Article Search
Volume 27.1
January–April 2024
Full table of contents
ISSN: 1094-8074, web version;
1935-3952, print version
Recent Research Articles
See all articles in 27.1 January-April 2024
See all articles in 26.3 September-December 2023
See all articles in 26.2 May-August 2023
See all articles in 26.1 January-April 2023
 Sangmin Lee. School of Life and Environmental Sciences, Burwood Campus, Deakin University, 221 Burwood Highway, Burwood, VIC 3125, Australia. sangminlee76@gmail.com
Sangmin Lee. School of Life and Environmental Sciences, Burwood Campus, Deakin University, 221 Burwood Highway, Burwood, VIC 3125, Australia. sangminlee76@gmail.com
Sangmin Lee is a postdoctoral research fellow at the Deakin University (Melbourne, Australia). His research work focuses on the taxonomy, palaeobiogeography, and phylogeny of late Palaeozoic brachiopods. In particular, since 2012 he has performed geological/palaeontological field trips to Spitsbergen, Arctic Norway for reconstructing the palaeogeography, palaeoclimate, and palaeoecology in the northern margin of Pangea during the late Palaeozoic. In addition, he is developing a 3-D geometric morphometric methodology applied to brachiopod external shells, using X-ray microtomographic and laser-scanning techniques.

 G.R. Shi. School of Life and Environmental Sciences, Burwood Campus, Deakin University, 221 Burwood Highway, Burwood, VIC 3125, Australia. grshi@deakin.edu.au
G.R. Shi. School of Life and Environmental Sciences, Burwood Campus, Deakin University, 221 Burwood Highway, Burwood, VIC 3125, Australia. grshi@deakin.edu.au
Guang R. Shi holds a Personal Chair in palaeontology at Deakin University, Melbourne, Australia, where he has been employed since 1992. His main research interests include Late Palaeozoic brachiopod taxonomy, biostratigraphy and global biogeography; and the applications of palaeontology to palaeogeographical, palaeoclimatic and plate tectonic reconstructions and macroevolution.

 Tae-Yoon S. Park. Division of Polar Earth-System Sciences, Korea Polar Research Institute, 26 Songdomirae-ro, Yeonsu-gu, Incheon 21990, Republic of Korea. typark@kopri.re.kr
Tae-Yoon S. Park. Division of Polar Earth-System Sciences, Korea Polar Research Institute, 26 Songdomirae-ro, Yeonsu-gu, Incheon 21990, Republic of Korea. typark@kopri.re.kr
Tae-Yoon Park is a senior research scientist at Korea Polar Research Institute. He works on the Paleozoic invertebrates from Anatarctica and Greenland, with special focus on the Cambrian arthropod evolution.

 Jae-Ryong Oh. Division of Polar Earth-System Sciences, Korea Polar Research Institute, 26 Songdomirae-ro, Yeonsu-gu, Incheon 21990, Republic of Korea; and Polar Sciences, University of Science and Technology, Yuseong-gu, Daejeon 34113, Republic of Korea. ggilli@kopri.re.kr
Jae-Ryong Oh. Division of Polar Earth-System Sciences, Korea Polar Research Institute, 26 Songdomirae-ro, Yeonsu-gu, Incheon 21990, Republic of Korea; and Polar Sciences, University of Science and Technology, Yuseong-gu, Daejeon 34113, Republic of Korea. ggilli@kopri.re.kr
Jae-Ryong Oh is Ph.D students at the University of Science and Technology. He is a sedimentologist studying on the late Paleozoic sedimentary rocks of Svalbard, Arctic Norway.

 Horng-Sheng Mii. Department of Earth Sciences, National Taiwan Normal University, No. 88, Section 4, Ting-zhou Road, Taipei 11677, Taiwan, Republic of China. t44006@ntnu.edu.tw
Horng-Sheng Mii. Department of Earth Sciences, National Taiwan Normal University, No. 88, Section 4, Ting-zhou Road, Taipei 11677, Taiwan, Republic of China. t44006@ntnu.edu.tw
Horng-Sheng Mii is a professor of the National Taiwan Normal University. His research interest is mainly to reconstruct paleoenvironment by analyzing the stable carbon and oxygen isotope compositions and elemental concentration of carbonate fossil shells. He is collaborating in many paleoenvironmental projects including studying fossil brachiopods collected from Devonian to Permian, foraminifera obtained from Quaternary marine cores, and Holocene molluscs collected from archaeological sites.

 Mirinae Lee. Department of Earth and Environmental Sciences, Andong National University, Andong 36729, Republic of Korea. amsassia@anu.ac.kr
Mirinae Lee. Department of Earth and Environmental Sciences, Andong National University, Andong 36729, Republic of Korea. amsassia@anu.ac.kr
Mirinae Lee is now a Ph.D. candidate of the Andong National University in Korea; she will graduate Ph. D. course in August, 2017. Her research interest is focused on paleoecology, paleobiogeography and paleobiology of Early Paleozoic corals and coral-like organisms, especially from China.
FIGURE 1. Thin section and cathodoluminescence (CL) photomicrographs: Timaniella harkeri (GSC26406) (1-4), Spiriferina sp.(BS-1) (5-8), Stenoscisma timorense (BS-2) (9-12), Cleiothyridina baracoodensis (ML32) (13-16) and Tylothyris transversa (TeP) (17-20). 1-2, transmitted light (TL) (1)and corresponding CL images (2) of nonluminescent shell and infilling sediment composed of fine sand grains. 3-4, TL (3) and CL (4) images of nonluminescent associated with partially luminescent shell in the cardinal area of dorsal valve. 5-8, TL (5,7) and CL (6, 8) images of nonluminescent associated with partially luminescent shell and luminescent infilling cement. 9-12, TL (9, 11) and CL (10, 12) images of luminescent shell with thin silicified layers and recrystallization along inner wall of brachiopod shell. 13, plane-polarized light (PPL) image of the original infilling sediment composed of calcareous sands. 14, PPL image showing the section of infilling cement. 15-16, TL (15) and CL (16) images of slightly luminescent shell. 17, PPL image of infilling comprising lime mud, calcite cement and relatively large, radially arranged, siliceous crystals. 18-19,, TL (18) and CL (19) images of nonluminescent associated with partially luminescent shell. 20, CL image showing the section of nonluminescent internal shell structure (spiralia) preserved within infilling (This structure is hardly recognized in the TL image). Abbreviations: bs, brachiopod shell; im, infilling material; sl, silicified layer; cc, calcite crystal; NL, nonluminescent; SL, slightly luminescent; L, luminescent.
