 Stereom microstructures of Cambrian echinoderms revealed by cathodoluminescence (CL)
Stereom microstructures of Cambrian echinoderms revealed by cathodoluminescence (CL)
Article number: 16.3.32A
https://doi.org/10.26879/397
Copyright Paleontological Society, December 2013
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 29 April 2013. Acceptance: 5 December 2013
{flike id=611}
ABSTRACT
Echinoderms possess a skeleton with a unique and distinctive meshlike microstructure called stereom that is underpinned by a specific family of genes. Stereom is thus considered the major echinoderm synapomorphy and is recognized already in some Cambrian echinoderm clades. However, data on the skeletal microstructures of early echinoderms are still sparse and come only from isolated ossicles of limited taxonomic value in which the primary calcium carbonate has been replaced or thinly coated by phosphates, silica or iron oxides.
Here, we applied cathodoluminescence (CL) to reveal stereom microstructures of the diagenetically altered calcitic skeletons of some Cambrian echinoderms (in particular Protocinctus, Stromatocystites and Dibrachicystidae). CL not only provides insights into the diagenesis of their skeletons but also reveals primary microstructural details that are not visible under transmitted light or SEM. Different stereom types (resembling labyrinthic, fascicular, galleried, microperforate and imperforate microfabrics) comparable to those observed in extant echinoderms have been recognized for the first time in these Cambrian taxa. Our results show that the stereom microstructures widely occur in various Cambrian echinoderm clades which suggest that they likely evolved the same genetically controlled biomineralization mechanisms as those observed in modern echinoderms.
These results underline that the CL technique can be a powerful tool in the detection of the microstructural organization in even severely recrystallized echinoderm specimens. Given the close association between the skeletal microstructure and the investing soft tissues, the method presented here opens new possibilites for revealing skeletal growth and soft tissue palaeoanatomy of fossil echinoderms.
Przemysław Gorzelak. Department of Biogeology, Institute of Paleobiology, Polish Academy of Sciences, Twarda Str. 51/55, PL 00-818 Warsaw, Poland, E-mail: pgorzelak@twarda.pan.pl
Samuel Zamora. Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington DC, 20013–7012, USA, samuel@unizar.es
Keywords: skeleton; biomineralization; Cambrian; echinoderms; stereom; cathodoluminescence
Final citation: Gorzelak, Przemysław and Zamora, Samuel. 2013. Stereom microstructures of Cambrian echinoderms revealed by cathodoluminescence (CL) Palaeontologia Electronica Vol. 16, Issue 3; 32A; 17p. https://doi.org/10.26879/397
palaeo-electronica.org/content/2014/611-cambrian-stereom
INTRODUCTION
 Echinoderms have a skeleton composed of hundreds to thousands of calcitic plates (the so-called ossicles) that are formed within syncytium through a highly controlled intracellular biomineralization process (e.g., Okazaki, 1960; Märkel, 1986). Each plate is composed of a unique three-dimensional meshwork of mineral trabeculae called stereom that is the first recognizable echinoderm synapomorphy (Figure 1.3). Smith (1980) recognized different stereom types and layers in various recent echinoderms and identified their relationship with associated soft tissues. For example, it has been shown that the galleried stereom is always associated with penetrative collagenous fibers whereas fine-meshed labyrinthic stereom is characteristic for the muscle fiber attachment. Despite clear functional aspects of each stereom type, their possible phylogenetic significances have also been emphasized (e.g., Simms, 2011). Morphogenesis of all stereom microfabrics is basically the same (Dubois and Jangoux, 1990). A recent study by Gorzelak et al. (2011) has shown that the growth of the stereom meshwork is a highly complex process of the initial, fast growth of thin open meshwork (the so-called "inner core") and a simultaneous, slow thickening process. This process involves different cellular activities and different types of organic components (the so-called intrastereomic organic matrix; IOM) that are incorporated into the crystal structure at various structural levels (e.g., Weiner, 1985; Gorzelak et al., 2013). At the nanoscale, echinoderm skeleton has a typical nanocomposite structure consisting of 30–100 nm spherical particles (the so-called 'nanograins' or 'nanobricks') (Stolarski et al., 2009; Gorzelak et al., 2013). Yet the genes responsible for the stereom formation are unique of echinoderms among living phyla (Bottjer et al., 2006) although some of these biomineralization genes have been also reported in their sister group - hemichordates (Cameron and Bishop, 2012).
Echinoderms have a skeleton composed of hundreds to thousands of calcitic plates (the so-called ossicles) that are formed within syncytium through a highly controlled intracellular biomineralization process (e.g., Okazaki, 1960; Märkel, 1986). Each plate is composed of a unique three-dimensional meshwork of mineral trabeculae called stereom that is the first recognizable echinoderm synapomorphy (Figure 1.3). Smith (1980) recognized different stereom types and layers in various recent echinoderms and identified their relationship with associated soft tissues. For example, it has been shown that the galleried stereom is always associated with penetrative collagenous fibers whereas fine-meshed labyrinthic stereom is characteristic for the muscle fiber attachment. Despite clear functional aspects of each stereom type, their possible phylogenetic significances have also been emphasized (e.g., Simms, 2011). Morphogenesis of all stereom microfabrics is basically the same (Dubois and Jangoux, 1990). A recent study by Gorzelak et al. (2011) has shown that the growth of the stereom meshwork is a highly complex process of the initial, fast growth of thin open meshwork (the so-called "inner core") and a simultaneous, slow thickening process. This process involves different cellular activities and different types of organic components (the so-called intrastereomic organic matrix; IOM) that are incorporated into the crystal structure at various structural levels (e.g., Weiner, 1985; Gorzelak et al., 2013). At the nanoscale, echinoderm skeleton has a typical nanocomposite structure consisting of 30–100 nm spherical particles (the so-called 'nanograins' or 'nanobricks') (Stolarski et al., 2009; Gorzelak et al., 2013). Yet the genes responsible for the stereom formation are unique of echinoderms among living phyla (Bottjer et al., 2006) although some of these biomineralization genes have been also reported in their sister group - hemichordates (Cameron and Bishop, 2012).
