 Partial skull and endocranial cast of the ankylosaurian dinosaur Hungarosaurus from the Late Cretaceous of Hungary: implications for locomotion
Partial skull and endocranial cast of the ankylosaurian dinosaur Hungarosaurus from the Late Cretaceous of Hungary: implications for locomotion
Article number: 17.1.1A
https://doi.org/10.26879/405
Copyright Palaeontological Association, January 2014
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 28 May 2013. Acceptance: 5 December 2013
{flike id=612}
ABSTRACT
A partial skull of ankylosaur from the Upper Cretaceous (Santonian) Csehbánya Formation in Iharkút and the endocranial cast taken from it are described. The morphology of the exoccipital, the elongated 'neck' region of the basioccipital, the shape of the occipital condyle, and the different flexure of the medulla relative to the forebrain unambiguously differentiate this specimen from the basicranium of Struthiosaurus, so it is assigned to Hungarosaurus sp. Whereas the endocranial cast reflects a brain generally similar to those of other ankylosaurs, the dorsally hypertrophied cerebellum (also present is Struthiosaurus transylvanicus) is quite unusual within the group suggesting a more sophisticated cerebral coordination of posture and movement, and perhaps a more cursorial locomotary habit than predicted for other ankylosaurs.
Attila Ősi. Hungarian Academy of Sciences – Eötvös Loránd University, Lendület Dinosaur Research Group, Pázmány Péter sétány 1/c, Budapest, 1117, Hungary hungaros@gmail.com
Xabier Pereda Suberbiola. Universidad del País Vasco/Euskal Herriko Unibertsitatea, Facultad de Ciencia y Tecnología, Dpto. Estratigrafía y Paleontología, Apartado 644, 48080 Bilbao, Spain xabier.pereda@ehu.es
Tamás Földes. Institute of Diagnostic Imaging and Radiation Oncology of the University of Kaposvár, Kaposvár, 7400 Guba S. 40, Hungary t.foldes@t-online.hu
Keywords: Santonian, Iharkút, Ankylosauria, Hungarosaurus, skull, endocranial cast
Final citation: Ősi, Attila, Pereda Suberbiola, Xabier, and Földes, Tamás. 2014. Partial skull and endocranial cast of the ankylosaurian dinosaur Hungarosaurus from the Late Cretaceous of Hungary: implications for locomotion, Palaeontologia Electronica Vol. 17, Issue 1; 1A; 18p. https://doi.org/10.26879/405
palaeo-electronica.org/content/2014/612-skull-of-hungarosaurus
INTRODUCTION
Cranial remains of ankylosaurs are among the rarest fossils from the Late Cretaceous of Europe. Until the discovery of the fossil assemblage assigned to the species Hungarosurus tormai Ősi, 2005 from the Santonian of Iharkút, western Hungary, which includes disarticulated cranial bones (Ősi, 2005; Ősi and Makádi, 2009), mainly the type material of Struthiosaurus, Struthiosaurus austriacus Bunzel, 1870 (Gosau beds, Lower Campanian, Austria) and a second species, Struthiosaurus transylvanicus Nopcsa, 1915 (Sinpetru beds, Maastrichtian, Romania), were available to provide information on the cranial morphology of European Late Cretaceous ankylosaurs. The ankylosaur material published in the last two decades from Campano-Maastrichtian sediments of southern France and northern Spain, including Struthiosaurus languedocensis Garcia and Pereda Suberbiola, 2003 ('Fuvelian' beds, Lower Campanian, France), is composed mainly of postcranial material (e.g., Pereda Suberbiola, 1993, 1999; Garcia and Pereda Suberbiola, 2003), and the cranial elements are represented only by edentulous maxillary and dentary fragments and isolated teeth (Pereda Suberbiola, 1992, 1999). A new partial skull from the Chera locality, in Valencia, eastern Spain is now under description (Company et al., 2009; work currently in progress).
Cranial remains of Struthiosaurus austriacus include a fragmentary braincase (PIUW 2349/6), two fragments of the orbital region (PIUW 2349/17), a distal fragment of right quadrate (PIUW 2349/uncatalogued), a fragmentary right dentary (PIUW 2349/5), the anterior end of left and right mandibles (PIUW 2349/uncatalogued) and about 18 teeth (PIUW 2349/7-9, 39, and uncatalogued). After the initial descriptions of Bunzel (1870, 1871), various authors reviewed these cranial remains, especially the braincase. These studies and opinions on the taxonomic position of this small specimen are summarized by Pereda Suberbiola and Galton (1992, 1994), so they are not repeated here. For today, the most accepted view is that the braincase of S. austriacus is from a subadult nodosaurid ankylosaur (Pereda Suberbiola and Galton, 1994).
The material of Struthiosaurus transylvanicus includes the posterior half of the skull with the skull roof, temporal region and, most importantly, the braincase (NHMUK R4966). This material, collected together with associated postcranial remains, was regarded as a primitive ankylosaurian (Nopcsa, 1915, 1929). The most informative and single overlapping cranial element known in both Central European species of Struthiosaurus is the braincase. In addition, the morphology of the endocranial cast is also available in both species. Pereda Suberbiola and Galton (1994) made a detailed comparison of the braincases of the two Struthiosaurus species and concluded that the Transylvanian material cannot be assigned certainly into a different species, and they regarded this material as Struthiosaurus cf. S. austriacus. This view was also suggested by Parish (2005). Comparison of the other remains, however, gives further support for distinguishing the two species (Pereda Suberbiola and Galton, 2001).
New discoveries of ankylosaur remains from the Santonian Csehbánya Formation in Iharkút resulted in an ankylosaur skull fragment, including the braincase and a small portion of the skull roof. Besides teeth, this is the only known, common cranial element in all European Late Cretaceous ankylosaurs (except in S. languedocensis) that can help to distinguish the different species from each other.
Institutional Abbreviations
AMNH – American Museum of Natural History, New York, NY, USA; CAMSM – Sedgwick Museum of Earth Sciences, University of Cambridge, Cambridge, UK; CEUM – College of Eastern Utath Prehistoric Museum, Price, Utah, USA; MCM – Mikasa City Museum, Mikasa, Japan; MTM – Hungarian Natural History Museum, Budapest, Hungary; NHMUK – The Natural History Museum, London, UK (formerly BMNH, British Museum of Natural History); PIUW – Paläontologisches Institut der Universität Wien, Vienna, Austria; ROM – Royal Ontario Museum, Toronto, Canada; UALVP – University of Alberta, Laboratory for Vertebrate Paleontology, Edmonton, AB, Canada; ZPAL – Institute of Palaeobiology (Zaklad Paleobiologii) of the Polish Academy of Sciences, Warsaw, Poland.
LOCALITY AND GEOLOGICAL SETTING
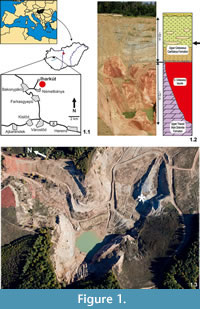 The Late Cretaceous Iharkút vertebrate locality is situated close to the villages of Németbánya and Bakonyjákó, in the heart of the Bakony Mountains, western Hungary (47° 13' 52'' N, 17° 39' 01'' E, see Figure 1.1). The locality is in an abandoned and recultivated open-pit bauxite mine (Figure 1.3) that now belongs to Dino Park Ltd. The discovery of the locality is mainly due to intensive mining activities in the area. Although it has been known since the first decades of the 20th century that bauxite occurs in various parts of the Bakony Mountains, the quest for the 'red gold', as the bauxite is frequently called by inhabitants of the region, was started in the late 1960s. Extensive search and mining provided huge open-pit mines, the only outcrops of the bone-yielding Csehbánya Formation.
