FIGURE 1. Sketched and simplified illustration of the life cycle of epicarideans. 1.1: Planktic epicaridium larva. 1.2: Microniscium larva feeding on a copepod (intermediate host). 1.3: Planktic cryptoniscium larva. 1.4: Adult feeding on a crustacean final host.

FIGURE 2. Confronting phylogenetic hypotheses in Epicaridea. Dashed lines represent supported monophyletic groups. 2.1: Molecular phylogeny from Boyko et al. (2013). 2.2: Phylogeny based on putative apomorphic morphological characters from Wägele (1989).
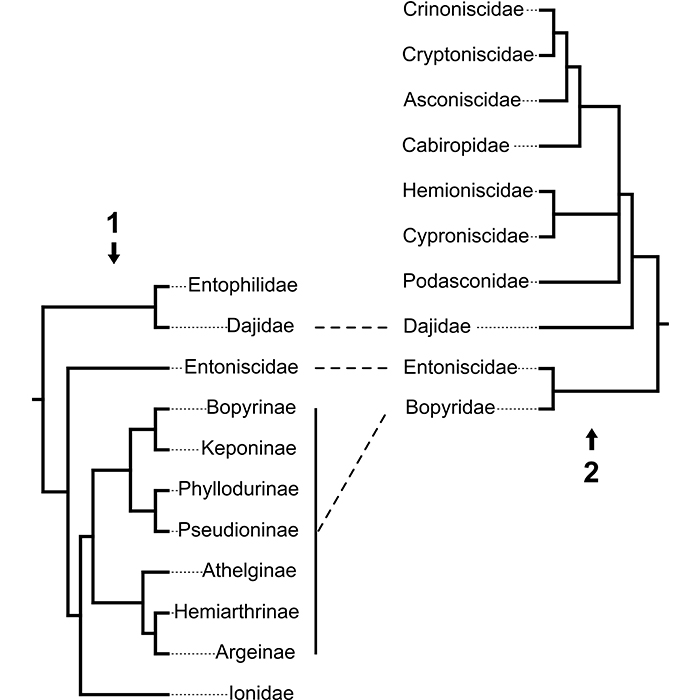
FIGURE 3. Illustration of possible entombment conditions suggested for Vendean amber: cryptoniscium larvae living in an aquatic environment close to the resin producing tree and getting trapped by making contact with submerged liquid resin.
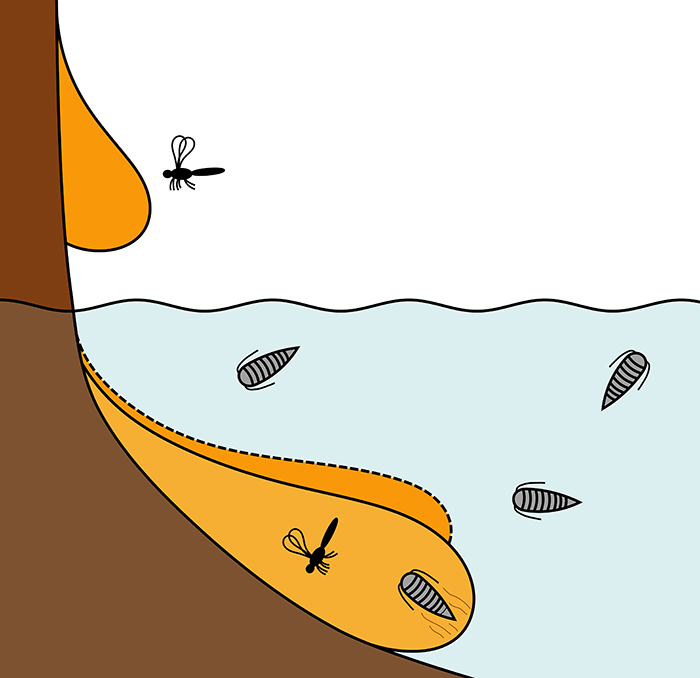
FIGURE 4. Map of France (4.1) and a detailed map of the Vendée department (reddish) (4.2). The fossil site is marked by a star. 4.3: Photograph of a piece of Vendean amber - notice the layered build-up of the resin.