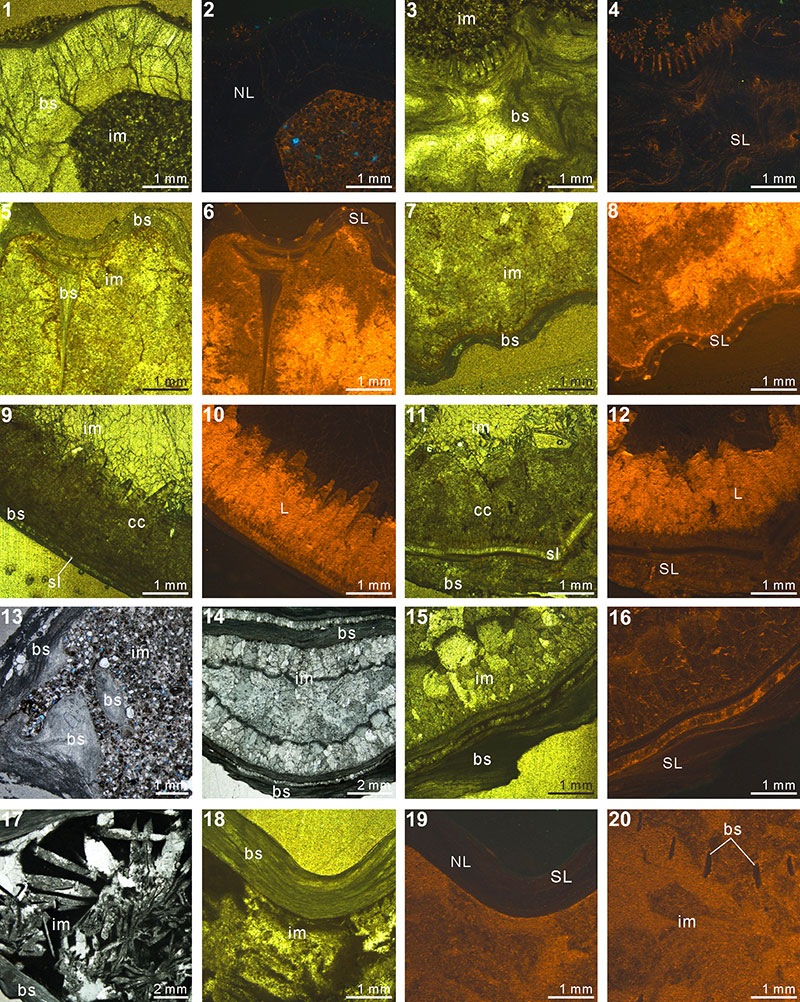
FIGURE 2. Thin section and cathodoluminescence (CL) photomicrographs: Indospirifer sp.(3229) (1-4), Cyrtospirifer whitneyi (CD) (5-8), Spiriferidae gen. sp. indet.(S1) (9-12), Spiriferella loveni (F8) (13-16), Meekella sangzhiensis (Q-1) (17-18) and Tyloplecta nanjingensis (Q-2) (19-20). 1, plane-polarized light (PPL) image of infilling micrite and shell fragments scattered. 2-3,transmitted light (TL) (2) and corresponding CL (3) images of luminescent shell. 4, CL image showing slightly luminescent shell. 5-8, TL (5, 7) and CL (6, 8) images of both luminescent shell and infilling sediment. 9-10, PPL images of brachiopod shell, infilling packstone and thin silicified layer along their boundary. 11-12, TL (11) and CL (12) images of nonluminescent shell. 13-16, TL (13, 15) and CL (14, 16) images showing a variety of shell luminescence and infilling sediment composed of calcitic skeletal grains (bryozoan in 15 and 16). 17-20, PPL (17, 19, 20) and TL (18) images of both silicified shells and infillings. Abbreviations: bs, brachiopod shell; im, infilling material; fs, fragmented shell; os, other shell material; sl, silicified layer; NL, nonluminescent; SL, slightly luminescent; L, luminescent.
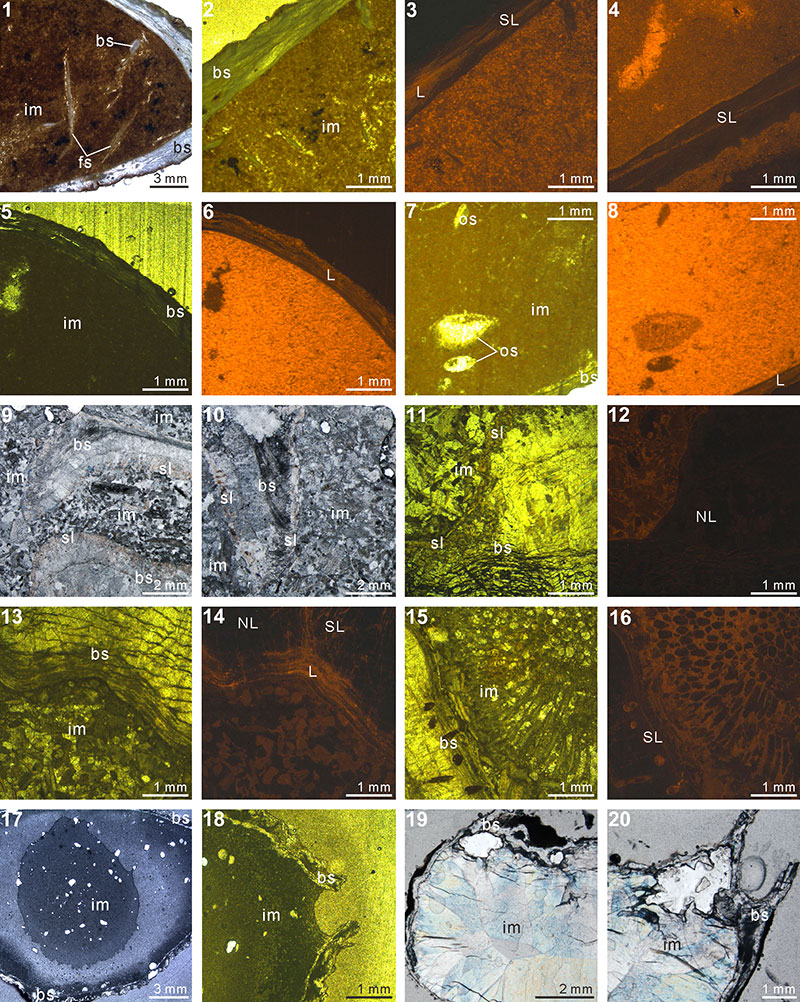
FIGURE 3. XMT result of Timaniella harkeri (GSC26406). 1-10, serial slices in the coronal plane (from posterior to anterior). 11-17, serial slices in the transverse plane (from ventral to dorsal). 18-22, serial slices in the sagittal plane (from lateral to middle). 23-25, lateral (23), ventral (24) and dorsal (25) views of the reconstructed 3-D model (external shell). 26, ventral view of the 3-D model in transparent mode. All the slice images were obtained under false-color lookup tables (Color 2 option in DataViewer). Abbreviations: tt, teeth; sp, spiralia; sk, socket.
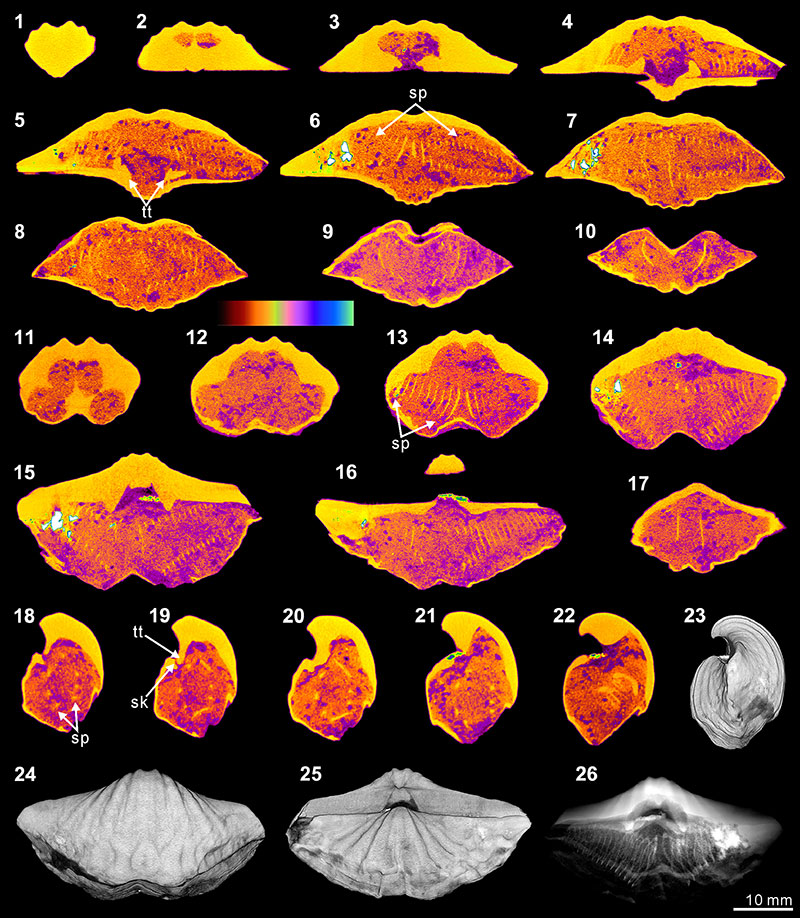
FIGURE 4. XMT result of Stenoscisma timorense (BS-2). 1-10, serial slices in the coronal plane (from posterior to anterior). 11-17, serial slices in the transverse plane (from ventral to dorsal). 18-22, serial slices in the sagittal plane (from lateral to middle). 23-25, lateral (23), ventral (24) and dorsal (25) views of the reconstructed 3-D model (external shell). 26, posterior view of the coronally sectioned 3-D model. 27, ventral view of the 3-D model in transparent mode. All the slice images were obtained under false-color lookup tables (Color 1 option in DataViewer). Abbreviations: sd, spondylium; tt, teeth.