The Late Cretaceous Iharkút vertebrate locality is situated close to the villages of Németbánya and Bakonyjákó, in the heart of the Bakony Mountains, western Hungary (47° 13' 52'' N, 17° 39' 01'' E, see Figure 1.1). The locality is in an abandoned and recultivated open-pit bauxite mine (Figure 1.3) that now belongs to Dino Park Ltd. The discovery of the locality is mainly due to intensive mining activities in the area. Although it has been known since the first decades of the 20th century that bauxite occurs in various parts of the Bakony Mountains, the quest for the 'red gold', as the bauxite is frequently called by inhabitants of the region, was started in the late 1960s. Extensive search and mining provided huge open-pit mines, the only outcrops of the bone-yielding Csehbánya Formation.
The Iharkút vertebrate locality is situated on the Transdanubian Central Range that was on the northern part of the triangular-shaped Apulian microplate between Africa and Europe during the Mesozoic. Southwards and westwards, this block had direct connection with the southern and eastern Alps (Csontos and Vörös, 2004), the latter yielding the early Campanian Austrian Muthmannsdorf vertebrate fauna (e.g., Bunzel, 1871; Seeley, 1881; Pereda Suberbiola and Galton, 2001; Buffetaut, Ősi and Prondvai, 2011). Eastwards, the Transdanubian Central Range could have been relatively close to the Moeasian corner and the famous Haţeg Basin from which an exceptional Maastrichtian terrestrial vertebrate fauna has been documented (e.g., Nopcsa, 1923; Csiki and Grigorescu, 2007; Weishampel et al., 2010 and references therein).
As in most parts of the Transdanubian Central Range, the thick basement of the Iharkút locality is formed by the Upper Triassic Main Dolomite Formation (Figure 1.2). Deep (50 to 90 m), tectonically controlled sinkholes on the karstified surface of this dolomite were filled up by the Cretaceous (pre-Santonian) bauxite (Figure 1.2). Palynological results indicate that this paleosurface including Triassic rocks and the accumulated bauxite lens were covered by fluvial deposits of the Csehbánya Formation no later than the Santonian (Bodor and Baranyi, 2012). Bone-yielding beds occur in this formation as an alluvial flood plain deposit consisting of alternating coarse basal breccia, sandstone, siltstone and paleosol beds (Jocha-Edelényi, 1988; Ősi and Mindszenty, 2009; Figure 1.2). Although isolated bones, teeth and plant remains appear in various stratigraphic horizons of the formation (including red paleosols, blackish, organic rich clay), the most productive sequence is a greyish, coarse basal breccia covered with sandstone and braunish siltstone. These beds produced a rich and diverse vertebrate fossil assemblage (Ősi et al., 2012 and references therein), including five published (Ősi, 2005; Ősi and Makádi, 2009) and two undescribed skeletons of Hungarosaurus.
MATERIAL AND METHODS
The partial skull (PAL 2013.23.1, Appendix) referred here to Hungarosaurus sp. is housed in the Hungarian Natural History Museum (MTM). Preparation, including the cleaning of the brain cavity of the specimen was done mechanically. Some anatomical features were studied with the help of computer tomograph scans at the Institute of Diagnostic Imaging and Radiation Oncology of the University of Kaposvár, a method that also helped to provide a 3D reconstruction and visualization of the specimen. For the CT scanning a Siemens Stomatom Definition Flash machine was used. The fossils were scanned using a resolution of 1.0×1.0×0.6 mm in three different directions (saggital, horizontal and coronal). CT scans were evaluated by the Medical Volume Explorer (MVE) Software. Osteological terminology, as much as possible, follows Baumel and Witmer (1993).
A silicone rubber mould of the endocranial cavity was made from the specimen using Oxam S1 two component silicone to show the approximate structure of the brain, the arrangement of the principal cranial nerves and part of the vascular system. Most parts and various features of the endocranial cavity (e.g., orientation of different parts of the brain) were more easily studiable by the silicone rubber mould in contrast to the CT scans. However, some inner structures (e.g., the diameter, morphology and orientation of pathways of cranial nerves) can be better reconstructed via CT slices.
Anatomical Abbreviations
bo, basioccipital; brca, brain cavity; bs, basisphenoid; bt, basal tubera; ca, canal; cbl, cerebellum; cer, cerebrum; cr, crest; cso, cartilage filled pit of the supraoccipital; de, depression; eo, exoccipital; fo, foramen; fr, frontal; gr/cso?, groove, perhaps the cartilage filled pit in the supraoccipital; la-sph, laterosphenoid-sphenethmoid; me, medulla; mes, mesencephalon; ob, olfactory bulbs; oc, occipital condyle; ol, olfactory lobes; op-pr, opisthotic-prootic complex; pa, parietal; pal ar, palatine artery; pit, pituitary fossa; po fl, pontine flexure; pp, paroccipital process; so, supraoccipital; tca, temporal cavity, II–XII, cranial nerves.
DESCRIPTION AND COMPARISION OF THE SKULL
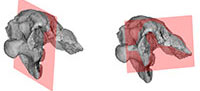
T he specimen (PAL 2013.23.1, Figure 2, Figure 3) preserves the posterior and central parts of the skull roof, most of the occipital region and the partial basicranium. This preservation is quite similar to that of Struthiosaurus austriacus (PIUW 2349/6), in the latter form only the frontal region is missing (Pereda Suberbiola and Galton, 1994). Due to diagenetic events, the Hungarian specimen was slightly compressed from the left lateral direction (Figure 2.5–2.6, Figure 3.1-3.4), thus the skull roof and the braincase lost their original shape being slightly damaged. The greatest length measured from the anteriormost point of the skull roof to the occipital condyle is 115 mm, the greatest height from the base of the occipital condyle to the top of the skull is approximately 80 mm (Table 1). In most cases, the cranial elements are completely ossified with each other, and the sutures cannot be recognized between them, so the individual bones were identified on the basis of the characteristic structures. PAL 2013.23.1 is broken anteriorly somewhere within the frontals, anteroventrally at the level of the prootic and basisphenoid–basipterygoid process, laterally in the frontals and parietal and the paroccipital processes (Figure 2, Figure 3.5-3.6).
he specimen (PAL 2013.23.1, Figure 2, Figure 3) preserves the posterior and central parts of the skull roof, most of the occipital region and the partial basicranium. This preservation is quite similar to that of Struthiosaurus austriacus (PIUW 2349/6), in the latter form only the frontal region is missing (Pereda Suberbiola and Galton, 1994). Due to diagenetic events, the Hungarian specimen was slightly compressed from the left lateral direction (Figure 2.5–2.6, Figure 3.1-3.4), thus the skull roof and the braincase lost their original shape being slightly damaged. The greatest length measured from the anteriormost point of the skull roof to the occipital condyle is 115 mm, the greatest height from the base of the occipital condyle to the top of the skull is approximately 80 mm (Table 1). In most cases, the cranial elements are completely ossified with each other, and the sutures cannot be recognized between them, so the individual bones were identified on the basis of the characteristic structures. PAL 2013.23.1 is broken anteriorly somewhere within the frontals, anteroventrally at the level of the prootic and basisphenoid–basipterygoid process, laterally in the frontals and parietal and the paroccipital processes (Figure 2, Figure 3.5-3.6).
