DESCRIPTION
Braincase
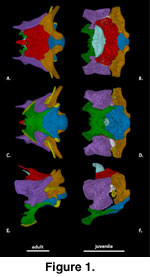 General Features of the
Braincase (Figure 1). The description of the individual braincase elements
provided here is based primarily on the adult specimens. Some observations on
the juveniles are also referenced in the description, but a summary of our
observations on ontogenetic differences in braincase features is provided in the
discussion. The ossified braincase of S. crocodilurus, like most
lizards, consists of an orbitotemporal region represented by the paired
orbitosphenoids and an otooccipital region consisting of a midline anteroventral
sphenoid (a composite element that includes the chondrocranial basisphenoid and
dermatocranial parasphenoid), a midline posteroventral basioccipital, a midline
dorsal supraoccipital, paired anterior prootics, and paired posterior
otooccipitals (opisthotic and exoccipital). The otooccipital elements fuse into
a single unit in the adult (Zhang 1991). The fusion of the opisthotic and
exoccipital occurs prenatally in most squamates (Estes et al. 1988;
Maisano
2001) but are partially separated in some juvenile S. crocodilurus.
The length of the braincase relative to the total length of the skull is similar
in both the juvenile and adult specimens we scanned (Table 1).
General Features of the
Braincase (Figure 1). The description of the individual braincase elements
provided here is based primarily on the adult specimens. Some observations on
the juveniles are also referenced in the description, but a summary of our
observations on ontogenetic differences in braincase features is provided in the
discussion. The ossified braincase of S. crocodilurus, like most
lizards, consists of an orbitotemporal region represented by the paired
orbitosphenoids and an otooccipital region consisting of a midline anteroventral
sphenoid (a composite element that includes the chondrocranial basisphenoid and
dermatocranial parasphenoid), a midline posteroventral basioccipital, a midline
dorsal supraoccipital, paired anterior prootics, and paired posterior
otooccipitals (opisthotic and exoccipital). The otooccipital elements fuse into
a single unit in the adult (Zhang 1991). The fusion of the opisthotic and
exoccipital occurs prenatally in most squamates (Estes et al. 1988;
Maisano
2001) but are partially separated in some juvenile S. crocodilurus.
The length of the braincase relative to the total length of the skull is similar
in both the juvenile and adult specimens we scanned (Table 1).
The braincase as a unit
articulates with the pterygoids via the basipterygoid processes of the sphenoid,
the epipterygoids via the alar processes of the prootics, the parietals via the
ascending process of the tectum synoticum (see Supraoccipital), the squamosals
via the paraoccipital processes (see Otooccipital), and the vertebral column via
the occipital condyle and the flexor and extensor musculature of the neck (Oelrich
1956).
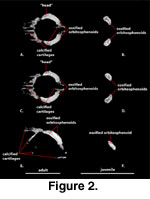 Orbitosphenoid (Figure 2).
Because of their relatively small size and lack of contact with other ossified
elements, the orbitosphenoids are often lost or overlooked in standard skeletal
preparations. The orbitosphenoids of S. crocodilurus were not
studied or described by
McDowell and Bogert (1954),
Wu and Huang (1986),
Zhang
(1991), or Conrad (2004). The presence of orbitosphenoids in Xenosaurus
is subject to variation, with bilateral asymmetry reported in some specimens
(Evans, personal commun., 2004). CT data sets reveal their presence in S.
crocodilurus and document that they are well developed even in the juvenile.
Orbitosphenoid (Figure 2).
Because of their relatively small size and lack of contact with other ossified
elements, the orbitosphenoids are often lost or overlooked in standard skeletal
preparations. The orbitosphenoids of S. crocodilurus were not
studied or described by
McDowell and Bogert (1954),
Wu and Huang (1986),
Zhang
(1991), or Conrad (2004). The presence of orbitosphenoids in Xenosaurus
is subject to variation, with bilateral asymmetry reported in some specimens
(Evans, personal commun., 2004). CT data sets reveal their presence in S.
crocodilurus and document that they are well developed even in the juvenile.
The paired orbitosphenoids,
which ossify from the embryonic pila metoptica and form the orbitotemporal
portion of the osseous braincase (Bellairs and Kamal 1981), lie anterior and
medial to the epipterygoids in a position that approximates the posterior margin
of the orbits. The orbitosphenoids consist of a pair of mediolaterally
flattened, rod-like bones that are oriented anterodorsally-posteroventrally, but
whose dorsal ends lie distinctly anterior and lateral to their ventral ends. A
convex posterodorsal margin, a concave anteroventral margin, and an
anteroposteriorly expanded dorsal ‘head’ give these bones a costiform appearance
when viewed as isolated elements (Figure 2A, C). They are approximately
symmetrical in size and shape. Several of the calcified cartilages that support
the membranous braincase are visible in the CT data set of the adult specimen (FMNH
215541).
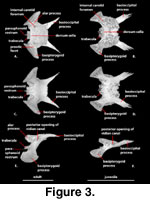 Sphenoid (Figure 3). The
sphenoid is an irregularly shaped compound bone that is composed of the chondrocranial basisphenoid underlain and tightly fused to the dermatocranial
parasphenoid. The sphenoid forms strong sutural contacts with the prootic
anterodorsally and the basioccipital posteriorly. The suture with the
basioccipital is transversely oriented medially and extends posterolaterally
along a pair of elongated basioccipital processes (‘triangular arms’ of
McDowell
and Bogert 1954) of the sphenoid that run along the lateral margin of the basioccipital and contribute to the anterior margins of the basal tubera
(‘muscular tuberosity’ of
McDowell
and Bogert 1954; ‘spheno-occipital tubercles’
of Oelrich 1956). This contribution to the basal tubera was considered a
synapomorphy of Xenosaurus + S. crocodilurus by
Rieppel
(1980). These processes account for nearly one-half of the total length of the
sphenoid and reach a point that is nearly level with the posterior margin of the fenestra ovalis
(McDowell
and Bogert 1954).
Sphenoid (Figure 3). The
sphenoid is an irregularly shaped compound bone that is composed of the chondrocranial basisphenoid underlain and tightly fused to the dermatocranial
parasphenoid. The sphenoid forms strong sutural contacts with the prootic
anterodorsally and the basioccipital posteriorly. The suture with the
basioccipital is transversely oriented medially and extends posterolaterally
along a pair of elongated basioccipital processes (‘triangular arms’ of
McDowell
and Bogert 1954) of the sphenoid that run along the lateral margin of the basioccipital and contribute to the anterior margins of the basal tubera
(‘muscular tuberosity’ of
McDowell
and Bogert 1954; ‘spheno-occipital tubercles’
of Oelrich 1956). This contribution to the basal tubera was considered a
synapomorphy of Xenosaurus + S. crocodilurus by
Rieppel
(1980). These processes account for nearly one-half of the total length of the
sphenoid and reach a point that is nearly level with the posterior margin of the fenestra ovalis
(McDowell
and Bogert 1954).
