 Dental ecomorphology and macroevolutionary patterns of North American Late Cretaceous metatherians
Dental ecomorphology and macroevolutionary patterns of North American Late Cretaceous metatherians
Article number: 26.3.a48
https://doi.org/10.26879/1177
Copyright Paleontological Society, November 2023
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 6 July 2021. Acceptance: 30 October 2023.
ABSTRACT
Metatherian mammals were taxonomically rich and abundant in Late Cretaceous faunas of North America. Although much attention has been paid to metatherian taxonomy, a comprehensive, quantitative study on the ecomorphology of this clade is lacking. Here, we predict the diets of a large sample of metatherians using three-dimensional dental topographic analysis, with the aim to better understand macroevolutionary patterns in dental morphology and dietary diversity. Contrary to their taxonomic diversity patterns, our results show that dental disparity and dietary diversity did not significantly change throughout the Late Cretaceous and that most metatherians were invertivorous (diets of insects and soft-bodied invertebrates). Nevertheless, we also found that metatherians occupied a wide range of dietary niches and were arguably the most dietarily diverse of any mammalian clade of the Late Cretaceous. Regarding the timing of metatherian ecomorphological diversification, our results indicate that it began by the mid-Cretaceous in-step with the Cretaceous Terrestrial Revolution and the taxonomic diversification of angiosperms, prior to the ecological diversifications of multituberculates and eutherians that began in the latest Cretaceous and Paleocene.
Alexandria L. Brannick. Department of Biology, University of Washington, Seattle, Washington, 98195-1800, USA. alexbrannick@gmail.com
Henry Z. Fulghum. Committee on Evolutionary Biology, University of Chicago, Chicago, Illinois, 60637, USA. hzf@uchicago.edu
David M. Grossnickle. Oregon Institute of Technology, Natural Sciences Department, Klamath Falls, Oregon, 97601, USA. david.grossnickle@oit.edu
Gregory P. Wilson Mantilla. Department of Biology, University of Washington, Seattle, Washington, 98195-1800, USA and Burke Museum of Natural History and Culture, Seattle, Washington, 98195-1800, USA. Corresponding author. gpwilson@uw.edu
Keywords: Metatheria; Cretaceous; ecomorphology; dental topographic analysis
Final citation: Brannick, Alexandria L., Fulghum, Henry Z., Grossnickle, David M., and Wilson Mantilla, Gregory P. 2023. Dental ecomorphology and macroevolutionary patterns of North American Late Cretaceous metatherians. Palaeontologia Electronica, 26(3):a48.
https://doi.org/10.26879/1177
palaeo-electronica.org/content/2023/4007-metatherian-ecomorphology
Copyright: November 2023 Paleontological Society
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Metatherian mammals (the stem-based clade of extant marsupials and their closest relatives; e.g., Rougier et al., 1998) were evolutionarily successful during the Late Cretaceous (ca. 100-66 million years ago [Ma]). They were geographically widespread, occupying all northern landmasses and possibly some southern ones (Rougier et al., 1998; Krause, 2001; Kielan-Jaworowska et al., 2004; Martin et al., 2005; Vullo et al., 2009; Averianov et al., 2010; Williamson et al., 2014; Goin et al., 2016). Late Cretaceous metatherians were also numerically abundant, making up as much as 45% of all mammalian fossil individuals within local faunas (e.g., Cifelli, 2004; Wilson, 2014), and were taxonomically rich (Bennett et al., 2018) – at least 68 species are known worldwide from the Late Cretaceous (Williamson et al., 2014). It has been hypothesized that during the early Late Cretaceous (Albian-Cenomanian-late Santonian; 100-85 Ma), metatherians underwent a taxonomic radiation that led to at least five major lineages (Glasbiidae, Pediomyidae, Alphadontidae, Stagodontidae, and Marsupialia; Clemens, 1966; Davis, 2007; Johanson, 1996; Wilson et al., 2016; see Benson et al., 2013; Newham et al., 2014; Grossnickle and Newham, 2016; and Bennett et al., 2018 for discussion on the possible effect of fossil sampling on mammalian taxonomic diversity patterns in the Late Cretaceous). Despite theoretical models and empirical data that indicate ecomorphological diversification often accompanies taxonomic radiation (e.g., Rabosky and Adams, 2012; Ramírez-Barahona et al., 2016), this pattern has never been explicitly demonstrated for Late Cretaceous metatherians.
Several studies, mostly qualitative in nature and focused on individual taxa, highlight ecomorphological diversity among Late Cretaceous metatherians. The postcranial fossil record of these taxa is sparse, and therefore few studies have measured their locomotor diversity and substrate use (Szalay, 1994; Szalay and Trofimov, 1996; Szalay and Sargis, 2006; DeBey and Wilson, 2017); instead, most studies have used the abundant craniodental fossil record to reconstruct feeding ecology. For example, the highly distinctive, broad-basined, bunodont molars of Glasbius have prompted interpretations that it was frugivorous (e.g., Clemens, 1966, 1979); the large, bulbous premolars of Didelphodon and its broad-basined molars with enhanced shearing facets and robust skull morphology have led to inferences of carnivory, omnivory, and durophagy (Clemens, 1966, 1968, 1979; Fox and Naylor, 1986, 2006); and the elongated, buccolingually compressed molars of Nanocuris with their exaggerated postvallum-prevallid shearing crest and a reduced talonid indicate adaptation to carnivory (Fox et al., 2007; Wilson and Riedel, 2010).
Other studies have taken more quantitative approaches to investigating metatherian dental ecomorphology. Gordon (2003) used three-dimensional (3D) geometric morphometrics and shearing-crest measurements to examine the dietary morphospace of 10 fossil mammal species (five metatherian species) in relation to a sample of extant mammals; she found that most metatherians and eutherians from the Lancian North American land mammal ‘age’ (NALMA; ca. 69–66 Ma; Woodburne, 2004) largely overlapped in morphospace with extant insectivores (i.e., invertivores). Using 2D geometric morphometrics, Wilson (2013) mapped morphospace occupation and quantified the morphological disparity of mammalian (including metatherian) teeth immediately before and after the Cretaceous-Paleogene (K-Pg) boundary. He found that metatherians exploited a wide range of body sizes and feeding ecologies in the Lancian but that local extinction contributed to a loss in ecological endmembers within the Hell Creek mammalian fauna (Wilson, 2013). Grossnickle and Newham (2016) took a more synoptic approach by investigating dental morphological disparity through time on a global scale and found that metatherian disparity increased throughout the Late Cretaceous. Nonetheless, studies utilizing 3D morphological data in concert with broader taxonomic sampling of Metatheria within a phylogenetic context are lacking.
Here, we quantify the ecomorphological diversity of metatherians through the Late Cretaceous, using the densely sampled North American dental fossil record (Cifelli et al., 2004; Williamson et al., 2014). We (i) apply dental topographic analyses (e.g., Boyer, 2008; Pampush et al., 2016; López- Torres et al., 2017) to upper molars of 42 species of Late Cretaceous metatherians; (ii) map and quantify the resulting dental morphological diversity and disparity of these metatherians by time bin and taxonomic family; (iii) infer diet in these fossil taxa, by comparing their dental topographic values to those of 30 extant mammalian species with known diets; and (iv) evaluate the resulting patterns of ecomorphological diversity and disparity through time relative to corresponding patterns of taxonomic richness and to possible evolutionary drivers, such as the Cretaceous Terrestrial Revolution (KTR; Lloyd et al., 2008), the ecological rise of angiosperms (e.g., Wing and Boucher, 1998), and the Cretaceous-Paleogene mass extinction (e.g., Simpson, 1937).
BACKGROUND
Two hypotheses provide a framework to discuss the timing of metatherian taxonomic and ecomorphological diversification (see Grossnickle et al., 2019 for review). “The Early Rise Hypothesis,” coined by Grossnickle et al. (2019), is related to the ecological radiation of crown-group angiosperms (flowering plants), which began after the KTR (ca. 85-80 Ma). Angiosperms experienced a taxonomic radiation during the KTR (125-80 Ma; Wing and Boucher, 1998; Anderson et al., 2005; Magallón et al., 2013; Magallón et al., 2015), but the Early Rise Hypothesis is more closely linked to the post-KTR ecological (not taxonomic) rise of angiosperms (beginning by ca. 85-80 Ma), which may have been a more critical driver of increases in mammalian diversity (Meredith et al., 2011; Eriksson, 2016). The Early Rise Hypothesis is supported by macroevolutionary patterns of some mammal groups (Wilson et al., 2012; Grossnickle and Polly, 2013; Grossnickle and Newham, 2016; Chen et al., 2019; Grossnickle et al., 2019); for example, multituberculates increased both their taxonomic and ecomorphological diversity during the late Late Cretaceous (ca. 83-66 Ma; Wilson et al. 2012). The angiosperm radiation likely spurred co-evolution and diversification of insects (Grimaldi, 1999) and provided novel food sources–such as new fruits and pollinating insects–for mammals. Angiosperms also evolved to provide a complex canopy structure by the Late Cretaceous or early Paleogene (Wing and Boucher, 1998; Crifò et al., 2014), allowing for more arboreal lifestyles among mammals (Chen et al., 2019). Thus, under the Early Rise Hypothesis, we predict that beginning in the late Late Cretaceous metatherians increased both the disparity of their dental morphologies (magnitude of morphological differences) and the diversity of their diets (number of dietary categories).
Alternatively, the downstream effects of the KTR might have manifested among non-therian mammals only (e.g., multituberculates; Wilson et al., 2012), and despite the increased diversity of food resources and novel evolutionary adaptations of tribosphenic molars (e.g., increased grinding capabilities), therians were ecomorphologically constrained until the extinction of non-avian dinosaurs (e.g., Simpson, 1937; Van Valen and Sloan, 1977; Archibald, 1983, 2011; Stucky, 1990; Alroy, 1999; Grossnickle et al., 2019). This hypothesis, called “the Suppression Hypothesis” (Grossnickle and Newham, 2016), is supported by evidence of sharp increases in origination rates (Alroy, 1999), body size (Alroy, 1999; Smith et al. 2010), and morphological disparity (Halliday and Goswami, 2016) in early Cenozoic mammalian faunas. For this hypothesis, we predict that throughout the Late Cretaceous the metatherian dental disparity and dietary diversity remained low and stable, or only increased very gradually with time.
METHODS
Previous Methodological Approaches
Diet is a critical component of an animal’s ecology and informs trophic relationships within ecosystems (e.g., Pineda-Munoz et al., 2016). Tooth shape correlates with diet (e.g., Kay, 1975; Boyer, 2008; Bunn and Ungar, 2009; Ungar, 2010; Evans, 2013), and a variety of methods have been developed to investigate this relationship. Here, we use dental topographic analysis (DTA; López-Torres et al., 2017) to quantify the shape of three-dimensional models of entire tooth crown surfaces. Our application of DTA encompasses three metrics–relief index (RFI; Ungar and M’Kirera, 2003; Boyer, 2008), Dirichlet normal energy (DNE; Bunn et al., 2011; Winchester, 2016), and orientation patch count rotated (OPCR; Evans et al., 2007; Evans and Jernvall, 2009), all of which have been shown to correlate with diet in extant mammals. Much of the research that has applied these dental topographic measures has focused on placental mammals, mainly Primates (e.g., Boyer, 2008; Boyer et al., 2010; Bunn et al., 2011; Winchester et al., 2014; Pampush et al., 2016; López- Torres et al., 2017), but also carnivorans (Evans et al., 2007; Evans and Jernvall, 2009), bats (Santana et al., 2011), rodents (Evans et al., 2007; Evans and Jernvall, 2009; Prufrock et al., 2016; Spradley, 2017), and other euarchontans (Boyer, 2008; Selig et al., 2019). Dietary interpretations of some fossil taxa, including multituberculates (Wilson et al., 2012) and meridiolestidans (Harper et al., 2018), have also been proposed using DTA. Metatherians (including marsupials) have been undersampled and understudied in DTA studies (but see Smits and Evans, 2012; Spradley, 2017; Smith, 2017); nevertheless, studies have shown that dental topographic metrics also correlate with diet in metatherians in a way that is consistent with the patterns seen in primates and other placentals (e.g., frugivores have lower DNE values than both folivores and invertivores do; Smith, 2017; Spradley, 2017).
We acknowledge that many abbreviations are used throughout this text; please see Table 1 for a complete list of abbreviations used.
Extant Mammal Sampling
 To provide a modern analog, we collected dental surface data for 56 upper molar specimens (and two upper fourth premolars of Lynx rufus and Crocuta crocuta) representing eight taxonomic orders, 27 genera, and 30 species of extant marsupials and placentals (Table 2; Figure 1). The species in our dataset were selected to provide diverse representation of dental morphology, diet, and phylogeny. We sampled only adult specimens and, whenever possible, both male and female specimens from each species. Although we tried to avoid sampling specimens of captive individuals, three species in our sample are represented by captive individuals because those were the only available specimens (see Appendix 3 Appendix 3). Any effects of captive diets on tooth morphology should be minimal because we selected teeth with little to no wear (see below). Because we are interested in the dental morphology and diet of Late Cretaceous metatherians with tribosphenic molars, extant mammals with derived dental morphology or dental formulae were not considered for our extant sample (i.e., homodont dentitions, enamel-less teeth) as we assumed these morphologies would be less informative for our fossil sample. Because most fossil metatherians in our sample were also relatively small (≤ 5 kg), we primarily sampled small-bodied, extant mammals (all but six species are ≤ 5 kg) to minimize potential biases related to differences in body sizes. We did not include folivores in our extant sample because small-bodied, folivorous mammals typically have extremely derived teeth (e.g., Phloeomys, the giant cloud rat); moreover, body-mass estimates for almost all of the fossil metatherian taxa in our sample are below what is considered the physiologically derived minimum body-size threshold for folivory (Kay, 1975, 1984). We included placental mammals in our sample to increase both the sample size and range of diets (Smith, 2017) and to form an extant phylogenetic bracket (Witmer, 1995) around our sample of fossil metatherians. Although placentals and marsupials possess dental morphological differences, the metrics used in our DTA are homology-free and based on overall crown shape (e.g., Evans et al., 2007; Boyer, 2008; Bunn et al., 2011; Evans, 2013; Berthaume et al., 2019), so including placental mammals in our extant sample should not negatively impact our interpretations.
To provide a modern analog, we collected dental surface data for 56 upper molar specimens (and two upper fourth premolars of Lynx rufus and Crocuta crocuta) representing eight taxonomic orders, 27 genera, and 30 species of extant marsupials and placentals (Table 2; Figure 1). The species in our dataset were selected to provide diverse representation of dental morphology, diet, and phylogeny. We sampled only adult specimens and, whenever possible, both male and female specimens from each species. Although we tried to avoid sampling specimens of captive individuals, three species in our sample are represented by captive individuals because those were the only available specimens (see Appendix 3 Appendix 3). Any effects of captive diets on tooth morphology should be minimal because we selected teeth with little to no wear (see below). Because we are interested in the dental morphology and diet of Late Cretaceous metatherians with tribosphenic molars, extant mammals with derived dental morphology or dental formulae were not considered for our extant sample (i.e., homodont dentitions, enamel-less teeth) as we assumed these morphologies would be less informative for our fossil sample. Because most fossil metatherians in our sample were also relatively small (≤ 5 kg), we primarily sampled small-bodied, extant mammals (all but six species are ≤ 5 kg) to minimize potential biases related to differences in body sizes. We did not include folivores in our extant sample because small-bodied, folivorous mammals typically have extremely derived teeth (e.g., Phloeomys, the giant cloud rat); moreover, body-mass estimates for almost all of the fossil metatherian taxa in our sample are below what is considered the physiologically derived minimum body-size threshold for folivory (Kay, 1975, 1984). We included placental mammals in our sample to increase both the sample size and range of diets (Smith, 2017) and to form an extant phylogenetic bracket (Witmer, 1995) around our sample of fossil metatherians. Although placentals and marsupials possess dental morphological differences, the metrics used in our DTA are homology-free and based on overall crown shape (e.g., Evans et al., 2007; Boyer, 2008; Bunn et al., 2011; Evans, 2013; Berthaume et al., 2019), so including placental mammals in our extant sample should not negatively impact our interpretations.