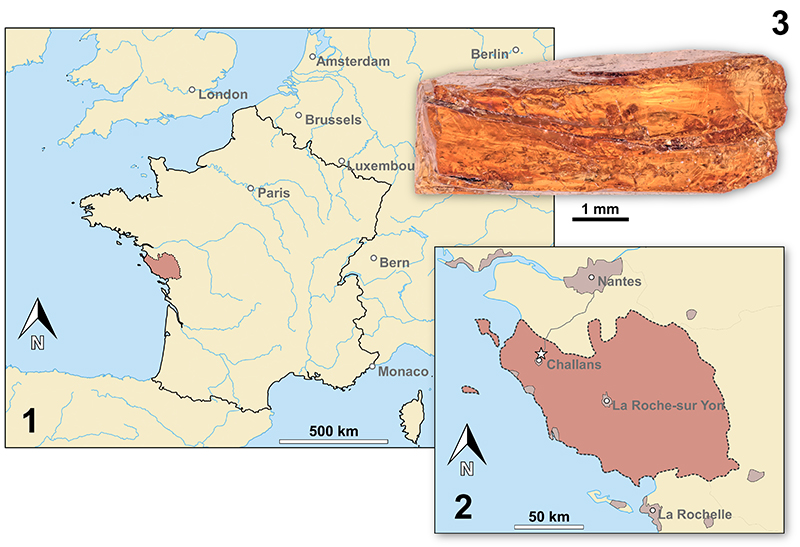
FIGURE 5. Vacuotheca dupeorum sp. nov., comparative overview of the type material sorted by collection number (same scale). 5.1: Paratype IGR.GAR-8.1-1, lateral view, epifluorescence. 5.2: Paratype IGR.GAR-8.1-2, latero-ventral view, reflected light. 5.3-5.6: Paratype IGR.GAR-8.2, lateral view (5.3-5.5) and lateral view of the opposite side (5.6), epifluorescence (5.3, 5.6), reflected light (5.4) and 3D red-cyan anaglyph of reflected light micrograph (5.5). 5.7-5.8: Holotype IGR.GAR-28, ventro-lateral view (5.7) and dorsal view (5.8), epifluorescence.
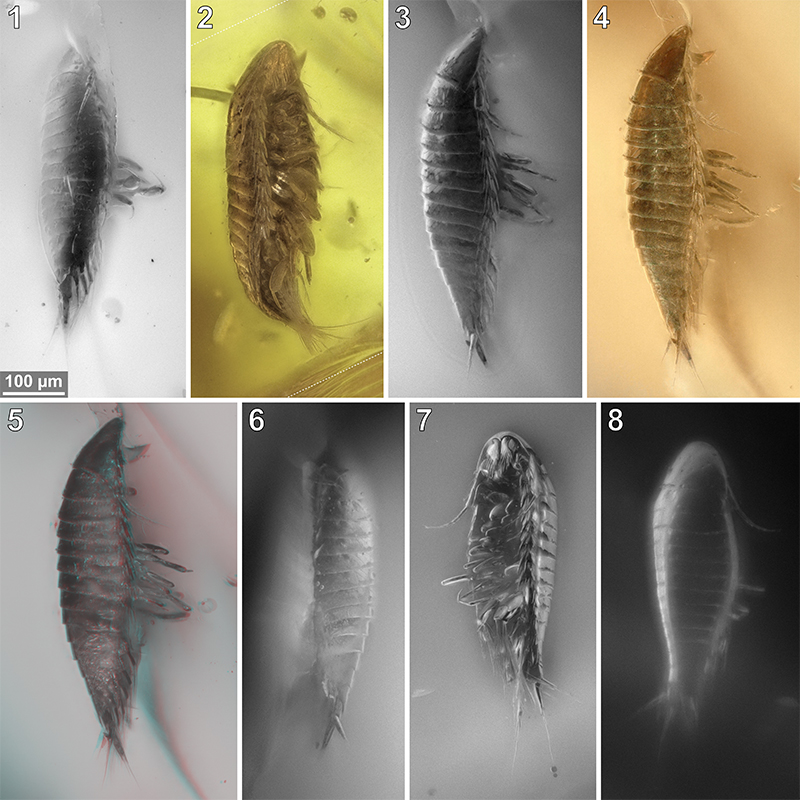
FIGURE 6. Vacuotheca dupeorum sp. nov., comparative overview of the type material sorted by collection number (same scale). 6.1-6.2: Paratype IGR.GAR-41-1, dorsal view, epifluorescence (6.1) and reflected light (6.2). 6.3: Paratype IGR.GAR-41-2, dorsal view, epifluorescence. 6.4-6.5: Paratype IGR.GAR-48, ventrolateral view (6.4) and dorsal view (6.5). 6.6-6.7: Paratype IGR.GAR-51, located at the surface of the amber piece and cracked in roughly frontal plane, ventral view of the dorsal surface (6.6) and dorsal view (6.7), reflected light (6.6) and epifluorescence (6.7). 6.8: Paratype IGR.GAR-53-1, ventral view, epifluorescence.
