APPENDIX 1. PALYNOLOGICAL DATING OF THE NEW ZEALAND MIOCENE
A regional marine regression was underway in New Zealand by the earliest Miocene and, therefore, coastal coal deposits tend to overlie dateable marine deposits, or are intercalated with them near the base of the sequence. In these cases, the dating of terrestrial sequences must be inferred (i.e., as somewhat younger) from the age of the underlying marine strata.
The Gore Lignite Measures lie in such a situation. These were zoned with palynology by Pocknall and Mildenhall (1984) with interfingering marine sediments having dateable foraminiferal assemblages providing time constraints (see summary in Isaac and Lindqvist, 1990). For the Rhoipites waimumuensis Zone, these fossils provide an Altonian age (16-19 Ma in Morgans et al., 2004). The overlying Proteacidites isopogiformis Zone is present in lignite at Kapuka, where it directly overlies marine sediment with early-mid Altonian forams (Pocknall, 1990). Despite this, the P. isopogiformis Zone was later inexplicably dated as early Otaian by Morgans et al. (2004). Pocknall (1990) also noted that the W6 coal seam at Newvale Coal Mine fell into the P. isopogiformis zone, and that the boundary of the next highest zone, the Tricolpites latispinosus Zone (later to become the Spinitricolpites latispinosus Zone, Mildenhall and Pocknall, 1989. For consistency, this form is used below), likely lay between the W6 and W7 seams. However, Lee et al. (2007, p. 566) stated that “Given the close association between marine strata of Waitakian age and the lower and middle Gore Lignite Measures in the Waimumu area (Isaac and Lindqvist, 1990), we consider that the [W6] lignite is probably Waitakian (Late Oligocene to early Miocene) in age.” The “close association” is the presence of marine facies (Chatton Formation) approximately 120 m stratigraphically below the W6 coal at the base of the Hedgehope Stream section. This has been dated with foraminifera as “Duntroonian or Waitakian” (Isaac and Lindqvist, 1990, p. 16) and placed in the Upper Nothofagidites matauraensis Zone by Pocknall and Mildenhall (1984). It is separated from the W6 seam by the R. waimumuesis Zone. The Hedgehope marine occurrence correlates with more Chatton Formation at the base of the Dolamore Park section, which has been dated with forams as “Duntroonian” (Isaac and Lindqvist 1990, p. 16) and also placed in the Upper N. matauraensis Zone. Therefore, the claim by Lee et al. (2007) of a Waitakian age for the W6 is far older than the likely late Altonian age of the P. isopogiformis Zone indicated at Kapuka. It is also unlikely, given that Waitakian marine is known to be associated with the Upper N. matauraensis Zone and is further separated from the P. isopogiformis Zone by the R. waimumuensis Zone. The assertion (Lee et al., 2007) that the “close” marine was Waitakian is unwarranted, given that it may be at least as likely, and perhaps more likely, Duntroonian.
 Two well-dated marine sequences in North Otago/South Canterbury have been palynologically documented and provide some input on dating the zonation. The Bluecliffs section of Otaian age contains (Pocknall, 1982a) Foveotriletes palaequetrus, a taxon defining the top of the Rhoipites waimumuensis Zone. Pocknall (1982a) also listed Proteacidites isopogiformis and Triporopollenites ambiguous, both defining the top of the P. isopogiformis Zone, as well as Rugulatisporites micraulaxis (noting it was a junior synonym of R. cowrensis ), a taxon defining the base of the Spinitricolpites latispinosus Zone. Pocknall himself regarded Bluecliffs as belong in the P. isopogiformis Zone. If this is the case, then added to the occurrence at Kapuka, the P. isopogiformis Zone includes both some Otaian and Altonian time (Figure 17). However, the other taxa indicate unresolved issues. Some, like F. palaequetrus, might be explained away by ‘recycling’, but may also be indicating the lack of clear knowledge of the ranges of relatively rare taxa.
Two well-dated marine sequences in North Otago/South Canterbury have been palynologically documented and provide some input on dating the zonation. The Bluecliffs section of Otaian age contains (Pocknall, 1982a) Foveotriletes palaequetrus, a taxon defining the top of the Rhoipites waimumuensis Zone. Pocknall (1982a) also listed Proteacidites isopogiformis and Triporopollenites ambiguous, both defining the top of the P. isopogiformis Zone, as well as Rugulatisporites micraulaxis (noting it was a junior synonym of R. cowrensis ), a taxon defining the base of the Spinitricolpites latispinosus Zone. Pocknall himself regarded Bluecliffs as belong in the P. isopogiformis Zone. If this is the case, then added to the occurrence at Kapuka, the P. isopogiformis Zone includes both some Otaian and Altonian time (Figure 17). However, the other taxa indicate unresolved issues. Some, like F. palaequetrus, might be explained away by ‘recycling’, but may also be indicating the lack of clear knowledge of the ranges of relatively rare taxa.
At the Riflebutts section of Altonian age Pocknall (1981) listed a variety of taxa thought to be restricted to the Spinitricolpites latispinosus Zone (Pocknall and Mildenhall 1984; Mildenhall and Pocknall, 1989), but also two taxa defining the top of the Proteacidites isopogiformis Zone; Monoporopolenites fossulus and Beaupredictes verrucosos. As with Bluecliffs, these later occurrences might indicate either recycling, or that they are not definitive of the top of the P. isopogiformis Zone.
The Manuherikia Group, as currently understood, does not contact any marine strata (Douglas, 1986), therefore dating is strongly reliant on palynological correlation with coastal sequences. It was first zoned palynologicaly by Mildenhall and Pocknall (1989). They recognized both the Proteacidites isopogiformis and Spinitricolpites latispinosus Zones that they had previously defined in the coastal Gore Lignite Measures. In addition, they introduced two, younger zones, the Chenopodipollis chenopodiaceoides Zone and the Podosporites erugatus Zone. Unlike their other zones, these were effectively abundance zones, not defined on first or last appearances of key taxa. This proceeded from their belief that there had been significant sediment loss (i.e., an ‘unconformity’) between the markedly different S. latispinosus and C. chenopodiaceoides palynological assemblages. Mildenhall and Pocknall (1989) inferred that this time gap was equivalent to much of the middle Miocene and, therefore, the C. chenopodiaceoides Zone was most likely late Miocene. In contrast, Pole and Douglas (1998) saw no sedimentological evidence of any significant erosional break across sections equivalent to the S. latispinosus and C. chenopodiaceoides Zones. The implication of this is that the Manuherikia Group is likely to span the Miocene, i.e., the middle Miocene is not absent. They also drew attention to significant environmental change, associated with a change in vegetation from widespread rainforest and coal-forming swamps, with acidic water and an absence of fire, to one where burning was extensive, herbfields were extensive, but coal-forming swamps absent, and waters were alkaline, leading to bone preservation and stromatolites. They suggested, and provided supporting evidence, that this change most likely correlated with the global middle Miocene cooling and drying event at around 14 Ma. On the basis that there was no stratigraphic loss in the sequence, Pole and Douglas (1998) proposed that a palynological zonation could be defined on the changing proportions of the major taxa. For example, this allowed definition of the base of an Asteraceae-Chenopodiaceae Zone, rather than the vague, overall composition definition of the Mildenhall and Pocknall (1989) C. chenopodiaceoides Zone.
Following this, Mildenhall et al. (2003) and Field et al. (2009) examined a series of palynological samples from marine sediment in the Bryce Burn in Southland, extending from high in the Cliffdenian, through the Lillburnian (c. 14.4-13.7 Ma). This is the type area for the middle Miocene New Zealand stages, and it might be expected to show evidence of any dramatic climate change around 14 Ma. However, no evidence of any significant vegetation change was found through the sequence and they, therefore, they concluded the 14 Ma date for the change argued by Pole and Douglas (1998) was either incorrect or represented only a local vegetation change.
The absence of any appreciable palynological change over the 14 Ma mark in the Bryce Burn sequence is extraordinary, as this appears to have been, globally, one of the most significant periods of change in the Neogene. It would be useful to have other, Southern Hemisphere, palynological sequences across this date for comparison, but so far these have been elusive. For example, a recent summary of Patagonian palynology (Palazzesi and Barreda, 2012) indicated that between 16 and 10 Ma, Nothofagus dropped significantly, Ephedra rose significantly, ‘amaranths’ and ‘asters’ increased slightly, but grasses hardly at all. However, sections covering 14 Ma appear to be absent. The uncertainty of just how this large climate shift played out around the world focusses attention on the results of Field et al. (2009). Their pollen counts are low and they cite an average of 52 and sometimes as low as 14. Full taxon lists were not given but at least 10 taxa (more were implied) were recognized as recycled from the Eocene as they were thought to be extinct by the Miocene. Despite the recycling, a further five taxa were considered to be genuine range extensions. The grounds for this must be tenuous, as Field et al. (2009) noted that all recovered spores and pollen are “badly etched and mechanically broken.” The presence of what Field et al. (2009) described as “abundant” terrestrial organic debris deposited into their estimated paleobathymetry of 1000-2000m, added to a significant amount of recognized palynological recycling, suggests the possibility that erosion of large amounts of peat/lignite occurred into the deep water Bryce Burn sequence. If this occurred, it may have overwhelmed any contemporaneous pollen influx and render any perceived palynological patterns meaningless. It is noted that Fleming et al. (1969) indicated detrital lignite and coal in part of the nearby Clifden section, a sequence with which Field et al. (2009) compare the palynological results of Bryce Burn. Though field observations of lignite and coal were restricted to the uppermost Altonian part of the Clifden section, the implications of this for palynological results on other parts of the section where lignite may have broken down to microscopic levels should be obvious.
