 Oriented microwear on a tooth of Edestus minor (Chondrichthyes, Eugeneodontiformes):
Oriented microwear on a tooth of Edestus minor (Chondrichthyes, Eugeneodontiformes):
Implications for dental function
Article number: 22.2.39
https://doi.org/10.26879/831
Copyright Society for Vertebrate Paleontology, July 2019
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 3 November 2018. Acceptance: 26 May 2019.
{flike id=2603}
ABSTRACT
The symphyseal tooth whorls of the Carboniferous chondrichthyan Edestus consist of files of teeth having sharply-pointed, serrated crowns, joined at their bases. A single tooth whorl was present in each jaw. How these tooth whorls functioned is unclear, since their convex curvature allows only a few of the most lingual crowns of opposing tooth whorls to occlude. Rather than working in opposition, like scissors, the more labial teeth might have been used to cut and disable prey with a vertical motion of the anterior part of the body. Provided the scratches observed on the surface of Edestus teeth can be inferred to have been generated in the process of feeding, their orientation might be used to distinguish whether the teeth were used mainly in occlusion, to cut prey trapped between the jaws, or mainly to cut prey situated outside the oral cavity. Edestus minor teeth having unusually good surface preservation were examined for microwear. The teeth are from the Strawn Group (Desmoinesian, Middle Pennsylvanian) of San Saba County, Texas, USA. The best-preserved crown surfaces display scratches 50 to 500 micrometers long. The scratches are oriented predominantly transversely to the basal-apical axis. This observation appears to support the vertical slashing hypothesis. However, the possibility that interaction with the substrate contributed to the observed wear cannot be discounted.
Wayne M. Itano. Museum of Natural History, University of Colorado, 1995 Dartmouth Ave., Boulder, Colorado 80305, USA. wayne.itano@aya.yale.edu
Keywords: Pennsylvanian; Texas; Chondrichthyes; Edestidae; sharks; functional morphology; dental microwear
Itano, Wayne M. 2019. Oriented microwear on a tooth of Edestus minor (Chondrichthyes, Eugeneodontiformes): Implications for dental function. Palaeontologia Electronica 22.2.39A 1-16. https://doi.org/10.26879/831
palaeo-electronica.org/content/2019/2603-edestus-minor-microwear
Copyright: July 2019 Society of Vertebrate Paleontology.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0/creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Edestus sensu lato (including Lestrodus Obruchev, 1953, and Edestodus Obruchev, 1953) is a chondrichthyan in the order Eugeneodontiformes Zangerl, 1981, that possessed symphyseal tooth whorls of similar sizes in both jaws. It is known from Pennsylvanian deposits ranging in age from Bashkirian to early Kasimovian and geographically from North America, Britain, and Russia (Itano et al., 2012).
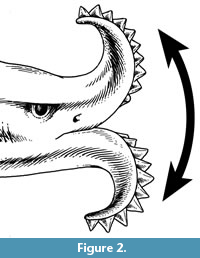
 Edestus sensu lato may be unique among chondrichthyans in possessing two large, symphyseal tooth whorls of similar sizes, with blade-like, sharply-pointed, serrated crowns. Teeth are shed from the outer, labial end and replaced at the inner, lingual end, as in a normal chondrichthyan tooth file. This unusual dentition is best known from the holotype of Edestus mirus Hay, 1912, which shows both tooth whorls of a single individual (Figure 1). The convex curvature of the tooth whorls prevents all but the two or three lingualmost crowns of each whorl from occluding with the corresponding crowns of the opposing whorl. Itano (2014) proposed that the tooth whorls were used in an unconventional way, not to cut prey caught between opposing tooth whorls (scissors-mode), but to slash prey situated outside the oral cavity, with a vertical motion of the whole head (Figure 2).
Edestus sensu lato may be unique among chondrichthyans in possessing two large, symphyseal tooth whorls of similar sizes, with blade-like, sharply-pointed, serrated crowns. Teeth are shed from the outer, labial end and replaced at the inner, lingual end, as in a normal chondrichthyan tooth file. This unusual dentition is best known from the holotype of Edestus mirus Hay, 1912, which shows both tooth whorls of a single individual (Figure 1). The convex curvature of the tooth whorls prevents all but the two or three lingualmost crowns of each whorl from occluding with the corresponding crowns of the opposing whorl. Itano (2014) proposed that the tooth whorls were used in an unconventional way, not to cut prey caught between opposing tooth whorls (scissors-mode), but to slash prey situated outside the oral cavity, with a vertical motion of the whole head (Figure 2).
Given that possession of a flexible neck, as in the Devonian tetrapod-like fish Tiktaalik (Daeschler et al., 2006), is unknown in any chondrichthyan, the vertical motion might have been carried out by (1) flexing of the whole body between a U and inverted-U shape or (2) vertical motion of the front part of the body by using the pectoral fins like wings (Itano, 2014, p. 215-216). At present, there is no postcranial fossil evidence of Edestus to either support or refute either of these two possibilities.
 Macrowear sustained during life has been reported on a tooth of E. minor from the Smithwick Shale (Pennsylvanian, late Atokan = Moscovian) of San Saba County, Texas, USA (Itano, 2015). The apex of the crown is truncated, and the surface of the remaining part is smooth and convex, as if worn by repeated contact with an abrasive surface, such as the skin of a large fish covered with scales or denticles. The fact that the abraded surface is roughly perpendicular to the basal-apical axis of the crown appears to support the slashing-mode hypothesis over the scissors-mode hypothesis. Similar apical wear has recently been reported on two crowns of a partial tooth whorl of E. heinrichi from a shale bed overlying the Herrin (No. 6) Coal (Pennsylvanian, late Desmoinesian = late Moscovian) of Randolph County, Illinois, USA (Itano, 2018).
