 Evidence of Binary Division in Mature Central Capsules of a Collosphaerid Colonial Radiolarians: Implications for Shell Ontogenetic Patterns in Modern and Fossil Species
Evidence of Binary Division in Mature Central Capsules of a Collosphaerid Colonial Radiolarians: Implications for Shell Ontogenetic Patterns in Modern and Fossil Species
O. Roger Anderson and Shyam M. Gupta
Article number: 1.1.2A
Copyright Palaeontological Assocation, 28 January 1998
https://doi.org/10.26879/98002
Plain-language and multi-lingual abstracts
PDF version
Submission: 8 August 1997. Acceptance: 28 October 1997
ABSTRACT
Evidence is presented from fossil shells and living species of the colonial radiolarian Acrosphaera that mature central capsules with shells can produce daughter central capsules and shells by binary fission. These data indicate that in colonial collosphaerid radiolaria, at least, proliferation of central capsules can occur after maturation and may account for rapid increase in biomass and population size in response to favorable environments. This augments prior evidence that central capsules of non-shelled colonial radiolaria can proliferate by binary fission. Also, these observations extend our understanding of the pattern of silicification and shell formation to include two possibilities: (1) the current view of "simultaneous shell deposition" where all of the skeletons are deposited at nearly the same time following multiple divisions of skeletonless central capsules and (2) "successive shell deposition," where mature central capsules with shells give rise to additional ones through delayed binary fission. These observations have interesting implications for life cycle dynamics of radiolaria in modern and paleo-oceanic environments.
O. Roger Anderson, Biological Oceanography, Lamont-Doherty Earth Observatory of Columbia University, Palisades, New York 10964, U.S.A. ora@ldeo.columbia.edu
Shyam M. Gupta, National Institute of Oceanography, Dona Paula, Goa, 403 004, India. smgupta@csnio.ren.nic.in
KEY WORDS: fossil shells, colonial radiolarian Acrosphaera, central capsules, binary fission, collosphaerid radiolaria, biomass, population size, non-shelled colonial radiolaria, silicification, life cycle, radiolaria, paleo-oceanic, Quaternary, ecology, shell ontogeny
Final citation: Anderson, O. Roger and Gupta, Shyam M. 1998. Evidence of Binary Division in Mature Central Capsules of a Collosphaerid Colonial Radiolarians: Implications for Shell Ontogenetic Patterns in Modern and Fossil Species. Palaeontologia Electronica Vol. 1, Issue 1; 2A; 13p. https://doi.org/10.26879/98002
palaeo-electronica.org/content/pe-1998-1-table-of-contents/172-1998-1/461-hetermorph-ammonites
INTRODUCTION
The skeletal remains of radiolaria and planktonic foraminifera are among the most important microfossils in interpreting marine sediments. Radiolaria are of particular interest because of their wide geographic distribution and diverse skeletal morphologies. Much radiolarian micropaleontological data is based on analyses of mature skeletons. Likewise, reconstructions of radiolarian phylogenies have largely used mature skeletons (e.g., Knoll and Johnson 1975, Goll 1979, Goll and Björklund 1980, Riedel and Sanfilippo 1981, Sanfilippo and Riedel 1992). However, a more thorough knowledge of skeletal ontogeny may be a useful source of additional evidence to decipher phylogenetic lineages and better interpret paleoenvironments based on a wider range of skeletal forms.
EVIDENCE FOR SKELETAL DEVELOPMENTAL PATTERNS
Evidence for skeletal development in radiolaria has been obtained by (1) observing living organisms with light optics to record events during skeletal deposition, (2) reconstructing skeletal ontogenesis by analysis of stages of development inferred from plankton or sedimentary samples, and (3) examining fine structural evidence based on scanning and transmission electron microscopy of living organisms fixed during varying stages of skeletal deposition (e.g., Anderson and Bennett 1985, Anderson et al. 1987, 1988, 1989, Thurow and Anderson 1986).
