 Themophysiology and Biology of Giganotosaurs: Comparison with Tyrannosaurus
Themophysiology and Biology of Giganotosaurs: Comparison with Tyrannosaurus
Article number: 2.2.12A
Copyright Paleontological Society, 22 October 1999
https://doi.org/10.26879/99012
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 11 June 1999. Acceptance: 5 October 1999.
ABSTRACT
Large carnivorous dinosaurs present many interesting biological and ecological questions. The most important information for understanding the ecology of these dinosaurs is their metabolism and thermal physiology. These factors in conjunction with their body mass determine the quantity of meat these animals needed to consume, how rapidly they grew and behavioral characteristics such as range size and reproduction. Oxygen isotope values of bone phosphate may be used to determine the relative temperature variations experienced by skeletal regions during bone deposition. Temperature variations relate to an animal’s thermal physiology and can be used to estimate their metabolic physiology. Previously, we reported (Barrick and Showers 1994) on the thermophysiology of Tyrannosaurus rex using this methodology. Here, we present the results of the even larger South American carnivorous dinosaur Giganotosaurus carolinii. Comparisons of the isotopic patterns are used as a basis for a preliminary discussion of the biology of these large theropods. Data support the interpretation that both theropods support homeothermy by means of intermediate metabolic rates.
Reese E. Barrick. Department of Marine, Earth and Atmospheric Sciences, North Carolina State University, Raleigh, North Carolina, 27695-8208, USA. reese_barrick@ncsu.edu
William J. Showers. Department of Marine, Earth and Atmospheric Sciences, North Carolina State University, Raleigh, North Carolina, 27695-8208, USA. w_showers@ncsu.edu
KEY WORDS: dinosaur, oxygen isotopes, metabolism, bones, vertebrate, Giganotosaurus,
Tyrannosaurus, diet, growth, physiology
Final citation: Barrick, Reese E. and William J. Showers 1999. Thermophysiology and Biology of Giganotosaurus: Comparison with Tyrannosaurus. Palaeontologia Electronica, 2.2.12, 21 pp. https://doi.org/10.26879/99012
https://palaeo-electronica.org/content/2-2-giganotosaurs-and-tyrannosaurus
Copyright: October 1999 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Giganotosaurus, at 12.5 m in length and ~8000 kg estimated mass (Coria and Salgado 1995) is similar in size to Tyrannosaurus rex (~6000 kg, Anderson et al., 1985) and is the largest theropod known. The type specimen of Giganotosaurus carolinii was recovered from the Upper Cretaceous fluvial deposits of the Rio Limay Formation (Albian-Cenomanian), Neuquen Group, deposited at southern mid-paleolatitudes in the eastern part of Neuquen Province, Argentina (Coria and Salgado, 1995; Scotese, 1997). It has been assigned to the Carcharodontosauridae, a group of advanced allosauroids also known from the middle Cretaceous of Africa and North America (Sereno et al. 1996; Harris 1998). The large size of this theropod suggests biological comparisons including thermal physiology and food requirements with the northern hemisphere tyrannosaurs.
Barrick and Showers (1994) used oxygen isotope values to delineate the thermal physiology of Tyrannosaurus rex. They concluded that T. rex was a homeotherm (maintained a pattern of stable core body temperatures) by virtue of a metabolic rate elevated above the modern reptilian level. It was argued that the data were the result of diagenetic alteration (Kolodny et al. 1996) and that homeothermy in dinosaurs is the result of gigantothermy (Paladino et al. 1997). The case against diagenetic alteration has been outlined in Barrick et al. (1996) and Barrick (1998). Two papers (Barrick and Showers 1995; Barrick et al., 1996) have shown that patterns of homeothermy may be found in juvenile and small adult dinosaurs (20-150 kg) as well as in large individuals (2000-4000 kg). These dinosaurs did not live in a completely homogenous environment with respect to temperature, thus simple mass homeothermy or gigantothermy (patterns of stable core body temperatures maintained in bradymetabolic animals utilizing large body size and homogenous environmental temperatures) is not an adequate explanation for isotopic patterns seen in these dinosaurs. Barrick et al. (1996) and Reid (1996) have suggested intermediate metabolic levels for the Dinosauria. However, the isotopic patterns in a large theropod have yet to be repeated. In this paper we report the results from a Giganotosaurus carolinii individual and compare them to the pattern of isotope distribution found in the previously studied T. rex individual.
METHODS
Eighty-two samples were analyzed from 13 bones in Giganotosaurus compared to 54 samples from 12 bones in T. rex (Barrick and Showers, 1994). The proximal and distal ends of a rib, femur, tibia and pubis were sampled to search for temperature trends along the length of these bones. Heterogeneity in the oxygen isotope value of bone phosphate (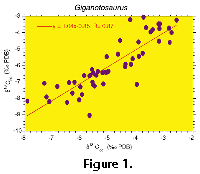 Δδp) within skeletal elements is used to calculate intrabone temperature variability while differences in the mean values between skeletal elements are used to determine interbone temperature differences. For these calculations, Δδp was multiplied by the slope of Longinelli and Nuti’s (1973) phosphate paleotemperature equation (i.e., 4.3).