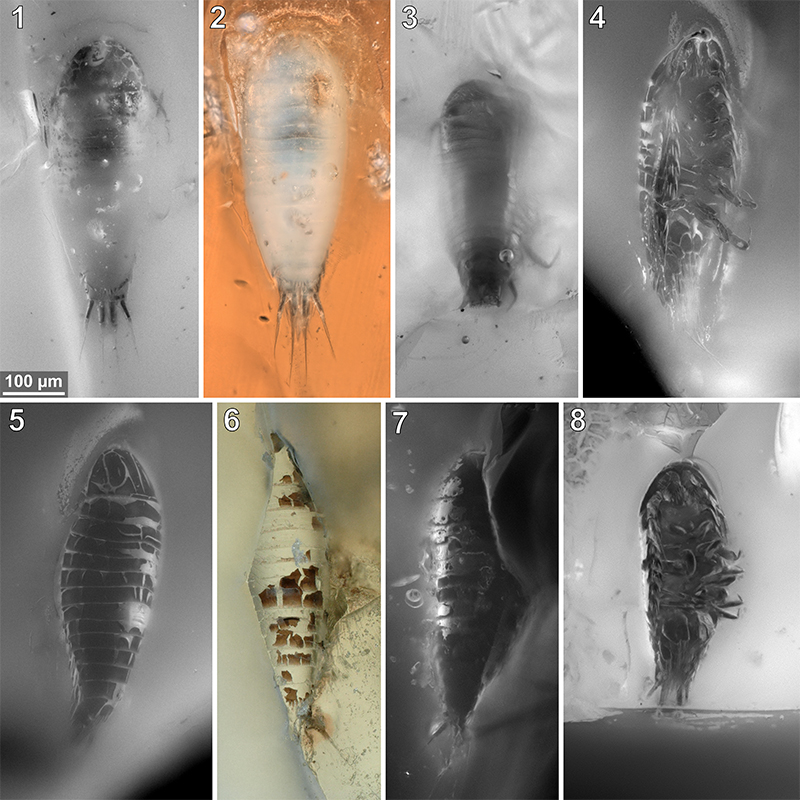
FIGURE 7. Vacuotheca dupeorum sp. nov., comparative overview of the type material sorted by collection number (same scale). 7.1-7.3: Paratype IGR.GAR-53-2, dorsal view (7.1, 7.2) and ventral view (7.3), reflected light with (7.1) and without (7.2) polarising filter and epifluorescence (7.3). 7.4-7.6: Paratype IGR.GAR-64, dorso-lateral view (7.4) and ventro-lateral view (7.5, 7.6), epifluorescence (7.4, 7.6) and transmitted light (7.5). 7.7: Paratype IGR.GAR-65, lateral view, epifluorescence. 7.8: Paratype IGR.GAR-89, ventro-lateral view, epifluorescence. Dashed lines mark areas with artificially created background.

FIGURE 8. Vacuotheca dupeorum sp. nov., comparative overview of the type material sorted by collection number (same scale). 8.1: Paratype IGR.GAR-89, ventro-lateral view, reflected light; pr7, pereopod 7. 8.2: Paratype IGR.GAR-90, located at the surface of the amber piece and cracked in roughly frontal plane, ventral view of the dorsal surface, 3D red-cyan anaglyph of reflected light micrographs. 8.3: Paratype IGR.GAR-92, lateral view, epifluorescence. 8.4-8.6: dorsal view (8.4) and ventral view (8.5, 8.6), epifluorescence (8.4, 8.5) and transmitted light (8.6). 8.7: Paratype IGR.GAR-94, lateral view, epifluorescence.

FIGURE 9. Vacuotheca dupeorum sp. nov., comparative overview of the type material sorted by collection number (same scale). 9.1-9.3: Paratype IGR.GAR-95-1, ventral view, reflected light (9.1), transmitted light (9.2) and epifluorescence (9.3). 9.4: Paratype IGR.GAR-95-2, lateral view, epifluorescence. 9.5: Paratype IGR.GAR-97, ventral view, epifluorescence. 9.6-9.7: Paratype IGR.GAR-98, dorsal view, epifluorescence (9.6) and reflected light (9.7). Dashed lines mark areas with artificially created background.