Tooth Position Sampled
Some DTA studies have assessed complete post-canine tooth rows (e.g., Evans et al., 2007; Wilson et al., 2012; Pineda-Munoz et al., 2016). They treat the post-canine tooth row as a functional unit and capture morphological differences along it that may more accurately determine feeding ecology (e.g., bone-cracking premolar morphology versus reduced upper molar morphology of hyenas; Figueirido et al., 2013). Nevertheless, obtaining a complete cheek tooth row for fossil taxa can be challenging–the fossil record for many extinct species included in this study does not include teeth from all post-canine tooth positions. To maximize our taxonomic sample, we sampled only one tooth position but acknowledge that this choice might impact the resolution and potentially the accuracy of our results; we note this issue for individual cases where it might be relevant. Whereas most DTAs have focused on lower molars, specifically the lower second molar (m2) or penultimate lower molar (e.g., Boyer, 2008; Selig et al., 2019), we focused on the penultimate upper molar (most commonly M3 in metatherians and M2 in eutherians; Table 2-Table 3). This tooth position is heavily involved in mechanical food processing and tends to be more representative of the general molar morphology of a taxon than are the first or last molars (Wilson, 2013). As the penultimate molar position, the M2 of eutherians and the M3 of metatherians occupy functionally analogous positions in the jaw (Janis, 1990; Wilson, 2013). Moreover, the M2 of eutherians (the stem-based clade of living placentals and their closest relatives; Wible et al., 2007) may be homologous to the M3 of metatherians (McKenna, 1975; Luckett, 1993; O’Leary et al., 2013), despite the predominant dental-formula convention. In two cases, we did not sample the M2 of eutherians: those species with a specialized carnassial pair and those species with a reduced dental formula. For those with a specialized carnassial pair, the ultimate premolar (part of the carnassial pair) was sampled because it is heavily involved in food processing (Van Valkenburgh, 2007); in both species with a sectorial carnassial pair (Crocuta crocuta and Lynx rufus), the ultimate premolar is also the penultimate tooth. For those species with a reduced dental formula, either the penultimate tooth (e.g., Procyon lotor) or the only molar (e.g., Spilogale putoris) was sampled–in both cases the M1 was sampled (Table 2).
For both extant and fossil taxa, we selected upper molars with as little wear as possible to avoid artifacts or possible confounding signals in dietary interpretations caused by dental wear (Selig et al., 2019). Although some extant mammal species have teeth in which dental wear is important for food processing function (e.g., ungulates; Fortelius, 1985)–and it has been hypothesized that some fossil species changed dietary habits as excessive amounts of dental wear accumulated (e.g., stagodontid metatherians; Fox and Naylor, 1995, 2006)–we assumed that in most cases unworn teeth would most accurately reflect the average lifetime dietary ecologies of both the extant and fossil taxa sampled here. We did not include in our sample any extant mammal species with teeth that have, to our knowledge, secondary wear-induced functionality. Specimens with cusps missing due to breakage were also excluded.
Dietary Categories
We classified each extant species in our dataset into one of six dietary categories: carnivory (carn), animal-dominated omnivory (ado), plant-dominated omnivory (pdo), frugivory (frug), invertivory (inv; i.e., ‘insectivory’), or soft-invertebrate specialist (sis) (Table 2). We used six specific dietary categories rather than the classic three-diet classification scheme (herbivory-omnivory-carnivory) to provide more detailed dietary information and to avoid oversimplification (Pineda-Munoz and Alroy, 2014). Our choice of diet categories follows Smith (2017), who used these six categories (along with folivory and hard-object invertivory) in DTA analyses of lower molars. Our ‘soft-invertebrate specialist’ group includes taxa that primarily consume soft invertebrates such as earthworms and slugs, whereas our ‘invertivory’ (i.e., ‘insectivory’) group includes taxa that primarily eat relatively harder-bodied insects, such as beetles and moths. Following Pineda-Munoz and Alroy (2014), we classified diets of each species, with emphasis on its primary food resource. A species was classified as a specialist (i.e., non-omnivore) if one food resource makes up 50% or more of its total diet. For dietary information, we used online archives (EltonTraits [Wilman et al., 2014] and Mammal DIET [Kissling et al., 2014]) and a natural history compendia (Nowak, 1999). We supplemented each classification with primary literature sources (see Table 2 for sources), which were especially important when species-level information was extrapolated from genus-level information in the online archives (see Kissling et al., 2014; Table 2).
We acknowledge that our decision to use six dietary categories rather than the classic ‘carnivore-omnivore-herbivore’ trophic classification could lead to greater overlap of categories in the morphospace and less power to predict diet. We classified the diet of some extant taxa in our sample differently than previous studies have. For example, Nasua narica (white-nosed coati) is known to eat insects, but it is strictly frugivorous when fruit is available (e.g., Nowak, 1999). Although some studies classified its diet as plant-dominated omnivory (Smith, 2017), we followed EltonTraits, which records its diet as 70% fruit and considered this taxon a frugivore. We recognize that in this and any large-scale study of mammalian feeding behaviors, decisions that reduce the complexity of dietary data into discrete categories could have an impact on the results.
Fossil Metatherian Sampling
We sampled 71 isolated upper molars of 42 species (22 genera; six major clades) of North American Late Cretaceous (NALK) metatherians from the Western Interior region (Table 3). Our sample includes two stagodontids, one deltatheriid, two glasbiids, eight pediomyids, six taxa classified as incertae sedis, four herpetotheriids, and 19 alphadontids. To increase our taxonomic sampling of Cretaceous metatherians, we substituted the M2 (which tends to be morphologically very similar to the M3) for some species that did not have an available M3, and we used upper molar specimens of uncertain position (i.e., “Mx”) for some species that did not have definitive M2 or M3 specimens available (see Table 3 for details). Our sample includes 62% of the known species of NALK metatherians (42 of 68 known species; Case et al., 2005; Williamson et al., 2014; Cohen, 2018; Cohen et al., 2020). Some species were omitted from our sample because of either a lack of a well-preserved upper molar in the fossil record or an appropriate specimen was not available for loan. Our sampling of deltatheriids and stagodontids is limited, and this likely artificially reduced both morphological disparity values and morphospace occupation (e.g., Nanocuris has been interpreted as a specialized carnivore), especially in the pre-Aquilan and Lancian time bins (see below). The absence of other taxa may have had a negligible effect on the results because their morphologies are approximated by other sampled taxa (e.g., the absence of the pediomyoid genus Aquiladelphis may be accounted for by the presence of other pediomyoid genera in our sample to some degree).
We assigned each fossil species in our sample to one (or more) of four time bins depending on the known temporal range of each species (Williamson et al., 2014), using a range-through approach. Three bins are Cretaceous NALMAs (Woodburne, 2004): Aquilan (ca. 86-79 Ma), Judithian (79-69 Ma), and Lancian (69-66 Ma). We binned the eight specimens from geologic units that pre-date the Aquilan NALMA into a “pre-Aquilan” time bin (ca.126-86 Ma). Most taxa that we assigned to the pre-Aquilan time bin are from 100-86 Ma, but we also include Atokatheridium, which has a range of ca. 126-100 Ma. Because the “Edmontonian” NALMA is poorly characterized and not well sampled (Cifelli et al., 2004), we lumped the “Edmontonian” taxa into the Judithian bin. We recognize that these time bins are uneven in duration and that the longer duration bins could artificially inflate measures of disparity and diversity; however, we were unable to more finely and precisely bin our data due to uneven sample sizes across time bins and the lack of high-precision ages for certain geologic units.
Collection of 3D Tooth Surface Data
Three-dimensional digital models of the sampled teeth were created using micro-computed tomography (μCT) scan data. We scanned original specimens of teeth, molds of teeth, and epoxy casts of teeth (Table 2-Table 3). López-Torres et al. (2017) found that OPCR values of epoxy casts tend to be higher than those from their original specimens due to potential for artificially rougher surfaces on the casts (both DNE and RFI are more robust to this effect). Thus, we interpret OPCR results for the relatively few casts in our sample (15 of 71 specimens) with caution. Specimens were scanned using either a Bruker Skyscan 1172, Skyscan 1173, or NSI X5000 scanner, all of which are housed on the University of Washington campuses. We also downloaded image stacks (TIFF format) of scan data for eight extant specimens (Table 2) from the MorphoSource online repository (morphosource.org) to bolster our modern comparative dataset (Appendix 1). For detailed information regarding scanner types and scan settings, see Appendix 2. Molds of extant teeth were made using Coltene President Plus polyvinylsiloxane (type 2, medium consistency), and epoxy casts were collected from the UWBM, University of California Museum of Paleontology, and Sam Noble Oklahoma Museum of Natural History collections. For specimens scanned with Bruker Skyscan scanners, scan data were reconstructed using NRecon (Bruker microCT, Belgium); scans completed using the NSI X5000 were reconstructed using efX Reconstruction (North Star Imaging, Inc.). We segmented raw scan data using Avizo Lite 9.2.0 (Thermo Fisher Scientific). We then removed artifacts (“cleaning”), cropped, and oriented tooth models using GeoMagic Studio (3DS Systems). Specimen models were cropped to include the entire enamel cap (EEC cropping method; see Berthaume et al., 2019 for details) and were oriented such that the occlusal plane is perpendicular to the Z-axis. We exported the cleaned and oriented 3D tooth models from GeoMagic Studio as PLY files. These PLY files were imported back into Avizo Lite 9.2.0, and the 3D tooth models were simplified to 20,000 faces using the Simplification Editor tool. We then used the “Remesh Surface” function to downsample the tooth models to ~10,000 faces. The remesh function was used because it reduces the chance that surfaces with extremely disparate polygon mesh face-sizes are produced during simplification (Spradley, personal comm., 2018). We then used the “Smooth Surface” function with 25 iterations and lambda = 0.6 (Spradley et al., 2017; Spradley, personal comm., 2018). Because the consistency of model creation and processing is extremely important for producing comparable DTA results (Spradley et al., 2017; Berthaume et al., 2019), we used the same workflow for the creation of all models in this study. The resulting smoothed tooth models were saved as PLY files and used in our DTA analyses.
Dental Topographic Analyses (DTA)
We computed RFI, DNE, and OPCR for all 3D tooth models using the molaR_Batch function from the package molaR, version 4.2 (Pampush et al., 2016), in R version 3.3.3 (R Core Team, 2017). RFI is the ratio between the 3D surface area of a tooth crown and the 2D “footprint” area of a tooth (Ungar and M’Kirera, 2003). We use a modified version of this ratio in which the entire tooth crown is more accurately considered (Boyer, 2008). The modified RFI calculation is:
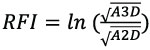
(A3D = 3D embedded surface area of the tooth crown, A2D = 2D tooth crown footprint area in occlusal view; Boyer, 2008; López-Torres et al., 2017). DNE represents the curvature of the tooth crown by calculating the sum energy values across the entire occlusal surface (Bunn et al., 2011; Winchester et al., 2014; Winchester, 2016). OPCR measures tooth crown complexity by calculating the total number of patches, or “tools,” on the crown of a tooth. A patch is a contiguous group of pixels that face the same cardinal direction on the tooth model (Evans et al., 2007; Evans and Jernvall, 2009; Wilson et al., 2012). Parameters for each metric were set as follows: RFI–alpha = 0.15; DNE–boundary discard = “Vertex”; and OPCR–step size = 8 and minimum patch size = 3 pixels (Evans et al., 2007; Pampush et al., 2016; Smith, 2017; Spradley, 2017). We ran a second DTA with the OPCR minimum patch size = 5 pixels to minimize any “noise” that might artificially inflate values for our extant and fossil samples, which include molds and casts, respectively (Winchester, 2016; López-Torres et al., 2017).
We log-transformed our DTA data to reduce skew. We generated scatter biplots of all possible combinations of the dental metrics to visualize morphospace occupation of extant dietary groups. We then plotted our fossil metatherian DTA values within the same morphospace of the extant dataset to examine both how fossil morphospace occupation compared to extant mammal morphospace occupation and how fossil morphospace occupation changed through time. We also tested for correlation between our DTA metrics by calculating Spearman’s rho and using least-squares linear regressions.
To test for differences between DTA values of the six dietary groups, we used one-way analysis of variance (ANOVA) and Tukey’s honest significant difference (HSD) post hoc test. We also performed a MANOVA using all three DTA metrics as independent variables.
Dietary Inference of Fossil Metatherians
To quantitatively infer diet in our sample of fossil metatherians, we conducted a discriminant function analysis (DFA) using the function lda() from the package MASS (Venables and Ripley, 2002). We first used the extant comparative dataset and a leave-one-out cross validation to assess the accuracy of discriminant functions in predicting diet (see MASS package documentation for more information). We then applied this DFA to the fossil metatherian DTA data (with fossils treated as having unknown diets), and we used posterior probabilities of dietary groupings to infer fossil diets. In a second permutation, we conducted a DFA on the extant comparative dataset using both the DTA data and mean body mass (compiled from the primary literature) to test whether this would significantly improve the discriminatory power of our model (Winchester et al., 2014). Because the resulting accuracy did not significantly improve discrimination, we only report the results from the first permutation. For fossil species in which different specimens were classified differently by the DFA (e.g., Didelphodon vorax), we based our dietary inferences on additional evidence, such as which diet was most commonly reconstructed by the specimens and evidence from previous studies, or we simply report two possible diet classifications for the species.
Dental Disparity of Fossil Metatherians
We calculated morphological disparity in our sample of fossil metatherians as: i) intra-family disparity and ii) total disparity per time bin. We did not calculate the intra-family disparity per time bin because sample sizes were too small. All disparity calculations used mean species values of each standardized, log-transformed DTA metric. We measured disparity as both the variance of each DTA metric and the sum of variances (Ciampaglio et al., 2001) using the morphol.disparity function in the geomorph package in R (Adams et al., 2020), which calculates a simulation-based p -value for statistical comparison between groups (i.e., between families or between time bins). We generated 95% confidence intervals using a custom bootstrapping function in R with 1,000 replicates.
Testing for Phylogenetic Signal
We tested for phylogenetic signal in the DTA results of our extant comparative dataset using a phylogenetic tree that we generated suing TimeTree (www.timetree.org; Kumar et al., 2017). We calculated Blomberg’s K (Blomberg et al., 2003) and Pagel’s lambda (Pagel, 1992) using the phylosig function in the package phytools in R (Revell, 2012). We did not test for phylogenetic signal in the DTA results of our fossil taxa because the most recent species-level phylogeny that includes all of the fossil taxa in our sample (Williamson et al., 2014) is highly unresolved with a large polytomy.
RESULTS
Phylogenetic Signal
Only OPCR shows a significant phylogenetic signal (Table 4); both DNE and RFI have a moderate but insignificant phylogenetic signal (p > 0.05 for both Blomberg’s K and Pagel’s lambda). The detected phylogenetic signal is likely due to the inclusion in our extant dataset of many species of Didelphimorphia, which have molars of similar gross morphology despite differing in dietary ecologies. Spradley (2017) also noted this gross morphological similarity among didelphimorph molars but still found them to be informative extant analogs for inferring dietary habits of fossil taxa.
