Volume 28.2
May–August 2025
Full table of contents
ISSN: 1094-8074, web version;
1935-3952, print version
Recent Research Articles
See all articles in 28.2 May-August 2025
See all articles in 28.1 January-April 2025
See all articles in 27.3 September-December 2024
See all articles in 27.2 May-August 2024
Interested in submitting a paper to Palaeontologia Electronica?
Click here to register and submit.
Article Search
 D. Cary Woodruff. Royal Ontario Museum; University of Toronto, Toronto, ON, Canada; and Great Plains Dinosaur Museum and Field Station, Malta, MT, United States of America. sauropod4@gmail.com
D. Cary Woodruff. Royal Ontario Museum; University of Toronto, Toronto, ON, Canada; and Great Plains Dinosaur Museum and Field Station, Malta, MT, United States of America. sauropod4@gmail.com
D. Cary Woodruff – Cary received his B.Sc. and M.Sc. in Earth Sciences with an emphasis in Paleontology at Montana State University under Dr. John "Jack" Horner. Cary's research ranges from actualistic taphonomy of burrowing dinosaurs, bovid vertebral anatomy, soft tissue preservation in the fossil record, with the majority dedicated to sauropod biomechanics and ontogeny. Cary is the Director of Paleontology at the Great Plains Dinosaur Museum in Malta, Montana, and is simultaneously a Ph.D. student at the University of Toronto with Dr. David Evans.

 Denver W. Fowler. Dickinson Museum Center, Dickinson, ND, United States of America. df9465@hotmail.com
Denver W. Fowler. Dickinson Museum Center, Dickinson, ND, United States of America. df9465@hotmail.com
Dr. Denver W. Fowler – Denver received his B.Sc. at the University of Durham, his M.Sc. at the University of Bristol, and recently his Ph.D. at Montana State University under Dr. John "Jack" Horner. Denver's diverse research topics range from taphonomy, biomechanics, paleobiology, biostratigraphy, to ontogeny. Denver's research regarding paleobiology and heterochrony is helping to better understand the intimate details surrounding dinosaur lives and evolution. Denver is currently the Curator of Paleontology at the Dickenson Dinosaur Museum in Dickinson, ND.

 John R. Horner. Burke Museum of Natural History and Culture, University of Washington, Seattle, WA, United States of America. johnrhorner@mac.com
John R. Horner. Burke Museum of Natural History and Culture, University of Washington, Seattle, WA, United States of America. johnrhorner@mac.com
Dr. John "Jack" R. Horner – Jack has received honorary doctorates from the University of Montana and Pennsylvania State University. In 1979 Jack and Bob Makela discovered the first dinosaur eggs in North America at a massive nesting colony known as "Egg Mountain". The discovery of "Egg Mountain" and the hadrosaur Maiasaura was the first evidence of dinosaurian parental care, and a major component to the "Dinosaur Renaissance". Since coming to the Museum of the Rockies in 1982, Jack has built one of the largest dinosaur research facilities in the world. Jack's research has spanned every aspect of paleontology, but his work on dinosaur ontogeny – via histologic examination – has re-written how we understand the growth and development of dinosaurs.
APPENDIX 1.
Terms, definition, and usage of pneutmatic morphology and histology used in this analysis.
Pneumatic Architecture (Wedel et al., 2000)
Camera - Round cavity, 5-150 mm in size, septal thickness of 2-10 mm, and a regular branching pattern.
Camella - Angular cavity, 2-20 mm in size, septal thickness of 1-3 mm, and an irregular branching pattern.
Acamerate - Pneumatic structures limited to fossae. Fossae do not significantly invade the centrum.
Procamerate - Deep fossae penetrate to median septum, but are not enclosed by osteal margins.
Camerate - Large, enclosed camerae with regular branching pattern; cameral generations usually limited to 3.
Camellate - Fine internal structures composed entirely of small scaled, thin-walled camellae; can produce a “honeycomb”-like network.
Histologic Terms (Francillon‐Vieillot et al., 1990; de Ricqlès et al., 1991; Castanet et al., 1992; Huttenlocker et al., 2013)
Line Of Arrested Growth (LAG) - Thin bands that represent temporary arrest of osteogenesis, and are considered osteological response to predictable environmental cues.
Annuli - Translucent to opaque bands, thicker than LAGs, represent a slowing (but not a cessation) of osteogenesis.
Woven Bone - Highly disorganized arrangement of collagen fibers, which reflects a high rate of osteogenesis.
Fibrolamellar Bone - Woven bone with intervening and randomly oriented primary osteons.
External Fundamental System (EFS) - Slowly deposited parallel-fibered or lamellar tissue along the outermost cortex (closely spaced outermost series of LAGs).
Haversian Bone - Bone that is completely remodeled by secondary osteons.
Primary Osteon - Central blood vessel and surrounding concentric bone tissue.
Secondary Osteon - Osteon formed by replacement of existing bone, surrounded by an outer cement sheath.
Cancellous Bone - Highly vascular bone that contains a higher surface area to mass ratio.
Trabeculae - Rod-shaped bone tissue in cancellous bone. Provides lightweight internal support.
Laminar Vascular Canal - Circumferentially oriented rows of vascular canals.
Longitudinal Vascular Canal - Canals oriented parallel to the long axis on the bone.
Reticular Vascular Canal - Obliquely oriented vascular canals.
APPENDIX 2.
Sauropod specimens examined in this analysis (H-MOS = Histo-Morph Ontogeny Scale [see Appendix 3]).
| Specimen Number | Material Examined | Taxonomy | H-MOS Estimation |
| AMNH 353 | Femur | Apatosaurus sp. (McIntosh, 1990) | Stage 4 |
| AMNH 435 | Femur | Apatosaurus sp. (this analysis) | Stage 2 or 3 |
| AMNH 606 | Femur | Apatosaurus sp. (this analysis) | Stage 4 |
| AMNH 613 | Femur | Diplodocidae (this analysis) | Stage 1 |
| AMNH 5855 | Femur | Diplodocus sp., Barosaurus (Mook, 1917; McIntosh, 2005) | Stage 2 |
| AMNH 6341 | Vertebrae | Barosaurus (Lull, 1919; Tschopp et al., 2015) | Stage 3 or 4 |
| AMNH 7530 | Vertebrae | Barosaurus, Kaatedocus (Michelis, 2004; Tschopp et al., 2015) | Stage 2 |
| AMNH 7535 | Vertebrae | Barosaurus (Michelis, 2004; Tschopp et al., 2015) | Stage 2 or 3 |
| AMNH 7539 | Femur | Diplodocus sp. (this analysis) | Stage 2 |
| ANS 21122 | Vertebrae, Femur, Skull, Dorsal Ribs | Suuwassea, Apatosaurinae, Dicraeosauridae (Harris, 2006a,b; Whitlock and Harris, 2010; Woodruff and Fowler, 2012; Tschopp et al., 2015) | Stage 3 |
| BYU 601-17103 | Femur | Apatosaurus sp. (Wilhite, 2003) | Stage 4 |
| BYU 725-4889 | Femur | Diplodocus sp. (Wilhite, 2003) | Stage 2 |
| BYU 725-9026 | Femur | Diplodocus sp. (Wilhite, 2003) | Stage 2 or 3 |
| BYU 725-11421 | Femur | Diplodocus sp. (Wilhite, 2003) | Stage 2 or 3 |
| BYU 725-12155 | Femur | Diplodocus sp. (Wilhite, 2003) | Stage 3 |
| BYU 725-13369 | Femur | Diplodocus sp. (Wilhite, 2003) | Stage 3 |
| BYU 725-13643 | Femur | Diplodocus sp. (Wilhite, 2003) | Stage 4 |
| BYU 725-13670 | Femur | Diplodocus sp. (Wilhite, 2003) | Stage 1 |
| BYU 725-16569 | Femur | Diplodocus sp. (Wilhite, 2003) | Stage 2 |
| BYU 725-16610 | Femur | Diplodocus sp. (Wilhite, 2003) | Stage 2 |
| BYU 17096 | Skull | Apatosaurus sp. (Balanoff et al., 2010) | Stage 2 |
| BYU Fe-4-DM197 | Femur | Diplodocus sp. (Wilhite, 2003) | Stage 3 |
| BYU Fe-5-DM172 | Femur | Diplodocus sp. (Wilhite, 2003) | Stage 3 |
| CM 84 | Vertebrae, Femur | Diplodocus carnegii (Hatcher, 1901; Tschopp et al., 2015) | Stage 4 |
| CM 85 | Femur | Apatosaurus sp. (McIntosh, 1981) | Stage 4 |
| CM 87 | Femur | Apatosaurus sp. (McIntosh, 1981) | Stage 4 |
| CM 94 | Vertebrae, Femur, Dorsal Ribs | Diplodocus carnegii (Hatcher, 1901; Tschopp et al., 2015) | Stage 4 |
| CM 555 | Vertebrae | Apatosaurus excelsus,Brontosaurus excelsus (McIntosh, 1981; Tschopp et al., 2015) | Stage 3 |
| CM 563 | Vertebrae | Apatosaurus excelsus, Brontosaurus parvus (Gilmore, 1936; Tschopp et al., 2015) | Stage 4 |
| CM 566 | Femur | Apatosaurus sp., Brontosaurus parvus (McIntosh, 1981; Tschopp et al., 2015) | Stage 1 |
| CM 572 | Vertebrae | Haplocanthosaurus priscus (Hatcher, 1903; Tschopp et al., 2015) | Stage 3 or 4 |
| CM 879 | Vertebrae | Haplocanthosaurus utterbacki (Hatcher, 1903; Tschopp et al., 2015) | Stage 2 or 3 |
| CM 3018 | Vertebrae, Femur, Skull | Apatosaurus louisae (Gilmore, 1936; Tschopp et al., 2015) | Stage 4 |
| CM 3390 | Vertebrae | Apatosaurus sp. (McIntosh, 1981) | Stage 2 |
| CM 11161 | Skull | Diplodocus longus; Diplodocinae Indeterminate (Berman and McIntosh, 1978; Tschopp et al. 2015) | Stage 4 |
| CM 11162 | Skull | Apatosaurus louisae (Berman and McIntosh, 1978; Tschopp et al., 2015) | Stage 4 |
| CM 11338 | Vertebrae | Camarasaurus lentus (Gilmore, 1925) | Stage 2 |
| CM 21785 | Femur | Apatosaurus sp. (McIntosh, 1981) | Stage 4 |
| CM 21788 | Femur | Diplodocus sp. (Wilhite, 2003) | Stage 1 or 2 |
| CM 30762 | Femur | Diplodocus sp. (Wilhite, 2003) | Stage 2 |
| CM 30766 | Femur | Apatosaurus sp., Apatosaurine (Wilhite, 2003; Tschopp et al., 2015) | Stage 3 |
