Article Search
Volume 27.1
January–April 2024
Full table of contents
ISSN: 1094-8074, web version;
1935-3952, print version
Recent Research Articles
See all articles in 27.1 January-April 2024
See all articles in 26.3 September-December 2023
See all articles in 26.2 May-August 2023
See all articles in 26.1 January-April 2023
TABLE 1. Measurements of Fistulipora enodata Gorjunova, 1970. Abbreviations: N = number of measurements; X = mean; SD = standard deviation; CV = coefficient of variation; MIN = minimal value; MAX = maximal value.
TABLE 2. Measurements of Fistulipora guttata Trizna and Klautzan, 1961. Abbreviations as for Table 1.
TABLE 3. Measurements of Fistulipora sakagamii n. sp. Abbreviations as for Table 1.
TABLE 4. Measurements of Dybowskiella hupehensiformis n. sp. Abbreviations as for Table 1.
TABLE 5. Measurements of Fistuliramus xianzaensis Liu and Wang, 1987. Abbreviations as for Table 1.
TABLE 6. Measurements of Eridopora uncata Yang and Lu, 1983. Abbreviations as for Table 1.
TABLE 7. Measurements of Cyclotrypa alexanderi Sakagami, 1963. Abbreviations as for Table 1.
TABLE 8. Measurements of Hexagonella kobayashii Sakagami, 1968. Abbreviations as for Table 1.
TABLE 9. Measurements of Goniocladia aff. indica Waagen and Pichl, 1885. Abbreviations as for Table 1.
TABLE 10. Measurements of Liguloclema meridianus (Etheridge, 1926). Abbreviations as for Table 1.
TABLE 11. Measurements of Etherella tibetensis n. sp. Abbreviations as for Table 1.
TABLE 12. Measurements of Tabulipora xinjiangensis Yang and Lu, 1983. Abbreviations as for Table 1.
TABLE 13. Measurements of Dyscritella lii n. sp. Abbreviations as for Table 1.
TABLE 14. Measurements of Ulrichotrypa omanica Ernst et al., 2008. Abbreviations as for Table 1.
TABLE 15. Measurements of Neoeridotrypella astrica (Linskaya, 1951). Abbreviations as for Table 1.
TABLE 16. Measurements of Streblotrypa (Streblotrypa ) parviformis n. sp. Abbreviations as for Table 1.
TABLE 17. Measurements of Streblotrypa (Streblascopora ) delicatula Sakagami, 1961. Abbreviations as for Table 1.
TABLE 18. Measurements of Streblotrypa (Streblascopora ) marmionensis (Etheridge, 1926). Abbreviations as for Table 1.
TABLE 19. Measurements of Rhabdomeson bretnalli Crockford, 1957. Abbreviations as for Table 1.
TABLE 20. Measurements of Primorella rotunda Gorjunova, 1975. Abbreviations as for Table 1.
TABLE 21. Measurements of Timanotrypa australis n. sp. Abbreviations as for Table 1.
TABLE 22. Measurements of Spinofenestella sp. Abbreviations as for Table 1.
TABLE 23. Measurements of Spinofenestella subquadratopora (Schulga-Nesterenko, 1952). Abbreviations as for Table 1.
TABLE 24. Measurements of Polypora consanguinea Bassler, 1929. Abbreviations as for Table 1.
TABLE 25. Measurements of Polypora brouweri Bassler, 1929. Abbreviations as for Table 1.
TABLE 26. Measurements of Polypora aff. voluminosa Trizna and Klautzan, 1961. Abbreviations as for Table 1.
TABLE 27. Measurements of Mackinneyella obesa (Crockford, 1957). Abbreviations as for Table 1.
TABLE 28. Measurements of Protoretepora irregularis n. sp. Abbreviations as for Table 1.
TABLE 29. Measurements of Tibetiporella ornata n. gen. n. sp. Abbreviations as for Table 1.
TABLE 30. Matrix of occurrences of bryozoan species from the Zhongba Formation used for the numerical plots in the Figure 23 (1 - present, 0 - absent). Thailand and Western Australia share the hightest number of species with the Zhongba area (five and four species, respectively), whereas other regions share three or fewer species with the studied area.
TABLE 31. Distribution of bryozoan growth forms in the Zhongba Formation.
FIGURE 1. Position of the sampling area and tectonic setting of the Tibetan Plateau and Southern China (simplified after Dai et al., 2011). GCT: Great Counter thrust; ZGT: Zhongba-Gyangze thrust.
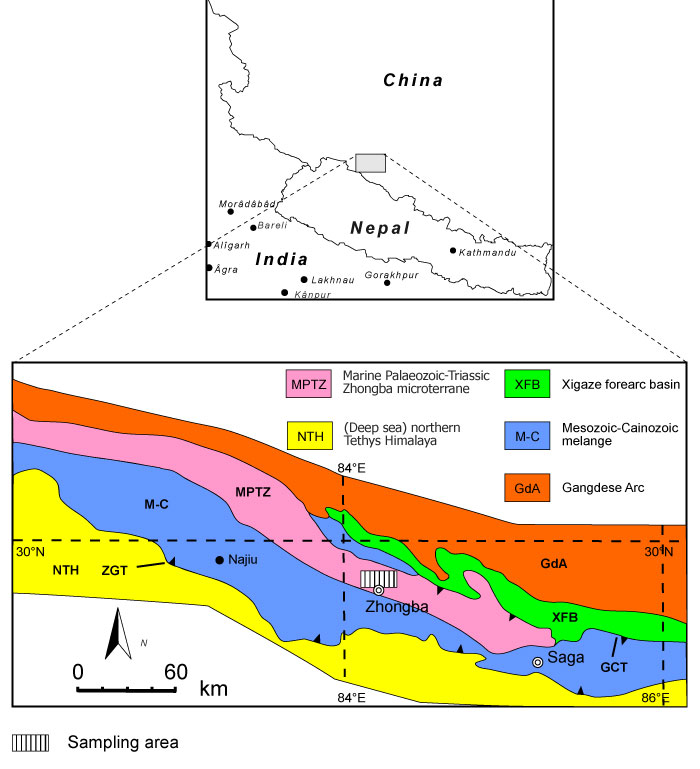
FIGURE 2. Lithological characteristics of the limestones from the Zhongba Formation. 1, limestone sample with abundant bryozoans (SMF 23.262); 2, rudstone containing crinoids, cystoporate (Fistulipora enodata Gorjunova, 1970) and fenestrate bryozoans (SMF 23.263); 3, bindstones with Fistulipora guttata Trizna and Klautzan, 1961 (SMF 23.264); and 4, grain- to packstones with bryozoan and crinoid fragments (SMF 23.265).
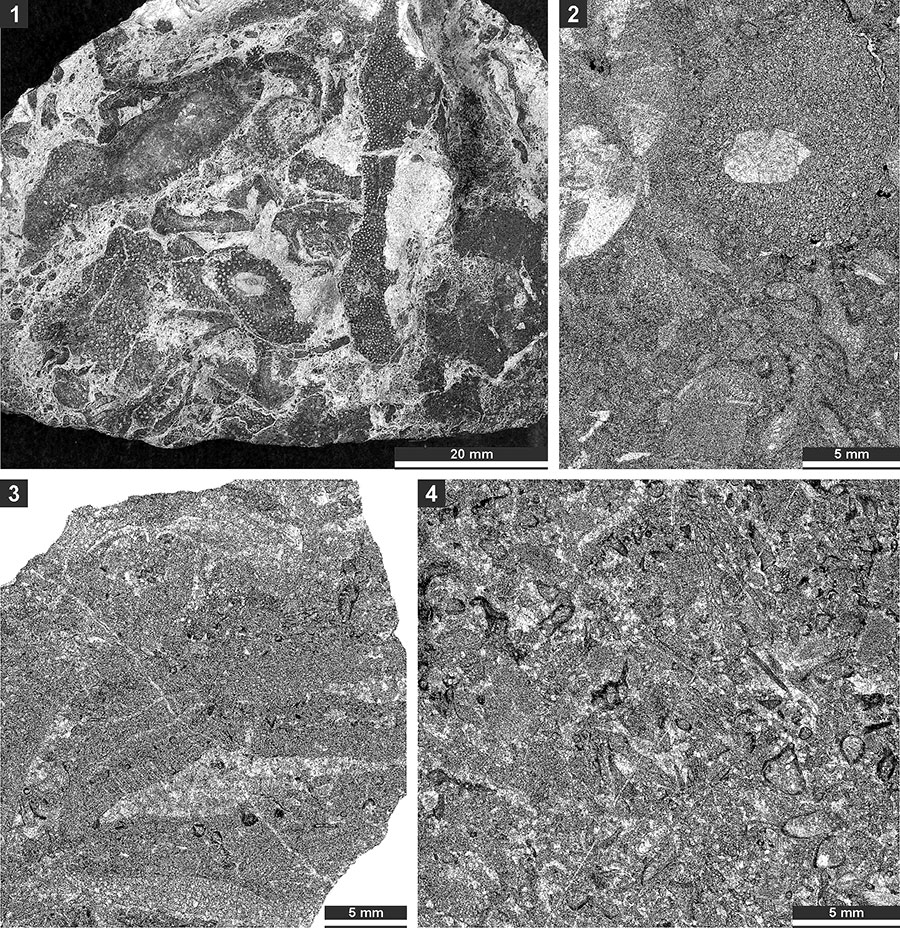
FIGURE 3. Thin section photographs of Fistulipora enodata Gorjunova, 1970, SMF 23.014 (1-4); Fistulipora guttata Trizna and Klautzan, 1961, SMF 23.025 (5-8); and Fistulipora sakagamii n. sp., holotype SMF 23.028 (9 and 10). 1 and 5, longitudinal section showing autozooecial chambers and vesicular skeleton; and 2-4, 6-8, 9, and 10, tangential sections showing autozooecial apertures and vesicles.
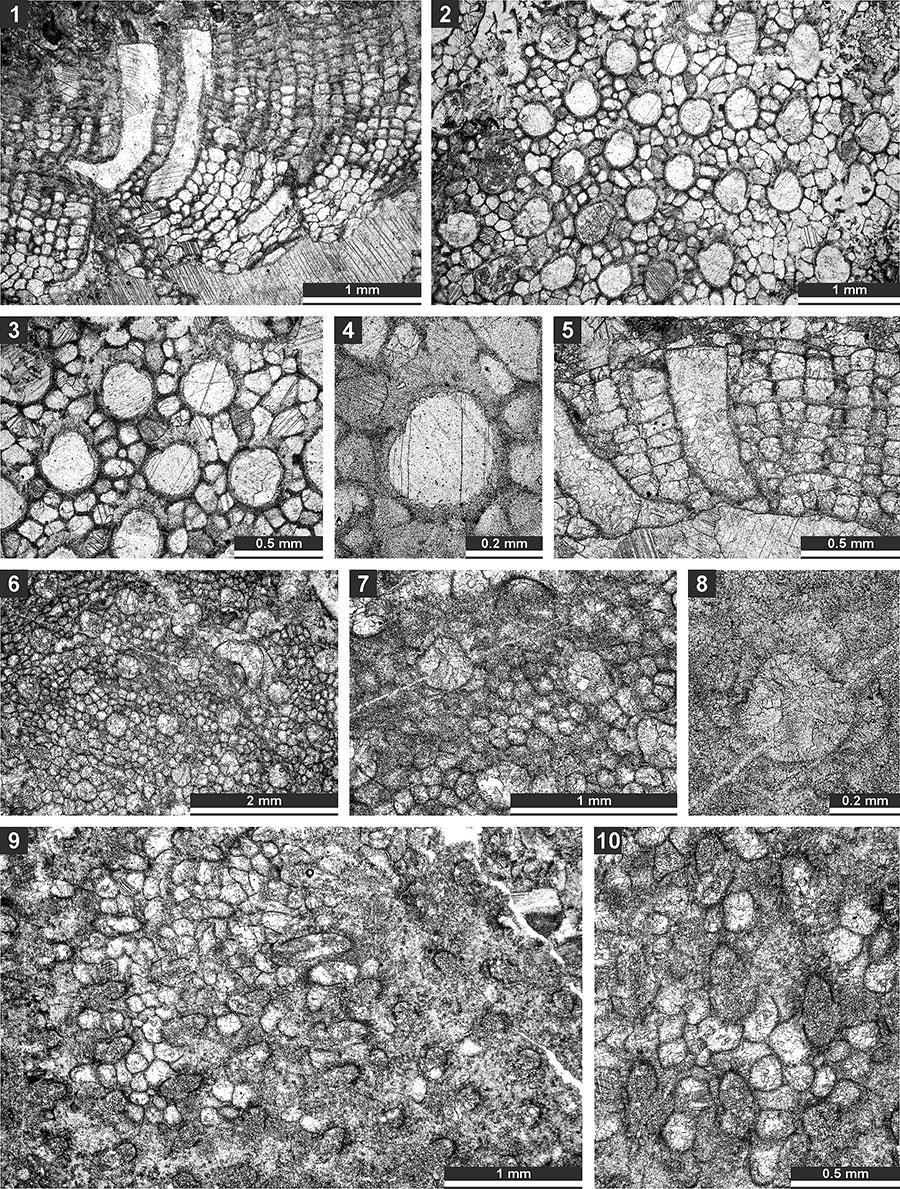
FIGURE 4. Thin section photographs of Fistulipora sakagamii n. sp., holotype SMF 23.028 (1 and 2); Dybowskiella hupehensiformis n. sp., holotype SMF 23.030 and paratype SMF 23.032 (3-7); and Fistuliramus xianzaensis Liu and Wang, 1987, SMF 23.037 (8). 1, 2, 5-7, tangential section showing autozooecial apertures and vesicles; 3 and 4, longitudinal section showing autozooecial chambers and vesicular skeleton; and 8, branch transverse section.