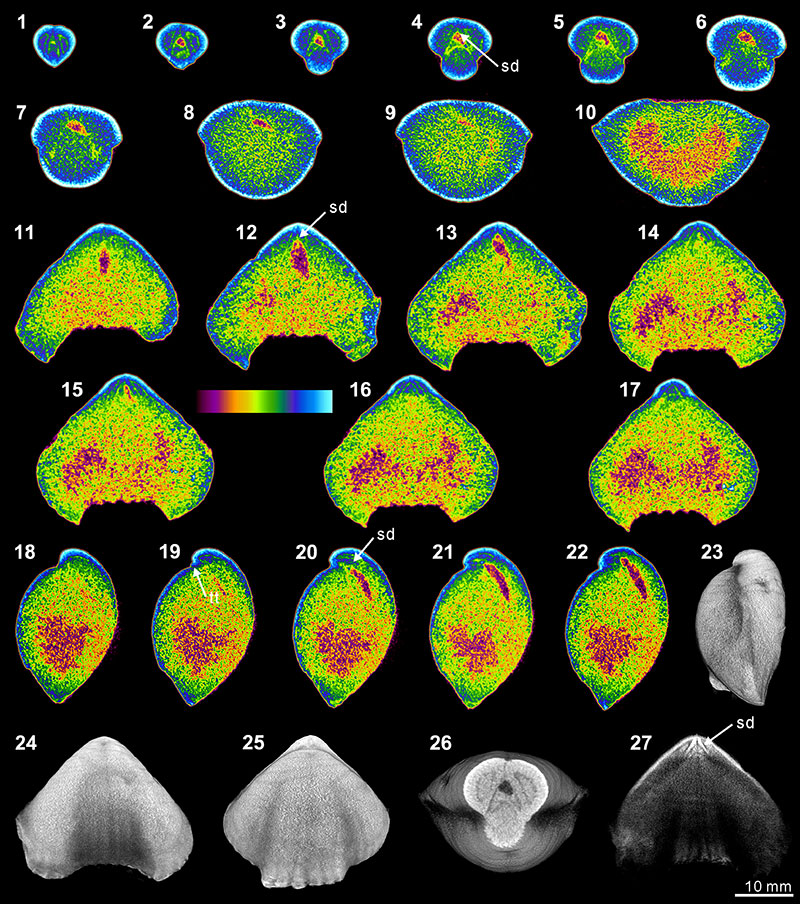
FIGURE 5. XMT result of Cleiothyridina baracoodensis (ML32). 1-11, serial slices in the coronal plane (from posterior to anterior). 12-21, serial slices in the transverse plane (from ventral to dorsal). 22-27, serial slices in the sagittal plane (from lateral to middle). 28-31, lateral (28), ventral (29), dorsal (30) and posterior (31) views of the reconstructed 3-D model (external shell). 32, ventral view of the 3-D model in transparent mode. All the slice images were obtained under false-color lookup tables (Color 2 option in DataViewer). Abbreviations: tt, teeth; sk, socket; cf, cardinal flanges.
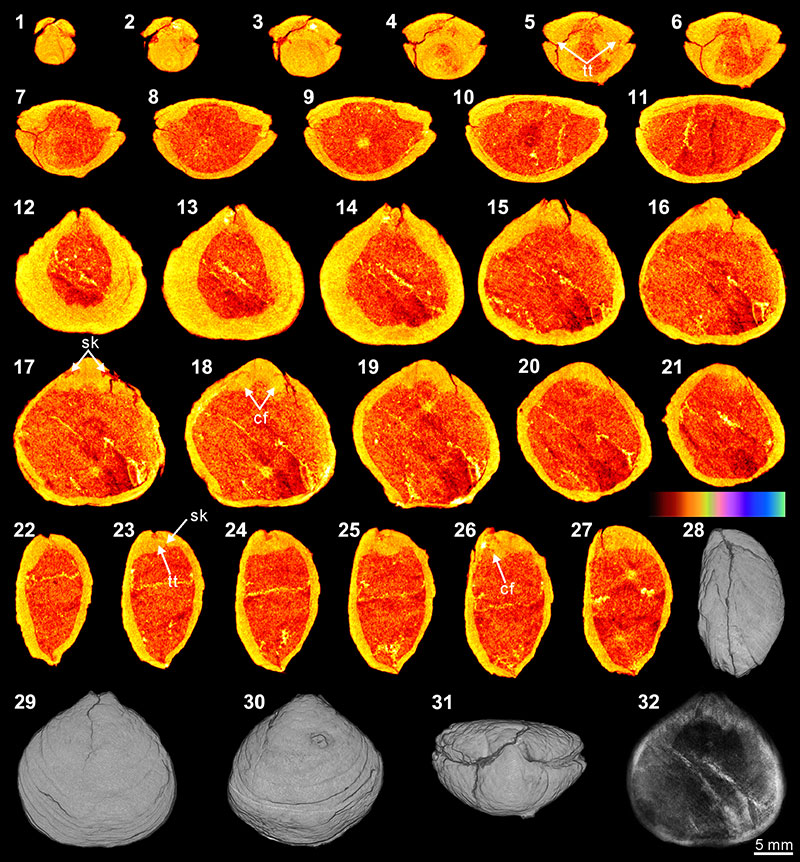
FIGURE 6. XMT result of Tylothyris transversa (TeP). 1-7, serial slices in the coronal plane (from posterior to anterior). 8-14, serial slices in the transverse plane (from ventral to dorsal). 15-19, serial slices in the sagittal plane (from lateral to middle). 20-22, lateral (20), ventral (21) and dorsal (22) views of the reconstructed 3-D model (external shell). 23, ventral view of the 3-D model in transparent mode. All the slice images were obtained under false-color lookup tables (Color 1 option in DataViewer). Abbreviation: sp, spiralia.
