DISCUSSION
E.
E. Williams (1972) stated why West Indian anoles are well-suited subjects
for the study of ecological principles: They are taxonomically well known. There
are large museum collections providing a source for metric data. They are
diverse. And they are distributed over a large number of islands with varying
levels of diversity, which allows each island fauna to be used as an experiment
of nature (his term) to test ecological hypotheses. While those attributes are
valid, complicated issues relevant to longer time scales (e.g., the
biogeographical stratification of island faunas and the phylogenetic
relationships among scattered island species) are often difficult to resolve.
Ecological, phylogenetic, and paleontological approaches have been applied, each
elucidating some aspect of diversity, origin, biogeography, or ecology, but none
alone or in aggregate is totally satisfactory. For example, Losos
and de Queiroz (1997) proposed that the ancestral Greater Antillean anole
may have been a trunk-crown ecomorph, which could be consistent with
phylogenetic analysis, with a South American origin, and with the meager fossil
record (de Queiroz et al. 1998).
But the fossil record, while suggestive, is insufficient to test this hypothesis
rigorously. Nevertheless, new data from this and other studies contribute to
understanding the historical and ecological development of the Caribbean fauna.
Taphonomy
Iturralde-Vinent
and MacPhee (1996) document an age of 15-20 Ma for Dominican amber. They
further demonstrate the likelihood that amber-bearing deposits in Hispaniola are
derived from a single sedimentary basin. Vegetation within the basin is known to
include the resin-producing legume Hymenaea protera, which gave rise to
the amber, Acacia eocaribbeanensis, half a dozen or so other angiosperms
represented by flowers in amber, two genera of bamboo, an epiphytic fern (Grammitis
succinea), two genera of mosses, and 10 liverworts (Hueber
and Langenheim 1986, Dilcher et
al. 1992, Graham 1992, Poinar
1991, 1992).
Hymenaea courbaril, an extant resin-producing species of Mexico, Central
America, South America, and the Antilles, grows in a variety of habitats and
reaches a height of 55 m (Poinar
1992). Resin is produced in all growing stages, but in mature trees resin
issues from openings to resin cavities in the cambial zone. Hymenaea
courbaril is pollinated by glossophagine bats.
The placement of the AMNH and NMBA specimens
within the trunk-crown ecomorph is consistent with exposure to weeping sap from Hymenaea
limbs, and therefore the ultimate incorporation of these particular lizards into
amber as inclusions. An ecology that facilitates the association of sap and
organisms is necessary because entombment must take place before introduction
into a sedimentary environment. For animals larger than insects, but still too
small to escape from the sticky sap, death and subsequent entombment is a
possibility; especially if their ecology places them in proximity.
The SMU 74976 specimen, as observed under a
dissecting microscope and with CT data, is substantially de-fleshed and has
missing bony elements. The nasals, right prefrontal, and the majority of the
palate are missing without damage to adjacent bones. In contrast, the damage to
the parietal is accompanied by breaks and many adjacent bones are missing,
including the right temporal arcade, right quadrate, and the right posterior
mandible. The pattern of damage appears to indicate trauma that excised a
portion of the right rear of the skull. None of the missing bones is present in
the amber specimen, although a portion of the left hyoid arch is still in place,
a delicate structure that could easily have been floated off. The pattern of
bone damage and loss as illustrated by CT images suggests to us an initial
trauma to the posterior portion of the head, followed by some degree of
decomposition and loss of flesh, resulting in further loss of bones from the
anterior and palatal regions. Subsequent total engulfment in resin preserved the
head, albeit with an unknown degree of further decomposition. 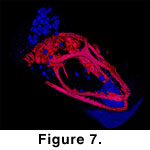 The
pattern and orientation of air bubbles entrapped in the amber of SMU74796
indicate the specimens skull was in an upright position and out-gassing due to
decompostion was primarily concentrated in the damaged rear portion of the skull
as illustrated in Figure 7. All three of
the amber anole specimens exhibit damage to the skull roof primarily in the
region of the parietal and temporal arcade.
The
pattern and orientation of air bubbles entrapped in the amber of SMU74796
indicate the specimens skull was in an upright position and out-gassing due to
decompostion was primarily concentrated in the damaged rear portion of the skull
as illustrated in Figure 7. All three of
the amber anole specimens exhibit damage to the skull roof primarily in the
region of the parietal and temporal arcade.
Damage to the skull roof could have been inflicted
by predators. Documented predators of modern Caribbean anoles are predominantly
birds, specifically American kestrels (Falco sparverius)
and pearly-eyed thrashers (Magarops fuscatus) (Adolf
and Roughgarden 1983, McLaughlin
and Roughgarden 1989, Roughgarden
1995). Andrews (1990) noted
that the kestrel Falco tinnunculus sometimes consumes only part of its
prey, pulling flesh through a hole at the top of the thorax, leaving skin with
some bone behind. Further, the damage exhibited in the three amber anoles is
consistent with damage patterns documented in bird predation on small mammals (Andrews
1990). Birds have been identified from Dominican amber from preserved
feathers. The only identified feather is tentatively referred to the Picidae,
woodpeckers and their relatives (Poinar
1992). Nevertheless, it is reasonable to assume that predatory birds played
a similar ecological role with respect to anoles in the Caribbean during the
Miocene as they do now. Although not conclusive, the common pattern of damage to
the three amber-preserved anoles appears indicative of aborted avian predation.
