 A perspective on the evidence for keratin protein preservation in fossils: An issue of replication versus validation
A perspective on the evidence for keratin protein preservation in fossils: An issue of replication versus validation
Article number: 22.3.2E
https://doi.org/10.26879/1017E
Copyright Paleontological Society, December 2019
Submission: 1 August 2019. Acceptance: 10 December 2019.
ABSTRACT
The preservation potential of biomolecules within vertebrate integument through deep time has recently been subject to much research and controversy. In particular, the preservation potential of proteins, such as collagen and keratin, is currently debated. Here, we examine claims from a recent study (Schweitzer et al., 2018, PLoS One), which concludes that feather keratin has a high preservation potential. We argue that this work provides insufficient evidence for protein preservation due to issues of methodology and data interpretation. Additionally, we contrast their approach and claims to those of other recently published studies in relation to the question of keratin protein preservation in fossils. We worry that most of the perceived evidence for Mesozoic polypeptide survival stems from repeated replication of methods prone to false detection, rather than triangulation by validating these claims with alternative methods that provide independent lines of evidence. When alternative explanations exist for the evidence cited as support for dinosaur proteins far exceeding their predicted preservation limits, it is most parsimonious to reject the more extreme taphonomic hypotheses. The evidence is instead more consistent with a mode of preservation in which keratinous structures do not fossilize organically as polypeptides, but rather as largely pigment and/or calcium phosphate remnants, which were originally held within the keratin matrix that is now lost. Unsupported taphonomic models (e.g., keratin polypeptide preservation) have the potential to influence our interpretation of fossil data, potentially resulting in erroneous paleobiological or evolutionary conclusions, as illustrated in another recent paper (Pan et al., 2019, PNAS) that we also discuss.
Evan T. Saitta. Integrative Research Center, Field Museum of Natural History, Chicago, Illinois, USA. evansaitta@gmail.com
Jakob Vinther. School of Earth Sciences and School of Biological Sciences, University of Bristol, Bristol, UK. jakob.vinther@bristol.ac.uk
Keywords: keratin, feathers, protein, maturation, taphonomy, immunochemistry
Final citation: Saitta, Evan T. and Vinther, Jakob. 2019. A perspective on the evidence for keratin protein preservation in fossils: An issue of replication versus validation. Palaeontologia Electronica 22.3.2E 1–30. https://doi.org/10.26879/1017E
palaeo-electronica.org/content/2019/2890-commentary-keratin-protein-preservation
Copyright: December 2019 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Proteins have commonly been thought to have low preservation potential through fossilization (Kriausakul and Mitterer, 1978; Armstrong et al., 1983; Lowenstein, 1985; Eglinton and Logan, 1991; Mitterer, 1993; Bada, 1998; Briggs and Summons, 2014), in large part due to the thermodynamic instability of peptide bonds and susceptibility for hydrolysis, among other degradation reactions. The oldest, arguably non-controversial peptide sequences are ~3.8 Ma, resulting from a unique molecular stabilization mechanism arising from close association of proteins involved in mineralization with the calcite of avian eggshell (Demarchi et al. 2016), and ~3.4 Ma, resulting from extremely low temperatures of the permafrost in which the bones derived (Rybczynski et al. 2013). Even when amino acids within calcite matrices have been reported in much older Palaeozoic fossils (Abelson, 1954), they only consist of a small subset of thermally stable amino acids, inconsistent with peptide sequence preservation. Claims of preserved proteins far older, including Mesozoic proteins (Asara et al., 2007; Schweitzer et al., 2007, 2009; Cleland et al., 2015; Schroeter et al., 2017), have been met with mixed support or criticism (Buckley et al., 2008, 2017; Kaye et al., 2008; Pevzner et al., 2008; Bern et al., 2009; Saitta et al., 2019).
Antibodies
In their recent article, Schweitzer et al. (2018) argue that keratin protein can preserve organically in fossils as old as the Mesozoic (e.g., feather fossils), with sequence and higher order protein structure preserving such that they can be detected using immunochemistry, which relies on the binding of antibodies with 3-dimensional epitopes that may be very specific to a certain protein (Schweitzer et al., 1999a, 1999b; Moyer et al., 2014, 2016a, 2016b; Pan et al., 2016). Note that keratinous structures, such as feathers (whose modern fractal structure consists of a central subcutaneous calamus and exposed rachis, second order branching barbs, and third order branching barbules [Lucas and Stettenheim 1972]), can contain keratin protein matrix (Fraser et al., 1971), melanin-bearing vesicles called melanosomes along with other types of pigments (Roy et al., 2019), calcium phosphates (Earland et al., 1962; Blakey et al., 1963), and surficial lipids (e.g., preening waxes [Jacob, 1978]). Also note that, unlike polypeptides, the high preservation potential of melanin and melanosomes in fossils has now largely been accepted (Roy et al., 2019), with the alternate hypothesis that these microbodies actually represent preserved bacteria (Pinheiro et al., 2012; Moyer et al., 2014; Lindgren et al., 2015a; Schweitzer et al., 2015) having been rejected along several lines of evidence using multiple analytical and experimental methods (Barden et al., 2015; Vinther, 2016).
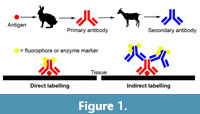 Some investigations into molecular fossil preservation are designed with a strong molecular biology approach, often without considering taphonomic factors such as time and diagenesis. These efforts rely heavily on immunostaining to identify putative protein fragments, often of collagen or keratin, in fossils (e.g., Schweitzer et al., 2007, 2009, 2013; Lindgren et al., 2018). For detailed reviews of immunochemistry see Coons et al. (1941), Ramos-Vara (2005), and Maity et al., (2013). Immunostaining is a molecular biology technique that uses antibodies, which are immune system proteins, raised against a target protein of interest, for example antibodies derived from a rabbit injected with the target protein. When immunostaining is applied to tissues, typically as thin sections, it is referred to as immunohistochemistry, and when immunostaining is applied to cells, it is referred to as immunocytochemistry. Specimens or regions of specimens to which the antibody binds can then be visualised in a variety of ways. In direct labelling (Figure 1), the primary antibody that is raised against the target protein is labelled directly with an attached fluorescent compound (i.e., fluorophore) or an enzyme that can catalyse the production of a coloured product. The fluorescence or colouration can then be visualised with light microscopy to determine where the antibodies bind and localise to on the specimen. Light absorbance at particular wavelengths can also be used as a quantitative measure of a sample’s antigenicity (e.g., Collins et al., 1992). It should be noted that, beyond the fluorophores or enzymes commonly used in conjunction with light microscopy, other visualisation markers, such as gold particles, can be used in conjunction with electron microscopy. In indirect labelling (Figure 1), the primary antibody raised against the target protein binds to the specimen. Then, a labelled secondary antibody raised against the immunoglobulin proteins of the primary antibody is applied to the specimen, which binds to the primary antibody in order to visualise the localisation of the primary antibody. For example, the secondary antibodies could be produced by a goat raised against rabbit antibodies, when rabbit was used to produce the primary antibodies raised against the target protein. Signal amplification can also be achieved (e.g., using secondary antibodies that have multiple labelling compounds attached to each antibody), but these may amplify nonspecific antibody localisation on the specimen (i.e., false positives) (Coons et al., 1941; Ramos-Vara, 2005; Maity et al., 2013). These immunochemistry approaches, particularly when performed on putative dinosaur proteins, cells, and tissues (e.g., Schweitzer et al., 2007, 2009, 2013), have had a tremendous impact on taphonomy research and public interest in palaeontology over the last decade or two.
Some investigations into molecular fossil preservation are designed with a strong molecular biology approach, often without considering taphonomic factors such as time and diagenesis. These efforts rely heavily on immunostaining to identify putative protein fragments, often of collagen or keratin, in fossils (e.g., Schweitzer et al., 2007, 2009, 2013; Lindgren et al., 2018). For detailed reviews of immunochemistry see Coons et al. (1941), Ramos-Vara (2005), and Maity et al., (2013). Immunostaining is a molecular biology technique that uses antibodies, which are immune system proteins, raised against a target protein of interest, for example antibodies derived from a rabbit injected with the target protein. When immunostaining is applied to tissues, typically as thin sections, it is referred to as immunohistochemistry, and when immunostaining is applied to cells, it is referred to as immunocytochemistry. Specimens or regions of specimens to which the antibody binds can then be visualised in a variety of ways. In direct labelling (Figure 1), the primary antibody that is raised against the target protein is labelled directly with an attached fluorescent compound (i.e., fluorophore) or an enzyme that can catalyse the production of a coloured product. The fluorescence or colouration can then be visualised with light microscopy to determine where the antibodies bind and localise to on the specimen. Light absorbance at particular wavelengths can also be used as a quantitative measure of a sample’s antigenicity (e.g., Collins et al., 1992). It should be noted that, beyond the fluorophores or enzymes commonly used in conjunction with light microscopy, other visualisation markers, such as gold particles, can be used in conjunction with electron microscopy. In indirect labelling (Figure 1), the primary antibody raised against the target protein binds to the specimen. Then, a labelled secondary antibody raised against the immunoglobulin proteins of the primary antibody is applied to the specimen, which binds to the primary antibody in order to visualise the localisation of the primary antibody. For example, the secondary antibodies could be produced by a goat raised against rabbit antibodies, when rabbit was used to produce the primary antibodies raised against the target protein. Signal amplification can also be achieved (e.g., using secondary antibodies that have multiple labelling compounds attached to each antibody), but these may amplify nonspecific antibody localisation on the specimen (i.e., false positives) (Coons et al., 1941; Ramos-Vara, 2005; Maity et al., 2013). These immunochemistry approaches, particularly when performed on putative dinosaur proteins, cells, and tissues (e.g., Schweitzer et al., 2007, 2009, 2013), have had a tremendous impact on taphonomy research and public interest in palaeontology over the last decade or two.
However, immunostaining techniques, especially those that treat fossil bone as they do fresh tissues, might be prone to false positives (True, 2008) and require careful and thorough comparison to positive and negative controls. Fossils pose particular challenges for immunostaining. One must control for excavation, handling, storage, and laboratory contamination. Fossils, especially open-system fossil bone, should also be considered as environmental samples that microbes can preferentially inhabit, even while buried (Saitta et al., 2019). Paleontological preparation techniques can introduce organic consolidants and preservatives, such as cyanoacrylates that can influence immunostaining (Saitta et al., 2018b). Even possibly endogenous ancient organics in fossils must be considered as potential sources of non-specific antibody binding, such as melanoidin-like polymers (Collins et al., 1992).
Purpose of This Perspective
Here, we first illustrate our concerns with the recent study by Schweitzer et al. (2018). We clarify some of our positions on taphonomy in general. We then raise concerns about the samples used, the methods employed, and the results and discussion as presented by Schweitzer et al. (2018). Next, we discuss the implications that unsupported taphonomic models can have on our attempts to study paleobiology and evolutionary biology as in Pan et al. (2019), for which we also present a critique. Finally, we present our view of keratinous structure fossilization and compare it to other types of organic preservation, along with discussion of broader themes in scientific epistemology, including replication versus validation. We also provide recommendations for future work on the question of keratin polypeptide preservation in fossils. In this perspective, we attempt to limit our discussion to studies of fossil keratinous structures as much as possible (e.g., as opposed to fossil bone).
AN EXPERIMENTAL INVESTIGATION INTO KERATIN PRESERVATION: SCHWEITZER ET AL. (2018)
The recent study by Schweitzer et al. (2018) analysed a coot specimen from a hot spring sinter reported to be 10 Ka, as well as extant feathers which were experimentally exposed to one of three conditions in an attempt to simulate degradation over long periods of time. Treatment 1 exposed feathers intermittently watered with distilled water to 60º C while buried in sediment (sand from the Judith River Formation in Montana, USA) for three years, then allowed them to dry, and finally stored them at room temperature for 14 years still buried. Treatment 2 exposed feathers to 350º C for 10 years while buried in sediment, and these were then stored for seven years at room temperature either still buried or in sealed laboratory tubes. Also included was a control feather sample that was covered but not buried at room temperature for 17 years. These natural and experimental samples were analysed by light and scanning electron microscopy (SEM), immunohistochemistry (including a combined immunogold labelling [secondary antibody/indirect labelling] and transmission electron microscopy [TEM]), and time-of-flight secondary ion mass spectrometry (TOF-SIMS). In all samples, they report structural preservation of feather anatomy as well as positive anti-keratin antibody binding, although the binding was observed at low levels and appears patchy in the sinter coot and the treatment 2 experimental feathers (350º C, 10 years). Melanosomes were not observed using TEM in the treatment 2 experimental feathers (350º C, 10 years) or the sinter coot. Furthermore, the control and treatment 2 (350º C, 10 years) experimental feathers both yielded N-containing secondary ions when analysed with TOF-SIMS (no other samples were analysed with TOF-SIMS). Schweitzer et al. (2018) interpreted all of these results as evidence for keratin protein preservation in the samples and an indication of its stability through fossilization, presumably exceeding that of melanosomes.
Comments on Taphonomy in General
Schweitzer et al. (2018) point to the extensive fossil record of soft tissues in exceptional fossils as an indication of natural mechanisms for their stabilization. Of course, it should first be kept in mind that the preservation of various non-biomineralised tissues and molecules in exceptional fossils deep into the geologic record should not be interpreted as an indication that all types of soft tissues or biomolecules have roughly equal fossilization potential, even when under conditions favourable to organic preservation (Eglinton and Logan, 1991; Briggs and Summons, 2014). In other words, inherent stability or instability of the molecule itself is a factor in preservation, beyond just favourable environmental conditions. It should also be noted that exceptional preservation might involve drastic decay or diagenetic breakdown/alteration of certain tissue components into more thermodynamically stable compounds such that their resulting residues are what make up the fossil (e.g., labile lipid in situ polymerization into kerogen [Stankiewicz et al., 2000; Gupta et al., 2009]), rather than chemical fixation from an external agent or environmental conditions preserving original chemical structures with high fidelity. For example, the black staining of a fossil feather might indicate the survival of diagenetically altered residues of certain thermally stable feather components (e.g., melanin [Vinther 2008; Saitta et al., 2018a]), rather than fixation of the proteinaceous feather as a whole (i.e., keratin matrix) through surficial mineral deposition as hypothesized by Schweitzer et al. (2018). Furthermore, resistance of a molecule to microbial or autolytic decay does not necessarily imply thermal stability through diagenesis (Parry et al., 2018).
In an attempt to dismiss kinetic models of biomolecule degradation (e.g., Allentoft et al., 2012), Schweitzer et al. (2018, p. 1) state that “... models are hypotheses, and thus subject to testing. Empirical data are more valid than models, and can be used to either support or overturn them.” Although empirical data are indeed used to ‘ground-truth’ scientific models, such data must still be properly interpreted to draw well-supported conclusions about the natural world. Does the methodology yield data appropriate for addressing the posited question and is it accurate (i.e., no false positives or false negatives)? Models become useful to inform our understanding of underlying natural processes and systems. Model development (e.g., experimental taphonomy or kinetic calculations) and empirical ‘ground-truthing’ (e.g., direct analyses of fossils) thereby work in conjunction to continuously refine our understanding of fossilization (Briggs and McMahon, 2016).
