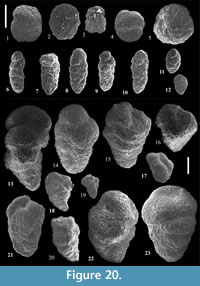 Material. Five specimens from three samples.
Material. Five specimens from three samples.
Description. Test free, low-trochospiral coil with 4 chambers in the dorsal side and 3 in the ventral one. Chambers subcircular and flattened; sutures depressed. Aperture interiomarginal, wall coarsely agglutinated.
Trochammina sp. 2
Figure 20.2
Material. Four specimens from three samples.
Description. Test free, with 4-5 chambers in the dorsal side, and 3-4 in the ventral one increasing rapidly in size. Wall thick with very coarse agglutination obliterating the sutures.
Trochammina sp. 3
Figure 20.3
Material. One specimen from the sample PC060601.
Description. Test free, small, trochospiral globigerina-type coil. A spire visible on the dorsal side, three globose chambers visible in the ventral side; sutures depressed. Aperture interiomarginal, wall thick coarsely agglutinated.
Trochammina sp. 4
Figure 20.4
Material. Three specimens from the samples PC060604 and PC060605.
Description. Test free, low trochospiral coil; three subcircular and flattened chambers in the ventral side separated by sutures depressed. Periphery lobate.
Trochammina sp. 5
Figure 20.5
Material. One specimen from sample PC060605.
Description. Test free, globorotalia-type coil. Chambers flattened and discoidal, periphery lobate. Aperture interiomarginal, wall coarsely agglutinated.
Trochammina spp.
Material. 22 specimens from five samples.
Description. Broken specimens referred to the genus Trochammina.
Suborder VERNEUILININA Mikhalevich and Kaminski, 2004 in Mikhalevich, 2004
Superfamily VERNEULINOIDEA Cushman, 1911
Family PROLIXOPLECTIDAE Loeblich and Tappan, 1985
Genus KARRERULINA Finlay, 1940
Karrerulina conversa (Grzybowski, 1901)
Figure 20.6-10
1901 Gaudryna conversa; Grzybowski, p. 285, pl. 7, figs. 15, 16.
1977 Plectina conversa (Grzybowski); Krasheninnikov and Pflaumann, p. 569, pl. 3, fig. 4a-b.
1992 Karrerulina conversa (Grzybowski); Morlotti and Kuhnt, p. 222, pl. 4, fig. 15.
2005 Karrerulina conversa (Grzybowski); Kaminski and Gradstein, p. 469, pl. 116, figs.1-11.
Material. 26 specimens from 10 samples.
Description. Test free, elongated, initial trochospiral coil, followed by a triseriate stage, and finally a biseriate adult stage. Aperture terminal and circular.
Distribution. Paleocene-Oligocene (Kaminski and Gradstein, 2005).
Karrerulina horrida (Mjatliuk, 1970)
Figure 20.11
1970 Karreriella horrida; Mjatliuk, p. 114-115, pl. 5, fig. 9, pl. 33, figs. 15-16c.
1996 Karrerulina horrida (Mjatliuk); Kaminski, Kuhnt, and Radley, p. 12, pl. 2, fig. 16.
2005 Karrerulina horrida (Mjatliuk); Kaminski and Gradstein, p. 473, pl. 117, figs. 1-11.
Material. 32 specimens from 15 samples.
Description. Test free elongated, rounded in trasversal section, composed of an initial trochospiral coil with 4-6 chambers per whorl, followed by a large-sized triseriate stage, and a final biseriate stage consisting of 1-2 whorls. Wall medium to finely agglutinated.
Distribution. Maastrichtian-Miocene (Kaminski and Gradstein, 2005).
Family REOPHACELLIDAE Mikhalevich and Kaminski, 2004 in Mikhalevich, 2004
Subfamily VERNEUILININAE Cushman, 1911
Genus GAUDRYINA d'Orbigny, 1839
Gaudryina sp.
Figure 20.13
Material. One single specimen from the sample PC1.
Description. Test conical, triangular in section, initially triseriate, latest biseriate with rounded section. Aperture an arc in the inner margin of the ultimate chamber.
Order LOFTUSIIDA Kaminski and Mikhalevich, 2004 in Mikhalevich, 2004
Superfamily Loftusiacea Brady, 1884
Suborder LOFTUSIINA Kaminski and Mikhalevich, 2004 in Mikhalevich, 2004
Superfamily HAPLOPHRAGMIOIDEA Eimer and Fickert, 1899
Family CYCLAMMINIDAE Marie, 1941
Subfamily ALVEOLOPHRAGMIINAE Saidova, 1981
Genus RETICULOPHRAGMIUM Maync, 1955
Reticulophragmium acutidorsatum (Hantken, 1868)
Figure 21.1-3
1868 Haplophragmium acutidorsatum; Hantken, p. 82, pl.1, fig. 1.
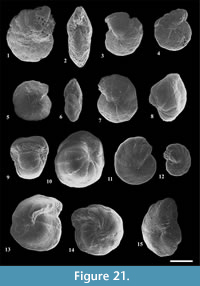 2004 Cyclammina acutidorsata (von Hantken); Green, Kaminski and Sikora, p. 127, pl. 4, fig. 1.
2004 Cyclammina acutidorsata (von Hantken); Green, Kaminski and Sikora, p. 127, pl. 4, fig. 1.
2006 Reticulophragmium acutidorsatum (Hantken); Kaminski, Silye, and Kender, p. 390, pl. 6, fig. 3.
2009 Reticulophragmium acutidorsatum (Hantken); Kender, Kaminski, and Jones, p. 504, pl. 8, fig. 9.
Material. 101 specimens from 20 samples.
Description. Test free, biconvex with acute periphery; planispiral involute coil with 12-14 chambers in the last whorl increasing gradually in size. Sutures few incise, aperture an arc at the base of the ultimate chamber; wall alveolar and finely agglutinated.
Distribution. Late Eocene-Miocene.
Reticulophragmium amplectens (Grzybowski, 1898)
Figure 21.4-7
1898 Cyclammina amplectens; Grzybowski, p. 292, pl. 12, figs. 1-3.
1954 Cyclammina amplectens Grzybowski; Geroch and Gradzinski, p. 39-40, tab. IV, figs. 10-13, tab. V, fig. 10a-d.
1992 Reticulophragmium amplectens (Grzybowski); Morlotti and Kuhnt, p. 223, pl. 4, figs. 9-11.
2005 Reticulophragmium amplectens (Grzybowski); Kaminski and Gradstein, p. 491, pl. 123, figs. 1-6.
Material. 18 specimens from four samples.
Description. Test free, biconvex, planispiral involute coil, with acute periphery and depressed umbilicus. Initial chambers are triangular and without alveoles which appear in the adult stage, simple and not branched.
Distribution. Eocene-Oligocene of the Thethys (Kaminski and Gradstein, 2005); signaled from the Miocene of the Greenland Sea (Osterman and Spiegler, 1996).
Reticulophragmium rotundidorsatum (Hantken, 1875)
Figure 21.8-10
1875 Haplophragmium rotundidorsatum; Hantken, p. 12-13, pl. 1, fig. 2.
1994 Reticulophragmium rotundidorsatus (von Hantken); Schröder-Adams and McNeil, p. 41, pl. 8, figs. 5-7.
2005 Reticulophragmium rotundidorsatum (Hantken); Kaminski and Gradstein, p. 503, pl. 126, figs. 1-7.
2009 Reticulophragmium rotundidorsatum (Hantken); Kender, Kaminski, and Jones, p. 505, pl. 9, fig. 3.
Material. 139 specimens from eight samples.
Description. Test free, subspheric, small to medium size, planispiral involute coil with rounded and thick periphery. Chambers increasing rapidly in size in the ultimate whorl, the last chamber, very large in size, tends to obliterate the umbilical region. Sutures straight and depressed; aperture multiples at the base of the last chamber. Wall alveolar and finely agglutinated.
Distribution. Middle Eocene-late Miocene (Kaminski and Gradstein, 2005).
Reticulophragmium projectum Schröder-Adams and McNeil, 1994
Figure 21.13-14
1994 Reticulophragmium projectus; Schröder-Adams and McNeil, p. 41, pl. 8, figs. 1-4.
Material. 100 specimens from four samples.
Description. Test free, subglobose, planispiral involute coil, with rounded periphery. Sutures straight or weakly sigmoidal starting tangentially from the umbilicus in radial direction.
Distribution. Oligocene-Miocene of Beaufort-Mackenzie basin (Schröder-Adams and McNeil, 1994).
Remarks. Differs from R. rotundidorsatum by the form and direction of the sutures, and in having a less inflated chambers.
Subfamily CYCLAMMININAE Marie, 1941
Genus CYCLAMMINA Brady, 1879
Cyclammina cancellata Brady, 1879
Figure 21.11-12
1879 Cyclammina cancellata; Brady, p. 62, pl. 37, figs. 8-16.
2005 Cyclammina cancellata Brady; Kaminski and Gradstein, p. 476, pl. 118a-b.
Material.Six specimens from three samples.