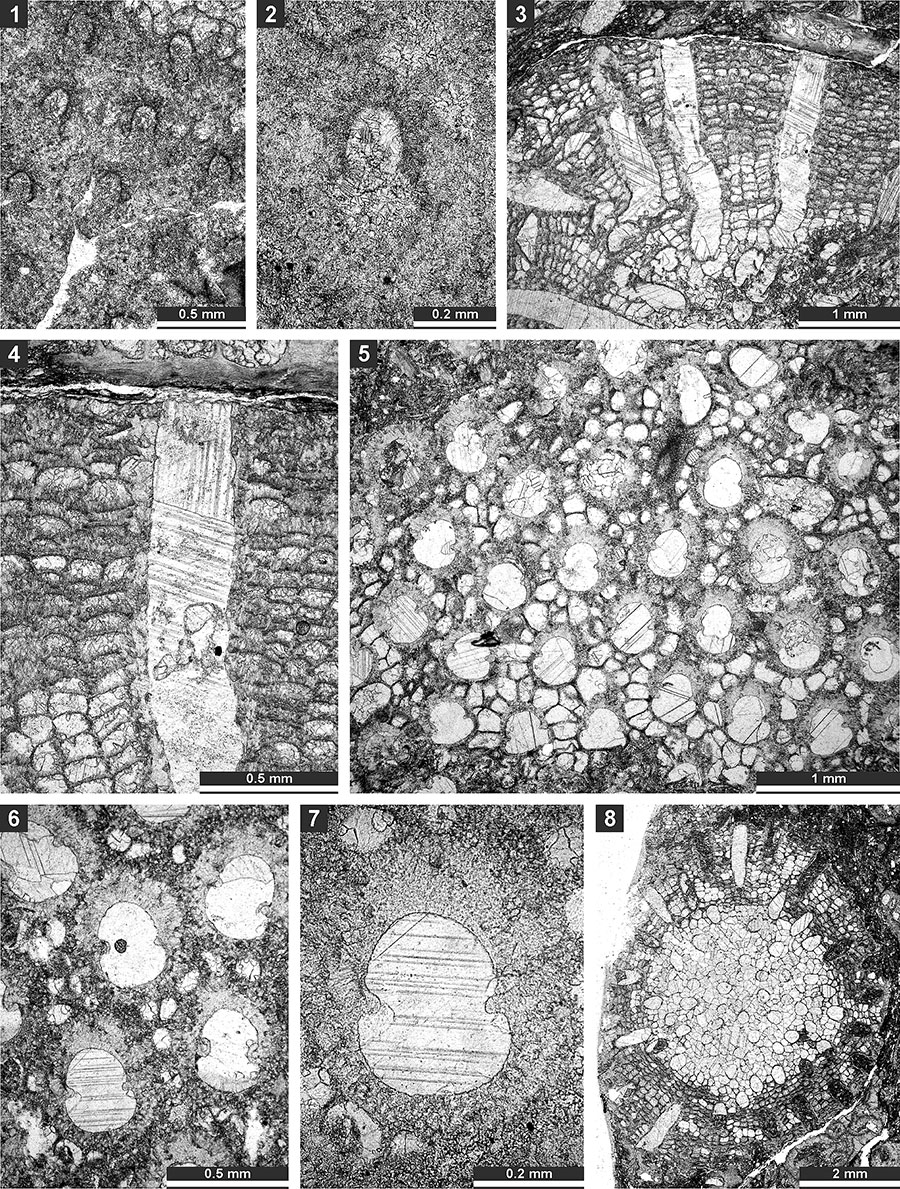
FIGURE 5. Thin section photographs of Fistuliramus xianzaensis Liu and Wang, 1987, SMF 23.042 (1) and SMF 23.038 (2 and 3); and Eridopora uncata Yang and Lu, 1983, SMF 23.052 (4 and 6) and SMF 23.051 (5). 1, longitudinal section; and 2-6, tangential section showing autozooecial apertures and vesicles.
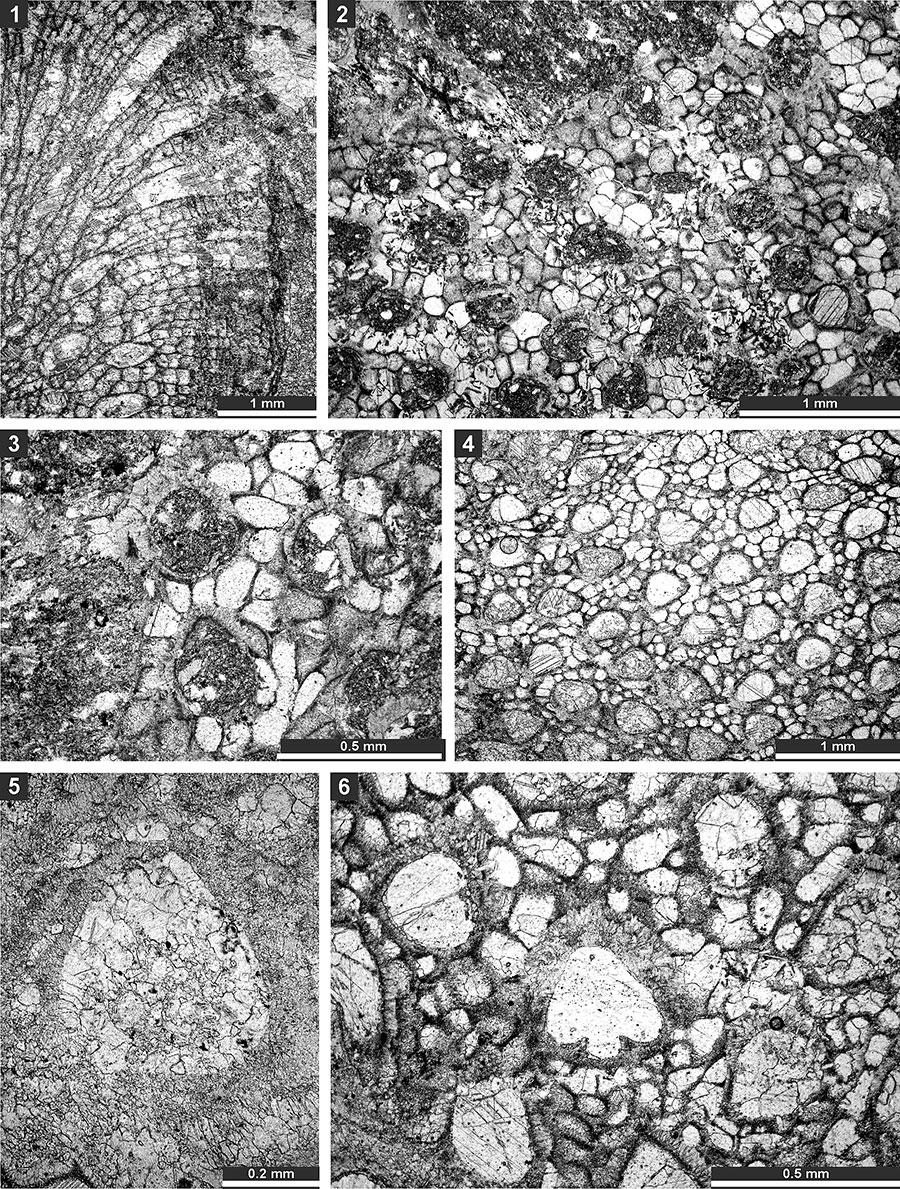
FIGURE 6. Thin section photographs of Eridopora uncata Yang and Lu, 1983, SMF 23.052 (1-3); Cyclotrypa alexanderi Sakagami, 1963 SMF 23.056 (4-5), SMF 23.061 (6), and SMF 23.056 (7); and Hexagonella kobayashii Sakagami, 1968, SMF 23.069 (8-9) and SMF 23.070 (10 and 11). 1-3, tangential section showing autozooecial apertures with lunarial ends indenting into the aperture (arrow); 7, tangential section showing autozooecia and macrozooecia (arrow); 8 and 9, branch transverse section showing autozooecial chambers and vesicular skeleton; and 10 and 11, longitudinal section showing autozooecial chambers and vesicular skeleton.
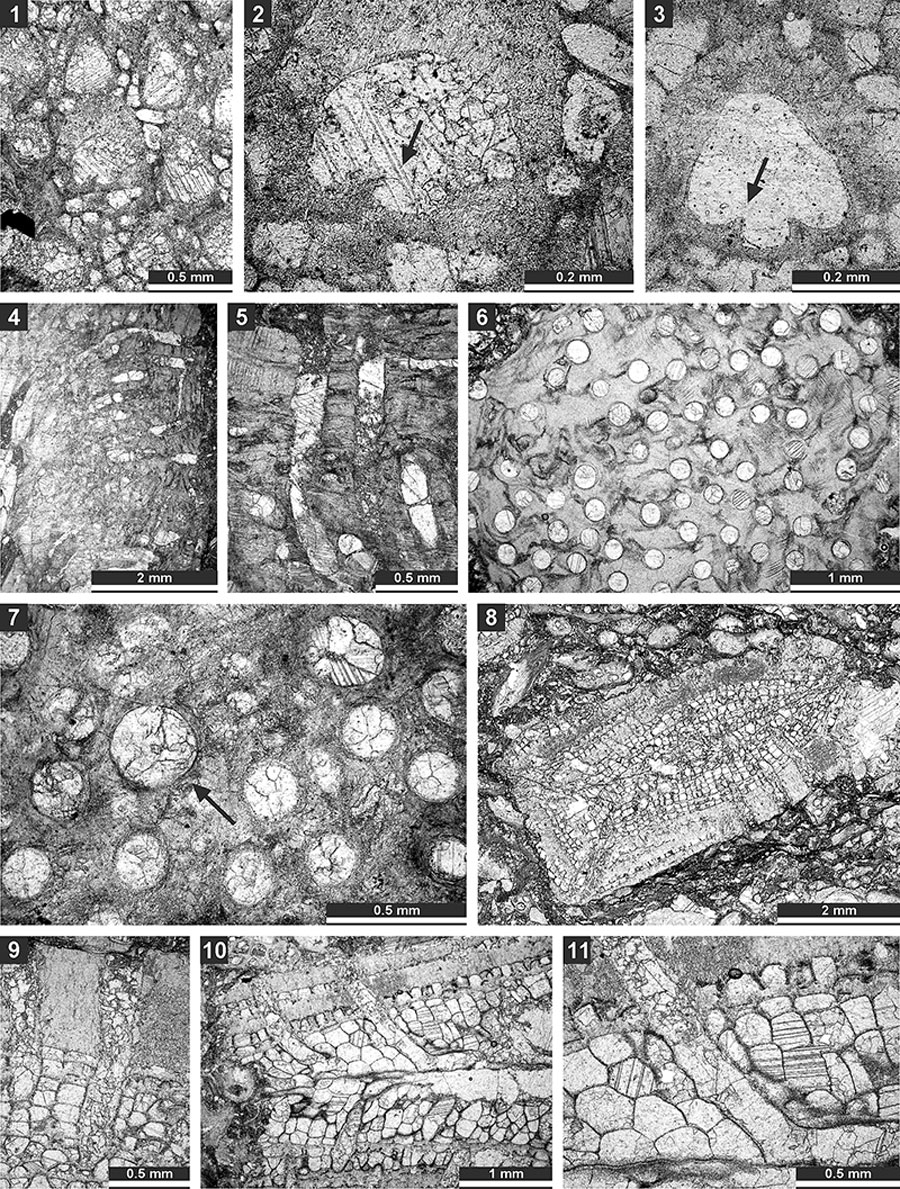
FIGURE 7. Thin section photographs of Hexagonella kobayashii Sakagami, 1968, SMF 23.067 (1-3) and SMF 23.072 (4); and Goniocladia aff. indica Waagen and Pichl, 1885, SMF 23.266 (5), SMF 23.077 (6), and SMF 23.073 (7). 1-3 and 6, tangential section; 7, mid-tangential section showing autozooecial chamber; 4, transverse section showing mesotheca with median tubules (arrow); and 5, external view of the colony form the reverse side.