Schweitzer et al. (2018) note that various pathways exist which might alter molecules and stabilize them through geologic time. It is true that molecular interactions and chemical reactions can slow the degradation of certain biomolecules (e.g., the ‘molecular cooling’ of short peptide sequences bound to ~3.8 Ma eggshell biocalcite [Demarchi et al., 2016]) or convert them into a substance that can survive in organic form through the conditions of deep-burial diagenesis and over long periods of time (e.g., the polymerization of resin terpenoids into amber [Lambert and Poinar,2002]). However, not all biomolecules undergo the same diagenetic alterations or have the same preservation potential, and our previous work, based on methods developed to study the formation of fossil fuel or related sedimentary organic matter (Rhead et al., 1971; Peters et al., 1981; Lewan, 1983; Monthioux et al., 1985; Lewan et al., 1986; Rullkötter and Marzi, 1988; Teerman and Hwang, 1991; Behar et al., 1995, 1997, 2003; Koopmans et al., 1996; Versteegh et al., 2004; Schimelmann et al., 2006), has supported the hypothesis that the keratin protein component of keratinous tissues is lost largely due to hydrolytic fragmentation, even when these anatomical structures recognizably preserve in exceptional fossils (Saitta et al., 2017a, 2018a). Note that partial shielding of peptide bonds between hydrophobic amino acid monomers from the hydrolytic action of water molecules (as discussed by Pan et al. [2019]) due to peptide folding, for example in globular proteins, does not halt protein fragmentation entirely as only the internal peptide bonds are shielded from hydrolysis and eventually these shielded peptide sequences should become exposed (Nielsen-Marsh, 2002). Also note that collagen and keratin are not globular proteins.
According to thermodynamics, keratinous tissues are expected to primarily preserve in fossils either organically via melanin pigment (Vinther, 2015) (non-protein organic geopolymers like N-heterocyclic polymers or kerogen that form through diagenesis could feasibly produce an amorphous matrix, but melanosome exposure in fossil integument is inconsistent with a predominance of such organics in at least some fossils) or inorganically via endogenous calcium phosphate (Saitta et al., 2018b). These organic and inorganic constituents have a demonstrably higher preservation potential than protein based on theoretical (e.g., reaction kinetics [Nielsen-Marsh, 2002]) and non-controversial empirical evidence (e.g., the fossil record of biomineralized tissues). On the other hand, Schweitzer et al. (2018) use the prevalence of keratinous structure preservation in exceptional fossils as a line of evidence in favour of keratin protein preservation, but their model of keratinous structure preservation does not consider that such tissues contain multiple molecular components of varying preservation potential, and that only a subset of these needs to preserve, not necessarily the keratin protein itself, for an originally keratinous structure to be identified as such in a fossil. For example, a keratinous structure such as a feather can contain both protein and pigment in vivo, which then results in a carbonaceous fossil after protein loss and pigment retention.
Comments on Materials
The coot specimen from a siliceous hot spring deposit studied by Schweitzer et al. (2018) is presented as having an age of 10 Ka. However, in the original description of the specimen, this is described as a maximum age based on the upper bound dictated by the mineralogy of the encrusting sinter (Channing et al., 2005). The silica phase of sinter transforms from opal-A to opal-C/CT about 10,000 years after deposition (Herdianita et al., 2000), and the encrusting silica phase of the sinter around the specimen was determined to consist of amorphous opal-A by X-ray diffraction. The minimum age of the specimen, based on seral developmental stage and trunk diameter of lodgepole pine trees growing on the inactive hot spring sinter from which the specimen was found (Despain, 1990; Kaufmann, 1996), was reported as 300 years. Clearly, the age of the specimen is poorly constrained, and the possibility that the specimen is far younger than the 10 Ka presented by Schweitzer et al. (2018) should be kept in mind. It is also difficult to constrain precisely what local elevated temperatures this specimen experienced since the encrusting sinter would have been deposited through evaporation and cooling of the hot spring water. The soft tissues associated with this specimen are also reported to be difficult to identify at the microscopic level, particularly among infiltrating organisms (e.g., fungal hyphae), and were interpreted as external moulds in the initial description of the specimen (Channing et al., 2005).
Comments on Methods
Possibility of sample contamination. Storing samples for 7–14 years before analysis (Schweitzer et al., 2018) provides an opportunity for and increases the risk of proteinaceous contamination (even if covered). This issue is further compounded by the fact that samples were buried in collected sediment, which could have contained contaminating organic material/organisms (unless sterilized beforehand) or been colonized during the years of storage (Salonius et al., 1967; also see Roll-Hansen [1979] for a discussion of Louis Pasteur’s refutation of ‘spontaneous generation’ by demonstrating microbial colonization of sterilized media).
Thermal maturation concerns. The two experimental treatments would be expected to lead to rapid dehydration. In treatment 1, the 60º C would promote evaporation and drying of the specimen, which was only watered intermittently as reported. After three years this specimen was then allowed to dry. Additionally, if this treatment was intended to promote microbial proliferation in order to simulate long-term decay (i.e., greater than 3-17 years), the 60º C conditions can be above the optimal growth conditions of many common bacteria. For example, optimum temperature for growth and keratinolytic enzyme production is about 20-30º C for at least four species of keratinolytic bacteria (Sangali and Brandelli, 2000; Allpress et al., 2002; Yamamura et al., 2002; Brandelli and Riffel, 2005) although other species of keratinolytic bacteria show optimal enzyme production at about 40-50º C (Lin et al., 1999; Kim et al., 2001; Balaji et al., 2008). In treatment 2, 350º C is well above the boiling point of water at atmospheric pressure, and after 10 years, this sample would have been very dehydrated. The loss of liquid water in the experimental samples is important since one of the most common degradation reactions for proteins and other biomacromolecules is hydrolysis, which requires the presence of water (Lang, 1986). Furthermore, dry conditions are not realistic of the sorts of burial environments that most fossil keratinous structures, and indeed fossils in general, are found (i.e., sediment deposited by the action of water or below a ground water table [Nichols, 2009; Boggs, 2014]). The formations that preserve keratinous structures as organic remains are often from marine or lacustrine environments with low sedimentation rates, indicating that carcasses would have been within highly water-logged and possibly reducing sediments for extended periods of time (Benton et al., 2008; Bergmann et al., 2010; Li et al., 2010; Brown et al., 2017). As such these experimental conditions are highly problematic.
In contrast, our simulation of deep-burial diagenesis over long periods of time (e.g., tens of millions of years) by thermal maturation in either sealed noble metal capsules (Saitta et al., 2017a) or compacted sediment (Saitta et al., 2018a) uses an approach commonly used to simulate the formation of fossil fuels and other diagenetically altered sedimentary organic matter (Rhead et al., 1971; Peters et al., 1981; Lewan, 1983; Monthioux et al., 1985; Lewan et al., 1986; Rullkötter and Marzi, 1988; Teerman and Hwang, 1991; Behar et al., 1995, 1997, 2003; Koopmans et al., 1996; Versteegh et al., 2004; Schimelmann et al., 2006). The conditions are often on the order of 250º C and 250 bar for 24 hours (McNamara et al., 2013a, 2013b, 2017). Raising the pressure during heating keeps water in the liquid phase and prevents dehydration of the sample from the boiling off of water (Poole et al., 1992). This maintains the proper aqueous chemistry required to not simply simulate geothermal heat during deep burial, but aqueous degradation over long periods of time under diagenetic conditions. Recent maturation experiments controlling for the same temperature and duration (200º C, 1 hour), either with or without elevated pressure (135 bar), appear to illustrate how the keratin matrix shows greater loss and melanosomes become more exposed when pressure is raised above the boiling point of water (see figure 2C-D of Slater et al., 2019).
Depending on burial depth as well as spatial and temporal variations in geothermal gradients (e.g., Hitchon, 1984), not all fossils will have been buried deeply enough to experience the exact temperatures used in thermal maturation experiments, but elevated temperatures kinetically simulate diagenetic conditions over millions of years within the timeframe of the experiment. Although high temperatures help to simulate extended durations of time (i.e., accounting for the kinetics of the reaction), care must be taken to not ascribe certain chemical reactions to natural fossilization processes when activation energies are only exceeded in the laboratory conditions (e.g., when compared to fossils of low thermal maturity). This concern can be mitigated with respect to certain questions. For example, the temperature conditions used by Saitta et al. (2018a) (i.e., 210-250º C, 225-300 bar, 12-23 hours within compacted sediment) can likely be assumed to exceed the limits of protein survival (contra Schweitzer et al. [2018]). Therefore, when the matured products are comparable to natural fossils at the macroscopic and ultrastructural scale, a model of keratinous structure fossilization that involves the loss of proteinaceous tissue is supported. On the other hand, moderate maturation at 100º C and 250 bar for 24 hours (Saitta et al., 2017b) allows for appreciable structural preservation in keratinous tissues that does not appear to be present in fossils (e.g., plumulaceous-barbule-like fibrous rachidial subunits [see discussion of claims of keratin fibers in fossils below]). Therefore, these low temperature conditions are likely insufficient to simulate fossilization, at least for fossils of appreciable age and/or thermal maturity.
Note that in addition to sufficient temperature, sufficient time is also needed in experimental maturation. Recent experiments have used temperatures of 200º C (either at 135 bar or atmospheric pressure) for only 1 hour (Slater et al., 2019), similar to some treatments in earlier experiments (McNamara et al., 2013b, 2017). However, this duration also appears to result in dramatic keratin structural preservation and, therefore, likely unrealistic simulation of fossil diagenesis when compared to the lack of structure apparent in fossils. To highlight this, consider that the treatment of feathers to conditions of 200º C and atmospheric pressure for 1 hour is equivalent to the conditions sometimes used in cooking, such as baking a potato. Since an edible baked potato would not likely be considered to be chemically equivalent to a hypothetical fossil potato, this would be an indication that these conditions are possibly insufficient at least with respect to relatively robust biomolecules such as starches. Of course, proteins are less stable than these carbohydrates and so this analogy is imperfect.
Melanosomes had been shown to survive thermal maturation in sealed, metal capsules under conditions commonly used to study deep burial diagenesis in fossils (Colleary et al., 2015). By running these maturation experiments on samples encased in compacted sediment, we were able to simultaneously and experimentally demonstrate the hypothesized dynamic of melanosome survival and keratin protein degradation and loss during the production of dark stains directly comparable to fossil feathers at the macroscopic and microscopic/ultrastructural level, likely as a result of a sediment filtration mechanism (Saitta et al., 2018a). The fact that our sediment-encased maturation experiments yield samples directly comparable to carbonaceous fossils down to the ultrastructural level observed by SEM (i.e., exposed melanosomes resting on the sediment [Vinther, 2008]) is a testament to the appropriateness of our simulation of fossilization in at least some aspects (Saitta et al., 2018a).
Immunochemistry concerns. The immunochemistry controls used by Schweitzer et al. (2018) include 1) primary antibody omission to control for nonspecific binding of secondary antibody, 2) attempting to block primary antibody binding sites through incubation with excess antigen source protein in order to control for nonspecific antibody accumulation on the samples, and 3) “incubating feather specimens with a non-relevant protein (human alpha keratin, [Moyer et al., 2016a]) [to control] for nonspecific binding of primary antibodies” (Schweitzer et al., 2018, p. 6). While these sorts of methodological controls are useful, as discussed below they are likely insufficient to fully rule out false positive results and their descriptions are spread across the supplemental material of more than one publication (Moyer et al., 2016a; Schweitzer et al., 2018), increasing the difficulty of their interpretation. Instead, we propose below that controls based on exposing non-keratinous biological samples to antibody techniques be used in addition to these methodological controls. A powerful control would be to experimentally treat non-keratin proteinaceous tissues, as well as non-protein-rich tissues (e.g., plant tissues), as the feathers were treated to see if the resulting browning products can induce anti-keratin antibody binding (Collins et al., 1992).
It should also be noted that the use of immunogold labelling can improve the resolution of immunochemical analyses, but that improved resolution of a technique does not necessarily mean improved accuracy, and the potential for false positives should still be considered. The assertion that immunochemistry has been used to distinguish between α- and β-keratins in fossils (Schweitzer et al., 2018) is dependent on these results representing true positives, notwithstanding the fact that these fossil analyses only examined tissues typically thought to be dominated by β-keratin (Schweitzer et al., 1999a, 1999b; Moyer et al., 2016a, 2016b; Pan et al., 2016), at least in extant organisms.
Comments on Results and Discussion
 Some of the results reported by Schweitzer et al. (2018) are unexpected. Recognizable structural preservation of the treatment 2 experimental feathers (350º C, 10 years) differs from our attempts to expose feathers to these sorts of high temperatures at atmospheric pressure. In Saitta et al. (2018b), we briefly described supplemental results of modern feathers placed into a laboratory oven set to 350º C in an attempt to replicate the temperature conditions of Schweitzer et al. (2018). Within minutes of turning on the oven, the feathers began to produce copious amounts of smoke, prompting us to halt the attempt, and were converted into a black ash-like material without readily discernible macroscopic structural preservation (Figure 2) (although running these conditions within compacted sediment would likely have helped to prevent disassociation of the ash). Structural preservation in the experimental feathers of Schweitzer et al. (2018) is also different from fossil feathers preserved as carbonaceous compression fossils, which are relatively 2-dimensional and largely consist of dark stains composed of exposed melanosomes resting on the sediment as evidenced by scanning electron microscopy (Vinther et al. 2008; also see our commentary on the counterargument of Pan et al. [2019] below).
Some of the results reported by Schweitzer et al. (2018) are unexpected. Recognizable structural preservation of the treatment 2 experimental feathers (350º C, 10 years) differs from our attempts to expose feathers to these sorts of high temperatures at atmospheric pressure. In Saitta et al. (2018b), we briefly described supplemental results of modern feathers placed into a laboratory oven set to 350º C in an attempt to replicate the temperature conditions of Schweitzer et al. (2018). Within minutes of turning on the oven, the feathers began to produce copious amounts of smoke, prompting us to halt the attempt, and were converted into a black ash-like material without readily discernible macroscopic structural preservation (Figure 2) (although running these conditions within compacted sediment would likely have helped to prevent disassociation of the ash). Structural preservation in the experimental feathers of Schweitzer et al. (2018) is also different from fossil feathers preserved as carbonaceous compression fossils, which are relatively 2-dimensional and largely consist of dark stains composed of exposed melanosomes resting on the sediment as evidenced by scanning electron microscopy (Vinther et al. 2008; also see our commentary on the counterargument of Pan et al. [2019] below).
The blackened ash-like material produced by our supplementary attempts (Saitta et al. 2018b) as well as the browned/blackened experimental and natural samples of Schweitzer et al. (2018) are consistent with the formation of melanoidin-like oxidation and condensation products (Pamplona, 2011). These N-heterocyclic polymers could, for example, represent advanced lipoxidation end products between the degradation and subsequent condensation of keratin protein and feather preening waxes, such as the waxes detected on the control feathers of Schweitzer et al. (2018) using TOF-SIMS. The increased electron density of the matrix surrounding the melanosomes in the treatment 1 experimental feather (60º C, three years) might therefore be indicative of condensation reactions producing N-heterocyclic polymers. If degradation of original biomolecules and subsequent polymerization of their products can occur without a loss of structural fidelity to the overall soft tissue, then these thermally stable polymers could explain the structural preservation reported by Schweitzer et al. (2018) as has been suggested in fossil bone soft tissues (Wiemann et al., 2018; with similar organic signatures reported by Kiseleva et al. [2019] and Ullmann et al. [2019]). However, note that issues of potential biofilm infiltration into bones still remain (Kaye et al., 2008; Saitta et al., 2019). Ultimately, there are three key issues with respect to the results of Schweitzer et al. (2018), as discussed below: 1) such polymers are not preserved proteins, 2) they can yield false positive immunochemistry results, and 3) their formation may not be favoured in the sorts of depositional environments that yield organically preserved fossil feathers.
1) Lack of evidence for protein preservation. To corroborate their findings, Schweitzer et al. (2018) performed TOF-SIMS to reveal certain positive secondary ions that might be consistent with protein fragments. While their control feather is comparable to previously published spectra for keratin (K0253, Sigma-Aldrich), their treatment 2 experimental feather (350º C, 10 years) did not yield a spectrum as consistent with keratin or an unaltered protein source.