FIGURE 10. Vacuotheca dupeorum sp. nov., reconstructions and drawings. 10.1: Reconstruction in dorsal view (based on multiple specimens) including the striation pattern (based on paratype IGR.GAR-93). 10.2: Reconstruction based on paratype IGR.GAR-95-1 and holotype IGR.GAR-28, head shield in ventral view, numbers refer to the elements of antennula (numbers on the left side) and antenna (numbers on the right side). 10.3: Drawing of the paratype IGR.GAR-41-1, uropod region in dorsal view. bs, basipod of the uropod; en, endopod of the uropod; ex, exopod of the uropod; pl5, pleon segment 5; pt, pleotelson (pleon segment 6 and telson). Drawing of the holotype IGR.GAR-28, coxal plates in ventro-lateral view, mirrored. per3 - per7, pereon segments 3-7; pl1, pleon segment 1.
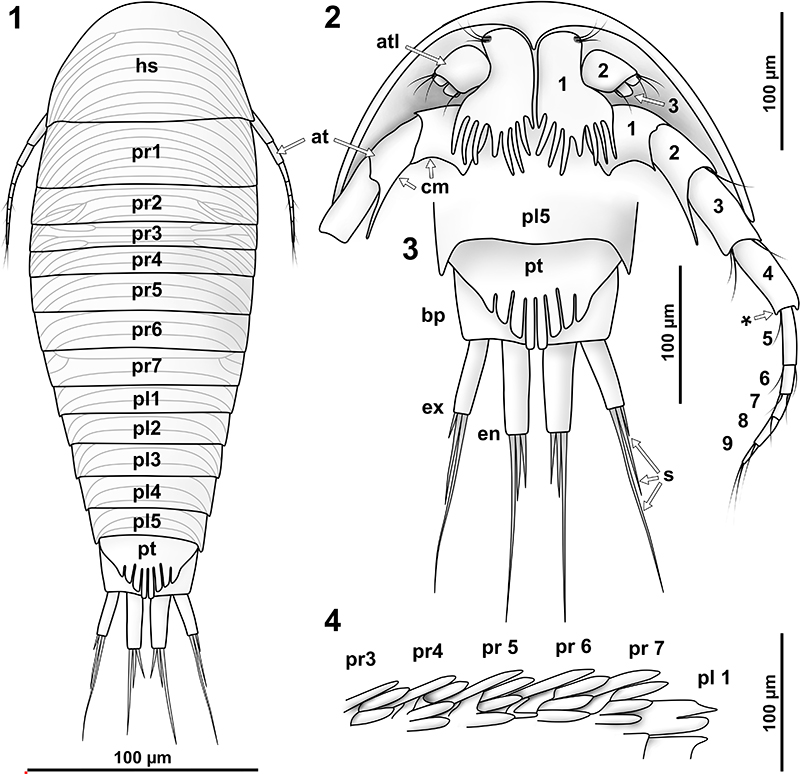
FIGURE 11. Vacuotheca dupeorum sp. nov., detailed images of the pleon and the uropod region (11.2-11.8 with same scale). 11.1: Holotype IGR.GAR-28, setulose setae on pleopod 1, ventro-lateral view, epifluorescence. 11.2: Holotype IGR.GAR-28, pleon and uropods in ventro-lateral view, epifluorescence. bas, basipod of pleopod 1; en, endopod of the pleopod 1; ex, exopod of the pleopod 1; pl1 and pl5, pleopod segment 1 and 5; up, uropod segment. 11.3: Paratype IGR.GAR-48, pleon region in ventral view, epifluorescence. pr7, propodus of pereopod 7. 11.4: Paratype IGR.GAR-64, uropod region in dorsal view, epifluorescence. 11.5-11.6: Paratype IGR.GAR-53-2, uropod region in dorsal view, reflected light (11.4) and epifluorescence (11.5). 11.7 - 11.8: Paratype IGR.GAR-41, uropod region in dorsal view, epifluorescence. bas, basipod of the uropod; en, endopod of the uropod; ex, exopod of the uropod; pl5, pleon segment 5; pt, pleotelson (pleon segment 6 and telson); st, setae.