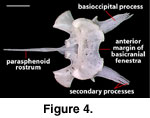 The distal ends of the basioccipital
processes of NAUQSP 17563 and TNHC 629987 have secondary, fingerlike processes
(Figure 4) that are absent in FMNH 215541 and asymmetrically reduced (right) or
lost (left) in MVZ 204291.
The distal ends of the basioccipital
processes of NAUQSP 17563 and TNHC 629987 have secondary, fingerlike processes
(Figure 4) that are absent in FMNH 215541 and asymmetrically reduced (right) or
lost (left) in MVZ 204291.
A distinct unossified area at
the sphenoid-basioccipital suture (basicranial fontanelle) was noted by
McDowell
and Bogert (1954) on AMNH 44928 (Rieppel 1980, figure 20).
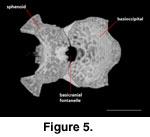 It is absent in FMNH
215541 and MVZ 204291, where the region is fully ossified and only a shallow
dimple is present on the ventral side of the suture. A large and distinct
basicranial fontanelle is present along the sphenoid-basioccipital suture in
TNHC 62987 (Figure 5). When the disarticulated sphenoid and basioccipital of
NAUQSP 17563 are articulated, the fontanelle also clearly is present; the
disarticulated elements clearly preserve the margins (Figure 4). Based on figure
20 of Rieppel (1980) and its associated scale bar, the condylobasal length of
AMNH 44928 is between 22 and 23 mm, which is considerably shorter than the same
measurement in FMNH 2155541 (31.25 mm) and MVZ 204291 (32.7 mm). The fact that
most authors who commented on the skull morphology of S. crocodilurus
were utilizing skeletally immature specimens was noted by
Conrad (2004). It is
possible that absence of the fontanelle can be used to mark skeletally mature
specimens; if so, skeletal maturity is reached between 23 mm and 32 mm.
It is absent in FMNH
215541 and MVZ 204291, where the region is fully ossified and only a shallow
dimple is present on the ventral side of the suture. A large and distinct
basicranial fontanelle is present along the sphenoid-basioccipital suture in
TNHC 62987 (Figure 5). When the disarticulated sphenoid and basioccipital of
NAUQSP 17563 are articulated, the fontanelle also clearly is present; the
disarticulated elements clearly preserve the margins (Figure 4). Based on figure
20 of Rieppel (1980) and its associated scale bar, the condylobasal length of
AMNH 44928 is between 22 and 23 mm, which is considerably shorter than the same
measurement in FMNH 2155541 (31.25 mm) and MVZ 204291 (32.7 mm). The fact that
most authors who commented on the skull morphology of S. crocodilurus
were utilizing skeletally immature specimens was noted by
Conrad (2004). It is
possible that absence of the fontanelle can be used to mark skeletally mature
specimens; if so, skeletal maturity is reached between 23 mm and 32 mm.
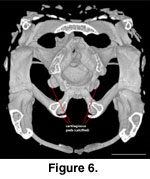 Anteroventrally the sphenoid
participates in the synovial palatobasal articulation with the pterygoids via
the well-developed basipterygoid processes (McDowell
and Bogert 1954). Pads of
calcified cartilage cover the distal surface of the basipterygoid processes in
the adult (Figure 6).
Anteroventrally the sphenoid
participates in the synovial palatobasal articulation with the pterygoids via
the well-developed basipterygoid processes (McDowell
and Bogert 1954). Pads of
calcified cartilage cover the distal surface of the basipterygoid processes in
the adult (Figure 6).
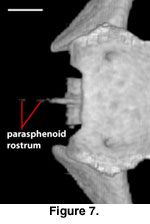
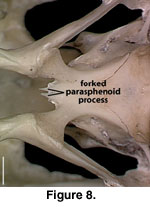
A distinct and well-ossified
parasphenoid rostrum is present in NAUQSP 17563 (Figure 4). A distinct process
also is discernible in the digital data set of FMNH 215541, but the bone was
thin enough that the CT renderings show gaps in part of the rostrum (Figure 7).
A well-developed rostrum was also depicted by
Zhang (1991, figures 5, 6). No
distinct parasphenoid rostrum is preserved on MVZ 204291; instead a short,
forked rostrum is present, with each arm of the fork situated beneath the medial
side of the trabeculae (Figure 8). A short, unforked rostrum that extends beyond
the anterior margin of the basipterygoid processes was illustrated by
Conrad
(2004, figure 14B). The range of variation in expression of the parasphenoid
rostrum in S. crocodilurus is unknown.
 Paired vidian canals extend
through the sphenoid, their margins formed by the basisphenoid (above) and
parasphenoid (below;
Bellairs and Kamal 1981). The anterior openings of these
canals are positioned ventrolaterally and just posterior to the bases of the
trabeculae cranii, and transmit the palatine artery and palatine branch of the
facial nerve (cranial nerve VII). From its anterior opening, the vidian canal
extends posterodorsally along a straight path that parallels and lies lateral to
the trabecula. At a position just posterior to the trabecula, the vidian canal
splits sending a short canal medially. This split is the osseous signature of
the bifurcation of the internal carotid artery from which an anterior branch
(palatine artery) and medial branch (cerebral carotid artery) are derived. The
position of this split is close enough to the pituitary fossa that the ‘canal’
communicating the cerebral carotid artery to the floor of the fossa is extremely
short. Behind this bifurcation, the vidian canal continues posterodorsally and
slightly laterally before opening onto the lateral wall of the braincase.
Paired vidian canals extend
through the sphenoid, their margins formed by the basisphenoid (above) and
parasphenoid (below;
Bellairs and Kamal 1981). The anterior openings of these
canals are positioned ventrolaterally and just posterior to the bases of the
trabeculae cranii, and transmit the palatine artery and palatine branch of the
facial nerve (cranial nerve VII). From its anterior opening, the vidian canal
extends posterodorsally along a straight path that parallels and lies lateral to
the trabecula. At a position just posterior to the trabecula, the vidian canal
splits sending a short canal medially. This split is the osseous signature of
the bifurcation of the internal carotid artery from which an anterior branch
(palatine artery) and medial branch (cerebral carotid artery) are derived. The
position of this split is close enough to the pituitary fossa that the ‘canal’
communicating the cerebral carotid artery to the floor of the fossa is extremely
short. Behind this bifurcation, the vidian canal continues posterodorsally and
slightly laterally before opening onto the lateral wall of the braincase.
 The posterior opening of the
vidian canal lies fully within the sphenoid, and is positioned a short distance
below and behind the suture of the alar process of the sphenoid with the
anterior inferior process of the prootic. Our
observations are consistent with those of
Conrad (2004) for the position of the
posterior opening in the sphenoid, but the dorsal border of the posterior
opening of the vidian canal was described as being formed by the prootic by
McDowell and Bogert (1954). The fact that this was likely in error was noted by
Underwood (1957), but S. crocodilurus was scored as having the
posterior opening of the vidian canal on the sphenoid-prootic suture by
Estes et
al. (1988, p. 223; see comment associated with synapomorphy 1 under Anguidae).