Δδp) within skeletal elements is used to calculate intrabone temperature variability while differences in the mean values between skeletal elements are used to determine interbone temperature differences. For these calculations, Δδp was multiplied by the slope of Longinelli and Nuti’s (1973) phosphate paleotemperature equation (i.e., 4.3).
An assessment of the isotopic integrity of the fossil bone material was made using comparisons of the co-existing structural carbonate (δsc), secondary calcite cements (δcc) with the bone phosphate (Fig. 1.1-3) as suggested by (Barrick and Showers 1995; Barrick et al. 1996; Barrick 1998). The carbon isotope signature indicates that after burial, during recrystallization of the bone apatite crystals, the structural carbonate ions were exchanged with carbonate ions from the diagenetic groundwater (r=0.87) and that oxygen atoms within the ions were exchanged (r=0.80). The solubility of apatite decreases significantly after recrystallization (Grupe 1988; Trueman and Benton 1997) essentially making the structural carbonate of the recrystallized carbonate fluorapatite impervious to secondary diagenetic events as it also does to trace elements incorporated during recrystallization (Williams 1988; Wright et al. 1987; Trueman and Benton 1997). The pronounced covariation of cement to structural carbonate carbon and oxygen isotope values precludes more than one major diagenetic event affecting the isotopic composition of the structural carbonate and carbonate cements. If the phosphate ions were significantly exchanged during this process, it is expected that δp will covary with δcc or δsc indicating re-equilibration with the diagenetic fluids. This is not the case as seen in Figure 1.3 where the covariance is very weak (r=0.02), similar to the case seen in T. rex. Thus, complete equilibration of δp with diagenetic fluids is precluded. In addition, an isotopic comparison was made between the cancellous and compact bone samples. Cancellous bone is more susceptible to alteration than compact bone because of its greater exposed surface area as shown in rare earth element studies (Grupe 1988). Thus, partial alteration of δp would result in different isotope ratios between the cancellous and compact bone. This is not apparent, for the mean δp for 48 dense samples is 17.4‰ with a s1 of 0.66 and 34 cancellous samples have a mean value of 17.5‰ with a σ1 of 0.50. There is a 2.2‰ total range in the isotopic values of Giganotosaurus while only a 0.1‰ difference in the mean dense and cancellous δ18Op values. This precludes partial alteration of the δ18Op values and thus in conjunction with the co-existing carbonate and phosphate data, isotopic preservation is interpreted for these bones.
RESULTS
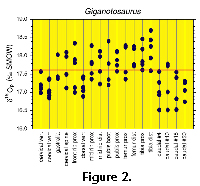 The δ18O values for each bone of Giganotosaurus are shown in Figure 2. Intrabone and interbone temperature variability calculated from the isotope values are shown in Figure 3. The vertebrae have the lowest δ18O values and thus represent the warmest skeletal elements. The cervical and dorsal centra show ~0.6‰ isotopic variability which corresponds to a 2.5°C intrabone temperature range. The caudal vertebrae have greater isotopic heterogeneities, ranging between 0.5-1.2‰ (2.5-5.0°C). There is less than 0.2‰ interbone isotopic difference between all of the vertebral centra, representing very little mean temperature differences between the vertebrae (<1°C). The neural spine of one cervical vertebra is ~0.5‰ more positive (~2°C cooler) than that of its associated centrum. The neural spine also has a slightly greater isotopic heterogeneity.
The δ18O values for each bone of Giganotosaurus are shown in Figure 2. Intrabone and interbone temperature variability calculated from the isotope values are shown in Figure 3. The vertebrae have the lowest δ18O values and thus represent the warmest skeletal elements. The cervical and dorsal centra show ~0.6‰ isotopic variability which corresponds to a 2.5°C intrabone temperature range. The caudal vertebrae have greater isotopic heterogeneities, ranging between 0.5-1.2‰ (2.5-5.0°C). There is less than 0.2‰ interbone isotopic difference between all of the vertebral centra, representing very little mean temperature differences between the vertebrae (<1°C). The neural spine of one cervical vertebra is ~0.5‰ more positive (~2°C cooler) than that of its associated centrum. The neural spine also has a slightly greater isotopic heterogeneity. 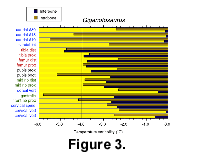 Thus, while the caudal 4 vertebra exhibits the most negative mean value, there is no significant difference in temperatures (±0.3°C) for the vertebral centra. The ribs have higher δ18O values with more intrabone variability than the vertebrae, including the neural spine. The two ribs sampled are between 2.5-3.0°C cooler than the vertebrae and intrabone temperature variability is ~3-4°C with no difference between ribs from the anterior and middle sections of the rib cage. Gastralia show a nearly 6°C intrabone temperature range. There is no apparent difference in mean temperature or variability between the proximal and distal ends of the rib from near the middle of the rib cage. The limbs show greater isotopic variability than the ribs. The femur displays 2-4°C intrabone temperature variability with no difference between the proximal and distal ends of the shaft, and displays a mean temperature over 3.5°C colder than the vertebrae. The tibia is 4-5°C cooler than the vertebrae and is coldest at its distal end. Heterogeneity in the δ18O values suggests an increase in temperature variability from the proximal to distal end of the shaft of 3.5 to 4.5°C. Pubis mean temperatures remained between 2.5-3.5°C cooler than the vertebrae with intrabone temperature variability of 2-5°C.