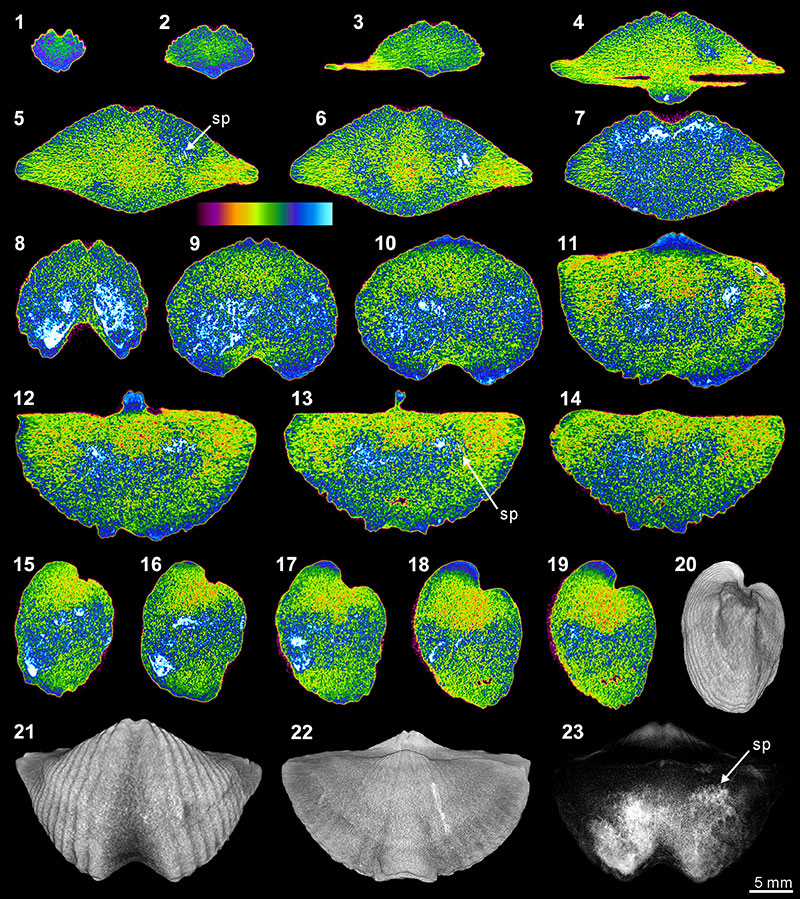
FIGURE 7. XMT result of Spiriferina sp. (BS-1). 1-7, serial slices in the coronal plane (from posterior to anterior). 8-14, serial slices in the transverse plane (from ventral to dorsal). 15-19, serial slices in the sagittal plane (from lateral to middle). 20-22, lateral (20), ventral (21) and dorsal (22) views of the reconstructed 3-D model (external shell). 23, ventral view of the 3-D model in transparent mode. All the slice images were obtained under false-color lookup tables (Color 1 option in DataViewer). Abbreviations: dp, dental plates; ms, median septum; sp, spiralia; tt, teeth; cr, crus; sk, socket.
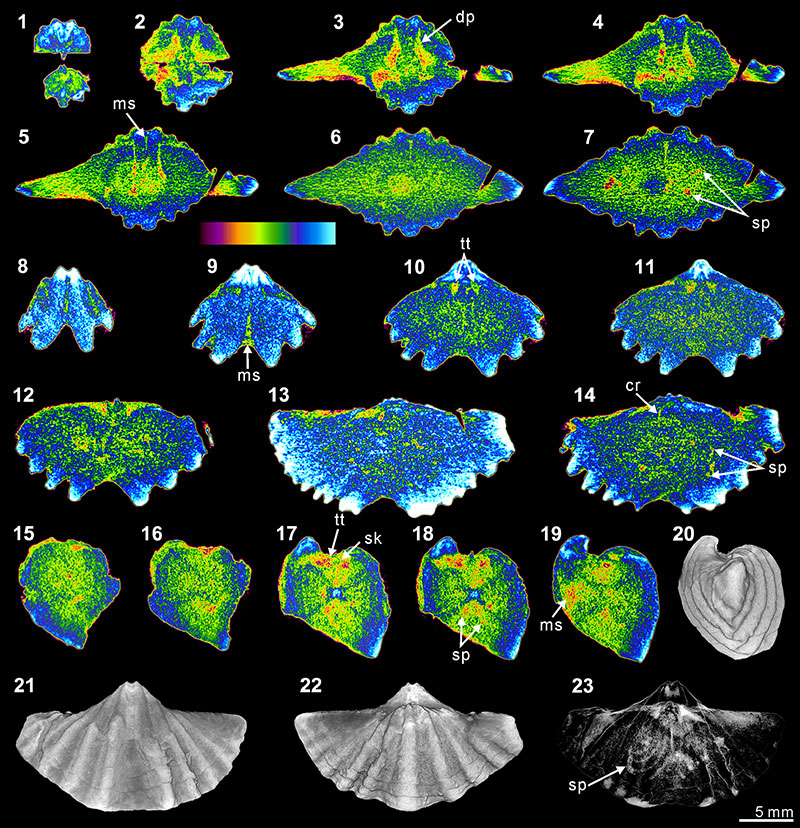
FIGURE 8. XMT result of Indospirifer sp. (3229). 1-8, serial slices in the coronal plane (from posterior to anterior). 9-15, serial slices in the transverse plane (from ventral to dorsal). 16-20, serial slices in the sagittal plane (from lateral to middle). 21-22, lateral (21) and ventral (22) views of the reconstructed 3-D model (external shell). 23-24, ventral (23) and ventroanterior (24) views of the 3-D model in transparent mode. All the slice images were obtained under false-color lookup tables (Color 1 option in DataViewer). Abbreviations: dp, dental plates; cp, cardinal process; hp, hinge plate; sp, spiralia.
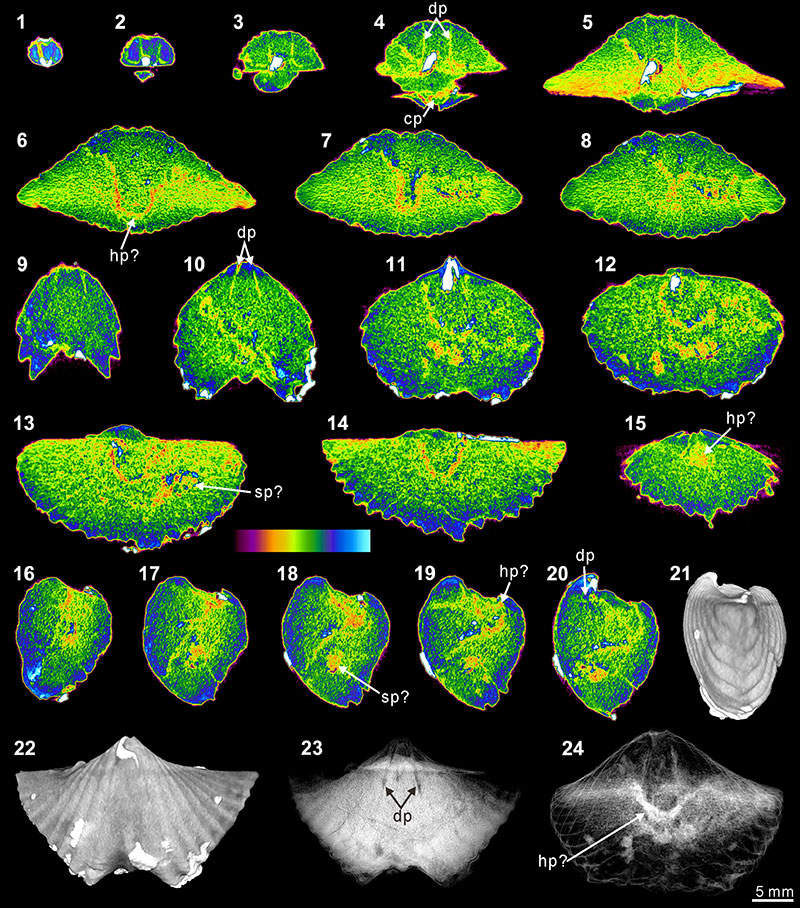
FIGURE 9. XMT result of Cyrtospirifer whitneyi (CD). 1-10, serial slices in the coronal plane (from posterior to anterior). 11-18, serial slices in the transverse plane (from ventral to dorsal). 19-24, serial slices in the sagittal plane (from lateral to middle). 25-28, lateral (25), ventral (26), dorsal (27) and posterior (28) views of the reconstructed 3-D model (external shell). 29, ventral view of the 3-D model in transparent mode. All the slice images were obtained under false-color lookup tables (Color 1 option in DataViewer). Abbreviations: dp, dental plates; tt, teeth.