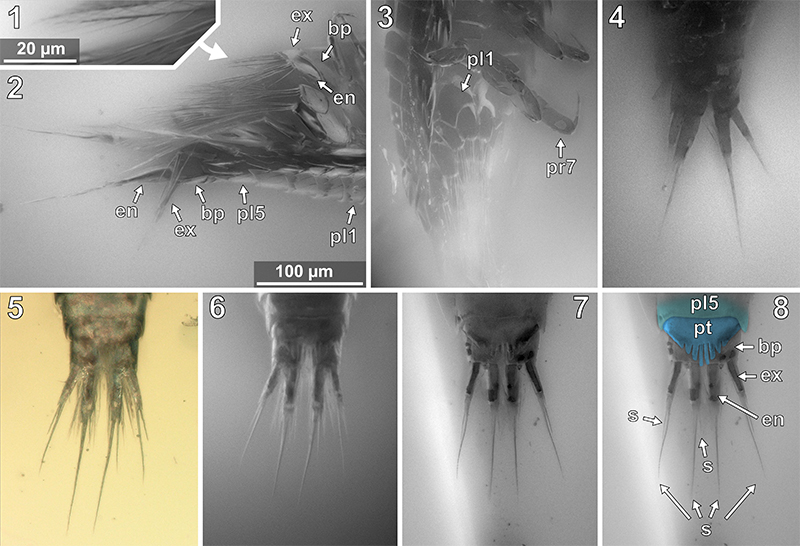
FIGURE 12. Vacuotheca dupeorum sp. nov., detailed images of the head region. 12.1-12.2: Paratype IGR.GAR-95-1, head region in ventral view, epifluorescence, numbers refer to the elements of antennula (atl) and antenna (ant), in blue colour (12.1), same scale as 12.4. 12.3: Paratype IGR.GAR-64, head region in ventro-lateral view, epifluorescence, same scale as 12.4. 12.4: Paratype IGR.GAR-53-1, head region in ventro-lateral view, epifluorescence. pr3, propodus of pereopod 3; *, junction between antennal peduncle and flagellum (element 4 and element 5). 12.5-12.6: Paratype IGR.GAR-95-1, head and pereon region in ventral view (12.5) and distal antenna elements in ventral view (12.6), epifluorescence, same scale. bs1, basipod of pereopod 1; cp1 - cp4, coxal plates of pereon segments 1 to 4; dc1 - dc2, dactsyli of pereopods 1 and 2; mp, mouthparts; s, seta. 12.7: Holotype IGR.GAR-28, head region in ventro-lateral view, epifluorescence, compare to 10.2 for identification of details.
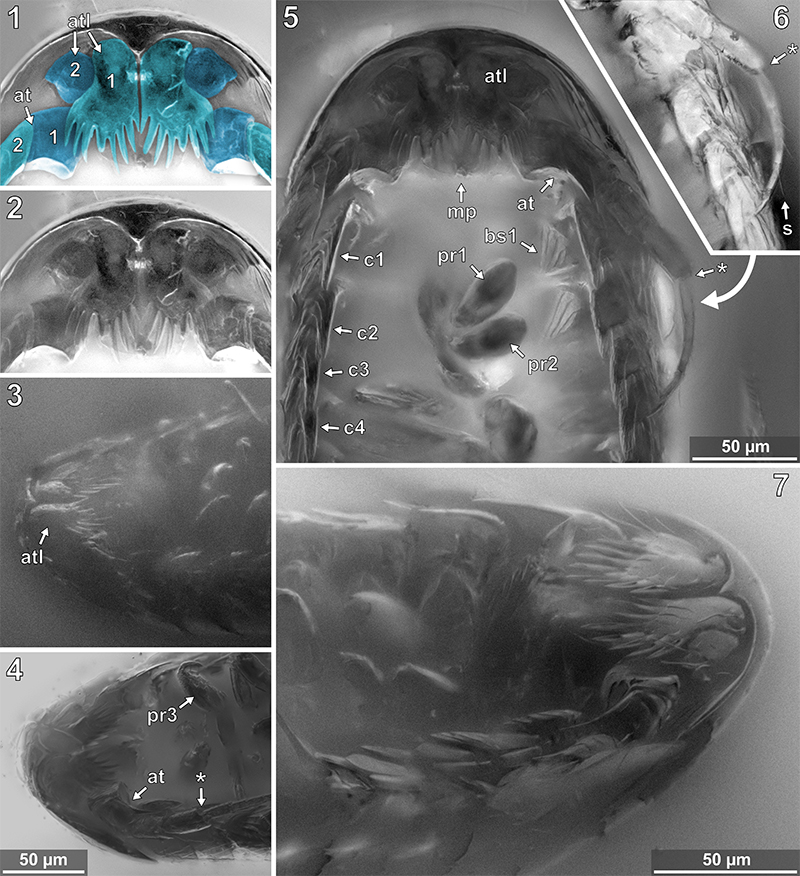
FIGURE 13. Vacuotheca dupeorum sp. nov., detailed images of the lateral body side. 13.1: Paratype IGR.GAR-53-1, ventro-lateral view, epifluorescence. at, antenna; pr3 - pr7, pereopod segments 3 to 7, arrows point to the corresponding coxal plates. 13.2: Paratype IGR.GAR-48, ventro-lateral view, epifluorescence. 13.3: Holotype IGR.GAR-28, ventro-lateral view, epifluorescence. bp, basipod of pleopod 1; en, endopod of pleopod 1; ex, exopod of pleopod 1.