Dental Topographic Analyses
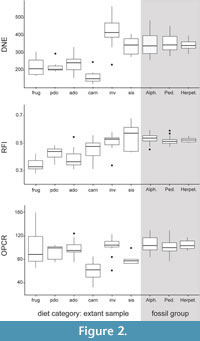 In our extant dataset, DNE values are positively correlated with both RFI and OPCR values (Table 5), which is consistent with the results of Spradley (2017). The dietary patterns for raw DNE data and raw RFI data are more similar to each other than they are for the raw OPCR data (Figure 2; Appendix 3). In both DNE and RFI, mean values are highest for invertivores and soft-invertebrate specialists, a pattern which seems to be driving the correlation between these dental metrics. The range of DNE values for other dietary categories overlap with each other, except carnivory, which has the lowest mean DNE values (Figure 2).
In our extant dataset, DNE values are positively correlated with both RFI and OPCR values (Table 5), which is consistent with the results of Spradley (2017). The dietary patterns for raw DNE data and raw RFI data are more similar to each other than they are for the raw OPCR data (Figure 2; Appendix 3). In both DNE and RFI, mean values are highest for invertivores and soft-invertebrate specialists, a pattern which seems to be driving the correlation between these dental metrics. The range of DNE values for other dietary categories overlap with each other, except carnivory, which has the lowest mean DNE values (Figure 2).
The RFI values are correlated with the DNE values. Mean RFI is lowest in frugivores and highest in soft-invertebrate specialists. Mean RFI generally increases with the percentage of animal material in the diet; however, we did not include folivores in our sample which are known to also have high RFI values (e.g., Boyer, 2008; Winchester, 2014). Invertivores and soft-invertebrate specialists have the highest mean RFI values, which is consistent with the tall, pointed cusps on their crowns (Figure 2).
The pattern of OPCR values is not as clear as those of the two other DTA metrics, and the range of values for most dietary categories overlap with each other. Mean OPCR is lowest in carnivores, as in other studies (Figure 2; Evans et al., 2007; Spradley, 2017), and highest in invertivores (Bunn et al., 2011), which contrasts with most studies in which frugivores typically have the highest OPCR (e.g., Santana et al., 2010; Pineda-Munoz et al., 2017). OPCR is most useful in distinguishing carnivory from other dietary categories in our sample.
In our fossil dataset, the most densely sampled clades (alphadontids, pediomyids, and herpetotheriids) have similar mean values and ranges for all three metrics. Mean DNE values of the fossil clades are similar to soft-invertebrate specialists, but their DNE ranges overlap with invertivores as well (Figure 2; Appendix 4). The RFI ranges of the fossil clades are not as large as for DNE or OPCR and overlap mostly with invertivore and soft-invertebrate specialists but also slightly with extant carnivores. The OPCR ranges of the fossil clades overlap with ranges of almost all the dietary categories, except carnivory.
The MANOVA, which incorporates all three DTA metrics, indicates significant differences among the six dietary groups (Wilks’ λ = 0.141, F = 9.165, p < 0.001; Table 6). The results of our ANOVA and post hoc pairwise comparisons recover DNE as the strongest performing metric, with a significant difference of means for 8 of 15 (53%) of the pairwise comparisons of DTA values for each dietary category (Table 6). This is followed by RFI with 6 of 15 (40%) and OPCR with 4 of 15 (27%). Among these comparisons, the means of four pairs of dietary categories (frug-ado, pdo-ado, pdo-frug, and sis-inv) were statistically indistinguishable by any metric. These results are consistent with the misidentification of specimens to these dietary categories as recovered by our DFA (see below).
Dental Morphospace Occupation
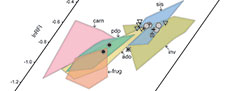
 In the bivariate scatterplots and the 3D scatterplot of the log-transformed DTA values of our extant sample, there is moderate separation between some dietary categories (Figure 3). Carnivores, with a combination of low DNE and OPCR values and wide range of RFI values, form a loose cluster that is mostly segregated from other groups, although some specimens overlap with plant-dominated omnivores and frugivores. Plant-dominated omnivores and animal-dominated omnivores largely overlap with each other, with intermediate values of all three dental topography metrics–we explore the effects of this overlap among the omnivore categories on our DFA (see below). Invertivores and soft-invertebrate specialists, with high DNE and RFI values and mid-range OPCR values, occupy similar regions of the morphospace. Frugivores largely overlap with the two omnivore categories but segregate on the basis of generally lower RFI values and some higher OPCR values. Consistent with other DTA studies (e.g., Smith, 2017), some areas of the morphospace are unoccupied, including the region with low DNE, high OPCR, and high RFI values and the region with high DNE, low OPCR, and low RFI values.
In the bivariate scatterplots and the 3D scatterplot of the log-transformed DTA values of our extant sample, there is moderate separation between some dietary categories (Figure 3). Carnivores, with a combination of low DNE and OPCR values and wide range of RFI values, form a loose cluster that is mostly segregated from other groups, although some specimens overlap with plant-dominated omnivores and frugivores. Plant-dominated omnivores and animal-dominated omnivores largely overlap with each other, with intermediate values of all three dental topography metrics–we explore the effects of this overlap among the omnivore categories on our DFA (see below). Invertivores and soft-invertebrate specialists, with high DNE and RFI values and mid-range OPCR values, occupy similar regions of the morphospace. Frugivores largely overlap with the two omnivore categories but segregate on the basis of generally lower RFI values and some higher OPCR values. Consistent with other DTA studies (e.g., Smith, 2017), some areas of the morphospace are unoccupied, including the region with low DNE, high OPCR, and high RFI values and the region with high DNE, low OPCR, and low RFI values.
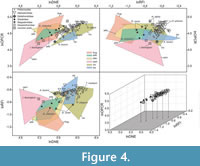 The NALK metatherians occupy a more restricted region of the morphospace than the extant sample does (Figure 4). Most NALK metatherian taxa have mid to high OPCR values, mid to high RFI values, and high DNE values and accordingly overlap with extant invertivores and soft-invertebrate specialists in the morphospace. In contrast, fewer fossils plot in the morphospace region that is occupied by extant plant-dominated and animal-dominated omnivores. The specimens of Glasbius have mid-range DNE, OPCR, and RFI values and thus fall among plant-dominated omnivores and frugivores. The two specimens of Didelphodon vorax plot in regions occupied by the extant animal-dominated omnivores and invertivores. Iugomortiferum thoringtoni and Dakotadens morrowi plot away from the main cluster of NALK metatherians; D. morrowi is near the edge of the omnivore-carnivore region of the morphospace, and I. thoringtoni is within the carnivore region of morphospace (Figure 4).
The NALK metatherians occupy a more restricted region of the morphospace than the extant sample does (Figure 4). Most NALK metatherian taxa have mid to high OPCR values, mid to high RFI values, and high DNE values and accordingly overlap with extant invertivores and soft-invertebrate specialists in the morphospace. In contrast, fewer fossils plot in the morphospace region that is occupied by extant plant-dominated and animal-dominated omnivores. The specimens of Glasbius have mid-range DNE, OPCR, and RFI values and thus fall among plant-dominated omnivores and frugivores. The two specimens of Didelphodon vorax plot in regions occupied by the extant animal-dominated omnivores and invertivores. Iugomortiferum thoringtoni and Dakotadens morrowi plot away from the main cluster of NALK metatherians; D. morrowi is near the edge of the omnivore-carnivore region of the morphospace, and I. thoringtoni is within the carnivore region of morphospace (Figure 4).
Dietary Inference
The first and second discriminant functions (DFs) account for most of the between-group variance, with DF1 most strongly linked to DNE and DF2 most strongly associated with RFI and OPCR (Table 7). The DFA correctly classified the diets of 58.9% (33 of 56) of the extant specimens (Table 8). The soft-invertebrate specialists and invertivores have the highest percentages of correctly classified taxa (80% and 75%, respectively), indicating that together the DTA metrics can differentiate between these similar dietary groups. Animal-dominated omnivores were most frequently misclassified (11 of 23 misclassifications; 48%) as either frugivores, invertivores, or plant-dominated omnivores (Table 8). Frugivores and carnivores were misclassified at the next highest rates (40% and 33.3%, respectively). For many of the incorrectly classified specimens (14 of 23), their DFA-predicted diet had a low posterior probability (< 0.50) that was often only slightly higher than the posterior probability for their assigned diet (Table 9).
Our DFA predicted the diets of the fossil taxa with about the same certainty as extant taxa, with 35.2% of fossil specimens classified with posterior probabilities > 0.60 for their predicted diet (Table 10; 33.9% of extant specimens had posterior probabilities of > 0.60, of which three specimens were misclassified). Almost all of the fossil specimens classified with posterior probabilities > 0.60 were classified as invertivores. Of the 41 fossil species sampled here, 20 species had more than one specimen sampled; nine species with more than one specimen sampled were classified with two different diets by the DFA. Classifications for these species were most often spread across invertivores, soft-invertebrate specialists, and plant-dominated omnivores (Table 10). Of the 71 fossil specimens sampled, 46.5% were classified as invertivores, 28.2% were classified as soft-invertebrate specialists, and 22.5% were classified as plant-dominated omnivores. One specimen of Didelphodon vorax was classified as an animal-dominated omnivore. Iugomortiferum thoringtoni was identified as the sole carnivore in the sample (although we urge caution about this assignment; see discussion below), and no frugivores were identified. The only deltatheriid, a specimen of Atokatheridium boreni, was identified as a soft-invertebrate specialist, and all four glasbiid specimens sampled were identified as plant-dominated omnivores. The DFA that used the OPCR data with a minimum patch count = 5 (rather than 3) (Appendix 5; Winchester, 2016; López- Torres et al., 2017) was slightly less accurate than our original DFA (OPCR minimum patch count = 3). The second DFA correctly classified 57.1% of the extant specimens, whereas the original DFA correctly classified 58.9% of the extant specimens. We compared the diet predictions between the two DFAs for both extant and fossil taxa and found minimal differences (Table 9-Table 10; Appendix 5). Hereafter, we focus only on the results of the analyses of the first DFA (OPCR minimum patch count = 3).
Dental Disparity and Diet Diversity through Time of NALK Metatherians
 Metatherian dental disparity, as calculated by the variance of each DTA metric and the sum of variances, does not significantly change throughout the Late Cretaceous (Figure 5; Table 11). The lnDNE, lnOPCR, and sum of variance values decrease from the Aquilan to Lancian bins, but in most cases these changes were not significant (Table 11). The dental disparity values of alphadontids are greater than the corresponding values of pediomyids, but again the differences are not statistically significant, except for lnDNE.
Metatherian dental disparity, as calculated by the variance of each DTA metric and the sum of variances, does not significantly change throughout the Late Cretaceous (Figure 5; Table 11). The lnDNE, lnOPCR, and sum of variance values decrease from the Aquilan to Lancian bins, but in most cases these changes were not significant (Table 11). The dental disparity values of alphadontids are greater than the corresponding values of pediomyids, but again the differences are not statistically significant, except for lnDNE.
Our DFA results suggest that the diversity of diets among NALK metatherians differed through the Late Cretaceous. Invertivores, soft-invertebrate specialists, and plant-dominated omnivores occur in all four Cretaceous time bins but in variable proportions (Table 12). The raw number of plant-dominated omnivore species does not vary much across time bins (from one to three), but the relative abundance of species of plant-dominated omnivores is high in the pre-Aquilan and low from the Aquilan through Lancian. The number of invertivore species is high in the Aquilan and younger Late Cretaceous time bins; likewise, their relative abundance (75%) is very high in the Aquilan but slightly lower thereafter. The number of soft-invertebrate specialists is low until the Judithian time bin, and their relative abundance follows a similar pattern. The only animal-dominated omnivore, Didelphodon vorax (see discussion below), occurs in the Lancian time bin and has the lowest relative abundance of all diet categories. The only taxon reconstructed as a carnivore by our DFA, Iugomortiferum thoringtoni (see discussion below), occurs in the Aquilan time bin. The DFA-reconstructed diets of several fossil species conflict with previous assessments, and we discuss other possible dietary classifications in the Discussion. However, using alternative diet reconstructions for these taxa does not significantly alter the overall dietary diversity patterns through time.
DISCUSSION
North American metatherians reached substantial taxonomic diversity during the Late Cretaceous (Williamson et al., 2014; Bennett et al., 2018). However, there is uncertainty surrounding the dietary diversity and ecomorphological patterns of NALK metatherians. It has been hypothesized that the novel food sources and habitats that arose with the angiosperm ecological diversification in the late Late Cretaceous (starting ca. 85-80 Ma) catalyzed an ecomorphological diversification (e.g., Grossnickle et al., 2019) and possibly a taxonomic diversification (Williamson et al., 2014; Wilson et al., 2016) of metatherians and additional mammal groups (e.g., Wilson et al., 2012; Grossnickle and Newham, 2016). Others contend that most mammalian clades, including metatherians, remained ecomorphologically constrained until the extinction of non-avian dinosaurs at the K-Pg boundary (e.g., Alroy, 1999). Below, we discuss the results of our dental topographic analyses (DTA) and how they and associated disparity measures, ecomorphospace plots, and dietary inferences shed light on those differing viewpoints of the evolutionary ecology of NALK metatherians.
Extant Mammal DTA Metrics and DFA Performance
Dental topographic analyses are still relatively new, having only been applied for the last 15 years and mostly on lower molars (but see Santana et al., 2011; Smits and Evans, 2012; Pineda-Munoz et al., 2017). Our study tests the validity and utility of applying those DTA methods to isolated upper molars and on a predominantly marsupial sample; the results are mostly congruent with previous studies on lower molars. Both types of invertivores in our extant sample (i.e., ‘invertivores’ and ‘soft-invertebrate specialists’) show characteristically high RFI and high DNE values (Figure 2; Boyer, 2008; Bunn et al., 2011; Winchester et al., 2014; López-Torres et al., 2017; Smith, 2017; Spradley, 2017), reflecting the tall, sharp cusps and high shearing-crest lengths used to puncture insect carapaces and shear soft-bodied invertebrates (Strait, 1993, 1997). Omnivores plot in the middle of the morphospace with low to mid-range values for DNE and mid-range values for RFI and OPCR, which is consistent with lower DTAs of lower molars in Boyer (2008), Bunn et al. (2011), Winchester et al. (2014), López-Torres et al. (2017), and Smith (2017). The intermediate values of omnivores reflect a morphology that is adapted to process a wide variety of food materials through a balance of shearing, crushing, and grinding (Ungar, 2010). The broad congruence of our upper-molar results with previous lower-molar results and the discrimination of diets via DTA lend support to the use of upper molars to infer diets of extinct mammals.
