DISCUSSION
In all of the horn locking positions, horn contact was made on the lateral and medial surfaces of the postorbital horncores. Thus, it appears that most of the force was applied to the horncores on their lateral and medial surfaces, rather than the rostral or caudal surfaces. Chasmosaurine ceratopsid postorbital horncores, particularly those of Triceratops, are often slightly mediolaterally compressed rather than perfectly round in cross section. This would have allowed a greater surface area on the medio-lateral surfaces of the horns, and hence a greater area of contact when the horns were locked.
Horn Locking and Paleopathologies
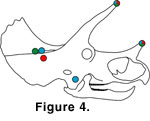 Based on the horn locking positions explored in this study, specific predictions can be made for sites on the skull where horn-induced trauma should occur
(Figure 4). In the single horn contact position, there was little danger of injury from the postorbital horns (unless the skulls were tilted down to a great degree
rostrally). The nasal horn was placed against the rostromedial portion of the squamosal bone of the frill, and the horn could possibly inflict injury here.
Based on the horn locking positions explored in this study, specific predictions can be made for sites on the skull where horn-induced trauma should occur
(Figure 4). In the single horn contact position, there was little danger of injury from the postorbital horns (unless the skulls were tilted down to a great degree
rostrally). The nasal horn was placed against the rostromedial portion of the squamosal bone of the frill, and the horn could possibly inflict injury here.
In the full horn locking position, both the nasal horn and the postorbital horns were possible sources of injury. The nasal horn was positioned very near the opponent's jugal, and the tips of the postorbital horns of both animals were quite near the rival's parietal portion of the frill, in the upper temporal fenestra region.
In the oblique horn locking position, the nasal horns once again were the major possible cause of injury. Here, injuries are predicted in the rostral portions of both the squamosals and the parietals.
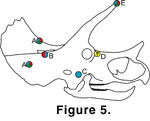 Some known pathologies in Triceratops skulls correspond to injuries predicted by these models
(Figure 5). Hatcher et al. (1907) noted a series of abnormal foramina along the medial border of the left squamosal of the specimen USNM 1201, which they interpreted as a traumatic injury. This corresponds to possible injury positions predicted by the FHL and OHL models. Additionally,
Erickson (1966) noted an anomalous foramen in the jugal of specimen SMM P62/1/1. This corresponds to the placement of the nasal horn against the jugal in the FHL model. The skull SMNH P1163.4 lacks a major portion of the right
squamosal, which occurred pre mortem based upon bone texture; the parietal bar of this specimen may display a healed fracture
(Tanke and Rothschild
2002). These injury locations correspond to those predicted by all three of the horn locking positions shown here.
Some known pathologies in Triceratops skulls correspond to injuries predicted by these models
(Figure 5). Hatcher et al. (1907) noted a series of abnormal foramina along the medial border of the left squamosal of the specimen USNM 1201, which they interpreted as a traumatic injury. This corresponds to possible injury positions predicted by the FHL and OHL models. Additionally,
Erickson (1966) noted an anomalous foramen in the jugal of specimen SMM P62/1/1. This corresponds to the placement of the nasal horn against the jugal in the FHL model. The skull SMNH P1163.4 lacks a major portion of the right
squamosal, which occurred pre mortem based upon bone texture; the parietal bar of this specimen may display a healed fracture
(Tanke and Rothschild
2002). These injury locations correspond to those predicted by all three of the horn locking positions shown here.
Injuries to the horncores themselves are also possible. Gilmore (1919) documented a pair of chasmosaurine (probably Triceratops) horncores, USNM 4708, in which the distal end of the right horncore is missing. Some evidence of healing is visible on the tip of this horn. Additionally, Rothschild and Tanke (1992) figured a cf. Anchiceratops (a chasmosaurine closely related to Triceratops) horncore, TMP 89.12.8, with pseudoarthrosis on its distal tip. This too may be due to trauma (Tanke, personal commun., 2003), whether from combat or other causes (e.g., a collision with a tree). Possibly such breaks occurred when stresses within horncore became too great for the bone to withstand. This could happen in any of the modeled horn positions, but horn injury would be particularly likely in those cases where the very tip of the horn was subjected to especially great stress, as in the OHL position (or if the SHC position were adopted with horn contact placed more distally). Injuries to the nasal horn are also possible in all three of the positions. However, this horn is typically much shorter and robust and thus less likely to break than the postorbital horns.
Some Triceratops specimens exhibit possible traumatic injuries not predicted by any of the models. For instance, the right frontal of the skull YPM 1823 preserves an anomalous foramen. None of the horn locking positions infer horn contact in this area. If this pathology is horn induced, it may have been caused by a slip of an opponent's horn.
It is tempting to suggest that the pathological specimens described here illustrate a stereotypy in lesion placement consistent with horn-induced injury, but the sample size simply isn't large enough to make this suggestion. Additionally, reports of pathology may be biased toward the horns and frill. Further work is needed to accurately map and define the occurrence of lesions in ceratopsian skulls.