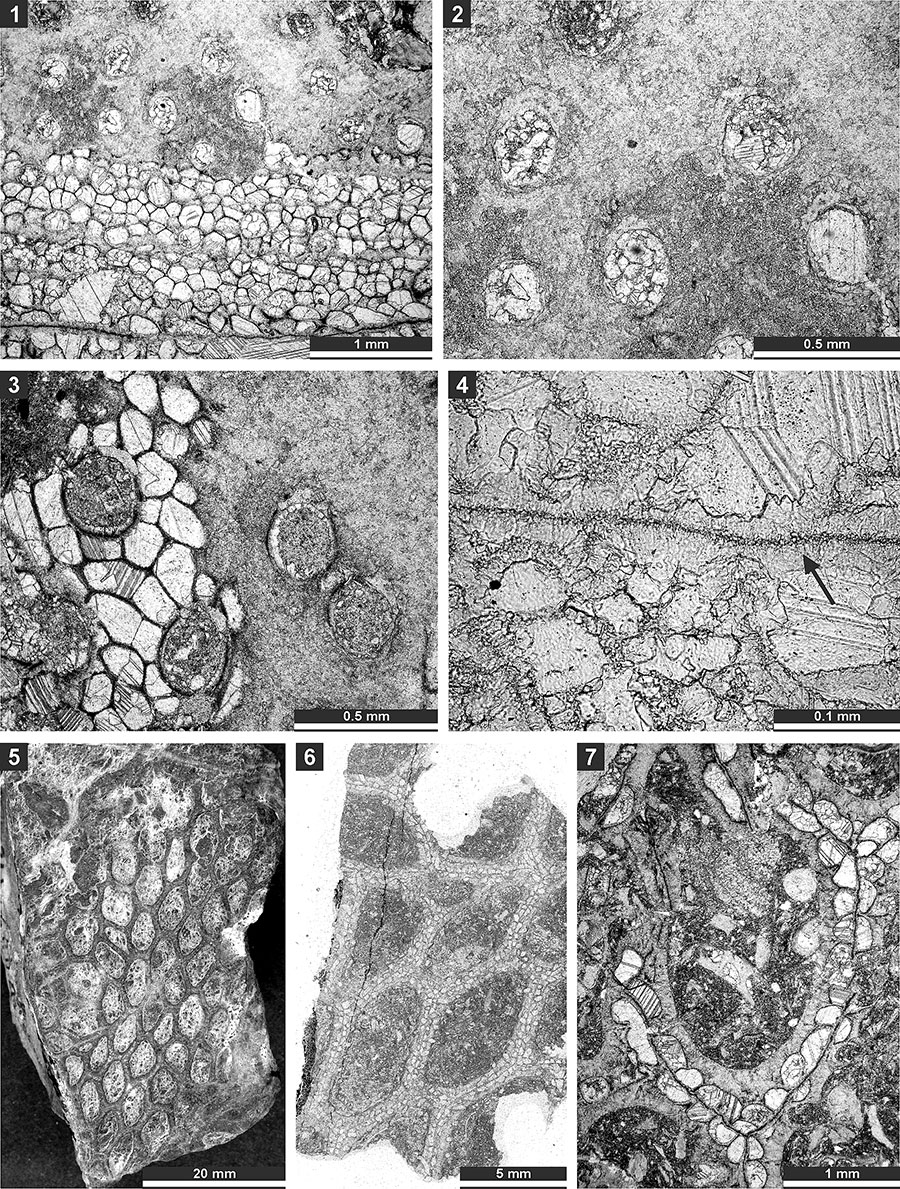
FIGURE 8. Thin section photographs of Goniocladia aff. indica Waagen and Pichl, 1885, SMF 23.076 (1 and 2), SMF 23.077 (3-5), and SMF 23.073 (6 and 7); and Liguloclema meridianus (Etheridge, 1926), SMF 23.093 (8). 1-2, and 6, branch transverse sections showing autozooecia chambers, mesotheca and hemisepta (arrow); 3-5, tangential sections showing autozooecial apertures; 7, longitudinal section showing autozooecial chamber with superior hemisepta (arrow); and 8, branch oblique section showing mesotheca, autozooecial chambers with hemisepta and vesicular skeleton.
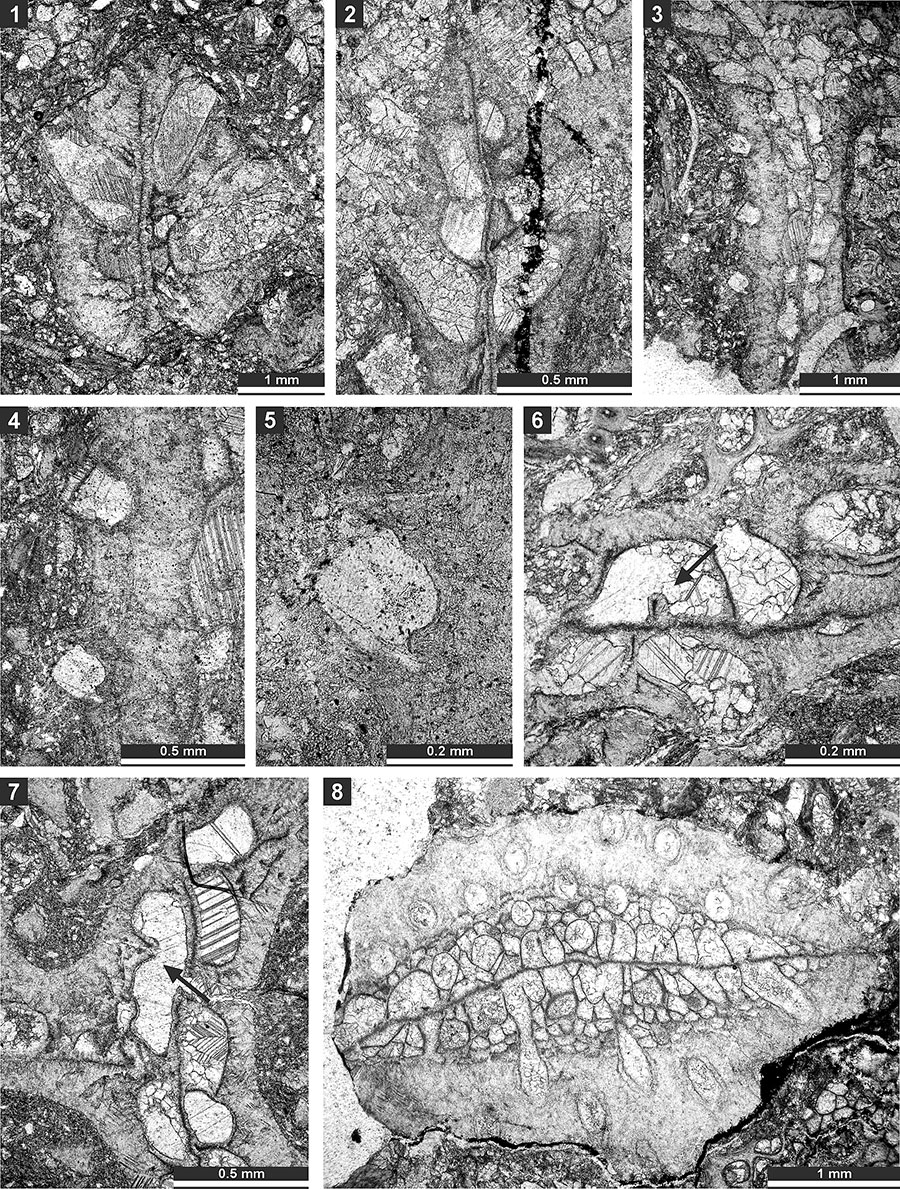
FIGURE 9. Thin section photographs of Liguloclema meridianus (Etheridge, 1926), SMF 23.093 (1), SMF 23.085 (2), and SMF 23.083 (3-5); and Etherella tibetensis n. sp., paratype SMF 23.217 (6). 1 and 2, branch oblique section showing mesotheca, autozooecial chambers with hemisepta and vesicular skeleton; 3 and 4, mid-tangential section showing autozooecial chambers with hemisepta; 5, tangential section showing autozooecial apertures; and 6, transverse section showing extrazooecial skeleton and sparse vesicles.
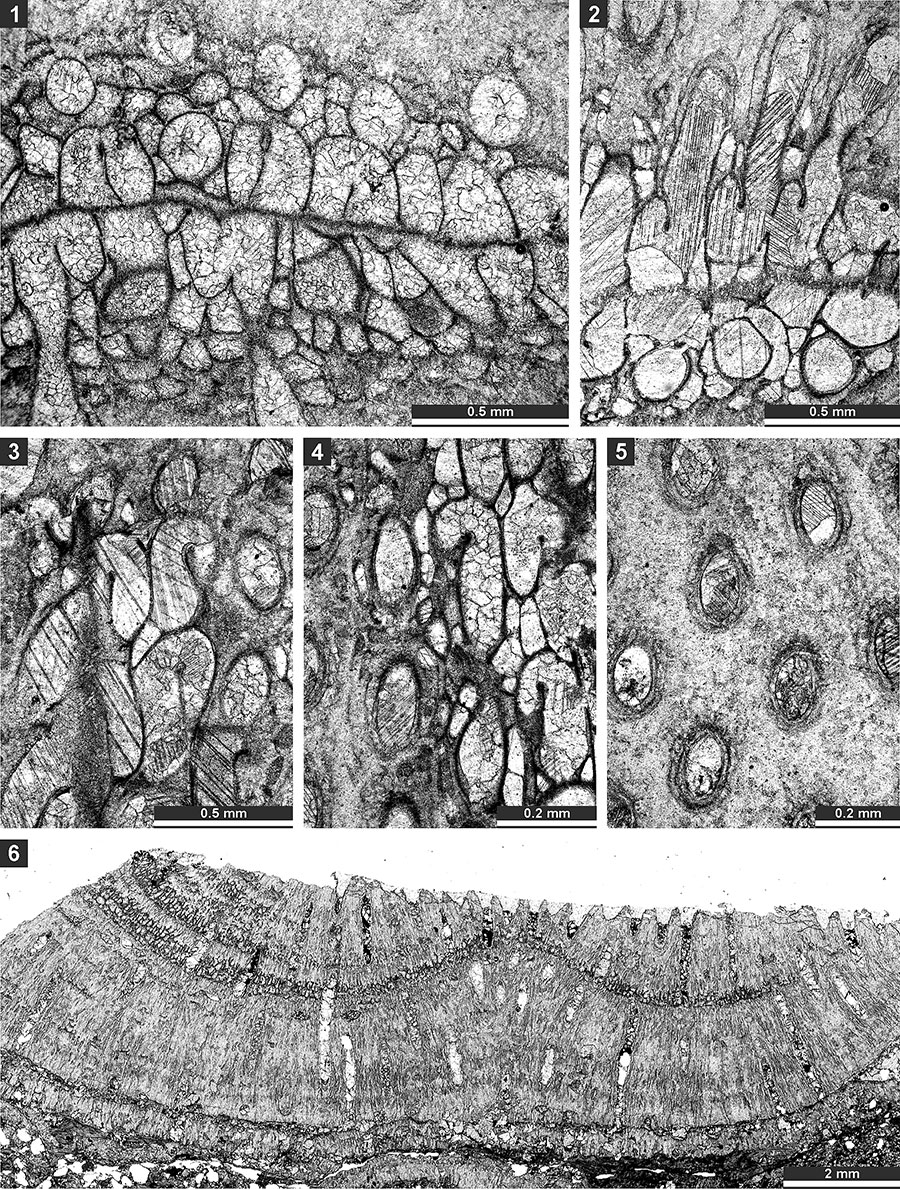
FIGURE 10. Thin section photographs of Etherella tibetensis n. sp., paratype SMF 23.267 (1), paratype SMF 23.216 (2), paratype SMF 23.219 (3 and 4), holotype SMF 23.214 (5-7), and paratype SMF 23.230 (8 and 9). 1, external view of the colony (split along mesotheca); 2-4, tangential section showing arrangement of autozooecia and wall microstructure; 5-7, oblique section showing hemisepta and mesotheca (arrows); and 8 and 9, branch transverse section showing autozooecial chambers and extrazooecial skeleton.
