 Termite nests in eolian backshore settings: An unusual record throughout the Quaternary in the Neotropical realm
Termite nests in eolian backshore settings: An unusual record throughout the Quaternary in the Neotropical realm
Article number: 24.1.a15
https://doi.org/10.26879/1146
Copyright Palaeontological Association, April 2021
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 21 December 2020. Acceptance: 20 April 2021.
ABSTRACT
In this paper, we report the unusual presence of extant termite nests in frontal beach eolian sandy substrates in the Rio Grande do Sul Coastal Plain (southernmost Brazil) and describe the nest architectures, aiming to compare them with termite fossil nests preserved in Pleistocene backshore deposits of this same coastal plain. Four sites were analyzed along the modern beach, and the architecture of the termite nests was described. The nests are composed of a rounded convex epigeal portion and a hypogeal portion with variable dimensions formed by interconnecting spherical to semispherical chambers. Considering that termites are assumed to colonize more mature, vegetated soils due to the need for abundant cellulosic material, the occurrence of termite nests in backshore dunes close to the shoreline opens a discussion about the distribution and habit of termites and the applicability of their trace fossils for paleoenvironmental reconstitutions.
Kimberly Silva Ramos. ICHNOS Research Group, Geology Graduate Program, Unisinos University, Av. Unisinos, 950, 93022-000, São Leopoldo, RS, Brazil. kimberlyr@edu.unisinos.br
Renata Guimarães Netto. ICHNOS Research Group, Geology Graduate Program, Unisinos University, Av. Unisinos, 950, 93022-000, São Leopoldo, RS, Brazil. nettorg@unisinos.br
Daniel Sedorko. ICHNOS Research Group, Geology Graduate Program, Unisinos University, Av. Unisinos, 950, 93022-000, São Leopoldo, RS, Brazil and Universidade Federal de Uberlândia, Laboratório de Paleontologia Aplicada, Campus Monte Carmelo. Av. XV de Novembro, 501, Boa Vista, Monte Carmelo - MG. 38500-000, Brazil. sedorko@ufu.br
Key words: actuopaleontology; coastal environments; paleobiogeography; eolian dunes; neoichnology; Termitichnus
Final citation: Ramos, Kimberly Silva, Netto, Renata Guimarães, and Sedorko, Daniel. 2021. Termite nests in eolian backshore settings: An unusual record throughout the Quaternary in the Neotropical realm. Palaeontologia Electronica, 24(1):a15. https://doi.org/10.26879/1146
palaeo-electronica.org/content/2021/3342-termite-nests-in-backshore-settings
Copyright: April 2021 Palaeontological Association. This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Termites are social insects within the Blattodea order that construct complex ground nests (Inward et al., 2007). This group occurs in several modern environments, including grasslands and vegetated zones close to or inside tropical forests, and is restricted to the latitudinal interval 0º to 50º in the Northern and Southern hemispheres (Lee and Wood, 1971; Su, 2003). Unlike ants that commonly nest in coastal dunes (e.g., Chen et al., 2015), termites are seldom registered in these settings. A few studies reported termite nests in sandy soils in coastal environments (e.g., Kaiser, 1953; Araújo and Fontes, 1979; Fontes, 1979, 1982, 1992; Vasconcellos et al., 2005; Diehl et al., 2014, 2015; Santos, 2015), mostly in denser vegetated areas of restinga ecosystems (e.g., Moura and Machado, 2017). However, these occurrences are rare compared with termite nests in mature soils (e.g., Martius, 1994; Bandeira et al., 2003; Oliveira et al., 2013; Cancello et al., 2014; Plaza and Galbiati, 2017). Indeed, termite nests in barely vegetated dunes in backshore settings are virtually unrecorded.
Abundant trace fossils attributed to ants and termites, along with other such insects like beetles, solitary wasps, and cicadas, occur in the Upper Pleistocene eolian deposits of the Rio Grande do Sul Coastal Plain (PCRS, as it is known in Brazil), southernmost Brazil (Gibert et al., 2006). This insect trace fossil assemblage descends from a well-marked Fe-rich surface that suggests a depositional hiatus, leading Netto et al. (2007) to preliminarily infer colonization of more mature soils in alluvial deposits that settled over the eolian dunes due to a significant sea-level fall. Among all insect groups represented in this trace fossil assemblage, only termites do not leave the burrow to feed and necessarily have to construct their nests in vegetated areas or nearby. Thus, they are the best biomarker to characterize the type of paleosol developed at the top of the Pleistocene deposits, which is crucial to understand better the sedimentary dynamics involved in the PCRS evolution during the terminal Pleistocene.
Thus, to test the hypothesis erected by Netto et al. (2007), we performed a search for termite nests in the modern coastal dune deposits of the PCRS (barrier-lagoon system IV, Holocene to recent), which is considered a modern analog for the Upper Pleistocene coastal settings (barrier-lagoon system III) (e.g., Tomazelli and Vilwock, 2000; Carassai, 2013). Surprisingly, this search revealed extant termite nests in the barely vegetated frontal eolian dunes in different latitudes along the PCRS and a few meters from the sea. Hence, the objective of this study is threefold: (i) to report the unusual occurrence of termite nests in sandy substrates from frontal beach eolian dunes; (ii) to characterize the architecture of these termite nests for actuopaleontological approaches; and (iii) to highlight the relevance of these occurrences for the insect trace fossil record in paleosols.
