 Soft-tissue anatomy of the Plesiosaur pectoral girdle inferred from basal Eosauropterygia taxa and the extant phylogenetic bracket
Soft-tissue anatomy of the Plesiosaur pectoral girdle inferred from basal Eosauropterygia taxa and the extant phylogenetic bracket
Article number: 18.1.8A
https://doi.org/10.26879/446
Copyright Society for Vertebrate Paleontology, March 2015
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 29 November 2013. Acceptance: 30 January 2015
{flike id=1062}
ABSTRACT
Plesiosaurians are highly derived secondarily-adapted organisms (if fishes are primarily-adapted) with a long evolutionary history, and they are closely related with basal eosauropterygians. Attempts to reconstruct soft-tissue anatomy can be complicated due to the lack of extant closely-related species, thus various lines of evidence must be considered. This study aims to reconstruct the pectoral girdle myology of eosauropterygians. Information derived from the extant phylogenetic bracket method was not sufficient to clarify muscle attachments in the pectoral girdle of plesiosaurians. To correctly infer muscle homologies, the extant phylogenetic bracket information had to be complemented with developmental and osteological information, and osteological transformations had to be traced back to Permian basal neodiapsids. The reconstructed pectoral girdle musculature presented here is, thus, significantly different from previous attempts. As in secondarily-adapted aquatic modern analogues, several muscles atrophied (e.g., pectoralis, episternocleidomastoideus) and others specialized (e.g., coracobrachialis, clavodeltoideus) in order to attain a more influential role to the stringent conditions of subaquatic locomotion. The subcoracoscapularis, scapulodeltoideus, scapulohumeralis and supracoracoideus are inferred to be glenohumeral stabilizers. The clavodeltoideus acted as the main protractor muscle and the coracobrachialis as a major retractor muscle, possibly in conjunction with the latissimus dorsi. Several heads of the triceps possibly atrophied, as in whales, serving mainly as a cubital joint stabilizer. The trapezius, serratus and levator scapulae served as pectoral girdle stabilizers.
Ricardo Araújo. Huffington Department of Earth Sciences, Southern Methodist University, Dallas, Texas 75275, USA; Museu da Lourinhã, Rua João de Luís de Moura, 95, 2530-158 Lourinhã, Portugal; Instituto de Plasmas e Fusão Nuclear, Instituto Superior Técnico, Universidade de Lisboa, Portugal and Museum für Naturkunde, Berlin, Germany. rmaraujo@smu.edu
Fernando Correia. Departamento de Biologia, Universidade de Aveiro, Campus Santiago, 3810-193 Aveiro, Portugal. fjorgescorreia@sapo.pt
KEY WORDS: myology; Eosauropterygia; basal neodiapsids; Extant phylogenetic bracket; pectoral girdle; plesiosaurians
Final citation: Araújo, Ricardo and Correia, Fernando. 2015. Soft-tissue anatomy of the Plesiosaur pectoral girdle inferred from basal Eosauropterygia taxa and the extant phylogenetic bracket. Palaeontologia Electronica 18.1.8A: 1-32. https://doi.org/10.26879/446
palaeo-electronica.org/content/2015/1062-plesiosaur-pectoral-myology
[Plésiosaures sont] les plus hétéroclites des habitants de l'ancien monde, ceux de tous qui paraissent le mieux mériter le nom de monstre.
-- G. Cuvier (in Piveteau 1955)
INTRODUCTION
The pectoral girdle of plesiosaurians is radically different from basal neodiapsids (e.g., Claudiosaurus, Acerosodontosaurus and Thadeosaurus). Eosauropterygians present secondary adaptations to a marine lifestyle unparalleled in other marine groups. For instance, the subaqueous locomotory mode of plesiosaurians possessing four long, sub-equal paddles remains a functional riddle (e.g., Connybeare, 1824; Watson, 1924; Robinson, 1975, 1977; Carpenter et al., 2010).
Although speciose, Eosauropterygia lack a fossil record that demonstrates the evolution of their derived pectoral girdle from the primitive basal neodiapsid condition. There are two important morphological gaps, one between the primitive condition in basal neodiapsids and pachypleurosaurs, and a second between nothosaurs-pistosaurs and plesiosaurians. The retention of the plesiomorphic condition in the pectoral girdle of basal neodiapsids, exemplified by the presence of an ossified sternum and retention of the infra- and supraglenoid buttresses, makes them fundamental to understanding the topological transformations that led to the unique configuration of the eosauropterygian pectoral girdle. Some previously considered Younginiformes and Claudiosaurus have been proposed as sister-taxa to Sauropterygia (Carroll, 1981; Storrs, 1991), although more recent studies have placed them at the base of Neodiapsida (Rieppel, 1993; Caldwell, 1996; Merck, 1997; Müller, 2003; Bickelmann, et al. 2009).
This paper will: (1) document areas of soft-tissue attachment utilizing an appropriate extant phylogenetic bracket; and (2) present transformational hypotheses from extinct taxa being constrained by developmental and myological observations on extant taxa. The soft-tissue anatomical hypotheses presented here are based on the fossil record and extant taxa that can be tested via histological studies of the muscle scars left on the periosteum (e.g., Benjamin et al., 2008; Klein, 2010).
The extant phylogenetic bracket is used here to reconstruct soft-tissue parts. Considering that plesiosaurians nest in Lepidosauromorpha, the upper bracket that serve for comparisons are Iguana (Russell and Bauer, 2008) and the gekkotan Eublepharis macularius (Zaaf et al., 1999). The lower bracket is the crocodilians (Cong et al., 1998; Meers, 2003). Carpenter et al. (2010) attempted a rigorous analysis using two end-members of extant taxa for comparison (turtles and squamates). However, the choice of turtles for a phylogenetic bracket seems imprudent for two reasons: (1) the phylogenetic relationships of turtles are greatly controversial (see Rieppel, 2008 for a summary of the debate), and (2) turtles have a highly modified body plan readily distinct from Eosauropterygia (Rieppel, 2001, 2008). Nevertheless, we still use turtles as an extra phylogenetic bracket, giving further confidence to our reconstructions and emphasizing the conservative patterns of muscle attachment even in highly derived reptilian clades. We also note that turtles have not yet been refuted as a related taxon to Sauropterygia (e.g., Rieppel, 2008).
ACRONYMS
MNHN - Muséum National d'Histoire Naturelle, Paris; SMNS - Staatliches Museum für Naturkunde Stuttgart; PIMUZ - Paläontologisches Institut & Museum der Universität Zürich; GPIT - Geologisch-Paläontologischen Institut und Museum Tübingen.
MATERIAL AND METHODS
Direct observation of the muscle insertions and origins of the pectoral girdle musculature was done using five dissected squamate Eublepharis macularius specimens. The myology of the squamate Iguana and crocodilians are derived from the literature (Cong et al., 1998; Meers, 2003; Russell and Bauer, 2008). All the specimens used for this study were photographed using a Canon® EOS 1000D and Canon® EF 18-55 mm lens. Detailed assessment of the osteological correlates required a macro lens (Tamron® SP f/2.8 90 mm Di). The fossil specimens used for this study are listed below.
Basal Neodiapsida
Hovasaurus sp. (MNHN 1908-11-15a, MNHN 1908-32-23, MNHN 1925-5-30a, MNHN 1908-32-22a)
Claudiosaurus germaini (MNHN 1908-11-5a, MNHN 1908-11-20a, MNHN 1978-11-8a)
Tangasaurus ? (MNHN 1908-11-16a)
Keichousaurus hui (SMNS 81780, SMNS 82044), Keichousaurus sp. (SMNS 59705)
Sauropterygia: Placodontia
Placodontia gen. et sp. indet. (SMNS 59824)
Placodus sp. (SMNS 54558, 16254a)
Simosaurus sp. (SMNS 16736)
Simosaurus gaillardoti (SMNS 820221, SMNS7862, SMNS 18373)
Cymatosaurus (GPIT-RE-1337)
Paraplacodus broilli (PIMUZ T4775)
Paraplacodus (PIMUZ T4827)
Eosauropterygia: Pachypleurosauria
Neusticosaurus edwardsii (PIMUZ T3438, PIMUZ T3460, PIMUZ T3749, PIMUZ T3758, PIMUZ T3775, PIMUZ T3935, PIMUZ T3769)
Neusticosaurus peyeri (PIMUZ T3403, PIMUZ T3445, PIMUZ T3615, PIMUZ T3932, PIMUZ T3705, PIMUZ T3789)
Neusticosaurus pusillus (PIMUZ T3672, PIMUZ T3442, PIMUZ T3750, PIMUZ T3934, PIMUZ T4289)
Pachypleurosaurus (PIMUZ T3399)
Serpianosaurus mergiolensis (PIMUZ T1071)
Serpianosaurus sp. (PIMUZ T3675)
Eosauropterygia: Nothosauria
Nothosauridae gen. et sp. indet. (MNHN AC9807, MNHN AC9250, MNHN AC9454, MNHN AC9247, MNHN 9244, MNHN 9454, SMNS 84508, SMNS 64509, SMNS 84526, SMNS 84073, SMNS 84518, SMNS84517, GPIT-RE-1533, GPIT-RE-01872, GPIT-RE-01673, GPIT-RE-01539)
Nothosaurus giganteus (SMNS 17215)
Nothosaurus sp. (SMNS17326, SMNS 59822, SMNS 420RF, SMNS 84067, SMNS 84069, SMNS 18689, SMNS 16256, GPIT-RE-01571, GPIT-RE-01863, GPIT-RE-01532, GPIT-RE-01127, GPIT-RE-01315)
Nothosaurus miriabilis (SMNS 13232)
Nothosaurus (Paranothosaurus) amsleri (PIMUZ T4829)
Ceresiosaurus calcagnii (PIMUZ T2461, PIMUZ T4835, PIMUZ T2464, PIMUZ T3983)
Eosauropterygia: Plesiosauria
Cryptoclidus oxonensis (MNHN 1909-10)
Cyamodus (SMNS 54556, SMNS 54562)
Pistosaurus (SMNS 81918)
Rhomaleosaurus (SMNS 53044)
Liopleurodonferox (GPIT uncatalogued)
Peloneustes philarcus (GPIT 1754-3)
Meyerasaurus (SMNS 12478)
HOMOLOGY STATEMENTS
Transformational Hypothesis of the Pectoral Girdle in Eosauropterygia
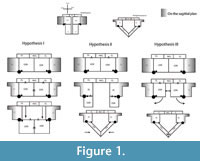 The pectoral girdle of eosauropterygians, even in basal forms, has unique adaptations. The development of a strong clavicular arch and ventral expansion of the coracoid as well as the most ventral portions of the scapula are characteristics unparalleled in other groups. The lack of fossils that document the transition between basal neodiapsid pectoral girdles and those of basal eosauropterygians hinders accurate reconstruction of limb myology. The morphological gap at the base of the clade represents a problem for tracking the evolution of the musculature across Eosauropterygia. In order to go beyond the lack of fossils, though, transformational hypotheses between basal neodiapsids and basal eosauropterygians are proposed below (Figure 1). The three alternatives are based on: (1) simple geometrical operations (e.g., rotation, translation) of the bones while maintaining the primitive topology as much as possible, (2) the maintenance of the relative orientation of the bone borders, and (3) the contacts between bones and the orientation of the glenoid. The location of the glenoid implies that the coracoid foramen is stable in position during the transformation because the supracoracoid nerve that passes through it supplying the supracoracoideus muscle.
The pectoral girdle of eosauropterygians, even in basal forms, has unique adaptations. The development of a strong clavicular arch and ventral expansion of the coracoid as well as the most ventral portions of the scapula are characteristics unparalleled in other groups. The lack of fossils that document the transition between basal neodiapsid pectoral girdles and those of basal eosauropterygians hinders accurate reconstruction of limb myology. The morphological gap at the base of the clade represents a problem for tracking the evolution of the musculature across Eosauropterygia. In order to go beyond the lack of fossils, though, transformational hypotheses between basal neodiapsids and basal eosauropterygians are proposed below (Figure 1). The three alternatives are based on: (1) simple geometrical operations (e.g., rotation, translation) of the bones while maintaining the primitive topology as much as possible, (2) the maintenance of the relative orientation of the bone borders, and (3) the contacts between bones and the orientation of the glenoid. The location of the glenoid implies that the coracoid foramen is stable in position during the transformation because the supracoracoid nerve that passes through it supplying the supracoracoideus muscle.
Hypothesis I. Formation of the pectoral vacuity by posterior displacement of the coracoid, without loss of the median contact between the coracoids and maintaining the orientation of its anterior border. This hypothesis implies a very loose connection with the scapula.
Hypothesis II.Initial detachment of the coracoid from the scapula, leaving the scapula on the sagittal plane and the coracoid on the horizontal plane. This implies the loss of the median contact between the coracoids on a first phase and further posterior displacement of these bones. There is later restitution of the median contact between the coracoids.
Hypothesis III. Rotation of the coracoid. In the final transformed model what was the anterior border of the coracoid becomes the medial border.
In all hypotheses it is observed that: (1) the coracoid loses contact with the clavicle, (2) the posterior process of the interclavicle is significantly reduced, (3) complete ventralization of the coracoid and clavicles, (4) partial ventralization of the scapula and formation of the dorsal process of the scapula and (5) loss or chondrification of the sternum.