Finding no marked palynological change in the Bryce Burn section, Mildenhall et al. (2003) suggested that the major vegetation change event apparent in the Manuherikia Group might correlate with “any one of 3 major Mid-late Miocene isotope events.” Miller et al. (1991) defined the Mi2 (16 Ma), Mi3 (13.6 Ma - i.e., the c. 14 Ma event) and Mi4 (12.6 Ma) events. Any of these dates would make no real difference to the basic claim of Pole and Douglas (1998) that there is an essentially continuous sedimentary record in the Manuherikia Group and the middle Miocene is well represented. However, correlation with the 16 Ma event would imply that the Spinitricolpites latispinosus Zone is almost entirely early Miocene and, therefore, there is a problem with the Mildenhall et al. (2003) application of it to the middle Miocene Bryce Burn. Correlation with the 12.6 Ma event, as well as Mildenhall et al.’s suggestion of a correlation with a “New Zealand-wide change in the vegetation which occurs in the late Miocene (Mildenhall 1980) at 8 Ma,” are simply incompatible with the Youngson et al. (1998) 13.4 Ma K-Ar date from well above the vegetation shift (see Pole, 2003, p.280-281)
A further development in the dating of the Miocene palynological zones was provided by Bannister et al. (2005). They provided results from a palynological sample of the Foulden Maar that is associated with volcanics radiometrically dated to 23-24 Ma, thus the Waitakian stage and close to the Oligocene-Miocene boundary. The palynological assemblage was suggested as being in the Spinitricolpites latispinosus Zone. Although none of the diagnostic taxa of the S. latispinosus Zone were listed, three taxa were present that are listed by Mildenhall and Pocknall (1989) as being restricted to that Zone: Ilex anguloclavatus. Podocarpidites puteus and P. torquatus. However, one taxon, Triporopollenites ambiguous defines the top of the Proteacidites isopogiformis Zone by its extinction, while two further taxa, Striatricolporites pseudostriatus and Tricolpites delicatulus, are only known from the younger Chenopodipollis chenopodiaceoides Zone or above. If the Foulden Maar does lie within the S. latispinosus Zone, its absolute age indicates serious issues with the palynological zonation system as summarised by Morgans et al. (2004) (the age makes it equivalent with what is thought to be the boundary of the earlier Rhoipites waimumuensis Zone and underlying Upper N. matauraensis Zone). It is likely that some of these issues derive from defining the S. latispinosus Zone by rare taxa, and then a “ballooning” of the Zone by including samples (and their taxa) into it, that do not include the key taxa.
Most recently, a novel approach to date the Manuherikia Group has been provided by Schwarzhans et al. (2012) in a study of fish otoliths from the sediments of fossil Lake Manuherikia. These come from the bone-rich beds that are one of the criteria Pole and Douglas (1998) used to recognize a major climate shift. Among the 14 otolith species described were two from marine fish. The most likely explanation for their presence in a fresh water deposit was that they were transported inland by predators, mostlikely fish-eating birds. This provides an independent method to link the fresh water Manuherikia Group with the marine realm. One otolith species, Lactarius sigmoidalis is known from Duntroonian-Lillburnian marine strata in New Zealand. The other, genus aff. Bleekeria sagittiformis, is known only from Altonian marine strata of New Zealand, although related taxa are known from the Clifdenian to Waiuan. Schwarzhans et al. (2012) therefore dated the bone bed as Altonian. The implication of this is that the bone-bearing unit, rather than being late Miocene, as implied by Mildenhall and Pocknall (1989 - it correlates with part of the Ranfurly d2019 drillhole and Gimmerburn/Haughton’s Hill drillhole shown in their figure 2 as falling within the Chenopodipollis chenopodiaceoides Zone), or middle Miocene as argued by Pole and Douglas (1998), it would be early to earliest middle Miocene. This would make the evidence of significant drying older than anywhere else it has ever been reported from. Although the results are intriguing, the apparent extinction of ‘genus aff. Bleekeria sagittiformis’ by the end of the Altonian is based on its absence from just four otolith assemblages of Clifdenian-Waiauan age. There is a reasonable chance that further collecting may extend its range. For now, on the bulk of the evidence, I remain in favour of a middle Miocene age (post 13.6 Ma) for the Casuarinaceae Zone (Figure 17).
The informal name for the location is followed by (in brackets) the New Zealand Geological Society locality number when available. Coordinates for leaf assemblages are given in terms of the New Zealand Transverse Mercator (NZTM) grid.
Manuherikia Group
Bannockburn-02 (F41/f208). A mudstone unit within sandstone of the Fiddlers Member, Dunstan Formation, Manuherikia Group. Much of this unit has been destroyed since it was collected by the construction of a water reservoir, although the unit continues further along strike (NZTM 1298658E, 5000414N). It is dominated by Nothofagus and has common Myrtaceae and Casuarinaceae (probably Allocasuarina ) (Campbell and Holden, 1984; Pole, 1993c, 1993d, 1993g).
Bannockburn-03 (F41/f214). A layer of shale flaking onto the road (NZTM 1298717E, 5000400N) is interdistributary bay sediment of the Cromwell Submember, Kawarau Member, of the Dunstan Formation, Manuherikia Group (see Douglas, 1986, figure 7.10). Although not specifically indicated on his diagram (figure 8.2 of Douglas, 1986), this location represents the gradation of distal Nevis Oil Shale Member with Kawarau Member (B.J. Douglas pers. comm.). The distinctive elements of the assemblage are Nothofagus, Araucaria, and Casuarinaceae (Pole, 1993b, 1993c, 1993g).
Bannockburn-04 (F41/f220). An assemblage from a clay-rich mud bed within the Cromwell Submenber, Kawarau Member, Dunstan Formation, Manuherikia Group (NZTM 1298698E, 5000780N). It has distinctly larger leaves than all other Bannockburn assemblages. It is dominated by Elaeocarpus, Myrtaceae (probably all “rainforest” Myrtaceae, including Metrosideros, Ripogonum and Lauraceae (Pole, 1993d, 1993e, 1993f). Conifers and Casuarinaceae are absent.
Nevis-01 (F42/f006). An isolated outcrop of mudstone in the Nevis Valley, Nevis Oil Shale Member, Dunstan Formation, Manuherikia Group (NZTM 1284257E, 4990227N). Legumes are common (Pole et al., 1989) and there is a single instance of Phyllocladus (Pole, 1992b) but Nothofagus is absent or at least rare, although it does occur in Nevis Oil Shale localities to the NE (see below).
Nevis-09 to Nevis-17. These are spot-collections along about 200 m of “Shale Ridge” in the Nevis Valley, Nevis Oil Shale Member, Dunstan Formation, Manuherikia Group (NZTM 1284508E, 4991035N to 1284432E, 4991079N, see Douglas, 1986, figure 5.1). They are grouped as they all appear to sample the same flora, and are noticeably different from Nevis-01 about 800 m to the SW (Pole et al., 1989) in the prominence of Nothofagus and rarity of legumes.
Blue Lake and Grey Lake. These are exposures of muddy beds within the St Bathans Member, Manuherikia Group. They are the oldest strata in the Manuherikia Group, but were not covered in the Mildenhall and Pocknall (1989) palynological work. There is, however, an unpublished report (Pocknall 1982b). The fossil beds comprise a mixture of intact compressed leaves and beds of reworked leaf and cuticle fragments (Pole, 2008). These compression assemblages commonly have prominent (c. 40-50 %) Nothofagus azureus (Pole, 1993c), which has extremely delicate cuticle and is therefore absent from the dispersed cuticle assemblages. Lauraceae and Myrtaceae are also prominent (Pole, 2007a; Pole et al., 2008). Conifers, principally Podocarpaceae, are common and diverse (Pole, 2007). No Casuarinaceae macrofossils are known. Leaf histograms are shown for Grey Lake-05 (NZTM 1349484E, 5027849N, listed as GL-05 in Pole (2008).
Lauder Hill Station (H41/F046). This falls into marginal Fiddlers Member-Kawarau Member, Manuherikia Group (NZTM 1340970E, 5019502N). The distinctive elements of this assemblage are very large leaves, probably of Euphorbiaceae (Pole 1993g), while Conifers, Nothofagus, and Casuarinaceae are absent.
Vinegar Hill. Very rare, small, and mostly fragmentary leaves of Nothofagus occur in a fissile mudstone of the Lauder Member, Bannockburn Formation, Manuherikia Group (NZTM 1343228E, 5025562N, at approximately the 63.5 m level in the measured section of Douglas, 1986, figure 7.12). A collection in the Otago University Geology Department includes a shoot of Araucaria. These are the only known leaf macrofossils in the Bannockburn Formation.
Gore Lignite Measures
This covers a large number of localities spread across the East Southland Group (Isaac and Lindqvist 1990). The palynology was documented by Pocknall and Mildenhall (1984) who established a Late Oligocene-early Miocene age. Documentation of plant macrofossils includes Campbell and Holden (1984), Lindqvist (1990), Pole (2007, 2008), Pole et al. (2008), Carpenter et al. (2010a, 2010b), and Jordan et al. (2011).
Longford Formation
Initial sedimentation within the Murchison Basin was marine (Lihou, 1993), but then progressively shallowed to estuarine conditions (upper Mangles Formation) by the early Miocene (Altonian). This passes up conformably into thick beds with common fluvial cobble conglomerate and finer grained sediment including leaf beds (Longford Formation) that are thus likely to be middle Miocene. The locality formed part of a thesis by A.M. Holden from which two papers were published (Holden, 1982a, 1982b). One bed at this locality (NZTM 616819E, 5378471N) is dominated by very large leafed Nothofagus, which Holden placed in N. novaezeelandiae. I regard these as a separate, unnamed species.
The Dunedin Volcano
Kaikorai Leaf Beds (I44/f145) and Taiaroa Head. These are fluvial-lacustrine units within the Dunedin Volcano, a feature of the Dunedin Volcanic Group (Coombs et al., 1986, 2008). Potassium-argon dating indicates the main phase of volcanic activity was 13-11 Ma, although some continued until 10 Ma (Coombs et al., 1986). Fossil leaves from Kaikorai Valley were first published by Oliver (1936), who described seven species of Fagaceae, including Fagus, Nothofagus, and an extinct Parafagus, as well as a variety of other taxa. Campbell (1985) placed all the fagaceous material into a new genus, Nothofagaphyllites, as well as documenting Casuarinaceae. Based on Campbell’s and Oliver’s material Pole (1993h) regarded two species of Nothofagus were present. As Oliver’s collection contains a variety of angiosperm remains, but Campbell’s collection is essentially all Nothofagus, it is likely they collected from slightly different horizons. Nothofagus leaves from Tairoa Head were illustrated by Pole (1993h).
Waipiata Volcanics
The Waipiata Volcanics, which surround the Dunedin Volcano, range from late Oligocene to late Miocene (Coombs et al., 2008). They include the Foulden Maar and Cornish Head fossil localties.
Foulden Maar (I43/f8503). An accumulation of diatomite within the Foulden Maar has a K-Ar date of 23.2 Ma from basanite that likely dates the explosive event (C. Timms pers. comm., 2007 to Lindqvist and Lee, 2009). This is earliest early Miocene, Otaian in the local timescale (Crundwell et al., 2004). A diverse macrofossil assemblage, in which there were no particular dominants, was described by Pole (1996). Nothofagus was absent and conifers were represented by one species of large-leaved Podocarpus (Pole, 1993b). Subsequently published material includes flowers (Bannister et al., 2005; Lee et al., 2010), orchids (Conran et al., 2009), ferns (Conran et al., 2010), Lauraceae (Bannister et al., 2012), and Laurelia (Conran et al., 2013).