BACKGROUND
Social insect nests are distributed in several continental sub-environments, from sparsely vegetated areas to humid soils associated with forests (Genise, 2017). This large distribution allows the application of nest architecture as an auxiliary tool to interpret depositional settings in continental paleoenvironments. Termites (Order Blattodea), which are the object of this study, live in colonies and are organized into castes formed by the breeding couple and sterile individuals, the latter subdivided into workers and soldiers. Workers are responsible for food capture and nest building while soldiers defend the colony (Brusca and Brusca, 2007).
Termites feed mainly from cellulosic material inside the nest. Thus, they tend to nidify in soils rich in grass, herbaceous plants, wood, fungi, excrement, animal remains, lichens, and organic matter. Termites can obtain energy from these materials due to the protozoa present in their intestine (e.g., Lima and Costa, 2007). They also depend on humidity to avoid body desiccation. Thus, unlike other social insects, the termite nest architecture guarantees internal microclimate maintenance, keeping the local humidity and controlling the relative heat (Redford, 1984; Noirot and Darlington, 2000; Genise et al., 2004; Korb, 2011). The termite's constructive behavior changes the substrate, increasing the porosity and mixing minerals, depositing in, or extracting them from the substrate (Wood and Sands, 1978). Termites are ecosystem “engineers”, influencing the availability of resources for other organisms and modifying the edaphic trophic relations (Lavelle et al., 1997).
The oldest putative record of termites is from the Lower Cretaceous (Jarzembowski, 1981; Martinez and Martinell, 1995). In the PCRS, social insect nests are common in the eolian deposits of the lagoon-barrier systems III (Upper Pleistocene), represented by the ichnogenera Termitichnus, Vondrichnus, and Krausichnus (Gibert et al., 2006), and IV (Holocene to Recent), represented by their modern counterparts. Ant nests are widespread throughout the east to west modern backshore extension and commonly occur in the foredunes. Termites are less frequent and show a more consistent distribution in the inner portion of the backshore, in vegetated areas that characterize the restinga ecosystem (Diehl et al., 2014, 2015). The occurrence of termite nests in the foredunes was unrecorded until now.
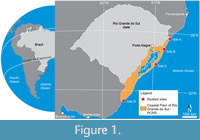 The PCRS is a geological province that characterizes the Atlantic coastal plain of the Rio Grande do Sul State (Figure 1) and comprises the exposed portion of the Pelotas Basin, a marginal rift basin formed due to the South Atlantic Ocean opening in the Cretaceous (Milani et al., 2007). The PCRS is composed mostly of sandy and minor silty deposits accumulated during four previous falling and rising sea-level cycles. Each cycle represents the establishment of a lagoon-barrier system (LBS), the oldest one (LBS I) formed at the end of the Pliocene, the following ones (LBS II-III) during the Pleistocene, and the last (LBS IV) during the Holocene (Tomazelli and Villwock, 2000). The sandy deposits represent beach barriers and show two main sedimentary facies associations: a marine facies that contains deposits formed by the wave and current action in shoreface and foreshore settings, and an eolian facies, representing backshore settings (Gibert et al., 2006).
The PCRS is a geological province that characterizes the Atlantic coastal plain of the Rio Grande do Sul State (Figure 1) and comprises the exposed portion of the Pelotas Basin, a marginal rift basin formed due to the South Atlantic Ocean opening in the Cretaceous (Milani et al., 2007). The PCRS is composed mostly of sandy and minor silty deposits accumulated during four previous falling and rising sea-level cycles. Each cycle represents the establishment of a lagoon-barrier system (LBS), the oldest one (LBS I) formed at the end of the Pliocene, the following ones (LBS II-III) during the Pleistocene, and the last (LBS IV) during the Holocene (Tomazelli and Villwock, 2000). The sandy deposits represent beach barriers and show two main sedimentary facies associations: a marine facies that contains deposits formed by the wave and current action in shoreface and foreshore settings, and an eolian facies, representing backshore settings (Gibert et al., 2006).
The LBS IV characterizes the modern beach deposits in the littoral. The LBS IV backshore deposits are composed exclusively of fine-grained, well-sorted quartz sand, except for shell fragments blown by the wind from the foreshore to the foredunes. The backshore extension is variable throughout the PCRS, narrower in the NNE and wider in SSW. Vegetation is generally scarce in the foredunes and restricted to areas where some water supply exists. Vegetation is represented mostly by xerophytic grasses, but other groups of herbaceous angiosperm plants and ferns from the dune pioneer vegetation also occur. The phreatic line is shallow in the backshore zone and oscillates according to storm surges and exceptional tides. Tides range from 30 to 50 cm on average, characterizing a micro-tidal regime (Netto and Grangeiro, 2009). Regarding climatic pattern, temperatures are variable throughout the year in the PCRS, with the minimal average recorded in site D (7.2 ºC in July, South Hemisphere winter) and maximum average record in site C (31.7 ºC in January, South Hemisphere summer) (Table 1). Rain is well-distributed, oscillating between 74 mm in the drier month and 164 mm in the rainiest month (Table 1). The winds are constant along the year in the whole coastal plain, with average annual winds between 7.0 to 8.0 m/s in 50 m height (Amarante and Silva, 2002).
