 Forelimb motion and orientation in the ornithischian dinosaurs Styracosaurus and Thescelosaurus, and its implications for locomotion and other behavior
Forelimb motion and orientation in the ornithischian dinosaurs Styracosaurus and Thescelosaurus, and its implications for locomotion and other behavior
Article number: 26.3.a41
https://doi.org/10.26879/1289
Copyright Society of Vertebrate Paleontology, October 2023
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 2 April 2023. Acceptance: 24 September 2023.
ABSTRACT
The range of motion (ROM) in the forelimb has previously been studied in many saurischian dinosaur species but only a few ornithischian dinosaur species. Here, we fill in some of the blanks in current knowledge of ornithischian forelimb function by investigating the range of shoulder motion and the orientation of the humerus, radius, and ulna in the centrosaurine ceratopsid Styracosaurus albertensis and the basal ornithopod Thescelosaurus sp. Manual manipulation of forelimb bones, using the margins of bony articular surfaces to delimit the range of motion, shows that humeral ROM and forearm orientation in S. albertensis resemble those previously found in chasmosaurine ceratopsians. Locomotion occurred with the elbows tucked in at the sides and with the radius anterior to the ulna, without pronation. The animal was also capable of splaying its forelimbs with the elbows strongly everted, so that elbow flexion and extension produced side-to-side or up-and-down movements of the torso and head. Thescelosaurus sp. had limited humeral ROM and could not swing its humerus forward through the parasagittal plane as far as a vertical orientation. While being swung upward through the transverse plane, the humerus could not move as high as a horizontal position. Skeletal proportions and spinal curvature indicate that the forelimbs of Thescelosaurus could contact the ground while the animal stood. However, the animal is unlikely to have used quadrupedal locomotion, because its palms faced medially, and its fingers would have flexed through the transverse plane and therefore would not have provided forward propulsion.
Philip J. Senter. Department of Biological and Forensic Sciences, Fayetteville State University, 1200 Murchison Road, Fayetteville, North Carolina 28301, U.S.A, corresponding author. psenter@uncfsu.edu
Jared J. Mackey. High Purity Standards, 7221 Investment Drive, North Charleston, South Carolina 29418, U.S.A. jared.mackey@antylia.com
Keywords: Styracosaurus; Thescelosaurus; Ceratopsia; Ornithopoda; Ornithischia; forelimb
Final citation: Senter, Philip J. and Mackey, Jared J. 2023. Forelimb motion and orientation in the ornithischian dinosaurs Styracosaurus and Thescelosaurus, and its implications for locomotion and other behavior. Palaeontologia Electronica, 26(3):a41.
https://doi.org/10.26879/1289
palaeo-electronica.org/content/2023/3978-forelimb-function
Copyright: October 2023 Society for Vertebrate Paleontology.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
The range of motion (ROM) in the limbs of animals constrains their movement, which in turn constrains their behavior. Studies of limb ROM in fossil animals are therefore useful in reconstructing their behavior. Studies of forelimb ROM have been published for numerous species of Mesozoic saurischian dinosaurs (Osmólska and Roniewicz, 1969; Galton, 1971; Osmólska et al., 1972; Nicholls and Russell, 1985; Sereno, 1993; Gishlick, 2001; Carpenter, 2002; Kobayashi and Barsbold, 2005; Senter, 2005, 2006a, b; Senter and Parrish, 2005, 2006; Senter and Robins, 2005; Bonnan and Senter, 2007; Langer et al., 2007; Mallison, 2010; Vargas-Peixoto et al., 2015; White et al., 2015; Senter and Sullivan, 2019), as have studies of pedal ROM in several species (Senter, 2009; White et al., 2016), enabling researchers to draw inferences regarding behavior involved in predation, locomotion, and other aspects of their lives. In contrast, limb ROM has been little studied in ornithischian dinosaurs. ROM within the manus has been studied only in the basal ceratopsian Leptoceratops gracilis (Senter, 2007) and the iguanodontian ornithopods Uteodon aphanoecetes (Carpenter and Wilson, 2008) and Iguanodon bernissartensis (Norman 1980). Shoulder and elbow motion have been studied in chasmosaurine ceratopsids (Johnson and Ostrom, 1995; Paul and Christiansen, 2000; Thompson and Holmes, 2007; Rega et al., 2010) and the iguanodontian ornithopod Uteodon aphanoecetes (Carpenter and Wilson, 2008), and measured in the basal ceratopsians Psittacosaurus neimongoliensis, Leptoceratops gracilis, and Protoceratops andrewsi (Senter, 2007).
Here, we augment current knowledge of limb ROM and orientation in ornithischians by reporting studies of shoulder ROM and forelimb orientation in Styracosaurus albertensis and Thescelosaurus sp. S tyracosaurus albertensis is a ceratopsian dinosaur of the subfamily Centrosaurinae within the family Ceratopsidae. It is known from the Dinosaur Park Formation (Upper Cretaceous: Campanian) of Alberta (Ryan et al., 2007). Thescelosaurus is a genus of ornithopod dinosaur (Dieudonné et al., 2020) of the family Thescelosauridae. It is known from the Frenchman Formation of Saskatchewan, the Hell Creek Formation of Montana and South Dakota, the Lance Formation of Wyoming, and the Scollard Formation of Alberta (all Upper Cretaceous: Maastrichtian) (Boyd et al. 2009).
The family Ceratopsidae has two subfamilies: Centrosaurinae and Chasmosaurinae. Before now, forelimb motion has been studied only in the Chasmosaurinae. The inclusion of the centrosaurine S. albertensis in this study therefore has the potential to add significantly to knowledge of the evolution of ceratopsid forelimb posture and movement. A particular point of contention in previous studies of ceratopsid forelimb orientation and movement is the degree of habitual pronation of the forearm (Paul and Christiansen, 2000; Thompson and Holmes, 2007; Fujiwara, 2009; Rega et al., 2010). In tetrapods generally, the distal end of the humerus has two condyles. The lateral condyle articulates with the radius, and the medial condyle articulates with the ulna. Distal to the radius and ulna are the wrist and manus, with the thumb on the radial side of the manus and the fifth finger on the ulnar side of the manus. Locomotion in most extant quadrupedal tetrapods involves positioning the manus so that during locomotion, the palm faces caudally (posteriorly), so that flexion of the fingers produces a caudally directed force that propels the animal forward. In most cases, this requires that the manus be pronated, which means that the distal end of the radius is crossed over the distal end of the ulna. Researchers studying forelimb orientation in ceratopsids have come to different conclusions about whether such was the case in the ceratopsid forelimb.
Johnson and Ostrom (1995) used manual manipulation of casts of the pectoral girdle, humerus, radius, and ulna of the chasmosaurine Torosaurus latus, to study forelimb posture and locomotion in that species. They concluded that T. latus habitually held its humerus out laterally in a sprawl, which produced the pronation necessary to orient the palm posteriorly (caudally). However, their study was flawed in that they incorrectly oriented the pectoral girdle with the costal (medial) surface of the scapula facing largely ventrally, which caused the glenoid to face laterally. In contrast, articulated ceratopsid specimens show that the medial surface of the scapula faces medially, which causes the glenoid to face posteroventrally (Senter, 2006c; Senter and Robins, 2015). The incorrect positioning of the pectoral girdle by Johnson and Ostrom (1995) affected the positions of the rest of the limb bones, which casts doubt upon the correctness of their orientations as reconstructed in that study. Lockley and Hunt (1995) examined ceratopsid tracks and concluded that during locomotion, the elbows of ceratopsids were tucked in at the sides, rather than being held outward in a sprawl (here and below, the phrase “tucked in at the sides” allows for a small amount of lateral eversion of the elbows, as per Thompson and Holmes (2007), due to the transverse width of the ribcage). They suggested that a ceratopsid could nevertheless sprawl the forelimbs to support the heavy head when the animal was not in locomotion. Paul and Christiansen (2000) combined information from ceratopsid tracks with examination of the articular surfaces of the bones of chasmosaurine pectoral girdles and forelimbs, and concluded that locomotion occurred with the elbows tucked in at the sides, so that the radius was anterior to the ulna instead of being held in a pronated position, with the humerus approaching but not achieving a vertical position when the animal moved its forelimb forward, and with the humerus approaching a horizontal position when the animal moved its forelimb rearward. Fujiwara (2009) examined an articulated specimen of the chasmosaurine Triceratops sp. and concluded that the radius was positioned anterior to the ulna, with the palmar surface of the wrist facing medially, and with the first through third metacarpals arranged in a tight arc, so that most of the palm faced medially but the first three fingers pointed forward instead of laterally. Thompson and Holmes (2007) studied forelimb movement in the chasmosaurine Chasmosaurus irvinensis by means of manual manipulation of scale models of scanned bones, orienting the bones to make the manus fit its positions in ceratopsid manus prints. They concluded that during locomotion, the forelimbs were oriented as reconstructed by Fujiwara (2009), with the elbows slightly everted but not sprawled. According to their reconstruction, the humerus was oriented approximately 45° to the horizontal in lateral view when the animal moved its forelimb forward, and the humerus moved into an approximately horizontal position when the animal moved its forelimb rearward. Rega et al. (2010) came to the same conclusion regarding forelimb movement in the same species, based on digital reanimation of the same set of scanned bones.
