FIGURE 1. General location, indicating Central Otago in New Zealand and the three ‘regions’ that were studied.
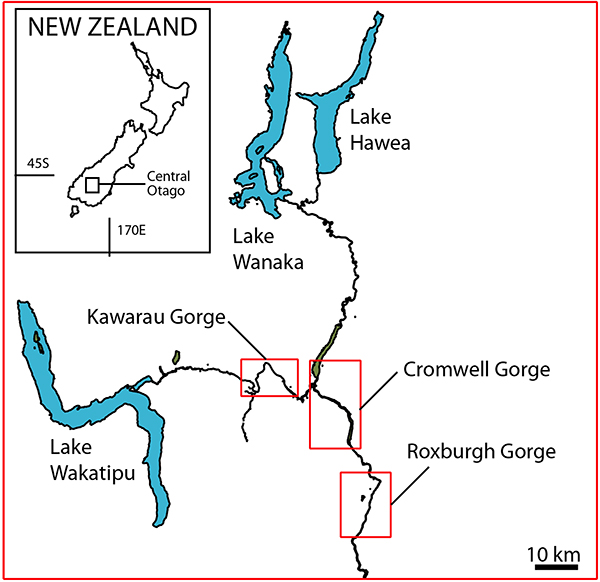
FIGURE 2. The Kawarau Region, showing shelter locations (red dots) along the Kawarau River.
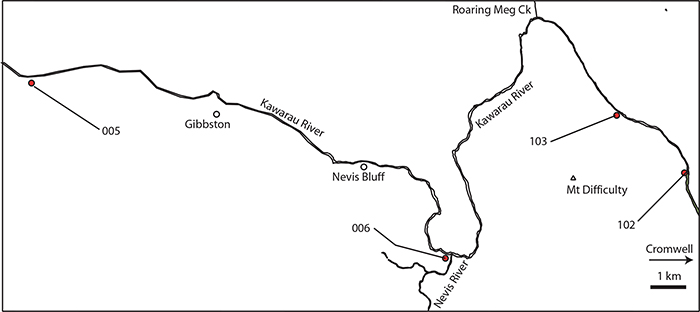
FIGURE 3. The Cromwell Region, showing shelter locations (blue dots) to the east of Lake Dunstan and within the Cromwell Gorge. Groups of closely spaced shelters are surrounded by an ellipse. Groups of closely spaced shelters are surrounded by an ellipse. Lines indicate the ‘sectors’.
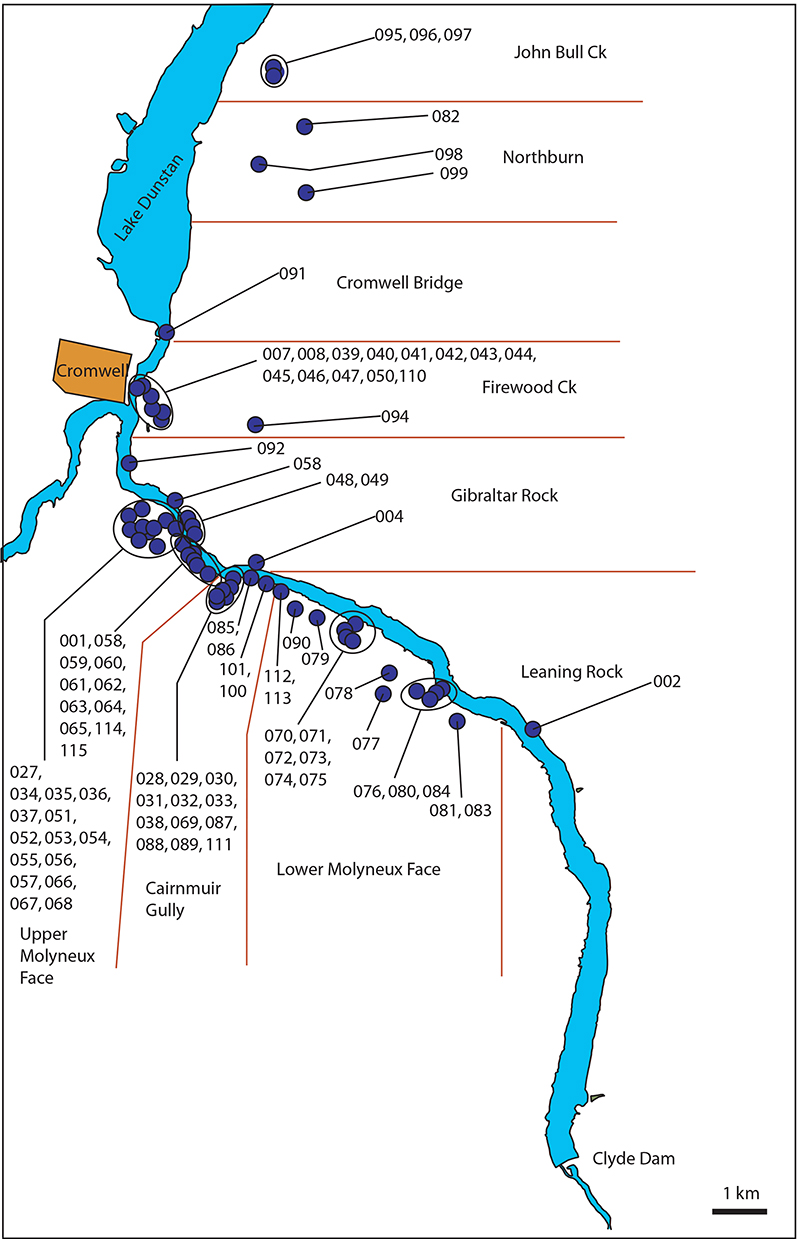
FIGURE 4. The Roxburgh Region, showing shelter locations (blue dots) centered on the Roxburgh Gorge. Groups of closely spaced shelters are surrounded by an ellipse. Lines indicate the ‘sectors’.