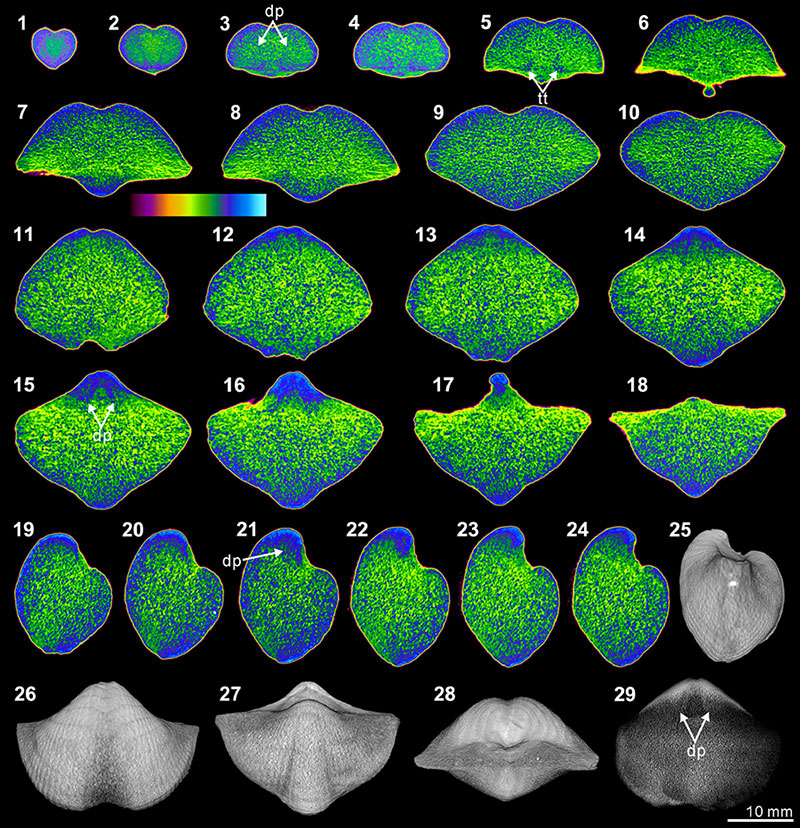
FIGURE 10. XMT result of Spiriferidae gen. sp. indet. (S1). 1-8, serial slices in the coronal plane (from posterior to anterior). 9-15, serial slices in the transverse plane (from ventral to dorsal). 16-21, serial slices in the sagittal plane (from lateral to middle). 22-24, lateral (22), ventral (23) and posterior (24) views of the reconstructed 3-D model (external shell). 25, ventral view of the 3-D model in transparent mode. All the slice images were obtained under false-color lookup tables (Color 1 option in DataViewer). Abbreviation: dp, dental plates.
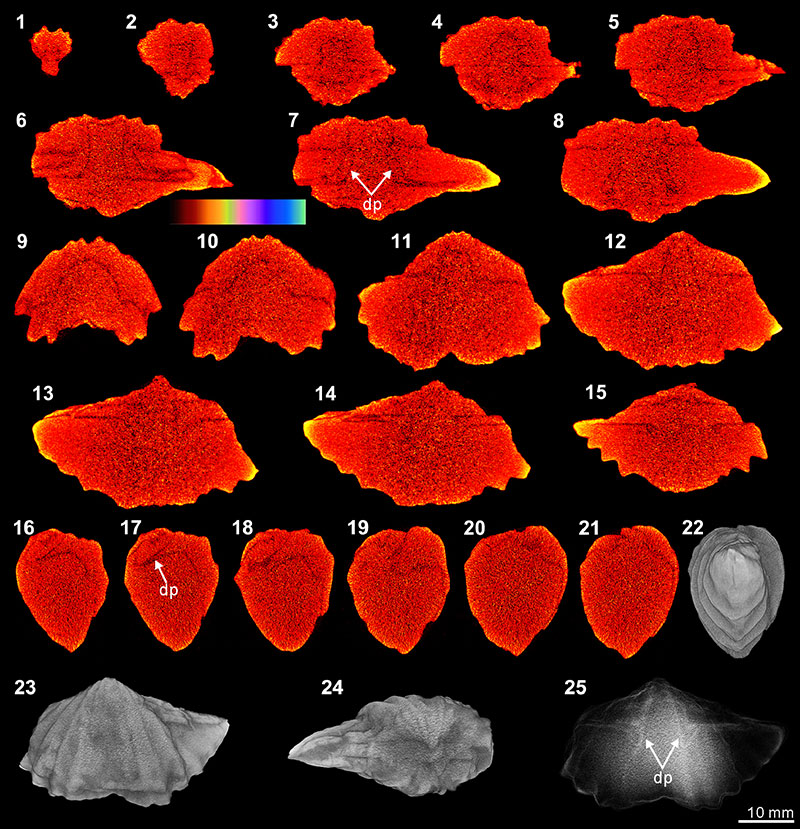
FIGURE 11. XMT result of Meekella sangzhiensis (Q-1). 1-7, serial slices in the coronal plane (from posterior to anterior). 8-15, serial slices in the transverse plane (from ventral to dorsal). 16-21, serial slices in the sagittal plane (from lateral to middle). 22-24, lateral (22), ventral (23) and dorsal (24) views of the reconstructed 3-D model (external shell). 25, ventral view of the 3-D model in transparent mode. All the slice images were obtained under false-color lookup tables (Color 1 option in DataViewer). Abbreviation: dp, dental plate.
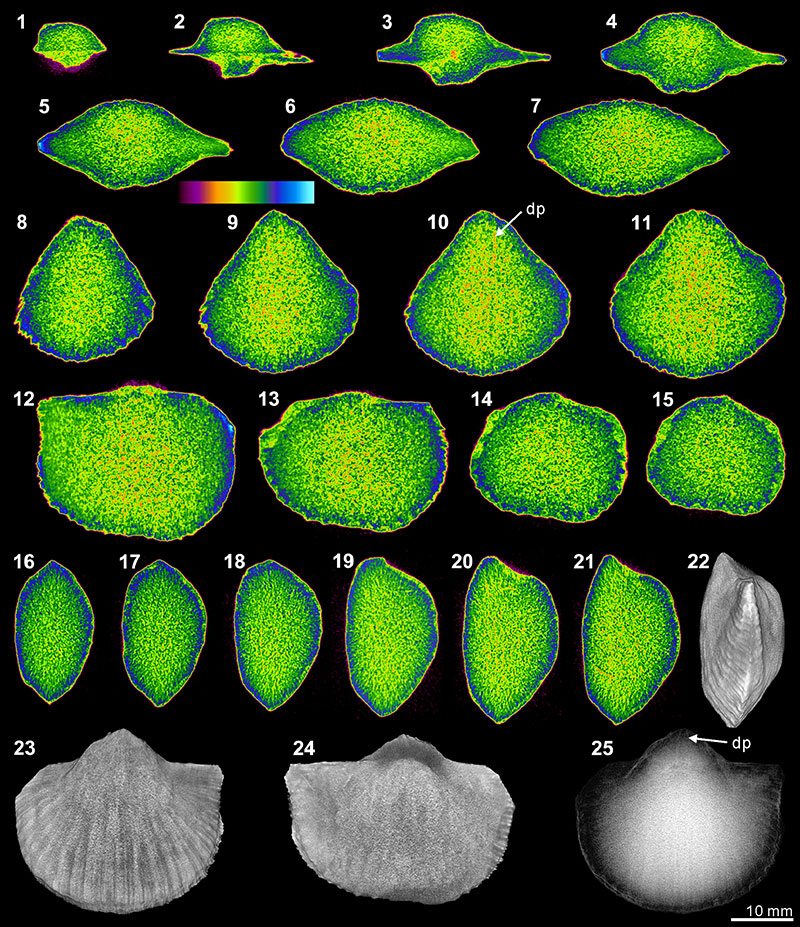
FIGURE 12. XMT result of Tyloplecta nankingensis (Q-2). 1-7, serial slices in the transverse plane (from dorsal to ventral). 8-14, serial slices in the coronal plane (from posterior to anterior). 15-18, serial slices in the sagittal plane (from lateral to middle). 19-21, lateral (19), ventral (20) and dorsal (21) views of the reconstructed 3-D model (external shell). 22, ventral view of the 3-D model in transparent mode. All the slice images were obtained under false-color lookup tables (Color 1 option in DataViewer). Abbreviations: cp, cardinal process; ap, adductor platform; ms, median septum; mc, muscle scar.