A general consensus has therefore emerged that in chasmosaurine ceratopsids, the radius was anterior to the ulna during locomotion, instead of being pronated, that the palmar surface of the wrist faced medially, and that the posterior part of the palm also faced medially, although the arched shape of the metacarpus oriented the fingers such that the first three were aimed forward and produced a rearward push when flexed. However, the same has not been confirmed with the ceratopsid subfamily Centrosaurinae. Fujiwara (2009) mentioned that he manually articulated of the radius and ulna of a specimen of the centrosaurine Styracosaurus albertensis and found support for a similar forearm orientation in that species, but his study did not examine movement or humeral orientation in the forelimb of S. albertensis. The study of forelimb orientation and movement in S. albertensis that is presented here includes those details.
Just as Styracosaurus fills an important taxonomic gap in current knowledge of forelimb function in ceratopsians, Thescelosaurus does the same for ornithopods. Thescelosaurus is a basal ornithopod, outside the ornithopod clade Iguanodontia (Boyd et al., 2009; Dieudonné et al., 2020). The clade Iguanodontia includes the families Rhabdodontidae, Dryosauridae, and Hadrosauridae, in addition to the ornithopod genera Tenontosaurus, Camptosaurus, Uteodon, Iguanodon, and several other genera (McDonald, 2012). Because Thescelosaurus is the first non-iguanodontian ornithopod for which forelimb ROM has been studied, the taxonomic gap that it fills is a major one in the study of ornithischian forelimb function.
In previous studies of limb ROM in Mesozoic dinosaurs, researchers used the edges of the articular surfaces of the bones to place limits on the inferred ROM. However, archosaur limb joint surfaces are often capped by substantial amounts of articular cartilage (Fujiwara et al., 2010; Holliday et al., 2010; Tsai and Holliday, 2015), and the influence of such cartilage and other soft tissues on ROM should be taken into account when studying fossil archosaur limb ROM. Recent studies have clarified the influence of soft tissues on archosaur limb ROM (Fujiwara et al. 2010; Hutson and Hutson 2012, 2013, 2014, 2015a, b; White et al. 2016; Senter and Sullivan, 2019). Here, we apply insights from such studies, to shed light on the evolution of forelimb function in ornithischian dinosaurs.
Institutional abbreviations–AMNH, American Museum of Natural History, New York City, New York, U.S.A.; CMN, Canadian Museum of Nature, Ottawa, Ontario, Canada; NCSM, North Carolina Museum of Natural Sciences, Raleigh, North Carolina, U.S.A.
MATERIALS AND METHODS
Styracosaurus albertensis
T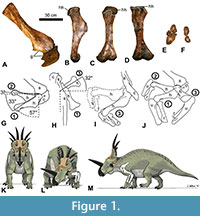 he right scapula, coracoid, and humerus of the holotype specimen of Styracosaurus albertensis (CMN 344) were used to study shoulder ROM in this species (Figure 1). The study was conducted in two stages. The first stage employed the “bare-bones” method of the previous studies cited above, in which the edges of the preserved articular surfaces were presumed to define the limits of motion. At the shoulder joint, this method involves the assumption that the proximal articular surface of the humerus (the humeral head) will remain in articulation with the articular surface of the scapula + coracoid (the glenoid cavity) as the humerus is posed in different positions. The second stage used the results of a previous study of shoulder ROM in extant archosaurs, including the differences in magnitude between bare-bones ROM and fully fleshed ROM (due to the influence of skin, muscle, and articular cartilage) (Hutson and Hutson, 2013), to infer how the shoulder ROM of a living S. albertensis may have differed from the bare-bones values.
he right scapula, coracoid, and humerus of the holotype specimen of Styracosaurus albertensis (CMN 344) were used to study shoulder ROM in this species (Figure 1). The study was conducted in two stages. The first stage employed the “bare-bones” method of the previous studies cited above, in which the edges of the preserved articular surfaces were presumed to define the limits of motion. At the shoulder joint, this method involves the assumption that the proximal articular surface of the humerus (the humeral head) will remain in articulation with the articular surface of the scapula + coracoid (the glenoid cavity) as the humerus is posed in different positions. The second stage used the results of a previous study of shoulder ROM in extant archosaurs, including the differences in magnitude between bare-bones ROM and fully fleshed ROM (due to the influence of skin, muscle, and articular cartilage) (Hutson and Hutson, 2013), to infer how the shoulder ROM of a living S. albertensis may have differed from the bare-bones values.
In the first stage of the study, the humerus was photographed in orthal views while being posed at three extremes of motion: full depression in the parasagittal plane (position 1), full elevation in the parasagittal plane (position 2), and partial elevation in the transverse plane with simultaneous full protraction (abduction) in the coronal plane (position 3). During the posing, the right radius and ulna were included in articulation with the distal end of the humerus, with the elbow flexed, to enable visualization of the entire limb in motion. During the posing, the scapula and coracoid were supported from beneath by sandbags on a table top. In position 1, the humerus was supported from beneath by sandbags, while the radius and ulna were supported from beneath by the hands of human volunteers. In position 2, the three limb bones were supported from beneath by sandbags. In position 3, the three limb bones were supported from beneath by human volunteers’ hands. In each position, the bones were photographed in lateral view, dorsal view, and anterior (cranial) view.
To position the bones for photography, the right scapula and coracoid were placed on a table as if the animal were lying on its left side, with the medial surface of the distal half of the scapular blade parallel to the plane of the tabletop (which approximated the midsagittal plane of the animal), because articulated specimens show that in non-avian dinosaurs the scapula was lateral and not dorsal to the ribcage (Senter, 2006c; Senter and Robins, 2015). The long axis of the scapular blade was oriented at approximately 45° to the edge of the table. This allowed the edge of the table to serve as a proxy for the animal’s horizontal anteroposterior (craniocaudal) axis in photos taken in lateral view. In ceratopsids, the animal’s horizontal anteroposterior (craniocaudal) axis was parallel with the sacrum, which was horizontal (parallel with the surface of the ground) when the animal was standing (Senter and Robins, 2015).
Senter and Robins (2015) found that the mean orientation of the scapula relative to the sacrum in articulated ceratopsid skeletons is 55° (Senter and Robins, 2015). However, that study also pointed out that the scapular blade was mobile in non-avian dinosaurs and that the ceratopsid scapula is oriented at 41° to 71° to the sacrum in various articulated specimens. The scapular orientation used in this study is close to that of articulated skeletons of Styracosaurus albertensis (AMNH 5376) and Centrosaurus nasicornis (AMNH 5351), in both of which the scapular blade is oriented at 41° to the sacrum (Senter and Robins, 2015).
Through the entire ROM at the shoulder, the glenoid cavity and the head of the humerus are separated by a gap that corresponds to the space that articular cartilage occupies in extant archosaurs. In extant archosaurs, the shape of the articular cartilage does not precisely match that of the underlying bony surface of the glenoid cavity (Holliday et al., 2010). However, in S. albertensis, the glenoid cavity and the humeral head are well-defined, with clearly demarcated edges, which allowed the protocol used in previous studies (Senter, 2005, 2006b; Senter and Robins, 2005; Senter and Parrish, 2006; Senter and Sullivan, 2019) to be applied in this study, as follows. The humerus was posed in position 1 with the anterior edge of the humeral head aligned with the coracoid lip of the glenoid cavity. It was posed in position 2 with the posterior edge of the humeral head aligned with the scapular lip of the glenoid cavity. It was posed in position 3 with the lateral edge of the humeral head aligned with the lateral edge of the glenoid cavity at the joint between the scapula and coracoid. For photography in position 3, a partial instead of full elevation was chosen, because fully elevating the humerus through the transverse plane entailed posing the humerus approximately perpendicular to the table top, with part of the humerus directly above the rim of the glenoid cavity, and the bone was too heavy to support in that position without the risk of damaging the glenoid cavity by having the humerus crush it from above. During manual manipulation of the humerus, we visually confirmed that the humeral head remained in articulation with the glenoid cavity when the humerus was elevated so that its long axis was approximately perpendicular to the animal’s midsagittal plane, but we refrained from holding the humerus in that position for photography, due to concern for the safety of the specimen.
The heaviness of the fossil bones may also have introduced a small amount of imprecision to the ROM measurements, because it is possible that muscle fatigue may have caused the supporting human hands to move slightly toward the floor during the time that it took the photographer (PJS) to move from one spot to the next to take photographs in the three views (lateral, dorsal, and anterior (cranial)). The resulting measurements of ROM should therefore be understood as close approximations with somewhat less precision than was possible in previous studies. Nevertheless, these approximations provide useful information on ROM in S. albertensis that are sufficiently informative to draw useful inferences about forelimb use in the animal and about differences in forelimb use between S. albertensis and other dinosaurs.