| CM 33976 | Femur | Apatosaurus sp., Diplodocus sp. (Wilhite, 2003, this analysis) | Stage 1 |
| CM 33991 | Femur | Diplodocus sp. (McIntosh, 1981) | Stage 1 |
| CMC VP 7747 | Femur | Diplodocidae, Diplodocus sp. (Meyers, 2004; Woodruff and Fowler, 2004) | Stage 2 |
| CMC VP14128 | Skull | Diplodocus sp. (this analysis) | Stage 2 |
| CMNH 10039 | Femur | Apatosaurus sp. (Wilhite, 2003) | Stage 2 |
| CMNH 10380 | Vertebrae | Haplocanthosaurus delfsi (Wilhite, 2003) | Stage 4 |
| DNM 3781 | Femur | Diplodocus sp. (Wilhite, 2003) | Stage 4 |
| GPDM 220 | Vertebrae | Camarasaurus sp. (this analysis) | Stage 4 |
| HMNS 175 | Femur | Diplodocus hayi, Galeamopus (Holland, 1924; Tschopp et al., 2015) | Stage 4 |
| KUVP 1351 | Femur | Apatosaurus sp. (Wilhite, 2003) | Stage 4 |
| MOR 592 | Vertebrae, Femur, Skull, Dorsal Ribs, Cervical Ribs | Amphicoelias altus, Dicraeosauridae, Diplodocus sp. (Wilson and Smith, 1996; Whitlock and Harris, 2010; Woodruff and Fowler, 2012) | Stage 3 |
| MOR 700
(2 specimens) |
Skull, Cervical Ribs | Apatosaurus sp. (Woodruff and Fowler, 2014) | Stage 2 and 4 |
| MOR 714 | Vertebrae | diplodocid indeterminate (this analysis) | Stage 1 |
| MOR 790
(15 specimens) |
Vertebrae, Femur, Dorsal Ribs, Cervical Ribs | Diplodocinae; Diplodocus sp. (Myers, 2004; Woodruff and Fowler, 2012) | Stage 2 |
| MOR 957 | Vertebrae, Femur | Apatosaurus sp. (this analysis) | Stage 4 |
| MOR 7029 | Vertebrae, Skull | Diplodocus sp. (Woodruff and Fowler, 2014) | Stage 3 |
| MWC 5439 | Femur | Apatosaurus sp. (this analysis) | Stage 1 |
| MWC
“Moffit Co. Apato.” |
Femur | Apatosaurus sp. (this analysis) | Stage 4 |
| NSMT-PV 20375 | Vertebrae, Femur | Apatosaurus ajax, Apatosaurinae Indeterminate (Upchurch et al., 2004b; Tschopp et al., 2015) | Stage 4 |
| OMNH 1793 | Femur | Diplodocus sp. (Wilhite, 2003) | Stage 1 |
| OMNH 01667 | Femur | Apatosaurus sp. (Wilhite, 2003) | Stage 4 |
| SMA 0003 | Vertebrae | Diplodocidae, Diplodocus sp. (Schwarz et al., 2007a; this analysis) | Stage 3 |
| SMA 0004 | Vertebrae, Skull | Kaatedocus (Tschopp and Mateus, 2012; Tschopp et al., 2015) | Stage 2 or 3 |
| SMA 0009 | Vertebrae, Femur, Cervical Ribs | Diplodocidae; Barosaurus, Brachiosaurus (Schwarz et al., 2007a; Woodruff and Fowler, 2012; Carballido et al., 2012; Tschopp et al., 2015) | Stage 1 |
| SMA 0011 | Vertebrae, Skull | Diplodocidae, Galeamopus (Klein and Sander, 2008; Tschopp et al., 2015) | Stage 3 |
| SMA 0014 | Femur | Diplodocidae (this analysis) | Stage 4 |
| SMM P84.15.2 | Femur | Apatosaurus sp. (this analysis) | Stage 1 |
| TMM 993-1 | Femur | Diplodocus sp. (Wilhite, 2003) | Stage 4 |
| USNM 2672 | Skull | Diplodocus sp., Diplodocinae Indeterminate (Berman and McIntosh, 1978; Tschopp et al., 2015) | Stage 4 |
| USNM 2673 | Skull | Diplodocus sp.; Galeamopus (Berman and McIntosh, 1978; Tschopp et al., 2015) | Stage 4 |
| USNM 4797 | Femur | Apatosaurus sp. (Wilhite, 2003) | Stage 4 |
| USNM 10865 | Femur | Diplodocus sp. (Wilhite, 2003) | Stage 4 |
| USNM 11162 | Skull | Apatosaurus louisae (Berman and McIntosh, 1978) | Stage 4 |
| USNM 337871 | Femur | Diplodocus sp. (Wilhite, 2003) | Stage 1 or 2 |
| WDC BS-157 | Femur | Apatosaurus sp. (Wilhite, 2003) | Stage 3 |
| YPM 429 | Vertebrae | Barosaurus (Lull, 1919; McIntosh, 2005; Tschopp et al., 2015) | Stage 4 |
| YPM 1980 | Vertebrae | Apatosaurus excelsus, Brontosaurus excelsus (Ostrom and McIntosh, 1966; Tschopp et al., 2015) | Stage 4 |
| YPM 5862 | Femur | Apatosaurus sp. (Wilhite, 2003) | Stage 1 |
APPENDIX 3.
The Histo-Morph Ontogeny Scale (H-MOS).
| Stage 1 | Stage 2 | Stage 3 | Stage 4 | |
| HOS | <4 | 5-7 | 8-10 | >10 |
| Postparietal aperture | ? | Present | Present | Absent |
| LAGs (minimum record via dorsal ribs) | <2 | 2-6 | 7 - 15* (15 is an estimated demarcation) |
>15 |
| Cervical pneumatic architecture |
Acamerate with shallow Fossae (4-8 mm) | Procamerate to Camerate with deepening Fossae (7-24 mm) |
Camerate with increasing depth and abundance of Fossae and Foramina | Camerate with extensive and numerous Fossae and Foramina |
| Cervical pneumaticity (CT scan) |
No internal structures | Camerae & Camellae | Thinning median septum with Camerae and Camellae | ? |
| Dorsal pneumaticity (CT scan) |
? | Thinning median septum with Camerae | Thinning median septum with Camerae and Camellae | Extensive Camerae in centrum and arch |
| Ant. cervical bifurcation | No bifurcation | No bifurcation | Notched to weakly bifurcated | Fully bifurcated |
| Post. cervical bifurcation | No bifurcation | Weakly bifurcated | Narrow bifurcation | Fully bifurcated |
| Ant. dorsal bifurcation | No bifurcation | Narrow bifurcation | Narrow bifurcation | Fully bifurcated |
| Post. dorsal bifurcation | No bifurcation | Narrow bifurcation | Bifurcated | Fully bifurcated |
| Ant. Caudal bifurcation | No bifurcation | No bifurcation | No bifurcation | Fully bifurcated |
| Neural spine Trabeculae (mm) |
? | ≤ 1 | ≥ 3 | ? |
| Femoral head orientation | ? | 20° - 11° | 10° - 5° | <5° |
| 4th Trochanter position | Proximal | Proximal | Proximal to mid-diaphysis | Mid-diaphysis |
| Medial Condyle | Not pronounced | Ventrally expanding | Laterally epanding | Greatly pronounced |
| Femur length (mm) | <800 | 800 - 1,200 | 1,200 - 1,450 | >1,450 |
| Body mass (kg) | <2,000 | 2,000 - 3,000 | 3,000 - 6,000 | >6,000 |
APPENDIX 4.
Diplodocidae body mass table using the allometry based body mass formula of Mazzetta (2004) (log Body Mass = 2.955 x log Femur Circumference − 4.166).
| Taxon | Specimen Number | Femur Circumference (mm) | Body Mass (kg) | Mass - Pneumaticity (kg) |
| Apatosaurus sp. | MWC "Moffat Co. Apato." | 908.05 | 49378.59631 | 44440.73667 |
| Apatosaurus | SMA 0014 | 810 | 26827.06928 | 24144.36235 |
| Apatosaurus | AMNH 613 | 805 | 26340.67055 | 23706.60349 |
| Apatosaurus | AMNH 606 | 795 | 25385.44993 | 22846.90494 |
| Apatosaurus | AMNH 7539 | 766 | 22745.52756 | 20470.9748 |
| Apatosaurus | AMNH 353 | 725 | 19332.98035 | 17399.68231 |
| A. louisae | CM 30766 | 701 | 17502.36514 | 15752.12863 |
| Brontosaurus | CM 21785 | 690 | 16703.17623 | 15032.8586 |
| Apatosaurus | CM 85 | 678 | 15859.29017 | 14273.36116 |
| Brontosaurus | CM 87 | 650 | 14000.9972 | 12600.89748 |
| Apatosaurus | CM 566 | 626 | 12527.86759 | 11275.08083 |
| Apatosaurus | CM 33976 | 600 | 11051.89995 | 9946.709959 |
| Apatosaurus | P25112 | 555 | 8777.801694 | 7900.021524 |
| Apatosaurus | OMNH 01667 | 513 | 6956.5965 | 6260.93685 |
| Apatosaurus | KUVP 1351 | 453 | 4816.928356 | 4335.235521 |
| Apatosaurus | BS 157 | 349 | 2228.687292 | 2005.818563 |
| Apatosaurus | CMNH 10039 | 310 | 1570.26809 | 1413.241281 |
| Apatosaurus | USNM 4797 | 288 | 1263.29507 | 1136.965563 |
| Apatosaurus | BYU 601-17103 | 216 | 539.896901 | 485.9072109 |
| Apatosaurus | YPM 5862 | 184.15 | 336.963175 | 303.2668575 |
| Apatosaurus | MWC 5439 | 148 | 176.6539566 | 158.9885609 |
| diplodocid indeterminate | SMM P84.15.2 | 120 | 95.05622917 | 85.55060625 |
| Galeamopus | HMNS 175 | 590 | 10516.4145 | 9464.77305 |
| Diplodocus | USMN 10865 | 564 | 9205.126313 | 8284.613681 |
| Diplodocus | TMM 993-1 | 551 | 8592.172245 | 7732.95502 |
| D. carnegii | CM 94 | 540 | 8095.12502 | 7285.612518 |
| Diplodocus | BYU 725-13643 | 536 | 7919.211712 | 7127.290541 |
| Diplodocus | BYU 725-13369 | 513 | 6956.5965 | 6260.93685 |
| D. carnegii | CM 84 | 510 | 6837.067544 | 6153.36079 |
| Diplodocus | BYU Fe-4-DM197 | 490 | 6074.764083 | 5467.287674 |
| Diplodocus | AMNH 435 | 458 | 4975.737808 | 4478.164027 |
| Diplodocus | BYU 725-11421 | 456 | 4911.804923 | 4420.624431 |
| Diplodocus | AMNH 7539 | 455 | 4880.043319 | 4392.038987 |
| Diplodocus | BYU 725-12155 | 455 | 4880.043319 | 4392.038987 |
| Diplodocus | BB 761 | 451 | 4754.355816 | 4278.920235 |
| Diplodocus | BYU Fe-5-DM172 | 435 | 4273.028154 | 3845.725339 |
| Diplodocus | AMNH 5855 | 410 | 3587.37103 | 3228.633927 |
| Diplodocus | MOR 592-35 | 409 | 3561.577304 | 3205.419574 |
| D. longus | CM 30762 | 387 | 3024.72504 | 2722.252536 |
| Diplodocus | BYU 725-16569 | 385 | 2978.766464 | 2680.889817 |
| Diplodocus | MOR 790 7-23-95-122 | 371.5 | 2680.577713 | 2412.519942 |
| Diplodocus | BYU 725-16610 | 365 | 2544.341575 | 2289.907418 |
| Diplodocus | MOR 790 7-5-95-7 | 362 | 2483.04063 | 2234.736567 |
| Diplodocus | BB 463 | 361 | 2462.826294 | 216.543665 |
| Diplodocus | BYU 725-4889 | 354 | 2324.36667 | 2091.930003 |
| Diplodocus | BYU 725-9026 | 350 | 2247.610597 | 2022.849537 |
| Diplodocus | USMN 337871 | 325 | 1805.574164 | 1625.016747 |
| D. longus | CM 21788 | 317 | 1677.374822 | 1509.63734 |
| D. longus | CM 33991 | 315 | 1646.295103 | 1481.665593 |
| Diplodocus | OMNH 1793 | 254 | 871.5325918 | 784.3793326 |
| Diplodocus | DNM 3781 | 243 | 764.6567709 | 688.1910938 |
| Diplodocus | BYU 725-13670 | 162 | 230.7368014 | 207.6631213 |
| diplodocid indeterminate | P.84.15.2 | 120 | 95.05622917 | 85.55060625 |
APPENDIX 5.
Supplemental Information.
The material contained herein serves to address some of the comments and questions raised by Wedel and Taylor (2013), along with some minor discussion on non-Apatosaurus and Diplodocus genera.
