|
|
|
INTRODUCTION
Ammonoid cephalopods are notable for their chambered shells, with the septal partitions often showing complex folding along their margins. Paleontologists have long used the suture lines formed by the intersection of the crinkled edges of the septa and the outer shell to classify ammonoids, and for more than a century have conducted various studies of suture and septal form and morphogenesis (Buckman 1892; Pfaff 1911; Spath 1919a; Westermann 1966; Mutvei 1967; Seilacher 1973; Ward and Westermann 1976; Bayer 1977a, 1977b, 1978a, 1978b; Hewitt 1985; Zaborksi 1986; Seilacher 1988; García-Ruiz et al. 1990; Hewitt et al. 1991; García-Ruiz and Checa 1993; Lutz and Boyajian 1995; Checa 1996; Checa and García-Ruiz 1996; Hammer 1999; Olóriz et al. 1999; Gildner 2003; Allen 2006) as well as their supposed function (Westermann 1971; Seilacher 1975; Westermann 1975; Henderson 1984; Hewitt and Westermann 1986, 1987; Jacobs 1990; Seilacher and LaBarbera 1995; Saunders 1995; Daniel et al. 1997; Hewitt and Westermann 1997; Hassan et al. 2002; Lewy 2002; Pérez-Claros 2005; Hammer and Bucher 2006; Pérez-Claros et al. 2007; De Blasio 2008). Given all the attention that has been paid to these features, it is surprising that we are still unsure of just how septa form or how they relate to other aspects of shell and soft part anatomy. How did the ammonoid create such a complexly folded, mineralized structure? Why are the suture lines (i.e., the folded margins of the septa) so consistently patterned that we can use sutures for fine-scale taxonomy? What process governs the addition of elements and increasing fold complexity of sutures through the ontogeny of an individual? Does the degree of sutural complexity relate to functional, constructional, developmental, or metabolic constraints on the septum? Models for Ammonoid Septal Formation
Several models for how septa and their folded edges form have been advanced within the paleontological literature. Living Nautilus provides a potentially observable analog, although the details of how Nautilus produces its septa are still a challenge to study. When constructing a new septal plate, Nautilus first shifts forward in the body chamber while growing new shell around the aperture, leaving a fluid-filled gap between its soft parts and the last septum (Ward 1987). The animal secretes a new septal membrane just behind its soft parts and then begins to mineralize it. Once the new septum is partially mineralized, the fluid is removed from the new chamber and replaced with gas (Ward 1987).
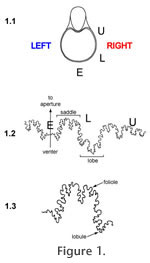
B Perhaps the most commonly-cited idea for septal formation is the tie-point model, best articulated by Seilacher (1973, 1975, 1988) and Westermann (1975). This model argues that the new, pre-calcified, septal membrane attaches to the outer shell wall at genetically defined tie-points, located at the tips of suture lobes (that is, the adapical-most flexure points; Figure 1). The membrane between the tie-points then is blown out toward the aperture by positive hydrostatic pressure, rather like a sheet pinned to a clothesline will billow out on a windy day, to form the saddles of the suture. The more tie-points present, the more complicated and crinkly the septal margin will be. The soft membrane then calcifies, preserving the crinkles and folds. Of some debate within the tie-point concept has been whether the posterior mantle of the ammonoid was involved in determining the shape of the septum (e.g., Hewitt et al. 1991), whether it was smooth or permanently fluted (e.g., Seilacher 1975 versus Westermann 1975 and Seilacher 1988), or even capable of changing form at will (Zaborski 1986; Lewy 2002, 2003; Hewitt and Westermann 2003). The viscous fingering model offers an alternative to the tie-point concept (García-Ruiz et al. 1990; García-Ruiz and Checa 1993; Checa and García-Ruiz 1996; Checa 2003). Here, the complex septal folding is due not to genetic constraints or the shape of the ammonoid posterior, but to the hydrostatic properties of the materials involved. Septa represent the interface between two fluids of differing viscosities, the mantle and the last, fluid-filled chamber. Such a situation will produce fractal "viscous fingering" of one fluid mass into the other; wall effects will produce greater complexity of interfingering near the shell margin and the siphuncle (García-Ruiz et al. 1990). Hammer (1999) challenged the viscous fingering model on the grounds that a purely hydrostatic model could not produce such consistent septal forms (but see response by Checa and García Ruiz 2000). He proposed a different way to think about the problem, using a reaction-diffusion model that explored how growth of the septal membrane could be guided by interactions between a slowly diffusing activator morphogen and a more rapidly diffusing inhibitor morphogen (Hammer 1999; see also Hammer and Bucher 1999 and Guex et al. 2003 for other applications of this concept). The reactions of these molecules would be capable of producing complex (and reproducible) structures within the septal membrane without requiring direct genetic control or a complex developmental regulatory system. Hammer (1999) noted that his model was consistent with the tie-point concept, as it was concerned with understanding why specific points on the septal margin would be consistently shaped in the same way. Careful study of ammonoid suture patterns can test some of the predictions of