Thus, while the caudal 4 vertebra exhibits the most negative mean value, there is no significant difference in temperatures (±0.3°C) for the vertebral centra. The ribs have higher δ18O values with more intrabone variability than the vertebrae, including the neural spine. The two ribs sampled are between 2.5-3.0°C cooler than the vertebrae and intrabone temperature variability is ~3-4°C with no difference between ribs from the anterior and middle sections of the rib cage. Gastralia show a nearly 6°C intrabone temperature range. There is no apparent difference in mean temperature or variability between the proximal and distal ends of the rib from near the middle of the rib cage. The limbs show greater isotopic variability than the ribs. The femur displays 2-4°C intrabone temperature variability with no difference between the proximal and distal ends of the shaft, and displays a mean temperature over 3.5°C colder than the vertebrae. The tibia is 4-5°C cooler than the vertebrae and is coldest at its distal end. Heterogeneity in the δ18O values suggests an increase in temperature variability from the proximal to distal end of the shaft of 3.5 to 4.5°C. Pubis mean temperatures remained between 2.5-3.5°C cooler than the vertebrae with intrabone temperature variability of 2-5°C.
DISCUSSION
There are several similarities in the isotopic patterns of Giganotosaurus and Tyrannosaurus with one major difference. In both dinosaurs, the vertebrae are the warmest (lowest ![]() 18O values) bones sampled. The cervicals and dorsal from Giganotosaurus centra show less variability than in the T. rex specimen. Previously, the ribs and dorsal vertebrae were considered as "core" bones representative of the warmest body temperatures. However, the mid section and distal end of ribs are in the outer shell of the body and may represent temperatures more similar to those of the extremities than the core body. In T. rex, values from the gastralia were similar to those from the femur (Barrick and Showers 1994). In both T. rex and Giganotosaurus, the ribs are 2-4°C cooler than the vertebra. The Giganotosaurus gastralia do have greater intrabone variability than in the T. rex individual. Both individuals display regional heterothermy in the limbs with cooler temperatures and greater isotopic variability occurring with greater distance from the core body. In both cases the femora are 3-4°C cooler than the vertebrae and the tibia 4-5°C cooler than the vertebrae. Only in Giganotosaurus were proximal and distal ends of these bones compared. No difference in mean temperature or variability was found along the femur while the tibia exhibited a trend of increasing temperature variability (1°C) and cooler mean temperature (1°C) distally along the shaft. Unfortunately there were no pedal elements preserved in Giganotosaurus. The consistency in trends of increasing isotopic variability and decreasing mean temperature distally along the limb elements between the Giganotosaurus and Tyrannosaurus suggest that the foot in Giganotosaurus was likely cooler and more variable than the tibia. Thus, the δ18O values between the dorsal/cervical vertebrae, ribs and limbs of both animals are strikingly similar suggesting similar thermoregulatory patterns. However, the δ18O values in the caudal vertebrae differ significantly. In T. rex, the caudal vertebrae exhibit similar intrabone patterns and gradually become more positive (~0.5-4°C cooler) with distance from the torso (Barrick and Showers 1994). In Giganotosaurus, however the caudal vertebrae exhibit the same mean temperature values as the dorsal and cervical vertebrae. The mean values from the proximal caudals resembled those of the dorsal vertebrae in both individuals. Yet in T. rex intrabone temperature variability in the 3 caudals analyzed (~2°C) was less than that in 4 Giganotosaurus caudals ranging from 2.5-5.5°C.