Based on these types of evidence, Anderson and Swanberg (1981) proposed that most skeletons of polycystinea can be explained as a product of two forms of skeletal development: (1) bridge growth yielding latticed structures and frameworks with somewhat polygonal pores, and (2) rim growth forming perforated shells with nearly round pores. However, the final shape of the pore may not be fully indicative of the developmental process. Further evidence of early stages, or regions with incomplete development of the skeleton, are often needed to confirm the type of ontogenetic process (e.g., Anderson et al. 1988). Using this paradigm of bridge and rim growth, several species of fossil and living radiolaria have been examined to determine the contribution of bridge and rim growth to the mature skeletal form (e.g., Anderson 1983, Thurow and Anderson 1986, Amon et al. 1990). Among the collosphaerid radiolaria, both types of skeletal growth are observed within different species (Anderson 1983).
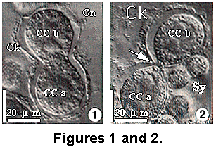 In addition, Anderson and Swanberg (1981) used fixed collosphaerid colonial radiolaria samples, to discover that the central capsules of these species divide by binary fission during proliferation within the colony. However, all of these stages of binary fission occurred early in development before silica deposition. The living cytoplasmic sheath (cytokalymma) that eventually deposits the silica was present during binary fission and exhibited a typical hour-glass shape surrounding the dividing central capsules (e.g., Figure 1).
In addition, Anderson and Swanberg (1981) used fixed collosphaerid colonial radiolaria samples, to discover that the central capsules of these species divide by binary fission during proliferation within the colony. However, all of these stages of binary fission occurred early in development before silica deposition. The living cytoplasmic sheath (cytokalymma) that eventually deposits the silica was present during binary fission and exhibited a typical hour-glass shape surrounding the dividing central capsules (e.g., Figure 1).
Skeletal deposition within the cytokalymma occurred later after all the central capsules had divided and filled the gelatinous envelope enclosing the colony. There was no evidence that further binary division occurred after the porous siliceous skeleton had been deposited enclosing the central capsule. This raised the interesting question of whether proliferation of the central capsules ceased after the skeleton was deposited. If so, this would be a serious limitation to further growth of the colony and could limit repair if there is disruption or trauma to the colony. If there were no further division, then the number of skeletal-bearing central capsules would be fixed until the colony dissipated during swarmer production (presumably sexual reproductive phase).
However, evidence is presented here, based on fossil specimens and laboratory observation of living species, that collosphaerid central capsules may divide after the porous skeleton has been deposited around the central capsule. These data raise the interesting questions of whether this process occurs widely in colonial and solitary species and the significance this process holds for the interpretation of radiolarian abundance and life cycle strategies.
MATERIALS AND METHODS
Shells of Acrosphaera cyrtodon were obtained from core samples collected from the central Indian Ocean Basin (-1010.032 South, 7520.328 East) at a water depth of 5,300 m. The shells were recovered from 20 cm core depth (Core AAS-176b) and prepared for light microscopic observation as a permanent mounted slide. Photomicrographs were made using a binocular microscope and photographed at 200 x magnification. All measurements of shell diameters and pore sizes were made with a Leica microscope and graduated reticule at 250x magnification.
Living colonial radiolaria were collected by SCUBA divers using hand-held polycarbonate jars to enclose the organisms as they drifted past or by plankton sampling using gentle net tows (1 mm mesh, 1 m diameter nets) according to previously published techniques (Anderson et al. 1991, Anderson 1992). The samples were collected at a location approximately 10 km southwest of Castle Harbour Bermuda during July, 1997. Living colonial radiolaria were observed and photographed with a dissecting microscope using dark field illumination.
RESULTS
Binary Fission in Living Colonial Radiolaria
Figures 1 and 2 show binary fission of central capsules in early stages of colony development in a living colonial radiolarian. These stages occur before deposition of the siliceous skeleton. Only the thin, living cytoplasmic envelope (cytokalymma), presenting an hour-glass appearance, encloses the dividing central capsules. Subsequent to repeated binary divisions of the central capsules, producing scores to hundreds of central capsules within a gelatinous sheath, the siliceous skeleton is deposited within the cytokalymma surrounding each central capsule.