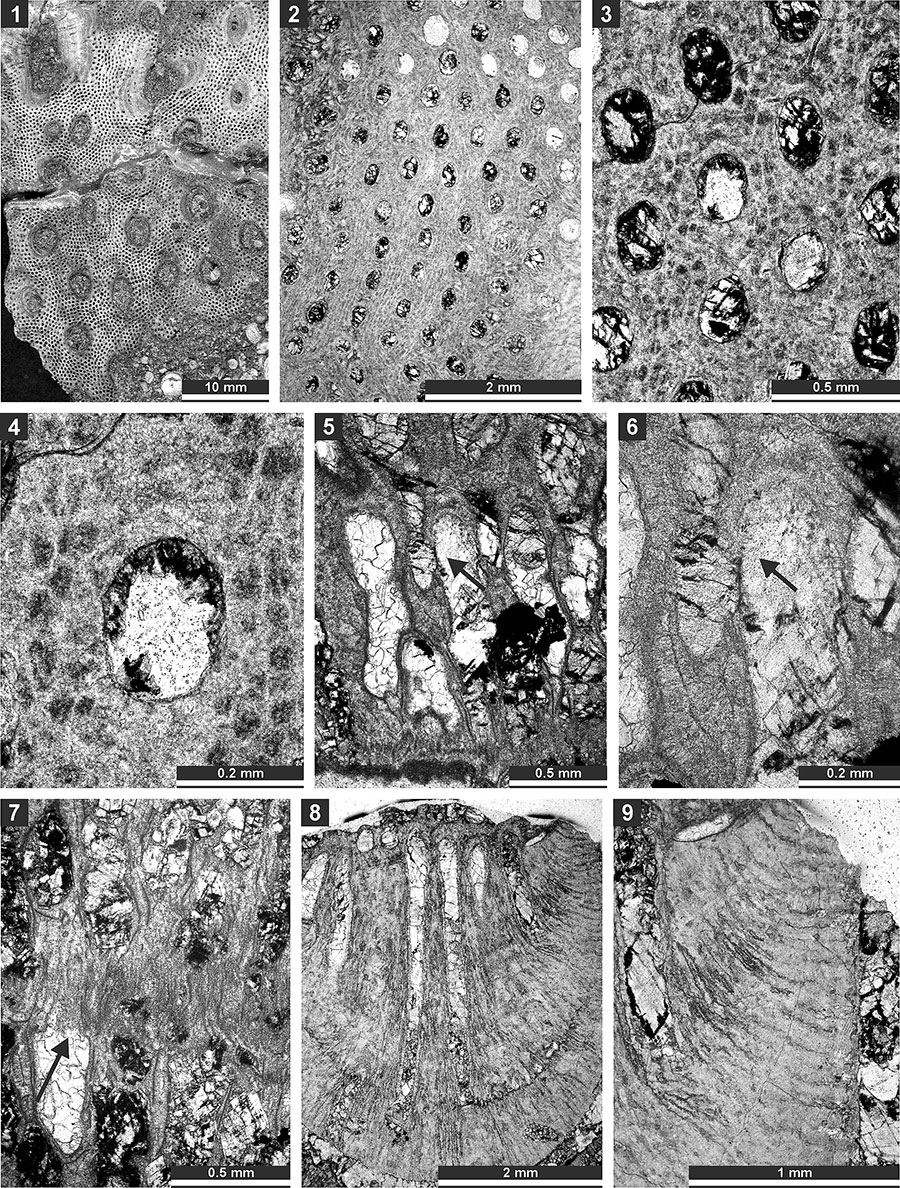
FIGURE 11. Thin section photographs of Tabulipora xinjiangensis Yang and Lu, 1983, SMF 23.233 (1 and 2); Dyscritella lii n. sp., holotype SMF 23.235 (3, 4, and 6) and paratype SMF 23.236 (5); and Ulrichotrypella omanica Ernst et al., 2008, SMF 23.242 (7 and 8). 1, longitudinal section showing ring septa; 2, oblique section showing macroacanthostyles and tubules; 3 and 4, oblique section through the colony; 5 and 6, tangential section showing autozooecial apertures and acanthostyles; and 7 and 8, oblique section.
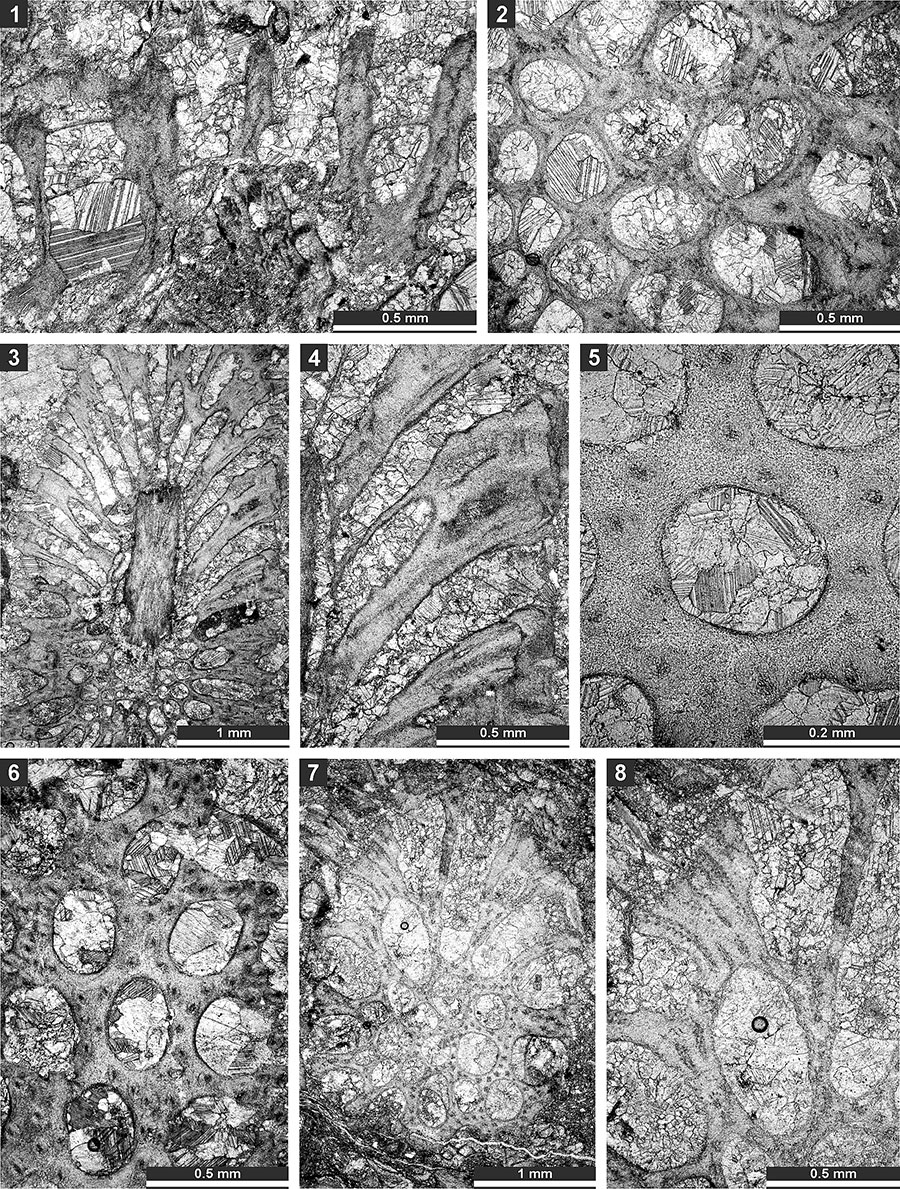
FIGURE 12. Thin section photographs of Ulrichotrypella omanica Ernst et al., 2008, SMF 23.242 (1); Neoeridotrypella astrica (Linskaya, 1951), SMF 23.245 (2 and 3), SMF 23.246 (4-6), and SMF 23.249 (7); and Streblotrypa (Streblotrypa) parviformis n. sp., holotype SMF 23.251 (8-10). 1, 6, 7, and 10, tangential section; 2-5, oblique branch section; and 8 and 9, longitudinal section.
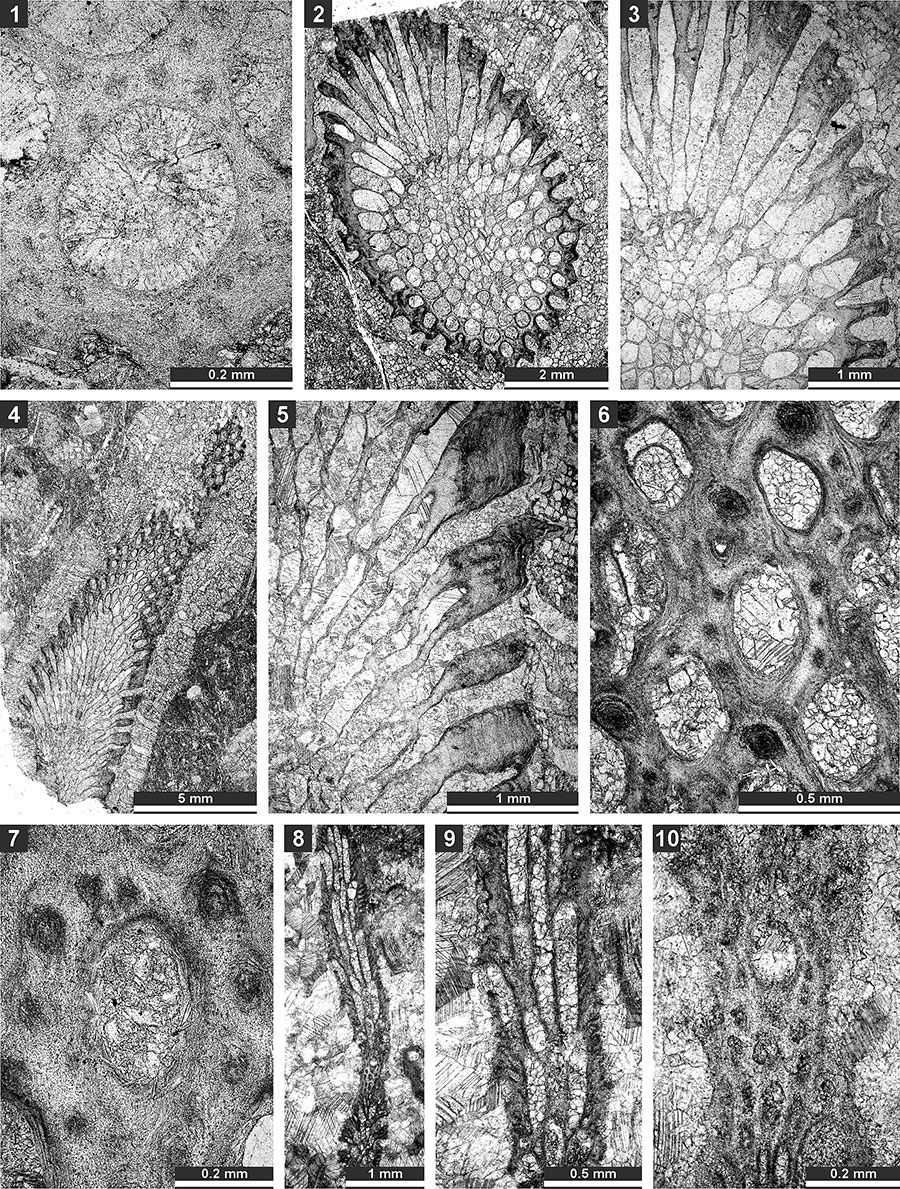
FIGURE 13. Thin section photographs of Streblotrypa ( Streblotrypa) parviformis n. sp., paratype SMF 23.260 (1) and paratype SMF 23.256 (2 and 3); Streblotrypa (Streblascopora) delicatula Sakagami, 1961, SMF 23.205 (4, 6, and 7), SMF 23.206 (5), and SMF 23.143 (8); and Streblotrypa (Streblascopora) marmionensis (Etheridge, 1926), SMF 23.145 (9). 1 and 8, branch transverse section; 2, 3, 5, and 9, longitudinal section; 4, branch oblique section; and 6 and 7, tangential section.