Differences between our DTA results and those of previous studies likely stem from differences in taxon sampling. Although the low OPCR values and low to mid-range RFI values of our extant carnivore sample are consistent with other studies (Figure 2; Evans et al., 2007; Evans and Jernvall, 2009; Pineda-Munoz et al., 2016; Smith, 2017), the relatively low DNE values of our carnivores are more similar to the values of hard-object feeders in other studies (Bunn et al., 2011; Winchester et al., 2014; Smith, 2017). Although this discrepancy could reflect different DTA patterns in upper and lower molars, it is likely due to the idiosyncrasies of our carnivore sample. Two of the six carnivore taxa (Crocuta crocuta, the spotted hyena and Sarcophilus harrisii, the Tasmanian devil) are known for their bone-cracking/durophagous habits (e.g., Werdelin, 1989; Wroe et al., 2005), and another taxon (Eira barbara, the tayra) supplements its carnivorous diet with fruit and honey (Bisbal, 1986). Increasing the sampling of hypercarnivorous taxa may add clarity to DTA patterns for carnivores and subsequent DFA carnivore classifications. Additionally, the DNE and OPCR values of our frugivore sample differ from those of previous studies: they are slightly higher and more variable (Bunn et al., 2011; Winchester et al., 2014). This discrepancy also likely reflects differences in taxon sampling. Whereas previous studies heavily sample primate frugivores, our sample includes one primate and four other taxa from Chiroptera, Carnivora, and Cetartiodactyla. Most of these other taxa incorporate small amounts of foods besides fruit into their diet (e.g., Pecari tajacu, the collared peccary, incorporates roots, insects, and small vertebrates in addition to fruit [Nowak, 1999; Desbiez et al., 2009]). The higher DNE and OPCR values in our frugivore sample may reflect dental adaptations, such as rugosities, for processing these other food materials (Santana et al., 2011; Smith, 2017), or other specialized features for processing poorly documented fallback foods (food consumed less often but are critical for survival during times of environmental stress)–an example of Liem’s paradox (e.g., Ungar, 2010).
The DFA correctly classified extant invertivores and soft-invertebrate specialists at the highest rate among the diet categories (Table 8). The few misclassified invertivore specimens were classified as soft-invertebrate specialists and vice versa. The DFA did not predict animal-dominated omnivores as reliably; some specimens were misclassified as frugivores, plant-dominated omnivores, and one as an invertivore. Among the frugivore sample, two specimens were misclassified as plant-dominated omnivores, one as a carnivore, and one as an animal-dominated omnivore. Among the plant-dominated omnivore sample, one specimen was misclassified as an animal-dominated omnivore and one as a frugivore. Often the assigned diet had the second highest posterior probability. These misclassifications likely stem in part from the overlapping range of DTA values among these dietary categories (Figure 2-Figure 3), which perhaps reflects some combination of dental morphological convergence among some animals in our extant sample, the incomplete and variable quality of the dietary data available, and the imperfect nature of the diet categorizations.
There were nine instances in which multiple specimens of the same extant species were classified into different dietary categories by the DFA and nine instances in which specimens of the same extinct species were classified differently from one another (Table 9-Table 10). In seven out of nine cases of the extant species, slight differences in wear among the specimens may have led to the different dietary assignments. In the other two cases, we did not detect differences in the amount of wear between the specimens of the same species. In both those cases, one specimen was classified as an invertivore and the other as a soft-invertebrate specialist, highlighting the substantial overlap in morphospace of these two invertebrate-eating diets (Figure 3). Thus, we highlight the need for further standardization and ground truthing of DTA methods. We recommend that whenever possible, studies should attempt to account for intraspecific variation by sampling more than one specimen per species and by controlling for wear across and within taxa.
Dietary Inferences and Dietary Diversity of NALK Metatherians
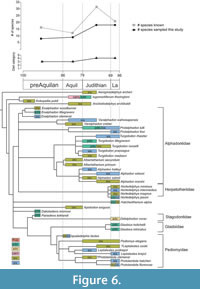 Although most Mesozoic mammals have conventionally been portrayed as small-bodied, terrestrial invertivores (e.g., Van Valen and Sloan, 1977; Kielan-Jaworowska et al., 2004), recent fossil discoveries and ecomorphological analyses have provided counterexamples, both among non-therians and therians, implying a much broader range of ecologies (e.g., Luo, 2007; Wilson et al., 2012; Grossnickle and Polly, 2013; Chen et al., 2019; Grossnickle et al., 2019). Our quantitative study of dental ecomorphology in part reinforces the conventional view by reconstructing most NALK metatherians (81%, 34 of 42 species) as either invertivores or soft-invertebrate specialists (Table 10, Table 12; Figure 6). These results are consistent with previous inferences from other studies using other methods (Gordon, 2003; Wilson, 2013; Williamson et al., 2014; Grossnickle and Newham, 2016) and with the observation that the most taxonomically rich families of Cretaceous metatherians (e.g., alphadontids and pediomyids) have conservative tribosphenic molar morphologies. Nevertheless, our DFA diet reconstructions predicted that a few NALK metatherians had diets beyond invertivory, indicating that NALK metatherians as a whole achieved greater dietary diversity than is conventionally portrayed. For example, our DFA reconstructed Glasbius as a plant-dominated omnivore, a prediction that is in line with previous interpretations that this taxon was either herbivorous or frugivorous (Clemens, 1966, 1979; Gordon, 2003; Kielan-Jaworowska et al., 2004; Wilson, 2013; Williamson et al., 2014). Overall, we see evidence of the following diets in NALK metatherians: invertivory, carnivory, animal- and plant-dominated omnivory (including durophagy), and likely frugivory.
Although most Mesozoic mammals have conventionally been portrayed as small-bodied, terrestrial invertivores (e.g., Van Valen and Sloan, 1977; Kielan-Jaworowska et al., 2004), recent fossil discoveries and ecomorphological analyses have provided counterexamples, both among non-therians and therians, implying a much broader range of ecologies (e.g., Luo, 2007; Wilson et al., 2012; Grossnickle and Polly, 2013; Chen et al., 2019; Grossnickle et al., 2019). Our quantitative study of dental ecomorphology in part reinforces the conventional view by reconstructing most NALK metatherians (81%, 34 of 42 species) as either invertivores or soft-invertebrate specialists (Table 10, Table 12; Figure 6). These results are consistent with previous inferences from other studies using other methods (Gordon, 2003; Wilson, 2013; Williamson et al., 2014; Grossnickle and Newham, 2016) and with the observation that the most taxonomically rich families of Cretaceous metatherians (e.g., alphadontids and pediomyids) have conservative tribosphenic molar morphologies. Nevertheless, our DFA diet reconstructions predicted that a few NALK metatherians had diets beyond invertivory, indicating that NALK metatherians as a whole achieved greater dietary diversity than is conventionally portrayed. For example, our DFA reconstructed Glasbius as a plant-dominated omnivore, a prediction that is in line with previous interpretations that this taxon was either herbivorous or frugivorous (Clemens, 1966, 1979; Gordon, 2003; Kielan-Jaworowska et al., 2004; Wilson, 2013; Williamson et al., 2014). Overall, we see evidence of the following diets in NALK metatherians: invertivory, carnivory, animal- and plant-dominated omnivory (including durophagy), and likely frugivory.
Dietary predictions for several taxa in our study conflicted with diet inferences from previous studies. In each case, however, the diet classification with the second highest posterior probability in our DFA matched with previous diet inferences. These taxa and their alternative diet classifications include (i) Iugomortiferum thoringtoni as a plant-dominated omnivore, (ii) Apistodon exiguus as an invertivore, and (iii) Alphadon halleyi, Alphadon wilsoni, and Protalphadon foxi as soft-invertebrate specialists. Below we discuss the diet reconstructions of these taxa in more detail.
Seven taxa (Pariadens kirklandi, Eoalphadon lillegraveni, Apistodon exiguus, Alphadon halleyi, Alphadon wilsoni, Turgidodon lillegraveni, Protalphadon foxi) were reconstructed in our DFA as plant-dominated omnivores. Most of these taxa lack most of the gross morphological features (e.g., large talonid basin, large protocone, bunodont cusps) characteristic of the crushing and grinding function necessary for most plant-based diets. Instead, most of these taxa have the conservative tribosphenic molar morphology (e.g., sharp shearing crests and unexpanded protocones) that is typically found among invertivores (e.g., Cifelli, 1990; Johanson, 1996; Davis, 2007; Williamson et al., 2014; Cohen, 2018). Such discrepancies between our diet reconstruction and those from previous studies are expected, considering the difficulty that the DFA model had in correctly predicting animal-dominated omnivory, and to a lesser extent, plant-dominated omnivory, frugivory, and carnivory. For Pariadens kirklandi, Eoalphadon lillegraveni, and Turgidodon lillegraveni, the second highest posterior probabilities were for the animal-dominated omnivore category, and posterior probabilities of other dietary categories were much lower (Table 10); this provides additional evidence for omnivory despite the dearth of supportive qualitative evidence. We consider there to be less overall evidence of omnivory for Apistodon exiguus, Alphadon halleyi, Alphadon wilsoni, and Protalphadon foxi; instead invertivore or soft-invertebrate specialist may be a more plausible diet reconstructions for these taxa. Evidence for Apistodon exiguus being an invertivore includes its very small body size, previous interpretations of its gross dental morphology (Williamson et al., 2014), and invertivory having the second highest posterior probability for this taxon in our DFA. The interpretations of Alphadon halleyi and Alphadon wilsoni as soft-invertebrate specialists are in line with analyses of the jaw morphology (Grossnickle and Polly, 2013; Brannick and Wilson, 2020; Morales-García et al., 2021), gross dental morphology (Gordon, 2003; Wilson, 2013; Grossnickle and Newham, 2016), and the DFA results, in which the soft-invertebrate specialist category has the second highest posterior probability for both of these species. Evidence for P. foxi as a soft-invertebrate specialist includes its dietary classification in a similar DTA study on lower molars (Smith, 2017) and our DFA results, in which the soft-invertebrate specialist category has the second highest posterior probability (within 0.10 of the highest posterior probability) for this taxon. Thus, we consider Apistodon exiguus, Alphadon halleyi, Alphadon wilsoni, and Protalphadon foxi to likely have had insect-dominated diets, but the DTA and DFA results indicate that their diets also had a plant component; this is consistent with the view of the ancestral tribosphenic molar morphology being adapted for consuming both animal and plant materials (Butler, 1972).
Our DFA reconstructed different diets for the two specimens of the relatively large-bodied Didelphodon vorax; one specimen as an invertivore and one as an animal-dominated omnivore. We favor the animal-dominated omnivore classification because it is in line with previous interpretations that D. vorax was a predator-scavenger with durophagous capabilities (Clemens, 1966, 1968, 1979; Fox and Naylor, 1986, 2006; Wilson et al., 2016; Brannick and Wilson, 2020) or an omnivore as indicated by dental microwear (Wilson et al., 2016). The bulbous premolars of Didelphodon are well suited for crushing hard objects, like bone and shells (Clemens, 1966; Fox and Naylor, 1995, 2006; Wilson et al., 2016; Cohen, 2018). One possible explanation for the invertivore reconstruction of one specimen is that we used relatively unworn molars (earlier ontogenetic wear stage) of Didelphodon in our analysis. That is, Didelphodon and other stagodontids may have experienced an ontogenetic shift in diet that tracks body size (Fox and Naylor, 1995, 2006; Peng et al., 2017) with younger individuals having been more faunivorous (e.g., molars with enhanced postvallum/prevallid shear and dentary shapes more capable of withstanding dorsoventral bending forces) and older individuals having been omnivorous/durophagous (e.g., horizontally worn grinding platforms and dentary shapes more capable of withstanding mediolateral forces; Fox and Naylor, 1995, 2006; Peng et al., 2017; Brannick and Wilson, 2020). Moreover, having analyzed only molar morphology, we did not account for critical dietary data from other tooth positions, such as premolars (Wilson, 2013; Smith, 2017). We suggest that future studies more deeply explore potential biases by comparing dietary inferences from DTA on a single tooth position to those from larger functional units like cheek tooth rows (Evans et al., 2007; Wilson et al., 2012). In a similar manner, further study of tooth wear as it relates to ontogenetic stage, functional efficiency, and dietary preference could lend important nuance to the dietary characterization of extinct taxa in studies using DTA (Ungar, 2010). Another productive line of inquiry for other taxa would be to compare dietary inferences from DTA to those from other quantitative methods that are independent of gross morphology of teeth (e.g., microwear, isotopic analyses, mandibular bending strength), as has been done for Didelphodon (Wilson et al., 2016; Brannick and Wilson, 2020).
Although our DFA classified Iugomortiferum thoringtoni as a carnivore, this taxon has low-crowned molar morphology with inflated cusps and weakly developed conules (Cifelli, 1990), all of which is inconsistent with interpretation of carnivory (de Muizon and Lange-Badré, 1997). The DNE value of I. thoringtoni is within the range of extant carnivores, plant-dominated omnivores, and frugivores, whereas its RFI value is within the range of extant carnivores, plant-dominated omnivores, and invertivores. Further, its low OPCR value is within the range of extant carnivores and invertivores. The OPCR value of I. thoringtoni may be underestimated because we used an epoxy cast of the specimen (OMNH 20936) and the small size of the specimen might have amplified any infidelities of the cast (although see discussion of cast fidelity and OPCR values in López-Torres et al., 2017). In addition, we analyzed only one specimen of I. thoringtoni, which has some wear and an uncertain identification of its position in the molar series (“M1?” in Cifelli, 1990). Taking these considerations into account, we consider it very likely that I. thoringtoni was a plant-dominated omnivore rather than a carnivore, and further studies are needed to resolve this issue.
Metatherian Ecomorphology through the Late Cretaceous
By the beginning of the Late Cretaceous (ca. 100 Ma) metatherians in North America had diversified into at least four clades (Deltatheriidae, Stagodontidae, Aquiladelphidae, Alphadontidae, and possibly Glasbiidae, Pediomyidae, and Marsupialia were also present, see Wilson et al., 2016). This higher-level taxonomic diversification was associated with moderate dietary diversity–three of the six dietary categories that we recognize here (plant-dominated omnivory, invertivory, and soft-invertebrate specialists; Figure 6; Table 10, Table 12). Raw species richness peaked in the Judithian (32 recognized species) and stayed relatively high in the Lancian leading up to the K-Pg mass extinction (22 species), although this peak might shift earlier in time or flatten if we account for differential sampling intensity through the Late Cretaceous (e.g., Grossnickle and Newham, 2016; Cohen, 2018; Bennett et al., 2018; Cohen et al., 2020). Nevertheless, according to our results, dental ecomorphological disparity did not significantly change throughout the Late Cretaceous and only in the Lancian did ecomorphological diversity (number of diet categories) increase slightly to include animal-dominated omnivory (Figure 5-Figure 6). Indeed, over 80% of the taxa sampled (34 of 42) were interpreted as either invertivores or soft-invertebrate specialists (Table 12; Figure 6). A literal reading of our results would thus suggest that ecomorphological diversity and disparity did not track increases in taxonomic richness of NALK metatherians. This decoupled pattern has also been found in other taxonomic groups, such as anomodont therapsids (Ruta et al., 2013), graptoloids (Bapst et al., 2012), and angiosperms (e.g., Wing and Boucher, 1998; Lupia et al., 1999). That said, we caution that additional sampling might change this pattern. We were unable to sample several important stagodontids, including the middle Turonian (pre-Aquilan) Hoodootherium, and Fumodelphodon, the Aquilan through possibly “Edmontonian” Eodelphis, and Judithian and “Edmontonian” members of Didelphodon. These taxa, which have previously been interpreted as carnivores and animal-dominated omnivores (e.g., Scott and Fox, 2015; Cohen, 2018; Brannick and Wilson, 2020), would have likely pushed back the appearance of those diet categories and increased disparity values earlier in the Late Cretaceous. The Lancian deltatheriid Nanocuris, which has also been considered carnivorous on the basis of its distinctive, sectorial molars with carnassial notches (Fox et al., 2007; Wilson and Riedel, 2010), would have further added to the range of Lancian ecomorphologies and would have likely increased disparity values. We also did not sample the middle Turonian Scalaridelphys and Aquilan Aquiladelphis, respectively, both of which are pediomyoids that have both been interpreted as plant-dominated omnivores (Cohen et al., 2020). Thus, we underscore that our results should be taken as minimum estimates both for the magnitude of dietary diversity and dental ecomorphological disparity achieved by NALK metatherians and for when they achieved it.