Description. Test free, planispiral coil with rounded periphery, umbilicus depressed. Eleven chambers in the last whorl, sutures depressed. Wall thick and finely agglutinated with sparse quartz grains.
Distribution. Late Eocene to Recent (Kaminski and Gradstein, 2005).
Cyclammina placenta (Reuss, 1851)
Figure 21.15
1851 Nonionina placenta; Reuss, p. 72, pl. 5, fig. 33.
1981 Cyclammina placenta Reuss; Gradstein and Berggren, p. 254, pl. 7, figs. 4-8.
1989 Reticulophragmium placenta (Reuss); Kaminski, Gradstein, and Berggren, pl. 5, fig. 3a-b.
2005 Cyclammina placenta (Reuss); Kaminski and Gradstein, p. 480, pl. 119, figs. 1-6.
Material. Eight specimens from four samples.
Description. Test free, flattened and narrow, planispiral coil with acute periphery. The number of chambers in the last whorl is variable and dependent to the dimension of the test. Sutures sigmoidal.
Distribution. Eocene-Miocene (Kaminski and Gradstein, 2005).
Cyclammina spp.
Material. 18 specimens from eight samples.
Description. In this group are included all the post mortem compressed or broken specimens not recognizable at specific rank.
Suborder ATAXOPHRAGMIINA Fursenko, 1958
Superfamily ATAXOPHRAGMIOIDEA Schwager, 1877
Family ATAXOPHRAGMIIDAE Schwager, 1877
Subfamily ATAXOPHRAGMIINAE Schwager, 1877
Genus ARENOBULIMINA Cushman, 1927
Arenobulimina sp.
Figure 20.17
Material. Two specimens from the samples PC060602 and PC22.
Family GLOBOTEXTULARIIDAE Cushman, 1927
Subfamily GLOBOTEXTULARIINAE Cushman, 1927
Genus GRAVELLINA Brönnimann, 1953
Gravellina sp.
Figure 20.21
Material. One single specimen from the sample PC060618.
Genus TETRATAXIELLA Seiglie, 1964
Tetrataxiella subtilissima Cetean and Kaminski, 2011
Figure 20.20
2011 Tetraxiella subtilissima; Cetean and Kaminski, p. 258, pl. 1, figs 16, 17; pl. 2, figs 1-4, 21, 22.
Material. One specimen from the sample PCs0.
Description. Test elongated, conical, trochospiral. The chambers increase rapidly in size and tend to be subcircular and flattened. Aperture indistinct due to the collapsed wall.
Distribution. Originally described for the Chattian of Angola (Cetean and Kaminski, 2011).
Subfamily LIEBUSELLINAE Saidova, 1981
Genus REMESELLA Vasicek, 1947
Remesella varians (Glaessner, 1937)
Figure 20.12
1937 Textulariella? varians; Glaessner, p. 366, pl. 2, fig. 15.
Material. One specimen from the sample PC060601.
Remesella sp.
Material. One broken specimen from the sample PC060601.
Order TEXTULARIIDA Delage and Hérouard, 1896
Suborder TEXTULARIINA Delage and Hérouard, 1896
Superfamily EGGERELLOIDEA Cushman, 1937
Family EGGERELLIDAE Cushman, 1937
Subfamily EGGERELLINAE Cushman, 1937
Genus EGGERELLA Cushman, 1937
Eggerella compressa (Andreae, 1884)
Figure 20.14
1884 Verneulina compressa; Andreae, p. 107, pl. 8, figs. 2, 3.
1937 Eggerella compressa (Andreae); Cushman, p. 47, pl. 5, fig. 7, 8 (cum syn.).
Material. One specimen from the sample PC13.
Description. Test free, large, initially 4-5 chambers per whorl, whereas the adult stage becomes triseriate. Wall coarsely agglutinated.
Distribution. Oligocene (Andreae, 1884)
Remarks. The specimens figured by Andreae are smaller (at least 1.3 mm) in respect to the collected specimen measuring 2.5 mm.
Eggerella sp. 1
Figure 20.15
Material. One single specimen from the sample PC3.
Description. Test subconical, initially planispirally coiled, chambers decreasing in number with the ontogeny. The last chamber of the collected specimen is broken; wall coarsely agglutinated.
Eggerella sp. 2
Figure 20.16
Material. One single specimen from the sample PC3.
Description. Test subconical, trochospiral coil, 3 chambers in the last whorl. Chambers are inflated and increase rapidly in size. Aperture at the end of the last chamber hemmed by a lip.
Family VALVULINIDAE Berthelin, 1880
Subfamily VALVULININAE Berthelin, 1880
Genus VALVULINA d'Orbigny, 1826
Valvulina flexilis Cushman and Renz, 1941
Figure 20.22
1941 Valvulina flexilis; Cushman and Renz, p. 7, pl. 1, figs. 16, 17.
Material. One single specimen from the sample PC060604.
Description. Test free, triseriate, extending rapidly from a subacute apex. Periphery lobate, chambers rounded and inflated increasing rapidly in size; sutures incise. Aperture at the base of the last chamber; wall thick and coarsely agglutinated.
Distribution. Late Oligocene of the Agua Salada Formation (Cushman and Renz, 1941).
Valvulina sp.
Figure 20.23
Material. One single specimen from the sample PC060621.
Description. Triseriate coiled test not attribuited to any known species.
Superfamily TEXTULARIOIDEA Ehrenberg, 1838
Family TEXTULARIIDAE Ehrenberg, 1838
Subfamily SIPHOTEXTULARIINAE Loeblich and Tappan, 1985
Genus SIPHOTEXTULARIA Finlay, 1939
Siphotextularia sp. 1
Figure 20.18
Material. One specimen from the sample PC060621.
Description. Large and flattened test; the early stage is triseriate, and measures 1/3 of the total lenght, later the chambers are biseriate arranged with a quadrangular section.
Siphotextularia sp. 2
Figure 20.19
Material. One specimen from the sample PC060621.
Description. Small test with a short triseriate stage. The chambers increase rapidly in size.
ACKNOWLEDGMENTS
Thanks to P. Di Stefano, G. Zarcone and M.S. Cacciatore (University of Palermo) for their help during the field work. I’m indebted to E. Fornaciari (Padova University) and M.G. Rossi (ISPRA, Rome) for the nannofossils analysis. G. Gaglianone (La Sapienza University, Rome) kindly supported me in the preparation of the material. Thanks to the editors, D. Hembree and Y. Nakamura, and to an anonymous reviewer for their constructive comments. P. Young kindly revised the English.
REFERENCES
Abate, B., Renda, P., and Tramutoli, M. 1988. Note illustrative della carta geologica dei Monti di Termini Imerese e delle Madonie occidentali (Sicilia centro-settentrionale). Memorie della Societa Geologica Italiana, 41:475-505. (In Italian)
Accordi, B. 1958. Il flysch oligocenico-aquitaniano dei Monti Nebrodi (Sicilia nordorientale). Eclogae Geologicae Helvetiae, 51(3):827-833.
Agnini, C., Fornaciari, E., Raffi, I., Catanzariti, R., Pälike, H., Backman, J., and Rio, D. 2014. Biozonation and biochronology of Paleogene calcareous nannofossils from low and middle latitudes. Newsletters on Stratigraphy, 47:131-181.
Akimoto, K., Hattori, M., Uematsu, K., and Kato, C. 2001. The deepest living foraminifera, Challenger Deep, Mariana Trench. Marine Micropaleontology, 42:95-97.
Alegret, L.,Cruz, L.E., Fenero, R., Molina, E., Ortiz, S., and Thomas, E. 2008. Effects of the Oligocene climatic events on the foraminiferal record from Fuente Caldera section (Spain, western Tethys). Palaeogeography, Palaeoclimatology, Palaeoecology , 269:94-102.
Alekseychik-Mitskevich, L.S. 1973. Klassifikatsii foraminifer semeystva Haplophragmiidae (Towards the classification of the foraminiferal family Haplophragmiidae). Trudy vsesoyuznogo neftyanogo nauchnoissledovatel'skogo geologorazvedochnogo instituta (VNIGRI), 343:12-44. (In Russian)
Andreae, A. 1884. Beitrag zur Kenntniss des Elsässer Tertiars; Theil II- Die Oligocän-schichten. Abhaundlungen zur Geologischen SpezialKarte von Elsass-Lothringen, Strassuburg, 2 (3):1-239. (In German)
Arapova, N.D. and Suleymanov, I.S. 1966. O foraminiferakh iz Konyakskikh otlozhenii zapadnogo Uzbekistan i Kyzylkumov. Tashkentskii Gosudarstvennyy Universitet im. V.I. Lenina, 273:121-127. (In Russian)
Arreguín-Rodríguez, G.J., Alegret, L., Sepúlveda, J., Newman, S., and Summons, R.E. 2014. Enhanced terrestrial input supporting the Glomospira acme across the Paleocene-Eocene boundary in Southern Spain. Micropaleontology, 60(1): 43-51.
