FIGURE 1. Protocols employed in this paper. (A) protocol for assessing the performance of surface scanning through glass. (B) schematics of the laser scaling device we describe in this paper. (C) photograph of the laser scaling device in action. (D) photogrammetric model of an hippuritid bivalve showing the laser dots clearly visible on the model texture.
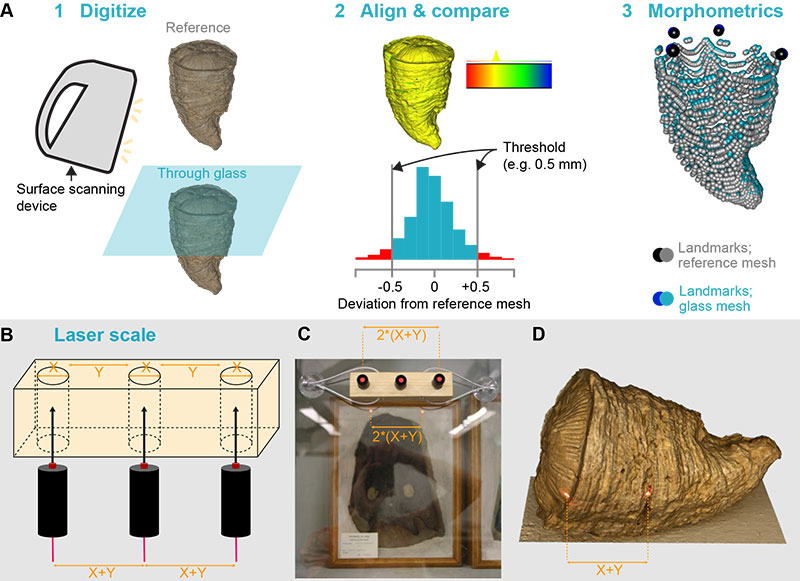
FIGURE 2. Difficulties and issues in data acquisition. (A) multiple surfaces created by reflections on the glass; these are frequent when using scanners requiring a close distance from the specimen, such as the Artec Spider. (B) light rings polluting the texture of the final model of NHMUK R1157, holotype of Temnodontosaurus eurycephalus. The 3D mesh (C) appears detailed, nonetheless. (D) 3D mesh of the extant goat ULgPA20230320-1, showing important errors in the orbit, palate, and basicranium. (E-H) 3D meshes of Lovenechinus sp. (ULgPA35401) produced using laser scanning with Creaform Handyscan 300 (E), white-light scanning with Artec Eva (F), white-light scanning with Artec Eva through flat glass (G), white-light scanning with Artec Eva through old glass (H), showing progressively fewer details of minute anatomical structures.
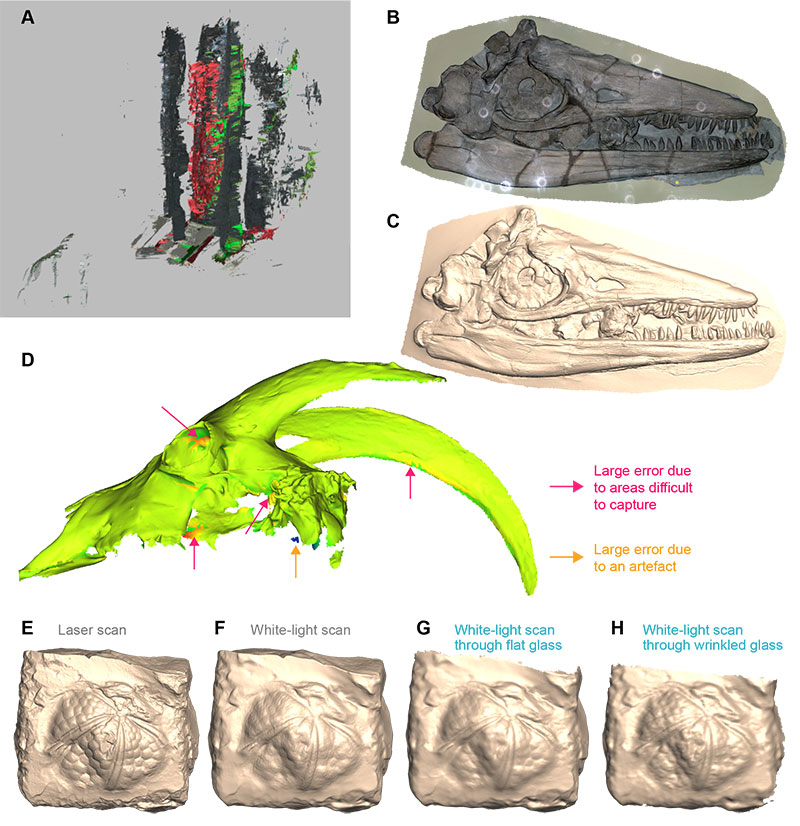
