 An overview of crawling water beetle larvae and a first possible record from 100-million-years-old Myanmar amber
An overview of crawling water beetle larvae and a first possible record from 100-million-years-old Myanmar amber
Article number: 26.3.a42
https://doi.org/10.26879/1290
Copyright Palaeontological Association, September 2023
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 7 April 2023. Acceptance: 11 September 2023.
ABSTRACT
The group Coleoptera contributes heavily to the modern-day species diversity and biomass. Most individuals in the modern fauna are present as larvae, since these live for quite long in some cases, and a lot of individuals never reach adulthood. Despite this fact, the larval stages often get less attention compared to the adults. The group of crawling water beetles, Haliplidae, is an ingroup of Adephaga, living mainly in freshwater as adults. The larvae also live in water, but do not swim like the adults; instead, they move over the surface of the ground and climb on water plants. The larvae develop through three larval stages; all have an elongated shape, and in most species the trunk end is strongly elongated, bearing numerous setae. Herein, we review the entire record of water crawling beetle larvae, report possible fossils, and compare the shape of their overall body outlines using an elliptic Fourier analysis. The fossils show a lower variation in comparison to modern fauna; the shapes of the fossils are well represented in the modern fauna. A minor difference is rather elongate thorax segments of the fossils in comparison to their extant counterparts. The new fossils expand the record of fossil adephagan larvae.
Simon Josef Linhart. Faculty of Biology, Ludwig-Maximilians-Universität München (LMU Munich), Großhaderner Str. 2, 82152 Planegg-Martinsried, Germany. S.Linhart@campus.lmu.de
Patrick Müller. Kreuzbergstr. 90, 66482 Zweibrücken, Germany. pat14789@web.de
Gideon T. Haug. Faculty of Biology, Ludwig-Maximilians-Universität München (LMU Munich), Großhaderner Str. 2, 82152 Planegg-Martinsried, Germany. gideon.haug@palaeo-evo-devo.info
Carolin Haug. Faculty of Biology, Ludwig-Maximilians-Universität München (LMU Munich), Großhaderner Str. 2, 82152 Planegg-Martinsried, Germany and GeoBio-Center at LMU, Richard-Wagner-Str. 10, 80333 München, Germany. carolin.haug@palaeo-evo-devo.info
Joachim T. Haug. Faculty of Biology, Ludwig-Maximilians-Universität München (LMU Munich), Großhaderner Str. 2, 82152 Planegg-Martinsried, German and GeoBio-Center at LMU, Richard-Wagner-Str. 10, 80333 München, Germany. joachim.haug@palaeo-evo-devo.info
Keywords: Haliplidae; Coleoptera; Cretaceous; diversity; Burmese amber; larva
Final citation: Linhart, Simon Josef, Müller, Patrick, Haug, Gideon T., Haug, Carolin, and Haug, Joachim T. 2023. An overview of crawling water beetle larvae and a first possible record from 100-million-years-old Myanmar amber. Palaeontologia Electronica, 26(3):a42.
https://doi.org/10.26879/1290
palaeo-electronica.org/content/2023/3964-new-cretaceous-beetle-larvae
Copyright: September 2023 Palaeontological Association.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
The group Holometabola represents nowadays a major share of the overall animal diversity; especially the group of Coleoptera (beetles) with about 380,000 formally described extant beetle species (McKenna et al., 2019) has an enormous individual richness and biomass. The majority of investigations on beetles, including for example formal descriptions, focus on adult specimens, the supposedly “important” life stage. Yet, given the sometimes short lifespan of the adult, the larval life phase is ecologically at least as important (possibly even more). Still, the larva (or even several larval stages) is known for only a minority of the formally described species of beetles.
Most beetles are important components of terrestrial ecosystems. However, there are also numerous groups of beetles with representatives specialized to aquatic ecosystems (Bouchard et al., 2017). One of these groups is Haliplidae, its representatives are known as crawling water beetles (Beutel et al., 2006). Haliplidae is an ingroup of Adephaga as demonstrated by morphological (Beutel and Haas, 1996; Beutel et al., 2006) as well as molecular studies (Ribera et al., 2002). So far, 238 extant species of Haliplidae have been formally described (Vondel, 2016). Larvae are only known for 21 species, only in few cases several different larval stages (instars) are known for one species, summing up to 31 larval specimens in the literature (details below).
Crawling water beetles spend most of their life in freshwater: during almost their entire larval phase, but also most of the time as adults. The adults carry air under their enlarged coxal plates (for details of air storage, see Matheson, 1912), which conceal the trochanter, parts of the femur and also parts of the abdomen (Matta, 1976).
The adults have been described as poor swimmers, living in shallow waters in habitats occupied by plants (Matheson, 1912; Hickman, 1931; Matta, 1976; Ghosh, 2021). When they swim, they use their setose legs (Matheson, 1912; Hickman, 1931), mostly the last pair, in an alternating pattern (Vondel, 2021). When not swimming, the adults crawl over the ground or the vegetation (Hickman, 1931; Matta, 1976) and hide when they are disturbed (Hickman, 1931). They seem to also walk on land (Hickman, 1931) and can also fly, but have rarely been observed to do so (Vondel, 1997). It seems that the adults are attracted by electric light; at least they have been found close to electric light sources (Hickman, 1931). Adult crawling water beetles have been considered omnivorous (Vondel, 1997), but also as mostly herbivorous (on algae; Hickman, 1931), feeding on animals only under the risk of starvation (Malcolm, 1971).
Crawling water beetles have three larval stages (instars) before pupating (see Haug, 2020 for terminological issues). Larval stages are comparably short-lived, with stage 1 only lasting 5 to 7 days, stage 2 lasting 6 to 8 days, and stage 3 lasting 5 to 10 days. Yet the duration of stage 3 can be prolonged (Hickman, 1931). Unlike in the adult, gas exchange is performed by filamentous tracheal gills (Vondel, 1997). Stage 3 larvae have functional spiracles that allow them to breath on land (Vondel, 1997).
The larvae of crawling water beetles live between algae, camouflaging between them (Vondel, 1997). Larvae are unable to swim (Leng, 1913; Vondel, 1997), only locomoting slowly between the algae (Vondel and Spangler, 2008). When getting disturbed, they curl up and remain in this position for some minutes (Hickman, 1931). The trunk end of the larva is prominently elongated (Vondel, 1997) and has been interpreted as derived from urogomphi (Klausnitzer, 1978; Vondel, 1997; Michat et al., 2020) or the last abdominal segment (Makarov and Prokin, 2015). It seems likely that this structure is a compound of several segments, yet it cannot be easily excluded that urogomphi are somehow participating in forming this structure; we will here use the neutral expression 'trunk end'.
The trunk end of the larvae is sometimes forked (Vondel, 1997; Makarov and Prokin, 2015), as for example in Haliplus subseriatus (Vondel, 2001) or in Haliplus kulleri (Vondel, 2011a), now considered as Haliplus abbreviatus (Vondel and Litovkin, 2017). The posterior trunk or abdomen of the larvae has been reported to have 9-10 visible units (Vondel, 1997; Makarov and Prokin, 2015), i.e., 8-9 abdominal segments plus the trunk end.
The mandibles form a suction-channel (Vondel, 1997) and are used to sting into the cells of water plants, mostly algae, and suck on these (Matta, 1976; Lawrence and Newton, 1982; Vondel, 1997). Hence, larvae are completely herbivorous (Hickman, 1931). The tarsi of the larvae consist of a single element, each bearing a single claw. The larvae go ashore and burrow into the ground to pupate (Vondel, 1997). This procedure seems necessary to develop normally (Hickman, 1931).
There are four well-recognised ingroups in Haliplidae: Peltodytes, Brychius, Phalilus, and Haliplus, with Peltodytes having been resolved as the sistergroup of a group including Brychius, Phalilus, and Haliplus (Beutel et al., 2006; Vondel, 2019). Haliplus contains most species of these three groups (Matheson, 1912) with about 161 species worldwide (Vondel and Alarie, 2016). Larvae of Haliplus, Brychius and Phalilus have short microtracheal gills, while larvae of Peltodytes have long tracheal gills for gas exchange (Vondel, 2012, 2016, 2019). Larvae of Peltodytes have long spines dorsally, larvae of Haliplus have shorter spines (Gundersen and Otremba, 1988). Larvae of Brychius live only in running waters as they need an oxygen-rich environment (Vondel, 1997).
The fossil record of Haliplidae is very scarce (Prokin and Ponomarenko, 2013) and restricted to finds of adults so far. The oldest records are from the Early Cretaceous (Prokin and Ponomarenko, 2013). Most of the fossils come from sedimentary rocks (Řiha, 1979; Prokin and Ponomarenko, 2013) and the specimens are often fragmentary (e.g., Prokop et al., 2004).
We here report new fossils preserved in about 100-million-years-old Kachin amber, Myanmar (Cruickshank and Ko, 2003; Shi et al., 2012; Yu et al., 2019). These fossils resemble larvae of modern crawling water beetles in many aspects. Fossil beetle larvae are still rare, but can be considered well known, for example in Cretaceous ambers (e.g., Grimaldi and Engel, 2005; Fikáček et al., 2014; Xia et al., 2015; Beutel et al., 2016; Zhang, 2017; Batelka et al., 2019, 2021; Gustafson et al., 2020; Zhao et al., 2019, 2020; Haug et al., 2021a, b, 2023; Zippel et al., 2022a, b, 2023; Liu et al., 2023). We compare the new fossils in a quantitative morphological frame with modern crawling water beetle larvae.
MATERIAL AND METHODS
Material
Three amber pieces are in the centre of this study, all from Cretaceous Kachin amber, Myanmar. Two specimens are part of the collection of one of the authors (PM) and are stored under repository numbers BUB 4436 and BUB 1222. They were legally purchased by one of the authors (PM) in the year 2016. One specimen is deposited in the Palaeo-Evo-Devo Research Group Collection of Arthropods, Ludwig-Maximilians-Universität München, Germany (LMU Munich), Germany, under repository number PED 1859. It was legally purchased on the trading platform ebay.com from the trader burmite-miner. In total, 13 beetle larvae are preserved in the three amber pieces, 11 of these in BUB 4436 and one larva in each of the other two pieces.
Extant comparative data comes from the literature. Extant larval specimens of the groups Haliplus, Brychius, and Peltodytes could be included. In total, 31 larvae of crawling water beetles from the literature (listed in detail below) and 10 of the fossils were analysed quantitatively (Suppl. Table 1). Images that were repetitively depicted were re-drawn from the version with the better representation in the available literature.
