 Likelihood estimation of the time of origin of Cetacea and the time of divergence of Cetacea and Artiodactyla
Likelihood estimation of the time of origin of Cetacea and the time of divergence of Cetacea and Artiodactyla
Article number: 1.2.8A
Copyright Palaeontological Association, 1 August 1998
https://doi.org/10.26879/98008
Plain-language and multi-lingual abstracts
PDF version
Submission: 30 April 1998, Acceptance: 7 July 1998
ABSTRACT
Continuity is important for tracing evolutionary lineages through geological time. Modern Odontoceti and Mysticeti can be traced backward in time to Eocene Archaeoceti, and before them to mesonychian Condylarthra. Within this shared continuum, the origin of Archaeoceti and the origin of Cetacea is marked by the first indication of a derived evolutionary transition-in-grade from terrestrial to aquatic life characteristic of later cetaceans. Archaeocetes are known from many fossil localities in Eocene marginal marine and shallow marine strata on six continents. These range in age from Priabonian (late Eocene; ca. 36 Ma) through late Ypresian (late early Eocene; ca. 49.5 Ma), a 13.5 m.y. time range, and they are widely distributed in North America (18 sites), Europe (5 sites), Asia (8 sites), Africa (8 sites), Australia (New Zealand; 2 sites), and Antarctica (1 site). Forty-two sites can be considered statistically-independent records.
With this information and a model sampling distribution of potential fossils, we can compare different hypothesized times of origin of archaeocetes by calculating relative likelihoods for each. The model sampling distribution reflects changing outcrop area of sedimentary rocks through geological time and changing numbers of archaeocetes during their diversification. The maximum-likelihood time of origin of archaeocetes is given by the age of the earliest fossil, which defines the beginning of the temporal range and requires no hypothesized extension. Likelihood ratios of 0.5 and 0.05 have associated probabilities less than or equal to 0.5 and 0.05, respectively, representing confidence limits equal to or greater than 50% and 95%. A critical likelihood l = 0.05 defines the maximum extension of range we can reasonably expect to find, and from this we estimate the time of origin of Archaeoceti to have been at or after about 51.6 Ma—within the early Eocene.
Fifty-six independent records of Mesonychidae and Hapalodectidae on the three northern continents range in age from Rupelian (early Oligocene; ca. 33 Ma) to Torrejonian (middle Paleocene; ca. 63 Ma). We estimate the time of origin of mesonychian condylarths to have been at or after about 66.7 Ma (virtually at the Cretaceous-Paleocene boundary). Artiodactyla, the extant sister-group of whales, has a fossil record extending to the beginning of the Eocene, with an arctocyonian ancestry extending into the latest Cretaceous. The fossil record as now known indicates evolutionary divergence of Mesonychia + Cetacea from Arctocyonia + Artiodactyla in the early Paleocene or, reasonably, at the very end of the Cretaceous.
Philip D. Gingerich. Department of Geological Sciences and Museum of Paleontology, The University of Michigan, Ann Arbor, Michigan 48109-1079, U.S.A. gingeric@umich.edu
Mark D. Uhen. Cranbrook Institute of Science, Bloomfield Hills, Michigan 48303-0801, U.S.A. muhen@pop.cranbrook.edu
KEY WORDS: Stratigraphic range, Cetacea, Mesonychia, Artiodactyla, Eocene
Final citation: Gingerich, Philip D. and Uhen, Mark D. 1998. Likelihood estimation of the time of origin of Cetacea and the time of divergence of Cetacea and Artiodactyla, Palaeontologia Electronica Vol. 1, Issue 2; 8A; 45p. https://doi.org/10.26879/98008
palaeo-electronica.org/content/1998-2/756-time-of-origin
INTRODUCTION
The geological time scale is based on the evolutionary succession of animal life found as fossils in superposed strata of sedimentary rocks covering large areas of the earth's surface. The Paleo-zoic, Meso-zoic, and Ceno-zoic eras are separated by mass extinctions and associated profound faunal change. Periods, epochs, ages, and biochrons are progressively finer subdivisions of the time scale distinguished by faunal differences and by extinction-origination turnovers of lesser magnitudes. The history of life through geological time is not a smooth and seamless history, but rather an episodic history. Times of turnover, whatever their scale, are critical events, often coming at a juncture of unusual extrinsic-environmental and intrinsic-biotic change, that challenges the survival of species. Those that survive may do so by moving or by changing at evolutionary rates that appear rapid in the context of geological time.
Dinosaurs and other reptiles dominated Mesozoic terrestrial and marine faunas. On land, dinosaurs were replaced by mammals that evolved to dominate succeeding terrestrial Cenozoic faunas. Condylartha or 'archaic ungulates' are common in terrestrial mammalian faunas of the Paleocene epoch, but were largely replaced by modern mammalian orders in the Eocene. In the sea, plesiosaurs, ichthyosaurs, and mosasaurs were replaced by mammals too, but marine mammals are not known from the Paleocene. The reign of dinosaurs, plesiosaurs, ichthyosaurs, and mosasaurs ended with the Mesozoic; mammals crossed the Cretaceous-Tertiary boundary and seemingly took advantage of new opportunities, diversifying broadly on land and eventually invading the sea. Most modern orders of mammals appeared at or near the Paleocene-Eocene boundary, replacing archaic orders from which they probably evolved, and the initial appearance of many orders closely spaced in time may reflect rapid evolution associated with a Paleocene-Eocene turnover event of some kind.
The geological time scale or evolutionary succession of life is calibrated in two ways that yield very different results. The conventional way in geology is to calibrate the evolutionary succession of animal life by finding interbedded crystaline rocks (principally basalts) that can be dated radiometrically (Dalrymple 1991). This assumes that the ejection or capture of energetic electrons in radioactive elements of rock-forming minerals happens randomly and independently of biotic evolution. A new and as yet unproven approach promoted by some biologists is to calibrate the evolutionary succession using molecular genetic differences between pairs of living plants or animals as clocks of divergence time (Zuckerkandl and Pauling 1962, 1965). This assumes that the genetic code changes randomly and independently of biotic evolution, which seems unlikely when development of each new generation depends so directly on the code it inherited.
Calibration of the evolutionary succession of life with molecular clocks is selective, meaning that divergences of animals like mammals that live today can be calibrated independently of faunal context, while divergences of and from animals like dinosaurs that are extinct cannot. This leads to faunal inconsistencies like wholesale overlap of otherwise Cenozoic mammalian orders with Mesozoic dinosaurs (Hedges et al. 1996, Kumar and Hedges 1998). Here we consistently use the geological radiometric time scale rather than molecular clocks to calibrate evolutionary succession. The numbers we use are drawn from the Haq et al. (1987) time scale because sea level change is sometimes important for interpreting shallow marine habitats of whales, but taking numbers from more recent timescales (e.g., Berggren et al. 1995, Gradstein and Ogg 1996, or Hardenbol et al. 1998) would not change our conclusions significantly.
Our focus is on Cetacea. Looking backward from the present, whales, like other mammalian orders, undoubtedly have a pedigree extending back to the earliest mammals known from the Triassic period, and before that to the earliest vertebrates of the Cambrian or Ordovician. The continuity of the cetacean germ line, whether followed backward in time or forward in time, is not in question because individuals propagate new individuals and new species necessarily evolve from old ones. Evolution is first and foremost a history of ancestors and their sometimes-divergent descendants. What in the complex genealogy of mammals makes a whale a whale? What are the characteristics by which whales are recognized? And when did whales first appear in the evolution of mammals?
WHAT, WHEN, AND WHERE IS A WHALE?
Living Cetacea are fully aquatic and share a hydrodynamically streamlined body form with forelimbs modified into flippers and loss of external hind limbs, while locomotion is powered by a heavily-muscled tail bearing a broad terminal horizontal fluke. Communication with other whales and sensory perception in an aquatic medium are largely sound-based, for which cetaceans have characteristically-dense tympanic bullae; isolated, highly modified periotic or petrosal bones; and large mandibular canals with thin lateral acoustic fenestrae. Feeding is accomplished by straining krill and small fish in the baleen whales (Mysticeti) or by catching larger fish, squid, and other animals and swallowing these whole or in large pieces in the toothed whales (Odontoceti). These divergent specialized adaptations to life in water, and the living suborders that have them, Mysticeti and Odontoceti, can be traced backward in the fossil record from the present to the Oligocene epoch (Fordyce and Barnes 1994).
Late Eocene Archaeoceti of the family Basilosauridae (especially Dorudontinae; Uhen 1996) resemble later mysticetes and odontocetes in having a hydrodynamically streamlined body form with forelimbs modified into flippers and locomotion powered by a heavily-muscled fluked tail, while retaining reduced but functional hind limbs (Gingerich et al. 1990). Basilosaurids have dense tympanic bullae, pterygoid sinuses, partially isolated periotics, and large mandibular canals with lateral acoustic fenestrae, which would have enabled them to hear directionally in water. Feeding was different in that cheek teeth retain complex morphology and functional occlusion, and heavy wear shows that food was chewed before swallowing. Thus, late Eocene basilosaurid archaeocetes have many, but not all, characteristics of later whales. Early mysticetes and odontocetes are difficult to distinguish from basilosaurids, and all are marine and fully aquatic. Temporal, morphological, and environmental/geographical continuity between late Eocene basilosaurids and following Oligocene mysticetes and odontocetes indicates basilosaurids are closely related to modern whales, and their derived aquatic characteristics affirm inclusion in Cetacea.