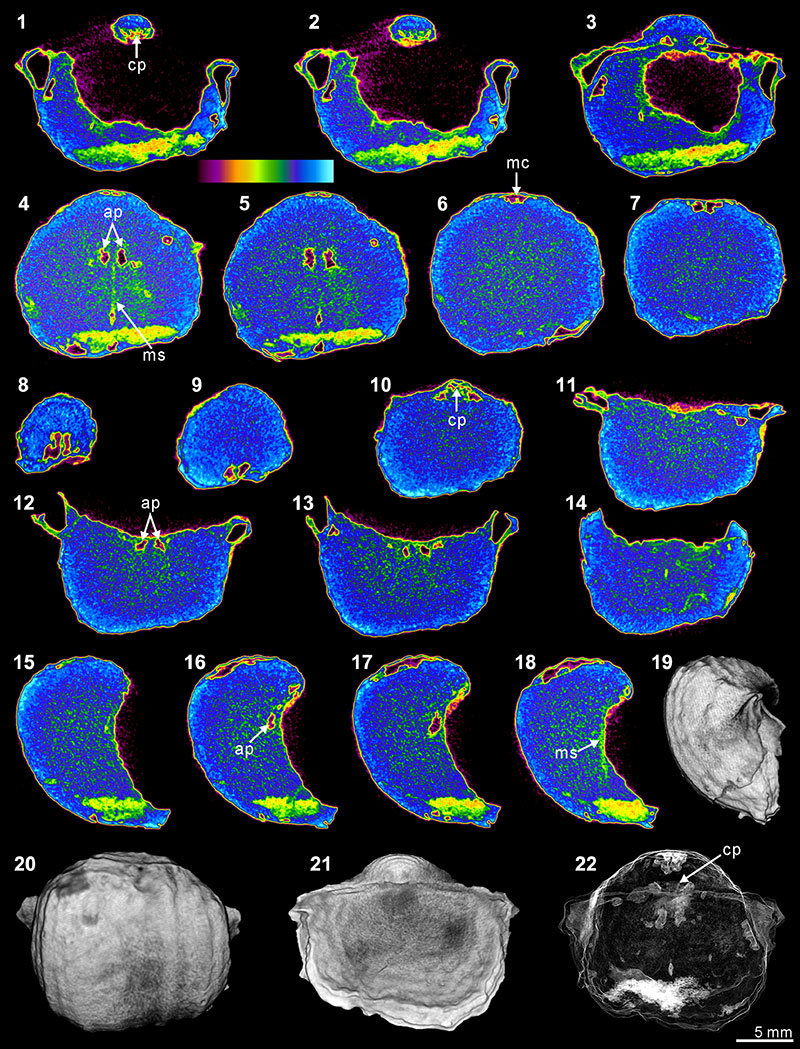
FIGURE 13. Three-dimensional reconstruction model of internal shell structures of Timaniella harkeri (GSC26406). 1-6, dorsal and anterior views of the whole shell interior through posteriorly continuous rotation. 7-12, dorsal and anterior views of shell interior without spiralia through posteriorly continuous rotation.
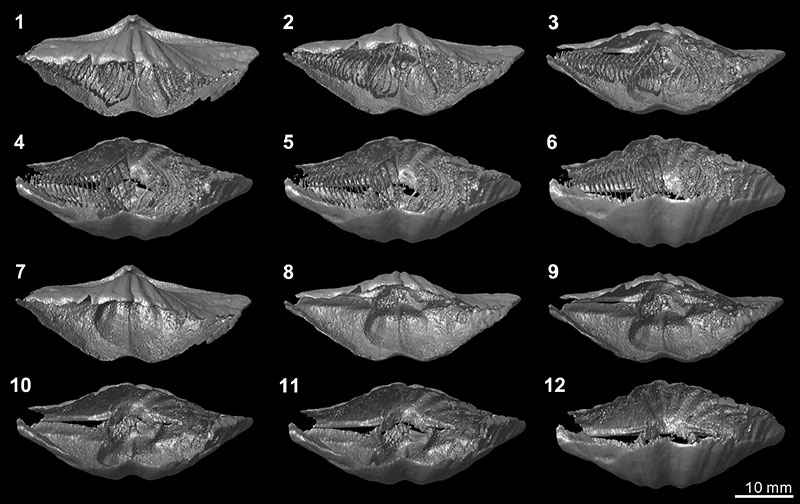
FIGURE 14. Three-dimensional rotational model (video) of Timaniella harkeri (GSC26406). Click on image to download or see video.
TABLE 1. Summary of brachiopod taxa, locality and stratigraphic details of selected specimens for the XMT experiments.
| Sample | Species | Locality | Formation | Age | References |
| GSC26406 | Timaniella harkeri Waterhouse in Bamber and Waterhouse, 1971 | Grinnell Peninsula, Devon Island, Canada | Assistance Fm. | Roadian | Waterhouse and Waddington (1982) |
| BS-1 | Spiriferina sp. | West Timor, Indonesia | Maubisse Fm. | Wuchiapingian | Charlton et al. (2002) |
| BS-2 | Stenoscisma timorense (Hayasaka and Gan, 1940) | West Timor, Indonesia | Maubisse Fm. | Wuchiapingian | Archbold and Bird (1989) |
| ML32 | Cleiothyridina baracoodensis (Etheridge, 1903) | Carnarvon Basin, WA, Australia | Callytharra Fm. | Artinskian | Etheridge (1903), Thomas (1958) |
| TeP | Tylothyris transversa Roberts, 1971 | Bonaparte Basin, WA, Australia | Enga Sandstone | Tournaisian | Roberts (1971) |
| 3229 | Indospirifer sp. | Unknown | Unknown | Devonian | No records so far |
| CD | Cyrtospirifer whitneyi (Hall, 1858) | Rockford, Iowa, USA | Lime Creek Fm. | Frasnian | Day (1988) |
| S1 | Spiriferidae gen. sp. indet. | Skansen, Spitsbergen, Norway | Wordiekammen Fm. | Pennsylvanian | Nakamura et al. (1992) |
| F8 | Spiriferella loveni (Diener, 1903) | Kapp Starostin, Spitsbergen, Norway | Kapp Starostin Fm. | Kungurian | Gobbett (1964), Nakamura et al. (1992) |
| Q-1 | Meekella sangzhiensis Liu and Zhao in Liu et al., 1982 |
Liuyang County, Hunan Province, China | Qixia Fm. | Kungurian | Liu et al. (1982) |
| Q-2 | Tyloplecta nankingensis (Frech, 1911) | Liuyang County, Hunan Province, China | Qixia Fm. | Kungurian | Liu et al. (1982) |
TABLE 2. Summary of XMT setting, nature of brachiopod shells and their infillings, CL results of brachiopod shells, and quality of XMT images.
