 Stenolaemate bryozoans from the Graham Formation, Pennsylvanian (Virgilian) at Lost Creek Lake, Texas, USA
Stenolaemate bryozoans from the Graham Formation, Pennsylvanian (Virgilian) at Lost Creek Lake, Texas, USA
Article number: 25.2.a15
https://doi.org/10.26879/1174
Copyright Paleontological Society, May 2022
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Appendix
Supplementary Material
Submission: 1 July 2021. Acceptance: 21 April 2022.
ABSTRACT
An exceptionally well-preserved bryozoan fauna has been described from the Finis Shale Member, Graham Formation, Pennsylvanian (Virgilian) at Lost Creek Lake, Texas, USA. Nineteen bryozoan species (four cystoporates, one trepostome, two rhabdomesine cryptostomes, and 12 fenestrates) have been identified in two profiles which cut the most vertical range, at the level of the outcrop-base, of the Finis Shale. Two species are new: a trepostome Dyscritella felixi n. sp. and a fenestrate Laxifenestella texana n. sp. The fauna was studied on a combined basis of external and internal morphology, using a SEM and thin sections, respectively. Bryozoans from the Finis Shale Member exhibit a variety of growth forms from encrusting unilaminar, erect ramose, erect reticulate robust, and erect reticulate delicate, to erect pinnate morphologies. The erect growth forms clearly dominate, and bryozoans become more robust in the upper level of the profiles. The distribution pattern of bryozoan growth forms indicates gradual shallowing in the profiles supporting the assumption of a transgressive-regressive cycle in the Finis Shale. Bryozoan richness, abundance, and α-diversity increase toward the top of the profiles. Palaeobiogeographic relations of the Finis Shale bryozoans are mostly restricted to the American realm, with some connections to the Pennsylvanian of Europe.
Andrej Ernst. Institut für Geologie, Universität Hamburg, Bundesstr. 55, 20146 Hamburg, Germany. Andrej.Ernst@uni-hamburg.de
Anna Lene Claussen. GeoZentrum Nordbayern, Friedrich-Alexander University Erlangen-Nürnberg (FAU), Loewenichstraße 28, D-91054 Erlangen, Germany. anna.lene.claussen@fau.de
Barbara Seuss. GeoZentrum Nordbayern, Friedrich-Alexander University Erlangen-Nürnberg (FAU), Loewenichstraße 28, D-91054 Erlangen, Germany. barbara.seuss@fau.de
Patrick N. Wyse Jackson. Department of Geology, Trinity College, Dublin 2, Ireland. wysjcknp@tcd.ie
Keywords: Finis Shale; cyclothem; North American Midcontinent; morphology; taxonomy; ecology
Final citation: Ernst, Andrej, Claussen, Anna Lene, Seuss, Barbara, and Wyse Jackson, Patrick N. 2022. Stenolaemate bryozoans from the Graham Formation, Pennsylvanian (Virgilian) at Lost Creek Lake, Texas, USA. Palaeontologia Electronica, 25(2):a15. https://doi.org/10.26879/1174
palaeo-electronica.org/content/2022/3608-pennsylvanian-bryozoan-fauna
Copyright: May 2022 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
http://zoobank.org/A4A07D50-3DDB-4E45-A255-1ECCF45F147A
INTRODUCTION
Bryozoans represent one of the most important animal groups in marine biotopes of the Carboniferous age showing high diversity and wide distribution (e.g., Ross, 1981; Bancroft, 1987). The available literature documents rich and abundant bryozoan faunas in Carboniferous rocks worldwide. Pennsylvanian Bryozoa from North America were studied in the pioneering works on palaeontology as, for example, by Meek (1872), Ulrich (1890), Keyes (1888, 1894), and many others. In the first half of the twentieth century many local faunas including bryozoans were described from Pennsylvanian localities in North America (e.g., Rogers, 1900; Condra, 1902, 1903a, b; Girty, 1911; Morningstar, 1922; Mather, 1915; Moore, 1929, 1930; Sayre, 1930). However, the study of bryozoans is hindered by the necessity of requiring extensive preparation of thin sections as well as by often inadequate descriptions of previously known records, which makes correlation of modern descriptions with these earlier taxonomic accounts difficult. Therefore, it is important to describe well-preserved bryozoan assemblages in order to expand the knowledge of bryozoan morphology, taxonomy, and distribution to raise the utility of bryozoans for various approaches in geological and biological sciences, notably for studies of evolution, palaeoecology, and palaeogeography.
The study of Palaeozoic bryozoans is challenging. Largely they belong to the Superorder Palaeostomata Ma et al., 2014, the group of stenolaemate bryozoans with calcitic skeletons that show a high potential for preservation. Their internal morphology is complex, and their study requires essential preparation of oriented thin sections. Bryozoan remnants are often imbedded in hard limestones. In that case preparation of serial thin sections from the rock appears to be the best method. On the other hand, bryozoans can be derived from soft deposits like shales from which bryozoan fragments can be isolated from the sediment. Such material allows investigation of the external morphology, often unseen in thin sections. A comprehensive study of the well-preserved bryozoan material by use of the dual modern methods - SEM processing and oriented thin sections - enables a comprehensive description of bryozoans and a better understanding of their morphology and taxonomy.
Deposits from the Finis Shale at the Lost Creek Lake outcrop near Jacksboro (i.e., “Spillway Section”, TXV-200), Texas, USA, provide the opportunity to study a rich and well-preserved bryozoan fauna of late Pennsylvanian age. The deposits from the Finis Shale are categorized as a Liberation Lagerstätte (Roden et al., 2019) containing fossils which are both extremely well preserved and at the same time can be easily removed from the surrounding material. The Finis Shale deposits are laterally widely distributed in Northern Texas (Barnes et al., 1987) and at TXV-200 mirror a diverse faunal assemblage from the upper Pennsylvanian. Lower parts of the shale contain abundant conodonts and foraminifers (especially fusulinids) (e.g., Nestell et al., 2019; personal observation BS). Deposits above, when sea level continuously retreats, comprise a fauna with remains of fish including teeth and bones (e.g., Maisey et al., 2017), various brachiopod and mollusc (cephalopod, gastropod, and bivalvia) species (e.g., Miller and Downs, 1950; Boardman et al., 1984; Grossman et al., 1991; Lobza et al., 1994; Forcino et al., 2010; Karapunar et al., 2022; Niko et al., 2022), skeletal parts of all kinds of echinoderms (personal observation BS), among others (compare Boston, 1988; Lobza et al., 1994) but also fragments of plants (e.g., Boston, 1988; McKinzie and McLeod, 2003; personal observation BS). Boston (1988) divided the Finis Shale into seven biofacies according to sedimentological and faunistic characters and Forcino et al. (2010) also recognized community patterns that are largely driven by the three most abundant brachiopod taxa at the outcrop.
Bryozoans from the Finis Shale have never been studied in detail so far. We present a comprehensive taxonomic description of 19 bryozoan species recorded from the outcrop TXV-200 and discuss their morphology as well as their ecology and palaeobiogeographic relations.
Geographical and Geological Setting
 Bryozoans in this study derive from an outcrop (AMNH locality #5562 = TXV-200) (Figure 1) at the Lost Creek Lake emergency spillway near its dam. TXV-200 (33° 14’ 12.2” N / 98° 7’ 12” W) is located approximately 4 km northeast of Jacksboro (Jack County, TX, USA) (Figure 1A), and this outcrop was created during the building of the emergency spillway. The outcrop consists of a flat plane with occurrences of conulariids and a main outcrop area, which is a horseshoe-shaped slope of Finis Shale (Figure 1B-E) mostly covered by Jacksboro Limestone (Figure 1F) (e.g., Boston, 1988; Forcino et al., 2010). Lower parts of the shale are blackish-deep gray and clayey (Figure 1E) while weathered parts commonly appear yellowish (Figure 1D). The entire Finis Shale at this locality has a thickness of approximately 30 m (Nestell et al., 2019), while the directly accessible part is only 5-6 m with a maximum of less than 10 m above the bedding plane (Figure 1C).
Bryozoans in this study derive from an outcrop (AMNH locality #5562 = TXV-200) (Figure 1) at the Lost Creek Lake emergency spillway near its dam. TXV-200 (33° 14’ 12.2” N / 98° 7’ 12” W) is located approximately 4 km northeast of Jacksboro (Jack County, TX, USA) (Figure 1A), and this outcrop was created during the building of the emergency spillway. The outcrop consists of a flat plane with occurrences of conulariids and a main outcrop area, which is a horseshoe-shaped slope of Finis Shale (Figure 1B-E) mostly covered by Jacksboro Limestone (Figure 1F) (e.g., Boston, 1988; Forcino et al., 2010). Lower parts of the shale are blackish-deep gray and clayey (Figure 1E) while weathered parts commonly appear yellowish (Figure 1D). The entire Finis Shale at this locality has a thickness of approximately 30 m (Nestell et al., 2019), while the directly accessible part is only 5-6 m with a maximum of less than 10 m above the bedding plane (Figure 1C).
 Both the Finis Shale and Jacksboro Limestone represent the basal parts of the Graham Formation (Cisco Group; Figure 2A) (Moore and Plummer, 1922; Grossman et al., 1991) of Virgilian (Pennsylvanian, Upper Carboniferous) age and thus are part of the Finis transgressive-regressive cycle (Boardman and Heckel, 1989; Boardman et al., 1984) representing sea level rise and fall during the Late Paleozoic Ice Age (e.g., Montañez and Poulsen, 2013). The regressive part of the cycle, based on the amount of filter feeders, is interpreted to represent a non-deltaic environment (Boston, 1988). The outcrop, at the time of deposition, was part of the eastern shelf on the western part of the Midland Basin on the North American Midcontinent with evolving orogens toward the North and East, i.e., the Amarillo and Arbuckle Mountains and the Ouachita Foldbelt, respectively (Yang and Kominz, 2003).
Both the Finis Shale and Jacksboro Limestone represent the basal parts of the Graham Formation (Cisco Group; Figure 2A) (Moore and Plummer, 1922; Grossman et al., 1991) of Virgilian (Pennsylvanian, Upper Carboniferous) age and thus are part of the Finis transgressive-regressive cycle (Boardman and Heckel, 1989; Boardman et al., 1984) representing sea level rise and fall during the Late Paleozoic Ice Age (e.g., Montañez and Poulsen, 2013). The regressive part of the cycle, based on the amount of filter feeders, is interpreted to represent a non-deltaic environment (Boston, 1988). The outcrop, at the time of deposition, was part of the eastern shelf on the western part of the Midland Basin on the North American Midcontinent with evolving orogens toward the North and East, i.e., the Amarillo and Arbuckle Mountains and the Ouachita Foldbelt, respectively (Yang and Kominz, 2003).
Material and Methods
During a field trip the Finis Shale at the TXV-200 locality was extensively bulk-sampled. Three transects were prepared allowing sampling from a fresh outcrop of shale (Figure 2B). Samples were collected every 25 cm, and each sample contained 2.5 kg of sediment, measured with a scale. In the laboratory (FAU-GZN Paläoumwelt, Erlangen) the samples were dried at 35°C before 1.5 kg of each sample was disarticulated using tap water. In part the samples contained a distinct amount of water so that only little more than 1.5 kg of shale was left after drying. Solvents were not required to break up the shales as they disintegrated easily in tap water. The samples were sieved using four mesh sizes (2 mm, 500 µm, 250 µm, and 125 µm). The split fractions again were dried at 35°C. The larger fractions (i.e., 2 mm and 500 µm) were picked for all fossils and identifiable fossil fragments and sorted (Figure 3). The bryozoan remains were closely examined, and from each species the best-preserved specimens were used for further analysis (thin sections and SEM).
 A selection of best-preserved bryozoan remains was prepared for visualization and study with the SEM. The fossils were glued onto SEM-stubs and sputter-coated (Cressington 108) before being investigated in the SEM (TESCAN VEGA\\xmu).
A selection of best-preserved bryozoan remains was prepared for visualization and study with the SEM. The fossils were glued onto SEM-stubs and sputter-coated (Cressington 108) before being investigated in the SEM (TESCAN VEGA\\xmu).
Separate bryozoan fragments were imbedded in epoxy resin (SpeciFix-20) under vacuum, then cut and polished for preparation of oriented thin sections. Thin sections were investigated using a binocular microscope in transmitted light. Measurements of morphological characters were partly adopted from Anstey and Perry (1970), Hageman (1991, 1993), and Snyder (1991a, b). Statistics were summarized using arithmetic mean, sample standard deviation, coefficient of variation, and minimum and maximum values (see Supplementary Material).
Studied material is deposited at the Bayerische Staatssammlung für Paläontologie und Geologie, Munich, Germany, under collection numbers SNSB-BSPG 2020 XCI 22-128 (thin sections: SNSB-BSPG 2020 XCI 22-96; SEM: SNSB-BSPG 2020 XCI 97-128).
SYSTEMATIC PALAEONTOLOGY
Phylum Bryozoa Ehrenberg, 1831
Class Stenolaemata Borg, 1926
Superorder Palaeostomata Ma, Buttler, and Taylor, 2014
Order Cystoporata Astrova, 1964
Suborder Fistuliporina Astrova, 1964
Family Fistuliporidae Ulrich, 1882
Genus Fistulipora M’Coy, 1849
Type species. Fistulipora minor M’Coy, 1849, by original designation. Mississippian (Lower Carboniferous); England.
Diagnosis. Massive, encrusting, or ramose colonies. Cylindrical autozooecia with thin walls and complete diaphragms. Apertures rounded, possessing horseshoe-shaped lunaria. Autozooecia separated by the extrazooidal vesicular skeleton.
Remarks. Fistulipora M’Coy, 1849 differs from Eridopora Ulrich, 1882 in having rounded, horseshoe-shaped lunaria instead of triangular ones. Furthermore, Eridopora develops persistently encrusting colonies, whereas Fistulipora may also develop massive and ramose colonies. Fistulipora differs from Dybowskiella Waagen and Wentzel, 1886, in the shape of lunaria, whose ends do not inflect autozooecial chambers.
Occurrence. Ordovician to Permian; worldwide.
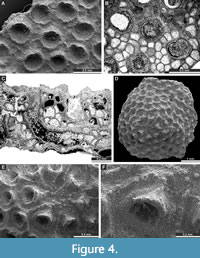
Fistulipora nodulifera Meek, 1872
Figure 4A-C; Appendix
 1872 Fistulipora nodulifera Meek, p. 143, pl. 5, 5a-d.
1872 Fistulipora nodulifera Meek, p. 143, pl. 5, 5a-d.
1894 Fistulipora nodulifera Meek, 1872; Keyes, pl. 34, fig. 3.
1903a Fistulipora nodulifera Meek, 1872; Condra, p. 30-31, pl. 1, figs. 1-5.
1929 Fistulipora nodulifera var. maculosa Moore, p. 5, pl. 1, figs. 9, 12.
1930 Fistulipora nodulifera Meek, 1872; Sayre, p. 87-88, pl. 2, figs. 4-6.
2017 Fistulipora nodulifera Meek, 1872; Ernst and Vachard, p. 18, figs. 6A-D.
2021 Fistulipora nodulifera Meek, 1872; Ernst, Krainer and Lucas, p. 220-222, figs. 4c-f.
Material. SNSB-BSPG 2020 XCI 29, SNSB-BSPG 2020 XCI 34, SNSB-BSPG 2020 XCI 56a-d, SNSB-BSPG 2020 XCI 57, SNSB-BSPG 2020 XCI 76, SNSB-BSPG 2020 XCI 97.
Description. Encrusting colonies, 0.63-0.80 mm thick. Autozooecia growing from thin epitheca, bending in the early exozone to the colony surface. Basal diaphragms rare. Autozooecial apertures circular to oval. Lunaria well-developed, rounded; ends of lunaria not indenting into autozooecia. Vesicles small to large, separating autozooecia in 1-2 rows, 10-15 surrounding each autozooecia aperture, with rounded to flat roofs, polygonal in tangential section. Microacanthostyles in outer layer of the calcite material, 0.020-0.035 mm in diameter. Autozooecial walls granular prismatic, 0.013-0.018 mm thick. Depressed maculae consisting of vesicles present, 0.81-1.26 mm in diameter, spaced 5-6 mm from centre to centre.
Remarks. Fistulipora nodulifera Meek, 1872, differs from F. vaccula Moore, 1929, from the Graham Formation (Virgilian) of Texas in possessing smaller autozooecial apertures (aperture width 0.22-0.35 mm vs. 0.35-0.50 mm in F. vaccula). Fistulipora nodulifera differs from F. distincta Schulga-Nesterenko, 1955, from the Pennsylvanian (Moscovian) of the Russian Platform in having smaller autozooecial apertures (aperture width 0.22-0.35 mm vs. 0.30-0.35 mm in F. distincta).
Moore (1929) distinguished a variety Fistulipora nodulifera var. maculosa Moore, 1929 from the Graham Formation (Virgilian) of Texas by presence of maculae. However, maculae were also mentioned in other materials of F. nodulifera.
Occurrence. Carboniferous, Pennsylvanian (Virgilian); Texas, Nebraska, Missouri, New Mexico (USA). Horquilla Formation, Carboniferous, Pennsylvanian, Desmoinesian (late Moscovian); Cerros de Tule, Sonora, Mexico. Gray Mesa Formation, Pennsylvanian (Desmoinesian); Fra Cristobal Mountains, New Mexico, USA. Graham Formation, Pennsylvanian (Virgilian); TXV-200 (“Spillway section at Lost Creek Lake”), Texas, USA.
Genus Eridopora Ulrich, 1882
Type species. Eridopora macrostoma Ulrich, 1882, by original designation. Mississippian (Lower Carboniferous); North America.
Diagnosis. Thin encrusting colonies. Oval apertures with strongly developed lunaria of distinct triangular shape. Cylindrical autozooecia with thin walls and complete diaphragms. Vesicular skeleton consists of angular vesicles.
Remarks. Eridopora Ulrich, 1882, differs from Fistulipora M’Coy, 1849, in having large triangular lunaria instead of horseshoe-shaped ones, and predominantly encrusting colonies.