That scoring may be based on the description provided by
McDowell
and Bogert (1954); alternatively, the position of this opening is polymorphic for S.
crocodilurus. These conflicting observations also may be due to the
complex configuration of the articulated skull in this region. The posterior
opening of the vidian canal is immediately dorsal to the posterior basioccipital
process of the sphenoid. This process obscures the sutural contacts of the
basioccipital, prootic, and sphenoid, and in the articulated skull the dorsal
border of the posterior vidian canal may appear superficially to be formed by
the prootic (Conrad 2004). The posterior opening of the vidian canal was
reported to be subject to individual variation across ontogenetic stages in some
lacertid lizards by
Barahona and Barbadillo (1998).
The posterior opening of the
vidian canal lies fully within the sphenoid, and is positioned a short distance
below and behind the suture of the alar process of the sphenoid with the
anterior inferior process of the prootic. Our
observations are consistent with those of
Conrad (2004) for the position of the
posterior opening in the sphenoid, but the dorsal border of the posterior
opening of the vidian canal was described as being formed by the prootic by
McDowell and Bogert (1954). The fact that this was likely in error was noted by
Underwood (1957), but S. crocodilurus was scored as having the
posterior opening of the vidian canal on the sphenoid-prootic suture by
Estes et
al. (1988, p. 223; see comment associated with synapomorphy 1 under Anguidae).
That scoring may be based on the description provided by
McDowell
and Bogert (1954); alternatively, the position of this opening is polymorphic for S.
crocodilurus. These conflicting observations also may be due to the
complex configuration of the articulated skull in this region. The posterior
opening of the vidian canal is immediately dorsal to the posterior basioccipital
process of the sphenoid. This process obscures the sutural contacts of the
basioccipital, prootic, and sphenoid, and in the articulated skull the dorsal
border of the posterior vidian canal may appear superficially to be formed by
the prootic (Conrad 2004). The posterior opening of the vidian canal was
reported to be subject to individual variation across ontogenetic stages in some
lacertid lizards by
Barahona and Barbadillo (1998).
The recessus vena jugularis
(Oelrich 1956) extends as a prominent groove from the posterior opening of the vidian canal posterodorsally and marks the path of the internal carotid artery,
the lateral head vein, and the vidian branch of the facial nerve (Oelrich 1956).
This groove extends only a short distance in the sphenoid with most of its
length occurring in the prootic beneath the crista prootica (see
Prootic).
The sella turcica, which houses
the hypophysis of the pituitary body, is deep but obscured in dorsal view by the
well-developed and widely overhanging dorsum sella. This overhang is especially
prominent laterally where distinct clinoid processes are drawn anteriorly. At
their greatest dorsal extent, the clinoid processes of the sphenoid fail to
contribute to the incisura prootica, the osseous margin of which is formed
completely by the prootic. A large canal housing the abducens nerve (VI)
penetrates each side of the dorsum sella along a straight, posterodorsal path.
These canals are positioned laterally with their anterior openings emerging
beneath the clinoid processes into rather deep lateral pockets. These lateral
pockets are more deeply invaginated than those in most other lizards (e.g.,
Chamaeleo, Stellio, Acanthasaura, Sauromalus, Iguana,
Sceloporus, Eublepharis, Mabuya, Platysaurus,
Gerrhosaurus, Tupinambis, Proctoporus, Elgaria,
Anguis, Ophisaurus, Varanus, and Heloderma), but are
approximately equivalent to those in an adult Xenosaurus grandis
(MVZ 128947). Some specimens of Cordylus also approach this condition.
Each pocket is separated from the pituitary fossa by the crista trabecularis, a
posterior extension of the trabecula cranii.
In the floor of the pituitary
fossa, directly posterior and medial to the trabeculae of the basisphenoid, is a
pair of foramina that transmit the dorsal branch of the internal carotid artery
(cerebral carotid artery). These foramina are smaller than the anterior openings
of the vidian canal and closely approximate the sagittal midline of the sella
turcica. The position of carotid foramina within the sella turcica is variable
in other anguimorphs (e.g., Varanus;
Rieppel and Zaher 2000).
The sella turcica narrows
anteriorly where the sphenoid forms a short rostrum. The basisphenoid
contribution to this rostrum (ossified trabeculae communis-tropibasic skull) is
easily discernible from the underlying parasphenoid, which extends further
anteriorly.
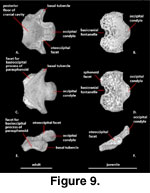 Basioccipital (Figure 9). The basioccipital ossifies within the posterior portion of the embryonic basal plate
and forms the posterior floor of the ossified braincase. The basioccipital
contacts the otooccipitals dorsally, the sphenoid anteriorly and
anterolaterally, and the prootics anterodorsally. In juvenile specimens, the
posterior margin of the large basicranial fontanelle is formed by the
basioccipital (Figure 5). Two prominent basal tubera extend from the
posterolateral margin. These tubercles receive the insertion of the longus
capitis muscles (Rieppel and Zaher 2000). The CT data set of the adult
clearly reveals the presence of calcified cartilages on the distal surfaces of
the basal tubera (Figure 6), but these are not visible in the juvenile.
Basioccipital (Figure 9). The basioccipital ossifies within the posterior portion of the embryonic basal plate
and forms the posterior floor of the ossified braincase. The basioccipital
contacts the otooccipitals dorsally, the sphenoid anteriorly and
anterolaterally, and the prootics anterodorsally. In juvenile specimens, the
posterior margin of the large basicranial fontanelle is formed by the
basioccipital (Figure 5). Two prominent basal tubera extend from the
posterolateral margin. These tubercles receive the insertion of the longus
capitis muscles (Rieppel and Zaher 2000). The CT data set of the adult
clearly reveals the presence of calcified cartilages on the distal surfaces of
the basal tubera (Figure 6), but these are not visible in the juvenile.
The basioccipital does not
contribute to the floor or the medial aperture of the recessus scala tympani. It
forms the ventral margin of the foramen magnum and the middle portion of the
occipital condyle. The occipital condyle is concave dorsally in posterior view
(Conrad 2004). The dorsal surface of the basioccipital houses a shallow
depression just anterior to the basal tubercle. That depression is confluent
with a triangular space formed between the crista interfenestralis and the
crista tuberalis in the otooccipital that terminates at the lateral aperture of
the recessus scala tympani (see Otooccipital). Near the junction of the
basioccipital, otooccipital, and prootic the dorsal surface of the basioccipital
shows another shallow depression that marks the bottom of the lagenar recess,
the ventral terminus of the cavum cochleare (Wever 1978).