Avnimelech, M. 1952. Revision of the tubular Monothalamia. Contributions from the Cushman Foundation for Foraminiferal Research, 3:60-68.
Bąk, K., Bąk, M., Geroch, S., and Manecki, M. 1997. Stratigraphy and palaeoenvironmental analysis of the benthic foraminifera and radiolarians in Palaeogene Variegated Shales in the Skole Unit, Polish Flysch Carpathians. Annales Societatis Geologorum Poloniae, 67:135-154.
Basilone, L. 2012. Litostratigrafia della Sicilia. Arti Grafiche Palermitane s.r.l., Palermo. (In Italian)
Bellagamba, M. and Coccioni, R. 1990. Deep-water agglutinated foraminifera from the Massignano section (Ancona, Italy), a proposed stratotype for the Eocene-Oligocene boundary . NATO ASI Series. Series C: Mathematical and Physical Sciences, 327:883-921.
Benedetti, A. 2010. Biostratigraphic remarks on the Caltavuturo Formation (Eocene-Oligocene) cropping out at Portella Colla (Madonie Mts., Sicily). Revue de Paléobiologie, 29(1):197-216.
Benedetti, A. 2015. Twin embryos in the larger benthic foraminifer Nephrolepidina praemarginata. Journal of Mediterranean Earth Sciences, 7:9-17.
Benedetti, A. and D'Amico, C. 2012. Benthic foraminifers and gastropods from the Gratteri Formation cropping out near Isnello (Madonie Mts., Sicily). Italian Journal of Geosciences, 131(1):47-65.
Benedetti, A. and Pignatti, J. 2008. Deep-water agglutinated foraminifers (DWAF) assemblages from the Priabonian-Rupelian of the Madonie Mountains (Sicily). Atti del Museo Civico di Storia Naturale di Trieste, 53(suppl.):97-109.
Benedetti, A. and Pignatti, J. 2009. Caudammina gutta, a new species of Caudammina (Hormosinellidae, Foraminiferida) from the Rupelian of Sicily. Rivista Italiana di Paleontologia e Stratigrafia, 115(3):337-348.
Benedetti, A. and Pignatti, J. 2013. Conflicting evolutionary and biostratigraphical trends in Nephrolepidina praemarginata (Douvillé, 1908) (Foraminiferida). Historical Biology, 25(3):363-383.
Berggren, W.A. and Kaminski, M.A. 1990. Abyssal agglutinates: Back to basics, p. 53-75. In Hemleben, C., Kaminski, M.A., Kuhnt, W., and Scott, D.B. (eds.), Paleoecology, Biostratigraphy, Paleoceanography and Taxonomy of Agglutinated Foraminifera. NATO ASI Series C, Volume 327. Kluwer Academic Publisher, Dordrecht.
Bermúdez, P. and De Rivero, F.C. 1963. Estudio sistematico de los Foraminiferos Quitinosos, Mikrogranulares y Arenaceos. Universidad Central de Venezuela, Ediciones de la Biblioteca. (In Spanish)
Berry, E.W. 1928. The smaller foraminifera of the middle Lobitos shales of northwestern Peru. Eclogae Geologae Helvetiae, 21:390-405.
Berthelin, G. 1880. Memoire sur les foraminiferes fossiles de l'etage Albien de Montcley (Doubs). Memoires de la Societe Geologique de France, Series 3, 1:1-84. (In French)
Blainville, H.M. Ducrotay de, 1827. Manuel de malacologie et de conchyliologie (1825). Levrault. Paris. (In French)
Brady, H.B. 1878. On the reticularian and radiolarian rhizopoda (Foraminifera and Polycystina) of the North-Polar Expedition of 1875, 1876. Annals and Magazine of Natural History, series 5, 1:1-425.
Brady, H.B. 1879. Notes on some of the reticularian Rhizopoda of the Challenger Expedition. Quarterly Journal of the Microscopical Science, 19:20-63.
Brady, H.B. 1881. Notes on some of the reticularian Rhizopoda of the "Challenger" Expedition; Part III. Quarterly Journal of the Microscopical Science, 21:31-71.
Brady, H.B. 1884. Report on the foraminifera dredged by H.M.S. Challenger during the years 1873-1876. Zoology, 9:1-814.
Brönnimann, P. 1953. Arenaceous foraminifera from the Oligo-Miocene of Trinidad. Contributions from the Cushman Foundation for Foraminiferal Research, 4:87-100.
Brouwer, J. 1965. Agglutinated foraminifera faunas from some turbiditic sequences. Proceedings, Koninklijke Nederlandse Akademie van Wetenschappen. Series B, 68(5):309-334.
Bubík, M. 1995. Cretaceous to Paleogene agglutinated foraminifera of the Bile Karpaty unit (West Carpathians, Czech Republic), p. 71-116. In Kaminski, M.A., Geroch, S., and Gasiński, M.A. (eds.), Proceedings of the Fourth International Workshop on Agglutinated Foraminifera. Grzybowski Foundation Special Publication, 3. Grzybowski Foundation, Krakow.
Calderone, S., Dongarrà, G., Leone, M., and Longinelli, A. 1980. Significato delle "Clay-ironstones" nella successione imerese (Sicilia). Rendiconti della Societa Italiana di Mineralogia e Petrologia, 36(1):207-233. (In Italian)
Carpenter, W.B. 1869. On the rhizopodal fauna of the deep sea. Proceedings of the Royal Society of London, 18:59-62.
Catanzariti, R., Rio, D., and Martelli, L. 1997. Late Eocene to Oligocene calcareous nannofossil biostratigraphy in the northern Appennines: the Ranzano sandstone. Memorie di Scienze Geologiche, 49:207-253.
Cetean, C.G. and Kaminski, M.A. 2011. New deep-water agglutinated foraminifera from the Upper Oligocene of offshore Angola. Micropaleontology, 57:255-262.
Coccioni, R. 1989. Deep-water agglutinated foraminifera at the Eocene-Oligocene boundary in the Massignano Section, Ancona, Italy. Acta Naturalia de "l'Ateneo Parmense", 25 (1-4):71-111.
Coltro, R. 1963. Le facies di Polizzi dell’Eocene alloctono della Sicilia centro-settentrionale. Rivista Italiana.di Paleontologia e Stratigrafia, 69(2):167-233. (In Italian)
Corliss, B.H. 1985. Microhabitats of benthic foraminifera within deep-sea sediments. Nature, 314(4):435-438.
Cotton, L.J. and Pearson, P. 2011. Extinction of larger benthic foraminifera at the Eocene-Oligocene boundary. Palaeogeography, Palaeoclimatology, Palaeoecology, 311:281-296.
Coxall, H.K. and Pearson, P. 2007. The Eocene-Oligocene transition, p. 351-387. In Williams, M., Haywood, A.M., Gregory, F.J., and Schmidt, D.N. (eds.), Deep-time Perspectives on Climate Change: Marrying the Signal from Computer Models and Biological Proxies. Micropaleontological Society Special Publication 2. The Geological Society, London.
Coxall, H.K., Wilson, P.A., Palike, H., Lear, C.H., and Backman J. 2005. Rapid stepwise onset of Antarctic glaciation and deeper calcite compensation in the Pacific Ocean. Nature, 433:53-57.
Cushman, J.A. 1910. A monograph of the foraminifera of the North Pacific Ocean. Pt. I - Astrorhizidae and Lituolidae. U.S. National Museum Bulletin, 71(1):1-134.
Cushman, J.A. 1911. A monograph of the foraminifera of the North Pacific Ocean. Pt. II - Textulariidae. U.S. National Museum Bulletin, 71(2):1-108.
Cushman, J.A. 1926. The foraminifera of the Velasco shale of the Tampico embayment. Bulletin of the American Association of Petroleum Geologists, 10:581-612.
Cushman, J.A. 1927. An outline of a re-classification of the foraminifera. Contributions from the Cushman Laboratory for Foraminiferal Research, 2:94-95.
Cushman, J.A. 1928. Foraminifera, their classification and economic use. Cushman Foundation for Foraminiferal Research, Special Publication, 1:1-401.
Cushman, J.A. 1933. Some new foraminiferal genera. Contributions from the Cushman Laboratory for Foraminiferal Research, 9:32-38.
Cushman, J.A. 1937. A Monograph of the Foraminiferal Family Valvulinidae. Cushman Foundation for Foraminiferal Research, Special Publication, 8. Norwood Press, Sharon.
Cushman, J.A. and Hanna, M.A. 1927. Foraminifera from the Eocene near San Diego, California. Transactions of the San Diego Society of Natural History , 5(4):45-64.
Cushman, J.A. and Jarvis, P.W. 1928. Cretaceous foraminifera from Trinidad. Contributions from the Cushman Laboratory for Foraminiferal Research, 4:85-103.