Occurrence. Devonian to Permian; worldwide.
Eridopora beilensis Perkins and Perry in Perkins et al., 1962
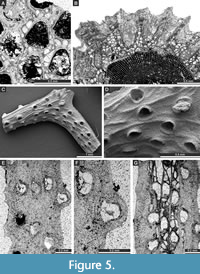
Figure 4D-F, Figure 5A-B; Appendix
1962 Eridopora beilensis Perkins and Perry in Perkins et al., p. 12, pl. 3, figs. 1-4.
 Material. SNSB-BSPG 2020 XCI 36, SNSB-BSPG 2020 XCI 59a, b, SNSB-BSPG 2020 XCI 98.
Material. SNSB-BSPG 2020 XCI 36, SNSB-BSPG 2020 XCI 59a, b, SNSB-BSPG 2020 XCI 98.
Description. Encrusting colonies, 0.20-1.26 mm thick. Autozooecia growing from thin epitheca, bending in the early exozone to the colony surface. Basal diaphragms rare to absent. Autozooecial apertures circular to oval. Lunaria well-developed, triangular; ends of lunaria not indenting autozooecia. Vesicles small to large, separating autozooecia in 1-2 rows, 13-16 surrounding each autozooecia aperture, with rounded roofs, polygonal in tangential section. Autozooecial walls granular prismatic, 0.015-0.023 mm thick. Maculae not observed.
Remarks. The species Eridopora cf. beilensis Perkins and Perry, 1962, from the Arnsbergian (= Serpukhovian; late Mississippian) of Northern Yorkshire, England, described by Bancroft (1984) in his unpublished PhD dissertation is very similar to the original description of this species and the present species. However, the present species has smaller autozooecial apertures (average aperture width 0.30 mm vs. 0.39 mm in Eridopora cf. beilensis in Bancroft’s PhD). Eridopora beilensis differs from E. macrostoma Ulrich, 1882, in having smaller autozooecial apertures (average aperture width 0.30 mm vs. 0.39 mm in E. macrostoma).
Occurrence. Lecompton Limestone, Pennsylvanian (Virgilian); Kansas, USA. Graham Formation, Pennsylvanian (Virgilian); TXV-200 (“Spillway section at Lost Creek Lake”), Texas, USA.
Family Cystodictyonidae Ulrich, 1884
Genus Cystodictya Ulrich, 1882
Type species. C. ocellata Ulrich, 1882, by original designation. Lower Mississippian; Kentucky, USA.
Diagnosis. Bifoliate colonies, strap-like, branching in plane of mesotheca. Autozooecia with peristomes and lunaria. Ridges between autozooecial rows lacking. Mesotheca thin to moderately thick, indistinctly laminated to granular-prismatic, with low ridges, running parallel to ranges of autozooecia. Autozooecia teardrop-shaped at their bases, quadrate in transverse section; partly isolated by boxlike vesicles; recumbent portion short; blunt proximolateral hemisepta at zooecial bend, indenting zooecial cavity and producing slight hook-shaped appearance of autozooecia in deep tangential section. Diaphragms lacking. Walls laminated; boundary serrated, tubules in cortex. Lunarium in exozone, light coloured, laminated, some with core and proximal rib. Compound range walls thin in endozone with dark boundary continuous into dark central layer of mesotheca; thick in exozone with many flexures and irregular tubuli. Vesicles small, boxlike, in endozone; low blisters in inner exozone; stereom in exozone; laminated with tubuli and flexures.
Remarks. Cystodictya Ulrich, 1882, differs from Sulcoretepora d’Orbigny, 1849, in possessing teardrop-shaped apertures, straight mesotheca and autozooecial walls, which are distinctly tripartite in Sulcoretepora and more homogenous in Cystodictya. Furthermore, Cystodictya possesses hemisepta, which are absent in Sulcoretepora. Cystodictya differs from Dichotrypa Ulrich in Miller, 1889, in lacking acanthostyles in its exterior stereom.
Occurrence. Middle Devonian - Pennsylvanian; worldwide.
Cystodictya formosa Moore, 1929
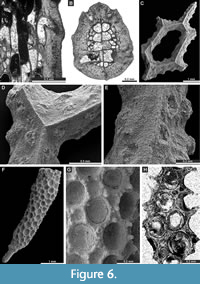
Figure 5C-G, Figure 6A-B; Appendix
 1929 Cystodictya formosa Moore, p. 150-151, pl. 18, figs. 4, 12, 13.
1929 Cystodictya formosa Moore, p. 150-151, pl. 18, figs. 4, 12, 13.
1929 Cystodictya formosa var. robusta Moore, p. 151, pl. 18, figs. 14, 29, 31.
1929 Cystodictya formosa var. striata Moore, p. 153, pl. 18, figs. 20-22.
Material. SNSB-BSPG 2020 XCI 23a, b, SNSB-BSPG 2020 XCI 27, SNSB-BSPG 2020 XCI 28, SNSB-BSPG 2020 XCI 33, SNSB-BSPG 2020 XCI 99.
Description. Bifoliate branches, 0.67-1.07 mm wide and 0.40-0.92 mm thick. Mesotheca 0.008-0.010 mm thick, granular. Autozooecia tubular, teardrop-shaped at their bases, trapezoidal to semicircular in transverse quadrate in cross-section, recumbent on the mesotheca for a relatively short distance, then bending upwards at low angles in exozone and intersecting the surface almost perpendicularly. Diaphragms lacking; long proximolateral hemisepta at zooecial bend present. Autozooecial apertures circular to oval, arranged in 4-6 alternating rows on the colony surface. Lunaria distinct, horseshoe-shaped. Vesicular skeleton well-developed, covered in exozone by thick stereom. Vesicles small, rectangular in tangential section, with rounded roofs, completely separating autozooecia in exozone in 1-2 rows. Autozooecial walls granular, 0.008-0.010 mm thick in endozone. Stereom well developed, 0.13-0.18 mm thick, consisting of laminated material, completely separating autozooecia in exozone.
Remarks. Moore (1929) established several varieties of the species Cystodictya formosa from the Graham Formation of Texas. Of these varieties, Cystodictya formosa var. robusta and C. formosa var. striata differ only in the branch width and thickness from the species C. formosa Moore, 1929. The differences in the branch width and thickness are indeed minimal and do not exceed normal variation within an assemblage from a restricted biotope (branch width 0.96 mm for C. formosa, 1.15 mm for C. formosa var. robusta, and 1.1 mm for C. formosa var. striata; branch thickness 0.72 mm for C. formosa, 1.3 mm for C. formosa var. robusta, and 0.72 mm for C. formosa var. striata, as given by Moore (1929).
Cystodictya formosa Moore, 1929, differs from C. modesta Moore, 1929, in possessing thicker and wider branches as well as in larger and wider spaced autozooecial apertures (aperture width 0.10-0.18 mm vs. 0.057-0.085 mm in C. modesta).
Occurrence. Upper Graham Formation, Pennsylvanian (Virgilian); Texas, USA. Graham Formation, Pennsylvanian (Virgilian); TXV-200 (“Spillway section at Lost Creek Lake”), Texas, USA.
Suborder Hexagonellina Morozova, 1970
Family Goniocladiidae Waagen and Pichl, 1885
Genus Goniocladia Etheridge, 1876
Type species. Carinella cellulifera Etheridge, 1873, subsequently designated by Etheridge (1876). Mississippian (Lower Carboniferous); Carluke (Scotland).
Diagnosis. Reticulate colonies with polygonal fenestrules. Branches bifoliate, joined by anastomoses or rarely by dissepiments. Autozooecia in two or more rows on each side of the mesotheca. Apertures with more or less developed lunaria and apertural styles. Thin mesotheca protruding as ridge on the circular reverse side and as sharp keel on peaked obverse side. Median rods in mesotheca usually lacking, in few species present. Thin-walled autozooecia usually separated by vesicular skeleton.
Remarks. Goniocladia Etheridge, 1876, differs from Aetomacladia Bretnall, 1926, and Goniocladiella Nekhoroshev, 1953, in having a reticulate colony shape rather than a pinnate one (main branch with diverging lateral branches).
Occurrence. Carboniferous - Permian; worldwide.
Goniocladia grahamensis Moore, 1929
Figure 6C-E; Appendix
1929 Goniocladia grahamensis Moore, p. 154-156, pl. 18, figs. 1-3, 8-11.
Material. SNSB-BSPG 2020 XCI 100.
Description. Reticulate colonies consisting of anastomosing bifoliate branches. Branches 0.54-0.70 mm wide. Fenestrules oval to polygonal, 1.00-1.36 mm wide and 2.32-2.66 mm long. Autozooecia budding in 2-3 rows from each side of thin mesotheca, opening on both sides of the sharp median carina. Lunaria present, horseshoe-shaped, directed toward fenestrule. Numerous microstylets on the colony surface, 0.008-0.011 mm in diameter.
Remarks. Goniocladia grahamensis Moore, 1929, differs from G. subpulchra Schulga-Nesterenko, 1955, from the Gzhelian of the Russian Platform, and from G. tenuis Schulga-Nesterenko, 1933, from the Gzhelian of Russia, in having smaller fenestrules (fenestrule width 1.00-1.36 mm vs. 1.25-2.00 in G. subpulchra and 1.75-2.00 mm in G. tenuis; fenestrule length 2.32-2.66 mm vs. 3.25-4.25 mm in G. subpulchra and 3.15-4.70 mm in G. tenuis).
Occurrence. Upper Graham Formation, Pennsylvanian (Virgilian); Texas, USA. Graham Formation, Pennsylvanian (Virgilian); TXV-200 (“Spillway section at Lost Creek Lake”), Texas, USA.
Order Trepostomata Ulrich, 1882
Suborder Amplexoporina Astrova, 1965
Family Dystritellidae Dunaeva and Morozova, 1967
Genus Dyscritella Girty, 1911
Type species. Dyscritella robusta Girty, 1911, by original designation. Mississippian; Arkansas, USA.
Diagnosis. Ramose and encrusting colonies with abundant acanthostyles and exilazooecia. Autozooecia parallel to longitudinal direction of the colony in endozone; gradually bending outward in exozone. Diaphragms in autozooecia lacking or very rare; lacking in exilazooecia. Exilazooecia circular to angular in cross section and separated from the autozooecia and from each other by thick walls. Two sizes of acanthostyles may be present. Zooecial walls thin in endozone, rapidly thickening in the exozone.
Remarks. Dyscritella Girty, 1911, generally lacks diaphragms, which are commonly developed in the similar genus Dyscritellina Morozova in Dunaeva and Morozova, 1967.
Occurrence. Devonian to Triassic; worldwide.
Dyscritella felixi n. sp.
Figure 6F-H, Figure 7A-B; Appendix
zoobank.org/B5FFF8E9-7B9E-4132-8C26-2AF97A7F089B
Etymology. The species is named after Felix, son of Barbara Seuss.
 Holotype. SNSB-BSPG 2020 XCI 101.
Holotype. SNSB-BSPG 2020 XCI 101.
Paratypes. Thin sections: SNSB-BSPG 2020 XCI 86, SNSB-BSPG 2020 XCI 87, SNSB-BSPG 2020 XCI 88, SNSB-BSPG 2020 XCI 89.
Type locality. TXV-200 (“Spillway section at Lost Creek Lake”), Texas, USA.
Type stratum. Graham Formation, Pennsylvanian (Virgilian).
Diagnosis. Thin encrusting colonies; 5-7 acanthostyles and 1-4 exilazooecia surrounding each autozooecial aperture; maculae absent.
Description. Encrusting colony, 0.40-0.50 mm in thickness. Autozooecial chambers tubular, growing from a thin epitheca. Acanthostyles common to abundant, 5-7 surrounding each autozooecial aperture, originating from base of exozone, moderate to large in size. Diaphragms absent. Exilazooecia small to moderate in size, rounded-angular, 1-4 surrounding each autozooecial aperture. Autozooecial walls granular, 0.008-0.013 mm thick in endozone; thick, merged, laminated without distinct zooecial boundaries, 0.033-0.055 mm thick in exozone. Maculae absent.
Remarks. Dyscritella felixi n. sp. is similar to D. inaequalis Girty, 1911, from the Fayetteville Shale (Mississippian) of Arkansas. The latter species developed ramose colony instead of encrusting ones as in the present species. Furthermore, it has smaller autozooecial apertures. Girty (1911, p. 194) measured 0.14 mm as the maximum size of the autozooecial apertures for his species in contrast to 0.15-0.24 mm in the studied material. Dyscritella felixi n. sp. differs from D. incrustans Dunaeva, 1964, from the Carboniferous (Serpukhovian-Bashkirian) of the Ukraine in possessing larger autozooecial apertures (aperture width. 0.15-0.24 mm vs. 0.12-0.15 mm in D. incrustans).
Order Cryptostomata Vine, 1884
Suborder Rhabdomesina Astrova and Morozova, 1956
Family Rhomboporidae Simpson, 1895
Genus Rhombopora Meek, 1872
[= Shishoviclema Gorjunova, 1985]
Type species. Rhombopora lepidodendroides Meek, 1872, by original designation. Pennsylvanian (Upper Carboniferous), North America.
Diagnosis. Ramose colonies. Autozooecia with oval apertures and regularly thickened walls in the exozone, intersecting the colony surface at low angles. Diaphragms present in exozone. One or two acanthostyles located on the distal end of each aperture. Abundant aktinotostyles arranged in a regular pattern around the apertures. Metazooecia few to absent. Exozonal walls thick, laminated.
Remarks. Rhombopora Meek, 1872, differs from Megacanthopora Moore, 1929, in having fewer metazooecia. Rhombopora Meek, 1872, differs from Primorella Romantchuk and Kiseleva, 1968, through the presence of acanthostyles.
Occurrence. Devonian - Permian; worldwide.
Rhombopora lepidodendroides Meek, 1872
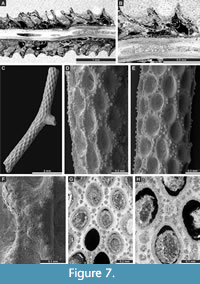
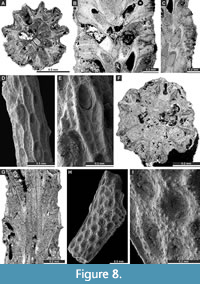
Figure 7C-H, Figure 8A-C; Appendix
1872 Rhombopora lepidodendroides Meek, p. 141-143, pl. 7, figs. 2a-2f.
 1877 Rbombipora lepidodendroides Meek, 1872; White, p. 99, pl. 6, figs. 5a-d.
1877 Rbombipora lepidodendroides Meek, 1872; White, p. 99, pl. 6, figs. 5a-d.
1884 Rhombopora lepidodendroidea Meek, 1872; Ulrich, p. 27, pl. 1, figs. 1-1b.
1887 Rhombopora lepidodendroidea Meek, 1872; Foerste, pl. 7, figs. 3a, b.
1888 Rhombopora lepidodendroides Meek, 1872; Keyes, p. 225.
1894 Rhombopora lepidodendroides Meek, 1872; Keyes, p. 35, pl. 33, figs. 4a, b.
1896 Rhombopora lepidodendroides Meek, 1872; Smith, p. 237.
1899 Rhombopora lepidodendroides ? Meek, 1872; Knight, p. 366.
1903a Rhombopora lepidodendroides Meek, 1872; Condra, p. 99, pl. 6, figs. 2-4, p. 7, figs. 1-12.
1903b Rhombopora lepidodendroides Meek, 1872; Condra, p. 22, pl. 2, figs. 1-11.
1908 Rhombopora aff. lepidodendroides Meek, 1872; Girty, p. 153, pl. 31, fig. 17.
1915 Rhombopora lepidodendroides Meek, 1872; Mather, p. 132, pl. 6, figs. 8, 9.
?1915 Rhombopora lepidodendroides Meek, 1872; Girty, p. 46-48.
1922 Rhombopora lepidodendroides Meek, 1872; Plummer and Moore, p. 169, pl. 23, figs. 20-27.
1922 Rhombopora lepidodendroidea Meek, 1872; Morningstar, p. 163-164.
1924 Rhombopora lepidodendroides Meek, 1872; Coryell in Morgan, pl. 38, figs. 3-5.
?1929 Rhombopora communis Moore, p. 139-140, pl. 17, fig. 12, text-figs. 4l, m.
1930 Rhombopora lepidodendroides Meek, 1872; Sayre, p. 92, pl. 1, figs. 6-8.
1935 Rhombopora lepidodendroides Meek, 1872; Twenhofel and Shrock, figs. 85K-L.
1944 Rhombopora lepidodendroides Meek, 1872; Shimer and Shrock, pl. 101, figs. 4-6.
1953 Rhombopora lepidodendroides Meek, 1872; Bassler, p. G134, figs. 95, 4a-c.
1953 Rhombopora lepidodendroides Meek, 1872; Shrock and Twenhofel, p. 246, fig. 7.
1962 Rhombopora lepidodendroides Meek, 1872; Perkins, Perry and Hattin, p. 18-20, pl. 3, figs. 5-7.
1970 Rhombopora lepidodendroides Meek, 1872; Huffman, p. 673, pl. 105, figs. 1-7, pl. 106, figs. 1-6.
1970 Rhombopora cf. lepidodendroides Meek, 1872; Fritz, p. 74-76, pl. 15, figs. 1, 4.
1971 Rhombopora lepidodendroides Meek, 1872; Newton, p. 28-29, pl. 1, figs. 1-6, 11, 12, pl. 2, 1-8, 11-16.
1985 Rhombopora lepidodendroides Meek, 1872; Gorjunova, p. 121, pl. 7, fig. 5.
1995 Rhombopora lepidodendroides Meek, 1872; Sakagami, 1995, p. 261-262, figs. 1.1-6.
2005 Rhombopora lepidodendroides Meek, 1872; Ernst, Schäfer, and Reijmer, p. 307, pl. 2, figs. 6-7.
2006 Rhombopora lepidodendroides Meek, 1872; Ernst and Minwegen, p. 579, figs. 5J-M.
2008 Rhombopora lepidodendroides Meek, 1872; Ernst and Winkler Prins, p. 24, pl. 12, 4-6.
2021 Rhombopora lepidodendroides Meek, 1872; Ernst, Krainer and Lucas, p. 225-227, figs. 6f-i, 7a-c.
Material. SNSB-BSPG 2020 XCI 44a-c, SNSB-BSPG 2020 XCI 45a, b, SNSB-BSPG 2020 XCI 58a, b, SNSB-BSPG 2020 XCI 63, SNSB-BSPG 2020 XCI 66, SNSB-BSPG 2020 XCI 80a, b, SNSB-BSPG 2020 XCI 102, SNSB-BSPG 2020 XCI 103.