Evidence for Unusual Binary Fission in Living and Fossil Specimens
Figures 3-5 are living Acrosphaera sp. showing possible stages of budding from mature central capsules  surrounded by a complete porous shell. Bud-like protruberances of cytoplasm (Figure 3) project from the surface of the central capsule, and gradually are
surrounded by a complete porous shell. Bud-like protruberances of cytoplasm (Figure 3) project from the surface of the central capsule, and gradually are  separated from it (Figure 4) but are connected to the mature central capsule by thin strands of cytoplasm. Other mature stages with binary pairs (Figure 5) are composed of a larger, shell-bearing central capsule with a smaller one in close proximity and still connected by strands of cytoplasm. Additional evidence of binary division of mature central capsules was obtained from fossil evidence. Figures 6 and 7 show a pair of porous skeletons (shells), obtained from fossil material, of Acrosphaera cyrtodon joined by a neck-like connection. One of the shells (Figure 6, a) is more spherical and larger (130 microns), whereas the other (Figure 6, b) is smaller (120 microns) and more prolate or bud-like. Shell B is connected to shell A by siliceous linkages that appear to be deposited by bridge growth. The shells are clearly united and are not an artifact of diagenesis or preparation for microscopic examination. A through focal series examination confirms that the connection is complete in all planes of focus and is approximately a cylindrical latticed connection uniting the two shells.
separated from it (Figure 4) but are connected to the mature central capsule by thin strands of cytoplasm. Other mature stages with binary pairs (Figure 5) are composed of a larger, shell-bearing central capsule with a smaller one in close proximity and still connected by strands of cytoplasm. Additional evidence of binary division of mature central capsules was obtained from fossil evidence. Figures 6 and 7 show a pair of porous skeletons (shells), obtained from fossil material, of Acrosphaera cyrtodon joined by a neck-like connection. One of the shells (Figure 6, a) is more spherical and larger (130 microns), whereas the other (Figure 6, b) is smaller (120 microns) and more prolate or bud-like. Shell B is connected to shell A by siliceous linkages that appear to be deposited by bridge growth. The shells are clearly united and are not an artifact of diagenesis or preparation for microscopic examination. A through focal series examination confirms that the connection is complete in all planes of focus and is approximately a cylindrical latticed connection uniting the two shells.  The bud-like appearance of shell B, and the asymmetrical arrangement of the connecting neck tapering from shell B to shell A suggests that shell B was produced by budding from shell A. It is noteworthy that the asymmetrical arrangement of the connecting neck is different from the nearly symmetrical connection in early stages of central capsule division prior to silicification (Figures 1 and 2).
The bud-like appearance of shell B, and the asymmetrical arrangement of the connecting neck tapering from shell B to shell A suggests that shell B was produced by budding from shell A. It is noteworthy that the asymmetrical arrangement of the connecting neck is different from the nearly symmetrical connection in early stages of central capsule division prior to silicification (Figures 1 and 2).
If the two shells were simply twin shells deposited simultaneously due to incomplete binary fission, we would expect a more symmetrical arrangement (e.g., Figure 2). Moreover, shell A is a complete spheroidal lattice and shell B is atypical in being elongated at the point of attachment (and smaller), further suggesting that shell B is a derived product from the central capsule of shell A. Since shell A is intact and shows no sign of damage or abnormal development, we do not think that shell B is a product of a repair process or adjustment to damage during growth of shell A.
One interpretation of the fossil binary shells is that shell B was produced by delayed binary fission of central capsule A subsequent to silicification of its shell. During subsequent silicification of shell B, the new shell became connected by bridge growth to shell A, perhaps due to the proximity of the daughter shell to the mother shell. If binary fission occurred subsequent to silicification of shell A, then the daughter nucleus within central capsule B must have passed through a pore of the mother shell (A) when the cytoplasm protruded to form central capsule B. The proliferating nuclei in a mother cell are approximately 8 to 10 microns (Anderson 1976, 1978). Hence, the pores in the presumed mother shell (A) must be approximately this dimension to allow the protruding cytoplasmic bud and daughter nucleus to pass. The mean size of pores in the presumed mother shell (A) is 14 microns (ranges 10–22 microns) and therefore are large enough to permit passage of a daughter nucleus.