The secondary ions detected by TOF-SIMS consistent with a protein source in the treatment 2 experimental feather (350º C, 10 years) could, of course, derive from contamination (e.g., from the surrounding sediment or the external environment), given the extended period of time the samples were stored prior to analysis. Additionally, even if these secondary ions indicate the presence of amino acids, they do not necessitate the presence of polypeptides capable of providing epitopes for antibodies. Furthermore, a N-heterocyclic polymer, without associated proteins, would presumably produce a variety of N-containing secondary ions. The presence of high mass (m/z = 100-500) secondary ions in the treatment 2 experimental feather (350º C, 10 years) was taken by Schweitzer et al. (2018) to represent polypeptide fragments of various lengths, but these could also represent fragments of various masses derived from a long N-heterocyclic polymer. These high mass, positive secondary ions are lacking, or at least extremely weak, in their control feather (room temperature, 17 years) compared to the treatment 2 experimental feather (350º C, 10 years) (see figure S5b in Schweitzer et al. [2018]). If these high mass secondary ions were indeed polypeptide fragments, then they should also appear in the control feather (room temperature, 17 years). Instead, the fact that they are more prevalent in the treatment 2 experimental feather (350º C, 10 years) is consistent with their formation through thermally induced polymerization. Additionally, the detection of organic secondary ions with low hydrogen content in the treatment 2 experimental feather (350º C, 10 years) could be indicative of condensation (i.e., dehydration) reactions that help to form N-heterocyclic polymers.
The secondary ion reported as m/z = 70 (without decimal places) and identified by Schweitzer et al. (2018) as C4H8N+, if indeed deriving from unaltered protein or amino acids, would actually correlate more closely with a secondary ion C3H4NO+ (m/z = 70.03) deriving from the amino acid Asn, rather than C4H8N+ from the amino acids Pro, Arg, Val, or Leu (m/z = 70.07) (Hedberg et al., 2012). Asn contains an amide-bearing R group side chain, but deamidation is a common protein degradation reaction (Robinson and Rudd, 1974). Therefore, preservation of this amino acid would be unexpected, particularly in the treatment 2 experimental feather (350º C, 10 years). Furthermore, in this highly heated feather, the prevalence of secondary ions m/z = 30 and 70 is not detected across the entirety of the preserved structure. Although an argument could be that such patchy secondary ion (as well as antibody) signatures are indicative of the degradation (or antiquity) of these purported proteins, if these structures are claimed to be keratin protein preservation, then the secondary ion signature should be present across the structure; otherwise, another explanation must be provided for the retention of their recognizable feather structure. In other words, if the structures consisted of protein that had undergone significant degradation such that only trace amounts of protein were preserved in a patchy manner, then an explanation must be provided as to how the structures persisted at all (see our discussion of how N-heterocyclic polymers are not proteins per se).
What is surprising is that Schweitzer et al. (2018) do not discuss the presence of many S-bearing secondary ions other than HSO3- and SO4- (possibly derived from breakdown products of the keratin protein through oxidation of S-bearing amino acids), but instead rely on a relatively small number of N-containing secondary ions to support their conclusion. If keratin protein was still present, one might expect S signatures deriving from cysteine in the form of secondary ions of such as C2H6NS+ (m/z = 76), S- (m/z = -32), or SH- (m/z = -33) (Wagner and Castner 2001), at least relative to non-keratin protein-derived material. The treatment 2 experimental feather (350º C, 10 years) had a reduction in peaks near m/z = -32 and -33 compared to the control feather and keratin standard, although note that the peak near m/z = 76 is fairly low even in the keratin reference spectrum (Schweitzer et al., 2018). Given that disulphide bonds provide structural integrity to keratin fibers (Strasser et al., 2015), if these fibers are claimed to be molecularly preserved such that their structure is still present, one might expect disulphide bonds to be preserved and detectable. Could some S-bearing breakdown products also have escaped from the sample via volatile products like H2S, thiols, or others (i.e., the sorts of gases that might have been responsible for the strong odour of the smoke produced during our attempt to heat feathers)? The 350º C conditions of treatment 2 exceed the reported thermal decomposition and melting points of some amino acids (Dunn and Brophy, 1932; Lien and Nawar, 1974), including cysteine (Weiss et al., 2018), which is the amino acid responsible for the formation of disulfide bridges that provides stability and mechanical resilience to keratin protein (Strasser et al., 2015). If thermal decomposition of individual amino acids is occurring, we stand by our previous assertion (Saitta et al., 2017a) that it is indeed likely impossible to preserve an amino acid sequence (a prerequisite for epitope survival), regardless of whether hydrolytic cleavage of peptide bonds has occurred.
Many types of ancient organismal remains often become darker with time and diagenesis due to chemical alterations (e.g., conodont elements [Epstein et al., 1977]), some which have been linked to the sorts of chemical reactions involved in the browning of food during cooking (Collins et al., 1992; Wiemann et al., 2018). But even if the initial reactants of complex geopolymer products include proteins, can these products be called ‘fossil proteins’? Proteins are defined by their complex structure and function, with some extreme biochemical perspectives even requiring that a denatured protein be described simply as a polypeptide due to a loss of metabolic function (Collins et al., 1998). To consider stable N-heterocyclic polymers, if these are indeed what is being produced in the experiments of Schweitzer et al., (2018), as preserved or fossilized protein per se would be ill-advised. Protein function/folding and various amino acid side chains are lost (e.g., deamidation) through diagenesis (Eglinton and Logan, 1991). Also lost are the amino acid sequences (Lang, 1986) and, in thermal conditions that exceed the decomposition points of some amino acids (Dunn and Brophy, 1932; Lien and Nawar, 1974; Weiss et al., 2018), some of the peptide monomers themselves. Additionally, these N-heterocyclic polymers form in association with the products of other types of biomolecules such as lipids or polysaccharides (Fu et al., 1996; Hidalgo et al., 1999). When melanoidins (e.g., those present in ancient carbonate skeletons [Collins et al., 1992]) are formed from protein-polysaccharide mixtures, crosslinking occurs at the N-terminal amino group of amino acids, meaning that peptide bonds, and therefore peptide sequences, are lost (Singh et al., 2001; Vistoli et al., 2013). In such an example, the resulting polymer might equally be referred to as ‘fossil polysaccharide’. Therefore, claiming that the results of Schweitzer et al. (2018) represent protein preservation would be inappropriate.
2) Potential false positive immunochemistry results. The reduced, patchy binding of antibodies in the treatment 2 experimental feather (350º C, 10 years) as well as the coot found in the sinter is indicative of protein degradation. Again, if the preserved, morphologically recognizable structures themselves are claimed to be keratin protein preservation, then the epitope signature should likely be present across the structure rather than patchy. The low levels of antibody reactivity in these samples could be from nonspecific binding (James and Tawfik, 2003) of antibodies to resulting N-heterocyclic polymers, not preserved polypeptide sequences. Cross reactivity of antibodies to such browning products has been reported (Collins et al., 1992). Diverse, heterogeneous, thermally resistant, and insoluble N-heterocyclic polymers as a cause of low levels of antibody binding could explain why patchy antibody staining can occur even when the larger feather structure is preserved in the experimental or sinter feathers (e.g., visible filamentous structures or recognizable feather morphology).
Calcified materials have also been reported to interfere in immunochemistry experiments (Sedivy and Battistutti, 2003), so any phosphatic ash resulting from thermally degraded feathers should also be considered as a potential explanation of their antibody results. The claim by Schweitzer et al. (2018) that most keratins are not biomineralized requires supporting evidence since calcium phosphates have been detected and quantified in a wide variety of extant keratinous tissue types from phylogenetically diverse taxa (Earland et al., 1962; Blakey et al., 1963; Pautard, 1963, 1964, 1966, 1970; Blakey and Lockwood, 1968; Baggott et al., 1988; Hieronymus et al., 2006; Szewciw et al., 2010). For example, whale baleen contains relatively high amounts of calcium phosphatein vivo for mechanical reinforcement (Pautard 1963; Szewciw et al., 2010), and Miocene fossil whale baleen preserves as phosphate (Gioncada et al., 2016). Even the mechanical properties and wear patterns of rhinoceros horn are influenced by melanin and calcium phosphate (Hieronymus et al., 2006). Specifically, their claim that extant feathers have not been shown to contain biominerals is in direct conflict with reported calcium phosphate in calami (Earland et al., 1962; Blakey et al., 1963). One method of quantifying calcium phosphate in extant keratinous structures even involves exposing the tissues to high heat in a laboratory oven to combust the organics (Pautard, 1964), a process analogous to cremation but using temperatures higher than the experimental conditions discussed here (e.g., 650º C), and then exposing the resulting ash to acid dissolution to observe weight changes. TOF-SIMS revealed Ca+ secondary ions in the control and treatment 2 experimental feathers (350º C, 10 years) despite the prevalence of dark organic material (Schweitzer et al., 2018), which could be consistent with the presence of calcium phosphates, bearing in mind that extant feathers can contain various metals, including trace metals (Howell et al., 2017). The fact that the material properties of keratinous tissues can also be influenced by inherent properties of the keratin protein component itself (e.g., through secondary structure or crosslinks [Fraser and Parry, 2014]) does not preclude a role for calcification to contribute to the tissue’s material properties and biomechanical function.
We also note that the samples need to be washed carefully to remove excess antibody, but we assume that this precaution would be standard procedure in any laboratory, and there is currently no reason to suspect this as an explanation.
Assuming this was a pigmented feather (the species used [Perdix perdix] is fairly pigmented in plumage), the failure of transmission electron microscopy to reveal melanosomes in the treatment 2 experimental feather (350º C, 10 years) might indicate that the extreme conditions resulted in significant degradation of even some relatively stable organics. For experimental illustrations of the relative thermal stability of melanin compared to keratin protein see Colleary et al. (2015) and Saitta et al. (2017a, 2018a). Note that melanin and melanosomes can also be susceptible to oxidation (Slater et al., 2019), which could have occurred in this treatment. The observation of a lack of melanosomes might further emphasize the potential role for highly altered organic polymers (Collins et al., 1992) or phosphates (Sedivy and Battistutti, 2003) to yield false positive antibody results. Another possible explanation is that altered melanosomes exist in the darkened mass of degraded keratin protein that has been converted into N-heterocyclic polymers of similar colour and electron density.
3) Environmental conditions that promote organic fossil preservation appear to differ from those that promote N-heterocyclic polymer formation. Organically preserved, as opposed to phosphatically preserved (e.g., Shuvuuia fibers [Saitta et al., 2018b]), fossil keratinous structures are found most often in marine or lacustrine environments, particularly euxinic or hypoxic/anoxic environments (e.g., Brown et al., 2017). The dry, oxic conditions in the experiments of Schweitzer et al. (2018) not only fail to simulate these environments, they are also the conditions that might be expected to limit rapid peptide hydrolysis and favour oxidation and condensation reactions to form N-heterocyclic polymers, such as through Maillard-like reactions (Singh et al., 2001; Vistoli et al., 2013). In contrast, our maturation conditions are run under elevated pressure to keep water from boiling off, and when run within compacted sediment, yield results directly comparable to carbonaceous fossils of keratinous tissues (e.g., the Jehol biota of China) at the macro- and ultrastructural level with a preferential loss of proteinaceous tissue that exposes melanosomes, as can be examined using SEM (Saitta et al., 2018a).
Assessment: Schweitzer et al. (2018)
We find it most probable that the organics detected by Schweitzer et al. (2018) are highly degraded, altered, and nitrogenous browning polymers rather than keratin polypeptide preservation.
UNSUPPORTED TAPHONOMIC MODELS MIGHT NEGATIVELY IMPACT PALEOBIOLOGY AND EVOLUTIONARY BIOLOGY: PAN ET AL. (2019)
A recent study by Pan et al. (2019) illustrates how failure to reject a particular taphonomic model can begin to muddy the waters of investigations into paleobiology and evolution. In this case, their model is that keratin protein can preserve organically in fossils with sequence survival of a degree capable of eliciting a specific immunological response. Without thorough examination of amino acid composition or racemization in fossil avian and non-avian dinosaurs, Pan et al. (2019) used SEM, TEM (including ChemiSTEM), immunofluorescence, and immunogold labelling to not only conclude organic preservation of keratin protein sequences, but to also reconstruct keratin molecular evolution and feather ultrastructural evolution in non-avian dinosaurs and early birds with arguably surprising results. Pan et al. (2019) suggest that early paravians like Anchiornis had non-embryonic feathers that not only contained detectable levels of α-keratin in addition to feather-specific β-keratin, unlike modern feathers, but were dominated by α-keratin filaments of diameters not seen in modern feathers. Furthermore, they concluded that feathers containing α-keratin were present even up through some early avians, with modern feathers dominated solely by feather-specific β-keratin appearing in neornithines. These unique molecular compositions were hypothesized by Pan et al. (2019) to influence the mechanical properties, and therefore biomechanics, of early feathers, making them less suitable for powered flight. Also, the outgroup feathers of the alvarezsaur Shuvuuia were reported as containing non-feather β-keratin, without α-keratin, as were the claw sheaths of the oviraptorosaur Citipati.
Seemingly, there are some contradictions within their interpretation of the purported fossil data. For example, the asserted dramatic and mosaic molecular evolution of feather protein composition might yield challenges in producing specific antibodies for those ancient proteins when antibodies are raised against extant proteins; this would make true positive immunological binding more difficult to conclude. Additionally, if a combination of feather-specific β-keratin and α-keratin is suspected to be the plesiomorphic condition in feathers, the results whereby the outgroup Shuvuuia feathers contained solely non-feather β-keratin are potentially unexpected. Contrary to Pan et al. (2019), without additional outgroups it cannot be determined phylogenetically based on this data alone whether Shuvuuia represents a loss of α-keratin in feathers. Furthermore, their conclusion that the ~160 Ma Anchiornis possessed feather-specific β-keratin is on the older end of or potentially in conflict with estimates from extant molecular phylogenies that place the diversification of this protein family at ~143 Ma (~176-110 Ma at 95% highest posterior density [Greenwold and Sawyer, 2011]) or even ~124 Ma (Greenwold and Sawyer, 2013).
However, there are more fundamental problems with this study involving the data itself, similar to those described in the largely experimental work of Schweitzer et al. (2018). For example, keratin, particularly α-keratin such as in human integument, is an extremely common laboratory contaminant (Keller et al., 2008). Any reports of such in unexpected samples (e.g., tissues typically dominated by β-keratin in extant taxa) should consider this possibility.
There is Likely no Keratin Preserved in Shuvuuia deserti Fibres
Pan et al. (2019) do not discuss the counterarguments of Saitta et al. (2018b) about the fibers from the Shuvuuia specimen that they claim consist of non-feather β-keratin, and as such, the topic deserves further discussion here. Our work has shown that previous reports of keratin protein preservation based on antibody binding in the feather fibres associated with this specimen of the dinosaur Shuvuuia deserti (Schweitzer et al., 1999b; reanalysed in Moyer et al., 2016a) likely represent false positive results (Saitta et al., 2018b). These findings can probably be extended to a very similar study of the claw sheaths of the oviraptorosaur Citipati from the same formation (Moyer et al., 2016b). By analysing a fibre from this Shuvuuia specimen using a combination of light microscopy, laser-stimulated fluorescence, focused ion beam SEM, energy dispersive X-ray spectroscopy, and TOF-SIMS, we showed that not only was this fiber inorganic and non-proteinaceous, but that it consisted primarily of calcium phosphate with some clay mineral infiltration and was covered in cyanoacrylate from preparation of the fossil (Amy R. Davidson [preparator of the Shuvuuia specimen] pers. comm., 2018; Saitta et al., 2018b). While the cyanoacrylate glue yielded several N-bearing secondary ions under TOF-SIMS, no distinct secondary ions for amino acids were localized to the fiber (Saitta et al., 2018b). Both the calcium phosphate and the applied organic polymers used to consolidate the specimen (i.e., cyanoacrylate, which was detected, or butvar, which was also used during preparation [Amy R. Davidson pers. comm., 2018; Saitta et al., 2018b]) could have influenced the immunochemical analyses and resulted in false positives.