Description. Ramose colonies, branches 0.73-1.48 mm in diameter, with 0.19-0.40 mm wide exozones and 0.27-0.68 mm wide endozones. Autozooecia short, growing in spiral pattern from a distinct median axis at angles of 32-55° in endozone, abruptly bending in exozones and intersecting colony surface at angles of 77-86°; triangular to rhombic, teardrop-shaped in transverse sections of endozone. Autozooecial apertures oval, arranged in quincunx on colony surface. Aktinotostyles abundant, arranged in a single row between autozooecial apertures forming relatively regular hexagons. One or two large acanthostyles between successive autozooecial apertures, with narrow hyaline core and wide laminated sheaths. Metazooecia rare to absent. Autozooecial walls laminated, without distinct boundaries in exozone. Autozooecial walls hyaline, 0.008-0.013 mm thick in endozone; laminated in exozone. Mural spines in outer exozonal walls, 0.01-0.02 mm in diameter.
Remarks. Rhombopora lepidodendroides Meek, 1872, differs from R. corticata Moore, 1929, in having smaller autozooecial apertures (average aperture width 0.15 mm vs. 0.17 mm in R. corticata; data from Ernst and Winkler Prins, 2008) and shorter distances between apertures along branch (average distance 0.47 mm vs. 0.72 mm in R. corticata; data from Ernst and Winkler Prins, 2008). Rhombopora lepidodendroides differs from R. vera Dunaeva, 1961 from the Moscovian of Ukraine in possessing larger autozooecial apertures (aperture width 0.11-0.17 mm vs. 0.07-0.09 mm in R. vera).
Rhombopora communis Moore, 1929, is apparently synonymous with R. lepidodendroides Meek, 1872. The original description of R. communis is short, and differences between the two species, noted by Moore (1929, p. 140) are minimal.
Occurrence. Studied material comes from the Graham Formation, Pennsylvanian (Virgilian) at the TXV-200 (“Spillway section at Lost Creek Lake”), Texas, USA. The majority of the records of Rhombopora lepidodendroides Meek, 1872 (see synonymy list), come from the Pennsylvanian to the lower Permian of the USA and Canada. This species has also been recorded from the Pennsylvanian of the Cantabrian Mountains, Spain: Valdeteja Formation (Bashkirian) of Valdeteja, León; San Emiliano Formation (Westphalian B/C) of Valverdín; Picos de Europa Formation (Moscovian) of La Hermida; ? Las Llacerias Formation (Kasimovian) of Sotres, Asturias. One record of R. lepidodendroides is known from the Pennsylvanian of Bolivia (Sakagami 1995).
Family Hyphasmoporidae Vine, 1885
Genus Streblotrypa Vine, 1885
Subgenus Streblotrypa (Streblotrypa) Vine, 1885
Type species. Streblotrypa nicklesi Vine, 1885, by original designation. Late Mississippian (Middle Carboniferous; Pendleian = Serpukhovian); Hurst, north Yorkshire, England.
Diagnosis. Ramose colonies with indistinct bundle of about 10 or fewer axial zooecia in the endozone. Autozooecia budding from axial bundle, having long inflated proximal parts, rounded-polygonal in transverse section in the endozone, bending abruptly at the transition between endo- and exozone. Autozooecial apertures rounded to oval. Diaphragms rare. Hemisepta usually present. Metazooecia usually restricted to rows between the autozooecial apertures; styles usually lacking but poorly developed acanthostyles sometimes occurring. Autozooecial walls laminated, without distinct autozooecial boundaries.
Remarks. Streblotrypa (Streblotrypa) Vine, 1885, differs from S. (Streblascopora) Bassler, 1929, by an indistinctly defined axial bundle of axial zooecia and well-developed hemisepta.
Occurrence. Carboniferous to Permian; worldwide.
Streblotrypa (Streblotrypa) multipora Warthin, 1930
Figure 8D-G; Appendix
1930 Streblotrypa multipora Warthin, p. 42, pl. 3, fig. 15.
Material. SNSB-BSPG 2020 XCI 39b, SNSB-BSPG 2020 XCI 40, SNSB-BSPG 2020 XCI 41, SNSB-BSPG 2020 XCI 43, SNSB-BSPG 2020 XCI 104.
Description. Ramose colonies, branches 0.43-0.76 mm in diameter, with 0.19-0.38 mm wide endozones and 0.10-0.20 mm wide exozones. Branch transverse sections rounded to oval. Autozooecia budding from the axial bundle in a regular spiral pattern at angles of 18-28°, bending in exozones and intersecting colony surface at angles of 60-80°, having long inflated proximal parts, rounded-polygonal in transverse section in the endozone. Axial bundle indistinct, formed by 2-5 axial zooecia, 0.11-0.15 mm in diameter. Autozooecial apertures oval, opening around the branches in regular diagonal rows. Autozooecial boundaries marked by sharp ridges on the colony surface, which form a roughly hexagonal pattern. Superior hemisepta absent; inferior hemisepta well-developed, thin, placed approximately in the middle of the chamber, curved proximally. Autozooecial diaphragms rarely occurring. Metazooecia oval to rounded, usually 7-10 arranged in 2-3 rows between apertures, but often clustered in parts of branches lacking apertures. Autozooecial walls hyaline, 0.010-0.015 mm thick in endozone; laminated in exozone.
Remarks. Streblotrypa (Streblotrypa) multipora Warthin, 1930, differs from S. (S.) merceri Morningstar, 1922, from the Pennsylvanian of Ohio by presence of 7-10 metazooecia between autozooecial apertures instead of four in the latter species. Streblotrypa (Streblotrypa) multipora differs from S. (S.) heltzelae Ernst et al., 2016, from the Boggy Formation of Oklahoma by its absence of acanthostyles.
Occurrence. Upper Wetumka - lower Wewoka Formation, Pennsylvanian (Desmoinesian), Oklahoma, USA. Graham Formation, Pennsylvanian (Virgilian); TXV-200 (“Spillway section at Lost Creek Lake”), Texas, USA.
Order Fenestrata Elias and Condra, 1957
Suborder Phylloporinina Lavrentjeva, 1979
Family Chainodictyonidae Nickles and Bassler, 1900
Genus Rhombocladia Rogers, 1900
Type species. Rhombocladia delicata Rogers, 1900, by original designation. Pennsylvanian (Upper Coal Measures); Kansas, USA.
Diagnosis. Ramose colonies. Flattened branches bearing 4-12 zooecial rows. Vestibule weakly developed. Diaphragms rare. Superior hemisepta usually developed. Oval apertures arranged in a diagonal pattern. Autozooecial chambers rhombic in mid-tangential section. Macroacanthostyles often occurring at distal ends of autozooecial apertures. Microacanthostyles present in zooecial walls, sometimes forming star-like accumulations. Leptozooecia rarely present on the frontal surface or on lateral parts of branches. Dorsal wall very thin.
Remarks. The genus Rhombocladia differs from Chainodictyon Foerste, 1887, by having a ramose instead of a reticulate colony form and by the development of hemisepta. From Kallodictyon Morozova, 1981, it differs in colony-form, the thin dorsal wall and the absence of leptozooecia on the dorsal surface of the colony.
Occurrence. Middle Devonian - Upper Permian; worldwide.
Rhombocladia delicata Rogers, 1900
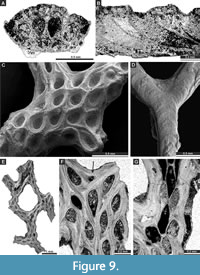
Figure 8H-I, Figure 9A-B; Appendix
1900 Rhombocladia delicata Rogers, p. 12, pl. 1, figs. 1-1d.
 pars 1906 Rhombocladia delicata Rogers, 1900; Johnsen, p. 58, pl. 11, fig. 30a [? non 30b].
pars 1906 Rhombocladia delicata Rogers, 1900; Johnsen, p. 58, pl. 11, fig. 30a [? non 30b].
pars 1929 Rhombocladia delicata Rogers, 1900; Moore, p. 149, pl. 17, figs. 26-28, 30, 31 [non 29, 32].
1930 Rhombocladia delicata Rogers, 1900; Warthin, p. 42, pl. 3, fig. 17.
1963 Rhombocladia delicata Rogers form A; Ceretti, p. 327, pl. 7, fig. 10.
1964 Rhombocladia delicata Rogers; Ceretti, p. 184, 185, pl. 33, fig. 2a, b, 4a, b.
2003 Rhombocladia delicata Rogers, 1900; Ernst, p. 63, 64, pl. 5, figs. 4-7; text-fig. 3.
2016 Rhombocladia delicata Rogers, 1900; Ernst et al., p. 530, figs. 6b-f, 8a, d, 9a.
2021 Rhombocladia delicata Rogers, 1900; Ernst, Krainer, and Lucas, p. 228, figs. 7d-h.
Material. SNSB-BSPG 2020 XCI 22a-c, SNSB-BSPG 2020 XCI 105.
Description. Ramose colonies, branches 0.74-1.07 mm wide, 0.40-0.50 mm deep, flattened, bearing 5-6 zooecial rows; dorsal wall rugose. Diaphragms not observed. Superior hemisepta present. Oval apertures arranged in a diagonal pattern. Autozooecial chambers rhombic in mid-tangential section. Single macroacanthostyle at the distal end of each autozooecial aperture present. Microacanthostyles arranged in few irregular rows between autozooecial apertures, 0.008-0.013 mm in diameter. Leptozooecia not observed in present material. Autozooecial walls in endozone 0.015-0.020 mm thick, hyaline; finely laminated in exozone.
Remarks. Rhombocladia delicata Rogers, 1900, differs from R. coronata Schulga-Nesterenko, 1955, from the Moscovian of Russian Platform by presence of macroacanthostyles. It differs from R. carnica Ceretti, 1964, from the Pennsylvanian (lower Gzhelian) of Italy by having wider branches (0.74-1.07 mm vs. 0.63-0.96 mm).
Occurrence. Carboniferous, Pennsylvanian; Kansas, USA. Carboniferous, Middle Pennsylvanian (Wewoka Formation); Oklahoma, USA. Carboniferous, Pennsylvanian, Corona Formation (early Gzhelian); Kron Alpe (Monte Corona), Carnic Alps (Austria). Carboniferous, Pennsylvanian, Auernig Formation (Lower Gzhelian); Auernig, Carnic Alps (Udine, Italy). Strata probably attributable to the Las Llacerias Formation, Pennsylvanian (Kasimovian); Cantabrian Mountains, Asturias, NW Spain. Carboniferous, Pennsylvanian, Missourian (Kasimovian), Deese Group, Boggy Formation; Buckhorn Asphalt Quarry near Sulphur, Oklahoma, USA. Gray Mesa Formation, Pennsylvanian (Desmoinesian); Fra Cristobal Mountains, New Mexico, USA. Graham Formation, Pennsylvanian (Virgilian); TXV-200 (“Spillway section at Lost Creek Lake”), Texas, USA.
Genus Chainodictyon Foerste, 1887
Type species. Chainodictyon laxum Foerste, 1887, by original designation. Pennsylvanian (Upper Carboniferous); USA, Ohio.
Diagnosis. Reticulate colonies, regularly anastomosing. Flattened branches bearing 3-12 rows of autozooecia. Vestibule weakly developed. Diaphragms rare. Hemisepta absent. Oval apertures arranged in a diagonal pattern. Autozooecial chambers rhombic in mid-tangential section. Styles absent. Leptozooecia common on the obverse surface of branches. Reverse wall very thin.
Remarks. Chainodictyon Foerste, 1887, differs from Rhombocladia Rogers, 1900, by having a reticulate colony form instead of a ramose one and by the absence of hemisepta. Chainodictyon differs from Kallodictyon Morozova, 1981, in its development of a thin reverse wall and by absence of leptozooecia on the reverse surface of branches.
Occurrence. Mississippian of Kazakhstan and Russia, Pennsylvanian of USA, and Russia, and Lower Permian of Russia.
Chainodictyon minor Ulrich, 1890
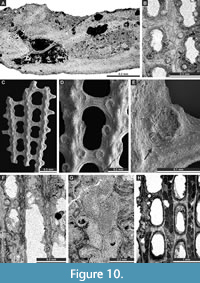
Figure 9C-G, Figure 10A; Appendix
1890 Chainodictyon laxum var. minor Ulrich, p. 640, pl. 62, figs. 3-3a.
 Material. SNSB-BSPG 2020 XCI 37, SNSB-BSPG 2020 XCI 38, SNSB-BSPG 2020 XCI 42, SNSB-BSPG 2020 XCI 106, SNSB-BSPG 2020XCI 125.
Material. SNSB-BSPG 2020 XCI 37, SNSB-BSPG 2020 XCI 38, SNSB-BSPG 2020 XCI 42, SNSB-BSPG 2020 XCI 106, SNSB-BSPG 2020XCI 125.
Description. Reticulate colonies formed by anastomosing branches. Branches frequently bifurcating, flattened, bearing 2-4 alternating rows of autozooecia; dorsal wall rugose, 0.30-0.79 mm wide. Fenestrules elongate, rounded-polygonal, 0.65-1.10 mm wide, and 1.26-1.90 mm long. Autozooecial apertures oval. Diaphragms not observed. Hemisepta absent. Styles absent. Leptozooecia common to abundant, small, occurring between autozooecia on the obverse side of branches. Autozooecial walls in endozone 0.013-0.020 mm thick, hyaline; finely laminated in exozone.
Remarks. Chainodictyon minor Ulrich, 1890, differs from C. laxum Foerste, 1887, in having a smaller size of fenestrules (fenestrule width 0.65-1.10 mm vs. ca. 1.3 mm in C. laxum; fenestrule length 1.26-1.90 mm vs. 2.5 mm in C. laxum). Chainodictyon minor differs from C. angustum Schulga-Nesterenko, 1952, from the Lower Permian (Asselian) of Russia in having smaller fenestrules (fenestrule width 0.65-1.10 mm vs. 1.2-2.0 mm in C. angustum; fenestrule length 1.26-1.90 mm vs. 3.6-4.1 mm in C. angustum).
Occurrence. Pennsylvanian; USA, Illinois. Graham Formation, Pennsylvanian (Virgilian); TXV-200 (“Spillway section at Lost Creek Lake”), Texas, USA.
Suborder Fenestellina Astrova and Morozova, 1956
Family Fenestellidae King, 1849
Subfamily Fenestellinae King, 1849
Genus Fabifenestella Morozova, 1974
Type species. Fenestella praevirgosa Schulga-Nesterenko, 1951, subsequently designated by Morozova (1974). Pennsylvanian (Upper Carboniferous, Gzhelian); Russia.
Diagnosis. Reticulate colonies of different shape, with moderately wide and thick branches and moderately wide dissepiments. Autozooecia arranged in two rows on the branches, rectangular to pentagonal in deep tangential section and fabiform in shallow to mid-tangential section. Axial wall between autozooecial rows weakly undulating. Both superior and inferior hemisepta present. Low and wide keel with alternating nodes developed (modified after Morozova, 2001, p. 53).
Remarks. Fabifenestella Morozova, 1974, differs from Exfenestella Morozova, 1974, in the presence of a low and wide keel with alternating nodes. Fabifenestella differs from Minilya Crockford, 1944, in having rectangular to fabiform autozooecial shape in mid-tangential section instead of triangular one.
Occurrence. Mississippian (Lower Carboniferous) - Upper Permian; worldwide.
Fabifenestella compactilis (Condra, 1902)
Figure 10B-G; Appendix
1902 Fenestella conradi var. compactilis Condra, p. 348, pl. 22, figs. 1-2.
1903a Fenestella conradi-compactilis Condra, 1902; p. 60-61, pl. 8, figs. 11-12.
non 1941 Fenestella conradi compactilis Condra, 1902; Schulga-Nesterenko, p. 98-99, pl. 17, fig. 3, pl. 18, figs. 2-3.
1957 Fenestella compactilis Condra, 1902; Elias and Condra, p. 93-94
1957 Fenestella compactilis var. plattsmouthensis; Elias and Condra, p. 95-96, pl. 12, figs. 6-7.
Material. SNSB-BSPG 2020 XCI 49, SNSB-BSPG 2020 XCI 62, SNSB-BSPG 2020 XCI 75, SNSB-BSPG 2020 XCI 107.
Exterior description. Reticulate colonies formed by straight branches joined by relatively wide dissepiments. Fenestrules oval to rectangular, about twice as long as wide. Autozooecia arranged in two rows on branches. Additional autozooecium often developed in the place of branch diversion. Autozooecial apertures circular, with low peristome, containing eight nodes (stellate structure); two to three apertures spaced per fenestrule length. Proximal pore present, positioned proximally to the autozooecial aperture, 0.025-0.037 mm in diameter. Keel wide, low, containing densely spaced alternating nodes. Nodes varying in size, elliptically shaped.
Interior description. Autozooecia relatively long, roughly pentagonal to rectangular in deep tangential section, becoming fabiform in mid-tangential section; with short to moderately long vestibule in longitudinal section. Axial wall between autozooecial rows weakly to strongly undulating; aperture positioned at distal end of chamber. Hemisepta present, positioned in the distal half of autozooecial chamber. External laminated skeleton well-developed on both obverse and reverse sides. Heterozooecia not observed.