The earliest fossil record of echinoderms dates back to the classic lower Cambrian, formerly Cambrian Series 2 (Zamora et al., 2013). However, they probably appeared by the end of the Terreneuvian (Smith et al., 2013) just before the major transitional phase in ocean geochemistry from aragonite to calcite seas due to the falling Mg/Ca ratio (Kouchinsky et al., 2012). It is concluded from the fact that modern echinoderms commonly have a skeleton composed of high-magnesium calcite, and that the newly evolved organisms produce the skeletal mineralogy whose formation is compatible with seawater chemistry (e.g., Stanley, 2006). Simultaneously, skeletal mineralogies are generally considered to be conserved within the phylogenetic lineages; i.e, the organisms appear to have retained the same type of skeletal polymorph throughout their remainder history (e.g., Zhuravlev and Wood, 2008 but see also Ries, 2004).
Despite a significant progress in understanding Cambrian echinoderms, information regarding their stereom microstructures is critically poor as their skeletons are commonly altered by diagenesis or totally dissolved. Typically, they are mostly preserved as natural molds (Figure 1.6) or recrystallized skeletons (Figure 1.4) in which pore spaces have been filled by secondary, strongly cemented sparry calcite, which obscures the primary three-dimensional morphology of their stereom. By contrast, isolated echinoderm elements replaced or thinly coated by silica, iron oxides or phosphates (Figure 1.1, 1.2, 1.7) when treated with acid etching reveal their primary microstructural details which have provided important palaeobiological and palaeogeographic information (e.g., Berg-Madsen, 1986; Clausen and Smith, 2005, 2008; Kouchinsky et al., 2011; Clausen and Peel, 2012; Kouchinsky et al., in press). Unfortunately, they are usually informative at class level or above because they are impossible to assign to a concrete species or even family (Zamora et al., 2013).
Here we provide a promising technique (cathodoluminescence) to reveal the original stereom microstructure in recrystallized calcitic Cambrian echinoderms that is invisible under conventional transmitted light or SEM. We believe that further investigations will provide important complementary information to the previous aforementioned ways of preservation and will contribute in a better understanding of stereom organization in Cambrian echinoderms.
MATERIALS
The middle Cambrian (or Cambrian Series 3) in the western Mediterranean region, is regionally subdivided into three stages, named Leonian, Caesaraugustan and Languedocian in the Iberian Peninsula and Montagne Noire (Liñán et al., 1993; Álvaro and Vizcaïno, 1998); and named Agdzian, Caesaraugustan and Languedocian (last revision in Geyer and Landing, 2004) in Morocco.
Specimens included in this study come from two different sections, one in the Anti Atlas (Morocco) and the other at the Iberian Chains (Spain). Specimens from Morocco belong to the edrioasteroid, Stromatocystites sp., recently introduced by Smith et al. (2013). Specimens have been collected from the Tarhouch Member of the Ourika Wawrmast Formation, near the small village of Tarhia. The beds where specimens occur are grey-green siltostones with rare trilobite fragments and only a few other faunal components including brachiopods and the edrioasteroid described herein. Specimens occur normally as natural molds, but fresh rocks also provide rare specimens preserving calcite skeletons. These levels correspond with the Cephalopyge notabilis Zone (see Geyer and Landing, 2004).
The material from Spain comes from a single stratigraphic level in the northern part of the Iberian Chains, northeast Spain, at the locality of Purujosa. In this area a well-developed section at the Borraca Creek displays the middle Cambrian Mesones Group (Liñán et al., 1992), which is divided into the Valdemiedes, Mansilla and Murero Formations (see Zamora et al., 2009; Álvaro et al., 2013 for more detailed geological information). Specimens of echinoderms have been collected from the Mansilla Formation where they occur as calcite skeletons associated with very common trilobites and brachiopods. The echinoderms have been sampled from purple to reddish nodular limestones and shales that occur at the top of the Mansilla Formation and include two different taxa, the cinctan Protocinctus mansillaensis Rahman and Zamora 2009 and an indeterminate species of dibrachicystid. These levels correspond with the regional Eccaparadoxides asturianus Zone, which is considered Upper Leonian in age (Sdzuy et al., 1999).
Both stratigraphic levels correspond with the regional Agdzian of Morocco and the Leonian of Spain, respectively. These correspond with the global Stage 5 of Cambrian Series 3 and are approximately 505 million years old (Peng et al., 2012).