MATERIAL AND METHODS
Four equidistant localities were selected for prospection throughout the eolian deposits of the PCRS (sites A, B, C, and D, Figure 1). Termite nests were found in all sites and analyzed in situ, being numbered according to the site of occurrence (e.g., A.1, A.2, A.3 for site A). The nest architecture was described, and internal morphological variations were accessed by successive cuts in the nest structure and casting. Nest casts were made in sites B and D using an orthophthalic resin catalyzed by butanox 5%. The entire nest architecture was characterized; however, the deeper portions received more attention for comparison purposes with the fossil nests due to their higher fossilization potential. Comparisons between the modern and ancient nest architectures were based on Genise (2017). Termites were collected and identified at the family level (identification key of Constantino, 1999).
RESULTS
 Field Observations
Field Observations
While ant nests are abundant and widespread in the eolian dunes of the PCRS, termite nests are less abundant and restricted to vegetated areas associated with some freshwater supply in the surrounding zone. All observed nests present a dome-shape with no open chimneys, varying in diameter, height, and depth as detailed below.
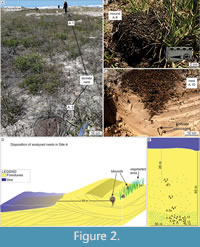
Site A. Termite nests from site A (Itapeva Beach; Figure 1) form dense patches of nests in frontal eolian dunes where water supply is available in the interdunes (Figure 2D), and the patches are ~30 m distant from the high tide line. Angiosperms from the families Gramineae, Hypericaceae, Araliaceae, Asteraceae, Juncaceae, and some ferns (family Equisetaceae and others) constitute the vegetation of the foredunes. Termite nest concentration is higher where vegetation is denser, with ferns predominating (Figure 3C).
 The hypogeal nest portion exhibits a gradual downward reduction in diameter, finishing in few horizontally-oriented passages that reach 60 cm in length. Some passages are produced inside the roots that the termites ate. The average nest mound diameter in site A is 21.27 cm, and the average height is 21.15 cm. The depth of the nest below the surface is 54.23 cm (Table 2). One nest showed a large and empty chamber in which coleopteran larvae were observed (Figure 3A-B). Termites were active in all levels of the nest.
The hypogeal nest portion exhibits a gradual downward reduction in diameter, finishing in few horizontally-oriented passages that reach 60 cm in length. Some passages are produced inside the roots that the termites ate. The average nest mound diameter in site A is 21.27 cm, and the average height is 21.15 cm. The depth of the nest below the surface is 54.23 cm (Table 2). One nest showed a large and empty chamber in which coleopteran larvae were observed (Figure 3A-B). Termites were active in all levels of the nest.
Site B. Only five termite nests sparsely distributed along the eolian dunes were observed in site B (Tramandaí Beach; Figure 1). The nest closest to the shoreline was casted, while the others were only measured (Table 2). The termite nests from site B also occur in the foredunes and are ~50 m from the high tide line (Figure 4D). Angiosperms from the families Gramineae, Araliaceae, and Juncaceae characterize the primary vegetation in site B (Figure 4A).
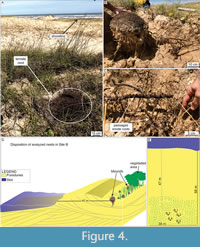 The hypogeal nest portion exhibits a gradual downward reduction in diameter, finishing in a few horizontally-oriented passages that can reach 70 cm in length, mostly produced inside roots consumed by the termites. The average mound diameter in site B is 23.42 cm, and the average height is 19.56 cm. The nest reaches 94.5 cm in depth below the surface (Table 2), but only one nest was excavated in this locality (Figure 4). Termites were active in all levels of the nest.
The hypogeal nest portion exhibits a gradual downward reduction in diameter, finishing in a few horizontally-oriented passages that can reach 70 cm in length, mostly produced inside roots consumed by the termites. The average mound diameter in site B is 23.42 cm, and the average height is 19.56 cm. The nest reaches 94.5 cm in depth below the surface (Table 2), but only one nest was excavated in this locality (Figure 4). Termites were active in all levels of the nest.
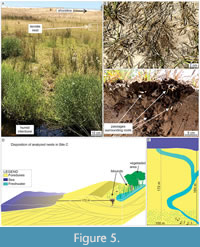 Site C. This site (Mostardas Beach; Figure 1) has the smallest density of termite nests among the studied areas; only three nests were found. The termite nest closest to the coastline was located 174 m from the high tide line, next to a freshwater stream in the interdune zone (Figure 5). The primary vegetation explored by termites in these nests is grass (Gramineae). However, plants from the family Juncaceae and other angiosperm taxa also occur on the stream edges.
Site C. This site (Mostardas Beach; Figure 1) has the smallest density of termite nests among the studied areas; only three nests were found. The termite nest closest to the coastline was located 174 m from the high tide line, next to a freshwater stream in the interdune zone (Figure 5). The primary vegetation explored by termites in these nests is grass (Gramineae). However, plants from the family Juncaceae and other angiosperm taxa also occur on the stream edges.
 The nest mounds are very small (4.36 cm high on average) compared to the other sites' nests (Table 2). However, the nests show a downward enlargement below the general funnel that characterizes the bottom of the termite nests in the PCRS, resembling a downward helicoidal pattern in passages distribution (Figure 6). This particular architectural detail was observed exclusively in site C. Termites were active in all nest levels.