| Sample | X-ray beam | Resolution (µm/pixel) |
Exposure (ms) | Rotation step (°) | No. of projections | Shell mineral composition |
Nature of material inside of valve |
Cathodoluminescence of brachiopod shell# |
Degree of visibility of shell internal structures in XMT images | |
| (keV) | (µA) | |||||||||
| GSC26406* | 89 | 112 | 31.82 | 316 | 0.70 | 271 | calcite | sandstone | NL, partially SL | All internal shell structures distinctly defined; Figures 3, 13, 14 |
| BS-1 | 85 | 118 | 26.98 | 316 | 0.70 | 275 | calcite | calcite cement | SL | Dental structures and median septum well recognized; Figure 7 |
| BS-2* | 78 | 125 | 34.58 | 474 | 0.90 | 209 | calcite | quartz cement | L | Dental structures (spondylium) recognized; Figure 4 |
| ML32 | 100 | 100 | 24.90 | 316 | 0.90 | 214 | calcite | mixed carbonate-silicate | SL | Shell articulation and cardinal structure recognized; Figure 5 |
| TeP* | 88 | 112 | 31.82 | 316 | 0.70 | 271 | calcite | mixed carbonate-silicate | NL, partially SL | Dental structures poorly detected, spiralia partly recognized; Figure 6 |
| 3229* | 78 | 125 | 34.58 | 474 | 0.70 | 269 | calcite | lime mudstone | SL | Dental structures well defined, but having possible mock structures; Figure 8 |
| CD* | 88 | 112 | 32.85 | 474 | 0.90 | 209 | calcite | wackestone | L | Dental structures weakly detected; Figure 9 |
| S1 | 100 | 100 | 34.58 | 474 | 0.90 | 209 | calcite | packstone | NL, partly SL | Dental structures not sharply bounded, but distinctly recognized; Figure 10 |
| F8* | 100 | 100 | 34.58 | 474 | 0.90 | 209 | calcite | grainstone | SL, partially L | No traces of internal structures |
| Q-1* | 80 | 124 | 34.58 | 474 | 0.90 | 209 | silica | microcrystalline quartz | Dental structures poorly and partly recognized; Figure 11 | |
| Q-2 | 80 | 124 | 34.58 | 474 | 0.70 | 269 | silica | quartz cement | Cardinal structures and muscle scars distinctly defined; Figure 12 | |
| *An additional specimen has been thin sectioned for CL analysis. #NL, SL and L indicate nonluminescent, slightly luminescent and luminescent, respectively. |
||||||||||
TABLE 3. Summary of EDS results, showing the elemental composition in brachiopod shell and infilling of each specimen.
| Composition of element (formula) (%) | |||||||||||
| Sample | EDS experiment area | C (CO2) | Ca (CaO) | Si (SiO2) | Mg (MgO) | Al (Al2 O3) | S (SO3) | K (K2O) | Mn (MnO) | Fe (FeO) | others |
| GSC26406* | Brachiopod shell | 59.02 | 40.98 | ||||||||
| Major grain in infilling sediment | 39.90 | 60.10 | |||||||||
| Minor grain in infilling sediment | 42.75 | 1.66 | 2.37 | 53.22 | |||||||
| BS-1 | Brachiopod shell | 58.14 | 41.86 | ||||||||
| Infilling cement | 55.34 | 44.66 | |||||||||
| BS-2* | Brachiopod shell | 53.25 | 43.94 | 1.53 | 0.22 | 1.06 | |||||
| Infilling cement | 33.06 | 66.94 | |||||||||
| ML32 | Brachiopod shell | 54.50 | 45.50 | ||||||||
| Major grain in infilling sediment | 27.27 | 72.73 | |||||||||
| Minor grain in infilling sediment | 37.85 | 8.89 | 2.45 | 5.63 | 45.19 | ||||||
| Infilling micrite | 43.23 | 55.51 | 1.25 | ||||||||
| TeP* | Brachiopod shell | 57.14 | 41.64 | 0.89 | |||||||
| Infilling material (by area) | 49.07 | 31.67 | 13.27 | 0.55 | 2.75 | 0.78 | 0.21 | 1.70 | |||
| 3229* | Brachiopod shell | 61.42 | 37.13 | 0.38 | 0.40 | 0.66 | |||||
| Infilling sediment | 54.35 | 38.30 | 3.72 | 0.45 | 1.09 | 0.32 | 0.91 | 0.86 | |||
| CD* | Brachiopod shell | 51.48 | 46.68 | 0.62 | 1.21 | ||||||
| Infilling sediment | 44.15 | 42.93 | 7.29 | 0.99 | 1.87 | 0.40 | 0.62 | 0.82 | 0.93 | ||
| S1 | Brachiopod shell | 51.44 | 48.56 | ||||||||
| Major grain in infilling sediment | 59.39 | 40.61 | |||||||||
| Thin layer between shell and infilling | 47.06 | 2.86 | 50.08 | ||||||||
| F8* | Brachiopod shell | 55.30 | 38.80 | 0.45 | 0.57 | 0.87 | |||||
| Infilling sediment (by area) | 59.72 | 23.82 | 10.95 | 3.66 | 0.72 | 1.13 | |||||
| Q-1* | Brachiopod shell | 35.17 | 63.61 | 0.25 | 0.97 | ||||||
| Infilling cement | 35.94 | 63.68 | 0.39 | ||||||||
| Q-2 | Major part of brachiopod shell | 34.76 | 65.24 | ||||||||
| Minor part of brachiopod shell | 52.01 | 29.37 | 18.62 | ||||||||
| Infilling cement | 33.96 | 66.04 | |||||||||
| *An additional specimen has been thin sectioned for EDS analysis. | |||||||||||
Virtual palaeontology: the effects of mineral composition
and texture of fossil shell and hosting rock on the quality
of X-ray microtomography (XMT) outcomes
using Palaeozoic brachiopods
Plain Language Abstract
Palaeontologists can create virtual models of fossils from cross sectional images taken by high-resolution scanning (X-ray microtomography). This process is being used to produce three-dimensional (3-D) reconstructions of the internal external details of fossils still enclosed in a host rock. Rocks and fossils can be made of different materials, and we set out to discover what effect this had on the quality of the 3-D models being produced.
We tested this by scanning 11 brachiopods infilled with sediment. Our results showed that there was very good contrast in the cross sections of original shell material infilled with sandy quartz grains, and they produced the most detailed 3-D models of the internal features of a brachiopod shell. However, the more shells changed in texture and composition after burial, particularly if they had become silica replaced or recrystallized, the increasingly less contrast there was with the host rock, and consequently the detail in the 3-D models was affected. Another observation was that it was more difficult to reconstruct the detailed internal features of a brachiopod shell when the sediment infilling the shell also contained fragments of other fossil materials.
In summary, our study shows that if there is not sufficient difference in mineral composition and texture between the host rock and fossil, then correspondingly there is little contrast in the cross sectional images. This ultimately impacts on the detail and quality of the 3-D model being produced.