The oldest known dental fossils of metatherians, which date to ca. 110 Ma (Davis et al., 2008; Davis and Cifelli, 2011 and see Williamson et al., 2014; Bi et al., 2018 for discussion regarding Sinodelphys szalayi and the earliest eutherians), strongly suggest that invertivory was plesiomorphic for the clade (e.g., Williamson et al., 2014; Grossnickle and Newham, 2016). Together, our dietary inferences and those for the taxa that we were not able to sample indicate that by the early Late Cretaceous (ca. 100 Ma) metatherians were exploiting other food sources beyond insects (Cohen, 2018; Cohen et al., 2020). Notably, the dietary shifts toward omnivory (plant-dominated and animal-dominated omnivory) and carnivory largely occurred in metatherian subclades other than the most taxonomically prolific clades (the Alphadontidae and Pediomyidae) (Figure 6). Plant-dominated omnivory first appeared by the late Cenomanian (ca. 96 Ma) in the Stagodontidae (Pariadens kirklandi) and possibly Aquiladelphidae (Dakotadens morrowi, see discussion of phylogenetic relationships in Cohen et al., 2020). Later in the middle Turonian, stagodontids began their more thorough exploration of the carnivore and animal-dominated omnivore regions of the dietary ecomorphospace, culminating in the Lancian with the relatively large-bodied, durophagous predator-scavenger Didelphodon vorax. Glasbiidae is another group that shows up in the fossil record only at the very end of the Cretaceous (last 300-500 ky; Wilson, 2005); this sister taxon to Pediomyidae has only two known species (Glasbius twitchelli and Glasbius intricatus), but they are the most morphologically distinctive examples of plant-dominated omnivory-frugivory among NALK metatherians. Finally, deltatheroidans were likely the most carnivorous among the NALK metatherians, culminating in the highly specialized, Lancian carnivore Nanocuris (Fox et al., 2007; Wilson and Riedel, 2010). (Note that some Aptian-Albian members with a relatively larger talonid and a less reduced metaconid likely had diets other than strict carnivory [Rougier et al., 2015].)
Nevertheless, the two most taxonomically rich clades of NALK metatherians, the Alphadontidae and Pediomyidae, show relatively little dietary diversity (Figure 6). Alphadontids originated by at least the Cenomanian (but probably earlier; Wilson et al., 2016) and peaked in taxonomic richness in the Judithian (15 species, including alphadontids not sampled here). The oldest known pediomyids are from the middle Turonian (Cohen et al., 2020), but like alphadontids, probably originated earlier and reached their highest taxonomic richness in the Judithian (five species, including pediomyids not sampled here) and sustained that level through the Lancian. Many of these alphadontid and pediomyid species were sympatric; for example, Protalphadon lulli, Alphadon marshi, Alphadon wilsoni, Turgidodon rhaister, Pediomys elegans, Leptalestes cooki, Leptalestes krejcii, Protolambda florencae, and Protolambda hatcheri are all found in the Lance Formation (see Williamson et al., 2014 for a tabulation of species occurrences per locality). Although previous studies have hypothesized that pediomyids had greater crushing and grinding capacity relative to other metatherian groups and, in turn, likely incorporated more plant material into their diets (Wilson, 2013; Cohen et al., 2020), our DFA shows that both pediomyids and alphadontids fed on mainly insects. Diet partitioning within the invertivore adaptive zone may help explain how alphadontids and pediomyids were able to maintain their tremendous taxonomic richness (e.g., eight species in the Hell Creek fauna) (Hardin, 1960). As more pediomyid taxa appear in the Judithian, alphadontids appear to experience a dietary shift from invertivory to soft-invertebrate specialization, whereas pediomyids were mostly invertivores (Table 12; Figure 6). It is possible that further dietary differences, such as specialization for particular species of insects, drove the niche partitioning, but that level of diet specificity cannot be detected by the methods utilized here. Other potential explanations of niche or resource partitioning include spatial separation (using different habitats), temporal avoidance, or separation along an ecological axis different from diet, such as locomotor mode or body size (e.g., Schoener, 1975; Keddy, 1989). For example, the two pediomyid species Protolambda florencae and Pediomys elegans are contemporaneous (Lance and Hell Creek faunas) and were both reconstructed by our DFA as invertivores. Resource partitioning may have occurred along the axis of body size (i.e., P. florencae is larger and so probably consumed larger insects than did Pediomys elegans), which might have enabled these pediomyids to co-exist. However, other potential ecological axes on which partitioning might have occurred are difficult to discern in this fossil record (e.g., locomotion/substrate use, diel activity pattern, etc.).
During the Late Cretaceous in North America, metatherians shared the ecospace with other mammalian groups, including eutriconodontans, multituberculates, spalacotherioids, and their sister taxon eutherians. Among those groups, metatherians were arguably the most dietarily diverse, having occupied up to five categories: invertivory, carnivory, animal- and plant-dominated omnivory, and likely frugivory. It has been suggested that the non-tribosphenic dentitions of most non-therian mammals were more morphologically constrained than tribosphenic dentitions were, and, consequently, non-therians attained less dietary diversity than therians did (Chen et al., 2019; but see Harper et al., 2019 on South American dryolestoids). For instance, spalacotherioids and eutriconodonts were likely restricted to invertivory and faunivory, respectively (Hu et al., 2005; Grossnickle and Polly, 2013; Chen et al., 2019; Morales-García et al., 2021). Multituberculates were the most dietarily diverse non-therian mammal group. Their diets ranged from invertivory to animal- and plant-dominated omnivory, and by the late Late Cretaceous (ca. 84 Ma) even herbivory (Wilson et al., 2012; Grossnickle and Polly, 2013; Weaver et al., 2019; Weaver and Wilson, 2021). Still, metatherians probably had a broader dietary range than multituberculates and attained that diversity earlier in the Cretaceous. However, unlike multituberculates, metatherians did not continue to diversify in North America after the K-Pg mass extinction (Wilson, 2014; Williamson et al., 2014). The early eutherians, which include many lineages that retain the plesiomorphic tribosphenic molar morphology, were mostly insectivorous during the Late Cretaceous, although some of the larger-bodied taxa, such as Altacreodus magnus (formerly Cimolestes magnus), were likely faunivorous (e.g., Wilson, 2013; Grossnickle and Newham, 2016; Chen et al., 2019). Additionally, zhelestid (Harper, 2012; Gheerbrant and Astibia, 2012; Harper et al., 2019) and gypsonictopid eutherians (Crompton and Kielan-Jaworowska, 1978), which both first appear in North America in the Campanian, and the Lancian taeniodont Schowalteria (Fox and Naylor, 2003) are inferred to have included plant material in their diets based on their tooth morphology. Archaic ungulates, which first appear in the very latest Cretaceous but very rarely, and plesiadapiform primates, which have lineages that are believed to extend back into the very latest Cretaceous, have both been interpreted as animal- and plant-dominated omnivores (e.g., Archibald et al., 2011; Fox and Scott, 2011; Wilson Mantilla et al., 2021). Whereas Late Cretaceous eutherians ranged from invertivory, faunivory, and animal- and plant-dominated omnivory, they were less dietarily diverse compared to contemporaneous metatherians and did not expand beyond invertivory until the Campanian (at least in North America; Harper, 2012; Harper et al., 2019), well after metatherians had.
Thus, our study does not exclusively support either the Suppression Hypothesis or the Early Rise Hypothesis. The Suppression Hypothesis predicts that the ecomorphological diversity (number of diets) and disparity (magnitude of morphological difference) in metatherians was low and stable throughout the Late Cretaceous. Whereas our quantitative results of dental disparity and ecomorphological diversity are consistent with this hypothesis– that is, dental disparity does not significantly change through the Late Cretaceous and most metatherians were invertivores and soft-invertebrate specialists (Figure 6); we posit that inclusion of, for example, the middle Turonian stagodontids (Fumodelphodon and Hoodootherium) and aquiladelphids (Scalaridelphys) and the Lancian Nanocuris would likely increase dental disparity and diversity of dietary categories recorded for at least those intervals. Moreover, the ecomorphological diversity and disparity values are likely greater than those of other contemporary mammalian clades, which exhibit a smaller range of diets and dental morphologies.
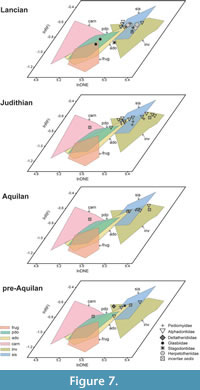 The Early Rise Hypothesis predicts that rapid increases in ecomorphological diversity and disparity of metatherians began in the late Late Cretaceous. Although our DFA shows that metatherians were mostly invertivores and soft-invertebrate specialists, it also shows that by the pre-Aquilan–prior to the ecological radiation of angiosperms–they had begun to exploit other diets as well, including plant-dominated omnivory (Figure 6-Figure 7). Whereas dietary diversity and disparity were both stable throughout the Late Cretaceous, they were elevated relative to contemporary mammalian groups; we hypothesize that the diversification that produced this relatively high dietary diversity and dental disparity arose during the late Early Cretaceous. As such, we would suggest that the ecomorphological expansion of NA metatherians was not temporally correlated with the ecological rise of angiosperms but perhaps with their earlier taxonomic diversification (Cohen et al., 2020), which occurred during the Cretaceous Terrestrial Revolution (ca. 125-80 Ma). As new species of angiosperms appeared during their taxonomic diversification, they may have provided new food resources for metatherians to exploit, thus catalyzing the ecomorphological expansion of metatherians. Or perhaps other possible co-occurring factors during the Cretaceous Terrestrial Revolution, such as the extinction of eutriconodontans and spalacotherioids (Grossnickle and Polly, 2013; Cohen et al., 2020), allowed metatherians to expand into newly vacated niches. Future studies should test this hypothesis by applying DTA to samples of Early Cretaceous metatherians; however, to achieve this, additional field work should be undertaken to bolster the sparse fossil record from this interval.
The Early Rise Hypothesis predicts that rapid increases in ecomorphological diversity and disparity of metatherians began in the late Late Cretaceous. Although our DFA shows that metatherians were mostly invertivores and soft-invertebrate specialists, it also shows that by the pre-Aquilan–prior to the ecological radiation of angiosperms–they had begun to exploit other diets as well, including plant-dominated omnivory (Figure 6-Figure 7). Whereas dietary diversity and disparity were both stable throughout the Late Cretaceous, they were elevated relative to contemporary mammalian groups; we hypothesize that the diversification that produced this relatively high dietary diversity and dental disparity arose during the late Early Cretaceous. As such, we would suggest that the ecomorphological expansion of NA metatherians was not temporally correlated with the ecological rise of angiosperms but perhaps with their earlier taxonomic diversification (Cohen et al., 2020), which occurred during the Cretaceous Terrestrial Revolution (ca. 125-80 Ma). As new species of angiosperms appeared during their taxonomic diversification, they may have provided new food resources for metatherians to exploit, thus catalyzing the ecomorphological expansion of metatherians. Or perhaps other possible co-occurring factors during the Cretaceous Terrestrial Revolution, such as the extinction of eutriconodontans and spalacotherioids (Grossnickle and Polly, 2013; Cohen et al., 2020), allowed metatherians to expand into newly vacated niches. Future studies should test this hypothesis by applying DTA to samples of Early Cretaceous metatherians; however, to achieve this, additional field work should be undertaken to bolster the sparse fossil record from this interval.
SUMMARY
Our study is the most comprehensive study to date to apply dental topographic analysis to a large sample of metatherian molars from the Late Cretaceous of North America. We provide a more detailed, quantitative understanding of the macroevolutionary patterns of dental morphology and diet during the early history of this important clade. Although dietary inferences from our DTA suggest that many NALK metatherians were invertivorous, the analyses also indicate that early metatherians exhibited a broad range of diets, including invertivory, soft-invertebrate specialists, carnivory, animal- and plant-dominated omnivory, and likely frugivory. Our morphological disparity results show that dental disparity did not significantly increase in the Late Cretaceous. However, our results do not meet predictions of the Suppression Hypothesis (i.e., that mammalian ecological diversity was suppressed until the K-Pg mass extinction event), as our diet reconstructions and those from previous studies of taxa not sampled in our study show that NALK metatherians diversified into a wider range of dietary niches–more so than any other contemporary mammalian clade. We argue that relative to other mammalian clades, both dental disparity and dietary diversity of metatherians were moderately high and stable throughout the Late Cretaceous. Our results indicate a pre-K-Pg ecological diversification that is distinct from that predicted by the Early Rise Hypothesis because it began prior to the diversification of angiosperms and was more correlated in time with the Cretaceous Terrestrial Revolution and the mid-Cretaceous taxonomic diversification of angiosperms.
ACKNOWLEDGEMENTS
We thank one anonymous reviewer and J. Samuels for their helpful feedback that improved this manuscript. Special thanks to S. Smith and J. Spradley for guidance in implementing DTA methodology and J. Cohen and J. Case for helpful advice and communications regarding specimens. We also thank J. Calede, M. Chen, J. Claytor, and A. Magee for coding support. We thank L. Weaver, P. Wilson Deibel, J. Claytor, B. Hovatter, and M. Whitney for helpful feedback on the manuscript and B. Hovatter for scanning support. For access to specimens and help with specimen loans, we are grateful to S. Santana (UWBM), J. Bradley (UWBM), P. Holroyd (UCMP), W.A. Clemens (UCMP), R.L. Cifelli (OMNH), J. Larsen (OMNH), M. Caldwell (UALVP), H. Gibbins (UALVP), J. Gillette (MNA), D. Gillette (MNA), A. King (MNA), R. Irmis (UMNH), C. Levitt-Bussian (UMNH), E. Sargis (YPM), C. Norris (YPM), D. Brinkman (YPM), and M. Fox (YPM). We are grateful to S. Santana and A. Summers for access to μCT scanners on the UW campuses and K. Cohen, J. Huie, and K. Hall for scanner training and support. We also thank A. Kinahan for help with CT scan segmentation of extant specimens and MorphoSource.org, G.S. Yapuncich, D.M. Boyer, P. Whitmer, E. Delson, and J. Shi for allowing access to μCT scans of some of the extant mammal specimens used here. We are grateful to the Paleontological Society N. Gary Lane Award and the UW Biology Department Walter and Margaret Sargent award for funding this research. We are also grateful to the editors of Palaeontolgica Electronica for their patience as we completed this manuscript and its submission.
REFERENCES
Adams, D.C., Collyer, M.L., and Kaliontzopoulou, A. 2020. Geomorph: software for geometric morphometric analyses. R package version 3.2.1.
Agosta, S.J. and Morton, D. 2003. Diet of the big brown bat, Eptesicus fuscus, from Pennsylvania and western Maryland. Northeastern Naturalist, 10:89-104.
https://doi.org/10.1656/1092-6194(2003)010[0089:DOTBBB]2.0.CO;2
Alroy, J. 1999. The fossil record of North American mammals: evidence for a Paleocene evolutionary radiation. Systematic Biology, 48:107-118.
https://doi.org/10.1080/106351599260472
Andersen, G.E., Johnson, C.N., Barmuta, L.A., and Jones, M.E. 2017. Dietary partitioning of Australia’s two marsupial hypercarnivores, the Tasmanian devil and the spotted-tailed quoll, across their shared distributional range. PLoS ONE, 12:e0188529.
https://doi.org/10.1371/journal. pone.0188529
Anderson, C.L., Bremer, K., and Friis, E.M. 2005. Dating phylogenetically basal eudicots using rbcL sequences and multiple fossil reference points. American Journal of Botany, 92:1737-1748.
https://doi.org/10.3732/ajb.92.10.1737
Archibald, D. 1983. Structure of the K-T mammal radiation in North America: speculations on turnover rates and tropic structure. Acta Palaeontologica Polonica, 28:7-17.