The nest mounds are very small (4.36 cm high on average) compared to the other sites' nests (Table 2). However, the nests show a downward enlargement below the general funnel that characterizes the bottom of the termite nests in the PCRS, resembling a downward helicoidal pattern in passages distribution (Figure 6). This particular architectural detail was observed exclusively in site C. Termites were active in all nest levels.
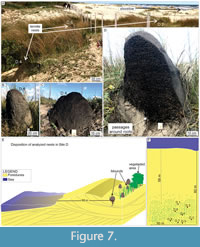 Site D. The termite nests from site D (Barra do Chuí Beach; Figure 1) are ~58 m from the high tide line and are common along the foredunes, mostly in lee side and interdunes, concentrated in the sand strip between 60 and 80 m from the sea (Figure 7E). The surrounding vegetation is primarily of plants from families Gramineae and Araliaceae.
Site D. The termite nests from site D (Barra do Chuí Beach; Figure 1) are ~58 m from the high tide line and are common along the foredunes, mostly in lee side and interdunes, concentrated in the sand strip between 60 and 80 m from the sea (Figure 7E). The surrounding vegetation is primarily of plants from families Gramineae and Araliaceae.
The nests present a bigger epigeal portion than the other sites’ nests, reaching 1.10 m in high and going deeper into the substrate (Table 2). The nest structure is characterized by dense passages regularly distributed in the epigeal portion. However, in the hypogeal portion, it assumes a less dense distribution with thicker passages. The black color of D.4 nest is due to the occurrence of Pleistocene peat deposits buried 2 m below the substrate surface (Figure 8). Termites were active in all levels of the nest.
Nest Morphology and Casts
 The hypogeal portion of nests B.1 and D.4 was cast by resin to highlight the underground structures that present higher fossilization potential. The casts revealed a similar architecture in both nests, except for more passages inside roots in nest B.1 (Figure 4C) than in D.4 (Figure 8).
The hypogeal portion of nests B.1 and D.4 was cast by resin to highlight the underground structures that present higher fossilization potential. The casts revealed a similar architecture in both nests, except for more passages inside roots in nest B.1 (Figure 4C) than in D.4 (Figure 8).
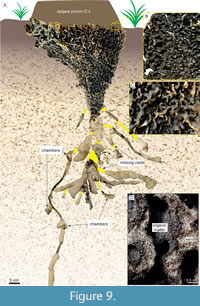 A general downward funnel-shaped structure characterizes the hypogeal portion in both casted nests, with condensed passages (Figure 9A-D) from epigeal portion converging to horizontally-oriented passages presenting larger diameters. In nest D.4, the deepest structures spread from this condensed part (Figure 9A), reaching 132.5 cm in depth. The nests present lateral projections that can reach 70 cm in length, following the root systems. Some of the horizontal passages contain small chambers connected (up to 5 cm in diameter; Figure 9A). Successive cuts evidenced the hypogeal architecture in the nest C.1, which differs from casted nests by the general helicoidal downward arrangement (Figure 6) and absence of lateral projections. In all observed nests, the sediment used by termites is predominantly quartz-sand, while the passages are revested by dark, thinner sediment with organic matter (Figure 9D). Termites were present in every level of the casted nests.
A general downward funnel-shaped structure characterizes the hypogeal portion in both casted nests, with condensed passages (Figure 9A-D) from epigeal portion converging to horizontally-oriented passages presenting larger diameters. In nest D.4, the deepest structures spread from this condensed part (Figure 9A), reaching 132.5 cm in depth. The nests present lateral projections that can reach 70 cm in length, following the root systems. Some of the horizontal passages contain small chambers connected (up to 5 cm in diameter; Figure 9A). Successive cuts evidenced the hypogeal architecture in the nest C.1, which differs from casted nests by the general helicoidal downward arrangement (Figure 6) and absence of lateral projections. In all observed nests, the sediment used by termites is predominantly quartz-sand, while the passages are revested by dark, thinner sediment with organic matter (Figure 9D). Termites were present in every level of the casted nests.
 Successive cuts allowed accessing the architecture of the epigeal portion of the nests. Except for nest A.3 (Figure 3A-B), the epigeal passages' disposition is similar in all analyzed nests. The nest A.3 presents a large chamber in the middle of the epigeal portion (Figure 3A). The main difference between all mounds resides in their dimensions, mostly the nest height, which varies from 4 cm (nest C.1) to 110 cm (nest D.6) (Table 2).
Successive cuts allowed accessing the architecture of the epigeal portion of the nests. Except for nest A.3 (Figure 3A-B), the epigeal passages' disposition is similar in all analyzed nests. The nest A.3 presents a large chamber in the middle of the epigeal portion (Figure 3A). The main difference between all mounds resides in their dimensions, mostly the nest height, which varies from 4 cm (nest C.1) to 110 cm (nest D.6) (Table 2).
The collected specimens were mostly composed of workers. However, the occasional presence of soldiers allowed attribution to the family Termitidae (Figure 10).