Macrowear sustained during life has been reported on a tooth of E. minor from the Smithwick Shale (Pennsylvanian, late Atokan = Moscovian) of San Saba County, Texas, USA (Itano, 2015). The apex of the crown is truncated, and the surface of the remaining part is smooth and convex, as if worn by repeated contact with an abrasive surface, such as the skin of a large fish covered with scales or denticles. The fact that the abraded surface is roughly perpendicular to the basal-apical axis of the crown appears to support the slashing-mode hypothesis over the scissors-mode hypothesis. Similar apical wear has recently been reported on two crowns of a partial tooth whorl of E. heinrichi from a shale bed overlying the Herrin (No. 6) Coal (Pennsylvanian, late Desmoinesian = late Moscovian) of Randolph County, Illinois, USA (Itano, 2018).
Observations of dental microwear have long been used to elucidate dental function and diet in extant and extinct animals. The first application of dental microwear in paleontology seems to have been by Simpson (1926), who used scratch directions to infer chewing movements in Mesozoic multituberculate mammals. More recently, 2D, feature-based dental microwear observations have been used to infer diet in mammals, as in distinguishing browsers from grazers, using a scanning electron microscope (SEM) (Walker et al., 1978) or a low-magnification binocular light microscope (Solounias and Semprebon, 2002). 2D microwear analysis has been applied to fishes in a few cases (Purnell et al., 2006; Purnell et al., 2007). A 2D microwear analysis relies on human identification of features such as scratches and pits and is subject to strong intra- and inter-observer errors (Mihlbachler and Beatty, 2012). A 3D dental microwear texture analysis uses a confocal profilometer and scale-sensitive fractal analysis to measure surface textures without requiring the counting of individual features (Ungar et al., 2003; Scott et al., 2006). A 3D dental microwear analysis is subject to smaller intra- and inter-observer errors (DeSantis et al., 2013; Arman et al., 2016) but requires specialized equipment. The method has been applied to studies of dental microwear in mammals (e.g., DeSantis, 2016) and fishes (Purnell et al., 2012; Purnell and Darras, 2016), including, very recently, sharks (McLennan, 2018).
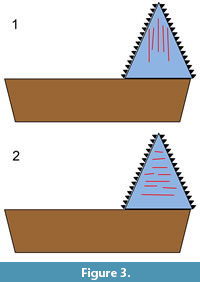 In the present study, a form of 2D microwear analysis was used. The orientation of microwear features on the crown surfaces of an Edestus tooth might help to distinguish between the two aforementioned alternative hypotheses (scissors vs. slashing). Scratches oriented mainly parallel to the basal-apical axis would support the hypothesis that the whorls were used in opposition, like scissors (Figure 3.1), while scratches oriented mainly perpendicular to that axis would support the vertical-slashing hypothesis (Figure 3.2). This presupposes that the observed wear is indeed feeding-related, which is not known a priori. There is also a possibility that scratches could have been generated through interaction with the substrate. In addition, wear acquired postmortem needs to be distinguished from wear acquired in vivo.
In the present study, a form of 2D microwear analysis was used. The orientation of microwear features on the crown surfaces of an Edestus tooth might help to distinguish between the two aforementioned alternative hypotheses (scissors vs. slashing). Scratches oriented mainly parallel to the basal-apical axis would support the hypothesis that the whorls were used in opposition, like scissors (Figure 3.1), while scratches oriented mainly perpendicular to that axis would support the vertical-slashing hypothesis (Figure 3.2). This presupposes that the observed wear is indeed feeding-related, which is not known a priori. There is also a possibility that scratches could have been generated through interaction with the substrate. In addition, wear acquired postmortem needs to be distinguished from wear acquired in vivo.
The two hypothesized functions of the teeth need not be mutually exclusive. Possibly teeth at an early stage of ontogeny might have been used in occlusion, while they were still within the oral cavity, and then used in a slashing, non-occlusal manner at a later stage of ontogeny. However, the possibility that the innermost teeth were used to cut prey, while the outer two-thirds to three-quarters of the teeth had no function runs into the objection that those outer teeth carry a heavy cost in terms of weight and water resistance, so it is not obvious why they would be retained.
Other chondrichthyans, both extant and extinct, have had non-occlusal tooth-like structures, but attached to their rostra. These chondrichthyans comprise the extant Pristidae (sawfish) and Pristiophoridae (sawsharks) and the extinct Sclerorhynchidae. Analogies in function between Edestus tooth whorls and the toothed rostrum of the extant sawfish Pristis were proposed soon after the discovery of Edestus (Hitchcock, 1856; Leidy, 1857). The rostrum of Pristis microdon is used both to sense and to capture prey, by stunning or impaling them or by pinning them to the substrate (Wueringer et al., 2012). Observations of microwear on rostral teeth of the sawshark Pristiophorus cirratus suggest that it also uses its rostrum to capture prey, though not necessarily to impale them (Nevatte et al., 2017). In that work, numbers of scratches were counted, but the directional distributions of the scratches were assessed only qualitatively. The extinct sclerorhynchid Schizorhiza stroemeri has rostral teeth that are triangular, with sharp cutting edges, so that its rostrum might have been used as a slashing weapon, in a similar manner to the function that is hypothesized here for the Edestus tooth whorls (Kirkland and Aguillón-Martinez, 2002; Smith et al., 2015).
The extant batoid Aetobatus narinari is an example of a chondrichthyan in which teeth are retained beyond the point at which they occlude with the teeth of the other jaw. Photographs of the upper and lower dentitions of a single individual were published previously (Itano, 2018, fig. 7A-C). It has been hypothesized that the projecting lower dentition is used like a shovel, to uncover prey in the substrate (Owen, 1840-1845, p. 47). Observations of this behavior are lacking. Also, the post-occlusal extremity of the lower dentition of the specimen examined does not show any increased wear. While the absence of increased wear does not rule out the “shovel” hypothesis, neither does it provide any support for that hypothesis. Another possibility is that the post-occlusal part of the lower dentition (five teeth out of 19 visible on the specimen examined) has no function. In that case, it might be that the lower symphyseal teeth are so rigidly cemented together that it takes some time for them to break off after they have ceased to be functional.