Despite recent insinuations by Schweitzer et al. (2019) that these organic consolidants were only applied to the specimen after the 1999 study (Schweitzer et al., 1999b), there is no evidence provided for this claim, and given that the original preparation of the specimen involved the use of cyanoacrylate and butvar, it seems very likely that such polymers could have elicited their observed antibody results. We are concerned that the ethanol used to rinse the fibers in the original study (Schweitzer et al., 1999b) would have been an insufficient organic solvent to remove these polymers. Despite their reporting of hollow fibers (Schweitzer et al., 1999b, 2019), the fiber we observed was not hollow nor did it appear to contain filaments within it.
Furthermore, the suggestion that atmospheric exposure could explain our failure to detect proteinaceous signatures in the Shuvuuia fiber (Schweitzer et al., 2019) fails to acknowledge that the specimen would also have been exposed to the atmosphere during the original study (Schweitzer et al., 1999b). Additionally, the specimen would have been exposed to oxidative weathering from the atmosphere during near-surface burial, excavation, and preparation due to the large pore size of the poorly consolidated sediment matrix (i.e., large grain size combined with little cementation) and likely also ground water during both shallow and deep burial. The ~17-year gap between the original study and the reanalysis of Saitta et al. (2018b) is insignificant in light of the ~75-million-year taphonomic history of the specimen; if proteins had become fixed or stabilized during fossilization, we should have detected them.
Finding no corroborating evidence for protein preservation in one of the pioneering studies using antibodies for protein detection in a paleontological sample highlights the well-known issues with antibodies frequently dealt with by neontologists and medical researchers (True, 2008). Antibodies can often yield false positives but can be a powerful tool when used appropriately.
Electron Microscopy and Pattern Seeking
 Ultrastructural data can often be at risk of misinterpretation. There is a risk of falsely perceiving patterns that might not actually be present in even random data, a common and normal psychological phenomenon known as apophenia (Conrad, 1958). Certain fossil organics, like melanosomes (when preserved organically and not as molds due to weathering), appear consistently under electron microscopy in exceptional fossils of tissues known to possess them in vivo (Figure 3) and show relatively limited, quantifiable variation in morphology and organization in line with expected biological variation (see Vinther, 2015 and Roy et al., 2019 for thorough reviews of published reports of fossil melanosomes). However, other structural data are open to subjective interpretation. One example is likely the recent report of rods, cones, and oil droplets from a fossil bird retina based on low-resolution electron micrographs (Tanaka et al., 2017). Other structures show high levels of variation in size and shape more consistent with abiotically self-organizing structures than biologically formed structures (Brasier et al., 2006), such as putative dinosaur erythrocytes (Bertazzo et al., 2015) which are most likely degraded organic material, possibly exogenous, shaped by the low pressure or vacuum conditions of electron microscopy (Saitta et al., 2017b).
Ultrastructural data can often be at risk of misinterpretation. There is a risk of falsely perceiving patterns that might not actually be present in even random data, a common and normal psychological phenomenon known as apophenia (Conrad, 1958). Certain fossil organics, like melanosomes (when preserved organically and not as molds due to weathering), appear consistently under electron microscopy in exceptional fossils of tissues known to possess them in vivo (Figure 3) and show relatively limited, quantifiable variation in morphology and organization in line with expected biological variation (see Vinther, 2015 and Roy et al., 2019 for thorough reviews of published reports of fossil melanosomes). However, other structural data are open to subjective interpretation. One example is likely the recent report of rods, cones, and oil droplets from a fossil bird retina based on low-resolution electron micrographs (Tanaka et al., 2017). Other structures show high levels of variation in size and shape more consistent with abiotically self-organizing structures than biologically formed structures (Brasier et al., 2006), such as putative dinosaur erythrocytes (Bertazzo et al., 2015) which are most likely degraded organic material, possibly exogenous, shaped by the low pressure or vacuum conditions of electron microscopy (Saitta et al., 2017b).
Pan et al. (2019) report filamentous structures in the fossil feathers, but these are not readily obvious to us based on their electron micrographs. Instead, the fossils appear to show impressions in the sediment where barbs once were (e.g., figure 1A of Pan et al. [2019]), consistent with experimental simulations of fossil diagenesis (Saitta et al., 2018a). Like the SEM data, the TEM data also appears to be at risk of non-patterned data being interpreted as patterned, explaining why Pan et al. (2019) report several ~8 nm diameter fibers amid a high-magnification image of electron density variation containing points of varied diameter (figure 2E of Pan et al. 2019). It is also not clear why such points of elevated electron density must represent the cross section of a long fiber without observing multiple slices of TEM data. Determining the relative proportions of β-keratin and α-keratin in the fossils based on the prevalence of thick versus thin filaments is therefore ill-advised. Furthermore, Pan et al. (2019) report an amorphous keratin matrix, but a rough ultrastructural texture is not unexpected when viewing objects like rock under such high magnifications and an amorphous matrix does not require the presence of polypeptides. The TEM data showing accumulated concentrations of melanosomes in all of the studied fossil feathers compared to modern feathers is most consistent with a model of feather fossilization whereby keratin protein degrades and is lost, while melanosomes are left behind.
Lindgren et al. (2015b) described a fossil feather where arguably fiber-like structures were relatively more prominent than in the Pan et al. (2019) images, but these consisted of calcium phosphate rather than organic material. Whether or not integumentary keratin protein undergoing authigenic mineralization through microbial decay to preserve fine structural details is not inconceivable based on the observed phosphatization of other soft tissues in both fossils and experiments (Briggs et al., 1993) but requires further evidence, particularly through experimental taphonomy (Saitta et al., 2018b).
Elemental Signatures: Organic Material Localized to Fossil Melanosomes
The ChemiSTEM results of Pan et al. (2019) showed elevated levels of S, N, and Cu in the fossil melanosomes compared to low levels of these elements in the surrounding matrix, while a modern feather showed a matrix enriched in S and N with Cu concentrated in the melanosomes. The elevated S in the fossil melanosomes could be explained by either high concentrations of S-bearing phaeomelanin within them or by taphonomic incorporation of S from external sources under a process known as sulfurization or vulcanization (McNamara et al., 2016). Both phaeo- and eumelanin contain N, so elevated N in the fossil melanosomes relative to the matrix is also unsurprising. Elevated Cu in the melanosomes is consistent with the fact that Cu can chelate to melanin in modern and fossil feathers (Wogelius et al., 2011; Vinther, 2015; Howell et al., 2017). What is most concerning is that the surrounding matrices of these fossil melanosomes are depleted in N (present in all proteins due to the amino group of their constituent amino acids), S (present in relatively high amounts in keratin protein compared to other proteins [Fraser and Parry, 2014]), and Cu (an element that can chelate to various organic geopolymers during fossilization [Vinther, 2015]). If present, any small amounts of organic matrix may instead be a kerogen-like substance that formed by polymerization of labile lipids (Stankiewicz et al., 2000; Gupta et al., 2009) around the melanosomes after keratin had degraded away, which would yield an aliphatic hydrocarbon signature. In contrast, note how the modern feather they analyzed shows a keratin matrix enriched in S and especially N, while Cu is concentrated in the melanosomes (figure 3B-D of Pan et al. [2019]). This chemical data, like the structural data, is consistent with a loss of keratin protein (as opposed to a prevalence of α-keratin over β-keratin), leaving behind a concentration of thermally stable melanosomes as the only remaining organic component of the fossil feather (Saitta et al., 2017a).
Low levels of Ca in the matrix surrounding some of the fossil melanosomes might be unsurprising since calcification (CaPO4) of feathers appears to be more heavily localized to the calamus/rachis (Earland et al., 1962; Blakey et al., 1963; Bergmann et al., 2010; Howell et al., 2017; Saitta et al., 2018b) (also see relatively low Ca concentrations of the modern feather, presumably a barbule based on size, in figure 3E of Pan et al. [2019]), while melanosomes can often be found in the barbs and barbules (Vinther, 2015). Ca that is present in the fossil matrix is likely indicative of the mineral composition of the rock.
Immunochemistry Issues are Still Present in Fossil Studies
Despite the methodological and sediment controls used in the immunochemical analyses of Pan et al. (2019), a more helpful control would be to examine fossils organically preserving non-keratinous tissues that would have originally been protein-rich, such as organically preserved fossil eyes consisting of melanosomes (Clements et al., 2016), or organic fossils preserving tissues that were originally non-protein-dominated, such as fossils of polysaccharide-dominated leaves or arthropod chitin. Fossil controls from the same specimen but of a different tissue type or of a different type of fossil from the same formation and lithology are crucial. For example, a study of Citipati fossil claw sheaths used modern, decalcified ostrich bone as a control (Moyer et al., 2016b), but a better control would have been the fossil bone from the specimen itself, which might contain recalcitrant geopolymers or applied organic consolidants from fossil collection/preparation capable of eliciting immunological false positives (Saitta et al., 2018b). In contrast, the modern, decalcified ostrich bone would lack the mature geopolymers, calcium phosphate, and any applied organic consolidants that could be causing the fossil claw sheath results. Exhaustive controls are important for immunochemistry because, although the immune system is a powerful and complex adaptation, the well-studied phenomenon of human allergies serves as a stark reminder that antibodies can show cross reactivity (Aalberse et al., 2001) in vivo within the biological system they evolved in (Morisset et al., 2003), let alone under laboratory circumstances (True 2008; Saitta et al., 2018b).
Pan et al. (2019) inadvertently illustrate the potential for raised antibodies to react with a variety of proteins in that the antiserum raised against modern feather extracts was able to react with more than one type of protein within the β-keratin family in modern tissues. This issue also appears to be present in their anti-pan cytokeratin antibodies used to search for epidermal α-keratin more broadly. Beyond these issues, all of the fossil samples showed very weak and patchy antibody binding under both immunofluorescence and immunogold labelling, far from an unambiguous indication that the carbonaceous residue of the fossil is formed primarily from ancient keratin protein matrix (compounded by figure 4 in the main text of Pan et al. [2019] lacking immunofluorescence overlay images under normal light microscopy, making a determination of the extent of fluorescence challenging). Assuming proper immunochemistry methodology (e.g., washing), there is no reason why such results could not be explained by antibody cross reactivity with non-proteinaceous organic geopolymers, such as diagenetically altered melanin or kerogen derived from in situ polymerization of feather waxes.
Although the sample size is small, the tendency for fossils to yield positive results for multiple types of antibodies might be an indication of the method’s propensity for false positives as opposed to evidence that these primitive structures contained unusual combinations of keratin proteins. Statistical analysis in which many different types of antibodies are applied to many different types of fossil tissues spanning multiple formations could help to identify any tendency for cross reactivity. It would be interesting to increase the number of antibodies from the several used in Pan et al. (2019) to a larger number with which more detailed statistical analysis can be applied. Different antibodies could be applied to various subsamples from a single specimen. Doing so could help determine if the staining patterns they ascribe to macroevolution are instead better explained as statistical noise of unpredicted antibody cross reactivity. Indeed, some fossil samples readily show positive staining with several different antibodies targeting different proteins (Lindgren et al., 2018), either an indication of exceptional and diverse protein preservation or a propensity for antibodies to cross react with organic geopolymers, calcium phosphate, or consolidants. This proposed immunological survey could be coupled with a detailed investigation into the nature of the binding between antibodies and fossil keratinous structures (e.g., the types of intermolecular bonds).
Revolutionizing Evolutionary Biology with Questionable Data?
Observations from the fossils inexplicable under the taphonomic model of Pan et al. (2019) are dismissed under nonspecific mechanisms such as “compression and degradation” (p. 2) or “various diagenetic influences” (p. 4). After roughly 20 years of such immunological reports of Mesozoic proteins, Pan et al. (2019) go the furthest in using such results to inform upon evolution (e.g., primitive feathers differed radically in keratin protein sequence and filament diameter from modern feathers) and paleobiology (e.g., the feathers of early birds were ill-suited for powered flight). Despite attempts to justify these conclusions within the context of evolutionary-development (i.e., evo-devo), they represent dramatic changes to our understanding of feather evolution that are potentially at odds with molecular divergence estimates of feather keratins (Greenwold and Sawyer, 2011, 2013) and also with other lines of evidence about the flight capabilities of early avians outside of neornithines (Feo et al., 2015). If such conclusions are based on faulty data (i.e., that the purported proteins are not actually preserved), then their effects on our understanding of paleobiology (e.g., biomechanics) and evolutionary biology can be of great concern.
Assessment: Pan et al. (2019)
We find it most probable that the fossils studied by Pan et al. (2019) show keratin protein loss and fossil melanin retention and that the antibody results are due to cross reactivity with substances such as stable organic geopolymers (e.g., fossil melanin or possibly kerogen) or consolidants from preparation rather than endogenous keratin polypeptides.
CONCLUSIONS: KERATINOUS STRUCTURE FOSSILIZATION AND SCIENTIFIC EPISTEMOLOGY IN RELATION TO MOLECULAR FOSSILS
So far, evidence for organic preservation over geological timescales has corroborated models proposed by researchers as far back as Abelson (1954) of protein and amino acid relative instability as well as the relative stability of a range of other organic molecules, as reviewed by Eglinton and Logan (1991). Under optimal conditions, embedded inside and stabilized by calcite crystal, a very short peptide sequence survived for ~3.8 million years (Demarchi et al., 2016). Over timescales covering most of the Phanerozoic, only a few isolated amino acids might preserve inside certain mineral matrices such as calcite (Abelson, 1954; Penkman et al., 2013). More stable molecules, such as polysaccharides like chitin, may reveal diagnostic residues in at least up to 25 Ma fossils (Stankiewicz et al., 1997), although occurrences of far older and diagenetically altered chitin (i.e., a highly nitrogenous and oxygenated organic geopolymer) have also been posited (Cody et al., 2011). Similarly, melanin pigment has been shown to survive with moieties of about 10% intact in Jurassic samples (Glass et al., 2012). Cholesterol may survive as saturated cholestanes in rocks dating back 700-800 Ma (Bobrovskiy et al., 2018), while unsaturated, intact cholesterols were detected in a Devonian fossil preserved within carbonate concretion (Melendez et al., 2013).
Keratinous Structure Preservation in Fossils
Our model of keratinous structure preservation (i.e., keratin protein breakdown and loss with largely pigment and/or calcium phosphate preservation) is not only supported experimentally, yielding results comparable to fossils (Saitta et al., 2017a, 2018a), but can also be explained mechanistically given our understanding of the thermal stability of the various components of keratinous tissues (Colleary et al., 2015; Saitta et al., 2018a), conforming to predications made from paleo-proteomics research (Wadsworth and Buckley, 2014). Although some mixed results have been reported in favor of the preservation of certain amino acids in fossil feathers within amber (McCoy et al., 2019), these would regardless be inconsistent with long polypeptide or protein sequence preservation.
Specifically, there appears to be two major modes of keratinous structure fossilization. 1) Preservation can be predominantly organic in anoxic, fine-grained sediments via melanosomes or amorphous melanin (with possibly an additional kerogen component deriving from in situ polymerization of labile lipids like waxes present on some keratinous structures such as feathers, potentially Fine-grained sediments can retainapparent in the occasional amorphous matrix observed alongside melanosomes on some fossils). Fine-grained sediments can retain diagenetically stable molecules due to smaller pore sizes, and anoxic conditions can limit microbial degradation or oxidative weathering of organic material. Clays, specifically, can also inhibit some bacterial decomposers (McMahon et al., 2016). Recent experiments (Slater et al., 2019) also suggest that melanin might be more susceptible to oxidative weathering prior to diagenetic alteration (see Roy et al., 2019 for descriptions of diagenetic alterations of pigments). 2) Preservation can also occur predominantly as calcium phosphate in coarse-grained, relatively oxic sediments, such as the feathers of Shuvuuia (Saitta et al., 2018b) or Miocene whale baleen (Gioncada et al. 2016). These open conditions and large pore sizes can allow for loss of organics through microbial degradation, aqueous hydrolysis and peptide fragmentation, or oxidative weathering. Questions still remain as to whether endogenous calcium phosphates are entirely responsible for this preservation, or if significant secondary calcium phosphate can precipitate onto endogenous calcium phosphate that acts as a nucleation site. Furthermore, the possibility of microbially mediated phosphatization of keratin protein has yet to be fully demonstrated (e.g., experimentally), although authigenic mineralization of soft tissues is a known preservation mechanism. As such, authigenic mineralization of keratin should be considered as a possibility, albeit not fully demonstrated, with endogenous calcium phosphate from in vivo calcification of keratinous structures being the main driver of phosphatic preservation under a more conservative hypothesis.