In all hypotheses the reorientation of the coracoid is the critical issue because it is what allows scapular reconfiguration. The hypothesis is seemingly best backed up by the fossil record (i.e., placodonts) is Hypothesis II. However, it requires acceptance of the pectoral girdle reconstructions by Drevermann (1933), Pinna (1980) and supported by Rieppel (2000) for placodonts, in which the coracoids are widely separated medially. Yet, contrasting with these reconstructions, medially-contacting coracoids seem to be present in Psephochelyspolyosteoderma (Li and Rieppel, 2002). Comparisons with placodonts are difficult because they evolved their own locomotion patterns that depart from those seen in Eosauropterygia (Mazin and Pinna, 1993). Therefore, until new discoveries are made, the other hypotheses appear to be more compelling. Similarly, not only are the bauplan of taxa related to Eosauropterygia radically different (e.g., Thallatosauria, Saurosphargidae), thus incurring similar problems as those pointed for turtles and placodonts, but also, the morphology of the pectoral girdle of these taxa is already specialized to a marine lifestyle in their own way. Therefore, the choice of Claudiosaurus, Acerosodontosaurus or Thadeosaurus for outgroup comparison is related to the ill-adapted condition of the pectoral girdle of these taxa resembling those of terrestrial neodiapsid taxa.
Between hypothesis I and II no significant changes in muscle attachment topology would be required, but in hypothesis III a radical transformation would have had to happen because all muscles would have to be reorganized due to the rotation of the coracoid. However, developmental biology can provide important clues. Developmental patterns are highly conservative across taxa (Kirschner and Gerhart, 1998) and are essential to make accurate homology statements (Shubin, 1995). The development and ossification patterns of the pectoral girdle are well-described for squamates (Schauinsland, 1900, 1903; Rieppel, 1994; Maisano 2001, 2002) and crocodilians (Kälin, 1929; Rieppel, 1993).
Despite the presence of the sternum in basal neodiapsids (e.g., Harris and Carroll, 1977; Carroll, 1981), Eosauropterygia have an uncalcified sternum as a result of paeodomorphosis (Rieppel, 2000). Both in squamates and crocodilians the coracoid and sternum have an intimately linked developmental pattern (Schauinsland, 1903, figure 33; Kälin, 1929, figure 5), and in early stages of embryogenesis distinct from the formation of the interclavicle and clavicle (latter absent on crocodilians). In Sphenodon, the left and right sterna are separated medially, butting on the medial border of the coracoid cartilage (Schauinsland, 1903, figure 33). Late development adjoins the coracoids medially, and the sternum is pushed towards the posterior of the medial border of the coracoids, now separated by the interclavicle (Schauinsland, 1903, figure 34). Furthermore, medially contacting coracoids and posteriorly-located sternum relative to the coracoid are present plesiomorphically in basal neodiapsid specimens (e.g., Claudiosaurus MNHN 1908-11-9a, Hovasaurus 1925-5-30a). Thus, developmental and paleontological evidence suggests that the median contact of the coracoids in Eosauropterygia is well-substantiated. The developmental information seems to refute hypothesis II, in which the coracoids were separate at some point in the evolution of the clade. That is not the case for hypothesis I in which the median contact of the coracoids is preserved through evolution. Thus, the preferred transformational hypothesis is I. The acceptance of a transformational hypothesis has implications on the attachment of muscles and ligaments, and the distribution of cartilages. The attachment of the scapulosternal ligament - consequently the triceps origin - would vary if hypothesis I or II are used, as will be discussed below. Similarly, the persistence of the sternum has important implications on the reconstruction of several muscles, namely the pectoralis, costocoracoideus and sternocoracoideus (see below).
Nothosaur-plesiosaurian Pectoral Girdle Topological Homologies
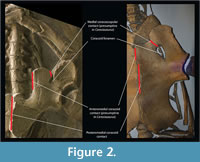 The comparison between nothosaur Ceresiosaurus calcagnii (PIMUZ T3983) and the basal plesiosaurian Meyerasaurus victor (SMNS12478) provides a good example for understanding the pectoral girdle homologies between these two groups. We chose the pectoral girdle of M. victor because it is not overprinted with more derived plesiosaurian anatomy on the one hand, and C. calcagnii has a very well-preserved, articulated girdle. In ventral view, the scapula of C. calcagnii (PIMUZ T3983) (Figure 2) has only one contact with the coracoid, the lateral coracoscapular contact. A second medial coracoscapular contact is eminent but does not materialize in this genus (Hänni, 2004). However, the medial coracoscapular contact is present in several other nothosaur species [e.g., Neusticosaurus edwardsii (PIMUZ T3758), Keichousaurus hui (SMNS 81780), Nothosaurus mirabilis (SMNS 13232)]. The coracoid foramen separates the two coracoscapular contacts. Similarly, in the basal plesiosaurian Meyerasaurus there are two coracoscapular contacts separated by what is homologized as being the coracoid foramen. This homology extends to all plesiosaurians although the pectoral girdle had undergone important transformations. For instance, in Hydrotherosaurus alexandrae (Welles, 1962) the scapulae only contact the coracoids laterally, while in Kronosaurus queenslandicus (White, 1940) and Peloneustes philarchus (Linder, 1913) the coracoid foramen remains reduced (i.e., the ventral area of the pectoral girdle bones is significantly larger than the area of the foramen). The implication of this homology statement is that what has been called the pectoral fenestra in plesiosaurians (e.g., White, 1940; Bardet et al., 1999; Smith and Vincent, 2010) is the coracoid “foramen”, as also previously suggested by Carroll (1981). Pistosaurs (e.g., Corosaurus Storrs, 1991, and Pistosaurus Sues, 1987) seem to have undergone a different evolutionary path because the coracoid foramen is absent. The pectoral ventral fenestra (Storrs, 1991; Rieppel, 2000), described for nothosaurs and pistosaurs, which is the area enclosed by the clavicular arch and the coracoscapular unit is therefore not homologous to what has been called “pectoral fenestra” of plesiosaurians. In plesiosaurians the pectoral fenestra is therefore absent; the only vacuity seen corresponds to the coracoid “foramen”, despite the misleading designation.
The comparison between nothosaur Ceresiosaurus calcagnii (PIMUZ T3983) and the basal plesiosaurian Meyerasaurus victor (SMNS12478) provides a good example for understanding the pectoral girdle homologies between these two groups. We chose the pectoral girdle of M. victor because it is not overprinted with more derived plesiosaurian anatomy on the one hand, and C. calcagnii has a very well-preserved, articulated girdle. In ventral view, the scapula of C. calcagnii (PIMUZ T3983) (Figure 2) has only one contact with the coracoid, the lateral coracoscapular contact. A second medial coracoscapular contact is eminent but does not materialize in this genus (Hänni, 2004). However, the medial coracoscapular contact is present in several other nothosaur species [e.g., Neusticosaurus edwardsii (PIMUZ T3758), Keichousaurus hui (SMNS 81780), Nothosaurus mirabilis (SMNS 13232)]. The coracoid foramen separates the two coracoscapular contacts. Similarly, in the basal plesiosaurian Meyerasaurus there are two coracoscapular contacts separated by what is homologized as being the coracoid foramen. This homology extends to all plesiosaurians although the pectoral girdle had undergone important transformations. For instance, in Hydrotherosaurus alexandrae (Welles, 1962) the scapulae only contact the coracoids laterally, while in Kronosaurus queenslandicus (White, 1940) and Peloneustes philarchus (Linder, 1913) the coracoid foramen remains reduced (i.e., the ventral area of the pectoral girdle bones is significantly larger than the area of the foramen). The implication of this homology statement is that what has been called the pectoral fenestra in plesiosaurians (e.g., White, 1940; Bardet et al., 1999; Smith and Vincent, 2010) is the coracoid “foramen”, as also previously suggested by Carroll (1981). Pistosaurs (e.g., Corosaurus Storrs, 1991, and Pistosaurus Sues, 1987) seem to have undergone a different evolutionary path because the coracoid foramen is absent. The pectoral ventral fenestra (Storrs, 1991; Rieppel, 2000), described for nothosaurs and pistosaurs, which is the area enclosed by the clavicular arch and the coracoscapular unit is therefore not homologous to what has been called “pectoral fenestra” of plesiosaurians. In plesiosaurians the pectoral fenestra is therefore absent; the only vacuity seen corresponds to the coracoid “foramen”, despite the misleading designation.
In nothosaurs the coracoid is a thickened bone that contacts medially with its counterpart (Rieppel, 2000). However, in some other pistosaurs(namely, Pistosaurus and Augustasaurus ) alongside this thickened portion there is a thin anterior and posterior expansion of the coracoids (Sues, 1987). The anterior coracoid expansion is also medially developed and would meet its other coracoid counterpart. On the other hand, the more basal nothosaur Ceresiosaurus does not share this anterior expansion, but instead the anteromedial portion of the coracoid contacting the scapula is significantly thinner than the anterolateral glenoidal portion (Figure 2). What is the key to understanding the developmental process leading to these two conditions? The medial margin of the anteromedial portion of the coracoid in nothosaurs is homologous to the medial margin of the anterior portion of the coracoid in some pistosaurs and all plesiosaurians. Meyerasaurus also shows an anterior expansion of the coracoid that meets the scapula anteriorly and conjoins its counterpart (Figure 2).This homology scheme, most importantly, maintains the topological relationships of the coracoid and scapula and their contacts.
CARTILAGES AND LIGAMENTS OF THE EOSAUROPTERYGIAN PECTORAL GIRDLE
Pectoral girdle Cartilages
Presternum and mesosternum cartilages. The sternum (presternum, mesosternum and xiphisternum) is present in crocodilians (Hyman, 1922) and squamates (Russell and Bauer, 2008). Thus, in non-plesiosaurian eosauropterygians, the presence of these cartilages can be inferred. In basal neodiapsids the presternum (=sternum; differentiation of the sternal bones is not commonly used in paleontological literature, e.g., in Harris and Carroll, 1977; Carroll, 1980; nor in developmental literature, e.g., Schauinsland, 1903) is ossified (Haughton, 1924; Piveteau, 1926; Harris and Carroll, 1977) even in young individuals (Currie and Carroll, 1984). However, the unique pectoral girdle configuration of Eosauropterygia, with a long coracoid longitudinal medial contact and reduced posterior process of the interclavicle, hinders the reconstruction of the sternal cartilages. According to the reconstruction presented here the sternal cartilages are absent in plesiosaurians (Level III of inference), but there was probably an atrophied presternum articulating in the posteromedial facet of the coracoid in pachypleurosaurs and nothosaurs (Level I of inference).
In both squamates and crocodilians the presternum articulates with the interclavicle and the coracoid. To maintain this topological connection would imply an attachment on the posterior border of the interclavicle and on the anterior border of the coracoid. Yet, the fossil record does not support a topological connection of the presternum on the anterior border of the coracoid, instead it articulates posteromedially (e.g., MNHN 1908-11-5a, MNHN 1908-11-16a, MNHN 1908-32-22a). This is particularly noticeable in the medial coracoid border of Thadeosarus (MNHN 1908-11-5a) which has two facets, one for articulation with the other coracoid and more posteriorly for articulation with the presternum. Furthermore, the coracoid in basal Eosauropterygia (pachypleurosaurs and nothosaurs) is teardrop-shaped in cross-section with the thinnest portion anteriorly (e.g., SMNS 84518, SMNS 84518, SMNS 17215, SMNS 420RF, SMNS 84067, SMNS 18248, SMNS 18689, PIMUZ T3983, PIMUZ T2464). Thus, the anterior border of the coracoid possesses no osteological correlates to host cartilage. In plesiosaurians, the anterior expansion of the coracoid linking medially with the scapula precludes the presence of any remnant of the presternum. Similarly, in the early development of Sphenodon pectoral girdle, the sternum does not articulate with the interclavicle, rather, the sternum is developed connected with the coracoid posteromedially, not anteriorly (Schauinsland, 1903).
An eventual posterior migration of the sternum implies the disruption of the topological connection between the interclavicle and coracoid. The posteromedial coracoid facets in Thadeosaurus (MNHN 1908-11-5a) seem to be present to a lesser degree in some well-preserved articulated eosauropterygian pectoral girdles [e.g., Neusticosaurus pusillus (PIMUZ T3672), Neusticosaurus edwardsii (PIMUZ T3460, PIMUZ T3758), Nothosaurus giganteus (PIMUZ T4829), Nothosaurus mirabilis (SMNS 13232)]. This reduced posteromedial facet of the coracoid is interpreted as an articulation site for a highly atrophied remnant of the presternum. Thus, a major trend in Eosauropterygia is, in fact, the anterior and posterior expansion of the coracoid (Sues, 1987) and not the extension of sternal cartilages. Furthermore, in Eosauropterygia, the gastralia are arranged immediately posterior to the coracoid, leaving no or very limited space for the articulation of any sternal cartilage. Some taxa that reify this assertion are, e.g., in pachypleurosaurs: Neusticosaurus edwardsi (PIMUZ T3460), Keichousaurus hui (SMNS 81780); in nothosaurs: Ceresiosaurus calcagnii (PIMUZ T2461), Nothosaurus giganteus (PIMUZ T4829); in the pistosaurs, Augustasaurus , see Sander et al. 1997, and Corosaurus , see Storrs 1991, the pectoral girdle is not articulated with the gastralia and in Yunguisaurus only the dorsal view of the articulated specimen is visible (Sato et al., 2011) or there is a high degree of disarticulation of the gastralia (Sato et al., 2014); plesiosaurians: Hydrorionbrachypterygius (GPITRE3185-RE-85), Meyerasaurus victor (SMNS 12478), Macropla tenuiceps (BMNH R5488), Hauffiosaurus zannoni Hauff uncatalogued type specimen. However, in basal neodiapsids the sternum articulates anteriorly with the coracoid, and gastralia are located immediately posteriorly (e.g., MNHN 1908-11-5a, MNHN 1908-11-16a, MNHN1908-32-22a). The lines of evidence presented suggest a possible articulation of the posteromedial facet of the coracoid with an atrophied presternum in pachypleurosaurs and nothosaurs, which is nevertheless absent in plesiosaurians. For a completely different opinion see Nicholls and Russell (1991). Nicholls and Russell’s (1991) hypothesis is not testable in the fossil record unless there is direct evidence of a sternal ossified cartilage. Their hypothesis does not preclude leaving any osteological correlates. Until now, there is no evidence for their hypothesis in Sauropterygia, even in well-preserved and articulated specimens. Therefore, it remains merely conjectural.