18O values) bones sampled. The cervicals and dorsal from Giganotosaurus centra show less variability than in the T. rex specimen. Previously, the ribs and dorsal vertebrae were considered as "core" bones representative of the warmest body temperatures. However, the mid section and distal end of ribs are in the outer shell of the body and may represent temperatures more similar to those of the extremities than the core body. In T. rex, values from the gastralia were similar to those from the femur (Barrick and Showers 1994). In both T. rex and Giganotosaurus, the ribs are 2-4°C cooler than the vertebra. The Giganotosaurus gastralia do have greater intrabone variability than in the T. rex individual. Both individuals display regional heterothermy in the limbs with cooler temperatures and greater isotopic variability occurring with greater distance from the core body. In both cases the femora are 3-4°C cooler than the vertebrae and the tibia 4-5°C cooler than the vertebrae. Only in Giganotosaurus were proximal and distal ends of these bones compared. No difference in mean temperature or variability was found along the femur while the tibia exhibited a trend of increasing temperature variability (1°C) and cooler mean temperature (1°C) distally along the shaft. Unfortunately there were no pedal elements preserved in Giganotosaurus. The consistency in trends of increasing isotopic variability and decreasing mean temperature distally along the limb elements between the Giganotosaurus and Tyrannosaurus suggest that the foot in Giganotosaurus was likely cooler and more variable than the tibia. Thus, the δ18O values between the dorsal/cervical vertebrae, ribs and limbs of both animals are strikingly similar suggesting similar thermoregulatory patterns. However, the δ18O values in the caudal vertebrae differ significantly. In T. rex, the caudal vertebrae exhibit similar intrabone patterns and gradually become more positive (~0.5-4°C cooler) with distance from the torso (Barrick and Showers 1994). In Giganotosaurus, however the caudal vertebrae exhibit the same mean temperature values as the dorsal and cervical vertebrae. The mean values from the proximal caudals resembled those of the dorsal vertebrae in both individuals. Yet in T. rex intrabone temperature variability in the 3 caudals analyzed (~2°C) was less than that in 4 Giganotosaurus caudals ranging from 2.5-5.5°C.
The features indicative of endothermy (defined as the maintenance a high and constant body temperature by metabolic means by Bennett and Ruben 1979) in Giganotosaurus include the homeothermy of the cervical and dorsal vertebra and regional heterothermy of the limbs. The pattern is even more suggestive than for T. rex as the core (vertebral) temperature range is nearly 1.5°C narrower in Giganotosaurus. Clarke and Jenkyns (1999) report maximum Albian –Cenomanian sea-surface temperatures of 16-18°C for southern latitudes of 49°-53°S. This strongly suggests that the terrestrial climate was temperate where Giganotosaurus roamed in the Neuquen basin. Such a climate provides evidence against the warm homogenous environment in which gigantothermy could provide stable core body temperatures. In order to maintain the thermoregulatory pattern seen in Giganototsaurus, an elevated metabolic rate is required. However, the pattern of high intrabone variability and no mean interbone differences in the tail is also typical of a mass homeotherm (Barrick et al. 1997). Core homeothermy and regional limb heterothermy indicate that both individuals maintained metabolic levels above those of modern reptiles. While core homeothermy within a temperate climate fits the definition of endothermy it is likely that these dinosaurs possessed intermediate metabolic levels rather than modern mammalian levels. A drop in mass-specific metabolic rates between juveniles and adults at this intermediate level would also explain the mass homeotherm-like isotopic pattern seen in the Giganotosaurus tail. In adults, bone turnover rates in vertebrae are much more rapid than in limb bones (Francillon-Vieillot et al. 1990) and reflect a narrower time of bone deposition. Thus, a change in thermoregulatory style would be presented in the vertebrae more rapidly than in the limbs. Endothermy has come to be synonymous with mammalian and avian tachymetabolism where metabolic rates are 10x greater than for ectotherms (e.g., Ruben 1995). Endogenous heat production through elevated metabolic rates is required for endothermy, but intermediate levels (e.g. 5x metabolic rate of ectotherms) may have been high enough for the maintenance of homeothermy in dinosaurs, thus making them at least intermediate endotherms.