METHODS
Thin sections of the specimens polished down to less than 25 µm were coated with carbon and examined with a cathodoluminescence (CL) microscope at the Institute of Paleobiology of the Polish Academy of Sciences in Warsaw. This microscope is equipped with a hot cathode integrated with UV-VIS spectograph and linked to a Kappa video camera for recording digital images of even very short-lived and dull luminescence phenomena. An electron energy of 14 keV and a beam current between 0.2-0.3 mA was used for both CL microscopy and spectroscopy. Integration times for CL-emission spectra of luminescent samples were commonly 100 s. Stereom dimensions (the maximum pore diameter and the thickness of trabeculae separating the pores) were measured directly from CL photographs.
Additional analyses of selected thin sections were conducted using Energy Dispersive Spectroscopy performed on a Scanning Electron Microscope Philips XL–20 coupled with the EDS detector ECON 6, system EDX-DX4i at the Institute of Paleobiology of the Polish Academy of Sciences in Warsaw (accelerating voltage = 25 kV, working distance = 34 mm, a beam diameter ~5 µm). Each sample was analyzed on three selected areas (~50x50 µm) on echinoderm plate and two selected areas (~50x50 µm) on the surrounding sediment. The back-scattered electron (BSE) detector of the Philips XL–20 microscope was also used for the distinction between materials of lower versus higher atomic number.
Thin sections are housed at the Institute of Paleobiology, Polish Academy of Sciences, Warsaw (ZPALV.42Cm/Cm/1-37).
RESULTS
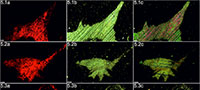
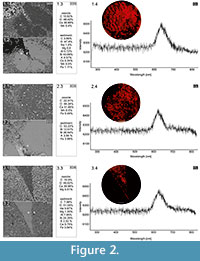 Cambrian echinoderms investigated in this study underwent a similar type of diagenetic transformation. They are preserved as recrystallized calcitic plates with low concentrations of Mg, Mn or Fe depending on the specimen (Figure 2). The pore spaces of these ossicles are infilled with sparry calcite cement secreted in optical continuity to the echinoderm plates. Thus, under polarizing microscopy, these specimens behave as a single calcite crystal and their stereom organization remains undetectable in transmitted light (details in Appendix 1, Appendix 2, Appendix 3, Appendix 4, and Appendix 5) or SEM (Figure 2). However, under CL calcitic specimens reveal relic "ghost" microstructures that are greatly enhanced under this technique. These specimens typically show orange to red luminescing stereom and commonly non-luminescent, likely ferroan, calcite infilling of the stereom pore system. The CL emission spectra showed emission maximum at about 610-620 nm, which corresponds to Mn2+ activation in calcite. Strong contrast between cement as well as host sediment and the skeleton under CL allows the primary microstructural organization of investigated specimens to be ascertained. Examples of CL photomicrographs are given in Figure 3 and are included in the Appendix. Although details about the three-dimensional microarchitecture are difficult to infer from presented thin sections, as mentioned above some generalizations about the stereom design of each ossicles can be made and their brief descriptions are given below.
Cambrian echinoderms investigated in this study underwent a similar type of diagenetic transformation. They are preserved as recrystallized calcitic plates with low concentrations of Mg, Mn or Fe depending on the specimen (Figure 2). The pore spaces of these ossicles are infilled with sparry calcite cement secreted in optical continuity to the echinoderm plates. Thus, under polarizing microscopy, these specimens behave as a single calcite crystal and their stereom organization remains undetectable in transmitted light (details in Appendix 1, Appendix 2, Appendix 3, Appendix 4, and Appendix 5) or SEM (Figure 2). However, under CL calcitic specimens reveal relic "ghost" microstructures that are greatly enhanced under this technique. These specimens typically show orange to red luminescing stereom and commonly non-luminescent, likely ferroan, calcite infilling of the stereom pore system. The CL emission spectra showed emission maximum at about 610-620 nm, which corresponds to Mn2+ activation in calcite. Strong contrast between cement as well as host sediment and the skeleton under CL allows the primary microstructural organization of investigated specimens to be ascertained. Examples of CL photomicrographs are given in Figure 3 and are included in the Appendix. Although details about the three-dimensional microarchitecture are difficult to infer from presented thin sections, as mentioned above some generalizations about the stereom design of each ossicles can be made and their brief descriptions are given below.
Stereom Organization in Protocinctus
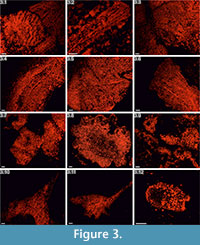 Integument plates. These ossicles are mostly made of various types of irregular, labyrinthic-like stereom, in which pores are variable in size and show no alignment. In transversal sections, coarse stereom with thin (3-10µm), irregular and elongated (up to ~30µm) pores bounded by medium-sized (10-20µm) trabeculae predominate in exterior areas (Figure 3.1-3.3, 3.5, Appendix 1.1a, 1.3a, Appendix 2.1a, Appendix 3.1a-3.3a, 3.5a), whereas finer trabeculae (<10µm), if present, occasionally occur in the interior parts of some ossicles (e.g., Figure 3.5, Appendix 1.4). Fascicular-like stereom is the second major stereom type that is visible in some of the investigated sections (e.g., Figure 3.2, 3.5, Appendix 1.5a, 1.7a, Appendix 2.2a, Appendix 3.2a). It is composed of branching, parallel and fine (2-8µm) to coarser (~15µm) trabecular rods without forming continuous galleries. Occasionally, the fascicles tend to form parallel compact and imperforate ridges (~10-25µm thick) resembling echinoid spine septa (Figure 3.2, Appendix 1.5a-1.7a, Appendix 2.2a, 2.4a, Appendix 3.2a) with narrow, vertically elongated pores (3-12µm in width) and smaller struts (3-10µm) occasionally uniting the main rods. One plate from the integument (Figure 3.1) is composed of large, irregular, typically tortuous and strongly ornamented (50-100µm thick) ridges separated by elongated (~20µm in width) pores. Additionally, at least some of the outer surfaces of certain ossicles (Figure 3.5, Appendix 3.2a, 3.3a, 3.5a) seem to be composed of micro- to imperforate compact layers (up to ~50µm thick). Galleried stereom is rarely present in these ossicles. It is hardly visible in the interior part of one ossicle (Figure 3.3, Appendix 1.3a) or in the exterior areas of transversal and axial sections of some ossicles (e.g., Appendix 3.3a).