Basioccipital
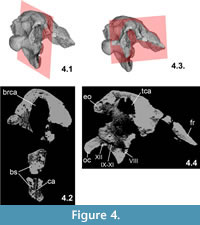
The basioccipital is a massive and thick element (Figure 2, Figure 3). It borders the posteroventral side of the braincase and forms the whole occipital condyle. The condyle is sub-spherical (Figure 3.3–3.4), in contrast to the much wider than high condyle of Struthiosaurus, almost identical in shape with that of Silvisaurus (based on the cast NHMUK 11189), and its greatest lateromedial width is 29.5 mm and the greatest dorsoventral height is 26.2 mm.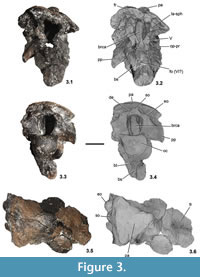 (These parameters of the condyle in the holotype material of Hungarosaurus [PAL 2013.23.1] are 32.2 mm and 27 mm, respectively; the greatest width in S. austriacus is 21 mm and 24 mm in S. transylvanicus [Pereda Suberbiola and Galton, 1994]). The greatest width of the foramen magnum in PAL 2013.23.1 is 19 mm but its lateral walls are slightly compressed medially, so originally it could have been approximately 5 mm wider. Ventrally, the rounded surface of the condyle is very slightly eroded. The 'neck' region of the basioccipital connecting the condyle with the anterior part of the basioccipital is relatively longer (Figure 2.3-2.6) than that of S. austriacus and that of Pawpawsaurus from the Early Cretaceous of Texas, USA (Lee, 1996). The ventral surface of the basioccipital is deeply concave and marginated laterally by the anteroposteriorly elongate basal tubera. A prominent, anteroposteriorly oriented central ridge on this concave surface seen in Pawpawsaurus (Lee, 1996) is present neither on this specimen nor in Struthiosaurus. A complex series of foramina pierces the ventrolateral wall of the braincase most probably in the area at the junction of the basioccipital and the opisthotic–exoccipital–paroccipital process. In the identification and description of these openings we mainly follow the work of Eaton (1960) and Carpenter and Kirkland (1998) on Silvisaurus, Pereda Suberbiola and Galton (1994) on Struthiosaurus, Lee (1996) on Pawpawsaurus, Norman and Faiers (1996) on cf. Polacanthus, and Vickaryous and Russell (2003) on Euoplocephalus. Other papers describe ankylosaurian braincases: Maryańska (1977) on Saichania; Tumanova (1987) on Talarurus; Sullivan (1999) on Nodocephalosaurus; Carpenter et al. (2001) on Cedarpelta; Averianov (2002) and Parish and Barrett (2004) on Bissektipelta; Parsons and Parsons (2009) on Tatankacephalus. Discussed from posterior to anterior direction, the first opening of PAL 2013.23.1, with a diameter of 4 mm and visible on both sides (Figure 2.4), is considered the exit of the hypoglossal nerve (CN XII). Based on parasagittal CT scans the canal of CN XII has a dorsomedial–lateroventral orientation. A 2–3 mm thick bony septum separates this aperture from the following one. On the better preserved right side, this second opening is slightly smaller (ca. 3 mm), but after a thin bony septum a third opening is in the row. On the left side the second and third foramina occur as a single, large opening and they appear to correspond to the exits of the accessory (CN XI), vagus (CN X) and glossopharyngeal (CN IX) nerves (Figure 2.4, Figure 4.4). Anterior to the third aperture, an almost vertically oriented crest of the paroccipital process connects with the basioccipital.
(These parameters of the condyle in the holotype material of Hungarosaurus [PAL 2013.23.1] are 32.2 mm and 27 mm, respectively; the greatest width in S. austriacus is 21 mm and 24 mm in S. transylvanicus [Pereda Suberbiola and Galton, 1994]). The greatest width of the foramen magnum in PAL 2013.23.1 is 19 mm but its lateral walls are slightly compressed medially, so originally it could have been approximately 5 mm wider. Ventrally, the rounded surface of the condyle is very slightly eroded. The 'neck' region of the basioccipital connecting the condyle with the anterior part of the basioccipital is relatively longer (Figure 2.3-2.6) than that of S. austriacus and that of Pawpawsaurus from the Early Cretaceous of Texas, USA (Lee, 1996). The ventral surface of the basioccipital is deeply concave and marginated laterally by the anteroposteriorly elongate basal tubera. A prominent, anteroposteriorly oriented central ridge on this concave surface seen in Pawpawsaurus (Lee, 1996) is present neither on this specimen nor in Struthiosaurus. A complex series of foramina pierces the ventrolateral wall of the braincase most probably in the area at the junction of the basioccipital and the opisthotic–exoccipital–paroccipital process. In the identification and description of these openings we mainly follow the work of Eaton (1960) and Carpenter and Kirkland (1998) on Silvisaurus, Pereda Suberbiola and Galton (1994) on Struthiosaurus, Lee (1996) on Pawpawsaurus, Norman and Faiers (1996) on cf. Polacanthus, and Vickaryous and Russell (2003) on Euoplocephalus. Other papers describe ankylosaurian braincases: Maryańska (1977) on Saichania; Tumanova (1987) on Talarurus; Sullivan (1999) on Nodocephalosaurus; Carpenter et al. (2001) on Cedarpelta; Averianov (2002) and Parish and Barrett (2004) on Bissektipelta; Parsons and Parsons (2009) on Tatankacephalus. Discussed from posterior to anterior direction, the first opening of PAL 2013.23.1, with a diameter of 4 mm and visible on both sides (Figure 2.4), is considered the exit of the hypoglossal nerve (CN XII). Based on parasagittal CT scans the canal of CN XII has a dorsomedial–lateroventral orientation. A 2–3 mm thick bony septum separates this aperture from the following one. On the better preserved right side, this second opening is slightly smaller (ca. 3 mm), but after a thin bony septum a third opening is in the row. On the left side the second and third foramina occur as a single, large opening and they appear to correspond to the exits of the accessory (CN XI), vagus (CN X) and glossopharyngeal (CN IX) nerves (Figure 2.4, Figure 4.4). Anterior to the third aperture, an almost vertically oriented crest of the paroccipital process connects with the basioccipital.
Basisphenoid
 Ventrally and anteriorly, the basioccipital continues within the basisphenoid (Figure 2, Figure 3), which is slightly broken and compressed dorsally, and anteriorly, so the basipterygoid process is very fragmentary. The most characteristic feature of this element is its ventral orientation with an angle of about 95° relative to the axis of the occipital condyle, a feature shared with Struthiosaurus (this angle is 105° in Struthiosaurus austriacus; Pereda Suberbiola and Galton, 1994), the only other ankylosaur with a markedly ventrally projecting basisphenoid. In other nodosaurids, for example in Panoplosaurus (Pereda Suberbiola and Galton, 1994) and cf. Polacanthus (Norman and Faiers, 1996), this angle is 125°, 135° in Pawpawsaurus (Lee, 1996), 160° in Silvisaurus (Eaton, 1960). On the other hand, this angle is usually higher in ankylosaurids, for example, it is about 150° in Euoplocephalus (Coombs, 1978a).
Ventrally and anteriorly, the basioccipital continues within the basisphenoid (Figure 2, Figure 3), which is slightly broken and compressed dorsally, and anteriorly, so the basipterygoid process is very fragmentary. The most characteristic feature of this element is its ventral orientation with an angle of about 95° relative to the axis of the occipital condyle, a feature shared with Struthiosaurus (this angle is 105° in Struthiosaurus austriacus; Pereda Suberbiola and Galton, 1994), the only other ankylosaur with a markedly ventrally projecting basisphenoid. In other nodosaurids, for example in Panoplosaurus (Pereda Suberbiola and Galton, 1994) and cf. Polacanthus (Norman and Faiers, 1996), this angle is 125°, 135° in Pawpawsaurus (Lee, 1996), 160° in Silvisaurus (Eaton, 1960). On the other hand, this angle is usually higher in ankylosaurids, for example, it is about 150° in Euoplocephalus (Coombs, 1978a).