Documentation Methods
Fossil specimens were documented on a Keyence VHX-6000 digital microscope. Each specimen was tested with different illumination settings: unpolarised low-angle ring light and cross-polarised co-axial light, each with black and white background. Each image is a composite image combing different focus layers, panoramas of adjacent image details, as well as HDR. Images were further processed in Adobe Photoshop CS2 (for more details, see Haug et al., 2021a).
Outlines to be used in the quantitative analysis were drawn in Adobe Illustrator CS2 or Inkscape. Only one half was used. Appendages were omitted, the body was artificially straightened (details in Haug et al., 2021a)
The extant specimens from the literature were re-drawn in Inkscape. Only one half was drawn (the better preserved one), mirrored, and compared with the original image, especially if the width is correctly represented (Haug et al., 2021a).
Quantitative Analysis
A morphometric analysis was performed in the free program package SHAPE. It combines an elliptic Fourier transformation with a principle component analysis (Iwata and Ukai, 2002; Braig et al., 2019).
In addition, different lengths were measured. For extant specimens from the literature, this was only possible when a scale was provided. For fossil specimens, it was only possible when the structures of interest were (completely) available. Measured lengths include: 1) the total body length, from the anterior middle of the head capsule to the tip of the trunk end; 2) the length of the head capsule from the anterior middle to the posterior middle; 3) the width of the head capsule at the broadest point.
Measurements could be performed on 26 specimens: all measurements could be performed on 14 extant specimens from the literature and on 10 fossil specimens. For one fossil specimen (BUB 4436 specimen 2), only the total body length and the length of the head capsule could be measured. On one specimen (BUB 44362 specimen 6), only the length of the head capsule could be measured.
RESULTS
Extant Larvae of the Group Haliplidae
All larvae of crawling water beetles in dorsal (or ventral) view with a sufficient degree of detail were considered for the analysis. All occurrences are listed chronologically; this is necessary to avoid considering the same specimen twice if having been re-figured (we used this approach already in previous studies, e.g., Haug et al., 2020, 2021b, c, 2022b).
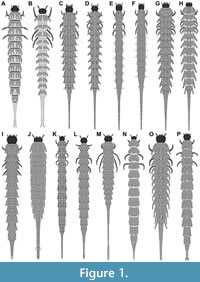 1) Böving and Craighead (1931 their p. 97 plate 5G; re-figured in Klausnitzer, 1977 p. 163 figure 16) provided a drawing of a larva of Haliplus confinis (specimen Hal 001; Figure 1A). The larval stage was not mentioned. No indication of size was provided. The authors also provided close-up drawings of the third leg (their p. 97 plate 5F) and of the head (their p. 97 plate 5H).
1) Böving and Craighead (1931 their p. 97 plate 5G; re-figured in Klausnitzer, 1977 p. 163 figure 16) provided a drawing of a larva of Haliplus confinis (specimen Hal 001; Figure 1A). The larval stage was not mentioned. No indication of size was provided. The authors also provided close-up drawings of the third leg (their p. 97 plate 5F) and of the head (their p. 97 plate 5H).
2) Bertrand (1933) provided drawings of three larvae of the group Haliplus and of one larva of the group Brychius. The first specimen was (presumably) a stage 3 larva (his p. 527 figure 9) of Haliplus lineatocollis (specimen Hal 025; Figure 2G). No indication of size was provided.
The second specimen was (presumably) a stage 2 larva (his p. 527 figure 10) of Haliplus lineatocollis (specimen Hal 026; Figure 2F). No indication of size was provided.
 The third specimen was (presumably) a stage 1 larva (his p. 527 figure 11) of Haliplus lineatocollis (specimen Hal 027; Figure 2E). No indication of size was provided.
The third specimen was (presumably) a stage 1 larva (his p. 527 figure 11) of Haliplus lineatocollis (specimen Hal 027; Figure 2E). No indication of size was provided.
The fourth specimen was a larva (his p. 529 figure 16) of Brychius elevatus (specimen Hal 028; Figure 1P). The larval stage was not mentioned. No indication of size was provided.
3) Peterson (1957 his p. 169 figure C42 H) provided a drawing of a larva of Haliplus (specimen Hal 003; Figure 1B). The species was not further determined. The larval stage was not mentioned. Total length of the larva was stated to have been 6 mm.
The author also provided a drawing of a larva of Peltodytes (his p. 169 figure C42F). The larva was shown in lateral view and could therefore not be further considered here (re-figured in Klausnitzer, 1977 p. 163 figure 15).
4) Klausnitzer (1978 his p. 269 plate H6) provided a drawing of a larva of Haliplus fulvus (specimen Hal 002; Figure 2M). The larval stage was not mentioned. No indication of size was provided. According to author the original drawing was provided by Schiödte (1864 his p. 46 plate 8 figure 16; the specimen was redrawn from Klausnitzer, 1978 as more details were accessible in the electronic version available to the authors).
5) Vondel (1986 his p. 129 figure 1) provided a drawing of a stage 3 larva of Haliplus laminatus (specimen Hal 020; Figure 1K). No indication of size was provided. The author also provided a lateral view (his p. 130 figures 5 and 6), close-up drawings of one half of the head and the pro- and mesothorax (his p. 129 figure 2), the antenna (his p. 130 figure 7), the mandible (his p. 130 figure 8), the left foreleg (his p. 130 figure 9), tibia and tarsus (his p. 130 figure 10) and abdominal segments 1 (his p. 129 figure 3), 8 and the trunk end (his p. 129 figure 4).
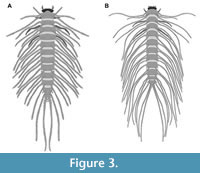 6) Spangler (1991) provided drawings of one larva of the group Peltodytes and of one larva of the group Haliplus. The first specimen was a larva of Peltodytes (his p. 311 figure 34.105; specimen Hal 014; Figure 3B). The species was not further determined. The larval stage was not mentioned. According to the provided scale bar, the overall length was 10.71 mm; the length of the head capsule was 0.16 mm; the width of the head capsule was 0.87 mm.
6) Spangler (1991) provided drawings of one larva of the group Peltodytes and of one larva of the group Haliplus. The first specimen was a larva of Peltodytes (his p. 311 figure 34.105; specimen Hal 014; Figure 3B). The species was not further determined. The larval stage was not mentioned. According to the provided scale bar, the overall length was 10.71 mm; the length of the head capsule was 0.16 mm; the width of the head capsule was 0.87 mm.
The second specimen was a larva (his p. 311 figure 34.106) of Haliplus (specimen Hal 015; Figure 1J). The species was not further determined. The larval stage was not mentioned. According to the provided scale bar, the overall length was 7.55 mm; the length of the head capsule was 0.29 mm; the width of the head capsule was 0.52 mm.
7) Vondel (1995 his p. 115 figure 2) provided a drawing of a stage 3 larva of Haliplus apicalis (specimen Hal 024; Figure 2J). No indication of size was provided.
8) Vondel (1996 his p. 10 figure 1) provided a drawing of a stage 3 larva of Haliplus varius (specimen Hal 021; Figure 1L). No indication of size was provided. The author also provided a lateral view of the head and thorax (his p. 10 figure 4), close-up drawings of the prothorax (his p. 10 figure 7), the antenna (his p. 10 figure 2), the mandible (his p. 10 figure 3), the first leg (his p. 10 figure 5) and the second leg (his p. 10 figure 6).
9) Vondel (1997) provided drawings of three larvae of the group Haliplus. The first specimen was a stage 1 larva of Haliplus apicalis (his p. 69 figure 38a; specimen Hal 029; Figure 2H). No indication of size was provided.
The second specimen was a stage 2 larva of Haliplus apicalis (his p. 69 figure 38b; specimen Hal 030; Figure 2I). No indication of size was provided.
The third specimen was a stage 3 larva of Haliplus variegatus (his p. 71 figure 40c; specimen Hal 031; Figure 1O). No indication of size was provided.
10) Vondel (2001) provided a drawing of a stage 3 larva of Haliplus subseriatus (his p. 15 figure 1; specimen Hal 022; Figure 1M). No indication of size was provided. The author also provided a lateral view of the head, thorax and the first two abdominal segments (his p. 15 figure 2), close-up drawings of the head and the thorax (his p. 15 figure 3), the antenna (his p. 15 figure 7), the mandible (his p. 15 figure 8) and different views of the legs (his p. 15 figures 4-6).
11) Vondel (2004) provided drawings of two larvae of the group Haliplus. The first specimen was a stage 3 larva of Haliplus testudo (his p. 58 figure 1; specimen Hal 009; Figure 1G). No indication of size was provided. The author also provided a ventral view (his p. 58 figure 2), close-ups of the antenna (his p. 58 figure 3), the mandible (his p. 58 figure 4), and of the legs (his p. 58 figures 5-7).
The second specimen was a stage 3 larva of Haliplus timmsi (his p. 60 figure 8; specimen Hal 010; Figure 1H). No indication of size was provided. The author also provided a ventral view (his p. 60 figure 9), close-ups of the antenna (his p. 60 figure 10), the mandible (his p. 60 figure 11), and of the legs (his p. 60 figures 12-14).
12) White (2009) provided drawings of two larvae of Haliplidae (his p. 148 figures 5, 6). No further information was included. Therefore, it was not further considered in our analyses.
13) Vondel (2011b) provided drawings of one larva of the group Peltodytes and of one larva of the group Haliplus. The first specimen was a stage 3 larva of Peltodytes caesus (his p. 128 figure 1; specimen Hal 011; Figure 3A). No indication of size was provided. According to the author, the original drawing was provided by Vondel (1997 his p. 71 figure 40a; the specimen was redrawn from Vondel, 2011b as more details were accessible in the electronic version available to the authors).
The second specimen was a stage 3 larva of Haliplus variomaculatus (his p. 129 figure 18; specimen Hal 012; Figure 1I). According to the provided scale bar, the overall length was 8.85 mm; the length of the head capsule was 0.53 mm; the width of the head capsule was 0.71 mm. The author also provided a lateral view (his p. 129 figure 19), close-ups of the head and thorax (his p. 129 figure 20), the antenna (his p. 129 figure 22), the mandible (his p. 129 figure 23) and the posterior abdomen (his p. 129 figure 21).