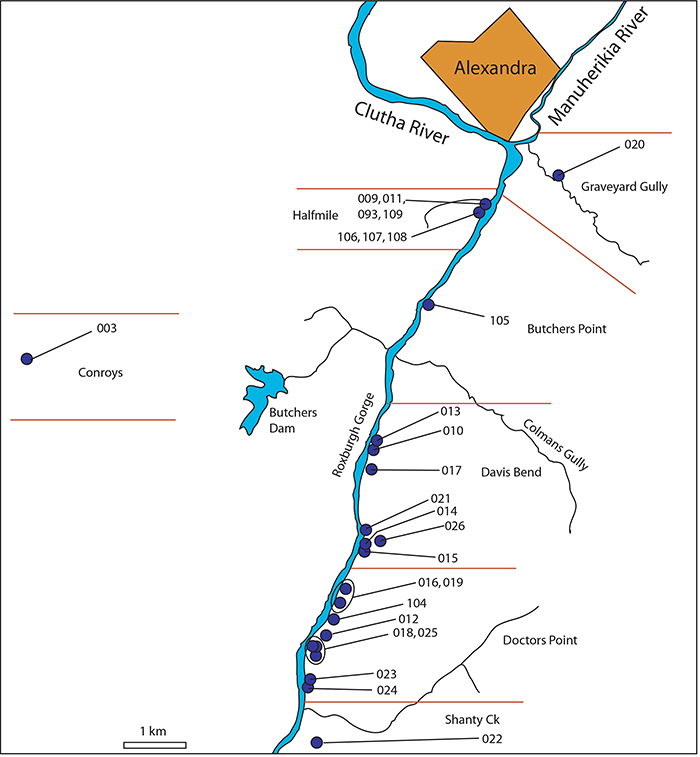
FIGURE 5. Examples of shelters the Cromwell Region. A. Shelter-29. Note relict Sophora microphylla tree (c. 350 mm diameter) outside. B. Shelter-71 (arrowed). This picture encapsulates the typical rocky and treeless environment of the Gorges today - and how the record of the rock shelters represents tiny windows into the very different vegetation of the past. C. Shelter-58, view from within the shelter, out over Lake Dunstan in the Cromwell Gorge. D. Shelter-69 (arrowed) above the vehicle track. E. Shelter-31. Vehicle track in the background. F. Shelter-43 (arrowed). Longest icicles are about 400 mm. G. Shelter-83. Trowel (260 mm) for scale. H. Shelter-83, internal view to show ‘piedmont’ of remaining sediment. Grey and brown mid-late Holocene material is overlain by a post-European cap of light material with rabbit and sheep coprolites. Trowel (260 mm) for scale.
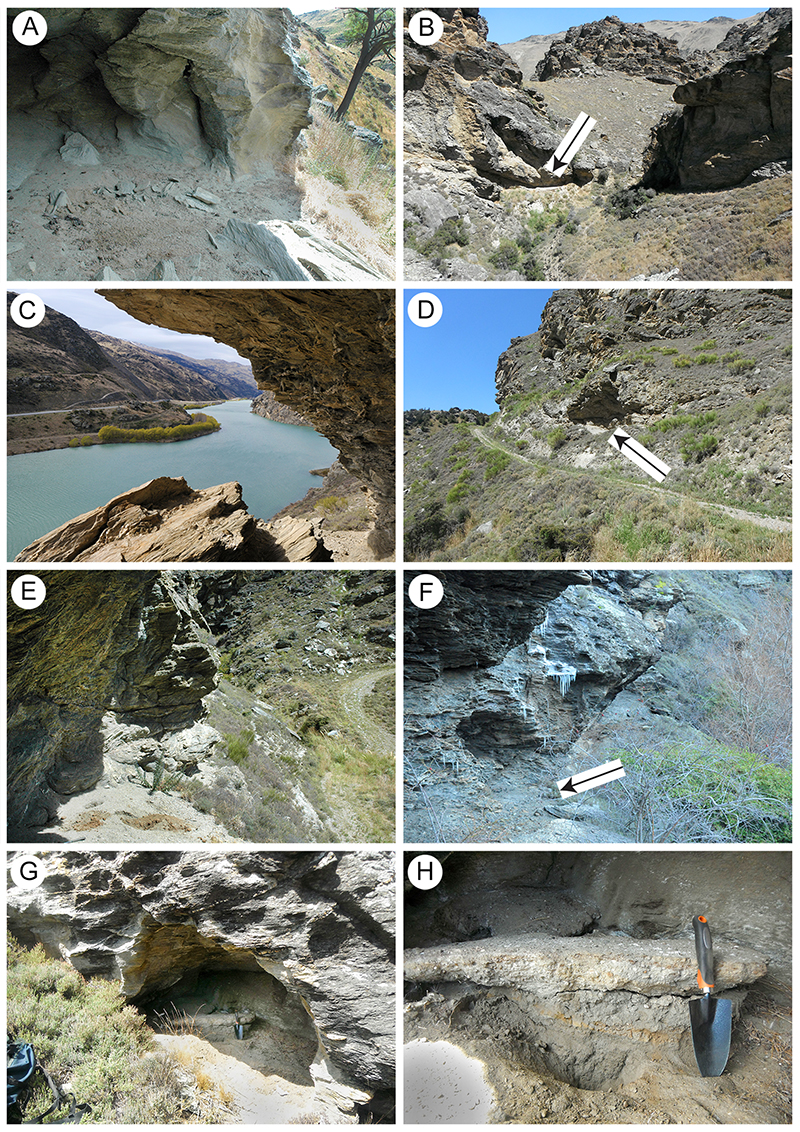
FIGURE 6. Examples of the dried vegetation zone in Cromwell Region shelters. A. Shelter-41. Surface view of an extracted mass of chewed twigs and leaf fragments. Ballpoint pen (140 mm) for scale. B. Shelter-40. Cross section showing a brown zone of dry vegetation (arrowed) below a grey zone of lithic rubble and capped by a zone within which sheep and rabbit coprolites are prominent. Ballpoint pen (140 mm) for scale. C. Shelter-46. Lens of twigs and leaf material below ballpoint pen (140 mm) and trowel, exposed in the face of a relict ‘piedmont’ of sediment. D. Shelter-71. A relatively thick brown, vegetation-rich zone adjacent trowel, capped by a pale lithic rubble. This is a close up of the floor of the shelter in Figure 5B. Trowel (260 mm) for scale. E. Shelter-78. Cross-section of an extracted lump of fine twigs and leaf material. Hand for scale. F. Shelter-28. Cross section of a sand-rich zone of dry vegetation and moa feathers. Rock hammer (handle 35 mm wide) for scale. G. Shelter-83. Surface view of extracted block of silt-rich sediment, with dried leaves on the partings. Ballpoint pen (140 mm) for scale. H. Shelter-88. Small relict ‘piedmont’ of sediment to left of trowel (260 mm), with brown zone of dry vegetation (arrowed).