To measure the range of parasagittal motion in lateral view and the coronal range of motion in dorsal view, photos of the bones at the estimated extremes of motion were digitally superimposed. A line was then drawn down the long axis of the scapular blade and of the humerus, and the ranges of motion were measured from the angles between the lines with a protractor. The long axis of the humerus was considered to be a line connecting the center of the articular surface of the humeral head to the center of the distal articular surface of the humerus.
To determine the correct orientation of the radius and ulna relative to the humerus and each other, the radius and ulna were posed in articulation with the humerus, while supported from beneath on sandbags, with the elbow strongly flexed (approximately 90°), and the shapes of the articular surfaces were noted. The range of elbow flexion and extension was not measured.
Thescelosaurus sp.
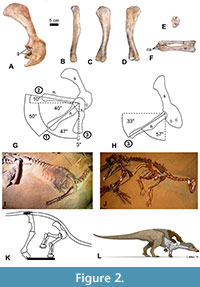 We used the right scapulocoracoid and humerus of a specimen of Thescelosaurus sp. (NCSM 15728) to study shoulder ROM in this species (Figure 2). We employed the same method used for S. albertensis, with the following differences. NCSM 15728 is preserved and on public display lying on its left side, with much of the skeleton still in articulation, as originally found, including the ribcage, the right pectoral and pelvic girdles, and the vertebral column from the neck to the base of the tail. The humerus, radius, and ulna are disarticulated from the rest of the skeleton, which allowed us to pose the humerus in the same three positions described above for S. albertensis (except that in position 3, we posed the humerus in full elevation through the transverse plane), with one of us (JJM) supporting it from beneath by hand. The nature of the display area prevented us from obtaining photos in dorsal view. Other logistical difficulties with the display area prevented us from using the protocol that was used in previous studies to minimize parallax by setting up camera stands so as to ensure that for each view, the photos are taken from the same point in space. We therefore improvised unique protocols for the specimen’s display area. For photos in lateral view, the photographer (PJS) stood over the skeleton, aimed the camera’s lens straight down, and centered the viewfinder over the center of the glenoid cavity. For photos in anterior (cranial) view, we made use of a grid for field mapping, composed of a square frame of plastic tubing 1 m on each side, with decimeters marked by strings across the frame. We positioned the frame so that one edge was parallel to the long axis of the skeleton’s sacrum, a proxy for the animal’s horizontal anteroposterior (craniocaudal) axis, and took photos down a string that was parallel to that axis to the best of our ability in the cramped space of the display area. In lateral view, the specimen’s scapula is approximately 60° to the sacrum, which agrees with the value of 60° that a previous study found between the scapula and the sacrum in another articulated specimen of Thescelosaurus (Senter and Robins, 2015). Difficulties in positioning ourselves for photography led to a small degree of parallax in the lateral-view photos and a larger degree of parallax in the anterior-view photos. Our measurements of ROM are therefore not precise and should be understood only as close approximations. Nevertheless, these approximations provide useful information on ROM in Thescelosaurus that are sufficiently informative to draw useful inferences about forelimb use in the animal and about differences in forelimb use between Thescelosaurus and other dinosaurs.
We used the right scapulocoracoid and humerus of a specimen of Thescelosaurus sp. (NCSM 15728) to study shoulder ROM in this species (Figure 2). We employed the same method used for S. albertensis, with the following differences. NCSM 15728 is preserved and on public display lying on its left side, with much of the skeleton still in articulation, as originally found, including the ribcage, the right pectoral and pelvic girdles, and the vertebral column from the neck to the base of the tail. The humerus, radius, and ulna are disarticulated from the rest of the skeleton, which allowed us to pose the humerus in the same three positions described above for S. albertensis (except that in position 3, we posed the humerus in full elevation through the transverse plane), with one of us (JJM) supporting it from beneath by hand. The nature of the display area prevented us from obtaining photos in dorsal view. Other logistical difficulties with the display area prevented us from using the protocol that was used in previous studies to minimize parallax by setting up camera stands so as to ensure that for each view, the photos are taken from the same point in space. We therefore improvised unique protocols for the specimen’s display area. For photos in lateral view, the photographer (PJS) stood over the skeleton, aimed the camera’s lens straight down, and centered the viewfinder over the center of the glenoid cavity. For photos in anterior (cranial) view, we made use of a grid for field mapping, composed of a square frame of plastic tubing 1 m on each side, with decimeters marked by strings across the frame. We positioned the frame so that one edge was parallel to the long axis of the skeleton’s sacrum, a proxy for the animal’s horizontal anteroposterior (craniocaudal) axis, and took photos down a string that was parallel to that axis to the best of our ability in the cramped space of the display area. In lateral view, the specimen’s scapula is approximately 60° to the sacrum, which agrees with the value of 60° that a previous study found between the scapula and the sacrum in another articulated specimen of Thescelosaurus (Senter and Robins, 2015). Difficulties in positioning ourselves for photography led to a small degree of parallax in the lateral-view photos and a larger degree of parallax in the anterior-view photos. Our measurements of ROM are therefore not precise and should be understood only as close approximations. Nevertheless, these approximations provide useful information on ROM in Thescelosaurus that are sufficiently informative to draw useful inferences about forelimb use in the animal and about differences in forelimb use between Thescelosaurus and other dinosaurs.
To determine the correct orientation of the radius and ulna relative to the humerus and each other, the radius and ulna were posed in articulation with the humerus while supported by hand, and the shapes of their articular surfaces were noted. This was hampered by the current configuration of the radius, ulna, and carpals. The radius and ulna are currently maintained in articulation with the radius slightly displaced proximally, so that when the radius is correctly articulated with the humerus, the ulna is not, and vice versa. In their current configuration, the distal end of the radius is slightly and unnaturally crossed over that of the ulna, and carpal bones are attached to the distal ends of the radius and ulna, partly obscuring the shapes of the distal ends of the radius and ulna (Figure 2F). Despite these challenges, the articular surfaces of the radius and ulna are sufficiently exposed to draw conclusions about their natural configuration and motion. The range of elbow flexion and extension was not measured.
RESULTS
Styracosaurus albertensis (Bare Bones)
The humeral head of S. albertensis is more extensive and bulbous on the posterior side of the humerus than on the anterior side (Figure 1B-D). This allows the humerus to be retracted to a sub-horizontal position but limits depression through the parasagittal plane, so that the humerus cannot approach a vertical orientation (Figure 1G). When swung laterally, the humerus can be abducted up to 32° anterior (cranial) to a position perpendicular to the anteroposterior (craniocaudal) axis of the body (Figure 1H). Manual manipulation confirmed that the humerus can be elevated laterally up to about 90° from the horizontal in anterior (cranial) view (Figure 1I).
When articulated with the humerus, the radius and ulna are parallel to each other, without the distal end of the radius crossing medially over the ulna. When the elbows are tucked in at the sides (positions 1 and 2), the entire radius is anterior (cranial) to the ulna, so that the palmar surface of the wrist faces medially (Figure 1J). When the elbows are swung out laterally so that the forelimb is sprawled (position 3), the radius is medial to the ulna, so that the palmar surface of the wrist would face posteriorly (Figure 1I, J). A lack of mutually opposed rolling surfaces at the distal ends of the radius and ulna (Figure 1F) prevents active supination and pronation.
Styracosaurus albertensis (Implications for ROM in the Intact Animal)
In the American alligator (Alligator mississippiensis), the ROM at the shoulder estimated from intact cadavers, in all dimensions, is similar to but slightly greater than that estimated using the bare-bones method (Hutson and Hutson, 2013). Because this animal represents one of the two major lineages of extant archosaurs, we can tentatively infer that the same may have been the case for Styracosaurus albertensis, another archosaur. This inference cannot be drawn with complete certainty, because the relationship between Styracosaurus and extant archosaurs is distant. However, if it is correct, the shoulder ROM as found with bare bones is a close approximation of the ROM in the shoulder of the live animal, which is only slightly greater.
Long-axis rotation of the humerus occurs in walking alligators and lizards (Baier and Gatesy, 2013), and the avian tibiotarsus and tarsometatarsus regularly undergo long-axis rotation during locomotion (Kambic et al., 2014, 2017). It is possible that long-axis rotation of the humerus took place also in Styracosaurus in some circumstances. If so, it would have influenced the positioning of the radius and ulna. The shapes of the bony articular surfaces at the shoulder joint of Styracosaurus do not entirely preclude long-axis rotation of the humerus. However, the semicircular shape of the glenoid cavity of Styracosaurus would not have allowed as great a degree of humeral long-axis rotation as the saddle-shaped glenoid cavity of an alligator or lizard allows.