14) Vondel (2011a) provided drawings of four larvae of the group Haliplus. The first specimen was a stage 3 larva of Haliplus abbreviatus, formerly named Haliplus kulleri (his p. 48 figure 1; specimen Hal 016; Figure 2D). According to the provided scale bar, the overall length was 8.63 mm; the length of the head capsule was 0.40 mm; the width of the head capsule was 0.68 mm. The author also provided close-ups of the head, thorax and the first abdominal segment (his p. 48 figure 2), the antenna (his p. 48 figure 4), the mandible (his p. 48 figure 5), different views of the legs (his p. 49 figure 6-11) and the posterior abdominal segments (his p. 48 figure 3).
The second specimen was a stage 3 larva of Haliplus maculatus (his p. 50 figure 12; specimen Hal 017; Figure 2C). According to the provided scale bar, the overall length was 11.31 mm; the length of the head capsule was 0.48 mm; the width of the head capsule was 0.67 mm. The author also provided close-ups of the head, thorax and the first abdominal segment (his p. 50 figure 13), the antenna (his p. 50 figure 15), the mandible (his p. 50 figure 16), different views of the legs (his p. 51 figures 17-22) and the posterior abdominal segments (his p. 50 figure 14).
The third specimen was a stage 1 larva either of H. abbreviatus or H. maculatus (his p. 52 figure 23; specimen Hal 018; Figure 2A). According to the provided scale bar the overall length was 1.70 mm; the length of the head capsule was 0.22 mm; the width of the head capsule was 0.34 mm. The author also provided close-ups of different views of the legs (his p. 52 figure 24-29).
The fourth specimen was a stage 2 larva either of H. abbreviatus or H. maculatus (his p. 53 figure 30; specimen Hal 019; Figure 2B). According to the provided scale bar, the overall length was 2.99 mm; the length of the head capsule was 0.29 mm; the width of the head capsule was 0.36 mm. The author also provided close-ups of different views of the legs (his p. 53 figures 31-36).
15) Vondel (2012) provided drawings of five larvae of the group Haliplus. The first specimen was a stage 1 larva of Haliplus halsei (his p. 195 figure 1; specimen Hal 004; Figure 2L). Total length of the larvae was stated to have been 3.70 mm. Based on this information, the length of the head capsule was 0.29 mm; the width of the head capsule was 0.48 mm. The author also provided close-ups of the head (his p. 195 figure 2), the antenna (his p. 195 figure 4), the mandible (his p. 195 figure 5), different views of the legs (his p. 199 figures 6-11) and the posterior abdominal segments (his p. 195 figure 3).
The second specimen was a stage 3 larva of Haliplus halsei (his p. 200 figure 12; specimen Hal 005; Figure 1C). According to the provided scale bar, the overall length was 13.67 mm; the length of the head capsule was 0.64 mm; the width of the head capsule was 0.83 mm. The author also provided a lateral view (his p. 200 figure 13), close-ups of the head (his p. 200 figure 14), the antenna (his p. 200 figure 17), the mandible (his p.200 figure 16), different views of the legs (his p. 201 figures 18-23) and the posterior abdominal segments (his p. 200 figure 15).
The third specimen was a stage 3 larva of Haliplus pilbaraensis (his p. 202 figure 24; specimen Hal 006; Figure 1D). According to the provided scale bar, the overall length was 11.82 mm; the length of the head capsule was 0.42 mm; the width of the head capsule was 0.65 mm. The author also provided a lateral view (his p. 202 figure 25), close-ups of the head (his p. 202 figure 26), the antenna (his p. 202 figure 29), the mandible (his p. 202 figure 28), different views of the legs (his p. 203 figures 30-35) and the posterior abdominal segments (his p. 202 figure 27).
The fourth specimen was a stage 3 larva of Haliplus fortescueensis (his p. 204 figure 36; specimen Hal 007; Figure 1E). According to the provided scale bar, the overall length was 10.18 mm; the length of the head capsule was 0.43 mm; the width of the head capsule was 0.49 mm. The author also provided a lateral view (his p. 204 figure 37), close-ups of the head (his p. 204 figure 38), the antenna (his p. 204 figure 41), the mandible (his p. 204 figure 40), different views of the legs (his p. 205 figures 43-48) and the posterior abdominal segments (his p. 204 figure 39).
The fifth specimen was a stage 3 larva of Haliplus pinderi (his p. 206 figure 49; specimen Hal 008; Figure 1F). According to the provided scale bar, the overall length was 9.19 mm; the length of the head capsule was 0.32 mm; the width of the head capsule was 0.46 mm. The author also provided a lateral view (his p. 206 figure 50), close-ups of the head (his p. 206 figure 51), the antenna (his p. 206 figure 54), the mandible (his p. 206 figure 53), different views of the legs (his p. 207 figures 55-60) and the posterior abdominal segments (his p. 206 figure 52).
16) Yee and Kehl (2015) provided a micrograph of a larva of Haliplidae (their p. 1025 figure 39.19). No further information was provided. The morphology with long protrusions indicates that the larva is a representative of the group Peltodytes. The specimen was depicted in lateral view and can therefore not be further considered here.
17) Glime (2017) provided a micrograph of a larva of Haliplidae (his. 11-9-5 figure 15). No further information was provided. The morphology indicates that the larva is a representative of Haliplus. The specimen was depicted in lateral view and can therefore not be further considered here.
18) Michat et al. (2020) provided a drawing of a stage 1 larva of Haliplus indistinctus (their p. 4 figure 1A; specimen Hal 013; Figure 2K). According to the provided scale bar, the overall length was 3.35 mm; the length of the head capsule was 0.20 mm; the width of the head capsule was 0.29 mm. The authors also provided close-ups of the head in dorsal (their p. 4 figure 1B) and ventral view (their p. 4 figure 1C), the antenna in different views (their p. 5 figure 2A, B), the mandible (their p. 5 figure 2C), the maxilla in different views (their p. 5 figure 2D, 2E), the labium in different views (their p. 5 figure 3A, 3B), different views of the legs (their p. 8 figure 4) and the trunk end (their p. 9 figure 5A).
19) Watanabe and Yamasaki (2020) provided an image of a stage 3 larva of Haliplus kamiyai (their p. 369 figure 3a left; specimen Hal 023; Figure 1N). According to the provided scale bar, the overall length was 11.45 mm; the length of the head capsule was 0.26 mm; the width of the head capsule was 0.53 mm. The authors also provided a lateral view (their p. 369 figure 3a mid), a dorsal view (their p. 369 figure 3a right) and a close-up of the first leg (their p. 369 figure 3b) (and an image of the larva feeding on algae; their p. 369 figure 3c; and an image of the pupae; their p. 369 figure 3d).
New Fossil Larvae Resembling those of Haliplidae
1) 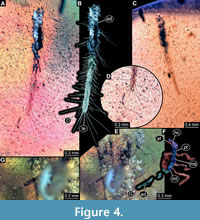 BUB 4436 specimen 1 (Hal 032) preserved together with 10 additional specimens in the same amber piece. Specimen accessible from both sides (Figure 4A-C). Unclear which side is dorsal and ventral. Body with long setae. Only parts of abdomen preserved. Other body parts missing, head and the tarsi not accessible. Number of abdominal segments can not be counted. The trunk end is elongated, bearing long setae (Figure 4D). Exact length of the specimen can not be measured due to missing parts.
BUB 4436 specimen 1 (Hal 032) preserved together with 10 additional specimens in the same amber piece. Specimen accessible from both sides (Figure 4A-C). Unclear which side is dorsal and ventral. Body with long setae. Only parts of abdomen preserved. Other body parts missing, head and the tarsi not accessible. Number of abdominal segments can not be counted. The trunk end is elongated, bearing long setae (Figure 4D). Exact length of the specimen can not be measured due to missing parts.
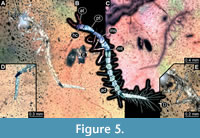 2) BUB 4436 specimen 2 (Hal 033) accessible from dorso-lateral view (Figure 5A-C). Body with long setae. It has 9 abdominal units, i.e., 8 true segments and the trunk end (Figure 5B). The trunk end is elongated, bearing long setae (Figure 5D). Each leg bears a single claw (Figure 5E). Overall length of larva is 2.55 mm. Length of head capsule is 0.15 mm. Width of head capsule is not measurable.
2) BUB 4436 specimen 2 (Hal 033) accessible from dorso-lateral view (Figure 5A-C). Body with long setae. It has 9 abdominal units, i.e., 8 true segments and the trunk end (Figure 5B). The trunk end is elongated, bearing long setae (Figure 5D). Each leg bears a single claw (Figure 5E). Overall length of larva is 2.55 mm. Length of head capsule is 0.15 mm. Width of head capsule is not measurable.
 3) BUB 4436 specimen 3 (Hal 034) accessible from dorsal (Figure 6A, B) and ventral (Figure 6C) view. Body with long setae. Parts of the abdomen are concealed. Number of abdominal segments can not be counted. The trunk end is elongated, bearing long setae (Figure 6E). Each leg bears a single claw (Figure 6D). Overall length of larva is 5.30 mm. The length of head capsule is 0.25 mm. Width of head capsule is 0.24 mm.
3) BUB 4436 specimen 3 (Hal 034) accessible from dorsal (Figure 6A, B) and ventral (Figure 6C) view. Body with long setae. Parts of the abdomen are concealed. Number of abdominal segments can not be counted. The trunk end is elongated, bearing long setae (Figure 6E). Each leg bears a single claw (Figure 6D). Overall length of larva is 5.30 mm. The length of head capsule is 0.25 mm. Width of head capsule is 0.24 mm.
 4) BUB 4436 specimen 4 (Hal 035) accessible from dorsal (Figure 7A, B) and partly in ventral (Figure 7C) view. Body with long setae. Mouthparts not accessible due to head orientation (Figure 7E). It has 9 abdominal units, 8 true segments and the trunk end (Figure 7B). The trunk end is elongated, bearing long setae (Figure 7D). Tarsi not accessible. Overall length of larva is 4.55 mm. Length of head capsule is 0.22 mm. Width of head capsule is 0.44 mm.
4) BUB 4436 specimen 4 (Hal 035) accessible from dorsal (Figure 7A, B) and partly in ventral (Figure 7C) view. Body with long setae. Mouthparts not accessible due to head orientation (Figure 7E). It has 9 abdominal units, 8 true segments and the trunk end (Figure 7B). The trunk end is elongated, bearing long setae (Figure 7D). Tarsi not accessible. Overall length of larva is 4.55 mm. Length of head capsule is 0.22 mm. Width of head capsule is 0.44 mm.