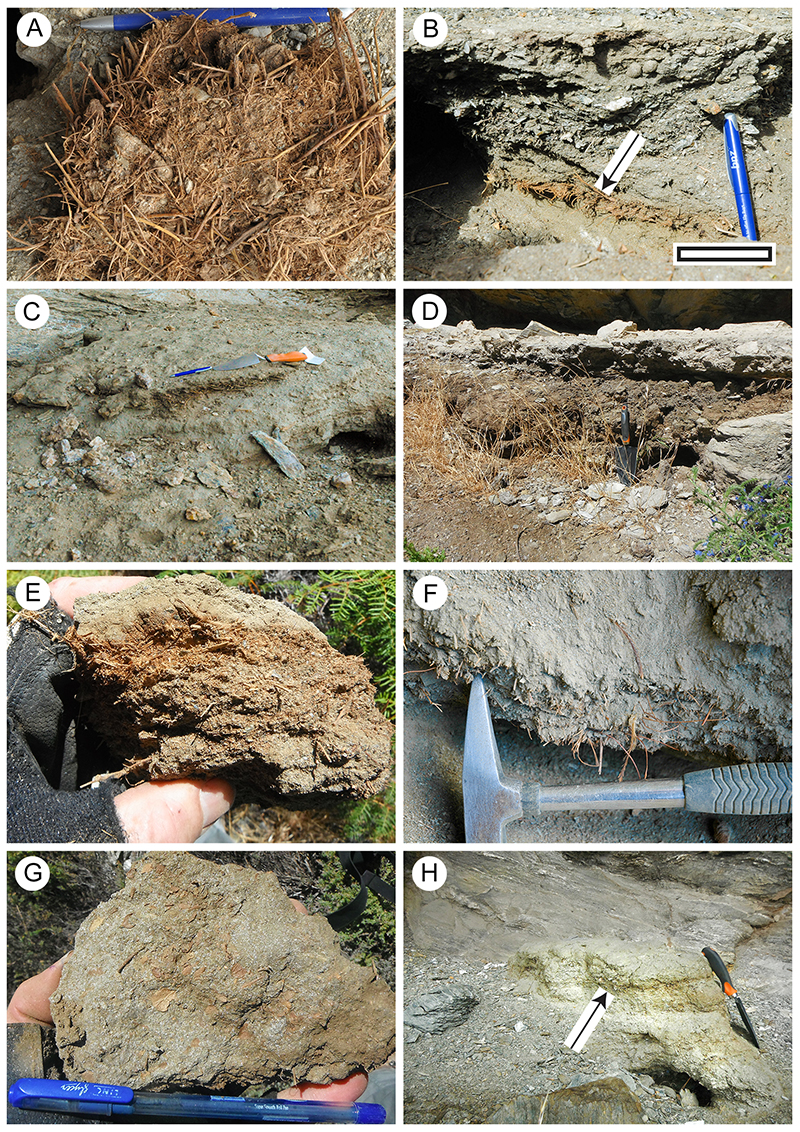
FIGURE 7. Poaceae cuticle morphology (Transmitted light microscopy). A. Showing characteristic long and short epidermal cells, and an (arrowed) stomatal complex (LX2529, Shelter-102, scale bar equals 100 μm). B. Detail, including a stomatal complex (LX2533, Shelter-80, scale bar equals 40 μm). C. A zone of long and short epidermal cells above, and a zone of epidermal cells with trichomes below (LX3184, Shelter-10, scale bar equals 100 μm). D. Two zone of long and short epidermal cells with a zone of purely long cells between (LX3224, Shelter-108, scale bar equals 100 μm).
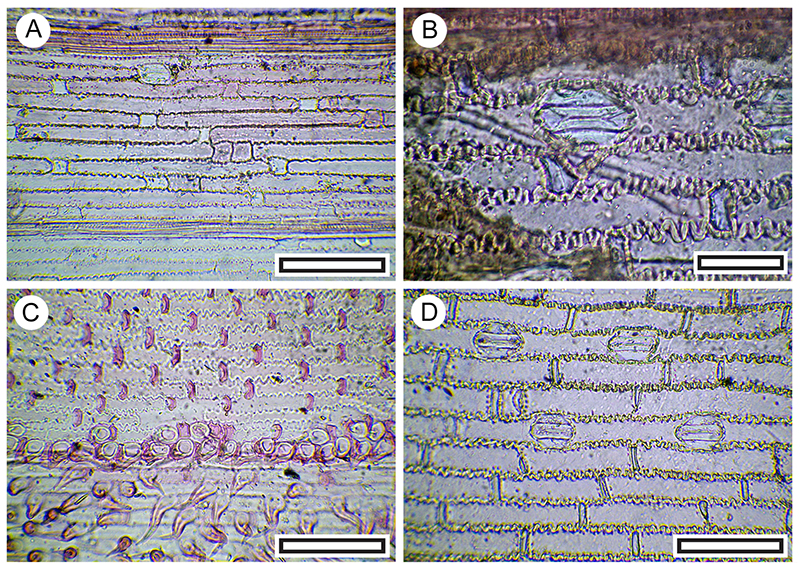
FIGURE 8. Carmichaelia sp. cuticle morphology (Transmitted light microscopy). A. Showing transversely oriented stomatal complexes along the stem (long-axis of the stem is left-right) (LX2570, Shelter-46, scale bar equals 100 μm). B. Also with transversely oriented stomatal complexes, but including trichome attachment (arrowed, SL6583, LX2478, Coprolite-11, scale bar equals 100 μm).
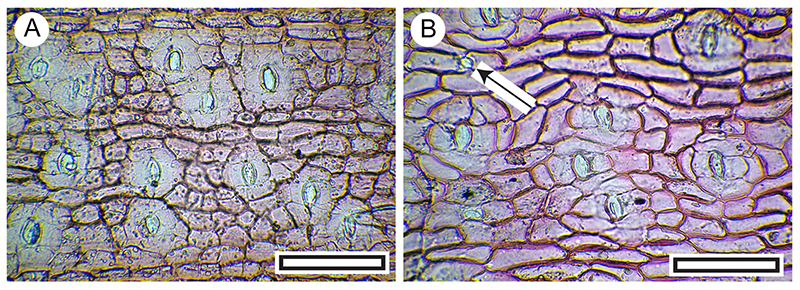
FIGURE 9. Sophora cuticle morphology (Transmitted light microscopy). A. View of S. microphylla stomatal (abaxial) surface showing deeply stained trichome bases, papillae, and stomatal complexes (LX2799, Shelter-96, scale bar equals 100 μm). B. View of S. microphylla stomatal (abaxial) surface of less-stained specimen, but still showing relatively well-stained trichome bases, papillae, and stomatal complexes (LX2590, Shelter-50, scale bar equals 100 μm). C. Detail to show stomatal complexes, partly obscured by irregular papillae (LX3052, from Coprolite-26, scale bar equals 40 μm). D. View of S. microphylla non-stomatal (adaxial) surface, with deeply staining trichome attachment at right (LX3052, Coprolite-26, scale bar equals 40 μm). E. View of S. microphylla non-stomatal (adaxial) surface, with four deeply staining trichome attachments. Note the characteristic radiating surrounding epidermal cells (LX3006, Shelter-76, scale bar equals 100 μm). F. View of basal portion of S. microphylla leaf mid-rib (abaxial surface), showing dense trichome attachment sites (LX3106, Shelter-39, scale bar equals 100 μm). G. View of another style of S. microphylla cuticle morphology from basal portion of the leaf mid-rib (abaxial surface), showing dense trichome attachment sites, and thinner cuticle on either side (LX3052, Coprolite-26, scale bar equals 100 μm). H. Abaxial cuticle of Sophora prostrata. Note clearly different from S. microphylla in absence of trichome attachment sites, and distinct ring of subsidiary cells around the stoma (OPH5491, scale bar equals 100 μm).