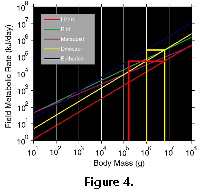 At an adult body mass of 8000 kg, even if Giganotosaurus began life with a modern avian metabolic level, it would have had an intermediate metabolic rate the equivalent of a 1000 kg mammalian carnivore. At 6000 kg, the metabolic rate of T. rex would have been equivalent to an 800 kg mammal. The dinosaurian metabolic rate is calculated by projecting the mass vs. field metabolic rate regression of modern avians, mammals, and reptilians (Nagy 1987) to a mass of 6000 and 8000 kg (Fig. 4) and projecting a hypothesized dinosaur regression midway between the reptilian and eutherian mammal regression lines. In fact, at this weight (~7000 kg), the avian regression line falls midway between the projected eutherian mammal and reptilian regression lines, intersecting the hypothesized intermediate dinosaurian regression line. Theropods are more closely related to birds (Ji-Qiang et al. 1998) than other dinosaurs and a projected intermediate metabolic level for 6000-8000 kg birds, fits well with the interpretation of intermediate metabolic levels for these large theropods based on their
At an adult body mass of 8000 kg, even if Giganotosaurus began life with a modern avian metabolic level, it would have had an intermediate metabolic rate the equivalent of a 1000 kg mammalian carnivore. At 6000 kg, the metabolic rate of T. rex would have been equivalent to an 800 kg mammal. The dinosaurian metabolic rate is calculated by projecting the mass vs. field metabolic rate regression of modern avians, mammals, and reptilians (Nagy 1987) to a mass of 6000 and 8000 kg (Fig. 4) and projecting a hypothesized dinosaur regression midway between the reptilian and eutherian mammal regression lines. In fact, at this weight (~7000 kg), the avian regression line falls midway between the projected eutherian mammal and reptilian regression lines, intersecting the hypothesized intermediate dinosaurian regression line. Theropods are more closely related to birds (Ji-Qiang et al. 1998) than other dinosaurs and a projected intermediate metabolic level for 6000-8000 kg birds, fits well with the interpretation of intermediate metabolic levels for these large theropods based on their ![]() p distribution. The thermoregulatory pattern in Giganotosaurus corroborates the interpretation based on the pattern seen in T. rex. If we assume that all adults of Giganotosaurus and Tyrannosaurus reflect the same thermoregulatory patterns as the two individuals sampled, they would have enjoyed the benefits of endothermy at metabolic levels intermediate between those of modern mammals and reptilians. An extension of this interpretation is that juvenile theropods also maintained intermediate if not high metabolic levels. Comparatively, an 8000 kg lizard or crocodile would have the same metabolic food requirements as a 200 kg carnivorous mammal (i.e., medium sized male African lion Panthera leo). Food requirements for various sized eutherian mammals can be calculated from the generalized equation y=axb where y is the food requirement in g/day, a=0.235, x is the mass of the individual in g, and b=0.822 (eq. 19 in Nagy 1987). By converting dinosaurian or reptilian metabolic rates to equivalent mammalian metabolic rates this single equation can be used to estimate food requirements for these dinosaurs. Using the intermediate metabolic rates for Giganotosaurus (8000 kg) and Tyrannosaurus (6000 kg), their food requirements would have been 20 and 17 kg/day respectively. This is the same food requirement for 3-4 large male tigers or African lions. Modern male lions (Panthera leo) reach only ~250 kg while large male tigers (Panthera tigris) may reach just over 300 kg (Walkers Mammal’s of the World, 1991). For comparative purposes, had Giganotosaurus maintained a strictly reptilian metabolic rate, it would have required 5.5 kg/day, whereas a Giganotosaurus individual with a eutherian metabolic rate would have required 111 kg/day of food. Thus, for under 4x rather than 20x the food requirements of a similarly sized bradymetabolic ectotherm or mass homeotherm, Giganotosaurus enjoyed the benefits of endothermy. These benefits include, core homeothermy 365 days a year, higher activity levels, and rapid growth rates. Concomitantly, these dinosaurs would also have kept some of the benefits typical of bradymetabolic ectotherms (e.g., lower food requirements, greater percentage of energy budget directed toward growth and reproduction). Table 1 records the food requirements of a tyrannosaur or giganotosaur at various body sizes throughout ontogeny assuming this intermediate metabolic rate. Each adult tyrannosaur or giganotosaur would have required the equivalent of 2 large ornithopods or 1 subadult sauropod (~7000 kg) per year. The amount of food required to sustain a metabolism high enough to maintain core homeothermy in these animals is much smaller than their stomach volume. Thus, these dinosaurs could have eaten much more than required for maintenance of their metabolism. A large male African lion (Panthera leo) weighing 250 kg may eat up to 40 kg of meat in one meal (Walker’s Mammals of the World, 1991) which is enough meat to support it for 7-8 days. 6000-8000 kg theropods could have held 10 times this amount of food in their stomachs. Thus, these dinosaurs could have stored 2-3 weeks food requirements in their stomachs with a single large meal. A truly bradymetabolic tyrannosaur or giganotosaur needing but 2000 kg of meat per year could have survived on 5 good meals/year. Evidence of relatively unaltered bone in a tyrannosaur coprolite indicates that residence time of food in these theropod digestive tracts was short (Chin et al. 1998; Bartlett et al. 1998) suggesting that these dinosaurs ate more often than once every 2-3 weeks. Evidence of soft tissue in dinosaur coprolites suggest even shorter residence times (Bertrand 1903; Chin 1999). Greater food intake would have supported increased activity levels and or growth rates. An intermediate metabolic level has important implications for growing juvenile theropods. For example, 100 to 1000 kg growing theropods would have needed 1-6 kg of food/day while 2000-5000 kg individuals would have needed 10-16 kg/day. Much greater amounts of food would have been available to these individuals and could have been held in their digestive systems. Energy from greater food acquisition greater than required by field metabolism could be put directly into growth, as the maintenance of homeothermy would have allowed continuous annual growth. Thus, greater amounts of energy were available for growth in these theropods than is available for mammalian style endotherms. More continuous, rapid growth due to maintenance of homeothermy would have been available for these dinosaurs than is available for bradymetabolic ectotherms. Curry (1998) and Ricqles et al. (1998) have suggested extremely rapid growth rates for Apatosaurus (=75% adult size in 7-10 yrs) and Maiasaura (adult size in 8 yrs or less) based upon histological analyses. Intermediate metabolic rates suggested here, allowing the maintenance of core homeothermy at all sizes, would have supported similarly rapid growth rates for these theropods as is suggested for Apatosaurus and Maiasaura. Intermediate metabolic rates are also consistent with the isotopic patterns of several herbivorous dinosaurs (Barrick et al. 1996, 1998). On the other hand, this intermediate metabolic strategy would have these individuals to survive longer periods during resource scarcity than true modern endotherms.
