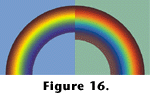OTHER DETERMINANTS OF VISION
Striking Oil in the Retina
 Color vision in animals is sculpted by filters that modify the spectrum of light before it reaches the photoreceptor outer segments. In humans
(Goldsmith 1990) and some fish (Thorpe et al. 1993) the lens and other structures absorb a fair amount of light, particularly at short wavelengths. This absorption makes us insensitive to UV light
(Stark and Tan 1982). Of greater potential interest are pigments contained in oil droplets within individual photoreceptors.
Color vision in animals is sculpted by filters that modify the spectrum of light before it reaches the photoreceptor outer segments. In humans
(Goldsmith 1990) and some fish (Thorpe et al. 1993) the lens and other structures absorb a fair amount of light, particularly at short wavelengths. This absorption makes us insensitive to UV light
(Stark and Tan 1982). Of greater potential interest are pigments contained in oil droplets within individual photoreceptors.
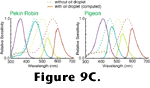
 Because different photoreceptors may have different oil droplets, these droplets provide another mechanism by which animals could have modified the absorption spectra of photoreceptors to extract spectral information from the light striking their eyes (see
Figure 9A-Figure 9B). However, animal evolution appears not to have taken this pathway in the development of color vision. Instead, oil droplets sharpen the tuning of photoreceptors reducing the window of the receptor's spectral responses
(Goldsmith 1990,
Bowmaker et al. 1997,
Vorobyev et al. 1998). This sharpening is graphically depicted in
Figure 9C.
Because different photoreceptors may have different oil droplets, these droplets provide another mechanism by which animals could have modified the absorption spectra of photoreceptors to extract spectral information from the light striking their eyes (see
Figure 9A-Figure 9B). However, animal evolution appears not to have taken this pathway in the development of color vision. Instead, oil droplets sharpen the tuning of photoreceptors reducing the window of the receptor's spectral responses
(Goldsmith 1990,
Bowmaker et al. 1997,
Vorobyev et al. 1998). This sharpening is graphically depicted in
Figure 9C.
 Oil droplets are known to exist in the retinas of a wide variety of animals, primarily terrestrial tetrapods
(Robinson 1994).
Walls (1942) and later
Robinson (1994) suggested that the presence of pigmented oil droplets in distantly-related groups of animals implies that they first appeared in basal sarcopterygians, approximately 400 million years ago. The patchy distribution of oil droplets and, more particularly, pigmented oil droplets was explained by Walls as an indication that when animals with oil droplets evolve nocturnal lifestyles they lose their oil droplets (or at least their droplets' pigmentation). Once lost the oil droplets (or their sequestered pigments) cannot be reacquired.
Oil droplets are known to exist in the retinas of a wide variety of animals, primarily terrestrial tetrapods
(Robinson 1994).
Walls (1942) and later
Robinson (1994) suggested that the presence of pigmented oil droplets in distantly-related groups of animals implies that they first appeared in basal sarcopterygians, approximately 400 million years ago. The patchy distribution of oil droplets and, more particularly, pigmented oil droplets was explained by Walls as an indication that when animals with oil droplets evolve nocturnal lifestyles they lose their oil droplets (or at least their droplets' pigmentation). Once lost the oil droplets (or their sequestered pigments) cannot be reacquired.
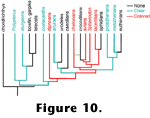 The homology of oil droplets among various clades cannot currently be supported with any firm data, though
Figure 10 depicts reasonable hypothetical relationships. Unlike that of the opsins, the molecular biology underlying the production of oil droplets is unknown, so we cannot evaluate Walls' suggestion that lost oil droplets are difficult to recover. If Walls was correct, though, his supposition suggests many interesting paleobiological conclusions. For instance, the MRCA of all tetrapods would have to have been diurnal, and metatherian and prototherian mammals must have been more active under the Mesozoic sunlight than their eutherian cousins who were apparently forced to spend their days sequestered in shaded hideouts. Similarly - particularly if the recent claim of a close relationship between chelonians and crocodilians
(Hedges and Poling, 1999) is correct - we could safely infer that most if not all dinosaurian lineages were well-endowed with brightly colored oil droplets in their cones.