Cornish Head. A late Miocene mudflow includes Nothofagus leaves with plicate vernation (Pole, 1994).
Great Barrier Island
Fossil leaves are common at Medlands Creek in the Beeson Island Volcanics (NZTM 1825710, 5981903). This has been dated as middle Miocene by Booden et al. (2012). The palynology was mentioned by Couper (1953b) but the macroflora, which includes Nothofagus, Phyllocladus and bipinnate legume leaflets (pers. obs.) remains unpublished.
Mataora (T13/f47). This is a late Miocene (6-6.5 Ma; Kapitean and latest Tongaporutuan stages) lens of diatomite in the Coromandel, North Island (Pole and Moore, 2011). A low diversity macroflora includes the conifers Mataoraphyllum and Phyllocladus.
APPENDIX 3. NOTES ON PLANT TAXA IN THE NEW ZEALAND MIOCENE
Ferns
The impression gathered from the few palynological lists of the Manuherikia Group and Gore lignite Measures (e.g., Pocknall, 1982b) is that ferns were neither particularly diverse nor common. The tree ferns (Cyatheaceae) are included, but probably with the prominence that they are met within New Zealand Quaternary assemblages. The tree ferns have been regarded as indicators of a distinct everwet climate today - the “Baumfarnklima” (Troll, 1970). Tree ferns tend to disappear from vegetation as total rainfall decreases or becomes markedly seasonal and additionally, they are rare in tropical forests. For instance, in Australia, tree ferns are absent from typical “dry rainforest” (e.g., Fensham, 1996), and in New Zealand one can observe a marked difference from abundant tree ferns from the very wet, western side of the Haast Pass in the Southern Alps to their virtual absence on the drier eastern side (dominated by Nothofagus ). GBIF data show they occur across a wide MAT (7, 4.5, 26, 28°C), to sub-zero minimum temperatures of the coldest month (-5, -2°C), but they are mostly absent below 1000 mm MAP (virtually no records < 650 mm MAP) and rainfall in the driest month is usually plentiful (0, 30, 280, 500 mm).
The only ferns known as macrofossils from the Manuherikia group are Blechnum sp. and Pneumatoperis sp. (Pole, 1992b) and these also occur in New Zealand today. Neither are particularly helpful climate indicators.
Conifers
Araucariaceae
Both Agathis and Araucaria macrofossils are known from a small number of Miocene deposits (Pole, 1992b, 1997, 2007b; Lee et al., 2007).
Libocedrus has an uncommon and scattered distribution in the lower Manuherikia Group. There are two species in New Zealand today, one which is widespread in cooler situations in the North and South Islands, and one species which is scattered in warmer parts of the North Island. It is not clear if the fossils represent either of these two species. Papuacedrus is also present in one assemblage (Pole, 2007b). This genus is now found only in very high rainfall areas of New Guinea, but it is also known as fossils from very conifer-rich fossil assemblages, (and probably also very high rainfall) in Tasmania (Hill and Carpenter, 1989) and Patagonia (Wilf, 2009), and the Antarctic Peninsula (Bastos et al., 2013; Zhou and Li, 1994).
Typically, the Podocarpaceae tend to be associated with cool and everwet conditions, but do have a presence in some distinctly dry areas, and even fire-prone ones. Despite this rather wide range of climate, over much of this climate-space, the richness is low - often just one species and one genus (typically Podocarpus ). The highest diversity of Podocarpaceae is clearly associated with cool and wet conditions in New Zealand, Tasmania, and New Caledonia. In megathermal vegetation, podocarps tend to be restricted to localized areas of unusual substrate, for instance the raised peat swamps of Borneo, where Podocarpus leaves can be exceptionally large (There is no evidence for leaves of such size in the New Zealand Miocene.). A diverse range of Podocarpaceae is now known from the Miocene in New Zealand (e.g., Pocknall and Mildenhall, 1984; Mildenhall and Pocknall, 1989; Pole 1992b, 1993b, 2007b; Jordan et al., 2011).
The family is not in New Zealand today, but is restricted to warmer latitudes. Fossil pollen grains representing the Gnetaceae have long been known in New Zealand, but they have been described either as Ephedra, a desert shrub of North America, or Ephedripites, a neutral name, but still with unfortunate taxonomic connotations. Cuticle evidence confirms that Gnetaceae were present in the Miocene (Pole, 2008), but as something more akin to, but still distinct from, the present Gnetum, more a genus of rainforests. GBIF data indicate Gnetum occurring across a wide rainfall gradient, only in warm conditions, and never where the minimum temperature of the coldest month is below freezing.
Angiosperms
Palm fossils are widely distributed in the Manuherikia Group and Gore Lignite measures, both as pollen and macrofossils and dispersed cuticle (Couper, 1960; Pocknall and Mildenhall, 1984; Mildenhall and Pocknall, 1989; Pole, 1993i, 2007c). Their presence in these locations is only slightly further south than the current global southern limit of palms, which lies at about 43° 45” on mainland New Zealand, and a little further south on the Chatham Island group, at 44° 20” (Parson, 2007). This corresponds to a MAT of a little over 10°C. Elsewhere, the southernmost limit of palms is 35° in South America, at 37° 47” in Victoria, Australia (they do not extend to Tasmania). Wing and Greenwood (1993) concluded that palms were good evidence for CMM being above 5°C and for average minimum temperatures above -10 to -4°C, although they were not so useful in estimating MAT. Greenwood and Wing (1995, p. 1044) added that “Frost sensitivity of palms restricts them to climates with MAT >10°C, CMM >5°C, and yearly minimum temperature >-10°C.” Palms are thus good indicators of warm conditions. In the current study, the 0.02 percentile limit of their MAT range is about 12.2°C.
Palynology indicates palm biodiversity from the lower Manuherikia Group was at least three-the pollen Arecipites otagoensis (Couper, 1960) Mildenhall and Pocknall 1989, A. waitakiensis Mildenhall and Pocknall 1989, and Dicolpopollis cf. D . metroxylonoides , (affinity to Metroxylon ). Perhaps other monocotyledonous pollen taxa unassigned to modern groups may also have been palms. This suggests the climate was well within the limits for the family.
The Arecaceae are far from ubiquitous in warm climates, and this suggests they may have further value as climatic indicators. For instance, in some parts of the world they dominate vast tracks of semi-swamp areas, whereas in some tropical rainforests they may be essentially absent, or represented only by scattered understory specimens.
The family has a relatively confined range of MAT, mostly moderate rainfall in the driest month, and few records where the min temperature of the coldest month falls below freezing. The family is represented in New Zealand today by Laurelia, which is also known as fossils (Conran et al., 2013). Dispersed cuticle (Pole, 2008) suggests at least six taxa of Atherospermataceae were present in the New Zealand Miocene.
The family is currently known only from the pollen type Ilexpollenites (Pocknall and Mildenhall, 1984; Mildenhall and Pocknall, 1989). Today there are no indigenous Aquifoliaceae in New Zealand, although Ilex extends into the tropics of Australia (see Martin, 1977), but the family is prominent in Tropical Montane Cloud Forests (e.g., Shi and Zhu, 2008).
This subfamily is what was long regarded as Bombacaceae, but has now been placed within an expanded Malvaceae (Angiosperm Phylogeny Group, 2009). It is represented by the pollen type Bombacacidites bombaxoides that has a close similarity with extant Bombax and is frequently used as an indicator of very warm conditions. GBIF data indicate a minimum MAT for Bombax of about 20.5°C and for the Bombacoideae of about 16°C. Bombacacidites.bombaxoides has been reported from some New Zealand Miocene deposits, although it is always rare or uncommon, prompting Pocknall (1982b) to suggest the pollen had come as long distance dispersal from warmer latitudes.
The pollen type Haloragacidites harrsii (or Myricipitesharrisii ) is usually associated with the Casuarinaceae, although other families perhaps cannot be ruled out. Mildenhall and Harris (1971) gave the affinities as Casuarinaceae ( Casuarina ), Loganiaceae ( Geniostoma ), and Myricaceae ( Canacomyrica. However, Casuarina was split into four genera by Johnson (1980, 1982, 1988), and these genera cannot reliably be distinguished on the basis of their pollen. The taxonomy highlights an important environmental distinction - three of the genera ( Casuarina , Allocasuarina, Ceuthostoma ) are plants of either arid conditions, or are restricted to sclerophyll vegetation (with fire as an important part of the ecology) in climates which may be very wet. The fourth genus is Gymnostoma, a plant which today only occupies more mesic conditions (rainforest, heath forest, cloud forest) where fire is normally absent although it may colonise areas after fire (Paijmans, 1976; Scriven and Hill, 1995). Australia today is marginal for its existence where Prider and Christophel (2000 p. 432) concluded that the local species is “restricted to low-nutrient sites where light and possibly water are not limiting factors.” These include the edges of a few watercourses within lowland rainforest and in more stunted vegetation on the adjacent very cloudy mountaintops. It is notable that the lowland rainforest it occurs within has strongly seasonal rainfall. It more extensive on the mountaintops, although it often grows there directly within deep standing water (pers. obs.). Gymnostoma, was clearly present in the New Zealand Miocene where it has been found as fruits at Landslip Hill (Campbell and Holden, 1984) and as “articles” in the Gore Lignite Measures (Pole, 2008). Its presence in the fossil record is a good indicator of MAT of at least 16°C. The presence in the Manuherikia Group fossil record of Allocasuarina contributes to the evidence that sclerophyll, nonrainforest vegetation was present (Campbell and Holden, 1984).
Elaeocarpaceae and Cunoniaceae
These two are closely related, widespread families, most diverse at lower latitudes, but often prominent in terms of biomass in cooler regions. They are both prominent in New Zealand today. The Miocene leaves identified by Pole (1993d) as Elaeocarpus/Sloanea are clearly Elaeocarpus, and comparable to extant species such as Elaeocarpus costatus of Lord Howe Island. I now regard the leaves from Foulden Hills that I identified as Euphorbiaceae (FOLD-10), as more likely a species of Elaeocarpus. The parataxa MANU-15 and MANU-16 from the Manuherikia Group (Pole, 1993g) are mostly likely, respectively, Cunoniaceae and Elaeocarpaceae.