Middle Eocene Archaeoceti of the family Protocetidae resemble basilosaurid archaeocetes, mysticetes, and odontocetes in having a hydrodynamically streamlined body form and locomotion powered by a heavily-muscled tail, while retaining large functional hind limbs (Gingerich et al. 1994). The form of the forelimbs is as yet poorly documented, and the presence of a fluke is only a possibility. Protocetids have dense tympanic bullae and large mandibular canals with lateral acoustic fenestrae, but isolation of the periotic was limited, making directional hearing questionable. Like basilosaurids, protocetids had cheek teeth different in detail but retaining complex morphology and functional occlusion, and here too heavy wear shows that food was chewed before swallowing. Protocetids were probably good swimmers and all are found in marine strata. Thus, middle Eocene protocetid archaeocetes have many, but not all, characteristics of basilosaurid archaeocetes and some characteristics of later whales. Here again, it is the relative continuity in time, form, and place between middle Eocene protocetids and late Eocene basilosaurids that indicates protocetid archaeocetes are closely related to basilosaurids, and their derived aquatic characteristics affirm inclusion in Cetacea.
Early Eocene Archaeoceti of the family Pakicetidae are poorly known postcranially. Pakicetus has a dense tympanic bulla with a characteristically cetacean sigmoid process (Gingerich and Russell 1981), but the periotic was firmly integrated in the basicranium, making directional hearing questionable (Gingerich et al. 1983). Pakicetus had no enlargement of the mandibular canal and the incus was intermediate in morphology between those of modern artiodactyls and cetaceans, suggesting not only that it could not hear directionally but it could not hear well in water (Thewissen and Hussain 1993). Pakicetus had cheek teeth retaining complex morphology and functional occlusion, with larger protocones but otherwise the same general pattern of cusps and crests as later protocetids and basilosaurids. Early Eocene pakicetid archaeocetes are found in river and estuarine deposits in association with land mammals, but these deposits are peripheral to the Tethys Sea and pakicetid-bearing deposits are overlain by protocetid-bearing marine strata. Pakicetids have some, but not all, characteristics of later protocetid and basilosaurid archaeocetes and they have but few characteristics of later whales. Relative continuity in time, form, and place indicates early Eocene pakicetids are closely related to middle Eocene protocetids, and the derived aquatic characteristics of pakicetids affirm inclusion in Cetacea (Gingerich et al. 1983, Thewissen and Hussain 1993, Thewissen 1994). Pakicetus, the remingtonocetid Remingtonocetus (Kumar and Sahni 1986; Gingerich et al. 1995a), and ambulocetid Ambulocetus (Thewissen et al. 1994), all discovered in recent years, have such important primitive and nonaquatic characteristics that all have forced us to expand our concept of Cetacea.
The Paleocene mammals most similar to pakicetids and later protocetids are Condylartha or Mesonychia of the family Mesonychidae (Van Valen 1966, 1969, 1978). Mesonychidae did fit comfortably in the archaic order Condylarthra until Van Valen suggested that dental similarities to later Protocetus were important. This possible connection to later whales now overshadows their clear connection to earlier Paleocene condylarths but the original temporal, morphological, and geographical resemblance has not changed. Protocetid teeth and mesonychid teeth have similarly-unusual proportions, and a protocetid of uncertain generic attribution, one pakicetid (Ichthyolestes), and a possibly Ambulocetus-like genus (Gandakasia) were originally described as mesonychids by Pilgrim (1940) and by Dehm and Oettingen-Speilberg (1958). These were included in Mesonychidae by Szalay and Gould (1966), although they are now known to be primitive archaeocetes rather than mesonychids.
Some Mesonychidae like Asian late Paleocene Sinonyx (Zhou et al. 1995) and North American early Eocene Pachyaena (Zhou et al. 1992; O'Leary and Rose 1995) are known from virtually complete skeletons showing them to be hoofed cursorial mammals with no aquatic specializations and no distinctively cetacean characteristics (they have ossified auditory bullae, but lack in particular the dense enlarged bullae with sigmoid processes seen in Pakicetus). Thus we distinguish Cetacea from non-Cetacea at a gap between Mesonychidae and Pakicetidae. This gap is not a rigid boundary, but one subject to revision in light of new discoveries (postcranial remains of pakicetids will be critical here): the connection may become weaker if similarities in form that we see now are in the future overshadowed by similarity to some other group, or the connection may become stronger if new similarities are discovered that reinforce it (similarity in any particular case, like continuity, is always relative to that in competing cases).
What makes a primitive archaeocete like Pakicetus (Gingerich and Russell 1981) or Ambulocetus (Thewissen et al. 1994) a whale, when mesonychids like Sinonyx and Pachyaena are not whales? Berta (1994) asked "what is a whale?". Gish (1994) asked "when is a whale a whale?" And a third question might be "Where is a whale a whale?" It is not possible to answer one question without the others, and the answer to all three is that a primitive early fossil whale is a whale when continuity in temporal, morphological, and geographical range connects it to living whales—ideally through a closely-connected series of temporal, morphological, and geographical intermediates—and its form shows one or more of the specializations of whales. The former reflects the "shared" and the latter the "derived" components of synapomorphy diagnosing Cetacea. In our view as paleontologists, whales became whales when they first showed evidence of the evolutionary transition in grade from terrestrial to aquatic life characteristic of living cetaceans.
ORIGIN OF WHALES
If everyone agreed that whales became whales when they first showed evidence of the evolutionary transition in grade from terrestrial to aquatic life, then it would still be possible to view the origin of whales in different ways, depending on whether one assumed a predominantly comparative or predominantly historical perspective. We do not view such a dichotomy as necessary so much as matter-of-factly descriptive of perspectives colleagues have.
To comparative biologists concerned solely with living organisms, a whale is a whale after the time of separation of Cetacea from its closest living noncetacean relative. Flower (1883) interpreted an elongated larynx, complex stomach, simple liver, reproductive organs, and fetal membranes as linking Cetacea to Artiodactyla anatomically, but later authors did not find this convincing (Kellogg 1936; Simpson 1945). We owe our present understanding that extant Artiodactyla are the sister-group of extant Cetacea to comparative immunology (Boyden and Gemeroy 1950), to the fossil record (Van Valen 1966, 1969), and more recently to molecular gene sequencing (Arnason et al. 1991; Irwin et al. 1991; Milinkovitch 1992; Krettek et al. 1995; D'Erchia et al. 1996). Claims that (1) sperm whales are mysticetes (Milinkovitch et al. 1993, 1994; Douzery 1993; Milinkovitch 1995; Milinkovitch et al. 1995; but see Ohland et al. 1995 and Messenger and McGuire 1998); (2) Cetacea originated within Artiodactyla as the sister-group of extant camels (Goodman et al. 1985), hippopotami (Sarich 1993; Irwin and Arnason 1994; Arnason and Gullberg 1996), ruminants (Graur 1993; Graur and Higgins 1994), or suids (Kumar and Hedges 1998); and (3) whales are the sister group of perissodactyls (McKenna 1987) cast doubt on the efficacy of molecular systematics, but we accept that Artiodactyla is probably the closest living sister-group to Cetacea. If true, then to a comparative biologist the time of origin of Cetacea is the time Cetacea diverged from Artiodactyla.
To paleontologists like us concerned with both living and extinct organisms, a whale is not a whale until it has both (1) separated from its closest living sister taxon (Artiodactyla), becoming a distinct clade, and (2) acquired one or more characteristics of Cetacea, achieving a distinctive grade. Cetacea and Artiodactyla can be traced back in geological time to different stem groups within Condylarthra (Mesonychia and Arctocyonia, respectively; Van Valen 1966, 1971, 1978; Rose 1996). Consequently, there is a possibly-significant interval of time between events (1) and (2) that we consider here. In the following analyses we first estimate the time of origin of whales as conceived by paleontologists, based on the fossil record, where Cetacea is not only a distinct clade but also a distinct grade. We then estimate the time of divergence of Cetacea from Artiodactyla by estimating the time of origin of Mesonychia and comparing that to the ranges of Arctocyonia and Artiodactyla in the fossil record.
One way to evaluate the time of origin of whales is to consider it to be close to the time of first preservation of whales in the fossil record. This is reasonable because whales are relatively large animals with well-ossified skeletons, most whales have distinctive dental and osteological characteristics related to aquatic or partially aquatic adaptations enabling them to be identified, and life in water enhances preservation potential in the fossil record. We can then consider the distribution of ages of known fossils and estimate an acceptable confidence limit for this distribution.
In the following calculations we use the fossil record of Archaeoceti to estimate the time of origin of Cetacea as a whole. We do this because Archaeoceti represents the Eocene initial diversification of whales and is thus the subgroup having the greatest bearing on the time of origin of the order. We concentrate on Archaeoceti too because this is the group for which we can best determine the number of independently-sampled site records. Occurrences of non-cetaceans, even when thought to be broadly ancestral (like mesonychians for example), contribute little in constraining the time of origin of cetaceans, just as the stratigraphic distribution of Archaeoceti contributes little to knowledge of the time of origin of later Odontoceti or Mysticeti.