Anterior Cervical Bifurcation. Wedel and Taylor (2013) suggest that there is no evidence of bifurcated neural spines more anteriorly than C6 in any known North American diplodocid. While it is correct that the anterior most neural spines are damaged and reconstructed from Diplodocus carnegii (CM 84), Brontosaurus excelsus (YPM 1980), and Brontosaurus parvus (UWGM 15556), resulting in unknown spine morphology for these specimens, other cervical series from North American diplodocids do show anterior bifurcation (Appendix 6). The anterior-most cervical neural spines from CM 555 (Apatosaurus or Brontosaurus excelsus) are damaged, yet C4 distinctly has weak bifurcation. C5 has weakly expressed bifurcation; while C6 and the remaining vertebrae have fully expressed bifurcation. Even within Barosaurus lentus we see similar anterior-most bifurcation. In AMNH 7530 (alternatively identified as Kaatedocus by Tschopp et al., 2015), the neural spine of C2 is not bifurcated, yet the neural spine of C3 clearly is. After C3, the spines of C4 and C5 are broad, but not bifurcated. The remaining cervical vertebrae are on display, and removal to examine the anterior morphology was not possible at the time of visitation. A slightly larger specimen AMNH 7535 exhibits similar spine morphology. The neural spine of C2 is slightly damaged at the apex, but the neural spine would appear to be bifurcated (if so it would be the anterior most documentation of spine bifurcation). C3 and C5 are missing, and the neural spine of C4 is badly damaged. The neural spine of C6 is clearly bifurcated, yet C7-C13 are un-bifurcated. The final vertebra from this specimen, C14, does have a broad neural spine with incipient or weakly expressed bifurcation.
The claim that no North American diplodocids possessed bifurcated neural spines farther anteriorly than C6 is now shown to be incorrect. Through examination of several complete or nearly complete cervical series, we now see that in diplodocids incipient to weak neural spine bifurcation can occur between C3-C5 (Appendix 6). It is interesting to see that in the anterior most cervical vertebrae there is a small span of bifurcation preceded and followed by a lack of bifurcation. If the hypothesis that bifurcation is developed as a means to maintain and sustain horizontal mobility (Woodruff, 2014) is correct, the presence of select anterior-most bifurcation could be indicative of active cranial mobility. As demonstrated by Wedel and Taylor (2013), serial position is critical. If serial position was unknown in these specimens then there would be a valid argument to place these bifurcated anterior cervicals more posteriorly. From the examined specimens it appears that diplodocids (at least North American taxa) could have neural spine bifurcation prior to C6, and that these damaged historic specimens could be reconstructed with such.
The “End” Of Diplodocid Ontogeny. With the information attained from many of these 2/3 sized diplodocids (such as MOR 592 MOR 7029 [both Diplodocus sp.]), it would appear that once a diplodocid reaches roughly 2/3 its maximum length that proportionally (and potentially mechanically) it is more equivalent to the skeletally mature form. Simply applying force per unit area or a Ponderal Index (Thompson, 1942), the gravitational forces acting upon a 15 meter and 27 meter long animal are more similar (approximately 2 times an increase in both body mass and body length) than those acting upon a 2 meter and a 15 meter long animal (approximately 38 times heavier and 7.5 times longer). Once a diplodocid reaches that 2/3 sized threshold it has achieved the skeletal adaptations needed to support the large vertebral column (i.e., bifurcation of the neural spines). From the 2/3 size through the remainder of its life, the animal then modifies the existing structure to deal with the increasing stresses enacted upon it. This explains why the spine bifurcation changes are less dramatic, and why the centrum begins to expand along with other proportional changes. At the point of dramatic weight increase, it is far easier to modify a pre-existing structure than to suddenly develop a new feature. It would appear that from hatching, an immature diplodocid is in a dramatic ontogenetic race to develop the skeletal features needed to support its eventual gigantic girth.
Woodruff and Fowler (2012) Clarification. In the discussion section Woodruff and Fowler (2012) say, “Just as particularly large diplodocid specimens... have been more recently recognized as large and potentially older individuals of already recognized taxa,... taxa defined on small specimens... might represent immature forms of Diplodocus or Apatosaurus.” Clarifying the original phrasing, the meaning of this passage is that ontogenetic and statigraphic analysis of the characters that diagnose particular taxa within Dipodocoidea (particularly those described based on immature holotypes) may significantly alter the structure of the phylogenetic tree. Further, isolated specimens currently attributed to a given taxon may instead turn out to be ontogenetic stages of a different taxon (without sinking the original designated taxon), these mis-assignments due to heterochronic shifts are only recognizable with stratigraphic and ontogenetic analysis.
Barosaurus. One possible contentious specimen to the ongoing discussion of ontogenetic development of neural spine bifurcation is the immature Barosaurus sp. (DINO 2921) described by Melstrom et al. (2016). While documenting numerous important ontogenetic vertebral characters (ranging from Elongation Index, neurocentral fusion, to pneumatic architecture; and thus further verification of the allometric development of the sauropod skeleton), Melstrom et al. (2016) claim that the morphology of the spine bifurcation in this ~1/3 adult sized individual is indistinguishable from that of a mature animal. The precise spinal morphologies and details are hopefully forthcoming (and respectfully such is not demonstrated nor documented in Melstrom et al. [2016]), but perhaps the specimen DINO 2921 could falsify the hypothesis of Woodruff and Fowler (2012).
Yet we would propose that if Melstrom et al. (2016) are indeed correct about the spine morphology of DINO 2921, this could be an incredibly important key to understanding sauropod evolution within the Morrison Formation. If the stratigraphic resolution is correct (see the informative works of K. Truillo), then DINO 2921 comes from the lower portion of the Brushy Basin Member (Turner and Peterson, 1999; Carpenter, 2013), while other immature Barosaurus specimens (such as AMNH 7530 and 7535; note Tschopp et al. [2015] identify AMNH 7530 as Kaatedocus) are non-bifurcated and come from the upper portion of the Salt Wash Member (Turner and Peterson, 1999; Michelis, 2004; Tschopp and Mateus, 2013). If this resolution, taxonomy, and morphology is correct, then this has substantial implications for Barosaurus heterochrony. Again, all of this hinges on the correct initial identification, but if so, this means that Lower and Upper Morrison specimens have differing vertebral biomechanics (i.e., distinct differing morphologies), and given the stratigraphic and calculated temporal range, this may be initial grounds to begin the examination and inquire into heterochrony, and therefore the possibility of two Morrison Barosaurus taxa.
Camarasaurus. A minor point we would like to bring to light is the recognition of neural spine bifurcation within the basal macronarian Camarasaurus. Woodruff and Fowler (2012) note that in specimens from the Kenton Quarry, the neural spines of immature Camarasaurus sp. are morphologically similar to immature diplodocids in that the neural spines are short and non-bifurcated. No in depth examination on Camarasaurus sp. was carried out in the preliminary analysis (but is currently underway by DCW), but the possibility of Camarasaurus spine ontogeny was favored by Wedel and Taylor (2013). While display features can be modified throughout ontogeny (i.e., male peacock plumage and cassowary and helmeted guinea fowl casques), it would seem unusual that a biomechanical feature could be ontogenetic in one clade, yet static in a closely related clade. In examination of the cervical and dorsal series from the presumed immature Camarasaurus lentus (CM 11338), the neural spines are bifurcated, but the depth of bifurcation is shallow and the neural spine apices are much closer together than in a fully mature animal. A relatively small Camarasaurus sp. specimen at the Great Plains Dinosaur Museum (GPDM 220) has cervical and dorsal neural spines that likewise exhibit shallow bifurcation and narrow neural spines (Woodruff and Foster, 2017). These features alone have been previously thought to be valid autapomorphies of a new genus (N. Murphy and K. Carpenter, personal commun., 2012). However, analysis by Woodruff and Foster (2017) has contrarily demonstrated that GPDM 220 is a maturationally old, small statured individual. Thus GPDM 220 would further verify the complex relationship between vertebral mechanics and ontogeny within sauropods (Woodruff and Foster, 2017).
Haplocanthosaurus. Wedel and Taylor (2013) perform a laudable job verifying that the genus Haplocanthosaurus is not a juvenile Apatosaurus or Diplodocus (the specific lines of reasoning will not be addressed here but we recommend referral to their text; Wedel and Taylor [2013] p. 23-27). Being one of the rarest of Morrison taxa, Haplocanthosaurus is known from three species: H. delfsi, H. priscus, and “H. utterbacki”. Collected from the lower Brushy Basin Member of the Morrison Formation, CM 572 (H. priscus) and CM 879 (“H. utterbacki”) both were found meters away from each other in the Marsh-Felch Quarry 1. While CM 879 has not been histologically sampled to assess maturity, the general consensus (largely based on the overall morphology) is that it represents an immature animal (the differences and validity between H. priscus and H. delfsi shall not be addressed here; McIntosh and Williams [1988], Wedel and Taylor [2013]). As Wedel (2009) illustrates, CM 572 exhibits widespread neurocentral synostosis, whereas CM 879 exhibits primarily completely unfused neural arches (remember from the manuscript that vertebral fusion is not conclusively indicative of maturity within dinosaurs). Though very weakly expressed and exceedingly rare, incipient neural spine bifurcation has been observed within some specimens of Haplocanthosaurus (Appendix 7). Neural spine bifurcation is observed in a posterior dorsal of CM 879, and within an anterior dorsal of CM 572. We are well aware of the importance of serial position and the morphological differences between such vertebrae, however due to the rarity of this feature and the relative proximity a comparison shall still be made. In a posterior cervical of CM 879, the bifurcation is formed by a connection of two closely spaced “humps” (reminiscent of bifurcation observed in CM 555). Within an anterior dorsal of CM 572 these “humps” are spaced and the bifurcation trough is a shallow “V”-shape. Based on the spinal morphology, it would appear that, unlike other members of Diplodocidae, Haplocanthosaurus did not biomechanically require neural spine bifurcation. In conjunction with McIntosh and Williams (1988) and Wedel and Taylor (2013), we would consider “H. utterbacki” as a nomen dubium, and further agree that it represents an immature form of H. priscus. In addition to this, the variation between the two specimens would suggest that while incipient, the neural spine bifurcation observed in Haplocanthosaurus may also be ontogenetic. Verification of this point requires further specimens and overlapping material.
In regards to the phylogenetic assignment of Haplocanthosaurus, we would again stress the need for the recognition of ontogenetic stages. In his analysis of Diplodocoidea, J. Whitlock (2011a) shows that the character matrix for Haplocanthosaurus is a combination of several specimens including CM 879 (“H. utterbacki”), CM 572 (H. priscus), and CMNH 10380 (H. delfsi). While these individuals were included to complete otherwise missing characters from other incomplete specimens, the taxonomic uncertainty of Haplocanthosaurus could be due to the fact that the characters states representing it are from a combination of varying ontogenetic stages and potentially separate species (sensu Mannion et al., 2012).
APPENDIX 6.
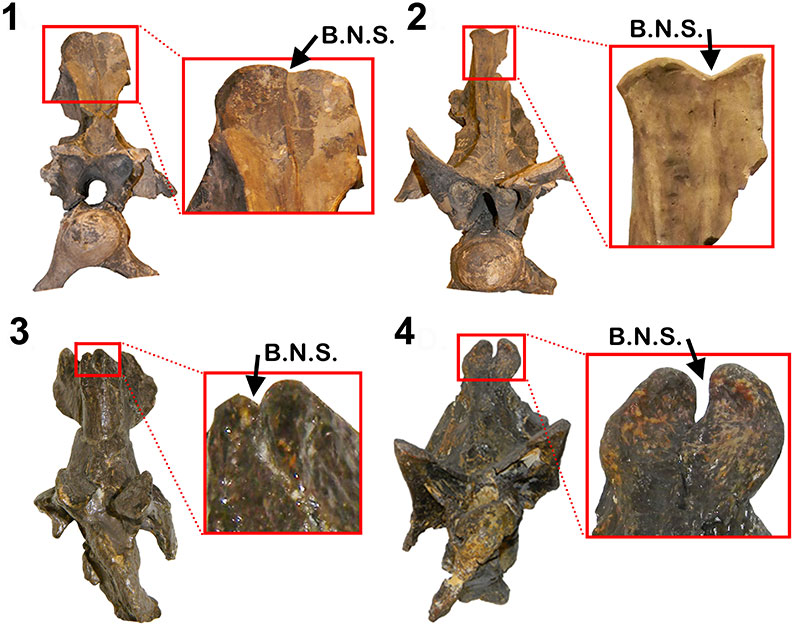
Neural spine bifurcation in diplodocid anterior most cervical vertebrae. 1-2, Apatosaurus CM 555; 3,Barosaurus AMNH 7530; 4,Diplodocus sp. MOR 592. B.N.S = Bifurcated Neural Spine. All cervical vertebrae in anterior view. Not to scale. Scale bars equal 10 cm.