The pollen type Nyssapollenites endobalteus has been found in situ in Euphorbiaceae flowers in the Foulden Maar (Lee et al., 2010), an assemblage also containing leaf fossils representing either Mallotus or Macaranga (Pole, 1996; Lee et al., 2010) or more definitely Mallotus (Nucete et al., 2012). Both genera are common in everwet rainforest (typically as “pioneers” in gaps) in Australia and in similar habitats in many other countries (Whitmore, 1980) and also range into seasonally dry vegetation. There are various reports in the paleobotanical literature to the effect that the genera indicate dry conditions. For example, some of the evidence leading to a conclusion that the Foulden Hills Diatomite vegetation experienced “at least seasonally dry periods,” was the claim by Bannister et al. (2005) that Mallotus and/or Macaranga “inhabited dry forest margins” in Australia. This is misleading as the genera have very wide ranges. The GBIF data of the precipitation of the driest month clearly illustrate the range of rainfall. The two genera are also little help in defining temperatures, but there are few records where the minimum temperature of the coldest month is below freezing.
The pollen Tricolpites reticulatus is present in many palynological samples, and is well established as representing Gunnera (Jarzen, 1980). This genus occurs today across a wide range of MAT, and although it is typically associated with very wet habitats (Wanntorp and Wanntorp, 2003). GBIF records suggest minimum rainfall of the driest months may be low, often around 10 mm across a wide range of MAT.
This endemic Australia family of six genera is mostly found in the drier, nonforest vegetation of today that has a strong annual rainfall deficit. The presence of Gyrostemonaceae in New Zealand’s past has been deduced from the pollen type Gyropollis psilatus. Although earlier discussions about this pollen grain gave its affinities as Didymotheca, Codonocarpus, and Gyrostemon (Mildenhall, 1989), Raine et al. (2008) list them only as Gyrostemon and Didymotheca. It is not clear how the affinities with Codonocarpus have been ruled out. This may be important, as New Zealand palynological works have cited the presence of Gyropollis psilatus as indicating “arid conditions” (Mildenhall, 1989) or “ periodic drier conditions” (Field et al., 2009). This is despite the pollen being found in broadly coal sequences in association with typical rainforest taxa in both Australia and New Zealand. However, the family today includes one species, Codonocarpus attenuatus occurs in dry rainforest (although typically with no seasonal drought). Given the normal associates of the fossil, it is more likely that it, too, represents a rainforest taxon. The Gyrostemonaceae may be a further example of a family that has radiated into drier habitats from rainforest in the late Cenozoic, and at the same time, lost diversity in the rainforest (Crisp et al., 2004).
The broad-leaved Lauraceae are a typical component of warm rainforest worldwide, and they may be diverse and an important component of the biomass. They are clearly warmth limited, with a southernmost limit in New Zealand well to the north of the Manuherikia Group and Gore Lignite Measures, at about 42 °S (Wardle, 1991). Near this limit there are only one or two species, and the very high diversity of Lauraceae in the southern New Zealand Miocene record suggests climatic conditions were well above the cooler limits for the family (Pole, 2007a; Bannister et al., 2012). The genera Cryptocarya and Endiandra were both present in the Miocene (both currently extinct in New Zealand) with Endiandra suggesting a MAT of at least 15°C. It is also notable that Lauraceae leaves at their southern limit in New Zealand today are still relatively large, whilst smaller leaved species exist in Australia. One might speculate that the real southern limit of Lauraceae in New Zealand might be further south, but is limited by what might be a fortuitous (perhaps Quaternary extinction) absence of small-leaved species. Small-leaved Lauraceae were present in the New Zealand Pliocene (Pole, 2007c) and Jordan (1997b) has noted similar small Lauraceae in the Pleistocene of Tasmania.
In megathermal dry forests, the legumes are a prominent and diverse component occupying tree, liane, and herb niches. There is no evidence yet for the legumes in the cuticle record in the lowest Manuherikia Group. Stratigraphicaly higher, they were certainly an important component of the Nevis Oil Shale (Pole et al. 1989; Pole, 1992c) and in a probable correlative at Bannockburn-03 (unpublished specimen). The pods and leaves in the Nevis Oil Shale were suggested to represent Serianthes, a genus that is entirely restricted to megathermal locations today (MATs at least 20.8°C). However, extant Serianthes have leaflets with a retuse apex, a feature that is not present on the fossils. The fossils may well be an extinct genus, related to Serianthes, perhaps with cooler climatic requirements.
The family consists mostly of lianas, but is currently extinct in New Zealand and dispersed cuticle is the first record of the family in New Zealand (Pole, 2008). Although it perhaps has a reputation as being tropical, GBIF data show it occurs across very wide rainfall and MAT regimes, the lower limit of MAT is about 9.5°C, and the minimum temp of the coldest month is often well below zero. Identification of the family alone is, therefore, not helpful in narrowing paleotemperature.
GBIF data indicate a wide range of MAT, but few records where the temperature of the coldest month is below freezing, but where driest month precipitation is mostly >30 mm. It is represented in New Zealand today by Hedycarya, and dispersed cuticle (Pole, 2008) suggests at least two species of Hedycarya were present in the Miocene.
The family has a wide climatic range, but tends to be prominent in cool, wet forests (it is represented by two genera in New Zealand today). A leaf from the Foulden Maar (FOLD-3, Pole, 1996) I regard now as clearly an Ardisia, a genus common in many SE Asian-Australian rainforests today, and restricted to warm MATs of at least 16°C, minimum temperatures well above freezing, and everwet conditions.
Myrtaceae occur commonly in the New Zealand Miocene, either as pollen (Mildenhall, 1980), although identification to genus is difficult, or as macrofossils, where identification to genus level is more feasible. Metrosideros is an important component of many forests in New Zealand and Pacific islands and even as far south as the Auckland Island in the Southern Ocean. In the Early-earliest Miocene of New Zealand it is present as leaf impressions, dispersed cuticle and fruits (Pole et al., 2008) along with Syzigium. The presence of Metrosideros in a fossil assemblage is probably a good control on the upper range of MAT, limiting it to about 20°C, and it is also a good indicator of perhumid conditions. Eucalyptus is known from the New Zealand Miocene as leaves, fruits (Pole, 1993), and probably pollen. Its presence is virtually certain evidence of pyrophylic forests, but it occurs across a very wide range of climate.
Nothofagus pollen is virtually ubiquitous in the New Zealand Miocene, and most palynological samples include species of least three of the extant subgenera (Dettmann et al., 1990). The Nothofagus classification of Hill and Read (1991) divided species producing “fusca-type” pollen into two subgenera, Nothofagus and Fuscospora. At present all Miocene species of fusca-type pollen are regarded as Fuscospora (McGlone et al., 1996). The relative proportions of these subgenera of Nothofagus appear to have some climatic significance. However, this is bedeviled by the fact that while they seem to have co-occurred in the past, they do not occur together today - subgenus Brassospora no longer co-exists with other subgenera. Additionally, there is probably an element of trying to force fossil Nothofagus pollen species into one of the four extant subgenera. Nevertheless, pollen grains that appear identical to extant Nothofagus menziesii and N. fusca - only become prominent compared to other Nothofagus pollen at specific periods in the Cenozoic (McQueen et al., 1968; Pocknall, 1989). These are thought to represent periods of deteriorated (cooler and/or drier) climate. However, the largest leaved Nothofagus in Patagonia, Nothofagus alpina (leaves that have an average length of around 90 mm) are deciduous, related to warm, humid conditions, and produce Menziesii -type pollen (Ramirez et al., 1997), a fact which McQueen (1977) noted was contrary to an earlier belief (McQueen et al., 1968) that the group was a consistent indicator of cool conditions. Conversely, in the mountains of New Guinea, those Nothofagus with the reputation of being the warmest indicators (subgenus Brassospora ) occur in forests with a MAT as low as 10.6°C (Read et al., 2005, 2010). They, therefore, range into microthermal conditions.
There are just two genera of Proteaceae in New Zealand today, Knightia and Toronia, and these have a southern limit similar to the Lauraceae (reaching only to the north of the South Island). In the Miocene they occurred further south and with much higher diversity (Couper, 1953a, 1960a; McIntyre, 1968; Pocknall and Mildenhall, 1984; Mildenhall and Pocknall, 1989). Although McQueen et al., (1968) suggested that a high diversity of Proteaceae in the pollen record might be an indicator of dryness (as diverse Proteaceae typify the Mediterranean-like climates of several parts of Australia), there is also a remarkable diversity of Proteaceae genera in the forests of northeast Queensland today. This is where the relationships of several of the Proteaceae in New Zealand represented by Miocene leaf cuticle lie. GBIF data for some of these genera are often sparse, or else do not reveal locality data for security reasons. Carpenter (1994) and Pole (1998) recorded cuticle of Helicia, Musgravea, Macadamia, the Gevuininae- Hicksbeachia, and Tribe Embothrieae, and Pole (2008) added Placospermum. Carpenter et al. (2010a) confirmed the placement of these later fossils into the subfamily Persoonioideae with their finding (p.7) that a group of characters are “now uniquely found in the subfamily in combination. This is the presence of parallel aligned brachyparacytic stomatal complexes and undulate anticlinal cell walls and the synapomorphy of large stomatal size.” At the same time they described a new genus from the Gore Lignite Measures, Persoonieaephyllum, that differs from Placospermum in its broad stomatal complexes and more or less parallel major venation.
Carpenter et al. (2010b) described Banksia novae-zelandiae from the Gore Lignite, a taxon said to be (p. 288) “best regarded as an extinct stem relative of Banksia ” and (p. 294)“best regarded as belonging to a species of Banksia.”The authors also noted the absence of Banksia pollen (Banksieaeidites elongates) not only at the location, but also in New Zealand since the late Eocene, and that the fossil comes from a fire-free environment. Extant Banksia are all intimately associated with fire. This suggests application of the genus Banksia to these fossils is somewhat forced and is unhelpful for ecological comparison.
Christophel (1984) discussed the climatic implications of fossil subtribe Musgraveinae, and drew comparison with “extant Simple Notophyll Vine Forest” and “might represent subtropical vegetation.”
Muller and Leenhouts (1976) indicate that the pollen genus Cupaniedites, known from the Manuherikia Group and Gore lignites (Pocknall and Mildenhall, 1984; Mildenhall and Pocknall, 1989), is equivalent to their pollen type B of the tribe Cupanieae, and includes genera such as Cupania, Cupaniopsis, Diploglottis, Mischocarpus, and Rhysotoechia. GBIF data indicate that together, these indicate warm MATs, minimum temperatures well above freezing, but precipitation in the driest month that may range from nearly 200 mm to zero. Leaves and dispersed cuticle from the New Zealand Miocene include Alectryon and members of the tribe Cupanieae (Pole, 1996, 2010b). Alectryon (tribe Nephelieae occurs across a wide range of MAT, precipitation of the driest month, but is essentially absent where the minimum temperature of the coldest month falls below freezing.
Mildenhall (1989) reported the pollen type Symplocoipollenites austellus, with affinities to Symplocos, at Shale Creek and Hawkdun. The pollen type was distinct enough for Frederiksen (1980) to regard it as the modern genus, Symplocos.