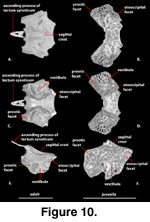 Supraoccipital (Figure 10). The supraoccipital forms the roof of the ossified braincase and the dorsal margin of
the foramen magnum. It contacts the otooccipital posteroventrally and the
prootic anteroventrally. The ossified base of the ascending process of the
tectum synoticum is present and contacts the parietal (McDowell
and Bogert 1954). The ascending process is heavily calcified in the adult but lacks a solid
contact with the main body of the supraoccipital (Figure 10A). A weakly
developed sagittal crest extends along the dorsal midline (McDowell
and Bogert 1954), declining in prominence posteriorly. The supraoccipital forms the top of
the otic chamber, housing the canals for the anterior and posterior semicircular
ducts as well as the osseous common crus where the two canals join within the
otic capsule (see Inner Ear).
Supraoccipital (Figure 10). The supraoccipital forms the roof of the ossified braincase and the dorsal margin of
the foramen magnum. It contacts the otooccipital posteroventrally and the
prootic anteroventrally. The ossified base of the ascending process of the
tectum synoticum is present and contacts the parietal (McDowell
and Bogert 1954). The ascending process is heavily calcified in the adult but lacks a solid
contact with the main body of the supraoccipital (Figure 10A). A weakly
developed sagittal crest extends along the dorsal midline (McDowell
and Bogert 1954), declining in prominence posteriorly. The supraoccipital forms the top of
the otic chamber, housing the canals for the anterior and posterior semicircular
ducts as well as the osseous common crus where the two canals join within the
otic capsule (see Inner Ear).
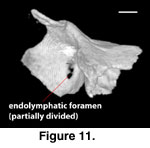 The opening for the
endolymphatic duct as it passes from the otic chamber is exposed in medial view
and lies completely within the supraoccipital. At its posterior edge, the margin
of the foramen is not sharply defined, but rather is formed by the angled slope
of the posterior portion of the tympanic bulla. The foramen appears to be
partially divided in FMNH 215541 (Figure 11). The overall appearance is of a
canal oriented anteroventrally to posterodorsally. Calcified endolymph is
present in both scanned specimens and appears in the CT data sets as bright
white masses lying along the ventral margin of the supraoccipital. In our
colored renderings of the braincase the endolymph appears in pale blue (Figure
1G, H). In the adult (FMNH 215541) the endolymph appears as approximately
symmetrical, small masses on either side of the bone. In the juvenile (TNHC
62987) the endolymph forms an extensive and somewhat asymmetrical mass that
extends anteriorly approximately one-fifth of the way along the total length of
the parietal.
The opening for the
endolymphatic duct as it passes from the otic chamber is exposed in medial view
and lies completely within the supraoccipital. At its posterior edge, the margin
of the foramen is not sharply defined, but rather is formed by the angled slope
of the posterior portion of the tympanic bulla. The foramen appears to be
partially divided in FMNH 215541 (Figure 11). The overall appearance is of a
canal oriented anteroventrally to posterodorsally. Calcified endolymph is
present in both scanned specimens and appears in the CT data sets as bright
white masses lying along the ventral margin of the supraoccipital. In our
colored renderings of the braincase the endolymph appears in pale blue (Figure
1G, H). In the adult (FMNH 215541) the endolymph appears as approximately
symmetrical, small masses on either side of the bone. In the juvenile (TNHC
62987) the endolymph forms an extensive and somewhat asymmetrical mass that
extends anteriorly approximately one-fifth of the way along the total length of
the parietal.
Prootic (Figure 12). The prootic
is a triradiate bone that ossifies in the anterior half of the otic capsule and
contributes the anterior half of the osseous otic chamber. The prootic contacts
the epipterygoid anterodorsally, the sphenoid anteroventrally, the basioccipital
posteroventrally, the otooccipital posteriorly, and the supraoccipital dorsally.
It does not contact the parietal or the quadrate in any of the specimens
available to us, but was noted to do so by
Conrad (2004) and
Zhang (1991). In
both cases, the prootic closely approaches these bones, but the CT data reveal
no contact.
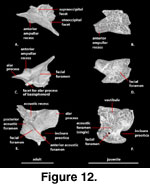 A pair of elongate, anteriorly directed, mediolaterally compressed
dorsal alar processes (‘dorsal anterior wing’ of
McDowell
and Bogert 1954) form
the anteriormost extent of the osseous neurocranium. Elongate, anterodorsally
directed alar processes of the prootic were considered a synapomorphy of
Scleroglossa by
Estes et al. (1988). The alar process of the prootic contacts
the dorsal tip of the epipterygoid and forms the dorsal margin of a prominent
incisura prootica (‘trigeminal notch’ of
McDowell
and Bogert 1954) with
contribution from the anterior ampullar recess (McDowell
and Bogert 1954). The
anterior ampullar recess extends far enough ventrally so that it appears to
partially divide the incisura prootica in lateral view in some specimens. The
anterior inferior process of the prootic forms the ventral margin of the
incisura prootica and contacts the dorsal margin of the sphenoid, but the
sphenoid does not participate in formation of the incisura prootica.
A pair of elongate, anteriorly directed, mediolaterally compressed
dorsal alar processes (‘dorsal anterior wing’ of
McDowell
and Bogert 1954) form
the anteriormost extent of the osseous neurocranium. Elongate, anterodorsally
directed alar processes of the prootic were considered a synapomorphy of
Scleroglossa by
Estes et al. (1988). The alar process of the prootic contacts
the dorsal tip of the epipterygoid and forms the dorsal margin of a prominent
incisura prootica (‘trigeminal notch’ of
McDowell
and Bogert 1954) with
contribution from the anterior ampullar recess (McDowell
and Bogert 1954). The
anterior ampullar recess extends far enough ventrally so that it appears to
partially divide the incisura prootica in lateral view in some specimens. The
anterior inferior process of the prootic forms the ventral margin of the
incisura prootica and contacts the dorsal margin of the sphenoid, but the
sphenoid does not participate in formation of the incisura prootica.
The path of the anterior
semicircular canal is visible as a prominent ridge on the lateral surface of the
prootic in front of the crista prootica and just behind the alar process. The
bone is extremely thin along the path of this canal, and in the 3-D digital
renderings of the juvenile CT dataset the thinnest areas appear as holes in the
bone; this is an artifact of the digital rendering process (see
Methods).
The crista prootica is present
as a prominent crest on the lateral surface of the prootic that extends
posterodorsally from a point above the clinoid processes of the sphenoid to a
point above the fenestra ovalis. This lamina of membrane bone (sensu
Rieppel
1993) is not as well developed in S. crocodilurus (or
Xenosaurus) as it is in Varanus (McDowell
and Bogert 1954; Gauthier
1982), in which it partially obscures the fenestra ovalis when viewed laterally
(Rieppel and Zaher 2000). In both Xenosaurus grandis and S.
crocodilurus, the crest is most extensive (somewhat more so in
Xenosaurus) at its posterodorsal end and is reduced in extent
anteroventrally. A slight raised area along the clinoid process of the sphenoid
near the suture with the prootic is continuous with the crista prootica is some
specimens. The recessus vena jugularis is formed ventral to this crest and
extends from the posterior opening of the vidian canal (in the sphenoid) to the
anterior margin of the fenestra ovalis. It delineates the medial wall of the
portion of the cranioquadrate space that transmits the lateral head vein and the
branches of the facial nerve. As a result of reduction in the crista prootica,
this groove is not as well developed below the lateral facial foramen as it is
above it.