Calculations similar to those presented here can be carried out for Cetacea as a whole (and for Artiodactyla and other orders of mammals) when counts of independently-sampled site records are known, but these are not yet available. If we can generalize based on what we demonstrate here about the statistical power of even modest numbers of independent sites, the temporal ranges of all of the better known orders of mammals are already closely constrained by the known fossil record.
CLASSICAL CONFIDENCE INTERVALS FOR TEMPORAL RANGES
Strauss and Sadler (1989) derived a one-tailed confidence interval for a taxon's stratigraphic or temporal range that we shall use here:
p1 = 1 - (1 + a)-(n - 1) (1)
where p1 is the confidence level (e.g., 0.95), their a is the range extension expressed as a proportion of the known stratigraphic or temporal range (not to be confused with conventional use of a to represent level of significance, see below), and n is the number of independently-sampled fossiliferous horizons. Solving for a by rearranging terms yields the required range extension as a function of the confidence level and number of samples (Marshall 1990). Derivation of equation 1 by Strauss and Sadler and discussion of this by Marshall both make it seem unduly complicated (due in part to the complexity of their notation), but the derivation is actually both simple and intuitive, as we discovered by deriving this independently (Gingerich and Uhen 1994).
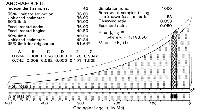
 Start by assuming that fossils are uniformly distributed throughout the unknown estimated temporal range or expected temporal density [ETD] of a taxon of interest (Figure 1). This assumption of uniformity is too simplistic, and we will relax it later (when the density associated with an area or volume need no longer correspond to the temporal range). Assume that sampling is random. A uniform ETD means that fossils from different times have an equal probability of being sampled. Construct a sample of some size n by drawing n independent samples at random from ETD. We can now sort these from oldest to youngest and they define the observed temporal range or observed temporal density [OTD]. We are interested in the time of origin of Archaeoceti and do not care about their time of extinction (or conversion into Odontoceti and Mysticeti), meaning that we are interested in a one-tailed range extinction. This is, in any case, more conservative (yielding a broader interval) in allocating all of the tail probability to one tail. One sample is required to define the youngest end of the OTD (t3, the time we are not interested in here), so n - 1 samples remain for estimation of ETD and t1 from OTD and t2.
Start by assuming that fossils are uniformly distributed throughout the unknown estimated temporal range or expected temporal density [ETD] of a taxon of interest (Figure 1). This assumption of uniformity is too simplistic, and we will relax it later (when the density associated with an area or volume need no longer correspond to the temporal range). Assume that sampling is random. A uniform ETD means that fossils from different times have an equal probability of being sampled. Construct a sample of some size n by drawing n independent samples at random from ETD. We can now sort these from oldest to youngest and they define the observed temporal range or observed temporal density [OTD]. We are interested in the time of origin of Archaeoceti and do not care about their time of extinction (or conversion into Odontoceti and Mysticeti), meaning that we are interested in a one-tailed range extinction. This is, in any case, more conservative (yielding a broader interval) in allocating all of the tail probability to one tail. One sample is required to define the youngest end of the OTD (t3, the time we are not interested in here), so n - 1 samples remain for estimation of ETD and t1 from OTD and t2.
We require one additional number a, the level of significance or error rate we are willing to accept. This determines the confidence interval 1 - a that we seek. By convention, a = 0.05 is the usual error rate, which corresponds to a 95% confidence interval for observed temporal density OTD.
The probability that any sample drawn from unknown ETD falls in OTD is the ratio of lengths (or areas or volumes) OTD/ETD, which cannot be greater than 1 (because ETD ³ OTD). If the probability that one sample drawn from ETD falls in OTD is OTD/ETD, then the probability that two samples drawn independently from ETD both fall in OTD is the product of OTD/ETD times OTD/ETD or (OTD/ETD)2. The probability that n - 1 samples drawn from ETD all fall in OTD is (OTD/ETD)n-1. Setting this quantity equal to the error rate a:
a = (OTD/ETD)n-1 (2)
which is, in simpler form, exactly the same as equation 1 [where p1 = 1 - a and (1 + a)-(n-1) = (OTD/OTD + (ETD-OTD)/OTD)-(n-1) = (OTD/ETD)n-1]. Solving for ETD yields:
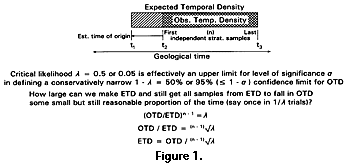
ETD = OTD / a1/(n-1) = OTD / (n-1)Öa (3)
With a = 0.05 and an observed range OTD based on two independent samples, meaning n - 1 = 1, the 95% confidence limit for ETD is 20 ´ OTD. When OTD is based on three independent samples, meaning n - 1 = 2, the 95% confidence limit for ETD is 4.47 ´ OTD. ETD/OTD is the inverse of the (n - 1)th root of a. This quantity converges rapidly to 1 as n increases, meaning ETD approximates OTD even for relatively small n. Further, this result is not very sensitive to a.
If we assume that the distribution of fossils representing the temporal duration of a group of organisms is uniform through time, and if we know (1) the beginning and end of the group's stratigraphic or observed temporal range (t2 and t3, respectively, in Figure 1), and (2) the number of independent samples this range is based on (n), then we can estimate the time of origin of the group (t1 in Figure 1). The beginning and end of the observed range encompass the observed temporal density OTD. OTD is necessarily represented by n ³ 2 samples. Estimated temporal range ETD can be calculated using equation 3, and the difference between ETD and OTD is added to the beginning of the observed temporal density. This yields a classical 1 - a confidence limit for the time of origin of the group.
LIKELIHOOD ESTIMATION
Probability is a way of comparing observed data or results for a given hypothesis. Likelihood on the other hand is a way of comparing hypotheses for a given set of data or results. The two are related, differing only by an arbitrary constant, with the likelihood of a hypothesis given the data or results being proportional to the probability of the data given the hypothesis (Edwards 1972). When hypotheses are compared, this is conveniently done in the form of likelihood ratios or relative likelihoods, L, ratios of any individual likelihood to the maximum, scaled from 0 to 1. With the maximum likelihood scaled to 1, as it is in the following calculations, the likelihood ratios are just the individual likelihoods, which are in turn upper limits of the individual probabilities of the data for each hypothesis.
Likelihood estimation involves comparison of the relative likelihoods of different hypotheses concerning t1 and ETD (Figure 1), where the likelihood of a particular hypothesis is proportional to the probability of the observed results, n samples in OTD, for that hypothesis: k á P(OTD,n|ETD), with k being an arbitrary constant. Given the geometric model shown here and the observed results, n samples falling in OTD, the hypothesis about t1 and ETD that has maximum likelihood is the hypothesis that t1 = t2 and ETD = OTD. No matter what the size of n, ETD = OTD has maximum likelihood because the exponentiated quotient (OTD/ETD)n = (OTD/OTD)n = 1n = 1, and by definition no probability of observed data can be greater than 1. Maximum likelihood, by convention, has an associated likelihood ratio L of k á P divided by itself: L = (k á P)/(k á P) = 1, and competing likelihood ratios are necessarily smaller, lying in the range 0 to 1. Note that while P ² 1, there is always a likelihood ratio L = 1 and L is consequently an upper bound for P.
It is useful to distinguish sets of hypotheses that satisfy some minimal likelihood criterion, and we are here interested in all hypotheses for which likelihoods exceed the critical likelihood l = 0.5 and all hypotheses for which likelihoods exceed l = 0.05, that is, hypotheses with at least 1 in 2 chances of occurrence (for which the betting odds are an even "50-50") or at least 1 in 20 chances of occurrence (for which the odds are "5-95"). These lambdas are upper limits for ordinary levels of significance a = 0.5 and a = 0.05 and hence define conservatively narrow 50% and 95% confidence limits for t2 and OTD in terms of an hypothesized origination time t1 and ETD. In the uniform case, as before,
l = (OTD/ETD)n-1 (4)
and
ETD = OTD / (n-1)Öl (5)
We are interested to know how large we can make ETD and still expect all samples from ETD to fall in OTD in 1 out of 2 or in 1 out of 20 trials—in other words, how large can ETD be and still yield OTD some small, but still reasonable, proportion of the time?
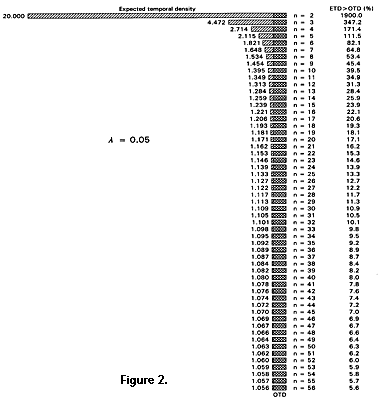 The hypotheses being compared are constructed to differ by some number of arbitrarily small increments i added to OTD, and there is in theory no limit on the fineness of the increment nor on the number of incremented hypotheses that can be compared. Increments of i are added to OTD until the sum ETD satisfies equations 4 and 5. OTD, n, and l are, of course, known or specified in advance. Relationship of ETD to OTD is not sensitive to n nor to l if n is in the range considered here (Figure 2 and Figure 3). Increments of i added to OTD correspond to addition of some amount of time to t2. The time involved is proportional to i in the uniform case, but not in the nonuniform models considered here.