Archibald, D. 2011. Extinction and Radiation: How the Fall of Dinosaurs Led to the Rise of Mammals. The Johns Hopkins University Press, Baltimore, Maryland.
Archibald, J.D., Zhang, Y., Harper, T., and Cifelli, R.L. 2011. Protungulatum, confirmed Cretaceous occurrence of an otherwise Paleocene eutherian (placental?) mammal. Journal of Mammalian Evolution, 18:153-161.
https://doi.org/10.1007/s10914-011-9162-1
Atramentowicz, M. 1988. La frugivorie opportuniste de trios marsupiaux didelphidé de Guyane. Revue d’Écologie, 43:47-57.
https://doi.org/10.3406/revec.1988.5412
Averianov, A.O., Archibald, D., and Ekdale, E.G. 2010. New material of the Late Cretaceous Deltatheroidan mammal Sulestes from Uzbekistan and phylogenetic reassessment of the metatherian-eutherian dichotomy. Journal of Systematic Palaeontology, 8:301-330.
https://doi.org/10.1080/14772011003603499
Baker, R.H. and Baker, M.W. 1975. Montane habitat used by the spotted skunk (Spilogale putorius) in Mexico. Journal of Mammalogy, 56:671-673.
https://doi.org/10.2307/1379480
Bapst, D.E., Bullock, P.C., Melchin, M.J., Sheets, H.D., and Mitchell, C.E. 2012. Graptoloid diversity and disparity became decoupled during the Ordovician mass extinction. PNAS, 109:3428-3433. https://doi.org/10.1073/pnas.1113870109
Bartoszewicz, M., Okarma, H., Zalewski, A., and Szczęsna, J. 2008. Ecology of the raccoon (Procyon lotor) from western Poland. Annales Zoologici Fennici, 45:291-298.
https://doi.org/10.5735/086.045.0409
Belcher, C.A., Nelson, J.L., and Darrant, J.P. 2007. Diet of the tiger quoll (Dasyurus maculatus) in south-eastern Australia. Australian Journal of Zoology, 55:117-122.
https://doi.org/10.1071/ZO06102
Bennett, C.V., Upchurch, P., Goin, F.J., and Goswami, A. 2018. Deep time diversity of metatherian mammals: implications for evolutionary history and fossil-record quality. Paleobiology, 44:171–198.
https://doi.org/10.1017/pab.2017.34
Benson, R.B.J., Mannion, P.D., Butler, R.J., Upchurch, P., Goswami, A., and Evans, S.E. 2013. Cretaceous tetrapod fossil record sampling and faunal turnover: implications for biogeography and the rise of modern clades. Palaeogeography, Palaeoclimatology, Palaeoecology, 372:88-107.
https://doi.org/10.1016/j.palaeo.2012.10.028
Berthaume, M.A., Winchester, J., and Kupczik, K. 2019. Effects of cropping, smoothing, triangle count, and mesh resolution on 6 dental topographic metrics. PLoS ONE, 14:e0216229.
https://doi.org/10.1371/journal.pone.0216229
Bi, S., Jin, X., Li, S., and Du, T. 2015. A new Cretaceous metatherian mammal from Henan, China. PeerJ, 3:e896.
https://doi.org/10.7717/peerj.896
Bisbal, E J. 1986. Food habits of some Neotropical carnivores in Venezuela (Mammalia, Carnivora). Mammalia, 50:329-339.
https://doi.org/10.1515/mamm.1986.50.3.329
Blomberg, S.P., Garland Jr., T., and Ives, A.R. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution, 57:717-745.
https://doi.org/10.1111/j.0014-3820.2003.tb00285.x
Boyer, D.M. 2008. Relief index of second mandibular molars is a correlate of diet among prosimian primates and other euarchontan mammals. Journal of Human Evolution, 55:1118-1137.
http://doi.org/10.1016/j.jhevol.2008.08.002
Boyer, D.M., Evans, A.R., and Jernvall, J. 2010. Evidence of dietary differentiation among Late Paleocene-Early Eocene plesiadapids (Mammalia, Primates). American Journal of Physical Anthropology, 142:194-210.
https://doi.org/10.1002/ajpa.21211
Brannick, A.L. and Wilson, G.P. 2020. New specimens of the Late Cretaceous metatherian Eodelphis and the evolution of hard-object feeding in the Stagodontidae. Journal of Mammalian Evolution, 27:1-16.
https://doi.org/10.1007/s10914-018-9451-z
Bunn, J.M. and Ungar, P.S. 2009. Dental topography and diets of four Old World monkey species. American Journal of Primatology, 71:466-477.
https://doi.org/10.1002/ajp.20676
Bunn, J.M., Boyer, D.M., Lipman, Y., St. Clair, E.M., Jernvall, J., and Daubechies, I. 2011. Comparing Dirichlet normal surface energy of tooth crowns, a new technique of molar shape quantification for dietary inference, with previous methods in isolation and in combination. American Journal of Physical Anthropology, 145:247-261.
https://doi.org/10.1002/ajpa.21489
Butler, P.M. 1972. Some functional aspects of molar evolution. Evolution, 26:474-483.
Cáceres, N.C. 2002. Food habits and seed dispersal by the white-eared opossum Didelphis albiventris in southern Brazil. Studies on Neotropical Fauna and Environment, 37:97-104.
https://doi.org/10.1076/snfe.37.2.97.8582
Cantor, M., Ferreira, L.A., Silva, W.R., and Setz, E.Z.F. 2010. Potential seed dispersal by Didelphis albiventris (Marsupialia, Didelphidae) in highly disturbed environment. Biota Neotropica, 10:45-51.
https://doi.org/10.1590/s1676-06032010000200004
Carey, A.B., Colgan III, W., Trappe, J.M., and Molina, R. 2002. Effects of forest management on truffle abundance and squirrel diets. Northwest Science, 76:148-157.
https://hdl.handle.net/2376/949
Case, J.A., Goin, F.J., and Woodburne, M.O. 2005. “South American” marsupials from the Late Cretaceous of North America and the origin of marsupial cohorts. Journal of Mammalian Evolution, 12:461-494.
https://doi.org/10.1007/s10914-005-7329-3
Casella, J. and Cáceres, N.C. 2006. Diet of four small mammal species from Atlantic forest patches in South Brazil. Neotropical Biology and Conservation, 1:5-11.
Charles-Dominique, P., Atramentowicz, M., Charles-Dominique, M., Gérard, H., Hladik, A., Hladik, C.M., and Prévost, M.F. 1981. Les mammiféres frugivorés arboricolés d'une foret Guyanaise: inter-relations plantes-animaux. Revue d’Écologie, 35:342-435.
https://doi.org/10.3406/revec.1981.4121
Chen, M., Strömberg, C.A.E., and Wilson, G.P. 2019. Assembly of modern mammal community structure driven by Late Cretaceous dental evolution, rise of flowering plants, and dinosaur demise. PNAS, 116:9931-9940.
https://doi.org/10.1073/pnas.1820863116
Ciampaglio, C.N., Kemp, M., and McShea, D.W. 2001. Detecting changes in morphospace occupation patterns in the fossil record: characterization and analysis of measures of disparity. Paleobiology, 27:695-715.
https://doi.org/10.1666/0094-8373(2001)027<0695:dcimop>2.0.co;2
Cifelli, R.L. 2004. Marsupial mammals form the Albian-Cenomanian (Early-Late Cretaceous) boundary, Utah. Bulletin of the American Museum of Natural History, 285:62-79.
https://doi.org/10.1206/0003-0090(2004)285<0062:C>2.0.CO;2
Cifelli, R.L., Eberle, J.J., Lofgren, D.L., Lillegraven, J.A., and Clemens, W.A. 2004. Mammalian biochronology of the Late Cretaceous, p. 21-42. In Woodburne, M.O. (ed.), Late Cretaceous and Cenozoic Mammals of North America: Biostratigraphy and Geochronology. Columbia University Press, New York.
https://doi.org/10.7312/wood13040-004
Clemens, W.A. 1966. Fossil mammals of the type Lance Formation Wyoming. Part II. Marsupialia. University of California Publications in Geological Sciences, 62:1-122.
Clemens, W.A. 1968. A mandible of Didelphodon vorax (Marsupialia, Mammalia). Los Angeles County Museum Contributions in Science, 133:1-11.
https://doi.org/10.5962/p.241122
Clemens, W.A. 1979. Marsupialia, p. 192-220. In Lillegraven, J.A., Kielan-Jaworowska, Z., and Clemens, W.A. (eds.), Mesozoic Mammals: The First Two-Thirds of Mammalian History. University of California Press, Berkeley, California.
Clotheir, R.R. 1955. Contribution to the life history of Sorex vagrans in Montana. Journal of Mammalogy, 36:214-221.
https://doi.org/10.2307/1375879
Cohen, J.E. 2018. Earliest divergence of stagodontid (Mammalia: Marsupialiformes) feeding strategies from the Late Cretaceous (Turonian) of North America. Journal of Mammalian Evolution, 25:165-177.
https://doi.org/10.1007/s10914-017-9382-0
Cohen, J.E., Davis, B.M., and Cifelli, R.L. 2020. Geologically oldest Pediomyoidea (Mammalia, Marsupialiformes) from the Late Cretaceous of North America, with implications for taxonomy and diet of earliest Late Cretaceous mammals. Journal of Vertebrate Paleontology, 40:e1835935.
https://doi.org/10.1080/02724634.2020.1835935
Cooper, S.M., Holekamp, K.E., and Smale, L. 1999. A seasonal feast: long-term analysis of feeding behaviour in the spotted hyaena (Crocuta crocuta). African Journal of Ecology, 37:149-160.
https://doi.org/10.1046/j.1365-2028.1999.00161.x
Crabb, W.D. 1941. Food habits of the prairie spotted skunk in southeastern Iowa. Journal of Mammalogy, 22:349-364.
https://doi.org/10.2307/1374928
Crifó, C., Currano, E.D., Baresch, A., and Jaramillo, C. 2014. Variations in angiosperm leaf vein density have implications for interpreting life form in the fossil record. Geology, 42:919-922.
https://doi.org/10.1130/g35828.1
Crompton, A.W. and Kielan-Jaworowska, Z. 1978. Molar structure and occlusion in Cretaceous therian mammals, p.249-287. In Butler, P.M. and Joysey, K.A. (eds.), Studies in the Development, Function and Evolution of Teeth, Academic Press, New York.
Davis, B.M. 2007. A revision of “pediomyid” marsupials from the Late Cretaceous of North America. Acta Palaeontologica Polonica, 52:217-256.
Debelica, A., Matthews, A.K., and Ammerman, L.K. 2006. Dietary study of big free-tailed bats (Nyctinomops macrotis) in Big Bend National Park, Texas. The Southwestern Naturalist, 51:414-418.
https://doi.org/10.1894/0038-4909(2006)51[414:DSOBFB]2.0.CO;2
DeBey L.B. and Wilson, G.P. 2017. Mammalian distal humerus fossils from eastern Montana, USA with implications for the Cretaceous-Paleogene mass extinction and the adaptive radiation of placentals. Palaeontologia Electronica, 20.3.49A.
https://doi.org/10.26879/694
De Carvalho, R.F., Passos, D.C., and Lessa, L.G. 2019. Diet variations in short-tailed opossum Monodelphis domestica (Didelphimorphia, Didelphidae) due to seasonal and intersexual factors. Mastozoología Neotropical, 26:340-348.
https://doi.org/10.31687/saremmn.19.26.2.0.14
Easterla, D.A. and Whitaker, Jr., J.O. 1972. Food habits of some bats from Big Bend National Park, Texas. Journal of Mammalogy, 53:887-890.
https://doi.org/10.2307/1379227
Eriksson, O. 2016. Evolution of angiosperm seed disperser mutualisms: the timing of origins and their consequences for coevolutionary interactions between angiosperms and frugivores. Biological Reviews, 91:168-186.
https://doi.org/10.1111/brv.12164
Evans, A.R. 2013. Shape descriptors as ecometrics in dental ecology. Hystrix, the Italian Journal of Mammalogy, 24:133-140.
https://doi.org/10.4404/hystrix-24.1-6363
Evans, A.R. and Jernvall, J. 2009. Patterns and constraints in carnivoran and rodent dental complexity and tooth size. Journal of Vertebrate Paleontology, 29:92A.
https://doi.org/10.1080/02724634.2009.10411818
Evans, A.R., Wilson, G.P., Fortelius, M., and Jernvall, J. 2007. High-level similarity of dentitions in carnivorans and rodents. Nature, 445:78-81.
https://doi.org/10.1038/nature05433
Figueirido, B., Tseng, Z.J., and Martín-Serra, A. 2013. Skull shape evolution in durophagous carnivorans. Evolution, 67:1975-1993.
https://doi.org/10.1111/evo.12059
Fortelius, M. 1985. Ungulate cheek teeth: developmental, functional, and evolutionary interrelations. Acta Zoologica Fennica, 185:1-76.
Fox, B.J. and Archer, E. 1984. The diets of Sminthopsis murina and Antechinus stuartii (Marsupialia: Dasyuridae) in sympatry. Australian Wildlife Research, 11:235-248.
https://doi.org/10.1071/wr9840235
Fox, R.C. and Naylor, B.G. 1986. A new species of Didelphodon Marsh (Marsupialia) from the Upper Cretaceous of Alberta, Canada: paleobiology and phylogeny. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 172:357-380.
https://doi.org/10.1127/njgpa/172/1986/357
Fox, R.C. and Naylor, B.G. 1995. The relationships of the Stagodontidae, primitive North American Late Cretaceous mammals, p. 247-250. In Sun, A. and Wang, Y. (eds.), Sixth Symposium on Mesozoic Terrestrial Ecosystems and Biota. China Ocean Press, Beijing.
Fox, R.C. and Naylor, B.G. 2003. A Late Cretaceous taeniodont (Eutheria, Mammalia) from Alberta, Canada. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 229:393-420.
https://doi.org/10.1127/njgpa/229/2003/393
Fox, R.C. and Naylor, B.G. 2006. Stagodontid marsupials from the Late Cretaceous of Canada and their systematic and functional implications. Acta Palaeontologica Polonica, 51:13-26.
Fox, R.C. and Scott, C.S. 2011. A new, early Puercan (earliest Paleocene) species of Purgatorius (Plesiadapiformes, Primates) from Saskatchewan Canada. Journal of Paleontology, 85:537-548. https://doi.org/10.1666/10-059.1
Fox, R.C., Scott, C.S., and Bryant, H.N. 2007. A new, unusual therian mammal from the Upper Cretaceous of Saskatchewan, Canada. Cretaceous Research, 28:821-829.
https://doi.org/10.1016/j.cretres.2006.12.005
Fritts, S.H. and Sealander, J.A. 1978. Diets of bobcats in Arkansas with special reference to age and sex differences. The Journal of Wildlife Management, 42:533-539.
https://doi.org/10.2307/3800815
Gheerbrant, E. and Astibia, H. 2012. Addition to the Late Cretaceous Laño mammal faunule (Spain) and to the knowledge of European “Zhelestidae” (Lainodontinae nov.). Bulletin de la Société géologique de France, 183:537-546.
https://doi.org/10.2113/gssgfbull.183.6.537
Glendenning, R. 1959. Biology and control of the coast mole, Scapanus orarius orarius True, in British Columbia. Canadian Journal of Animal Science, 39:34-44.
https://doi.org/10.4141/cjas59-006
Goin, F.J., Woodburne, M.O., Zimicz, A.N., Martin, G.M., and Chornogubsky, L. 2016. A Brief History of South American Metatherians: Evolutionary Contexts and Intercontinental Dispersals. Springer, Dordrecht Heidelberg, New York, London.