DISCUSSION
The ontogeny of the shells in modern and ancient radiolarian species is poorly understood, although we are gaining insight into the dynamic role that the cytoplasm plays in depositing silica and determining the elaborate geometry of the product (e.g., Anderson 1983, Anderson and Swanberg 1981). A more complete analysis of shell ontogeny including observations of the kind presented here may help to clarify phylogenetic relationships based on developmental stages in addition to current evidence that is largely based on morphology of mature shells observed in different geological strata. The data presented here suggest that in collosphaerid radiolaria, at least, shells may develop either by simultaneous silicification of central capsules after all of them have divided (simultaneous deposition, Anderson and Swanberg 1981), or by delayed budding and formation of central capsules surrounded by siliceous shells after some of the central capsules have fully matured (successive deposition). The evidence for simultaneous division of the central capsules and skeletal deposition is well documented for some colonial radiolaria based on light microscopy (e.g., Anderson 1983, Anderson and Swanberg 1981). Moreover, nuclei of some colonial species divide by binary fission in advance of reproductive stages (e.g., Anderson 1983, p. 160) thus providing numerous nuclei in the intracapsular cytoplasm that can serve as reproductive nuclei. These may become the nuclei of swarmers or could serve as nuclei for bud formation.
Limitations of Current Data and Need for Additional Exemplars
Binary fission in some species of radiolaria has been reported in living individuals beginning in the nineteenth century (e.g., Hertwig 1879, Borgert 1909, 1922, Hollande et al. 1953) and discussed in the general context of interpreting radiolarian reproductive strategies. Haeckel (1887, p. xcix) noted that while the general course of individual development begins with swarmer production, there exisits in some groups a different form of ontogeny, introduced by simple division of the unicellular organism, and coming under the term "regeneration" in its wider sense. "This spontaneous division occurs quite commonly in the Polycyttaria and produces their colonies." "In all these cases the increase by division is nothing else than an ordinary case of cell-division, in which bisection of the nucleus precedes that of the central capsule." During culturing studies in our laboratories, colonies of Collozoum inerme (a species lacking skeletal elements) collected alive frequently contain central capsules that occur as binary pairs or appear elongated with two central deposits of reserve material suggesting a late stage of binary division. Central capsules of shell-bearing species are known to undergo binary fission during early stages of colony development (e.g., Figures 1 and 2).
We have only indirect evidence, however, for budding of mature central capsules in shelled species. The clearest evidence is from fossil and modern skeletons showing the bud-like attachment of a smaller shell to a larger shell (i.e., Figures 6 and 7). Although bud-like bodies have been observed in living colonial radiolaria (e.g, Figures 3, 4, and 5), we have not found the earliest stages where the bud is just protruding from the central capsule. It would be very important to perform fine structural analyses of these bud like stages to determine if they are indeed products of binary fission, to complete the suite of data supporting budding of mature central capsules, and to document the cytological events accompanying budding.
Examples of binary shells have been reported for a wide range of shelled colonial and solitary radiolaria. Kling (1971) presented photographs of paired shells with varying degrees of separation and an interconnected skeletal framework that appeared to be "stretched" suggesting deposition during binary fission, but the species were not identified. Takahashi (1981) presented scanning electron microscopic evidence of paired shells in living radiolaria, collected in sediment traps, many with narrow connecting skeletal frameworks. These included colonial radiolaria: Acrosphaeramurrayana (plate 1), Disolenia collina (plate 3), Disolenia zanguebarica (plate 3), and Siphonosphaera socialis (plate 4), and quite interestingly phaeodaria: Castanidium abundiplanatum (plate 58). Takahashi (1981, plate 58) notes in the figure legend "Two specimens splitting apart" suggesting that the phaeodarian was undergoing binary fission. Haeckel (1887, p. xcix) commented that "Among the Phaeodaria division is commonly observed in the order Phaeocystina, and also in the Phaeoconchia." "In all these cases the increase by division is nothing else than an ordinary case of cell-division, in which bisection of the nucleus precedes that of the central capsule." Examples of apparent division in fossil skeletons of solitary spumellaria have been reported by deWever (1985) who examined Paleozoic radiolaria, and Sugiyama et al. (1992) who reported Pliocene fossil skeletons of Spongaster tetras with a bud-like smaller skeleton clearly attached to the surface (plate 7). During several years of culturing solitary radiolaria in the laboratory we have observed only a few instances where additional individuals of the same species were observed in the culture vessels that originally contained one mature radiolarian. We cannot say if these came from binary fission of the mature individual or were adult stages of immature individuals originally included with the culture water. We also suspect that laboratory culture conditions are not always optimal for some species, though many individuals grow and mature to release swarmers. However, it is not uncommon to observe closely-joined binary pairs of shells (usually one shell slightly smaller than the other) connected by strands of cytoplasm in living colonies (as in Figure 5) .