Many of the reported lesions are consistent with horn-thrust injuries, but it is extremely important to note that traumatic injury has not been conclusively demonstrated in many of these specimens. Careful examination, perhaps coupled with bone histology work, is necessary to support the claims that these cranial anomalies are due to trauma and not other factors (as in the case of many squamosal fenestrae, long attributed to horn injuries but now reinterpreted as a disease or other bone remodeling process possibly analogous to that seen in some modern turtle carapaces; Tanke and Farke 2002). Bone fractures (as seen in SMNH P1163.4) are the only truly unambiguous indication of trauma. Indications of osteomyelitis or other conditions are less reliable, as they may be associated with non-traumatic disease processes. Even if the pathologies can be shown to result from trauma, it is difficult to conclusively demonstrate that these injuries resulted from horn locking behavior and not environmental obstacles (e.g. rocks, trees) or predator encounters.
Difficulties in Modeling Behavior
Horn shape, size, and orientation are variable in Triceratops (well illustrated in Lehman 1990), complicating interpretation and application of this study's results. The nasal horn is a particularly important variable. This horn is relatively long in YPM 1822, and there was some danger of the horn piercing a rival animal's skull during horn locking. This may have limited the range of movement for fighting animals. The nasal horn is reduced in size in many other Triceratops specimens (e.g., SDSM 2670), so these animals may have had a greater range of movement during combat. In fact, the nasal horn was so blunt in some specimens (e.g., USNM 1201) that it could have been used to butt against opponents' flanks without risk of major injury to either animal. Additionally, a blunt nasal horn would presumably reduce the risk of injury to the frill in any of the hypothesized positions.
Postorbital horn length and orientation also vary in Triceratops. Forster (1996) noted that YPM 1822 has comparatively short horns relative to its basal skull length, contrasting with the relatively longer horns seen in other specimens (e.g., USNM 1201). Additionally, the horns of YPM 1822 are at a smaller angle relative to horizontal than in some other Triceratops specimens (e.g., USNM 4928). This variation could affect horn locking in several ways. If the horns were relatively longer, but at the same orientation as in the models used here, horn locking positions would remain much the same. However, in cases of extreme elongation, horn contact would have to occur more distally on the horn core to reduce risk of injury to the frill. If the horns were at a greater angle to vertical, the relative orientation of the combatants' skulls would also have to be at a greater angle to allow effective horn locking in some positions. If the horns were at a greater angle to each other in the sagittal plane, the horns would contact each other quite proximally in the SHC position, but the horns would contact each other more distally in the OHL or FHL positions. Based upon experiments with simple clay models, all of the same horn locking positions were repeatable with these different horn orientations. However, much more experimentation is needed to determine how the full spectrum of Triceratops horn variation would have affected horn locking positions. If Triceratops wrestled in life, it likely employed any number of variations upon the positions found here.
The inferred presence of a keratinous sheath on the outside of the horns also complicates modeling, particularly if the sheath significantly lengthened the horn or altered horn shape. Anecdotally, Hatcher et al. (1907, p. 32) reported that when the Triceratops specimen YPM 1821 was discovered, "a portion of the investing horny material was still in place about the left horn core, though in such a decomposed condition that it was impossible to preserve it." Happ and Morrow (2000) also reported possible remnants of the horn sheath in a Triceratops specimen from the Hell Creek Formation of Montana, but this specimen has not been fully described. However, none of these fossils reveal the length or shape of the sheath, or if it was indeed a keratinous sheath and not just a layer of skin. Any speculation upon sheath morphology is difficult. But, it is highly unlikely that Triceratops had complicated, curled horns such as seen in the mountain sheep, Ovis canadensis. In this and other sheep and goats with curled horns, the horncore itself is also curled, although not to the degree of the sheath (Schaffer and Reed 1972). This contrasts with the relatively straight horncores seen in Triceratops. The curled horn morphology is also apparently related to head-butting behavior (Lundrigan 1996), a behavior that was clearly difficult if not impossible in Triceratops. In any case, the addition of horn length by a sheath would affect horn locking behavior in the manner discussed above for variations in horncore length. Horn locking positions would remain much the same, regardless of sheath length.
A major simplification of this study is the use of two identical cranial models. In actuality, it is highly unlikely that any two interacting Triceratops had identical cranial morphology. Horn locking would still be possible, but variations in skull morphology must be investigated with further modeling.
Triceratops Horn Locking Compared with Bovid Mammal Horn Locking
The supraorbital horns of Triceratops and many of its close relatives are frequently compared to those of bovid mammals such as bison, goats, and African antelope (e.g., Farlow and Dodson 1975). Indeed, the gross horn morphology of both groups is quite similar, in that the horns are paired, placed posteriorly on the skull, and unbranched. However, horn orientation is quite different between the two groups (Farlow 1990). In Triceratops and other known chasmosaurines, the postorbital horns are directed rostro-dorsally relative to the rest of the skull, with only a small lateral component in most taxa. The horns of bovid mammals may point laterally (as in the American bison or the African buffalo), caudally (as in gazelle and oryx), or laterally and rostrally (e.g., some domesticated cattle). Also, the horns of bovids may be "curled" (as in bighorn sheep) or twisted (as in kudu).