Remarks. Fabifenestella compactilis (Condra, 1902) differs from F. praevirgosa (Schulga-Nesterenko, 1951) from the Pennsylvanian (Gzhelian) of the Russian Platform in possessing smaller fenestrules (fenestrule width 0.15-0.27 mm vs. 0.28-0.35 mm in F. praevirgosa; fenestrule length 0.38-0.63 mm vs. 0.75-0.90 mm in F. praevirgosa). Fabifenestella compactilis differs from F. almazani Ernst and Vachard, 2017, from the Pennsylvanian (Moscovian) of Mexico in the closer spacing of fenestrules (average distance between dissepiment centres 0.67 mm vs. 0.74 mm in F. almazani), and in wider spacing of autozooecial apertures (average distance between aperture centres along branches 0.28 mm vs. 0.23 mm in F. almazani).
Morozova (2001) listed the species Minilya conradi-compactilis (Condra, 1902). However, the placement of this species in the genus Minilya Crockford, 1944, appears incorrect. The original description, as well as the following ones, was performed without use of thin sections, therefore, the generic assignment could not be clarified with certainty. However, Elias and Condra (1957, p. 94) described the shape of the autozooecia as follows: “Outline of zooecial chamber subpentagonal at base, but transverse sides of pentagons strongly inclined distad; at slightly higher level central zigzag line becomes nearly straight, pentagons inclined forwardly become parallelograms,...”. They also depicted images of polished slabs showing the internal shape of autozooecia on the pl. 12, fig. 5 (Fenestella compactilis) and fig. 7 (Fenestella compactilis var. plattsmouthensis). The shape of the autozooecia in both species is identical and typically fabiform, not triangular or trapezoid as in Minilya.
Occurrence. Cass Limestone, Douglas Group, Pennsylvanian (Virgilian); Nebraska, USA. Graham Formation, Pennsylvanian (Virgilian); TXV-200 (“Spillway section at Lost Creek Lake”), Texas, USA.
Genus Laxifenestella Morozova, 1974
Type species. Fenestella sarytshevae Schulga-Nesterenko, 1951, subsequently designated by Morozova (1974). Mississippian, Serpukhovian; Moscow Syncline, Russia.
Diagnosis. Reticulate colonies consisting of relatively wide and thick branches and moderately wide dissepiments. Autozooecia arranged in two rows on the branches. Autozooecial chambers rectangular to pentagonal in mid-tangential section. Axial wall between autozooecial rows weakly undulating. Both superior and inferior hemisepta present. Narrow keel with single row of nodes developed (modified after Morozova 2001, p. 44).
Remarks. Laxifenestella Morozova, 1974, differs from Fenestella Lonsdale, 1839, in the rectangular to pentagonal shape of autozooecia in mid-tangential section and the presence of well-developed hemisepta.
Occurrence. Lower Devonian - Upper Permian; worldwide.
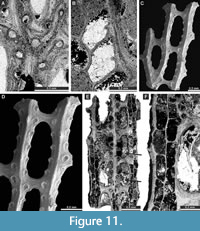
Laxifenestella placida Moore, 1929
Figure 10H, Figure 11A-D; Appendix
1929 Laxifenestella placida Moore, p. 17, pl. 2, figs. 5-7.
 Material. SNSB-BSPG 2020 XCI 35, SNSB-BSPG 2020 XCI 65, SNSB-BSPG 2020 XCI 67, SNSB-BSPG 2020 XCI 108.
Material. SNSB-BSPG 2020 XCI 35, SNSB-BSPG 2020 XCI 65, SNSB-BSPG 2020 XCI 67, SNSB-BSPG 2020 XCI 108.
Exterior description. Reticulate colonies formed by straight branches joined by relatively narrow flat dissepiments. Fenestrules oval to rectangular. Autozooecia arranged in two rows on branches. Autozooecial apertures circular, with low peristome; four to five apertures spaced per fenestrule length. Rounded nodes on the low keel, widely spaced. Reverse side finely striated.
Interior description. Autozooecia relatively long, roughly pentagonal to rectangular in mid-tangential section; with short to moderately long vestibule in longitudinal section. Axial wall between autozooecial rows straight to weakly undulating; aperture positioned at distal end of chamber. Both superior and inferior hemisepta long. External laminated skeleton well-developed on both obverse and reverse sides traversed by abundant microstylets. Microstylets 0.005-0.008 mm in diameter. Heterozooecia not observed.
Remarks. Laxifenestella placida Moore, 1929, differs from L. stuckenbergi (Nikiforova, 1938) from the Upper Carboniferous - Lower Permian of Russia in having wider branches (branch width 0.32-0.54 mm vs. 0.23-0.28 mm in L. stuckenbergi), and in larger fenestrules (fenestrule width 0.30-0.53 mm vs. 0.15-0.22 in L. stuckenbergi; fenestrule length 0.82-1.35 mm vs. 0.35-0.45 mm in L. stuckenbergi). Laxifenestella placida Moore, 1929, differs from L. benskiensis (Schulga-Nesterenko, 1951, from the Mississippian (Serpukhovian) of Russia in having wider branches (0.32-0.54 mm vs. 0.30-0.35 mm in L. benskiensis), and in larger fenestrules (fenestrule width 0.30-0.53 mm vs. 0.30-0.45 in L. benskiensis; fenestrule length 0.82-1.35 mm vs. 0.70-0.80 mm in L. benskiensis).
Occurrence. Upper Graham Formation, Pennsylvanian (Virgilian); Texas, USA. Graham Formation, Pennsylvanian (Virgilian); TXV-200 (“Spillway section at Lost Creek Lake”), Texas, USA.
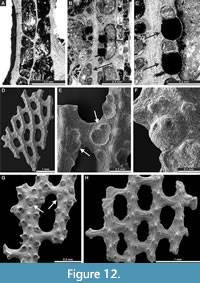
Laxifenestella texana n. sp.
Figure 11E-F, Figure 12A-G; Appendix
zoobank.org/A7A6097D-A051-4B20-A58B-8FA7576AB66B
Etymology. The species is named after Texas where it was found for the first time.
 Holotype. SNSB-BSPG 2020 XCI 81.
Holotype. SNSB-BSPG 2020 XCI 81.
Paratypes. SNSB-BSPG 2020 XCI 109, SNSB-BSPG 2020 XCI 110, SNSB-BSPG 2020 XCI 111.
Type locality. TXV-200 (“Spillway section at Lost Creek Lake”), Texas, USA.
Type stratum. Graham Formation, Pennsylvanian (Virgilian).
Diagnosis. Laxifenestella species with straight branches joined by relatively narrow dissepiments, oval to rectangular fenestrules, three to four circular apertures per fenestrule length and single node in peristome, directed into the next fenestrule; densely spaced rounded nodes on the low keel; reverse side finely striated; long superior and inferior hemisepta; apparent reproductive abundant heterozooecia in form of isolated zooecia with enlarged endozonal chambers.
Exterior description. Reticulate colonies formed by straight branches joined by relatively narrow dissepiments. Fenestrules oval to rectangular. Autozooecia arranged in two rows on branches. Autozooecial apertures circular, with low peristome; three to four apertures spaced per fenestrule length. Single node in peristome, directed into the next fenestrule (Figure 12G). Rounded nodes on the low keel, closely spaced. Reverse side finely striated.
Interior description. Autozooecia relatively long, roughly pentagonal to rectangular in mid-tangential section; with short to moderately long vestibule in longitudinal section. Axial wall between autozooecial rows straight to weakly undulating; aperture positioned at distal end of chamber. Both superior and inferior hemisepta long. External laminated skeleton well-developed on both obverse and reverse sides traversed by microstylets. Microstylets 0.003-0.005 mm in diameter. Apparent reproductive heterozooecia in form of isolated zooecia with enlarged endozonal chambers abundant (Figure 12B-C, E-F). Chambers rounded to oval, 0.11-0.16 mm in diameter. Nanozooecia present (Figure 12G).
Remarks. Laxifenestella texana n. sp. differs from L. placida Moore, 1929, in possessing narrower branches (branch width 0.23-0.34 mm vs. 0.32-0.54 mm in L. placida), and in smaller fenestrules (fenestrule width 0.20-0.33 mm vs. 0.30-0.53 in L. placida; fenestrule length 0.44-0.70 mm vs. 0.82-1.35 mm in L. placida). Laxifenestella texana n. sp. differs from L. stuckenbergi (Nikiforova, 1938) from the Upper Carboniferous - Lower Permian of Russia in having larger fenestrules (fenestrule width 0.20-0.33 mm vs. 0.15-0.22 in L. stuckenbergi; fenestrule length 0.44-0.70 mm vs. 0.35-0.45 mm in L. stuckenbergi). Furthermore, Laxifenestella texana n. sp. has smaller and closer nodes on the obverse keel.
Genus Cavernella Morozova, 1974
Type species. Fenestella dvinensis Schulga-Nesterenko, 1951 = junior subjective synonym of Fenestella praecavifera Schulga-Nesterenko, 1951, subsequently designated by Morozova (1974). Pennsylvanian (Upper Carboniferous, Kasimovian); Russia.
Diagnosis. Colony fan-shaped or conical. Branches straight, connected by thin dissepiments. Two rows of autozooecia on branches, overlapped basally. Autozooecial chambers pentagonal to triangular in mid-tangential section. Axial wall between autozooecial rows strongly undulating. Superior hemisepta absent or poorly developed; inferior hemisepta absent. Low keel with a single row of nodes on the observed surface. Pairs of heterozooecia (cavernozooecia) on the reverse side of branches or on dissepiments, opening into the fenestrule (modified after Morozova 2001, p. 46).
Occurrence. Pennsylvanian (Upper Carboniferous) - Upper Permian; Eurasia, North America.
Remarks. Cavernella Morozova, 1974, differs from other fenestrates in the presence of cavernozooecia.
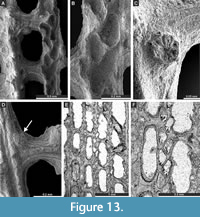
Cavernella praecavifera (Schulga-Nesterenko, 1951)
Figure 12H, Figure 13A-F, Figure 14A-D; Appendix
1951 Fenestella praecavifera Schulga-Nesterenko, p. 44-45, pl. 9, fig. 4, text-fig. 9.
 1951 Fenestella dvinensis Schulga-Nesterenko, p. 45-48, pl. 9, figs. 2, 5, text-fig. 10.
1951 Fenestella dvinensis Schulga-Nesterenko, p. 45-48, pl. 9, figs. 2, 5, text-fig. 10.
non 1983 Fenestella dvinensis Schulga-Nesterenko, 1951; Yang and Lu, p. 273-274, pl. 5, figs. 11-14.
2001 Cavernella dvinensis (Schulga-Nesterenko, 1951); Morozova, pl. 7, fig. 2.
Material. SNSB-BSPG 2020 XCI 69, SNSB-BSPG 2020 XCI 71, SNSB-BSPG 2020 XCI 73, SNSB-BSPG 2020 XCI 78, SNSB-BSPG 2020 XCI 79, SNSB-BSPG 2020 XCI 112, SNSB-BSPG 2020 XCI126, SNSB-BSPG 2020 XCI 127, SNSB-BSPG 2020 XCI 128.
Exterior description. Reticulate colonies formed by straight branches joined by relatively narrow dissepiments. Fenestrules oval to rectangular. Autozooecia arranged in two rows on branches. Autozooecial apertures circular, with low peristome and stellate structure; two to four apertures spaced per fenestrule length. Rounded nodes on the low keel, regularly spaced. Reverse side finely striated containing rounded nodes 0.025-0.040 mm in diameter.
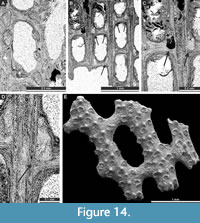 Interior description. Autozooecia relatively long, roughly pentagonal to triangular in mid-tangential section; with short to moderately long vestibule in longitudinal section. Axial wall between autozooecial rows strongly undulating; aperture positioned at distal end of chamber. Superior hemisepta long; inferior hemisepta indistinct. External laminated skeleton well-developed on both obverse and reverse sides traversed by microstylets. Microstylets 0.008-0.010 mm in diameter. Heterozooecia are cavernozooecia in form of elongated chambers, arranged in pairs on the reverse side of branches or on dissepiments, opening into the fenestrule, 0.06-0.10 mm in width and with rounded openings 0.04-0.05 mm in diameter.
Interior description. Autozooecia relatively long, roughly pentagonal to triangular in mid-tangential section; with short to moderately long vestibule in longitudinal section. Axial wall between autozooecial rows strongly undulating; aperture positioned at distal end of chamber. Superior hemisepta long; inferior hemisepta indistinct. External laminated skeleton well-developed on both obverse and reverse sides traversed by microstylets. Microstylets 0.008-0.010 mm in diameter. Heterozooecia are cavernozooecia in form of elongated chambers, arranged in pairs on the reverse side of branches or on dissepiments, opening into the fenestrule, 0.06-0.10 mm in width and with rounded openings 0.04-0.05 mm in diameter.
Remarks. Differences between Cavernella dvinensis (Schulga-Nesterenko, 1951) and C. praecavifera (Schulga-Nesterenko, 1951) are minimal, therefore, C. dvinensis is regarded as being junior synonym of C. praecavifera. As far as Morozova (1974, p. 65) designated Fenestella dvinensis Schulga-Nesterenko, 1951, as the type species of the genus Cavernella, this name is retained for the type species of the genus, despite its junior status (Code of Zoological Nomenclature, Article 67.1.2).
The species identified as Fenestella dvinensis Schulga-Nesterenko, 1951, from the Kankerin Formation of Kalpin of western Xinjiang, China (Yang and Lu, 1983) does not belong to this species because it lacks cavernozooecia. It differs also in some other morphological characters. This bryozoan has stellate nodes instead of rounded ones in C. dvinensis. Furthermore, the Chinese material has significantly smaller fenestrules (fenestrule length 0.40-0.45 mm vs. 0.60-0.65 mm in the material from the Russian Platform and 0.53-0.71 mm in the studied material from the Finis Shale).
Cavernella praecavifera (Schulga-Nesterenko, 1951) differs from Cavernella supercarbonica (Schulga-Nesterenko, 1951) from the Pennsylvanian (Gzhelian) of the Russian Platform in having smaller fenestrules (fenestrule width 0.19-0.39 mm vs. 0.27-0.40 mm in C. supercarbonica; fenestrule length 0.53-0.71 mm vs. 0.50-0.75 mm in C. supercarbonica).
Occurrence. Pennsylvanian (Upper Carboniferous, Kasimovian); Russia. Graham Formation, Pennsylvanian (Virgilian); TXV-200 (“Spillway section at Lost Creek Lake”), Texas, USA.
Subfamily Polyporinae Vine, 1884
Genus Acupipora Gorjunova and Weiss, 2012
Type species. Polypora subborealis Schulga-Nesterenko, 1951 (= Polypora krasnopolskyi Schulga-Nesterenko, 1951), subsequently designated by Gorjunova and Weiss (2012). Pennsylvanian, Gzhelian; Russia.
Diagnosis. Reticulate colonies of different shapes built by straight or slightly undulating, bifurcating branches, joined at regular intervals by straight and short dissepiments without autozooecia. Autozooecia arranged in 3-5 alternating rows on branches, and 2-3 after bifurcation. Autozooecial chambers polygonal tubular, proximally recumbent on budding plate, with long axis directed toward obverse surface; rhomboidal, hexagonal to tetragonal in tangential sections through endozone, superior hemisepta present, blunt; inferior hemisepta present, positioned at the base of the autozooecia chamber in its middle-distal part. Vestibules moderately long. Diaphragms absent. Autozooecial apertures rounded. Microacanthostyles and nodes usually present on obverse surface. Heterozooecia in form of isolated zooecia with enlarged endozonal chambers present. Nanozooecia (autozooecia sealed by perforated terminal diaphragm) present (modified after Gorunova and Weiss, 2012).
Remarks. Acupipora Gorunova and Weiss, 2012, differs from the genus Polyporella Simpson, 1895, in the shape of autozooecia (rhomboidal, hexagonal to tetragonal vs. hexagonal to tetragonal-pentagonal in Polyporella). Acupipora differs from Polypora M’Coy, 1844 in shape of autozooecia (rhomboidal, hexagonal to tetragonal vs. hexagonal in Polypora) and in presence of inferior hemisepta.
Occurrence. Pennsylvanian, Gzhelian; Russia. Pennsylvanian, Desmoinesian (late Moscovian); Mexico. Graham Formation, Pennsylvanian (Virgilian); Texas, USA.
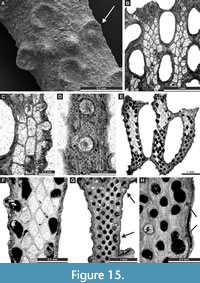
Acupipora elliptica (Rogers, 1900)
Figure 14E, Figure 15A-D; Appendix
1900 Polypora elliptica Rogers, p. 7, pl. 4, fig. 2.
 1924 Polypora elliptica Rogers, 1900; Morgan, p. 116, pl. 37, fig. 9.
1924 Polypora elliptica Rogers, 1900; Morgan, p. 116, pl. 37, fig. 9.
1929 Polypora elliptica Rogers, 1900; Moore, p. 23-24, pl. 3, figs. 7, 8, 20.
1930 Polypora elliptica Rogers, 1900; Sayre, p. 89-90, pl. 3, figs 2-4.
1930 Polypora elliptica Rogers, 1900; Moore, p. 155-156.
1937 Polypora elliptica Rogers, 1900; Elias, p. 327-328, fig. 3m.
1980 Protoretepora elliptica (Rogers, 1900); Simonsen and Cuffey, p. 15, figs. 3F, 4F, 6F, 7F.
Material. SNSB-BSPG 2020 XCI 60, SNSB-BSPG 2020 XCI 64, SNSB-BSPG 2020 XCI 70, SNSB-BSPG 2020 XCI 77, SNSB-BSPG 2020 XCI 113.
Exterior description. Reticulate colonies composed of moderately wide branches joined by moderately wide dissepiments. Autozooecia arranged in 3-5 alternating rows on branches, 2-3 after bifurcation. Autozooecial apertures rounded to oval, having a prominent rim with spine-like projection toward nearest fenestrule, 3-5 spaced per length of fenestrule. Fenestrules oval to slightly rectangular. Nodes large, elliptical, spaced regularly between autozooecial apertures.