Cushman, J.A. and Renz, H.H. 1941. New Oligocene-Miocene foraminifera from Venezuela. Contributions from the Cushman Laboratory for Foraminiferal Research, 17(1):1-27.
Cushman, J.A. and Renz, H.H. 1946. The foraminifeal fauna of the Lizard Springs Formation of Trinidad, British West Indies. Cushman Foundation for Foraminiferal Research, Special Publication 18:1-48.
Cushman, J.A. and Waters, J.A. 1927 Some arenaceous foraminifera from the Upper Cretaceous of Texas. Contributions from the Cushman Laboratory for Foraminiferal Research, 2:81-85.
Dallan, L. 1962. Contributo alla geologia dell’Appennino Tosco-Emiliano II.- Ricerche micropaleontologiche nei flysch dei dintorni di Pievelago (Appennino Modenese). Bollettino della Società Geologica Italiana, 81(3):77-127. (In Italian)
Dallan Nardi, L. 1968. I microforaminiferi del «Macigno» di Calafuria (Monti Livornesi). Bollettino della Società Geologica Italiana, 87(4):611-621. (In Italian)
de Folin, L. 1883. Recherches sur quelques foraminifères à l’effet d’obtenir des preuves à l’appui de la classification de certaines organisms vaseux. Congrès Scientifique de Dax, Sess. 1(1882):1-320. (In French)
Delage, Y. and Hérouard, E. 1896. Traité de Zoologia Concrète, 1: La cellule at le protozoaires. Schleicher Frères. Paris. (In French)
Dongarrà, G. and Ferla, P. 1982. Le argille di Portella Colla e del Flysch Numidico auct. (M. Madonie; Sicilia); Aspetti deposizionali e diagenetici. Rendiconti della Societa Italiana di Mineralogia e Petrologia, 38(3):1119-1133. (In Italian)
d'Orbigny, A. 1826. Tableau méthodique de la classe des Céphalopodes. Annales des Sciences Naturelle, (1), 7: 96-314. (In French)
d'Orbigny, A. 1839. Foraminiféres, in Ramon de la Sagra,Histoire physique, politique et naturelle de l'Île de Cuba. Arthus Bertrand, Paris. (In French)
d'Orbigny, A. 1846. Foraminiféres Fossiles du Bassin Tertiare de Vienne (Autriche). Gide et Compagnie, Paris. (In French)
Dylążanka, M. 1923. Warstwy inoceramowe z łómu w Szymbarku koło Gorlic. Rocznik Polskiego Towarzystwa Geologicznego, 1:36-80. (In Polish)
Earland, A. 1934. Foraminifera; Part III - the Falklands sector of the Antarctic (excluding South Georgia). Discovery Reports, 10:1-208.
Ehrenberg, G.C. 1838. Über dem blossen Auge unsichtbare Kalkthierchen und Kieselthierchen als Hauptbestandtheile der Kreidegebirge. Bericht uber die zur Bekanntmachung geeigneten Verhanlungen del Königlichen Preussischen Akademie der Wissenschaften zu Berlin, 1838:192-200. (In German)
Eimer, G.H.T. and Fickert, C. 1899. Die Artbildung und Verwandtschaft bei den Foraminiferen. Entwurf einer natuerlichen Eintheilung derselben. Zeitschrift fuer Wissenschaftliche Zoologie, 65:599-708. (In German)
Emiliani, C. 1954. The Oligocene microfaunas of the central part of the northern Apennines. Palaeontographia Italica, 48 [1952]:77-184.
Finlay, H.J. 1939. New Zealand Foraminifera: Key species in stratigraphy- No. 2. Transactions of the Royal Society of New Zealand, 69:89-128.
Finlay, H.J. 1940. New Zealand Foraminifera: Key species in stratigraphy- No. 4. Transactions of the Royal Society of New Zealand, 69:448-472.
Friedberg, W. 1901. The foraminifera from the Inoceramus beds near Rzeszów and Dębice. Rozprawy Akademii Umięjetnoœci w Krakowie, Wydział Matematyczno-Przyrodniczy, Kraków, ser. 2, 41:601-668. (In Polish with German summary)
Fursenko, A.V. 1958, Osnovnye etapy razvitiya faun foraminifer v geologicheskom proshlom [Fundamental state of development of foraminiferal faunas in the geological past]. Trudy lnstituta Geologicheskikh Nauk. Akademiia Nauk Belorusskoy SSR, 1:10-29. (In Russian)
Galeotti, S., Kaminski, M.A., Coccioni, R., and Speijer, P. 2004. High resolution deep-water agglutinated foraminiferal record across the Paleocene/Eocene transition in the Contessa Road Section (central Italy), p. 83-103. In Bubìk, M. and Kaminski, M.A. (eds.), Proceedings of the Sixth International Workshop on Agglutinated Foraminifera. Grzybowski Foundation Special Publication,8. Grzybowski Foundation, Krakow.
Geroch, S. 1960. Microfaunal assemblages from the Cretaceous and Paleogene Silesian Unit in the Beskid Śląski Mts. (Western Carpathians). Biuletyn Instytutu Geologicznego, 153:7-138.
Geroch, S. 1966. Lower Cretaceous small foraminifera of the Silesian series, Polish Carpathians. Rocznik Polskiego Towarzystwa Geologicznego, 36:413-480.
Geroch, S. and Gradzinski, R. 1954. Stratigrafia serii podslàskiej zywieckiego okna tektonicznego. Rocznik Polskiego Towarzystwa Geologicznego, 24:3-62. (In Polish)
Geroch, S. and Kaminski, M.A. 1993. The morphology and systematics of Nothia excelsa (Grzybowski), a deep-water agglutinated foraminifer. Rocznik Polskiego Towarzystwa Geologicznego, 62[1992]:255-265.
Geroch, S. and Nowak, W. 1984. Proposal of zonation for the Late Tithonian-Late Eocene, based upon Arenaceous Foraminifera from the Outher Carpathians, Poland, p. 225-239. In Oertli, H.J. (ed.), Benthos '83, 2nd International Symposium on Benthic Foraminifera, (Pau 1983). Elf Aquitaine, Esso REP, and Total CFP, Pau and Bordeaux.
Glaessner, M.F. 1937. Studien über Foraminiferen aus der Kreide und dem Tertiär des Kaukasus; 1. Die Foraminiferen der ältesten Tertiärschichten des Nordwest-Kaukasus. Problems of Paleontology, 2-3:349-410. (In German)
Gooday, A.J. 1996. Epifaunal and shallow infaunal foraminiferal communities at three abyssal NE Atlantic sites subject to differing phytodetritus regimes. Deep-Sea Research, 43:1395-1431.
Govindan, A. 2004. Miocene deep-water agglutinated foraminifera from the offshore Krishna-Godavari Basin, India. Micropaleontology, 50(3):213-252.
Gradstein, F.M. and Berggren, W.A. 1981. Flysch-type agglutinated foraminifera and the Maastrichtian to Paleogene history of the Labrador and North Seas. Marine Micropaleontology, 6:211-268.
Gradstein, F.M. and Kaminski, M.A. 1997. New species of Paleogene deep-water agglutinated foraminifera from the North Sea and Norwegian Sea. Journal of the Geological Society of Poland, 67:217-229.
Green, R.C., Kaminski, M.A., and Sikora, P.J. 2004. Miocene deep-water agglutinated foraminifera from Viosca Knoll, offshore Louisiana (Gulf of Mexico), p. 119-144. In Bubìk, M. and Kaminski, M.A. (eds.), Proceedings of the Sixth International Workshop on Agglutinated Foraminifera. Grzybowski Foundation Special Publication, 8. Grzybowski Foundation, Krakow.
Grzybowski, J. 1896. Otwornice czerwonych ilów z Wadowic. Rozprawy Wydzialu Matematyczno-Przyrodniczego, AkademiaUmiejetnosci w Krakowie, serya 2, 30:261-308. (In Polish)
Grzybowski, J. 1898. Otwornice pokladów naftonosnych okolicy Krosna. Rozprawy Wydzialu Matematyczno-Przyrodniczego, AkademiaUmiejetnosci w Krakowie, serya 2, 33:257-305. (In Polish)
Grzybowski, J. 1901. Otwornice warstw inoceramowych okolicy Gorlic. Rozprawy Wydzialu Matematyczno-Przyrodniczego, Akademia Umiejetnosci w Krakowie, serya 2, 41: 219-286. (In Polish)
Gümbel (1862). Die Streitberger Schwammlager und ihre Foraminiferen-Einschlüsse. Jahreshefte des Vereins für vaterländische Naturkunde in Württemberg, 18:192-238. (In German)
Haeckel, E. 1894. Systematische Phylogenie: Entwurf eines natürlichen Systems der organismen auf Grund ihrer Stammesgeschichte. 1Systematische Phylogenie der Protisten und Pflanzen: erster Theil des Entwurfs einer systematischen Stammesgeschichte. Reimer. Berlin. (In German)
Hammer, Ø. and Harper, D.A.T. 2006 . Paleontological Data Analysis. Blackwell Publishing, Oxford.