Logically, horn morphology should be correlated to fighting style (e.g., Geist 1966). But, few morphometric studies have investigated the relationship (if any) between horn orientation, horn shape, and horn use in bovid mammals. Lundrigan (1996) studied this problem for 21 bovid species representing 11 of the 12 bovid tribes. Interestingly, she found that neither the vertical angle of the horns nor their angle with the sagittal plane was significantly correlated to any fighting mode. This would suggest that horn orientation has little bearing on the behavior for which these horns are used. Instead, Lundrigan (1996) found that the overall horn shape and length (e.g., the degree of curvature and the greatest straight-line length of the horn) were significantly correlated to fighting mode.
Bovids with very short horns and short catching arches (the recurved region between the base and the tip of the horn) tended to engage in stabbing behavior (e.g., mountain goats). Neither of these qualities applies to Triceratops, but it may apply to other ceratopsid dinosaurs (see next section).
Lundrigan (1996) determined that bovids employing ramming behavior (e.g., bighorn sheep) tended to have "curled" horns with a large basal circumference. Such behavior clearly was not possible in Triceratops, due to the near-vertical horn orientation that prevented any frontal contact. Also, this horn orientation would have made a high-speed head-to-head charge quite hazardous.
According to Lundrigan's (1996) analysis, bovids that employ "fencing" behavior (in which the horns are clashed against each other, without sustained locking) usually possess a long horn "reach" (greatest "straight" length of horn, exclusive of curves). Such behavior would have been physically possible in Triceratops.
Bovids engaging in sustained wrestling (horn locking) behavior generally have a large "catching arch," (Lundrigan 1996). In many of these animals, the dorsal surface of the skull is held nearly against the ground during horn locking. This specific position could not have occurred in Triceratops, due to limitations in mobility imposed by its vastly different horn orientation, probably more limited neck mobility, and the large bony frill. If Triceratops locked horns, it would have done so in a manner different from that seen in bovids, as illustrated by the models in this study (Figure 1, Figure 2, Figure 3). The complex motions required to interlock the horns of Triceratops suggest that the individual combatants had to carefully and deliberately orchestrate horn locking.
Caro et al. (2003) also discussed the relationship between horn shape and behavior. However, their study primarily focused upon qualitative variables such as horn tip orientation or horn sheath morphology, and information such as absolute horn size and orientation was not considered. Thus, their results are not directly comparable to Lundrigan's (1996) results. Nonetheless, Caro et al. (2003) also found significant correlations between horn morphology and fighting style, most of which broadly match Lundrigan's results.
It is quite probable that relationships between horn morphology and behavior existed in ceratopsid dinosaurs, as discussed by other workers (e.g., Farlow and Dodson 1975). But ultimately, ceratopsid behavior cannot be directly inferred from that of bovids due to the disparate phylogenetic origin, unique horn orientation, and other unusual cranial features (such as nasal horns and frills) found in ceratopsids.
Some extant chameleons (e.g., Chamaeleo jacksoni) superficially resemble Triceratops in horn number (up to three horns), position (one nasal and two supraorbital), and orientation (directed rostrally). Most importantly, three-horned chameleons are known to lock horns in intraspecific combat. During these confrontations, male Jackson's chameleons will face off and then rush forward to interlock horns. Then, they may twist their heads back and forth in an effort to throw each other off balance. The combatants may also attempt to stab each other in the body or limbs (Carpenter and Ferguson 1977). Some illustrated chameleon horn locking positions even resemble those modeled for Triceratops (e.g., Farlow 2001). Unfortunately, the analogy is not perfect. For instance, the horns are directed almost horizontally in chameleons, contrasting with the more vertical orientation of ceratopsids, and chameleons are much smaller than ceratopsids. Nonetheless, chameleons provide an extant precedent for wrestling with three horns.
Horn Use in Other Chasmosaurines
The observations made for Triceratops likely apply to most other chasmosaurines, particularly those with elongated postorbital horns (including Anchiceratops, Arrhinoceratops, Diceratops, Pentaceratops, and Torosaurus). The only major difference occurs in some individuals of the genus Chasmosaurus; many specimens of this taxon (e.g., ROM 843) have very small or non-existent postorbital horns. Clearly, horn locking did not occur here (barring the presence of extremely elongated horn sheaths which have not been preserved in the fossil record). If intraspecific fighting did occur in these individuals without postorbital horns, it was probably quite different relative to Triceratops, perhaps with head or flank butting or stabbing behavior. Further work on Chasmosaurus cranial models may elucidate this.
Few cranial pathologies have been described in chasmosaurines outside of Triceratops (excluding squamosal fenestrae, of dubious origin as mentioned above), but those that have been are consistent with horn locking models discussed above. For instance, Torosaurus specimen MPM VP8149 displays a lesion on the medio-rostral margin of the left squamosal (Marshall and Barretto 2001), a location consistent with all three of the hypothetical horn locking positions. Diceratops specimen USNM 2412 displays an area of calloused bone on the left squamosal, also suggesting horn-induced injury. Careful study of other chasmosaurine specimens may show additional evidence of cranial pathology.
The results of this study are not applicable to any described centrosaurine taxa, because most of these taxa do not possess elongated postorbital horns.