The medial surface of the
prootic contains the acoustic recess, a deep invagination that is penetrated by
a single opening for the facial nerve (VII) and a pair of foramina for the
acoustic (VIII) nerve. The facial foramen is ventrally located and much smaller
than either the anterior or posterior acoustic foramina. The relative size of
the auditory foramina exhibits considerable variation within and between
anguimorph lizard species (Oelrich 1956;
Norell and Gao 1997;
Rieppel and Zaher
2000). The canal housing the facial nerve extends ventrolaterally through the
prootic and exits into the cranioquadrate space via a single foramen. This
lateral opening is positioned within the recessus vena jugularis anteroventral
to the fenestra ovalis, slightly less than halfway between the fenestra ovalis
and the prootic-sphenoid suture.
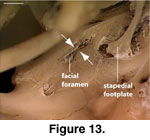 The lateral opening for the
facial nerve was reported to be bifurcated in some anguimorphan squamates (some
Varanus, some mosasaurs;
Rieppel and Zaher
2000) by a strut of bone that
extends across the groove, thus delineating separate paths for the anterior
palatine and posterior hyomandibular branches of the facial nerve. Although none
of our specimens show such a bifurcation, its presence in some S.
crocodilurus was reported by
Conrad (2004) who noted the potential
importance of documenting variation in this feature. In MVZ 204291 a small
extension of the crista prootica extends ventrally towards a bony upgrowth on
the lateral wall of the prootic just anterior to the fenestra ovalis (Figure
13). This helps explain the presence of a bifurcation in some specimens, but
regarding the resultant morphology as a bifurcation of the foramen is somewhat
misleading. We examined two specimens of Varanus exanthematicus
(California Academy of Sciences [CAS] 228520 and CAS 228521) and in both the
facial nerve exits the braincase via a normal, single foramen. The appearance of
a ‘bifurcation’ in the adult is a result only of the meeting of a ventral
downgrowth of the crista prootica with a dorsally directed flange of bone from
the lateral wall of the prootic, clearly visible in the younger specimen (CAS
228521). The eventual fusion of the two flanges of bone creates a canal,
positioned lateral to the facial foramen proper. It is through this canal that
the two branches of the facial nerve pass. This is similar to the development of
an alisphenoid canal (or alar canal) in some carnivoran mammals in which a strut
of bone obscures the foramen rotundum in lateral view (e.g., Canis;
Evans
1993).
The lateral opening for the
facial nerve was reported to be bifurcated in some anguimorphan squamates (some
Varanus, some mosasaurs;
Rieppel and Zaher
2000) by a strut of bone that
extends across the groove, thus delineating separate paths for the anterior
palatine and posterior hyomandibular branches of the facial nerve. Although none
of our specimens show such a bifurcation, its presence in some S.
crocodilurus was reported by
Conrad (2004) who noted the potential
importance of documenting variation in this feature. In MVZ 204291 a small
extension of the crista prootica extends ventrally towards a bony upgrowth on
the lateral wall of the prootic just anterior to the fenestra ovalis (Figure
13). This helps explain the presence of a bifurcation in some specimens, but
regarding the resultant morphology as a bifurcation of the foramen is somewhat
misleading. We examined two specimens of Varanus exanthematicus
(California Academy of Sciences [CAS] 228520 and CAS 228521) and in both the
facial nerve exits the braincase via a normal, single foramen. The appearance of
a ‘bifurcation’ in the adult is a result only of the meeting of a ventral
downgrowth of the crista prootica with a dorsally directed flange of bone from
the lateral wall of the prootic, clearly visible in the younger specimen (CAS
228521). The eventual fusion of the two flanges of bone creates a canal,
positioned lateral to the facial foramen proper. It is through this canal that
the two branches of the facial nerve pass. This is similar to the development of
an alisphenoid canal (or alar canal) in some carnivoran mammals in which a strut
of bone obscures the foramen rotundum in lateral view (e.g., Canis;
Evans
1993).
The acoustic foramina are
situated within the acoustic recess and are positioned dorsal to the facial
foramen. The anterior acoustic foramen is single, not paired as described in
Ctenosaura pectinata (Oelrich 1956). It lies above and slightly
anterior to the facial foramen, whereas the posterior acoustic foramen lies
distinctly behind and dorsal to both the facial and anterior acoustic foramen.
The anterior acoustic foramen is slightly smaller than the posterior acoustic
foramen, but both are distinctly larger than the facial foramen. The posterior
foramen opens directly into the vestibule of the cavum capsularis of the inner
ear. The anterior foramen pierces the ventromedial wall of the anterior ampullar
recess and transmits vestibular branches of the vestibulocochlear nerve (VIII)
to the semicircular canals.
The fenestra ovalis (‘foramen
ovale’ of Oelrich 1956; ‘fenestra vestibuli’ of
Jollie 1960) is a subelliptical
opening whose anterior margin is formed by the prootic and posterior margin by
the otooccipital. It is distinctly asymmetrical in S. crocodilurus,
with more than half of its diameter enclosed by the otooccipital. The fenestra
ovalis is positioned above and anterior to the small lateral aperture of the
recessus scala tympani. The fenestra also is anterior to the tip of the basal
tubercle, which is in direct line ventral to the recessus scala tympani (in the
plesiomorphic condition for Squamata the fenestra ovalis is in direct line
dorsal to the tip of the basal tubercle [Norell and Gao 1997]; the relative
shift in position of the fenestra ovalis in S. crocodilurus is
shared with mosasaurs, but Varanus exhibits the plesiomorphic condition).
Ventral to the fenestra ovalis,
the prootic and otooccipital are in contact again, forming, respectively, the
anterior and posterior margins of the cavum cochleare. This cavity takes the
form of an inverted pyramid, with the apex oriented ventrally. The ventral
extremity (lagenar recess) houses the lagena of the ear (Wever 1978).