Episcapular (= suprascapular) cartilage - Andrews (1910) suggests the presence of the suprascapular cartilage in Cryptoclidus due to the presence of a concave dorsal border of the scapula. The dorsal extension of the scapula by means of a cartilaginous tissue is supported by the phylogenetic bracket because both crocodilians (Cong et al., 1998; Meers, 2003) and squamates (Russell and Bauer, 2008) have a well-developed suprascapular cartilage. However, the dorsal process of the scapula in non-plesiosaurian eosauropterygians is rod-like shaped and very reduced dorsally. The presence of this cartilage would thus have a vestigial functional role on these taxa.
Epicoracoid cartilage - The epicoracoid cartilage extends from the coracoid and scapular rays of squamates anteriorly, linking ventrally both coracoids (Russell and Bauer, 2008). In Alligator mississippiensis the coracoids are also linked by the epicoracoid cartilage. In crocodilians the epicoracoid cartilage serves as the origin for the coracohyoideus muscle as well as part of the coracobrachialis (Cong et al., 1998). In crocodiles and squamates the epicoracoid cartilage is mostly on the medial border of the coracoids meeting the presternum. Due to the median contact of the coracoids on the sagittal plane it implies that it is not present in Eosauropterygia (Level III of inference), but it could be present in juveniles or in taxa that do not bear this condition (e.g., Simosaurus ).
Capsular cartilage - Cartilaginous tissue is ubiquitous in all amniotes at the glenohumeral joint, thus, the capsular cartilage can be confidently reconstructed in Eosauropterygia.
On the Presence of Cartilage on the Plesiosaurian Coracoid Foramen (=pectoral fenestra)
Robinson (1975, figure 20) and Storrs (1986) reconstructed the subcoracoscapularis and the supracoracoideus as originating from the pectoral fenestra, implying that it would be covered with cartilage although they offer no further discussion. But neither the epicoracoid cartilage nor the presternum is supported by the lines of evidence presented above. Furthermore, as mentioned, the so called “pectoral fenestra” is homologous with the coracoid “foramen” in plesiosaurians (see nothosaur-plesiosaurian pectoral girdle topological homologies). The actual pectoral fenestra described in pachypleurosaurs and nothosaurs is closed by expansions of the coracoid and scapula in plesiosaurians. Also, importantly, the enlargement of the coracoid foramen is an artifact of the amplification of the coracoscapular muscle attachment area and may have concomitantly increased rigidity of the pectoral girdle via the medial scapular contact and the long longitudinal bar of the coracoid. Thus, the reconstruction of cartilage on the plesiosaurian coracoid “foramen” seems, under this homology hypothesis, implausible.
Pectoral Girdle and Brachial Ligaments
Medial scapulosternal ligament. This ligament can be divided in two parts (Howell, 1936): the scapulohumeral and scapulosternal ligaments. As a whole this ligament prevents anterior displacement of the pectoral girdle, and it is the origin of some extrinsic limb muscles, e.g., triceps heads (Peterson, 1973). The topology of the ligament and respective attachments seen in lizards is also verified in crocodilians (see Russell and Bauer, 2008; Jasinoski et al., 2006).
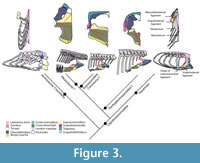 Scapulohumeral ligament. In lizards it originates dorsal to the glenoid fossa on the scapula and inserts on the humerus. Subsequently this ligament continues as the scapulosternal ligament (see below; Figure 3). This ligament serves as an origin of the triceps to the scapular head, but also helps maintain the integrity of the glenohumeral joint (Peterson, 1973). Jasinoski et al. (2006) describe this tendon in crocodilians just as in lizards, thus we can infer an equivalent topology to Eosauropterygia (Level I of inference, Figure 3). Considering Hypothesis I or II for the topological change from basal neodiapsids and eosauropterygians, which implies assuming the glenoid as a fixed point, the reconstruction suggested here corresponds topologically to that of crocodilians and squamates.
Scapulohumeral ligament. In lizards it originates dorsal to the glenoid fossa on the scapula and inserts on the humerus. Subsequently this ligament continues as the scapulosternal ligament (see below; Figure 3). This ligament serves as an origin of the triceps to the scapular head, but also helps maintain the integrity of the glenohumeral joint (Peterson, 1973). Jasinoski et al. (2006) describe this tendon in crocodilians just as in lizards, thus we can infer an equivalent topology to Eosauropterygia (Level I of inference, Figure 3). Considering Hypothesis I or II for the topological change from basal neodiapsids and eosauropterygians, which implies assuming the glenoid as a fixed point, the reconstruction suggested here corresponds topologically to that of crocodilians and squamates.
Scapulosternal ligament. The scapulosternal ligament is the continuation of the scapulohumeral ligament circling around the humerus and then spreading into a sheath that has three points of attachment. In squamates, the three points of attachment are located: (i) on the ventral area of the coracoid near the sternum, (ii) on the posteroventral border of the coracoid and another (iii) extending all the way to the scapula, passing medially to the coracoid and inserting on the area dorsal to the glenoid (Russell and Bauer, 2008).
This ligament besides stabilizing the glenohumeral joint it also serves as an origin of the coracoidal head of the triceps. The scapulosternal component of the medial scapulosternal ligament attaches in a similar manner in crocodilians (Jasinoski et al., 2006). Due to the highly derived condition in eosauropterygians the reconstruction of the attachments of this ligament (Figure 3) is difficult. The scapular attachment is not troublesome to reconstruct because the homologous region corresponds to the attachment on the dorsal process of the scapula on the region dorsal to the glenoid; thus it may have arisen from the scapula dorsally on the rim of the glenoid. The second attachment is on the ventral border of the coracoid in crocodilians and lizards, and its homologous part corresponds to the medial border in plesiosaurians (hypothesis I or II). In many plesiosaurian taxa the thickened glenoidal region of the coracoid forms a distinct ventral process or expansion (personal observation in: Wapuskanectes, Callawayasaurus, Cryptoclidus, Meyerasaurus, Liopleurodon, Peloneustes ), which could serve as an attachment for this ligament. Finally, considering the fact that there was an anteroposterior expansion of a ventrally-located coracoid in pistosaurs (see Storrs, 1986; Sues, 1987; Sander et al., 1997), it is assumed that the posteromedial border of the coracoid served as the origin of this head of the scapulosternal ligament, as in crocodilians and lizards. However there is no macroscopic osteological correlates that substanciate this origin. The phylogenetic bracket allows a Level I inference of the position of this ligament taking into consideration the transformational hypothesis presented here.
Mesocleidosternal ligament. This is a simple ligament that connects the lateral-most tips of the interclavicle to the presternum (Russell and Bauer, 2008). Thus, the reconstruction of this ligament is dependent on the presence of the presternum in Eosauropterygia. Above several arguments were provided for the absence or atrophy of sternal cartilages. In fact, a reduced presternum on the coracoid posteromedial border of basal Eosauropterygia, separate from the interclavicle, seems to be indicative of the absence of this ligament (Level III of inference; Figure 3). The case is more obvious in plesiosaurians due to the enlargement of the coracoid “foramen” as well as the long median contact of the coracoids and scapulae implying that the mesocleidosternal ligament is absent (Level III of inference).
PECTORAL GIRDLE AND BRACHIAL MYOLOGY IN EOSAUROPTERYGIA
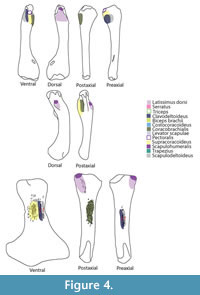 Although the episternocleidomastoideus has insertions on the squamate pectoral girdle, it will not be considered in this analysis because it does not play a fundamental role in locomotion (Russell and Bauer, 2008; Meers, 2003). Conversely, the latissimus dorsi does not insert or originate in any pectoral girdle element, but it will be considered (Figure 3). A summary of the insertions on the humerus are depicted in Figure 4. The nomenclature and homology scheme presented here is consistent with Diogo and Abdala’s (2010) comprehensive anatomical review.
Although the episternocleidomastoideus has insertions on the squamate pectoral girdle, it will not be considered in this analysis because it does not play a fundamental role in locomotion (Russell and Bauer, 2008; Meers, 2003). Conversely, the latissimus dorsi does not insert or originate in any pectoral girdle element, but it will be considered (Figure 3). A summary of the insertions on the humerus are depicted in Figure 4. The nomenclature and homology scheme presented here is consistent with Diogo and Abdala’s (2010) comprehensive anatomical review.
Shoulder Musculature
M. subcoracoscapularis (= m. subscapularis): Origin. The nomenclature of this muscle differs in squamates and crocodilians. In Iguana this muscle originates from the medial surface of the episcapular cartilage, from the medial and posterior areas of the scapula, as well as on the coracoids, onto large surfaces of the anteromedial part of the epicoracoid and medial aspect of coracoid (Russell and Bauer, 2008). In crocodilians, the subscapularis muscle inserts on the medial side of the scapula occupying the anteroposterior extension almost completely, including from the constriction of the scapula to its posterior one third (Meers, 2003; Cong et al., 1998). In turtles the subscapularis originates on the scapular prong medially (Walker, 1973).
 The subcoracoscapularis is reconstructed along the medial surface of the scapula in basal Eosauropterygia (Level I; Figure 3, Figure 5), but in the medial aspect of the coracoids this implies a Level II inference, only supported by the squamates. Due to the morphological transformations that occurred in plesiosaurians, this insertion corresponds to the dorsal side of the scapula and coracoid. This muscle is divided in squamates into a subscapular and subcoracoid component, each playing different roles (Jenkins and Goslow, 1983; Russell and Bauer, 2008). This division is followed here in Eosauropterygia reconstruction.
The subcoracoscapularis is reconstructed along the medial surface of the scapula in basal Eosauropterygia (Level I; Figure 3, Figure 5), but in the medial aspect of the coracoids this implies a Level II inference, only supported by the squamates. Due to the morphological transformations that occurred in plesiosaurians, this insertion corresponds to the dorsal side of the scapula and coracoid. This muscle is divided in squamates into a subscapular and subcoracoid component, each playing different roles (Jenkins and Goslow, 1983; Russell and Bauer, 2008). This division is followed here in Eosauropterygia reconstruction.
Isolated nothosaur coracoids (e.g., SMNS 420RF, SMNS 17326) show osteological correlates demonstrated by faint striae running along the mediolateral extention of the coracoid. The striae are not as deeply grooved as on the ventral side. In medial view, the dorsal part of the scapula receives the clavicle medially and dorsally in all non-plesiosaurian eosauropterygians, but ventrally the medial side of the scapula is concave and completely striated, most conspicuously in the ventral portion with dorsoventrally orientated striae (e.g., SMNS 84069, SMNS84517). In plesiosaurians osteological correlates on the dorsal side of the coracoid are not evident; the expanded ventral part of the scapula (apart from the dorsal blade) serves as the origin of the scapular portion of the subcoracoscapularis. In pachypleurosaurs, due to the articulation of most specimens, the dorsal surface of the coracoid and medioventral side of the scapula is rarely visible.
This muscle has a poor mechanical advantage and it mainly assists stabilization of the glenohumeral joint in crocodilians (Meers, 2003; Cong et al., 1998), similar to the case in Varanus (Jenkins and Goslow, 1983).
Insertion. The insertion in lizards is lateral to the glenoid buttress of the humerus (Gregory and Camp, 1918; Howell, 1936). In sea turtles the powerful subsapularis inserts on the medial process of the humerus dorsally (Walker, 1973). In crocodiles the subscapularis inserts on the medial protuberance of the humerus via the capsular joint (Meers, 2003; Cong et al., 1998). The reconstruction in basal neodiapsids is therefore on the medial process of the humerus proximally and dorsally (Holmes, 1977). For Keichousaurus, Lin and Rieppel (1998) reconstructed on the Y-shaped crest on the dorsal surface of the proximal portion of the humerus. Similarly, in plesiosaurians the dorsal and proximal surface of the humeral capitulum served as the attachment area for the subcoracoscapularis (Figure 4).