An opening is visible on both sides anterodorsally on the basisphenoid. These foramina open into the pituitary fossa, thus they are most probably the exits for the abducens nerve (CN VI, Figure 3.2). In anterior view, the pituitary fossa is relatively not as wide as that seen in Struthiosaurus (PIUW 2349/6). A 'large opening for the internal carotid artery' as described in Struthiosaurus austriacus (Pereda Suberbiola and Galton, 1994, p. 178) cannot be recognized on the Hungarian specimen. This is probably due to the damaged anterolateral margins of the basisphenoid. Posterior to the opening of the abducens nerve (CN VI) an elongated groove extends from the ventral part of the basisphenoid up to a large opening with rounded margins, just in the connection of the basisphenoid–prootic. This large, partially preserved opening, better preserved on the left side (Figure 3.2), is considered the aperture of the trigeminal nerve (CN V). If preserved, then only the dorsalmost part of the basipterygoid process is present (no sutures can be observed with the basisphenoid). Centrally, on the posteroventral surface a marked, dorsally directed 2 mm wide foramen enters into the basisphenoid. CT imaging, however, do not reveal any pathway continuing into deeper parts of the bone. Nevertheless, axial CT scans through the basisphenoid demonstrated a thin and approximately 2 cm long canal (Figure 4.2); the function or connections of it is, however, unknown.
Exoccipital-opisthotic-prootic Complex
Although the individual cranial elements are in most cases massively fused, on the basis of comparisons with other ankylosaurs the preserved sidewall of the braincase in the Hungarian specimen is formed by the exoccipital-opisthotic-prootic complex (Figure 2.1-2.4). The more anteriorly positioned laterosphenoid-sphenethmoid region is almost completely missing, only their dorsalmost edges are preserved at the connection to the parietal-frontal (Figure 1.5-1.6). The exoccipital-opisthotic-prootic complex forms the dorsal border of the row of exits for the cranial nerves. The exoccipital forms the lateral border of the foramen magnum and dorsolaterally it bears a thickened, oval-shaped, posteromedially pointed protuberance. These protuberances are suggested to be 'tubercles for neural arches of the atlas' sensu Parish and Barrett (2004). The left and right protuberances are closer together (but still positioned dorsal to the occipital condyle, Figure 3.3-3.4) than those of S. austriacus. These protuberances are relatively smaller than in S. transylvanicus and, in the latter form, they are interpreted as remains of the proatlases (Pereda Suberbiola and Galton, 1994). Anterolateral to these protuberances a deep, anteromedial-posterolaterally oriented depression extends slightly below the skull roof, but there is no posterodorsal overhang by the skull roof, as seen e.g. in ankylosaurids (Vickaryous et al., 2004). The paroccipital process is massive and it is oriented lateroventrally and slightly posteriorly, similar to that of S. austriacus (Pereda Suberbiola and Galton, 1994). Anteriorly it is fused with the opisthotic and ventrally a crest extends towards the basal tubera. On the right side, anterior to this crest, at least one opening is present that is slightly damaged. This opening is probably the foramen ovale for the vestibulocochlear nerve (CN VIII). CT scans indicate that the pathway of CN VIII is a robust, ca. 4 mm wide canal (Figure 4.4) and has a dorsomedial to lateroventral orientation. The opisthotic and the prootic are relatively poorly preserved but one foramen (2 mm in diameter), which is visible on each side is probably present on the opisthotic. Foramina in similar positions have been identified as possible openings of veins in Silvisaurus (Eaton, 1960). The skull roof is slightly compressed dorsally, so that the connection of the parietal with the opisthotic-prootic complex cannot be studied.
Supraoccipital
The supraoccipital is co-ossified with the skull roof. Because no sutures marginating the supraoccipital can be detected, the extent of this bone is unclear. Nevertheless, a 6 to 8 mm wide, shallow bony ridge, present on the posterodorsally facing nuchal shelf in various nodosaurids (e.g., Struthiosaurus, Pawpawsaurus, Silvisaurus), extends anteroposteriorly and merges anteriorly with the presumed posterior margin of the parietal, and posteriorly ends between the two protuberances of the exoccipitals to form the dorsalmost margin of the foramen magnum (Figure 3.3-3.4). The nuchal region above the foramen magnum was the insertion surface for the musculus trapezius group among others; the complex surface of this margin (exoccipital-supraoccipital) indicates strongly developed boundles of these muscles. This is related to the downward angle of the head relative to the neck (supported by a posteroventrally angled occipital condyle); 'this posture facilitated cropping low vegetation but required strong muscles to sustain the head posture' (Lee, 1996, p. 244). Although in most ankylosaurids the supraoccipital is covered dorsally by the parietal (see Maryańska, 1977), in the skeletally immature specimen of Pinacosaurus (ZPAL MgD II/1) the anteroposteriorly extended ridge of the supraoccipital can still be observed.
Parietal
Although the skull roof is slightly compressed from the dorsal direction, the posterior part of the parietal shows its originally convex, slightly domed dorsal surface (Figure 2.3-2.4). At the posterior end of the parietal in the anteriormost margin of the steeply inclined nuchal shelf, a transverse, shallow crest can be observed that might represent the sutural boundaries of the parietal with the supraoccipital and exoccipital (Figure 3.5-3.6). Another option is that this suture is the posterior margin of a dermal osteoderm co-ossified with the parietal, similar to that seen in Struthiosaurus austriacus (Pereda Suberbiola and Galton, 1994, figure 2a). The dorsal surface of the parietal is ornamented by small pits and shallow grooves. The broken lateral edges of the skull roof indicate that the supratemporal fenestrae were closed as in all ankylosaurs. It is uncertain whether some small parts of the squamosal and postorbital are preserved in this specimen being fused to the parietal-frontal part of the skull roof, or the preserved part of the skull roof is exclusively formed by the parietal and frontal.
Frontal
The anterior portion of the preserved skull roof is formed by a slightly domed element suggested here to be the frontal (Figure 2, Figure 3.5-3.6). This approximately 9 mm thick bone possesses two roughly parallel crests ventrally, which laterally border the olfactory lobes (CN I) and probably formed the contact between the frontal and the laterosphenoid-sphenethmoid complex. In addition, on the ventral side of the anteriormost part of the frontal, a shallow, eroded crest that becomes wider anteriorly can be observed (Figure 2.5-2.6). We suggest that this crest served to separate from dorsally the olfactory lobes, which were divergent anteriorly, though not as strongly as described in ankylosaurids (Coombs, 1978a). Witmer and Ridgely (2008) pointed out that divergent olfactory tracts are also present in the derived nodosaurid Panoplosaurus (ROM 1215). If Hungarosaurus also had divergent olfactory tracts, a feature that was suggested to be associated with the complex nasal passages (Coombs, 1978a; Norman and Faiers, 1996), then not only in ankylosaurids (Coombs, 1978a; Witmer and Ridgely, 2008), but also nodosaurids including the highly derived (Panoplosaurus; Witmer and Ridgely, 2008) and the more basal (Hungarosaurus) forms could have had this feature, and perhaps the complex looping pathway of the airway was more general within the group.
A wide and shallow, anteroposteriorly oriented groove is present on the dorsal surface that is quite similar to that one separating the two trapezoid posterior dermal plates in the frontal region of Pawpawsaurus (Lee, 1996, figure 4). If this interpretation is correct, then the frontal region of Hungarosaurus could have been similarly ornamented by two, relatively large dermal plates as seen in the North American form.
Description and comparison of the endocranial cast
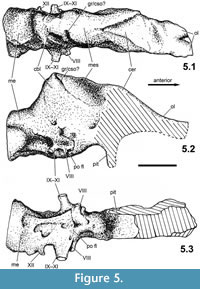 The endocranial cast taken from the braincase of PAL 2013.23.1 reveals the main structures and proportions of the hindbrain and partially those of the midbrain; the absence of the anterior walls of the braincase (i.e., laterosphenoid-sphenethmoid) prevents the reconstruction of the anterior, anteroventral and ventral sides of the telencephalon and those of the olfactory lobes and bulbs (Figure 5). Only the ventral side of the parietal and frontal provides some information on the dorsal surface of the forebrain, and the dorsal side of the basisphenoid provides some information of the posteroventral surface of the diencephalon. The endocranial cast clearly shows that the brain cavity was compressed transversely due to taphonomic processes, thus the lateral extension of the different brain regions cannot be precisely determined. However, this deformation did not significantly change the orientation and dorsoventral extension of the cavity. As in the endocranial casts of other ankylosaurs (see Table 2), the relatively smooth surface indicates that the brain was not closely applied to the braincase wall, thus the original form and sharp boundaries of the different regions of the brain cannot be precisely determined. Nevertheless, the endocranial cast provides significant information on the orientation and approximate extent of the principal regions (fore-, mid- and hindbrain).