 5) BUB 4436 specimen 5 (Hal 036) accessible from dorsal (Figure 8A, B) and ventral (Figure 8C) view. Body with long setae. Partly concealed from both views. Mouthparts not accessible (Figure 8D). Number of abdominal segments can not be counted. The trunk end is elongated, bearing long setae (Figure 8F). Tarsi are not accessible (Figure 8E). Overall length of larva is 4.58 mm. Length of head capsule is 0.14 mm. Width of head capsule is 0.35 mm.
5) BUB 4436 specimen 5 (Hal 036) accessible from dorsal (Figure 8A, B) and ventral (Figure 8C) view. Body with long setae. Partly concealed from both views. Mouthparts not accessible (Figure 8D). Number of abdominal segments can not be counted. The trunk end is elongated, bearing long setae (Figure 8F). Tarsi are not accessible (Figure 8E). Overall length of larva is 4.58 mm. Length of head capsule is 0.14 mm. Width of head capsule is 0.35 mm.
6) BUB 4436 specimen 6 (Hal 037) accessible only from lateral view (Figure 4E, F). No setae apparent. Number of abdominal segments can not be counted. Trunk end missing (Figure 4G). Tarsi are not accessible. Exact length can not be measured due to the missing parts. Length of head capsule is 0.31 mm. Width of head capsule is not measurable.
 7) BUB 4436 specimen 7 (Hal 038) accessible from dorsal (Figure 9A, B) and ventral (Figure 9C) view. Body with long setae. Mouthparts not accessible (Figure 9F). It has 10 abdominal units, 9 true segments and the trunk end (Figure 9B). The trunk end is elongated, bearing long setae (Figure 9E). Each leg bears a single claw (Figure 9D). Overall length of larva is 5.16 mm. Length of head capsule is 0.20 mm. Width of head capsule is 0.34 mm.
7) BUB 4436 specimen 7 (Hal 038) accessible from dorsal (Figure 9A, B) and ventral (Figure 9C) view. Body with long setae. Mouthparts not accessible (Figure 9F). It has 10 abdominal units, 9 true segments and the trunk end (Figure 9B). The trunk end is elongated, bearing long setae (Figure 9E). Each leg bears a single claw (Figure 9D). Overall length of larva is 5.16 mm. Length of head capsule is 0.20 mm. Width of head capsule is 0.34 mm.
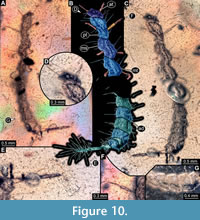 8) BUB 4436 specimen 8 (Hal 039) accessible from dorso-lateral (Figure 10B, C) and ventro-lateral (Figure 10A) view. Body with long setae. Mouthparts not accessible (Figure 10D). Parts of abdomen concealed. Number of abdominal segments can not be counted (Figure 10G). The trunk end is elongated, bearing long setae (Figure 10E). Each leg bears a single claw (Figure 10F). Overall length of larva is 5.73 mm. Length of head capsule is 0.25 mm. Width of head capsule is 0.44 mm.
8) BUB 4436 specimen 8 (Hal 039) accessible from dorso-lateral (Figure 10B, C) and ventro-lateral (Figure 10A) view. Body with long setae. Mouthparts not accessible (Figure 10D). Parts of abdomen concealed. Number of abdominal segments can not be counted (Figure 10G). The trunk end is elongated, bearing long setae (Figure 10E). Each leg bears a single claw (Figure 10F). Overall length of larva is 5.73 mm. Length of head capsule is 0.25 mm. Width of head capsule is 0.44 mm.
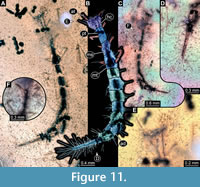 9) BUB 4436 specimen 9 (Hal 040) accessible from dorsal (Figure 11A, B) and ventral (Figure 11C) view. Body with long setae. Head with few details (Figure 11E). It has 9 abdominal units, 8 true segments and the trunk end (Figure 11B). The trunk end is elongated, bearing long setae (Figure 11D). Each leg bears a single claw (Figure 11F). Overall length of larva is 4.49 mm. Length of head capsule is 0.29 mm. The width of head capsule is 0.38 mm.
9) BUB 4436 specimen 9 (Hal 040) accessible from dorsal (Figure 11A, B) and ventral (Figure 11C) view. Body with long setae. Head with few details (Figure 11E). It has 9 abdominal units, 8 true segments and the trunk end (Figure 11B). The trunk end is elongated, bearing long setae (Figure 11D). Each leg bears a single claw (Figure 11F). Overall length of larva is 4.49 mm. Length of head capsule is 0.29 mm. The width of head capsule is 0.38 mm.
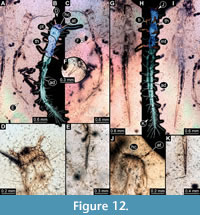 10) BUB 4436 specimen 10 (Hal 041) accessible from dorsal (Figure 12A, B) and ventral (Figure 12C) view. Body with long setae. Antennae stout. Mouthparts not accessible (Figure 12D). The number of abdominal segments can not be counted. The trunk end is elongated, bearing long setae (Figure12E). Each leg bears a single claw (Figure 12F). Overall length of larva is 4.40 mm. Length of head capsule is 0.22 mm. Width of head capsule is 0.39 mm.
10) BUB 4436 specimen 10 (Hal 041) accessible from dorsal (Figure 12A, B) and ventral (Figure 12C) view. Body with long setae. Antennae stout. Mouthparts not accessible (Figure 12D). The number of abdominal segments can not be counted. The trunk end is elongated, bearing long setae (Figure12E). Each leg bears a single claw (Figure 12F). Overall length of larva is 4.40 mm. Length of head capsule is 0.22 mm. Width of head capsule is 0.39 mm.
11) BUB 4436 specimen 11 (Hal 042) accessible from dorsal (Figure 12I) and ventral (Figure 12G, H) view. Body with long setae. Mouthparts not accessible (Figure 12J). It has 9 abdominal units, 8 true segments and the trunk end (Figure 12H). The trunk end is elongated, bearing long setae (Figure 12K). Each leg bears a single claw (Figure 12G). Overall length of larva is 5.31 mm. Length of head capsule is 0.10 mm. Width of head capsule is 0.26 mm.
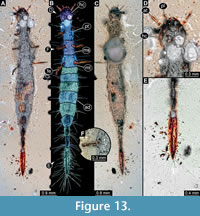 12) BUB 1222 (Hal 043) accessible from dorsal (Figure 13C) and ventral (Figure 13A, B) view. Body with long setae. Mandibles prominent. Maxillae appear elongated (Figure 13D). It has 9 abdominal units, 8 true segments and the trunk end (Figure 13B). The trunk end is elongated, bearing long setae (Figure 13E). Each leg bears a single claw (Figure 13F). Overall length of larva is 8.31 mm. Length of head capsule is 0.54 mm. Width of head capsule is 0.64 mm.
12) BUB 1222 (Hal 043) accessible from dorsal (Figure 13C) and ventral (Figure 13A, B) view. Body with long setae. Mandibles prominent. Maxillae appear elongated (Figure 13D). It has 9 abdominal units, 8 true segments and the trunk end (Figure 13B). The trunk end is elongated, bearing long setae (Figure 13E). Each leg bears a single claw (Figure 13F). Overall length of larva is 8.31 mm. Length of head capsule is 0.54 mm. Width of head capsule is 0.64 mm.
 13) PED 1859 (Hal 045) accessible from dorsal (Figure 14C) and ventral (Figure 14A, B) view. Body with long setae. The mouthparts are accessible only in frontal view (Figure 14D). It has 9 abdominal units, 8 true segments and the trunk end (Figure 14B). The trunk end is elongated, bearing long setae (Figure 14F). Each leg bears a single claw (Figure 14E). Overall length of larva is 4.63 mm. Length of head capsule is 0.22 mm. Width of head capsule is 0.52 mm.
13) PED 1859 (Hal 045) accessible from dorsal (Figure 14C) and ventral (Figure 14A, B) view. Body with long setae. The mouthparts are accessible only in frontal view (Figure 14D). It has 9 abdominal units, 8 true segments and the trunk end (Figure 14B). The trunk end is elongated, bearing long setae (Figure 14F). Each leg bears a single claw (Figure 14E). Overall length of larva is 4.63 mm. Length of head capsule is 0.22 mm. Width of head capsule is 0.52 mm.
Shape Analysis
The shape analysis of the body outline resulted in five principle components that describe about 96% of the overall variation (Suppl. Text 1; Suppl. Figure 1; Suppl. Files 1-5).
PC1 explains 44.21% of the overall variation. It is mainly influenced by the broadness in the middle of the body. A smaller value indicates a slender and a higher value a broader body.
PC2 explains 31.83% of the overall variation. It is mainly influenced by the degree of tapering of the body. A smaller value indicates a conical form with the broader side being anterior and a higher value indicates a slender linear form.
PC3 explains 11.76% of the overall variation. It is mainly influenced by the broadness of the head. A smaller value indicates a slender and a higher value indicates a broader head.
PC4 explains 6.08% of the overall variation. It is mainly influenced by the positioning of the lateral extensions of the segments and the anterior end of the head. Smaller and larger values indicate different pronounced extensions, values around 0 indicate only little protruding extensions.
PC5 explains 3.03% of the overall variation. It is mainly influenced by the positioning of the extensions of the segments in combination with the body width. Extensions are similarly distributed to PC4; low values indicate a more slender body, high values a broader one.
Total Head Length vs. Total Body Length
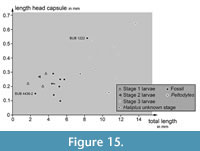 Plotting the length of the head capsule versus the total body length reveals certain clusters (Figure 15). The three larvae of stage 1 and the single larva of stage 2 from the modern fauna plot together. The shortest head of an extant larva is represented by a stage 3 larva of the group of Peltodytes, which is partly surprising.
Plotting the length of the head capsule versus the total body length reveals certain clusters (Figure 15). The three larvae of stage 1 and the single larva of stage 2 from the modern fauna plot together. The shortest head of an extant larva is represented by a stage 3 larva of the group of Peltodytes, which is partly surprising.
