| |
MATERIALS AND METHODS
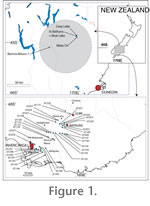 Fossils were collected from two geological basins of which the sedimentary fill is now represented by two stratigraphic groups (Figure 1). The Manuherikia Group (MG) is an extensive (>5600 km2) deposit of Miocene fluvial (Dunstan Formation) to lacustrine (Bannockburn Formation) basin fill in Central Otago, southern N.Z. The stratigraphy and sedimentology has been detailed by
Douglas (1986), the palynology by
Mildenhall (1989) and
Mildenhall and Pocknall (1989), who dated the Group as Early-Late Miocene, and the macropaleobotany by
Pole (1993c and references therein). The sediments which yield dispersed cuticle come from outcrops in the oldest unit, the incised valley fill of the St Bathans Member (sample numbers prefixed with "BL" and "GL"). Fossils were collected from two geological basins of which the sedimentary fill is now represented by two stratigraphic groups (Figure 1). The Manuherikia Group (MG) is an extensive (>5600 km2) deposit of Miocene fluvial (Dunstan Formation) to lacustrine (Bannockburn Formation) basin fill in Central Otago, southern N.Z. The stratigraphy and sedimentology has been detailed by
Douglas (1986), the palynology by
Mildenhall (1989) and
Mildenhall and Pocknall (1989), who dated the Group as Early-Late Miocene, and the macropaleobotany by
Pole (1993c and references therein). The sediments which yield dispersed cuticle come from outcrops in the oldest unit, the incised valley fill of the St Bathans Member (sample numbers prefixed with "BL" and "GL").
The second geological unit is the Gore Lignite Measures, of the East Southland Group, which accumulated on a coastal delta (Isaac and Lindqvist 1990). Palynology of this unit has been detailed by
Pocknall (1982) and
Pocknall and Mildenhall (1984). Samples were taken from drill core housed in the Crown Minerals Dunedin core library (sample number prefixed with "Sthd"). Full sample details for both basins are provided in
Pole (2007) including precise grid references for the MG samples and drill core depths for the East Southland Group. Detailed stratigraphic sections of the East Southland Group are present in
Isaac and Lindqvist (1990). Precise stratigraphic relationships within the St Bathans Member, where there is complex fluvial channel cross-cutting, are still being resolved and cannot be shown on a simple section. Stratigraphic details at that resolution are not relevant at this stage. The only additional sample here is Bannockburn-1, which is the only sample found in the Bannockburn region, which has good cuticular preservation. It was collected at F41 087626 (grid reference based on the New Zealand 1:50 000 Topographic Map 260 series). The outcrop has since been covered over by lake-shore development after the completion of the Clyde Hydroelectric scheme.
Cuticle preparation followed a standard method whereby sediment was disaggregated by covering with hot water and adding some 40% hydrogen peroxide. The organic fraction was removed by sieving and cuticle further cleaned by immersion for several hours in warm aqueous chromium trioxide. After washing a staining in Crystal Violet or Safranin, cuticle fragments were mounted on microscope slides (specimen numbers prefixed with "SB" or "SL") in Thymol Glycerine Jelly for Transmitted Light Microscopy (TLM), or on aluminium stubs (specimen numbers prefixed with "S-") with double-sided tape and coated with platinum for Scanning Electron Microscopy (SEM). Material is lodged in the State Herbarium of Queensland.
Cuticle morphologies are described as parataxa. They are given a non-hierarchical parataxon code. For pragmatism this consists of the prefix 'CUT-Mo-' (for "cuticle-monocot") followed by a string of three letters. These letters have no meaning, but together with the prefix, form a unique text string. For previous use of parataxa for fossil cuticles, see
Carpenter and Pole (1995),
Pole (1996,
1998,
2007). For each parataxon, a 'reference specimen' is nominated. Specimens are stored in the Queensland State Herbarium. The "typical" monocot parataxa – those with aligned stomata – are distinguished on the basis of a key (Appendix) and to keep morphological similar types together, they are described in the general order of the key. Rhipogonum is the exception, being one of the 'net-veined' monocots, which is identifiable by direct comparison with the distinctive cuticle of extant species. It is described first.
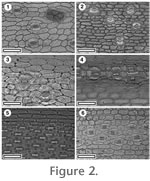 Cuticle identification was based on the published guides listed above as well as the author's reference collection of extant monocot cuticle (specimens prefixed with "OPH"). This has been prepared from the collections of the Queensland Herbarium (AQ) and the Allan Herbarium (CHR). The numbers of the parent herbarium specimens are given to allow relocation of the specimen from which the cuticle fragment was prepared. Cuticle preparation initially followed the same aqueous chromium trioxide method as for fossils, but more recently has switched to boiling in a 6:1 mixture of hydrogen peroxide and glacial acetic acid. This was found to give better results for the relative fragile monocot cuticle. The reference collection currently includes over 230 species of monocot, mainly Arecaceae. The cuticle of a range of common extant New Zealand monocots is shown in
Figure 2. Cuticle identification was based on the published guides listed above as well as the author's reference collection of extant monocot cuticle (specimens prefixed with "OPH"). This has been prepared from the collections of the Queensland Herbarium (AQ) and the Allan Herbarium (CHR). The numbers of the parent herbarium specimens are given to allow relocation of the specimen from which the cuticle fragment was prepared. Cuticle preparation initially followed the same aqueous chromium trioxide method as for fossils, but more recently has switched to boiling in a 6:1 mixture of hydrogen peroxide and glacial acetic acid. This was found to give better results for the relative fragile monocot cuticle. The reference collection currently includes over 230 species of monocot, mainly Arecaceae. The cuticle of a range of common extant New Zealand monocots is shown in
Figure 2.
The taxonomic details are presented in the
Appendix.
|