The hypotheses being compared are constructed to differ by some number of arbitrarily small increments i added to OTD, and there is in theory no limit on the fineness of the increment nor on the number of incremented hypotheses that can be compared. Increments of i are added to OTD until the sum ETD satisfies equations 4 and 5. OTD, n, and l are, of course, known or specified in advance. Relationship of ETD to OTD is not sensitive to n nor to l if n is in the range considered here (Figure 2 and Figure 3). Increments of i added to OTD correspond to addition of some amount of time to t2. The time involved is proportional to i in the uniform case, but not in the nonuniform models considered here.
MODEL DISTRIBUTION OF POTENTIAL FOSSILS
 A uniform distribution of fossils is a good initial assumption, but there are several reasons not to expect the distribution of fossils representing the temporal duration of a group of organisms to be uniform through geological time: (1) the number of organisms in the group and their geographic distribution may have changed through time, changing the probability of preservation as fossils; (2) the outcrop area accessible for sampling today is different for rocks of different ages; and (3) the proportions of marine and non-marine environments on the surface of the earth have changed through time. All of these may affect distributions of fossils and make them nonuniform. Here we consider diversity to have increased at a constant rate during the interval between the time of origin of archaeocetes and their first appearance in the fossil record. We consider that the outcrop area of fossil-bearing sedimentary strata decreases exponentially for rocks of increasing age through the course of Phanerozoic time, as shown in Figure 2 (based on data in Blatt and Jones 1975). We use an exponential model fit to data for the Phanerozoic because we lack detailed knowledge of changing exposure of sedimentary strata during epochs of Cenozoic time—when detailed information about Cenozoic strata is available it can be substituted to permit a more refined calculation.
A uniform distribution of fossils is a good initial assumption, but there are several reasons not to expect the distribution of fossils representing the temporal duration of a group of organisms to be uniform through geological time: (1) the number of organisms in the group and their geographic distribution may have changed through time, changing the probability of preservation as fossils; (2) the outcrop area accessible for sampling today is different for rocks of different ages; and (3) the proportions of marine and non-marine environments on the surface of the earth have changed through time. All of these may affect distributions of fossils and make them nonuniform. Here we consider diversity to have increased at a constant rate during the interval between the time of origin of archaeocetes and their first appearance in the fossil record. We consider that the outcrop area of fossil-bearing sedimentary strata decreases exponentially for rocks of increasing age through the course of Phanerozoic time, as shown in Figure 2 (based on data in Blatt and Jones 1975). We use an exponential model fit to data for the Phanerozoic because we lack detailed knowledge of changing exposure of sedimentary strata during epochs of Cenozoic time—when detailed information about Cenozoic strata is available it can be substituted to permit a more refined calculation.
 Our nonuniform model distribution of potential fossils is shown graphically in Figure 4 and Figure 6. It has a geological age or time dimension, a diversity dimension, and a fossiliferous strata dimension. The fossiliferous strata and time dimensions define an area of potential fossils under the exponential curve (stippled), and this area plus the diversity dimension define a volume of potential fossils. Regions of interest within the whole volume of potential fossils have been lettered A, B, C, D, and E: A is the partially shaded volume at the left representing the distribution of sedimentary rocks lacking the taxon of interest because it had not yet originated and diversified; B is the narrow, hatched, wedge-shaped volume representing diversification after origination but before any appearance in the known fossil record; C is the cross-hatched volume of observed temporal density OTD representing the known fossil record of archaeocetes; D is not used here (because the temporal extension is left-tailed); and E is the partially shaded volume at the right representing the distribution of sedimentary rocks lacking the taxon of interest because it had by now given rise to something else or become extinct. Volumes B and C together correspond to the expected temporal density ETD. Total volumes and normalized proportions of A, B, C, D, and E are given in Figures 4 and 6 for the 95% confidence limit calculation shown graphically.
Our nonuniform model distribution of potential fossils is shown graphically in Figure 4 and Figure 6. It has a geological age or time dimension, a diversity dimension, and a fossiliferous strata dimension. The fossiliferous strata and time dimensions define an area of potential fossils under the exponential curve (stippled), and this area plus the diversity dimension define a volume of potential fossils. Regions of interest within the whole volume of potential fossils have been lettered A, B, C, D, and E: A is the partially shaded volume at the left representing the distribution of sedimentary rocks lacking the taxon of interest because it had not yet originated and diversified; B is the narrow, hatched, wedge-shaped volume representing diversification after origination but before any appearance in the known fossil record; C is the cross-hatched volume of observed temporal density OTD representing the known fossil record of archaeocetes; D is not used here (because the temporal extension is left-tailed); and E is the partially shaded volume at the right representing the distribution of sedimentary rocks lacking the taxon of interest because it had by now given rise to something else or become extinct. Volumes B and C together correspond to the expected temporal density ETD. Total volumes and normalized proportions of A, B, C, D, and E are given in Figures 4 and 6 for the 95% confidence limit calculation shown graphically.
It is important to emphasize that a model distribution of potential fossils is a model assuming 'all else' not represented here to be equal. Fossils may or may not be evenly distributed though time, and we have explicitly built some of the ways that they are not evenly distributed into our model. When there is knowledge of additional structure shaping the fossil record such factors can and should be built into a better model. All inference about stratigraphic ranges depends on random sampling in the context of some model, explicit or not, and explicit models are always better than vaguely-conceived implicit models. The actual ages of most fossil samples and the temporal differences between most samples are often poorly known or not known at all, but only two ages are important in our calculations: the age of the oldest known sample (t2), the age of the youngest known sample (t3). We require, in addition, some estimate of the number of independently-sampled fossiliferous horizons (n). Finally, the purpose of this exercise is less calculation of precise cut-off ages than comparison of the relative likelihoods of different possibilities.
TIME OF ORIGIN OF ARCHAEOCETES
There are approximately 42 independently sampled sites yielding archaeocete whales distributed on six continents (Table 1). These range in age from late Priabonian (t3, ca. 36 Ma) to late Ypresian (t2, ca. 49.5 Ma), for an observed temporal range t2 - t3 = 13.5 m.y. Calculation of ETD from equation 3 yields an expected temporal range of 14.78 m.y., and adding the difference between expected and observed ranges to the oldest stratigraphic record at 49.5 yields a classical 95% confidence limit for the time of origin of archaeocetes and hence all whales at 50.78 Ma, an extension of about 1.3 m.y. beyond the earliest stratigraphic record known to date.
 Likelihood calculation of the time of origin of whales is outlined in the work sheet of Table 2 and shown graphically in Figure 4. Here the origination volume OTD is successively incremented by 0.001 units until critical likelihoods l = 0.5 and l = 0.05 are reached and exceeded. Hypothesized times of origin are interpolated at 49.99 and 51.64 Ma, respectively, with the latter, an extension of about 2.1 m.y. beyond the earliest stratigraphic record, being the likelihood equivalent of a 95% confidence limit. This limit at 51.64 Ma is almost 1 m.y. earlier in time than the classical confidence interval of Strauss and Sadler (1989) based on a uniform distribution of potential fossils. The difference is due to assumptions built into our geometric model of increasing density and diversity of whales during their initial diversification and to the exponentially increasing availability of fossil-bearing sedimentary rocks in more recent geological times.
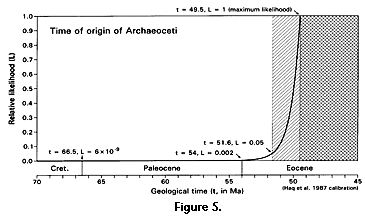
Likelihood calculation of the time of origin of whales is outlined in the work sheet of Table 2 and shown graphically in Figure 4. Here the origination volume OTD is successively incremented by 0.001 units until critical likelihoods l = 0.5 and l = 0.05 are reached and exceeded. Hypothesized times of origin are interpolated at 49.99 and 51.64 Ma, respectively, with the latter, an extension of about 2.1 m.y. beyond the earliest stratigraphic record, being the likelihood equivalent of a 95% confidence limit. This limit at 51.64 Ma is almost 1 m.y. earlier in time than the classical confidence interval of Strauss and Sadler (1989) based on a uniform distribution of potential fossils. The difference is due to assumptions built into our geometric model of increasing density and diversity of whales during their initial diversification and to the exponentially increasing availability of fossil-bearing sedimentary rocks in more recent geological times.
The likelihood function calculated for the time of origin of Archaeoceti is shown in Figure 5. Both of the confidence limits calculated here, a 50% limit at 49.99 Ma and a 95% limit at 51.64 Ma, fall comfortably within the early Eocene on the Haq et al. (1987) time scale used here. Thus we can be confident, given present evidence, that whales originated in the early Eocene. Any discovery older than the known temporal range of archaeocetes would of course extend both the range and the ages associated with all critical likelihoods.
TIME OF ORIGIN OF MESONYCHIA
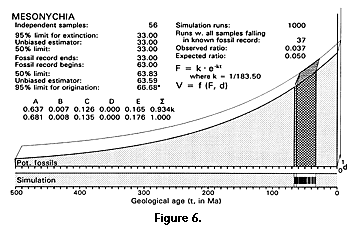 There are approximately 56 independently sampled sites yielding mesonychian condylarths distributed on the three northern continents (Table 3). These range in age from Rupelian early Oligocene (t2, ca. 33 Ma) to Torrejonian middle Paleocene (t3, ca. 63 Ma), for an observed temporal range t2 - t3 = 30 m.y. Likelihood calculation of the time of origin of mesonychians is outlined in the work sheet of Table 4 and shown graphically in Figure 6. Critical likelihoods l = 0.5 and l = 0.05 correspond to hypothesized times of origin at 63.83 and 66.68 Ma, respectively, with the latter, an extension of about 3.7 m.y. beyond the earliest stratigraphic record, being the likelihood equivalent of a 95% confidence limit.