One fossil specimen (BUB 4436 specimen 2) plots clearly in the area of the extant stage 1 and 2 larval specimens. The other specimens of BUB 4436 and the specimen of PED 1859 plot together between the area occupied by the extant stage 1 and 2 larvae and the area occupied by the extant stage 3 larvae. BUB 1222 plots closely to the area occupied by the extant stage 3 larvae.
PC2 vs. PC1
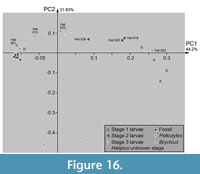 Plotting PC2 vs. PC1 reveals certain clusters. Extant stage 3 larvae of Haliplus and the single larval specimen of Brychius plot widely scattered along PC1 and mostly in the positive range of PC2 (Figure 16). The three specimens of the stage 2 larvae of Haliplus plot near or in the area occupied by the specimens of the stage 3 larvae. Stage 2 larvae Hal 019 and Hal 030 plot slightly outside the area occupied by stage 3 larvae, Hal 026 inside. The extant stage 1 larvae of Haliplus occupy a different area than the extant stage 2 and 3 larvae of Haliplus. The specimens of Peltodytes plot clearly outside of the area of Haliplus and Brychius. The stage of the Haliplus larvae of Hal 001, Hal 002, Hal 003 and Hal 015 was not mentioned in the original sources. Hal 001, Hal 003 and Hal 015 plot in or close to the area occupied by the stage 3 larvae of Haliplus. Hal 002 plots outside the areas occupied by the other larvae, closely to the area occupied by the stage 1 larvae.
Plotting PC2 vs. PC1 reveals certain clusters. Extant stage 3 larvae of Haliplus and the single larval specimen of Brychius plot widely scattered along PC1 and mostly in the positive range of PC2 (Figure 16). The three specimens of the stage 2 larvae of Haliplus plot near or in the area occupied by the specimens of the stage 3 larvae. Stage 2 larvae Hal 019 and Hal 030 plot slightly outside the area occupied by stage 3 larvae, Hal 026 inside. The extant stage 1 larvae of Haliplus occupy a different area than the extant stage 2 and 3 larvae of Haliplus. The specimens of Peltodytes plot clearly outside of the area of Haliplus and Brychius. The stage of the Haliplus larvae of Hal 001, Hal 002, Hal 003 and Hal 015 was not mentioned in the original sources. Hal 001, Hal 003 and Hal 015 plot in or close to the area occupied by the stage 3 larvae of Haliplus. Hal 002 plots outside the areas occupied by the other larvae, closely to the area occupied by the stage 1 larvae.
All fossil specimens plot closely to the extant stage 3 larvae of Haliplus and also closely together among each other. They occupy an area at the most negative edge of the morphospace occupied by the extant stage 3 larvae.
PC1 vs. Total Body Length
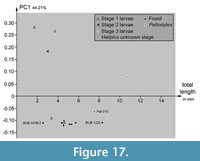 Plotting PC1 vs. the total body length reveals some differences between extant and fossil specimens (Figure 17). Specimens of stage 3 larvae of Haliplus plot together. The different lengths of these specimens scatter over a range of about 6 mm. The single stage 2 larva of Haliplus plots in the same area as the three stage 1 larvae. The specimens of stage 1 and 2 larvae occupy a bigger range of PC1 than the stage 3 larvae specimens. The single Peltodytes specimen plots in the area of the stage 3 larvae specimens of Haliplus. The larval stage of the Haliplus specimen Hal 015 was not mentioned. Hal 015 is a bit shorter than the stage 3 larvae, but plots in the same range of PC1 like the stage 3 larvae.
Plotting PC1 vs. the total body length reveals some differences between extant and fossil specimens (Figure 17). Specimens of stage 3 larvae of Haliplus plot together. The different lengths of these specimens scatter over a range of about 6 mm. The single stage 2 larva of Haliplus plots in the same area as the three stage 1 larvae. The specimens of stage 1 and 2 larvae occupy a bigger range of PC1 than the stage 3 larvae specimens. The single Peltodytes specimen plots in the area of the stage 3 larvae specimens of Haliplus. The larval stage of the Haliplus specimen Hal 015 was not mentioned. Hal 015 is a bit shorter than the stage 3 larvae, but plots in the same range of PC1 like the stage 3 larvae.
The range of PC1 occupied by the fossil specimens is smaller than the range occupied by the extant specimens. BUB 4436 specimen 2 (Hal 033) plots inside the area occupied by extant stage 1 and 2 larvae of Haliplus. All other specimens of BUB 4436 (Hal 035, Hal 036, Hal 038, Hal 039, Hal 040, Hal 041, Hal 042) included in the plot and the specimen of PED 1859 plot together. They occupy an area with a longer length than the extant stage 1 and 2 larvae, but shorter than the extant stage 3 larvae. BUB 1222 (Hal 043) is a bit shorter than the shortest determined extant stage 3 larva.
DISCUSSION
Are the Fossil Specimens Larvae of the Group Haliplidae?
Larvae of Haliplidae share a number of different characters. The larvae have an elongated trunk end (Vondel, 1997; Makarov and Prokin, 2015) and an overall slender body shape (Vondel, 1997). Larvae of Haliplidae have nine to ten abdominal units including the elongated trunk end (Vondel, 1997; Makarov and Prokin, 2015). The legs (locomotory appendages) have tarsi with just a single element and bear a single claw. Each mandible has a suction channel. The maxillae and labium have rather small palps (Vondel, 1997). While larvae of certain other beetle groups have large prominent trunk ends (e.g., Haug and Haug, 2019; Zippel et al., 2022a), in these the overall appearance is less slender. Hence, for larvae of Haliplidae it is the combination of characters mentioned above that needs to be looked for in the fossils.
In the new fossil specimens, in which the trunk end is preserved, it is prominently elongated, bearing numerous setae. In BUB 4436 specimen 5 the trunk end is elongated and bears setae, but it is remarkably more slender compared to the rest of the specimens (Figure 8F). The trunk end of the other eleven specimens look quite similar, elongated but not significantly more slender than the remaining body. One specimen (BUB 4436 specimen 6) does not have the trunk end preserved, but otherwise resembles the other specimens in their overall appearance.
In six of the new fossil specimens, nine abdominal units (eight segments plus trunk end) are apparent, while one specimen seems to have ten (BUB 4436 specimen 7; Figure 9). Segments numbers can sometimes be hard to recognise in fossil larvae; subdivisions can be obscured or folds can appear like true subdivisions. It is therefore unclear whether this variation in the fossils is preservational or reflects true original morphology. In any case, the range of abdominal units is compatible with that reported in extant larvae of Haliplidae (Vondel, 1997; Makarov and Prokin, 2015).
Nine of the new fossil specimens show tarsi bearing one single claw as in modern larvae of Haliplidae. In the other specimens, this detail is not accessible.
The mouthparts are only accessible in two of the fossils, BUB 1222 and PED 1859. In BUB 1222, the mandible and a part of a palp of one maxilla are accessible (Figure 13D). A further determination of different elements is not possible, and a possible suction channel in the mandible could not be observed due to the limited accessibility. The mouthparts of PED 1859 are only accessible in frontal view (Figure 14D) and it is not possible to distinguish the different parts of the mouthparts. Hence, this aspect remains rather non-informative. Yet, none of the specimens appears to have prominent palps (of maxillae and labium) which is compatible with short palps in modern larvae of Haliplidae.
Almost all new fossil specimens bear prominent setae or setae-like structures largely comparable to those in modern larvae of Haliplidae (e.g., Vondel, 2011b; Michat et al., 2020). However, it seems that relatively more setae are present in the fossil forms. This aspect remains challenging to evaluate, as almost all specimens of the literature are only provided as drawings, which might provide a different impression in comparison to a micrograph.
Other important features being characteristic for modern larvae of Haliplidae could, unfortunately, not be observed in the fossils. This applies, for example, to the gills, which are very rarely preserved (but see Zippel et al., 2022c).
The long thorax segments in the fossils are different from modern larvae of Haliplidae, although there is a certain variation also in this character. Yet, this factor of difference and the elongate body of the larvae also reminds of that of larvae of quite some other groups and should lead us to consider also some alternative interpretations.
Larvae of raphidiopterans (snakeflies), for example, also have a very elongate trunk and in addition have a relatively long prothorax, as seen in the fossils. In contrast to larvae of Haliplidae (and the fossils), raphidiopteran larvae have two claws (Kluge, 2003). Also, in raphidiopteran larvae there is no example of a trunk end known to be as elongated as it is in Haliplidae or in the fossils, even not in fossil snakefly larvae (Haug et al., 2022c). Likewise elongated trunks are also known in megalopteran larvae (Baranov et al., 2022). Yet, here differences are even more expressed to the fossils, as megalopteran larvae have either a terminal filament or a pair of claw-shaped protrusions at the trunk end (absent in the fossils at hand), they bear lateral gills on the trunk (absent in the fossils at hand), and there is no example with an elongated trunk end. Also, both raphidiopteran and megalopteran larvae have prominent forward-protruding mouthparts, while in the fossils the mouthparts are neither prominent nor appear to protrude necessarily forward (an original antero-ventral orientation seems likely).
In summary, the fossils strongly resemble each other (allowing to make statements also for the more incomplete specimens); many of the fossil specimens show some characteristics well known in modern larvae of Haliplidae. There is also no character directly contradicting an interpretation of the new fossils as larvae of Haliplidae, besides the rather long thorax segments. There are some cases in other neuropteriformian larvae in which the modern forms have rather short thorax segments (Haug et al., 2022d), but some fossil forms have elongate thorax segments (Haug et al., 2021d). The difference between the extant larvae of Haliplidae and the new fossils may represent a similar phenomenon. We therefore interpret the specimens as possible fossil larvae of the group Haliplidae.
There is so far no formally described species of Haliplidae from Myanmar amber. Therefore, it would in principle be possible to erect a new species based on the here reported specimens. Yet, as many details are not accessible, it is very difficult to find usable diagnostic characters for properly characterising such a species. We therefore decided to not formally erect a new species.
One Amber Piece with Eleven Specimens
The amber piece BUB 4436 includes eleven different specimens of fossil larvae of the group Haliplidae. Such cases immediately raise the question whether this is indicative of certain aspects of the life style (Hörnig et al., 2016, 2022; Schädel et al., 2021).