Hammer, Ø., Harper, D.A.T., and Ryan, P.D. 2001. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica 4.1.4A: 1-9 palaeo-electronica.org/2001_1/past/issue1_01.htm
Hantken, M. 1868. A kis-czelli talyag foraminiferai. Magyar. Földt. Tars., Munk, Pest, Kot , 4:82. (In Hungarian)
Hantken, M. 1875. Die fauna der Clavulina Szaboi Schichten. Theil 1, Foraminiferen. Mittheilungen aus dem Jahrbuche der Koniglich-Ungarische Geologische Anstalt, Budapest, 4(1):1-93. (In German)
Hanzlíková, E. 1972. Carpathian Upper Cretaceous Foraminiferida of Moravia (Turonian-Maastrichtian). Rozpravy Ústředního Ústavu Geologického, 39:5-160.
Haq, B.U. 1981. Paleogene paleoceanography: Early Cenozoic oceans revisited. Oceanologixa Acta, special volume:71-82.
Haynes, J. and Nwabufo-Ene, K. 1998. Foraminifera from the Paleocene phosphate beds, Sokoto, Nigeria. Revista Espanola de Micropaleontologia, 30:51-76.
Holbourn, A.E. and Henderson, A.S. 2002. Re-illustration and Revised Taxonomy for Selected Deep-sea Benthic Foraminifers. Palaeontologia Electronica, 4.2: 1-34 palaeo-electronica.org/paleo/2001_2/foram/issue2_01.htm
Holbourn, A.E.L. and Kaminski, M.A. 1995. Lower Cretaceous benthic foraminifera from DSDP Site 263: micropaleontological constraints for the evolution of the Indian Ocean. Marine Micropaleontology, 26:425-460.
Jones, R.W. and Charnock, M.A. 1985. «Morphogroups» of agglutinating foraminifera, their life position and feeding habits and potential applicability in (paleo) ecological studies. Revue de Paléobiologie, 4(2):311-320.
Jones, T.R. and Parker, W.K. 1860. On the rhizopodal fauna of the Mediterranean, compared with that of the Italian and some older Tertiary deposits. Quarterly Journal of the Geological Society of London, 16:292-307.
Jurkiewicz, H. 1960. Otwornice z lupkow czarnorzeckich wschodniej czesci jednostki slaskiej. Annales de la Société géologique de Pologne, 30:333-343. (In Polish)
Kaminski, M.A. 2004. The Year 2000 classification of agglutinated foraminifera, p. 237-255. In Bubik, M. and Kaminski, M.A. (eds.), Proceedings of the Sixth International Workshop on Agglutinated Foraminifera. Grzybowski Foundation Special Publication, 8. Grzybowski Foundation, Krakow.
Kaminski, M.A. 2005. The utility of Deep-Water Agglutinated Foraminiferal acmes for correlating Eocene to Oligocene abyssal sediments in the North Atlantic and Western Tethys. Studia Geologica Polonica, 124:325-339.
Kaminski, M.A. 2014. The year 2010 classification of the agglutinated foraminifera. Micropaleontology, 60:89-108.
Kaminski, M.A. and Austin, W.E.N. 1999. Oligocene Deep Water Agglutinated Foraminifers at Site 985, Norwegian Basin, southern Norwegian Sea. Proceedings of the Ocean Drilling Program, Scientific Results, 162:169-177.
Kaminski, M.A., Boersma, A., Tyszka, J., and Holbourn, A.E.L. 1995. Response of deep-water agglutinated foraminifera to dysoxic conditions in the California Borderland basins, p. 131-140. In Kaminski, M.A., Geroch, S., and Gasiński, M.A. (eds.), Proceedings of the Fourth International Workshop on Agglutinated Foraminifera, Grzybowski Foundation Special Publication, 3. Grzybowski Foundation, Krakow.
Kaminski, M.A. and Filipescu, S. 2000. Praesphaerammina, a new genus of Cenozoic deep-water agglutinated foraminifera from the Carpathian flysch deposits. Micropaleontology, 46(4):353-359.
Kaminski, M.A. and Geroch, S. 1992. Trochamminoides grzybowskii, nom. nov., a new name from Trochamminia elegans Gryzbowski, 1898; Foraminiferida. Journal of Micropalaeontology, 11:64.
Kaminski, M.A. and Geroch, S. 1993. A revision of foraminiferal species in the Grzybowski Collection, p. 239-323. In Kaminski, M.A., Geroch, S., and Kaminski, D. (eds.), The Origins of Applied Micropaleontology: The School of Jozef Grzybowski. Grzybowski Foundation Special Publication, 1. Alden Press, Oxford.
Kaminski, M.A. and Gradstein, F.M. 2005. Atlas of Paleogene Cosmopolitan Deep-water Agglutinated Foraminifera. Grzybowski Foundation Special Pubulication, 10. Grzybowski Foundation, Krakow.
Kaminski, M.A., Gradstein, F.M., Goll, R.M., and Grieg, D. 1990. Biostratigraphy and paleoecology of deep-water agglutinated foraminifera at ODP Ste 643, Norwegian-Greenland Sea, p. 345-386. In Hemleben, C., Kaminski, M.A., Kuhnt, W., and Scott, D.B. (eds.), Paleoecology, Biostratigraphy, Paleoceanography and Taxonomy of Agglutinated Foraminifera, NATO ASI Series. Kluwer Academic Publisher, Dordrecht.
Kaminski, M.A. and Huang, Z. 1991. Biostratigraphy of Eocene to Oligocene deep-water agglutinated foraminifers in the red clays from Site 767, Celebes Sea . Proceedings of the Ocean Drilling Program, Scientific Results , 124:171-180.
Kaminski, M.A. and Kuhnt, W. 2004. What, if anything, is a Paratrochamminoides ? A key to the morphology of the Cretaceous to Cenozoic species of Conglophragmium and Paratrochamminoides (Foraminifera), p. 273-285. In Bubík, M. and Kaminski, M.A. (eds.), Proceedings of the Sixth International Workshop on Agglutinated Foraminifera. Grzybowski Foundation Special Publication, 8. Grzybowski Foundation, Krakow.
Kaminski, M.A. and Schröder, C.J. 1987. Environmental analysis of deep-sea agglutinated foraminifera: Can we distunguish tranquil from disturbed environments?, p. 90-93. In Gulf Coast Section SEPM Foundation Eighth Annual Research Conference, Selected Papers and Illustrated Abstracts . Editor unknown, Houston.
Kaminski, M.A., Gradstein, F.M., and Berggren, W.A. 1989. Paleogene benthic foraminifer biostratigraphy and paleoecology at site 647, southern Labrador Sea. Proceedings of the Ocean Drilling Program, Scientific Results, 105:705-730.
Kaminski, M.A., Kuhnt, W., and Radley, J.D. 1996. Paleocene-Eocene deep water agglutinated foraminifera from the Numidian Flysch (Rif, Northern Morocco); their significance for the palaeoceanography of the Gibraltar gateway. Journal of Micropaleontology, 15:1-19.
Kaminski, M.A. and Ortiz, S. 2014. The Eocene-Oligocene turnover of deep-water agglutinated foraminifera at odpsite 647, southern labrador sea (North Atlantic). Micropaleontology, 60:53-66.
Kaminski, M.A., Silye, L., and Kender, S. 2006. Miocene deep-water agglutinated foraminifera from ODP Hole 909c: Implications for the paleoceanography of the Fram Strait Area, Greenland Sea. Micropaleontology, 51[2005]:373-403.
Kaminski, M.A., Silye, L., and Kender, S. 2009. Miocene deep-water agglutinated foraminifera from the Lomonosov Ridge and the opening of the Fram Strait. Micropaleontology , 55:117-135.
Kaminski, M.A., Uchman, A., Neagu, T., and Cetean, C.G. 2008. A larger agglutinated foraminifer originally described as a marine plant: The case of Arthrodendron Ulrich, 1904 (Foraminifera), its synonyms and homonyms. Journal of Micropalaeontology, 27:103-110.
Karrer, F. 1866. Über das Auftreten von Foraminiferen in der älteren Schichten des Wiener Sandsteins. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften Wein, Mathematisch-Naturwissenschaft-liche Klasse, 52:121-193. (In German)
Katz, M.E., Miller, K.G., Wright, J.D., Wade, B.S., Browning, J.V., Cramer, B.S., and Rosenthal, Y. 2008. Stepwise transition from the Eocene greenhouse to the Oligocene icehouse. Nature Geoscience, 1:329-334.
Kender, S., Kaminski, M.A., and Cieszkowski, M. 2005. Foraminifera from the Eocene variegated shales near Barwinek (Magura unit, outer Carpathians), the type locality of Noth (1912) revisited. Annales Societatis Geologorum Poloniae, 75:249-271.