The idea that keratin protein has a higher preservation potential than melanosomes is contrary to several reports from various experimental and observational studies of melanin/melanosome stability through fossilization (Glass et al., 2012; Lindgren et al., 2012; Field et al., 2013; Colleary et al., 2015; Vinther et al., 2008; Gabbott et al., 2016; Vinther 2015, 2016; Saitta et al., 2017a, 2018a). The fact that macroscopic carbonaceous staining patterns in fossil integument can present as stripes (Zhang et al., 2010), spots (Li et al., 2010), and countershading (Vinther et al., 2016) is evidence that pigmentation is driving organic preservation of feathers and scales as opposed to protein because such gaps would be inconsistent with the idea that protein or the integumentary structures as a whole produce these stains. The lack of observed melanosomes in the coot preserved in the sinter in Schweitzer et al. (2018) could either be due to non-pigmented feathers (although the species has predominantly pigmented plumage), the fact that aqueous conditions during thermal alteration have been known to eliminate melanosome morphology (Colleary et al., 2015; Brown et al., 2017), or simply having been missed during inspection.
If Schweitzer et al. wish to provide an alternative, radical model of protein preservation, then a detailed and convincing mechanism for long-term polypeptide preservation through diagenesis must be presented beyond claiming that “the processes that result in this preservation are not completely known” (Schweitzer et al., 2018, p. 11). Schweitzer et al. (2018) propose three hypotheses for keratin protein stabilization: 1) macromolecular aggregation and hydrophobic interactions, 2) macromolecular crosslinking (e.g., with Fe), and 3) post-mortem mineral deposition onto the keratin surface.
However, 1) aggregation and hydrophobic interactions cannot completely halt hydrolysis, and any protected internal hydrophobic regions of proteins are predicted to eventually become exposed as proteins fragment and denature through diagenesis (see the globular protein predictions of Nielsen-Marsh [2002]). 2) Although crosslinking during the production of N-heterocyclic polymers does produce a thermally resistant residue (Wiemann et al., 2018) that cannot be said to represent surviving protein per se, proposed crosslinking with Fe to preserve amino acid sequences over geologic time has yet to be fully demonstrated (e.g., the purported Fe-mediated fossil bone collagen polypeptide evidence proposed by Boatman et al., 2019 relies on problematic microscopy, immunological, and FITR data [see Saitta et al., 2019 on FTIR considerations]), especially for proteins such as keratin. Given the conditions, Fe crosslinking might not be an explanation for surviving residues in the experimental feathers of Schweitzer et al. (2018) since no exogenous Fe was added in their treatments. Experiments on ostrich blood vessels exposed to Fe-bearing haemoglobin were reported to survive for over two years at room temperature conditions (Schweitzer et al., 2014). However, such durations are still about eight orders of magnitude younger than Mesozoic samples that additionally would have experienced some degree of geothermal heat during burial. Furthermore, hypoxia appeared to play a role in vessel preservation in those experiments (Schweitzer et al., 2014) such that redox conditions might be driving the observed preservation patterns as opposed to any molecular crosslinking of proteins by Fe. Molecular crosslinking of mussel foot protein 1 (mfp-1) via a coordination complex of Fe with three amino acid residues of 3,4-dihydroxyphenylalanine (dopa) in the granular cuticles of the byssal threads of marine mussels confers hardness and abrasion resistance to these structures (Harrington et al., 2010); but an influence on these mechanical properties does not necessitate that such Fe crosslinking additionally confers diagenetic stability. 3) The suggestion that keratin protein might be preferentially preserved in exceptional fossils due to stabilization from surficial mineral deposition onto the negatively charged protein does not consider that proteins other than keratin can carry a negative electric charge under certain pH conditions (Xia 2007). Therefore, it does not fully explain why other types of proteinaceous soft tissues do not preserve in fossils with more frequency than currently observed, in a manner similar to integumentary structures. Furthermore, if such a mechanism was responsible for preserving fossil protein, then one might predict negatively charged nucleic acids like DNA to persist deep into the geologic record as well, a notion which has been near universally rejected (Cooper and Poinar, 2000), although dubious reports have since appeared in the literature from a minority of researchers in rare instances (Schweitzer et al., 2013).
An interesting hypothesis might be that thermally stable, insoluble organic geopolymers (e.g., fossil melanin or N-heterocyclic polymers from protein and lipid/polysaccharide degradation and condensation) shield polypeptides from hydrolysis or other breakdown pathways and prevent them from leaching from the fossil. Indeed, amino acid residues can be found in tight association with such recalcitrant organics like kerogen (Mongenot et al., 2001; Riboulleau et al., 2003) or potentially in ancient eggshell (Crisp et al., 2013). However, even within closed systems, polypeptides fragment and only the more thermally stable amino acids tend to be detected (Crisp et al., 2013; Riboulleau et al., 2003); therefore it is unclear to us what thorough evidence exists that protein-derived material within such recalcitrant organics would persist as polypeptides, especially polypeptides long enough and with higher order folding preservation to elicit a genuine (i.e., specific) antibody response. Unless precautions are taken to expose such putative shielded polypeptides from an organic polymer matrix, which can involve treatments as extreme as thermochemolysis (Mongenot et al., 2001; Riboulleau et al., 2003), then how can antibody binding be interpreted other than cross reactivity to the surface of this matrix? Would such a treatment also degrade any polypeptides supposedly held within this organic matrix, and if so, how would one confirm their presence? Furthermore, in exposed, open systems such as bone (Saitta et al., 2019) and especially integument, how could an N-heterocyclic polymer and any putative protein-derived material within it be confidently attributed to endogenous biomolecules as opposed to microbes during decay or within the subsurface biome? If controversial evidence for Mesozoic keratin polypeptides were only to occur in the presence of, for example, fossil melanin, at what point would we suspend our belief in the presence of labile biomacromolecules alongside this fossil melanin and simply conclude the presence of fossil melanin on its own? While a negative feedback loop, whereby protein breakdown leads to the formation of a resilient, insoluble polymer that slows the breakdown of the remaining unaltered protein within it is an intriguing idea, one would have to demonstrate why such a process would occur in these fossils as opposed to hydrolytic fragmentation and loss of protein-derived material (e.g., as in collagen gelatinization [Pfretzschner, 2006]). With this in mind, we still would be somewhat skeptical of an explanation for supposed trace amounts of polypeptides, only detectable with highly sensitive and potentially low specificity methods, that depends on dramatic breakdown and condensation of the very substance claimed to be exceptionally preserved.
Currently, we posit that there is no unambiguous evidence for protein preservation (i.e., a sequence of amino acids bound together in a polypeptide chain) older than ~3.8 Ma (Demarchi et al., 2016). Fragmentary protein sequences with preferential loss of certain amino acids (e.g., hydrophilic or thermally unstable amino acids) would likely have lost quaternary and tertiary structure and would not be amenable for yielding a specific immunoreaction with an antibody. Furthermore, N-heterocyclic polymers, which may be derived in part from proteins, are so altered from their protein precursors that their presence and potential cross reactivity with antibodies do not provide evidence for preserved or stabilized proteins.
Scientific Epistemology in Relation to Molecular Fossils
When purported exceptionally preserved biomacromolecules become ever more difficult to detect with alternative analytical methods, we worry that the claim begins to take on the nature of Carl Sagan’s ‘invisible dragon’ (Sagan, 1995).
Contrary to the argument of Schweitzer et al. (2019), there is a difference between scientific replication and validation: “Saitta et al., did not attempt similar studies; thus they did not directly test the original hypothesis of preservation” (p. 7). Replication of a scientific protocol can support the idea that the steps of the protocol were carried out as intended. However, if the method itself is flawed in some circumstances (e.g., yielding false positives) or does not provide the proper evidence by which to evaluate a particular hypothesis, then replicating the method does nothing to further support the conclusion of a study. Instead, alternative methods can be used to provide independent lines of evidence in favor of (i.e., corroborating) or against (i.e., rejecting) a hypothesis; this is what was done by Saitta et al. (2018b) when re-examining Shuvuuia fibers as well as Saitta et al. (2019) when re-examining purported dinosaur bone collagen. For example, if immunochemistry is suspected to yield false positives for a sample, then simply repeating this method would be of limited use, if not uninformative. Furthermore, high sensitivity of a method can actually be an impediment when there is concern about a lack of specificity. If fossil feathers such as the fibers of Shuvuuia are suggested to preserve via keratin protein, then that might imply that the structure as a whole would be protein based rather than containing trace amounts of degraded peptides, and as such, it might not require highly sensitive analytical techniques to detect the corresponding chemical signatures; the fibers should have an obvious proteinaceous signature under a variety of methods if supposed keratin polypeptide preservation is producing the large-scale structure of the fossils.
The importance of validation, also referred to as triangulation, in addition to simple replication of scientific studies has recently come to the forefront. Munafò and Smith (2019) state:
These efforts [to replicate results] are laudable, but insufficient. If a study is skewed and replications recapitulate that approach, findings will be consistently incorrect or biased... replication alone will get us only so far. In some cases, routine replication might actually make matters worse. Consistent findings could take on the status of confirmed truths, when they actually reflect failings in study design, methods or analytical tools. We believe that an essential protection against flawed ideas is triangulation. This is the strategic use of multiple approaches to address one question. Each approach has its own unrelated assumptions, strengths and weaknesses. Results that agree across different methodologies are less likely to be artefacts (p. 399-401).
Methodological triangulation has been advocated for many years by researchers across disciplines, including the social sciences (e.g., Heesen et al., 2019). Even the famous sociologist and civil rights activist W.E.B. Du Bois championed the approached in the late nineteenth century (Du Bois, 1899).
Another potential fallacy worth noting, although not quite explicitly stated by researchers in support of Mesozoic proteins, would be a ‘holy grail’ mentality. As paleontologists, we often remain hopeful that taxa known only from minimal skeletal material might eventually yield a complete or even articulated skeleton upon additional field work. Such optimism is often warranted given that bones, consisting of apatite, are known to be thermally stable through diagenesis and capable of fossilization. However, ‘getting lucky’ might not always be an option for biomolecules or tissues that are inherently unstable through diagenesis, like proteins. Given that both polar ice caps are far younger than the Mesozoic (Barker and Thomas 2004) as well as our understanding of geothermal gradients and diagenetic histories of specific depositional basins (e.g., Hitchon 1984), we can constrain to some degree the temperatures these fossils would have been exposed to, leading to kinetic models that are not very encouraging of Mesozoic protein preservation (Nielsen-Marsh 2002). Any defense that invokes the idea that certain fossil specimens show unexpectedly high quality of protein preservation (e.g., Schweitzer et al., 2007, 2009) while fossils of similar geographic location and geologic age do not (e.g., Saitta et al., 2019) would be an illiberal position when access to the potentially exceptional samples is restricted or contingent upon collaboration (John R. Horner pers. email comm., 2015, 2016; Mary H. Schweitzer pers. email comm., 2015).
It is also worth discussing recent Mesozoic DNA claims, since these likely suffer from the same issue of faulty replication as do the claims of Mesozoic polypeptides. Recently, nuclear DNA has been suggested to preserve in dinosaur fossil bones based on propidium iodide (PI) staining (Schweitzer et al., 2013; Bailleul et al., 2019) despite the fact that PI cannot permeate the nuclear membrane and recent work has shown that PI staining can reveal abundant environmental (i.e., microbial) DNA contamination on ‘soft tissue’ structures from demineralized dinosaur fossils (Saitta et al., 2019). Contrasting the results shown by Bailleul et al. (2019) to those of Saitta et al. (2019) likely demonstrates that the instances in which the PI staining only appears on a small portion of an osteocyte-shaped structure are simply a select subset of instances in which PI can adhere more broadly across the whole structure. These recent claims also highlight an important issue in extreme claims of ancient biomolecule preservation - describing the PI stains as evidence for a “compound chemically consistent with DNA” (Bailleul et al. 2019, p. 22) comes across as obscurantism, conveying a sense of hesitancy (through the use of moderate language) to reach extreme conclusions. However, DNA is a molecule, so the implication in their statement is that a substance chemically consistent with DNA would be DNA, and therefore the claim is not moderate despite its language. Using faulty methods (e.g., PI to search for nuclear dinosaur DNA) to make vaguely worded claims can confuse researchers unfamiliar in molecular methods (e.g., many paleontologists), leading to false impressions about biomolecule preservation.
The belief that keratin polypeptides preserve in fossils continues to be a widespread belief in dinosaur paleontology influencing the interpretation of fossil data (e.g., Barbi et al., 2019). When unsupported taphonomic models are used as the lens by which to view the fossil record, erroneous conclusions can be drawn on matters of paleobiology or evolutionary biology. The utility of the fossil record as a data source for paleontology and related fields of science is dependent upon correct interpretation of such data, emphasizing the importance of taphonomic research.
Future Work
With respect to the question of keratin polypeptide preservation in fossils, particularly Mesozoic fossils, further work can still be done to help resolve some of the concerns we raise in this commentary. 1) The use of improved controls in immunological studies, particularly the inclusion of modern, experimental, and fossil tissues that are non-keratinous or non-proteinaceous, as well as consolidating organic polymers (e.g., cyanoacrylate or butvar). 2) Increasing the sample sizes of both applied antibodies and samples/controls in immunological studies in order to statistically examine the possibility of cross reactivity. 3) Considering the effects of pressure and compacted sediment matrix in thermal maturation experiments on organic preservation. 4) The open access of potentially key specimens so that independent research groups can attempt to validate (rather than simply replicate) immunological claims using additional lines of evidence derived from alternative analytical methods (e.g., amino acid analysis using high performance liquid chromatography or pyrolysis gas chromatography-mass spectrometry), each with their own benefits and drawbacks.
ACKNOWLEDGMENTS
We thank the two anonymous reviewers for their helpful comments.
REFERENCES
Aalberse, R.C., Akkerdaas, J., and Van Ree, R. 2001. Cross‐reactivity of IgE antibodies to allergens. Allergy, 56:478-490. https://doi.org/10.1034/j.1398-9995.2001.056006478.x
Abelson, P.H. 1954. Amino acids in fossils. Science, 119:576-576.