The protractor pterygoidei,
pseudotemporalis profundus, pseudotemporalis superior, and the
3c-layer of the adductor mandibulae externus all take their origin (at
least in part) from the lateral surface of the prootic (Haas 1960;
Rieppel 1980). The origin of the protractor pterygoidei lies largely along the
lower margin of the incisura prootica and includes the inferior process of the
prootic and the alar process of the sphenoid (some fibers continue along a
tendon to take their origin on the posterodorsal edge of the basipterygoid
process; Rieppel 1980). The pseudotemporalis profundus originates
largely from the epipterygoid with a posterior continuation of fibers onto the
ventral edge of the alar process of the prootic (Haas 1960;
Rieppel 1980). The
m. pseudotemporalis superficialis (generally given as m.
pseudotemporalis superior by
Haas [1960], but see p. 29 of his paper)
also originates from the alar process of the prootic, although from a more
dorsal position just behind the contact surface for the epipterygoid, as well as
from the ligamentous attachment of the alar process of the prootic to the
descending process of the parietal (Rieppel 1980). The positioning in S.
crocodilurus of the points of origin for the m.
pseudotemporalis superficialis was considered to be derived by
Rieppel (1980). The 3c-layer of the m. adductor mandibulae
externus takes its origin on the alar process and crista prootica
(Rieppel 1980).
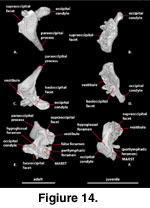 Otooccipital (Figure 14). The otooccipital is formed from a fusion between the opisthotic and exoccipital.
This fusion generally occurs during prenatal ontogeny and is considered a
synapomorphy of Squamata (Estes et al. 1988;
Maisano 2001). The original
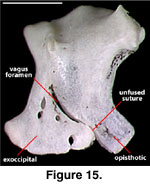
separation of the two elements is marked by the position of the vagus foramen
(Figure 15; Conrad [2004:421] erroneously stated that the vagus foramen lies at
the suture of the supraoccipital and the otooccipital, but his figure 15C
accurately depicts and labels the vagus foramen). The bones are already fused in
one juvenile specimen (TNHC 629987), but in NAUQSP 17563 the separation of the
two elements is distinct ventrally (as it is in some hatchling lacertids and
xantusiids; Rieppel 1992;
Maisano 2001,
2002;
Figure 15).
The otooccipital is sutured to the prootic anteriorly, the supraoccipital
dorsally, and the basioccipital ventromedially. Posterolaterally it contacts
(via connective tissues) the quadrate, supraoccipital, and the postparietal
process of the parietal. It does not contact the squamosal. The otooccipitals
contribute the lateral and dorsolateral portions of the occipital condyle, form
the lateral margins of the foramen magnum, and contribute substantially to the
posterior braincase via strongly developed lateral extensions, the paraoccipital
processes. The otooccipital forms more than half of the diameter of the fenestra
ovalis.
Otooccipital (Figure 14). The otooccipital is formed from a fusion between the opisthotic and exoccipital.
This fusion generally occurs during prenatal ontogeny and is considered a
synapomorphy of Squamata (Estes et al. 1988;
Maisano 2001). The original
separation of the two elements is marked by the position of the vagus foramen
(Figure 15; Conrad [2004:421] erroneously stated that the vagus foramen lies at
the suture of the supraoccipital and the otooccipital, but his figure 15C
accurately depicts and labels the vagus foramen). The bones are already fused in
one juvenile specimen (TNHC 629987), but in NAUQSP 17563 the separation of the
two elements is distinct ventrally (as it is in some hatchling lacertids and
xantusiids; Rieppel 1992;
Maisano 2001,
2002;
Figure 15).
The otooccipital is sutured to the prootic anteriorly, the supraoccipital
dorsally, and the basioccipital ventromedially. Posterolaterally it contacts
(via connective tissues) the quadrate, supraoccipital, and the postparietal
process of the parietal. It does not contact the squamosal. The otooccipitals
contribute the lateral and dorsolateral portions of the occipital condyle, form
the lateral margins of the foramen magnum, and contribute substantially to the
posterior braincase via strongly developed lateral extensions, the paraoccipital
processes. The otooccipital forms more than half of the diameter of the fenestra
ovalis.
 Anteriorly the otooccipital
contacts the prootic above and below the fenestra ovalis. The dorsal
articulation facet between the two bones is pierced by the opening of the
horizontal semicircular canal. The ventral articulation between the two bones is
less expansive and forms the margin of the lagenar recess. On the lateral
surface of the otooccipital just posterior and posteroventral to the fenestra
ovalis is a broad, low ridge of bone, the crista interfenestralis (Säve-Söderbergh
1947). Its expression is reduced anteroventrally and does not extend as far as
the prootic-otooccipital suture. Posterodorsally it merges with a sharply
defined crest, the crista tuberalis (Säve-Söderbergh
1947) that runs
approximately dorsoventrally from the ventral surface of the paroccipital
process to the dorsal margin of the basal tubercle. These two crests delimit a
roughly triangular space near the dorsal apex of which is situated the small
lateral aperture of the recessus scala tympani (LARST; ‘foramen rotundum’ of
Conrad 2004). Immediately posterior to the crista tuberalis the otooccipital is
pierced by three foramina. The most dorsal of these is a crescent-shaped vagus
foramen transmitting cranial nerve X (according to
Hu [1980] the accessory nerve
[XI] merges with the vagus as soon as it leaves the brain). Ventral to this are
two small hypoglossal foramina transmitting branches of cranial nerve XII. A
third hypoglossal foramen is present posterior to these at approximately a level
just ventral to the base of the vagus foramen.
Anteriorly the otooccipital
contacts the prootic above and below the fenestra ovalis. The dorsal
articulation facet between the two bones is pierced by the opening of the
horizontal semicircular canal. The ventral articulation between the two bones is
less expansive and forms the margin of the lagenar recess. On the lateral
surface of the otooccipital just posterior and posteroventral to the fenestra
ovalis is a broad, low ridge of bone, the crista interfenestralis (Säve-Söderbergh
1947). Its expression is reduced anteroventrally and does not extend as far as
the prootic-otooccipital suture. Posterodorsally it merges with a sharply
defined crest, the crista tuberalis (Säve-Söderbergh
1947) that runs
approximately dorsoventrally from the ventral surface of the paroccipital
process to the dorsal margin of the basal tubercle. These two crests delimit a
roughly triangular space near the dorsal apex of which is situated the small
lateral aperture of the recessus scala tympani (LARST; ‘foramen rotundum’ of
Conrad 2004). Immediately posterior to the crista tuberalis the otooccipital is
pierced by three foramina. The most dorsal of these is a crescent-shaped vagus
foramen transmitting cranial nerve X (according to
Hu [1980] the accessory nerve
[XI] merges with the vagus as soon as it leaves the brain). Ventral to this are
two small hypoglossal foramina transmitting branches of cranial nerve XII. A
third hypoglossal foramen is present posterior to these at approximately a level
just ventral to the base of the vagus foramen.