APPENDIX 7.
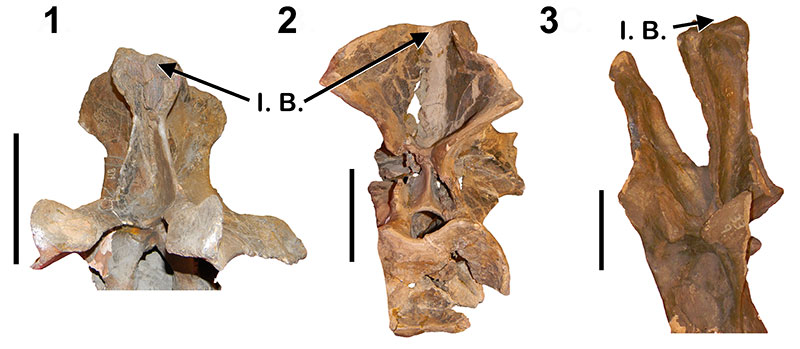
Haplocanthosaurus incipient neural spine bifurcation. 1, Haplocanthosaurus “utterbacki ” (CM 879) anterior dorsal in anterior view; 2,Hapalocanthosauruspriscus (CM 529) anterior dorsal in posterior view; 3,H. “utterbacki ” (CM 879) posterior dorsal in anterior view. I.B. = Incipient Bifurcation. Not to scale. Scale bar equals 10 cm.
APPENDIX 8.
Elongation Index of diplodocid cervical vertebrae (EI = centrum length divided by cotyle diameter).
| Diplodocus | |||
| HQ 1 SMA 0003 | |||
| Centrum Length (mm) | Cotyle Diameter (mm) | EI | |
| C2 | 84 | 37 | 2.27027 |
| C3 | 105.5 | 31 | 3.40323 |
| C4 | 131 | 46.5 | 2.8172 |
| C5 | 184 | 30.5 | 6.03279 |
| C6 | 228 | 46 | 4.95652 |
| C7 | 280 | 44 | 6.36364 |
| C8 | 376 | 55.5 | 6.77477 |
| C9 | 375 | 71 | 5.28169 |
| C10 | 385 | 49 | 7.85714 |
| C11 | 431 | 75 | 5.74667 |
| C12 | 458 | 74 | 6.18919 |
| C13 | 454 | 58.5 | 7.76068 |
| C14 | 463 | 77 | 6.01299 |
| C15 | 474 | 77 | 6.15584 |
| HQ 2 SMA 0004 | |||
| Centrum Length (mm) | Cotyle Diameter (mm) | EI | |
| C2 | 83.5 | 35 | 2.38571 |
| C3 | 107 | 27 | 3.96296 |
| C4 | 132 | - | - |
| C5 | 158 | 28 | 5.64286 |
| C6 | 192 | 26.5 | 7.24528 |
| C7 | 218 | 37 | 5.89189 |
| C8 | 247 | 34.5 | 7.15942 |
| C9 | 264 | 38 | 6.94737 |
| C10 | 296 | 53 | 5.58491 |
| C11 | 295 | 51 | 5.78431 |
| C12 | 316 | 56 | 5.64286 |
| C13 | 326 | 66 | 4.93939 |
| C14 | 314 | 61 | 5.14754 |
| MOR 592 | |||
| Centrum Length (mm) |
Cotyle Diameter (mm) |
EI | |
| C2 | 114.4 | 41.2 | 2.7767 |
| C3 | 128.3 | 41.9 | 3.06205 |
| C4 | - | - | - |
| C5 | 163.6 | 49 | 3.33878 |
| C6 | - | - | - |
| C7 | 247 | 37.5 | 6.58667 |
| C8 | 256.5 | 47.4 | 5.41139 |
| C9 | 271.6 | 67.8 | 4.0059 |
| C10 | 291 | 62.4 | 4.66346 |
| C11 | 279.4 | 103.5 | 2.69952 |
| C12 | 266.7 | 112.5 | 2.37067 |
| C13 | 239.2 | 180.8 | 1.32301 |
| C14 | 349.3 | 73.2 | 4.77186 |
| C15 | 304.8 | 96.2 | 3.1684 |
| CM 84 | |||
| Centrum Length (mm) |
Cotyle Diameter (mm) |
EI | |
| C2 | 165 | 54 | 3.05556 |
| C3 | 243 | 69 | 3.52174 |
| C4 | 289 | 81 | 3.5679 |
| C5 | 372 | 94 | 3.95745 |
| C6 | 442 | 99 | 4.46465 |
| C7 | 485 | 114 | 4.25439 |
| C8 | 512 | 120 | 4.26667 |
| C9 | 525 | 159 | 3.30189 |
| C10 | 595 | 175 | 3.4 |
| C11 | 605 | 210 | 2.88095 |
| C12 | 627 | 225 | 2.78667 |
| C13 | 638 | 231 | 2.7619 |
| C14 | 642 | 295 | 2.17627 |
| C15 | 595 | 245 | 2.42857 |
| Apatosaurus | |||
| CM 555 | |||
| Centrum Length (mm) |
Cotyle Diameter (mm) |
EI | |
| C2 | 140 | 63 | 2.22222 |
| C3 | 221 | 84 | 2.63095 |
| C4 | 274 | 96 | 2.85417 |
| C5 | 270 | 60 | 4.5 |
| C6 | 295 | 114 | 2.58772 |
| C7 | 316 | - | - |
| C8 | 344 | 113 | 3.04425 |
| C9 | 380 | - | - |
| C10 | - | - | - |
| C11 | 480 | 193 | 2.48705 |
| C12 | 460 | 245 | 1.87755 |
| C13 | - | - | - |
| C14 | 378 | 260 | 1.45385 |
| C15 | - | - | - |
| CM 563 | |||
| Centrum Length (mm) |
Cotyle Diameter (mm) |
EI | |
| C3 | 250 | 80 | 3.125 |
| C4 | 300 | 125 | 2.4 |
| C5 | 342 | 134 | 2.55224 |
| C6 | - | - | - |
| C7 | 415 | 170 | 2.44118 |
| C8 | 415 | 205 | 2.02439 |
| C9 | 445 | 215 | 2.06977 |
| C10 | 475 | 250 | 1.9 |
| NSMT-PV 20375 | |||
| Centrum Length (mm) |
Cotyle Diameter (mm) |
EI | |
| C3 | 352 | 97 | 3.62887 |
| C4 | - | - | - |
| C5 | 375 | 155 | 2.41935 |
| C6 | 395 | 195 | 2.02564 |
| C7 | 420 | 220 | 1.90909 |
| C8 | 395 | 195 | 2.02564 |
| C9 | 380 | 235 | 1.61702 |
| C10 | 390 | - | - |
| C11 | - | - | - |
| C12 | 475 | 240 | 1.97917 |
| C13 | - | - | - |
| C14 | 450 | 305 | 1.47541 |
| CM 3018 | |||
| Centrum Length (mm) |
Cotyle Diameter (mm) |
EI | |
| C2 | 190 | 85 | 2.23529 |
| C3 | 280 | 100 | 2.8 |
| C4 | 370 | 100 | 3.7 |
| C5 | - | - | - |
| C6 | 440 | 150 | 2.93333 |
| C7 | 450 | 190 | 2.36842 |
| C8 | 485 | 225 | 2.15556 |
| C9 | 510 | 230 | 2.21739 |
| C10 | 530 | 250 | 2.12 |
| C11 | 550 | 240 | 2.29167 |
| C12 | 490 | 265 | 1.84906 |
| C13 | 480 | - | - |
| Barosaurus | |||
| AMNH 7530 | |||
| Centrum Length (mm) |
Cotyle Diameter (mm) |
EI | |
| C2 | 80 | 14 | 5.71429 |
| C3 | 75 | 26 | 2.88462 |
| C4 | 97 | 37 | 2.62162 |
| C5 | 123 | 62 | 1.98387 |
| C6-C12 are on display and could not be measured first hand | |||
| AMNH 7535 | |||
| Centrum Length (mm) |
Cotyle Diameter (mm) |
EI | |
| C2 | 87 | 15 | 5.8 |
| C3 | - | - | - |
| C4 | 102.5 | 23 | 4.45652 |
| C5 | - | - | - |
| C6 | 135 | 25 | 5.4 |
| C7 | 140 | 21 | 6.66667 |
| C8 | 166 | 36 | 4.61111 |
| C9 | - | - | - |
| C10 | - | - | - |
| C11 | 234 | 32 | 7.3125 |
| C12 | - | - | - |
| C13 | 281 | 30 | 9.36667 |
| C14 | 323 | 47 | 6.87234 |
| AMNH 6341 (from McIntosh, 2005) | |||
| Centrum Length (mm) |
Cotyle Diameter (mm) |
EI | |
| C8 | 618 | 130 | 4.75385 |
| C9 | 685 | 123 | 5.56911 |
| C10 | 737 | 168 | 4.3869 |
| C11 | 775 | 145 | 5.34483 |
| C12 | 813 | 155 | 5.24516 |
| C13 | 850 | 180 | 4.72222 |
| C14 | 865 | 155 | 5.58065 |
| C15 | 840 | 160 | 5.25 |
| C16 | 750 | 250 | 3 |
| YPM 429 (from McIntosh, 2005) | |||
| Centrum Length (mm) |
Cotyle Diameter (mm) |
EI | |
| C13 | 930 | 220 | 4.22727 |
| C14 | 890 | 345 | 2.57971 |
| C15 | - | 300 | - |
| C16 | 720 | 365 | 1.9726 |
FIGURE 1. Elements histologically sampled for this analysis. 1, Digital reconstruction of Apatosaurus louisae (by K. Stevens) with sampled elements highlighted in red. Approximate location of sampling in dorsal ribs (2; from Gilmore, 1936), neural spines (3; from Hatcher, 1901), and femora (4; from Gilmore, 1936).
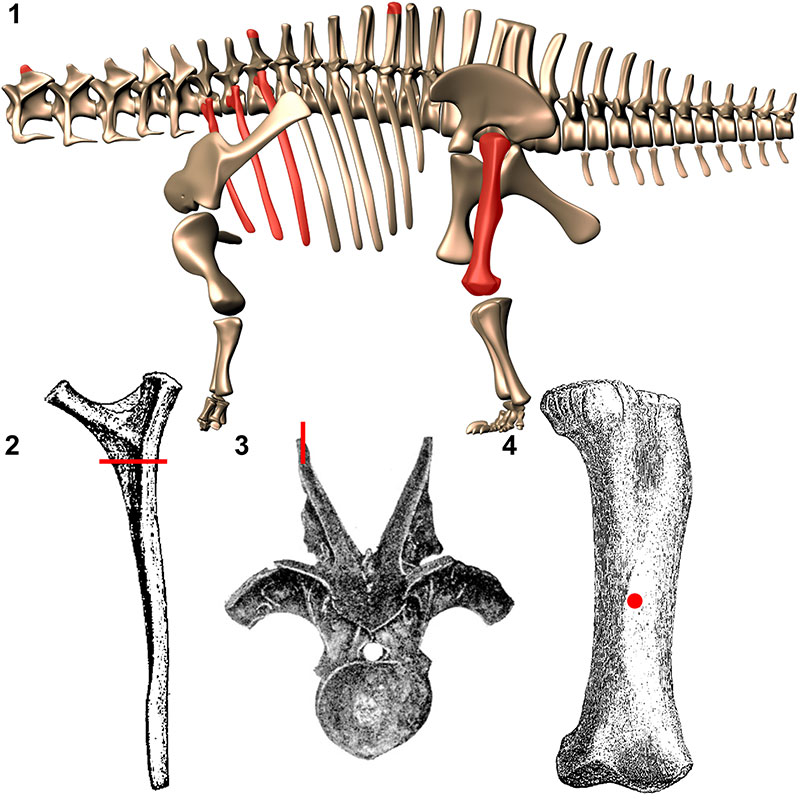
FIGURE 2. Schematic of coring bit method used in this analysis. A two part Bosch ™ core bit (1) with a coin placed on the bone surface prior to coring (2), the core bit separated post coring, with a bolt inserted to push on the coin (3) to extract the bone core (4).