The homology of oil droplets among various clades cannot currently be supported with any firm data, though
Figure 10 depicts reasonable hypothetical relationships. Unlike that of the opsins, the molecular biology underlying the production of oil droplets is unknown, so we cannot evaluate Walls' suggestion that lost oil droplets are difficult to recover. If Walls was correct, though, his supposition suggests many interesting paleobiological conclusions. For instance, the MRCA of all tetrapods would have to have been diurnal, and metatherian and prototherian mammals must have been more active under the Mesozoic sunlight than their eutherian cousins who were apparently forced to spend their days sequestered in shaded hideouts. Similarly - particularly if the recent claim of a close relationship between chelonians and crocodilians
(Hedges and Poling, 1999) is correct - we could safely infer that most if not all dinosaurian lineages were well-endowed with brightly colored oil droplets in their cones.
Double Vision
In
1942, Walls wrote (p. 103):
In truth, the working out of the photochemical system of the cone may long continue to seem the most difficult branch of the physiology of the eye. [...] with the most careful methods, we can succeed in seeing living cones only as completely colorless structures, whose bland innocence conceals invisible traces of three important somethings - to our utter exasperation.
On this topic, much has changed in the intervening years. As discussed above, we now have a fairly-detailed understanding of the photochemistry of retinal cells and its relation to color vision. We also know that despite his comparative genius, Walls was too parochial in presuming that other animals would, like us, be restricted to having only three visual pigments. In the hopes of stimulating further research in a particular direction, I would like to contrast what we've learned since Walls wrote the above quote to what we've learned since he wrote the following (p. 58-59):
...the puzzle [double cones] represent is particularly irritating to the curious investigator because they are so very widespread among vertebrates. If they occurred in only one or two animals, we might dismiss them as a curiosity. Perhaps if they occurred in the human retina we would before now have gained some clue to their role in visual processes; but their functional significance, their exact mode of formation in the developing retina, and the probable time and manner of their evolutionary origin have yet to be determined. Next to amacrine cells, the double cones are physiologically the most obscure elements in any and all retinae They have unfortunately not greatly interested visual physiologists, since the latter have their attention focused upon the human retina, in which double cones are lacking.
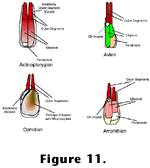 Little to no editing would be required to make these statements an accurate reflection of our current state of knowledge about "double cones". In addition to those of
Figure 9A,
Figure 11 contains schematic drawings of these structures in a variety of vertebrates to show some of their diversity as well as their common features. Whether or not all double cones - or more generally, paired photoreceptors - are homologous, and whether or not they serve similar functions are open
questions.
Little to no editing would be required to make these statements an accurate reflection of our current state of knowledge about "double cones". In addition to those of
Figure 9A,
Figure 11 contains schematic drawings of these structures in a variety of vertebrates to show some of their diversity as well as their common features. Whether or not all double cones - or more generally, paired photoreceptors - are homologous, and whether or not they serve similar functions are open
questions.
One potential clue about the function of paired photoreceptors is that they frequently inhabit retinas in the form of regular mosaics.
Figure 12 is a photomicrograph of the retina from a sunfish (Lepomis cyanellus). Clicking on the image highlights the orderliness of the orientations of the double cones. Such mosaics are common among fish
(Ali and Anctil 1976), and qualitatively similar patterns have been demonstrated in one bird
(Engström 1958) and one lizard (Dunn 1966). Most tetrapods that have double cones appear to have irregular mosaics (i.e., no pattern), but close inspection of the chicken retina indicates that its double cones are not randomly oriented
(Morris 1970). It is possible that some semblance of order will be found upon close examination in all retinas containing double cones.
 A few hypotheses have been put forward to explain the functional significance of paired photoreceptors and their mosaics. Many explanations revolve around electrical connections between the two constituent photoreceptors of each double cone (e.g.,
Richter and Simon 1974). Such explanations are not compelling given that electrical coupling between photoreceptors can be accomplished with a much smaller areal contact via gap junctions near the synapses of the cells. Many nonpaired photoreceptors are electrically coupled via such junctions, and at least sometimes double cones do not have any demonstrable electrical connections between their constituent photoreceptors
(Mariani 1986). Others have argued that double cone mosaics somehow aid the detection of movement (Lyall 1957,
Wagner 1972,
Wagner 1978). Acceptance of this explanation suffers from the fact that the two halves of double cones appear to be optically coupled in such a way that absorption in one of a double cone's outer segments may have been due to light which first passed through either of the double cone's constituent inner segments (M.P. Rowe et al.