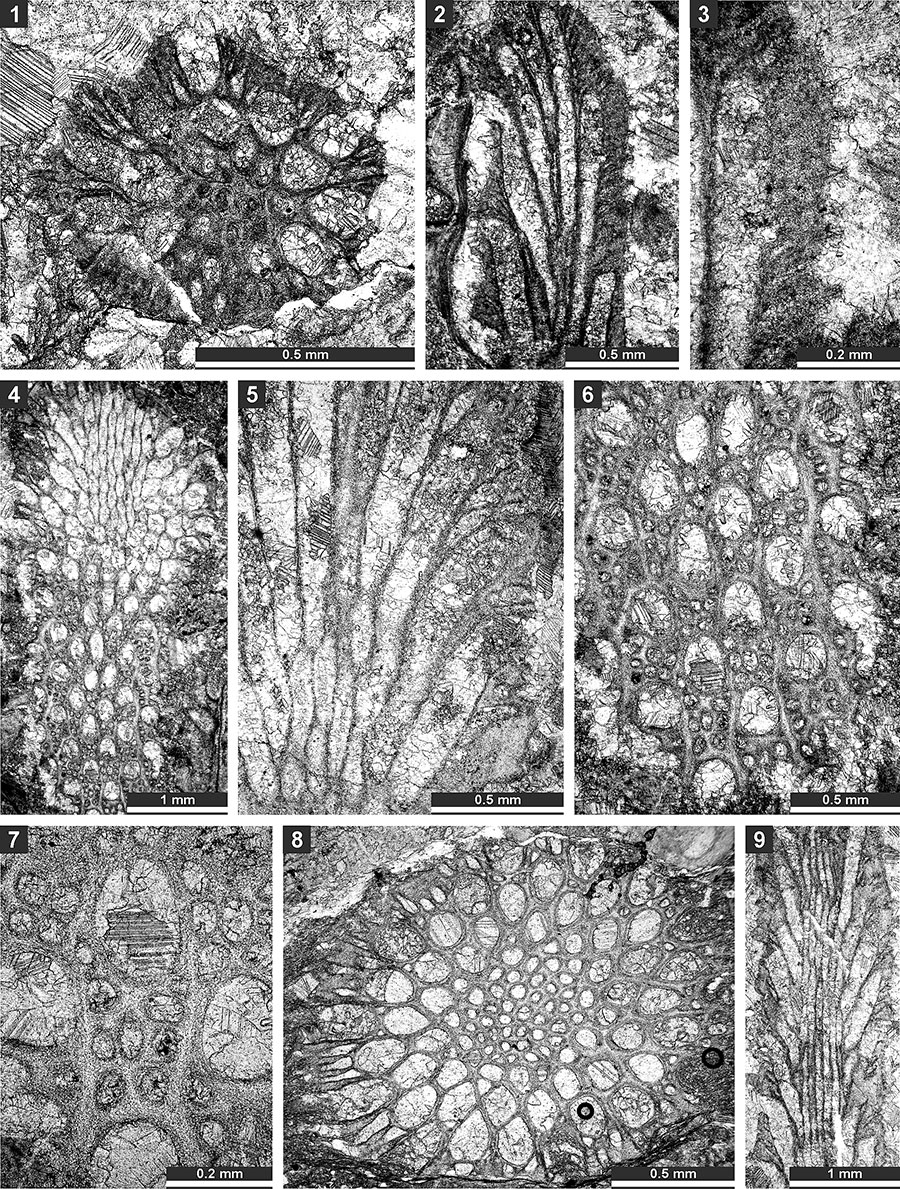
FIGURE 14. Thin section photographs of Streblotrypa ( Streblascopora) marmionensis (Etheridge, 1926), SMF 23.145 (1 and 2), and SMF 23.148 (3); and Rhabdomeson bretnalli Crockford, 1957, SMF 23.155 (4-6) and SMF 23.157 (7-9). 1, longitudinal section; 2 and 7, tangential section; 3 and 4, branch transverse section; and 5, 6, 8 and 9; branch oblique section.
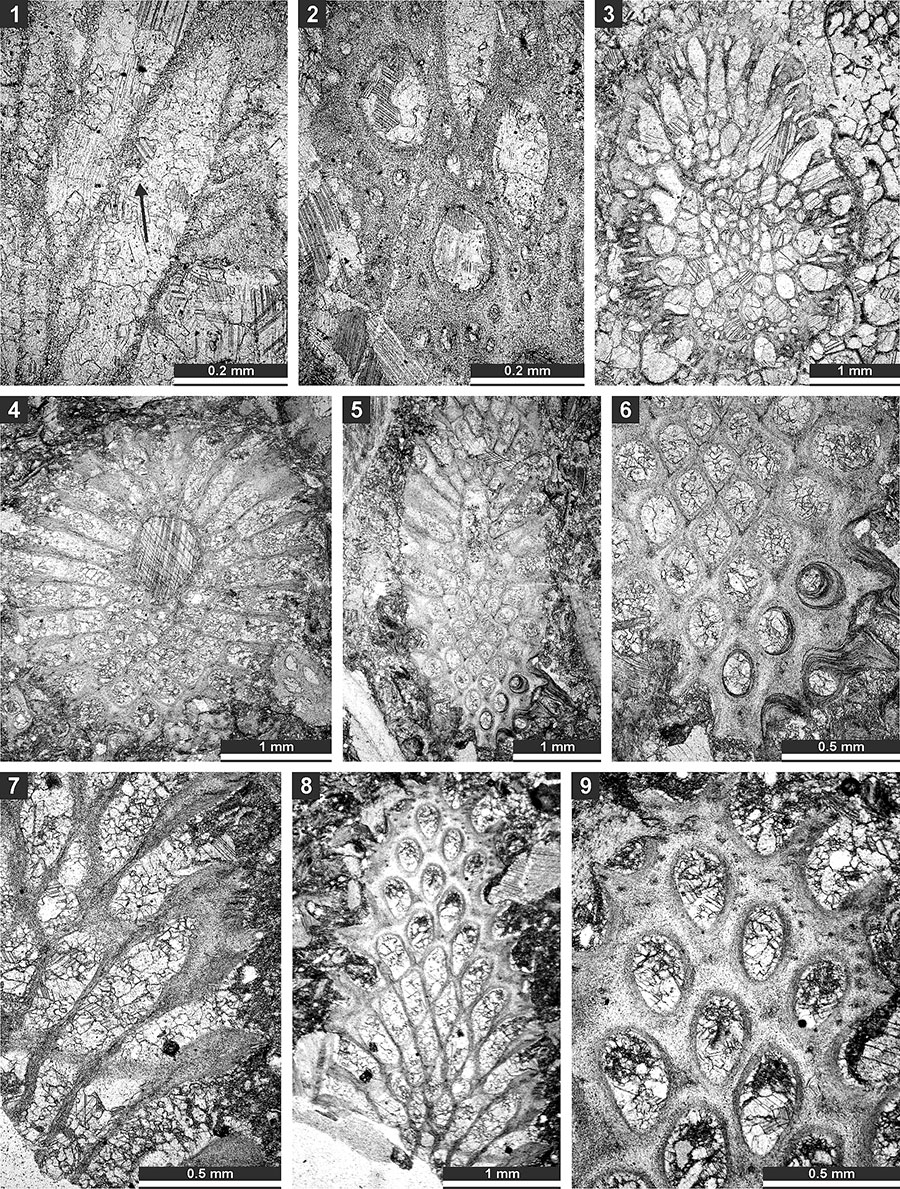
FIGURE 15. Thin section photographs of Rhabdomeson sp. SMF 23.160 (1-4) and SMF 23.158 (5); and Primorella rotunda Gorjunova, 1985, SMF 23.163 (6) and SMF 23.162 (7-9). 1-3, branch oblique section; 4 and 5, longitudinal section; 6, tangential section; and 7-9, oblique section.
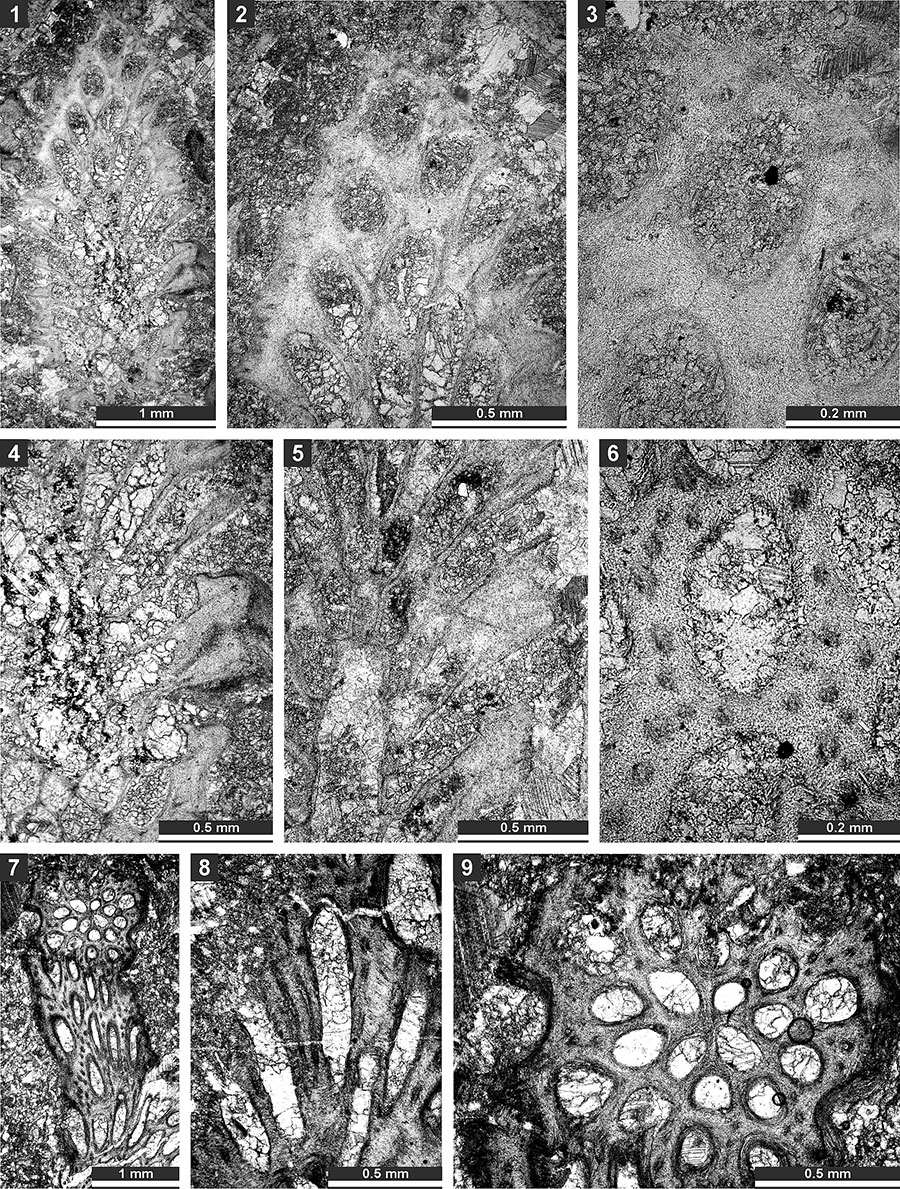
FIGURE 16. Thin section photographs of Timanotrypa australis n. sp., holotype SMF 23.165 (1), paratype SMF 23.166 (2 and 3), paratype SMF 23.169 (4), and paratype SMF 23.171 (5). 1, oblique section of the pinnate branch; 2 and 3, tangential section showing autozooecial apertures, nodes (arrows) and microstyles; and 4 and 5, branch transverse section.