The possibility that Edestus tooth whorls were used to uncover or scrape prey, such as mussels, from surfaces, has been suggested (Eaton, 1962). While the tongue-like, projecting lower dentition of Aetobatus forms a plausible shovel, the Edestus tooth whorls (Figure 1) seem less well adapted to either digging or scraping, but well adapted to cutting through flesh. Zangerl and Jeremiah (2004) suggested that Edestus might have rushed at prey, with mouth wide open and teeth exposed, to cut and disable prey. While this mode, which is roughly analogous to extant billfish (Istiophoridae and Xiphiidae), cannot be ruled out, it would seem that narrow, spike-like teeth would better serve that function than serrated, triangular teeth.
Cursory examination by the author of teeth of E. heinrichi Newberry and Worthen, 1870, by optical microscopy and by SEM revealed a few scratches, but these were not consistently present and lacked any consistent orientation. Whether any of the scratches were generated in vivo, rather than postmortem, e.g., by abrasion or by damage during preparation, was impossible to determine. Teaford (1988) has discussed the problem of distinguishing wear acquired during life on mammalian teeth from postmortem wear. In vivo wear is “generally laid down in a regular fashion at specific locations on the teeth” (Teaford, 1988). The scratches observed on the teeth of E. heinrichi do not appear to pass this test. Many, probably most, Edestus teeth in museum collections originate from carbonaceous shales associated with coal deposits. Crowns and bases of such teeth are commonly stained black. The crown surfaces are diagenetically altered and are sometimes coated with crystals of marcasite. This form of preservation seems to be detrimental to the preservation of in vivo microwear.
A group of Edestus teeth was located in the course of a review of North American occurrences of Edestus (Itano et al., 2012). These teeth are all from the same locality and display various states of preservation. The bases and crowns are light-colored and clearly have a different taphonomic history compared to the ones originating from carbonaceous shales. The crowns were examined for the presence of scratches by optical microscopy.
Institutional Abbreviations
TMM, Vertebrate Paleontology Laboratory (formerly with the Texas Memorial Museum), University of Texas, Austin, Texas, USA; USNM, National Museum of Natural History, Washington, District of Columbia, USA.
MATERIAL AND METHODS
Material
 One fragmentary crown of Edestus sp., TMM 40234-19; 13 complete or partial crowns of Edestus minor, TMM 40234-8, TMM 40234-13, TMM 40234-14, TMM 40234-15, TMM 40234-16, TMM 40235-17, TMM 40234-18, TMM 40234-20, TMM 40234-21, TMM 40234-22, TMM 40234-23, TMM 40234-24, and TMM 40234-25, all from the same locality. Previously, some were given the common number TMM 40234-1. All now have individual specimen numbers. Some are shown in Figure 4.1-7.
One fragmentary crown of Edestus sp., TMM 40234-19; 13 complete or partial crowns of Edestus minor, TMM 40234-8, TMM 40234-13, TMM 40234-14, TMM 40234-15, TMM 40234-16, TMM 40235-17, TMM 40234-18, TMM 40234-20, TMM 40234-21, TMM 40234-22, TMM 40234-23, TMM 40234-24, and TMM 40234-25, all from the same locality. Previously, some were given the common number TMM 40234-1. All now have individual specimen numbers. Some are shown in Figure 4.1-7.
Locality and Age
Upper Strawn Group, Pennsylvanian, middle Desmoinesian (Moscovian global stage), approximately 310 Ma, 5 km west of Richland Springs, San Saba County, Texas, USA.
Remarks on Material
Although they have been in the collections of the TMM for a long time, these Edestus teeth appear not to have been reported in the published literature until noted by Itano et al. (2012). The collector and date of collection are unknown. They were transferred from the Texas Bureau of Economic Geology some time ago, with no provenance information other than what is stated here in the “Locality and age” section. They might have been collected during the surveys of E. Cope and W.F. Cummins in the late nineteenth century (J.C. Sagebiel, pers. comm., 2011).
Methods
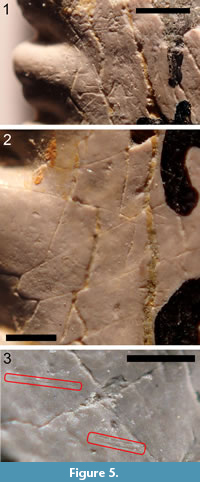 Measurement of scratches. All of the teeth listed in the Material section were examined under a binocular microscope for the presence of scratches that could be attributed to in vivo wear. Attempts were made to distinguish such scratches from cracks, preparation marks, and other types of postmortem damage. Figure 5.1-3 shows various linear features on the surface of TMM 40234-17 that are and that are not attributed to in vivo wear. Figure 5.1 shows gouges and deep scratches in a crossed pattern that are interpreted as the result of a preparator removing matrix with a sharp implement. Figure 5.2 shows a region where most of the linear features form an angular network of cracks. These are interpreted as the result of differential expansion of the inner dentine and the outer, hypermineralized layer. (In the absence of histological studies of the hypermineralized outer layer, it is not here referred to as enameloid. Duffin (2016) has shown that the outer, hypermineralized layer teeth of E. heinrichi and of several other eugeneodontiform chondrichthyans are composed not of enameloid, but rather of a compact form of dentine made up of very small crystallites, 0.5 µm in length.) Figure 5.3 shows a small region where some shallowly incised scratches, which are interpreted as in vivo wear, can be seen among the more prominent cracks. The longer scratch is isolated, but the other one consists of a pair of parallel scratches separated by 35 µm.