Gompper, M.E. 1996. Sociality and asociality in white-nosed coatis (Nasua narica): foraging costs and benefits. Behavioral Ecology, 7:254-263.
https://doi.org/10.1093/beheco/7.3.254
Gordon, C.L. 2003. Functional morphology and diet of Late Cretaceous mammals of North America. Unpublished PhD Thesis, University of Oklahoma, Norman, Oklahoma, USA.
Grimaldi, D. 1999. The co-radiations of pollinating insects and angiosperms in the Cretaceous. Annals of the Missouri Botanical Garden, 86:373-406.
https://doi.org/10.2307/2666181
Grossnickle, D.M. and Newham, E. 2016. Therian mammals experience an ecomorphological radiation during the Late Cretaceous and selective extinction at the K-Pg boundary. Proceedings of the Royal Society B, 283:20160256.
https://doi.org/10.1098/rspb.2016.0256
Grossnickle, D.M. and Polly, P.D. 2013. Mammal disparity decreases during the Cretaceous angiosperm radiation. Proceedings of the Royal Society B, 280:20132110.
https://doi.org/10.1098/rspb.2013.2110
Grossnickle, D.M., Smith, S.M., and Wilson, G.P. 2019. Untangling the multiple ecological radiations of early mammals. Trends in Ecology and Evolution, 34:936-949.
https://doi.org/10.1016/j.tree.2019.05.008
Hall, E.R. and Dalquest, W.W. 1963. The mammals of Veracruz. University of Kansas Publications Museum of Natural History, 14:165-362.
Halliday, T.J.D. and Goswami, A. 2016. Eutherian morphological disparity across the end Cretaceous mass extinction. Biological Journal of the Linnean Society, 118:152-168.
https://doi.org/10.1111/bij.12731
Hardin, G. 1960. The competitive exclusion principle. Science, 131:1292-1297.
https://doi.org/10.1126/science.133.3450.391.a
Harper, A.M. 2012. Three dimensional analysis of large scale dental wear in Late Cretaceous eutherians, Dzharakuduk region, Uzbekistan. Unpublished Masters Thesis, San Diego State University, San Diego, California.
Harper, T., Parras, A., and Rougier, G.W. 2019. Reigitherium (Meridiolestida, Mesungulatoidea) an enigmatic Late Cretaceous mammal from Patagonia, Argentina: morphology, affinities, and dental evolution. Journal of Mammalian Evolution, 26:447-478.
https://doi.org/10.1007/s10914-018-9437-x
Hopkins, D.D. and Forbes, R.B. 1980. Dietary patterns of the Virginia opossum in an urban environment. The Murrelet, 61:20-30.
https://doi.org/10.2307/3536187
Hu, Y., Meng, J., Wang, Y., and Li, C. 2005. Large Mesozoic mammals fed on young dinosaurs. Nature, 433:149-152.
https://doi.org/10.1038/nature03102
Janis, C.M. 1990. Correlation of cranial and dental variables with body size in ungulates and macropodoids, p. 255-300. In Damuth, J. and MacFadden, B.J. (eds.), Body Size in Mammalian Paleobiology: Estimation and Biological Implications. Cambridge University Press, New York.
Johanson, Z. 1996. Revision of the Late Cretaceous North American marsupial genus Alphadon. Palaeontographica Abteilung A: Palaeozoologie-Stratigraphie, 242:127-184.
https://doi.org/10.1127/pala/242/1996/127
Jones, M.E. and Barmuta, L.A. 1998. Diet overlap and relative abundance of sympatric dasyurid carnivores: a hypothesis of competition. Journal of Animal Ecology, 67:410-421.
https://doi.org/10.1046/j.1365-2656.1998.00203.x
Joshi, A.R., David Smith, J.L., and Cuthbert, F.J. 1995. Influence of food distribution and predation pressure on spacing behavior in palm civets. Journal of Mammalogy, 76:1205-1212.
https://doi.org/10.2307/1382613
Julien-Laferrière, D. and Atramentowicz, M. 1990. Feeding and reproduction of three didelphid marsupials in two neotropical forests (French Guiana). Biotropica, 22:404-415.
https://doi.org/10.2307/2388558
Kay, R.F. 1975. The functional adaptations of primate molar teeth. American Journal of Physical Anthropology, 43:195-216.
https://doi.org/10.1002/ajpa.1330430207
Kay, R.F. 1984. On the use of anatomical features to infer foraging behavior in extinct primates, p. 21-53. In Rodman, P.S. and Cant, J.G.H. (eds.), Adaptations for Foraging in Nonhuman Primates: Contributions to an Organismal Biology of Prosimians, Monkeys, and Apes. Columbia University Press, New York.
https://doi.org/10.7312/rodm90184-003
Keddy, P.A. 1989. Competition. Chapman and Hall, New York.
https://doi.org/10.1007/978-94-010-9011-7
Kielan-Jaworowska, Z., Cifelli, R.L., and Luo, Z. 2004. Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure. Columbia University Press, New York.
Kissling, W.D., Dalby, L., Fløjgaard, C., Lenoir, J., Sandel, B., Sandom, C., Trøjelsgaard, K., and Svenning, J. 2014. Establishing macroecological trait datasets: digitization, extrapolation, and validation of diet preferences in terrestrial mammals worldwide. Ecology and Evolution, 4:2913-2930.
https://doi.org/10.1002/ece3.1136
Krause, D.W. 2001. Fossil molar from a Madagascan marsupial. Nature, 412:497-498.
https://doi.org/10.1038/35087649
Kruuk, H. 1972. The Spotted Hyena: A Study of Predation and Social Behavior. University of Chicago Press, Chicago.
Kumar, S., Stecher, G., Suleski, M., and Hedges, S.B. 2017. TimeTree: a resource for timelines, timetrees, and divergence times. Molecular Biology and Evolution, 34:1812-1819.
https://doi.org/10.1093/molbev/msx116
Lessa, L.G. and Geise, L. 2014. Food habits of Metachirus nudicaudatus (Didelphimorphia, Didelphidae) in a Brazilian Cerrado: diet composition and dietary seasonality. Studies on Neotropical Fauna and Environment, 49:75-78.
https://doi.org/10.1080/01650521.2014.924805
Linley, G.D., Rypalski, A., Story, G., and Ritchie, E.G. 2020. Run rabbit run: spotted-tailed quoll diet reveals invasive prey is top of the menu. Australian Mammalogy, 43:221-225.
https://doi.org/10.1071/am19069
Lloyd, G.T., Davis, K.E., Pisani, D., Tarver, J.E., Ruta, M., Sakamoto, M., Hone, D.W.E., Jennings, R., and Benton, M.J. 2008. Dinosaurs and the Cretaceous Revolution. Proceedings of the Royal Society B, 275:2483-2490.
https://doi.org/10.1098/rspb.2008.0715
López-Torres, S., Selig, K.R., Prufrock, K.A., Lin, D., and Silcox, M.T. 2017. Dental topographic analysis of paromomyid (Plesiadapiformes, Primates) cheek teeth: more than 15 million years of changing surfaces and shifting ecologies. Historical Biology, 30:76-88.
http://doi.org/10.1080/08912963.2017.1289378
Luckett, W.P. 1993. An ontogenetic assessment of dental homologies in therian mammals, p.182-204. In Szalay, F.S., Novacek, M.J., and McKenna, M.C. (eds.), Mammal Phylogeny: Mesozoic Differentiation, Multituberculates, Monotremes, Early Therians, and Marsupials. Springer, New York.
Luo, Z. 2007. Transformation and diversification in early mammal evolution. Nature, 450:1011-1019.
https://doi.org/10.1038/nature06277
Lupia, R., Lidgard, S., and Crane, P.R. 1999. Comparing palynological abundance and diversity: implications for biotic replacement during the Cretaceous angiosperm radiation. Paleobiology, 25:305-340.
https://doi.org/10.1017/s009483730002131x
Magallón, S., Hilu, K.W., and Quandt, D. 2013. Land plant evolutionary timeline: gene effects are secondary to fossil constraints in relaxed clock estimation of age and substitution rates. American Journal of Botany, 100:556-573.
https://doi.org/10.3732/ajb.1200416
Magallón, S., Gómez-Acevedo, S., Sánchez-Reyes, L.L., and Hernández-Hernández, T. 2015. A metacalibrated time-tree documents the early rise of flowering plant phylogenetic diversity. New Phytologist, 207:437-453.
https://doi.org/10.1111/nph.13264
Martin, J.E., Case, J.A., Jagt, J.W.M., Schulp, A.S., and Mulder, E.W.A. 2005. A new European marsupial indicates a Late Cretaceous high-latitude transatlantic dispersal route. Journal of Mammalian Evolution, 12:495-511.
https://doi.org/10.1007/s10914-005-7330-x
McCracken, K.E. 1990. Microhabitat and dietary partitioning in three species of shrews at Yellow Bay Montana. Unpublished Masters Thesis, University of Montana, Missoula, Montana, USA.
McKenna, M.C. 1975. Toward a phylogenetic classification of the Mammalia, p. 21-46. In Luckett, W.P. and Szalay, F.S. (eds.), Phylogeny of the Primates. Plenum, New York.
https://doi.org/10.1007/978-1-4684-2166-8_2
Medellin, R.A. 1994. Seed dispersal of Cecropia obtusifolia by two species of opossums in the Selva Lacandona, Chiapas, Mexico. Biotropica, 26:400-407.
https://doi.org/10.2307/2389234
Meredith, R.W, Janečka, J.E., Gatesy, J., Ryder, O.A., Fisher, C.A., Teeling, E.C., Goodbla, A., Eizirik, E., Simão, T.L.L., Stadler, T., Rabosky, D.L., Honeycutt, R.L., Flynn, J.J., Ingram, C.M., Steiner, C., Williams, T.L., Robinson, T.J., Burk-Herrick, A., Westerman, M., Ayoub, N.A., Springer, M.S., and Murphy, W.J. 2011. Impacts of the Cretaceous Terrestrial Revolution and KPg extinction on mammal diversification. Science, 334:521-524.
https://doi.org/10.1126/science.1211028
Meserve, P.L. 1981. Trophic relationships among small mammals in a Chilean semiarid thorn scrub community. Journal of Mammalogy, 62:304-314.
https://doi.org/10.2307/1380707
Mondolfi, E. and Padilla, G.M. 1958. Contribución al conocimiento del “perrito de agua” (Chironectes minimus Zimmermann). Memoria de la Sociedad de Ciencias Naturales La Salle, 17:41-155.
Moore, A.W. 1933. Food habits of Townsend and coast moles. Journal of Mammalogy, 14:36-40.
https://doi.org/10.2307/1374030
Morales-García, N.M., Gill, P.G., Janis, C.M., and Rayfield, E.J. 2021. Jaw shape and mechanical advantage are indicative of diet in Mesozoic mammals. Communications Biology, 4:242.
https://doi.org/10.1038/s42003-021-01757-3
Nakashima, Y., Inoue, E., Inoue-Murayama, M., and Abd. Sukor, J. 2010. High potential of a disturbance-tolerant frugivore, the common palm civet Paradoxurus hermaphroditus (Viverridae), as a seed disperser for large-seeded plants. Mammal Study, 35:209-215.
https://doi.org/10.3106/041.035.0307
Newham, E., Benson, R., Upchurch, P., and Goswami, A. 2014. Mesozoic mammaliaform diversity: the effect of sampling corrections on reconstructions of evolutionary dynamics. Palaeogeography, Palaeoclimatology, and Palaeoecology, 412:32-44.
https://doi.org/10.1016/j.palaeo.2014.07.017
Nowak, R.M. 1999. Walker’s Mammals of the World, Sixth Edition. The Johns Hopkins University Press, Baltimore, Maryland.
O’Leary, M.A., Bloch, J.I., Flynn, J.J., Gaudin, T.J., Giallombardo, A., Giannini, N.P., Goldberg, S.L., Kraatz, B.P., Luo, Z., Meng, J., Ni, X., Novacek, M.J., Perini, F.A., Randall, Z.S., Rougier, G.W., Sargis, E.J., Silcox, M.T., Simmons, N.B., Spaulding, M., Velazco, P.M., Weksler, M., Wible, J.R., and Cirranello, A.L. 2013. The Placental mammal ancestor and the post-K-Pg radiation of placentals. Science, 339:662-667.
https://doi.org/10.1126/science.1229237
Pagel, M.D. 1992. A method for the analysis of comparative data. Journal of Theoretical Biology, 156:431-442.
https://doi.org/10.1016/s0022-5193(05)80637-x
Pampush, J.D., Winchester, J.M., Morse, P.E., Vining, A.Q., Boyer, D.M., and Kay, R.F. 2016. Introducing molaR: a new R package for quantitative topographic analysis of teeth (and other topographic surfaces). Journal of Mammalian Evolution, 23:397-412.
https://doi.org/10.1007/s10914-016-9326-0
Parolin, L.C., Bianconi, G.V., and Mikich, S.B. 2016. Consistency in fruit preferences across the geographical range of the frugivorous bats Artibeus, Carollia and Sturnira (Chiroptera). Iheringia, Série Zoologia, 106: e2016010.
https://doi.org/10.1590/1678-4766e2016010
Pemberton, D., Gales, S., Bauer, B., Gales, R., Lazenby, B., and Medlock, K. 2008. The diet of the Tasmanian Devil, Sarcophilus harrisii, as determined from analysis of scat and stomach contents. Papers and Proceedings of the Royal Society of Tasmania, 142:13-22.
https://doi.org/10.26749/rstpp.142.2.13
Peng, A., Toews, N., and Wilson, G.P. 2017. An ontogenetic investigation of a Cretaceous North American mammal, Didelphodon vorax (Metatheria: Marsupialiformes: Stagodontidae), through quantitative and descriptive analysis of the dentary. Geological Society of America Annual Meeting Abstracts with Programs, 49.
https://doi.org/10.1130/abs/2017AM-300648
Pineda-Munoz, S. and Alroy, J. 2014. Dietary characterization of terrestrial mammals. Proceedings of the Royal Society B, 281:20141173.
https://doi.org/10.1098/rspb.2014.1173
Pineda-Munoz, S., Lazagabaster, I.A., Alroy, J., and Evans, A.R. 2016. Inferring diet from dental morphology in terrestrial mammals. Methods in Ecology and Evolution, 8:481-491.
https://doi.org/10.1111/2041-210X.12691
Prufrock, K.A., López- Torres, S., Silcox, M.T., and Boyer, D.M. 2016. Surfaces and spaces: troubleshooting the study of dietary niche space overlap between North American stem primates and rodents. Surface Topography: Metrology and Properties, 4:024005.
https://doi.org/10.1088/2051-672X/4/2/024005
Rabosky, D.L. and Adams, D.C. 2012. Rate of morphological evolution are correlated with species richness in salamanders. Evolution, 66:1807-1818.
https://doi.org/10.1111/j.1558-5646.2011.01557.x
Ramírez-Barahona, S., Barrera-Redondo, J., and Eguiarte, L.E. 2016. Rates of ecological divergence and body size evolution are correlated with species diversification in scaly tree ferns. Proceedings of the Royal Society B, 283:20161098.
https://doi.org/10.1098/rspb.2016.1098
Revell, L.J. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3:217-223.
https://doi.org/10.1111/j.2041-210x.2011.00169.x
Robinson, J.G. and Redford, K.H. 1986. Body size, diet, and population density of neotropical forest mammals. The American Naturalist, 128:665-680.
https://doi.org/10.1086/284596
Rose, C. and Prange, S. 2015. Diet of the recovering Ohio bobcat (Lynx rufus) with a consideration of two subpopulations. The American Midland Naturalist, 173:305-317.
https://doi.org/10.1674/amid-173-02-305-317.1
Rostovskaya, M.S., Zhukova, D.V., Illarionova, A.E., Ustyugovia, S.V., Borissenko, A.V., and Sviridoz, A.V. 2000. Insect prey of the long-eared bat Plecotus auritus (L.) (Chiroptera: Vespertilionidae). Russian Entomological Journal, 9:185-189.