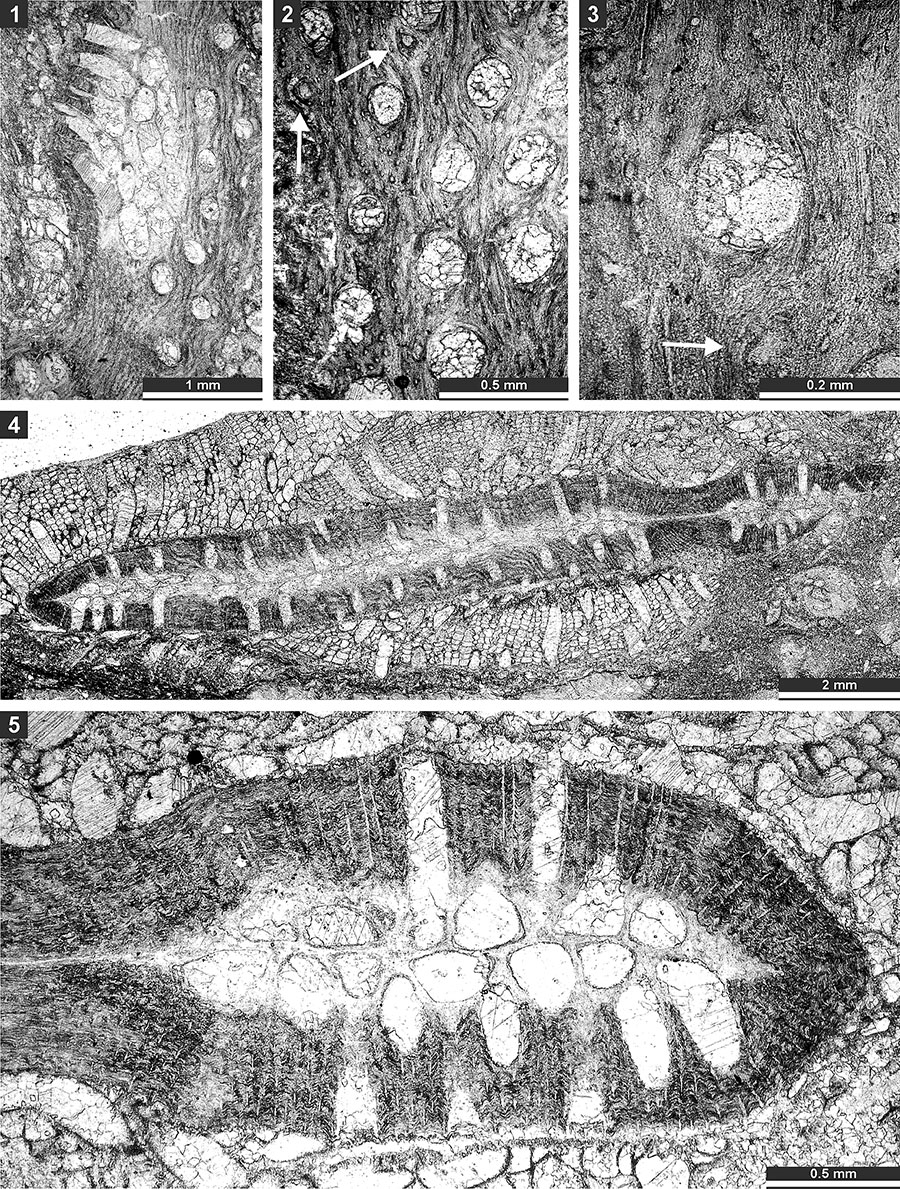
FIGURE 17. Thin section photographs of Spinofenestella sp., SMF 23.178 (1-5); and Spinofenestella subquadratopora (Schulga-Nesterenko, 1952), SMF 23. 179 (6-8). 1-3, tangential section of the reverse side; 4-7, tangential section of the obverse side; and 8, mid- tangential section.
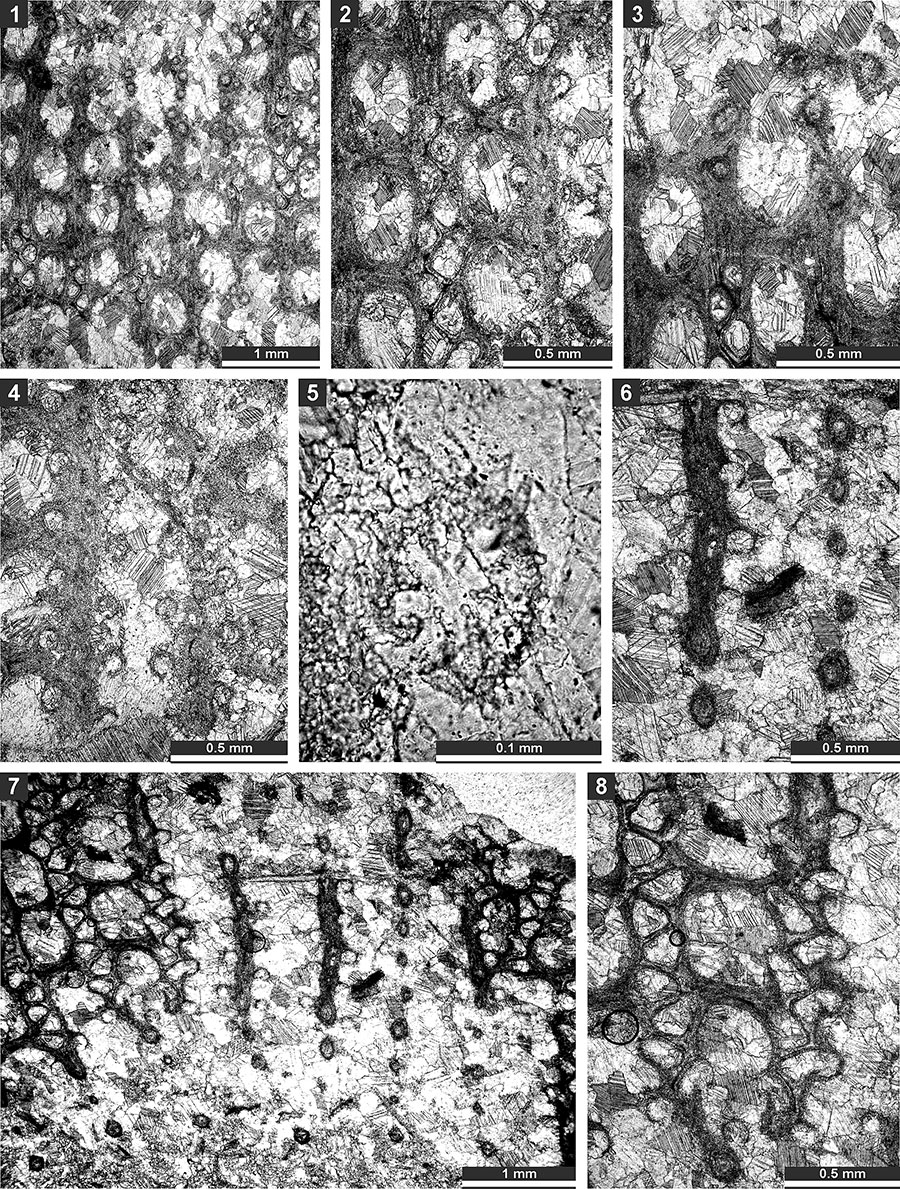
FIGURE 18. Thin section photographs of Polypora consanguinea Bassler, 1929, SMF 23.182 (1-4); and Polypora brouweri Bassler, 1929, SMF 23.183 (5-9). 1, oblique section; 2-5, tangential section showing autozooecial apertures, nodes and autozooecial chambers; 6, reverse side of the colony; and 7-9, tangential section showing autozooecial apertures and chambers.
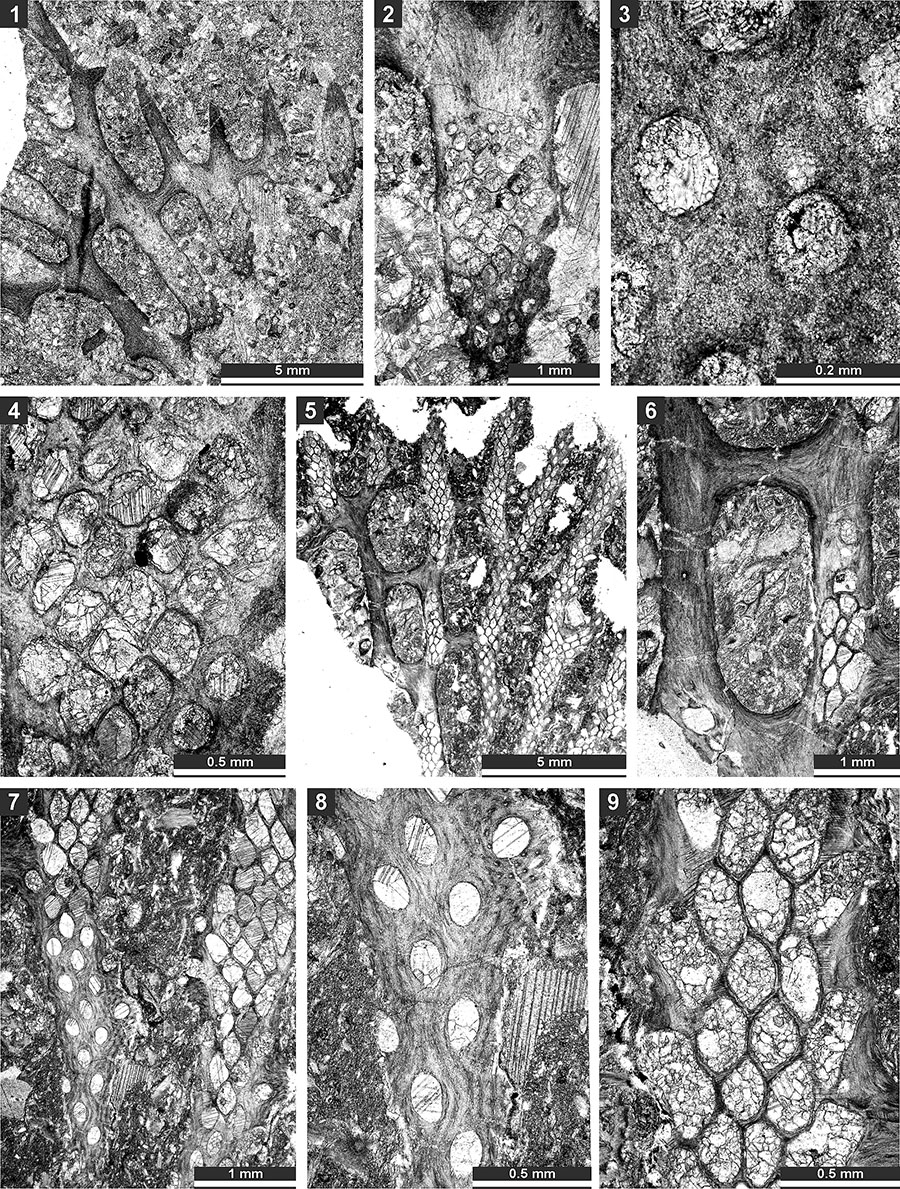
FIGURE 19. Thin section photographs of Polypora aff. voluminosa Trizna and Klautzan, 1961, SMF 23.187 (1-3, and 5) and SMF 23.185 (4 and 6); and Mackinneyella obesa (Crockford, 1957), SMF 23.191 (7) and SMF 23.192 (8). 1 and 2, tangential section branches and fenestrules; 3, tangential section of the reverse side showing nodes; 4, tangential section showing autozooecial apertures and chambers with hemisepta (arrow); 5, tangential section showing autozooecial apertures, chambers and heteromorph (arrow); 6, tangential section showing autozooecial apertures and nodes; and 7 and 8, tangential section showing autozooecial apertures and chambers.