Measurement of scratches. All of the teeth listed in the Material section were examined under a binocular microscope for the presence of scratches that could be attributed to in vivo wear. Attempts were made to distinguish such scratches from cracks, preparation marks, and other types of postmortem damage. Figure 5.1-3 shows various linear features on the surface of TMM 40234-17 that are and that are not attributed to in vivo wear. Figure 5.1 shows gouges and deep scratches in a crossed pattern that are interpreted as the result of a preparator removing matrix with a sharp implement. Figure 5.2 shows a region where most of the linear features form an angular network of cracks. These are interpreted as the result of differential expansion of the inner dentine and the outer, hypermineralized layer. (In the absence of histological studies of the hypermineralized outer layer, it is not here referred to as enameloid. Duffin (2016) has shown that the outer, hypermineralized layer teeth of E. heinrichi and of several other eugeneodontiform chondrichthyans are composed not of enameloid, but rather of a compact form of dentine made up of very small crystallites, 0.5 µm in length.) Figure 5.3 shows a small region where some shallowly incised scratches, which are interpreted as in vivo wear, can be seen among the more prominent cracks. The longer scratch is isolated, but the other one consists of a pair of parallel scratches separated by 35 µm.
It is common in dental microwear studies to examine small areas of tooth surfaces, typically a few hundred micrometers across (Walker et al., 1978; Ungar, 1996; Williams et al., 2009). Scratches apparently generated in vivo were only sparsely observed on the Edestus tooth studied here. For that reason, and also to avoid possible bias due to the choice of an area of the tooth surface that did not represent the tooth surface as a whole, an area as large as was practicable was surveyed (several millimeters across). Compare the studied area (Figure 6) to the entire side of the tooth (Figure 4.3).
 In order to count all of the scratches within the studied area and to avoid double-counting, it is best to obtain a single image having sufficient resolution to view the scratches. In order to do this with the photographic equipment that was available, it was necessary to obtain many overlapping images and to merge them into a single image. The procedure for doing this is described in Appendix 1.
In order to count all of the scratches within the studied area and to avoid double-counting, it is best to obtain a single image having sufficient resolution to view the scratches. In order to do this with the photographic equipment that was available, it was necessary to obtain many overlapping images and to merge them into a single image. The procedure for doing this is described in Appendix 1.
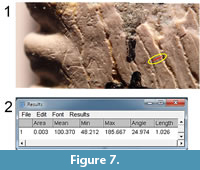
Once a blended single image is obtained, it can be imported into a computer program and viewed on the computer monitor at any desired magnification. Viewing of small sections of the blended single image was sometimes supplemented by viewing other images containing that same area. The ImageJ program (version 1.50b, Fiji distribution) (Schneider et al., 2012) was used for this purpose. In ImageJ, the operator can mark a line segment, as in Figure 7.1 (red mark).  The program automatically collects the numerical parameters defining the line segment, including the position, length, and angle with respect to horizontal (Figure 7.2). For the present study, only the angle is of importance.
The program automatically collects the numerical parameters defining the line segment, including the position, length, and angle with respect to horizontal (Figure 7.2). For the present study, only the angle is of importance.
Statistical methods. The angular data of the scratches are analyzed according to circular statistics (Mardia and Jupp, 2000). Rao’s equal spacing test for uniformity (Mardia and Jupp, 2000, p. 108) is used to test the null hypothesis that the scratches are drawn from a uniform distribution. The test measures the deviation of the differences between angles and the uniform case, in which the spacings would be 360º/n, where n is sample size. To apply the test, it is necessary to make a simple adjustment to the data, which was made also by Varriale (2016) in his study of dinosaur dental microwear. There is no distinction between a scratch with angle θ and θ + 180º. Angles in the data set are mapped into the range from -90º to +90º. If each angle is multiplied by 2, the data set is mapped into the range from -180º to +180º. If 180º is added to each angle, which does not affect the angular spacing, the data set is mapped into the range from 0º to 360º, and the formula (Mardia and Jupp, 2000, eq. [6.3.45]) for the quantity L that characterizes the deviation from angular uniformity can be applied directly:
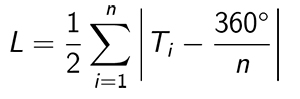
where Ti is the i th angular interval in the data set. The mean direction of the scratches is calculated according to Eqs. 2.21-2.24 of Mardia and Jupp (2000). The sample circular standard deviation is calculated according to Eq. 2.3.11 of Mardia and Jupp (2000). Since the calculations of the mean direction and the circular standard deviation are carried out on doubled angles, the values obtained in this manner must be divided by two in order to obtain the final results.
RESULTS
Initial Survey of All Teeth
 All of the teeth listed in the Material section were examined for the presence of scratches that could be attributed to in vivo wear. The tooth from the sample that at first appeared to be the best-preserved, TMM 40234-8 (Figure 4.2; Itano et al., 2012, figure 12), was unsuitable for this study because of the presence of a transparent coating, possibly shellac. The coating caused reflections that partially obscured small surface features, including scratches. Removal of the coating (Williams and Doyle, 2010) was not attempted, since exploratory microscopic examination indicated that, while some scratches were present, the density of such scratches was less than on other teeth, in particular, TMM 40234-17. Also, the teeth were on loan from another institution, and such cleaning would have had the potential to cause damage. Many of the teeth in the sample were considered not useful for this study because the apical parts of the crown were truncated (broken off). In none of the cases in which the crowns were apically truncated was the surface of the remaining part of the crown smoothly polished, as for the Edestus minor tooth described by Itano (2015). Others, e.g., TMM 40234-18 (Figure 4.4), have had much of the outer, hypermineralized layer removed. On TMM 40234-18, the outer layer is so reduced in thickness that the darker, inner, dentine layer shows through. Whether the removal of the outer layer occurred in vivo or postmortem is not known. The other teeth having preserved apical parts (TMM 40234-17, TMM 40234-24, TMM 40234-25, and TMM 40234-23) (Figure 4.3, 4.5, 4.6, and 4.7, respectively) exhibit varying degrees of removal of the outer layer in the region of the apices.