There are approximately 56 independently sampled sites yielding mesonychian condylarths distributed on the three northern continents (Table 3). These range in age from Rupelian early Oligocene (t2, ca. 33 Ma) to Torrejonian middle Paleocene (t3, ca. 63 Ma), for an observed temporal range t2 - t3 = 30 m.y. Likelihood calculation of the time of origin of mesonychians is outlined in the work sheet of Table 4 and shown graphically in Figure 6. Critical likelihoods l = 0.5 and l = 0.05 correspond to hypothesized times of origin at 63.83 and 66.68 Ma, respectively, with the latter, an extension of about 3.7 m.y. beyond the earliest stratigraphic record, being the likelihood equivalent of a 95% confidence limit.
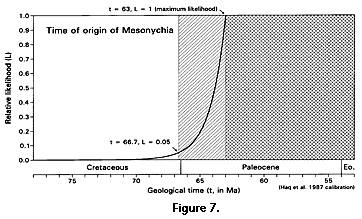
The likelihood function calculated for the time of origin of Mesonychia is shown in Figure 7. The 50% confidence limit at 63.83 Ma falls within the early Paleocene, and the 95% confidence limit at 66.68 Ma falls within the very latest Cretaceous close to the Cretaceous-Tertiary boundary at 66.5 on the Haq et al. (1987) time scale used here. Considering what we know of their fossil record, mesonychians evidently originated at or near the Cretaceous-Tertiary boundary. Any discovery older than the known temporal range of mesonychians would of course extend both the range and the ages associated with all critical likelihoods.
DISCUSSION
 The time of origin of archaeocetes calculated here takes nonuniform aspects of the distribution of potential fossils into account. The resulting estimate at ca. 51.64 Ma, is a little more than 2 m.y. before the first fossil archaeocetes appeared in the fossil record at ca. 49.50 Ma. This is a substantial 16% increase in the estimated temporal range of archaeocetes compared to their observed temporal range (15.64/13.50 = 1.16), but it is a rather small 4% increase in the estimated temporal range of cetaceans as a whole (51.64/49.50 = 1.04). The fossil record of early cetaceans is not yet adequate to answer all questions we ask of it, but it is adequate to constrain the time of origin of Cetacea. Further, the early Eocene time of origin we calculate here is consistent with the very primitive transitionally-aquatic remains of early Eocene fossil cetaceans found in recent years.
The time of origin of archaeocetes calculated here takes nonuniform aspects of the distribution of potential fossils into account. The resulting estimate at ca. 51.64 Ma, is a little more than 2 m.y. before the first fossil archaeocetes appeared in the fossil record at ca. 49.50 Ma. This is a substantial 16% increase in the estimated temporal range of archaeocetes compared to their observed temporal range (15.64/13.50 = 1.16), but it is a rather small 4% increase in the estimated temporal range of cetaceans as a whole (51.64/49.50 = 1.04). The fossil record of early cetaceans is not yet adequate to answer all questions we ask of it, but it is adequate to constrain the time of origin of Cetacea. Further, the early Eocene time of origin we calculate here is consistent with the very primitive transitionally-aquatic remains of early Eocene fossil cetaceans found in recent years.
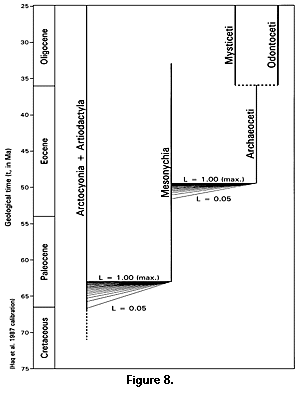 The temporal range of mesonychians, from at least 63 to 33 Ma (middle Paleocene through early Oligocene) and possibly from 66.7 Ma to about 33 Ma (latest Cretaceous through early Oligocene), precedes and overlaps the origin of archaeocetes in the early Eocene. The temporal range of arctocyonians plus artiodactyls, from at least 67 to 0 Ma (latest Cretaceous to the present), coincides with or overlaps the origin of mesonychians in the latest Cretaceous or early Paleocene. These temporal relationships, with times of origin of Archaeoceti and Mesonychia constrained to a range of reasonable likelihoods, are shown in Figure 8. Phylogenetic relationships shown in Figure 8 are the same as those outlined in a cladistic analysis by Geisler and O'Leary (1997). Mesonychia are monophyletic in the sense that all are thought to be descendants of a single common ancestor, but paraphyletic in the sense that some descendants (principally those in Cetacea) are not included.
The temporal range of mesonychians, from at least 63 to 33 Ma (middle Paleocene through early Oligocene) and possibly from 66.7 Ma to about 33 Ma (latest Cretaceous through early Oligocene), precedes and overlaps the origin of archaeocetes in the early Eocene. The temporal range of arctocyonians plus artiodactyls, from at least 67 to 0 Ma (latest Cretaceous to the present), coincides with or overlaps the origin of mesonychians in the latest Cretaceous or early Paleocene. These temporal relationships, with times of origin of Archaeoceti and Mesonychia constrained to a range of reasonable likelihoods, are shown in Figure 8. Phylogenetic relationships shown in Figure 8 are the same as those outlined in a cladistic analysis by Geisler and O'Leary (1997). Mesonychia are monophyletic in the sense that all are thought to be descendants of a single common ancestor, but paraphyletic in the sense that some descendants (principally those in Cetacea) are not included.
Claims that whales originated or whale ancestors separated from artiodactyls 80-90 million years ago, based on cladistic analyses (Novacek 1992; Archibald 1996) and/or molecular clocks (Hedges et al. 1996, Kumar and Hedges 1998), conflict with our results. It may be possible to shape the cladograms in question to conform more closely to the fossil record (since cladistic analyses are rarely constrained by geological time or the age of fossils in any case), but there appears to be no way to reconcile our results with early divergence times hypothesized from molecular studies. The many conflicting hypotheses of cetacean relationship to and within Artiodactyla in the current literature suggest that times of divergence based on present molecular 'clocks' cannot be taken seriously either. From the point of view of the fossil record and geological time scale, the idea that whales might be found in the mid-Cretaceous involves vanishingly small likelihoods (on the order of 1 to 10 in a billion). This does not prove that a mid-Cretaceous origin of whales is impossible, but shows the conjecture to be beyond reasonable expectation given what we know about the fossil record of whales (in the same way that the expectation of 28 heads and no tails is beyond reasonable expectation when a coin is tossed 28 times).
A distinction is sometimes drawn between the beginning of an estimated stratigraphic or temporal range and the 'true' time of origin of a taxon, and it is possible that there is a slight difference. However the geological time scale that is almost universally used as a context for discussion of animal history is itself based on the fossil record, and it necessarily takes account of whatever this slight difference might be, meaning, possibly, that the whole Phanerozoic time scale should be inflated by some small percentage. However, this would affect all times calibrated from geological evidence uniformly, it would affect all inferences concerning evolutionary time and rates uniformly, and the difference, consequently, is negligible.
It will undoubtedly be possible to discover and quantify additional factors influencing estimation of the time of origin of Cetacea, but these are not likely to add more than possibly another 0.5 to 1.0 m.y. to the estimate given here. There is a high probability that additional fossil whales will be found intermediate in time between known records, and there is a reasonable probability that the fossil record can be extended earlier than any record known at present. There is of course some chance that a fossil whale could be found at any time in the geological past, but this chance diminishes with predictable rapidity farther back in time. In Figure 5 we provide quantitative estimates of how likely we are to find fossil whales older than any known at present. The importance of earlier discoveries would be inversely proportional to such vanishing likelihoods (that is, of great importance), and we hope skeptics will channel their energy and attention into a search for earlier fossil whales.
ACKNOWLEDGMENTS
We thank P. M. Sadler and D. J. Strauss for helpful comments during development of this extension of their pioneering work on confidence intervals for stratigraphic ranges. Our results were first presented at the invitation of L. G. Barnes at the 1996 North American Paleontological Convention in Washington, D.C., and the paper was completed with support from National Science Foundation grant EAR-9714923. We thank N. MacLeod and two anonymous reviewers for reading and improving the manuscript.
REFERENCES
Albright, L. B. 1996. A protocetid cetacean from the Eocene of South Carolina. Journal of Paleontology, 70:519-523.
Andrews, C. W. 1906. A Descriptive Catalogue of the Tertiary Vertebrata of the Fayum, Egypt. British Museum (Natural History), London.
Andrews, C. W. 1919. A description of new species of zeuglodont and of leathery turtle from the Eocene of Southern Nigeria. Proceedings of the Zoological Society of London, 1919:309-319.
Archibald, J. D. 1996. Fossil evidence for a Late Cretaceous origin of 'hoofed' mammals. Science, 272:1150-1153.
Arnason, U., and A. Gullberg. 1996. Cytochrome b nucleotide sequences and the identification of five primary lineages of extant cetaceans. Molecular Biology and Evolution, 13:407-417.