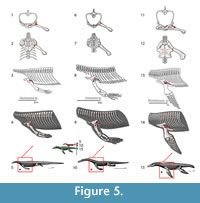
 M. scapulodeltoideus (= m. deltoideus scapularis, = m. dorsalis scapulae, = m. teres major). Origin. The scapulodeltoideus is here considered as a synonym of the deltoideus scapularis and teres major (Meers, 2003), and dorsalis scapulae (Cong et al., 1998). The origin in the squamate phylogenetic bracket is in the lateral side of the dorsalmost part of the scapula (Russell and Bauer, 2008). In the crocodilian bracket the origin of the scapulodeltoideus is on the posterodorsal quadrant of the lateral side of the scapula; with some fibers on the episcapular cartilage. Thus, the origin in Eosauropterygia is a Level I of inference, on the lateral side of the dorsal blade of the scapula (Figure 3, Figure 6). Although the dorsal blade of the scapula is very difficult to observe in lateral view in most pachypleurosaurs, in Serpianosaurus (PIMUZ T3675) a discernable smooth surface is evident. In larger nothosaur specimens, but especially in the isolated scapulae of SMNS 84069 and GPIT RE-1533, faint but visible striae and pitting run along the dorsal blade extension of the scapula, particularly on the dorsal tip. The striation density contrast between the lateral side and the medial side of the scapula is notable.
M. scapulodeltoideus (= m. deltoideus scapularis, = m. dorsalis scapulae, = m. teres major). Origin. The scapulodeltoideus is here considered as a synonym of the deltoideus scapularis and teres major (Meers, 2003), and dorsalis scapulae (Cong et al., 1998). The origin in the squamate phylogenetic bracket is in the lateral side of the dorsalmost part of the scapula (Russell and Bauer, 2008). In the crocodilian bracket the origin of the scapulodeltoideus is on the posterodorsal quadrant of the lateral side of the scapula; with some fibers on the episcapular cartilage. Thus, the origin in Eosauropterygia is a Level I of inference, on the lateral side of the dorsal blade of the scapula (Figure 3, Figure 6). Although the dorsal blade of the scapula is very difficult to observe in lateral view in most pachypleurosaurs, in Serpianosaurus (PIMUZ T3675) a discernable smooth surface is evident. In larger nothosaur specimens, but especially in the isolated scapulae of SMNS 84069 and GPIT RE-1533, faint but visible striae and pitting run along the dorsal blade extension of the scapula, particularly on the dorsal tip. The striation density contrast between the lateral side and the medial side of the scapula is notable.
Insertion. The scapulodeltoideus has a topologically similar insertion with the clavodeltoideus. As a matter of fact, Walker (1973) describes a common insertion for the deltoideus on the dorsal surface of the deltopectoral crest. In Squamata, the scapulodeltoideus inserts on the preaxial surface of the deltopectoral crest, tightly associated with the clavodeltoideus insertion (Russell and Bauer, 2008). The insertion for crocodiles is tendinous, attaching on the dorsalmost portion of the deltopectoral crest (Meers, 2003). The insertion for basal neodiapsids and similarly for nothosaurs is on the deltopectoral crest, dorsally to the clavodeltoideus insertion (Figure 4, Figure 6). For plesiosaurians, if the insertion is on the ventral surface of the bone it is located preaxially relatively to the clavodeltoideus. In the case that the insertion is on the preaxial surface of the humerus it is located dorsally relative to the clavodeltoideus. The reconstructed origin and insertion of this muscle in Eosauropterygia implies a glenohumeral stabilizer function.
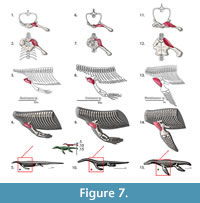 M. clavodeltoideus (= m. deltoideus clavicularis, = m. deltoideus). Origin. The clavodeloideus arises from the median half of the clavicle in Iguana and similarly in Eublepharis (Zaaf et al., 1999; Russell and Bauer, 2008). This muscle originates from the acromion process of the anterior scapula margin with some fibers in roughly the same area as the trapezius (Cong et al., 1998; Meers, 2003). Once again, the nomenclature of this muscle is disparate between the taxa here analyzed. Clavodeltoideus was the term used for squamates (Russell and Bauer, 2008), deltoideus clavicularis is used by Meers (2003) and deltoideus is used by Cong et al. (1998). The clavicle is absent in crocodiles (Hyman, 1922; Kälin, 1929), which leads to the idea that the clavodeltoideus can operate, in the absence of the clavicle, on the scapula. In arboreal forms such as the chameleon, with no clavicle, the clavodeltoideus originates on the coracoid and sternum (Peterson, 1973). Therefore the clavicular origin in most generalized squamates leads to a Level II of inference in Eosauropterygia. However, the notable decrease of the relative size of the clavicle in plesiosaurians may have led to a posterior migration of this muscle on the scapula, which is still supported by the archosaur branch of the extant phylogenetic bracket (Figure 3). In more basal Eosauropterygia, this muscle had a mostly clavicular component (Figure 7). We infer that the origin is posterior to that of the trapezius muscle, which maintains the expected topological relationships (Figure 3, Figure 7).
M. clavodeltoideus (= m. deltoideus clavicularis, = m. deltoideus). Origin. The clavodeloideus arises from the median half of the clavicle in Iguana and similarly in Eublepharis (Zaaf et al., 1999; Russell and Bauer, 2008). This muscle originates from the acromion process of the anterior scapula margin with some fibers in roughly the same area as the trapezius (Cong et al., 1998; Meers, 2003). Once again, the nomenclature of this muscle is disparate between the taxa here analyzed. Clavodeltoideus was the term used for squamates (Russell and Bauer, 2008), deltoideus clavicularis is used by Meers (2003) and deltoideus is used by Cong et al. (1998). The clavicle is absent in crocodiles (Hyman, 1922; Kälin, 1929), which leads to the idea that the clavodeltoideus can operate, in the absence of the clavicle, on the scapula. In arboreal forms such as the chameleon, with no clavicle, the clavodeltoideus originates on the coracoid and sternum (Peterson, 1973). Therefore the clavicular origin in most generalized squamates leads to a Level II of inference in Eosauropterygia. However, the notable decrease of the relative size of the clavicle in plesiosaurians may have led to a posterior migration of this muscle on the scapula, which is still supported by the archosaur branch of the extant phylogenetic bracket (Figure 3). In more basal Eosauropterygia, this muscle had a mostly clavicular component (Figure 7). We infer that the origin is posterior to that of the trapezius muscle, which maintains the expected topological relationships (Figure 3, Figure 7).
The ventral side of the scapula is heavily pitted and porous in the pristinely preserved SMNS 84069, but this texture is also present in Nothosaurus miriabilis (SMNS13232) or Ceresiosaurus calcagnii (PIMUZ T2461). Similarly the clavicles are striated ventrally with the striae oriented mediolaterally, although on the median (=medial?) part of the bone they seem to fade in some specimens (e.g., SMNS 59822, SMNS 84526), but not in SMNS 13232. In Ceresiosaurus calcagnii (PIMUZ T2464) the clavicle is deeply grooved laterally and only very fine striations continue medially.
Insertion. The reconstruction of the clavodeltoideus is on the deltopectoral crest for all taxa analysed (Level I inference), inserting proximally and on its preaxial surface (Walker, 1973; Meers, 2003; Russell and Bauer, 2008). The clavodeltoideus is thus reconstructed on the preaxial surface of the deltopectoral crest in basal neodiapsids, and similarly in nothosaurs (Figure 4, Figure 7). For plesiosaurians the reconstruction is not trivial because there are muscle scars in the preaxial side of the humerus, as well as on the preaxial portion of the homologous structure of the deltopectoral crest (Figure 4, Figure 7). Assuming the protractor function of this muscle in the extant taxa was maintained through Eosauropterygia evolution, it is reasonable to postulate that the insertion of the clavodeltoideus shifted towards the preaxial surface of the humerus. In plesiosaurians, the clavodeltoideus inserts more distally than other eosauropterygians, as for other muscles inserting on the humerus.
M. scapulohumeralis (= m. scapulohumeralis caudalis, = m. scapulohumeralis posterior). Origin. In squamates, when discernable, it arises from the posterior scapular margin (Zaaf et al., 1999; Russell and Bauer, 2008). In crocodilians, it originates on the posterior border of the scapula dorsal to the glenoid rim, along about half of the scapula (Cong et al., 1998; Meers, 2003).
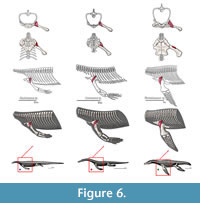
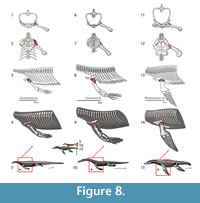 The recession of the dorsal component of the scapula implies that this muscle had to migrate to a more ventral position, and expanded anteroposteriorly in Eosauropterygia, occupying a region near the glenoid (Figure 3). In Eosauropterygia it is thus reconstructed as having an origin along the dorsal surface of the glenoid on the scapula (Figure 3, Figure 8). Given the most likely homology of this region with respect to the extant phylogenetic bracket it implies a Level I of inference. In lateral view, the scapular body is ventrally concave in all non-plesiosaurian eosauropterygians. In pachypleurosaurs and some small nothosaurs, this region of the scapula is smooth [e.g., C. calcagnii (PIMUZ T4835), N. edwardsii (PIMUZ T3758, PIMUZ T3775), N. peyeri (PIMUZ T3932)], but in larger nothosaurs there are dorsoventral striae ventrally (SMNS 84517). In Nothosaurus miriabilis (SMNS1323)fine striae run both dorsoventrally in the ventral portion and anteroposteriorly in the more dorsal regions of the concavity. However the pattern is most clear in the well-preserved scapula of SMNS 84069, in which the striae are denser along the edges of the concavity, converging to its center.
The recession of the dorsal component of the scapula implies that this muscle had to migrate to a more ventral position, and expanded anteroposteriorly in Eosauropterygia, occupying a region near the glenoid (Figure 3). In Eosauropterygia it is thus reconstructed as having an origin along the dorsal surface of the glenoid on the scapula (Figure 3, Figure 8). Given the most likely homology of this region with respect to the extant phylogenetic bracket it implies a Level I of inference. In lateral view, the scapular body is ventrally concave in all non-plesiosaurian eosauropterygians. In pachypleurosaurs and some small nothosaurs, this region of the scapula is smooth [e.g., C. calcagnii (PIMUZ T4835), N. edwardsii (PIMUZ T3758, PIMUZ T3775), N. peyeri (PIMUZ T3932)], but in larger nothosaurs there are dorsoventral striae ventrally (SMNS 84517). In Nothosaurus miriabilis (SMNS1323)fine striae run both dorsoventrally in the ventral portion and anteroposteriorly in the more dorsal regions of the concavity. However the pattern is most clear in the well-preserved scapula of SMNS 84069, in which the striae are denser along the edges of the concavity, converging to its center.
This muscle functions as a humeral elevator and glenohumeral stabilizer in alligator and Varanus (Jenkins and Goslow, 1983; Meers, 2003). In Eosauropterygia it possibly assisted in glenohumeral stabilization given the lack of a dorsal component of the scapula.
Insertion. In lizards the scapulohumeralis inserts on the dorsal aspect of the medial process (Russell and Bauer, 2008). However, in turtles this muscle inserts, together with the subscapularis, on the medial process, but on the ventral aspect of the bone (Walker, 1973). In crocodiles, the medial protuberance is the insertion for the subscapularis (Meers, 2003), thus the scapulohumeralis inserts on the dorsal surface of the humerus proximally. The extant phylogenetic bracket used here consists of crocodiles and lizards, therefore the reconstruction is unambiguous, Level I inference (Figure 4, Figure 8). The site of insertion of the scapulohumeralis for basal neodiapsids is thus, proximal to the insertion of the latissimus dorsi, on the dorsal surface of the humerus (Figure 4). Similarly, the protuberance on the dorsal surface of the nothosaur humerus served for the attachment of the scapulohumeralis; and for plesiosaurians the heavily striated dorsal surface of the humerus, possibly even on the proximal aspect of the tuberosity, served for the attachment of this muscle.
M. supracoracoideus (= m. coracobrachialis superior , = m. coracobrachialis brevis dorsalis). Origin. Supracoracoideus originates from the anterior surface of the epicoracoid and ventral surface of the coracoids (Zaaf et al., 1999; Russell and Bauer, 2008). The nomenclature of this muscle in crocodilians is rather confusing and disparate. To make the descriptions conform the subdivisions of the supracoracoideus into longus, intermedius and brevis and the coracobrachialis brevis dorsalis of Meers (2003) correspond respectively to the coracobrachialis superior medial portion, coracobrachialis superior lateral portion, supracoracoideus coracoid portion/coracobrachialis superior lateral portion and supracoracoideus scapular portion of Cong et al. (1998). Simplifying the nomenclature in crocodilians results in an origin on the anterior portion of the coracoid extending dorsally to the constriction of the scapula and anteriorly to the coracoid foramen; and another portion originating medially on the coracoid and ventral-most area of the scapula, anterior to the coracoid foramen.
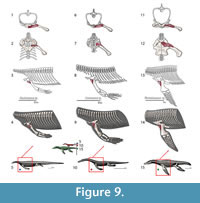 The scapular portion of this muscle insertion in crocodilians would imply a Level II of inference, thus we restrain the origin to the coracoids which is Level I. In Eosauropterygia the origin is reconstructed along the anterior portion of the coracoids, extending along most of its mediolateral length (Figure 3, Figure 9). This reconstruction makes use of the transformational hypothesis I (or II) in which the anterior border of the coracoid remains in the same topological position across the evolution of the clade (Figure 1). In basal Eosauropterygia, the coracoid is anteriorly very thin and does not present conspicuous osteological correlates, despite the presence of pitting in isolated coracoids of the nothosaur MNHN AC9454 or convoluted periosteum in MNHN 9244. However, in plesiosaurians (e.g., Meyerasaurus ) and some pistosaurs (Sues, 1987), a thick glenoidal section that extends all the way medially separates the preglenoidal from the postglenoidal section of the coracoid. The preglenoidal (or anterior) section of the coracoid is reconstructed here as the origin of the supracoracoideus.
