| |
THE LIVING PARADIGM: THE PEARLY NAUTILUS
The sole living representatives of the coiled, chambered cephalopod shell, apart from Spirula, are species of Nautilus. With the pearly nautilus as a standard of reference, the temptation to extrapolate thoughts and observations to the extinct group of ammonites is understandable. In the 50 years that have elapsed since the appearance of my monograph on the subject of post-mortem distribution of cephalopod shells (Reyment 1958), I have seen opinions wax and wane until it now seems clear that experts in the field have embraced the view that the general rule must be that dispersal of shells by marine currents is a major factor, notwithstanding that in situ deposition can occur and has no doubt taken place (the "gastropod-mode"). Results reported in
Wani (2004,
2007) and
Wani et al. (2005) have brought to light a possible explanation for the segregation of mature shells from juvenile shells, sometimes observed Recently defunct juvenile shells may become more readily waterlogged than adults and hence could be buried in sediment near to where they lived. This forms the basis of a model, which might be applicable to some ammonite occurrences and would explain the atypical age-pyramid from time to time encountered in shell accumulations. However, the model for age-differentiation reflected in the occurrence of ammonite shells contains an element of unreality in that it presupposes, tacitly, that juvenile mortality in shelled cephalopods, past and present, is exceptionally high and that all growth phases share an equal opportunity of being preserved post-mortem. This hypothesis has yet to be objectively tested. There is also the differentiation in morphology usually seen in micromorphic and macromorphic ammonite shells. In species of the Cretaceous genus Knemiceras, to cite an example, dimorphism is pronounced yet size-related differentiation with respect to preservation in occurrences is not observed in any of the large collections known to me. However, the late Professor Tove Birkelund once showed me material of heteromorphic Cretaceous ammonites from Greenland, which I now believe could have represented macromorphs and micromorphs in two size groups.
Kobayashi seems to have been the first to formalize the concept of the post-mortem distribution of cephalopod shells when he introduced the term "palaeoflumenology" into the scientific literature (Kobayashi 1954). Kobayashi was concerned with explaining the occurrence of Aturia in the Miocene of Japan. Part of his reasoning was based on an analogy with the post-mortem distribution of living Nautilus, shells of which are carried by the Kuroshio current from the Philippines. Kobayashi (op. cit.) also reported the occurrence of drifted Nautilus shells at Misaki near Tokyo (NB. one of the marine biological stations of Tokyo University is located at Misaki, Tokyo Bay) and stressed the fact that these occurrences are several thousand kilometers from their natural habitat. Other exotic occurrences noted by him are Lao Chao Island, New Zealand beaches, New South Wales, the Nicobar Islands and Sunday Islands.
Wani (2004,
2007), who in commenting on the evidence for the nekroplanktonic dispersal of nautiloid shells (and occasional living individuals) as far as Japan, thought that the distance involved was too great to be reasonable and that some nearer natural habitat would be more likely. He appears to be unaware of the evidence for wide dispersal of Nautilus spp. extending to the western Indian Ocean, registered in the pertinent literature, and summarized and annotated by
House (1987). A similar remark can be applied to the opinion expressed in
Machalski et al. (2007) with respect to the dispersal of scaphitids, at least as far as can be concluded from the brief discussion in their text.
Toriyama et al. (1965) summarized information on drifted shells stranded on the islands of Ko Phe Tra and Ko Tarutao on the western coast of Thailand. The finds at Ko Tarutao occur high up on the beach, together with driftwood, Sepia shells and in the fringing zone delineated by shrubs and grass. The relatively large proportion of partially broken shells accords well with what occurs along the western coast of Malaya (Reyment 1973, p. 36) and with what
Teichert (1970) recorded for islands off the Burmese coast in the Bay of Bengal. While on this subject, the interpretation of shell breakage caused by collisions between drifters, as promoted by
Wani (2004), although possible in rare cases, should be kept within reasonable perspective. A common source of shell damage is due to wave action smashing shells against reefs and to swash abrasion in a pebbly shore-zone.