Arnason, U., A. Gullberg, and B. Widegren. 1991. The complete nucleotide sequence of the mitochondrial DNA of the fin whale, Balaenoptera physalus. Journal of Molecular Evolution, 33:556- 568.
Ball, O. M. 1931. A contribution to the palaeobotany of the Eocene of Texas. Bulletin of the Agricultural and Mechanical College of Texas, 2(5):1-173.
Berggren, W. A., D. V. Kent, C. C. Swisher, M.-P. Aubry. 1995. A revised Cenozoic geochronology and chronostratigraphy. SEPM Special Publication, 54:129-212.
Berta, A. 1994. What is a whale? Science, 263:180-181.
Blanckenhorn, M. 1900. Neues zur Geologie und PalŠontologie Aegyptens. II. Das Palaeogen. Zeitschrift der Deutschen Geologischen Gesellschaft, 52:403-479.
Blatt, H., and R. L. Jones. 1975. Proportions of exposed igneous, metamorphic, and sedimentary rocks. Geological Society of America Bulletin, 86:1085-1088.
Borsuk-Bialynicka, M. 1988. New remains of Archaeoceti from the Paleogene of Antarctica. Polish Polar Research, 9:437-445.
Boule, M. 1903. Le Pachyaena de Vaugirard. Memoirs de la SociŽtŽ GŽologique de France, 28:5-16.
Bown, T. M. 1982. Geology, paleontology, and correlation of Eocene volcaniclastic rocks, southeast Absaroka range, Hot Springs County, Wyoming. U.S. Geological Survey Professional Paper, 1201A:1-75.
Boyden, A., and D. Gemeroy. 1950. The relative position of the Cetacea among the orders of Mammalia as indicated by precipitin tests. Zoologica, New York Zoological Society, 35:145-151.
Case, G. R. 1975. [Zeuglodont remains reported from Twiggs Clay Member, Barnwell Formation, central Georgia]. Society of Vertebrate Paleontology News Bulletin, 103:24.
Chow, M. 1965. Mesonychids from the Eocene of Honan. Vertebrata PalAsiatica, 9:286-291.
Chow, M., and T. Qi. 1978. Paleocene mammalian fossils from Nomogen Formation of Inner Mongolia. Vertebrata PalAsiatica, 16: 77-85.
Chow, M., Y. Chang, B. Wang, and S. Ting. 1973. New mammalian genera and species from the Paleocene of Nanhsiung, N. Kwangtung. Vertebrata PalAsiatica, 11:31-35.
Cooke, C. W., and H. K. Shearer. 1918. Deposits of Claiborne and Jackson age in Georgia. U. S. Geological Survey Professional Paper, 120:41-81.
Cope, E. D. 1872a. Descriptions of some new Vertebrata from the Bridger group of the Eocene. Paleontological Bulletin, Philadelphia, 1:1-6.
Cope, E. D. 1872b. Notices of new Vertebrata from the upper waters of Bitter Creek, Wyoming Territory. Paleontological Bulletin, Philadelphia, 6:1-6.
Cope, E. D. 1874. Report upon vertebrate fossils discovered in New Mexico, with descriptions of new species. Annual Report of the Chief of Engineers for 1874. Government Printing Office, Washington, 589-606.
Corgan, J. X. 1976. Vertebrate fossils of Tennessee. Tennessee Department of Conservation, Division of Geology, Bulletin, 77:1-100.
Crusafont Pair—, M., and J. M. Golpe-Posse. 1968. Dissacus progressus, nova sp., el primer creodonto de Espa–a. Boletin del Instituto Geologico y Minero de Espa–a, Madrid, 79(4):354-357.
Crusafont Pair—, M. 1973. Yacimientos del Eoceno prepirenaico (nuevas localidades del Cuisiense). Acta Geologica Hispanica, 8:145-147.
D'Erchia, A. M., C. Gissi, G. Presole, C. Saccone, and U. Arnason. 1996. The guinea-pig is not a rodent. Nature, 381:597- 600.
Daly, E. 1992. A list, bibliography, and index of the fossil vertebrates of Mississippi. Mississippi Department of Environmental Quality, Office of Geology, Bulletin, 128:1-47.
Dalrymple, G. B. 1991. The Age of the Earth. Stanford University Press, Stanford, 474 pp.
Dashzeveg, D. 1976. Novyye mezonikyidy (Condylarthra, Mesonychidae) iz paleogena Mongolii [New mesonychids (Condylarthra, Mesonychidae) from the early Paleogene of Mongolia]. In N. N. Kramarenko (ed.), Paleontologiya i Biostratigrapfiya Mongolii. Transactions of the Joint Soviet-Mongolian Paleontological Expedition, Moscow, 3:14-31.
Dehm, R., and T. z. Oettingen-Spielberg. 1958. PalŠontologische und geologische Untersuchungen im TertiŠr von Pakistan. 2. Die mitteleocŠnen SŠugetiere von Ganda Kas bei Basal in Nordwest- Pakistan. Abhandlungen der Bayerische Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Klasse, MŸnchen, Neue Folge, 91:1-54.
Dockery, D. T. 1974. An Archaeoceti from the Moodys Branch Formation (upper Eocene) of Mississippi. The Compass of Sigma Gappa Epsilon, 51:61-64.
Douzery, E. 1993. Evolutionary relationships among Cetacea based on the sequence of the mitochondrial 12S rRNA gene: possible paraphyly of toothed-whales (odontocetes) and long separate evolution of sperm whales (Physeteridae). Comptes Rendus de l'AcadŽmie des Sciences, Paris, Sciences de la Vie, 316: 1511-1518.
Edwards, A. W. F. 1972. Likelihood. Cambridge University Press, Cambridge.
ƒlouard, P. 1981. DŽcouverte d'un archŽocte dans les environs de Kaolack. Notes Africaines, Dakar, 109:8-10.
Flower, W. H. 1883. Cetacea. Encyclopedia Britannica, 9th edition, Edinburgh, 15:391-400.
Fordyce, R. E. 1985. Late Eocene archaeocete whale (Archaeoceti: Dorudontinae) from Waihao, South Canterbury, New Zealand. New Zealand Journal of Geology and Geophysics, 28:351- 357.
Fordyce, R. E., and L. G. Barnes. 1994. The evolutionary history of whales and dolphins. Annual Review of Earth and Planetary Sciences, 22:419-455.
Fraas, E. 1904. Neue Zeuglodonten aus dem unteren MitteleocŠn vom Mokattam bei Cairo. Geologische und PalŠontologische Abhandlungen, Jena, Neue Folge, 6:197-220.
Gabunia, L. K. 1982. A first find of a mesonychid in the Eocene of the Soviet Union [Russian with English summary]. Soobshcheniya Akademii Nauk Gruzinskoi SSR [Bulletin of the Academy of Sciences of the Georgian SSR], 106:525-528.
Gazin, C. L. 1952. The lower Eocene Knight Formation of western Wyoming and its mammalian fauna. Smithsonian Miscellaneous Collections, 117:1-82.
Geisler, J. H., and M. A. O'Leary. 1997. A phylogeny of Cetacea, Artiodactyla, Perissodactyla, and archaic ungulates: the morphological evidence (abstract). Journal of Vertebrate Paleontology, Abstracts of Papers, 17(3):48.
Gimbrede, L. d. A. 1962. The Hurricane Lentil: source of many Claiborne fossils. Journal of Paleontology, 36:1116-1120.
Gingerich, P. D. 1991. Partial skeleton of a new archaeocete from the earliest middle Eocene Habib Rahi limestone, Pakistan (abstract). Journal of Vertebrate Paleontology, Abstracts of Papers, 11(3):31.
Gingerich, P. D. 1992. Marine mammals (Cetacea and Sirenia) from the Eocene of Gebel Mokattam and Fayum, Egypt: stratigraphy, age, and paleoenvironments. University of Michigan Papers on Paleontology, 30:1-84.
Gingerich, P. D., M. Arif, and W. C. Clyde. 1995a. New archaeocetes (Mammalia, Cetacea) from the middle Eocene Domanda Formation of the Sulaiman Range, Punjab (Pakistan). Contributions from the Museum of Paleontology, University of Michigan, 29:291-330.
Gingerich, P. D., M. Arif, M. A. Bhatti, H. A. Raza, and S. M. Raza. 1995b. Protosiren and Babiacetus (Mammalia, Sirenia and Cetacea) from the middle Eocene Drazinda Formation, Sulaiman Range, Punjab (Pakistan). Contributions from the Museum of Paleontology, University of Michigan, 29:331-357.
Gingerich, P. D., H. Cappetta, and M. Traverse. 1992. Marine mammals (Cetacea and Sirenia) from the middle Eocene of KpogamŽ- HahotoŽ in Togo (abstract). Journal of Vertebrate Paleontology, 12A:29-30.
Gingerich, P. D., S. M. Raza, M. Arif, M. Anwar, and X. Zhou. 1994. New whale from the Eocene of Pakistan and the origin of cetacean swimming. Nature, 368:844-847.
Gingerich, P. D., and D. E. Russell. 1981. Pakicetus inachus, a new archaeocete (Mammalia, Cetacea) from the early-middle Eocene Kuldana Formation of Kohat (Pakistan). Contributions from the Museum of Paleontology, University of Michigan, 25:235-246.