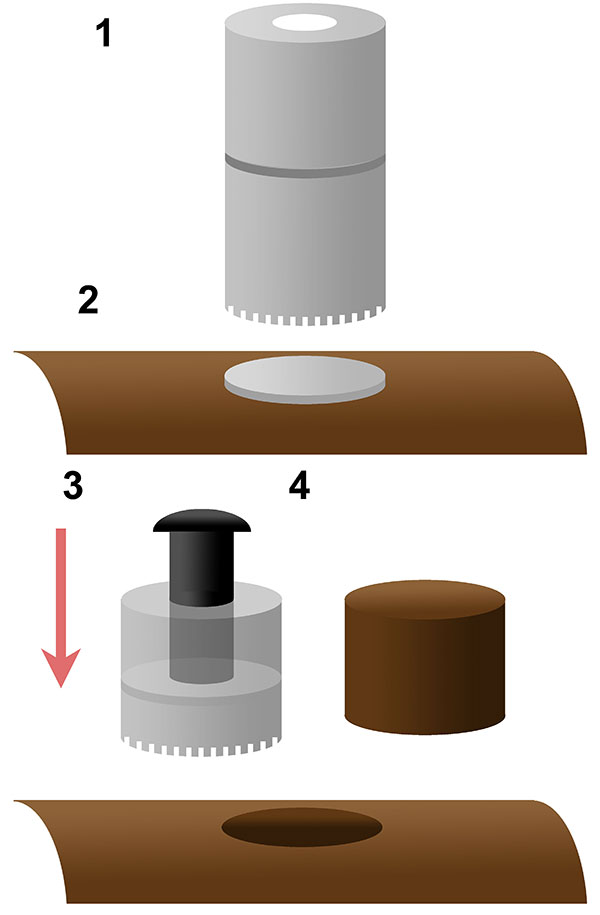
FIGURE 3. Ontogenetic development of neural spine bifurcation in Morrison Formation sauropods. Apatosaurinae - represented by the immature individuals CM 555 (Apatosaurus excelsus or Brontosaurus excelsus) and CM 3390 (Apatosaurus sp.) compared to the mature 3018. Diplodocus - represented by the immature MOR 592 (Diplodocus sp.) compared to the mature CM 84 (Diplodocus carnegii). Camarasaurus - represented by the immature CM 11338 (Camarasaurus lentus) compared to the mature AMNH 5761 (Camarasaurus supremus). Barosaurus - represented by the immature AMNH 7535 compared to the mature AMNH 6341. Images not to scale. Scale bars equal 10 cm.
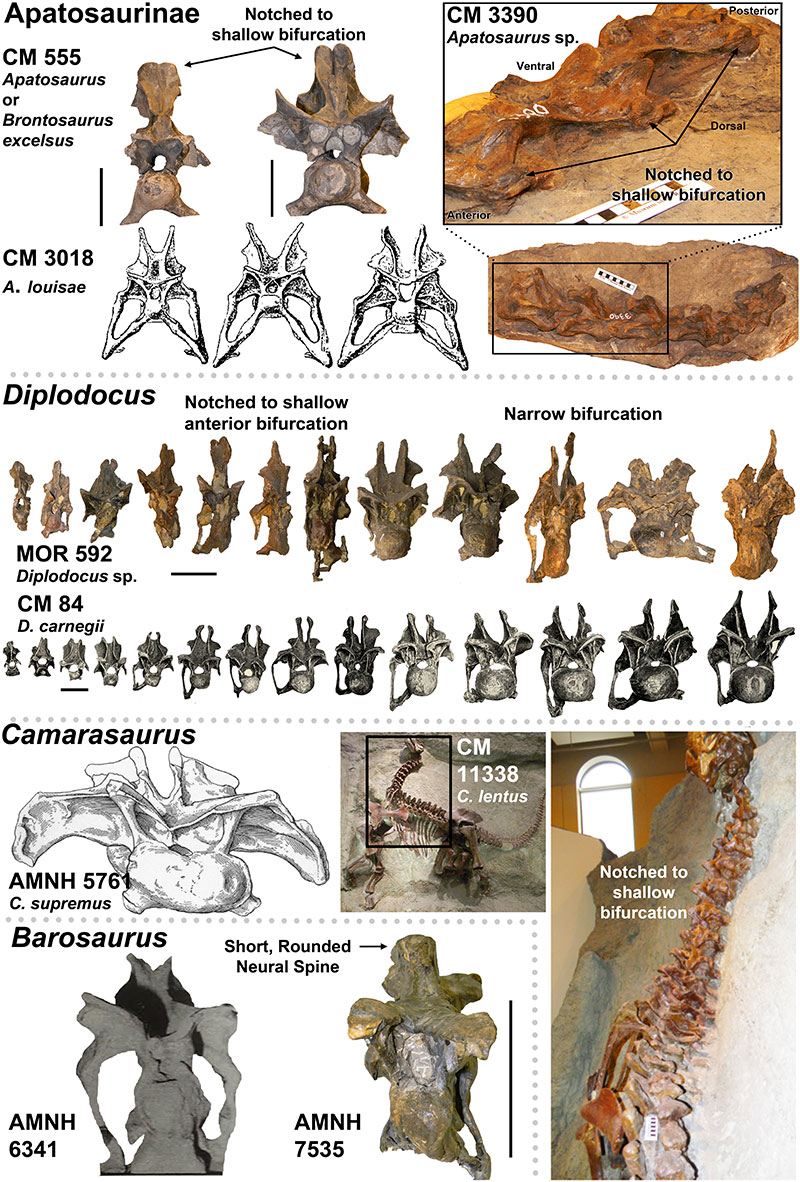
FIGURE 4. Presence of the Postparietal Aperture within Diplodocidea (indicated by red arrows). 1-4, Diplodocid specimens with the Postparietal Aperture: 1, Suuwassea (ANS 21122); 2, Galeamopus (SMA 0011); 3, Apatosaurus sp. (BYU 17096) (Balanoff, et al., 2010); 4, Kaatedocus (SMA 0004). Note 1 and 3 are dorsal views while 2 and 4 are posterior views. 5-6, Diplodocid skulls illustrating the presence of the Postparietal Aperture in immature individuals (5-image courtesy of the Science Museum of Minnesota), and its absence in mature individuals (6). For 5, from left to right: Kaatedocus (SMA 0004), Diplodocus sp. (SMM P. 84.15.3), Apatosaurus sp. (MOR 700), Galeamopus (SMA 0011; this skull is partially damaged, so the morphology of the foramen may be distorted), Suuwassea (ANS 21122), and Diplodocus sp. (MOR 592). For 6, note that the Diplodocus and Apatosaurus skulls are stylized renderings based on multiple specimens (from Whitlock [2011b]; Diplodocus sp. to scale of USNM 2672, and Apatosaurus louisae to CM 11162). Skulls in 5 and 6 to scale.
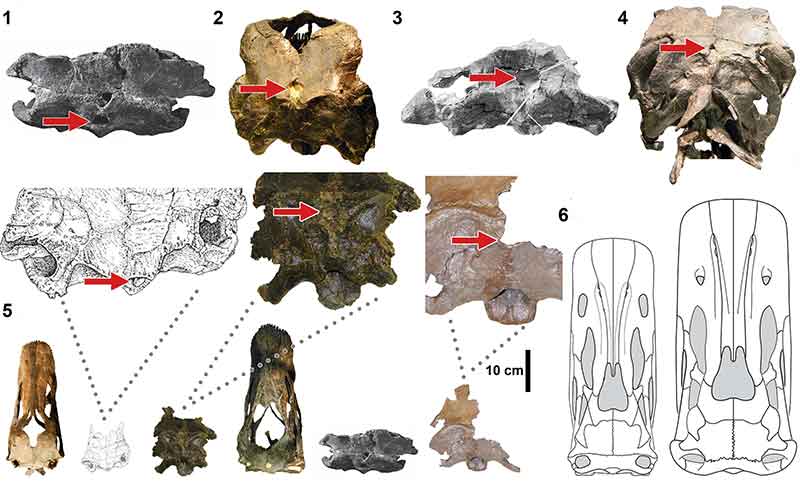
FIGURE 5. Macroscopic pneumatic architecture in diplodocid cervical vertebrae. 1, diplodocid indeterminate MOR 714 7-22-3-53; 2, Diplodocus sp. MOR 790 8-10-96-204; 3, D. carnegii CM 84 (from Hatcher, 1901). Increasing pneumatic complexity from 1 to 3. pfs = Pneumatic Fossa, acl = Accessory Lamina, pfm = Pneumatic Foramen (Wedel, 2003). Not to scale. Scale bar equals 10 cm.
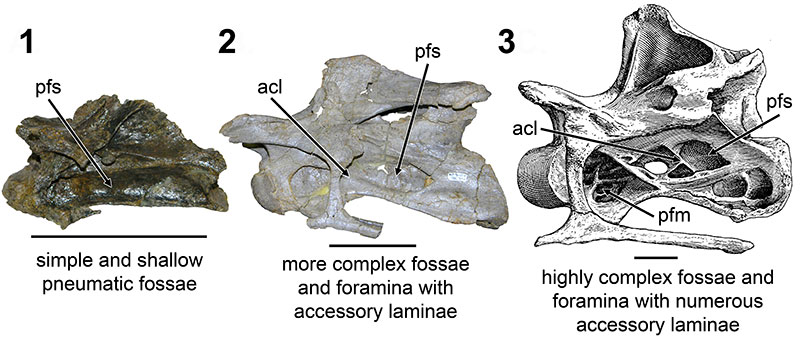
FIGURE 6. CT scans of diplodocid anterior cervical vertebrae. 1, diplodocid indeterminate MOR 714 7-22-3-53; 2, Diplodocus sp. MOR 790 8-10-96-204. Colored planes in 1 and 2 correspond to CT scan sections for each respective vertebrae. pfs = Pneumatic Fossa, cmr = Camera (Wedel, 2003). Red = frontal plane through the centrum, Blue = transverse plane through the anterior portion of the pfs, Green = transverse plane through the posterior portion of the pfs. Ant = anterior; Pos = posterior.
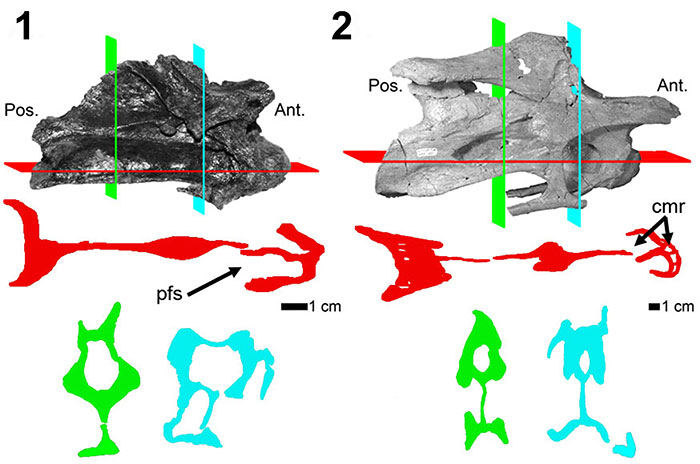
FIGURE 7. CT scans of diplodocid anterior dorsal vertebrae. 1, Diplodocus sp. MOR 790 8-21-95-238; 2, Diplodocus sp. MOR 592 8-22-90-15. Colored planes in 1 and 2 correspond to CT scan sections for each respective vertebrae. pfs = Pneumatic Fossa, cmr = Camera, cml = Camella (Wedel, 2003). Red = frontal plane through the centrum, Blue = transverse plane through the pfs.