Allentoft, M.E., Collins, M., Harker, D., Haile, J., Oskam, C.L., Hale, M.L., Campos, P.F., Samaniego, J.A., Gilbert, M.T.P., Willerslev, E., and Zhang, G. 2012. The half-life of DNA in bone: measuring decay kinetics in 158 dated fossils. Proceedings of the Royal Society of London B: Biological Sciences, 279:4724-4733. https://doi.org/10.1098/rspb.2012.1745
Allpress, J.D., Mountain, G., and Gowland, P.C. 2002. Production, purification and characterization of an extracellular keratinase from Lysobacter NCIMB 9497. Letters in Applied Microbiology, 34:337-342. https://doi.org/10.1046/j.1472-765x.2002.01093.x
Armstrong, W.G., Halstead, L.B., Reed, F.B., and Wood, L. 1983. Fossil proteins in vertebrate calcified tissues. Philosophical Transactions of the Royal Society of London B, 301:301-343. https://doi.org/10.1098/rstb.1983.0026
Asara, J.M., Schweitzer, M.H., Freimark, L.M., Phillips, M., and Cantley, L.C. 2007. Protein sequences from mastodon and Tyrannosaurus rex revealed by mass spectrometry. Science, 316:280-285. https://doi.org/10.1126/science.1137614
Bada, J.L. 1998. Biogeochemistry of organic nitrogen compounds, p. 64-73. In Stankiewicz, B.A. and van Bergen, P.F. (eds.), Nitrogen-Containing Macromolecules in the Bio- and Geosphere. Oxford University Press, Oxford. https://doi.org/10.1021/bk-1998-0707.ch005
Baggott, D.G., Bunch, K.J., and Gill, K.R. 1988. Variations in some inorganic components and physical properties of claw keratin associated with claw disease in the British Friesian cow. British Veterinary Journal, 144:534-542. https://doi.org/10.1016/0007-1935(88)90023-1
Bailleul, A.M., O’Connor, J., and Schweitzer, M.H. 2019. Dinosaur paleohistology: review, trends and new avenues of investigation. PeerJ, 7:p.e7764. https://doi.org/10.7717/peerj.7764
Balaji, S., Kumar, M.S., Karthikeyan, R., Kumar, R., Kirubanandan, S., Sridhar, R., and Sehgal, P.K. 2008. Purification and characterization of an extracellular keratinase from a hornmeal-degrading Bacillus subtilis MTCC (9102). World Journal of Microbiology and Biotechnology, 24:2741-2745. https://doi.org/10.1007/s11274-008-9782-7
Barbi, M., Bell, P.R., Fanti, F., Dynes, J.J., Kolaceke, A., Buttigieg, J., Coulson, I.M., and Currie, P.J. 2019. Integumentary structure and composition in an exceptionally well-preserved hadrosaur (Dinosauria: Ornithischia). PeerJ, 7:p.e7875. https://doi.org/10.7717/peerj.7875
Barden, H.E., Bergmann, U., Edwards, N.P., Egerton, V.M., Manning, P.L., Perry, S., van Veelen, A., Wogelius, R.A., and van Dongen, B.E. 2015. Bacteria or melanosomes? A geochemical analysis of micro-bodies on a tadpole from the Oligocene Enspel Formation of Germany. Palaeobiodiversity and Palaeoenvironments, 95:33-45. https://doi.org/10.1007/s12549-014-0177-5
Barker, P.F. and Thomas, E. 2004. Origin, signature and palaeoclimatic influence of the Antarctic Circumpolar Current. Earth-Science Reviews, 66:143-162. https://doi.org/10.1016/j.earscirev.2003.10.003
Behar, F., Lewan, M.D., Lorant, F., and Vandenbroucke, M. 2003. Comparison of artificial maturation of lignite in hydrous and nonhydrous conditions. Organic Geochemistry, 34:575-600. https://doi.org/10.1016/s0146-6380(02)00241-3
Behar, F., Tang, Y., and Liu, J. 1997. Comparison of rate constants for some molecular tracers generated during artificial maturation of kerogens: influence of kerogen type. Organic Geochemistry, 26:281-287. https://doi.org/10.1016/s0146-6380(97)00003-x
Behar, F., Vandenbroucke, M., Teermann, S.C., Hatcher, P.G., Leblond, C., and Lerat, O. 1995. Experimental simulation of gas generation from coals and a marine kerogen. Chemical Geology, 126:247-260. https://doi.org/10.1016/0009-2541(95)00121-2
Benton, M.J., Zhonghe, Z., Orr, P.J., Fucheng, Z., and Kearns, S.L. 2008. The remarkable fossils from the Early Cretaceous Jehol Biota of China and how they have changed our knowledge of Mesozoic life: Presidential Address, delivered 2nd May 2008. Proceedings of the Geologists' Association, 119:209-228. https://doi.org/10.1016/s0016-7878(08)80302-1
Bergmann, U., Morton, R.W., Manning, P.L., Sellers, W.I., Farrar, S., Huntley, K.G., Wogelius, R.A., and Larson, P. 2010. Archaeopteryx feathers and bone chemistry fully revealed via synchrotron imaging. Proceedings of the National Academy of Sciences, 107:9060-9065. https://doi.org/10.1073/pnas.1001569107
Bern, M., Phinney, B.S., and Goldberg, D. 2009. Reanalysis of Tyrannosaurus rex mass spectra. Journal of Proteome Research, 8:4328–4332. https://doi.org/10.1021/pr900349r
Blakey, P.R., Earland, C., and Stell, J.G.P. 1963. Calcification of keratin. Nature, 198:481. https://doi.org/10.1038/198481a0
Blakey, P.R. and Lockwood, P. 1968. The environment of calcified components in keratins. Calcified Tissue Research, 2:361-369. https://doi.org/10.1007/bf02279224
Boatman, E.M., Goodwin, M.B., Holman, H.Y.N., Fakra, S., Zheng, W., Gronsky, R., and Schweitzer, M.H. 2019. Mechanisms of soft tissue and protein preservation in Tyrannosaurus rex. Scientific Reports, 9:1-12. https://doi.org/10.1038/s41598-019-51680-1
Bobrovskiy, I., Hope, J.M., Ivantsov, A., Nettersheim, B.J., Hallmann, C., and Brocks, J.J. 2018. Ancient steroids establish the Ediacaran fossil Dickinsonia as one of the earliest animals. Science, 361:1246-1249. https://doi.org/10.1126/science.aat7228
Boggs Jr., S. 2014. Principles of Sedimentology and Stratigraphy. Pearson Education, London.
Brandelli, A. and Riffel, A. 2005. Production of an extracellular keratinase from Chryseobacterium sp. growing on raw feathers. Electronic Journal of Biotechnology, 8:35-42.
Brasier, M., McLoughlin, N., Green, O., and Wacey, D. 2006. A fresh look at the fossil evidence for early Archaean cellular life. Philosophical Transactions of the Royal Society B: Biological Sciences, 361:887-902. https://doi.org/10.1098/rstb.2006.1835
Briggs, D.E.G., Kear, A.J., Martill, D.M., and Wilby, P.R. 1993. Phosphatization of soft-tissue in experiments and fossils. Journal of the Geological Society, 150:1035-1038. https://doi.org/10.1144/gsjgs.150.6.1035
Briggs, D.E. and McMahon, S. 2016. The role of experiments in investigating the taphonomy of exceptional preservation. Palaeontology, 59:1-11. https://doi.org/10.1111/pala.12219
Briggs, D.E. and Summons, R.E. 2014. Ancient biomolecules: their origins, fossilization, and role in revealing the history of life. BioEssays, 36:482-490. https://doi.org/10.1002/bies.201400010
Brown, C.M., Henderson, D.M., Vinther, J., Fletcher, I., Sistiaga, A., Herrera, J., and Summons, R.E. 2017. An exceptionally preserved three-dimensional armored dinosaur reveals insights into coloration and Cretaceous predator-prey dynamics. Current Biology, 27:2514-2521. https://doi.org/10.1016/j.cub.2017.06.071
Buckley, M., Walker, A., Ho, S.Y.W., Yang, Y., Smith, C., Ashton, P., Oates, J.T., Cappellini, E., Koon, H., Penkman, K., Elsworth, B., Ashford, D., Solazzo, O.C., Andrews, P., Strahler, J., Shapiro, B., Ostrom, P., Gandhi, H., Miller, W., Raney, B., Zylber, M.I., Gilbert, M.T.P., Prigodich, R.V., Ryan, M., Rijsdijk, K.F., Janoo, A., and Collins, M.J. 2008. Technical comment: protein sequences from Mastodon and Tyrannosaurus rex revealed by mass spectrometry. Science, 310:33c. https://doi.org/10.1126/science.1147046
Buckley, M., Warwood, S., van Dongen, B., Kitchener, A.C., and Manning, P.L. 2017 A fossil protein chimera; difficulties in discriminating dinosaur peptide sequences from modern cross-contamination. Proceedings of the Royal Society B, 284:20170544. https://doi.org/10.1098/rspb.2017.0544
Channing, A., Schweitzer, M.H., Horner, J.R., and McEneaney, T. 2005. A silicified bird from Quaternary hot spring deposits. Proceedings of the Royal Society of London B: Biological Sciences, 272:905-911. https://doi.org/10.1098/rspb.2004.2989
Cleland, T.P., Schroeter, E.R., Zamdborg, L., Zheng, W., Lee, J.E., Tran, J.C., Bern, M., Duncan, M.B., Lebleu, V.S., Ahlf, D.R., and Thomas, P.M. 2015. Mass spectrometry and antibody-based characterization of blood vessels from Brachylophosaurus canadensis. Journal of Proteome Research, 14:5252-5262. https://doi.org/10.1021/acs.jproteome.5b00675
Clements, T., Dolocan, A., Martin, P., Purnell, M.A., Vinther, J., and Gabbott, S.E. 2016. The eyes of Tullimonstrum reveal a vertebrate affinity. Nature, 532:500-503. https://doi.org/10.1038/nature17647
Cody, G.D., Gupta, N.S., Briggs, D.E., Kilcoyne, A.L.D., Summons, R.E., Kenig, F., Plotnick, R.E., and Scott, A.C. 2011. Molecular signature of chitin-protein complex in Paleozoic arthropods. Geology, 39:255-258. https://doi.org/10.1130/g31648.1
Colleary, C., Dolocan, A., Gardner, J., Singh, S., Wuttke, M., Rabenstein, R., Habersetzer, J., Schaal, S., Feseha, M., Clemens, M., and Jacobs, B.F. 2015. Chemical, experimental, and morphological evidence for diagenetically altered melanin in exceptionally preserved fossils. Proceedings of the National Academy of Sciences, 112:12592-12597. https://doi.org/10.1073/pnas.1509831112
Collins M. J., Walton, D., and King, A. 1998. The geochemical fate of proteins, p. 74-77. In Stankiewicz, B.A. and Van Bergen, P.F. (eds.), Nitrogen-Containing Macromolecules in the Bio- and Geosphere. Oxford University Press, Oxford. https://doi.org/10.1021/bk-1998-0707.ch006
Collins, M.J., Westbroek, P., Muyzer, G., and De Leeuw, J.W. 1992. Experimental evidence for condensation reactions between sugars and proteins in carbonate skeletons. Geochimica et Cosmochimica Acta, 56:1539-1544. https://doi.org/10.1016/0016-7037(92)90223-6
Conrad, K. 1958. Die beginnende Schizophrenie: Versuch einer Gestaltsanalyse des Wahns. Thieme, Stuttgart.
Coons, A.H., Creech, H.J., and Jones, R.N. 1941. Immunological properties of an antibody containing a fluorescent group. Proceedings of the Society for Experimental Biology and Medicine, 47:200-202. https://doi.org/10.3181/00379727-47-13084p
Cooper, A. and Poinar, H.N. 2000. Ancient DNA: do it right or not at all. Science, 289:1139-1139. https://doi.org/10.1126/science.289.5482.1139b
Crisp, M., Demarchi, B., Collins, M., Morgan-Williams, M., Pilgrim, E., and Penkman, K. 2013. Isolation of the intra-crystalline proteins and kinetic studies in Struthio camelus (ostrich) eggshell for amino acid geochronology. Quaternary Geochronology, 16:110-128. https://doi.org/10.1016/j.quageo.2012.09.002
Demarchi, B., Hall, S., Roncal-Herrero, T., Freeman, C.L., Woolley, J., Crisp, M.K., Wilson, J., Fotakis, A., Fischer, R., Kessler, B.M., and Jersie-Christensen, R.R. 2016. Protein sequences bound to mineral surfaces persist into deep time. Elife, 5:e17092. https://doi.org/10.7554/elife.17092
Despain, D.G. 1990. Yellowstone Vegetation: Consequences of Environment and History in a Natural Setting. Robert Rinehart, Boulder, Colorado.
Du Bois, W.E.B. 1899. The Philadelphia Negro: A Social Study. University of Pennsylvania Press, Philadelphia, Pennsylvania.
Dunn, M.S. and Brophy, T.W. 1932. Decomposition points of the amino acids. Journal of Biological Chemistry, 99:221-229.
Earland, C., Blakey, P.R., and Stell, J.G.P. 1962. Molecular orientation of some keratins. Nature, 196:1287. https://doi.org/10.1038/1961287a0
Eglinton, G. and Logan, G.A. 1991. Molecular preservation. Philosophical Transactions of the Royal Society of London B, 333:315-328. https://doi.org/10.1098/rstb.1991.0081
Epstein, A.G., Epstein, J.B., and Harris, L.D. 1977. Conodont color alteration: an index to organic metamorphism. U.S. Geological Survey Professional Paper, 995:1-27. https://doi.org/10.3133/pp995
Feo, T.J., Field, D.J., and Prum, R.O. 2015. Barb geometry of asymmetrical feathers reveals a transitional morphology in the evolution of avian flight. Proceedings of the Royal Society B, 282:20142864. https://doi.org/10.1098/rspb.2014.2864
Field, D.J., D’Alba, L., Vinther, J., Webb, S.M., Gearty, W., and Shawkey, M.D. 2013. Melanin concentration gradients in modern and fossil feathers. PLoS One, 8:e59451. https://doi.org/10.1371/journal.pone.0059451
Fraser, R.D.B., MacRae, T.P., Parry, D.A.D., and Suzuki, E. 1971. The structure of feather keratin. Polymer, 12:35-56. https://doi.org/10.1016/0032-3861(71)90011-5
Fraser, R.B. and Parry, D.A. 2014. Amino acid sequence homologies in the hard keratins of birds and reptiles, and their implications for molecular structure and physical properties. Journal of Structural Biology, 188:213-224. https://doi.org/10.1016/j.jsb.2014.10.012
Fu, M. X., Requena, J. R., Jenkins, A. J., Lyons, T. J., Baynes, J. W., and Thorpe, S. R. 1996. The advanced glycation end product, N-(carboxymethyl) lysine, is a product of both lipid peroxidation and glycoxidation reactions. Journal of Biological Chemistry, 271:9982-9986. https://doi.org/10.1074/jbc.271.17.9982
Gabbott, S.E., Donoghue, P.C., Sansom, R.S., Vinther, J., Dolocan, A., and Purnell, M.A. 2016. Pigmented anatomy in Carboniferous cyclostomes and the evolution of the vertebrate eye. Proceedings of the Royal Society B, 283:20161151. https://doi.org/10.1098/rspb.2016.1151
Gioncada, A., Collareta, A., Gariboldi, K., Lambert, O., Di Celma, C., Bonaccorsi, E., Urbina, M., and Bianucci, G. 2016. Inside baleen: exceptional microstructure preservation in a late Miocene whale skeleton from Peru. Geology, 44:839-842. https://doi.org/10.1130/g38216.1
Glass, K., Ito, S., Wilby, P.R., Sota, T., Nakamura, A., Bowers, C.R., Vinther, J., Dutta, S., Summons, R., Briggs, D.E., and Wakamatsu, K. 2012. Direct chemical evidence for eumelanin pigment from the Jurassic period. Proceedings of the National Academy of Sciences, 109:10218-10223. https://doi.org/10.1073/pnas.1118448109
Greenwold, M.J. and Sawyer, R.H. 2011. Linking the molecular evolution of avian beta (β) keratins to the evolution of feathers. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 316:609-616. https://doi.org/10.1002/jez.b.21436
Greenwold, M.J. and Sawyer, R.H. 2013. Molecular evolution and expression of archosaurian β‐keratins: Diversification and expansion of archosaurian β‐keratins and the origin of feather β‐keratins. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 320:393-405. https://doi.org/10.1002/jez.b.22514
Gupta, N.S., Cody, G.D., Tetlie, O.E., Briggs, D.E., and Summons, R.E. 2009. Rapid incorporation of lipids into macromolecules during experimental decay of invertebrates: Initiation of geopolymer formation. Organic Geochemistry, 40:589-594. https://doi.org/10.1016/j.orggeochem.2009.02.005
Harrington, M.J., Masic, A., Holten-Andersen, N., Waite, J.H., and Fratzl, P. 2010. Iron-clad fibers: a metal-based biological strategy for hard flexible coatings. Science, 328:216-220. https://doi.org/10.1126/science.1181044
Hedberg, Y.S., Killian, M.S., Blomberg, E., Virtanen, S., Schmuki, P., and Odnevall Wallinder, I. 2012. Interaction of bovine serum albumin and lysozyme with stainless steel studied by time-of-flight secondary ion mass spectrometry and X-ray photoelectron spectroscopy. Langmuir, 28:16306-16317. https://doi.org/10.1021/la3039279
Heesen, R., Bright, L.K., and Zucker, A. 2019. Vindicating methodological triangulation. Synthese, 196:3067-3081. https://doi.org/10.1007/s11229-016-1294-7
Herdianita, N.R., Browne, P.R.L., Rodgers, K.A., and Campbell, K.A. 2000. Mineralogical and textural changes accompanying ageing of silica sinter. Mineralium Deposita, 35:48-62. https://doi.org/10.1007/s001260050005
Hidalgo, F.J., Alaiz, M., and Zamora, R. 1999. Effect of pH and temperature on comparative nonenzymatic browning of proteins produced by oxidized lipids and carbohydrates. Journal of Agricultural and Food Chemistry, 47:742-747. https://doi.org/10.1021/jf980732z
Hieronymus, T.L., Witmer, L.M., and Ridgely, R.C. 2006. Structure of white rhinoceros (Ceratotherium simum) horn investigated by X-ray computed tomography and histology with implications for growth and external form. Journal of Morphology, 267:1172-1176. https://doi.org/10.1002/jmor.10465
Hitchon, B. 1984. Geothermal gradients, hydrodynamics, and hydrocarbon occurrences, Alberta, Canada. AAPG Bulletin, 68:713-743. https://doi.org/10.1306/ad461377-16f7-11d7-8645000102c1865d
Howell, N.R., Lavers, J.L., Uematsu, S., Paterson, D., Howard, D.L., Spiers, K., de Jonge, M.D., Hanley, T., Garrett, R., and Banati, R.B. 2017. The topobiology of chemical elements in seabird feathers. Scientific Reports, 7:1998. https://doi.org/10.1038/s41598-017-01878-y
Jacob, J. 1978. Uropygial gland secretions and feather waxes, p. 165-211. In Florkin, M., Sheer, B.T., and Brush, A.H., (eds.), Chemical Zoology X, Aves. Academic Press, New York, New York.