All of the teeth listed in the Material section were examined for the presence of scratches that could be attributed to in vivo wear. The tooth from the sample that at first appeared to be the best-preserved, TMM 40234-8 (Figure 4.2; Itano et al., 2012, figure 12), was unsuitable for this study because of the presence of a transparent coating, possibly shellac. The coating caused reflections that partially obscured small surface features, including scratches. Removal of the coating (Williams and Doyle, 2010) was not attempted, since exploratory microscopic examination indicated that, while some scratches were present, the density of such scratches was less than on other teeth, in particular, TMM 40234-17. Also, the teeth were on loan from another institution, and such cleaning would have had the potential to cause damage. Many of the teeth in the sample were considered not useful for this study because the apical parts of the crown were truncated (broken off). In none of the cases in which the crowns were apically truncated was the surface of the remaining part of the crown smoothly polished, as for the Edestus minor tooth described by Itano (2015). Others, e.g., TMM 40234-18 (Figure 4.4), have had much of the outer, hypermineralized layer removed. On TMM 40234-18, the outer layer is so reduced in thickness that the darker, inner, dentine layer shows through. Whether the removal of the outer layer occurred in vivo or postmortem is not known. The other teeth having preserved apical parts (TMM 40234-17, TMM 40234-24, TMM 40234-25, and TMM 40234-23) (Figure 4.3, 4.5, 4.6, and 4.7, respectively) exhibit varying degrees of removal of the outer layer in the region of the apices.
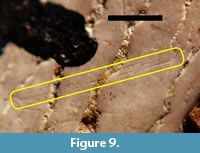
 The tooth having the highest number of visible scratches interpreted as having been acquired in vivo was TMM 40234-17. The labeled side (Figure 4.3) had more scratches than the unlabeled side and was chosen for detailed study. Figure 8 shows a part of the surface of TMM 40234-17 that has several shallowly incised scratches that are interpreted as in vivo wear. Some of these are pairs of parallel or nearly parallel scratches separated by approximately 45 µm to 70 µm. Most of the scratches are roughly transverse to the deep, sinuous cracks, so they are also roughly transverse to the basal-apical axis. Figure 9 shows one of the rare cases in which three nearly parallel scratches are present on TMM 40234-17. The separation between the two most widely separated scratches is approximately 54 µm.
The tooth having the highest number of visible scratches interpreted as having been acquired in vivo was TMM 40234-17. The labeled side (Figure 4.3) had more scratches than the unlabeled side and was chosen for detailed study. Figure 8 shows a part of the surface of TMM 40234-17 that has several shallowly incised scratches that are interpreted as in vivo wear. Some of these are pairs of parallel or nearly parallel scratches separated by approximately 45 µm to 70 µm. Most of the scratches are roughly transverse to the deep, sinuous cracks, so they are also roughly transverse to the basal-apical axis. Figure 9 shows one of the rare cases in which three nearly parallel scratches are present on TMM 40234-17. The separation between the two most widely separated scratches is approximately 54 µm.
 An unexpected observation was that the apices of several of the teeth show localized wear to the apical region, in the form of loss of some of the outer, hypermineralized layer. The localized wear can be seen, in varying degrees, on TMM 40234-17, TMM 40234-18, TMM 40234-24, TMM 40234-25, and TMM 40234-23 (Figure 4.3-7). In fact, of all the teeth in the sample that retain their apices, the only one that does not show some degree of localized wear to the apical region is TMM 40234-8 (Figure 4.2).
An unexpected observation was that the apices of several of the teeth show localized wear to the apical region, in the form of loss of some of the outer, hypermineralized layer. The localized wear can be seen, in varying degrees, on TMM 40234-17, TMM 40234-18, TMM 40234-24, TMM 40234-25, and TMM 40234-23 (Figure 4.3-7). In fact, of all the teeth in the sample that retain their apices, the only one that does not show some degree of localized wear to the apical region is TMM 40234-8 (Figure 4.2).
Detailed Study of TMM 40234-17
Approximately 62% of the portion of the numbered side of TMM 40234-17 that has a relatively intact surface (approximately 79 mm2 out of approximately 130 mm2) was imaged at high resolution by creating a blended mosaic of 63 images by the method described in Appendix 1.  The 63 images were arranged in nine horizontal strips of seven images each. Figure 6 shows the blended mosaic image. Figure 10 shows the same mosaic image with scratches marked in red. Secondary or tertiary scratches that are parallel to and associated with another nearby scratch are marked in green. The images in Figure 6 and Figure 10 are 11,951 pixels wide and 12,768 pixels high (approximately 145 megapixels, where 1 megapixel equals 1,048,576 pixels). The files have been reduced in resolution for publication. Higher-resolution versions of Figure 6 and Figure 10 are included as supplementary material (Supplement S1 and Supplement S2, respectively). A total of 107 scratches interpreted as having been acquired in vivo were recorded. Sets of two or three parallel scratches were counted as one scratch. A file containing the parameters of the scratches, in the format shown in Figure 7.2, is included as supplementary material (Supplement S3).
The 63 images were arranged in nine horizontal strips of seven images each. Figure 6 shows the blended mosaic image. Figure 10 shows the same mosaic image with scratches marked in red. Secondary or tertiary scratches that are parallel to and associated with another nearby scratch are marked in green. The images in Figure 6 and Figure 10 are 11,951 pixels wide and 12,768 pixels high (approximately 145 megapixels, where 1 megapixel equals 1,048,576 pixels). The files have been reduced in resolution for publication. Higher-resolution versions of Figure 6 and Figure 10 are included as supplementary material (Supplement S1 and Supplement S2, respectively). A total of 107 scratches interpreted as having been acquired in vivo were recorded. Sets of two or three parallel scratches were counted as one scratch. A file containing the parameters of the scratches, in the format shown in Figure 7.2, is included as supplementary material (Supplement S3).
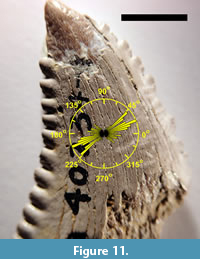
Figure 11 shows a bidirectional rose plot of the orientations of the 107 observed scratches overlaid on an image of TMM 40234-17. For this data set, L = 175.89. The null hypothesis (that the directions of the scratches are uniform) is rejected (p < 0.001) (Russell and Levitin, 1995, table II). Since the directions of the scratches are not uniformly distributed, the mean direction and the sample circular standard deviation can be calculated. The mean direction of the scratches is 14°. The sample circular standard deviation is 36°. The orientations of the observed scratches are thus predominantly transverse to the basal-apical axis.