The endocranial cast taken from the braincase of PAL 2013.23.1 reveals the main structures and proportions of the hindbrain and partially those of the midbrain; the absence of the anterior walls of the braincase (i.e., laterosphenoid-sphenethmoid) prevents the reconstruction of the anterior, anteroventral and ventral sides of the telencephalon and those of the olfactory lobes and bulbs (Figure 5). Only the ventral side of the parietal and frontal provides some information on the dorsal surface of the forebrain, and the dorsal side of the basisphenoid provides some information of the posteroventral surface of the diencephalon. The endocranial cast clearly shows that the brain cavity was compressed transversely due to taphonomic processes, thus the lateral extension of the different brain regions cannot be precisely determined. However, this deformation did not significantly change the orientation and dorsoventral extension of the cavity. As in the endocranial casts of other ankylosaurs (see Table 2), the relatively smooth surface indicates that the brain was not closely applied to the braincase wall, thus the original form and sharp boundaries of the different regions of the brain cannot be precisely determined. Nevertheless, the endocranial cast provides significant information on the orientation and approximate extent of the principal regions (fore-, mid- and hindbrain).
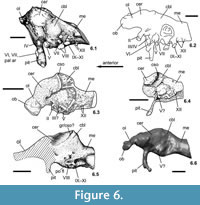
The endocranial cast taken from PAL 2013.23.1 shows a significant flexure along its long axis (Figure 5.2). The medulla is flexed ventrally relative to the forebrain by about 25°; this flexure is 15° in Euoplocephalus, 16° in cf. Polacanthus, 32° in Panoplosaurus, and 45° in Struthiosaurus transylvanicus. The Iharkút specimen is most similar to the endocasts of Struthiosaurus and Panoplosaurus in having a relatively compact and high mid- and hindbrain (compare Figure 6.5 to Figure 6.3, 6.4, 6.6). The pontine flexure is well developed as in other ankylosaurs.
Forebrain (prosencephalon)
 The dorsal surface of the olfactory lobes seen on the endocast of PAL 2013.23.1 indicates that they were divergent by about 25° (Figure 5.1). In Panoplosaurus (Witmer and Ridgely, 2008, figure 7d) and especially in Euoplocephalus (Coombs, 1978a), they are much more divergent, with an angle of about 80° in the latter genus. The dorsoventral extension and anteroposterior length of the lobes and the form of the bulbs cannot be determined. The cerebral region is slightly doomed dorsally and, though the braincase is transversely compressed, the cerebral lobes slightly expand laterally compared to the width of the mid- and hindbrain regions. The cerebrum of PAL 2013.23.1 is not as expanded dorsally and do not form the dominant portion of the brain as was noted in cf. Polacanthus by Norman and Faiers (1996). The main body of the diencephalon (posterior segment of the forebrain) cannot be recognized on the endocast, mostly because of the posterior expansion of the cerebral lobes.
The dorsal surface of the olfactory lobes seen on the endocast of PAL 2013.23.1 indicates that they were divergent by about 25° (Figure 5.1). In Panoplosaurus (Witmer and Ridgely, 2008, figure 7d) and especially in Euoplocephalus (Coombs, 1978a), they are much more divergent, with an angle of about 80° in the latter genus. The dorsoventral extension and anteroposterior length of the lobes and the form of the bulbs cannot be determined. The cerebral region is slightly doomed dorsally and, though the braincase is transversely compressed, the cerebral lobes slightly expand laterally compared to the width of the mid- and hindbrain regions. The cerebrum of PAL 2013.23.1 is not as expanded dorsally and do not form the dominant portion of the brain as was noted in cf. Polacanthus by Norman and Faiers (1996). The main body of the diencephalon (posterior segment of the forebrain) cannot be recognized on the endocast, mostly because of the posterior expansion of the cerebral lobes.
Midbrain (mesencephalon)
Only a slightly concave, 5-7 mm wide, posterodorsal-anteroventrally oriented surface can be observed laterally (better seen on the right lateral side). It is situated between the cerebral lobes and a posterodorsal-anteroventrally oriented groove, the latter of which was also described in Struthiosaurus as the 'cartilage-filled pit in the supraoccipital' (Hopson, 1979; Pereda Suberbiola and Galton, 1994, p. 182). The 'epiphysis' (Nopcsa, 1929) or protuberance on the dorsal side of the midbrain described in Struthiosaurus (Pereda Suberbiola and Galton, 1994; i.e. the 'cotylus on the laterosphenoid', Galton, 1989) cannot be seen on the Hungarian specimen.
Hindbrain (rhombencephalon)
The anterior portion of the hindbrain is dominated by the cerebellum that is strongly expanded dorsally. The posterior side of the cerebellum is narrow and steeply inclined, at an angle of 55° relative to the long axis of the medulla (Figure 5.2). In ankylosaurs, a similar, dorsally expanded cerebellum is documented only in Struthiosaurus transylvanicus (Nopcsa, 1929; Pereda Suberbiola and Galton, 1994). In S. austriacus, the dorsal expansion of this region is not as prominent, however, this feature might be explained by the suggested subadult nature of the specimen (PIUW 2349/6, Pereda Suberbiola and Galton, 1994). A relatively low, little expanded cerebellum is present in cf. Polacanthus, Panoplosaurus and Euoplocephalus; on the other hand, this feature is more typical of ornithopod dinosaurs (e.g., Kritosaurus [Ostrom, 1961], Dryosaurus [Galton, 1989]). As a posterior continuation of the pontine region, the myelencephalon (posterior portion of the hindbrain) is a relatively short segment with an oval, originally probably subcircular cross section. The ventral side of this portion of the endocast shows the openings on the different cranial nerves poorly. However, on the posterior part of the pontine flexure the casts of canals of the cranial nerves IX–XI and the XII can be recognized (Figure 5.3). The number of canals for the branches of the hypoglossal (CN XII) nerves is two in nodosaurids (Hopson, 1979; Norman and Faiers, 1996) and in some Asian ankylosaurids (Kurzanov and Tumanova, 1978; Parish and Barrett, 2004), but three in Euoplocephalus (Coombs, 1978a) and Bissektipelta (Parish and Barrett, 2004). In PAL 2013.23.1 only one larger opening can be certainly referred to the aperture of CN XII (Figure 5.3), but due to the slightly damaged region of the exoccipital-basioccipital contact, the presence of an additional exit cannot be excluded.
Taxonomic position of PAL 2013.23.1
Following Thompson et al. (2012) and earlier works used therein (e.g., Coombs and Maryanskya, 1990; Lee, 1996; Vickaryous et al., 2004), the basicranium described above can be referred to the Ankylosauria on the basis of the obliterated cranial sutures, the presence of well developed cranial ornamentation, and the closed supratemporal fenestrae. The nodosaurid status is supported by the slightly domed parietal (Thompson et al., 2012) and the posteroventrally oriented occipital condyle that is formed exclusively by the basioccipital (Vickaryous et al., 2004; this feature defines Group 'C' within Nodosauridae in Thompson et al., 2012). The 'single large median polygon of ornamentation present in the parietal region', regarded as a synapomorphic feature under ACCTRAN for Group 'D' within Nodosauridae by Thompson et al. (2012, suppl. information), is ambiguous in PAL 2013.23.1. This region in the Hungarian specimen is apparently covered with a slightly thickened, ornamented surface but the margins of a single large median polygon cannot be recognized.