Integument plates. These ossicles are mostly made of various types of irregular, labyrinthic-like stereom, in which pores are variable in size and show no alignment. In transversal sections, coarse stereom with thin (3-10µm), irregular and elongated (up to ~30µm) pores bounded by medium-sized (10-20µm) trabeculae predominate in exterior areas (Figure 3.1-3.3, 3.5, Appendix 1.1a, 1.3a, Appendix 2.1a, Appendix 3.1a-3.3a, 3.5a), whereas finer trabeculae (<10µm), if present, occasionally occur in the interior parts of some ossicles (e.g., Figure 3.5, Appendix 1.4). Fascicular-like stereom is the second major stereom type that is visible in some of the investigated sections (e.g., Figure 3.2, 3.5, Appendix 1.5a, 1.7a, Appendix 2.2a, Appendix 3.2a). It is composed of branching, parallel and fine (2-8µm) to coarser (~15µm) trabecular rods without forming continuous galleries. Occasionally, the fascicles tend to form parallel compact and imperforate ridges (~10-25µm thick) resembling echinoid spine septa (Figure 3.2, Appendix 1.5a-1.7a, Appendix 2.2a, 2.4a, Appendix 3.2a) with narrow, vertically elongated pores (3-12µm in width) and smaller struts (3-10µm) occasionally uniting the main rods. One plate from the integument (Figure 3.1) is composed of large, irregular, typically tortuous and strongly ornamented (50-100µm thick) ridges separated by elongated (~20µm in width) pores. Additionally, at least some of the outer surfaces of certain ossicles (Figure 3.5, Appendix 3.2a, 3.3a, 3.5a) seem to be composed of micro- to imperforate compact layers (up to ~50µm thick). Galleried stereom is rarely present in these ossicles. It is hardly visible in the interior part of one ossicle (Figure 3.3, Appendix 1.3a) or in the exterior areas of transversal and axial sections of some ossicles (e.g., Appendix 3.3a).
Marginal plates. These ossicles are largely made of coarse labyrinthic stereom (Figures 3.4, 3.6, Appendix 2.3a, 2.5a, 2.6a, Appendix 3.4a, 3.6a), in which pores are irregular in size (5-25µm) and shape. Trabecular thickness varies from 10 to 21µm. The outer surfaces of a single ossicle (Figure 3.4, Appendix 3.4a) seem to be composed of micro- to imperforate compact layers (up to ~30µm thick). Galleried stereom, in which pores are aligned in one direction, is visible in the exterior areas of transversal to axial sections of this ossicle (see also Appendix 3.4a). It is composed mainly of sub-parallel, sometimes radiating and only rarely branching trabeculae perpendicular to the ossicle surface whose diameter (8-20µm) occasionally increases toward the periphery.
Stereom Organization in Stromatocystites
Ambulacral plates. These ossilces are made almost entirely of coarse labyrinthic-like stereom (Figure 3.7, 3.9, Appendix 4.1a, 4.2a, 4.4a, 4.6a) with irregular pores (8-20µm) bounded by medium- to coarse-sized trabeculae (8-30µm). In some ossicles, possible insertion-pits for the ligament(?) covered by coarse (Figure 3.9, Appendix 4.4a) or fine (Figure 3.7, Appendix 4.6a) labyrinthic stereom are visible.
Thecal plates. These ossicles are composed of different types of possible labyrinthic stereom (Figure 3.8, Appendix 4.3a, 4.5a, 4.7a) with irregular pores (8-20µm) bounded by medium- to coarse-sized trabeculae (10-26µm). In some of the interior regions, an irregular stereom with fine trabeculae (2-10µm) separated by 4 to 15µm pores is present (Figure 3.8, Appendix 4.7a).
Stereom Organization in Dibrachicystidae
Thecal plates. These ossicles are largely made of medium- to coarse-sized trabeculae (10-25µm, rarely 5-7µm) and irregular pores (5-10µm in maximal diameter) (Figure 3.10, 3.11, Appendix 5.1a-5.4a) resembling labyrinthic stereom. Occasionally outer surfaces of these ossicles are composed of micro- to imperforate layers (20-40µm in thick) (Figure 3.10, Appendix 5.3a, 5.4a). Possible galleried stereom with aligned 5 to 12µm thick trabeculae and 3 to 10µm thick pores is hardly visible in the interior part of some ossicles (e.g., Figure 3.11, Appendix 5.2a, 5.3a).