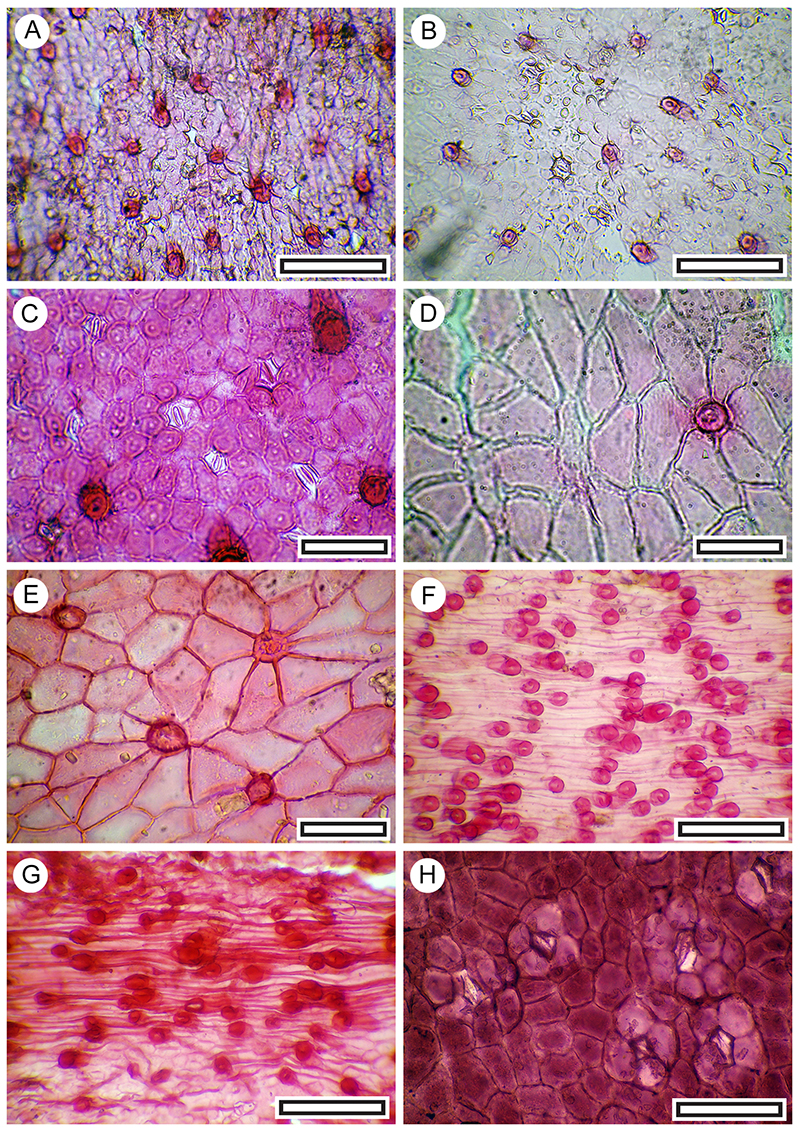
FIGURE 10. Rubus sp. cuticle morphology (Transmitted light microscopy). A. Abaxial surface, showing a typical massive, flanged and hollow trichome attachment, at left, and more poorly staining cuticle with stomatal complexes in the upper right (LX2775, Coprolite-8, scale bar equals 100 μm). B. A patch of stomatal complexes, showing their typically poorly defined outlines and the sinuous walls of the epidermal and subsidiary cells (LX2818, Shelter-96, scale bar equals 100 μm).
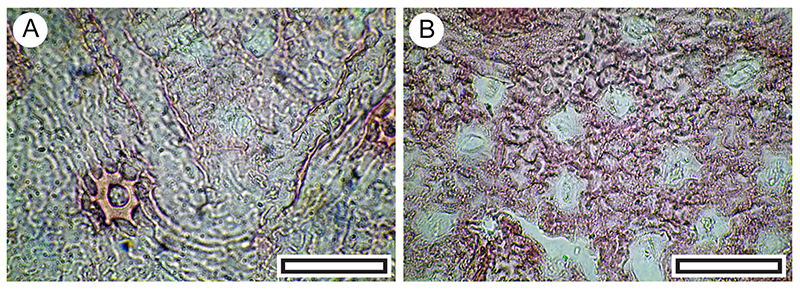
FIGURE 11. Melicytus alpinus. A. Abaxial surface, showing typical range of stomatal complexes and orientation (LX5560, Shelter-6, scale bar equals 100 μm). B. Detail of stomatal complexes showing the prominent outer stomatal ledges and thickened polar T-pieces (LX5338, Coprolite-106, scale bar equals 40 μm).
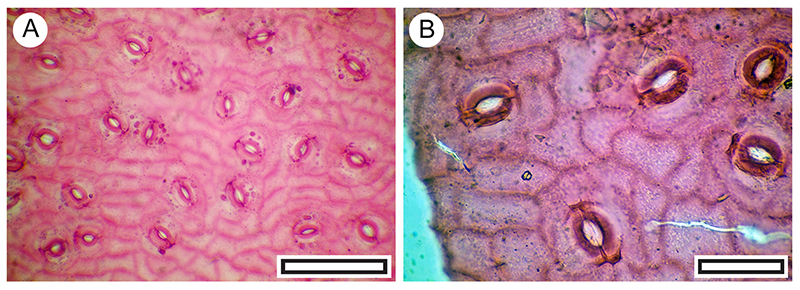
FIGURE 12. Kunzea ericoides. A. Abaxial surface, showing lid cell to left and stomatal complexes to right (LX3256, Shelter-62, scale bar equals 40 μm). B. Single lid cell (LX3253, Shelter-62, scale bar equals 40 μm).