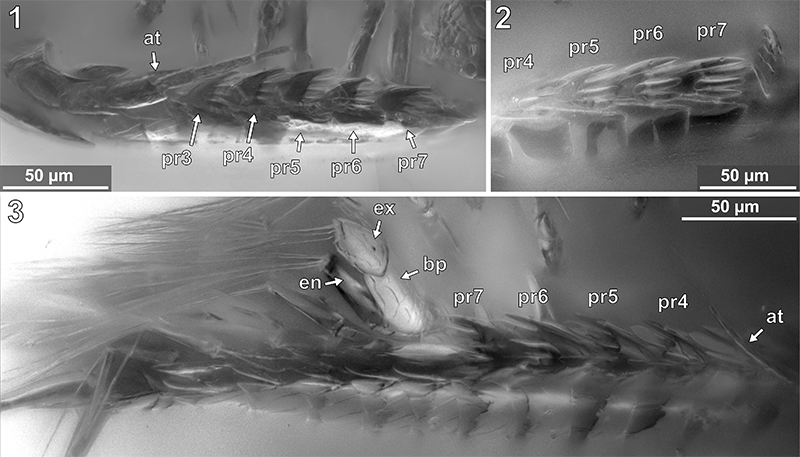
FIGURE 14. Vacuotheca dupeorum sp. nov., detailed images of the pereopods. 14.1: Holotype IGR.GAR-28, ventro-lateral view, epifluorescence. pr1-2 and pr4-6, pereopods 1-2 and 4-6. 14.2: Paratype IGR.GAR-53-1, pereopods in lateral view, right side of the image is anterior, epifluorescence, for labels see Figure 15 (corresponding drawing with labels). 14.3: Paratype IGR.GAR-8.1-1, posterior pereopods in lateral view, right side of the image is anterior, epifluorescence. pr6 - pr7, pereopods 6 and 7. 14.4-14.5: Paratype IGR.GAR-95-1, pereopods in ventral view, upper side of the image is anterior, same scale. pr4 - pr7, pereopods 4 to 7.
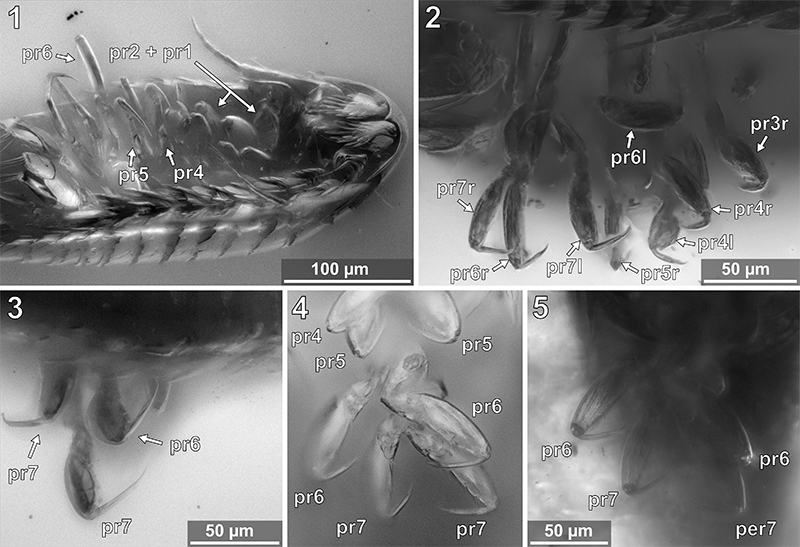
FIGURE 15. Vacuotheca dupeorum sp. nov., paratype IGR.GAR-53-1, drawing of pereopods 3 to 7 (pr3–pr7). (r), right body side; (l), left body side; bs, basipod; is, ischium; mr, merus; cp, carpus; pr, propodus; dc, dactylus. Notice the setae on the propodi of pereopods 3 and 4.