 The LARST is reduced in size
relative to that of most other lizards. The medial extension of this space is
similar to the normal condition for lizards, and a well-developed and fairly
large medial aperture (MARST) is present. The recessus scala tympani appears to
be contained entirely within the otooccipital in the CT data sets, although the
basioccipital closely approaches the ventral margin. The MARST can be seen in an
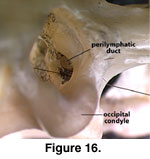
oblique posterolateral view looking through the foramen magnum. Just lateral to
that opening, the perilymphatic foramen opens into the recessus from the
vestibule. In the view through the foramen magnum (Figure 16), the two openings
are essentially indistinguishable, a situation also found in other lizards
(e.g., see caption to figure 11 in
Bell et al. 2003:295). The perilymphatic
foramen is positioned just ventral to an anteriorly projecting ledge of bone at
the base of the vestibule that marks the recess for the ampulla of the posterior
semicircular canal. Just dorsal to the vestibule, the articulation facet for the
supraoccipital contains a penetration marking the passage of the posterior
semicircular canal.
The LARST is reduced in size
relative to that of most other lizards. The medial extension of this space is
similar to the normal condition for lizards, and a well-developed and fairly
large medial aperture (MARST) is present. The recessus scala tympani appears to
be contained entirely within the otooccipital in the CT data sets, although the
basioccipital closely approaches the ventral margin. The MARST can be seen in an
oblique posterolateral view looking through the foramen magnum. Just lateral to
that opening, the perilymphatic foramen opens into the recessus from the
vestibule. In the view through the foramen magnum (Figure 16), the two openings
are essentially indistinguishable, a situation also found in other lizards
(e.g., see caption to figure 11 in
Bell et al. 2003:295). The perilymphatic
foramen is positioned just ventral to an anteriorly projecting ledge of bone at
the base of the vestibule that marks the recess for the ampulla of the posterior
semicircular canal. Just dorsal to the vestibule, the articulation facet for the
supraoccipital contains a penetration marking the passage of the posterior
semicircular canal.
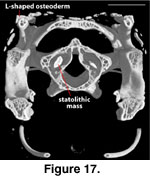 In the CT data sets each
vestibule is partly filled with a dense statolithic mass (Wever 1978) that
appear as a bright white mass in the digital data sets. In the adult the statolithic mass is larger on the right side than on the left (Figure 17).
In the CT data sets each
vestibule is partly filled with a dense statolithic mass (Wever 1978) that
appear as a bright white mass in the digital data sets. In the adult the statolithic mass is larger on the right side than on the left (Figure 17).
The paroccipital process forms
the posterior portion of the lateral braincase and the ventral margin of the
post-temporal fenestra. In the adult the lateral edge of the paroccipital
process bears a dorsally oriented process with a slight medial inflection at its
dorsal tip. This process contacts the supratemporal bone and the postparietal
process of the parietal. At its lateral most edge, the paroccipital process
contacts the cephalic condyle of the quadrate.
Cephalic Osteoderms
The presence of osteoderms in
S. crocodilurus was noted previously by several authors (e.g.,
Borsuk-Bialynicka 1986;
Wu and Huang 1986;
Zhao et al. 1999;
Hofmann 2000), but
detailed discussion of their distribution and general morphology is lacking. The
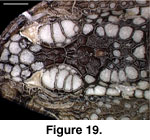
cephalic osteoderms in S. crocodilurus (Figure 18) do not contact
each other and are not compound. They are not fused to the skull, and even in
relatively large individuals they will pull free with the skin (the preserved
skin of MVZ 204291 provides a clear view of the ventral surface of the cephalic
osteoderms; Figure 19).
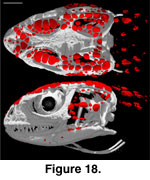 They are concentrated along the dorsolateral margin of
the head, with more limited distribution in the temporal region and neck and
along the lower jaw. Osteoderms are mostly absent from the dorsomedial and
ventral aspects of the head. They are generally flat, plate-like structures with
irregular margins and vary greatly in size, the largest being those that roof
the orbit.
They are concentrated along the dorsolateral margin of
the head, with more limited distribution in the temporal region and neck and
along the lower jaw. Osteoderms are mostly absent from the dorsomedial and
ventral aspects of the head. They are generally flat, plate-like structures with
irregular margins and vary greatly in size, the largest being those that roof
the orbit.
The osteoderms in the snout
region exhibit a regular arrangement. Two small, subcircular osteoderms overly
the nasal process of the premaxilla at the midline and an array of larger
osteoderms extend along each nasal/prefrontal and nasal/frontal contact onto the
anterior end of the frontal; together these approximate a posteriorly pointing
arrow. Two smaller osteoderms occur lateral and posterolateral to the tip of
this arrow along each prefrontal/frontal contact.
 Two linear series of osteoderms
roof each orbit. The first runs just lateral to, and parallels the margin of,
the frontal. It consists of eight small, subcircular osteoderms, the most
anterior of which is the largest and overlies the palpebral, and the most
posterior of which is the smallest and lies just anterior to the
postorbitofrontal. The second series of osteoderms roofing the orbit parallels
the first and consists of five osteoderms. The first and last are small and
subcircular, whereas the middle three are plate-like and are the largest
cephalic osteoderms.
Two linear series of osteoderms
roof each orbit. The first runs just lateral to, and parallels the margin of,
the frontal. It consists of eight small, subcircular osteoderms, the most
anterior of which is the largest and overlies the palpebral, and the most
posterior of which is the smallest and lies just anterior to the
postorbitofrontal. The second series of osteoderms roofing the orbit parallels
the first and consists of five osteoderms. The first and last are small and
subcircular, whereas the middle three are plate-like and are the largest
cephalic osteoderms.
Beginning where the
postorbitofrontal clasps the frontoparietal suture, a field of mostly
plate-like, moderately large osteoderms, extends posteriorly over the
supratemporal fenestra and canthal crest, then medially to meet its opposite
dorsal to the posterior margin of the parietal. This field can be broken down
into three approximately linear series of osteoderms. The first series,
consisting of roughly 12 osteoderms, lies along the lateral margin of the
frontoparietal suture and parietal table. It extends over the parietal near the
base of the supratemporal process to meet its opposite, the bilaterally
symmetrical series thereby forming the margins of a U-shaped osteoderm-free zone
above the parietal. The second series, consisting of approximately seven
osteoderms, lies entirely dorsal to the supratemporal fenestra and parallels the
canthal crest. The third series, consisting of six osteoderms, actually clasps
the canthal crest from its anterior margin to just anterior to the supratemporal.
The latter series help to anchor an aponeurosis covering the upper temporal
fenestra (Haas 1960). The posteriormost osteoderm in that series is the largest
of the six, and these osteoderms are uniquely L-shaped in cross-section (Haas
1960:24; Figure 17).
In the temporal region ventral
to the canthal crest is an array of osteoderms that is roughly E-shaped in
left-lateral view. The dorsal arm of the E consists of approximately 11 small,
subcircular osteoderms that parallel the posterior margin of the jugal and the
ventral margin of the canthal crest. The posteriormost of these is the largest
and lies just dorsal to the cephalic condyle of the quadrate. The middle arm of
the E consists of six small to moderately large, plate-like osteoderms extending
from the midpoint of the posterior margin of the jugal to the anterodorsal
corner of the quadrate. The ventral arm of the E consists of approximately three
small, plate-like osteoderms that parallel the posteroventral corner of the
jugal and extend posteriorly.