Kender, S., Kaminski, M.A., and Jones, R.W. 2008. Oligocene Deep-Water agglutinated foraminifera from the Congo Fan, offshore Angola: paleoenvironments and assemblages distributions, p. 107-156. In Kaminski, M.A. and Coccioni, R. (eds.), Proceedings of the Seventh International Workshop on Agglutinated Foraminifera.Grzybowski Foundation Special Publication, 13. Grzybowski Foundation, Krakow.
Kender, S., Kaminski, M.A., and Jones, R.W. 2009. Early to middle Miocene foraminifera from the deep-sea Congo Fan, offshore Angola. Micropaleontology, 54[2008]:477-568.
Krasheninnikov, V.A. and Pflaumann, U. 1978. Cretaceous agglutinated foraminifera of the Atlantic Ocean off west Africa (Leg 41, Deep Sea Drilling Project), p. 565-580. In Lancelot, Y., Seibold, E., Gardner, J.V. (eds.), Initial Reports of the Deep Sea Drilling Project, 41. U.S. Government Printing Office, Washington.
Kuhnt, W. 1990. Agglutinated foramnifera of western Mediterranean Upper Cretaceous pelagic limestones (Umbrian Apennines, Italy, and Betic Cordillera, Southern Spain). Micropaleontology, 36(4):297-330.
Kuhnt, W. and Collins, E.S. 1996. Cretaceous to Paleogene benthic foraminifers from the Iberia Abyssal Plain. Proceedings of the Ocean Drilling Program, Scientific Results, 149:203-216.
Kuhnt, W. and Kaminski, M.A. 1989. Upper Cretaceous deep-water agglutinated foraminiferal assemblages from the western Mediterranean and adjacent areas, p. 91-120. In Wiedmann, J. (ed.) , Cretaceous of the Western Tethys. Proceedings of the 3rd International Cretaceous Symposium, Tübingen, 1987. Schweizerbart'sche Verlagsbuchahandlung.
Kuhnt, W. and Kaminski, M.A. 1993. Changes in the community structure of deep water agglutinated foraminifers across the K/T boundary in the Basque Basin (northern Spain). Revista Española de Micropaleontología, 25(1):57-91.
Kuhnt, W. and Urquhart, E. 2001. Tethyan flysch-type benthic foraminiferal assemblages in the North Atlantic: Cretaceous to Palaeogene deep water agglutinated foramnifers from the Iberia abyssal plain (ODP Leg 173). Revue de Micropaléontologie , 44(1):27-59.
Kuhnt, W., Holbourn, A., and Zhao, Q. 2002. The early history of the South China Sea; evolution of Oligocene-Miocene deep water environments. Revue de Micropaléontologie, 45(2):99-159.
Kuhnt, W., Kaminski, M.A., and Moullade, M. 1989. Late Cretaceous deep-water agglutinated foraminiferal assemblages from the North Atlantic and its marginal seas. International Journal of Earth Sciences : 78(3):1121-1140.
Kuhnt, W., Moullade, M., and Kaminski, M.A. 1996. Ecological structuring and evolution of deep sea agglutinated foraminifera - A review . Revue de Micropaléontologie , 39(4):271-281.
Lankester, E.R. 1885. Protozoa. T he Encyclopaedia Britannica(9 th ed.), 19;830-866.
Lipparini, T. 1951. Foraminiferi dell’Oligocene nel Flysch di Cortona (Arezzo). Bollettino del Servizio Geologico d’Italia, 71(11):3-8. (In Italian)
Loeblich, A.R. and Tappan, H. 1964. Part C. Protista 2. Chiefly "Thecamoebians" and Foraminiferida, p. 1-900. In Moore, R.C. (ed.), Treatise on Invertebrate Paleontology . The Geological Society of America and the University of Kansas, Lawrence, Kansas.
Loeblich, A.R. and Tappan, H. 1984. Some new proteinaceous and agglutinated genera of Foraminiferida. Journal of Paleontology, 58:1158-1163.
Loeblich, A.R. and Tappan, H 1985. Some new and redefined genera and families of agglutinated foraminifera 2. Journal of Foraminiferal Researches, 15:175-217.
Loeblich, A.R. and Tappan, H. 1987. Foraminiferal Genera and Their Classification. Van Nostrand Reinhold Co., New York.
Loeblich, A.R. and Tappan, H. 1992. Present status of foraminiferal classification, p. 93-102. In Takayanagi, Y. and Saito, T. (eds.), Studies in Benthic Foraminifera . Tokai University Press, Tokai.
Majzon, L. 1943. Adatok egyes Karpataljai flis- retegekhez tekintettel a Globotruncanakra. A magyar Királyi Földtani Intézet, Évkönnyve, 37:1-170. (In Hungarian)
Marie, P. 1941. Les foraminiferes de la Craie a Belemnitella mucronaia du Bassin de Paris. Memoires du Museum Nationale d'Histoire Naturelle, n. ser. 12( I):1-296. (In French)
Martini, E. 1971. Standard Tertiary and Quaternary calcareous nannoplankton zonation, p. 739-785. In Farinacci, A. (ed.), Proceedings 2nd International Conference Planktonic Microfossils Roma. Edizione Tecnoscienze, Rome.
Maslakova, N.I. 1955. Stratigrafiya i fauna melkikh foraminifer paleogenovykh otlozhenii Vostochnykh Karpat. Materialy po Biostratigrafi zapadnykh oblastii Ukrainskoi SSR:5-132. (In Russian)
Maync, W. 1952. Critical taxonomic study and nomenclatural revision of the Lituolidae based upon the prototype of the fam ily, Lituola nautiloidea Lamarck, 1804. Contributions from the Cushman Foundation for Foraminiferal Research, 3:35-56.
Maync, W. 1955. Reticulophragmium, n. gen., a new name for Alveolophragmium Stschedrina, 1936 (pars) . Journal of Paleontology, 29:553-557.
McNeil, D.H. 1996. Distribution of Cenozoic agglutinated benthic foraminifera in the Beaufort-MacKenzie Basin. In Dixon, J. (ed.), Geological Atlas of the Beaufort-MacKenzie Area. Geological Survey of Canada Miscellaneous Report, 59, fig. 70. Geological Survey of Canada, Ottawa.
Mikhalevich, V. I. 1980. Taxonomy and evolution of the Foraminifera in the light of the new data on their cytology and ultrastructure. Trudy Zoologicheskogo Instiuta, Akademiya Nauk SSSR, 94:42-61. (In Russian)
Mikhalevich, V.I. 2004. On the heterogeneity of the former Textulariina (Foraminifera), p. 317-349. In Bubik, M. and Kaminski, M.A. (eds.), Proceedings of the Sixth International Workshop on Agglutinated Foraminifera. Grzybowski Foundation Special Publication, 8. Grzybowski Foundation, Krakow.
Mikhalevich, V.I. 2013. New insight into the systematics and evolution of the foraminifera. Micropaleontology, 59:493-527.
Milam, R.W. and Anderson, J.B. 1981. Distribution and ecology of recent benthonic foraminifera of the Adelie-George V continental shelf and slope, Antarctica. Marine Micropaleontology, 6:297-325.
Miller, K.G., Gradstein, F.M., and Berggren, W.A. 1982. Late Cretaceous to Early Tertiary agglutinated benthic foraminifera in the Labrador Sea. Micropaleontology, 28(1):1-30.
Miller, K.G. and Katz, M.E. 1987. Oligocene to Miocene benthic foraminiferal and abyssal circulation changes in the North Atlantic. Micropaleontology, 33(2):97-149.
Molina, E., Gonzalvo, C., Ortiz, S., and Cruz, L.E. 2006. Foraminiferal turnover across the Eocene-Oligocene transition at Fuente Caldera, southern Spain: No cause-effect relationship between meteorite impacts and extinctions. Marine Micropaleontology , 58:270-286.
Montanaro Gallitelli, E. 1943. Per la geologia delle argille ofiolitifere appenniniche. Nota II - Foraminiferi dell’argilla scagliosa di Varana. Atti della Società Toscana di Scienze Naturali, Memorie, 52:37-148. (In Italian)
Montanaro Gallitelli, E. 1955. Foraminiferi cretacei delle marne a fucoidi di Serramazzoni (Appennino modenese). Accademia di Scienze Lettere ed Arti di Modena, (ser. 5), 13:3-32. (In Italian)
Montanaro Gallitelli, E. 1958. Specie nuove e note di foraminiferi del Cretaceo superiore di Serramazzoni (Modena). Accademia di Scienze Lettere ed Arti di Modena , (ser. 5), 16:127-150. (In Italian)
Montfort, P. Denys, de. 1808. Conchyliologie systématique, et classification méthodique des coquilles. vol. I. F. Schoell. Paris. (In French)
Morgiel, J. and Olszewska, B. 1981. Biostratigraphy of the Polish External Carpathians based on agglutinated foraminifera. Micropaleontology, 27(1):1-30.