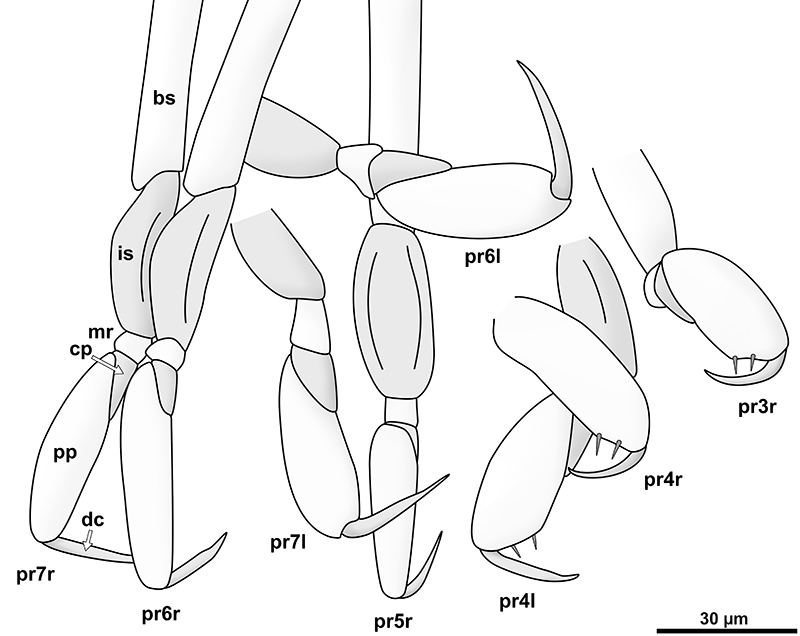
FIGURE 16. Phylogenetic tree of Epicaridea (Boyko et al., 2013) (topology on the left side, species names and taxonomic groups on the right side) mapped with characters gathered from descriptions and illustrations of literature. Characters are coded in colour as depicted in the illustration at the top. Beige is reserved for character states in Vacuotheca dupeorum gen. et sp. nov. (at the very top). Antenna: five flagellum elements (beige), four flagellum elements (orange), three flagellum elements (red), one flagellum elements (orange). Uropod: endopods longer or equal as exopods (beige), endopods shorter than exopods (red). Antennula: with posterior extension and teeth (beige), with posterior extension and without teeth (orange), without posterior extension (red). Coxa: coxal plates with teeth (beige), coxal plates without teeth (red). Telson: posterior margin with teeth (beige), posterior margin without teeth (red). (Fraisse, 1878; Giard and Bonnier, 1887; Bonnier, 1900; Thompson, 1901; Caullery, 1907; Miyashita, 1940; Nielsen and Strömberg, 1965; Bresciani, 1966; Bourdon, 1972, 1976, 1981; Holdich, 1975; Schultz, 1975, 1980; Kensley, 1979; Bourdon and Bruce, 1980; Anderson and Dale, 1981; Coyle and Mueller, 1981; Dale and Anderson, 1982; Adkinson and Collard, 1990; Rybakov, 1990; Pascual et al., 2002; Torres Jordá, 2003; Shimomura et al., 2005; Hosie, 2008; Boyko, 2015).

FIGURE 17. Body lengths of fossil epicaridean cryptoniscium larvae over time (all specimens) compared to cryptoniscium larvae and other developmental stages of modern epicaridean species and potential sistergroup taxa (smallest record of each species). The fading dotted lines at the left mark the earliest occurrences of trace fossils (precarious and affirmed) (Soergel, 1913; Klompmaker et al., 2014). The data from extant species is expanded for better visibility and is outlined by a dotted bracket. (Fraisse, 1878; Bonnier, 1900; Thompson, 1901; Caullery, 1907; Miyashita, 1940; Nielsen and Strömberg, 1965; Bresciani, 1966; Nielsen, 1967; Bourdon, 1972, 1972, 1976, 1981; Holdich, 1975; Schultz, 1975, 1980; Kensley, 1979; Bourdon and Bruce, 1980; Anderson and Dale, 1981; Coyle and Mueller, 1981; Dale and Anderson, 1982; Strömberg, 1983; Adkinson and Collard, 1990; Rybakov, 1990; Shields and Ward, 1998; Pascual et al., 2002; Torres Jordá, 2003; Shimomura et al., 2005; Hosie, 2008; Román-Contreras and Romero-Rodríguez, 2013; An et al., 2015; Serrano-Sánchez et al., 2016; Adlard and Lester, 1995; Atkins, 1933; Bruce, 2009; Brusca, 1978; McDermott, 2002; Romero-Rodríguez and Román-Contreras, 2008; Strömberg, 1971; Thamban et al., 2015; Truesdale and Mermilliod, 1977; Tsukamoto, 1981).