Interior description. Autozooecial chambers moderately long, rectangular to roughly hexagonal in the shallow tangential section, becoming rhombic in the mid and deep tangential sections. Superior hemisepta blunt, placed at the proximal part of the vestibule. Inferior hemisepta well-developed, situated in the distal third of autozooecial chambers. Interior hyaline skeleton well-developed, wrinkled on the reverse side of the branches. External laminated skeleton well-developed, traversed by abundant small microstylets. Microstylets 0.005-0.010 mm in diameter. Apparent reproductive heterozooecia in form of isolated zooecia with enlarged endozonal chambers present. Chambers rounded, 0.15 mm in diameter. Nanozooecia present.
Remarks. Acupipora elliptica (Rogers, 1900) is similar to A. mexicana Ernst and Vachard, 2017, from the Pennsylvanian (Moscovian-Kasimovian) of Mexico but differs from the latter due to the closer spacing of branches (average distance between branch centres 0.88 mm vs. 1.10 mm in A. mexicana). Acupipora elliptica differs from A. subborealis (Schulga-Nesterenko, 1951) in having larger fenestrules (fenestrule width 0.27-0.60 mm vs. 0.30-0.40 mm in A. subborealis; fenestrule length 0.70-0.99 mm vs. 0.70-0.80 mm in A. subborealis).
Occurrence. Drum Limestone, Pennsylvanian (Missourian); Kansas, USA. Deese Group, Boggy Formation, Pennsylvanian (Missourian); Oklahoma, USA. Topeka Limestone, Pennsylvanian (Virgilian); Kansas, USA. Upper Graham Formation, Pennsylvanian (Virgilian); Texas, USA. Graham Formation, Pennsylvanian (Virgilian); TXV-200 (“Spillway section at Lost Creek Lake”), Texas, USA.
Genus Polypora M’Coy, 1844
Type species. Polypora dendroides M’Coy, 1844, by original designation. Mississippian (Lower Carboniferous); Ireland.
Diagnosis. Colonies conical or fan-shaped, planar to longitudinally pleated; branches broad, linear, essentially parallel, spacing intermediate, dichotomously dividing; dissepiments narrow, perpendicular or at oblique angle to branches, regularly spaced at intermediate distance; three or more rows autozooecia per branch, up to at least six rows below branch bifurcations; autozooecial apertures aligned longitudinally and diagonally in alternating rows; no keel present on branches, although large styles may extend through obverse laminated skeleton; reverse surface typically smooth and finely pustulose; autozooecial chambers tubular, some proximally recumbent on budding plate, with long axis directed toward obverse surface but inclined distally at acute or obtuse angle; autozooecial chamber cross-sections rhomboidal, hexagonal, or irregularly polygonal in deep section parallel to base; single hemiseptum may be present on proximal wall at base of distal tube; diaphragms absent; intermediate- to large-diameter distal tube typically moderate length to long, with apertures of some surrounded by up to 16 stylets indenting apertural outline; terminal diaphragms may have a central perforation, representing secondary nanozooecia; in some species bowl-like cavities on dissepiments, linked to autozooecia by one or two meandering surficial grooves may be reproductive heterozooecia; no superstructure present; granular skeleton present in basal plate and axial wall but locally absent in transverse and lateral walls; extrazooecial skeleton laminated, traversed by abundant, moderate-size styles (Wyse Jackson et al., 2006).
Remarks. Polypora M’Coy, 1844, is similar to Paucipora Termier and Termier, 1971. The latter has well-developed hemisepta and shorter autozooecia. Polypora differs from Polyporella Simpson, 1895, in the presence of four rows of autozooecia on branches instead of three in the latter genus.
Occurrence. Lower Devonian to Upper Permian; worldwide.
Polypora triangularis Rogers, 1900
Figure 15E-H, Figure 16A-G; Appendix
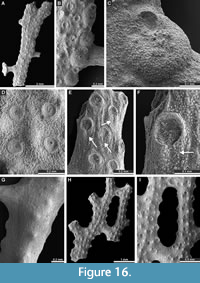 1900 Polypora triangularis Rogers, p. 8-9, pl. 4, figs. 3-3c.
1900 Polypora triangularis Rogers, p. 8-9, pl. 4, figs. 3-3c.
1929 Polypora aestacella Moore, p. 24-25, pl. 3, figs. 9, 10.
1929 Polypora sigillaria Moore, p. 121-122, pl. 15, figs. 12, 14.
Material. SNSB-BSPG 2020 XCI 24, SNSB-BSPG 2020 XCI 25, SNSB-BSPG 2020 XCI 26, SNSB-BSPG 2020 XCI 30, SNSB-BSPG 2020 XCI 48, SNSB-BSPG 2020 XCI 50, SNSB-BSPG 2020 XCI61, SNSB-BSPG 2020 XCI 72, SNSB-BSPG 2020 XCI 114, SNSB-BSPG 2020 XCI 115, SNSB-BSPG 2020 XCI 116, SNSB-BSPG 2020 XCI 117.
Exterior description. Reticulate colonies composed of moderately wide branches joined by moderately wide dissepiments. Autozooecia arranged in 5-7 alternating rows on branches. Autozooecial apertures rounded to oval, 7-10 spaced per length of fenestrule. Proximal pores at apertures developed, 0.025-0.027 mm in diameter, often continued in the proximal slit (Figure 16E-F). Fenestrules subrectangular to oval, longer than wider. Nodes on the reverse side of branches, 0.06-0.11 mm in diameter.
Interior description. Autozooecial chambers generally rhombic to roughly hexagonal in the mid-tangential section. Hemisepta absent. Extrazooidal skeleton well-developed, protruded by abundant microstylets. Microstylets 0.008-0.015 mm in diameter, spaced densely. Circular to elliptical nodes irregularly arranged between autozooecial rows, 0.045-0.115 mm in diameter. Apparent reproductive heterozooecia in the form of isolated zooecia with enlarged endozonal chambers present. Chambers rounded, 0.18-0.30 mm in diameter, covered by roofs with slightly depressed central parts. Skeletal material of roofs covered by abundant pustules. Nanozooecia present.
Remarks. Polypora sigillaria Moore, 1929, and P. aestacella Moore, 1929, are identical with P. triangularis Rogers, 1900. Their sizes are similar, and they both possess characteristic proximal pores at apertures. Polypora triangularis Rogers, 1900, differs from P. gzhelensis Schulga-Nesterenko, 1951, from the Pennsylvanian (Gzhelian) of the Russian Platform in having longer fenestrules (fenestrule length 2.30-3.25 mm vs. 1.30-1.45 mm in P. gzhelensis)
Polypora triangularis differs from P. vereyensis Schulga-Nesterenko, 1951, from the Pennsylvanian (Moscovian) of the Russian Platform in having narrower branches (branch width 0.54-1.03 mm vs. 0.95-1.05 mm in P. vereyensis) and in possessing longer fenestrules (fenestrule length 2.30-3.25 mm vs. 1.12-1.75 mm in P. vereyensis).
Occurrence. Pennsylvanian (Virgilian); Kansas, USA. Upper Graham Formation, Pennsylvanian (Virgilian); Texas, USA. Graham Formation, Pennsylvanian (Virgilian); TXV-200 (“Spillway section at Lost Creek Lake”), Texas, USA.
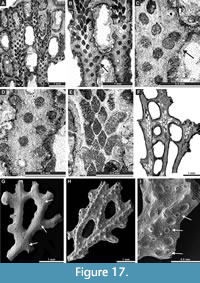
Polypora aff. hexagona Moore, 1929
Figure 16H-I, Figure 17A-E; Appendix
1929 Polypora hexagona Moore, p. 122, pl. 15, figs. 13, 17.
 Material. SNSB-BSPG 2020 XCI 32, SNSB-BSPG 2020 XCI 55, SNSB-BSPG 2020 XCI 118.
Material. SNSB-BSPG 2020 XCI 32, SNSB-BSPG 2020 XCI 55, SNSB-BSPG 2020 XCI 118.
Exterior description. Reticulate colonies composed of moderately wide branches joined by moderately wide dissepiments. Autozooecia arranged in 3-5 alternating rows on branches. Autozooecial apertures rounded to oval, 5-6 spaced per length of fenestrule. Fenestrules subrectangular to oval, longer than wider.
Interior description. Autozooecial chambers generally rhombic to roughly hexagonal in the mid-tangential section. Hemisepta absent. Extrazooidal skeleton well-developed, protruded by abundant microstylets. Microstylets 0.008-0.010 mm in diameter, spaced densely. Circular to elliptical nodes irregularly arranged between autozooecial rows, 0.040-0.065 mm in diameter. Apparent reproductive heterozooecia in form of isolated zooecia with enlarged endozonal chambers present. Chambers rounded, 0.18-0.30 mm in diameter, covered by roofs that are slightly depressed in their central parts. Skeletal material of roofs covered by abundant pustules. Nanozooecia present.
Remarks. The present species is very close to the species Polypora hexagona Moore, 1929, but has longer fenestrules (fenestrule length 1.25-1.52 mm vs. 0.9 mm in P. hexagona). The present species differs from P. aspera Rogers, 1900, from the Pennsylvanian of Kansas in having shorter fenestrules (fenestrule length 1.25-1.52 mm vs. 2.0 mm in P. aspera). The present species differs from P. gzhelensis Schulga-Nesterenko, 1951, from the Gzhelian of the Russian Platform in having narrower branches (branch width 0.38-0.70 mm vs. 0.70-0.95 in P. gzhelensis).
Occurrence. Graham Formation, Pennsylvanian (Virgilian); TXV-200 (“Spillway section at Lost Creek Lake”), Texas, USA.
Family Septoporidae Morozova, 1962
Genus Septopora Prout, 1859
Type species. Septopora cesteriensis Prout, 1859, by original designation. Mississippian (Lower Carboniferous); North America.
Diagnosis. Reticulate colonies consisting of moderately thick branches and broad, often curved dissepiments. New branches originate by bifurcation or fusion of pinnae. Fenestrules irregularly shaped. Autozooecia arranged in two rows on branches and pinnae, rectangular to pentagonal in mid-tangential section. Hemisepta absent. Keel broad, low, carrying a single row of nodes. Cyclozooecia spaced irregularly through the colony (modified after Morozova, 2001 and McKinney, 2002).
Remarks. Septopora Prout, 1859, differs from the similar genus Synocladiella Lisitsyn in Lisitsyn and Ernst, 2004 in having two rows of autozooecia on branches instead of 6-8 in Synocladiella. Septopora differs from Synocladia King, 1949 in having two rows of autozooecia on branches instead of 3-5 in Synocladia, as well as in presence of cyclozooecia.
Occurrence. Mississippian (Lower Carboniferous) to Upper Permian; North America, Europe, Asia.
Septopora blanda Moore, 1929
Figure 17F-I, Figure 18A-C; Appendix
1929 Septopora blanda Moore, p. 130, pl. 16, figs. 6, 12, pl. 17, fig. 2.
1930 Septopora blanda Moore, 1929; Warthin, p. 39, pl. 3, figs. 8a-b.
2016 Septopora blanda Moore, 1929; Ernst et al., p. 532-534, figs. 7b-d, 9g.
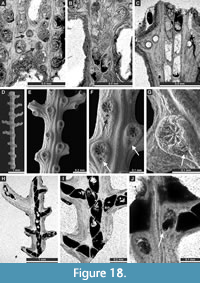 Material. SNSB-BSPG 2020 XCI 47, SNSB-BSPG 2020 XCI 54, SNSB-BSPG 2020 XCI 119, SNSB-BSPG 2020 XCI 120.
Material. SNSB-BSPG 2020 XCI 47, SNSB-BSPG 2020 XCI 54, SNSB-BSPG 2020 XCI 119, SNSB-BSPG 2020 XCI 120.
Exterior description. Reticulate colonies formed by straight or slightly undulating branches fused by pinnae. Fenestrules oval to reversed V-shaped. Autozooecia arranged in two rows on branches. Four to five apertures spaced per fenestrule length. Autozooecial apertures circular to oval. Elliptical nodes regularly arranged in a single row on the low keel between apertures, 0.08-0.10 mm wide and spaced 0.55-0.63 mm from centre-to-centre.
Interior description. Autozooecial chambers tetragonal, parallelogram-shaped in the mid-tangential section. Hemisepta absent. Extrazooidal skeleton well-developed. Cyclozooecia common, spaced irregularly on obverse surface, 0.05-0.08 mm in diameter.
Remarks. Septopora blanda Moore, 1929, differs from S. interporata Rogers, 1900, from the Pennsylvanian of Kansas, in having wider spaced nodes on keel (distance between node centres 0.55-0.63 mm vs.0.3-0.4 mm in S. interporata).
Occurrence. Wewoka Formation, middle Pennsylvanian (upper Desmoinesian); Oklahoma, USA. Deese Group, Boggy Formation, Pennsylvanian (Missourian); Oklahoma, USA. Upper Graham Formation, Pennsylvanian (Virgilian); Texas, USA. Graham Formation, Pennsylvanian (Virgilian); TXV-200 (“Spillway section at Lost Creek Lake”), Texas, USA.
Family Acanthocladiidae Ulrich, 1890
Genus Penniretepora d`Orbigny, 1849
[= Acanthopora Young and Young, 1875; Pinnatopora Vine, 1883]
Type species. Retepora pluma Phillips, 1836, subsequently designated by d`Orbigny (1849). Mississippian; Yorkshire, England.
Diagnosis. Colonies consisting of straight main branches with frequent lateral branches (pinnate); two rows of autozooecia both on main and lateral branches; autozooecia rectangular to pentagonal or trapezoid in mid-tangential section; hemisepta absent; superstructure absent; keel low with or without nodes.
Remarks. Penniretepora d`Orbigny, 1849, differs from Filites Počta in Barrande, 1894, in the shape of zooecia in mid-tangential section (rectangular to pentagonal or trapezoid vs. triangular in Filites). Penniretepora differs from Gorjunopora Ernst et al., 2015, in absence of hemisepta.
The genus Penniretepora d`Orbigny, 1849, needs critical re-evaluation. Many species were placed to Penniretepora because of their pinnate shape and two rows of zooecia on the branches. However, they have different internal morphology (shape of autozooecial chambers and presence/absence of hemisepta). Moreover, many such species were described without use of thin sections, therefore their internal morphology is unknown.
Occurrence. Devonian to Permian; worldwide.
Penniretepora flexistriata Richards, 1959
Figure 18D-J; Appendix
1959 Penniretepora flexistriata Richards, p.1116, text-figs. A7, A8.
1980 Penniretepora flexistriata Richards, 1959; Simonsen and Cuffey, p. 23-24, figs. 3K, 4K, 5K, 6K, 7K.
Material. SNSB-BSPG 2020 XCI 82, SNSB-BSPG 2020 XCI 83, SNSB-BSPG 2020 XCI 84, SNSB-BSPG 2020 XCI 85, SNSB-BSPG 2020 XCI 91, SNSB-BSPG 2020 XCI 93, SNSB-BSPG 2020 XCI 121, SNSB-BSPG 2020 XCI 122, SNSB-BSPG 2020 XCI 123.
Exterior description. Pinnate colonies consisting of straight main branches with frequent lateral branches. Main branches 0.22-0.43 mm wide, lateral branches 0.12-0.24 mm wide, diverging at angles 59-82° from main branches, spaced 0.59-0.85 mm from centre to centre. Autozooecia having circular apertures with stellate structure, arranged in two rows both on main and lateral branches; regularly one aperture at the base of each lateral branch and one aperture between two neighbouring lateral branches. Median keels low, undulating, nodes absent.
Interior description. Autozooecial chambers arranged in two alternating rows on branches, triangular to pentagonal or trapezoid in mid-tangential section both on main and secondary branches, short, inflated, with moderately long vestibules. Axial wall strongly undulating to zigzag from base to crest. Hemisepta absent. Apertural pore present, positioned proximally to the autozooecial aperture, 0.02-0.03 mm in diameter. Extrazooecial skeleton moderately developed, traversed by abundant microstylets; microstylets without hyaline core, regularly spaced across entire colony surface, 0.005-0.008 mm in diameter. Coarse longitudinal striation developed both on main and lateral branches.
Remarks. Penniretepora flexistriata Richards, 1959, is similar to P. cyclotriangulata Shishova, 1959, from the Upper Carboniferous (Moscovian) of Russia but differs from it in having narrower lateral branches (lateral branch width 0.12-0.24 mm vs. 0.20-0.25 mm in P. cyclotriangulata) and smaller apertures (aperture width 0.06-0.08 mm vs. 0.15-0.20 mm in P. cyclotriangulata).
Occurrence. Lower Virgilian to upper Wolfcampian; Kansas, USA. Graham Formation, Pennsylvanian (Virgilian); TXV-200 (“Spillway section at Lost Creek Lake”), Texas, USA.
Penniretepora oculata (Moore, 1929)
Figure 19A-E; Appendix
1929 Pinnatopora oculata Moore, p. 125-126, pl. 15, figs. 4, 5, 9.
1930 Pinnatopora oculata Moore, 1929; Warthin, p. 38, pl. 3, fig. 5.
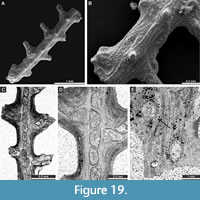 Material. SNSB-BSPG 2020 XCI 31, SNSB-BSPG 2020 XCI 51, SNSB-BSPG 2020 XCI 92, SNSB-BSPG 2020 XCI 94, SNSB-BSPG 2020 XCI 95, SNSB-BSPG 2020 XCI 96, SNSB-BSPG 2020 XCI 124.
Material. SNSB-BSPG 2020 XCI 31, SNSB-BSPG 2020 XCI 51, SNSB-BSPG 2020 XCI 92, SNSB-BSPG 2020 XCI 94, SNSB-BSPG 2020 XCI 95, SNSB-BSPG 2020 XCI 96, SNSB-BSPG 2020 XCI 124.
Exterior description. Pinnate colonies consisting of straight main branches with frequent lateral branches. Main branches 0.27-0.49 mm wide, lateral branches 0.19-0.30 mm wide, diverging at angles 68-81° from main branches, spaced 0.68-1.00 mm from centre to centre. Autozooecia having oval apertures, arranged in two rows both on main and lateral branches; regularly one aperture at the base of each lateral branch and one aperture between two neighbouring lateral branches. Median keels low, straight, containing widely spaced elliptical nodes.