DISCUSSION
The architecture of the termite nests constructed in the modern foredunes comprises three main elements: a thick-walled mound, one or more chambers, and a root-like tunnel system. The number of chambers per nest and their size and the size of the nest are variable. According to Korb (2011), the mound’s architecture is conditioned by balancing the need for ventilation and thermoregulation. Some fungus-growing termites construct thick-walled mounds to reduce heat loss to the outside, an adaptation to cooler conditions. The ventilation mechanism is more debated; in general, it is triggered by temperature gradients within the mound and between the mound and its exterior environment, resulting in heat convection (Korb, 2011). Considering that the average temperature in the studied sites is low compared with most soils where termites occur in Brazil, the thick walls found in the epigean mound might be a strategy for thermoregulation. However, these variations might also be linked to colony’s size and the time of colonization, as size variations are observed in nests constructed in the same site (e.g., sites A and B).
The smallest mounds occur in site C, which is also the less dense site. Although this site is located close to a large dune field (Mostardas dunes), the humidity was not a restricting factor for the colonies’ maturity because site C has a perennial stream nearby. Similarly, the substrate composition is mostly quartz-sand in all sites. We attribute the small dimensions to the dominance of grasses and an inferred absence of organic bed below the surface. The general low density can be attributed to intermittent dune migration, which precludes perennial colonization of the mounds. Similarly, site B also presents a few termite nests and is located close to a dune field (Tramandaí dunes). We discard anthropic degradation as responsible for the small size because all four sites occur in low anthropized dune fields in the periphery of urban areas.
Medium-sized mounds occur in sites A and B, and although they are also close to dune fields, the vegetation is more diverse than in site C. Thus, we infer that this richness in cellulosic source allowed better development of the colonies. The central chamber in nest A.3, a feature not observed in any of the other described nests, suggests a ventilation structure.
The larger mound is from site D, reaching 1.10 m in height. We attributed it to the association of taller and denser vegetation in site D than in other sites and an organic-rich bed 2 m below the surface, from where the colony catches sediments to build the nests or feed the larvae. These attributes allowed the development of long-term colonization.
Contrary to the general assumption that termites are consumers of wood, they can feed from different organic content disposed in the substrate, in various stages of decomposition, such as wood (live or dead), grasses, herbaceous plants, fungi, nests built by other species of termites, animal droppings and carcasses, and organic material present in the soil (Noirot, 1992; Sleaford et al., 1996; Miura and Matsumoto, 1997; Donovan et al., 2001). Thus, termite nests are not restricted to vegetated areas; well-developed nests also occur in organic-rich soils (as described for site D). The reworking of deeper sediments to the surface has been reported in Rhinotermitidae and Termitidae families (Nutting et al., 1987), but in all sites we only identified Termitidae specimens.
Dune pioneer vegetation is the main food source for termites in all studied sites, although ferns also occur. Their root systems and rhizomes are frequently found inside the nests, allowing us to infer that the termites nesting in the PCRS modern foredunes are mostly cellulosic feeders. According to Diehl et al. (2015), the termites that inhabit the areas close to the shoreline in the surroundings of site A are wood-feeders, humus-feeders, and intermediate in feeding habits. Thus, the presence of roots and rhizomes inside the nests and the poor-organic richness of the sandy substrate suggest a wood-feeder trophic habit. However, the thick-walled mounds point to a fungus-growing habit for these termites as well (Korb, 2011).
The distribution of termite nests in sandy, nutrient-poor substrates along the ~600 km of extension of the PCRS suggests that this is not an accidental occurrence. Termites have been recorded in Brazilian coastal areas, in the so-called restinga ecosystems, but always associated with arboreal vegetation (e.g., Kaiser, 1953; Araújo and Fontes, 1979; Fontes, 1979, 1982, 1992; Vasconcellos et al., 2005; Santos, 2015). Termites from the families Kalotermitidae and Termitidae were also reported in the PCRS by Diehl et al. (2014, 2015), mostly in the non-marine zones, except in Torres (Figure 1) where termites occur closer to the shoreline in vegetated areas. The areas sampled by Diehl et al. (2014, 2015) in Torres are a few kilometers north of Itapeva Beach (site A, Figure 1) and contain termites from the species Rugitermes sp., Anoplotermes sp., Aparatermes sp., and Araujotermes caissara (Diehl et al., 2015). However, no detail about the nest architecture of these species was mentioned. In our study, only termitids were found associated with dunes close to the shoreline.
Termite nests were confidently reported in the fossil record since the Late Cretaceous (e.g., Genise, 2017). Based on the average distribution and biology of extant termites, they have been used as a bioindicator of mature paleosols (e.g., Smith et al., 1993; Genise et al., 2000). The presence of termite nests in loose sediments of foredunes places new light on this paradigm and expands the paleoecological discussion regarding this insect group.
Termite nests have a high potential for fossilization, considering that they can reach up to 1 m depth. The mixture of sediment, feces, and spittle used to construct the nest chambers provides a strong cement that protects the nest from erosion, even in loose substrates (Ferreira et al., 2011). Unlike ants, termites rarely go outside the nest to collect their food. Thus, the nests need to be constructed in substrates that provide their food, mostly root systems or organic-rich soils, and a fair amount of humidity for fungi cultivation (Ferreira et al., 2011). However, in eolian substrates, humidity is low, and root systems are superficial and have a low preservation potential. Then, with rare exceptions, the presence of termite nests in ancient eolian deposits is not considered.