While working at Kyushu University, Fukuoka, in 1973, I learned that some primary schools support a project for recording occurrences of nautilus drifts, not only on the southern coastline of Kyushu but also further north on the eastern seaboard of Honshu. This information, and
Kobayashi's (1954) results, paved the way for an analysis of the occurrence of nautiloids in the Paleocene of Nigeria. It was concluded that the few shells found so far had been nekroplanktonically transported by the Benguela Current from the Cabinda region (Reyment 1967).
Chirat (2000, p. 72) arrived at an analogous explanation for the post-mortem distribution of Cenozoic Aturia in the Neogene deposits of the Aquitaine Basin (France).
Reyment (1958, p. 112) quoted Mr. D. Hall of the Singapore Fisheries who supplied information on finds of nautilus shells found during trawling. In 300 trawls in the South China Sea by the R. V. Manihine no live individuals were brought up, and only three damaged shells were found in the nets.
Teichert (1970) reported on the nekroplanktonic dispersal of shells of N. pompilius in the Bay of Bengal; he estimated the distance of post-mortem transport as being around 3000 km from the nearest known habitat of the species. It is now known that the pearly nautilus occurs in waters off the western coast of Thailand and Malaya, thus reducing the estimate of 3000 km for the nekroplanktonic dispersal of shells in the Bay of Bengal, given by Teichert, to around 800 km. In the same note he brought together some little-known published information on drifted shells, including the occurrence of a shell found floating 200 km SW of Sri Lanka and one collected from north of Madagascar.
Stenzel (1964, p. K88) referred to drifted shells found on the eastern coastline of Madagascar (see also
Dautzenberg 1923). References to living individuals and drifted shells recorded in the western Indian Ocean are documented in
House (1987, figure 1), and there is a reference in
Lehmann (1964, p. 192).
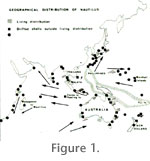 My sketch-map for the distribution of Nautilus spp., living and transported (Reyment 1973, p. 38), is based on information in
Stenzel (1964),
Teichert (1970) and, with respect to the western reaches of the Indian Ocean, a letter dated December 5, 1967 received from Dr. Anna Bidder (Cambridge). Dr. Bidder informed me that she "now had evidence of living Nautilus from Mauritius and Zanzibar." The biogeographical status of these occurrences is still poorly understood; it is, however, clear that they simplify the interpretation of post-mortem distribution of shells in the western Indian Ocean. The sketch-map (Reyment 1973) shows the occurrence of living Nautilus off South Australia. That observation is based on the report of the capture of a single living specimen off Foul Bay in 1911 (Iredale 1944).
Stenzel (1964, p. K88) referred to two stranded individuals found of the coast of southern Australia. Moreover, living Nautilus occur off Albany in the western extremity of the Great Australian Bight. In the normal case, Nautilus is believed to be restricted to warm tropical waters, but the southern Australian data indicate that even cooler waters can be tolerated. An updated version of the sketch-map from 1973 referred to above (Figure
1), based on new observations, and augmented with data from
House (1987, figures 1 and 3), gives the impression that outside the main area of distribution of species of Nautilus in the western Pacific Ocean, there is a large area in which living individuals and drifted shells occur much more sparsely. Examination of
Figure 1 possibly provides a clue to this seeming anomaly, notably, that the regions of greater population density lie in coral seas. The lesser densities are located in the outskirts of the geographical range of the genus where analogous underwater structures to coral reefs are scarce or lacking. This is the case for Japanese waters and to a fair degree, the eastern zones of the western Indian Ocean and the Great Australian Bight.
House (1987, figure. 3) related the pattern of drifted shells, and living distributions, to the ocean currents of the Indian and Pacific Oceans and could recognize the existence of a clear relationship between the shell-drift pattern and known main oceanic currents. Shells of Nautilus are reported to be well known to inhabitants of the eastern African coastline; evidence is provided by the issue of a postage stamp by Kenya depicting a stranded Nautilus shell (pictured by
House 1987, p. 56, figure 2). References on experiments for flotation times of chambered cephalopod shells are noted by
Saunders and Spinosa (1979) and
Hewitt (2006).