1994). As such, double cones appear poorly suited for motion detection; they throw away spatial information which would be useful for that task.
Van der Meer (1992) argued that the noncircular cross-sections of double cones allow receptors to be optimally packed while preserving the extracellular space that is necessary for their optical functioning. However, as indicated above, there appears to be no optical isolation between the two halves of a double cone. Furthermore, since many animals retain double cones, but do not have mosaics as regular as those shown in
Figure 12 (i.e., the double cones are not optimally packed), van der Meer's argument is at best only partial.
Cameron and Pugh (1991) argued that double cone mosaics are responsible for analysis of the linear polarization state of light. Unfortunately, the behavioral data supporting this conclusion in sunfish could not be replicated, and further experiments strongly indicated that these animals cannot be trained to discriminate
light on the basis of its polarization state even though the same fish in the same apparatus can be trained to make color discriminations (Pugh, personal commun., 1997). Any explanation of the functional significance of double cones should take into consideration the optical coupling of their inner segments since their ultrastructure suggests such coupling might be the primary reason for their existence. At present, no such explanation has withstood critical testing.
A few hypotheses have been put forward to explain the functional significance of paired photoreceptors and their mosaics. Many explanations revolve around electrical connections between the two constituent photoreceptors of each double cone (e.g.,
Richter and Simon 1974). Such explanations are not compelling given that electrical coupling between photoreceptors can be accomplished with a much smaller areal contact via gap junctions near the synapses of the cells. Many nonpaired photoreceptors are electrically coupled via such junctions, and at least sometimes double cones do not have any demonstrable electrical connections between their constituent photoreceptors
(Mariani 1986). Others have argued that double cone mosaics somehow aid the detection of movement (Lyall 1957,
Wagner 1972,
Wagner 1978). Acceptance of this explanation suffers from the fact that the two halves of double cones appear to be optically coupled in such a way that absorption in one of a double cone's outer segments may have been due to light which first passed through either of the double cone's constituent inner segments (M.P. Rowe et al.
1994). As such, double cones appear poorly suited for motion detection; they throw away spatial information which would be useful for that task.
Van der Meer (1992) argued that the noncircular cross-sections of double cones allow receptors to be optimally packed while preserving the extracellular space that is necessary for their optical functioning. However, as indicated above, there appears to be no optical isolation between the two halves of a double cone. Furthermore, since many animals retain double cones, but do not have mosaics as regular as those shown in
Figure 12 (i.e., the double cones are not optimally packed), van der Meer's argument is at best only partial.
Cameron and Pugh (1991) argued that double cone mosaics are responsible for analysis of the linear polarization state of light. Unfortunately, the behavioral data supporting this conclusion in sunfish could not be replicated, and further experiments strongly indicated that these animals cannot be trained to discriminate
light on the basis of its polarization state even though the same fish in the same apparatus can be trained to make color discriminations (Pugh, personal commun., 1997). Any explanation of the functional significance of double cones should take into consideration the optical coupling of their inner segments since their ultrastructure suggests such coupling might be the primary reason for their existence. At present, no such explanation has withstood critical testing.
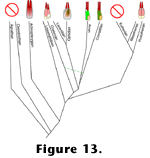 Irrespective of the function of double cones, their presence and potential homology in actinopterygians, metatherians, prototherians, lissamphibians, and reptiles (including birds) suggest that their first appearance - like that of the four reptilian photopigments - preceded the divergence of actinopterygians and sarcopterygians. Eutherian mammals apparently lost their ancestral double cones just as they lost two of their ancestral visual pigments. Although there is a strong possibility that these losses were functionally linked, they most probably were not identical events since one of the pigments eutherians retained is likely orthologous to the long wave sensitive pigment generally found in the double cones of birds
(Bowmaker et al.
1997). A diagram outlining some of the double cone types found in extant vertebrates is overlain on their potential phylogeny in
Figure 13. Assuming that double cones arose only once in vertebrate evolution, eutherians are unique in having ultimately discarded them. Thus, whatever advantages double cones might confer it is reasonable to conclude that they conferred them upon most extinct vertebrates as well.