Morlotti, E. 1998a. Alcune osservazioni paleoecologiche sul Flysch di Monte Sporno (Val Baganza, Appennino Parmense). Ateneo Parmense, Acta Naturalia, 34:31-36. (In Italian)
Morlotti, E. 1998b. I foraminiferi agglutinati di mare profondo come traccianti paleoecologici: alcuni problemi aperti. Ateneo Parmense, Acta Naturalia, 34:37-48. (In Italian)
Morlotti, E. and Kuhnt, W. 1992. Agglutinated deep-water foraminifera of the Eocene Monte Piano formation (Northern Apennines, Italy). Journal of Foraminiferal Research, 22:214-228.
Moullade, M., Kuhnt, W., and Thuro, J. 1988. Agglutinated benthic foraminifers from Upper Cretaceous variegated clays of the North Atlantic Ocean (DSDP Leg 93 and ODP Leg 103). Proceedings of the Ocean Drilling Program, Scientific Results, 103: 349-377.
Murray, J. W. and Alve, E. 2011. The distribution of agglutinated foraminifera in NW European seas: Baseline data for the interpretation of fossil assemblages. Palaeontologia Electronica 14.2.14: 1-41 palaeo-electronica.org/2011_2/248/index.html
Murray, J.W., Alve, E., and Cundy, A. 2003. The origin of modern agglutinated foraminiferal assemblages: evidence from a stratified fjord. Estuarine, Coastal and Shelf Science, 58:677-697.
Murray, J.W. and Pudsey, C. 2004. Living (stained) and dead foraminifera from the newly ice-free Larsen Ice Shelf, Weddell Sea, Antarctica: ecology and taphonomy. MarineMicropaleontology, 53:67-81.
Nagy, J., Gradstein, F.M., Kaminski, M.A., and Holbourn A.E.L. 1995. Foraminiferal morphogroups, paleoenvironments and new taxa from Jurassic and Cretaceous strata of Thakkhola, Nepal, p. 181-209. In Kaminski, M.A., Geroch, S., and Gasinski, M.A. (eds.), Proceedings of the Fourth International Workshop on Agglutinated Foraminifera, Grzybowski Foundation Special Publication, 3. Grzybowski Foundation, Krakow.
Nagy, J., Kaminski, M.A., Johnsen, K., and Mitlehner, A.G. 1997. Foraminiferal, palynomorph, and diatom biostratigraphy and paleoenvironments of the Torsk Formation: A reference section for the Paleocene-Eocene transition in the western Barents Sea, p. 15-38. In Hass, H.C. and Kaminski, M.A. (eds.), Contributions to the Micropaleontology and Paleoceanography of the Northern North Atlantic, Grzybowski Foundation Special Publication, 5. Grzybowski Foundation, Kiel.
Nagy, J., Kaminski, M.A., Kuhnt, W., and Bremer, M.A. 2000. Agglutinated foraminifera from neritic to bathyal facies in the Palaeogene of Spitsbergen and the Barents Sea, p. 333-361. In Hart, M.B., Kaminski, M.A., and Smart, C.W. (eds.), Proceedings of the Fifth International Workshop on Agglutinated Foraminifera. Grzybowski Foundation Special Publication, 7. Grzybowski Foundation, London.
Nicosia, M.L. 1952. Foraminiferi Oligocenici delle “Argille Rosse” di Catenanuova (F° 269 “Paternò”-Sicilia). Bollettino del Servizio Geologico d’Italia, 84:393-405. (In Italian)
Nigam, R., Mazumder, A., and Saraswat, R. 2004. Ammolagena clavata (Jones and Parker, 1860), an agglutinated benthic foraminiferal species-first report from the recent sediments, Arabian Sea, Indian Ocean region. Journal of Foraminiferal Research, 34:74-78.
Noth, R. 1912. Die Foraminiferenfauna der roten Tone von Barwinek und Karmarnók. Beiträge zur Paläontologie und Geologie Österreich-Ungarns und des Orients, 25:1-24. (In German)
Odin, G.S. and Matter, A. 1981. De glauconiarum origine. Sedimentology, 28:611-641.
Olszewska, B. 1997. Foraminiferal biostratigraphy of the Polish Outer Carpathians: a record of basin geohistory. Annales Societatis Geologorum Poloniae, 67:325-337.
Ortiz, S. and Kaminski, M.A. 2012. Record of deep-sea, benthic elongate-cylindrical foraminifera across the Eocene-Oligocene transition in the North Atlantic Ocean (ODP Hole 647A). Journal of ForaminiferalResearch, 42:345-368.
Osterman, L.E. and Spiegler, D. 1996. Agglutinated benthic foraminiferal biostratigraphy of Sites 909 and 913, northern North Atlantic. Proceedings of the Ocean Drilling Program, Scientific Results, 151:169-185.
Pälike, H., Norris, R.D., Herrie, J.O., Wilson, P.A., Coxall, H.K., Lear, C.H., Shackleton, N.J., Tripati, A.K., and Wade, B.S. 2006. The heartbeat of the Oligocene climate system. Science, 314:1894-1898.
Parisi, G. and Coccioni, R. 1988. Deep-water benthic foraminifera at the Eocene-Oligocene boundary in the Massignano section (Ancona, Italy), p. 97-109. In Premoli Silva, I., Coccioni, R., and Montanari, A. (eds.), The Eocene-Oligocene Boundary in the Marche-Umbria Basin (Italy). International Subcommission of Paleogene Stratigraphy, Special Publication II, 3. International Union of Geological Sciences, Ancona.
Parker, W.K. and Jones, T.R. 1859. On the nomenclature of the foraminifera. II. On the species enumerated by Walker and Montagu. A nnals and Magazine of Natural History , series 3, 4:333-351.
Pawlowski, J., Holzman, M., and Tyszka, J. 2013. New supraordinal classification of Foraminifera: molecules meet morphology. Marine Micropaleontology, 100:1-10.
Pescatore, T., Renda, P., and Tramutoli, M. 1987. Facies ed evoluzione sedimentaria del bacino Numidico nelle Madonie occidentali (Sicilia). Memorie della Societa Geologica Italiana, 38:297-316. (In Italian)
Petters, V. and Gandolfi, R. 1948. Contributo alla conoscenza dei foraminiferi oligocenici del versante nord dell’Appennino Settentrionale. Rivista Italiana di Paleontologia e Stratigrafia, 54(3):97-115. (In Italian)
Pflaumann, U. 1964. Geologisch-mikropalaontologische Untersuchungen in der Flysch-Oberkreide zwischen Wertach und Chiemsee in Bayern. Inaugural dissertation. Ludwig Maximilian Universität. München. (In German)
Podobina, V.M. 1978. Sistematika i filogeniya Gaplofragmiidey [Systematics and phylogeny of the Haplophragmiidae]. Tomsk Universitet, Tomsk. (In Russian)
Pokorny, V. 1951. Thalmannammina n.g. (Foraminifera) from the Carpathian flysch. Sbornik Ústredniho Ústavu Geologickeho, 18:469-480.
Preece, R.C., Kaminski, M.A., and Dignes, T.W. 1999. Miocene benthonic foraminiferal morphogroups in an oxygen minimum zone offshore Cabinda, p. 267-282. In Cameron, N.R., Bate, R.H., and Clure, V.S. (eds.), Oil and Gas Habitats of the South Atlantic.Geological Society Special Publication, 153 . The Geological Society, London.
Prothero, D.R., Ivany, L.C., and Nexbitt, A. 2003. From Greenhouse to Icehouse: The Marine Eocene-Oligocene Transition. Columbia University Press, New York.
Rauser-Chernousova, O.M. and Reytlinger, E.A. 1986, On the suprageneric systematics of the Order Hormosinida (Foraminifera). Paleontologicheskii Zhurnal, 4:15-20.
Rea, D.K. and Lyle, M.W. 2005. Paleogene calcite compensation depth in the eastern subtropical Pacific: answers and questions. Paleoceanography, 20:PA1012. doi:10.1029/2004PA001064
Reuss, A.E. 1845. Die Versteinerungen der böhmischen Kreideformation. E. Schweizerbart’sche Verlagsbuchhaundlung, Stuttgart.
Reuss, A.E. 1851. Uber die fossilen Foraminiferen und Entomostraceen der Septareinthone der Umgegend von Berlin. Zeitschrift der Deutschen Geologischen Gesellschaft, Berlin, 3:49-91.
Reuss, A.E. 1860. Die foraminiferen der westphälischen Kreideformation. Sitzungsberichte der K. Akademie der Wissenschaften in Wien, Mathematisch-Naturwissenschaftliche Klasse, 40:147-238.
Reuss, A.E. 1862. Entwurf einer systematischen Zusammenstellung der Foraminiferen. Sitzungsberichte der Kaiserliche Akademie der Wissenschaften in Wien, Mathematisch-Naturwissenschaftliche Klasse, 44:355-396.