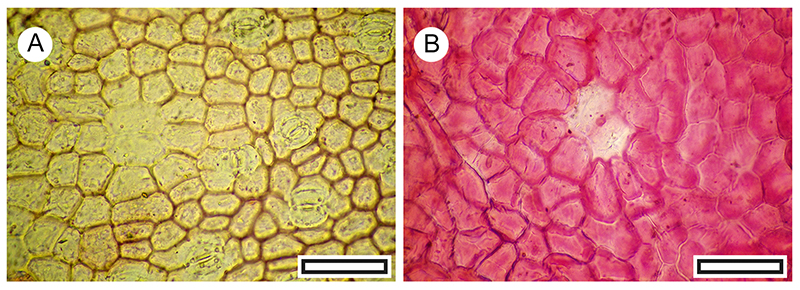
FIGURE 13. Loranthaceae (Korthalsella and Illiostylus) cuticle morphology (Transmitted light microscopy). A. Korthalsella sp., showing generally aligned stomatal complexes (LX2450, Shelter-82, scale bar equals 100 μm). B. Korthalsella sp., detail showing a single stomatal complex (LX2450, Shelter-82, scale bar equals 40 μm). C. Korthalsella sp., showing stomatal orientation perpendicular to epidermal cell rows (LX3371, Coprolite-79, scale bar equals 100 μm). D. Korthalsella sp., detail of stomatal complex (LX3371, Coprolite-79, scale bar equals 40 μm). E. Korthalsella salicornioides for comparison (Herbarium specimen, OPH9239, scale bar equals 100 μm). F. Korthalsella salicornioides for comparison (Herbarium specimen, OPH9239, scale bar equals 40 μm). G. Illiostylus micranthus for comparison (Herbarium specimen, OPH8301, scale bar equals 100 μm). H. Illiostylus micranthus for comparison (Herbarium specimen, OPH8301, scale bar equals 100 μm).
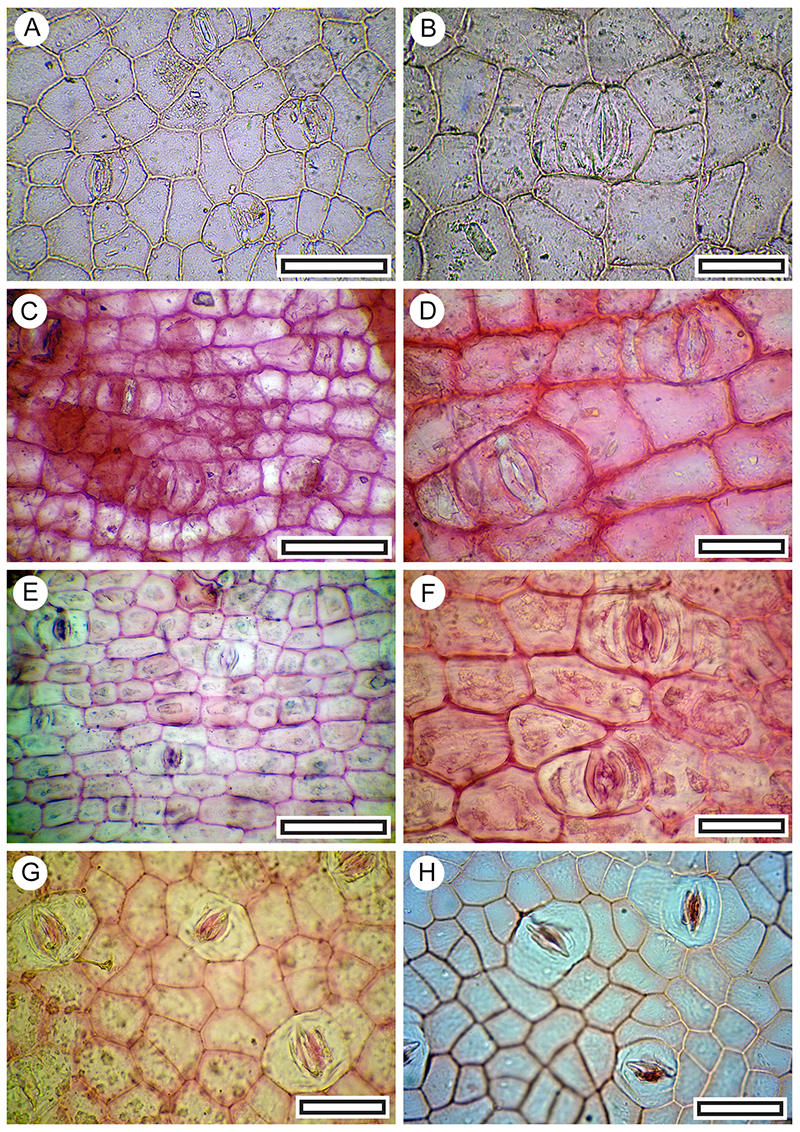
FIGURE 14. Muehlenbeckia sp. A. Abaxial surface with stomatal complexes below and peltate trichome with divided base above (LX2891, Shelter-95, scale bar equals 40 μm). B. Three stomatal complexes among fine surface ridging and peltate trichome with divided base at lower left (LX3100, Coprolite-36, scale bar equals 40 μm).
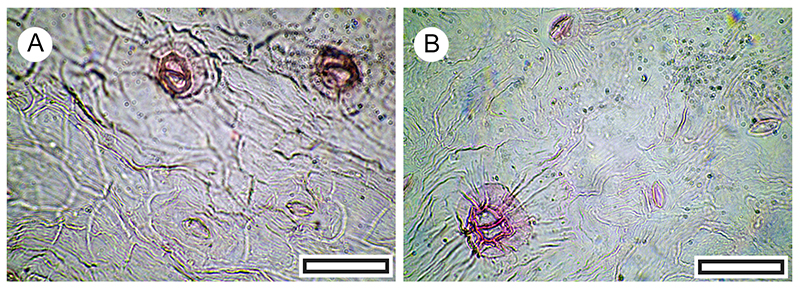
FIGURE 15. Malvaceae (Plagianthus sp. or Hoheria sp.) abaxial surface cuticle morphology (Transmitted light microscopy). A. Stellate trichome at upper right (LX2520, Shelter-103, scale bar equals 40 μm. B. Stellate trichome at upper right, group of stomatal complexes at lower left (LX2951, Shelter-33, scale bar equals 40 μm). C. ‘balloon’ trichome (LX2520, Shelter-103, scale bar equals 40 μm). D. two-armed trichome (LX2520, Shelter-103, scale bar equals 40 μm).