The scapular portion of this muscle insertion in crocodilians would imply a Level II of inference, thus we restrain the origin to the coracoids which is Level I. In Eosauropterygia the origin is reconstructed along the anterior portion of the coracoids, extending along most of its mediolateral length (Figure 3, Figure 9). This reconstruction makes use of the transformational hypothesis I (or II) in which the anterior border of the coracoid remains in the same topological position across the evolution of the clade (Figure 1). In basal Eosauropterygia, the coracoid is anteriorly very thin and does not present conspicuous osteological correlates, despite the presence of pitting in isolated coracoids of the nothosaur MNHN AC9454 or convoluted periosteum in MNHN 9244. However, in plesiosaurians (e.g., Meyerasaurus ) and some pistosaurs (Sues, 1987), a thick glenoidal section that extends all the way medially separates the preglenoidal from the postglenoidal section of the coracoid. The preglenoidal (or anterior) section of the coracoid is reconstructed here as the origin of the supracoracoideus.
Functionally in crocodilians and squamates this muscle is a limb protractor, however, given the posterior location of the coracoids relative to the glenoid and the great anteroposterior expansion of the coracoids restricted to the ventral portion of the body, such a function could not be developed in most Eosauropterygia. From a functional point of view, contrary to their extant relatives, the supracoracoideus is a retractor or glenohumeral joint stabilizer.
Insertion. In turtles the supracoracoideus inserts on the lateral process of the humerus ventrally, which is homologous with the deltopectoral crest (Walker, 1973). The insertion is on the deltopectoral crest for crocodiles and lizards, inserting on its apex (Meers, 2003) and on proximal border (Russell and Bauer, 2008), respectively. Yet, the ventrally-oriented deltopectoral crest in basal neodiapsids and in nothosaurs presents served as the area of attachment of this muscle (Level I of inference). However, in the absence of a deltopectoral crest in plesiosaurians makes the reconstruction of this muscle less straightforward (Figure 4, Figure 9). However, the presence of heavy muscle scars on the ventral surface of the humeri in many plesiosaurians defines the insertion of this muscle (Figure 4).
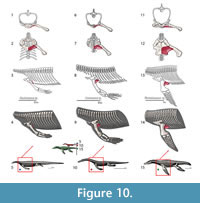 M. coracobrachialis longus and brevis. Origin. Coracobrachialis brevis and longus originate from the ventromedial surface of the coracoid and posterior coracoid process, respectively (Russell and Bauer, 2008); an identical attachment is present in Eublepharis (Zaaf et al. 1999). Cong et al. (1998) consider this muscle to be a single unit that synonymizes with the coracobrachialis brevis ventralis described by Meers (2003). It originates on the lateral surface of the coracoid along its dorsoventral extension to the coracoid foramen (Cong et al., 1998; Meers, 2003). We reconstruct the coracobrachialis in Eosauropterygia as extending along the ventral surface of the coracoid to the glenoid: Level I of inference (Figure 3, Figure 10). Among other functions, coracobrachialis mainly retracts the forelimb (Meers, 2003; Cong et al., 1998), and the action should have been similar in Eosauropterygia. The macroscopic osteological evidence for a strong coracobrachialis is clear in all clades. In the pachypleurosaurids Keichousaurus hui (SMNS81780, SMNS 82044), the coracoid is covered in striations along its mediolateral extension and they are perpendicularly arranged to its medial and lateral borders. Striations are also present in the larger species of the genus Neusticosaurus, namely N. edwardsii (PIMUZ T3769, PIMUZ T3758) and N. peyeri (PIMUZ T3445), although the median portion of the coracoid is finely pitted. A similar pattern occurs in nothosaurs with the medial and lateral extremeties of the coracoid more heavily striated than the median part (e.g., SMNS 1323, SMNS 420RF, SMNS 17326, SMNS 84518, PIMUZ T4829, PIMUZ T3983). However, in other specimens this section may be convoluted (MNHN 9244) or heavily pitted (MNHN AC9454).
M. coracobrachialis longus and brevis. Origin. Coracobrachialis brevis and longus originate from the ventromedial surface of the coracoid and posterior coracoid process, respectively (Russell and Bauer, 2008); an identical attachment is present in Eublepharis (Zaaf et al. 1999). Cong et al. (1998) consider this muscle to be a single unit that synonymizes with the coracobrachialis brevis ventralis described by Meers (2003). It originates on the lateral surface of the coracoid along its dorsoventral extension to the coracoid foramen (Cong et al., 1998; Meers, 2003). We reconstruct the coracobrachialis in Eosauropterygia as extending along the ventral surface of the coracoid to the glenoid: Level I of inference (Figure 3, Figure 10). Among other functions, coracobrachialis mainly retracts the forelimb (Meers, 2003; Cong et al., 1998), and the action should have been similar in Eosauropterygia. The macroscopic osteological evidence for a strong coracobrachialis is clear in all clades. In the pachypleurosaurids Keichousaurus hui (SMNS81780, SMNS 82044), the coracoid is covered in striations along its mediolateral extension and they are perpendicularly arranged to its medial and lateral borders. Striations are also present in the larger species of the genus Neusticosaurus, namely N. edwardsii (PIMUZ T3769, PIMUZ T3758) and N. peyeri (PIMUZ T3445), although the median portion of the coracoid is finely pitted. A similar pattern occurs in nothosaurs with the medial and lateral extremeties of the coracoid more heavily striated than the median part (e.g., SMNS 1323, SMNS 420RF, SMNS 17326, SMNS 84518, PIMUZ T4829, PIMUZ T3983). However, in other specimens this section may be convoluted (MNHN 9244) or heavily pitted (MNHN AC9454).
Insertion. In turtles, the coracobrachialis inserts on the proximal border of the medial process as well as on the intertubercular fossa on the ventral side of the humerus (Walker, 1973). In lizards, the coracobrachialis inserts on the posteroventral margin of the humeral shaft extending from the more proximal surface to nearly half the length (Russell and Bauer, 2008). The coracobrachialis inserts on the ventral and postaxial side of the deltopectoral crest in crocodiles (Meers, 2003). Congruently, the insertion for the extinct taxa can be reconstructed as on the ventral and postaxial portion of the humerus shaft proximally (Level I of inference). MAP6 has a discernable groove on the ventral and postaxial surface of the humerus (Figure 4, Figure 10). In Nothosaurus humeri (Bickelmann and Sander, 2008) the area ventral to the deltopectoral crest of insertion corresponds to the insertion of this muscle. Plesiosaurians do not have a demarcated deltopectoral crest. However, conspicuous muscle scars are present in the postaxial side at about mid-length of the humerus in many taxa.
M. biceps brachii. Origin. In Sphenodon , as in Eublepharis , the biceps brachii arises exclusively from the coracoid, presumably showing the plesiomorphic condition (Romer, 1956; Zaaf et al., 1999; Russell and Bauer, 2008). The origin in the Iguana is more complex: fibers overlie the secondary coracoid fenestra and medial border of the coracoid (Russell and Bauer, 2008). Cong et al. (1998) and Meers (2003) agree that the origin is via a tendinous attachment on the anterolateral border of the coracoid shaft ventral to the coracoid foramen. In Eosauropterygia, the phylogenetic bracket indicates a Level II of inference because the condition in crocodilians seems to have departed from the plesiomorphic condition, i.e., insertion on the posteromedial regions of the coracoid. The reconstructed biceps brachii on the posterior region of the coracoid is topologically posterior to the supracoracoideus and coracobrachialis origins. In crocodilians the coracobrachialis is posterior relative to the biceps brachii origin. The medial region of the coracoid in several eosauropterygian taxa is heavily striated interpreted here as part of the coracobrachialis origin, but in some specimens the posteromedial portion has deeper and wider grooves (SMNS 42012RF, MNHN AC9454), which can be associated to the origin of the biceps. Importantly, this muscle has a common developmental origin with the coracobrachialis and supracoracoideus comprising the muscle ventral mass (Sewertzoff, 1904). Thus, it may have not differentiated at its origin from the larger coracobrachialis, sharing a common area of attachment with this muscle.
This muscle is a forearm flexor in crocodilians and Varanus (Cong et al., 1998; Meers, 2003; Jenkins and Goslow, 1983). In Varanus it also stabilizes the glenohumeral joint. The forearm flexor function is very limited if not absent in plesiosaurians, thus this muscle might have adopted other functions. The lateral projections of the posterior part of the coracoids in some plesiosaurians (e.g., Cryptoclidus ) may be linked with the function of the biceps brachii.
Insertion. The biceps brachii inserts together with the biceps anticus on the proximomedial surface of the radius tendinously and on the proximal end of the shaft of the ulna (Zaaf et al., 1999; Russell and Bauer, 2008). It inserts on the humeroradialis tubercle of the radius on the posterior side (Meers, 2003).
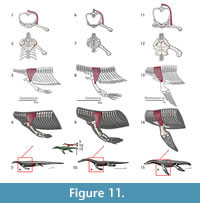 M. triceps brachii. Origin. Despite inconsistent division of the triceps into various constituent heads among authors, its origins and insertions are congruent. Meers (2003) divides the muscle into five heads whereas Russell and Bauer (2008) and Cong et al. (1998) cite only four heads but use consistent nomenclature. Zaaf et al. (1999) only divides the triceps into scapular and medial heads. The medial humeral head of the triceps is subdivided in Meers (2003) into the triceps brevis intermedius and triceps brevis caudalis; the triceps brevis cranialis can be synonymized with the triceps lateral humeral head. The scapular head of the triceps originates on the dorsal glenoid buttress in both squamates (Zaaf et al., 1999; Russell and Bauer, 2008) and crocodilians (Meers, 2003). The coracoid head originates on part of the sternoscapular ligament, which subsequently connects the scapula and the sternal region in squamates (Zaaf et al., 1999; Russell and Bauer, 2008). In crocodilians (Meers, 2003; Cong et al., 1998) it originates on the glenoid buttress of the scapula via a tendon that subsequently connects to the scapula (possibly equivalent to the sternoscapular ligament of squamates). The lateral humeral head of the triceps originates on a concavity dorsal to the deltapectoral crest of the humerus in squamates (Russell and Bauer, 2008), but in crocodilians on a thin band along the anterodorsal surface of the humerus (Meers, 2003; Cong et al., 1998). The medial lateral head originates on a concavity of the posterodorsal margin of the proximal humerus in squamates (Russell and Bauer, 2008) and crocodilians, although it covers the entire extension of the dorsal surface (Meers, 2003; Cong et al., 1998). Given the Level I of inference for all its origins, the reconstruction of the four heads of the triceps can be directly applied for Eosauropterygia (Figure 3, Figure 11). Caution should be taken concerning the path of the medial sternoscapular ligament, namely the sternoscapular portion, because its attachment locations rely on a transformational hypothesis of the pectoral girdle during the peculiar evolution of the Eosauropterygia.
M. triceps brachii. Origin. Despite inconsistent division of the triceps into various constituent heads among authors, its origins and insertions are congruent. Meers (2003) divides the muscle into five heads whereas Russell and Bauer (2008) and Cong et al. (1998) cite only four heads but use consistent nomenclature. Zaaf et al. (1999) only divides the triceps into scapular and medial heads. The medial humeral head of the triceps is subdivided in Meers (2003) into the triceps brevis intermedius and triceps brevis caudalis; the triceps brevis cranialis can be synonymized with the triceps lateral humeral head. The scapular head of the triceps originates on the dorsal glenoid buttress in both squamates (Zaaf et al., 1999; Russell and Bauer, 2008) and crocodilians (Meers, 2003). The coracoid head originates on part of the sternoscapular ligament, which subsequently connects the scapula and the sternal region in squamates (Zaaf et al., 1999; Russell and Bauer, 2008). In crocodilians (Meers, 2003; Cong et al., 1998) it originates on the glenoid buttress of the scapula via a tendon that subsequently connects to the scapula (possibly equivalent to the sternoscapular ligament of squamates). The lateral humeral head of the triceps originates on a concavity dorsal to the deltapectoral crest of the humerus in squamates (Russell and Bauer, 2008), but in crocodilians on a thin band along the anterodorsal surface of the humerus (Meers, 2003; Cong et al., 1998). The medial lateral head originates on a concavity of the posterodorsal margin of the proximal humerus in squamates (Russell and Bauer, 2008) and crocodilians, although it covers the entire extension of the dorsal surface (Meers, 2003; Cong et al., 1998). Given the Level I of inference for all its origins, the reconstruction of the four heads of the triceps can be directly applied for Eosauropterygia (Figure 3, Figure 11). Caution should be taken concerning the path of the medial sternoscapular ligament, namely the sternoscapular portion, because its attachment locations rely on a transformational hypothesis of the pectoral girdle during the peculiar evolution of the Eosauropterygia.
Insertion. The typical insertion is tendinous having the four heads of the triceps congregating on the olecranon process of the ulna (Zaaf et al., 1999; Russell and Bauer, 2008; Meers, 2003; Cong et al., 1998). However, there is no olecranon process on plesiosaurians, which suggests that this muscle was atrophied. Similarly, in whales the forelimb has an immobile cubital joint (Cooper et al., 2007). Although the triceps could still be present in plesiosaurians, its function could be to simply restrict the mobility of the cubital joint instead of flexing it as it happens in most reptiles (Russell and Bauer, 2008; Meers, 2003; Cong et al., 1998) - Level III of inference (Figure 4, Figure 11).