Although there is now direct evidence for two sympatric species of ankylosaurs (Hungarosaurus and cf. Struthiosaurus) in the Iharkút fauna (Ősi and Prondvai, 2013), the new braincase can be referred to Hungarosaurus on the basis of the following characters:
- The size, height/width ratio and shape of the occipital condyle of PAL 2013.23.1 is very similar to the occipital condyle preserved in the holotype specimen of Hungarosaurus tormai (MTM Gyn/404).
- The specimen shows several important differences compared to the basicrania of Struthiosaurus spp. The more elongated 'neck' region of the basioccipital, the shape of the occipital condyle, the morphology of the exoccipital, and the more ventral flexure (45°) of the medulla relative to the forebrain clearly distinguish the new specimen from the basicrania of Struthiosaurus.
- The new ankylosaur from Iharkút is represented by a complete, 21 cm long, skeletally mature humerus that suggests a total body length of 2–2.5 m (Ősi and Prondvai, 2013). The estimated size of this small bodied ankylosaur is almost half the length of Hungarosaurus (humerus length: 45.5 cm, adult body length ca. 4 m; Ősi and Makádi, 2009). The new braincase and its preserved occipital condyle (width: 29.5 mm) is from an animal much closer in size to that of Hungarosaurus (occipital condyle width: 32.2 mm) than from the small bodied ankylosaur referred to cf. Struthiosaurus, thus herein we tentatively refer the new specimen to Hungarosaurus sp. Accepting this hypothesis, the braincase is perhaps not from a fully grown, old individual.
IMPLICATIONS FOR ANKYLOSAUR LOCOMOTION
Limb Morphology and Paravertebral Elements
The discovery of the fifth skeleton revealed gracile, elongate forelimb elements and an unusual 1:1 forelimb-hindlimb ratio in Hungarosaurus (Ősi and Makádi, 2007, 2009).
The total length of the humerus+radius is 86.5 cm, whereas the length of the femur+fibula is 85.8 cm. Although the humerus (45.5 cm) is shorter than the femur (49 cm), the length ratio between them is 0.92. This ratio is 0.7 in Sauropelta (Ostrom, 1970), 0.5 in Niobrarasaurus (Carpenter et al., 1995), and approximately 0.54 in Gastonia (Gaston et al., 2001). We assume that in Hungarosaurus the anterior part of the body was in a more elevated position (at least 25% higher) than in other ankylosaurs, suggesting a different, more erect posture. This may be further supported by the extremely small deltopectoral crest of the humerus. This crest does not extend as far distally as in other ankylosaurs, and its lateral extension is also smaller than usual in ankylosaurs. Its relative surface for the attachment of forelimb muscles is almost half that in Sauropelta or in Euoplocephalus (Coombs, 1978b). Sauropsids with a well- developed deltopectoral crest are usually regarded as sprawling animals in which some of the muscles attaching onto this crest are essential to provide support for wide-gauge forelimb posture. However, as noted by Paul and Christiansen (2000), various perissodactyl mammals, including rhinos, also have a well-developed deltopectoral crest, so its development is not necessarily an indicator of sprawling posture. Significant reduction of this crest is, however, a better indicator of an erected forelimb posture.
Long limbs and erected forelimb posture of Hungarosaurus might be associated with a more cursorial habit than in other ankylosaurs. Coombs (1978c) listed cursorial adaptations that would be expected in most cursors and regarded ankylosaurs as 'low-grade to intermediate grade mediportal' animals. In Hungarosaurus, none of the listed adaptations can be seen that do not occur in other ankylosaurs. This indicates that Hungarosaurus was most probably not even a subcursorial or cursorial animal in terms of Coombs (1978c, p. 395). Nevertheless, the relatively more lightly built appendicular skeleton and the elongate forelimb suggest significant differences in posture and locomotion between Hungarosaurus and other ankylosaurs, especially massively built ankylosaurids.
This interpretation is further supported by the presence of paravertebral elements in Hungarosaurus, which are similar in morphology to those described in Minmi (Molnar and Frey, 1987). In the biomechanical model introduced by these authors for Minmi, it was suggested that these paravertebral elements are constructed to withstand flexion and tension, and to stiffen the vertebral column. They concluded that Minmi, in contrast with other massive, heavily armoured ankylosaurs, was probably more of a long distance cursor that obtained speed primarily 'by the action of limbs, rather than by flexion of the vertebral column' (Molnar and Frey, 1987, p. 33). We assume that this model is also applicable for Hungarosaurus, and suggest that the latter, in contrast with other ankylosaurs except for Minmi, was probably more cursorial, with fast limb movement and long stride.
Development of the Cerebellum
As was summarized by Paulin (1993, and references therein), the main function of the cerebellum is to control and coordinate movements. Pearson and Pearson (1976) noted that there is a correlation between the relative size and histological and morphological complexity of the cerebellum and the agility of an animal. Additionally, in mammals (especially in humans) it may also be involved in some cognitive functions such as attention and in regulating fear and pleasure responses, but its movement-related functions are the most solidly established. The cerebellum does not initiate movement, but it contributes to coordination, precision and accurate timing (Fine et al., 2002). Larsell (1967) pointed out that in the turtle Trionyx japonica, having a sensitive carapace, the medial region of the cerebellar cortex is extremely developed and that is related to 'massive tactile sensory input' from the carapace, not to the limited motor capabilities of the dorsal musculature (Paulin, 1993). Studies by Larsell (1967) show that in hummingbirds the legs, used mainly for perching, are not an important sensory organ, so the cerebellar regions related to the use of the legs are poorly developed. The same can be found in the tail feathers: they are extremely important for controlling and stabilizing flight, but it does not seem to be an important sensory structure. Therefore, the lobule I of the cerebellum, related to innervation of the tail, is relatively small (Larsell, 1967; Paulin, 1993).
The endocast taken from the braincase of Hungarosaurus unambiguously indicates that this ankylosaur can be characterized by a dorsally hypertrophied cerebellum in contrast to most ankylosaurs (Coombs, 1978a; Norman and Faiers, 1996; Witmer and Ridgely, 2008). Struthiosaurus transylvanicus and perhaps the adult individuals of S. austriacus are the only other taxa among ankylosaurs to show a similar development of the cerebellum (Nopcsa, 1929; Pereda Suberbiola and Galton, 1994). This neuroanatomical feature, along with the strongly ventrally pointed basisphenoid, might be a synapomorphic feature of Struthiosaurus and Hungarosaurus.
Here we suggest that the gracile and elongate forelimbs, the presence of paravertebral elements along the epaxial musculature and the dorsally hypertrophied cerebellum are all the consequence of a more erected posture and an agile behaviour for Hungarosaurus. That is further supported by the calculated, relatively low body mass (650 kg for an adult, 4 m long animal; Ősi and Makádi, 2009). The elongate and most probably quite erect forelimbs in a lightly built ankylosaur resulted in a relatively more dorsally positioned head than in other ankylosaurs, with short and wider gauge forelimb posture. This demands a more sophisticated cerebral coordination of posture and movement than is expected in other ankyloaurs.
CONCLUSIONS
Partial skull discovered from the Upper Cretaceous (Santonian) of Iharkút is referred to Hungarosaurus sp. on the basis of its similar size and the shape and size of the occipital condyle compared to that of the holotype. In addition, it differs from that of Struthiosaurus spp. in the morphology of the exoccipital, the more elongated 'neck' region of the basioccipital, the shape of the occipital condyle and the more ventral flexure (45°) of the medulla relative to the forebrain. The endocranial cast taken from this specimen of Hungarosaurus has a dorsally hypertrophied cerebellum similarly to that of Struthiosaurus. This feature, along with the ventrally oriented basisphenoid, might be synapomorpies shared by these European ankylosaurs.