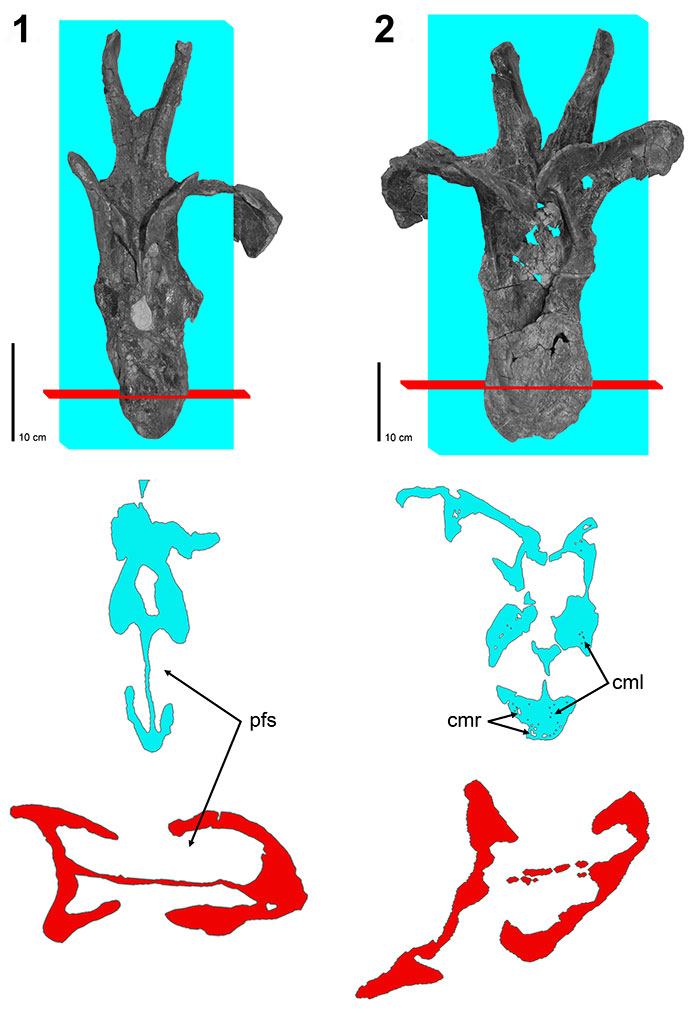
FIGURE 8. CT scans of diplodocid posterior dorsal vertebrae. 1, Diplodocus sp. MOR 790 7-8-95-17; 2, Diplodocus sp. MOR 592 8-22-90-77; 3, Apatosaurus sp. MOR 957 6-29-92#1. Colored planes in 1, 2, and 3 correspond to CT scan sections for each respective vertebrae. pfs = Pneumatic Fossa, cmr = Camera, cml = Camella (Wedel, 2003). Red = frontal plane through the centrum, Blue = transverse plane through the pfs.
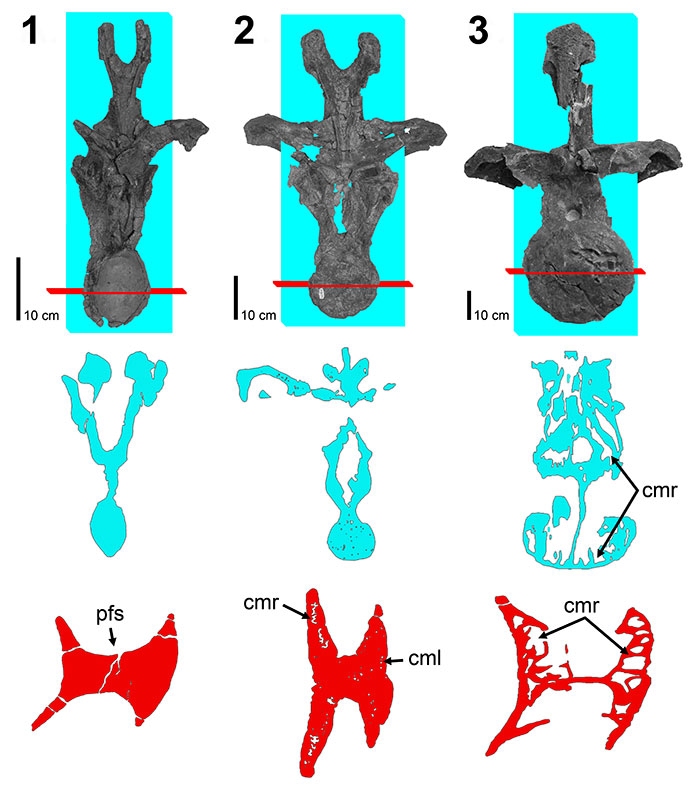
FIGURE 9. Coronal histologic sections of diplodocid posterior cervical and anterior dorsal vertebrae neural spines. 1, Posterior cervical Diplodocus sp. MOR 790 (un-numbered 1); 2, Anterior dorsal Diplodocus sp. MOR 790 8-21-95-238; 3, Posterior cervical Diplodocus sp. MOR 592 8-24-90-91; 4, Anterior dorsal Diplodocus sp. MOR 592 8-22-90-15. R.L. (right lateral), L.L (left lateral), M. (medial), L (lateral). Not to scale. Scale bar equals 1 cm.
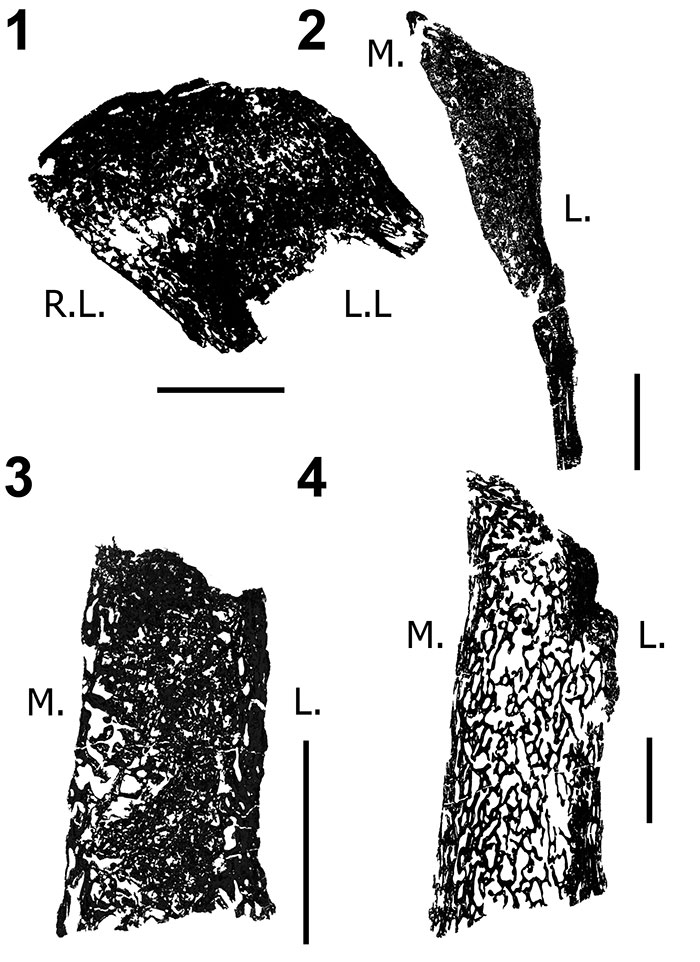
FIGURE 10. Transverse histologic sections of the H-MOS Stage 2 Diplodocus sp. 1, MOR 790 7-24-96-95 (10x) and 2, MOR 790 7-27-8-96 dorsal ribs (4x). 1, MOR 790 7-24-96-95 records a minimum of two annuli (blue lines). Red insert box highlights bone microstructure differences between the anterior intercostal ridge and lateral margins with longitudinal vascular canals. Green insert box highlights a sample of the segments that comprise one of the growth markers (an annulus, highlighted in blue). 2, MOR 790 7-27-8-96 records a minimum of six LAGs (blue lines; note some of these LAGs do extend past the demarcated blue lines). Red insert box highlights the microstructure and shows the vascularity patterns while the white arrows denote two of the LAGs present.
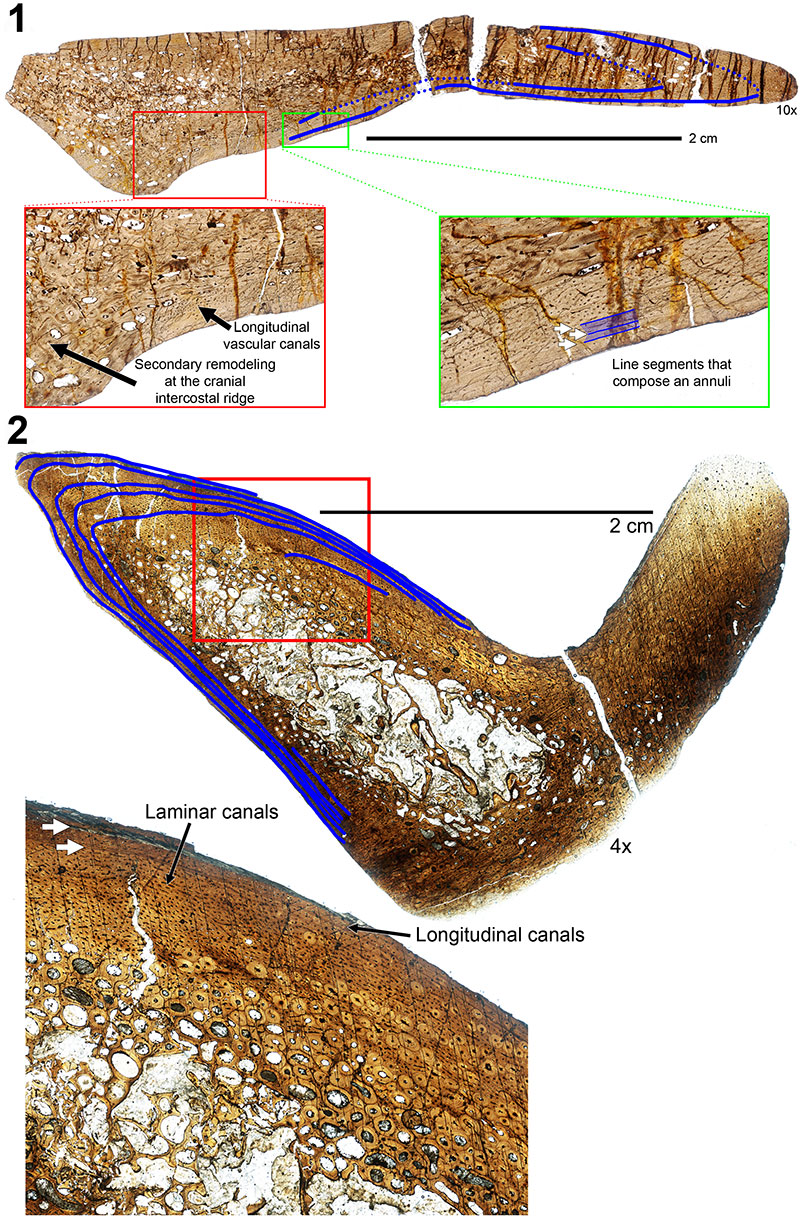
FIGURE 11. Transverse histologic section of the H-MOS Stage 3 Diplodocus sp. MOR 592 dorsal rib at 4x. MOR 592 records a minimum of eight LAGs (blue lines). Red insert box highlights the organization of longitudinal vascular canals in radial rows (alternating rows highlighted in blue). Green insert box highlights the vascularity patterns with white arrows denoting four of the LAGs present.