Ripogonum scandens is the most prominent climber in the present forests of NZ, and related species are found in Australian and New Guinean rainforests. The genus occurs over a relatively cool range of MAT 7.5-20°C, and minimum temperatures of the coldest month may be 2-3°C below freezing. Thus Ripogonum may be useful in limiting the upper estimates of MAT in fossil assemblages. Ripogonum is known as impressions in the Manuherikia Group (Pole, 1993) and compressions in Foulden Hills (Pole, 1996).
Martin (2000) identified the pollen grain Tricolpites trioblatus with Wilsonia. In the Manuherikia Group, the fossil is only present at mid-levels. The genus today grows only in habitats with a minimum month precipitation <55 mm, and total MAP mostly <1100 mm. It is also an indicator of a relatively tight range of MAT (12-18.5°C), where minimum temperatures of the coldest month are above freezing.
APPENDIX 4: NEW LEAF PARATAXA
Descriptive terminology follows Hickey (1973, 1979), Dilcher (1974), and Pole (1991). Specimens prefixed with “OU” are stored in the Geology Department of the University of Otago. Specimens prefixed with “LX” are stored in the Geology Department of the University of Auckland.
Taxon MANU-36
Figure 18.1
 Reference Specimen: OU13926 (Bannockburn-02)
Reference Specimen: OU13926 (Bannockburn-02)
Referred specimens and occurrence: OU13353, OU13356, OU13415 (Bannockburn-02); LX048, LX051, LX081, LX103, LX111 (Bannockburn-03); LX377 (Nevis-17); LX444 (Nevis-04).
Diagnostic features: The important characters are the narrowly elliptical shape and the prominent petiole. MANU-36 differs from MANU-19 in having a more prominent petiole, and from MANU-21 in the more prominent petiole and more closely spaced lateral veins.
Description. Size: length 44-75 mm, width 8-20 mm, microphyll. Shape: elliptic, symmetrical, apex acute, base convex. Petiole prominent, a straight, thicker continuation of the midrib (about 26 % of lamina length), length 19 mm. Margin entire. First order venation pattern externodromous. Development basic. Midrib massive (c. 4.5 % width of mid-lamina) and thickens prominently towards the base. First order lateral veins thin, irregularly and closelyspaced (c. every 2 mm), course almost straight, then curving towards the looping zone, angle of divergence moderate (c. 60°). Lateral loops indistinct.
Taxon MANU-37
Figure 18.2
Reference Specimen: OU12955 (Bannockburn-03)
OU13826, OU13827 (Bannockburn-02).
Diagnostic features: The important characters are the rounded apex and acute base. MANU-37 differs from MANU-39 in having a thinner midrib and lateral veins.
Description. Size: length 50-70 mm, width 27-32 mm, microphyll. Shape: elliptical, symmetrical, apex rounded, base concavo-convex. Petiole distinctly thicker than the midrib, and at an angle to it (about 10% length of laminae), length 6 mm. Margin entire. First order venation pattern externodromous although unclear. Development basic. Midrib weak (c. 1.1 % width of mid-lamina). First order lateral veins indistinct, angle of divergence narrow (c. 38°). Lateral loops indistinct.
Taxon MANU-38
Figure 18.3
Reference Specimen: OU29867 (Bannockburn-03)
Referred specimens and occurrence: OU13103, OU13305, OU13421 (Bannockburn-02); LX034, LX057, LX096 (F41/f214); LX485 (Nevis-07).
Diagnostic features: The important character is the truncate base. MANU-38 differs from MANU-4 in its stout petiole.
Description. Size: length 30-105 mm, width 22-58 mm, notophyll. Shape: ovate, symmetrical, apex probably acute, base truncate. Petiole appears abruptly thicker than the midrib (less than 10% of the lamina length), length 9 mm. Margin entire. First order venation pattern unclear. Development basic. Midrib stout (c. 2 % width of mid-lamina). First order lateral veins indistinct, angle of divergence moderate (c. 46°). Lateral loops indistinct.
Taxon MANU-39
Figure 18.4
Reference Specimen: LX071 (Bannockburn-03)
Referred specimens and occurrence: OU13308, OU13475 (Bannockburn-02).
Diagnostic features: The important characters are the rounded apex and acute base, but differing from MANU-37 in having a stronger midrib and lateral venation.
Description. Size: length 59-65 mm, width 28-30 mm, microphyll. Shape: elliptic, symmetrical, apex rounded, base convex. Petiole a simple, thickening, continuation of the midrib (about 20% of the lamina length), length 11 mm. Margin entire. First order venation pattern. Development basic. Midrib stout (c. 3.9 % width of mid-lamina) and thickening prominently towards the base. First order lateral veins, irregularly spaced, about 4-6 mm, course curved, angle of divergence narrow (c. 32° at the midrib but then flexing to about 45°), relatively thick proximally but thinning rapidly. Lateral loops indistinct.
Taxon MANU-40
Figure 18.5
Reference Specimen: OU13792 (Bannockburn-02)
Referred specimens and occurrence: OU137910 (Bannockburn-02).
Diagnostic features: The important character is the prominent series of lateral loops. MANU-40 differs from MANU-20 and FOLD-28 in having a wider looping zone - about one quarter of the distance from lamina margin to midrib.
Description. Size: length 60 mm, width 24 mm, microphyll. Shape: probably elliptic, symmetrical, apex unknown, base unknown. Petiole length unknown. Margin entire. First order venation pattern. Development basic. Midrib massive (c. 4.3 % width of mid-lamina). First order lateral veins, spacing regular, about 5-10 mm, possibly decreasing both proximally and distally, course strongly curved, angle of divergence moderate to wide (c. 53-81°), relatively thick. Lateral loops prominent, about the same height as breadth. Looping zone about 0.25 the distance from margin to midrib.
Taxon MANU-41
Figure 19.1
 Reference Specimen: LX541 (Nevis-14)
Reference Specimen: LX541 (Nevis-14)
Referred specimens and occurrence: LX392 (Nevis-17); LX408 (Nevis-09); LX479, LX492 (Nevis-07); LX511 (Nevis-15); LX564 (Nevis-13); LX597, LX615 (Nevis-11).
Diagnostic features: The important character is the asymmetry of the base. MANU-41 differs from the other asymetrically based, entire margined leaf or leaflet, FOLD-12, in not having a very acute base on one side, and having much more indistinct lateral veins near the margins.
Description. Size length 35-70 mm, width 11-28 mm, microphyll. Shape: ovate, asymmetrical at the base, apex acute, base truncate, on one side, rounded on the other. Petiole very short, c. 1 mm, possibly a pulvinus. Margin entire. First order venation pattern externodromous. Development basic. Midrib moderate (c. 1.7 % width of mid-lamina). First order lateral veins, very irregularly spaced, about 5-9 mm, with very irregular course, thin, angle of divergence moderate (c. 45°) on one side of the lamina, wide (c. 88-90°) on the other. Lateral loops indistinct.
Taxon MANU-42
Figure 19.2
Reference Specimen: LX431 (Nevis-04)
Referred specimens and occurrence: OU30066, LX021, LX134, LX781 (Bannockburn-03).
Diagnostic features: The important character is the presence of (uncommon) very small teeth. MANU-42 differs from MANU-37 and MANU-39 in the presence of these teeth.
Description. Size: length 26-c. 69 mm, width 15-45 mm, microphyll-notophyll. Shape: probably elliptic, symmetrical, apex rounded, base unknown. Petiole unknown, length unknown. Margin serrate. One small tooth noted, apparently widely spaced (less than 1 per cm), c. 0.5 mm high. First order venation pattern externodromous. Development basic. Midrib moderate (c. 1.4 % width of mid-lamina). First order lateral veins, regularly spaced, about 10-12 mm, course curving markedly towards the margin, angle of divergence narrow (c. 40-42°), moderately thick proximally. Lateral loops indistinct.
Taxon MANU-43
Figure 19.3
Reference Specimen: LX393 (Nevis-17)
Diagnostic features: The most important characteristic is the large number of closely spaced lateral veins, at a high angle to the midrib. This is shared with MANU-36, which is much narrower and with a much more prominent petiole.
Description. Size: length probably 125 mm or more, width 88 mm, mesophyll. Shape: symmetry, and apex unknown, base convex. Petiole, on the only specimen the petiole appears narrower than the midrib and at right angles to it, short (c. 5 mm). Margin probably entire. First order venation pattern probably externodromous, lateral veins closely spaced (c. 3-4 mm), approximately perpendicular to the midrib, with a straight course. Development basic. Midrib stout (c. 2.5 % width of mid-lamina). First order lateral veins, spacing distinctly closely spaced, about 2-3 mm, relatively regular, angle of divergence wide, departing the midrib at almost right angles and running in almost straight lines towards the margin. Lateral loops indistinct.
Taxon MANU-44
Figure 19.4
Reference Specimen: LX745 (Bannockburn-03)
Diagnostic features: The important characters are the lateral loops that almost touch a wavy margin. MANU-44 differs from MANU-46 in having lateral loops that almost touch the lamina margins.
Description. Size: length c. 38 mm, width c. 32 mm, microphyll. Shape: ovate, symmetrical, apex probably acute, base unknown. Petiole and length unknown. Margin undulate. First order venation pattern probably brochidodromous. Development basic. Midrib weak (c. 1.2 % width of mid-lamina), some deflection of course distally at lateral vein junctions. First order lateral veins, spacing regular, about 6-10 mm, decreasing distally , basal laterals unknown, but probably paired at the base, course curving only slightly towards the sinuses, then deflecting apically and almost meeting the margin, angle of divergence narrow to moderate (c. 43-55°), moderately thick. Lateral loops indistinct and looping zone very reduced.
Identification. Based on the marginal characters and basal lateral veins that are probably paired, this is most likely a Macaranga or Mallotus.
Taxon MANU-45
Figure 19.5
Reference Specimen: LX029 (Bannockburn-03)
Referred specimen and occurrence: LX758 (Bannockburn-03).
Diagnostic features: The important character is the depression of midrib and lateral veins into the lamina surface.
Description. Size: length 47 mm, width 34 mm, microphyll. Shape: elliptic, symmetry possibly asymmetrical, apex uncertain but probably rounded, base truncate. Petiole a simple continuation of the midrib (about 20% of lamina length), length 13 mm. Margin apparently entire. First order venation pattern externodromous. Development basic. Midrib stout (c. 2.4 % width of mid-lamina), depressed below leaf surface. First order lateral veins, spacing irregular, about 5-10 mm, decreasing proximally, curving towards the margin, angle of divergence moderate (c. 53-63°), prominent, depressed into lamina surface. Lateral loops indistinct, looping zone narrow.