All of this points to a need for better documentation of evidence for binary division in radiolaria from fossil and living material. It would be particularly advantageous if micropaleontologists and biostratigraphers could document each time they observe apparent budding in fossil shells of radiolaria including the geological time markers for the strata where they were found. Likewise, as additional researchers examine plankton samples and living radiolaria in culture, any evidence of binary fission would be especially helpful to further document its occurrence in extant species. Until we have more substantial evidence about the number of species that undergo binary fission, and the frequency of occurrence in a population, it will be difficult to make inferences about its significance for radiolarian ecology and population dynamics.
Moreover, if we have a well-documented record of occurrences such as binary shell formation from fossil and living material we can be more confident about making inferences from biological observations from the present to the past. There are few variables in the fossil record that correlate well with biological data from living individuals since only the hard parts typically are fossilized. The occurrence of fossil binary shells, and evidence of skeletal morphogenesis in shells that are incompletely formed, provide one line of evidence linking observations of living individuals with those in the past (e.g., Anderson and Swanberg 1981, Thurow and Anderson 1986, Anderson et al., 1988, Amon et al. 1990). Micropaleontolgical observations of variations in skeletal geometry may provide some interesting clues to skeletal morphogenesis linking modern and ancient species.
Simultaneous and Successive Patterns of Skeletal Deposition
The abnormal, asymmetrical pair of shells (Figures 6 and 7) from the fossil record and the sequence of views for a living Acrosphaera colony (Figures 3-5) support the possibility of successive deposition at least in some collosphaerid colonial radiolaria. It should be noted, however, that the rather convincing evidence of binary fission in Figures 6 and 7 occurred in Acrosphaera cyrtodon. The living colony is a different species of Acrosphaera. We do not know to what extent successive division may occur in different species of Acrosphaera. Paired shells observed in other colonoial radiolaria vary in geometry from two hemispherical shells fused at a common mid point to pairs of shells connected only by spines or an isthmus (e.g., Takahashi 1981, Paverd 1995). These observations conform to either incomplete binary fission (paired hemispheres attached due to incomplete division of the central capsule before skeletogenesis) or incomplete successive deposition of shells by delayed budding (united pairs of complete shells, e.g., Figure 6). The information presented here for fossil and living preparations is the first time these data have been synthesized into an explanation of binary fission during successive deposition of shells around central capsules. Moreover, if successive deposition occurs with daughter cells produced after maturation of the central capsule and deposition of the surrounding shell, this would alter our current understanding of reproductive cycles in radiolaria. According to our current understanding (e.g., Anderson 1983), the life cycle of radiolaria includes a single mature stage with intervening production of swarmers. The swarmers are released by the mature radiolarian and disperse in the environment. Subsequent maturation of the swarmers (either as asexual propagules or after fusion, if they are sexual gametes) yields a central capsule that becomes surrounded by a skeleton. The mature individual lives and feeds for a while and then releases swarmers, fully dissipating the cells, to produce the next generation, thus completing the life cycle. However, if there are further stages of binary fission within each generation in some radiolaria (as suggested previously in the literature and reported further here), this would permit additional stages of asexual proliferation of the radiolaria without swarmer production.