James, L.C. and Tawfik, D.S. 2003. The specificity of cross-reactivity: promiscuous antibody binding involves specific hydrogen bonds rather than nonspecific hydrophobic stickiness. Protein Science, 12:2183-2193. https://doi.org/10.1110/ps.03172703
Kaufmann, M.R. 1996. To live fast or not: growth, vigor and longevity of old-growth ponderosa pine and lodgepole pine trees. Tree Physiology, 16:139-144. https://doi.org/10.1093/treephys/16.1-2.139
Kaye, T.G., Gaugler, G., and Sawlowicz, Z. 2008. Dinosaurian soft tissues interpreted as bacterial biofilms. PLoS One, 3:e2808. https://doi.org/10.1371/journal.pone.0002808
Keller, B.O., Sui, J., Young, A.B., and Whittal, R.M. 2008. Interferences and contaminants encountered in modern mass spectrometry. Analytica Chimica Acta, 627:71-81. https://doi.org/10.1016/j.aca.2008.04.043
Kim, J.M., Lim, W.J., and Suh, H.J. 2001. Feather-degrading Bacillus species from poultry waste. Process Biochemistry, 37:287-291. https://doi.org/10.1016/s0032-9592(01)00206-0
Kiseleva, D., Shilovsky, O., Shagalov, E., Ryanskaya, A., Chervyakovskaya, M., Pankrushina, E., and Cherednichenko, N. 2019. Composition and structural features of two Permian parareptile (Deltavjatia vjatkensis, Kotelnich Site, Russia) bone fragments and their alteration during fossilisation. Palaeogeography, Palaeoclimatology, Palaeoecology, 526:28-42. https://doi.org/10.1016/j.palaeo.2019.04.015
Koopmans, M.P., De Leeuw, J.W., Lewan, M.D., and Damste, J.S.S. 1996. Impact of dia-and catagenesis on sulphur and oxygen sequestration of biomarkers as revealed by artificial maturation of an immature sedimentary rock. Organic Geochemistry, 25:391-426. https://doi.org/10.1016/s0146-6380(96)00144-1
Kriausakul, N. and Mitterer, R.M. 1978. Isoleucine epimerization in peptides and proteins: Kinetic factors and application to fossil proteins. Science, 201:1011-1014. https://doi.org/10.1126/science.201.4360.1011
Lambert, J.B. and Poinar, G.O. 2002. Amber: the organic gemstone. Accounts of Chemical Research, 35:628-636. https://doi.org/10.1021/ar0001970
Lang, E.W. 1986. Physical-chemical limits for the stability of biomolecules. Advances in Space Research, 6:251-255. https://doi.org/10.1016/0273-1177(86)90093-1
Lewan, M.D. 1983. Effects of thermal maturation on stable organic carbon isotopes as determined by hydrous pyrolysis of Woodford Shale. Geochimica et Cosmochimica Acta, 47:1471-1479. https://doi.org/10.1016/0016-7037(83)90306-x
Lewan, M.D., Bjorøy, M., and Dolcater, D.L. 1986. Effects of thermal maturation on steroid hydrocarbons as determined by hydrous pyrolysis of Phosphoria Retort Shale. Geochimica et Cosmochimica Acta, 50:1977-1987. https://doi.org/10.1016/0016-7037(86)90253-x
Li, Q., Gao, K.Q., Vinther, J., Shawkey, M.D., Clarke, J.A., D’alba, L., Meng, Q., Briggs, D.E., and Prum, R.O. 2010. Plumage color patterns of an extinct dinosaur. Science, 327:1369-1372. https://doi.org/10.1126/science.1186290
Lien, Y.C. and Nawar, W.W. 1974. Thermal decomposition of some amino acids. Valine, leucine and lsoleucine. Journal of Food Science, 39:911-913. https://doi.org/10.1111/j.1365-2621.1974.tb07274.x
Lin, X., Inglis, G.D., Yanke, L.J., and Cheng, K.J. 1999. Selection and characterization of feather-degrading bacteria from canola meal compost. Journal of Industrial Microbiology and Biotechnology, 23:149-153. https://doi.org/10.1038/sj.jim.2900706
Lindgren, J., Moyer, A., Schweitzer, M.H., Sjövall, P., Uvdal, P., Nilsson, D.E., Heimdal, J., Engdahl, A., Gren, J.A., Schultz, B.P., and Kear, B.P. 2015a. Interpreting melanin-based coloration through deep time: a critical review. Proceedings of the Royal Society B: Biological Sciences, 282:20150614. https://doi.org/10.1098/rspb.2015.0614
Lindgren, J., Sjövall, P., Carney, R.M., Cincotta, A., Uvdal, P., Hutcheson, S.W., Gustafsson, O., Lefèvre, U., Escuillié, F., Heimdal, J., and Engdahl, A. 2015b. Molecular composition and ultrastructure of Jurassic paravian feathers. Scientific Reports, 5:13520. https://doi.org/10.1038/srep13520
Lindgren, J., Sjövall, P., Thiel, V., Zheng, W., Ito, S., Wakamatsu, K., Hauff, R., Kear, B.P., Engdahl, A., Alwmark, C, and Eriksson, M.E. 2018. Soft-tissue evidence for homeothermy and crypsis in a Jurassic ichthyosaur. Nature, 564:359-365. https://doi.org/10.1038/s41586-018-0775-x
Lindgren, J., Uvdal, P., Sjövall, P., Nilsson, D.E., Engdahl, A., Schultz, B.P., and Thiel, V. 2012. Molecular preservation of the pigment melanin in fossil melanosomes. Nature Communications, 3:824. https://doi.org/10.1038/ncomms1819
Lowenstein, J.M. 1985. Molecular Approaches to the identification of species: immunological analysis of proteins from living or fossil organisms can be used to identify unrecognizable species and to obtain new information on taxonomic relationships and dates of evolutionary divergence. American Scientist, 73:541-547.
Lucas, A. M. and Stettenheim, P. R. 1972. Avian Anatomy: Integument, Part 2. US Department of Agriculture, Washington, D.C.
Maity, B., Sheff, D., and Fisher, R.A. 2013. Immunostaining: detection of signaling protein location in tissues, cells and subcellular compartments. Methods in Cell Biology, 113:81-105. https://doi.org/10.1016/b978-0-12-407239-8.00005-7
McCoy, V.E., Gabbott, S.E., Penkman, K., Collins, M.J., Presslee, S., Holt, J., Grossman, H., Wang, B., Kraemer, M.M.S., Delclòs, X., and Peñalver, E. 2019. Ancient amino acids from fossil feathers in amber. Scientific Reports, 9:6420. https://doi.org/10.1038/s41598-019-42938-9
McMahon, S., Anderson, R.P., Saupe, E.E., and Briggs, D.E. 2016. Experimental evidence that clay inhibits bacterial decomposers: implications for preservation of organic fossils. Geology, 44:867–870. https://doi.org/10.1130/G38454.1
McNamara, M.E., Briggs, D.E., Orr, P.J., Field, D.J., and Wang, Z., 2013b. Experimental maturation of feathers: implications for reconstructions of fossil feather colour. Biology Letters, 9:p.20130184. https://doi.org/10.1098/rsbl.2013.0184
McNamara, M.E., Briggs, D.E., Orr, P.J., Field, D.J., and Wang, Z. 2017. Correction to ‘Experimental maturation of feathers: implications for reconstructions of fossil feather colour’. Biology Letters, 13. https://doi.org/10.1098/rsbl.2017.0128
McNamara, M.E., Briggs, D.E., Orr, P.J., Gupta, N.S., Locatelli, E.R., Qiu, L., Yang, H., Wang, Z., Noh, H., and Cao, H. 2013a. The fossil record of insect color illuminated by maturation experiments. Geology, 41:487-490. https://doi.org/10.1130/g33836.1
McNamara, M.E., van Dongen, B.E., Lockyer, N.P., Bull, I.D., and Orr, P.J. 2016. Fossilization of melanosomes via sulfurization. Palaeontology, 59:337-350. https://doi.org/10.1111/pala.12238
Melendez, I., Grice, K., and Schwark, L. 2013. Exceptional preservation of Palaeozoic steroids in a diagenetic continuum. Scientific Reports, 3:2768. https://doi.org/10.1038/srep02768
Mitterer, R.M. 1993. The diagenesis of proteins and amino acids in fossil shells, p. 739-753. In Engel, M.H. and Macko, S.A. (eds.), Organic Geochemistry: Principles and Applications. Springer, Boston, Massachusetts. https://doi.org/10.1007/978-1-4615-2890-6_35
Mongenot, T., Riboulleau, A., Garcette-Lepecq, A., Derenne, S., Pouet, Y., Baudin, F., and Largeau, C. 2001. Occurrence of proteinaceous moieties in S-and O-rich late Tithonian kerogen (Kashpir oil shales, Russia). Organic Geochemistry, 32:199-203. https://doi.org/10.1016/s0146-6380(00)00154-6
Monthioux, M., Landais, P., and Monin, J.C. 1985. Comparison between natural and artificial maturation series of humic coals from the Mahakam delta, Indonesia. Organic Geochemistry, 8:275-292. https://doi.org/10.1016/0146-6380(85)90006-3
Morisset, M., Moneret-Vautrin, D.A., Kanny, G., Guenard, L., Beaudouin, E., Flabbee, J., and Hatahet, R. 2003. Thresholds of clinical reactivity to milk, egg, peanut and sesame in immunoglobulin E-dependent allergies: evaluation by double-blind or single-blind placebo-controlled oral challenges. Clinical and Experimental Allergy, 33:1046-1051. https://doi.org/10.1046/j.1365-2222.2003.01734.x
Moyer, A.E., Zheng, W., Johnson, E.A., Lamanna, M.C., Li, D.Q., Lacovara, K.J., and Schweitzer, M.H. 2014. Melanosomes or microbes: testing an alternative hypothesis for the origin of microbodies in fossil feathers. Scientific Reports, 4:4233. https://doi.org/10.1038/srep04233
Moyer, A.E., Zheng, W., and Schweitzer, M.H. 2016a. Keratin durability has implications for the fossil record: results from a 10 year feather degradation experiment. PloS One, 11:e0157699. https://doi.org/10.1371/journal.pone.0157699
Moyer, A.E., Zheng, W., and Schweitzer, M.H. 2016b. Microscopic and immunohistochemical analyses of the claw of the nesting dinosaur, Citipati osmolskae. Proceedings of the Royal Society B, 283:20161997. https://doi.org/10.1098/rspb.2016.1997
Munafò, M.R. and Smith, G.D. 2018. Robust research needs many lines of evidence. Nature, 553:339-401. https://doi.org/10.1038/d41586-018-01023-3
Nichols, G. 2009. Sedimentology and Stratigraphy. Wiley-Blackwell, Hoboken, New Jersey.
Nielsen-Marsh, C. 2002. Biomolecules in fossil remains-multidisciplinary approach to endurance. Biochemist, 24:12-14. https://doi.org/10.1042/bio02403012
Pamplona, R. 2011. Advanced lipoxidation end-products. Chemico-Biological Interactions, 192:14-20. https://doi.org/10.1016/j.cbi.2011.01.007
Pan, Y., Zheng, W., Moyer, A.E., O’Connor, J.K., Wang, M., Zheng, X., Wang, X., Schroeter, E.R., Zhou, Z., and Schweitzer, M.H. 2016. Molecular evidence of keratin and melanosomes in feathers of the Early Cretaceous bird Eoconfuciusornis. Proceedings of the National Academy of Sciences, 113:E7900-E7907. https://doi.org/10.1073/pnas.1617168113
Pan, Y., Zheng, W., Sawyer, R.H., Pennington, M.W., Zheng, X., Wang, X., Wang, M., Hu, L., O’Connor, J., Zhao, T., Li, Z., Schroeter, E.R., Wu, F., Xu, X., Zhou, Z., and Schweitzer, M.H. 2019. The molecular evolution of feathers with direct evidence from fossils. Proceedings of the National Academy of Sciences, 116:3018-3023. https://doi.org/10.1073/pnas.1815703116
Parry, L.A., Smithwick, F., Nordén, K.K., Saitta, E.T., Lozano-Fernandez, J., Tanner, A.R., Caron, J.B., Edgecombe, G.D., Briggs, D.E., and Vinther, J. 2018. Soft-bodied fossils are not simply rotten carcasses—toward a holistic understanding of exceptional fossil preservation. BioEssays, 40:1700167. https://doi.org/10.1002/bies.201700167
Pautard, F.G.E. 1963. Mineralization of keratin and its comparison with the enamel matrix. Nature, 199:531-535. https://doi.org/10.1038/199531a0
Pautard, F.G.E. 1964. Calcification of keratin. Progress in the Biological Sciences in Relation to Dermatology, 2:227-240.