In extant archosaurs, it is not possible to achieve active pronation by crossing the distal end of the radius over the distal end of the ulna, unlike in many mammals, which are able to actively produce such motion. A lateral expansion of the proximal ulna prevents passive radioulnar mobility in ostriches and other extant birds (Hutson and Hutson, 2015b), whereas soft and bony anatomy allow passive radioulnar mobility in the alligator (Hutson and Hutson, 2015b). In S. albertensis, as in birds, a lateral expansion of the proximal ulna is present, preventing passive radioulnar mobility. Therefore, as in birds, S. albertensis was unable to achieve active or passive pronatory movement of the distal radius over the distal ulna.
Thescelosaurus sp. (Bare Bones)
The humeral head of Thescelosaurus sp. is more extensive and bulbous on the posterior side of the humerus than on the anterior side (Figure 2B-D). This allows the humerus to be retracted to a sub-horizontal position but limits depression through the parasagittal plane, so that the humerus cannot approach a vertical orientation (Figure 2G). When swung laterally, the humerus can be abducted up to a position approximately perpendicular to the anteroposterior (craniocaudal) axis of the body (Figure 2G). When perpendicular to the anteroposterior axis of the body, the humerus can be elevated laterally between 50° and 60° from the horizontal in anterior (cranial) view (Figure 2H).
When articulated with the humerus, the radius and ulna are configured such that the palmar surface of the wrist faces medially when the elbows are tucked in (positions 1 and 2), and the palmar surface of the wrist faces caudally when the elbows are swung forward (cranially) through the transverse plane (position 3). There are no mutually opposed rolling surfaces at the proximal or distal ends of the radius and ulna. A broad, flat articular surface between the radius and ulna at the proximal end (Figure 1E) prevents both active and passive supination and pronation of the bones.
Thescelosaurus sp. (Implications for ROM in the Intact Animal)
As with Styracosaurus, we can tentatively infer that shoulder ROM in intact Thescelosaurus was similar to but slightly greater than that estimated using the bare-bones method, because such is the case in extant Alligator mississippiensis, another archosaur (Hutson and Hutson, 2013). As with Styracosaurus, this inference cannot be drawn with complete certainty, because the relationship between Thescelosaurus and extant archosaurs is distant. However, if it is correct, the shoulder ROM as found with bare bones is a close approximation of the ROM in the shoulder of the live animal, which is only slightly greater.
As with Styracosaurus, the shapes of the bony articular surfaces at the shoulder joint of Thescelosaurus do not preclude a small degree of long-axis rotation of the humerus, which would have influenced the positioning of the radius and ulna. As with Styracosaurus, the semicircular shape of the glenoid cavity of Thescelosaurus would not have allowed as great a degree of humeral long-axis rotation as the saddle-shaped glenoid cavity of an alligator or lizard allows. The shapes of the articular surfaces of the radius and ulna also indicate that without long-axis rotation of the humerus, the forearm is configured such that the palm faces medially when the elbows are tucked in at the sides (positions 1 and 2).
The lack of rolling articular surfaces between the radius and ulna was not conducive to pronation and supination. Furthermore, the flat shape of the proximal articular surface between the two bones prevented both active and passive radioulnar mobility. The palms could be aimed posteriorly only by means of humeral motion at the shoulder.
DISCUSSION
Quadrupedal ability was achieved differently in ceratopsians and ornithopods. Skeletal morphology indicative of moment arms for different muscles shows that the achievement of quadrupedal ability involved evolutionary changes to different sets of forelimb muscles between the two groups (Dempsey et al., 2023). In ceratopsians, the transition was accompanied by osteological changes that shifted pectoral and forelimb muscles so as to decrease the moment arm for humeral retraction (Maidment and Barrett, 2012; Barrett and Maidment, 2017) and to decrease the moment arm for humeral abduction (Barrett and Maidment, 2017). In ceratopsids, an increase in the moment arm for elbow extension suggests more splaying of the elbows than in other ornithischian taxa, although not a habitual full sprawl (Dempsey et al., 2023). In ornithopods, the moment arms of several posturally important pectoral and forelimb muscles underwent no major change (Maidment and Barrett, 2012), and the ancestral ornithischian moment arm for humeral abduction was retained in hadrosaurs (Barrett and Maidment, 2017). In both ceratopsians and ornithopods, an increase in the moment arm for humeral adduction occurred during the transition to quadrupedal ability (Dempsey et al., 2023). Iguanodon differs from other quadrupedal ornithopods in having undergone a reduction in the moment arm of humeral adduction and an increase in the moment arm for humeral abduction (Dempsey et al., 2023). In Styracosaurus and Thescelosaurus, osteological data and the results of this study additionally provide the following insights.
Forelimb Function in Styracosaurus albertensis
Skeletal proportions indicate that the manus of S. albertensis reached the ground when the hindlimbs were upright (Senter and Robins, 2015). Limb proportions and the front-heavy nature of the animal precluded habitual bipedal locomotion. The animal was therefore a habitual quadruped. During quadrupedal locomotion, the elbows were most likely tucked in at the sides, rather than sprawled outwards laterally. This is because the orientation of the forearm was such that with the humeri sprawled out to the sides, the forearms would have moved in the transverse plane, so that they would have produced a side-to-side rocking motion instead of forward propulsion. Only with the elbows tucked in at the sides would the forearms have moved in the parasagittal plane, producing forward motion. This interpretation agrees with trackway data (Lockley and Hunt, 1995; Lockley and Tempel, 2014) and with reconstructions of chasmosaurine ceratopsids (Thompson and Holmes, 2007; Fujiwara, 2009; Rega et al., 2010). Tucking the elbows in at the sides would also have produced the narrow spacing between left and right ceratopsian manus tracks that is present in ceratopsian trackways (Lockley and Hunt, 1995; Lockley and Tempel, 2014), whereas a pose in which the humeri were sprawled out to the sides would have produced wider spacing between the left and right manus prints. These findings are consistent with the previous finding that the moment arm for humeral adduction is greater in ceratopsians than in bipedal ornithischians (Dempsey et al., 2023), because a greater moment arm for humeral adduction would have facilitated the tucking in of the elbows.
Forelimb Function in Thescelosaurus
The anterior dorsal vertebrae in Thescelosaurus articulate in a downward curve that positions the shoulder sufficiently near the ground for the manus to contact the ground when the animal’s sacrum is horizontal and its hindlimbs are upright (Figure 2I-L). Its manus could therefore have contacted the ground during standing or walking. However, it is unlikely to have used its manus in locomotion. When the elbows are tucked in at the sides, the palms face medially, and finger flexion is not caudally but medially directed, because the metacarpus of Thescelosaurus lacks the tight arc on the pollucal side that is present in ceratopsids and orients the first three fingers of the ceratopsid hand anteriorly. The orientation of the fingers of Thescelosaurus would not have been conducive to forward propulsion during locomotion. To orient the palms such that the fingers flexed in a posterior (caudal) direction, producing forward propulsion, the humerus would have had to swing forward through the coronal plane, so that the elbows were held out from the body in a sprawl. In such a position, elbow flexion and extension would have occurred through the transverse plane, not the parasagittal plane, and would therefore not have produced a posteriorly directed force that generated forward propulsion.
Evolution of Forelimb Function in Ornithischia
A limited range of motion at the shoulder appears to be plesiomorphic for the Dinosauria. During movement of the humerus through the parasagittal plane, an inability to swing the humerus forward beyond a sub-vertical position is present in basal (non-coelurosaurian) theropods (Carpenter, 2002; Senter and Robins, 2005; Senter and Sullivan, 2019), sauropodomorphs (Bonnan and Senter, 2007; Langer et al., 2007), and basal (non-ceratopsid) ceratopsians (Senter, 2007). As shown here, the same is the case in the basal ornithopod Thescelosaurus. During movement of the humerus through the transverse plane, an inability to laterally elevate the humerus to a horizontal position is present in basal (non-coelurosaurian) theropods (Carpenter, 2002; Senter and Robins, 2005; Senter and Sullivan, 2019), sauropodomorphs (Bonnan and Senter, 2007; Langer et al., 2007), and Psittacosaurus (Senter, 2007). As shown here, the same is the case in the basal ornithopod Thescelosaurus. This indicates that the greater ability to protract the humerus in Dromaeosauridae (Senter, 2006b), as well as the greater ability to laterally elevate the humerus in Paraves (Senter, 2006b), Ornithomimosauria (Senter, 2006c), and quadrupedal ceratopsians (Senter, 2007; this study), represent derived conditions. The increases in maximal humeral protraction and elevation in Paraves, Ornithomimosauria, and basal (non-ceratopsid) quadrupedal ceratopsians are due to an extension of the glenoid cavity onto the lateral surface of the scapula and coracoid (Senter, 2006b, 2006c, 2007). As shown here, these abilities are maintained in Styracosaurus albertensis, due to an increase in size and bulbosity of the humeral head, despite its loss of the lateral extension of the glenoid. The morphology of the glenoid and humeral head in other members of the Ceratopsidae resembles that of S. albertensis (Paul and Christiansen, 2000; Thompson and Holmes, 2007; Fujiwara, 2009; Mallon and Holmes, 2010). It is therefore plausible that in the Ceratopsidae generally, as in S. albertensis, the humerus could be swung into a subhorizontal position both by being swung posteriorly through the parasagittal plane and by being swung laterally through the transverse plane.