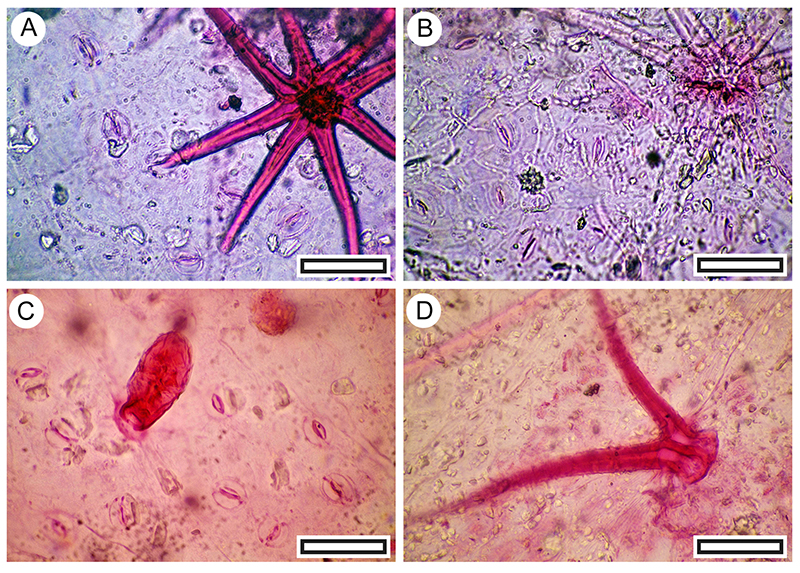
FIGURE 16. Myrsine divaricata cuticle morphology (Transmitted light microscopy). A. Margin of a leaf showing dark glandular structures, situated just inside the margin, and veins, projecting from below (LX2515, Shelter-103, scale bar equals 1 mm). B. Typical abaxial surface cuticle showing scattered stomatal complexes and the stem of a peltate trichome (the cap has detached) at right (LX2557, Shelter-50, scale bar equals 100 μm). C. Detail showing an intact peltate trichome at left and two stomatal complexes at right. Note the highly sinuous margins of the epidermal and subsidiary cells (LX2789, Coprolite-21, scale bar equals 40 μm). D. Typical non-stomatal (adaxial) surface showing the distinctive ridged ornamentation and obscured outlines of the epidermal cells (LX2811 Shelter-96, scale bar equals 40 μm).
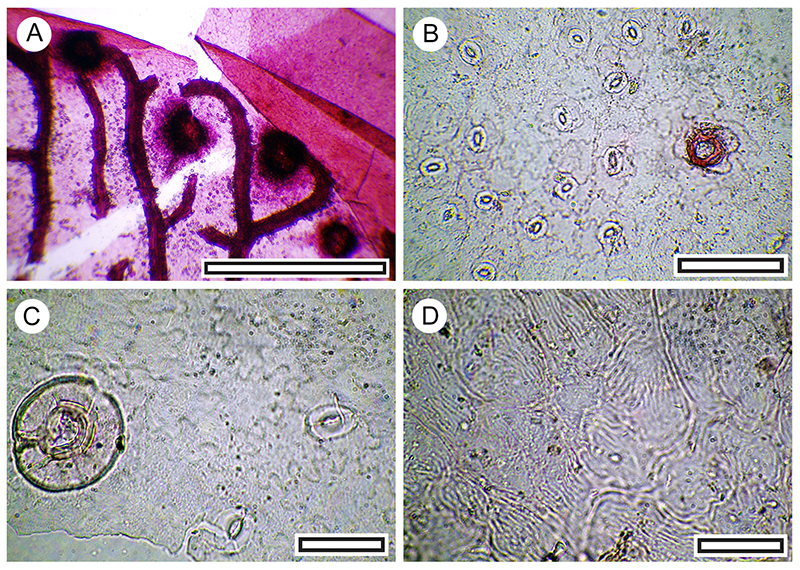
FIGURE 17. Coprosma propinqua (both LX3272, Shelter-62, scale bars equals 40 μm). A. Four stomatal complexes on abaxial surface with prominent fine ridges perpendicular to the stomatal pore. B. Zone of simple trichomes.
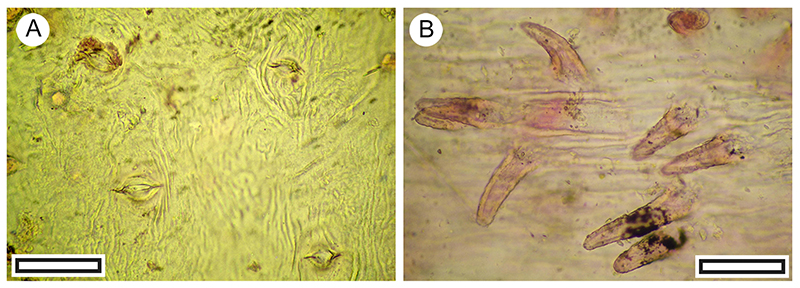
FIGURE 18. Hebe cupressoides cuticle morphology (Transmitted light microscopy). A. Section of an intact stem with paired leaves (LX5392, Shelter-7, scale bar equals 1 mm). B. Detail of leaf pair on a stem (LX2569, Shelter-46, scale bar equals 1 mm). C. Two paired leaves illustrating the ‘marginal frill’ at the leaf apices (LX2569, Shelter-46, scale bar equals 1 mm). D. Cuticle showing generally aligned stomatal complexes on adaxial surface and an ornamentation of dense ridges (SL6473, Shelter-78, scale bar equals 100 μm). E. Detail of cuticle showing generally aligned stomatal complexes and an ornamentation of dense ridges (LX2558, Shelter-50, scale bar equals 40 μm).
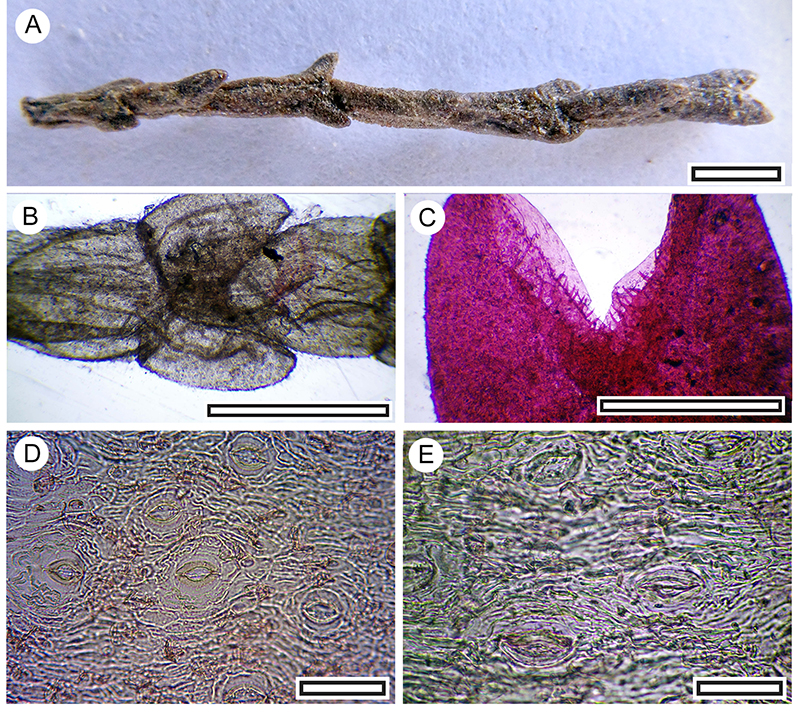
FIGURE 19. Hebe pimeleoides cuticle morphology (Transmitted light microscopy). A. Abaxial surface showing relatively small papillae and stomatal complexes (LX2468, Shelter-92, scale bar equals 100 μm). B. Abaxial surface showing relatively large papillae and stomatal complexes (LX2960, Shelter-33, scale bar equals 100 μm). C. Abaxial surface detail showing slightly flanged papillae and stomatal complexes (LX2938, Shelter-39, scale bar equals 40 μm). D. Abaxial surface detail showing slightly smoother papillae and stomatal complexes (LX2947, Shelter-33, scale bar equals 40 μm). E. Adaxial surface showing lack of papillae and trichome bases (one is arrowed, LX2959, Shelter-33, scale bar equals 100 μm). F. Abaxial surface detail showing two stomatal complexes (LX2997, Shelter-51, scale bar equals 40 μm).