DISCUSSION AND CONCLUSIONS
 Shallow, linear features were recorded as scratches acquired in vivo unless they were interpreted as preparation marks, cracks, or some other form of postmortem damage. Other than preparation marks, such features are expected to have random orientations. For example, scratches induced by tumbling in sediment, either pre-burial or post-exposure, would have random orientations. Experimental studies have shown that the main effect of abrasion of teeth by tumbling in sediment is to obliterate microwear features rather than to modify or to create new ones (King et al., 1999). In particular, generation of microwear features having a preferred orientation was not observed in those experiments. Inclusion of scratches with random orientations in the data set should not bias the calculated mean direction but would increase the circular standard deviation. The lengths of the features, though recorded, were not thought to be of significance, particularly since many, if not most, of the scratches could have been reduced in apparent length by postmortem weathering or abrasion. Positional dependence on the tooth of scratches acquired in vivo is to be expected. However, the sample size was too small to divide into subsets from different regions of the examined tooth surface.
Shallow, linear features were recorded as scratches acquired in vivo unless they were interpreted as preparation marks, cracks, or some other form of postmortem damage. Other than preparation marks, such features are expected to have random orientations. For example, scratches induced by tumbling in sediment, either pre-burial or post-exposure, would have random orientations. Experimental studies have shown that the main effect of abrasion of teeth by tumbling in sediment is to obliterate microwear features rather than to modify or to create new ones (King et al., 1999). In particular, generation of microwear features having a preferred orientation was not observed in those experiments. Inclusion of scratches with random orientations in the data set should not bias the calculated mean direction but would increase the circular standard deviation. The lengths of the features, though recorded, were not thought to be of significance, particularly since many, if not most, of the scratches could have been reduced in apparent length by postmortem weathering or abrasion. Positional dependence on the tooth of scratches acquired in vivo is to be expected. However, the sample size was too small to divide into subsets from different regions of the examined tooth surface.
One of the few mechanisms that could lead to linear features having a preferred orientation is preparation with a sharp tool. Preparation-related features would be of recent origin and hence would have sharp edges and be unworn. A few marks fitting this description are present, as in Figure 5.1, but these are limited to a small part of the surface. More commonly, the linear features thought to be scratches acquired in vivo show signs of weathering or abrasion, as in Figure 5.3, Figure 8, and Figure 9. Also, the double and triple scratches, which have varying amounts of separation, are difficult to attribute to tool marks.
The sharp, serrated teeth of Edestus strongly suggest that it was a predator. If the prey were other fish, then the scratches might have been caused by contact with dermal denticles or scales on the skin of such prey.
The observed localized wear to the apices of some teeth is unexplained but might be a result of the apices being abraded by repeatedly being dragged through the outer covering of prey. Such wear would be consistent with the use of the tooth whorls to slash. In order for such wear to occur, the prey would have had to have been relatively heavy. Such a mode of predation would tend to cause an increase in the density of scratches toward the tooth apices. Such an increase in scratch density might be observable if more and better-preserved material can be located.
Due to the paucity of information on the paleoenvironment and fauna associated with these teeth, the monograph by Zangerl and Richardson (1963) on the paleoecology of two Pennsylvanian black shales might be informative. The invertebrate fauna of these shales indicates marine conditions. Edestus remains, including those of juveniles, are known to occur in these shales (Zangerl and Richardson, 1963; Taylor and Adamec, 1977; Zangerl and Jeremiah, 2004). Zangerl and Richardson (1963, p. 136-137) noted the presence in these shales of remains of fish, mainly chondrichthyans and “palaeoniscoids,” that showed evidence of damage due to predation. They noted “essentially whole individuals, near-perfectly articulated, except for local, usually linear, areas of disturbance.” Often, body parts were nearly severed, but apparently still connected to each other by connective tissue. In addition to noting the fish having linear wounds, they remarked on the presence of many specimens of “near-perfectly articulated partial individuals.” These consist of separated heads and tails or bodies lacking heads, tails, or both. There is no proof that the “amputation” observed is not the result of ordinary taphonomic processes, e.g., decay. However, that seems unlikely, since the partial bodies are described as being near-perfectly articulated. Both the linear wounds and the amputated remains might be the result of predation by Edestus, either by a bite with the inner, lingual parts of the tooth whorls, or by a sweeping blow with the outer, labial parts. Predation by the former mode would tend to create scratches on the Edestus teeth oriented mainly parallel to the basal-apical axis (Figure 3.1), while the latter mode would tend to create scratches oriented mainly perpendicular to that axis (Figure 3.2). Since the partial bodies are articulated, they may represent prey that was cut, but not ingested, by the predator.
The observation that scratches on a lateral face of a crown of an Edestus tooth are oriented transversely to the basal-apical axis supports the hypothesis that the tooth whorls were used to kill or disable prey by slashing them, rather than to cut prey caught between the two whorls. However, the teeth might have served other functions, e.g., for the few most lingual teeth, cutting prey trapped between the jaws. The exploratory nature of this study, based mainly on examination of a single tooth, is acknowledged. Alternative interpretations of the same observations are possible, even if the scratches were acquired in vivo rather than postmortem.
Two such alternative interpretations are:
1) “Reciprocating saw” hypothesis: Zangerl and Jeremiah (2004) hypothesized that the lower jaw of Edestus might have been capable of linear forward and backward motions, so that it could operate like the human tool, the reciprocating saw, to cut prey trapped between the jaws. Such a mode of predation could result in tooth microwear consisting of scratches oriented transversely to the basal-apical axis, either from interaction between the teeth and the prey or between teeth of opposing jaws. This interpretation, while not ruled out by the observations, suffers from the same drawback as the “scissors” mode, which is that only a few of the innermost teeth seem to be effective in cutting, while the majority of the teeth do not seem to be functional, despite the fact that they carry a cost in terms of weight and water resistance. A true “circular saw” action, which might make use of more of the teeth, is difficult to imagine.