Irrespective of the function of double cones, their presence and potential homology in actinopterygians, metatherians, prototherians, lissamphibians, and reptiles (including birds) suggest that their first appearance - like that of the four reptilian photopigments - preceded the divergence of actinopterygians and sarcopterygians. Eutherian mammals apparently lost their ancestral double cones just as they lost two of their ancestral visual pigments. Although there is a strong possibility that these losses were functionally linked, they most probably were not identical events since one of the pigments eutherians retained is likely orthologous to the long wave sensitive pigment generally found in the double cones of birds
(Bowmaker et al.
1997). A diagram outlining some of the double cone types found in extant vertebrates is overlain on their potential phylogeny in
Figure 13. Assuming that double cones arose only once in vertebrate evolution, eutherians are unique in having ultimately discarded them. Thus, whatever advantages double cones might confer it is reasonable to conclude that they conferred them upon most extinct vertebrates as well.
To Peer Inside a Dinosaur's Eyes
 In outlining the EPB method,
Witmer (1995) discussed osteological correlates as tests of deductions about soft tissue anatomy in extinct organisms. A potential osteological correlate exists for the retinal features here under discussion for terrestrial vertebrates. Scleral ossicles - rings of bony plates inside the sclera - are found in the eyes of most extant reptiles.
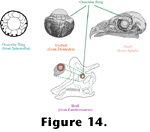
Figure 14 depicts the arrangement of these bony plates as found in the eye of a tuatara, and their positioning within the eyeball of an albatross and the skulls of an eagle and a Lambeosaurus.
Figure 15 documents their presence in individuals from several other extinct reptilian groups. Walls thought the ossicles' primarily function was to reinforce the indentation at the interface between the cornea and sclera (see the albatross eye in the middle of
Figure 14). Were it not for these ossicles, this corneal scleral sulcus would balloon outward as a consequence of the intraocular pressure, particularly when the pressure rises during accommodation.
In outlining the EPB method,
Witmer (1995) discussed osteological correlates as tests of deductions about soft tissue anatomy in extinct organisms. A potential osteological correlate exists for the retinal features here under discussion for terrestrial vertebrates. Scleral ossicles - rings of bony plates inside the sclera - are found in the eyes of most extant reptiles.
Figure 14 depicts the arrangement of these bony plates as found in the eye of a tuatara, and their positioning within the eyeball of an albatross and the skulls of an eagle and a Lambeosaurus.
Figure 15 documents their presence in individuals from several other extinct reptilian groups. Walls thought the ossicles' primarily function was to reinforce the indentation at the interface between the cornea and sclera (see the albatross eye in the middle of
Figure 14). Were it not for these ossicles, this corneal scleral sulcus would balloon outward as a consequence of the intraocular pressure, particularly when the pressure rises during accommodation.
 Furthermore, at least in birds, the ossicles allow the animal to adjust the shape of its cornea in order to modify its focussing power
(Walls 1942). Walls hypothesized that the animals that secondarily lost the sclerotic rings in their eyes did so because in their acquired nocturnal or fossorial lifestyles they no longer required the ability to accurately focus light upon their retinas - animals that live in low-light environments generally have low visual acuity; in the hypothetical design of eyes, there is a tradeoff between acuity and sensitivity. If an animal's vision is constantly blurry due to features that heighten sensitivity, fine adjustments in focus will not provide the enhancement necessary to make them adaptively favorable. Snakes no longer have scleral ossicles, and neither do crocodiles (except as a cartilaginous ring). In owls the ossicles are reduced.
Furthermore, at least in birds, the ossicles allow the animal to adjust the shape of its cornea in order to modify its focussing power
(Walls 1942). Walls hypothesized that the animals that secondarily lost the sclerotic rings in their eyes did so because in their acquired nocturnal or fossorial lifestyles they no longer required the ability to accurately focus light upon their retinas - animals that live in low-light environments generally have low visual acuity; in the hypothetical design of eyes, there is a tradeoff between acuity and sensitivity. If an animal's vision is constantly blurry due to features that heighten sensitivity, fine adjustments in focus will not provide the enhancement necessary to make them adaptively favorable. Snakes no longer have scleral ossicles, and neither do crocodiles (except as a cartilaginous ring). In owls the ossicles are reduced.
If Walls' hypothesis is correct, then we can presume that any animal that retained scleral ossicles was diurnal. Logically we would also conclude that an animal that had secondarily lost these ossicles
was either nocturnal or (like us) the descendent of a lineage that had gone through a nocturnal phase.