Orientation of the forearms such that with the elbows tucked in at the sides, the radius is anterior to the ulna, without pronation, so that the palms face medially, appears to be plesiomorphic for the Dinosauria. It is present in non-avian theropods (Sereno, 1993; Carpenter, 2002; Senter and Robins, 2005; Senter, 2006b; Senter and Sullivan, 2019), basal (non-sauropod) sauropodomorphs (Bonnan and Senter, 2007; Langer et al., 2007; Mallison, 2010), the basal (non-ceratopsid) ceratopsians Psittacosaurus and Leptoceratops (Senter, 2007), and the basal ornithopod Thescelosaurus (this paper). Changes in the orientation of the manus are present in quadrupedal ceratopsians. In Leptoceratops, the metacarpus is arched in proximal view such that the first two fingers are aimed forward, so that during flexion they produce a rearward push that provides forward propulsion (Senter, 2007). In Protoceratops, the distal radius is crossed over the ulna, pronating the entire manus, so that all fingers produce a rearward push that provides forward propulsion during flexion (Senter, 2007). In chasmosaurine ceratopsids, the metacarpus is arched in proximal view such that the first three fingers are aimed forward, so that during flexion they produce a rearward push that provides forward propulsion (Fujiwara, 2009). The configuration of the centrosaurine metacarpus has not been studied, but it is plausible that it matches the configuration in the Chasmosaurinae. As shown in this study, the configuration of the radius and ulna in the centrosaurine Styracosaurus albertensis matches that of chasmosaurine ceratopsids. Changes in the orientation of the manus are also present in Iguanodon and hadrosaurid ornithopods of the family Hadrosauridae. In these ornithopods, the first finger is held off the ground (Iguanodon) or lost (Hadrosauridae), and the second finger is reoriented such that it flexes toward the third finger instead of toward the palm, as revealed both by skeletal anatomy (Norman, 1980; Senter, 2012) and by trackway evidence (Wright 1999; Lockley and Wright 2001).
Locomotion and Combat in Ceratopsians
The results of this study have implications for locomotion in the Ceratopsidae. As previous studies have shown, chasmosaurine locomotion occurred with the radius anterior to the ulna, without pronation, and with the propulsive force from the manus provided by the first three fingers (Thomson and Holmes, 2007; Fujiwara, 2009; Mallon and Holmes, 2010), a conclusion that is consistent with data from ceratopsid tracks (Lockley and Hunt, 1995; Lockley and Tempel, 2014). This study is the first to confirm with manual manipulation of bones that the same was the case in the Centrosaurinae. Articulated skeletons of the centrosaurines Centrosaurus apertus (AMNH 5351) and Styracosaurus albertensis (AMNH 5372) provide a further line of evidence that the radius was anterior to the ulna, without pronation, when the elbows were tucked in at the sides (figure 3 of Senter and Robins, 2015). It is plausible that both ceratopsid subfamilies inherited their identical forearm configuration from the common ceratopsid ancestor. It is also plausible that among ceratopsians, the forearm pronation that is present in Protoceratops is a trait that is unique to the protoceratopsid lineage and therefore not an evolutionary stage through which quadrupedal ceratopsians passed on the way to becoming ceratopsids.
In ceratopsid specimens for which the complete manus is known, the first three fingers have hoof-like unguals and unreduced phalangeal counts (two phalanges in the first finger, three in the second, and four in the fifth), and the fourth and fifth fingers have reduced phalangeal counts (three phalanges in the fourth finger, and two in the fifth), lack unguals, and have an ovoid nub as the terminal phalanx (Brown, 1917, 1937; Fujiwara, 2009; Mallon and Holmes, 2010)–although exceptions in which the fifth finger has an unguliform terminal phalanx are known (Rega et al., 2010). The reduced phalangeal counts and degenerate fingertip morphology in the fourth and fifth fingers are consistent with vestigialization (Senter, 2010), which in turn is consistent with a lack of use in locomotion. Furthermore, in the specimens for which the original position of the fifth finger (as originally found in articulation) has been maintained and is therefore known, its position suggests that it may have been held off the ground (Fujiwara, 2009; Mallon and Holmes, 2010), which is further consistent with locomotor propulsion by the first through third fingers only.
Fossil tracks confirm that the palm of the ceratopsid manus was oriented caudomedially (Lockley and Hunt, 1995; Milner et al., 2006; Lockley and Tempel, 2014). The relative locations of the manus and pes prints in these tracks indicate that the forelimbs were not sprawled during locomotion, and that instead the elbows were tucked in at the sides.
The results of this study also have implications for combat behavior in the Ceratopsidae. As previously mentioned, quadrupedal ceratopsians, including ceratopsids, were capable of moving their forelimbs into a sprawling position. In this position, elbow flexion and extension did not generate forward propulsion but instead raised and lowered the torso and head if done simultaneously on both sides, and rocked the torso and head left and right if done alternately on both sides. Many extant lizard species raise and lower the torso and head during agonistic displays (Carpenter and Ferguson, 1977), and it is possible that ceratopsians engaged in similar behavior. It is also possible that ceratopsians used their ability to sprawl the forelimbs for lateral head-shoving contests, in which side-to-side rocking would have been important. The motive power in such movement would have been provided by elbow extensors, which is consistent with the increase in the moment arm of elbow extension musculature that is present in ceratopsid forelimbs (Dempsey et al., 2023). The head is enlarged in quadrupedal ceratopsians and especially in ceratopsids. This enlargement is consistent both with a display function and with the use of the head as a weapon and a shoving instrument in dominance contests. The ability to sprawl the forelimbs and rock the torso from side to side would have facilitated the latter use. Previous researchers have noted that the heads of ceratopsians were suited to combat involving head shoving, with or without horn locking (Farlow and Dodson, 1975; Molnar, 1977; Farke, 2004; Krauss et al., 2010), and that a sprawling posture would have facilitated stability during head-shoving contests (Farlow and Dodson, 1975). The increase in the moment arm of the musculature for elbow extension that is present in ceratopsids and facilitates sprawling (Dempsey et al., 2023) would have been conducive to such behavior. Such contests need not have resulted in punctures caused by horns. Few pathologies that are attributable to horn punctures are known in ceratopsids (Tanke and Rothschild, 2010). This is consistent more with attempts to shove opponents laterally with the head than with attempts to gore opponents with the horns. Furthermore, the locations of most combat-attributed cranial lesions in Triceratops are consistent with head-shoving contests involving horn-locking, rather than with attempts to gore each other with the horns (Farke, 2004). Likewise, cranial lesions in Centrosaurus are inconsistent with the use of the nasal horn in goring attempts (Farke et al., 2009). An example of a Triceratops frill that appears to have been punctured by a horn approaching from the rear (D’Anastasio et al., 2022) is consistent with a goring attempt, but a goring attempt is not the only possible scenario.
Locomotion in Ornithopods
It is uncontroversial that limb proportions in basal ornithischians and basal ornithopods suggest bipedal carriage (Norman et al., 2004; Butler and Barrett, 2012). In Thescelosaurus, the forelimb is sufficiently short to have been clear of the ground when the humerus was swung posteriorly through the parasagittal plane (Figure 2K, L). Thescelosaurus was therefore capable of bipedal locomotion. As previously mentioned, its forelimb joint orientation was not ideal for quadrupedal locomotion. It did not preclude quadrupedal locomotion, but quadrupedal locomotion would have been awkward, with the fingers pointing laterally while the distal metacarpus contacted the ground and the elbow flexed and extended through the parasagittal plane, so that finger flexion did not contribute to locomotion. Instead, the fingers would have been held in hyperextension, so as to avoid flexion, because their flexion would have been medially directed and would therefore have impeded locomotion.
A similar situation is present in the iguanodontian ornithopod Uteodon aphanoecetes, a basal member of the iguanodontian clade Styracosterna (McDonald, 2012). Its forelimb could reach the ground, and its elbow flexed and extended in the parasagittal plane when the elbows were tucked in at the sides. In that position, its palms faced medially, and finger flexion occurred through the transverse plane instead of the parasagittal plane (Carpenter and Wilson, 2008), as in Thescelosaurus. A previous study attributed quadrupedal locomotion to the species (Carpenter and Wilson, 2008). If Uteodon engaged in quadrupedal locomotion, it would have been as described above for Thescelosaurus, with the fingers pointing laterally and being held in hyperextension, because their flexion would have been medially directed and therefore would not have contributed to locomotion.