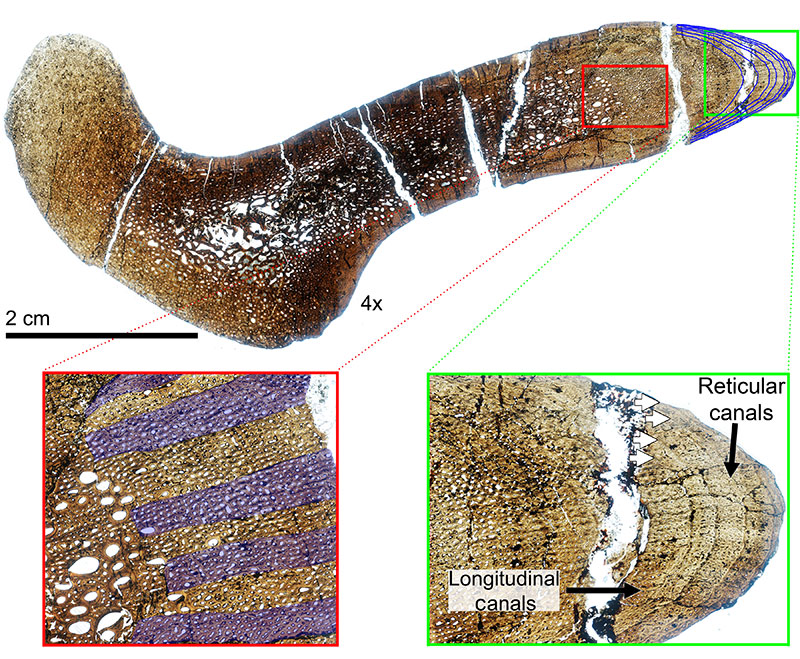
FIGURE 12. Transverse histologic section of the H-MOS Stage 4 Diplodocus CM 94 (Diplodocus carnegii paratype) dorsal rib at 4x. CM 94 records a minimum of 24 LAGs (blue lines). Red insert box highlights the episodic zones of large (LDC) and small (SDC) diameter longitudinal vascular canals with white arrows denoting some of the LAGs present. Green insert box highlights the outermost cortex which records the presence of an EFS. White arrows denote LAGS present, with the EFS bracketed.
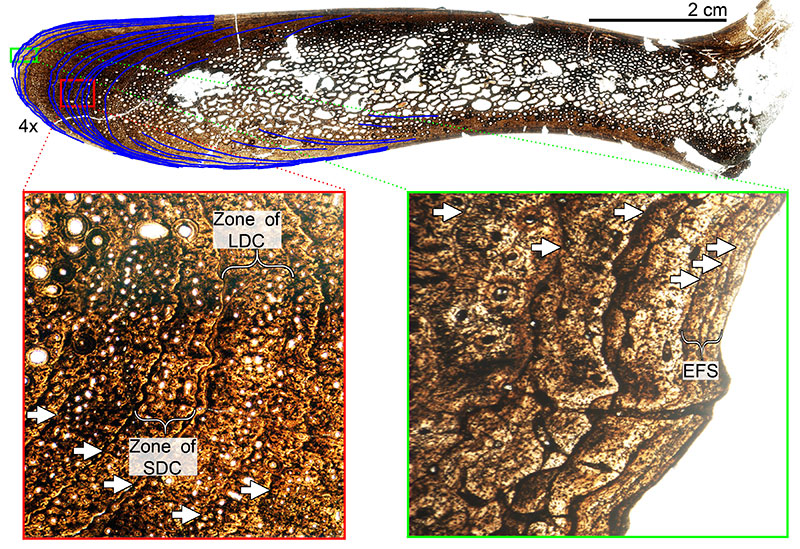
FIGURE 13. Transverse histologic section of the H-MOS Stage 2 Diplodocus sp. MOR 790 7-23-95-122 femur at 4x. MOR 790 7-23-95-122 is HOS 7 out of 13. No LAGs are present.

FIGURE 14. Transverse histologic section of the H-MOS Stage 3 Diplodocus sp. MOR 592-35 femur at 4x. White arrow marks one observable LAG. MOR 592 is HOS 9 out of 13.

FIGURE 15. Selection of the diplodocid femoral dataset used throughout this ontogenetic analysis. Top row diplodocines, from left to right: CM 33991 (Diplodocus longus), CM 21788 (D. longus), CM 30762 (D. longus), MOR 790 7-5-97-7 (Diplodocus sp.), MOR 790 7-23-95-122 (Diplodocus sp.), MOR 592-35 (Diplodocus sp.), CM 21752 (Diplodocus sp.), SMA 0013 (Diplodocus sp.), CM 84 (D. carnegii); Bottom row apatosaurines, from left to right: AMNH 613 (Apatosaurus sp.), OMNH 1279 (Apatosaurus sp.), AMNH 606 (Apatosaurus sp.), MWC 5439 (Apatosaurus sp.), CM 21784 (A. louisae), CM 33997 (A. louisae), MOR 700 7-24-91-31 (Apatosaurus sp.), AMNH 353 (Apatosaurus sp.), SMA 0014 (Apatosaurus sp.), CM 85 (Apatosaurus sp.), MOR 857 7-16-92-30 (Apatosaurus sp.), MWC “Moffit Co. Apato.” (Apatosaurus sp.). Scale bar equals 1 m. Note the colored line on CM 84 and MWC “Moffit Co. Apato.” illustrate how femoral positions and trends were examined. Colored lines to the far right of each row indicate the general allometric changes of each femur scaled to the same length (Black line = femoral length). Diplodocus row: Yellow lines = MOR 790 7-5-97-7, Blue lines = MOR 592-35, Red lines = CM 84. Apatosaurus row: Green lines = AMNH 613, Yellow lines = CM 33997, Blue lines = MOR 700 7-24-91-31, Red lines = MWC “Moffit Co. Apato.”
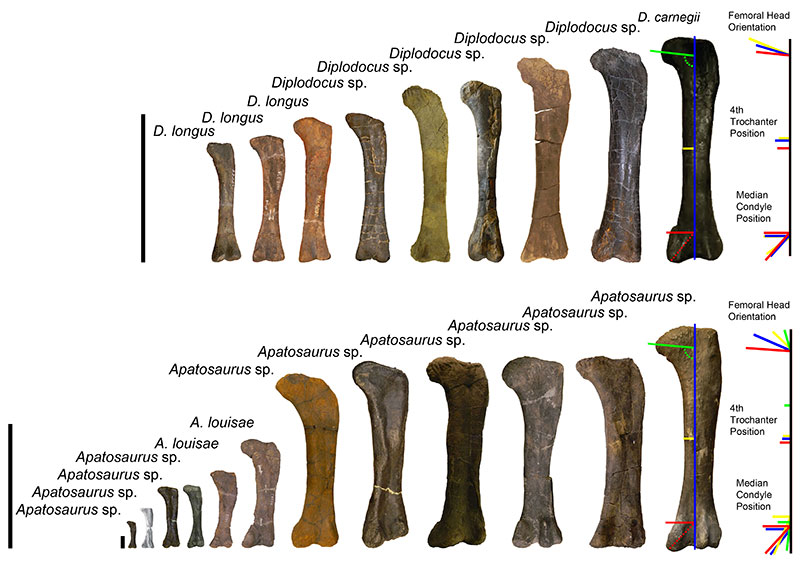
FIGURE 16. Calculated body masses for the Morrison diplodocids Apatosaurus (1) and Diplodocus (2) using the formula of Mazzetta et al. (2004) (log Body Mass = 2.955 x log Femur Circumference − 4.166); 3, Dorsal rib LAG count vs. femur circumference for Diplodocus; 4, Femur length vs. femur circumference for Diplodocus; 5, Dorsal rib LAG count vs. body mass for Diplodocus with posterior cervical vertebrae marking each size range to illustrate the correlation between mass and spine morphology. Data for all diplodocid specimens can be found in Appendix 3.
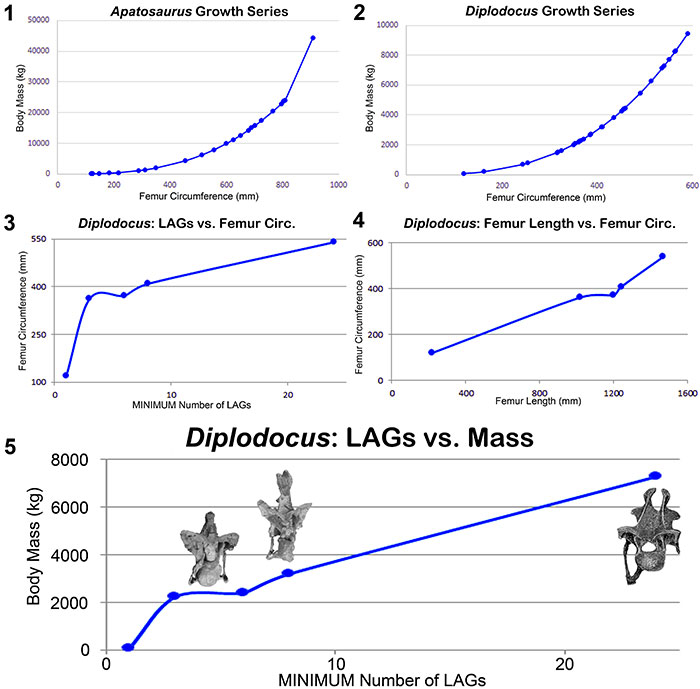
FIGURE 17. Histologic and morphologic commonalities of Diplodocus sp. (MOR 592) and Suuwassea emilieae (ANS 21122) which demonstrate immature maturational states for both animals (H-MOS Stage 3). Histologic section of Suuwassea tibia from Hedrick et al. (2014). The Suuwassea tibia histologic section is from Acta Palaeontologica Polonica, articles are distributed under the terms of the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. For licence details please see http://creativecommons.org/licenses/by/4.0/.
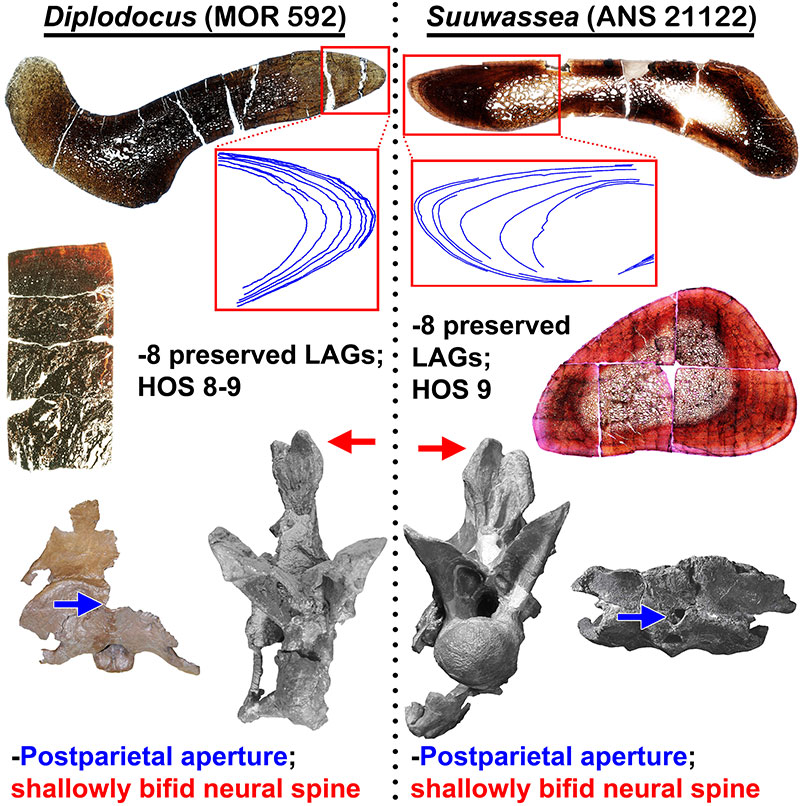
FIGURE 18. The degrees and variation of neurocentral fusion in diplodocid cervical vertebrae indicate that fusion is not uniformly indicative of maturity. 1, The posteriorly unfused H-MOS Stage 2 Diplodocus sp. MOR 790 8-10-96-204; 2, The completely fused H-MOS Stage 1 diplodocid indeterminate MOR 714 7-22-3-53; 3, The completely unfused H-MOS Stage 3 Apatosaurus excelsus (or Brontosaurus excelsus) CM 555; 4, The anteriorly unfused H-MOS Stage 3 Diplodocus sp. MOR 592. Red inset boxes highlight the visible sutures (in blue). Not to scale. Scale bar equals 10 cm.