Only a small number osteoderms
occur along the lower jaw. These include a few small, sub-circular ones lateral
to the jaw near the posterior margin of the dentary, a subcircular one near the
ventromedial margin of the jaw at the level of contact between the palatine and
maxilla, and a few plate-like ones along the dorsolateral margin of the
surangular just behind the coronoid process.
Osteoderms are limited to the
dorsal and dorsolateral aspects of the neck. There is a central field of
approximately six small to moderately large plate-like osteoderms just posterior
to the braincase. This field is bounded by two linear series of large,
plate-like osteoderms that run from the posterior margin of the squamosal
posteromedially beyond the boundary of the CT dataset.
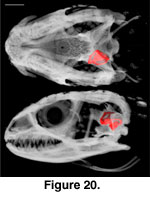 The inner ear cavities of S.
crocodilurus are described, from anterior to posterior and relative to
external landmarks of the braincase, based on a digital endocast derived from
FMNH 215541 (Figure 20,
Figure 21). The anterior semicircular canal emerges from the
anterior ampulla of the anterior ampullar recess in the prootic, near the level
of the origin of the basipterygoid process. It abruptly turns posterodorsally,
continuing to eventually meet the recessus crus communis in the supraoccipital.
At the level of the facial foramen the anterior acoustic foramen, which
transmits the anterior branch of the auditory nerve (Oelrich 1956), opens into
the cranial cavity from the ventromedial wall of the anterior ampullar recess.
The horizontal semicircular canal then emerges from the external ampulla of the
anterior ampullar recess, continuing posteriorly to eventually meet the
posterior ampullar recess in the otooccipital. The anterior ampullar recess then
opens into the vestibule, which houses the statolithic mass, saccule, utricle,
and sinuses (Oelrich 1956).
The inner ear cavities of S.
crocodilurus are described, from anterior to posterior and relative to
external landmarks of the braincase, based on a digital endocast derived from
FMNH 215541 (Figure 20,
Figure 21). The anterior semicircular canal emerges from the
anterior ampulla of the anterior ampullar recess in the prootic, near the level
of the origin of the basipterygoid process. It abruptly turns posterodorsally,
continuing to eventually meet the recessus crus communis in the supraoccipital.
At the level of the facial foramen the anterior acoustic foramen, which
transmits the anterior branch of the auditory nerve (Oelrich 1956), opens into
the cranial cavity from the ventromedial wall of the anterior ampullar recess.
The horizontal semicircular canal then emerges from the external ampulla of the
anterior ampullar recess, continuing posteriorly to eventually meet the
posterior ampullar recess in the otooccipital. The anterior ampullar recess then
opens into the vestibule, which houses the statolithic mass, saccule, utricle,
and sinuses (Oelrich 1956).
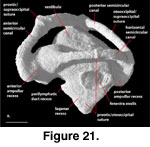 The lagenar recess starts to
differentiate from the ventral portion of the vestibule at the level of the
prootic-otooccipital suture, eventually extending ventrally to the level of the
base of the basal tubercle. This recess houses the lagena and perilymphatic
cistern and duct (Oelrich 1956). Further posteriorly, near the dorsal-most
extension of the supraoccipital, the anterior semicircular canal opens into the
recessus crus communis. At the same level the endolymphatic canal and posterior
acoustic foramen open into the cranial cavity from the dorsomedial and
ventromedial portions of the vestibule, respectively; the latter transmits the
posterior branch of the auditory nerve (Oelrich 1956).
The lagenar recess starts to
differentiate from the ventral portion of the vestibule at the level of the
prootic-otooccipital suture, eventually extending ventrally to the level of the
base of the basal tubercle. This recess houses the lagena and perilymphatic
cistern and duct (Oelrich 1956). Further posteriorly, near the dorsal-most
extension of the supraoccipital, the anterior semicircular canal opens into the
recessus crus communis. At the same level the endolymphatic canal and posterior
acoustic foramen open into the cranial cavity from the dorsomedial and
ventromedial portions of the vestibule, respectively; the latter transmits the
posterior branch of the auditory nerve (Oelrich 1956).
The laterally facing fenestra
ovalis straddles the prootic-otooccipital suture
and is approximately two-thirds filled by the stapedial footplate. The posterior
semicircular canal emerges from the recessus crus communis at the level of the
base of the basal tubercle and continues posteroventrally to meet the posterior
ampullar recess. Just posterior to the level of the recessus crus communis, the
perilymphatic duct recess opens into the cranial cavity from the otooccipital
via the medial aperture of the recessus scala tympani. The posterior ampullar
recess starts to differentiate from the posterior end of the vestibule at the
level of the stapedial footplate and is met by the posterior semicircular canal
just anterior to the base of the occipital condyle.

 General Features of the
Braincase (Figure 1). The description of the individual braincase elements
provided here is based primarily on the adult specimens. Some observations on
the juveniles are also referenced in the description, but a summary of our
observations on ontogenetic differences in braincase features is provided in the
discussion. The ossified braincase of S. crocodilurus, like most
lizards, consists of an orbitotemporal region represented by the paired
orbitosphenoids and an otooccipital region consisting of a midline anteroventral
sphenoid (a composite element that includes the chondrocranial basisphenoid and
dermatocranial parasphenoid), a midline posteroventral basioccipital, a midline
dorsal supraoccipital, paired anterior prootics, and paired posterior
otooccipitals (opisthotic and exoccipital). The otooccipital elements fuse into
a single unit in the adult (Zhang 1991). The fusion of the opisthotic and
exoccipital occurs prenatally in most squamates (Estes et al. 1988;
Maisano
2001) but are partially separated in some juvenile S. crocodilurus.
The length of the braincase relative to the total length of the skull is similar
in both the juvenile and adult specimens we scanned (Table 1).
General Features of the
Braincase (Figure 1). The description of the individual braincase elements
provided here is based primarily on the adult specimens. Some observations on
the juveniles are also referenced in the description, but a summary of our
observations on ontogenetic differences in braincase features is provided in the
discussion. The ossified braincase of S. crocodilurus, like most
lizards, consists of an orbitotemporal region represented by the paired
orbitosphenoids and an otooccipital region consisting of a midline anteroventral
sphenoid (a composite element that includes the chondrocranial basisphenoid and
dermatocranial parasphenoid), a midline posteroventral basioccipital, a midline
dorsal supraoccipital, paired anterior prootics, and paired posterior
otooccipitals (opisthotic and exoccipital). The otooccipital elements fuse into
a single unit in the adult (Zhang 1991). The fusion of the opisthotic and
exoccipital occurs prenatally in most squamates (Estes et al. 1988;
Maisano
2001) but are partially separated in some juvenile S. crocodilurus.
The length of the braincase relative to the total length of the skull is similar
in both the juvenile and adult specimens we scanned (Table 1).


