Rögl, F. 1995. A late Cretaceous flysch-type agglutinated fauna from the Trochamminoides proteus type locality (Wien-Hütteldorf, Austria), p. 249-263. In Kaminski, M.A., Geroch, S., and Gasiński, M.A. (eds.), Proceedings of the Fourth International Workshop on Agglutinated Foraminifera, 1993. Grzybowski Foundation Special Publication, 3. Grzybowski Foundation, Krakow.
Rhumbler, L. 1895. Entwurf eines natürlichen Systems der Thalamophoren. Nachtrichten der K. Gesellschaft der Wissenschaften zu Göttingen, mathematisch-physikalische Klasse , 1:4-98. (In German)
Rzehak, A., 1885. Bemerkungen über ei nige Foraminiferen der Oligocän Formation. Verhandlungen des Naturforschenden Vereins in Brünn (1884), 23:123-129. (In German)
Saidova, H.M. 1961. Ekologia foramnifer i paleogeografia dalnevostočnych morej SSSR i severno-zapadnoj časti Tikhogo okeana. Inst. Okeanol. Akad. Nauk. S.S.S.R ., Moscow. (In Russian)
Saidova, H.M. 1970. Bentosnye Foraminifery rayona KuriloKamchatskogo zheloba (po materialam 39-go reysa e/s "Vityaz") [Benthic foraminifera in the Kurile-Kamchatka region based on the data of the 39th cruise of the R/V "Vityaz."] Trudy lnstituta Okeanologii, 86:134-161. (In Russian)
Saidova, H.M. 1975. Bentosnye Foraminifery Tikhogo Okeana [Benthonic foraminfera of the Pacific Ocean]. Institut Okeanologii P.P. Shirshova, 3. Akademiya Nauk SSSR, Moscow. (In Russian)
Saidova, H.M. 1981. O sovremennom sostoyanii sistemy nadvidovykh taksonov Kaynozoyskikh bentosnykh foraminifer [On an up-to-date system of supraspecific taxonomy of Cenozoic benthonic foraminifera]. Institut Okeanologii P. P. Shirshova, Akademiya Nauk SSSR, Moscow. (In Russian)
Samuel, O. 1977. Agglutinated foramnifers from Paleogene flysch formations in West Carpathians of Slovakia. Západné Karpaty, sér. paleontológia, 2-3:7-70.
Sars, G.O. 1872. Undersøgelser over Hardangerfjordens Fauna. Fordhandlinger i Videnskasselskabet i Kristiania, 1871:246-255.
Schmidt Di Friedberg, P., Barbieri, F., and Giannini, C. 1960. La geologia del gruppo montuoso delle Madonne (Sicilia centro-settentrionale). Bollettino del Servizio Geologico d’Italia, 91:73-140. (In Italian)
Schröder-Adams, C.J. and McNeil, D.H. 1994. Oligocene to Miocene agglutinated foraminifers in deltaic and deep-water facies of the Beaufort-Mackenzie Basin. Bulletin - Geological Survey of Canada, Report, 477:1-75.
Schubert, R.J. 1902. Neue und interessante Foraminiferen aus dem südtiroler Altteriär. Beiträge zur Paläontologie und Geologie Österreich-Ungarns und des Orients, 14:9-26. (In German)
Schulze, F.E. 1875. Rhizopodienstudien. Archiv für Mikroskop Anatomie, 11:583-596.
Schwager, C. 1877. Quadro del proposto sistema di classificazione dei foraminiferi con guscio. Bolletino del Regio Comitato Geologico d'Italia, 8:18-27. (In Italian)
Seiglie, G.A. 1964. Algunos foraminíferos arenáceos recien-tes de Venezuela. Boletín del Instituto Oceanográfico, Universidad de Oriente, 3:5-14. (In Spanish)
Smith, R.K. 1971. Foraminiferal studies in the Lower and Middle Tertiary of Soquel Creek, Santa Cruz County, California. University of California Publications in Geological Sciences, 91:1-111.
Soliman, H.A. 1972. New Upper Cretaceous foraminifera from Soviet Carpathian (USSR). Revue de Micropaléontologie, 15:35-44.
Stainforth, R.M. 1952. Ecology of arenaceous foraminifera . The Micropaleontologist, 6:1-42.
Suleymanov, I.S. 1963. Noviy rod i dva novykh vida iz semeystva Ammodiscidae [A new genus and two new species from the family Ammodiscidea]. Doklady Akademi Nauk UzSSR, 11:41-43. (In Russian)
Tendal, O.S. and Hessler, R.R. 1977. An introduction to the biology and systematics of Komokiacea (Textulariina, Foraminiferida). Galathea Report,14:165-194.
Thalmann, H.E. 1947. Index to new genera, species and varieties of foraminifera for the year 1945 with supplements for the period 1939-1944, and addenda (1942-1945). Journal of Paleontology, 21:355-395.
Uchio, T. 1960. Ecology of living benthonic foraminifera from the San Diego, California area. Cushman Foundation for Foraminiferal Research Special Publication, 5:1-72.
Ulrich, E.C. 1904. Fossils and age of the Yakutat Formation. Description of the collections made chiefly near Kadiak, Alaska, p. 125-146. In Emerson, B.K., Palache, C., Dall, W.H., Ulrich, E.O., and Knowlton, F.H. (eds.), Alaska, vol. 4, Geology and Paleontology. Doubleday, Page & Co, New York.
Van den Akker, T.J.H.A., Kaminski, M.A., Gradstein, F.M., and Wood, J. 2000. Campanian to Palaeocene biostratigraphy and palaeoenvironments in the Foula Sub-basin, west of the Shetland Islands, UK. Journal of Micropalaeontology, 19:23-43.
Van der Zwaan, G.J., Duijnstee, I.A.P., Dulk, M. den, Ernst, S.R., Jannink, N.T., and Kouwenhoven, T.J. 1999. Benthic foraminifers: proxies or problems? A review of paleoecological concepts. Earth Science Reviews, 46:213-236.
van Morkhoven, F.P.C.M., Berggren, W.A., and Edwards, A.S. 1986. Cenozoic cosmopolitan deep-water benthic foraminifera. Bulletin des Centres Recherches Exploration-Production Elf-Aquitaine, Memoir, 11:1-423.
Vašiček, M. 1947. Poznámky k microbiostratigrafii magurského flyše na Moravě. Věstník Státního Geologického Ústavu Československé Republiky, 22:235-256. (In Czech)
Voloshinova, N.A. and Budasheva, A.I. 1961. Lituolids and trochamminids from the Tertiary deposits of Sakhalin Island and the Kamchatka Peninsula. Trudy Vsesoyuznogo Nauchno-Issledovatel’skogo Geologorazvedochnogo Instituta VNIGRI, 170: 170-272. (In Russian)
Vyalov, O.S. 1966. Remarks on foraminifera with Siliceous test. Pnleontologicheskiy Sbornik, 3(2): 27-36.
Vyalov, O.S. 1968. Deyaki mirkuvannya pro klasitikatsiyu kremenistikh foraminifer [Certain considerations on classification of siliceous foraminiferans]. Dopovidi Akademit Nauk Vkrains'kot RSR. Ser. B. Geologiya, Geofizika, Khimiya ta Biologiya, 1:3-6. (In Russian)
Wade, B.S. and Pearson, P.N. 2008. Planktonic foraminiferal turnover, diversity fluctuations and geochemical signals across the Eocene/Oligocene boundary in Tanzania. Marine Micropaleontology, 68:244-255.
Waśkowska, A. 2014. Distribution of the agglutinated foraminifer Ammolagena clavata (Jones and Parker) in Western Tethyan Upper Cretaceous and Paleogene deep-water deposits (Outer Carpathians, Poland). Micropaleontology , 60:77-88.
Wezel, F.C. 1966. La sezione tipo del Flysh Numidico: Stratigrafia preliminare della parte sottostante il complesso Panormide (Membro di Portella Colla). Atti dell’Accademia Gioenia di Scienze Naturali, 18:71-92. (In Italian)
White, M.P. 1928. Some index foraminifera from the Tampico Embayment area of Mexico (Part 2.). Journal of Palaeontology, 2:280-317.
Wickenden, R.T.D. 1932. New species of foraminifera from the Upper Cretaceous of the prairie provinces. Transactions Royal Society Canada, 26:85-91.
Wiesner, H. 1931. Die Foraminiferen der Deutsche Südpolar Expedition 1901-1903. Deutsche Südpolar Expedition, vol 20, Zoologie, 12:53-165.
Zachos, J.C., Quinn, T.M., and Salamy, K.A. 1996. High-resolution (104 years) deep-sea foraminiferal stable isotope records of the Eocene-Oligocene climate transition. Paleoceanography, 11(3):251-266.
Zachos, J., Pagani, M., Sloan, L., Thomas, E., and Billups, K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to Present. Science, 292:686-693.