2) Substrate interaction: it has been hypothesized that the Edestus tooth whorls might have been used to plow through the substrate to locate prey (Eaton, 1962). There seems to be no way to definitively refute this hypothesis. However, the sharply-pointed, serrated teeth of Edestus would seem to be better adapted to slice through flesh than to plow through sediment. In particular, there would seem to be no need for serrations if the teeth were to be used for the latter function. That does not rule out probing through the substrate as a secondary function.
ACKNOWLEDGMENTS
H. Blom (University of Uppsala) first suggested that tooth microwear might indicate how Edestus teeth were used. I thank J.C. Sagebiel (TMM) for the loan of specimens. I thank two anonymous reviewers and the Handling Editor for their comments, which led to improvements in the manuscript. Photographic equipment and software were purchased with the aid of a Karl Hirsch Memorial Research Grant from the Western Interior Paleontological Society (Denver). SEM imaging was performed by D.K. Elliott (Northern Arizona University). Photoshop is a registered trademark of Adobe Systems International.
REFERENCES
Arman, S.D., Ungar, P.S., Brown, C.A., DeSantis, L.R.G., Schmidt, C., and Prideaux, G.J. 2016. Minimizing inter-microscope variability in dental microwear texture analysis. Surface Topography: Metrology and Properties, 4:024007. https://doi.org/10.1088/2051-672X/4/2/024007
Daeschler, E.B., Shubin, N.H., and Jenkins, F.A., Jr. 2006. A Devonian tetrapod-like fish and the evolution of the tetrapod body plan. Nature, 440:757-763. https://doi.org/10.1038/nature04639
DeSantis, L.R.G. 2016. Dental microwear textures: Reconstructing diets of fossil mammals. Surface Topography: Metrology and Properties, 4:023002. https://doi.org/10.1088/2051-672X/4/2/023002
DeSantis, L.R.G., Scott, J.R., Schubert, B.W., Donohue, S.L., McCray, B.M., Van Stolk, C.A., Winburn, A.A., Greshko, M.A., and O'Hara, M.C. 2013. Direct comparisons of 2D and 3D dental microwear proxies in extant herbivorous and carnivorous mammals. PLOS One, 8:e71428. https://doi.org/10.1371/journal.pone.0071428
Duffin, C.J. 2016. Cochliodonts and chimaeroids: Arthur Smith Woodward and the Holocephalians. Geological Society, London, Special Publications, 430:137-154. https://doi.org/10.1144/SP430.9
Eaton, T.H., Jr. 1962. Teeth of edestid sharks. University of Kansas Publications, Museum of Natural History, 12:347-362.
Hay, O.P. 1912. On an important specimen of Edestus; with description of a new species, Edestus mirus. Proceedings of the United States National Museum, 42:31-38. https://doi.org/10.5479/si.00963801.42-1884.31
Hitchcock, E. 1856. Account of the discovery of the fossil jaw of an extinct family of sharks, from the Coal Formation. Proceedings of the American Association for the Advancement of Science, 9:229-230.
Itano, W.M. 2014. Edestus, the strangest shark? First report from New Mexico, North American paleobiogeography, and a new hypothesis on its method of predation. Mountain Geologist, 51:201-221.
Itano, W.M. 2015. An abraded tooth of Edestus (Chondrichthyes, Eugeneodontiformes): Evidence for a unique mode of predation. Transactions of the Kansas Academy of Science, 118:1-9. https://doi.org/10.1660/062.118.0101
Itano, W.M. 2018. A tooth whorl of Edestus heinrichi (Chondrichthyes, Eugeneodontiformes) displaying progressive macrowear. Transactions of the Kansas Academy of Science, 121:125-133. https://doi.org/10.1660/062.121.0214
Itano, W.M., Houck, K.J., and Lockley, M.G. 2012. Systematics and occurrences of Edestus (Chondrichthyes) worldwide and new occurrences from Colorado and Texas. Historical Biology, 24:397-410. https://doi.org/10.1080/08912963.2012.658569
King, T., Andrews, P., and Boz, B. 1999. Effect of taphonomic processes on dental microwear. American Journal of Physical Anthropology, 108:359-373. https://doi.org/10.1002/(sici)1096-8644(199903)108:3<359::aid-ajpa10>3.0.co;2-9
Kirkland, J.I. and Aguillón-Martinez 2002. Schizorhiza: A unique sawfish paradigm from the Difunta Group, Coahuila, Mexico. Revista Mexicana de Ciencias Geológicas, 19:16-24.
Leidy, J. 1857. Remarks on certain extinct species of fishes. Proceedings of the Academy of Natural Sciences of Philadelphia, 8:301-302.
Mardia, K.V., and Jupp, P.E. 2000. Directional Statistics. John Wiley and Sons, Ltd., Chichester, UK. https://doi.org/10.1002/9780470316979
McLennan, L. 2018. Tooth Wear, Microwear and Diet in Elasmobranchs. Ph.D. Thesis, University of Leicester, Leicester, UK.
Mihlbachler, M.C. and Beatty, B.L. 2012. Magnification and resolution in dental microwear analysis using light microscopy. Palaeontologia Electronica, 15.2.25A:1-14. https://doi.org/10.26879/317
Nevatte, R.J., Wueringer, B.E., Jacob, D.E., Park, J.M., and Williamson, J.E. 2017. First insights into the function of the sawshark rostrum through examination of rostral tooth microwear. Jounal of Fish Biology, 91:1582-1602. https://doi.org/10.1111/jfb.13467
Newberry, J.S. 1866. Edestus minor, Newb., p. 84-85. In Newberry, J.S. and Worthen, A. H.(eds.), Descriptions of New Species of Vertebrates, Mainly from the Sub-Carboniferous Limestone and Coal Measures of Illinois. Geological Survey of Illinois, 2:9-134. https://doi.org/10.5962/bhl.title.61379
Newberry, J.S. and Worthen, A.H. 1870. Descriptions of vertebrates. Geological Survey of Illinois, 4:343-374. https://doi.org/10.5962/bhl.title.23610
Obruchev, D.V. 1953. Izuchenie edestid i raboty A.P. Karpinskogo [A study of the edestids and the work of A.P. Karpinskii]. Trudy Paleontologicheskogo Instituta Akademii Nauk SSSR [Transactions of the Palaeontological Institute], 45:1-85.