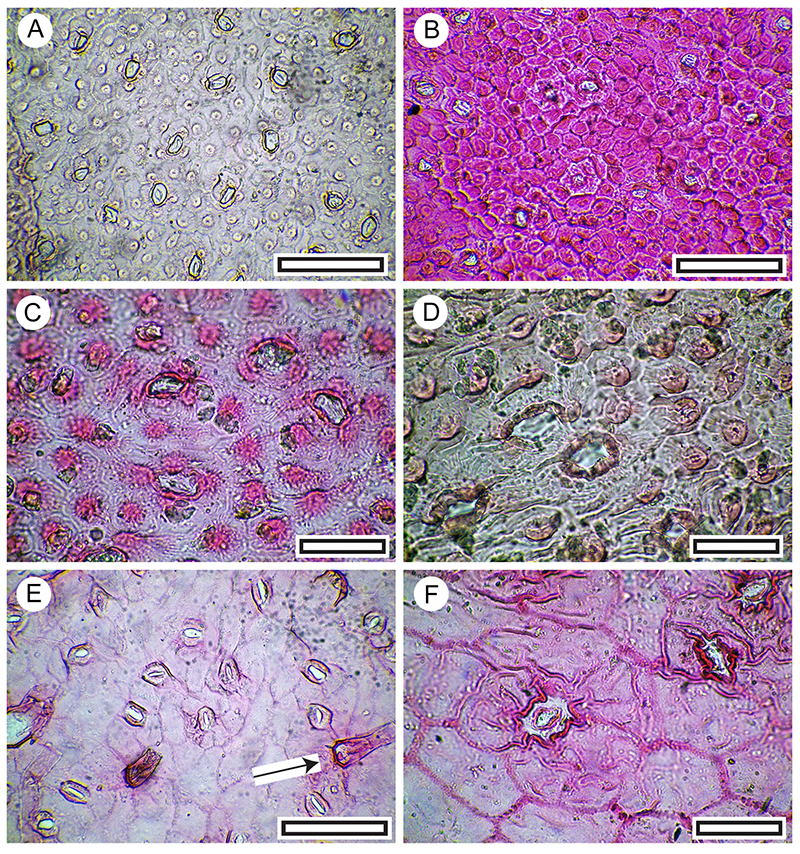
FIGURE 20. Olearia sp. cuticle morphology (Transmitted light microscopy). A. Abaxial surface showing a dense mass of trichome bases obscuring most stomatal complexes (LX2495, Coprolite-13, scale bar equals 40 μm). B. Non-stomatal (adaxial) surface showing a trichome attachment scar extending over several epidermal cells (LX3147, Shelter-12, scale bar equals 40 μm).
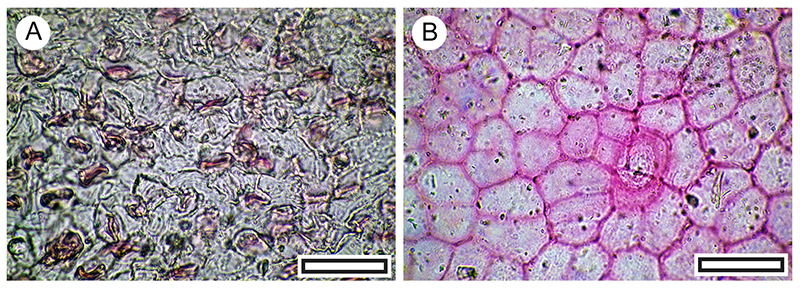
FIGURE 21. Pittosporum tenuifolium cuticle morphology (Transmitted light microscopy). A. Paracytic stomatal complexes on adaxial surface with a relatively sharply bounded trichome base at lower left (LX2576, Shelter-63, scale bar equals 40 μm). B. Paracytic stomatal complexes on abaxial surface with a more typical indistinctly bounded trichome base at upper right (LX2455, Shelter-111, scale bar equals 40 μm).
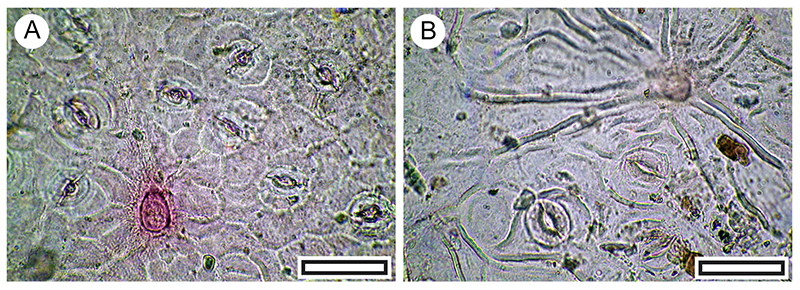
FIGURE 22. Pseudopanax leaf (reflected light) and cuticle morphology (transmitted light microscopy). A. Intact Pseudopanax ferox leaf (LX3111, Shelter-37, scale bar equals 10 mm). B. Pseudopanax ferox stomatal complexes on abaxial surface (LX2711, Shelter-1, scale bar equals 100 μm). C. Pseudopanax ferox (LX5490, Coprolite-15, scale bar equals 100 μm). D. Pseudopanax crassifolious, abaxial surface showing much larger and more irregular epidermal cells (modern reference material, OPH5338, scale bar equals 100 μm). E. Pseudopanax crassifolious, abaxial surface also showing much larger and more irregular epidermal cells (modern reference material, OPH9835, scale bar equals 100 μm).
