APPENDIX 1.
Reference list of material used in this study.
Abbas, E.M., Abdelsalam, K.M., Mohammed-Geba, K., Ahmed, H.O., and Kato, M. 2016. Genetic and morphological identification of some crabs from the Gulf of Suez, Northern Red Sea, Egypt. The Egyptian Journal of Aquatic Research, 42(3):319-329.
https://doi.org/10.1016/j.ejar.2016.08.003
Anosov, S. 2000. Keys to the identification of brachyuran larvae of the Black Sea. Crustaceana, 73(10):1239-1246.
https://www.jstor.org/stable/20106395
Bedini, R. 2006. Polymorphism of colour patterns related to environmental factors: correlation between habitat, sight, and mimicry in Sirpus zariquieyi Gordon, 1953. Crustaceana, 79(1):53-67.
Верещака, А.Л. 1989. Новый вид краба - Sirpus ponticus (Crustacea, Pirimelidae) в Черном море. Зоологический журнал, 68(8):41-47.
Beschin, C., and Checchi, A. 2018. Nuovo genere e nuova specie di Carcinidae (Crustacea, Decapoda, Portunoidea) nell’Eocene dei monti lessini (Italia nordorientale). Studi e Ricerche - Associazione Amici del Museo - Museo Civico “G. Zannato” Montecchio Maggiore (Vicenza), 25:27-31.
Beschin, C., Busulini, A., Tessier, G., and Zorzin, R. 2016. I crostacei associati a coralli nell'Eocene inferiore dell'area di Bolca: Verona e Vicenza, Italia nordorientale. Memorie del Museo Civico di Storia Naturale di Verona - 2. serie. Sezione Scienze della Terra - N. 9.
Van Bakel, B.W., Jagt, J.W., Fraaije, R.H., and Wille, E.R. 2004. Piacenzian (Pliocene) decapod crustacean faunules from northwest Belgium. Bulletin of the Mizunami Fossil Museum, 30:97-108.
Crosnier, A. 1962. XVI Crustacés Décapodes - Portunidae. In Faune de Madagascar. Publiée sous les auspices du Gouvernement de la République Malgache.
Crosnier, A. 2002. Révision du genre Parathranites Miers, 1886 (Crustacea, Brachyura, Portunidae). Zoosystema, 24(4):799-824.
Evans, N. 2018. Molecular phylogenetics of swimming crabs (Portunoidea Rafinesque, 1815) supports a revised family-level classification and suggests a single derived origin of symbiotic taxa. PeerJ, 6:e4260.
https://doi.org/10.7717/peerj.4260
Feldmann, R.M., Schweitzer, C.E., and Goedert, J.L. 2018. Complex taphonomic and depositional history of a new species of Carcinidae (Decapoda: Brachyura: Portunoidea) from Washington state, USA. Journal of Crustacean Biology, 38(5):579-585.
https://doi.org/10.1093/jcbiol/ruy066
Flores, A.A., and Paula, J. 2000. Larval and early juvenile stages of Pirimela denticulata (Montagu, 1808)(Crustacea, Brachyura, Pirimelidae) reared in the laboratory. Journal of Natural History, 34(11):2123-2143.
https://doi.org/10.1080/002229300750022367
Fransen, C.H.J.M. 2014. True crabs, pp. 249-358. FAO, Rome.
Froglia, C., and Manning, R.B. 1982. Notes on Liocarcinus pusillus (Leach) and related species. Quaderni del Laboratorio di Tecnologia della Pesca, 3(2-5):257-266.
Gillespie, G.E., Phillips, A.C., Paltzat, D.L., and Therriault, T.W. 2007. Status of the European Green Crab, Carcinus maenas, in British Columbia, 2006. Canadian Technical Reports of Fisheries and Aquatic Sciences, 2700:7.
Gordon, I. 1952. On a new crab from Cadaqués on the north east coast of Spain (Sirpus zariquieyi ng and. sp.). Eos, Rev. Esp. Ento., 28(4):303-314.