Implications of Alternative Life Cycles for Population Dynamics
The occurrence of asexual reproductive stages (in addition to swarmer production) within the life cycle of some radiolaria may provide greater plasticity in responding to changing environmental variables including food sources, water chemistry, physical structure of the water mass, temperature, etc. If proliferation can occur by binary fission, without swarmer production, these radiolaria can respond rapidly to favorable environments and increase population densities substantially without going through a more perilous intervening stage of reproduction by flagellated swarmers. Most species have a central capsular wall composed of numerous individual organic plates that fit against each other much like pieces of a jigsaw puzzle. Since the wall is not solid, there is the possibility of expansion and protrusion of central capsular cytoplasm to produce a bud by disarticulation of the plates at the site of budding.
Further work is needed with additional living species to determine if there is similar evidence of binary fission of mature stages of individuals as reported here. Moreover, if it is limited to colonial radiolaria, it at least helps to explain how colonies can become so very prolific (up to several hundred per cubic meter) in modern oceans (e.g., Swanberg 1979, Anderson 1983). Under favorable conditions, mature colonies of shelled species may be able to generate additional central capsules, thus increasing the number of colonies and their total biomass in oceanic planktonic communities. The fossil evidence and data from extant species presented here add additional evidence that central capsules can divide by binary fission and that this may be a long established reproductive mode especially among collosphaerid radiolaria. The data support previously published findings (Anderson and Swanberg 1981), and suggest that a successive mode of division, not reported previously, may be more common, in colonial radiolaria at least, than previously realized.
ACKNOWLEDGMENTS
We express appreciation to the staff at the Bermuda Biological Station for Research where living colonial radiolaria were collected. This study was funded in part by the EIA program of the Department of Ocean Development, New Delhi. This is Lamont-Doherty Earth Observatory Contribution number 5736.
REFERENCES
Amon, E. O., Braun, A. and Chuvashov, B. I. 1990. Lower Permian (Artinskian) Radiolaria from the Sim type section, Southern Urals, Geologica et Palaeontologica, 24:115-137.
Anderson, O. R. 1976. Ultrastructure of a colonial radiolarian Collozoum inerme (Müller) and a cytochemical determination of of the role of its zooxanthellae, Tissue and Cell, 8:195-208.
Anderson, O. R. 1978. Fine structure of a symbiont-bearing colonial radiolarian, Collosphaera globularis, and 14C isotopic evidence for assimilation of organic substances from its zooxanthellae, Journal of Ultrastructure Research, 62:181-189.
Anderson, O. R. 1983. Radiolaria. Springer-Verlag, Heidelberg, Germany.
Anderson, O. R. 1992. Laboratory maintenance cultures of planktonic foraminfera and radiolaria. pp. A-35.1–A-35.9. In Lee, J. J. and Soldo, A. T. (ed.), Protocols in Protozoology, Allen Press, Lawrence, KS.
Anderson, O. R. and Bennett, P. 1985. A conceptual and quantitative analysis of skeletal morphogenesis in living Euchitonia elegans and Spongaster tetras. Marine Micropaleontology, 9:441-454.
Anderson, O. R. and Swanberg, N. R. 1981. Skeletal morphogenesis in some living collosphaerid radiolaria, Marine Micropaleontology, 6:385-396.
Anderson, O. R., Moss, M. L. and Skalak, R. 1987. The cytoskeletal and biomineralized supportive structures in radiolaria. p. 200-214. In Bereiter-Hahn, J., Anderson, O. R. and Reif, W. (ed.), Cytomechanics, Springer-Verlag, Heidelberg.
Anderson, O. R., Hays, J. D. and Gross, M. 1988. An ontogenetic analysis of changes in morphology during phylogeny of some Lamprocyrtis spp. from sedimentary samples, Micropaleontology, 34: 41-55.
Anderson, O. R., Bennett, P. and Bryan, M. 1989. Experimental and observational studies of radiolarian physiological ecology: 1. Growth, abundance and opal productivity of the spongiose radiolarian Spongaster tetras tetras,Marine Micropaleontology, 14: 257-265.