 We can thus draw a line from the first terrestrial tetrapod to almost any extant bird, turtle or lizard and see an unbroken chain of animals that retained all of the features associated with the retinas of such animals today - four or more classes of cones (note this argument is independent of the argument presented above based on gene sequences), colored oil droplets, and probably double cones. Similarly we can conclude that many extinct side branches (e.g., ichthyosaurs, pterosaurs and most extinct dinosaurs) were also so endowed.
Figure 16 depicts some of the perceptual consequences we might predict from this endowment. In
Witmer's (1995) parlance, it is a Level I inference to say that, for example, Deinonychus had four cone classes, oil droplets and double cones in its retina. That is, we are justified in making a decisive and positive assertion that Deinonychus had these soft tissue features because they are present in birds and turtles, and they are associated with scleral ossicles that Deinonychus also retained (see:
the Tree of Life,
Chordata
section). Dinosaurs are generally restored as having been diurnal creatures. To the best of my knowledge, the above chain of reasoning is the first attempt at providing positive evidence that such a restoration is reasonable for essentially all lineages of reptiles (extinct or extant) other than modern crocodiles and snakes.
We can thus draw a line from the first terrestrial tetrapod to almost any extant bird, turtle or lizard and see an unbroken chain of animals that retained all of the features associated with the retinas of such animals today - four or more classes of cones (note this argument is independent of the argument presented above based on gene sequences), colored oil droplets, and probably double cones. Similarly we can conclude that many extinct side branches (e.g., ichthyosaurs, pterosaurs and most extinct dinosaurs) were also so endowed.
Figure 16 depicts some of the perceptual consequences we might predict from this endowment. In
Witmer's (1995) parlance, it is a Level I inference to say that, for example, Deinonychus had four cone classes, oil droplets and double cones in its retina. That is, we are justified in making a decisive and positive assertion that Deinonychus had these soft tissue features because they are present in birds and turtles, and they are associated with scleral ossicles that Deinonychus also retained (see:
the Tree of Life,
Chordata
section). Dinosaurs are generally restored as having been diurnal creatures. To the best of my knowledge, the above chain of reasoning is the first attempt at providing positive evidence that such a restoration is reasonable for essentially all lineages of reptiles (extinct or extant) other than modern crocodiles and snakes.
Potential Bones of Contention
Two cautionary notes must be raised in regard to the chain of logic linking scleral ossicles and circadian patterns of activity. First, it is possible to erroneously conclude that a structure did not exist in a lineage merely because it has not been found. For instance, ossicles are almost never found in large theropod dinosaurs, so one might deduce that in order for a theropod to grow to a large size it had to become nocturnal. However, scleral ossicles have been found in one specimen of Tyrannosaurus bataar (Sabath, personal commun., 1999). Ossicles likely existed in all theropods and are only missing in many forms due to preservation and/or preparation artifacts. The conclusion that the structures did not exist in an animal will always be more tentative than the conclusion that they did in forms for which positive evidence is known. On the other hand, when living forms do not have the structures it is reasonable to infer that the last animals known to have them may well have been the last animals in that lineage to have actually had them.
One group of extant animals that does not have scleral ossicles is mammals. Scleral ossicles have been found in the remains of some early synapsids
(T. Rowe 1988), so their absence in extant mammals represents a secondary loss. This is consistent with Walls' view that Mesozoic mammals were nocturnal. Using the argument from the previous paragraph, we can infer that scleral ossicles were lost from synapsid lineages at some point in the Late Triassic, since tritylodonts had them (T. Rowe, personal commun., 1999), but they are not known from later mammal or mammal-like forms.
We thus have a basis for reasoned speculation about the precise ecological forces which
led to nocturnality in our ancestors. For instance, what were the exact competitors, predators and sources of food for Late Triassic mammals? Or conversely, what flora and fauna permitted (if not encouraged) diurnality in Early Triassic mammalian ancestors? Similar arguments can be made for amphibians, crocodilians and any other group of animals that have lost or reduced their scleral ossicles.
The claim that it is a Level I inference that the retina of Deinonychus had four cone classes, double cones and oil droplets rests on the correlation between these structures and scleral ossicles. However, it is not necessarily true that all "scleral ossicles" are homologous, and it is certainly not true that all structures named "scleral ossicles" perform the same function. Included in
Figure 15 is a photo of the skull of a specimen of Dunkleosteus. This and other fish
dating back to at least the Silurian had small plates of bones in (or at least closely associated with) their eyes.