Guerao, G., Abelló, P., and Dos Santos, A. 2006. Morphological variability of the megalopa of Liocarcinus depurator (Brachyura: Portunidae) in Mediterranean and Atlantic populations. Journal of Natural History, 40(32-34):1851-1866.
https://doi.org/10.1080/00222930601046584
Guerao, G., and Abelló, P. 2011. Early juvenile development of Mediterranean Liocarcinus depurator (Crustacea: Decapoda: Brachyura: Portunidae). Journal of Natural History, 45(35-36):2171-2189.
https://doi.org/10.1080/00222933.2011.590948
Hogarth, P.J. 1978. Variation in the carapace pattern of juvenile Carcinus maenas. Marine Biology, 44(4):337-343.
https://doi.org/10.1007/BF00390898
Karasawa, H., and Fudouji, Y. 2000. Palaeogene decapod Crustacea from the Kishima and Okinoshima Groups, Kyushu, Japan. Paleontological Research, 4(4):239-253.
https://doi.org/10.2517/prpsj.4.239
Kato, H., and Karasawa, H. 1994. Minohellenus macrocheilus sp. nov. (Decapoda: Crustacea) from the Oligocene Ashiya Group, Kyushu, Japan. Bulletin of Kitakyushu Museum of Natural Hsitory, 13:51-58.
Kim, K.B., and Hong, S.Y. 1999. Larval development of the wrinkled swimming crab Liocarcinus corrugatus (Decapoda: Brachyura: Portunidae) reared in the laboratory. Journal of Crustacean Biology, 19(4):792-808.
https://doi.org/10.1163/193724099X00510
Kocataş, A. 1982. On the occurrence of Sirpus zariquieyi Gordon (Decapoda brachyura) in the Black Sea and Sea of Marmara. Crustaceana, 43(2):177-180.
https://doi.org/10.1163/156854082X00506
Koch, M., and Duris, Z. 2016. Notes on distribution of some portunid crabs in the Mediterranean Sea (Decapoda: Brachyura: Portunidae). Acta Musei Silesiae. Scientiae Naturales, 65(2):117.
https://doi.org/10.1515/cszma-2016-0015
Kondylatos, G., Crocetta, F., Corsini-Foka, M., and Froglia, C. 2020. Crustacea Decapoda from the Rhodes Island area (eastern Mediterranean): new records and an updated checklist. Diversity, 12(6):246.
https://doi.org/10.3390/d12060246
Kristensen, T., Nielsen, A.I., Jørgensen, A.I., Mouritsen, K.N., Glenner, H., Christensen, J.T., Lützen, J., and Høeg, J. T. 2012. The selective advantage of host feminization: a case study of the green crab Carcinus maenas and the parasitic barnacle Sacculina carcini. Marine biology, 159(9):2015-2023.
https://doi.org/10.1007/s00227-012-1988-4
Langeneck, J., and Di Franco, D. 2013. Further records of two uncommon Crustaceans in Italian seas: Maja goltziana D'Oliveira, 1888 (Decapoda Brachyura Majidae) and Xaiva biguttata (Risso, 1816)(Decapoda Brachyura Portunidae). Biodiversity Journal, 4(2):281-284.
Lebour, M.V. 1944. The larval stages of Portumnus (Crustacea Brachyura) with notes on some other genera. Journal of the Marine Biological Association of the United Kingdom, 26(1):7-15.
https://doi.org/10.1017/S0025315400014429
Lörenthey, E., and Beurlen, K. 1929. Die Fossilen Dekapoden der Länder der ungarischen Krone. Geologica Hungarica Series Palaeontologica, 3:1-420.
Macpherson, E. 1989. The identity of Xaiva pulchella MacLeay, 1838 (Decapoda, Portunidae). Crustaceana, 57(1):107-110.
https://doi.org/10.1163/156854089X00428
Marco-Herrero, E. 2015. Aplicación de técnicas morfológicas y moleculares en la identificación de la megalopa de decápodos braquiuros de la península ibérica. Thesis, Facultat de Ciències Biològiques, Universidad de Valencia.
Ng, P. 2002. On the unusual swimming crab, Coelocarcinus foliatus Edmondson, 1930, with description of a new species from the Indian Ocean (Decapoda, Brachyura, Portunidae). Crustaceana, 75(1):51-60.
http://www.jstor.org/stable/20105385
Nunes, D.M., Ferreira, R.C.P., Hazin, F.H., Travassos, P.E., and Souza-Filho, J.F. 2017. Deep sea decapod crustaceans of São Pedro and São Paulo Archipelago, Equatorial Atlantic, Brazil. Zootaxa, 4324(2):331-347.
https://doi.org/10.11646/zootaxa.4324.2.6
Ossó, A., and Stalennuy, O. 2011. Description of the first fossil species of Bathynectes (Brachyura, Polybiidae) in the Badenian (middle Miocene) of the Medobory Hills (Ukraine, Central Parathetys), with remarks on its habitat ecology. Treballs del Museu de Geologia de Barcelona, 18:37-46.
https://doi.org/10.32800/tmgb.2011.18.0037
Özcan, T., and Ateş, A.S. 2018. Presence of Knobby Swim Crab, Macropipus tuberculatus (Roux, 1830) (Decapoda: Brachyura: Polybiidae) on the Levantine Sea Coast of Turkey. Proceeding Book, International Marine & Freshwater Sciences Symposium, Kemer-Antalya, Turkey, p. 402.
