|
|
|
INTRODUCTIONIt has long been theorized that armored dinosaurs, especially stegosaurs, used their tails for defense, swinging them at attackers (e.g., Marsh 1880; Lull 1910; Bakker 1986). Other researchers claimed that this was impossible, because of supposedly insufficient mobility (Gilmore 1914; Hennig 1925; Janensch 1925). However, a plethora of stegosaur spikes with their tips broken off and with callus growths indicate that forceful collisions between stegosaur tails and other objects were not rare occurrences. Of a sample of 51 stegosaur spikes from the Morrison Formation of North America, about 10% showed broken tips (McWhinney et al. 2001; Carpenter et al. 2005). For the African genus Kentrosaurus Hennig, 1915 from the Tendaguru Formation of Tanzania (Hennig 1915) the ratio is unknown, because most material is lost or destroyed (Mallison 2011). However, Hennig (1925, table on p. 232) lists a surprisingly large number of specimens for which the maximum length can either not be given, or only estimated. These missing data may indicate that a significant percentage showed broken tips similar to those of American stegosaur spikes, but it is unclear which of these spikes are really tail tip spikes of Kentrosaurus and which were located further anteriorly. Carpenter et al. (2005) detailed the impact-related pathologies on stegosaur tail spikes and an exceptional find, which was a pathological caudal of Allosaurus Marsh, 1877 (Marsh 1877a) that apparently was struck at high velocity by a stegosaur spike tip. The impact destroyed part of the transverse process and the tip of the spike pierced the neural spine (Carpenter et al. 2005, figure 17.2). Carpenter et al. (2005) also assessed the forces involved, concluding that the tail of Stegosaurus Marsh, 1877 (Marsh 1877b) was strong enough to cause the damage. They also detailed two different methods for calculating the forces involved in causing damage to the spikes, concluding that both slashing and spearing actions took place. The forces involved both in slashing and spearing were sufficient to break spikes in the manner observed in the fossil record and sufficient to cause the damage to the Allosaurus vertebra (Carpenter et al. 2005). To date, this report remains the single published detailed biomechanical analysis of stegosaur tails.
The other main group of thyreophorans, ankylosaurs, has seen barely more attention.
Coombs (1978,
1979) studied their myology in detail and speculated that the tail was occasionally used to club predators, but did not attempt to assess forces and accelerations involved.
Maleev (1952) regarded the tail club as a 'mace', and
Arbour (2009) investigated tail mobility and impact forces in detail, distinguishing three categories of caudal vertebrae in ankylosaurs with three different functions: free vertebra form the flexible base of the tail, handle vertebra form a rigid club, and a transitional vertebra lies between the two. In the derived stegosaur Stegosaurus, the tail may have been functionally sectioned not by difference in vertebra shape, but by the large osteoderm plates, which inhibited motion so that five near-rigid sections were formed (Carpenter 1998;
Carpenter et al. 2005). This condition is a marked contrast to basal thyreophorans such as Scelidosaurus and stegosaurs other than Stegosaurus, where a functional partitioning is,
where the tail is known, absent because there were no osteoderms with a large anteroposterior extension present on the tail (e.g., Huayangosaurus
Dong et al., 1982; [Maidment et al. 2006]; Kentrosaurus
[Hennig 1925;
Mallison 2010a]). For some species the tail is insufficiently known (e.g., Miragaia
Mateus et al., 2009). In Kentrosaurus, the caudal vertebrae change significantly in shape, with the neural spines of the anterior caudals inclined posteriorly, those in the middle part of the tail sub-vertical, and those in the distal third hook-shaped and inclined anteriorly (Hennig 1925;
Mallison 2010a, figure 5).
The use of the tail as a weapon is common in extant reptiles, e.g., monitor lizards (Holland 1915), lacertilians (Carpenter 1961; Milstead 1970) and crocodylians (e.g., Pooley and Gans 1976). All crocodylians use vigorous swings of their tails to reverse the direction their snout points in, but alligators especially use powerful swipes of their tails to strike approaching antagonists (or other objects perceived as such; personal observation). Mammals usually do not have long, thick tails, so that other body parts are employed as weapons. Horns and antlers, e.g., usually are used in intraspecific fights between males (see, e.g., Geist 1971; Clutton-Brock 1982) and may be involved in resource competition between female ruminants (Roberts 1996). However, glyptodonts apparently used their tails (some of them club shaped) in intraspecific fights, delivering blows sufficiently strong to fracture and dent the opponent's carapace (Alexander et al. 1999). Considering everything, it seems highly likely that Kentrosaurus employed its tail as an active weapon, very likely for defense against predators, and potentially also for intraspecific fights.