Anderson, O. R., J. J. Lee, and W. W. Faber, Jr. 1991. Collection, maintenance and culture methods for the study of living foraminifera. pp. 335-357. In Lee, J. J. and Anderson, O. R. (ed.), Biology of Foraminifera. Academic Press, London.
Borgert, A. 1909. Die tripyleen Radiolarien der Plankton-Expedition. Circoporidae. Ergebnis Plankton-Expedition der Humbolt-Stiftung, 3:319-352.
Borgert, A. 1922. Die tripyleen Radiolarien der Plankton-Expedition, II. Allgemeiner Teil. Bau und Fortpflanzung der Tripyleen. 200 p. Ergebnis Plankton-Expedition der Humbolt-Stiftung, Vol. 3.
De Wever, P. 1985. Sur l'existence, des le Paleozoique, de radiolaires siamois, Revue de Paléobiologie, 4:111-116.
Goll, R. M. 1979. The Neogene evolution of Zygocircus, Neosemantis and Callimitra: Their bearing on nassellarian classification. A revision of the Plagiacanthoidea, Micropaleontology, 25:365-396.
Goll, R. M. and Björklund, K. R. 1980. The evolution of Eucornis fridtjofnanseni and its application to the Neogene biostratigraphy of the Norwegian-Greenland Sea, Micropaleontology, 26:356-371.
Haeckel, E. 1887. Report on Radiolaria collected by H. M. S. Challenger during the years 1873-1876. Volume XVIII (Part 1) p. xcix-c. In Thompson, C. W. and Murray, J. (ed.), The Voyage of H. M. S. Challenger. London, Her Majesty's Stationary Office.
Hertwig, R. 1879. Der Organismus der Radiolarien. Fischer Verlag, Jena.
Hollande, A., Enjumet, M. and Manciet, J. 1953. Les péridiniens parasites des phaeodariés et le problème de la sporogénese chez ces Radiolaires, Comptes Rendues de l'Academie des Sciences, Paris, 236:1607-1609.
Kling, S. A. 1971. Dimorphism in Radiolaria. pp. 663-672. In Farinacci, A. (ed.), Proceedings of the II Planktonic Conference, Roma 1970. Edizioni Tecnoscienza, Rome.
Knoll, A. W. and Johnson, D. A. 1975. Late Pleistocene evolution of the collosphaerid radiolarian Buccinosphaera invaginata Haeckel, Micropaleontology, NY, 21:60-68.
Paverd, van de, P. J. 1995.Recent Polycystine Radiolaria from the Snellius-II Expedition. Ph.D. Thesis, Free University, Amsterdam, The Netherlands.
Riedel, W. R. and Sanfilippo, A. 1981. Evolution and diversity of form in radiolaria. p. 323-346. In Simpson, T. L. and Volcani, B. E. (ed.), Silicon and Siliceous Structures in Biological Systems. Srpinger-Verlag, New York.
Sanfilippo, A. and Riedel, W.R. 1992. The origin and evolution of Pterocorythidae (Radiolaria): A Cenozoic phylogenetic study, Micropaleontology, 38:1-36.
Sugiyama, K., Nobuhara, T. and Inoue, K. 1992. Preliminary report on Pliocene radiolarians form the Nobori Formation, Tonohara Group, Shikoku, Southwest Japan, The Journal of Earth and Planetary Sciences, Nagoya University, 39:1-30.
Swanberg, N. R. 1979. The Ecology of Colonial Radiolarians: Their Colony Morphology, Trophic Interactions, and Associations, Behavior Distribution and the Photosynthesis of their Symbionts. Ph.D. thesis. Woods Hole Oceanographic Institution, Woods Hole, Massachusetts, U.S.A.
Takahashi, K. 1981. Vertical Flux, Ecology and Dissolution of Radiolaria in Tropical Oceans: Implications for the Silica Cycle. Ph.D. thesis. Woods Hole Oceanographic Institution, Woods Hole, Massachusetts, U.S.A.
Thurow, J. and Anderson, O. R. 1986. An interpretation of skeletal growth patterns of some middle Cretaceous and modern radiolarians, Micropaleontology, 32:289-302.