Pautard, F.G.E. 1966. A biomolecular survey of calcification, p. 108-122. In Fleisch, H., Blackwood, H.J.J., and Owen, M. (eds.), Calcified Tissues 1965. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-85841-3_22
Pautard, F.G.E. 1970. The mineral phase of calcified cartilage, bone and baleen. Calcified Tissue Research, 4:34-36. https://doi.org/10.1007/bf02152340
Penkman, K.E., Preece, R.C., Bridgland, D.R., Keen, D.H., Meijer, T., Parfitt, S.A., White, T. S., and Collins, M.J. 2013. An aminostratigraphy for the British Quaternary based on Bithynia opercula. Quaternary Science Reviews, 61:111-134. https://doi.org/10.1016/j.quascirev.2012.10.046
Peters, K.E., Rohrback, B.G., and Kaplan, I.R. 1981. Carbon and hydrogen stable isotope variations in kerogen during laboratory-simulated thermal maturation. AAPG Bulletin, 65:501-508. https://doi.org/10.1306/2f9197fb-16ce-11d7-8645000102c1865d
Pevzner, P.A., Kim, S., and NG, J. 2008. Technical comment: protein sequences from Mastodon and Tyrannosaurus rex revealed by mass spectrometry. Science, 321:1040b. https://doi.org/10.1126/science.1155006
Pfretzschner, H.U. 2006. Collagen gelatinization: the key to understand early bone-diagenesis. Palaeontographica Abteilung A, 278:135-148. https://doi.org/10.1127/pala/278/2006/135
Pinheiro, F.L., Horn, B.L., Schultz, C.L., de Andrade, J.A., and Sucerquia, P.A. 2012. Fossilized bacteria in a Cretaceous pterosaur headcrest. Lethaia, 45:495-499. https://doi.org/10.1111/j.1502-3931.2012.00309.x
Poole, P.H., Sciortino, F., Essmann, U., and Stanley, H.E. 1992. Phase behaviour of metastable water. Nature, 360:324-328. https://doi.org/10.1038/360324a0
Ramos-Vara, J.A. 2005. Technical aspects of immunohistochemistry. Veterinary Pathology, 42:405-426. https://doi.org/10.1354/vp.42-4-405
Rhead, M.M., Eglinton, G., and Draffan, G.H. 1971. Hydrocarbons produced by the thermal alteration of cholesterol under conditions simulating the maturation of sediments. Chemical Geology, 8:277-297. https://doi.org/10.1016/0009-2541(71)90022-2
Riboulleau, A., Mongenot, T., Baudin, F., Derenne, S., and Largeau, C. 2002. Factors controlling the survival of proteinaceous material in Late Tithonian kerogens (Kashpir Oil Shales, Russia). Organic Geochemistry, 33:1127-1130. https://doi.org/10.1016/s0146-6380(02)00081-5
Robinson, A.B. and Rudd, C.J. 1974. Deamidation of glutaminyl and asparaginyl residues in peptides and proteins. Current Topics in Cellular Regulation, 8:247-295. https://doi.org/10.1016/b978-0-12-152808-9.50013-4
Roll-Hansen, N. 1979. Experimental method and spontaneous generation: the controversy between Pasteur and Pouchet, 1859-64. Journal of the History of Medicine and Allied Sciences, 34:273-292. https://doi.org/10.1093/jhmas/xxxiv.3.273
Roy, A., Pittman, M., Saitta, E.T., Kaye, T.G., and Xu, X., 2019. Recent advances in amniote palaeocolour reconstruction and a framework for future research. Biological Reviews. https://doi.org/10.1111/brv.12552
Rullkötter, J. and Marzi, R. 1988. Natural and artificial maturation of biological markers in a Toarcian shale from northern Germany. Organic Geochemistry, 13:639-645. https://doi.org/10.1016/0146-6380(88)90084-8
Rybczynski, N., Gosse, J.C., Harington, C.R., Wogelius, R.A., Hidy, A.J., and Buckley, M. 2013. Mid-Pliocene warm-period deposits in the High Arctic yield insight into camel evolution. Nature Communications, 4:1550. https://doi.org/10.1038/ncomms2516
Sagan, C. 1995. The Demon-Haunted World: Science as a Candle in the Dark. Ballantine Books, New York, New York.
Saitta, E.T., Fletcher, I., Martin, P., Pittman, M., Kaye, T.G., True, L.D., Norell, M.A., Abbott, G.D., Summons, R.E., Penkman, K., and Vinther, J. 2018b. Preservation of feather fibers from the Late Cretaceous dinosaur Shuvuuia deserti raises concern about immunohistochemical analyses on fossils. Organic Geochemistry, 125:142-151. https://doi.org/10.1016/j.orggeochem.2018.09.008
Saitta, E.T., Kaye, T.G., and Vinther, J. 2018a. Sediment‐encased maturation: a novel method for simulating diagenesis in organic fossil preservation. Palaeontology, 62:135-150. https://doi.org/10.1111/pala.12386
Saitta, E.T., Liang, R., Lau, M.C., Brown, C.M., Longrich, N.R., Kaye, T.G., Novak, B.J., Salzberg, S.L., Norell, M.A., Abbott, G.D., and Dickinson, M.R. 2019. Cretaceous dinosaur bone contains recent organic material and provides an environment conducive to microbial communities. eLife, 8:e46205. https://doi.org/10.7554/elife.46205
Saitta, E.T., Rogers, C., Brooker, R.A., Abbott, G.D., Kumar, S., O’Reilly, S.S., Donohoe, P., Dutta, S., Summons, R.E., and Vinther, J. 2017a. Low fossilization potential of keratin protein revealed by experimental taphonomy. Palaeontology, 60:547-556. https://doi.org/10.1111/pala.12299
Saitta, E.T., Rogers, C.S., Brooker, R.A., and Vinther, J. 2017b. Experimental taphonomy of keratin: a structural analysis of early taphonomic changes. Palaios, 32:647-657. https://doi.org/10.2110/palo.2017.051
Salonius, P.O., Robinson, J.B., and Chase, F.E. 1967. A comparison of autoclaved and gamma-irradiated soils as media for microbial colonization experiments. Plant and Soil, 27:239-248. https://doi.org/10.1007/bf01373392
Sangali, S. and Brandelli, A. 2000. Feather keratin hydrolysis by a Vibrio sp. strain kr2. Journal of Applied Microbiology, 89:735-743. https://doi.org/10.1046/j.1365-2672.2000.01173.x
Schimmelmann, A., Sessions, A.L., and Mastalerz, M. 2006. Hydrogen isotopic (D/H) composition of organic matter during diagenesis and thermal maturation. Annual Review of Earth and Planetary Sciences, 34:501-533. https://doi.org/10.1146/annurev.earth.34.031405.125011
Schroeter, E.R., DeHart, C.J., Cleland, T.P., Zheng, W., Thomas, P.M., Kelleher, N.L., Bern, M., and Schweitzer, M.H. 2017. Expansion for the Brachylophosaurus canadensis collagen I sequence and additional evidence of the preservation of cretaceous protein. Journal of Proteome Research, 16:920-932. https://doi.org/10.1021/acs.jproteome.6b00873
Schweitzer, M.H., Lindgren, J., and Moyer, A.E. 2015. Melanosomes and ancient coloration re-examined: A response to Vinther 2015 (DOI 10.1002/bies. 201500018). BioEssays, 37:1174-1183. https://doi.org/10.1002/bies.201500061
Schweitzer, M.H., Schroeter, E.R., Cleland, T.P., and Zheng, W. 2019. Paleoproteomics of Mesozoic dinosaurs and other Mesozoic fossils. Proteomics, 19:1800251. https://doi.org/10.1002/pmic.201800251
Schweitzer, M.H., Suo, Z., Avci, R., Asara, J.M., Allen, M.A., Arce, F.T., and Horner, J.R. 2007. Analyses of soft tissue from Tyrannosaurus rex suggest the presence of protein. Science, 316:277-280. https://doi.org/10.1126/science.1138709
Schweitzer, M.H., Watt, J.A., Avci, R., Forster, C.A., Krause, D.W., Knapp, L., Rogers, R.R., Beech, I., and Marshall, M. 1999a. Keratin immunoreactivity in the Late Cretaceous bird Rahonavis ostromi. Journal of Vertebrate Paleontology, 19:712-722. https://doi.org/10.1080/02724634.1999.10011183
Schweitzer, M.H., Watt, J.A., Avci, R., Knapp, L., Chiappe, L., Norell, M., and Marshall, M. 1999b. Beta-keratin specific immunological reactivity in feather-like structures of the Cretaceous Alvarezsaurid, Shuvuuia deserti. Journal of Experimental Zoology, 285:146-157. https://doi.org/10.1002/(sici)1097-010x(19990815)285:2%3C146::aid-jez7%3E3.3.co;2-1
Schweitzer, M.H., Zheng, W., Cleland, T.P., and Bern, M. 2013. Molecular analyses of dinosaur osteocytes support the presence of endogenous molecules. Bone, 52:414-423. https://doi.org/10.1016/j.bone.2012.10.010
Schweitzer, M.H., Zheng, W., Cleland, T.P., Goodwin, M.B., Boatman, E., Theil, E., Marcus, M.A., and Fakra, S.C. 2014. A role for iron and oxygen chemistry in preserving soft tissues, cells and molecules from deep time. Proceedings of the Royal Society B: Biological Sciences, 281:20132741. https://doi.org/10.1098/rspb.2013.2741
Schweitzer, M.H., Zheng, W., Moyer, A.E., Sjövall, P., and Lindgren, J. 2018. Preservation potential of keratin in deep time. PloS one, 13:e0206569. https://doi.org/10.1371/journal.pone.0206569
Schweitzer, M.H., Zheng, W., Organ, C.L., Avci, R., Suo, Z., Freimark, L.M., Lebleu, V.S., Duncan, M.B., Vander Heiden, M.G., Neveu, J.M., and Lane, W.S. 2009. Biomolecular characterization and protein sequences of the Campanian hadrosaur B. canadensis. Science, 324:626-631. https://doi.org/10.1126/science.1165069
Sedivy, R. and Battistutti, W.B. 2003. Nanobacteria promote crystallization of psammoma bodies in ovarian cancer. Apmis, 111:951-954. https://doi.org/10.1034/j.1600-0463.2003.1111006.x
Singh, R., Barden, A., Mori, T., and Beilin, L. 2001. Advanced glycation end-products: a review. Diabetologia, 44:129-146. https://doi.org/10.1007/s001250051591
Slater, T.S., McNamara, M.E., Orr, P.J., Foley, T.B., Ito, S., and Wakamatsu, K. 2019. Taphonomic experiments resolve controls on the preservation of melanosomes and keratinous tissues in feathers. Palaeontology. https://doi.org/10.1111/pala.12445
Stankiewicz, B.A., Briggs, D.E., Evershed, R.P., Flannery, M.B., and Wuttke, M. 1997. Preservation of chitin in 25-million-year-old fossils. Science, 276:1541-1543. https://doi.org/10.1126/science.276.5318.1541
Stankiewicz, B.A., Briggs, D.E.G., Michels, R., Collinson, M.E., Flannery, M.B., and Evershed, R.P. 2000. Alternative origin of aliphatic polymer in kerogen. Geology, 28:559-562. https://doi.org/10.1130/0091-7613(2000)28%3C559:aooapi%3E2.0.co;2
Strasser, B., Mlitz, V., Hermann, M., Tschachler, E., and Eckhart, L. 2015. Convergent evolution of cysteine-rich proteins in feathers and hair. BMC Evolutionary Biology, 15:82. https://doi.org/10.1186/s12862-015-0360-y
Szewciw, L.J., De Kerckhove, D.G., Grime, G.W., and Fudge, D.S. 2010. Calcification provides mechanical reinforcement to whale baleen α-keratin. Proceedings of the Royal Society of London B: Biological Sciences, 277:2597-2605. https://doi.org/10.1098/rspb.2010.0399
Tanaka, G., Zhou, B., Zhang, Y., Siveter, D.J., and Parker, A.R. 2017. Rods and cones in an enantiornithine bird eye from the Early Cretaceous Jehol Biota. Heliyon, 3:e00479. https://doi.org/10.1016/j.heliyon.2017.e00479
Teerman, S.C. and Hwang, R.J. 1991. Evaluation of the liquid hydrocarbon potential of coal by artificial maturation techniques. Organic Geochemistry, 17:749-764. https://doi.org/10.1016/0146-6380(91)90019-g
True, L.D. 2008. Quality control in molecular immunohistochemistry. Histochemistry and Cell Biology, 130:473-480. https://doi.org/10.1007/s00418-008-0481-0
Ullmann, P.V., Pandya, S.H., and Nellermoe, R. 2019. Patterns of soft tissue and cellular preservation in relation to fossil bone tissue structure and overburden depth at the Standing Rock Hadrosaur Site, Maastrichtian Hell Creek Formation, South Dakota, USA. Cretaceous Research, 99:1-13. https://doi.org/10.1016/j.cretres.2019.02.012
Versteegh, G.J., Blokker, P., Wood, G.D., Collinson, M.E., Damsté, J.S.S., and de Leeuw, J.W. 2004. An example of oxidative polymerization of unsaturated fatty acids as a preservation pathway for dinoflagellate organic matter. Organic Geochemistry, 35:1129-1139. https://doi.org/10.1016/j.orggeochem.2004.06.012
Vinther, J. 2015. A guide to the field of palaeo colour. BioEssays, 37:643-656. https://doi.org/10.1002/bies.201500018
Vinther, J. 2016. Fossil melanosomes or bacteria? A wealth of findings favours melanosomes. BioEssays, 38:220-225. https://doi.org/10.1002/bies.201500168
Vinther, J., Briggs, D.E., Prum, R.O., and Saranathan, V. 2008. The colour of fossil feathers. Biology Letters, 4:522-525. https://doi.org/10.1098/rsbl.2008.0302
Vinther, J., Nicholls, R., Lautenschlager, S., Pittman, M., Kaye, T.G., Rayfield, E., Mayr, G., and Cuthill, I.C. 2016. 3D camouflage in an ornithischian dinosaur. Current Biology, 26:2456-2462. https://doi.org/10.1016/j.cub.2016.06.065
Vistoli, G., De Maddis, D., Cipak, A., Zarkovic, N., Carini, M., and Aldini, G. 2013. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radical Research, 47:3-27. https://doi.org/10.3109/10715762.2013.815348
Wadsworth, C. and Buckley, M. 2014. Proteome degradation in fossils: investigating the longevity of protein survival in ancient bone. Rapid Communications in Mass Spectrometry, 28:605-615. https://doi.org/10.1002/rcm.6821
Wagner, M.S. and Castner, D.G. 2001. Characterization of adsorbed protein films by time-of-flight secondary ion mass spectrometry with principal component analysis. Langmuir, 17:4649-4660. https://doi.org/10.1021/la001209t
Weiss, I.M., Muth, C., Drumm, R., and Kirchner, H.O. 2018. Thermal decomposition of the amino acids glycine, cysteine, aspartic acid, asparagine, glutamic acid, glutamine, arginine and histidine. BMC Biophysics, 11:2. https://doi.org/10.1186/s13628-018-0042-4
Wiemann, J., Fabbri, M., Yang, T.R., Stein, K., Sander, P.M., Norell, M.A., and Briggs, D.E. 2018. Fossilization transforms vertebrate hard tissue proteins into N-heterocyclic polymers. Nature Communications, 9:4741. https://doi.org/10.1038/s41467-018-07013-3
Wogelius, R.A., Manning, P.L., Barden, H.E., Edwards, N.P., Webb, S.M., Sellers, W.I., Taylor, K.G., Larson, P.L., Dodson, P., You, H., and Da-Qing, L. 2011. Trace metals as biomarkers for eumelanin pigment in the fossil record. Science, 333:1622-1626. https://doi.org/10.1126/science.1205748
Xia, X. 2007. Bioinformatics and the Cell: Modern Computational Approaches in Genomics, Proteomics and Transcriptomics. Springer, New York, New York. https://doi.org/10.1007/978-0-387-71337-3
Yamamura, S., Morita, Y., Hasan, Q., Rao, S.R., Murakami, Y., Yokoyama, K., and Tamiya, E. 2002. Characterization of a new keratin-degrading bacterium isolated from deer fur. Journal of Bioscience and Bioengineering, 93:595-600. https://doi.org/10.1016/s1389-1723(02)80243-2
Zhang, F., Kearns, S.L., Orr, P.J., Benton, M.J., Zhou, Z., Johnson, D., Xu, X., and Wang, X. 2010. Fossilized melanosomes and the colour of Cretaceous dinosaurs and birds. Nature, 463:1075-1078. https://doi.org/10.1038/nature08740