The elongate and most probably quite erect forelimbs, the presence of paravertebral elements along the epaxial musculature and the dorsally hypertrophied cerebellum indicate that the posture and movement of Hungarosaurus could have been more advanced, and perhaps this form was more cursorial than in other ankylosaurs.
ACKNOWLEDGEMENTS
The authors wish to thank T.A. Tumanova and S. Brusatte for their useful comments that highly improved the standards of the manuscript. We thank the 2000–2012 field crew for their assistance in the fieldwork. We are especially grateful to the Bakony Bauxite Mining Company and the Geovolán Zrt. for their logistic help, and T. Németh for his cooperation. We thank G. Zboray (Eötvös University), E. Prondvai (ELTE Lendület Dinosaur Research Group) and L. Makádi (MTA–ELTE Dinosaur Research Group) for useful discussions, Zs. Hajdu, D. Csengődi (MTA–ELTE Dinosaur Research Group) and L. Makádi for their technical assistance, and R. Tokai (Diagnostic Institute of University of Kaposvár, Hungary) for making the CT scans on the braincase and J. Abel for creating the Medical Volume Explorer visualization program. Field and laboratory work was supported by the MTA–ELTE Lendület Dinosaur Research Group (Grant no. 95102), Hungarian Scientific Research Fund (OTKA T–38045, PD 73021, NF 84193), National Geographic Society (Grant No. 7228–02, 7508–03), Bolyai Fellowship, Hungarian Natural History Museum, Eötvös Loránd University, Jurassic Foundation and Hantken Foundation. Research of X.P.S. is supported by the Ministerio de Economía y Competitividad of Spain (project CGL2010-18851/BTE) and the Gobierno Vasco/Eusko Jaurlaritza (group IT834-13).
REFERENCES
Averianov, A.O. 2002. An ankylosaurid (Ornithischia: Ankylosauria) braincase from the Upper Cretaceous Bissekty Formation of Uzbekistan. Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, Sciences de la Terre, 72:97–110.
Baumel, J.J. and Witmer, L.M. 1993. Osteologia, p. 45–132. In Baumel J.J. (ed), Handbook of Avian Anatomy: Nomina Anatomica Avium, 2nd edition. Cambridge, Massachusetts, Nuttall Ornithological Society.
Bodor, E.R. and Baranyi, V. 2012. Palynomorphs of the Normapolles group and related plant mesofossils from the Iharkút vertebrate site, Bakony Mountains (Hungary). Central European Geology 55/3:259–292.
Buffetaut, E., Ősi, A., and Prondvai, E. 2011. The pterosaurian remains from the Grünbach Formation (Campanian, Gosau Group) of Austria: a reappraisal. Geological Magazine, 148:334–339.
Bunzel, E. 1870. Notice of a fragment of a reptilian skull from the Upper Cretaceous of Grünbach. Quarterly Journal of the Geological Society London, 24:394.
Bunzel, E. 1871. Die Reptilfauna der Gosau Formation in der Neuen Welt bei Wiener-Neustadt. Abhandlungen der k. k. geologische Reichsanstalt, 5:1–18.
Carpenter, K., Dilkes, D., and Weishampel, D.B. 1995. The dinosaurs of the Niobrara Chalk Formation (Upper Cretaceous, Kansas). Journal of Vertebrate Paleontology, 15:275–297.
Carpenter, K. and Kirkland, J.I. 1998. Review of Lower and Middle Cretaceous ankylosaurs from North America, p. 249–270. In Lucas, S.G., Kirkland, J.I., and Estep, J.W. (eds.), Lower and Middle Cretaceous Terrestrial Ecosystems. New Mexico Museum of Natural History and Science Bulletin.
Carpenter, K., Kirkland, J.I., Burge, D., and Bird, J. 2001. Disarticulated skull of a new primitive ankylosaurid from the Lower Cretaceous of eastern Utah, p. 211–238. In Carpenter, K. (ed.), The Armored Dinosaurs. Indiana University Press, Bloomington.
Company, J., Pereda Suberbiola, X., and Ruiz-Omeñaca, J.I. 2009. Ankylosaurian remains from the Late Cretaceous of Chera (Valencia, Spain), p. 19. In Schwarz-Wings, D., Wings O., and Sattler F. (eds.), Abstract Volume of the 7th Annual Meeting of the European Association of Vertebrate Paleontology. Berlin.
Coombs, W.P., Jr. 1978a. An endocranial cast of Euoplocephalus (Reptilia, Ornithischia). Palaeontographica Abteilung A, 161:176–182.
Coombs, W.P., Jr. 1978b. Forelimb muscles of the Ankylosauria (Reptilia, Ornithischia). Journal of Paleontology, 52:642–657.
Coombs, W.P., Jr. 1978c. Theoretical aspects of cursorial adaptations in dinosaurs. Quarterly Review of Biology, 53:393–418.
Coombs, W.P., Jr. and Maryańska, T. 1990. Ankylosauria, p. 456–483. In Weishampel, D.B, Dodson, P., and Osmólska, H. (eds.), The Dinosauria. California University Press, Berkeley.
Csiki, Z. and Grigorescu, D. 2007. The dinosaur island – new interpretation of the Haţeg Basin vertebrate fauna after 110 years. Sargetia, 20:5–26.
Csontos, L. and Vörös, A. 2004. Mesozoic plate tectonic reconstruction of the Carpathian region. Palaeoclimatology, Palaeoecology, Palaeogeography, 210:1–56.
Eaton, T.H., Jr. 1960. A new armored dinosaur from the Cretaceous of Kansas. University of Kansas, Paleontological Contributions, 25:1–24.
Fine, E.J., Ionita, C.C., and Lohr, L. 2002. The history of the development of the cerebellar examination. Seminars in Neurology, 22:375–384.
Galton, P.M. 1989. Crania and endocranial casts from ornithopod dinosaurs of the families Dryosauridae and Hypsilophodontidae (Reptilia: Ornithischia). Geologica et Palaeontologica, 23:217–239.
Garcia, G. and Pereda Suberbiola, X. 2003. A new species of Struthiosaurus (Dinosauria: Ankylosauria) from the Upper Cretaceous of Villeveyrac (Southern France). Journal of Vertebrate Paleontology, 23:156–165.
Gaston, R.W., Schellenbach, J., and Kirkland, J.I. 2001. Mounted skeleton of the polacanthine ankylosaur Gastonia burgei, p. 386–398. In Carpenter, K. (ed.), The Armored Dinosaurs. Indiana University Press, Bloomington.
Hayakawa, H., Manabe, M., and Carpenter, K. 2005. Nodosaurid ankylosaur from the Cenomanian of Japan. Journal of Vertebrate Paleontology, 25:240–245.
Hopson, J.A. 1979. Paleoneurology. In Biology of the Reptilia, vol. 9: Neurology A, p. 39–146. In Gans, C., Northcutt, R.G., and Ulinski, P. (eds.), Academic Press, New York and London.
Jocha-Edelényi, E. 1988. History of evolution of the Upper Cretaceous Basin in the Bakony Mts at the time of the terrestrial Csehbánya Formation. Acta Geologica Hungarica, 31(1-2):19–31.
Kurzanov, S.M. and Tumanova, T.A. 1978. The structure of the endocranium in some Mongolian ankylosaurs. Paleontological Journal, 1978(3):369–374.
Larsell, O. 1967. The comparative anatomy and histology of the cerebellum from myxinoids through birds. University of Minnesota Press, Minneapolis.
Lee, Y.-N. 1996. A new nodosaurid ankylosaur (Dinosauria: Ornithischia) from the Paw Paw Formation (late Albian) of Texas. Journal of Vertebrate Paleontology, 16:232–345.
Maryańska, T. 1977. Ankylosauridae (Dinosauria) of Asia. Palaeontologia Polonica, 37:85–151.
Miyashita, T., Arbour, V.M., Witmer L.M., and Currie, P.J. 2011. The internal cranial morphology of an armoured dinosaur Euoplocephalus corroborated by X-ray computed tomographic reconstruction. Journal of Anatomy, 219:661–675.