Interior description. Autozooecial chambers arranged in alternating order in one row on branches, trapezoid to roughly pentagonal in mid-tangential section both on main and secondary branches, relatively long, inflated, with moderately long vestibules. Axial wall strongly undulating from base to crest. Hemisepta absent. Extrazooecial skeleton moderately developed, traversed by abundant microstylets; microstylets with distinct hyaline cores and dark laminated sheaths, diverging from inner hyaline skeleton, regularly spaced across entire colony surface, 0.010-0.015 mm in diameter. Reverse side containing longitudinal rows of microstylets. Nanozooecia present.
Remarks. Penniretepora oculata (Moore, 1929) is similar to P. rossica Shishova, 1959, from the Upper Carboniferous (Gzhelian) of Russia but differs in having narrower branches (main branch width 0.27-0.49 mm vs. 0.45-0.50 mm in P. rossica) and smaller apertures (aperture width 0.05-0.08 mm vs. 0.09-0.12 mm in P. rossica). Penniretepora oculata differs from P. mariae (Shishova, 1959) from the Upper Carboniferous (Gzhelian) of Russia in possessing large widely spaced nodes on branches instead of a narrow keel with a row of small closely spaced nodes in P. mariae. Furthermore, lateral branches in Penniretepora oculata are spaced wider than in P. mariae (distance between branch centres 0.68-1.00 mm vs. 0.67-0.71 in P. mariae).
Occurrence. Pennsylvanian of Oklahoma, USA. Upper Graham Formation, Pennsylvanian (Virgilian); Texas, USA. Graham Formation, Pennsylvanian (Virgilian); TXV-200 ("Spillway section at Lost Creek Lake"), Texas, USA.
DISCUSSION
The studied bryozoan assemblage from the Finis Shale Member, Graham Formation, Pennsylvanian (Virgilian) at Lost Creek Lake, Texas, USA, contains 19 species. It includes four cystoporates: Fistulipora nodulifera, Eridopora beilensis, Cystodictya formosa, and Goniocladia grahamensis; a single trepostome: Dyscritella felixi n. sp.; two rhabdomesine cryptostomes: Rhombopora lepidodendroides and Streblotrypa (Streblotrypa) multipora; and 12 fenestrates: Rhombocladia delicata, Chainodictyon minor, Fabifenestella compactilis, Laxifenestella placida, L. texana n. sp., Cavernella praecavifera, Acupipora elliptica, Polypora triangularis, P. aff. hexagona, Septopora blanda, Penniretepora flexistriata, and P. oculata. The fauna is of significance in a number of respects: firstly, the material allowed for a comprehensive investigation of both external and internal morphology on account of its excellent preservation. Secondly, abundant material was collected from two profiles through the upper part of the Finis Shale that permitted the documentation of the distribution of bryozoan species, and their growth forms within the member. Thirdly, the identified taxa indicated relationships to other palaeogeographical units of the Pennsylvanian.
Morphology
Moore (1929) published a comprehensive study of a bryozoan fauna from the Wayland Shale of the Upper Graham Formation in north-central Texas. He described 65 species and varieties, including 12 cystoporates, 4 trepostomes, 19 rhabdomesines and 30 fenestrates. Unfortunately, only a few of these descriptions were supported by the use of thin sections, whereas none of the fenestrate species in that paper were investigated using thin sections. However, this technique and methodology is absolutely necessary for the study of Palaeostomata, a superorder of stenolaemate bryozoans which occur within the Paleozoic (Wyse Jackson and Buttler, 2015). Except for some Russian specialists (e.g., Schulga-Nesterenko, 1933, 1952, 1955; Nikiforova, 1938) thin sections had a restricted use in the study of Palaeozoic bryozoans until the second half of the twentieth century (e.g., Condra, 1902, 1903a; Girty, 1908, 1915; Elias, 1937; Elias and Condra, 1957) and especially for the detailed investigation of fenestrate bryozoans the use of thin section has been avoided. Morozova (1974) attempted a revision of the genus Fenestella, which included the majority of fenestrate bryozoans with two rows of zooecia on branches. She recognized the importance of the shape of zooecial chambers in mid-tangential section as well as the presence and type of heterozooecia for the discrimination of fenestrate bryozoans. In general, it is difficult to compare fenestrate taxa primarily established on the outer morphology and without knowledge of their internal morphology with those that were studied by using thin sections. However, some external characters as for example the type of nodes on the keel (singular or double row as in case of determination of the genera Minilya and Fabifenestella), type of aperture (aperture nodes, pores, stellate structure), as well as numerical characters can be used as indirect clues for comparison of such taxa.
As mentioned above, the descriptions of fenestrate taxa in Moore’s paper are quite comprehensive but no information on their internal morphology is provided. We tried to link internal morphological characters discovered in the thin sections to their external morphology by the comparison of external and internal features. In the case of four species of fenestrate species with biserial arrangement of autozooecia on branches joined by sterile dissepiments (Fabifenestella compactilis, Laxifenestella placida, L. texana, and Cavernella praecavifera) we found a set of external characters usable for determination of those species in bryozoan fragments. The following characters can be used for the separation of these species in the studied material: node arrangement (single or double row), node size and spacing (distance from centre to centre as well as number of nodes per fenestrule length), shape of aperture (presence of apertural nodes, proximal pores, or stellate structure), number of apertures per fenestrule length. Additionally, such characters as the shape of dissepiments (flat or rounded) and that of the keel (high or low, narrow or wide) as well as the character of the reverse side (striated, smooth, or tuberculate) can be involved in the comparison. Below is a synopsis of those external characters through which the four species can be identified:
Fabifenestella compactilis. Double row of densely spaced nodes (average 0.14 mm apart), 2-3 apertures per fenestrule length, stellate structure and proximal pore present, flat dissepiments.
Laxifenestella placida. Single row of widely spaced nodes (on average 0.98 mm apart), 4-5 apertures per fenestrule length, apertures rounded with low peristome (neither nodes nor stellate structure present), flat dissepiments.
Laxifenestella texana. Single row of closely spaced nodes (on average 0.19 mm apart), 3-4 apertures per fenestrule length, apertures rounded with low peristome and single apertural node directed into the next fenestrule (stellate structure absent), rounded dissepiments.
Cavernella praecavifera. Single row of moderately spaced nodes (on average 0.34 mm apart), 2-4 apertures per fenestrule length, stellate structure present, flat dissepiments with longitudinal striae.
Moreover, Laxifenestella texana usually possesses abundant reproductive heterozooecia (Figure 12B-C, E-F). Cavernella praecavifera is distinguished by the presence of cavernozooecia. They represent elongate, distally tapered, somewhat distorted pyriform cavities with their long axis oriented parallel with branch axes (Morozova, 1974, 2001; McKinney and Wyse Jackson, 2015). These structures are best seen in thin sections, whereas they are generally difficult to detect externally, except in some weathered or partly destroyed fragments (Figure 13D).
The majority of fenestrate bryozoans in the Finis Shale possess apparent reproductive heterozooecia which represent rounded chambers attached at the proximal end of a vestibule. They correspond to the “type C ovicell” of Bancroft (1986a), who described them as small, rounded cavities representing distal extensions of autozooecial vestibules. Such heterozooecia are found in many fenestrate taxa from the Devonian to Permian (e.g., Southwood, 1985; Tavener-Smith, 1966). In weathered material they appear often as rounded depressions attached to apertures (Figure 12E). In well-preserved specimens, complete chambers are visible (Figure 12F, Figure 16A-C).
Another type of heterozooecia often found in fenestrate taxa represents secondary nanozooecia (Bancroft 1986b). These are perforated terminal diaphragms (Figure 12G, Figure 15A, Figure 16D-E, Figure 18E). They are compared with similar structures in modern cyclostomes, and their apparent function could be cleaning of the surface by action of a long single tentacle (Silén and Harmelin, 1974). Secondary nanozooecia were observed in Laxifenestella texana, Acupipora elliptica, Polypora triangularis, Polypora aff. hexagona, and Penniretepora oculata.
Stellate structures represent variably developed scallops around the aperture margin, with the septa between adjacent scallops pointed toward the midpoint of the aperture (Figure 10D-E, Figure 18E-G). These are usually eight septae, but a range from six to 16 septae is known (McKinney and Wyse Jackson, 2015). Their function is unclear as the structure appears only sporadically in many taxa. It is assumed that they may represent a stage of ontogenetic development of a zooecium. All the stellate structures in Finis Shale bryozoans are composed of eight septae. They occur in Fabifenestella compactilis, Cavernella dvinensis, and in Penniretepora flexistriata.
Three of the fenestrates described here, possess structures called “proximal pores”: Fabifenestella compactilis, Polypora triangularis, and Penniretepora flexistriata. Such pores are found consistently in various fenestrate taxa (e.g., McKinney and Wyse Jackson, 2015). In some taxa the pore is not completely closed by skeletal material forming a kind of a fossula (Gautier et al., 2013; Figure 16E-F). Their apparent function is assumed to facilitate the exchange of gases with the external environment, hydrostatic compensation, or as passageway for fecal products.
Palaeoecology
The studied bryozoan assemblage contains species with various growth forms, which can be summarized as encrusting unilaminar (Fistulipora nodulifera, Eridopora beilensis, Dyscritella felixi), erect ramose (Cystodictya formosa, Rhombopora lepidodendroides, Streblotrypa (Streblotrypa) multipora, Rhombocladia delicata), erect reticulate robust (Goniocladia grahamensis, Chainodictyon minor, Acupipora elliptica, Polypora triangularis, P. aff. hexagona), erect reticulate delicate (Fabifenestella compactilis, Laxifenestella placida, L. texana, Cavernella praecavifera, Septopora blanda), and erect pinnate (Penniretepora flexistriata, P. oculata). The term “reticulate” is used here instead of the popular definition “fenestrate” (cf. Nelson et al., 1988; Amini et al., 2004; Taylor and James, 2013) in order to avoid confusion in regard to the taxonomic unit (Order Fenestrata).
It is noteworthy that pronounced robust bryozoan species are absent in the two sections of the Finis Shale. Of the robust reticulate bryozoans, the taxa with branch width exceeding 0.5 mm are most abundant. Cystodictya formosa and Rhombopora lepidodendroides are the most robust taxa with branches which do not exceed 1.5 mm in diameter. In the majority of encrusting Fistulipora nodulifera and Eridopora beilensis the layer thickness does not exceed 1 mm, whereas Dyscritella felixi has colony thickness less than 0.5 mm.
Among the branching forms, Rhombopora lepidodendroides and S treblotrypa (Streblotrypa) multipora can be distinguished on account of the cylindrical form of the colony, with apertures opening around the stem. Cystodictya formosa possesses lancet- or strap-shaped bifoliate stems, with apertures opening on both sides of bifoliate-symmetrical branches (Figure 6B). Such colonies are also called “palmate” (Taylor and James, 2013). Rhombocladia delicata has a ramose colony produced by stems in which zooecia bud in one plane with apertures opened onto only one side of a flattened branch (Figure 9A-B).
In the erect reticulate robust group, Goniocladia grahamensis and Chainodictyon minor have reticulate colonies produced by fusing of branches (reteporiform after Stach, 1936). Branches of Goniocladia are bifoliate-symmetrical, with autozooecial apertures opening into the space of fenestrules (Figure 6C). In contrast, branches of Chainodictyon have the same form as those in Rhombocladia but are fused to form a reticulate meshwork (Figure 9E).
The reticulate robust taxa Acupipora elliptica, Polypora triangularis, and P. aff. hexagona, as well as the reticulate delicate Fabifenestella compactilis, Laxifenestella placida, L. texana, and Cavernella praecavifera possess classical colonies produced by branches joined by sterile dissepiments (e.g., Figure 10C, Figure 11C-D, Figure 12D, G-H, Figure 14E, Figure 16H). The species Septopora blanda possesses colonies produced by pinnate branches which are connected by fusion of lateral pinnae (Figure 17F-H). The pinnate Penniretepora flexistriata and P. oculata represent colonies consisting of a wider main branch with narrower lateral branches (Figure 18D, Figure 19A).
The colony form is an important morphological feature of bryozoans. In rare cases it can vary within species, but otherwise is firmly determinate (e.g., Hageman et al., 1997, 1998). The shape of the colony is significant for feeding and support (resistance against physical impacts) (e.g., Cowen and Rider, 1972; McKinney and Jackson, 1989; Taylor and James, 2013; Taylor, 2020). Bryozoan colonies, with some constraints, may thus be regarded as very important instrument for palaeoecological studies (e.g., Stach, 1936; Schopf, 1969; Nelson et al., 1988; Hageman et al., 1997, 1998; Amini et al., 2004).
Distribution of the five main growth forms (encrusting, ramose, reticulate delicate and robust, pinnate) in both of the studied profiles displays certain patterns. The erect growth forms clearly dominate in this assemblage (Figure 20). In total, bryozoans become more robust in the upper levels of the profiles. This pattern does not seem to have been influenced by selective taphonomic processes (e.g., Smith et al., 1992; Smith and Nelson, 1994). The studied material shows no or very few traces of abrasion or dissolution. In these samples smaller and more fragile skeletal fragments occur, e.g., diverse echinoderms or foraminifers which would disappear if mechanical and chemical destruction had been strong. The Finis Shale was deposited in the tropical to subtropical realm (https://deeptimemaps.com/north-america/), therefore, dissolution of zoaria can be discounted as a taphonomic process (Smith et al., 1992).
Encrusting Fistulipora nodulifera and Eridopora beilensis, both quite robust species, appear in samples B14 (Fistulipora) and B15 (Eridopora) of profile B, and in samples C9 (Eridopora) and C11 (Fistulipora) of profile C. Robust Polypora triangularis is most abundant in the upper part of profile B (especially in B15-B20).
Ramose and pinnate taxa are rather evenly distributed, but they show a distinct taxonomic shift. Ramose Cystodictya formosa dominates in the lower half of the profiles, whereas the abundance of Rhombopora lepidodendroides increases in the upper part. Cystodictya formosa is very rare in the uppermost part of the profile B (B18-B20). This change occurs quite abruptly. The species Penniretepora oculata gradually replaces P. flexistriata in the profile, a trend observed in both profiles. The abundance of reticulate growth forms, both robust and delicate, increases against that of the ramose ones in the upper parts of profiles.
Observed patterns can be interpreted as consequences of environmental changes. The occurrence of encrusting growth forms and overall increase of robustness may indicate shallowing and increase of water energy (e.g., Nelson et al., 1988; Amini et al., 2004). Additionally, several fragments of tabulate corals were found in the profile B16 (Graham Young, pers. comm. 2021). Along with the observed changes in the distribution of bryozoan growth forms the presence of a transgressive-regressive cycle suggested by Lobza et al. (1994) can be confirmed.
Numerical Estimation of the Faunal Composition
Determining and counting of the number of bryozoans in the studied material is tied with some reservations. Firstly, this material is mostly represented by bryozoan fragments rather than by complete colonies. Only Dyscritella felixi is found almost exclusively as complete (Figure 6F). Fistulipora nodulifera and Eridopora beilensis are partly represented by complete colonies (Figure 4D), otherwise these are recovered as quite large fragments. All other bryozoans occur in form of fragments of various sizes. Presumably, robust colonies are broken in fewer (and usually larger) fragments than the delicate ones; otherwise, the robust colonies might be larger than the delicate colonies. Therefore, the number of fragments counted has only limited use for the estimation of the species richness and common indices of local diversity such as Shannon index and Fisher’s α (Figure 21) that generally describe relationship between the number of species and the number of individuals in those species (Hammer et al., 2001).
Secondly, the studied material contains different amounts of bryozoan fragments within the single samples. The amount of rock material dissolved is constant for all samples. The bryozoan content in the samples increases strongly in the upper part of the profile. Whereas the samples B1-B10 and C1-C9 usually contain fewer than 100 fragments each, the samples from higher level often contain up to several thousand fragments. Counting of such numbers of fragments appeared unfeasible, therefore, a representative portion of these samples was taken for counting. The results are summarized in the Table 1 and Table 2 in which samples with more than one species are included. These estimations show that bryozoan richness and diversity increase in the profiles (Figure 21). The highest richness is observed at the level of B16-B17 and C14-C15. These data correspond also to the highest number of bryozoan fragments in those samples (not quantified but visually estimated).
Fragments of Cystodictya formosa are most abundant throughout the studied profiles found in all samples. However, its fragments become rare in the uppermost part of the profile B (samples B18-B20; Table 1). Fragments of Rhombopora lepidodendroides are also quite common occurring in most samples.
Fragments of pinnate bryozoans are quite abundant throughout the section, but these taxa show a taxonomic shift (see above). Fragments of encrusting cystoporates Fistulipora nodulifera and Eridopora beilensis occur in the upper parts of the profiles (see above).
Colonies and fragments of Dyscritella felixi, Chainodictyon minor, Laxifenestella placida, and Septopora blanda are relatively common throughout the sections, whereas fragments of Fabifenestella compactilis, Cavernella praecavifera, Acupipora elliptica, and Polypora aff. hexagona tend to be more abundant in the upper part of the profiles. Abundant fragments of Laxifenestella texana are found in the lower half of the profiles but are completely missing in the upper part of the profile B. Fragments of Polypora triangularis are common in the majority of samples, and especially abundant in the upper part of the profile B.
Fragments of Goniocladia grahamensis, Rhombocladia delicata, and Streblotrypa (Streblotrypa) multipora are very rare in the studied material tending to occur in the upper part of the profiles.
Palaeobiogeography
The bryozoan fauna from the Finis Shale displays palaeobiogeographic relationships mainly within the North American region. Five species are endemic in the Graham Formation of Texas: Cystodictya formosa, Goniocladia grahamensis, Dyscritella felixi, Laxifenestella placida, and L. texana. Polypora aff. hexagona is the species tentatively compared with the species Polypora hexagona, which is only known from the Graham Formation and might represent a sixth endemic taxon.