Taxon MANU-46
Figure 19.6
Reference Specimen: LX761 (Bannockburn-03)
Diagnostic features: The important character is the wavy margin. MANU-46 differs from MANU-44 in having the lateral loops further back from the lamina margins.
Description. Size: length 45 mm, width 30 mm, microphyll. Shape: probably ovate, symmetrical, apex unknown, base unclear, but tending truncate. Petiole unknown. Margin undulate. First order venation pattern probably externodromous, loops indistinct. Development basic. Midrib stout (c. 2.7 % width of mid-lamina), deflected at lateral vein junctions. First order lateral veins, irregularly spaced, about 5-8 mm, slightly curved to the looping zone, then curving strongly, angle of divergence narrow to wide (27 to 56°), relatively thin, not distinctly thicker than finer venation. Lateral loops distinct.
Taxon MANU-47
Figure 19.7
Reference Specimen: OU13829 (Bannockburn-02)
Referred specimen and occurrence: OU13828 (Bannockburn-02).
Diagnostic features: The important characters are the toothed margin and externodromous venation. Differs from FOLD-7 in having a more clearly defined, and narrower looping zone.
Description. Size: length 58-65 mm, width 25-28 mm, microphyll. Shape: ovate, symmetrical, apex acute, base not clear, possibly convex. Petiole unknown. Margin: toothed to crenate. One order of teeth, c. 1.5 mm high, sinus rounded, regularly spaced (c. 1 per 5 mm). First order venation pattern externodromous. Development basic. Midrib stout (c. 2.6 % width of mid-lamina). First order lateral veins, irregularly spaced, about 4-5 mm, with very irregular course, angle of divergence moderate to wide (c. 53-73°), relatively thin. Lateral loops distinct.
Taxon MANU-48
Figure 19.8
Reference Specimen: LX115 (Bannockburn-03)
Diagnostic features: An asymmetrical lamina with a probable pulvinate base.
Description. Size: length 19 mm, width 6.5 mm, nanophyll. Shape: oblong, whole lamina asymmetrical, apex essentially obtuse (but with a slight point), base truncate, slightly auriculate. Petiole absent, replaced by a pulvinus. Margin entire. First order venation pattern externodromous. Development basic. Midrib massive (c. 4.1 % width of mid-lamina). First order lateral veins, irregularly spaced, about 2 mm, about 6-7 clear lateral loops down each side of midrib, angle of divergence narrwo to wide (c. 30 to 90°). At the base lateral veins appear radiate from the midrib.
Identification. This leaflet is inferred to belong with the legumes that were described in (Pole et al. 1989b; Pole 1992c) as possible Serianthes.
APPENDIX 5: KEY TO ALL MANUHERIKIA GROUP AND FOULDEN MAAR LEAF PARATAXA
Morphological terms follow Hickey (1973, 1979), Dilcher (1974), and Pole (1991).
1 Not thought to be from a pinnately compound leaf 2
1 Clearly leaflets of pinnately compound leaf MANU-48
2 Leaves known to be palmately compound MANU-21
2 Leaves or possibly leaflets 3
3 Lamina tri-lobed FOLD-13 [Sterculiaceae or Hernandiaceae]
3 Lamina not lobed, or not known to be lobed 4
4 Margins entire or some rare small teeth or undulate 5
6 Venation brochidodromous MANU-10 [ Ripogonum scandens ]
6 Venation not brochidodromous 7
7 Basal laterals not paired, or this character not known 14
8 Basal laterals paired above base of lamina 9
8 Basal laterals paired at base of lamina 10
10 Leaf notophyll or mesophyll 12
12 Second Order Externals prominent near base of leaf. MANU-22
12 Second Order Externals not prominent, or absent. 13
13 Lamina wide elliptic, strong pulvinus FOLD-14 [Sterculiaceae or Tiliaceae]
13 Lamina wide ovate, no clear pulvinus FOLD-11 [Euphorbiaceae or Leguminosae]
14 Leaves, leaflets known to be asymmetrical at base 15
14 Leaves, leaflets symmetrical (or assumed so) at base 17
15 Margin entire, base acute on one side and oblique on other 16
15 Margin with rare very fine teeth, base acute on both sides FOLD-17
16 One side of base very acute, laterals strong to margins FOLD-12 [Rutaceae or Meliaceae]
16 One side of base moderately acute, laterals not strong to margins MANU-41
17 First order venation not visible 18
18 Lamina narrow elliptic, size microphyll FOLD-15 [?Winteraceae]
18 Lamina elliptical, size nanophyll FOLD-25
19 Venation longitudinal (intramarginal) 20
20 Leaf lanceolate MANU-7 Eucalyptus
21 More than one longitudinal vein at base MANU-9
21 Only a single longitudinal vein 22
22 Lateral veins relatively closely spaced FOLD-4[Myrtaceae]
22 Lateral veins relatively widely spaced MANU-8[Myrtaceae]
23 Veins not markedly impressed or raised 24
23 Veins strongly indented or raised MANU-45
24 Second order venation (markedly) percurrent 25
24 Second order venation not markedly percurrent 26
25 Leaf microphyll FOLD-2 [Lauraceae]
25 Leaf notophyll MANU-3 [Lauraceae]
27 Lamina narrow-elliptical 28
27 Lamina not narrow-elliptical 29
28 Lateral veins closely spaced, petiole long, stout MANU-36
28 Lateral veins prominently looped, petiole short, thin MANU-21 [leaflet]
29 Prominent veins parallel and below First Order Laterals MANU-18
29 No prominent veins parallel and below First Order Laterals 30
30 Lateral veins markedly decurrent on mid-vein 31
30 Lateral veins not markedly decurrent on mid-vein 32
31 Basal segment developed MANU-13
31 Basal segment not developed MANU-28
32 Two or three orders of lateral veins MANU-11
32 Only one order of lateral veins, or preservation unclear 33
33 Vein network within lateral like fine mesh MANU-12
33 Vein network within lateral not like fine mesh, or preservation unclear 34
34 Midrib and lateral veins appear "sheathed" with different coulour/texture FOLD-9 [?Euphorbiaceae]
34 Midrib and lateral veins normal, no evidence of a "sheath" 35
35 Lateral loops with bases equal to or longer than height 36
35 Lateral loops with bases shorter than height 38
36 Looping zone more than one quarter lamina width MANU-40
36 Looping zone less than one quarter lamina width 37
37 Internal network possibly cascade (Pole 1991, fig. 6c) but not clear MANU-20
37 Internal network cascade (Pole 1991, fig. 6c) FOLD-28
38 Lateral vein angle of divergence >45° and not closely spaced 39
38 Lateral vein angle of divergence <45° and closely spaced 52
39 Lateral vein angle of divergence <70° 40
39 Lateral vein angle of divergence >80° MANU-43
40 Base not truncate, or base unknown 41
41 Leaf size microphyll-notophyll 42
42 Lateral veins form prominent loops MANU-31
42 Lateral veins not forming prominent loops 43
43 Shape narrow-ovate to narrow-obovate FOLD-20
43 Shape not narrow-ovate to narrow-obovate 44
45 Leaf size notophyll or larger 46
45 Leaf size microphyll FOLD-23
46 Interlateral segments irregular FOLD-16
46 Interlateral segments regular FOLD-19
47 Margin with rare, very small teeth MANU-42
47 Margin completely entire 48
48 Petiole thin, lateral veins faint MANU-37
48 Petiole stout, lateral veins visible MANU-39
49 Shape ovate or rounded MANU-25
50 Apex rounded, no petiole FOLD-27
50 Apex emarginate, base petiolate FOLD-22
51 lateral veins thin, looping zone broad, petiole thin MANU-4
51 lateral veins unclear, no clear looping zone, petiole stout MANU-38
52 Apex acute, lateral veins flexuous, little looping zone FOLD-3 [cf. Ardisia]
52 Apex rounded, lateral veins basically straight, complex looping zone FOLD-18
53 Lateral veins run to margin MANU-44
53 Lateral veins loop well within margin MANU-46
54 Domatia in axils of veins 55
55 Basal laterals not paired MANU-5 [ Elaeocarpus ]
56 Base acute FOLD-1 [Euphorbiaceae]
57 Venation craspedodromous 58
57 Venation externodromous or mixed 60
58 Lateral veins regularly spaced 59
58 Lateral veins irregularly spaced FOLD-8 [Cunoniaceae or Elaeocarpaceae]
59 Leaf elliptical or ovate MANU-6 [ Nothofagus ]
60 Venation mixed (craspedodromous-externodromous) 70
61 Teeth obscure and/or irregular 62
62 Teeth broad, few, apical margin only, length of lateral loops bases more than half of the loop height FOLD-24
63 Teeth numerous, length of lateral loops bases less than one third of the loop height FOLD-26
63 Teeth numerous, length of lateral loops bases about one half of the loop height, internal venation cascade MANU-17
64 Teeth sharp, probably no looping zone FOLD-5 [Sapindaceae]
66 Second Order Venation percurrent MANU-16[Cunoniaceae or Elaeocarpaceae]
66 Second Order Venation at high angle to lateral veins MANU-26
67 Lateral veins form prominent loops. MANU-27
67 Lateral veins do not form prominent loops. 68
68 Looping zone wide FOLD-10 [?Euphorbiaceae]
68 Looping zone narrow or absent 69
69 Looping zone narrow MANU-47
69 Looping zone not clearly defined FOLD-7 [Cunoniaceae or Elaeocarpaceae]
71 Veins to center of sharp teeth MANU-29
71 Veins to apical margin of teeth or sinus 72
73 Teeth small, venation distinct FOLD-6 [Cunoniaceae or Elaeocarpaceae]
73 Teeth large, venation indistinct MANU-30
APPENDIX 6. SUMMARY OF PARATAXA FROM THE MANUHERIKIA GROUP AND FOULDEN MAAR
Distribution of all angiosperm leaf parataxa in the Manuherikia Group and Foulden Maar in assemblages used for physiognomic investigation. The number of specimens is given. The associated presence of conifers and Casuarinaceae is indicated by an asterix.
| Taxon | Margin | F41/f208 | F41/f214 | F41/f220 | Nevis | Foulden |
|---|---|---|---|---|---|---|
APPENDIX 7. VARIATION IN THE METHOD OF CO-EXISTENCE
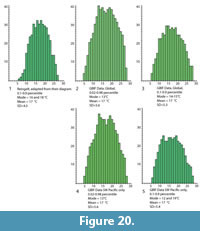 The Co-Existence Method attempts to find the maximum overlap in the climate ranges of an assemblage of taxa. The amount of overlap can be represented as a histogram. Figure 20 shows a variety of histograms that can be created for MAT data for the Foulden Maar taxa listed by Reichgelt et al. (2013). In each case the MAT range (x-axis, in°C) has been rounded to the nearest degree. The y-axis is the total number of overlaps. The first diagram (Figure 20.1) redraws the data from Reichgelt et al. (2013, Supplementary data). The following four histograms are based on GBIF data, using either the global distribution of taxa (Figure 20.2-3), or restricted to the southwest Pacific (Figure 20.4-5) area as per Reichgelt et al. (2013). For the global data, histograms are given based on 0.02-0.98 percentile (Figure 20.2, 20.4), as used in the current paper, and 0.1-0.9 percentile (Figure 20.3, 20.5) as used by Reichgelt et al. (2013).