Owen, R. 1840-1845. Odontography, Vol. 1. Hippolyte Bailliere, London. https://doi.org/10.5962/bhl.title.16281
Purnell, M.A., Bell, M.A., Baines, D.C., Hart, P.J.B., and Travis, M.P. 2007. Correlated evolution and dietary change in fossil stickleback. Science, 317:1887. https://doi.org/10.1126/science.1147337
Purnell, M.A. and Darras, L.P.G. 2016. 3D tooth microwear texture analysis in fishes as a test of dietary hypotheses of durophagy. Surface Topography: Metrology and Properties, 4:014006. https://doi.org/10.1088/2051-672x/4/1/014006
Purnell, M.A., Hart, P.J.B., Baines, D.C., and Bell, M.A. 2006. Quantitative analysis of dental microwear in threespine stickleback: a new approach to analysis of trophic ecology in aquatic vertebrates. Journal of Animal Ecology, 75:967-977. https://doi.org/10.1111/j.1365-2656.2006.01116.x
Purnell, M.A., Seehausen, O., and Galis, F. 2012. Quantitative three-dimensional microtextural analyses of tooth wear as a tool for dietary discrimination in fishes. Journal of the Royal Society Interface, 9:2225-2233. https://doi.org/10.1098/rsif.2012.0140
Russell, G.S. and Levitin, D.J. 1995. An expanded table of probability values for Rao’s spacing test. Communications in Statistics - Simulation and Computation, 24:879-888. https://doi.org/10.1080/03610919508813281
Schneider, C.A., Rasband, W.S., and Eliceiri, K.W. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9:671-675. https://doi.org/10.1038/nmeth.2089
Scott, R.S., Ungar, P.S., Bergstrom, T.S., Brown, C.A., Childs, B.E., Teaford, M.F., and Walker, A. 2006. Dental microwear texture analysis: technical considerations. Journal of Human Evolution, 51:339-349. https://doi.org/10.1016/j.jhevol.2006.04.006
Simpson, G.G. 1926. Mesozoic Mammalia. IV. The multituberculates as living animals. American Journal of Science, 11:228-250. https://doi.org/10.2475/ajs.s5-11.63.228
Smith, M.M., Riley, A., Fraser, G.J., Underwood, C., Welton, M., Kriwet, J., Pfaff, C., and Johanson, Z. 2015. Early development of rostrum saw-teeth in a fossil ray tests classical theories of the evolution of vertebrate dentitions. Proceedings of the Royal Society B, 282:20151628. https://doi.org/10.1098/rspb.2015.1628
Solounias, N. and Semprebon, G. 2002. Advances in the reconstruction of ungulate ecomorphology with application to early fossil equids. American Museum Novitates, 3366:1-49. https://doi.org/10.1206/0003-0082(2002)366<0001:aitrou>2.0.co;2
Taylor, K. and Adamec, T. 1977. Tooth histology and ultrastructure of a Paleozoic shark, Edestus heinrichii. Fieldiana: Geology, 33:441-470. https://doi.org/10.5962/bhl.title.5199
Teaford, M.F. 1988. Scanning electron microscope diagnosis of wear patterns versus artifacts on fossil teeth. Scanning Microscopy, 2:1167-1175.
Ungar, P.S. 1996. Dental microwear of European Miocene catarrhines: evidence for diets and tooth use. Journal of Human Evolution, 31:335-366. https://doi.org/10.1006/jhev.1996.0065
Ungar, P.S., Brown, C.A., Bergstrom, T.S., and Walker, A. 2003. Quantification of dental microwear by tandem scanning confocal microscopy and scale-sensitive fractal analyses. Scanning, 25:185-193. https://doi.org/10.1002/sca.4950250405
Varriale, F.J. 2016. Dental microwear reveals mammal-like chewing in the neoceratopsian dinosaur Leptoceratops gracilis. PeerJ, 4:e2132. https://doi.org/10.7717/peerj.2132
Walker, A., Hoeck, H.N., and Perez, L. 1978. Microwear of mammalian teeth as an indicator of diet. Science, 201:908-910. https://doi.org/10.1126/science.684415
Williams, V.S., Barrett, P.M., and Purnell, M.A. 2009. Quantitative analysis of dental microwear in hadrosaurid dinosaurs, and the implications for hypotheses of jaw mechanics and feeding. Proceedings of the National Academy of Sciences, 106:11194-11199. https://doi.org/10.1073/pnas.0812631106
Williams, V.S. and Doyle, A.M. 2010. Cleaning fossil tooth surfaces for microwear analysis: Use of solvent gels to remove resistant consolidant. Palaeontologia Electronica, 13.3.2T:1-12.
Wueringer, B.E., Squire, L., Jr., Kajiura, S.M., Hart, N.S., and Collin, S.P. 2012. The function of the sawfish's saw. Current Biology, 22:R150-R151. https://doi.org/10.1016/j.cub.2012.01.055
Zangerl, R. 1981. Chondrichthyes I: Paleozoic Elasmobranchii, p. 1-115. In Schultze, H.-P. (ed.), Handbook of Paleoichthyology 3A. Gustav Fischer Verlag, Stuttgart, Germany.
Zangerl, R. and Jeremiah, C. 2004. Notes on the tooth “saw blades” of Edestus, a late Paleozoic chondrichthyan. Mosasaur, 7:9-18.
Zangerl, R. and Richardson, E.S., Jr. 1963. The paleoecological history of two Pennsylvanian black shales. Fieldiana: Geology, 4:1-352. https://doi.org/10.5962/bhl.title.7199