Özcan, T., Irmak, E., Ateş, A.S., and Katağan, T. 2009. The occurrence of Bathynectes maravigna (Decapoda: Brachyura: Portunidae) in the Turkish part of the Levantine Sea. Marine Biodiversity Records, 2:e101.
https://doi.org/10.1017/S1755267209000955
Pancucci-Papadopoulou, M.A., and Naletaki, M. 2007. A new alien species in the Mediterranean? On the presence of Sirpus monodi Gordon, 1953 (Brachyura, Pirimelidae) in Greece. Mediterranean Marine Science, 8(2):91-96.
https://doi.org/10.12681/mms.157
Pastore, M.A. 1977. Presenza di Thia scutellata (Fabricius) e Xaiva biguttata (Risso) nel golfo di Taranto (mar Jonio). Thalassia Salentina, 7:83-90.
https://doi.org/10.1285/i15910725v7p83
Paula, J. 1987. Planktonic stages of brachyuran crabs from the southwestern Iberian coast (Crustacea, Decapoda, Brachyura). Journal of Natural History, 21(3):717-756.
https://doi.org/10.1080/00222938700770411
Paula, J. 1988. The larval and post-larval development of Pennant's swimming crab, Portumnus latipes (Pennant)(Brachyura, Portunidae), reared in the laboratory. Crustaceana, 55(2):202-216.
https://doi.org/10.1163/156854088X00537
Raso, J.E.G., and Manjón-Cabeza, E. 1996. New record of Liocarcinus mcleayi (Barnard, 1947), new combination (Decapoda, Brachyura, Portunidae) from south Europe. Crustaceana, 69(1):84-93. https://doi.org/10.1163/156854096X00114
Rathbun, M.J. 1926. The fossil stalk-eyed Crustacea of the Pacific slope of North America. Bulletin of the United States National Museum, 138:155 pp.
Reyes, C.C. 2015. Biology, ecology and dynamics of Pennant's swimming crab (Portumnus latipes) in the south of Portugal (Doctoral dissertation). Thesis, Universidade do Algarve.
Salas, C., Tirado, C., and Manjón-Cabeza, M.E. 2001. Sublethal foot-predation on Donacidae (Mollusca: Bivalvia). Journal of Sea Research, 46(1):43-56.
https://doi.org/10.1016/S1385-1101(01)00064-8
Schweitzer, C.E., and Feldmann, R.M. 2000. New fossil portunids from Washington, USA, and Argentina, and a re-evaluation of generic and family relationships within the Portunoidea Rafinesque, 1815 (Decapoda: Brachyura). Journal of Paleontology, 74(4):636-653.
https://doi.org/10.1666/0022-3360(2000)074<0636:NFPFWU>2.0.CO;2
Schweitzer, C.E., and Feldmann, R.M. 2002. New Eocene decapods (Thalassinidea and Brachyura) from Southern California. Journal of Crustacean Biology, 22(4):938-967.
https://doi.org/10.1163/20021975-99990304
Schweitzer, C.E., and Feldmann, R.M. 2010. New fossil decapod crustaceans from the Remy Collection, Muséum national d'Histoire naturelle, Paris. Geodiversitas, 32(3):399-415.
https://doi.org/10.5252/g2010n3a3
Schweitzer, C.E., Feldmann, R.M., and Karasawa, H. 2021. Part R, Revised, Volume 1, Chapter 8T15: Systematic descriptions: Superfamily Portunoidea. Treatise Online, 151:1-40.
https://doi.org/10.17161/to.vi.15392
Spitzner, F., Meth, R., Krüger, C., Nischik, E., Eiler, S., Sombke, A., Torres, G., and Harzsch, S. 2018. An atlas of larval organogenesis in the European shore crab Carcinus maenas L. (Decapoda, Brachyura, Portunidae). Frontiers in Zoology, 15(1):1-39.
https://doi.org/10.1186/s12983-018-0271-z
Todd, P.A., Briers, R.A., Ladle, R.J., and Middleton, F. 2006. Phenotype-environment matching in the shore crab (Carcinus maenas). Marine Biology, 148(6):1357-1367.
https://doi.org/10.1007/s00227-005-0159-2
APPENDIX 2.