p distribution. The thermoregulatory pattern in Giganotosaurus corroborates the interpretation based on the pattern seen in T. rex. If we assume that all adults of Giganotosaurus and Tyrannosaurus reflect the same thermoregulatory patterns as the two individuals sampled, they would have enjoyed the benefits of endothermy at metabolic levels intermediate between those of modern mammals and reptilians. An extension of this interpretation is that juvenile theropods also maintained intermediate if not high metabolic levels. Comparatively, an 8000 kg lizard or crocodile would have the same metabolic food requirements as a 200 kg carnivorous mammal (i.e., medium sized male African lion Panthera leo). Food requirements for various sized eutherian mammals can be calculated from the generalized equation y=axb where y is the food requirement in g/day, a=0.235, x is the mass of the individual in g, and b=0.822 (eq. 19 in Nagy 1987). By converting dinosaurian or reptilian metabolic rates to equivalent mammalian metabolic rates this single equation can be used to estimate food requirements for these dinosaurs. Using the intermediate metabolic rates for Giganotosaurus (8000 kg) and Tyrannosaurus (6000 kg), their food requirements would have been 20 and 17 kg/day respectively. This is the same food requirement for 3-4 large male tigers or African lions. Modern male lions (Panthera leo) reach only ~250 kg while large male tigers (Panthera tigris) may reach just over 300 kg (Walkers Mammal’s of the World, 1991). For comparative purposes, had Giganotosaurus maintained a strictly reptilian metabolic rate, it would have required 5.5 kg/day, whereas a Giganotosaurus individual with a eutherian metabolic rate would have required 111 kg/day of food. Thus, for under 4x rather than 20x the food requirements of a similarly sized bradymetabolic ectotherm or mass homeotherm, Giganotosaurus enjoyed the benefits of endothermy. These benefits include, core homeothermy 365 days a year, higher activity levels, and rapid growth rates. Concomitantly, these dinosaurs would also have kept some of the benefits typical of bradymetabolic ectotherms (e.g., lower food requirements, greater percentage of energy budget directed toward growth and reproduction). Table 1 records the food requirements of a tyrannosaur or giganotosaur at various body sizes throughout ontogeny assuming this intermediate metabolic rate. Each adult tyrannosaur or giganotosaur would have required the equivalent of 2 large ornithopods or 1 subadult sauropod (~7000 kg) per year. The amount of food required to sustain a metabolism high enough to maintain core homeothermy in these animals is much smaller than their stomach volume. Thus, these dinosaurs could have eaten much more than required for maintenance of their metabolism. A large male African lion (Panthera leo) weighing 250 kg may eat up to 40 kg of meat in one meal (Walker’s Mammals of the World, 1991) which is enough meat to support it for 7-8 days. 6000-8000 kg theropods could have held 10 times this amount of food in their stomachs. Thus, these dinosaurs could have stored 2-3 weeks food requirements in their stomachs with a single large meal. A truly bradymetabolic tyrannosaur or giganotosaur needing but 2000 kg of meat per year could have survived on 5 good meals/year. Evidence of relatively unaltered bone in a tyrannosaur coprolite indicates that residence time of food in these theropod digestive tracts was short (Chin et al. 1998; Bartlett et al. 1998) suggesting that these dinosaurs ate more often than once every 2-3 weeks. Evidence of soft tissue in dinosaur coprolites suggest even shorter residence times (Bertrand 1903; Chin 1999). Greater food intake would have supported increased activity levels and or growth rates. An intermediate metabolic level has important implications for growing juvenile theropods. For example, 100 to 1000 kg growing theropods would have needed 1-6 kg of food/day while 2000-5000 kg individuals would have needed 10-16 kg/day. Much greater amounts of food would have been available to these individuals and could have been held in their digestive systems. Energy from greater food acquisition greater than required by field metabolism could be put directly into growth, as the maintenance of homeothermy would have allowed continuous annual growth. Thus, greater amounts of energy were available for growth in these theropods than is available for mammalian style endotherms. More continuous, rapid growth due to maintenance of homeothermy would have been available for these dinosaurs than is available for bradymetabolic ectotherms. Curry (1998) and Ricqles et al. (1998) have suggested extremely rapid growth rates for Apatosaurus (=75% adult size in 7-10 yrs) and Maiasaura (adult size in 8 yrs or less) based upon histological analyses. Intermediate metabolic rates suggested here, allowing the maintenance of core homeothermy at all sizes, would have supported similarly rapid growth rates for these theropods as is suggested for Apatosaurus and Maiasaura. Intermediate metabolic rates are also consistent with the isotopic patterns of several herbivorous dinosaurs (Barrick et al. 1996, 1998). On the other hand, this intermediate metabolic strategy would have these individuals to survive longer periods during resource scarcity than true modern endotherms.