Gingerich, P. D., B. H. Smith, and E. L. Simons. 1990. Hind limbs of Eocene Basilosaurus: evidence of feet in whales. Science, 249:154-157.
Gingerich, P. D., and M. D. Uhen. 1994. Time of origin of primates. Journal of Human Evolution, 27:443-445.
Gingerich, P. D., N. A. Wells, D. E. Russell, and S. M. I. Shah. 1983. Origin of whales in epicontinental remnant seas: new evidence from the early Eocene of Pakistan. Science, 220:403-406.
Gish, D. T. 1994. When is a whale a whale? Impact, El Cajon, California, 250:i-iv.
Godinot, M., J. Crochet, J. Hartenberger, B. Lange-BadrŽ, D. E. Russell, and B. SigŽ. 1987. Nouvelles donnŽes sur les mammifres de Palette (Eocne infŽrieur, Provence). MŸnchner Geowissenschaftliche Abhandlungen, Reihe A, Geologie und PalŠontologie, 10:273-288.
Goodman, M., J. Czelusniak, and J. E. Beeber. 1985. Phylogeny of primates and other eutherian orders: a cladistic analysis using amino acid and nucleotide sequence data. Cladistics, 1:171-185.
Gradstein, F. M., and J. Ogg. 1996. A Phanerozoic time scale. Episodes, 19:3-5.
Granger, W. 1917. Notes on Paleocene and lower Eocene mammal horizons of northern New Mexico and southern Colorado. Bulletin of the American Museum of Natural History, 37:821-830.
Graur, D. 1993. Molecular phylogeny and the higher classification of eutherian mammals. Trends in Ecology and Evolution, 8:141-147.
Graur, D., and D. G. Higgins. 1994. Molecular evidence for the inclusion of cetaceans within the order Artiodactyla. Molecular Biology and Evolution, 11:357-364.
Gromova, V. 1952. O primitivnykh khishchnikakh iz paelogena Mongolii i Kazakhstana [On primitive carnivores of the Paleogene of Mongolia and Kazakhstan]. Akademiia Nauk SSSR, Trudy Paleontologicheskii Institut, Moscow, 41:51-77.
Gustafson, E. P. 1986. Carnivorous mammals of the late Eocene and early Oligocene of trans-Pecos Texas. Texas Memorial Museum Bulletin, 33:1-66.
Haq, B., J. Hardenbol, and P. R. Vail. 1987. Chronology of fluctuating sea levels since the Triassic. Science, 235:1156-1167.
Hardenbol, J. A., J. Thierry, M. B. Farley, T. Jacquin, P.-C. Graciansky, and P. R. Vail. 1998. Mesozoic-Cenozoic sequence chronostratigraphic framework. In P.-C. Graciansky, J. A. Hardenbol, T. Jacquin, P. R. Vail, and M. B. Farley (eds.), Sequence Stratigraphy of European Basins. Society of Economic Paleontologists and Mineralogists Special Publication, in press.
Hedges, S. B., P. H. Parker, C. G. Sibley, and S. Kumar. 1996. Continental breakup and the ordinal diversification of birds and mammals. Nature, 381:226-229.
Hooker, J. J., A. N. Insole, R. T. J. Moody, C. A. Walker, and D. J. Ward. 1980. The distribution of Cartilaginous fish, turtles, birds and mammals in the British Palaeogene. Tertiary Research, Leiden, 3:1-45.
Irwin, D. M., and U. Arnason. 1994. Cytochrome b gene of marine mammals: phylogeny and evolution. Journal of Mammalian Evolution, 2:37-55.
Irwin, D. M., T. D. Kocher, and A. C. Wilson. 1991. Evolution of the cytochrome b gene of mammals. Journal of Molecular Evolution, 32:128-144.
Kellogg, R. 1936. A review of the Archaeoceti. Carnegie Institution of Washington Publications, 482:1-366.
Kihm, A. J. 1984. Early Eocene mammalian faunas of the Piceance Creek Basin, northwestern Colorado. Ph.D. dissertation, University of Colorado, Boulder, pp. 1-381.
Krettek, A., A. Gullberg, and U. Arnason. 1995. Sequence analysis of the complete mitochondrial DNA molecule of the hedgehog, Erinaceus europaeus, and the phylogenetic position of the Lipotyphla. Journal of Molecular Evolution, 41:952-957.
Kuhn, O. 1935. ArchŠoceten aus dem norddeutschen AlttertiŠr. Zentralblatt fŸr Mineralogie, Geologie und PalŠontologie, Abteilung B, Stuttgart, 1935:219-226.
Kumar, S., and S. B. Hedges. 1998. A molecular timescale for vertebrate evolution. Nature, 392:917-920.
Kumar, K., and A. Sahni. 1985. Eocene mammals from the upper Subathu group, Kashmir Himalaya, India. Journal of Vertebrate Paleontology, 5:153-168.
Kumar, K., and A. Sahni. 1986. Remingtonocetus harudiensis, new combination, a middle Eocene archaeocete (Mammalia, Cetacea) from western Kutch, India. Journal of Vertebrate Paleontology, 6:326-349.
Lancaster, W. C. 1986. The taphonomy of an archaeocete skeleton and its associated fauna. In J. A. Schiebout and W. van den Bold (eds.), Montgomery Landing Site, Marine Eocene (Jackson) of Central Louisiana (Proceedings of a Symposium, Gulf Coast Association of Geological Societies). Louisiana State University Museum of Geosciences, Baton Rouge, 119-131.
Lemoine, V. 1891. ƒtude d'ensemble sur les dents des mammifres fossiles des environs de Reims. Bulletin de la SociŽtŽ GŽologique de France, 19:263-290.
Maher, J. C., and P. H. Jones. 1949. Ground-water Exploration in the Natchitoches area, Louisiana. U. S. Geological Survey Water-Supply Paper, 968D:159-211.
Marshall, C. R. 1990. Confidence intervals on stratigraphic ranges. Paleobiology, 16:1-10.
Matthew, W. D. 1909. The Carnivora and Insectivora of the Bridger Basin, Middle Eocene. Memoirs of the American Museum of Natural History, 9:289-567.
Matthew, W. D., and W. Granger. 1925. New mammals from the Irdin Manha Eocene of Mongolia. American Museum Novitates, 198:1-10.
McKenna, M. C. 1960. Fossil Mammalia from the early Wasatchian Four Mile fauna, Eocene of northwest Colorado. University of California Publications in Geological Sciences, 37:1-130.
McKenna, M. C. 1987. Molecular and morphological analysis of high-level mammalian interrelationships. In C. Patterson (ed.), Molecules and Morphology in Evolution: Conflict or Compromise?, Cambridge University Press, Cambridge, 55-93.
Messenger, S. L., and J. A. McGuire. 1998. Morphology, molecules, and the phylogenetics of cetaceans. Systematic Biology, 47:90-124.
Milinkovitch, M. C. 1992. DNA-DNA hybridizations support ungulate ancestry of Cetacea. Journal of Evolutionary Biology, 5: 149-160.
Milinkovitch, M. C. 1995. Molecular phylogeny of cetaceans prompts revision of morphological transformations. Trends in Ecology and Evolution, 10:328-334.
Milinkovitch, M. C., A. Meyer, and J. R. Powell. 1994. Phylogeny of all major groups of cetaceans based on DNA sequences from three mitochondrial genes. Molecular Biology and Evolution, 11:939-948.
Milinkovitch, M. C., G. Ort’, and A. Meyer. 1993. Revised phylogeny of whales suggested by mitochondrial ribosomal DNA sequences. Nature, 361:346-348.
Milinkovitch, M. C. 1995. Novel phylogeny of whales revisited but not revised. Molecular Biology and Evolution, 12:518-520.
Morgan, G. S. 1978. The fossil whales of Florida. Plaster Jacket, 29:1-20.
Novacek, M. J. 1992. Mammalian phylogeny: shaking the tree. Nature, London, 356:121-125.
Novacek, M. J., I. Ferrusqu’a-Villafranca, J. J. Flynn, A. R. Wyss, and M. Norell. 1991. Wasatchian (early Eocene) mammals and other vertebrates from Baja California, Mexico: the Lomas las Tetas de Cabra fauna. Bulletin of the American Museum of Natural History, 208:1-88.
O'Leary, M. A., and K. D. Rose. 1995. Postcranial skeleton of the early Eocene mesonychid Pachyaena (Mammalia: Mesonychia). Journal of Vertebrate Paleontology, 15:401-430.
Ohland, D. P., E. H. Harley, and P. B. Best. 1995. Systematics of cetaceans using restriction site mapping of mitochondrial DNA. Molecular Phylogenetics and Evolution, 4:10-19.
Osborn, H. F. 1895. Fossil mammals of the Uinta Basin. Expedition of 1894. Bulletin of the American Museum of Natural History, 7:71-105.
Osborn, H. F., and C. Earle. 1895. Fossil mammals of the Puerco beds. Collection of 1892. Bulletin of the American Museum of Natural History, 7:1-70.
Osborn, H. F., and J. L. Wortman. 1892. Fossil mammals of the Wahsatch and Wind River beds. Collection of 1891. Bulletin of the American Museum of Natural History, 4:81-147.
Palmer, K. V. W. 1939. Basilosaurus in Arkansas. Bulletin of the American Association of Petroleum Geologists, 23:1228-1229.