Table of material used in the study with grouping variables and references. (Available for download)
APPENDIX 3.
Updated morphological character matrix following Karasawa et al. (2008).(Available for download)
APPENDIX 4.
Character list, indicating characters and their states as published by Karasawa et al. (2008).
[1] Carapace proportion: much wider than long (0), slightly wider than long or longer than wide (1)
[2] Front with median notch: present (0), absent (1)
[3] Front with median lobe: absent (0), present (1)
[4] Frontal teeth: present (0), absent (1)
[5] Front forming T-shape: absent (0), present (1)
[6] Lower orbital tooth: low (0), long, visible dorsally (1)
[7] Inner orbital angle defined as lobe or tooth: present (0), absent (1)
[8] Upper orbital fissures: present (0), absent (1)
[9] Epibranchial spine: short (0), long (1)
[10] Carapace dorsal ridge: absent (0), present (1)
[11] Carapace surface: smooth (0), with tubercles (1)
[12] Anterolateral teeth: 1-5 (0), 6-9 (1)
[13] Orbital length: normal (0), wide (1)
[14] Basal article of antenna reaching front: present (0), absent (1)
[15] Basal article of antenna: fixed (0), free (1)
[16] Laterodistal area of basal article of antenna: absent (0), spine or lobed (1)
[17] Laterodistal expansion of basal article of antenna: absent (0), present (1)
[18] Epistomial spine: absent (0), present (1)
[19] Portunid lobe of maxilliped 1: absent (0), present (1)
[20] Telson of male pleon reaching: posterior of sternite 4 (0), anterior of sternite 4 (1)
[21] Telson of male pleon about as long as wide (0), much longer than wide (1)
[22] Telson shape of male pleon: triangular (0), semicircular (1)
[23] Male pleomere 6: wide (0), narrow (1)
[24] Lateral margin of male pleomeres 4-5: nearly straight (0), sinuous or concave (1)
[25] Male pleomere 3: narrow (0), wider than somite 4 (1), wide with rectangular corner (2)
[26] Male pleomere 3 with keel: absent (0), present (1)
[27] Sutures of male pleomeres: distinct (0), indistinct (1)
[28] Sutures of male pleomeres, if present: movable (0), immovable (1)
[29] Sternum width: distinctly narrow (0), relatively narrow (1), wide (2)
[30] Sternum shape: narrowly ovate (0), ovate (1), rather rectangular posteriorly (2)
[31] Sulcus delimiting sternites 3 and 4: well marked (0), indistinct (1)
[32] Sulcus delimiting sternites 6 and 7: complete (0), interrupted medially (1)
[33] Sulcus delimiting sternites 7 and 8: complete (0), interrupted medially (1)
[34] Secondary sulcus delimiting sternites 6 and 7: absent (0), present (1)
[35] Median transverse ridge between sternites 6/7: present (0), absent (1)
[36] Median line on thoracic sternites: up to sternite 7 (0), up to sternite 6 (1)
[37] Median groove on male thoracic sternite 3: present (0), absent (1)
[38] Episternites 4-7: narrow (0), wide (1)
[39] Posterolateral prolongation of male episternite 7: not marked (0), well developed (1)
[40] Sternite 8: reduced (0), expanded laterally (1), well developed (2)
[41] Penial groove on male sternite 8: absent (0), present (1)
[42] Male sternite 8 visible posteriorly: indistinct (0), distinct (1)
[43] Male sternite 8 visible ventrally: indistinct (0), distinct (1)
[44] Cheliped fingers dark in color: present (0), absent (1)
[45] Inner margin of cheliped merus with spines: absent (0), present (1)
[46] Outer surface of cheliped palm: smooth (0), transversely ridged (1)
[47] Cheliped length: longer than pereiopods (0), shorter than pereiopods (1)
[48] Pereiopods 2-4 propodi: normal (0), foliaceous-like (1)
[49] Dactyli 2-4 with corneous tip: present (0), absent (1)
[50] Pereiopod 5 with foliaceous propodus: absent (0), present (1)
[51] Pereiopod 5 dactyli: ensiform (0), narrow, lanceolate (1), lanceolate (2), ovate-elliptic (3)
[52] Pereiopod 5 merus with postero-distal spine: absent (0), present (1)
[53] Proximal insertion of pereiopod 5 propodus: absent (0), present (1)
[54] Pereiopod 5 merus: equal or longer than propodus (0), shorter than propodus (1)
[55] Gonopod 1 with subterminal spines: absent (0), present (1)
APPENDIX 5.