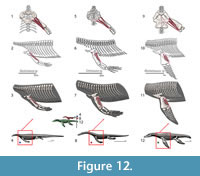 M. latissimus dorsi. Origin. In squamates, this superficial muscle originates dorsally on the neural spines of the last cervical and first seven dorsal vertebrae (Zaaf et al., 1999; Russell and Bauer, 2008), whereas the origin in crocodilians is on the first to the sixth dorsal vertebrae neural spines (Meers, 2003). In Cong et al. (1998), the last cervical neural spine through the fourth dorsal serves as the attachment, contrary to Meers (2003). The origin in Eosauropterygia is reconstructed with limited confidence (Level I of inference), from the first to the fourth thoracic; a more speculative reconstruction (Level II) could range from the last cervical to the seventh dorsal (Figure 3, Figure 12).
M. latissimus dorsi. Origin. In squamates, this superficial muscle originates dorsally on the neural spines of the last cervical and first seven dorsal vertebrae (Zaaf et al., 1999; Russell and Bauer, 2008), whereas the origin in crocodilians is on the first to the sixth dorsal vertebrae neural spines (Meers, 2003). In Cong et al. (1998), the last cervical neural spine through the fourth dorsal serves as the attachment, contrary to Meers (2003). The origin in Eosauropterygia is reconstructed with limited confidence (Level I of inference), from the first to the fourth thoracic; a more speculative reconstruction (Level II) could range from the last cervical to the seventh dorsal (Figure 3, Figure 12).
Insertion. The insertion of this muscle is highly conservative in Squamata (Zaaf et al., 1999; Russell and Bauer, 2008) and coincides in both crocodilian reconstructions (Cong et al., 1998; Meers, 2003). In turtles, lizards and crocodiles the tendinous insertion of the latissimus dorsi is on the proximal and dorsal surface of the humerus (Walker, 1973; Meers, 2003; Russell and Bauer, 2008). The reconstruction for the extinct forms is, thus, unambiguous (Level I inference). In nothosaurs, a distinct prominence on the dorsal surface of the humerus corresponds to the insertion of the latissimus dorsi (Bickelman and Sander, 2008). Proximally, in plesiosaurians the tuberosity of the humerus is detached by an isthmus from the humeral head (Figure 4, Figure 11). The dorsal surface of the tuberosity is frequently heavily striated thus corresponding to the insertion of the latissimus dorsi (Figure 4, Figure 11).
According to Cong et al. (1998) this muscle flexes the glenohumeral joint and according to Meers (2003) it also elevates the humerus. Cong et al. (1998) find an additional function to this muscle: it may cause posterior rotation while the arm is pronating. In most terrestrial squamates this muscle acts as a humeral retractor (Russell and Bauer, 2008).
M. pectoralis. Origin. In squamates it originates on the interclavicle: midventral and posterior border of the lateral process, along the lateral sheath of the rectus abdominis muscles and mesosternum, and on the posterior-most mesosternal rib (Zaaf et al., 1999; Russell and Bauer, 2008). Cong et al. (1998), Meers (2003) and Claessens (2004) agree that this muscle originates on the midline of the interclavicle (= episternum), on the posterolateral area of the interclavicle, as well as on the sternal portions of the thoracic ribs; but Cong et al. (1998) also considers the origin on the lateral side of the rectus abdominis. Reconstruction of the origin of this muscle in Eosauropterygia is difficult and relies on the presence of the presternum. Nevertheless, the hypothesis of creating a completely new origin for this muscle along the coracoid midline can be confidently rejected, because it is not supported at all by the phylogenetic bracket. Developmentally this muscle is one of the earliest to differentiate in crocodilians originating from the sternum (Kälin, 1929, figure 10). In squamates, Sewertzoff (1904) does not detail the pectoral girdle element from which the pectoralis (cited as superficial ventral mass) originates from, referring only the Rumpfregion (trunk region; p. 487). However, in Figure 6 (Sewertzoff, 1904, p. 490) Sewertzoff clearly shows that the origin of the superficial ventral mass (presumptive pectoralis) arises from an element medial to the coracoid, presumably the sternum. As a corollary, the pectoralis should be much reduced in pachypleurosaurs and nothosaurs originating from the atrophied presternum located on the posteromedial margin of the coracoid (Level I of inference), and it is absent or highly atrophied in pistosaurs and plesiosaurians (Level III of inference). A presumed origin from the gastralia is not substantiated developmentally and the myology of the extant phylogenetic bracket used here. Along these lines, Storrs (1986, 1991) and Hänni (2004) reported a weak or reduced insertion of this muscle in the humerus for Corosaurus alcovensis and Ceresiosaurus lanzi, respectively.
Insertion. The insertion of the pectoralis in the examined taxa is disparate topologically. Although it inserts unambiguously on the deltopectoral crest in turtles it attaches on the proximodorsal surface of the humerus (Walker, 1973), in crocodiles on the postaxial aspect of the deltopectoral crest (Meers, 2003), and in lizards on the preaxial aspect of the deltopectoral crest (Russell and Bauer, 2008). This implies that the reconstruction of the pectoralis is at best a Level II inference. Given that the pectoral girdle origin arises unambiguously from the posterior process of the interclavicle and this structure has been severely reduced in Eosauropterygia, this muscle is only interpretable for basal neodiapsids. For nothosaurs and plesiosaurians this muscle is thought to be atrophied (Level III of inference). This muscle is typically considered a humeral adductor and retractor, but some portions may be active during protraction and humeral rotation (Russell and Bauer, 2008).
Visceral Arch Musculature
M. episternocleidomastoideus. In Iguana and Gekko, this muscle originates on the posterolateral margin of the paroccipital process and inserts on the lateral process of the interclavicle (Russel and Bauer, 2008). The origin of this muscle could have also been reconstructed for plesiosaurians but the functional implications for locomotion are negligible. The osteological correlates for insertion of interclavicular muscles are insignificant; as this bone is essentially smooth [Keichousaurus hui (SMNS 81780), Nothosaurus miriabilis (SMNS1323), Ceresiosaurus calcagnii (PIMUZ T2464)].
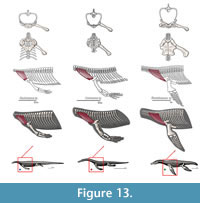 M. trapezius. Origin. In squamates it originates from the third cervical to the fourth dorsal vertebra (Russell and Bauer, 2008). Meers (2003) describes the origin of the trapezius on the thoracodorsal fascia. In Alligator sinensis the trapezius also originates on a fascia deeper to the dermis, from the anterior margin of the second row of cervical osteoderms to the surface of the first row of dorsal osteoderms (Cong et al., 1998). However, Cong et al. (1998) divides this muscle into deep and shallow portions. The deep portion originates on the dorsal zygapophyses of cervical vertebrae 3-8. In Eosauropterygia the trapezius originates from the last cervical vertebrae, thus accommodating the origins of the squamate and crocodilian bracket (Level I of inference; Figure 3 and Figure 13).
M. trapezius. Origin. In squamates it originates from the third cervical to the fourth dorsal vertebra (Russell and Bauer, 2008). Meers (2003) describes the origin of the trapezius on the thoracodorsal fascia. In Alligator sinensis the trapezius also originates on a fascia deeper to the dermis, from the anterior margin of the second row of cervical osteoderms to the surface of the first row of dorsal osteoderms (Cong et al., 1998). However, Cong et al. (1998) divides this muscle into deep and shallow portions. The deep portion originates on the dorsal zygapophyses of cervical vertebrae 3-8. In Eosauropterygia the trapezius originates from the last cervical vertebrae, thus accommodating the origins of the squamate and crocodilian bracket (Level I of inference; Figure 3 and Figure 13).
Insertion. In squamates, the trapezius inserts from the fourth to the last sternal rib and, on the scapula’s acromion, on the anterodorsal surface of the clavicle. In Sphenodon there is a thin insertion on the scapula’s acromion and a main insertion on the clavicle (Nash and Tanner, 1970; Robison and Tanner, 1962; Secoy, 1971). In crocodilians, it inserts on the anterior edge of the scapula dorsal to the acromion (Cong et al., 1998), whereas Meers (2003) considers it on the the anterior margin of the acromion, extending as a thin strip dorsoventrally. The clavicle in basal Eosauropterygia is divided by a ridge into an anterior and a posterior portion; this is especially evident in larger specimens [e.g., Ceresiosaurus calcagnii (PIMUZ T2464), nothosaur isolated clavicle SMNS 59822, Nothosaurus miriabilis (SMNS 1323)]. This ridge seems to divide the insertion of the trapezius from the origin of the clavodeltoideus; both sides have mediolaterally-oriented striations. In Eosauropterygia, we reconstruct the trapezius inserting on the anteroventral portion of the clavicle and perhaps extended posteriorly onto the scapula (Level I of inference, Figure 4 and Figure 13).This muscle reconstruction for Eosauropterygia seems to imply that the trapezius assisted in scapular girdle stabilization.
Somatic Musculature
M. episternohyoideus, M. omohyoideus, M. sternocoracoideus internus and externus. In Iguana, episternohyoideus (indistinguishable from sternohyoideus) originates from the central column of the interclavicles and inserts on the ceratobranchial and basihyal; M. omohyoideus originates on parts of the anterior surface of the clavicle and inserts on the ventromedial surface of the first ceratobranchial, but its attachment differs considerably from author to author; sternocoracoideus internus originates from the presternum and parts of the mesosternum and inserts tendinously on the secondary coracoid ray; sternocoracoideus externus originates on parts of the presternum and on adjacent areas to the sternocoracoideus internus (Russell and Bauer, 2008). The inference level for Eosauropterygia is I, because they all bear the interclavicle.
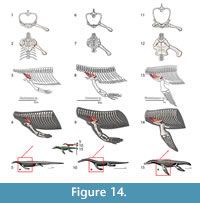 M. serratus. Origin. Cong et al. (1998) divides the serratus muscle in crocodilians into serratus superficialis ventralis (= serratus ventralis thoracis) and serratus profundus ventralis (= serratus ventralis cervicis). In squamates, the serratus muscle is divided into serratus anterior and serratus anterior superficialis. In squamates, the serratus anterior originates on the dorsolateral surface of the first three cervical ribs and the serratus anterior superficialis originates from the ventrolateral border of the third and fourth cervical ribs (Russell and Bauer, 2008). In Alligator sinensis, serratus ventralis originates on the lateral-most surface of the posterior cervical rib and on the three anterior dorsal ribs (Cong et al., 1998). Serratus profundus ventralis originates on the posterior-most five cervical ribs and on the lateral side of the first thoracic rib. The reconstruction in Eosauropterygia can be Level I if reconstructed only on the two first dorsal ribs, or Level II if extended to the third and fourth dorsal ribs (Figure 3, Figure 14).
M. serratus. Origin. Cong et al. (1998) divides the serratus muscle in crocodilians into serratus superficialis ventralis (= serratus ventralis thoracis) and serratus profundus ventralis (= serratus ventralis cervicis). In squamates, the serratus muscle is divided into serratus anterior and serratus anterior superficialis. In squamates, the serratus anterior originates on the dorsolateral surface of the first three cervical ribs and the serratus anterior superficialis originates from the ventrolateral border of the third and fourth cervical ribs (Russell and Bauer, 2008). In Alligator sinensis, serratus ventralis originates on the lateral-most surface of the posterior cervical rib and on the three anterior dorsal ribs (Cong et al., 1998). Serratus profundus ventralis originates on the posterior-most five cervical ribs and on the lateral side of the first thoracic rib. The reconstruction in Eosauropterygia can be Level I if reconstructed only on the two first dorsal ribs, or Level II if extended to the third and fourth dorsal ribs (Figure 3, Figure 14).
Insertion. The serratus anterior superficialis inserts on the episcapular cartilage (=suprascapular cartilage) both on the posterior border and on the posteromedial surface. The serratus anterior inserts on the medial face of the episcapula in Iguana (Russell and Bauer, 2008). In Alligator sinensis and on other crocodilians the serratus superficialis ventralis inserts medially on the scapula, on the episcapular cartilage and along the posterior margin of the scapula as a thin strip. M. serratus profundus ventralis inserts on the area where the episcapular cartilage and the dorsal end of the scapula meet extending anteroposteriorly. In all Eosauropterygia the level of inference is Level I if we reconstruct the episcapular cartilage, however, the attachment on the posterior margin of the scapula that it implies is a Level II inference, because it is only supported by the crocodilian bracket (Figure 4, Figure 14). Although the dorsal blade of the scapula is very difficult to observe in lateral view in most pachypleurosaurs, in Serpianosaurus (PIMUZ T3675) a discernable smooth surface is evident. In larger nothosaur specimens, but especially in the isolated scapulae of SMNS 84069 and GPIT RE-1533, faint but visible striae and pitting run along the dorsal blade extention of the scapula, particularly on the dorsal tip. The striation density contrast between the lateral side and the medial side of the scapula is notable. Similarly to the trapezius m., the serratus m. seems to have assisted in the scapular girdle stabilization in Eosauropterygia.