CONCLUSIONS
Giganotosaurus and Tyrannosaurus were two of the largest terrestrial carnivores ever to walk the Earth. Living, respectively in South America and North America, these creatures represent separate end members of large theropod evolution (Sereno et al. 1996). Yet the two large theropods sampled here and in Barrick and Showers (1994) that lived at similar latitudes (~50°) on opposite sides of the equator exhibit remarkably similar bone oxygen isotope patterns. This would be a very difficult pattern for random diagenetic processes to replicate even without considering coexisting phase and dense/cancellous tests, which also indicate preservation of the biologic signal in these individuals. The ![]() p values suggest that both individuals displayed (with the exception of the distal end of the tail) very similar heat distribution and thus thermoregulatory patterns. Given their body sizes, the thermoregulatory patterns (core homeothermy and greater limb heterothermy) seen in these two individuals strongly suggest to us that, as adults, they maintained metabolic levels midway between those of present mammals and reptiles. These intermediate metabolic levels would have supported homeothermy in the greenhouse world of the Cretaceous and would have supported very rapid growth rates in these theropods.
p values suggest that both individuals displayed (with the exception of the distal end of the tail) very similar heat distribution and thus thermoregulatory patterns. Given their body sizes, the thermoregulatory patterns (core homeothermy and greater limb heterothermy) seen in these two individuals strongly suggest to us that, as adults, they maintained metabolic levels midway between those of present mammals and reptiles. These intermediate metabolic levels would have supported homeothermy in the greenhouse world of the Cretaceous and would have supported very rapid growth rates in these theropods.
ACKNOWLEDGMENTS
The authors wish to thank Rodolfo Coria for his gracious help with access for sampling Giganotosaurus and discussions on dinosaurian paleontology of Argentina. We also wish to thank Dale Russell for extensive discussions on theropod biology.
REFERENCES
Anderson, J.F., Hall-Martin, A., and Russell, D.A., 1985. Long-bone circumference and weight in mammals, birds and dinosaurs: Journal of Zoology, v. 207, p. 53-61.
Barrick, R.E., and Showers, W.J., 1994. Thermophysiology of Tyrannosaurus rex: Evidence from oxygen isotopes: Science v. 265, p. 222-224.
Barrick, R.E. and Showers, W.J., 1995. Oxygen isotope variability in juvenile dinosaurs (Hypacrosaurus): Evidence for thermoregulation: Paleobiology v. 21, p. 552-560.
Barrick, R.E., Showers, W.J., and Fischer, A.G., 1996. Comparison of Thermoregulation of four ornithischian dinosaurs and a varanid lizard from the Cretaceous Two Medicine Formation: Evidence from oxygen isotopes: PALAIOS v. 11, p. 295-305.
Barrick, R.E., Stoskopf, M. and Showers, W.J. 1997. Oxygen Isotopes in Dinosaur Bones. In; The Complete Dinosaur, Farlow, J.O. and Brett- Surman, M., eds., Indiana University Press, Bloomington, p.474-490.
Barrick, R.E., 1998. Isotope Paleobiology of the Vertebrates: Ecology, Physiology, and Diagenesis, in Isotope Paleobiology and Paleoecology, The Paleontological Society Papers v. 4, p. 101-137.
Bartlett, J.A., Barrick, R.E., Lamb, J.P., Russell, D.A. and Straight, W.J., 1998. Digestive evidence for metabolism from carnivore coprolites: Abstracts of Papers, 58th Annual meeting of the Society of Vertebrate Paleontology, 26A.
Bennett, A.F., and Ruben, J.A., 1979. Endothermy and activity in vertebrates: Science, v. 206, p. 649-654.
Bertrand, C.E., 1903. Les Coprolithes de Bernissart. Mem. Mus. R. Hist Nat. Belg., v. 1, p. 1-154.