Walls (1942) argued, based upon his understanding of the structures from which the ossicles evolved in the two lineages, that the ossicles of such actinopterygian fish could not be homologous
wtih those of reptiles. It has been generally believed that the ossicles of modern fish form via ossification of scleral cartilages, which in reptiles coexist with the ossicles that form by direct ossification
(Hall and Miyake 1992,
Canavese et al. 1994). However,
Andrews (1996) examined ossicular development in turtles and concluded that it was more like that of fish than that of birds. Whether or not the ossicles are homologous in birds and fish, they cannot perform the same functions in both groups - as mentioned previously, the corneas of aquatic animals do not aid in focussing light. Thus, unlike birds, fish cannot (and of course, do not) accommodate by changing the shape of their corneas. Extant fish also do not squeeze their lenses as do reptiles, so the ossicles would not help them to accommodate in that fashion. Finally, in modern fish, there is no corneal-scleral sulcus as there is in reptilian eyes. However, this condition is similar to that of penguins which have well-developed ossicles in spite of their lack of a corneal-scleral sulcus (Suburo and Scolaro 1990). Frequently the corneas of fish are flattened probably to improve streamlining around the eye, so the junction between the cornea and sclera is convex. In extant actinopterygians, scleral ossicles are most prominently associated with large, swift-swimming scombrids, which may have retained them as supports for their relatively large extraocular muscles
(Nakamura and Yamaguchi 1991).
If it could be established that (contra
Walls 1942) fish and reptile scleral ossicles are, in fact, homologous then some of the previous conclusions would have to be modified pending the establishment that the ossicles of reptiles took on their present functions prior to the timing of the split between the lineages leading to the various major tetrapod groups. Consequently, further examinations of the nature of these structures in both extinct and extant forms are probably warranted. However, to put this objection into perspective, if we ultimately reject the logical linkage between scleral ossicles and diurnal activity patterns - and hence the retinal characteristics that appear linked to diurnality in reptiles - then my inferences about Deinonychus' retina merely become Level I´ in
Witmer's (1995) terminology. That is, we would still have good reason to infer that dinosaurs had retinas more similar to those of modern birds than those of say, modern mammals, but our confidence in the inference would not be quite as strong. If scleral ossicles are not reliable osteological correlates of double cones, oil droplets and four visual pigments it would be harder to argue against the hypothesis that some or all of these features were secondarily lost in particular lineages. Such secondary losses would be evolutionary changes for which we have no direct evidence in most lineages, though, and in such instances (e.g., for Deinonychus), it would be more parsimonious to conclude that the ancestral features were retained.
 Color vision in animals is sculpted by filters that modify the spectrum of light before it reaches the photoreceptor outer segments. In humans
(Goldsmith 1990) and some fish (Thorpe et al. 1993) the lens and other structures absorb a fair amount of light, particularly at short wavelengths. This absorption makes us insensitive to UV light
(Stark and Tan 1982). Of greater potential interest are pigments contained in oil droplets within individual photoreceptors.
Color vision in animals is sculpted by filters that modify the spectrum of light before it reaches the photoreceptor outer segments. In humans
(Goldsmith 1990) and some fish (Thorpe et al. 1993) the lens and other structures absorb a fair amount of light, particularly at short wavelengths. This absorption makes us insensitive to UV light
(Stark and Tan 1982). Of greater potential interest are pigments contained in oil droplets within individual photoreceptors.
 Because different photoreceptors may have different oil droplets, these droplets provide another mechanism by which animals could have modified the absorption spectra of photoreceptors to extract spectral information from the light striking their eyes (see
Figure 9A-Figure 9B). However, animal evolution appears not to have taken this pathway in the development of color vision. Instead, oil droplets sharpen the tuning of photoreceptors reducing the window of the receptor's spectral responses
(Goldsmith 1990,
Bowmaker et al. 1997,
Vorobyev et al. 1998). This sharpening is graphically depicted in
Figure 9C.
Because different photoreceptors may have different oil droplets, these droplets provide another mechanism by which animals could have modified the absorption spectra of photoreceptors to extract spectral information from the light striking their eyes (see
Figure 9A-Figure 9B). However, animal evolution appears not to have taken this pathway in the development of color vision. Instead, oil droplets sharpen the tuning of photoreceptors reducing the window of the receptor's spectral responses
(Goldsmith 1990,
Bowmaker et al. 1997,
Vorobyev et al. 1998). This sharpening is graphically depicted in
Figure 9C.