Fossil termite nests were reported in the Late Pleistocene deposits of the PCRS by Gibert et al. (2006) and Netto et al. (2012), represented by the ichnogenera Krausichnus (later discussed by Genise et al., 2013, as potential ant structures), ?Vondrichnus, and Termitichnus. These trace fossils occur together with other ichnotaxa representing insect nests or breeding chambers (e.g., ichnogenera Celliforma, Coprinisphaera, Tesseirei) in eolian sandy deposits formed in backshore settings. Rhizobioturbation is discrete in the stratigraphic levels containing insect trace fossils. However, root casts are abundant in upper levels (Netto et al., 2012, figure 17A-B). The morphology of the epigeal portion of the extant nests found in the backshore deposits of the PCRS and the downward funnel-shaped condensed passages resemble Termitichnus. Additionally, they are similar in size and share the same sub-spherical to ovoid chamber pattern connected in the bottom by primary and secondary burrows (e.g., Genise, 2017) (Figure 9). Nests with architecture similar to the ichnogenus Vondrichnus were not found in the modern deposits of the PCRS.
Although modern architecture and building strategies of termite nests are known, few studies focused on comparison with analogous trace fossils (e.g., Genise, 2017). Modern nests are investigated mostly in search of similarities between structures and taxonomic groups to infer whether morphology reflects the taxa. For example, Amitermes meridionalis and A. laurensis from Australia have mounds oriented to the solar light to keep the structures warm (Jacklyn, 1992). However, the potential of fossilization of the epigeal portion is low. If preserved, this ichnoassemblage would be represented by the prevalence of termite nests (ant nests are usually shallow in this environment, reworking less than 20 cm).
Ichnoassemblages dominated by termite structures were referred to represent the Termitichnus ichnofacies and are assumed as restricted to paleosols of forest environments (e.g., Genise and Bown, 1994; Genise et al., 2000). However, considering that termite nests can occur in several sub-environments (for example, as part of Coprinisphaera ichnofacies), the occurrence exclusively of termite structures is not an unequivocal indication of the Termitichnus ichnofacies (Genise, 2017). Thus, more neoichnological studies involving termite nests are crucial to improve their value for paleoenvironmental reconstructions and stratigraphic applications, and also to refine the significance of the Termitichnus ichnofacies.
CONCLUSION
This study reports termite nests associated with foredunes in shoreline settings along the PCRS (southern Brazil). The presence of nests with similar architecture and located in a similar environment along ~600 km corroborates that termites can colonize poorly vegetated sandy substrates close to high-tide level. Besides the biological significance, the reported Termitichnus -like nests in modern backshore dunes close to the shoreline opens a discussion related to the Termitichuns ichnofacies, for now defined by the dominance of termite trace fossils in well-vegetated continental settings.
ACKNOWLEDGMENTS
The authors thank the Brazilian Council for Scientific and Technological Development (CNPq) for the research grant 424237/2018-0 that supported this research; to itt Fossil - UNISINOS for resin and casts storage; to J. Villegas-Martín for fieldwork support; to L.A. Hartmann for the valuable suggestions in the first draft; to the two anonymous reviewers and to the editor J. Smith for valuable suggestions. KSR thanks to CNPq for the Master of Science fellowship (process 130470/2019-8) and to FAPERGS/CAPES Consortium (Edital 03/2018 - PRÓ-EQUIPAMENTOS (proc. 18/2551-0000429-4), which provided logistical support for this work. RGN thanks CNPq for the research fellow grants 303863/2016-1 and 310377/2019-6. DS thanks to CNPq for post-doctoral fellowship (process 159548/2018-7). This paper is a contribution to the BRASILEX Project from Unisinos University.
REFERENCES
Amarante, O.A.C. and Silva, F.J.L. 2002. Atlas eólico: Rio Grande do Sul. Secretaria de Energia Minas e Comunicações, Porto Alegre. http://www.isl2024.org.br/sistema/uploads/postagens/55/arquivos/atlas-eolico-rs.pdf
Araújo, R.L. and Fontes, L.R. 1979. Notes on the Neotropical genus Tauritermes, with a new species from Brazil (Isoptera, Kalotermitidae). Revista Brasileira de Entomologia, 23:20-34.
Bandeira, A.G., Vasconcellos, A., Silva, M.P., and Constantino, R. 2003. Effects of habitat disturbance on the termite fauna in a highland humid forest in the caatinga domain. Sociobiology, 42:117-127.
Brusca, R.C. and Brusca, G.J. 2007. Invertebrados, Segunda edição. Editora Guanabara-Koogan, Rio de Janeiro.
Cancello, E.M., Silva, R.R., Vasconcellos, A., Reis, Y.T., and Oliveira, L.M. 2014. Latitudinal variation in termite species richness and abundance along the Brazilian Atlantic Forest hotspot. Biotropica, 46:441-450. https://doi.org/10.1111/btp.12120
Carassai, J.J. 2013. A deriva litorânea e suas implicações na gênese e orientação de barreiras arenosas pleistocênicas (região de Osório), estado do Rio Grande do Sul, sul do Brasil. Unpublished Master's Thesis, Unisinos University, São Leopoldo, RS, Brazil.
Chen, X., Adams, B., Bergeron, C., Sabo, A., and Hooper-Bu'i, L. 2015. Ant community structure and response to disturbances on coastal dunes of Gulf of Mexico. Journal of Insect Conservation, 19:1-13. https://doi.org/10.1007/s10841-014-9722-9
Constantino, R. 1999. Chave ilustrada para identificação dos gêneros de cupins (Insecta: Isoptera) que ocorrem no Brasil. Papéis Avulsos de Zoologia, 25:387-448.