Ten species are distributed in the Pennsylvanian of North America. Fistulipora nodulifera was found in the Pennsylvanian (Desmoinesian) of Mexico and New Mexico. Eridopora beilensis was originally found in the Pennsylvanian (Virgilian) of Kansas. Streblotrypa (Streblotrypa) multipora was recorded from the Pennsylvanian (Desmoinesian) of Oklahoma, USA. Chainodictyon minor was previously known from the Pennsylvanian of Illinois. Fabifenestella compactilis was originally recorded from the Pennsylvanian (Virgilian) of Nebraska. Polypora triangularis is known from the Pennsylvanian (Virgilian) of Kansas and Texas, USA. Septopora blanda is known from the Pennsylvanian (Missourian to Virgilian) of Oklahoma and Texas. Penniretepora flexistriata was previously known from the Early Virgilian to late Wolfcampian of Kansas, USA. Penniretepora oculata was also recorded from the Pennsylvanian of Oklahoma. The species Acupipora elliptica is known from the Pennsylvanian (Missourian) of Kansas and Oklahoma, as well as from the Pennsylvanian (Virgilian) of Kansas and Texas. Additionally the genus Acupipora is also known with one species from the Pennsylvanian (Desmoinesian) of Mexico and from the Pennsylvanian (Gzhelian) of Russia, respectively.
Three species from the studied bryozoan assemblage were also recorded outside of the American realm. Rhombopora lepidodendroides is widely distributed in the Pennsylvanian of North America, with a few records from the lower Permian of USA. Moreover, this species is also known from the Pennsylvanian of Europe (NW Spain). A single record of this species was documented from the Pennsylvanian of South America (Bolivia).
Rhombocladia delicata has wide distribution in the Pennsylvanian of North America and Europe (Italy, Spain).
Cavernella praecavifera was previously known from the Upper Pennsylvanian (Kasimovian) of Russia. The genus Cavernella is distributed from the Pennsylvanian (Moscovian) to the upper Permian of Eurasia. It includes 13 genera, from which four are known from the Pennsylvanian of Russia and Hungary. The Permian species of Cavernella are known from Russia, Mongolia, and China. Cavernella praecavifera i s the first species of this genus identified in North America.
CONCLUSIONS
The faunal association of bryozoans from the Finis Shale displays relatively high diversity and abundance. The studied bryozoan fauna shows exceptional preservation and was studied through a combination of the examination and characterisation of external and internal morphology in detail. The distribution of bryozoan growth forms indicates gradual shallowing in the profiles and supports the assumption of a transgressive-regressive cycle in the Finis Shale (e.g., Lobza et al., 1994). Richness, abundance, and α-diversity support the conclusion of a shallowing sea. The bryozoan fauna from the studied locality displays palaeobiogeographic relations mainly within the North American region. However, five species are endemic to the Graham Formation of Texas whereas only three species are reported outside of the American realm, identifying relations with contemporary strata in Europe (Italy, Spain, Russia).
ACKNOWLEDGEMENTS
B. Seuss needs to thank members of the Dallas Paleontological Society for helping with the field work. Deutsche Forschungsgemeinschaft is appreciated for financial support (projects DFG ER 278/6.1 for AE, MU 2352/5-1 for ALC, and SE 2283/2-1 for BS). We are grateful to C. Buttler, Cardiff, and H. Nakrem, Oslo, for their useful and comprehensive reviews.
REFERENCES
Amini, Z.Z., Adabi, M.H., Burrett, C.F., and Quilty, P.G. 2004. Bryozoan distribution and growth form associations as a tool in environmental interpretation, Tasmania, Australia. Sedimentary Geology, 167(1-2):1-15. https://doi.org/10.1016/j.sedgeo.2004.01.010
Anstey, R.L. and Perry, T.G. 1970. Biometric procedures in taxonomic studies of Paleozoic bryozoans. Journal of Paleontology, 44(3):383-398. https://www.jstor.org/stable/1302549
Astrova, G.G. 1964. New order of the Paleozoic Bryozoa. Paleontologicheskii Zhurnal, 1964 (2):22-31. (In Russian)
Astrova, G.G. 1965. Morphology, history of development and system of the Ordovician and Silurian Bryozoa. Trudy Paleontologicheskogo Instituta Akademii Nauk SSSR, 106:1-432. (In Russian)
Astrova, G.G. and Morozova, I.P. 1956. On systematics of the order Cryptostomata. Doklady Akademii Nauk SSSR, 110(4):661-664. (In Russian)
Bancroft, A.J. 1984. Studies in Carboniferous Bryozoa. Unpublished PhD thesis, Durham University, Durham, UK. http://etheses.dur.ac.uk/7498/
Bancroft, A.J. 1986a. Ovicells in the Palaeozoic bryozoan order Fenestrata. Palaeontology, 29(1):155-164.
Bancroft, A.J. 1986b. Secondary nanozooecia in some Upper Palaeozoic fenestrate Bryozoa. Palaeontology, 29(1):207-212.
Bancroft, A.J. 1987. Biostratigraphical potential of Carboniferous Bryozoa. Courier Forschungsinstitut Senckenberg, 98:193-197.
Barnes, V.E., Hentz, T.F., and Brown, L.F.Jr. 1987, Geologic atlas of Texas, Wichita Falls-Lawton sheet: Austin, University of Texas, Bureau of Economic Geology, 1 sheet.
Bassler, R.S. 1929. The Permian Bryozoa of Timor. Paläontologie von Timor, 16:37-90.
Bassler, R.S. 1953. Bryozoa. Part G, p. 1-253. In Moore, R.C. (ed.), Treatise on Invertebrate Paleontology. Geological Society of America and University of Kansas Press, Lawrence, Kansas. https://doi.org/10.17161/dt.v0i0.5177
Boardman, D.R. II and Heckel, P.H. 1989. Glacial-eustatic sea-level curve for early Late Pennsylvanian sequence in north-central Texas and biostratigraphic correlation with curve for midcontinent North America. Geology 17:802-805. https://doi.org/10.1130/0091-7613(1989)017<0802:geslcf>2.3.co;2
Boardman, D.R. II, Mapes, R.H., Yancey, T.E., and Malinky, J.M. 1984. A new model for the depth-related allogenic community succession within North American Pennsylvanian cyclothems and implications on the black shale problem, p. 141-182. In Hyne N.J. (ed.), Limestones of the Mid-Continent: Tulsa Geological Society, Spec. Pub. 2.
Borg, F. 1926. Studies on Recent cyclostomatous Bryozoa. Zoologiska Bidrag från Uppsala, 10:181-507.
Boston, W.B. 1988. The surficial geology, paleontology, and paleoecology of the Finis Shale (Pennsylvanian, lower Virgilian) in Jack County, Texas. Unpublished Masters Thesis, The College of Arts and Sciences of Ohio University, Athens, Ohio, USA.
Bretnall, R.W. 1926. Description of some western Australian fossil Polyzoa. Bulletin of Western Australia Geological Survey, 88:7-33.
Ceretti, E. 1963. Briozoi carboniferi della Carnia. Giornale di Geologia, Annali del Museo Geologico di Bologna (series 2), 30 (for 1962):254-360.
Ceretti, E. 1964. Su alcuni Briozoi criptostomi delle Alpi Carniche. Giornale di Geologia, Annali del Museo Geologico di Bologna (series 2), 32:175-199.
Condra, G.E. 1902. New Bryozoa from the Coal Measures of Nebraska. The American Geologist, 30:337-358.
Condra, G.E. 1903a. The Coal Measure Bryozoa of Nebraska. Nebraska Geological Survey, 2:1-168.
Condra, G.E. 1903b. On Rhombopora lepidodendroides Meek. The American Geologist, 31:22-24.
Cowen, R. and Rider, J. 1972. Functional analysis of fenestellid bryozoan colonies. Lethaia, 5(2):145-164. https://doi.org/10.1111/j.1502-3931.1972.tb00848.x
Crockford, J. 1944. Bryozoa from the Permian of Western Australia. Part I. Cyclostomata and Cryptostomata from the north-west basin and Kimberly district. Proceedings of the New South Wales Linnean Society, 69:139-175.
d’Orbigny, A.D. 1849. Prodrome de paléontologie stratigraphique universelle des animaux mollusques rayonnés, faisant suite ou cours élémentaire de paléontologie et géologie stratigraphique. Volume 1. Victor Masson, Paris. https://doi.org/10.5962/bhl.title.45605
Dunaeva, N.N. 1961. Upper Carboniferous Bryozoans of the West Donetz Basin. Izdelie Akademii Nauk Ukrainskoi SSR, 38:1-142. (In Ukrainian)
Dunaeva, N.N. 1964. The Early Carboniferous trepostome fauna from the Donetz Basin. Trudy Instituta Geologicheskikh Nauk Akademii Nauk Ukrainskoi SSR, seriya stratigraphii i paleontologii, 48(2):104-141. (In Russian)
Dunaeva, N.N. and Morozova, I.P. 1967. Evolution and systematic position of certain Late Paleozoic Trepostomata. Paleontologicheskii Zhurnal, 1967(4):86-94. (In Russian)
Ehrenberg, C.G. 1831. Symbolae Physicae, seu Icones et descptiones Corporum Naturalium novorum aut minus cognitorum, quae ex itineribus per Libyam, Aegiptum, Nubiam, Dongalaam, Syriam, Arabiam et Habessiniam, studia annis 1820-25, redirent. Pars Zoologica, 4, Animalia Evertebrata exclusis Insectis. Berolini, 10 pls.
Elias, M.K. 1937. Stratigraphic significance of some late Paleozoic fenestrate bryozoans. Journal of Paleontology, 11(4):306-334. https://www.jstor.org/stable/1298324
Elias, M.K. and Condra, G.E. 1957. Fenestella from the Permian of west Texas. Geological Society of America Memoir, 70:1-158. https://doi.org/10.1130/mem70
Ernst, A. 2003. Upper Palaeozoic bryozoans from the Carnic Alps (Austria). Freiberger Forschungshefte, C499:55-77.
Ernst, A., Schäfer, P., and Reijmer, J.J.G. 2005. Stenolaemate Bryozoa from the Upper Carboniferous of the Cantabrian Basin, Northern Spain. Senckenbergiana lethaea, 85(2):307-317. https://doi.org/10.1007/bf03043613
Ernst, A. and Minwegen, E. 2006. Late Carboniferous Bryozoa from La Hermida, Spain. Acta Palaeontologica Polonica, 51(3):569-588.
Ernst, A. and Winkler Prins, C.F. 2008. Pennsylvanian bryozoans from the Cantabrian Mountains (northwestern Spain). Scripta Geologica, 137:1-123.
Ernst, A., Wyse Jackson, P.N., and Aretz, M. 2015. Bryozoan fauna from the Mississippian (Viséan) of Roque Redonde (Montagne Noire, southern France). Geodiversitas, 37(2):151-213.
Ernst, A., Seuss, B., Taylor, P.D., and Nützel, A. 2016. Bryozoan fauna of the Boggy Formation (Deese Group, Pennsylvanian) of the Buckhorn Asphalt Quarry, Oklahoma, USA. Palaeobiodiversity and Palaeoenvironments, 96:517-540. https://doi.org/10.1007/s12549-016-0231-6
Ernst, A. and Vachard, D. 2017. Middle Pennsylvanian bryozoans of Cerros de Tule, Sonora, Mexico. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 285(1):11-38. https://doi.org/10.1127/njgpa/2017/0660
Ernst, A., Krainer, K., and Lucas S.G. 2021. Stenolaemate bryozoans from the Gray Mesa Formation (Pennsylvanian) of the Fra Cristobal Mountains, New Mexico, USA. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 300(2):215-233. https://doi.org/10.1127/njgpa/2021/0986
Etheridge, R. Jr. 1873. Description of Carinella, a new genus of Carboniferous Polyzoa. Geological Magazine, 10:433-434. https://doi.org/10.1017/s0016756800466574
Etheridge, R. Jr. 1876. Notes on some Upper Palaeozoic Polyzoa. Transactions and Proceedings of the Royal Society of Victoria, 12:66-68.
Foerste, A.F. 1887. The Clinton Group of Ohio (Bryozoa). Bulletin of Scientific Laboratory, Denison University, 2(1/2):71-88, 149-176.
Forcino, F.L., Stafford, E.S., Warner, J.J., Webb, A.E., Leighton, L.R., Schneider, C.L., Michlin, T.S., Palazzolo, L.M., Morrow, J.R., and Schellenberg, S.A. 2010. Effects of data categorization on paleocommunity analysis: a case study from the Pennsylvanian Finis Shale of Texas. Palaios, 25(3):144-157. https://doi.org/10.2110/palo.2009.p09-011r
Fritz, M.A. 1970. Pennsylvanian Bryozoa (Ectoprocta) from the Tellevak Limestone, northwestern Ellesmere Island, Arctic Canada. Geological Survey of Canada Bulletin, 187:67-77. https://doi.org/10.4095/102333
Gautier, T.G., Wyse Jackson, P.N., and McKinney, F.K. 2013. Adlatipora, a distinctive new acanthocladiid bryozoan from the Permian of the Glass Mountains, Texas, U.S.A., and its bearing on fenestrate astogeny and growth. Journal of Paleontology, 87(3):444-455. https://doi.org/10.1666/12-128.1
Girty, G.H. 1908. The Guadalupian Fauna. US Geological Survey, Professional Paper, 58:1-649. https://doi.org/10.3133/pp58
Girty, G.H. 1911. New genera and species of Carboniferous fossils from the Fayetteville Shale of Arkansas. Annals of the New York Academy of Science, 20:189-238. https://doi.org/10.1111/j.1749-6632.1910.tb55149.x
Girty, G.H. 1915. Fauna of the Wewoka Formation of Oklahoma. US Geological Survey Bulletin 544:1-353. https://doi.org/10.1017/S0016756800203816
Gorjunova, R.V. 1985. Morphology, system and phylogeny of Bryozoa (Order Rhabdomesida). Trudy Paleontologicheskogo Instituta Akademii Nauk SSSR, 208:1-152. (In Russian)
Gorjunova, R.V. and Weiss, O.B. 2012. A new genus Acupipora gen. nov. from the Upper Carboniferous of the East European Platform and problem of classification of bryozoans of the Order Fenestellida. Paleontological Journal, 46(1):16-28. https://doi.org/10.1134/S0031030112010054
Grossman, E., Zhang, C., and Yancey, T.E. 1991. Stable-isotope stratigraphy of brachiopods from Pennsylvanian shales in Texas. Geological Society of America Bulletin, 103:953-965. https://doi.org/10.1130/0016-7606(1991)103<0953:sisobf>2.3.co;2
Hageman, S.J. 1991. Approaches to systematic and evolutionary studies of perplexing groups: an example using fenestrate Bryozoa. Journal of Paleontology, 65(4):630-647. https://doi.org/10.1017/S0022336000030729
Hageman, S.J. 1993. Effects of nonnormality on studies of the morphological variation of a rhabdomesine bryozoan, Streblotrypa (Streblascopora) prisca (Gabb and Horn). The University of Kansas Paleontological Contributions, 4:1-13. https://doi.org/10.17161/PCNS.1808.3767
Hageman, S., Bone, Y., McGowran, B., and James, N.P. 1997. Bryozoan colonial growth-forms as paleoinvernmental indicators: evaluation of methodology. PALAIOS, 12(5):405-419. https://doi.org/10.2307/3515380
Hageman, S.J., Bock, P.E., Bone, Y., and McGowran, B. 1998. Bryozoan growth habits: Classification and analysis. Journal of Paleontology, 72(3):418-436. https://doi.org/10.1017/S0022336000024161
Hammer, Ø., Harper, D.A.T., and Ryan, P.D. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4:1-9. http://palaeo-electronica.org/2001_1/past/issue1_01.htm
Huffman, S.F. 1970. The ectoproct (bryozoan) Rhombopora lepidodendroides Meek, Late Pennsylvanian (Virgilian), Nebraska. Journal of Paleontology, 44(4):673-679. https://www.jstor.org/stable/1302661
Johnsen, A. 1906. Bryozoen aus dem karnischen Fusulinenkalk. Neues Jahrbuch für Geologie und Paläontologie, 2:135-166.
Karapunar, B., Nützel, A., Seuß, B., and Mapes, R.H. 2022. Taxonomy and diversity of slit-band gastropods (order Pleurotomariida) from the Pennsylvanian of the U.S.A. Papers in Palaeontology, e1417:1-95. https://doi.org/10.1002/spp2.1417
Keyes, C.R. 1888. On the fauna of the Lower Coal Measures of central Iowa. Proceedings of the Academy of Natural Sciences of Philadelphia, 40:222-246.
Keyes, C.R. 1894. Paleontology of Missouri (Part II). Missouri Geological Survey, 5:1-226. https://doi.org/10.5962/bhl.title.52350
King, W. 1849. On some families and genera of corals. Annals and Magazine of the Natural History, 2:388-390. https://doi.org/10.1080/03745485909494780
Knight, W.C. 1899. The Nebraska Permian. Journal of Geology, 7:357-374. https://doi.org/10.1086/608385
Lavrentjeva, V.D. 1979. Phylloporinina - new Suborder of Palaeozoic Bryozoa. Paleontologicheskii Zhurnal, 1979(1):59-68. (In Russian).
Lisitsyn, V.P. and Ernst, A. 2004. Revision of the Paleozoic genera Thamniscus and Synocladia (Bryozoa). Paleontologicheskii Zhurnal, 2004(3):53-59. (In Russian)
Lobza, V., Schieber, J., and Nestell, M. 1994. The influence of sea level changes and possible pycnocline shifts on benthic communities in the Finis Shale (Virgilian) near Jacksboro, North-Central Texas, p. 927-947. In Embry, A.F., Beauchamp, B., and Glass, D.J. (eds.), Pangea: Global Environments and Resources, Canadian Society of Petroleum Geologists, Memoir 17.