Columnal? plates. Three possible stereom microfabrics can be distinguished in these ossicles (Figure 3.12). Peripheral areas are composed mainly of microperforated stereom layers underlain by coarse labyrinthic-like stereom composed of medium- to fine-sized trabeculae (5-20µm) (see also Appendix 5.5a, 5.6a). The inner part is made of poorly visible galleried stereom with thin (3 to 10µm) trabeculae. One ossicle (Appendix 5.7a) in oblique section display labyrinthic stereom composed of variously sized trabeculae (5-20µm) and pores (10-40µm).
DISCUSSION
Preservation and Preparation Methods – Review and New Perspectives
Despite an extensive fossil record of echinoderms that dates back to the lower Cambrian, information about the skeletal microstructure of early echinoderms is still poorly understood. This is due to a common alternation of high to low magnesium calcite during the early diagenesis, which leads to a significant degradation or loss of the primary microstructural features of echinoderm skeleton (Dickson, 2001; Gorzelak, 2012). Nevertheless, several methods have been applied to investigate stereom microstructures of fossil echinoderms. For example, it has been argued that etching with dilute acid or direct observations of petrologic thin sections via staining may provide good results, especially in fine-grained matrix (e.g., Laphan et al., 1976; Smith, 1990). However, these methods do not always provide consistent results and cannot be applied when the matrix is made of sparry calcite with a chemical composition similar to that of the stereom. Another interesting method was reported by Sevastopulo and Keegan (1980) who obtained excellent results after treatment with HF acid of echinoderm ossicles preserved in a siliceous matrix. In this case, the primary calcitic skeletons transformed into CaF2 without major changes in the skeletal microarchitecture, simultaneously dissolving the clastic matrix. Occasionally, original calcitic echinoderm skeletons were replaced or thinly coated by silica, phosphates, iron oxides or glauconite preserving their three-dimensional stereom (Smith, 1990; Clausen and Smith, 2008). Such ossicles can be extracted from rocks using 10% acetic acid and/or HCl and observed directly under SEM. Indeed, nearly all of the data on the microstructures of Cambrian echinoderms comes from fossils preserved in this way (see some of the most prominent examples in a Table 1).
CL, a characteristic type of luminescence (visible radiation) produced in a mineral subjected to bombardment by electrons, is widely applied in petrographic studies (e.g., Richter et al., 2003). The luminescence properties of carbonates are largely determined by incorporation of Mn2+ and REEs (Rare Earth Elements), which constitute the most significant activator elements, wheras Fe2+ is the most important quencher element. The activation of Mn2+ in calcite can easily be recognized by a broad emission band at 605-620 nm (e.g., Habermann et al., 2000; Richter et al., 2003). Despide its wide applications in sedimentary petrology, CL technique is an important but less widespread tool in palaeontological studies. For example some recrystallized fossils, in particular some Triassic foraminifers and corals, in which their microstructural details are not readily visible under transmitted light, are enhanced by CL (e.g., Martini et al., 1987; Rittel and Stanley, 1993). Cathodoluminesence imaging also revealed intralensar structures in the Devonian trilobite Phacops rana milleri (Miller and Clarkson, 1980; see also Marshall, 1988). More recently Kołodziej et al. (2011) showed that this method greatly improved detection of Jurassic foraminifers in thin sections.
Our results show that the cathodoluminescence is a powerful technique that can also be used to determine the skeletal microstructures of the oldest calcitic echinoderms that have not undergone significant recrystallization (for example, diagenetic dolomitization). Athough recent echinoderms may reveal weak luminescence (e.g., Richter et al., 2003, figure 4a), it is clear that the observed strong contrast in luminescence between cement with host sediment and the skeletons is a consequence of the distinct differences in concentrations of trace elements, like Fe2+ and Mn2+, during diagenesis. This is confirmed by recorded characteristic CL emission spectra of orange-red luminescing echinoderm plates and EDS geochemical analyses of selected samples (Figure 2). The latter indicates that echinoderm plates were preserved in siliceous and/or clay matrix enriched in Fe2+ and their pores were subsequently infilled by calcite (likely ferroan) cement. Although, an accurate interpretation of the geochemical data from these ossicles is difficult due to our inability to distinguish the stereom or its replacement from the cement infills in SEM, it seems that the differences in concentration of Fe2+ and Mn2+ between the cement and the stereom must be slight as suggested by homogeneous BSE images (Figure 2). In that respect, it is noteworthy that Walker et al. (1989), on the basis of theoretical considerations, suggested that there is no lower limit of Mn2+ activation in calcite. Other authors suggested that calcite requires a few ppm Mn2+ to show CL under hot cathode (Richter et al., 2003).
Stereom Design of Investigated Cambrian Echinoderms
There was a great advance in the knowledge of stereom microstructures of Cambrian echinoderms in the last years, especially after the study of isolated ossicles recovered after acid etching from tempestitic limestones (e.g., Berg-Madsen, 1986; Smith, 1990; Clausen and Smith, 2008; Kouchinsky et al., 2011; Elicki, 2011; Clausen and Peel, 2012). These ossicles are important in several aspects. First, they provide the occurrence of some groups in places where there is no record of complete echinoderm fossils and they certainly improve the palaeobiogeographic distribution of some major echinoderm groups. Second, they provide important palaeobiological information because they preserve stereom microstructure that can be studied under SEM. On the other hand, there is a serious drawback of these fossils because these ossicles can be assigned only to class level or above. This makes it difficult to transfer the information obtained from isolated elements into phylogenetic trees. Our data provides for the first time information on the stereom from specific taxa of echinoderms. Although still not perfect, because we only obtain data in two dimensions, we believe that further investigations of serial sections will reveal more accurate data of stereom distribution in complete specimens.