In later members of the clade Styracosterna, such as Iguanodon and hadrosaurids, the manus is reconfigured such that digit I (when present) is held off the ground, and digit II flexes toward digit III instead of toward the palm (Norman, 1980; Senter, 2012). This would have produced forward propulsion when the palms were facing sub-medially, as indicated by trackways from the Cretaceous Period (Wright, 1999; Lockley et al., 2003; Senter, 2012). These later ornithopods therefore solved the problem of quadrupedal locomotion with sub-medially facing palms in a way similar to the ceratopsid solution: reorientation of the leading finger(s).
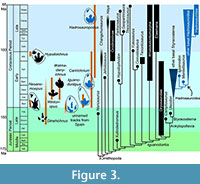 When forelimb configuration in Thescelosaurus and Uteodon are considered along with the known record of ornithopod tracks (e.g. Lockley and Wright, 2001; Lockley et al., 2003, 2009; Díaz-Martínez et al., 2015; Salisbury et al., 2016), it is evident that a substantial portion of the ornithopod footprint record has yet to be discovered. Manus prints in these two taxa, and possibly an array of ornithopods outside the clade Hadrosauroidea, would most likely have shown clawed fingers that pointed laterally during locomotion. Instead, the known manus prints of Cretaceous ornithopods usually indicate a manus with the fingertips contained within a mitten-like pad of flesh (Figure 3), as in hadrosaur “mummies” (cf. figure 7.8 of Murphy et al., 2007; figure 4 of Senter, 2012). The ichnogenus Neoanomoepus shows clawed fingers but is currently known only from the Berriasian (Lockley et al., 2009). The ichnogenus Hypsiloichnus shows clawed fingers but is currently known only from the Albian (Stanford et al., 2004). The presence of basal ornithopods such as Thescelosaurus as late as the Maastrichtian suggests that ornithopod traces with clawed fingers were made through the Late Cretaceous. If any survive, they still await discovery.
When forelimb configuration in Thescelosaurus and Uteodon are considered along with the known record of ornithopod tracks (e.g. Lockley and Wright, 2001; Lockley et al., 2003, 2009; Díaz-Martínez et al., 2015; Salisbury et al., 2016), it is evident that a substantial portion of the ornithopod footprint record has yet to be discovered. Manus prints in these two taxa, and possibly an array of ornithopods outside the clade Hadrosauroidea, would most likely have shown clawed fingers that pointed laterally during locomotion. Instead, the known manus prints of Cretaceous ornithopods usually indicate a manus with the fingertips contained within a mitten-like pad of flesh (Figure 3), as in hadrosaur “mummies” (cf. figure 7.8 of Murphy et al., 2007; figure 4 of Senter, 2012). The ichnogenus Neoanomoepus shows clawed fingers but is currently known only from the Berriasian (Lockley et al., 2009). The ichnogenus Hypsiloichnus shows clawed fingers but is currently known only from the Albian (Stanford et al., 2004). The presence of basal ornithopods such as Thescelosaurus as late as the Maastrichtian suggests that ornithopod traces with clawed fingers were made through the Late Cretaceous. If any survive, they still await discovery.
CONCLUSIONS
Forelimb orientation and shoulder motion in the centrosaurine ceratopsid Styracosaurus albertensis resembled that of chasmosaurine ceratopsids. Locomotion was accomplished with the elbows tucked in at the sides, with the radius anterior to the ulna, and without pronation. The shapes of the glenoid cavity and the bulbous humeral head enabled ceratopsids to rock the torso left and right with the forelimbs sprawled, possibly during head-shoving contests.
The ornithopod Thescelosaurus was most likely a habitual biped. The humerus could be swung forward only as far as a position perpendicular to the animal’s anteroposterior axis in dorsal view, and it could be elevated through the transverse plane somewhat but could not thereby achieve a horizontal position.
This study encountered logistical difficulties but nevertheless produced useful information about ceratopsid and ornithopod forelimb function. It therefore shows that an experimental setup with problems that preclude the precision that was possible in previous studies can still provide a useful contribution toward the filling of major blanks in current knowledge of forelimb function in dinosaurs.
ACKNOWLEDGMENTS
Several individuals deserve thanks for their help with this study, Part 18 of the Dinosaur Forelimb Project. The anonymous reviewers provided helpful suggestions that improved this paper. K. Shepherd and M. Feuerstack provided access to the Styracosaurus albertensis specimen at the Canadian Museum of Nature. M. Feuerstack, R. Holmes, and A. Prieto-Marquez used their hands to support the specimen’s bones during photography. V. Schneider provided access to the Thescelosaurus specimen at the North Carolina Museum of Natural Sciences and provided assistance with logistical issues during photography. S. Allen of Fayetteville State University served as coordinator for BIOL 430, the undergraduate student research course under the auspices of which the study of Thescelosaurus began. D. Okunbor and F. King of Fayetteville State University served as coordinators for the Institute of Undergraduate Research, under the auspices of which the study of Thescelosaurus continued. Paleoartist L. Walters created the fleshed-out illustrations of Styracosaurus and Thescelosaurus.
REFERENCES
Baier, D.B. and Gatesy, S.M. 2013. Three-dimensional skeletal kinematics of the shoulder girdle and forelimb in walking Alligator. Journal of Anatomy, 223:462–473.
https://doi.org/10.1111/joa.12102
Barrett, P.M. and Maidment, S.C.R. 2017. The evolution of ornithischian quadrupedality. Journal of Iberian Geology, 43:363–377.
https://doi.org/10.1007/s41513-017-0036-0
Bonnan, M.F. and Senter, P. 2007. Were the basal sauropodomorph dinosaurs Plateosaurus and Massospondylus habitual quadrupeds? Special Papers in Palaeontology, 77:139–155.
Boyd, C.A., Brown, C.M., Scheetz, R.D., and Clarke, J.A. 2009. Taxonomic revision of the basal neorithischian taxa Thescelosaurus and Bugenasaura. Journal of Vertebrate Paleontology, 29:758–770.
https://doi.org/10.1671/039.029.0328
Brown, B. 1917. A complete skeleton of the horned dinosaur Monoclonius, and description of a second specimen showing skin impressions. Bulletin of the American Museum of Natural History, 37:281–306.
Brown, B. 1937. The skeleton of Styracosaurus with the description of a new species. American Museum Novitates, 955:2–12.
Butler, R.J. and Barrett, P.M. 2012. Ornithopods, pp. 551–566. In Brett-Surman, M.K., Holtz, T.R. Jr., and Farlow, J.O. (eds.), The Complete Dinosaur, 2nd ed. Indiana University Press, Bloomington, USA.
Carpenter, K. 2002. Forelimb biomechanics of nonavian theropod dinosaurs in predation. Senckenbergiana Lethaea, 82:59–76.
https://doi.org/10.1007/bf03043773
Carpenter, C.C. and Ferguson, G.W. 1977. Variation and evolution of stereotyped behavior in reptiles. Part I. A survey of stereotyped reptilian behavioral patterns, pp. 355-554. In Gans, C. and Tinkle, D.W. (eds.), Biology of the Reptilia, Vol. 7. Ecology and Behaviour. Academic Press, New York, USA.
Carpenter, K. and Wilson, Y. 2008. A new species of Camptosaurus (Ornithopoda: Dinosauria) from the Morrison Formation (Upper Jurassic) of Dinosaur National Monument, Utah, and a biomechanical analysis of its forelimb. Annals of the Carnegie Museum. 76:227–263.
https://doi.org/10.2992/0097-4463(2008)76[227:ansoco]2.0.co;2
D’Anastasio, R., Cilli, J., Bacchia, F., Fanti, F., Gobbo, G., and Capasso, L. 2022. Histological and chemical diagnosis of a combat lesion in Triceratops. Scientific Reports, 12:1–8.
https://doi.org/10.1038/s41598-022-08033-2
Dempsey, M., Maidment, S.C.R., Hedrick, B.P., and Bates, K.T. 2023. Convergent evolution of quadrupedality in ornithischian dinosaurs was achieved through disparate forelimb muscle mechanics. Proceedings of the Royal Society B, 290:1–12.
https://doi.org/10.1098/rspb.2022.2435
Díaz-Martínez, I., Pereda-Suberbiola, X., Pérez-Lorente, F., and Canudo, J.I. 2015. Ichnotaxonomic review of large ornithopod dinosaur tracks: temporal and geographic implications. PLoS ONE, 10(2):e0115477.
https://doi.org/10.1371/journal.pone.0115477
Dieudonné, P.-E., Cruzado-Caballero, P., Godefroit, P., and Tortosa, T. 2020. A new phylogeny of cerapodan dinosaurs. Historical Biology, 33:2335–2355.
https://doi.org/10.1080/08912963.2020.1793979
Farke, A.A. 2004. Horn use in Triceratops (Dinosauria: Ceratopsidae): Testing behavioral hypotheses using scale models. Palaeontologia Electronica, 7.1:1–10.
https://palaeo-electronica.org/2004_1/horn/issue1_04.htm
Farke, A.A., Wolff, E.D.S., and Tanke, D.H. 2009. Evidence of combat in Triceratops. PLoS ONE, 4(1):e4252.
https://doi.org/10.1371/journal.pone.0004252
Farlow, J.O. and Dodson, P. 1975. The behavioral significance of frill and horn morphology in ceratopsian dinosaurs. Evolution, 29:353–361.
https://doi.org/10.1111/j.1558-5646.1975.tb00214.x
Fujiwara, S. 2009. A reevaluation of the manus structure in Triceratops (Ceratopsia: Ceratopsidae). Journal of Vertebrate Paleontology, 29:1136–1147.
https://doi.org/10.1671/039.029.0406
Fujiwara, S., Taru, H., and Suzuki, D. 2010. Shape and articular surface of crocodilian (Archosauria) elbow joints and its relevance to sauropsids. Journal of Morphology, 271:883–896.
https://doi.org/10.1002/jmor.10846
Galton, P.M. 1971. Manus movements of the coelurosaurian dinosaur Syntarsus and opposability of the theropod hallux. Arnoldia (Rhodesia), 15:1–8.