For any modeling of a potential tail strike, the geometry of the impact is of importance. Three types of impacts must be distinguished: penetrating, slashing, and blunt. In penetrating impacts the spike tip works like a spear tip, for slashing it works like a scimitar, and in blunt impacts the spike acts like a club or mace. The impact types differ in the angles between the spike's long axis, its direction of travel, and the target surface.
The tail spikes of Stegosaurus (e.g.,
Marsh 1880, pl. X;
McWhinney et al. 2001, figure 7.1;
Carpenter et al. 2005, figure 17.3) have a form intermediate between a spear (causing penetrating trauma) and a club (causing blunt trauma). The sharp tips are suitable for slashing and for easily penetrating soft tissues, but the conical form means that deeper penetration pushes a much larger cross section into the opponent, increasing the energy needed to increase penetration depth significantly. If an impact occurs at a shallow angle to the long axis of the spike, the contact area is large, as with a club, and penetration requires a much higher impulse. Compared to the North American genus, the distal tail spikes of Kentrosaurus are slimmer, being only about half as wide at the base, proportionally (Hennig 1915, figure 3,
1925, figure 54). The distribution of osteoderms on the body of Kentrosaurus is not entirely clear, with only a few found semiarticulated (Hennig 1925; Janensch
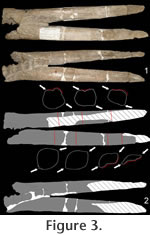
1925). Fortunately, the shape of the most distal spike pair is known with certainty, because one pair was found articulated with five distal caudal vertebrae (MB.R.3803;
Hennig 1925;
Figure 3).
However, the spikes on the tail tip apparently angled out from the long axis of the tail by only a small angle, as indicated by the angle between their bases and shafts. The angles may be as low as 20° (Hennig 1925), while spikes positioned further anteriorly showed increasingly larger angles and stouter form (Figure 2.4). Together, the arrangement and shape change of the spikes may indicate that the tail tip functioned primarily as a slashing weapon, with the occurrence of deeply penetrating strikes unlikely, while the slower moving spikes further anteriorly served mostly as defensive pikes, shaped to penetrate a predator that tried to attack the tail base. For this purpose, the spikes would need to stick out steeply, pointing their ends at the target. On the other hand, the spikes of MB.R.3803 may today be angled much more strongly than they were in life, and the angle between base and spike shaft may not be indicative of the true orientation of the spike. The tail tip of Kentrosaurus may then have been highly similar in overall appearance to that of Stegosaurus, in which the spikes stood out at nearly a 45° angle (Carpenter 1998, figure 3). Even a more laterally directed orientation is possible, because decay of soft tissues before complete burial of the articulated finds (e.g., the Stegosaurus stenops specimen shown in Carpenter [1998, figure 3]) could have led to the spikes being folded closer to the vertebral column than during life. Overall, geometry appears to indicate that blunt impacts of the tail tip spikes were most likely, with slashing impact also occurring, while deep penetrating trauma was rare. The large number of broken spikes (McWhinney et al. 2001; Carpenter et al. 2005), in contrast, may indicate that the above assessment of the geometry is wrong, and that the spikes may have "hooked into" the target's body. Therefore, all types of impacts must be taken into account when assessing stegosaur defense behavior. Besides the geometry of the impact, the speed of the tail tip is the main factor determining the damage a tail strike can cause. It depends on the tail's motion geometry and on the forces that the musculature can generate, which in turn depend directly on the available muscle cross section. Previously published reconstructions of dinosaur tail muscle cross sections usually differ significantly from the tail morphology of extant monitor lizards and alligators, as shown, among others, by Persons (2009) in a dissection study. A detailed assessment of tail volume and the influence of its reconstruction on COM position by Allen et al. (2009) found, for non-avian sauropsids, on average 158% mediolaterally, 133% dorsally, 186% ventrally, 91% dorsal diagonally, and 112% ventral diagonally greater dimensions in reality than in a simple, bone-determined elliptical model. Allen et al. (2009) found that their most detailed and realistic models closely approximated body mass, whereas the elliptical models underestimated total body mass by nearly 14%.
Here, I present the results of NASTRAN-based computer-aided engineering (CAE) modeling of tail motions of Kentrosaurus, which allow estimating the tail tip speeds and impact forces across the entire motion range of the tail in higher detail than the mathematical methods of Carpenter et al. (2005), using musculature reconstructions based both on previous methods, on data from extant alligators, and an intermediate version. Institutional AbbreviationsDMNS, Denver Museum of Nature and Science, Denver (US) MFN, Museum für Naturkunde – Leibniz-Institut für Evolutions- und Biodiversitätsforschung an der Humboldt-Universität zu Berlin, Berlin (Germany) Collection numbers MB.R.#### NMS, Naturmuseum Senckenberg, Frankfurt (Germany) SMA, Sauriermuseum Aathal, Aathal (CH) |
|