If so, their final entombment in resin occurred passively after death, followed
by incorporation into the depositional system of a single sedimentary basin some
15 to 20 million years ago.
Historical Biogeography
The age of the sediments containing amber as
determined by Iturralde-Vinent
and MacPhee (1996) provides the younger age limit for the first occurrence
of anoles on Hispaniola and also the oldest fossil evidence for anoles anywhere
in the Caribbean. However, because the earliest fossil evidence does not
necessarily correspond clearly to the time of introduction especially where the
fossil record is poor, the Dominican anoles do not set an acceptable older age
limit for anoles first entering the Caribbean region. Nor do they provide
definitive information as to the biogeographical route or mechanism utilized.
Evidence relevant to those issues must be garnered from other sources.
Iturralde-Vinent
and MacPhee (1999 and references therein) elaborated a biogeographical model
based on geological evidence, which provides both a mechanism and an older age
limit for the introduction of anoles to Hispaniola. Fundamental to their model
is the recognition that continual emergence of any current Caribbean island does
not extend older than 35 million years ago. Before that time islands certainly
existed, but subsidence and sea level rise prior to 35 million years ago drowned
all existing land surfaces. The land fauna of early Cenozoic Caribbean islands
would have been extirpated by inundation. Between 35 and 33 Ma, the
Eocene-Oligocene transition, tectonic uplift coincided with a drop in sea level,
which according to Iturralde-Vinent
and MacPhee (1999) resulted in exposure of what they term the "GAARlandia
landspan." A landspan is simply a subaerial connection between a continent,
in this case South America, and off-shelf islands, specifically the Greater
Antilles joined by an emergent Aves Ridge, hence the name GAARlandia. Subsequent
to 33 million years ago, general subsidence and sea level rise caused
connections of GAARlandia to founder and existing islands to form. Originally,
the landspan connection with South America provided a dispersal route to
Hispaniola and other presumptive islands of the Greater Antilles, followed by
the drowning of the connection, which facilitated alternately continent-island
and island-island vicariance. This model is certainly extreme compared to
concepts of Caribbean biogeography that attribute a greater permanency to
islands and rely on over-water dispersal to distribute island fauna (e.g., Hedges
1996). However, the extremes are not mutually exclusive in the context of
geologic time.
The important points for this study are that the
uplift and sea level drop between 35 and 33 million years ago provide a maximum
age for the introduction of anoles to any existing island. Subsequent
biogeographical events would involve vicariance certainly and over-water
dispersal possibly. In any event, anoles arrived on Hispaniola between 35 and 15
million years ago at most. Given the uncertainties involved, anoles could have
colonized what is now Hispaniola between 33 and 20 million years ago. This
represents a maximum span of 20 million years and a minimum of 13, which seems a
rather long interval in which to constrain a biogeographical event except when
compared to the previous less constrained interval that could accommodate 50
million years or more if the origin of Caribbean anoles was taken back into the
Cretaceous.
Evolutionary Pattern
Within that 13 million-year interval, T-clade and
probably A. chlorocyanus-group anoles became established, at least on
that portion of Hispaniola where the amber-producing sedimentary basin is
located. The three specimens available differ among themselves and may belong to
more than one species. Moreover, they could belong to one or more living
species. This extent of morphological variability is not limited to the fossils
in question, but has long been recognized in extant anoles and is still not
completely resolved even with large samples of living species. Nevertheless,
phylogenetic analyses are contributing to defining clades. For example, Poe's
(1998) study focused on the twig dwarf clade, the geographic distribution of
which does not appear to contradict the biogeographical model of Iturralde-Vinent
and MacPhee (1999). Cladistic resolution of the other anoles considered in
Poe's study is less reliable and not well supported. His results are consistent
with the molecular study of Jackman
et al. (1999) that concluded the deep branches characteristic of Anolis
phylogeny in their study reflected early and rapid diversification. That may
well explain part of the complexity of anole evolution, but it cannot explain
all of the inter- and intra-island evolution that has occurred in the Caribbean
since the Miocene. Individual variation in anoles on islands apparently
manifests rapidly and adaptively as shown by colonization experiments (Losos
et al. 1997, see also Thomson
1997), bringing about morphological differentiation within a population in a
matter of decades.
Anolis is a long-lived genus with a high
degree of specific diversity, a confusing amount of inter- and intra-specific
morphological variability and homoplasy, but a low level of morphological
disparity. Those attributes combine to obfuscate the phylogenetic and
biogeographical history of the group. But this same pattern of attributes
contributed to the viability of the genus over time. The fossil anoles from the
Dominican Republic, while few in number, suggest that the
evolutionary-ecological strategy this pattern reflects has not changed
significantly over the past 15 million years.
 The
pattern and orientation of air bubbles entrapped in the amber of SMU74796
indicate the specimens skull was in an upright position and out-gassing due to
decompostion was primarily concentrated in the damaged rear portion of the skull
as illustrated in Figure 7. All three of
the amber anole specimens exhibit damage to the skull roof primarily in the
region of the parietal and temporal arcade.
The
pattern and orientation of air bubbles entrapped in the amber of SMU74796
indicate the specimens skull was in an upright position and out-gassing due to
decompostion was primarily concentrated in the damaged rear portion of the skull
as illustrated in Figure 7. All three of
the amber anole specimens exhibit damage to the skull roof primarily in the
region of the parietal and temporal arcade.