Lonsdale, W. 1839. Corals, p. 675-694. In Murchison, R.I. (ed.), The Silurian System, founded on geological researches in the counties of Salop, Hereford, Radnor, Montgomery, Caermarthen, Brecon, Pembroke, Monmouth, Gloucester, Worcester, and Stafford; with Descriptions of the Coal-fields and Overlying Formations. John Murray, London. https://doi.org/10.5962/bhl.title.88029
M’Coy, F. 1844. A synopsis of the characters of the Carboniferous Limestone fossils of Ireland. Dublin University Press, Dublin. https://doi.org/10.5962/bhl.title.11559
M’Coy, F. 1849. On some new genera and species of Palaeozoic corals and Foraminifera. Annals and Magazine of Natural History, 3(2):130-131. https://doi.org/10.1080/03745485909494606
Ma, J.Y., Buttler, C.J., and Taylor, P.D. 2014. Cladistic analysis of the 'trepostome' Suborder Esthonioporina and the systematics of Palaeozoic bryozoans. In Rosso, A., Wyse Jackson P.N., and Porter, J.S. (eds.), Bryozoan Studies 2013. Studi Trentini di Scienze Naturali, 94:153-161.
Maisey, J.G., Bronson, A.W., Williams, R.R., and McKinzie, M. 2017. A Pennsylvanian ‘supershark’ from Texas. Journal of Vertebrate Paleontology, 37:3, e1325369. https://doi.org/10.1080/02724634.2017.1325369
Mather, K.F. 1915. The fauna of the Morrow Group of Arkansas and Oklahoma. Bulletin of Scientific Laboratories of the Denison University, 18:59-284.
McKinney, F.K. 2002. Chesterian (Carboniferous) Septopora (Order Fenestrida), eastern North America, p. 183-189. In Wyse Jackson, P.N., Buttler, C.J., and Spencer Jones, M.E. (eds.), Bryozoans Studies 2001. A.A. Balkema, Lisse, Netherlands.
McKinney, F.K. and Jackson, J.B.C. 1989. Bryozoan Evolution. Unwin Hyman, Boston.
McKinney, F.K., and Wyse Jackson, P.N. 2015. Order Fenestrata: Morphology and Growth. Treatise Online, 66 (1): Part G, Revised, Volume 2, Chapter 8A: 1-91. No. 64: Part G, Revised, Volume 2, Chapter 8H. https://doi.org/10.17161/to.v0i0.4952
McKinzie, M.G. and McLeod, J. 2003. Pennsylvanian fossils of North Texas. Occasional Papers of the Dallas Paleontological Society, 6:1-154.
Meek, F.B. 1872. Report on the paleontology of eastern Nebraska, p. 81-239. In Hayden, F.V. (ed.), Final report on the United States Geological Survey of Nebraska and Portions of adjacent Territories. U.S. Government Printing Office, Washington. https://doi.org/10.3133/70039703
Miller, A.K. and Downs, H.R. 1950. Ammonoids of the Pennsylvanian Finis Shale of Texas. Journal of Paleontology, 24(2):185-218. https://www.jstor.org/stable/1299501
Miller, S.A. 1889. North American geology and palaeontology for the use of amateurs, students and scientists. Western Methodist Book Concern, Cincinnati. https://doi.org/10.5962/bhl.title.28778
Montañez, I.P. and Poulsen, C.J. 2013. The Late Paleozoic Ice Age: An evolving paradigm. Annual Reviews Earth Planetary Sciences, 41:629-656. https://doi.org/10.1146/annurev.earth.031208.100118
Moore, R.C. 1929. A bryozoan faunule from the Upper Graham Formation, Pennsylvanian, of north central Texas. Journal of Paleontology, 3(1-2):1-27, 121-156. https://www.jstor.org/stable/1298043
Moore, R.C. 1930. New species of bryozoans from Pennsylvanian of Texas. Denison University Bulletin, Scientific Laboratories, 25:147-163.
Moore, R.C. and Plummer, F.B. 1922. Pennsylvanian stratigraphy of north central Texas. The Journal of Geology, 30(1):18-42. https://doi.org/10.1086/622838
Morgan, G.D. 1924. Geology of the Stonewall Quadrangle, Oklahoma. Bulletin of Bureau of Geology, Norman, Oklahoma, 2:1-248.
Morningstar, H. 1922. Pottsville fauna of Ohio. Bulletin (Geological Survey of Ohio), 25:1-312. https://doi.org/10.5962/bhl.title.17706
Morozova, I.P. 1962. On the systematics and phylogeny of Fenestelloidea. Paleontologicheskii Zhurnal 1962(4):104-115. (In Russian). https://doi.org/10.1080/00206816409474032
Morozova, I.P. 1970. Late Permian Bryozoa. Trudy Paleontologicheskogo Instituta Akademii Nauk SSSR, 122:1-347. (In Russian)
Morozova I.P. 1974. Revision of the genus Fenestella. Paleontologicheskii Zhurnal, 1974(2):54-67. (In Russian)
Morozova, I.P. 1981. Late Paleozoic bryozoans of the nord-west of the USSR. Trudy Paleontologicheskogo Instituta Akademii Nauk SSSR, 188:1-119. (In Russian)
Morozova, I.P. 2001. Bryozoans of the order Fenestellida (morphology, system, historical development). Trudy Paleontologicheskogo Instituta Rossiiskoi Akademii Nauk, 277:1-177. (In Russian)
Nekhoroshev, V.P. 1953. Lower Carboniferous Bryozoa of Kazakhstan. Akademia Nauk SSSR, Moscow. (In Russian)
Nelson, C.S., Hyden, F.M., Keane, S.L., Leask, W.L., and Gordon, D.P. 1988. Application of bryozoan zoarial growth-form studies in facies analysis of non-tropical carbonate deposits in New Zealand. Sedimentary Geology, 60:301-322. https://doi.org/10.1016/0037-0738(88)90126-1
Nestell, M.K., Nestell, G.P., and Barrick, J.E. 2019. The Kasimovian-Gzhelian boundary and associated microfauna of the transgressive part of the Finis Shale Cycle (Graham Formation, Cisco Group, Pennsylvanian) in North-Central Texas, USA. Paleontological Journal 53(8):832-836. https://doi.org/10.1134/S001030119080124
Newton, G.B. 1971. Rhabdomesid bryozoans of the Wreford Megacyclothem (Wolfcampian, Permian) of Nebraska, Kansas, and Oklahoma. University of Kansas Paleontological Contributions, 56:1-71.
Nickles, J.M. and Bassler, R.S. 1900. A synopsis of American fossil Bryozoa, including bibliography and synonymy. United States Geological Survey Bulletin, 173:1-663. https://doi.org/10.5962/bhl.title.14907
Nikiforova, A.I. 1938. Types of Carboniferous Bryozoa of the European part of the USSR. Paleontologiya SSSR (Paleontology of the USSR). Volume 4, Part 5. Paleontologicheskii Institut Academii Nauk SSSR, Moscow. (In Russian)
Niko, S., Seuss, B., and Mapes, R. H. 2022. Virgilian (Late Pennsylvanian) coiled nautiloids from the Finis Shale Member of the Graham Formation in Texas, southern Midcontinent North America. Bulletin of the Tohoku University Museum, 21:1-19.
Perkins, R.D., Perry, T.G., and Hattin, D.E. 1962. Some bryozoans from the Bell Limestone Member of the Lecompton Limestone (Virgilian) of Kansas. State Geological Survey of Kansas Bulletin, 157(5):1-25.
Phillips, J. 1836. Illustrations of the geology of Yorkshire. Pt. 2. The Mountain Limestone District. John Murray, London. https://doi.org/10.5962/bhl.title.127948
Plummer, F.B. and Moore, R.C. 1922. Stratigraphy of the Pennsylvanian formations of north-central Texas. Texas University Bulletin, 2132:1-237. https://doi.org/10.26153/tsw/5051
Počta, P. 1894. Bryozoaires, hydrozoaires et partie des anthozoaries. In Barrande, J. (ed.), Systême Silurien du Centre de la Bohême, 8:1-230. https://doi.org/10.5962/bhl.title.8122
Prout, H.A. 1859. Third series of descriptions of Bryozoa from the Palaeozoic rocks of the western states and territories. Transactions of the St Louis Academy of Science, 1:443-452.
Richards, H.G. 1959. New Virgilian and Wolfcampian fenestrate bryozoans from Kansas. Journal of Paleontology, 33(6):1115-1119. https://www.jstor.org/stable/1300839
Roden, V.J., Hausmann, I.M., Nützel, A., Seuss, B., Reich, M., Urlichs, M., Hagdorn, H., and Kiessling, W. 2019: Fossil liberation: a model to explain high biodiversity in the Triassic Cassian Formation. Palaeontology 63(1):85-102. https://doi.org/10.1111/pala.12441
Rogers, A.F. 1900. A new genus and species of Bryozoa from the Coal Measures of Kansas and Missouri. The Kansas University Quarterly, A9:1-12.
Romantchuk, T.V. and Kiseleva, A.V. 1968. New Late Permian bryozoans of the Far East. Paleontologicheskii Zhurnal, 1968(2):55-60. (In Russian)
Ross, J.R.P. 1981. Biogeography of Carboniferous ectoproct Bryozoa. Palaeontology, 24(2):313-341.
Sakagami, S. 1995. Upper Paleozoic bryozoans from the Lake Titicaca region, Bolivia. Part 2. Systematic paleontology. Transactions and Proceedings of the Palaeontological Society of Japan new series, 180:261-281. https://doi.org/10.14825/prpsj1951.1995.180_261
Sayre, A.N. 1930. The fauna of the Drum Limestone of Kansas and western Missouri. Bulletin of the Kansas Geological Survey, 31(17):75-203. https://doi.org/10.5962/bhl.part.10872
Schopf, T.J.M. 1969. Paleoecology of ectoprocts (bryozoans). Journal of Paleontology, 43(2):234-244. https://www.jstor.org/stable/1302306
Schulga-Nesterenko, M.I. 1933. Bryozoa from the coal-bearing and subjacent series of Pechora Land: Goniocladia Etheridge and Ramipora Toula, Carboniferous and Permian representatives of the family Cystodictyonidae. Trudy Vsesoyuznogo geologo-razvedochnogo obyedineniya NKTP SSSR, 259:1-63. (In Russian)
Schulga-Nesterenko, M.I. 1951. Carboniferous Fenestellida of the Russian Platform. Trudy Paleontologicheskogo Instituta, 32:1-157. (In Russian)
Schulga-Nesterenko, M.I. 1952. New Lower Permian bryozoans of Ural Mountains. Trudy Paleontologicheskogo Instituta, 37:1-79. (In Russian)
Schulga-Nesterenko, M.I. 1955. Carboniferous Bryozoa of the Russian Platform. Trudy Paleontologicheskogo Instituta Akademii Nauk SSSR, 57:1-207. (In Russian)
Shimer, H.W. and Shrock, R.R. 1944. Index Fossils of North America: A New Work Based on the Complete Revision and Reillustration of Grabau and Shimer’s “North American Index Fossils”. J. Wiley, and Sons, New York; Chapman and Hall, London.
Shishova, N.A. 1959. New species of the genus Penniretepora from the Carboniferous of the Moscow region. Materialy k Osnovam Paleontologii, 3:16-27. (In Russian)
Shrock, R.R. and Twenhofel, W.H. 1953. Principles of Invertebrate Palaeontology: New second edition. McGraw Hill, New York.
Silén, L. and Harmelin, J.-G. 1974. Observations on living Diastoporidae (Bryozoa, Cyclostomata), with special regard to polymorphism. Acta Zoologica Stockholm, 55:81-96. https://doi.org/10.1111/j.1463-6395.1974.tb00182.x
Simonsen, A.H. and Cuffey, R.J. 1980. Fenestrate, pinnate and ctenostome bryozoans and associated barnacle borings in the Wreford Megacyclothem (Lower Permian) of Kansas, Oklahoma, and Nebraska. University of Kansas Paleontological Contributions, 101:1-38.
Simpson, G.B. 1895. A discussion of the different genera of the Fenestellidae. Thirteenth Annual Report of the State Geologist of New York for the year 1893:687-727.
Smith, J.P. 1896. Marine fossils from the Coal Measures of Arkansas. Proceedings of the American Philosophical Society, 35(152):213-285.
Smith, A.M., Nelson, C.S. and Danaher, P.J. 1992. Dissolution behavior of bryozoan sediments: taphonomic implications for non tropical carbonates. Palaeogeography Palaeoclimatology Palaeoecology, 93:213-226. https://doi.org/10.1016/0031-0182(92)90098-P
Smith, A.M. and Nelson, C.S. 1994. Selectivity in sea-floor processes: taphonomy of bryozoans, p. 177-180. In Hayward, P.J., Ryland, J.S., and Taylor, P.D. (eds.), Biology and Palaeobiology of Bryozoans. Olsen & Olsen, Fredensborg.
Snyder, E.M. 1991a. Revised taxonomic procedures and paleoecological applications for some North American Mississippian Fenestellidae and Polyporidae (Bryozoa). Palaeontographica Americana, 57:1-275.
Snyder, E.M. 1991b. Revised taxonomic approach to acanthocladiid Bryozoa. In Bigey, F.P. (ed.), Bryozoaires actuels et fossil: Bryozoa living and fossil. Bulletin de la Société des Sciences Naturelles de l`Quest de la France, Mèmore HS, 1:431-445.
Southwood, D.A. 1985. Ovicells in some Fenestrata from the Permian of N. E. England, p. 301-310. In Nielsen, C. and Larwood, G.P. (eds.), Bryozoa: Ordovician to Recent. Olsen & Olsen, Fredensborg.
Stach, L.W. 1936. Correlation of zoarial form with habitat. Journal of Geology, 44(1):60-65.
https://doi.org/10.1086/624395
Tavener-Smith, R. 1966. Ovicells in fenestrate cryptostomes of Visean age. Journal of Paleontology, 40(1):190-198. https://www.jstor.org/stable/1301785
Taylor, P.D. 2020. Bryozoan Paleobiology. Wiley Blackwell, Hoboken, Chichester. https://doi.org/10.1002/9781118454961
Taylor, P.D. and James, N.P. 2013. Secular changes in colony-forms and bryozoan carbonate sediments through geological history. Sedimentology, 60(5):1184-1212. https://doi.org/10.1111/sed.12032
Termier H. and Termier G. 1971. Bryozoaires du Paléozoique supérieur de l´Afganistan. Documents des Laboratoires de Géologie de la Faculté des Sciences de Lyon, 47:1-52.
Twenhofel, W.H. and Shrock, R.R. 1935. Invertebrate Paleontology. McGraw-Hill Publishing Co, New York and London.
Ulrich, E.O. 1882. American Palaeozoic Bryozoa. The Journal of the Cincinnati Society of Natural History, 5:121-175, 232-257.
Ulrich, E.O. 1884. American Palaeozoic Bryozoa. The Journal of the Cincinnati Society of Natural History, 7:24-51.
Ulrich, E.O. 1890. Palaeozoic Bryozoa: III. Geological Survey, 8:283-688.
Vine, G.R. 1883. Notes on the Carboniferous Polyzoa of West Yorkshire and Derbyshire. Proceedings of the Yorkshire Geological and Polytechnic Society, 8(2):161-174. https://doi.org/10.1144/pygs.8.2.161
Vine, G.R. 1884. Fourth report of the committee consisting of Dr. H. R. Sorby and Mr. G. R. Vine, appointed for the purpose of reporting on fossil Polyzoa. Reports of the 53rd Meeting of the British Association for the Advancement in Sciences:161-209.
Vine, G.R. 1885. Further notes on new species and other Yorkshire Carboniferous fossil Polyzoa described by Prof. John Phillips. Proceedings of the Yorkshire Geological and Polytechnic Society, new series, 8:377-393. https://doi.org/10.1144/pygs.8.3.377
Waagen, W. and Pichl, J. 1885. Salt-Range fossils. Productus -Limestone fossils. Memoirs of the Geological Survey of India, Paleontologica Indica, 13(1):771-834.
Waagen, W. and Wentzel, J. 1886. Salt-Range fossils. Productus -Limestone fossils: Coelenterata. Memoirs of the Geological Survey of India, Paleontologica Indica, 13(1):835-924. https://doi.org/10.1017/S0016756800182548
Warthin, A.S., Jr. 1930. Micropalaeontology of the Wewoka, and Holdenville Formations. Bryozoa. Oklahoma Geological Survey Bulletin, 53:35-43.
White, C.A. 1877. Report upon the invertebrate fossils collected in portions of Nevada, Utah, Colorado, New Mexico, and Arizona by parties of the expeditions of 1871, 1872, 1873, and 1874. In Wheeler, G.M. (ed.), Report upon U.S Geographical Surveys west of the one hundredth meridian, v. 4, Paleontology, pt. l, 219 p. Washington, D.C., U.S. Government Printing Office. https://doi.org/10.5962/bhl.title.51607
Wyse Jackson, P.N., McKinney, F.K., and Bancroft, A.J. 2006. Fenestrate bryozoan genera based on species from Ireland originally described by Frederick M‘Coy in 1844. Palaeontology, 49:741-767. https://doi.org/10.1111/j.1475-4983.2006.00572.x
Wyse Jackson, P.N. and Buttler, C.J. 2015. Part G, Revised, Volume 2, Chapter 3: preparation, imaging and conservation of Paleozoic bryozoans for study. Treatise Online, 63:1-15.
Yang, J. and Lu, L. 1983. Upper Carboniferous and Lower Permian bryozoans from Kalpin of Western Xinjiang. Palaeontologia Cathayana, 1:259-317.
Yang, W. and Kominz, M.A. 2003. Characteristics, stratigraphic architecture, and time framework of multi-order mixed siliciclastic and carbonate depositional sequences, outcropping Cisco Group (Late Pennsylvanian and Early Permian), Eastern Shelf, north-central Texas. Sedimentary Geology, 154:53-87. https://doi.org/10.1016/S0037-0738(02)00100-8
Young, J. and Young, J. 1874. On a new genus of Carboniferous Polyzoa. Annals and Magazine of Natural History, Series 4(13):335-339. https://doi.org/10.1080/00222937408680876
Young, J. and Young, J. 1875. New species of Glauconome from Carboniferous limestone strata of the west of Scotland. Proceedings of the Natural History Society of Glasgow, 2:325-335.