Abstract in Korean
현대 과학기술의 발전과 함께, 미세 X선 단층촬영 기법은 다양한 화석 연구에 활발히 이용되고 있다. 하지만, 이 미세 X선 단층촬영을 통해 얻는 영상의 해상도는 화석과 그를 둘러싼 암석 사이의 밀도 차이에 비례하기 때문에, 항상 만족할만한 결과를 낳지는 못한다. 이 연구에서는 화석과 그 주변 물질의 광물 조성과 구조 차이가 미세 X선 단층촬영 영상 결과의 해상도에 어떤 영향을 미치는지를 알아보기 위해 다양한 퇴적암내에서 보존된 11개의 고생대 완족동물 시료들을 분석하였다. 그 결과, 고화질의 내부구조 복원을 위해선 화석 껍질과 그 주변 물질을 이루는 광물간의 조성과 구조에 충분한 차이가 필요하다는 점을 확인하였고, 탄산칼슘으로 이루어진 화석과 석영 입자들로 이루어진 주변 퇴적물의 조합이 최상의 화석 내부구조 복원을 가능하게 한다는 것을 알아내었다. 또한, 규화나 재결정과 같은 속성작용도 영상 결과물의 해상도에 영향을 미친다는 것과, 퇴적물내에 다른 생물의 파편이 포함되면서 내부구조 복원시 왜곡이 발생할 수도 있다는 것을 발견하였다.
Resumen en Español
Paleontología virtual: los efectos de la composición mineral y la textura de la concha fósil y la roca matriz en la calidad de los resultados de la microtomografía de rayos X (XMT) utilizando braquiópodos paleozoicos
La microtomografía de rayos X (XMT) se ha convertido en una herramienta común para investigaciones detalladas de una amplia gama de fósiles. Sin embargo, la XMT no siempre ha garantizado un resultado satisfactorio, ya que la resolución de las imágenes XMT depende críticamente del contraste entre el fósil y la roca matriz. En este trabajo se aplicó la XMT a 11 ejemplares de braquiópodos paleozoicos seleccionados de una serie de diferentes rocas sedimentarias con el fin de investigar el alcance en la calidad de los resultados con los efectos de la composición mineral y la textura en la roca y en la concha fósil. Nuestro estudio muestra que un contraste suficiente en la composición mineral y la textura entre la concha del braquiópodo y su material de relleno es necesario para obtener resultados XMT de alta calidad. Específicamente, los especímenes de braquiópodos con carbonato de calcio original, rellenos principalmente con granos de cuarzo, parecen producir los mejores resultados XMT caracterizados por estructuras internas de la concha claramente definidas. También descubrimos que la diagénesis es significativa para obtener resultados de calidad. Los procesos diagenéticos que incluyen silicificación y recristalización en la concha de los braquiópodos y/o en el material de relleno generalmente tienden a disminuir la resolución de los resultados con XMT, aunque este impacto es considerablemente complicado por el grado y aspecto de la diagénesis. Otro factor de menor importancia se refiere a la presencia de bioclastos dispersos en la roca matriz sedimentaria que potencialmente podrían confundirse con estructuras internas de concha genuina.
Palabras clave: tomografía computarizada; estructuras internas; relleno sedimentario; diagénesis; reconstrucción tridimensional
Traducción: Enrique Peñalver (Sociedad Española de Paleontología)
Résumé en Français
Paléontologie virtuelle : les effets de la composition et de la texture minérales des coquilles fossiles et des roches encaissantes sur la qualité des reconstructions par microtomodensitométrie par rayons X (XMT) en utilisant les brachiopodes paléozoïques
La microtomodensitométrie par rayons X (XMT) est devenue un outil populaire pour des études détaillées d’une grande variété de fossiles. La XMT n’a cependant pas toujours garanti un résultat satisfaisant, car la résolution des images générées par XMT dépend fortement du contraste entre le fossile et la roche encaissante. Dans ce papier, la XMT a été appliquée à 11 spécimens de brachiopodes paléozoïques sélectionnés dans une gamme variée de roches sédimentaires afin d’explorer l’étendue des effets de la composition et de la texture minérales de la roche et de la coquille fossile sur la qualité des reconstructions par XMT. Notre étude montre qu’un contraste suffisant de composition et de texture minérales entre la coquille du brachiopode et le matériel de remplissage est nécessaire pour produire des résultats de XMT de haute qualité. Plus précisément, il s’avère que les spécimens de brachiopodes avec leur coquille originelle en carbonate de calcium, principalement remplie de grains de quartz, produisent les meilleurs résultats de XMT caractérisés par des structures internes des coquilles définies très finement. Nous avons aussi montré que la diagénèse est importante pour déterminer la qualité de la XMT. Les processus diagénétiques, notamment la silicification et la recristallisation de la coquille du brachiopode et/ou du matériel de remplissage, ont tendance à diminuer la résolution des résultats de la XMT, bien que cet impact soit considérablement compliqué par le degré et l’aspect de la diagénèse. Un autre facteur moins important concerne la présence de bioclastes dispersés dans le sédiment encaissant qui pourraient potentiellement être confondus avec des structures internes de la coquille.
Mots-clés : tomodensitométrie ; structures internes ; remplissage sédimentaire ; diagénèse ; reconstruction tridimensionnelle
Translator: Antoine Souron
Deutsche Zusammenfassung
Virtuelle Paläontologie: Die Effekte von Mineralzusammensetzung und Struktur fossiler Schalen und Matrix auf die Qualität von Röntgen-Mikrotomographie-Ergebnissen mit paläozoischen Brachiopoden
Röntgen-Mikrotomographie ist zu einem beliebten Tool für detaillierte Untersuchungen einer großen Bandbreite an Fossilien geworden. Ein zufriedenstellendes Ergebnis ist jedoch nicht immer garantiert, da die Auflösung der Röntgen-Mikrotomographie-Abbildung entscheidend vom Kontrast zwischen Fossil und Matrix abhängt. In dieser Arbeit wurde Röntgen-Mikrotomographie bei 11 paläozoischen Brachiopoden aus verschiedenen Sedimentgesteinen angewandt, um die Auswirkungen von Mineralzusammensetzung und Struktur des Gesteins und der fossilen Schale auf die Qualität der Röntgen-Mikrotomographie-Ergebnisse zu untersuchen. Unsere Untersuchung zeigt, dass ein ausreichender Kontrast in Mineralzusammensetzung und Struktur zwischen Brachiopodenschale und Matrix nötig ist, um hochauflösende Röntgen-Mikrotomographie-Ergebnisse zu erzielen. Insbesondere Brachiopoden mit ihrem ursprünglichen Kalziumkarbonat, verfüllt hauptsächlich mit Quarzkörnchen, scheinen die besten Röntgen-Mikrotomographie-Ergebnissen zu produzieren, die durch klar definierte interne Schalenstrukturen charakterisiert sind. Wir haben außerdem herausgefunden, dass auch die Diagenese wichtig zur Bestimmung der Röntgen-Mikrotomographie Qualität ist. Diagenetische Prozesse wie Silifizierung und Rekristallisation der Schale und/oder des Füllmaterials tendieren dazu die Auflösung der Röntgen-Mikrotomographie-Ergebnisse zu verringern, auch wenn dieser Effekt deutlich durch Grad und Dimension der Diagenese verkompliziert wird. Ein anderer Faktor mit geringerer Bedeutung sind Bioklasten im umgebenden Sediment, die potentiell mit den originalen internen Schalenstrukturen verwechselt werden können.
Schlüsselwörter: Computertomographie; interne Strukturen; Sedimentfüllung; Diagenese; dreidimensionale Rekonstruktion
Translator: Eva Gebauer
Arabic
in progress
Translator: Ashraf M.T. Elewa
-
-
-
Review: The Princeton Field Guide to Mesozoic Sea Reptiles
 The Princeton Field Guide to Mesozoic Sea Reptiles
The Princeton Field Guide to Mesozoic Sea ReptilesArticle number: 26.1.1R
April 2023


 Poster Winners 2024
Poster Winners 2024