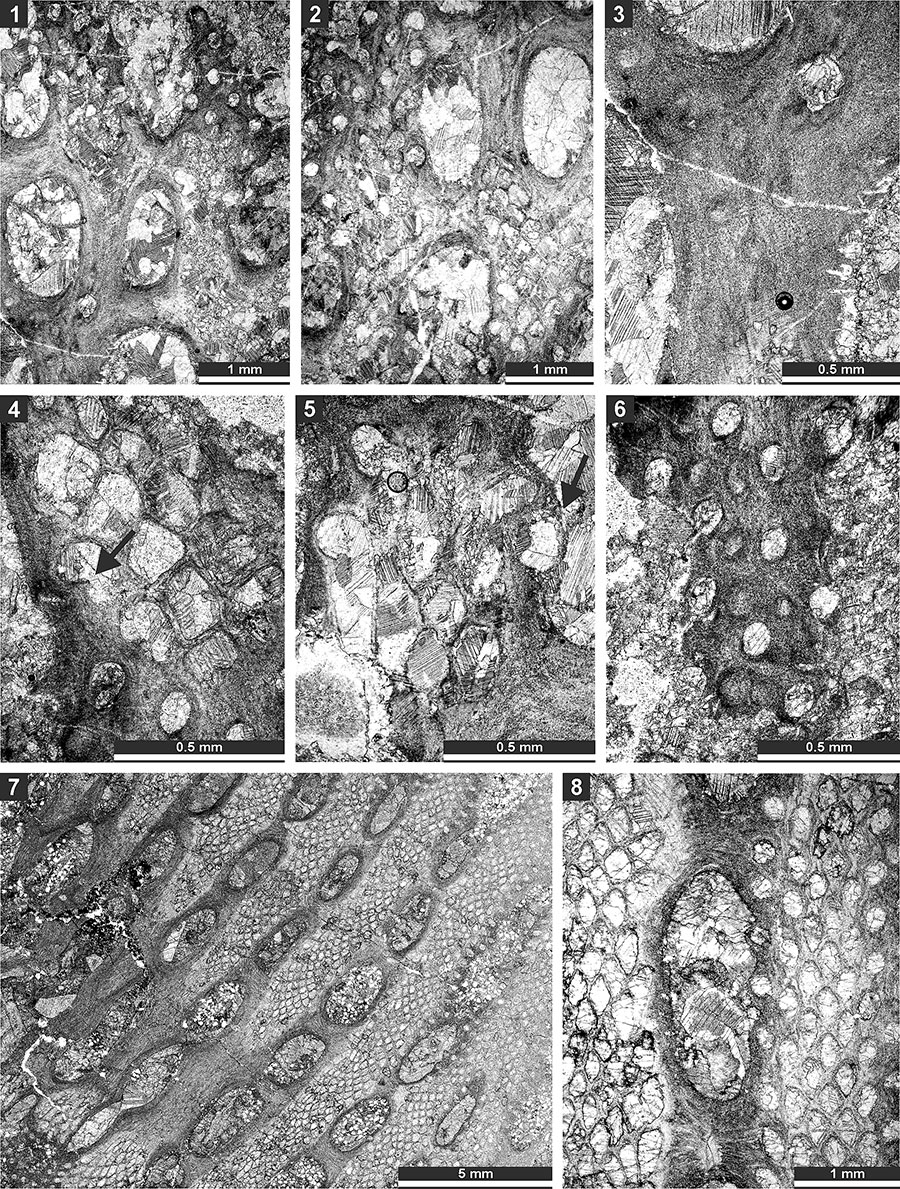
FIGURE 20. Thin section photographs of Mackinneyella obesa (Crockford, 1957), SMF 23.194 (1, 3), SMF 23.191 (2), and SMF 23.189 (4); and Protoretepora irregularis n. sp., paratype SMF 23.198 (5), SMF 23.268 (6), and SMF 23.195 (7). 1-3, and 7, tangential section showing autozooecial chambers; 4, branch transverse section; 5, branch longitudinal section; and 6, external view of the reverse side of the colony.
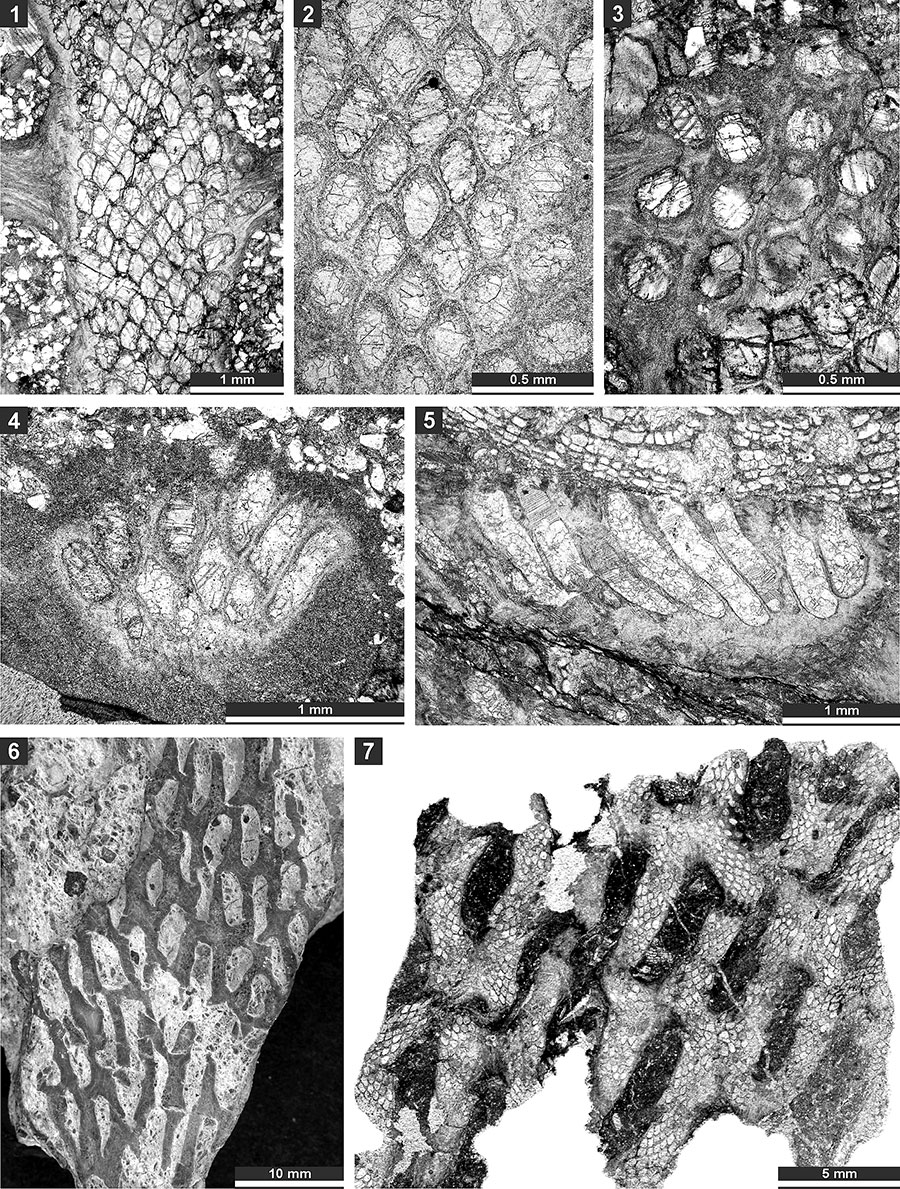
FIGURE 21. Thin section photographs of Protoretepora irregularis n. sp., paratype SMF 23.201 (1, 3, and 4), paratype SMF 23.202 (2), and holotype SMF 23.195 (5); and Tibetiporella ornata n. gen. n. sp., paratype SMF 23.224 (6 and 7) and holotype SMF 23.227 (8). 1, tangential section showing autozooecial apertures and chambers; 2, mid-tangential section showing autozooecial chambers; 3, 4, 7, and 8, tangential section showing autozooecial apertures, microstyles and nodes; 5, tangential section of obverse colony side with nodes; and 6, tangential section of the reverse side of the colony.
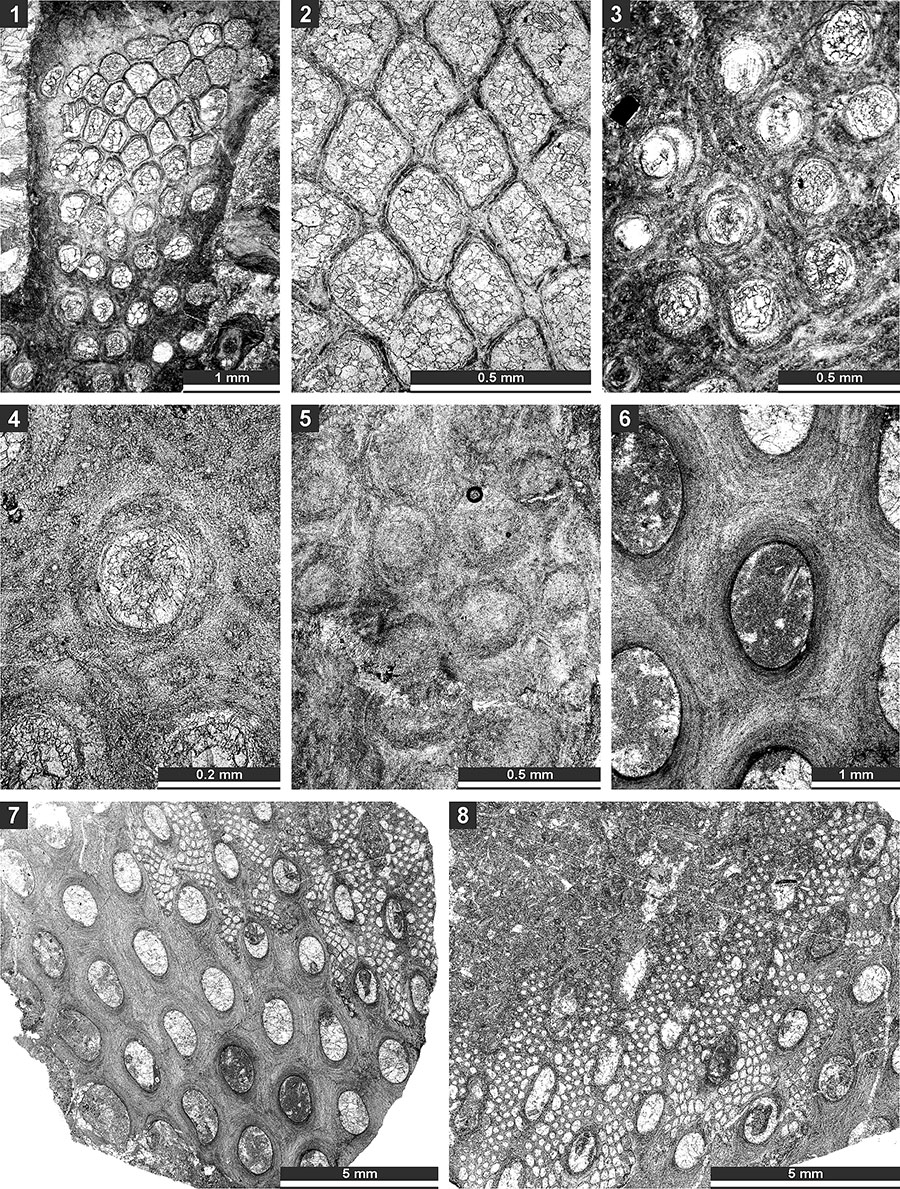
FIGURE 22. Thin section photographs of Tibetiporella ornata n. gen. n. sp., holotype SMF 23.227 (1 and 2), paratype SMF 23.211 (3 and 4), paratype SMF 23.210 (5 and 6), paratype SMF 23.213 (7 and 8), and paratype SMF 23.225 (9). 1 and 2, tangential section showing autozooecial apertures, nodes and heteromorphs; 3 and 4, mid-tangential section showing autozooecial chambers; 5 and 6, tangential section showing autozooecial apertures, nodes and heteromorphs; 7 and 8, longitudinal section showing autozooecial chambers and heteromorphs; and 9, transverse section showing autozooecial chambers.