Diehl, E., Diehl-Fleig, E., Albuquerque, E.Z. de, and Junqueira, L.K. 2014. Richness of termites and ants in the state of Rio Grande do Sul, Southern Brazil. Sociobiology, 61:145-154.
https://doi.org/10.13102/sociobiology.v61i2.145-154
Diehl, E., Diehl-Fleig, E., and Junqueira, L.K. 2015. Absence of relationship among termite (Insecta: Isoptera) richness, functional groups and environmental variables in Southern Brazil. EntomoBrasilis, 8:168-173. https://doi.org/10.12741/ebrasilis.v8i3.491
Donovan, S.E., Eggleton, P., and Bignell, D.E., 2001. Gut content analysis and a new feeding group classification of termites. Ecological Entomology, 26:356-366. http://doi.org/10.1046/j.1365-2311.2001.00342.x
Ferreira, E.V.O., Martins, V., Inda Jr., A.V., Giasson, E., and Nascimento, P.C. 2011. Ação dos térmitas no solo. Ciência Rural, 41:804-811. https://doi.org/10.1590/S0103-84782011005000044
Fontes, L.R. 1979. Atlantitermes, novo gênero de cupim, com duas novas espécies do Brasil (Isoptera, Termitidae, Nasutitermitinae). Revista Brasileira de Entomologia, 23:219-227.
Fontes, L.R. 1982. Novos táxons e novas combinações nos cupins nasutos geófagos da região Neotropical (Isoptera, Termitidae, Nasutitermitinae). Revista Brasileira de Entomologia, 26:99-108.
Fontes, L.R. 1992. Key to the genera of New World Apicotermitinae (Isoptera: Termitidae), p. 242-248. In Quintero, D. and Aiello A. (eds.), Insects of Panama and Mesoamerica: Selected studies. Oxford University Press, Oxford.
Genise, J.F. 2017. Ichnoentomology: insect traces in soils and paleosols. Topics in Geobiology, 37. Springer, New York. http://doi.org/10.1007/978-3-319-28210-7
Genise, J.F. and Bown, T.M. 1994. New trace fossils of termites (Insecta: Isoptera) from the Late Eocene-Early Miocene of Egypt, and the reconstruction of ancient isopteran social behavior. Ichnos, 3:155-183. https://doi.org/10.1080/10420949409386386
Genise, J.F., Mángano, M.G., Buatois, L.A., Laza, J.H., and Verde, M. 2000. Insect trace fossil associations in paleosols: The Coprinisphaera Ichnofacies. Palaios, 15:49-64. https://doi.org/10.2307/3515591
Genise, J.F., Bellosi, E.S., Gonzalez, M.G. 2004. An approach to the description and interpretation of ichnofabrics in paleosols. The application of ichnology to palaeoenvironmental and stratigraphic analysis. Geological Society, London, Special Publication, 228:355-382.
Genise, J.F., Cuezzo, F., González, M.G., and Krause, M. 2013. Organic linings in nests of the fire ants Solenopsis electra Forel and Solenopsis nr. macdonaghi Santschi from Argentina. Insectes Sociaux, 60:87-91.
Gibert, J.M. de, Netto, R.G., Tognoli, F.M.W., and Grangeiro, M.E. 2006. Commensal worm traces and possible juvenile thalassinidean burrows associated with Ophiomorpha nodosa, Pleistocene, southern Brazil. Palaeogeography, Palaeoclimatology, Palaeoecology, 230:70-84. https://doi.org/10.1016/j.palaeo.2005.07.008
Inward, D., Beccaloni, G., and Eggleton, P. 2007. Death of an order: a comprehensive molecular phylogenetic study confirms that termites are eusocial cockroaches. Biology Letters, 3:331-335. https://doi.org/10.1098/rsbl.2007.0102
IRGA. Instituto Riograndense do Arroz, 2020. Médias climatológicas de 30 anos (1981-2010). Online data available at https://irga.rs.gov.br/medias-climatologicas.
Jacklyn, P.M. 1992. “Magnetic” termite mound surfaces are oriented to suit wind and shade conditions. Oecologia, 91:385-395.
Jarzembowski, E.A. 1981. An early Cretaceous termite from southern England (Isoptera: Hodotermitidae). Sistematic Entomology, 6:91-96.
Kaiser, P. 1953. Anoplotermes pacificus eine mit Pflanzenwurzeln vergeselschaftet lebende Térmite. Hamburg, Zoologisch Museum (z.Zt.São Paulo, Brasilien) (Mit 16 Albildungen): Mitteilungen aus dem Zoologischen Museum Hamburg, 52:77-92.
Korb, J. 2011. Termite mound architecture, from function to construction, p. 349-374. In Bignell, D.E., Roisin, Y., and Lo, N. (eds.), Biology of termites: a modern synthesis. Springer, New York.
Lavelle, P., Bignell, D., and Lapage, M. 1997. Soil function in changing world: the role of invertebrate ecosystems engineers. European Journal of Soil Biology, 33:159-193.
Lee, K.E. and Wood, T.G. 1971. Termites and Soils. Academic Press, London.