Chin, K., Tokaryk, T.T., Erickson, G.M., and Calk, L.C., 1998. A king-sized theropod coprolite: Science v. 393, p. 680-682.
Chin, K., 1999. Exceptional soft-tissue preservation in a theropod coprolite from the upper Cretaceous Dinosaur Park Formation of Alberta: Journal of Vertebrate Paleontology Abstracts of Papers, 59th Annual Mtg., p. 37A.
Clarke, L.J., and Jenkyns, H.C., 1999. New oxygen isotope evidence for long-term Cretaceous climatic change in the Southern Hemisphere: Geology, v. 27, p. 699-702.
Coria, R. A. and L. Salgado 1995. A new giant carnivorous dinosaur from the Cretaceous of Patagonia. Nature 377: 225-226.
Curry, K., 1998. Histological quantification of growth rates in Apatosaurus: Abstracts of Papers, 58th Annual meeting of the Society of Vertebrate Paleontology, 36A.
Francillon-Vieillot, H. et al., 1990. Microstructure and mineralization of vertebrate skeletal tissues, in J. Carter (ed.), Skeletal Biomineralization, Van Nostrand-Reinhold, New York, p. 471-530.
Grupe, G., 1988. Impact of the choice of bone samples on trace element data in excavated human skeletons: Journal of Archaeological Science v. 15, p. 123-129.
Harris, J. D., 1998. A re-analysis of Acrocanthosaurus atokensis, its phylogenetic status, and paleobiogeographic implications, based on a new specimen from Texas. New Mexico Museum of Natural History and Science, Bulletin 13, iii + 75 p.
Kolodny, Y, Luz, B, Sander, M., and Clemens, W.A., 1996. Dinosaur bones: fossils or pseudomorphs? The pitfalls of physiology reconstruction from apatitic fossils: Palaeogeography, Palaeoclimatology, Palaeoecology v. 126, p. 161-171.
Longinelli, A., Nuti, S., 1973. Revised phosphate-water isotopic temperature scale: Earth Planetary Science Letters, v. 19, p. 373-376.
Nagy, K.A., 1987. Field metabolic rate and food requirement scaling in mammals and birds: Ecological Monographs, v. 57,p.111-128.
Person, A., Bocherens, H., Mariotti, A., Renard, M., 1996. Diagenetic evolution and experimental heating of bone phosphate: in, Biogenic phosphates as paleoenvironmental indicators, Longinelli, A., ed., Palaeogeography, Palaeoclimatology, Palaeoecology v.126, p. 135-149.
Paladino, F.V., Spotila, J.R., and Dodson, P., 1997. A blueprints for giants: Modeling the physiology of large dinosaurs: In; The Complete Dinosaur, Farlow, J.O. and Brett-Surman, M., eds., Indiana University Press, Bloomington, p. 491-504.
Ji-Qiang; Currie-Philip-J; Norell-Mark-A; Ji-Shuan , 1998. Two feathered dinosaurs from northeastern China: Nature, v. 393, p. 753-761.
Reid, R.E., 1996. Dinosaurian physiology: the case for intermediate dinosaurs, in Farlow, J.O., and Brett-Surman, M.K., eds., The Complete Dinosaur, Indiana Univ. Press, p. 449-473.
Ricqles, A. de, Horner, J.R., and Padian, K., 1998. Growth dynamics of the hadrosaurid dinosaur Maiasaura peeblesorum: Abstracts of Papers, 58th Annual meeting of the Society of Vertebrate Paleontology, 72A.
Ruben, J., 1995, The evolution of endothermy in mammals and birds: from physiology to fossils: Annual Reviews of physiology v. 57, p. 69-95.
Scotese, C. R. 1997. Paleogeographic Atlas, PALEOPMAP Progress Report 90-0497, PALEOMAP Project, University of Texas, Arlington.
Sereno, P. C., D. B. Dutheil, M. Iarochene, H. C. E. Larsson, G. H. Lyon, P. M. Magwene, C. A. Sidor, D. J. Varricchio and J. A. Wilson 1996. Predatory dinosaurs from the Sahara and Late Cretaceous faunal differentiation: Science 272 : 986-991..
Trueman, C.N., and Benton, M.J., 1997. A geochemical method to trace the taphonomic history of reworked bones in sedimentary settings: Geology v. 25, p. 263-266.
Walker’s Mammals of the World, 5th edition, volume II, p. 1209-1218, Johns Hopkins Univ. Press, Baltimore, 1991.
Williams, C.T., 1988. Alteration of chemical composition of fossil bones by soil processes and groundwater: in Grupe, G., and Herrmann, B., ed., Trace Elements in Environmental History: Springer-Verlag, p. 27-40.
Wright, J., Schrader, H., and Holser, W.T., 1987. Palaeoredox variations in ancient oceans recorded by rare earth elements in fossil apatite: Geochimica et Cosmochimica Acta v. 51, p. 631-644.