Extant representatives of Haliplidae are often very abundant at some places, but they do not occur abundantly everywhere (Matheson, 1912). Often a single habitat contains more than one species of Haliplidae occurring together (Vondel, 1986). Hickman (1931) recognized, while collecting larvae of Haliplidae, that at a particular spot almost all larvae were of the same larval stage. He also recognized that the larvae at such a spot had all moulted to the next larval stage when he returned to the spot a few days later. He stated that first and third stage larvae were never found together (Hickman, 1931). Additionally, larvae can occur without any adults and also adults without any larvae (Vondel, 1986).
Gyrinidae is the sistergroup to a group of other adephagan beetles including Haliplidae, and likewise has aquatic larvae (Ribera et al., 2002; Beutel et al., 2006). For the the species Andogyrus seriatopunctatus it has also been reported that all larvae moult from stage 1 to stage 2 at about the same time within a few days (Archangelsky and Michat, 2007). In fact, synchronised moulting leading to all individuals at a spot being in the same developmental stage is a widespread phenomenon throughout Euarthropoda and has been recognised in the fossil record (Haug et al., 2013).
Regarding the total body length, one specimen (BUB 4436 specimen 2) is shorter than the other specimens. This specimen probably represents a stage 1 larva. The other seven measurable specimens and BUB 4436 specimen 3, which could not be included in the shape analysis, have a length between 4.40 mm (BUB 4436 specimen 10) and 5.73 mm (BUB 4436 specimen 8). All these are probably stage 2 larvae. BUB 4436 specimen 6 has a length of at least 1.56 mm, but is incomplete. Still the preserved part indicates that the larvae could not have been larger than about 3 mm. This is still noticeably smaller than the smallest supposed stage 2 larva. Hence BUB 4436 specimen 6 is probably also a stage 1 larva, which would mean that there are two stage 1 larvae and eight stage 2 larvae (and one specimen of unclear stage). This would fit to the observations from the modern fauna that most larvae at a single spot could be stage 2 larvae with few representatives of stage 1 larvae (Matheson, 1912; Hickman, 1931; Vondel, 1986).
One could argue that the supposed stage 2 larvae (or at least some of them) are in fact stage 3 larvae as Vondel (1997) stated that the length of stage 3 larvae is at least 5 mm. Four of the supposed stage 2 fossils are over 5 mm long. This interpretation would be also consistent with the principle component analysis. The fossil specimens are very slender, they are as slender as the most slender extant specimens. The extant stage 2 larvae included in the analysis are all broader than the fossil specimens (Figure 16). However, all fossil specimens are very slender, the long and the short ones, including supposed stage 1 larvae. The latter would need to be interpreted at least as stage 2 larvae, if considering the supposed stage 2 larvae as stage 3 larvae, and they would still deviate from the modern morphology. This could either mean that we have only stage 3 larvae of very several different-sized species, or that the slenderness might be a general pattern of larvae of Haliplidae of the Cretaceous.
All extant stage 3 larvae included in the measurements are larger than supposed fossil stage 2 larvae, with over 8 mm. Also, one fossil specimen is clearly larger than the others (Hal 043) and is presumably a stage 3 larva, still it is smaller than the extant stage 3 larvae. We see it as most likely that the specimens represent three different larval stages possibly of a single species.
Fossil vs. Extant Larvae
The extant specimens occupy a rather large area of the morphospace; the fossil specimens cluster tightly together. This is still true if the larvae of Peltodytes, which are quite different in appearance, are not considered. The fossils are all quite slender and linear shaped, while the extant larvae show a larger variation in the body shape, but also showing slender and linear forms similar to the fossils.
The small area occupied by the fossils is coupled to the fact that all three supposed stages are very similar. In the extant larvae, stage 3 individuals are slightly differentiated. This may be coupled to their habit to go ashore and burrow into the ground to pupate (Vondel, 1997). Hence, there is a different selection pressure to the different larval stages possibly leading to different morphologies. The fossils may still have followed a different morphology.
Overall we can only assume that the fossil larvae lived in water based on the comparison to the extant forms. While it may seem unlikely to have aquatic animals preserved in amber, more and more such cases demonstrate that it is indeed possible that animals get trapped in amber while still being in water.
Yet, the fact that in the fossils all three supposed larval stages have a body shape with which modern larvae can crawl on land may indicate that the fossils did not yet live in water for the entire time, if at all. Still there is one modern stage 1 larva that also plots closer to the fossils (Figure 16). We might not know all variations of behaviour among the modern larvae; as often, we generalise for larger groups based on directly observable species. Also, as no distinct gills are preserved in the fossils, the overall lifestyle of these remains open to speculation.
Adephagan Larvae in the Fossil Record
Adephaga has been considered to represent an early diversifying lineage of beetles (e.g., Beutel et al., 2013). The larvae of the group have a broad spectrum of different morphologies, which should have contributed to the early diversification.
Larvae resembling those of modern ground beetles (Carabidae) have been reported, for example from the Triassic (Prokin et al., 2013), but also from Myanmar amber (Liu et al., 2023). Larvae of Gyrinidae have also been reported from Myanmar amber (Gustafson et al., 2020; Zhao et al., 2019). The now extinct group Coptoclavidae has been interpreted as an ingroup of Adephaga, and its aquatic larvae are well known in different Mesozoic deposits (Ponomarenko, 1993; Wang et al., 2009, 2010; Ponomarenko et al., 2015). Larvae of Dytiscidae have been found exquisitely preserved in the silicified Miocene lake deposits of Barstow, California, USA (Palmer et al., 1957) and Eocene Baltic amber (e.g., Wichard et al., 2009; Gröhn, 2015), but relatives are also known from older strata (Ghosh et al., 2007).
Despite these records, we should expect in fact many more fossils of adephagan larvae. The modern fauna has more than 40,000 species of adephagan beetles, and in the past these beetles must have been even more prominently represented in some of the faunas.
The here reported larvae represent another piece in the puzzle of the fossil record reflecting the evolution of specialised larval forms in Adephaga. The overall morphology of the fossils appears very modern, but the differences in thorax segment length might hint to at least some differences in comparison to their modern counterparts.
ACKNOWLEDGEMENTS
We thank Bernhard van Vondel and two anonymous reviewers for helpful comments, which improved the manuscript, and the editors Brooke Long-Fox and Russell Bicknell for handling the manuscript. The study is kindly supported by the German Research Foundation (DFG Ha 6300/6-1) and by the Volkswagen Foundation with a Lichtenberg professorship to JTH. We are grateful to all people providing low-cost, open-access or open-source software. J. Matthias Starck, Munich, is thanked for long-time support. This is LEON publication #55.
REFERENCES
Archangelsky, M. and Michat, M.C. 2007. Morphology and chaetotaxy of the larval stages of Andogyrus seriatopunctatus Régimbart (Coleoptera: Adephaga: Gyrinidae). Zootaxa, 1645(1):19-33.
https://doi.org/10.11646/zootaxa.1645.1.2
Baranov, V., Haug, C., Fowler, M., Kaulfuss, U., Müller, P. & Haug, J.T. 2022. Summary of the fossil record of megalopteran and megalopteran-like larvae, with a report of new specimens. Bulletin of Geosciences, 97:89-108.
https://doi.org/10.3140/bull.geosci.1840
Batelka, J., Engel, M.S., and Prokop, J. 2021. The complete life cycle of a Cretaceous beetle parasitoid. Current Biology, 31:R118-R119.
https://doi.org/10.1016/j.cub.2020.12.007
Batelka, J., Prokop, J., Pohl, H., Bai, M., Zhang, W., and Beutel, R.G. 2019. Highly specialized Cretaceous beetle parasitoids (Ripiphoridae) identified with optimized visualization of microstructures. Systematic Entomology, 44:396-407.
https://doi.org/10.1111/syen.12331
Bertrand, H. 1933. Les larves aquatiques des Coléoptères. La Terre et la Vie, 9:523-534.
https://doi.org/10.3406/revec.1933.2971
Beutel, R.G. and Haas, A. 1996. Phylogenetic analysis of larval and adult characters of Adephaga (Coleoptera) using cladistic computer programs. Insect Systematics & Evolution, 27:197-205.
https://doi.org/10.1163/187631296x00043
Beutel, R.G., Balke, M., and Steiner, W.E.Jr. 2006. The systematic position of Meruidae (Coleoptera, Adephaga) and the phylogeny of the smaller aquatic adephagan beetle families. Cladistics, 22:102-131.
https://doi.org/10.1111/j.1096-0031.2006.00092.x
Beutel, R.G., Wang, B., Tan, J.J., Ge, S.Q., Ren, D., and Yang, X.K. 2013. On the phylogeny and evolution of Mesozoic and extant lineages of Adephaga (Coleoptera, Insecta). Cladistics, 29:147-165.
https://doi.org/10.1111/j.1096-0031.2012.00420.x
Beutel, R.G., Zhang, W. W., Pohl, H., Wappler, T., and Bai, M. 2016. A miniaturized beetle larva in Cretaceous Burmese amber: reinterpretation of a fossil “strepsipteran triungulin”. Insect Systematics & Evolution, 47:83-91.
https://doi.org/10.1163/1876312x-46052134
Bouchard, P., Smith, A.B.T., Douglas, H., Gimmel, M.L., Brunke, A.J., and Kanda, K. 2017. Biodiversity of Coleoptera, p. 337-417. In Foottit, R.G. and Adler, P.H. (eds.), Insect Biodiversity: Science and Society. Second edition. John Wiley & Sons Ltd., Hoboken, New Jersey.
https://doi.org/10.1002/9781118945568.ch11
Böving, A.G. and Craighead, F.C. 1931. An Illustrated Synopsis of the Principal Larval Forms of the Order Coleoptera. Brooklyn Entomological Society, Brooklyn, New York.
https://doi.org/10.5962/bhl.title.6818
Braig, F., Haug, J.T., Schädel, M., and Haug, C. 2019. A new thylacocephalan crustacean from the Upper Jurassic lithographic limestones of southern Germany and the diversity of Thylacocephala. Palaeodiversity, 12:69-87.
https://doi.org/10.18476/pale.v12.a6
Cruickshank, R.D. and Ko, K. 2003. Geology of an amber locality in the Hukawng Valley, northern Myanmar. Journal of Asian Earth Sciences, 21:441-455.
https://doi.org/10.1016/s1367-9120(02)00044-5
Fikáček, M., Prokin, A., Yan, E., Yue, Y., Wang, B., Ren, D., and Beattie, R. 2014. Modern hydrophilid clades present and widespread in the Late Jurassic and Early Cretaceous (Coleoptera: Hydrophiloidea: Hydrophilidae). Zoological Journal of the Linnean Society, 170:710-734.
https://doi.org/10.1111/zoj.12114
Ghosh, S.C., Pal, T.K., and Nandi, A. 2007. First record of an aquatic beetle larva (Insecta: Coleoptera) from the Parsora formation (Permo‐Triassic), India. Palaeontology, 50:1335-1340.
https://doi.org/10.1111/j.1475-4983.2007.00701.x
Ghosh, S.K. 2021. Insecta: Coleoptera: Adephaga: Gyrinidae, Haliplidae, Hoteridae and Dytiscidae. State Fauna Series, 26(1):567-596.