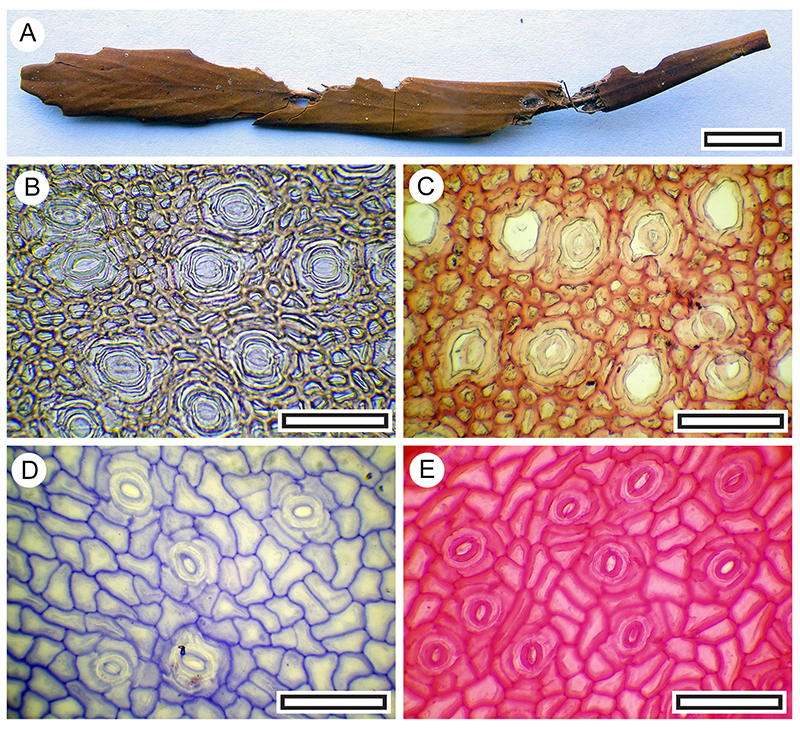
FIGURE 23. Coprolites regarded here as moa, based on their size (scale bar equals 30 mm). A. Coprolite-25, containing Poaceae (Shelter-32). B. Coprolite-26, containing Pseudopanax ferox, Pittosporum tennuifolium, Sophora microphylla (Shelter-32). C. Coprolite-15, containing Coprosma sp., Olearia sp, Pittosporum tennuifolium, Pseudopanax ferox, Rubus sp., Sophora microphylla (Shelter-102). D. Coprolite-111, containing Sophora microphylla, Pittosporum tennuifolium, Rubus sp., ? Hebe (Shelter-50). E. Coprolite-16, containing Sophora microphylla, Hebe ? cupressoides (Shelter-102). F. Coprolite-62, containing Sophora microphylla (Shelter-70). G. Coprolite-14, containing Hebe cupressoides, Sophora microphylla, Rubus sp., Pittosporum tennuifolium (Shi.e. elter-102). H. Coprolite-60, containing Sophora microphylla (Shelter-103).
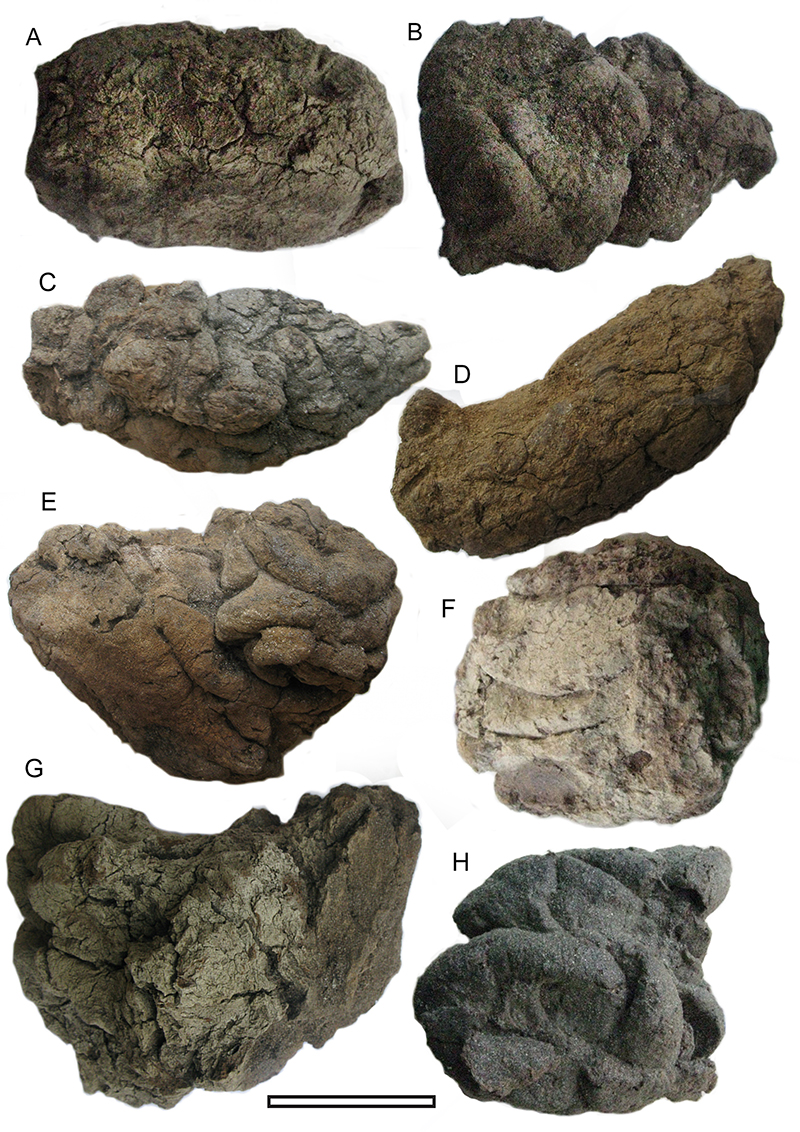
FIGURE 24. Length and width dimensions for coprolites investigated for cuticle in this work (red dots), overlain on a summary of Wood and Wilmshurst (2014, fig. 3). The blue curve contains the range of genetically confirmed moa coprolites in their study, and the small yellow dot indicates the size of their one genetically determined kakapo coprolite, and the green are those of their ‘putative’ kakapo coprolites. There is a clear group of coprolites in the present study which are well beyond the range of even ‘putative’ kakapo, and are regarded as moa (Coprolites-11, 14, 15, 16, 25, 26, 27, 28, 29, 51, 58, 60, 62, 78, 110, 111).