these models for septal formation and provide some constraints on their parameters. For example, the tie-point model implies that suture lobes should be more constrained in their position than suture saddles, and also suggests that saddle folioles should be more rounded while lobules are more incised. In addition, at least as presented by their authors, all three models make the assumption that septa are symmetrical across the shell's dorsal-ventral midline, so that right and left suture patterns match. To evaluate aspects like constraint and asymmetry in septal form requires a method for comparing and quantifying subtle differences in these complex structures. This paper makes use of a relatively new technique for analyzing ammonoid sutures, one that capitalizes on the power of Geographic Information Systems (GIS), to evaluate the merits of the various models for septal formation. Geographic Information Systems (GIS) as a Morphometrics ToolAllen (2007) provided an excellent review of previous approaches to the morphometric analysis of ammonoid sutures. She identified key reasons why suture shapes are difficult to quantify. These forms are both morphologically and mathematically complex, limiting the application of equation-based techniques such as Fourier analysis or mathematically-based simulated suture curves. Homology of features along a suture line is difficult to assess, making the use of techniques requiring extensive inferences about homology (such as landmark-based analyses) problematic. Traditional measures of sutural fold complexity, such as fractal dimension and the suture complexity index (SCI) of Saunders (1995), can yield the same values for sutures of very different shapes and can be difficult to apply to some suture morphotypes. Geographic Information Systems offer novel approaches to the quantitative study of anatomical form. GIS provides powerful tools for the storage, visualization, manipulation, and quantitative analysis of spatial data. While GIS is typically used to analyze geographic information, any spatially interesting phenomenon, such as an object with spatially arrayed features, is amenable to GIS techniques (Guo and Onasch 2001). In particular, many complex anatomical features of organisms, such as the occlusal surfaces of mammal molars (Zuccotti et al. 1998; Jernvall and Selänne 1999; M'Kirera and Ungar 2003; Ungar 2004; Evans et al. 2007; Plyusnin et al. 2008), conodont elements (Manship et al. 2006), and the sutures of ammonoids (Manship 2004; Waggoner and Manship 2004), are amenable to spatial analysis via GIS. Most previous work utilizing GIS for morphometric analysis has focused on mammal teeth. As one example, Plyusnin et al. (2008) applied GIS approaches to develop an automated system for classifying mammal teeth. They used 3D surface scans to create digital elevation models (DEMs) of the teeth. Applying techniques similar to those used to study landforms, different combinations of feature selection schemes and classification models were used to find the most effective method for identifying an unknown tooth. Manship (2004) developed a new method for inputting and analyzing ammonoid suture patterns within a GIS. This method provides a user-friendly way to compare ammonoid suture patterns visually and quantitatively as an aid to classification and the study of septal construction and function. Using GIS software, one can input, scale, and overlay suture patterns to better compare them visually. GIS technology can also pool a set of suture patterns together to make a polygon encompassing them all. This polygon represents an envelope of possible forms, useful for classifying new specimens into the correct species. The quantitative functions within GIS software enable one to calculate measures such as line lengths and areas of overlap with ease. Manship (2004) addressed the issue of error in recording suture patterns by quantitatively comparing the same suture pattern as published in the literature and as re-recorded from the fossil specimen by herself. The quantified mismatch between the two digitized versions of the same suture pattern was substantially smaller than mismatches between different sutures, indicating that human error in tracing and flattening suture patterns is not significant relative to biologically relevant variation in suture form. The primary advantage of GIS over more conventional morphometric tools, such as simple univariate or multivariate analyses, landmark-based approaches, eigenshape or Fourier analysis, is that GIS does not require the suture pattern to be simplified in order to quantify it. Rather than reducing a complex form to a few linear measurements, a set of landmarks, or a simplified "best-fit" curve, GIS can quantify shape in its original state, capturing the overall form of the anatomical feature being investigated and allowing for more powerful analyses. Rohlf and Marcus (1993) make a similar claim for geometric morphometrics, arguing that its ability to archive the original form of a feature makes it a more effective analytical tool than traditional multivariate morphometric techniques like principal component analysis and canonical variate analysis. However, whereas geometric morphometrics can only assess the geometric relationships among defined (and presumably homologous) landmarks, the GIS technique includes information from the entire complex form, making it potentially even more powerful for exploring shape variation. |
|