Our results obtained herein from CL provided insights into the general appearance of the skeletal microstructures of cinctans and dibrachicystids, and complement the previous data on edrioasteroids.
In cinctans (like Protocinctus), marginal plates are almost entirely composed of coarse, clearly structural, labyrinthic-like stereom. In extant echinoderms, this stereom type is commonly found on internal surfaces and/or externally where plates are embedded by connective tissue (Smith, 1990). The galleried stereom oriented perpendicular to the plate margins may be indicative of collagenous sutural fibers for binding adjacent plates. Much more differentiated stereom microfabrics are observed in integument plates of Protocinctus. Various stereom types (resembling labyrinthic, fascicular to galleried) and layers (imperforate) were distinguished. Most of them can be interpreted as a construction stereom rather than stereom associated with muscle or ligamentous tissue insertion. Possible fascicular stereom is indicative of a strictly unidirectional growth whereas the presence of compact, imperforate and thick ridges suggests that the secondary deposition of mineral infilling on the previously formed meshes must be involved.
Dibrachicystids possess thecal plates externally composed of either constructional, irregular stereom or micro- to imperforate layers that were likely developed for increasing the plate strengthening and resistance to abrasion. The presence of poorly visible galleried stereom may be a region of sutural collagenous fibers. Available sections of possible columnal plates indicate that they are similarly constructed externally as the thecal plates. Possible presence of the galleried stereom in the inner region may be indicative of the penetrative collagen fibers which bounded columnals together, as observed in extant crinoids and some Cambrian columnals.
Representatives of edrioasteroids (Stromatocystites) developed thecal and ambulacral plates almost entirely composed of different types of constructional labyrinthic-like stereom. Similar stereom organization has been reported in edrioasteroid ambulacral flooring plates by Sumrall and Parsley (2003, text-figs. 5, 7) and Clausen and Peel (2012, fig. 12)
The precise reconstruction of the stereom organization in each ossicle of a given animal requires more materials of precisely orientated thin sections, and this is clearly beyond the scope of this paper. Further recognition of the stereom from specific taxa will be important in order to incorporate such information into phylogenetic trees and then explore the evolution and significance of stereom in different groups.
Stereom - an Iconic Echinoderm Synapomorphy
Our results greatly complement data on the stereom microstructures of Cambrian echinoderms. Taken together our new data and published information (Smith, 1980, 1982; 1990; Berg-Madsen, 1986; Clausen and Smith, 2005, 2008; Kouchinsky et al., 2011; Clausen and Peel, 2012), it is clear that significant differentiation in stereom design, comparable to those observed in Recent echinoderms, had already evolved in the earliest echinoderm clades (Table 1). This suggests that the same genes that are involved in biomineralization process in extant echinoderms and/or the same constraints that determine extant stereom (nature of the investing soft tissue), likely characterized the earliest echinoderms. Given the strong structural similarities between the stereom of Cambrian echinoderms and their extant descendents, the unusual structure of the stereom and its unique genetic signature, it seems highly improbable that the stereom was independently acquired. Indeed, the stereom is considered to be the major echinoderm synapomorphy, and there is no evidence for its occurrence in any other phyla (e.g., Bottjer et al., 2006).
CONCLUSIONS
The cathodoluminescence (CL) technique, applied here for the first time for the diagenetically altered calcitic Cambrian echinoderms, showed that this method can successfully be used to highlight their primary microstructures. Presented method provided new insights into the stereom organization of some Cambrian clades that were not hitherto investigated. Different stereom microfabrics, comparable to those observed in extant echinoderms, were distinguished. These data suggest an evolutionary relationship between the genetic programmes of biomineralization in basal echinoderms. In analogy to the modern examples, it seems that even the earliest echinoderms were capable of controlling spatially and dynamically the formation of their stereom via an extremely high level of cellular control over the biomineralization process. We hope that our results will provide a stimulus for further investigation with the aid of the CL technique that may provide important information about the skeletal growth and soft tissue palaeoanatomy of not only Cambrian but also other fossil calcitic echinoderms.
ACKNOWLEDGEMENTS
This work was funded by the National Science Centre (NCN) grant no DEC-2011/03/N/ST10/04798 and was performed in the NanoFun laboratory co-financed by the European Regional Development Fund within the Innovation Economy Operational Programme POIG.02.02.00-00-025/09. SZ was funded by the Spanish Project CGL2011-24516) and a Post Doctoral grant at the Smithsonian Institution. We are grateful to O. Elicki (Germany) and S. Clausen (France) for allowing us to use some of their photographies. We thank the editor S. Gerber (France) and two anonymous reviewers for their constructive comments. Additional comments by A.B. Smith (United Kingdom) and J. Stolarski (Poland) greatly helped to improve the resulting manuscript. I. Pérez (Spain) provided help in preparing Figure 1.
REFERENCES
Álvaro, J.J. and Vizcaïno, D. 1998. Révision biostratigraphique du Cambrien moyen du versant méridional de la Montagne Noire (Languedoc, France). Bulletin de la Société géologique de France, 169:233-242.