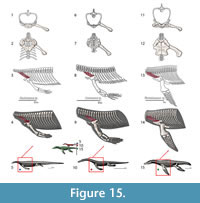 M. levator scapulae. Origin. Cong et al. (1998) divide the muscle into a profundus and superficialis portion. Meers (2003) considers this muscle a single unit. In squamates, the levator scapulae originates on the transverse process of the atlas. In crocodilians, on the medial and lateral sides of the scapula, it also originates on the anterior cervical ribs, more specifically, according to Cong et al. (1998), the profundus portion on the fourth cervical rib and the superficialis portion on the lateral surface of the first to the third cervical ribs. The origin in the atlas and on the anterior cervical ribs seems unlikely, given the elongation of the cervical region in Eosauropterygia. This is a small muscle related to the stabilization of the pectoral girdle, a function of diminished importance in the aquatic realm. If not completely atrophied, this muscle would have originated in the last cervical ribs, which implies a Level III of inference (Figure 3, Figure 15).
M. levator scapulae. Origin. Cong et al. (1998) divide the muscle into a profundus and superficialis portion. Meers (2003) considers this muscle a single unit. In squamates, the levator scapulae originates on the transverse process of the atlas. In crocodilians, on the medial and lateral sides of the scapula, it also originates on the anterior cervical ribs, more specifically, according to Cong et al. (1998), the profundus portion on the fourth cervical rib and the superficialis portion on the lateral surface of the first to the third cervical ribs. The origin in the atlas and on the anterior cervical ribs seems unlikely, given the elongation of the cervical region in Eosauropterygia. This is a small muscle related to the stabilization of the pectoral girdle, a function of diminished importance in the aquatic realm. If not completely atrophied, this muscle would have originated in the last cervical ribs, which implies a Level III of inference (Figure 3, Figure 15).
Insertion. In squamates, the levator scapulae inserts on the anterolateral and anteroventral face of the episcapular cartilage, and on the medial-most portion of the clavicular bar; in crocodilians, on the anterior margin of the scapula extending dorsoventrally as a thin strip, but Cong et al. (1998) extend the insertion of the superficialis dorsally to the episcapular cartilage. In Eosauropterygia, we reconstruct the levator scapulae on the dorsal blade of the scapula. It is inferred in Eosauropterygia as Level I for the fibers inserting on the episcapular cartilage and Level II on the anterior margin of the scapula (Figure 4, Figure 15). In Eosauropterygia, the levator scapulae m. - as the trapezius m. and serratus m. - aided in scapular girdle stabilization.
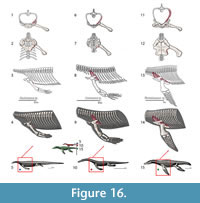 M. costocoracoideus. Origin. Meers (2003) divides into two bundles the costocoracoideus pars superficialis and pars profundus, but Cong et al. (1998) only consider the muscle as a single unit. In squamates, it originates on the costal cartilage of the first sternal rib (Russell and Bauer, 2008). It is possible that the costocoracoideus got atrophied in Eosauropterygia, given its functional role as a stabilizer of the coracoid (Level III of inference), thus of potentially reduced role in an aquatic medium. By using the extant phylogenetic bracket, it seems unlikely that the costocoracoideus would have arisen from the costal cartilage because this cartilage is not present in Eosauropterygia (see above). We reconstructed the origin of the costocoracoideus on the first dorsal rib (Level II of inference; Figure 3 and Figure 16).
M. costocoracoideus. Origin. Meers (2003) divides into two bundles the costocoracoideus pars superficialis and pars profundus, but Cong et al. (1998) only consider the muscle as a single unit. In squamates, it originates on the costal cartilage of the first sternal rib (Russell and Bauer, 2008). It is possible that the costocoracoideus got atrophied in Eosauropterygia, given its functional role as a stabilizer of the coracoid (Level III of inference), thus of potentially reduced role in an aquatic medium. By using the extant phylogenetic bracket, it seems unlikely that the costocoracoideus would have arisen from the costal cartilage because this cartilage is not present in Eosauropterygia (see above). We reconstructed the origin of the costocoracoideus on the first dorsal rib (Level II of inference; Figure 3 and Figure 16).
Insertion. In squamates, the costocoracoideus inserts on the scapulosternal ligament posteriorly. In crocodilians, the costocoracoideus pars profundus inserts on the proximoposterior edge of the coracoids and on the anterolateral border of the sternal plate (Meers, 2003). The insertion, as described by Cong et al. (1998), corresponds to that of costocoracoideus pars superficialis in Meers (2003). In Eosauropterygia, the insertion of the costocoracoideus extends anteroposteriorly from the median coracoid contact to the glenoid, if we follow the insertion of the costocoracoideus pars superficialis in crocodilians. The insertion point of this muscle is unclear in plesiosaurians. On one hand, the insertion in squamates is ligamentous and, for Eosauropterygia, the presence of this ligament is well supported by both ends of the phylogenetic bracket; but on the other hand this muscle has a bony origin in crocodilians. A more conservative reconstruction is adopted here where only bony attachments are considered, thus we reconstructed this muscle to insert on the lateral edge of the coracoid (Figure 3, Figure 16). In Eosauropterygia, the costocoracoideus m. served most likely for the stabilization of the coracoid, given the reconstruction of its attachments.
DISCUSSION
Comparison with Previous Reconstructions
 Figure 17 depicts all previously published plesiosaurian pectoral girdle reconstructions (Watson, 1924; Tarlo, 1958; Robinson, 1975; Lingham-Soliar, 2000; Carpenter et al., 2010). None of the previous reconstructions included ligaments, the coracoid origin of part of the triceps, the trapezius or the costocoracoideus. Tarlo (1958) was the only author to consider the origin of the serratus, levator scapulae and scapulodeltoideus, but via a mistaken topological homology. Carpenter et al. (2010) are the only authors to consider the biceps, but mistakenly reconstructed it on the scapula, which is unsupported by both branches of the extant phylogenetic bracket. It is important to note, however, that the Carpenter et al.’s (2010) reconstruction considers a different phylogenetic bracket (turtles and squamates) than the one proposed here (archosaurs and squamates). Nevertheless, the origin of the biceps in turtles is on the posterior coracoid (Lutz et al., 1996) and not on the scapula.
Figure 17 depicts all previously published plesiosaurian pectoral girdle reconstructions (Watson, 1924; Tarlo, 1958; Robinson, 1975; Lingham-Soliar, 2000; Carpenter et al., 2010). None of the previous reconstructions included ligaments, the coracoid origin of part of the triceps, the trapezius or the costocoracoideus. Tarlo (1958) was the only author to consider the origin of the serratus, levator scapulae and scapulodeltoideus, but via a mistaken topological homology. Carpenter et al. (2010) are the only authors to consider the biceps, but mistakenly reconstructed it on the scapula, which is unsupported by both branches of the extant phylogenetic bracket. It is important to note, however, that the Carpenter et al.’s (2010) reconstruction considers a different phylogenetic bracket (turtles and squamates) than the one proposed here (archosaurs and squamates). Nevertheless, the origin of the biceps in turtles is on the posterior coracoid (Lutz et al., 1996) and not on the scapula.
In previous publications the musculature was not reconstructed using Cryptoclidus as a model; however, it is here used in order to facilitate comparison. All reconstructions consider the supracoracoideus and the coracobrachialis. Both these muscles are reconstructed as originating on the ventral surface of the coracoid with the coracobrachialis topologically posterior to the supracoracoideus. The coracobrachialis is congruently reconstructed with largest area of attachment in all reconstructions. Robinson’s reconstruction of the supracoracoideus assumes the presence of cartilage on the pectoral vacuity and extends the origin of the supracoracoideus to the scapula. Thus the topological homology through the evolution of the coracoid foramen is misinterpreted, and also reconstruction of the scapular portion of the supracoracoideus is not supported by the extant phylogenetic bracket.
The M. pectoralis is reconstructed as originating from the gastralia both in Watson (1924) and Tarlo (1958), however, this origin is not present in crocodilians or squamates. Other reconstructions depict the medial contact of the coracoids as the origin of this muscle, but Robinson’s (1975) reconstruction extends to the scapula. Neither of these reconstructions is congruent with the extant phylogenetic bracket because in both crocodilians and squamates the pectoralis originates from the interclavicle.
Tarlo’s (1958) reconstruction of the scapular muscles does not seem to have tracked through Eosauropterygia evolution muscle attachments following homologous anatomical parts. This is important because: (1) the dorsal process of the scapula in plesiosaurians is homologous to the dorsal process of the scapula in pachypleurosaurs, nothosaurs and pistosaurs; and (2) according to the extant phylogenetic bracket, muscles such as the scapulodeltoideus, levator scapulae and serratus attach at the dorsal portion of the scapula, which is homologous to the dorsal process of the scapula in Eosauropterygia. Thus, the scapulodeltoideus, the serratus and the levator scapulae cannot originate from the anterior portion of the scapula, which is non-homologous with the dorsal process of the scapula in Eosauropterygia, as proposed by Tarlo (1958).
Most reconstructions of the clavodeltoideus place its origin on the clavicle, with the exception of Carpenter et al.’s (2010) reconstruction, which considers the origin of the muscle only on the scapula. The extant phylogenetic bracket supports both hypotheses, however, a scapular portion of the clavodeltoideus is also present in squamates (Russell and Bauer, 2008).
The scapulohumeralis is considered to have originated on the medial portion of the scapula near the contact with the adjacent scapula in Watson’s (1940) and Robinson’s (1975) reconstructions, but is reconstructed on the anterior tip of the scapula by Tarlo (1958). Lingham-Soliar (2000), probably following the squamate branch of the extant phylogenetic bracket, reconstructed two divisions of the scapulohumeralis, an anterior and a posterior division. However, a two-division scapulohumeralis is not supported by the crocodilian branch of the extant phylogenetic bracket.
In contrast, reconstructions for non-plesiosaurian eosauropterygians are not so disparate (Storrs 1986, 1991; Lin and Rieppel, 1998). Lin and Rieppel’s (1998) reconstruction is essentially similar to what is presented here with a difference consisting of the origin of the pectoralis and clavodeltoideus, which have been discussed above. However, Storrs (1986, 1991) proposes “large amounts of cartilage” on the pectoral girdle are neither supported by the extant phylogenetic bracket nor in the light of developmental patterns in squamates or crocodilians.
Major Evolutionary Trends of the Pectoral Girdle of Eosauropterygia
The stringent physical conditions imparted by a re-adaptation to an aquatic lifestyle led to a reorganization of the locomotor apparatus in Eosauropterygia. As Carroll and Gaskill (1985) correctly noted, the formation of a large pectoral fenestra in eosauropterygians is not indicative of the primitive condition, but rather a specialization. There was a functional specialization in the types of movement operated for swimming materialized by a physical specialization of particular muscle groups. Penguins (Schreiweis, 1982), pinnipeds (Murie, 1871) and other secondarily-adapted paraxial swimmers have also specialized pectoral girdle musculature with atrophy of certain muscles and increased muscle mass in others. In eosauropterygians, the atrophy of the scapular dorsal blade muscles (i.e., developmentally called somatic musculature) is a conspicuous manifestation of such specialization. But on the other hand, the dramatic increase of attachment area for the shoulder musculature [ventral mass in Sewertzoff’s (1904) classification] on the coracoid, plus, in plesiosaurians the radical increase of attachment surface for the scapular portion of the clavodeltoideus, counterbalances the effects of muscle atrophy with an optimization of other specific muscle groups. This re-arrangement of the pectoral girdle in Eosauropterygia and the myology thereof has striking functional implications. Contra Lin and Rieppel (1998), the functional re-arrangement of the muscles is not adductors in detriment of the abductors. Instead, the novel reorganization of the eosauropterygian pectoral girdle favors protraction and retraction movements (contra Carpenter et al., 2010). Contrary to the rationale presented by Carpenter et al. (2010), the glenoid architecture reflects the motion to the extent the glenoid must resist forces applied to the limb, thus, should also reflect the dominant motion vectors.
ACKNOWLEDGEMENTS
This work is dedicated to the memory of RA’s brother. RA is very grateful to B.D. Gomes for the help and companionship during the museum research trips entailed in this project. C. Hendrickx, E. Tschopp for useful discussions. Thanks to P. Havlik, H. Furrer, N. Bardet and R. Schoch for accessing to specimens. D.Winkler and L.L. Jacobs perused the manuscript and help me to greatly improve earlier versions. I would also like to thank M. Polcyn for the many discussions on marine reptile myology, from which some ideas expressed here derived. The comments by two anonymous reviewers improved the manuscript additionally.
REFERENCES
Andrews, C.W. 1910. A Catalogue of the Marine Reptiles of the Oxford Clay, Part I. British Museum (Natural History),London.
Bardet, N. 1999. A new elasmosaurid plesiosaur from the Lower Jurassic of Southern France. Paleontology, 42:927-952.
Benjamin, M., Kaiser, E., and Milz, S. 2008. Structure-function relationships in tendons: a review. Journal of Anatomy, 212:211-228.
Bickelmann, C., Müller, J., and Reisz, R.R. 2009. The enigmatic diapsid Acerosodontosaurus piveteaui (Reptilia: Neodiapsida) from the Upper Permian of Madagascar and the paraphyly of ‘‘younginiform’’ reptiles. Canadian Journal of Earth Sciences, 46:651-661.
Caldwell, M. 1996. Ichthyosauria: a preliminary phylogenetic analysis of diapsid affinities. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 200:361-386.