Glime, J.M. 2017. Aquatic insects: Holometabola - Coleoptera, Suborder Adephaga. Chapt. 11-9. In Glime, J.M. (ed.), Bryophyte Ecology. Volume 2. Bryological Interaction. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 19 July 2020.
Grimaldi, D. and Engel, M.S. 2005. Evolution of the Insects. Cambridge University Press, Cambridge, England.
Gröhn, C. 2015. Einschlüsse im baltischen Bernstein. Wachholtz Verlag-Murmann Publishers, Fleethörn, Germany.
Gundersen, R.W. and Otremba, C. 1988. Haliplidae of Minnesota. Science Museum of Minnesota, New Series, 6(3):1-43.
Gustafson, G.T., Michat, M.C., and Balke, M. 2020. Burmese amber reveals a new stem lineage of whirligig beetle (Coleoptera: Gyrinidae) based on the larval stage. Zoological Journal of the Linnean Society, 189:1232-1248.
https://doi.org/10.1093/zoolinnean/zlz161
Haug, C., Haug, G.T., Zippel, A., van der Wal, S., and Haug, J.T. 2021a. The earliest record of fossil solid-wood-borer larvae--immature beetles in 99 million-year-old Myanmar amber. Palaeoentomology, 4:390-404.
https://doi.org/10.11646/palaeoentomology.4.4.14
Haug, C., Zippel, A., Müller, P., and Haug, J.T. 2022a. A modern type of ant-like stone beetle larva preserved in 99-million-year-old Kachin amber. Fragmenta entomologica, 54:193-200.
https://doi.org/10.13133/2284-4880/706
Haug C., Zippel, A., Müller, P., and Haug, J.T. 2023. Unusual larviform beetles in 100-million-year-old Kachin amber resemble immatures of trilobite beetles and fireflies. PalZ, 97: 485-496.
https://doi.org/10.1007/s12542-023-00648-8
Haug, G.T., Baranov, V., Wizen, G., Pazinato, P.G., Müller, P., Haug, C., and Haug, J.T. 2021c. The morphological diversity of long-necked lacewing larvae (Neuroptera: Myrmeleontiformia). Bulletin of Geosciences, 96:431-457.
https://doi.org/10.3140/bull.geosci.1807
Haug, G.T., Haug, C., Pazinato, P.G., Braig, F., Perrichot, V., Gröhn, C., Müller, P., and Haug, J.T. 2020. The decline of silky lacewings and morphological diversity of long-nosed antlion larvae through time. Palaeontologia Electronica, 23(2):a39.
https://doi.org/10.26879/1029
Haug, G.T., Haug, C., van der Wal, S., Müller, P., and Haug, J.T. 2022b. Split-footed lacewings declined over time: indications from the morphological diversity of their antlion-like larvae. PalZ, 96:29-50.
https://doi.org/10.1007/s12542-021-00550-1
Haug, J.T. 2020. Why the term “larva” is ambiguous, or what makes a larva? Acta Zoologica, 101:167-188.
https://doi.org/10.1111/azo.12283
Haug, J.T. and Haug, C. 2019. Beetle larvae with unusually large terminal ends and a fossil that beats them all (Scraptiidae, Coleoptera). PeerJ, 7:e7871.
https://doi.org/10.7717/peerj.7871
Haug, J.T., Caron, J.-B., and Haug, C. 2013. Demecology in the Cambrian: synchronized molting in arthropods from the Burgess Shale. BMC Biology, 11:64.
https://doi.org/10.1186/1741-7007-11-64
Haug, J.T., Zippel, A., Haug, G.T., Hoffeins, C., Hoffeins, H.-W., Hammel, J.U., Baranov, V., and Haug, C. 2021b. Texas beetle larvae (Brachypsectridae) - the last 100 million years reviewed. Palaeodiversity, 14:161-183.
https://doi.org/10.18476/pale.v14.a8
Haug, J.T., Baranov, V., Müller, P., and Haug, C. 2021d. New extreme morphologies as exemplified by 100 million-year-old lacewing larvae. Scientific Reports, 11:20432.
https://doi.org/10.1038/s41598-021-99480-w
Haug, J.T., Engel, M.S., Santos, P.M. dos, Haug, G.T., Müller, P., and Haug, C. 2022c. Declining morphological diversity in snakefly larvae during last 100 million years. PalZ, 96:749-780.
https://doi.org/10.1007/s12542-022-00609-7
Haug, J.T., Linhart, S., Haug, G.T., Gröhn, C., Hoffeins, C., Hoffeins, H.-W., Müller, P., Weiterschan, T., Wunderlich, J., and Haug, C. 2022d. The diversity of aphidlion-like larvae over the last 130 million years. Insects, 13:336.
https://doi.org/10.3390/insects13040336
Hickman, J.R. 1931. Contribution to the biology of the Haliplidae (Coleoptera). Annals of the Entomological Society of America, 24(1):129-142.
https://doi.org/10.1093/aesa/24.1.129
Hörnig, M.K., Sombke, A., Haug, C., Harzsch, S., and Haug, J.T. 2016. What nymphal morphology can tell us about parental investment - a group of cockroach hatchlings in Baltic Amber documented by a multi-method approach. Palaeontologia Electronica, 19.1.6A:1-20.
https://doi.org/10.26879/571
Hörnig, M.K., Haug, C., Müller, P., and Haug, J.T. 2022. Not quite social - possible cases of gregarious behaviour of immatures of various lineages of Insecta preserved in 100-million-year-old amber. Bulletin of Geosciences, 97:69-87.
https://doi.org/10.3140/bull.geosci.1818
Iwata, H. and Ukai, Y. 2002. SHAPE: A computer program package for quantitative evaluation of biological shapes based on elliptic Fourier descriptors. Journal of Heredity, 93:384-385.
https://doi.org/10.1093/jhered/93.5.384
Klausnitzer, B. 1977. Bestimmungstabellen für die Gattungen der aquatischen Coleopteren-Larven Mitteleuropas. 22. Beitrag zur Kenntnis der mitteleuropäischen Coleopteren-Larven. Beiträge zur Entomologie, 27(1):145-192.
Klausnitzer, B. 1978. Ordnung Coleoptera (Larven). Springer Science+Business Media, Dordrecht, Netherlands.
https://doi.org/10.1007/978-94-009-9975-6
Kluge, N.J. 2003. Larval leg structure of Nannochorista Tillyard, 1917 and characteristics of Mecoptera. Russian Entomological Journal, 12(4):349-354.
Lawrence, J.F. and Newton Jr, A.F. 1982. Evolution and classification of beetles. Annual Review of Ecology and Systematics, 13(1):261-290.
https://doi.org/10.1146/annurev.es.13.110182.001401
Leng, C.W. 1913. Aquatic Coleoptera. Journal of the New York Entomological Society, 21(1):32-42.
Liu, H., Beutel, R.G., Makarov, K.V., Jarzembowski, E.A., Xiao, C., and Luo, C. 2023. The first larval record of Migadopinae (Coleoptera: Adephaga: Carabidae) from mid-Cretaceous Kachin amber, northern Myanmar. Cretaceous Research, 142:105413.
https://doi.org/10.1016/j.cretres.2022.105413
Makarov, K.V., and Prokin, A.A. 2015. About homology of Haliplus Latreille, 1802 larvae postanal process (Coleoptera, Haliplidae). Acta Entomologica Musei Nationalis Pragae, 55:879-881.
Malcolm, S.E. 1971. The water beetles of Maine: including the families Gyrinidae, Haliplidae, Dytiscidae, Noteridae, and Hydrophilidae. Technical Bulletin, Life Sciences and Agriculture Experiment Station, University of Maine at Orono, 48:1-49.
Matheson, R. 1912. The Haliplidæ of North America, North of Mexico. Journal of the New York Entomological Society, 20(3):156-193.
Matta, J.F. 1976. The Haliplidae of Virginia (Coleoptera: Adephaga). The insects of Virginia 10, Research Division Bulletin, 109:1-26.
McKenna, D.D., Shin, S., Ahrens, D., Balke, M., Beza-Beza, C., Clarke, D.J., Donath, A., Escalona, H.E., Friedrich, F., Letsch, H., Liu, S., Maddison, D., Mayer, C., Misof, B., Murin, P.J., Niehuis, O., Peters, R.S., Podsiadlowski, L., Pohl, H., Scully, E.D., Yan, E.V., Zhou, X., Ślipiński, A., and Beutel, R.G. 2019. The evolution and genomic basis of beetle diversity. Proceedings of the National Academy of Sciences, 116:24729-24737.
https://doi.org/10.1073/pnas.1909655116
Michat, M.C., Archangelsky, M., and Alarie, Y. 2020. Morphology and chaetotaxy of Neotropical Haliplus larvae (Coleoptera: Haliplidae). Revista Mexicana de Biodiversidad, 91:e913541.
Palmer, A.R., Carvalho, J.C.M., Cook, D.R., O’Neill, K., Petrunkevitch, A., and Sailer, R.I. 1957. Miocene arthropods from the Mojave desert, California. US Government Printing Office, Washington.
https://doi.org/10.3133/pp294g
Peterson, A. 1957. Larvae of insects an introduction to Nearctic species part 2 Coleoptera, Diptera, Neuroptera, Siphonaptera, Mecoptera, Trichoptera. Edwards Brothers INC, Ann Arbor, Michigan, USA.
Ponomarenko, A.G. 1993. Two new species of Mesozoic dytiscoid beetles from Asia. Paleontological Journal, 27(1A):182-191.