FIGURE 3. Mesh deviations from reference (laser scanned without glass), invertebrate fossils. (A-D) Caninophyllum patulum (ULgPA20230303-5); mapping of deviations (A, C) and associated histograms (B, D). (E-H) Siphonophyllia cylindrica (ULgPA20230303-4); mapping of deviations (E, G) and associated histograms (F, H). (I-L) Proterocidaris sp. (ULgPA35400); mapping of deviations (I, K) and associated histograms (J, L). (M-P) Lovenechinus sp. (ULgPA35401); mapping of deviations (M, O) and associated histograms (N, P). (Q-T) Hippurites radiosus (ULgPA3727); mapping of deviations (Q, S) and associated histograms (R, T). (U, V) aggregated data (invertebrates and vertebrates) for new (Q) and old (V) glass.
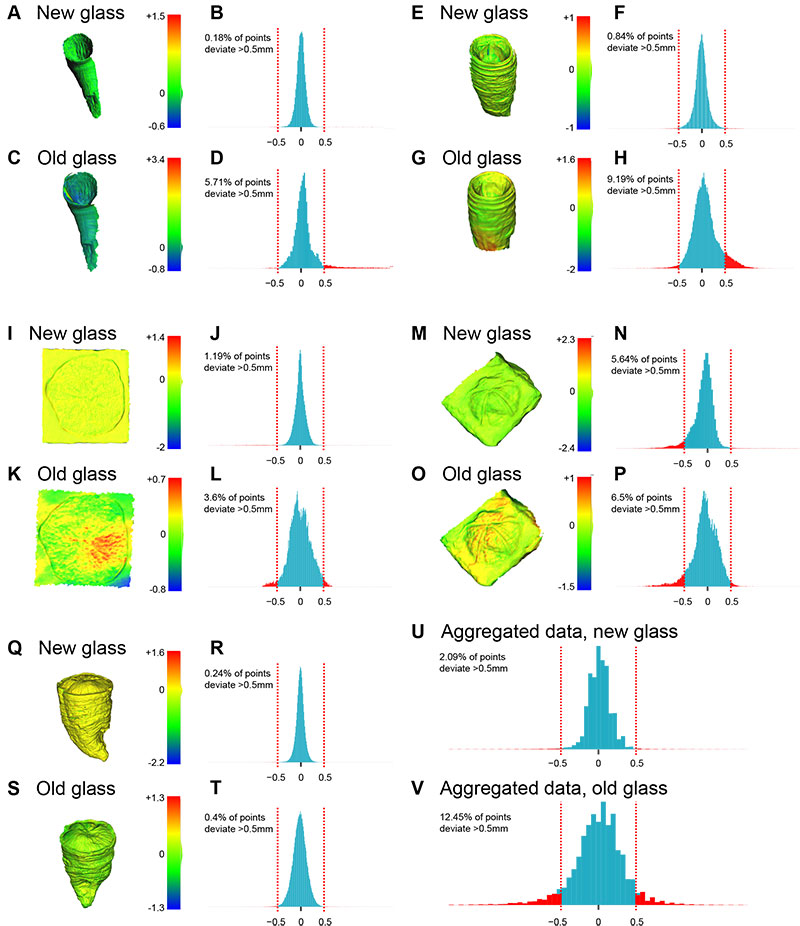
FIGURE 4. Mesh deviations from reference (laser scanned without glass), vertebrate fossils. (A-D) skull of Capra hircus (ULgPA20230320-1); mapping of deviations (A, C) and associated histograms (B, D). (E-H) femur of Ursus spelaeus (ULgPA12766); mapping of deviations (E, G) and associated histograms (F, H). (I-L) cast of Pleurosaurus thiollieri (ULgPA25056); mapping of deviations (I, K) and associated histograms (J, L). (M-N) Ichthyosaurus sp. (ULgPA13413); mapping of deviations (M) and associated histogram (N). (O-P) skull of Crocuta crocuta spelaea (ULgPA1806); mapping of deviations (O) and associated histogram (P). Abbreviation: horiz, horizontal.