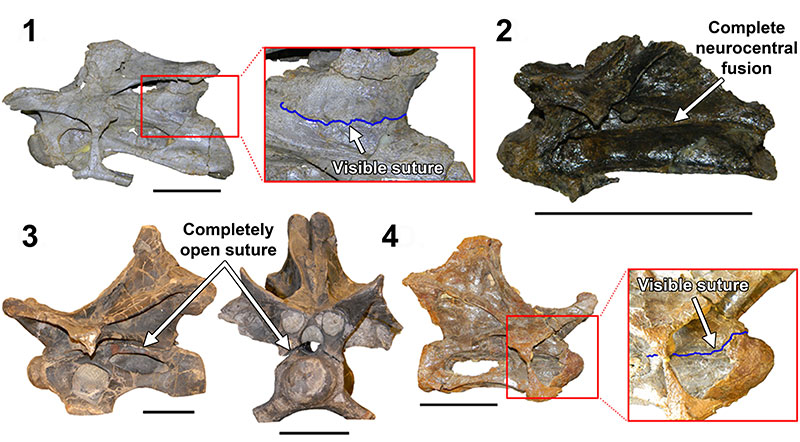
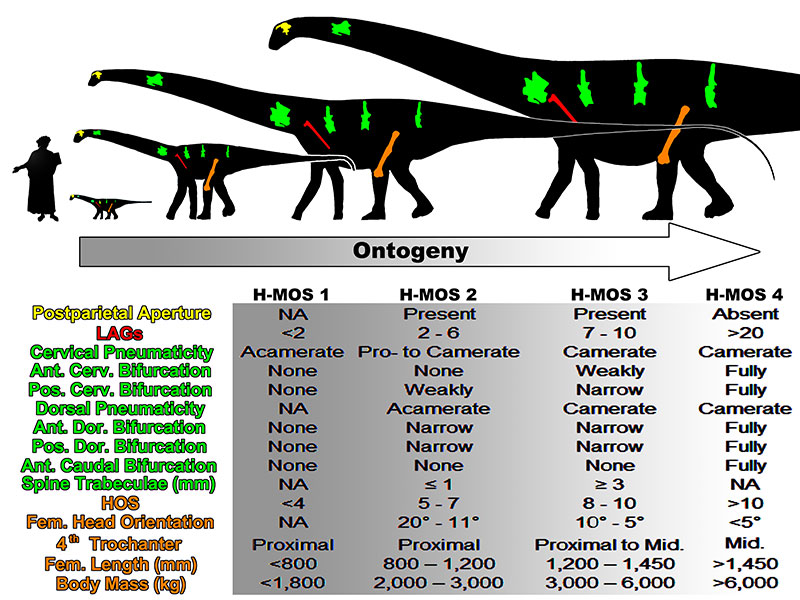
FIGURE 19. An ontogenetic trajectory in consideration of morphologic and histologic attributes for the Morrison diplodocid Diplodocus. Note diplodocids, and possibly all other sauropods, did not skeletally develop along an isometric trajectory. Human scale bar is Dante Alighieri from Domenico di Michelino’s “La commedia illumina Firenze”, depicting Dante as 1.63 m tall. Modified silhouette of Diplodocus carnegii CM 84 by S. Hartman and available via PhyloPic under the Creative Commons Attribution-ShareAlike 3.0 Unported License.
A new multi-faceted framework for deciphering diplodocid ontogeny
Plain Language Abstract
Studying the growth of sauropod dinosaurs has historically been problematic because 1) these dinosaurs lack crests, horns, and fills – features commonly examined in growth studies, and 2) they grew so fast that traditional methodologies could only determine relative maturity (i.e., a specimen is either more or less mature than another). However, several recent studies have shown that sauropod growth can in fact be more accurately tracked. Numerous skeletal features have been shown to change through growth, and new histologic methodologies are allowing us to calculate individual life ages. All of these new analyses are revealing that sauropods underwent radical changes through their growth and development. Yet in spite of these new findings, we still do not have an in-depth or overall picture of these collective allometric changes within sauropods. Within the discipline of paleontology, morphology and histology have independently been used to assess growth, and in many cases the argument has been made that one methodology is better suited than the other. For this study, we examined numerous histologic and morphologic characters within diplodocid sauropods (sauropods such as Apatosaurus and Diplodocus). By examining a broad range of histologic and morphologic features within an ontogenetic series, we recognized that suites of corroborating characters are found throughout diplodocid growth (here called the Histo-Morph Ontogeny Scale). Opposed to previous findings that could say how an individual feature changed through growth, we can now track how the animal itself changed through growth. This study is preliminary, and far from complete, but such combined and inclusive methodologies will allow us better resolution of these complex and amazing dinosaurs.
Resumen en Español
Un nuevo marco multifacético para descifrar la ontogenia de los diplodócidos
La determinación histológica de la madurez de los dinosaurios saurópodos es problemática ya que el rápido crecimiento conduce a la remodelación de las Líneas de Detención del Crecimiento (LDC; Lines of Arrested Growth o LAGs). Aunque se ha ideado un sistema complementario que utiliza varios factores, incluyendo cantidades relativas de remodelación (Histologic Ontogenetic Stage [HOS]), la mayoría de las evaluaciones de la madurez de los saurópodos se basa en indicadores morfológicos. Para evaluar mejor la madurez esquelética y el cambio morfológico a través de la ontogenia, se examinó el material craneal y postcraneal de más de 20 individuos de diplodócidos (Apatosaurus y Diplodocus) de la Formación Morrison, Jurásico Superior. Aquí se describen combinaciones coherentes de características morfológicas e histológicas que se pueden utilizar para determinar la madurez. Los diplodócidos pequeños (longitudes femorales ≤ 120 cm) muestran espinas neurales cervicales y dorsales débilmente bifurcadas o no bifurcadas, centra con cámaras o sin ellas, dos a seis LCDs conservadas en las costillas dorsales, y una designación femoral máxima de HOS 7. Los individuos más grandes (longitud femoral ~125 cm) tienen estructuras neumáticas internas más desarrolladas, mayor bifurcación de la columna neural, preservan hasta ocho LCDs y una designación femoral de HOS 9. En contraste, los saurópodos esqueléticamente maduros (longitudes femorales >150 cm) tienen estructuras neumáticas complejas, bifurcación de la columna neural extendida (también dentro de las caudales anteriores), y un HOS femoral entre 11-13. Además, todos los pequeños cráneos diplodócidos conservados exhiben un agujero postparietal (previamente se ha sugerido que corresponde con una apomorfía de Dicraeosauridae) que está ausente en grandes cráneos (cuando se conserva), lo que sugiere que es un carácter ontogenético. Estos hallazgos apoyan la hipótesis de un cambio morfológico ontogenético significativo en saurópodos diplodócidos y sugieren que hay que ser precavidos cuando se describen nuevos taxones basados en holotipos de individuos de cuerpo pequeño.
Palabras clave: diplodócido; ontogenia; morfología; histología
Traducción: Enrique Peñalver (Sociedad Española de Paleontología)
Résumé en Français
Un nouveau cadre à multiples facettes pour déchiffrer l'ontogénie des diplodocidés
Déterminer la maturité chez les dinosaures sauropodes en utilisant l'histologie est problématique car une croissance rapide entraine un remodelage des Lignes d'Arrêt de Croissance (LAC). Bien qu'un système complémentaire ait été conçu en se basant sur plusieurs facteurs, notamment le degré relatif de remodelage (Stade Ontogénétique Histologique [SOH]), la plupart des estimations de maturité chez les sauropodes sont basées sur des indicateurs morphologiques. Afin de mieux estimer la maturité squelettique et les changements morphologiques au cours de l'ontogénie, nous avons étudié du matériel crânien et postcrânien de plus de 20 individus de diplodocidés (Apatosaurus et Diplodocus) provenant du Jurassique supérieur de la formation de Morrison. Nous décrivons dans cet article des combinaisons cohérentes de caractères morphologiques et histologiques qui peuvent être utilisées pour vérifier la maturité. Les petits diplodocidés (longueur fémorale ≤ 120 cm) présentent des épines neurales cervicales et dorsales qui sont peu ou pas bifurquées, des centres avec ou sans cavités, deux à six LAC préservées dans les côtes dorsales, et une estimation maximale du SOH du fémur de 7. Les individus de plus grande taille (longueur fémorale ~ 125 cm) présentent des structures pneumatiques internes plus développées, une bifurcation des épines neurales plus importante, jusqu'à huit LAC préservées, et une estimation du SOH du fémur de 9. Au contraire, les sauropodes matures au niveau de leur squelette (longueur fémorale > 150 cm) montrent des structures pneumatiques complexes, une bifurcation des épines neurales plus importante (également dans les vertèbres caudales antérieures), et un SOH fémoral compris entre 11 et 13. De plus, tous les crânes préservés de petits diplodocidés présentent un foramen postpariétal (suggéré précédemment comme étant une synapomorphie des Dicraeosauridae). Ce foramen est absent sur les crânes de grande taille (quand la région en question est préservée), ce qui suggère que c'est un caractère ontogénétique. Ces découvertes soutiennent l'hypothèse de changements morphologiques ontogénétiques importants chez les sauropodes diplodocidés et appellent à la prudence lors de la description de nouveaux taxons basés sur des holotypes de petite taille.
Mots-clés : diplodocidé ; ontogénie ; morphologie ; histologie
Translator: Antoine Souron
Deutsche Zusammenfassung
Ein neuer vielseitiger Rahmen zur Entschlüsselung der Ontogenie der Diplodocidae
Es ist problematisch das Erwachsenenalter von sauropoden Dinosauriern histologisch zu bestimmen, da schnelles Wachstum die Harris-Linien verformt. Obwohl ein freies Verfahren entwickelt wurde, das einige Faktoren wie relative Mengen von Remodelierung (Histologic Ontogenetic Stage [HOS]) nutzt, beziehen sich die meisten Abschätzungen zum Erwachsenenalter von Sauropoden auf morphologische Indikatoren. Um ein voll ausgewachsenes Skelett und morphologische Änderungen während der Ontogenie beurteilen zu können, untersuchten wir craniales und postcraniales Material von über 20 diplodociden Individuen (Apatosaurus und Diplodocus) aus der oberjurassischen Morrison Formation. Hier beschreiben wir die konsistente Kombination morphologischer und histologischer Merkmale, die zur Bestimmung des Erwachsenenalters herangezogen werden können. Bei kleinen Diplodociden (Femurlänge ≤120 cm) sind die Dornfortsätze der Hals-und Dorsalwirbel nicht oder nur schwach gegabelt, die Wirbelzentren sind acamerat bis camerat, die Dorsalrippen haben zwei bis sechs erhaltene Harris-Linien und eine maximale Oberschenkel-Kennzeichnung von HOS 7. Bei größeren Individuen (Femurlänge ~125 cm) sind die internen pneumatischen Strukturen starker entwickelt, die Dornfortsätze sind stärker gegabelt, sie haben bis zu acht LAGs und eine Oberschenkel-Kennzeichnung von HOS 9. Degegen weisen ausgewachsene Sauropoden (Femurlänge >150 cm) komplexe pneumatische Strukturen auf, ausgeprägt gegabelte Dornfortsätze (ebenso an den anterioren Kaudalwirbeln) und eine Oberschenkel-Kennzeichnung zwischen 11 bis 13. Zusätzlich zeigen alle der erhaltenen kleinen Diplodociden-Schädel ein postparietales Foramen (was bislang für eine Apomorphie der Dicraeosauridae gehalten wurde), das (wenn erhalten) bei größeren Schädeln nicht vorkommt und somit ein ontogenetisches Merkmal nahelegt. Diese Funde unterstützen die Hypothese von signifikanten ontogenetisch-morphologischen Veränderungen bei diplodociden Sauropoden und mahnen zur Vorsicht wenn neue Taxa auf der Basis von kleinen Holotypen beschrieben werden.
Keywords: Diplodocidae; Ontogenie; Morphologie; Histologie
Translator: Eva Gebauer
Arabic

Translator: Ashraf M.T. Elewa
-
-
PE: An influential journal
 Palaeontologia Electronica among the most influential palaeontological journals
Palaeontologia Electronica among the most influential palaeontological journalsArticle number: 27.2.2E
July 2024



 A Review of Handbook of Paleoichthyology Volume 8a: Actinopterygii I, Palaeoniscimorpha, Stem Neopterygii, Chondrostei
A Review of Handbook of Paleoichthyology Volume 8a: Actinopterygii I, Palaeoniscimorpha, Stem Neopterygii, Chondrostei