Peterson, O. A. 1931. New mesonychids from the Uinta. Annals of Carnegie Museum, 20:333-340.
Petkewich, R. M., and W. C. Lancaster. 1984. Middle Eocene archaeocete whales from the McBean Formation of Burke County, Georgia (abstract). Georgia Journal of Science, 42:21.
Pilgrim, G. E. 1940. Middle Eocene mammals from north-west India. Proceedings of the Zoological Society of London, Series B, 110:127-152.
Pilleri, G. 1989. An archaeocete vertebra from the upper Eocene of Taradell, Catalonia, Spain. In Giorgio Pilleri (ed.), Contributions to the Paleontology of some Tethyan Cetacea and Sirenia (Mammalia), II. Brain Anatomy Institute, University of Berne, Ostermundingen, Switzerland, 7-12.
Pilleri, G., and F. Cigala Fulgosi. 1989. First Archaeoceti record from the Eocene of Italy (Varano, northern Apennines). In Giorgio Pilleri (ed.), Contributions to the Paleontology of some Tethyan Cetacea and Sirenia (Mammalia), II. Brain Anatomy Institute, University of Berne, Ostermundingen, Switzerland, 87-102.
Qi, T. 1975. An early Oligocene mammalian fauna of Ningxia. Vertebrata PalAsiatica, 13:217-224.
Qi, T.. 1987. The middle Eocene Arshanto fauna (Mammalia) of Inner Mongolia. Annals of Carnegie Museum, 56:1-73.
Qi, T., and X. Huang. 1982. A mesonychid (Mammalia) skull from the Paleocene of Laonan, Shaanxi. Vertebrata PalAsiatica, 20:18-25.
Ranga Rao, A. 1973. Notices of two new mammals from the upper Eocene Kalakot beds, India. Special Paper of the Directorate of Geology, Oil and Natural Gas Commission, Dehra Dun, 2:1-6.
Robinson, P. 1966. Fossil Mammalia of the Huerfano Formation, Eocene, of Colorado. Yale University Peabody Museum of Natural History Bulletin, 21:1-95.
Rose, K. D. 1981. The Clarkforkian land-mammal age and mammalian faunal composition across the Paleocene-Eocene boundary. University of Michigan Papers on Paleontology, 26:1-197.
Rose, K. D. 1996. On the origin of the order Artiodactyla. Proceedings of the National Academy of Sciences USA, 93:1705-1709.
Russell, D. E. 1982. Tetrapods of the northwest European Tertiary basin. Geologisches Jahrbuch, Reihe A, 60:5-74.
Russell, D. E., and R. Zhai. 1987. The Paleogene of Asia: mammals and stratigraphy. MŽmoires du MusŽum National d'Histoire Naturelle, Sciences de la Terre, 52:1-488.
Sanders, A. E. 1974. A paleontological survey of the Cooper Marl and Santee Limestone near Harleyville, South Carolina (preliminary report). Geologic Notes, South Carolina State Development Board, Division of Geology, 18:4-12.
Sarich, V. M. 1993. Mammalian systematics: twenty-five years among their albumins and transferrins. In F. S. Szalay, M. J. Novacek, and M. C. McKenna (eds.), Mammal Phylogeny: Placentals. Springer-Verlag, New York, 103-114.
Shikama, T. 1943. A new Eocene creodont from the H™san coal-mine, Ty™sen. Bulletin of the Biogeographical Society of Japan, 13:7-11.
Simpson, G. G. 1929. A collection of Paleocene mammals from Bear Creek, Montana. Annals of Carnegie Museum, 19:115-122.
Simpson, G. G. 1937. Fort Union of Crazy Mountain field, Montana, and its mammalian faunas. Bulletin of the United States National Museum, 169:1-287.
Simpson, G. G. 1945. The principles of classification and a classification of mammals. Bulletin of the American Museum of Natural History, 85:1-350.
Stehlin, H. G. 1926. Une espce lutŽtienne de Dissacus. Bulletin de la SociŽtŽ GŽologique de France, SŽrie 4, 26:185-189.
Strauss, D., and P. M. Sadler. 1989. Classical confidence intervals and Bayesian probability estimates for ends of local taxon ranges. Mathematical Geology, 21:411-427.
Stromer von Reichenbach, E. 1903. Bericht Ÿber eine von den Privatdozenten Dr. Max Blanckenhorn und Dr. Ernst Stromer von Reichenbach ausgefŸhrte Reise nach Aegypten. Einleitung. Ein SchŠdel und Unterkiefer von Zeuglodon osiris Dames. Sitzungsberichte der Mathematisch-physikalischen Classe der Kšniglichen Bayerischen Akademie der Wissenschaften, MŸnchen, 32: 341-352.
Szalay, F. S., and S. J. Gould. 1966. Asiatic Mesonychidae (Mammalia, Condylarthra). Bulletin of the American Museum of Natural History, 132:127-174.
Szalay, F. S., and M. C. McKenna. 1971. Beginning of the age of mammals in Asia: the late Paleocene Gashato fauna, Mongolia. Bulletin of the American Museum of Natural History, 144:269-318.
Thewissen, J. G. M. 1994. Phylogenetic aspects of cetacean origins: a morphological perspective. Journal of Mammalian Evolution, 2:157-184.
Thewissen, J. G. M., and S. T. Hussain. 1993. Origin of underwater hearing in whales. Nature, 361:444-445.
Thewissen, J. G. M., S. T. Hussain, and M. Arif. 1994. Fossil evidence for the origin of aquatic locomotion in archaeocete whales. Science, 263:210-212.
Ting, S., and C. Li. 1987. The skull of Hapalodectes (?Acreodi, Mammalia), with notes on some Chinese Paleocene mesonychids. Vertebrata PalAsiatica, 25:161-186.
Uhen, M. D. 1996. Dorudon atrox (Mammalia, Cetacea): form, function, and phylogenetic relationships of an archaeocete from the late mdidle Eocene of Egypt. Ph.D. dissertation, University of Michigan, 608 pp.
Van Valen, L. M. 1966. Deltatheridia, a new order of mammals. Bulletin of the American Museum of Natural History, 132:1-126.
Van Valen, L. M. 1969. The multiple origins of the placental carnivores. Evolution, 23:118-130.
Van Valen, L. M. 1971. Toward the origin of artiodactyls. Evolution, 25: 523-529.
Van Valen, L. M. 1978. The beginning of the age of mammals. Evolutionary Theory, 4:45-80.
Wang, B. 1975. Paleocene mammals of Chaling Basin, Hunan. Vertebrata PalAsiatica, 13:154-162.
Wang, B. 1976. Late Paleocene mesonychids from Nanxiong Basin, Guangdong. Vertebrata PalAsiatica, 14:259-262.
West, R. M. 1980. Middle Eocene large mammal assemblage with Tethyan affinities, Ganda Kas region, Pakistan. Journal of Paleontology, 54:508-533.
Westgate, J. W. 1994. Paleoecology of an Eocene coastal community from Georgia (abstract). Journal of Vertebrate Paleontology, 14A:52.
Xu, Y., D. Yan, S. Zhou, S. Han, and Y. Zhong. 1979. Subdivision of the red beds of Li-Guan-Qiao Basin with description of fossil mammals therefrom. Mesozoic and Cenozoic Red Beds of South China. Academia Sinica, Science Press, Beijing, 416-432.
Yan, D., and Y. Tang. 1976. Mesonychids from the Paleocene of Anhui. Vertebrata PalAsiatica, 14:252-285.
Zhang, Y., Y. You, H. Ji, and S. Ding. 1978. Cenozoic stratigraphy of Yunnan. Professional Papers on Stratigraphy and Paleontology, Beijing, 7:1-21.
Zhang, Y., J. Zheng, and S. Ding. 1979. Description of some condylarths from the Paleocene of Jiangxi. Mesozoic and Cenozoic Red Beds of South China. Academia Sinica, Science Press, Beijing, 382-386.
Zheng, J., Y. Tang, R. Zhai, S. Ding, and X. Huang. 1978. Early Tertiary strata of Lunan Basin, Yunnan. Professional Papers on Stratigraphy and Paleontology, Beijing, 7:22-29.
Zhou, X. 1995. Evolution of Paleocene-Eocene Mesonychidae (Mammalia, Mesonychia). Ph.D. dissertation, University of Michigan, Ann Arbor, 1-402.
Zhou, X., W. J. Sanders, and P. D. Gingerich. 1992. Functional and behavioral implications of vertebral structure in Pachyaena ossifraga (Mammalia, Mesonychia). Contributions from the Museum of Paleontology, University of Michigan, 28:289-319.
Zhou, X., R. Zhai, P. D. Gingerich, and L. Chen. 1995. Skull of a new mesonychid (Mammalia, Mesonychia) from the late Paleocene of China. Journal of Vertebrate Paleontology, 15:387-400.
Zuckerkandl, E., and L. Pauling. 1962. Molecular disease, evolution, and genic heterogeneity. In M. Kasha and B. Pullman (eds.), Horizons in Biochemistry, Academic Press, New York, 189-225.
Zuckerkandl, E., and L. Pauling. 1965. Evolutionary divergence and convergence in proteins. In V. Bryson and H. J. Vogel (eds.), Evolving Genes and Proteins, Academic Press, New York, 97-166.