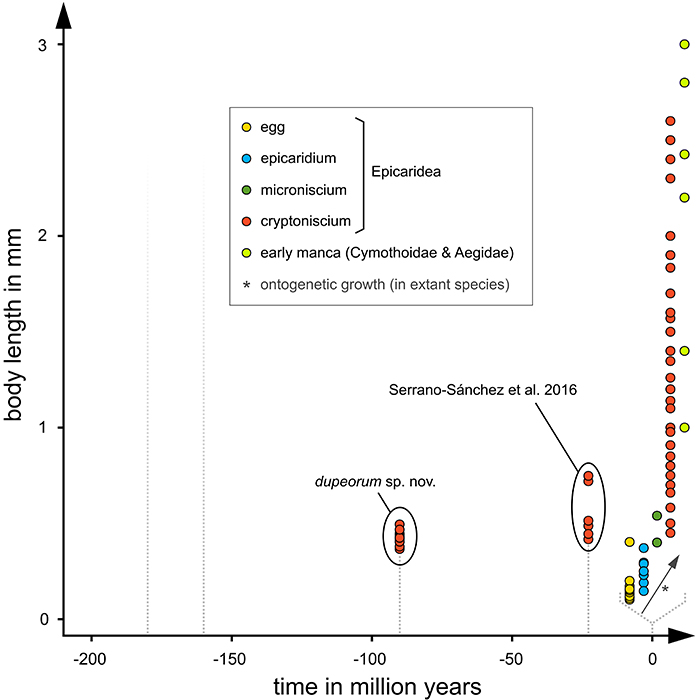
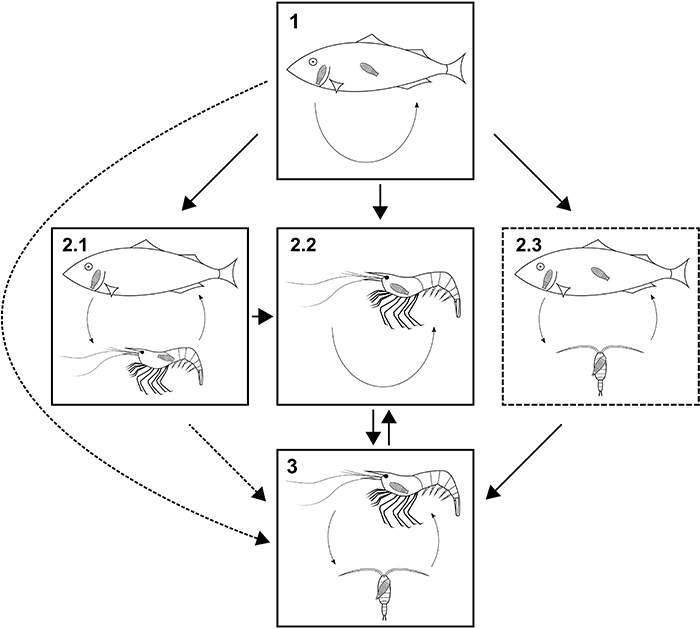
FIGURE 18. Possible evolutionary transitions between parasitic lifestyles in isopods (Cymothoida). Solid boxes, lifestyles with modern analogue; dashed box, lifestyle without modern analogue; solid arrows, likely transitions; dashed arrows, possible but less likely transitions. 18.1: Strict fish parasites, larvae or larvae and adults are parasiting fish (Aegidae, Cymothoidae, Gnathiidae). 18.2.1: Mixed fish and crustacean parasites, adult females parasitic to fish (some cymothoids). 18.2.2: Crustacean parasites without copepod intermediate host (Entoniscoides okadai, Epicaridea). 18.2.3: Larvae feed on copepods, adult females feed on fish. 18.3: Larvae feed on copepods and adults are parasitic to other crustaceans.