Ekman (1953) summarized the role of large-scale oceanic currents for the dispersal of various organisms. My sketch-map for the distribution of Nautilus spp., living and transported (Reyment 1973, p. 38), is based on information in
Stenzel (1964),
Teichert (1970) and, with respect to the western reaches of the Indian Ocean, a letter dated December 5, 1967 received from Dr. Anna Bidder (Cambridge). Dr. Bidder informed me that she "now had evidence of living Nautilus from Mauritius and Zanzibar." The biogeographical status of these occurrences is still poorly understood; it is, however, clear that they simplify the interpretation of post-mortem distribution of shells in the western Indian Ocean. The sketch-map (Reyment 1973) shows the occurrence of living Nautilus off South Australia. That observation is based on the report of the capture of a single living specimen off Foul Bay in 1911 (Iredale 1944).
Stenzel (1964, p. K88) referred to two stranded individuals found of the coast of southern Australia. Moreover, living Nautilus occur off Albany in the western extremity of the Great Australian Bight. In the normal case, Nautilus is believed to be restricted to warm tropical waters, but the southern Australian data indicate that even cooler waters can be tolerated. An updated version of the sketch-map from 1973 referred to above (Figure
1), based on new observations, and augmented with data from
House (1987, figures 1 and 3), gives the impression that outside the main area of distribution of species of Nautilus in the western Pacific Ocean, there is a large area in which living individuals and drifted shells occur much more sparsely. Examination of
Figure 1 possibly provides a clue to this seeming anomaly, notably, that the regions of greater population density lie in coral seas. The lesser densities are located in the outskirts of the geographical range of the genus where analogous underwater structures to coral reefs are scarce or lacking. This is the case for Japanese waters and to a fair degree, the eastern zones of the western Indian Ocean and the Great Australian Bight.
House (1987, figure. 3) related the pattern of drifted shells, and living distributions, to the ocean currents of the Indian and Pacific Oceans and could recognize the existence of a clear relationship between the shell-drift pattern and known main oceanic currents. Shells of Nautilus are reported to be well known to inhabitants of the eastern African coastline; evidence is provided by the issue of a postage stamp by Kenya depicting a stranded Nautilus shell (pictured by
House 1987, p. 56, figure 2). References on experiments for flotation times of chambered cephalopod shells are noted by
Saunders and Spinosa (1979) and
Hewitt (2006).
Ekman (1953) summarized the role of large-scale oceanic currents for the dispersal of various organisms.
Sinclair et al. (2007) reviewed aspects of the population dynamics of Nautilus spp. in a molecular biological analysis using the cytochrome oxidase subunit I (Cox I). In their explanation of the biogeographical elements found in their study, they thought that it might be possible, but unlikely, for animals to survive for months in cooler water. The fact that N. pompilius descends readily to depths of several hundred metres for foraging can be taken to indicate that it is a eurythermal animal. The solution to this enigma, as noted above, is contained in House's study, which says that at least some species of Nautilus are able to survive under presumably less than optimal conditions, such as pertain, for example, in the Great Australian Bight.
In a fisheries paper describing the exploitation of living molluscs of the Philippines,
Talavara and Faustino (1931) considered commercial aspects of the biology of Nautilus pompilius. They reported the species to be abundant off the southern coast of Negros, Tanon Strait, Bantayan, Gebu Palaman, Cuyo, Basilan, Leyte and Mindoro in a coral-bottom environment. Although animals were caught in traps set as deep as 400-600 m, most catches were made at depths around 60 m. Empty shells are commonly picked up along beaches or in shallows.
Moribund State
The question arises as to the final phase of life in a dying nautilus. Rather than just giving up the ghost in a few seconds, as might be surmised from some interpretations, it is far more likely that the animals go through a phase of death throes. It is known that moribund nautilus individuals (Karnovsky scale 10<20 units) have difficulty mustering the energy for maintaining zero, respectively negative buoyancy. Cameral liquid is therefore dissipated during the death-phase. None of the shells I dissected during my stay in the western Pacific Ocean in 1968 were found to contain significant amounts of cameral liquid in any of the chambers. This evidence accords with the observations recorded by
Bidder (1962) and
Denton and Gilpin-Brown (1967). It seems evident, therefore, that cameral liquid is quickly lost in the shells of dying individuals in consequence of which perishing animals embark automatically on a last journey to the surface.
|