Gishlick, A.D. 2001. The function of the manus and forelimb of Deinonychus antirrhopus and its importance for the origin of avian flight, pp. 301–318. In Gauthier, J. and Gall, L.F. (eds.), New Perspectives on the Origin and Early Evolution of Birds. Yale Peabody Museum, New Haven, USA.
Holliday, C.M., Ridgely, R.C., Sedlmayr, J.C., and Witmer, L.M. 2010. Cartilaginous epiphyses in extant archosaurs and their implications for reconstructing limb function in dinosaurs. PLoS ONE, 5(9):e13120.
https://doi.org/10.1371/journal.pone.0013120
Hutson, J.D. and Hutson, K.N. 2012. A test of the validity of range of motion studies of fossil archosaur elbow mobility using repeated-measures analysis and the extant phylogenetic bracket. Journal of Experimental Biology, 215:2030–2038.
https://doi.org/10.1242/jeb.069567
Hutson, J.D. and Hutson, K.N. 2013. Using the American alligator and a repeated-measures design to place constraints on in vivo shoulder joint range of motion in dinosaurs and other fossil archosaurs. Journal of Experimental Biology, 216:275–284.
https://doi.org/10.1242/jeb.074229
Hutson, J.D. and Hutson, K.N. 2014. A repeated-measures analysis of the effects of soft tissues on wrist range of motion in the extant phylogenetic bracket of dinosaurs: implications for the functional origins of an automatic wrist-folding mechanism in Crocodylia. Anatomical Record, 297:1228–1249.
https://doi.org/10.1002/ar.22903
Hutson, J.D. and Hutson, K.N. 2015a. Inferring the prevalence and function of finger hyperextension in Archosauria from finger-joint range of motion in the American alligator. Journal of Zoology (London), 296:189–199.
https://doi.org/10.1111/jzo.12232
Hutson, J.D. and Hutson, K.N. 2015b. An examination of forearm bone mobility in Alligator mississippiensis (Daudin, 1802) and Struthio camelus Linnaeus, 1758 reveals that Archaeopteryx and dromaeosaurs shared an adaptation for gliding and/or flapping. Geodiversitas, 37:325-344.
https://doi.org/10.5252/g2015n3a3
Johnson, R. and Ostrom, J.H. 1995. The forelimb of Torosaurus and an analysis of the posture and gait of ceratopsian dinosaurs, pp. 205–218. In Thomason, J.J. (ed.), Functional Morphology in Vertebrate Paleontology. Cambridge University Press, Cambridge, UK.
Kambic, R.E., Roberts, T.J., and Gatesy, S.M. 2014. Long-axis rotation: a missing degree of freedom in avian bipedal locomotion. Journal of Experimental Biology, 217:2770–2782.
https://doi.org/10.1242/jeb.101428
Kambic, R.E., Roberts, T.J. and Gatesy, S.M. 2017. 3-D range of motion envelopes reveal interacting degrees of freedom in avian hind limb joints. Journal of Anatomy, 231:906–920.
https://doi.org/10.1111/joa.12680
Kobayashi, K. and Barsbold, R. 2005. Anatomy of Harpymimus okaldnikovi Barsbold and Perle 1984 (Dinosauria; Theropoda) of Mongolia, pp. 97–126. In Carpenter, K. (ed.), The Carnivorous Dinosaurs. Indiana University Press, Bloomington, USA.
Kobayashi, Y., Takasaki, R., Kubota, K., and Fiorillo, A.R. 2021. A new basal hadrosaurid (Dinosauria: Ornithischia) from the latest Cretaceous Kia-ama Formation in Japan implies the origin of hadrosaurids. Scientific Reports, 11(8547):1–15.
https://doi.org/10.1038/s41598-021-87719-5
Krauss, D.A., Pezon, A., Nguyen, P., Salame, I., and Rywkin, S.B. 2010. Evolutionary interactions between horn and frill morphology in chasmosaurine ceratopsians, pp. 282–292. In Ryan, M.J., Chinnery-Allgeier, B.J., and Eberth, D.A. (eds.), New Perspectives on Horned Dinosaurs. The Royal Tyrrell Museum Ceratopsian Symposium. Indiana University Press, Bloomington, USA.
Langer, M.C., França, M.A.G., and Gabriel, S. 2007. The pectoral girdle and forelimb anatomy of the stem-sauropodomorph Saturnalia tupiniquim (Upper Triassic, Brazil). Special Papers in Palaeontology, 77:113–137.
Lockley, M.G. and Hunt, A.P. 1995. Ceratopsid tracks and associated ichnofauna from the Laramie Formation (Upper Cretaceous: Maastrichtian) of Colorado. Journal of Vertebrate Paleontology, 15:592–614.
https://doi.org/10.1080/02724634.1995.10011251
Lockley, M.G. and Tempel, J. 2014. “Fossil Trace” trace fossils: The historic, scientific and educational significance of Triceratops Trail - a controversial Upper Cretaceous tracksite complex in the Laramie Formation, Golden, Colorado. New Mexico Museum of Natural History and Science Bulletin, 62:441–458.
Lockley, M.G. and Wright, J.L. 2001. Trackways of large quadrupedal ornithopods from the Cretaceous: A review, pp. 428–442. In Tanke, D.H. and Carpenter, K. (eds.), Mesozoic Vertebrate Life. Indiana University Press, Bloomington, USA.
Lockley, M.G., McCrea, R.T., and Matsukawa, M. 2009. Ichnological evidence for small quadrupedal ornithischians from the basal Cretaceous of SE Asia and North America: implications for a global radiation, pp. 255–269. In Buffetaut, E., Cuny, G., Le Loeuff, J., and Suteethorn, V. (eds.), Late Palaeozoic and Mesozoic Ecosystems in SE Asia. The Geological Society, London.
https://doi.org/10.1144/sp315.18
Lockley, M.G., Nadon, G., and Currie, P.J. 2003. A diverse dinosaur-bird footprint assemblage from the Lance Formation, Upper Cretaceous, eastern Wyoming: Implications for ichnotaxonomy. Ichnos, 11:229–249.
https://doi.org/10.1080/10420940490428625
Maidment, S.C.R. and Barrett, P.M. 2012. Does morphological convergence imply functional similarity? A test using the evolution of quadrupedalism in ornithischian dinosaurs. Proceedings of the Royal Society B, 279:3765–3771.
https://doi.org/10.1098/rspb.2012.1040
Mallison, H. 2010. The digital Plateosaurus II: an assessment of the range of motion of the limbs and vertebral column and of previous reconstructions using a digital skeletal mount. Acta Palaeontologica Polonica, 55:433–458.
https://doi.org/10.4202/app.2009.0075
Mallon, J.C. and Holmes, R. 2010. Description of a complete and fully articulated chasmosaurine postcranium previously assigned to Anchiceratops (Dinosauria: Ceratopsia), pp. 189–202. In Ryan, M.J., Chinnery-Allgeier, B.J., and Eberth, D.A. (eds.), New Perspectives on Horned Dinosaurs. The Royal Tyrrell Museum Ceratopsian Symposium. Indiana University Press, Bloomington, USA.
McDonald, A.T. 2012. Phylogeny of basal iguanodonts (Dinosauria: Ornithischia): An update. PLoS ONE, 7(5):e36745.
https://doi.org/10.1371/journal.pone.0036745
Milner, A.R.C., Vice, G.S., Harris, J.D., and Lockley, M.G. 2006. Dinosaur tracks from the Upper Cretaceous Iron Springs Formation, Iron County, Utah. New Mexico Museum of Natural History and Science Bulletin, 35:105–113.
https://doi.org/10.1130/abs/2019am-336126
Molnar, R.E. 1977. Analogies in the evolution of combat and display structures in ornithopods and ungulates. Evolutionary Theory, 3:165–190.