Ponomarenko, A.G., Prokin, A.A., and Bashkuev, A.S. 2015. Coptoclavid beetles (Insecta: Coleoptera: Adephaga) from the Triassic of Lower Franconia, Germany. Paleontological Journal, 49:1334-1345.
https://doi.org/10.1134/s0031030115120096
Prokin, A.A. and Ponomarenko, A.G. 2013. The first record of crawling water beetles (Coleoptera, Haliplidae) in the Lower Cretaceous of Mongolia. Paleontological Journal, 47:89-93.
https://doi.org/10.1134/s0031030113010115
Prokin, A.A., Makarov, K.V., Ponomarenko, A.G., and Bashkuev, A.S. 2013. New beetle larvae (Coleoptera: Coptoclavidae, Carabidae, Polyphaga) from the Upper Triassic of Germany. Russian Entomological Journal, 22(4):259-274.
Prokop, J., Nel, A., Hájek, J., and Bubík, M. 2004. First record of a fossil beetle (Coleoptera, Haliplidae) from the basal Paleocene Flysch sediments in the Magura unit (Outer Western Carpathians, Moravia). Geologica Carpathica, 55:469-473.
Ribera, I., Hogan, J.E., and Vogler, A.P. 2002. Phylogeny of hydradephagan water beetles inferred from 18S rRNA sequences. Molecular Phylogenetics and Evolution, 23(1):43-62.
https://doi.org/10.1006/mpev.2001.1080
Řiha, P. 1979. Bibliographie der tertiären Haliplidae, Hygrobiidae, Dytiscidae (inklusive Palaeogyrinidae) und Gyrinidae der Welt (Coleoptera). Beiträge zur Entomologie, 29(1):267-270.
Schädel, M., Hörnig, M.K., Hyžný, M., and Haug, J.T. 2021. Mass occurrence of small isopodan crustaceans in 100-million-year-old amber: an extraordinary view on behaviour of extinct organisms. PalZ, 95:429-445.
https://doi.org/10.1007/s12542-021-00564-9
Schiödte, J.C. 1864. De metamorphosi eleutheratorum observationes: bidrag til insekternes udviklingshistorie. Thieles Bogtrykkeri, Copenhagen, Denmark.
https://doi.org/10.5962/bhl.title.8797
Shi, G., Grimaldi, D.A., Harlow, G.E., Wang, J., Wang, J., Yang, M., Lei, W., Li, Q., and Li, X. 2012. Age constraint on Burmese amber based on U-Pb dating of zircons. Cretaceous Research, 37:155-163.
https://doi.org/10.1016/j.cretres.2012.03.014
Spangler, P.J. 1991. Haliplidae (Adephaga), p. 311. In Stehr, F.W. (ed.), Immature insects: Volume 2. Kendall Hunt Pub Co, Dubuque, Iowa, USA.
Vondel, B.J. van. 1986. Description of the second and third-instar larvae of Haliplus laminatus (Schalter) with notes on the subgeneric status (Coleoptera: Haliplidae). Entomologische Berichten, 46(9):128-132.
Vondel, B.J. van. 1995. Haliplidae: Review of the Haliplidae of China, p. 111-154. In Jäch, M.A. and Ji, L. (eds.), Water Beetles of China Vol. 1. Zoologisch-Botanische Gesellschaft in Österreich und Wiener Coleopterologen-Verein, Vienna, Austria.
Vondel, B.J. van. 1996. Description of the second and third instar larva of Haliplus varius with notes on the subgeneric status (Coleoptera: Haliplidae). Entomologische Berichten, 56(1):9-11.
Vondel, B.J. van. 1997. Insecta: Coleoptera: Haliplidae, p. 1-95. In Schwoerbel, J. and Zwick, P. (eds.), Süßwasserfauna von Mitteleuropa 20. Gustav Fischer Verlag, Stuttgart, Jena, Lübeck, Ulm, Germany.
Vondel, B.J. van. 2001. Description of the third instar larva of Haliplus subseriatus (Coleoptera: Haliplidae). Entomologische Berichten, 61(1):14-16.
Vondel, B.J. van. 2004. First description of larvae of Haliplus -species from Australia (Coleoptera: Haliplidae). Tijdschrift Voor Entomologie, 147(1):57-61.
https://doi.org/10.1163/22119434-900000140
Vondel, B.J. van. 2011a. Description of Haliplus larvae from Lebanon (Coleoptera: Haliplidae). Koleopterologische Rundschau, 81:41-54.
Vondel, B.J. van. 2011b. Description of the third instar larva of Haliplus variomaculatus Brigham & Sanderson with notes on larvae of Nearctic Haliplidae (Coleoptera). Tijdschrift Voor Entomologie, 154(1):127-133.
https://doi.org/10.1163/22119434-900000310
Vondel, B.J. van. 2012. Description of larvae of four Haliplus species from Australia (Coleoptera: Haliplidae). Tijdschrift voor Entomologie, 155(2-3):193-208.
https://doi.org/10.1163/22119434-00002012
Vondel, B.J. van. 2016. 7.2 Haliplidae Aubé, 1836, p. 481-486. In Beutel, R.G. and Leschen, R.A.B. (eds.), Coleoptera, Beetles. Morphology and Systematics, Vol.1(2). Walter de Gruyter GmbH & Co KG, Berlin, Boston.
Vondel, B.J. van. 2019. Features of the metacoxal air-storage space as additional characters for reconstructing the phylogeny of Haliplidae (Coleoptera). Tijdschrift voor Entomologie, 162:13-32.
https://doi.org/10.1163/22119434-20192081
Vondel, B.J. van. 2021. Revision of the Nearctic Haliplidae (Coleoptera). Tijdschrift voor Entomologie, 163(2-3):101-298.
https://doi.org/10.1163/22119434-20202093
Vondel, B.J. van and Alarie, Y. 2016. A new species of Haliplus Latreille, 1802 (Coleoptera: Adephaga: Haliplidae) from Canada. The Coleopterists Bulletin, 70:801-804.
https://doi.org/10.1649/0010-065x-70.4.801
Vondel, B.J. van and Litovkin, S.V. 2017. Five new synonymies in Haliplus subgen. Liaphlus Guignot, based on the variability of the left paramere. Koleopterologische Rundschau, 87:31-35.
Vondel, B.J. van and Spangler, P.J. 2008. Revision of the Haliplidae of the Neotropical region including Mexico. Koleopterologische Rundschau, 78:69-194.
Wang, B., Ponomarenko, A.G., and Zhang, H.C. 2009. A new coptoclavid larva (Coleoptera: Adephaga: Dytiscoidea) from the Middle Jurassic of China, and its phylogenetic implication. Paleontological Journal, 43:652-659.
https://doi.org/10.1134/s0031030109060082
Wang, B., Ponomarenko, A.G., and Haichun, Z. 2010. Middle Jurassic Coptoclavidae (Insecta: Coleoptera: Dytiscoidea) from China: a good example of mosaic evolution. Acta Geologica Sinica - English Edition, 84:680-687.
https://doi.org/10.1111/j.1755-6724.2010.00272.x
Watanabe, R., and Yamasaki, S. 2020. Discovery of larvae of Haliplus kamiyai Nakane, 1963 (Coleoptera, Haliplidae) and implications for its life Cycle. Elytra, Tokyo, New Series, 10(2):365-371.
White, D.S. 2009. Coleoptera (beetles) in aquatic ecosystems, p. 144-156. In Likens, G.E. (ed.), Encyclopedia of Inland Waters. Elsevier, Academic Press, Cambridge, Massachusetts, USA.
https://doi.org/10.1016/b978-012370626-3.00160-5
Wichard, W., Gröhn, C., and Seredszus, F. 2009. Aquatic insects in Baltic amber: Wasserinsekten im baltischen Bernstein. Verlag Kessel, Remagen-Oberwinter, Germany.
Xia, F., Yang, G., Zhang, Q., Shi, G., and Wang, B. 2015. Amber: life through time and space. Science Press, Beijing.
Yee, D.A. and Kehl, S. 2015. Order Coleoptera, p. 1003-1042. In Thorp, J.H. and Rogers, D. C. (eds.), Thorp and Covich's freshwater invertebrates. Academic Press Books Elsevier, London, Oxford, Boston, New York, San Diego.
https://doi.org/10.1016/b978-0-12-385026-3.00039-5
Yu, T., Kelly, R., Mu, L., Ross, A., Kennedy, J., Broly, P., Xia, F., Zhang, H., Wang, B., and Dilcher, D. 2019. An ammonite trapped in Burmese amber. Proceedings of the National Academy of Sciences, 116:11345-11350.
https://doi.org/10.1073/pnas.1821292116
Zhang, W.W. 2017. Frozen Dimensions. The fossil insects and other invertebrates in amber. Chongqing University Press, Chongqing (in Chinese).
Zhao, X., Zhao, X., Jarzembowski, E.A., and Wang, B. 2019. The first whirligig beetle larva from mid-Cretaceous Burmese amber (Coleoptera: Adephaga: Gyrinidae). Cretaceous Research, 99:41-45.
https://doi.org/10.1016/j.cretres.2019.02.015
Zhao, X., Zhao, X., Jarzembowski, E., Tian, Y., and Chen, L. 2020. The first record of brachypsectrid larva from mid-Cretaceous Burmese amber (Coleoptera: Polyphaga). Cretaceous Research, 113:104493.
https://doi.org/10.1016/j.cretres.2020.104493
Zippel, A., Haug, C., Hoffeins, C., Hoffeins, H.-W., and Haug, J.T. 2022a. Expanding the record of larvae of false flower beetles with prominent terminal ends. Rivista Italiana di Paleontologia e Stratigrafia, 128:81-104.
https://doi.org/10.54103/2039-4942/17084
Zippel, A., Haug, C., Müller, P., and Haug, J.T. 2022b. First fossil tumbling flower beetle-type larva from 99 million-year-old amber. PalZ, 96:219-229.
https://doi.org/10.1007/s12542-022-00608-8
Zippel, A., Baranov, V.A., Hammel, J.U., Hörnig, M.K., Haug, C., and Haug, J.T. 2022c. The first fossil immature of Elmidae: an unusual riffle beetle larva preserved in Baltic amber. PeerJ, 10:e13025.
https://doi.org/10.7717/peerj.13025
Zippel, A., Haug, C., Müller, P., and Haug, J.T. 2023. The first fossil false click beetle larva preserved in amber. PalZ, 97:209-215.
https://doi.org/10.1007/s12542-022-00638-2