Álvaro, J.J., Zamora, S., Vizcaïno, D., and Ahlberg, P. 2013. Guzhangian (mid Cambrian) trilobites from silica concretions of the Valtorres Formation, Iberian Chains, NE Spain. Geological Magazine, 150 (1):123-142.
Berg-Madsen, V. 1986. Middle Cambrian cystoid (sensu lato) stem columnals from 720 Bornholm, Denmark. Lethaia, 19:67-80.
Bottjer, D.J., Davidson, E.H., Peterson, K.J., and Cameron, R.A. 2006. Paleogenomics of echinoderms. Science, 314:956-960.
Cameron, C.B. and Bishop, C.D. 2012. Biomineral ultrastructure, elemental constitution and genomic analysis of biomineralization-related proteins in hemichordates. Proceedings of the Royal Society B: Biological Sciences, 279 (1740):3041-3048.
Clausen, S. and Peel, J. 2012. Middle Cambrian echinoderm remains from the Henson Gletscher Formation of North Greenland.GFF, 134(3):173-200.
Clausen, S. and Smith, A.B. 2005. Palaeoanatomy and biological affinities of a Cambrian deuterostome (Stylophora). Nature 438:351-354.
Clausen, S. and Smith, A.B. 2008. Stem structure and evolution in the earliest pelmatozoan echinoderms. Journal of Paleontology, 82:737-748.
Dickson, J.A.D. 2001. Diagenesis and crystal caskets: echinoderm Mg calcite transformation, Dry Canyon, New Mexico, USA. Journal of Sedimentary Research, 71:764-777.
Dubois, Ph. and Jangoux, M. 1990. Stereom morphogenesis and differentiation during regeneration of fractured adambulacral spines of Asterias rubens (Echinodermata, Asteroidea). Zoomorphology, 109:263-272.
Elicki, O. 2011. First skeletal microfauna from the Cambrian Series 3 of the Jordan Rift Valley (Middle East). Memoirs of the Association of Australasian Palaeontologists, 42: 153-173.
Geyer, G. and Landing, E. 2004. A unified Lower-Middle Cambrian chronostratigraphy for West Gondwana. Acta Geologica Polonica, 54:179-218.
Gorzelak. P. 2012. Biomineralization and diagenesis of the stereom in extant and fossil echinoderms. Unpublished PhD Thesis, Institute of Paleobiology of Polish Academy of Sciences, Warsaw, Poland.
Gorzelak, P., Stolarski, J., Dubois, P., Kopp, Ch., and Meibom, A. 2011. 26Mg labeling of the sea urchin regenerating spine: insights into echinoderm biomineralization process. Journal of Structural Biology, 176:119-126.
Gorzelak, P., Stolarski, J., Mazur, M., and Meibom, A. 2013. Micro- to nanostructure and geochemistry of extant crinoidal echinoderm skeletons. Geobiology, 11(1):29-43.
Habermann, D., Neuser, R.D., and Richter, D.K. 2000. Quantitative high resolution spectral analysis in sedimentary calcite, p. 331-358. In Pagel, M., Barbin, V., Blanc, P., and Ohnenstetter, D. (eds.), Cathodoluminescence in geosciences. Springer, Berlin Heidelberg, New York, Tokyo,
Kołodziej, B., Jurkowska, A., Banaś, M., and Ivanova, D. 2011. Improving detection of foraminifera by cathodoluminescence. Facies, 57:571-578.
Kouchinsky, A., Bengtson, S., Clausen, S., Gubanov, A., Malinky, J.M., and Peel, J.S. 2011. A middle Cambrian fauna of skeletal fossils from the Kuonamka Formation, northern Siberia. Alcheringa, 35:123-189.
Kouchinsky, A., Bengtson, S., Clausen, S., and Vendrasco, M.J. in press. A lower Cambrian fauna of skeletal fossils from the Emyaksin Formation, northern Siberia. Acta Palaeontologica Polonica, 5X(X): XXX-XXX. http://dx.doi.org/10.4202/app.2012.0004
Kouchinsky, A., Bengtson, S., Runnegar, B., Skovsted, C., Steiner, M., and Vendrasco, M. 2012. Chronology of early Cambrian biomineralization. Geological Magazine, 149:221-251.
Lapham, K.E., Ausich, W.I., and Lane, N.G. 1976. A technique for developingthe stereom of fossil crinoid ossicles. Journal of Paleontology, 50:245-248.
Liñán, E., Gozalo, R., Gámez Vintaned, J.A., and Álvaro, J.J. 1992. Las formaciones del Grupo Mesones (Cámbrico Inferior-Medio) en las Cadenas Ibéricas. III Congreso Geológico de España y VIII Congreso Latinoamericano de Geología, Salamanca, Actas, 1:517-523.
Liñán, E., Perejón, A., and Sdzuy, K. 1993. The Lower-Middle Cambrian stages and stratotypes from the Iberian Peninsula: a revision. Geological Magazine, 130:817-833.
Märkel, K. 1986. Ultrastructural investigation of matrix-mediated biomineralization in echinoids (Echinodermata, Echinoidea). Zoomorphology, 106:232-243.
Marshall, D.J. 1988. Cathodoluminescence of Geological Materials. Boston, Unwin Hyman.