Lima, J.T. and Costa, A.M L. 2007. Recursos Alimentares Explorados Pelos Cupins. Universidade Estadual Paulista, Rio Claro.
Martinez, D. and Martinell, J. 1995. The oldest known record of social insects. Journal of Paleontology, 69:594-599. https://doi.org/10.1017/S0022336000034983
Martius, C. 1994. Diversity and ecology of termites in Amazonian forests. Pedobiologia, 38:407-428.
Milani, E.J., Melo, J.H.G., Souza, P.A., Fernandes, L.A., and França, A.B. 2007. Bacia do Paraná. Boletim de Geociências da Petrobrás, 15:265-287.
Miura, T. and Matsumoto, T. 1997. Open-air litter foraging in the nasute termite Longipeditermes longipes (Isoptera: Termitidae). Journal of Insect Behavior, 11:179-189.
Moura, D.M.D.S. and Machado, S.A. 2017. Occurrence of termites (Isoptera) in natural and anthropized sand dunes areas in southern Brazil with biological notes on the species. International Journal of Development Research, 7:14739-14745.
Netto, R.G., Tognoli, F.M.W., Gibert, J.M., and Oliveira, M.Z. 2007. Paleosol evolution in nearshore fluviatile Pleistocene deposits of the Chuí Formation, south of Brazil. Resúmenes de la 5ª Reunión Argentina de Icnología/3rd Reunión de Icnología del Mercosur, Ushuaia, Argentina, p. 55.
Netto, R.G. and Grangeiro, M.E. 2009. Neoichnology of the seaward side of Peixe Lagoon in Mostardas, southernmost Brazil: the Psilonichnus ichnocoenosis revisited. Revista Brasileira de Paleontologia, 12:211-224. http://doi.org/doi:10.4072/rbp.2009.3.04
Netto, R.G., Tognoli, F.M.W., Gandini, R., Lima, J.H.D., and Gibert, J.M. de 2012. Ichnology of the Phanerozoic deposits of southern Brazil, p. 37-68. In Netto, R.G., Carmona, N.B., and Tognoli, F.M.W. (eds.), Ichnology of Latin America - Selected Papers. Publicação Especial da Sociedade Brasileira de Paleontologia v. 2, Porto Alegre, RS.
Noirot, C. 1992. From wood- to humus-feeding: an important trend in termite evolution, p. 107-119. In Billen, J. (ed.), Biology and evolution of social insects. Leuven University Press, Leuven.
Noirot, C. and Darlington, J.P.E.C. 2000. Termite nests: architecture, regulation and defence, p. 121-139. In Abe, T., Bignell, D.E., and Higashi, M. (eds.), Termites: evolution, sociability, symbiosis, ecology. Kluwer Academic.
Nutting, W.L., Haverty, M.I., and LaFage, J.P. 1987. Physical and chemical alteration of soil by two subterranean termite species in Sonoran Desert grassland. Journal of Arid Environments, 12:233-239.
Oliveira, D.E., Carrijo, T.F., and Brandão, D. 2013. Species composition of termites (Isoptera) in different Cerrado vegetation physiognomies. Sociobiology, 60:190-197. https://doi.org/10.13102/sociobiology.v60i2.190-197
Plaza, T.G.D. and Galbiati, C. 2017. Influence of flood pulse on termite diversity (Insecta: Isoptera) in the Pantanal. Sociobiology, 64:310-316. http://doi.org/10.13102/sociobiology.v64i3.1371
Redford, K.H. 1984. The termitaria of Cornitermes cumulans (Isoptera, Termitidae) and their role in determining a potential keystone species. Biotropica, 16:112-119.
Santos, A.B. 2015. Termitofauna (Blattodea: Termitidae) associada a espécies arbóreas em área de Reserva da Ilha do Catalão da UFRJ, RJ. Unpublished Master's Dissertation. Universidade Federal Rural do Rio de Janeiro, Seropédica, RJ, Brazil.
Sleaford, F., Bignell, D.E., and Eggleton, P. 1996. A pilot analysis of gut contents in termites from the Mbalmayo Forest Reserve, Cameroon. Ecological Entomology, 21:279-288.
Smith, R.M.H., Mason, T.R., and Ward, J.D. 1993. Flash-flood sediments and ichnofacies of the Late Pleistocene Homeb Silts, Kuiseb River, Namibia. Sedimentary Geology, 85:579-599. https://doi.org/10.1016/0037-0738(93)90103-C
Su, N-Y. 2003. Overview of the global distribution and control of the Formosan subterranean termite. Sociobiology, 41:7-16.
Tomazelli, L.J. and Villwock, J.A. 2000. O Cenozóico no Rio Grande do Sul: Geologia da Planície Costeira, p. 375-406. In Holz, M. and De Ros, L.F. (eds.), Geologia do Rio Grande do Sul. Publicação Especial do Centro de Investigações do Gondwana (CIGO/UFRGS), Porto Alegre, RS.
Vasconcellos, A., Melo, A.C.S., Vasconcelos, E.M., and Bandeira, A.G. 2005. Cupins de duas florestas de restinga do nordeste brasileiro. Iheringia, Série Zoologia, 95:127-131.
Wood, T.G. and Sands, W.A. 1978. The role of termites in ecosystems, p. 245-292. In Brian, M.V. (ed.), Production ecology of ants and termites. Cambridge University Press, Cambridge.