Murphy, N.L., Trexler, D., and Thompson, M. 2007. “Leonardo”, a mummified Brachylophosaurus (Ornithischia: Hadrosauridae) from the Judith River Formation of Montana, pp. 117–133. In Carpenter, K. (ed.), Horns and Beaks. Ceratopsian and Ornithopod Dinosaurs. Indiana University Press, Bloomington, USA.
https://doi.org/10.2307/j.ctt1zxz1md.12
Nicholls, E.L. and Russell, A.P. 1985. Structure and function of the pectoral girdle and forelimb of Struthiomimus altus (Theropoda: Ornithomimidae). Palaeontology, 28:643–677.
Norman, D.B. 1980. On the ornithischian dinosaur Iguanodon bernissartensis of Bernissart (Belgium). Mémoire de l’Institut Royal des Sciences Naturelles de Belgique, 178:1–103.
Norman, D.B., Sues, H.-D., Witmer, L.M., and Coria, R.A. 2004. Basal Ornithopoda, pp. 393–412. In Weishampel, D.B., Dodson, P., and Osmólska, M. (eds.), The Dinosauria, 2nd ed. University of California Press, Berkeley, USA.
https://doi.org/10.1525/california/9780520242098.003.0021
Osmólska, H. and Roniewicz, E. 1969. Deinocheiridae, a new family of theropod dinosaurs. Palaeontologia Polonica, 27:5–19.
Osmólska, H., Roniewicz, E., and Barsbold, R. 1972. A new dinosaur, Gallimimus bullatus n. gen., n. sp. (Ornithomimidae) from the Upper Cretaceous of Mongolia. Palaeontologia Polonica, 27:103–143.
Paul, G.S. and Christiansen, P. 2000. Forelimb posture in neoceratopsian dinosaurs: implications for gait and locomotion. Paleobiology, 26:450–465.
https://doi.org/10.1666/0094-8373(2000)026%3C0450:fpindi%3E2.0.co;2
Pérez-Lorente, F., Cuenca-Bescós, G., Aurell, M., Canudo, J.I., Soria, A.R., and Ruiz-Omeñaca, J.I. 1997. Las Cerradicas tracksite (Berriasian, Galve, Spain): Growing evidence for quadrupedal ornithopods. Ichnos, 5:109–120.
https://doi.org/10.1080/10420949709386410
Rega, E., Holmes, R., and Tirabasso, A. 2010. Habitual locomotor behavior inferred from manual pathology in two Late Cretaceous chasmosaurine ceratopsian dinosaurs, Chasmosaurus irvinensis (CMN 41357) and Chasmosaurus belli (ROM 843), pp. 340–354. In Ryan, M.J., Chinnery-Allgeier, B.J., and Eberth, D.A. (eds.), New Perspectives on Horned Dinosaurs. The Royal Tyrrell Museum Ceratopsian Symposium. Indiana University Press, Bloomington, USA.
Ryan, M.J., Holmes, R., and Russell, A.P. 2007. A revision of the late Campanian centrosaurine ceratopsid genus Styracosaurus from the Western Interior of North America. Journal of Vertebrate Paleontology, 27:944–962.
https://doi.org/10.1671/0272-4634(2007)27[944:arotlc]2.0.co;2
Salisbury, S.W., Romilio, A., Herne, M.C., Tucker, R.T., and Nair, J.P. 2015. The dinosaurian ichnofauna of the Lower Cretaceous Broome Sandstone of the Walmadany area (James Price Point), Dampier Peninsula, Western Australia. Society of Vertebrate Paleontology Memoir, 16:1–152.
https://doi.org/10.1080/02724634.2016.1269539
Senter, P. 2005. Function in the stunted forelimbs of Mononykus olecranus (Theropoda), a dinosaurian anteater. Paleobiology, 31:373–381.
https://doi.org/10.1666/0094-8373(2005)031[0373:fitsfo]2.0.co;2
Senter, P. 2006a. Forelimb function in Ornitholestes hermanni Osborn (Dinosauria, Theropoda). Palaeontology, 49:1029–1034.
https://doi.org/10.1111/j.1475-4983.2006.00585.x
Senter, P. 2006b. Comparison of forelimb function between Deinonychus and Bambiraptor (Theropoda: Dromaeosauridae). Journal of Vertebrate Paleontology, 26:897–906.
https://doi.org/10.1671/0272-4634(2006)26[897:coffbd]2.0.co;2
Senter, P. 2006c. Scapular orientation in theropods and basal birds, and the origin of flapping flight. Acta Palaeontologica Polonica, 51:305–313.
Senter, P. 2007. Analysis of forelimb function in basal ceratopsians. Journal of Zoology (London), 273:305–314.
https://doi.org/10.1111/j.1469-7998.2007.00329.x
Senter, P. 2009. Pedal function in deinonychosaurs (Dinosauria: Theropoda): a comparative study. Bulletin of Gunma Museum of Natural History, 13:1–14.
Senter, P. 2010. Vestigial skeletal structures in dinosaurs. Journal of Zoology, 280:60–71.
https://doi.org/10.1111/j.1469-7998.2009.00640.x
Senter, P. 2012. Forearm orientation in Hadrosauridae (Dinosauria: Ornithopoda) and implications for museum mounts. Palaeontologia Electronica, 15.3.30A:1–10.
https://doi.org/10.26879/330
Senter, P. and Parrish, J.M. 2005. Functional analysis of the hands of the theropod dinosaur Chirostenotes pergracilis: evidence for an unusual paleoecological role. PaleoBios, 25(2):9–19.
Senter, P. and Parrish, J.M. 2006. Forelimb function in the theropod dinosaur Carnotaurus sastrei, and its behavioral implications. PaleoBios, 26(3):7–17.
Senter, P. and Robins, J.H. 2005. Range of motion in the forelimb of the theropod dinosaur Acrocanthosaurus atokensis, and implications for predatory behaviour. Journal of Zoology (London), 266:307–318.
https://doi.org/10.1017/s0952836905006989
Senter, P. and Robins, J.H. 2015. Resting orientations of dinosaur scapulae and forelimbs: a numerical analysis, with implications for reconstructions and museum mounts. PLoS ONE, 10(12):e0144036.
https://doi.org/10.1371/journal.pone.0144036
Senter, P.J. and Sullivan, C. 2019. Forelimbs of the theropod dinosaur Dilophosaurus wetherilli: range of motion, influence of paleopathology and soft tissues, and description of a distal carpal bone. Palaeontologia Electronica, 22.2.30A:1–19.
https://doi.org/10.26879/900
Sereno, P.C. 1993. Pectoral girdle and forelimb of the basal theropod Herrerasaurus ischigualastensis. Journal of Vertebrate Paleontology, 13:425–450.
https://doi.org/10.1080/02724634.1994.10011524
Stanford, R., Weems, R.E., and Lockley, M.G. 2004. A new dinosaur ichnotaxon from the Lower Cretaceous Patuxent Formation of Maryland and Virginia. Ichnos, 11:251–259.
Tanke, D.H. and Rothschild, B.M. 2010. Paleopathologies in Albertan ceratopsids and their behavioral significance, pp. 355–384. In Ryan, M.J., Chinnery-Allgeier, B.J., and Eberth, D.A. (eds.), New Perspectives on Horned Dinosaurs. The Royal Tyrrell Museum Ceratopsian Symposium. Indiana University Press, Bloomington, USA.
Thompson, S. and Holmes, R. 2007. Forelimb stance and step cycle in Chasmosaurus irvinensis (Dinosauria: Neoceratopsia). Palaeontologia Electronica, 10.1.5A:1–17.
https://palaeo-electronica.org/2007_1/step/index.html
Tsai, H.P. and Holliday, C.M. 2015. Articular soft tissue anatomy of the archosaur hip joint: structural homology and functional implications. Journal of Morphology, 276:601–630.
https://doi.org/10.1002/jmor.20360
Vargas-Peixoto, D., Stock Da-Rosa, A.A., and Gallo de França, M.A. 2015. Functional and biomechanic aspects of the scapular girdle and forelimbs of Unaysaurus tolentinoi Leal et al., 2004 (Saurischia: Sauropodomorpha). Journal of South American Earth Sciences, 61:129–133.
https://doi.org/10.1016/j.jsames.2014.09.024
White, M.A., Bell, P.R., Cook, A.G., Barnes, D.G., Tischler, T.R., Bassam, B.J., and Elliott, D.A. 2015. Forearm range of motion in Australovenator wintonensis (Theropoda, Megaraptoridae). PLoS ONE, 10(9):e0137709.
https://doi.org/10.1371/journal.pone.0137709
White, M.A., Cook, A.G., Klinkhamer, A.J., and Elliott, D.A. 2016. The pes of Australovenator wintonensis (Theropoda: Megaraptoridae): analysis of the pedal range of motion and biological restoration. PeerJ, 4:e2312.
https://doi.org/10.7717/peerj.2312
Wright, J.L. 1999. Ichnological evidence for use of the forelimb in iguanodontid locomotion. Special Papers in Palaeontology, 60:209–219.