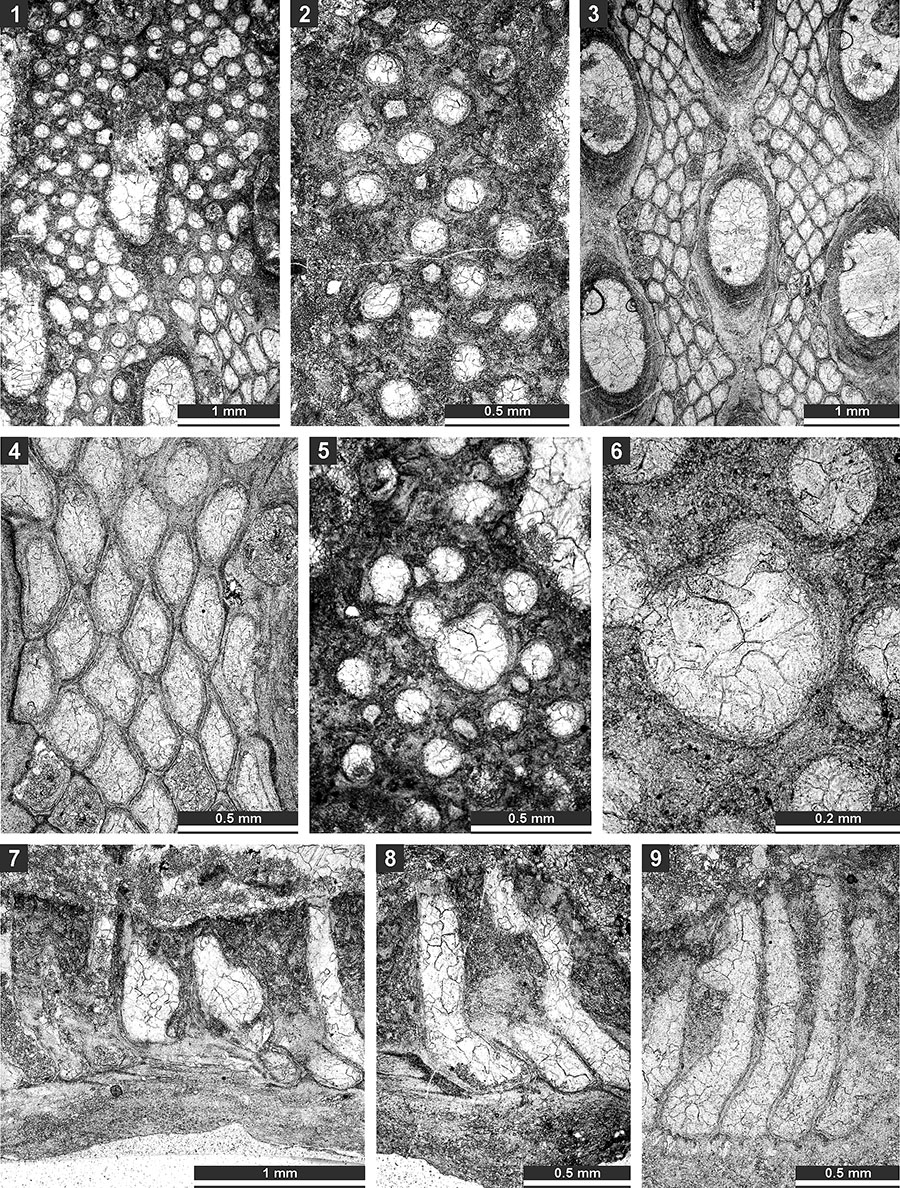
FIGURE 23. Palaeobiogeographical affinities of the bryozoan assemblage from the Zhongba Formation (Zhongba). Top: dendrogram of the cluster analysis using Jacquard's similarity index (unweighted pair-group average algorithm), and bottom: plot of non-metric MDS analysis made with Jacquard's similarity index. Areas: Pamir (Tajikistan), Oman (Batain Coast), Iran (central Iran, Chili Formation), Xainza (southwestern Tibet, Xiala Fm.), Thailand, Urals, Western Xinjiang (Baliqliq Group), and W. Australia (Noonkanbah Fm.). Japan, Malaysia, and Rutog (southwestern Tibet) were omitted as sharing only one species with Zhongba assemblage.
 Andrej Ernst. Institut für Geologie, Universität Hamburg, Bundesstr. 55, 20146 Hamburg, Germany. Andrej.Ernst@uni-hamburg.de
Andrej Ernst. Institut für Geologie, Universität Hamburg, Bundesstr. 55, 20146 Hamburg, Germany. Andrej.Ernst@uni-hamburg.de
Dr. Andrej Ernst is a specialist for bryozoan taxonomy, evolution, biogeography and ecology, as well as for carbonate sedimentology. His PhD at the University of Kiel (1999) was devoted Permian bryozoans of Zechstein Basin and NW Tethys. After a short post-doc project he spent one year at the Geological Museum in Oslo (2002-2003), then acted at the University of Kiel. In 2013 Andrej Ernst has moved to Hamburg. The range of his research includes study of Palaeozoic and some post-Palaeozoic bryozoans faunas worldwide.
Bryozoan fauna from the Permian (Artinskian-Kungurian) Zhongba Formation of southwestern Tibet
Plain Language Abstract
Bryozoans are marine colonial animals and represent a phylum known from the Early Ordovician to the present. The majority of bryozoans produce a stable calcitic skeleton which has a high preservation potential. Therefore, bryozoans have multiple uses for stratigraphy, palaeogeography and palaeoecology. In the present paper an Early Permian bryozoan fauna from the Zhongba Formation of southwestern Tibet is described. The Zhongba Formation was deposited in a relatively deep marine environment of the tropical part of the Tethys. The studied bryozoan fauna includes 30 species from 25 genera. Seven species are new: Fistulipora sakagamii n. sp., Dybowskiella hupehensiformis n. sp., Etherella tibetensis n. sp., Dyscritella lii n. sp., Streblotrypa (Streblotrypa) parviformis n. sp., Timanotrypa australis n. sp., and Protoretepora irregularis n. sp. One new genus with one species is also new: Tibetiporella ornata n. gen. n. sp. The majority of species is known from other regions with faunas of late early Permian age, which is older than the previously defined age of the Zhongba Formation as stated by other fossils like brachiopods (middle – late Permian). Furthermore, the identified species are distributed in such regions as Thailand, Oman, Western Australia, Timor, central Pamir, Iran, Urals, and other Tibetan localities. So far, this fauna shows an intermixture of species from the Northern and Southern Hemispheres, and implies more extensive faunal migrations into the tropical region from both the north and south than previously known.
Resumen en Español
Fauna de briozoos del Pérmico (Artinskiano-Kunguriano) de la Formación Zhongba, sudoeste tibetano
La asociación de briozoos de la Formación Zhongba del sudoeste de Tibet incluye 30 especies de 25 géneros. Siete especies son nuevas: Fistulipora sakagamii sp. n., Dybowskiella hupehensiformis sp. n., Etherella tibetensis sp. n., Dyscritella lii sp. n., Streblotrypa (Streblotrypa) parviformis sp. n., Timanotrypa australis sp. n., y Protoretepora irregularis sp. n. Un género con una especie también es nueva: Tibetiporella ornata gen. n. sp. n. La fauna descripta implica una edad cisuraliana (artinskiana-kunguriana) para la Formación Zhongba y muestra relaciones con las faunas cisuralianas de Tailandia, Australia Occidental, Omán, Timo, Pamir central, Irán, los Urales y otras localidades tibetanas. La fauna de briozoos de la Formación Zhongba muestra una intermezcla de elementos boreales y gondwánicos e implica migraciones faunísticas más intensas hacia la región tropical tanto desde el norte como desde el sur. El análisis paleoecológico sugiere que la depositación de la Formación Zhongba ocurrió en un escenario de plataforma media a cierta distancia de la línea de costa, probablemente influenciada por corrientes locales.
Palabras clave: Bryozoa; taxonomía; paleobiogeografía; género nuevo; especie nueva
Traducción: Diana Elizabeth Fernández
Résumé en Français
La faune de bryozoaires du Permien (Artinskien-Kungurien) de la formation de Zhongba, sud-ouest du Tibet
L'assemblage de bryozoaires de la formation de Zhongba, sud-ouest du Tibet, inclut 30 espèces appartenant à 25 genres. Sept espèces classifiées dans des genres déjà connus sont nouvelles : Fistulipora sakagamii n. sp., Dybowskiella hupehensiformis n. sp., Etherella tibetensis n. sp., Dyscritella lii n. sp., Streblotrypa (Streblotrypa) parviformis n. sp., Timanotrypa australis n. sp., et Protoretepora irregularis n. sp. Une nouvelle espèce appartenant à un nouveau genre est également décrite : Tibetiporella ornata n. gen. n. sp. La faune décrite indique un âge cisuralien (artinskien-kungurien) pour la formation de Zhongba et démontre des relations avec les faunes cisuraliennes de la Thaïlande, de l'Australie occidentale, du Sultanat d'Oman, du Timor, du Pamir central, de l'Iran, de l'Oural, et d'autres localités tibétaines. La faune de bryozoaires de la formation de Zhongba montre un mélange d'éléments boréaux et gondwaniens, et indique des dispersions fauniques fortes vers les régions tropicales à la fois depuis le nord et le sud. L'analyse paléoécologique suggère que la formation de Zhongba s'est déposée dans un milieu de plateforme moyenne, à une certaine distance de la côte, probablement sous l'influence des courants locaux.
Mots-clés : Bryozoa ; taxinomie ; paléobiogéographie ; nouveau genre ; nouvelle espèce
Translator: Antoine Souron
Deutsche Zusammenfassung
Die Bryozoen-Fauna aus der permischen (Artinskium-Kungurium) Zhongba Formation von Südwest-Tibet
Die Bryozoen-Assemblage aus der Zhongba Formation von Südwest-Tibet beinhaltet 30 Arten und 25 Gattungen. Sieben Arten sind neu: Fistulipora sakagamii n. sp., Dybowskiella hupehensiformis n. sp., Etherella tibetensis n. sp., Dyscritella lii n. sp., Streblotrypa (Streblotrypa) parviformis n. sp., Timanotrypa australis n. sp. und Protoretepora irregularis n. sp. Eine Gattung mit einer Art ist ebenfalls neu: Tibetiporella ornata n. gen. n. sp. Die beschriebene Fauna weist auf ein cisurales (Artinskium-Kungurium) Alter der Zhongba Formation hin und zeigt Verwandtschaften mit den cisuralen Faunen von Thailand, Westaustralien, Oman, Timor, Zentral-Pamir, Iran, Ural und anderen tibetischen Fundstellen. Die Bryozoen-Fauna der Zhongba-Formation zeigt eine Mischung aus borealen und gondwanischen Elementen und weist auf eine stärkere Faunen-Migration, sowohl von Norden als auch von Süden, in die tropischen Gebiete hin. Paläoökologische Analysen legen nahe, dass die Zhongba Formation in einiger Entfernung zur Küste an einem mittleren Schelf abgelagert wurde und möglicherweise durch lokale Strömungen beeinflusst war.
Schlüsselwörter: Bryozoa; Taxonomie; Paläobiogeographie; neue Gattung; neue Art
Translator: Eva Gebauer
Arabic

Translator: Ashraf M.T. Elewa
-
-
-
Review: The Princeton Field Guide to Mesozoic Sea Reptiles
 The Princeton Field Guide to Mesozoic Sea Reptiles
The Princeton Field Guide to Mesozoic Sea ReptilesArticle number: 26.1.1R
April 2023


 Poster Winners 2024
Poster Winners 2024