Table of occurrence dates, based on literature research for species included in the phylogeny. Abbreviations: FAD: first occurrence; LAD: last occurrence.
| Group | FAD | LAD |
| Thia scutella | 5.33 | 0 |
| Liocarcinus corrugatus | 5.33 | 0 |
| Liocarcinus zariquieyi | 2.58 | 0 |
| Liocarcinus heintzi | 33.9 | 27.82 |
| Liocarcinus pusillus | 2.58 | 0 |
| Polybius henslowii | 2.58 | 0 |
| Liocarcinus holsatus | 3.6 | 0 |
| Liocarcinus vernalis | 2.58 | 0 |
| Liocarcinus marmoreus | 2.58 | 0 |
| Liocarcinus depurator | 5.33 | 0 |
| Liocarcinus navigator | 2.58 | 0 |
| Liocarcinus maculatus | 0 | 0 |
| Parathranites tuberogranosus | 2.58 | 0 |
| Parathranites tuberosus | 2.58 | 0 |
| Parathranites ponens | 2.58 | 0 |
| Parathranites orientalis | 2.58 | 0 |
| Parathranites intermedius | 2.58 | 0 |
| Parathranites granosus | 2.58 | 0 |
| Parathranites hexagonus | 2.58 | 0 |
| Macropipus australis | 2.58 | 0 |
| Macropipus tuberculatus | 2.58 | 0 |
| Necora puber | 5.33 | 0 |
| Megokkos sp. | 48.6 | 33.9 |
| Megokkos alaskensis | 33.9 | 23.03 |
| Bathynectes piperitus | 2.58 | 0 |
| Bathynectes muelleri | 15.97 | 13.82 |
| Bathynectes maravigna | 2.58 | 0 |
| Baythnectes longispina | 2.58 | 0 |
| Coelocarcinus foliatus | 0 | 0 |
| Coelocarcinus aff. foliatus | 0 | 0 |
| Sirpus zariquieyi | 0 | 0 |
| Pirimela denticulata | 2.58 | 0 |
| Xaiva pulchella | 2.58 | 0 |
| Xaiva mcleayi | 2.58 | 0 |
| Xaiva biguttata | 2.58 | 0 |
| Portumnus tricarinatus | 23.03 | 5.33 |
| Portumnus lysianassa | 2.58 | 0 |
| Portumnus latipes | 2.58 | 0 |
| Carcinus aestuarii | 2.58 | 0 |
| Carcinus maenas | 5.33 | 0 |
APPENDIX 6.
Code used for MrBayes analysis. (Available for download)
APPENDIX 7.
Nexus format tree file. (Available for download)
APPENDIX 8.
Code used for R-statistics environment analysis. (Available for download)
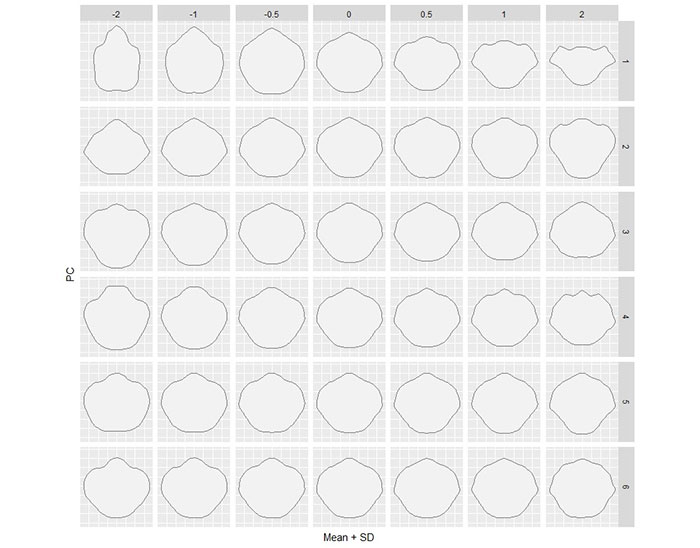
APPENDIX 9.
Graphical component loadings of PCA on outline analysis of the whole data set.