Molnar, R.E. and Frey, E. 1987. The paravertebral elements of the Australian ankylosaur Minmi (Reptilia: Ornithischia, Cretaceous). Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 175:19–37.
Nopcsa, F. 1915. Die Dinosaurier der Siebenbürgischen Landesteile Ungarns. Mitteilungen aus dem Jahrbuch der Ungarische Geologische Reichsanstalt, 23:1–26.
Nopcsa, F. 1923. On the geological importance of the primitive reptilian fauna in the uppermost Cretaceous of Hungary, with a description of a new tortoise (Kallokibotion). Quarterly Journal of the Geological Society London, 72:100–116.
Nopcsa, F. 1929. Dinosaurierreste aus Siebenbürgen, V. Geologica Hungarica, series Palaeontologica, 4:1–76.
Norman, D.B. and Faiers, T. 1996. On the first partial skull of an ankylosaurian dinosaur from the Lower Cretaceous of the Isle of Wight, southern England. Geological Magazine, 133:299–310.
Ősi, A. 2005. Hungarosaurus tormai, a new ankylosaur (Dinosauria) from the Upper Cretaceous of Hungary. Journal of Vertebrate Paleontology, 25:370–383.
Ősi, A. and Makádi, L. 2007. Unusual limb proportions in the Late Cretaceous Hungarosaurus (Ankylosauria), p. 48. In LeLoeuff, J. (ed.), Abstract volume of the 5th Annual Meeting of the European Association of Vertebrate Paleontology.
Ősi, A. and Makádi, L. 2009. New remains of Hungarosaurus tormai (Ankylosauria, Dinosauria) from the Upper Cretaceous of Hungary: skeletal reconstruction and body mass estimation. Paläontologische Zeitschrift, 83:227–245.
Ősi, A. and Mindszenty, A. 2009. Iharkút, Dinosaur-bearing alluvial complex of the Csehbánya Formation, p. 51–63. In Babinszky E. (ed.), Cretaceous sediments of the Transdanubian Range. Field guide of the geological excursion organized by the Sedimentological Subcommission of the Hungarian Academy of Sciences and the Hungarian Geological Society, Budapest, Hungary.
Ősi, A. and Prondvai, E. 2013. Sympatry of two ankylosaurs (Hungarosaurus and cf. Struthiosaurus) in the Santonian of Hungary. Cretaceous Research 44:58–63.
Ősi, A., Rabi, M., Makádi, L., Rabi, M., Szentesi, Z., Botfalvai, G., and Gulyás, P. 2012. The Late Cretaceous continental vertebrate fauna from Iharkút (Western Hungary): a review, p. 532–569. In Godefroit, P. (ed.), Bernissart Dinosaurs and Early Cretaceous Terrestrial Ecosystems. Indiana University Press, Bloomington.
Ostrom, J.H. 1961. Cranial morphology of the hadrosaurian dinosaurs of North America. Bulletin of the American Museum of Natural History, 122:33–186.
Ostrom, J.H. 1970. Stratigraphy and paleontology of the Cloverly Formation (Lower Cretaceous) of the Bighorn Basin area, Wyoming and Montana. Bulletin of the Peabody Museum of Natural History, 35:1–234.
Parish J.C. 2005. The Evolution and Palaeobiology of the Armoured Dinosaurs (Ornithischia: Ankylosauria). Unpublished PhD dissertation, University of Oxford, 471 pp.
Parish, J.C. and Barrett, P.M. 2004. A reappraisal of the ornithischian dinosaur Amtosaurus magnus Kurzanov and Tumanova 1978, with comments on the status of A. archibaldi Averianov 2002. Canadian Journal of Earth Sciences, 41:299–306.
Parsons, W.L. and Parsons, K.M. 2009. A new ankylosaur (Dinosauria: Ankylosauria) from the Lower Cretaceous Cloverly Formation of central Montana. Canadian Journal of Earth Sciences, 46:721–738.
Paul, G.S. and Christiansen, P. 2000. Forelimb posture in neoceratopsian dinosaurs: Implications for gait and locomotion. Paleobiology, 26:450–465.
Paulin, M.G. 1993. The role of the cerebellum in motor control and perception. Brain, Behaviour and Evolution, 41:39–50.
Pearson, R. and Pearson, L. 1976. The Vertebrate Brain. London: Academic Press.
Pereda Suberbiola, X. 1992. A revised census of European Late Cretaceous nodosaurids (Ornithischia: Ankylosauria): last occurrence and possible extinction scenarios. Terra Nova, 4:641–648.
Pereda Suberbiola, X. 1993. Armoured dinosaurs from the Late Cretaceous of southern France: a review. Revue de Paléobiologie, vol. spéc. 7:163–172.
Pereda Suberbiola, X. 1999. Ankylosaurian dinosaur remains from the Upper Cretaceous of Laño (Iberian Peninsula). Estudios del Museo de Ciencias Naturales de Alava, 14 (Núm. Espec. 1):273–288.
Pereda Suberbiola, J. and Galton, P.M. 1992. On the taxonomic status of the dinosaur Struthiosaurus austriacus from the Late Cretaceous of Austria. Comptes Rendus de l'Académie des Sciences Paris, 315 (II):1275–1280.
Pereda Suberbiola, J. and Galton, P.M. 1994. Revision of the cranial features of the dinosaur Struthiosaurus austriacus Bunzel (Ornithischia: Ankylosauria) from the Late Cretaceous of Europe. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen,191:173–200.
Pereda Suberbiola, J. and Galton, P.M. 2001. Reappraisal of the nodosaurid ankylosaur Struthiosaurus austriacus Bunzel from the Upper Cretaceous Gosau beds of Austria, p. 173–210. In Carpenter, K. (ed.), The Armored Dinosaurs., . Indiana University Press, Bloomington.
Seeley, H.G. 1881. On the reptile fauna of the Gosau Formation preserved in the Geological Museum of the University of Vienna. Quarterly Journal of the Geological Society London, 37:620–707.
Sullivan, R.M. 1999. Nodocephalosaurus kirtlandensis, gen. et sp. nov., a new ankylosaurid dinosaur (Ornithischia: Ankylosauria) from the Upper Cretaceous Kirtland Formation (Upper Campanian), San Juan Basin, New Mexico. Journal of Vertebrate Paleontology, 19:126–139.
Tumanova, T.A. 1987. The armored dinosaurs of Mongolia. Transactions of the joint Soviet-Mongolian Paleontological Expedition, 32: 1–76.
Thompson, R.S., Parish, J.C., Maidment, S.C.R., and Barrett, P.M. 2012. Phylogeny of the ankylosaurian dinosaurs (Ornithischia: Thyreophora). Journal of Systematic Palaeontology, 10:301–312.
Vickaryous, M.K. and Russell, A.P. 2003. A redescription of the skull of Euoplocephalus tutus (Archosauria: Ornithischia): a foundation for comparative and systematic studies of ankylosaurian dinosaurs. Zoological Journal of the Linnean Society, 137:157–186.
Vickaryous, M.K., Maryánska, T., and Weishampel, D.B. 2004. Ankylosauria, p. 363–392. In Weishampel, D.B., Dodson, P., and Osmólska, H. (eds.), The Dinosauria, 2nd edition. University of California Press, Berkeley.
Weishampel, D.B., Csiki, Z., Benton, M.J., Grigorescu, D., and Codrea, V. 2010. Palaeobiogeographic relationships of the Hateg biota - Between isolation and innovation. Palaeogeography, Palaeoclimatology, Palaeoecology, 293:419 p. 173–210437.
Witmer, L.M. and Ridgely, R.C. 2008. The paranasal air sinuses of predatory and armored dinosaurs (Archosauria: Theropoda and Ankylosauria) and their contribution to cephalic structure. Anatomical Record, 291:1362–1388.