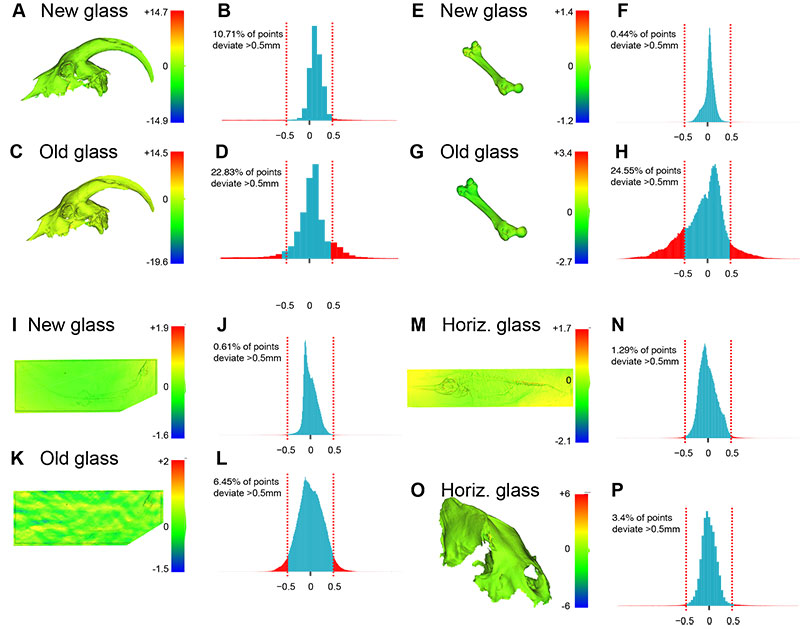
FIGURE 5. Case studies of morphometric analyses (A-C linear morphometrics; D-F high-density 3D geometric morphometrics). (A) 3D mesh of Ichthyosaurus sp. (ULgPA13413) produced by laser scanning showing the traits (linear measurements) we took. (B) relative differences in measurement lengths for each trait, comparing methods (grey) and the presence of glass (blue). (C) morphospace resulting from Principal Component Analysis of the z-transformed trait data. (D) 3D mesh of Hippurites radiosus (ULgPA3727) produced by white-light scanning, with fixed (black) and automatically placed surface semi-landmarks (grey) superimposed. (E) euclidean distance per landmark, comparing methods (grey) and the presence of new and old glass (blue). (F) morphospace resulting from Principal Component Analysis of the Procrustes coordinates.