Rougier, G.W., Wible, J.R., and Novacek, M.J. 1998. Implications of Deltatheridium specimens for early marsupial history. Nature, 396:459-463. https://doi.org/10.1038/24856
Rulison, E.L., Luiselli, L., and Burke, R.L. 2012. Relative impacts of habitat and geography on raccoon diets. The American Midland Naturalist, 168:231-246.
https://doi.org/10.1674/0003-0031-168.2.231
Ruta M., Angielczyk K.D., Fröbisch J., and Benton M.J. 2013 Decoupling of morphological disparity and taxic diversity during the adaptive radiation of anomodont therapsids. Proceedings of the Royal Society B, 280:20131071.
https://doi.org/10.1098/rspb.2013.1071
Sánchez-González, R., Hernández-Saint Martin, A.D., Rosas-Rosas, O.C., and García-Chávez, J. 2018. Diet and abundance of bobcat (Lynx rufus) in the Potosino-Zacatecano Plateau, Mexico. Therya, 9:107-112.
https://doi.org/10.12933/therya-18-498
Sánchez-Villagra, M.R. 2013. Why are there fewer marsupials than placentals? On the relevance of geography and physiology to evolutionary patterns of mammalian diversity and disparity. Journal of Mammal Evolution, 20:279-290.
https://doi.org/10.1007/s10914-012-9220-3
Sandidge, L.L. 1953. Food and dens of the opossum (Didelphis virginiana) in northeastern Kansas. Transactions of the Kansas Academy of Science, 56:97-106.
https://doi.org/10.2307/3626198
Santana, S.E., Strait, S., and Dumont, E.R. 2011. The better to eat you with: functional correlates of tooth structure in bats. Functional Ecology, 25:839-847.
https://doi.org/10.1111/j.1365-2435.2011.01832.x
Santori, R.T., Astúa de Moraes, D., and Cerqueira, R. 1995. Diet composition of Metachirus nudicaudatus and Didelphis aurita (Marsupialia, Didelphoidea) in Southeastern Brazil. Mammalia, 59:511-516.
https://doi.org/10.1515/mamm.1995.59.4.511
Schoener, T.W. 1974. Resource partitioning in ecological communities. Science, 185:27-39. https://doi.org/10.1126/science.185.4145.27
Schoonover, L.T. and Marshall, W.H. 1951. Food habits of the raccoon (Procyon lotor hirtus) in North-Central Minnesota. Journal of Mammalogy, 32:422-428.
https://doi.org/10.2307/1375790
Scott, C.S. and Fox, R.C. 2015. Review of Stagodontidae (Mammalia, Marsupialia) from the Judithian (Late Cretaceous) Belly River Groups of southeastern Alberta, Canada. Canadian Journal of Earth Sciences, 52:682-695.
https://doi.org/10.1139/cjes-2014-0170
Scott, L.K., Hume, I.D., and Dickman, C.R. 1999. Ecology and population biology of long-nosed bandicoots (Perameles nasuta) at North Head, Sydney Harbour National Park. Wildlife Research, 26:805-821.
https://doi.org/10.1071/wr98074
Selig, K.R., Sargis, E.J., and Silcox, M.T. 2019. The frugivorous insectivores? Functional morphological analysis of molar topography for inferring diet in extant treeshrews (Scandentia). Journal of Mammalogy, 100:1901-1917.
https://doi.org/10.1093/jmammal/gyz151
Simpson, G.G. 1937. The beginning of the age of mammals. Biological Reviews, 12(1):1-46.
https://doi.org/10.1111/j.1469-185X.1937.tb01220.x
Smith, F.A., Boyer, A.G., Brown, J.H., Costa, D.P., Dayan, T., Ernest, S.K.M., Evans, A.R., Fortelius, M., Gittleman, J.L., Hamilton, M.J., Harding, L.E., Lintulaakso, K., Lyons, S.K., McCain, C., Okie, J.G., Saarinen, J.J., Sibly, R.M., Stephens, P.R., Theodor, J., and Uhen, M.D. 2010. The evolution of maximum body size of terrestrial mammals. Science, 330:1216-1219.
https://doi.org/10.1126/science.1194830
Smith, S.M. 2017. Mammalian faunal recovery following the Cretaceous-Paleogene mass extinction: a multifaceted investigation. Unpublished PhD Thesis, University of Washington, Seattle, Washington, USA.
Smits, P.D. and Evans, A.R. 2012. Functional constraints on tooth morphology in carnivorous mammals. BMC Evolutionary Biology, 12:146.
https://doi.org/10.1186/1471-2148-12-146
Spradley, J.P. 2017. Dental ecometrics as a proxy of paleoenvironment reconstruction in the Miocene of South America. Unpublished PhD Thesis, Duke University, Durham, North Carolina, USA.
Spradley, J.P., Pampush, J.D., Morse, P.E., and Kay, R.F. 2017. Smooth operator: The effects of different 3D mesh retriangulation protocols on the computation of Dirichlet normal energy. American Journal of Physical Anthropology, 163:94-109.
https://doi.org/10.1002/ajpa.23188
Streilein, K.E. 1982. Behavior, ecology, and distribution of South American marsupials. Special Publication of the Pymatuning Laboratory of Ecology, 6:231-250.
Steiner, K.E. 1981. Nectarivory and potential pollination by a neotropical marsupial. Annals of the Missouri Botanical Garden, 68:505-513.
https://doi.org/10.2307/2398884
Strait, S.G. 1993. Molar morphology and food texture among small-bodied insectivorous mammals. Journal of Mammalogy, 74:391-402.
https://doi.org/10.2307/1382395
Strait, S.G. 1997. Tooth use and the physical properties of food. Evolutionary Anthropology, 5:199-211.
https://doi.org/10.1002/(sici)1520-6505(1997)5:6<199::aid-evan2>3.0.co;2-8
Stucky, R.K. 1990. Evolution of land mammal diversity in North America during the Cenozoic. Current Mammalogy, 2:375-432.
Szalay, F.S. 1994. Evolutionary History of the Marsupials and an Analysis of Osteological Characters. Cambridge University Press, New York.
Szalay, F.S. and Sargis, E.J. 2006. Cretaceous therian tarsals and the metatherian-eutherian dichotomy. Journal of Mammalian Evolution, 13:171-210.
https://doi.org/10.1007/s10914-006-9024-4
Szalay, F.S. and Trofimov, B.A. 1996. The Mongolian Late Cretaceous Asiatherium, and the early phylogeny and paleobiology of Metatheria. Journal of Vertebrate Paleontology, 16:474-509.
https://doi.org/10.1080/02724634.1996.10011335
Thums, M., Klaassen, M., and Hume, I.D. 2005. Seasonal changes in diet of the long-nosed bandicoot (Perameles nasuta) assessed by analysis of faecal scats and of stable isotopes in blood. Australian Journal of Zoology, 53:87-93.
https://doi.org/10.1071/zo04030
Tiffney, B.H. and Mazer, S.J. 1995. Angiosperms growth habit, dispersal and diversification reconsidered. Evolutionary Ecology, 9:93-117.
https://doi.org/10.1071/zo04030
Trombulak, S.C. 1985. The influence of interspecific competition on home range size in chipmunks (Eutamias). Journal of Mammalogy, 66:329-337.
https://doi.org/10.2307/1381245
Ungar, P.S. 2010. Mammal Teeth: Origin, Evolution, and Diversity. The Johns Hopkins University Press, Baltimore, Maryland, USA.
https://doi.org/10.5860/choice.48-3875
Ungar, P.S. and M’Kirera, F. 2003. A solution to the worn tooth conundrum in primate functional anatomy. Proceedings of the National Academy of Sciences, 100:3874-3877.
https://doi.org/10.1073/pnas.0637016100
Van Valen, L. and Sloan, R.E. 1997. Contemporaneity of late Cretaceous extinctions. Nature, 270:193.
https://doi.org/10.1038/270193a0
Van Valkenburgh, B. 2007. Déjà vu: the evolution of feeding morphologies in the Carnivora. Integrative and Comparative Biology, 47:147-163.
https://doi.org/10.1093/icb/icm016
Venables, W.N. and Ripley, B.D. 2013. Modern applied statistics with S-PLUS. Springer Science and Business Media, Berlin, Germany.
Vullo, R., Gheerbrant, E., de Muizon, C., and Néraudeau, D. 2009. The oldest modern therian mammal from Europe and its bearing on stem marsupial paleobiology. Proceedings of the National Academy of Sciences, 106:19910-19915.
https://doi.org/10.1073/pnas.0902940106
Weaver, L.N. and Wilson, G.P. 2021. Shape disparity in the blade-like premolars of multituberculate mammals: functional constraints and the evolution of herbivory. Journal of Mammalogy, 102:967-985.
https://doi.org/10.1093/jmammal/gyaa029
Weaver, L.N., Wilson, G.P., Krumenacker, L.J., McLaughlin, K., Moore, J.R., and Varricchio, D.J. 2019. New multituberculate mammals from the mid-Cretaceous (Lower Cenomanian) Wayan Formation of southeastern Idaho and implications for the early evolution of Cimolodonta. Journal of Vertebrate Paleontology, 39:e1604532.
https://doi.org/10.1080/02724634.2019.1604532
Werdelin, L. 1989. Constraint and adaptation in the bone-cracking canid Osteoborus (Mammalia: Canidae). Paleobiology, 15:387-401.
https://doi.org/10.1017/s009483730000957x
Whitaker, Jr. J.O. 1995. Food of the big brown bat Eptesicus fuscus from maternity colonies in Indiana and Illinois. The American Midland Naturalist, 134:346-360.
https://doi.org/10.2307/2426304
Whitaker, J.O. and Karataş, A. 2009. Food and feeding habits of some bats from Turkey. Acta Chiropterologica, 11:393-403.
https://doi.org/10.3161/150811009x485611
Wible, J.R., Rougier, G.W., Novacek, M.J, and Sher, R.J. 2007. Cretaceous eutherians and Laurasian origin for placental mammals near the K/T boundary. Nature, 447:1003-1006.
https://doi.org/10.1038/nature05854
Williamson, T.E., Brusatte, S.L., and Wilson, G.P. 2014. The origin and early evolution of metatherian mammals: The Cretaceous record. ZooKeys, 76:1-76.
https://doi.org/10.3897/zookeys.465.8178
Wilman, H., Belmaker, J., Simpson, J., De La Rosa, C., Rivadeneira, M.M., and Jetz, W. 2014. EltonTraits 1.0: species-level foraging attributes of the world’s birds and mammals. Ecology, 95:2027.
https://doi.org/10.1890/13-1917.1
Wilson, G.P. 2005. Mammalian faunal dynamics during the last 1.8 million years of the Cretaceous in Garfield County, Montana. Journal of Mammalian Evolution, 12:53-76.
https://doi.org/10.1007/s10914-005-6943-4
Wilson, G.P. 2013. Mammals across the K/Pg boundary in northeastern Montana, U.S.A.: dental morphology and body-size patterns reveal extinction selectivity and immigrant-fueled ecospace filling. Paleobiology, 39:429-469.
https://doi.org/10.1666/12041
Wilson, G.P. 2014. Mammalian extinction, survival, and recovery dynamics across the Cretaceous-Paleogene boundary in northeastern Montana, USA, p. 1-28. In Wilson, G.P., Clemens, W.A., Horner, J.R., and Hartman, J.H. (eds.), Through the End of the Cretaceous in the Type Locality of the Hell Creek Formation in Montana and Adjacent Areas.
https://doi.org/10.1130/2014.2503(15)
Wilson, G.P. and Riedel, J.A. 2010. New specimen reveals deltatheroidan affinities of the North American Late Cretaceous mammal Nanocuris. Journal of Vertebrate Paleontology, 30:872-884.
https://doi.org/10.1080/02724631003762948
Wilson, G.P., Evans, A.R., Corfe, I.J., Smits, P.D., Fortelius, M., and Jernvall, J. 2012. Adaptive radiation of multituberculate mammals before the extinction of dinosaurs. Nature, 483:457-460.
https://doi.org/10.1038/nature10880
Wilson, G.P., Ekdale, E.G., Hoganson, J.W., Calede, J.J., and Vander Linden, A. 2016. A large carnivorous mammal from the Late Cretaceous and the North American origin of marsupials. Nature Communications, 7:13734.
https://doi.org/10.1038/ncomms13734
Wilson Mantilla, G.P., Chester, S.G., Clemens, W.A., Moore, J.R., Sprain, C.J., Hovatter, B.T., Mitchell, W.S., Mans, W.W., Mundil, R., and Renne, P.R. 2021. Earliest Paleocene purgatoriids and the initial radiation of stem primates. Royal Society Open Science, 8:210050.
https://doi.org/10.1098/rsos.210050
Winchester, J.M. 2016. MorphoTester: an open source application for morphological topographic analysis. PLoS ONE, 11(2):e0147649.
https://doi.org/10.1371/journal.pone.0147649
Winchester, J.M., Boyer, D.M., St. Clair, E.M., Gosselin-Ildari, A.D., Cooke, S.B., and Ledogar, J.A. 2014. Dental topography of platyrrhines and prosimians: convergence and contrasts. American Journal of Physical Anthropology, 153:29-44.
https://doi.org/10.1002/ajpa.22398
Wing, S.L. and Boucher, L.D. 1998. Ecological aspects of the Cretaceous flowering plant radiation. Annual Review of Earth and Planetary Sciences, 26:379-421.
https://doi.org/10.1146/annurev.earth.26.1.379
Witmer, L.M. 1995. The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils, p. 19-33. In Thomason, J. (ed.), Functional morphology in Vertebrate Paleontology. Cambridge University Press, New York.
Woodburne, M.O. 2004. Definitions, p. xi-xvi, In Woodburne, M.O. (ed.), Late Cretaceous and Cenozoic Mammals of North America: Biostratigraphy and Geochronology. Columbia University Press, New York.
https://doi.org/10.7312/wood13040
Wroe, S., McHenry, C., and Thomason, J. 2005. Bite club: comparative bite force in big biting mammals and the prediction of predatory behaviour in fossil taxa. Proceedings of the Royal Society B, 272:691-625.
https://doi.org/10.1098/rspb.2004.2986
Zortéa, M. and Mendes, S.L. 1993. Folivory in the big fruit-eating bat, Artibeus lituratus (Chiroptera: Phyllostomidae) in eastern Brazil. Journal of Tropical Ecology, 9:117-120.
https://doi.org/10.1017/s026646740000705