Carpenter, K., Sanders, F., Reed, B., Reed, J., and Larson, P. 2010. Plesiosaur swimming as interpreted from skeletal analysis and experimental results. Transactions of the Kansas Academy of Sciences, 13(1-2):1-34.
Carrano, M.T. and Hutchinson, J.R. 2002. Pelvic and Hindlimb Musculature of Tyrannosaurus rex (Dinosauria: Theropoda). Journal of Morphology, 253:207-228.
Carroll, R. 1981. Plesiosaur ancestors from the Upper Permian of Madagascar. Philosophical Transactions of the Royal Society B, 293:315-383.
Carroll, R.L. and Gaskill, P. 1985. The nothosaur Pachypleurosaurus and the origin of plesiosaurs. Philosophical Transactions of the Royal Society of London B, 309:343-393
Claessens, L.P.A.M. 2004. Dinosaur gastralia; origin, morphology, and function. Journal of Vertebrate Paleontology, 24:89-106.
Cong, L., Hou, L.-H., and Wu, X.C. 1998. The gross anatomy of Alligator sinensis Fauvel [in Chinese with English summary]. Beijing: CIP, China.
Conybeare, W.D. 1824. Additional notices on the fossil genera Ichthyosaurus and Plesiosaurus. Transactions of the Geological Society of London, 1,2:103-123.
Cooper, L.S., Dawson, S.D., Reidenberg, J.S., and Berta, A. 2007. Neuromuscular anatomy and evolution of the cetacean forelimb. Anatomical Record, 290:1121-1137.
Currie, P.J. and Carroll, R.L. 1984. Ontogenetic changes in the eosuchian reptile Thadeosaurus. Journal of Vertebrate Paleontology, 4: 68-84.
Diogo, R. and Abdala, V. 2010. Muscles of Vertebrates: Comparative Anatomy, Evolution, Homologies and Development. Science Publishers, New Hampshire.
Drevermann, F.R. 1933. Die Placodontier. 3. Das Skelett von Placodus gigas Agassiz im Senckenberg-Museum. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft, 38:319-364.
Hänni, K. 2004.Die Gattung Ceresiosaurus. Hochschulverlag AG an der ETH Zürich.
Harris, J.M. and Carroll, R.L. 1977. Kenyasaurus, a new eosuchian reptile from the Early Triassic of Kenya. Journal of Paleontology, 51: 139-149.
Howell, A.B. 1936. Morphogenesis of the shoulder architecture. Part IV. Reptilia. Quarterly Review Biology, 11:183-208.
Hyman, L.H. 1922 Comparative vertebrate anatomy. University of Chicago Press.
Hieronymus, T. 2006. Quantitative microanatomy of jaw muscle attachment in extant diapsids. Journal of Morphology, 267: 954-967.
Jasinoski, S.C., Russell, A.P., and Currie, P.J. 2006. An integrative phylogenetic and extrapolatory approach to the reconstruction of dromaeosaur (Theropoda: Eumaniraptora) shoulder musculature. Zoological Journal of the Linnean Society, 146:301-344.
Jenkins, F.A. Jr. and Goslow, G.E. Jr. 1983. The functional anatomy of the shoulder of the savannah monitor lizard(Varanus exanthematicus). Journal of Morphology, 175:195-216.
Kälin, J.A. 1929. Über den Brutschuterapparat der Krokodile. Acta Zoologica, Stokholm, 10: 343-399.
Kirschner, M. and Gerhart, J. 1998. Evolvability. Proceedings of the National Academy of Sciences, 95:8420-8427.
Klein, N. 2010. Long bone histology of Sauropterygia from the Lower Muschelkalk of the Germanic Basin provides unexpected implications for phylogeny. PLoS ONE 5(7):e11613. doi:10.1371/journal.pone.0011613.
Li, C. and Rieppel, O. 2002. A new cyamodontoid placodont from Triassic of Guizhou, China. Chinese Science Bulletin, 47:403-407.
Lin, K. and Rieppel, O. 1998. Functional morphology and ontogeny of Keichousaurus hui (Reptilia, Sauropterygia). Fieldiana (Geology), 39:1-35.
Linder, H. 1913. Beitrage zur Kenntnis der Plesiosauria-Gattungen Peloneustes und Plesiosaurus. Geologische und Palæontologische Abhandlungen, 11: 339-409.
Lingham-Soliar T. 2000. Plesiosaur locomotion: is the four-wing problem real or merely an atheoretical exercise? Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 217:45-87.
Lutz, P.L., Musick, J.A., and Wyneken, J. 1996. The biology of sea turtles Volume II. Washington: CRC Press.
Mazin, J.-M. and Pinna, G. 1993. Paleoecology of the armoured placodonts. Paleontologia Lombarda, 2:83-91.
Meers, M.B. 2003. Crocodylian forelimb musculature and its relevance to Archosauria. Anatomical Record A, 274A:891-916.
Merck, J.W. 1997. A phylogenetic analysis of the euryapsid reptiles. Journal of Vertebrate Paleontology, 17:65A.
Motani, R. 2009. The Evolution of Marine Reptiles. Evolution: Education and Outreach, 2:224-235.
Müller, J. 2003. Early loss and multiple return of the lower temporal arcade in diapsid reptiles. Naturwissenschaften, 90: 473-476. doi:10.1007/s00114-003-0461-0.
Murie, J. 1871. Researches upon the anatomy of the Pinnipedia. Part I. On the walrus (Trichechus rosmarus Linn.). Transactions of the Zoological Society London, 7:411-464
Nash, D.E. and Tanner, W.W. 1970. A comparative study of the head and thoracic osteology and myology of the skinks Eumeces gilberti Van Denburgh and Eumeces skiItonianus (Baird and Girard). Brigham Young University Science Bulletin Series, 12:1-32.
Nicholls, E.L. and Russell, A.P. 1991. The plesiosaur pectoral girdle: the case for a sternum. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 182:161-185.
Owen, R. 1860. Palaeontology; or, a systematic summary of extinct animals and their geologic remains. Adam and Charles Black, Edinburgh.
Peterson, J.A. 1973. Adaptation for arboreal locomotion in the shoulder region of lizards. Ph.D. Thesis, University of Chicago.
Pinna, G. 1980. Lo scheletro postcraniale di Cyamodus hildegardis Peyer, 1931, descrito su un esemplare del Triassico Medio Lombardo. Atti della Societa Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano, 1 21:275-306.
Piveteau, J. 1926. Paléontologie de Madagascar, XIII -- Amphibiens et reptiles Permiens. Annales Paléontologie, 15:55-178.
Piveteau, J. 1955. Existence d’un reptile du groupe Procolophonides à Madagascar. Conséquences stratigraphiques et paleontologiques. Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences, 241:1325-1327.
Rieppel, O. 1993. Euryapsid relationships: a preliminary analysis. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 188:241-264.
Rieppel, O. 1993. Studies on skeleton formation in reptiles. IV. The homology of the reptilian (amniote) astragalus revisited. Journal of Vertebrate Paleontology, 13:31-47.
Rieppel, O. 1994. Studies on skeleton formation in reptiles. VI. Patterns of ossification in the skeleton of Lacerta agilis exigua Eichwald (Reptilia: Squamata). Journal of Herpetology, 28:145-153.
Rieppel, O. 2001. Turtles as hopeful monsters. BioEssays, 23:987-991.
Rieppel, O. 2008. The relationships of turtles within amniotes, p. 345-353. In Wyneken J., Godfrey H., and Bels V. (eds.), Biology of turtles. CRC press, Taylor and & Francis Group, Boca Raton.
Robinson, J.A. 1975. The locomotion of plesiosaurs. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 149: 286-332.
Robinson, J.A. 1977. Intercorporal force transmission in plesiosaurs. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 153: 86-128.
Robison, W.G. and Tanner W.W. 1962. A comparative study of the species of the genus Crotaphytus Holbrook (Iguanidae). Brigham Young University Science Bulletin Biological Series, 2:1-31.
Romer, A.S. 1956. Osteology of the Reptiles. Chicago, Chicago University Press.
Russell, A.P. and Bauer, A. 2008. The appendicular locomotor apparatus of Sphenodon and normal-limbed squamates, p. 1-465. In Gans, C., Gaunt, A.S., and Adler, K., (eds.), Biology of the Reptilia, vol. 21 (The skull and appendicular locomotor apparatus of Lapidosauria) Society for the study of amphibians and reptiles, Ithaca, New York.
Sander, P.M., Rieppel, O.C., and Bucher, H. 1997. A new pistosaurid (Reptilia: Sauropterygia) from the Middle Triassic of Nevada and its implications for the origin of the plesiosaurs. Journal of Vertebrate Paleontology, 17:526-533.
Sato, T., Cheng, Y.-N., Wu, X.-C., and Li, C. 2011. Osteology of Yunguisaurus Cheng et al., 2006 (Reptilia; Sauropterygia), a Triassic pistosauroid from China. Paleontological Research, 14:179-195
Sato T., Zhao L.-J., Wu X.-C., and Li C. 2014. A new specimen of the triassic pistosauroid Yunguisaurus, with implications for the origin of plesiosauria (Reptilia, Sauropterygia). Palaeontology, 57:55-76.
Schauinsland, H. 1900. Weitere Beiträge zur Entwicklungsgeschichte der Hatteria. Skelettsystem, schallleitender Apparat, Hirnnerven etc. Archiv fur Mikroskopische Anatomie und Entwicklungsgeschichte, 56:747–867.
Schauinsland, H. 1903 Beitrage zur Entwicklungsgeschichte und Anatomie der Wirbeltiere Sphenodon, Callorhynchus, Chamiileo. Zoologica, 16:99-143.
Schreiweis, D.O.A. 1982. Comparative study of the appendicular musculature of Penguins (Aves, Sphenisciformes). Smithsonian Contributions Zoology, 341.
Secoy, D. 1971. Myology of Sceloporus c. clarki Baird and Girard. Brigham Young University Sciences Bulletin Biological Series, 14:1-22.
Sewertzoff, Von A.N. 1904. Die Entwickelung der pentadactylen Extremität der Wirbeltiere. Anatomical Anzeiger, 25:472-495.
Shubin, N. 1995. The evolution of paired fins and the origin of tetrapod limbs: phylogenetic and transformational Approaches, p. 39-86. In Hecht, M.K., Macintyre, R.J., and Clegg, M.T. (eds.), Evolutionary Biology. Plenum Press, New York.
Smith, A. and Vincent, P. 2010. A new genus of pliosaur (Reptilia: Sauropterygia) from the lower Jurassic of Holzmaden, Germany. Palaeontology 53:1049-1063.
Storrs, G. 1986. Anatomy and relationships of Corosaurus alcovensis (Reptilia: Nothosauria) and the Triassic Alcova Limestone of Wyoming. PhD dissertation, Yale University.
Storrs, G.W. 1991. Anatomy and relationships of Corosaurus alcovensis (Diapsida: Sauropterygia) and the Triassic Alcova Limestone of Wyoming. Bulletin of the Peabody Museum of Natural History, 44:1-151.
Storrs, G.W. 1993. Function and phylogeny in sauropterygians (Diapsida) evolution. American Journal of Science, 293A:63-90.
Sues, H.-D. 1987. Postcranial skeleton of Pistosaurus and interrelationships of Sauropterygia (Diapsida). Zoological Journal of the Linnean Society, 90:109-131.
Tarlo, L.B. 1958. The scapula of Pliosaurus macromerus Phillips. Palaeontology, 1:193-199.
Tsuihiji, T. 2010. Reconstructions of the axial muscle insertions in the occipital region of dinosaurs: evaluations of past hypotheses on Marginocephalia and Tyrannosauridae using the Extant Phylogenetic Bracket Approach. Anatomical Record, 293:1360-1386.
Tumarkin-Deratzin, A.R. 2009. Evaluation of long bone surface textures as ontogenetic indicators in centrosaurine ceratopsids. Anatomical Record, 292:1485-1500.
Tumarkin-Deratzian, A.R., Vann, D.R., and Dodson, P. 2006. Bone surface texture as an ontogenetic indicator in long bones of the Canada goose Branta canadensis (Anseriformes: Anatidae). Zoological Journal of the Linnean Society, 148:133-168.
Tumarkin-Deratzian, A.R., Vann, D.R., and Dodson, P. 2007. Growth and textural ageing in long bones of the American alligator Alligator mississippiensis (Crocodylia: Alligatoridae). Zoological Journal of the Linnean Society, 150:1-39.
Watson, D.S. 1924 The elasmosaur shoulder girdle and fore-limb. Proceedings of the Zoological Society of London, 2:885-917.
Welles, S.P. 1962. A new species of elasmosaur from the Aptian of Colombia and a review of the Cretaceous plesiosaurs. University of California Publications Geological Sciences,44:1-89.
White, T.E. 1940. Holotype of Plesiosaurus longirostris Blake and classification of the plesiosaurs. Journal of Paleontology, 14:451-467.
Witmer, L. 1995. The Extant Phylogenetic Bracket and the importance of reconstructing soft tissues in fossils, p. 19-33. Thomason, J. (ed.). Functional morphology in vertebrate paleontology. Cambridge University Press, Cambridge.
Zaaf, A., Herrel, A., Aerts, P., and De Vree, F. 1999. Morphology and morphometrics of the appendicular musculature in geckoes with different locomotor habits (Lepidosauria). Zoomorphology, 119:9-22.
Zumwalt, A. 2005. A new method for quantifying the complexity of muscle attachment sites. Anatomical Record B: New Anatomy, 286B:21-28.