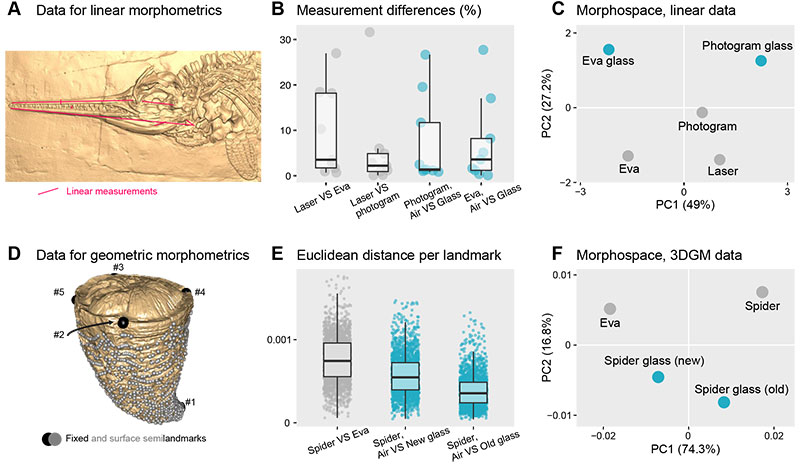
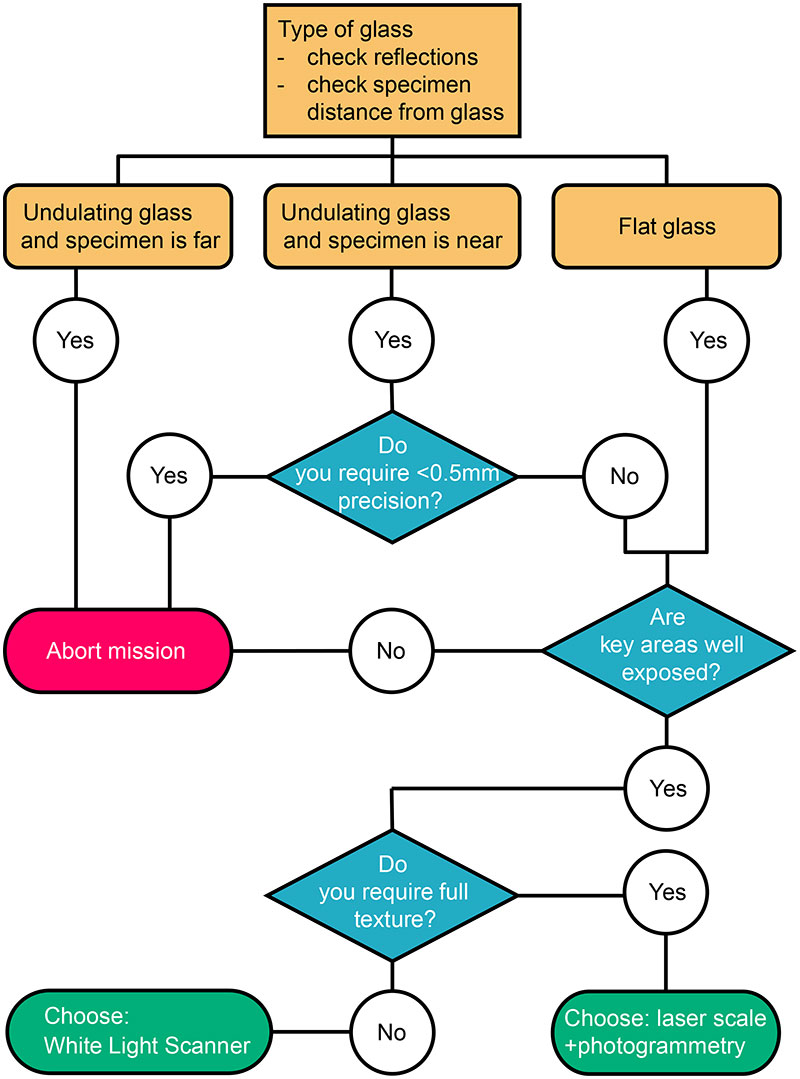
FIGURE 6. Decision tree to assess the usefulness and select the 3D digitization method when specimens are behind glass.