Martini, R., Amieux, P., Gandin, A., and Zaninetti. L. 1987. Triassic foraminifers from Punta Tonnara (SW Sardinia) observed in cathodoluminescence. Revue de Paléobiologie, 6:3-27.
Miller, J. and Clarkson, E.N.K. 1980. Post-Ecdysial Development of the Cuticle and the Eye of the Devonian Trilobite Phacops rana milleri Stewart 1927. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 288:461-480.
Okazaki, K. 1960. Skeleton formation of sea urchin larvae. II. Organic matrix of the spicule. Embryologia, 5:283-320.
Peng, S., Babcock, L.E., and Cooper, R.A. 2012. The Cambrian Period, p. 437-488. In Gradstein, F., Ogg, J., Schmitz, M.D., and Ogg, G.M. (eds.), The Geologic Time Scale 2012, Vol. 2. Elsevier BV, Amsterdam.
Rahman, I.A. and Zamora, S. 2009. The oldest cinctan carpoid (stem-group Echinodermata) and the evolution of the water vascular system. Zoological Journal of the Linnean Society, 157:420-432.
Richter, D.K., Goette, T., Goetze, J., Neuser, R.D., and Neuser, R.D. 2003. Progress in application of cathodoluminescence (CL) in sedimentary petrology. Mineralogy and Petrology, 79:127-166.
Ries, J.B. 2004. Effect of ambient Mg/Ca ratio on Mg fractionation in calcareous marine invertebrates: A record of the oceanic Mg/Ca ratio over the Phanerozoic. Geology, 32:981-984.
Rittel, J.F. and Stanley, G.D.Jr. 1993. Enhanced skeletal details and diagenetic processes of Triassic corals revealed by cathodoluminescence. Courier Forschungsinstitut Senckenberg, 164:339-346.
Sdzuy, K., Liñán, E., and Gozalo, R. 1999. The Leonian Stage (early Middle Cambrian): a unit for Cambrian correlation in the Mediterranean subprovince. Geological Magazine, 136:39-48.
Sevastopulo, G.D. and Keegan, J.B. 1980. A technique revealing the stereom microstructure of fossil crinoids. Palaeontology, 23:749-756.
Simms, M.J. 2011. Stereom microstructure of columnal latera: a character for assessing phylogenetic relationships in articulate crinoids. Swiss Journal of Palaeontology, 130:143-154.
Smith, A.B. 1980. Stereom microstructure of the echinoid test. Special Paper in Palaeontogy, 25:1-81.
Smith, A.B. 1982. The affinities of the Middle Cambrian Haplozoa (Echinodermata), Alcheringa: An Australasian Journal of Palaeontology, 6(2):93-99.
Smith, A.B. 1990. Biomineralization in echinoderms, p. 413-443. In Carter, J.G. (ed.), Skeletal biomineralization: patterns, processes, and evolutionary trends. Van Nostrand Reinhold, New York.
Smith, A B., Zamora, S., and Alvaro, J.J. 2013. The oldest echinoderm faunas deom Gondwana show that echinoderm body plan diversification was rapid. Nature Communications, 4: 1358. doi: 10.1038/ncomms2391.
Stanley, S.M. 2006. Influence of seawater chemistry on biomineralization throughout Phanerozoic time: Paleontological and experimental evidence: Palaeogeography Palaeoclimatology Palaeoecology, 232:214-236.
Stolarski, J., Gorzelak, P., Mazur, M., Marrocchi, Y., and Meibom, A. 2009. Nanostructural and geochemical features of the Jurassic isocrinid columnal ossicles. Acta Palaeontologica Polonica, 54:69-75.
Sumrall, C.D. and Parsley, R.L. 2003. Morphology and biomechanical implications of isolated discocystinid plates (Edrioasteroidea, Echinodermata) from the Carboniferous of North America. Palaeontology, 46:113-138.
Walker, G., Abumere, O.E., and Kamaluddin, B. 1989. Luminescence spectroscopy of Mn2+ centres in rock forming carbonates. Mineralogical Magazine, 53:201-211.
Weiner, S. 1985. Organic matrix-like macromolecules associated with the mineral phase of sea urchin skeletal plates and teeth. Journal of Experimental Zoology, 234:7-15.
Zamora, S. 2013. Morphology and phylogenetic interpretation of a new Cambrian edrioasteroid (Echinodermata) from Spain. Palaeontology, 56(2):421-431.
Zamora, S., Gozalo, R., and Liñán E. 2009. Middle Cambrian gogiid echinoderms from Northeast Spain: Taxonomy, palaeoecology and palaeogeographic implications. Acta Paleontologica Polonica, 54(2):253-265.
Zamora, S., Lefebvre, B., Álvaro, J.J., Clausen, S., Elicki, O., Fatka, O., Jell, P., Kouchinski, A., Lin, J.P., Nardin, E., Parsley, R., Rozhnov, S., Sprinkle, J., Sumrall, C.D., Vizcaïno, D., and Smith, A.B. 2013. Global Cambrian echinoderm diversity and palaeobiogeography. In Harper, D.A.T. snf Servais, T. (eds.), Early Palaeozoic Biogeography and Palaeogeography. Geological Society, London, Memoirs, 38, 151-171. dx.doi.org/10.1144/M38.13
Zhuravlev, A.Y. and Wood, R.A. 2008. Eve of biomineralization: controls on skeletal mineralogy. Geology, 36:923-926.