The Co-Existence Method attempts to find the maximum overlap in the climate ranges of an assemblage of taxa. The amount of overlap can be represented as a histogram. Figure 20 shows a variety of histograms that can be created for MAT data for the Foulden Maar taxa listed by Reichgelt et al. (2013). In each case the MAT range (x-axis, in°C) has been rounded to the nearest degree. The y-axis is the total number of overlaps. The first diagram (Figure 20.1) redraws the data from Reichgelt et al. (2013, Supplementary data). The following four histograms are based on GBIF data, using either the global distribution of taxa (Figure 20.2-3), or restricted to the southwest Pacific (Figure 20.4-5) area as per Reichgelt et al. (2013). For the global data, histograms are given based on 0.02-0.98 percentile (Figure 20.2, 20.4), as used in the current paper, and 0.1-0.9 percentile (Figure 20.3, 20.5) as used by Reichgelt et al. (2013).
Reichgelt et al. (2013) arrived at a MAT (0.1-0.9 percentile) of 18-20°C, though on the histogram here (A) there appear to be two modes around 16°C and 18°C. In the other histograms, modes appear several (up to five) degrees cooler than the mean, which is 17°C in each case. Skewed modes, plus the existence of ‘twin-peaks,’ suggests caution in interpreting precise values of MAT from these data.
APPENDIX 8: GBIF DATA SOURCE ACKNOWLEDGMENTS
Academia Sinica; Actualización del banco de datos florístico de la Península de Yucatán (BAFLOPY); Administración de Parques Nacionales, Argentina (APN-AR); Agentes Bioactivos de Plantas Desérticas de Latinoamérica (ICBG); American Museum of Natural History (AMNH), Arctos (MVZ), Avian Knowledge Network (AUDCLO, EBIRD_CL, BSC-EOC, EBIRD_NZ, EBIRD_YARD, EBIRD_AK, EBIRD_CA, CORBIDI, EBIRD_WI, VCE, AUDUBON_MA, EBIRD_PR, EBIRD_COL, EBIRD_CB, EBIRD_HISP, EBIRD_TX, EBIRD_MEX, CLO, EBIRD_KLAM_SISK), Bernice Pauahi Bishop Museum (BPBM), , Biologiezentrum Linz Oberoesterreich (LI), Borror Laboratory of Bioacoustics (BLB), Canadian Biodiversity Information Facility (PMAE), Canadian Museum of Nature (CMN), Field Museum (FMNH), Finnish Museum of Natural History (MZH), GBIF-Spain (ARM), GBIF-Spain (BDBCV-General), GBIF-Sweden (GBIF-SE; Andes to Amazon Biodiversity Program, Botanical Research Institute of Texas; Angiosperm specimens of Shoji Sasamura of Iwate Prefectural Museum; Angiospermatatophytina Collection of Saitama Museum of Natural History; Antarctic Plant Database, British Antarctic Survey (AAS); Aranzadi Zientzi Elkartea (ARAN Herbarioa); Árboles de la Península de Yucatán, Flora del Distrito de Tehuantepec, Oaxaca y Familia Asteraceae en México (IBUNAM); Árboles de la Península de Yucatán, Flora del Distrito de Tehuantepec, Oaxaca y Familia Asteraceae en México (IBUNAM); Árboles y Arbustos Nativos para la Restauración Ecológica y Reforestación de México (IE-DF,UNAM); Arizona State University Vascular Plant Herbarium (ASU); Arnold Arboretum, Harvard University Herbaria (A); Australian Antarctic Data Centre (ADT); Australian National Herbarium (CANB); Avian Knowledge Network (AUDCLO, EBIRD_NZ, BSC-EOC), National Museum of Natural History (USNM), OZCAM (Online Zoological Collections of Australian Museums) Provider (NMV), Yale University Peabody Museum (YPM), Arctos (MVZ), Bernice Pauahi Bishop Museum (BPBM), American Museum of Natural History (AMNH), University of Kansas Biodiversity Research Center (KU), Conservation InternationalAvian Knowledge Network (EBIRD_CA), Finnish Museum of Natural History (MZH), , data.gbif.org, 2010-12-24).; Banco Nacional de Germoplasma Vegetal; Berkeley Natural History Museums (BMHM); Bernice Pauahi Bishop Museum (BPBM); Biodiversidad de Costa Rica, Instituto Nacional de Biodiversidad (INBio), Costa Rica; Biodiversity Information System for Cheshire; Biodiversity Research Center (TaiBIF); BIOECO; Biologiezentrum Linz Oberoesterreich (LI); Bishop Museum Natural Sciences Data; Botanic Garden and Botanical Museum Berlin-Dahlem (BoGART); Botanic Garden and Botanical Museum Berlin-Dahlem, Herbarium Berolinense (B); Botanic Garden of the Finnish Museum of Natural History; Botãnica, Universidad de Leon (LEB); Botánica, Universidad de León: LEB-Brasil; Botanical Museum, Copenhagen (C); Botanical Research Institute of Texas (BRIT); Botanical Society of the British Isles - Vascular Plants Database; Botanical specimens database of Mr. Jiro Ito collection; Bristol Regional Environmental Records Centre - October 2009 (BRERC); California Academy of Sciences Botany (BOT); California State University; Cameroon National Herbarium (YA); Canadian Museum of Nature (CANM); Catálogos florísticos de México por entidad federativa e información etnobotánica de la Colección del Herbario Nacional Biól. Luciano Vela Gálvez (INIF); CeDoc de Biodiversitat Vegetal: BCN-Seeds; CeDoc of Plant Biodiversity (CeDocBIV), Univ. Barcelona; Central- and East African Plants Databases (Senckenberg); Centre d'Observation de Surveillance et d'Information Environnementales (COSIE); Chungnam University Natural History Museum- Plant (NHMC-PL); CIBIO (Institute of Biodiversity), Univ. Alicante; CIBIO, Universidad de Alicante, Herbario ABH; Colección científica del Museo de Historia Natural Alfredo Dugés; Colección de Monocotiledóneas Mexicanas (UAM-I); Colecciones Instituto Alexander von Humboldt (FMB); Colorado State University Herbarium (CSU); Comisión Nacional para el Conocimiento y uso de la Biodiversidad (CP-CT); Comisión Nacional para el Conocimiento y uso de la Biodiversidad (IE-XAL); Comisión Nacional para el Conocimiento y uso de la Biodiversidad (UNIBIO, IBUNAM); Comisión Nacional para el Conocimiento y uso de la Biodiversidad(ND); Comisión nacional para el conocimiento y uso de la biodiversidad, Herbarium de Geo. B. Hinton, Mexico; condoncollection, Museum of Natural and Cultural History - University of Oregon; Conservatoire botanique national du Bassin parisien; Consortium of California Herbaria; DAO Herbarium Type Specimens; Departamento de Biología de Organismos y Sistemas, Universidad de Oviedo; Department of Environment and Conservation, Western Australia; Department of Natural Resources, Environment, the Arts and Sport, Northern Territory of Australia; Department of Organisms and Systems Biology. University of Oviedo, FCO-Briof; Department of Premier and Cabinet representing the State of New South Wales (OEH Atlas of NSW Wildlife); Desarrollo Tecnológico e Innovación de la Junta de Extremadura(DGIDTI); Dibujos de la Real Expedición Botánica del Nuevo Reino de Granada (1783-1816), Real Jardín Botánico (CSIC); Dirección General de Investigación, Desarrollo Tecnológico e Innovación de la Junta de Extremadura(DGIDTI): (HSS); Dr Masatomo Suzuki collection; DSMZ Collection on Plant Cell Cultures ( Leibniz Institute DSMZ); Ecología y Fisiología Vegetal (herbario_cofc), Facultad de Ciencias, Universidad de Córdoba; EcoRecord - Vascular plant records held by EcoRecord for the Birmingham and the Black Country area collated prior to March 2013; Ejemplares tipo de plantas vasculares del Herbario de la Escuela Nacional de Ciencias Biológicas (ENCB); EKY_Darwincore (University of Tennessee, Knoxville); Endemic Species Research Institute (TaiBIF); Environment and Heritage Service - EHS Species Datasets; Escuela Técnica Superior de Ingenieros de Montes, UPM (EMMA); Espèces vasculaires endémiques et orchidées (CITES); Estudio de la avifauna y de las interacciones ave-planta en la Reserva de la Biosfera de la Barranca de Metztitlán Hidalgo; Estudio Florístico de la Sierra de Pachuca (ENCB, IPN); European Environment Agency (EEA); Factual Database of Native Flora Seeds in Korea, Korea Institute of Science and Technology Information; Fairchild Tropical Botanic Garden (FTG); Fairchild Tropical Botanic Garden Virtual Herbarium Darwin Core format; Field Museum (FMNH); Flora Atlas N.T. (Department of Natural Resources, Environment, The Arts and Sport, Northern Territory of Australia); Flora del Valle de Tehuacán-Cuicatlán: II Fase; Florística y biogeografía de algunos bosques mesófilos de la Huasteca Hidalguense; Folklore and Natural History Museum- Plant (JNHM-PL); Forest Research Institute, Department of Natural Forests (IFR-DNF); Formación del banco de datos del herbario (UCAM); Forster herbarium, Göttingen (GOET); Fundación Biodiversidad, Real Jardín Botánico (CSIC); Fundación Carl Faust: Herbario del Jardí Botànic Marimurtra: HMIM; Garhwal University Herbarium (HNB); GBIF-Spain Real Jardin Botanico (Madrid); GBIF-Sweden (UPS); GBR, Bioversity International; GEO-Tag der Artenvielfalt; Gesellschaft fur wissenschaftliche Datenverarbeitung mbH Gottingen (GWDG); Ghana Biodiversity Information Facility (GhaBIF); Ghana Herbarium, Department of Botany University of Ghana (LEGON-GC); Global Mountain Biodiversity Assessment (GMBA); Gothenburg Herbarium - General (GBIF:IH:GB:Herbarium); Green Plant Herbarium (TRT); Guadeloupe_Herbier (GUAD); Gyeongsangnam-do forest environment Research Institute-Plant (GFEI-PL); Halla Arboretum, Plant (JJHA-PL); Harvard University (MCZ); Harvard University Herbaria (HU); Herbaria of the University and ETH Zürich (Z+ZT); Herbario Amazónico Colombiano (COAH); Herbario CDMB - Jard?n Bot?nico Eloy Valenzuela; Herbario de la Escuela Nacional de Ciencias Biológicas del IPN (ENCB); Herbario de la Universidad de Arizona, EUA; Herbario de la Universidad de Salamanca (SALA); Herbario de la Universidad de Sevilla (SEV-Historico); Herbario del CIBNOR; Herbario del Instituto de Ecología (XAL); Herbario del Jardín Botánico-Histórico La Concepción (HBC); Herbario Los Tuxtlas (MEXU); Herbario Museo de La Salle Bogotá (MLS); Herbario Nacional de Plantas Vasculares - Museo Argentino de Ciencias Naturales 'Bernardino Rivadavia' (MACN); Herbario Nacional, Museo Nacional de Costa Rica (CR); Herbario SANT, Universidade de Santiago de Compostela (SANT); Herbario UNAP (Herbarium Amazonense - AMAZ); Herbário, Instituto de Investigação Científica Tropical (IICT, LISC); Herbarium - Las Cruces Biological Station (HLDG); Herbarium Amsterdam (AMD); Herbarium GJO ( Steiermärkisches Landesmuseum Joanneum); Herbarium Hamburgense (HBG); Herbarium of Kitakyushu Museum of Natural History and Human History; Herbarium of Oskarshamn (OHN); Herbarium of Taiwan Forestry Research Institute; Herbarium of the California Academy of Sciences (CAS); Herbarium of the Institute of Ecology, A. C., Mexico (IE-BAJIO); Herbarium of The New York Botanical Garden; Herbarium of the Universite Libre de Bruxelles (BRLU); Herbarium of the University of Aarhus (AAU); Herbarium of Université de Montpellier 2, Institut de Botanique (UM2); Herbarium of Vascular Plants Collection of the University of Extremadura (Spain); Herbarium of Vascular Plants Collection of the University of Extremadura (Spain) (UNEX Herbarium); Herbarium Senckenbergianum (FR); Herbarium specimen from "EA"; Herbarium Specimen of the Institute of Traditional Medicine Tanzania (TanBIF); Herbarium Specimens of Bonin and Ryukyu IslandsNational Institute of Genetics, ROIS; Herbarium specimens of Faculty of Symbiotic Systems Science, Fukushima University; Herbarium specimens of Museum national d'Histoire Naturelle - Vascular plants (P); Herbarium Specimens of Museum of Nature and Human Activities, Hyogo Pref., Japan; Herbarium Specimens of Tokushima Prefectural Museum; Herbarium specimens of Université de Montpellier 2 (MPU); Herbarium togoense (TOGO); Herbarium Universitat Ulm (ULM),; Herbarium W, Natural History Museum, Vienna; Herbarium Willing, Botanic Garden and Botanical Museum Berlin-Dahlem; Herbarium, University of Alabama Biodiversity and Systematics (UNA); Herbier de la Guyane (CAY); Herbier de Nouvelle Caledonie (NOU); Herbier de Wallis et Futuna; Herbier du Bénin; Hortus Botanicus Sollerensis Herbarium (FBonafè); Ibaraki Nature Museum; inatura - Erlebnis Naturschau Dornbirn ; iNaturalist research-grade observations, iNaturalist.org; Institut Botanic de Barcelona (BC); Institut Botanic de Barcelona, BC-Histórico; Institute of Botany, University of Hohenheim (HOH); Institute of Nature Conservation, Polish Academy of Sciences (IOP-PAS); Instituto de Botanica Darwinion (CONICET); Instituto de Investigación de Recursos Biológicos Alexander von Humboldt; Instituto de Investigaciones de la Amazonía Peruana, Herbario Herrerense; Instituto Nacional de Pesquisas da Amazonia (INPA); Inventaire national du Patrimoine naturel (INPN); Inventario florístico y faunístico del Parque Nacional Barranca del Cupatitzio; Israel Nature and Parks Authority (INPA); Jardín Botánico de Córdoba (COA); Jardin botanique de Montréal (JBM); Karl Franzens University of Graz, Insitute for Botany - Herbarium (GZU); KBIF Data Repository; Kochi Prefectural Makino Botanical Garden; Korea National Arboretum (Korea Forest Service), Plant (KNA_PL); Korean Ethnobotany Database; Kurashiki Museum of Natural History; Kyung Hee University Natural History Museum- Plant (NHMK-PL); Landcare Research Allan Herbarium (CHR); Las especies endémicas de plantas en el estado de Jalisco; Leiner-Herbar Konstanz; L'herbier de l'Université Louis Pasteur de Strasbourg (STR); Louisiana State University Herbarium (LSU); Lund Botanical Museum (LD); Marie-Victorin Herbarium (MT); MEXU/Colección de Plantas Acuáticas; MEXU/Colección Histórica; MEXU/Flora de Oaxaca; MEXU/Herbario Los Tuxtlas; MEXU/Plantas Vasculares; MEXU/Tipos de plantas vasculares; Mokpo Museum of Natural History; Mokpo Museum of Natural History-Plant (MNHM-PL); Muhimbili University of Health and Allied Sciences Herbarium (ITMH); Musée national d'Histoire naturelle Luxembourg (MNHNL); Museum für Naturkunde Berlin (MfN); Museum of Comparative Zoology, Harvard University; Museum of Natural and Cultural History - University of Oregon (MNCH); Nationaal Herbarium Nederland; National Biodiversity Data Centre (NBDC); National Chemical Laboratory (NCL); National Herbarium of New South Wales (NSW); National Herbarium of Tanzania (TanBIF); National Museum of Natural Science NMNH (USNM); National Museum of Nature and Science, Japan (HYO), (INM), (KYUM), (OSA); National Science Museum of Korea; National Science Museum of Korea- Plant (NSMK-PL); National Taiwan University, Herbarium (TAI); National Vegetable Germplasm Bank, Mexico (BANGEV, UACH); National vegetation diversity inventory and mapping plan, Taiwan Biodiversity Information Facility (TaiBIF); Natural History Museum Maastricht (NL) - Herbarium; Natural History Museum, University of Oslo; Natural History Museum, Vienna (Herbarium W); NatureServe Network Species Occurrence Data; Netherlands Centre for Biodiversity Naturalis, section National Herbarium of the Netherlands (L); New Mexico Biodiversity Collections Consortium database; New York Botanical Garden (NYBG); New Zealand Biodiversity Recording Network, New Zealand National Vegetation Survey Databank (GBIF New Zealand); New Zealand National Vegetation Survey Databank (LCR) (GBIF New Zealand); NHT_flora (TanBIF); Northern Arizona University (NAU); NSW Department of Environment, Climate Change, and Water representing the State of New South Wales; Oaxaca y Familia Asteraceae en México (IBUNAM); Observations du Conservatoire botanique national du Bassin parisien.; Oklahoma Vascular Plants Database Provider; Organization for Tropical Studies (OTS); Oxford University Herbaria; Papua New Guinea National Herbarium (LAE); Parcela Permanente en el Pantano Martos (Fundación Estación Biológica Guayacanal); Paris Service du Patrimoine naturel (SPN); Peabody Botany DiGIR Service; Phanérogames recensés aux Monts Nimba, Centre d'Observation de Surveillance et d'Information Environnementales (COSIE); Plant Observation Records of Japan, National Institute of Genetics, ROIS; Plant Specimen Database of Tama Forest Science Garden; Plant Specimens collections of the Kyushu UNniversity Museum; Plant specimens depodited in Osaka Museum of Natural History; Plant Specimens of Kurashiki Museum of Natural History; Plant Specimens of Oiso Municipal Museum; Plant Specimens of The Shimane Nature Museum of Mt. Sanbe; Plants of Papua New Guinea (National Herbarium of New South Wales); PRECIS (KwaZulu-Natal Herbarium); Private collection of Helmut Dalitz, Juergen Homeier, Juergen Homeier, Rainer Bussmann, Vindas Jorge, Institute of Botany, University of Hohenheim; Queensland Herbarium (AQ); Rapid Assessment Program (RAP) Biodiversity Survey Database, Conservation International; RBGE Herbarium (E); Real Jardin Botanico (Madrid), Vascular Plant Herbarium (MA); Registros biológicos en áreas protegidas obtenidos de documentos impresos, Administración de Parques Nacionales, Argentina; Rio de Janeiro Botanical Garden Herbarium Collection (RB); Royal Botanic Garden Edinburgh (RBGE); Royal Botanic Gardens, Kew (K); Royal Horticultural Society - RHS monitoring of native and naturalised plants and animals at its gardens and surrounding areas; Royal Museum of Central Africa (RMCA); Santa Barbara Museum of Natural History (SBMNH); SBT-Living (GBIF-Sweden); Scientific Research Centre of the Slovenian Academy of Sciences and Arts, Institute of Biology (ZRC-SAZU); Senckenberg (SMF); Seodaemun Museum of Natural History-Plant (SMNH-PL); Service du Patrimoine naturel, Muséum national d'Histoire naturelle, Paris (SPN-MNHN); Shizuoka Prefecture Museum of Natural History; Siamazonia Provider (IIAPPoa); 'Sistema de apoyo a la toma de decisiones para la reforestación rural en México', 'Recuento de la diversidad florística de Veracruz', 'Lista florística preliminar de Tamaulipas', 'Inventario florístico de la Sierra de San Carlos', ' Inventario florístico de la Frailescana (zona focal), Chiapas, México', 'Historia natural del parque ecológico estatal de Omiltemi Chilpancingo, Guerrero, México', 'Flora del Parque Nacional Cumbres de Monterrey', 'Flora del Valle de Tehuacán-Cuicatlán, Il Fase', 'Actualización e incremento del banco de datos de la colección de herbario del Jardín Etnobotánico de Oaxaca', 'Banco de información sobre características tecnológicas de maderas mexicanas', 'Base de datos de las regiones prioritarias 113 y 120 en los municipios de Zirándaro y Coahuayutla', 'Base de datos para la xiloteca del Instituto de Biología de la UNAM', 'Base de datos sobre la flora de Durango', 'Catálogo y base de datos preliminar de la flora de Sinaloa', 'Computarización de la xiloteca Dr. Faustino Miranda del Instituto de Ecología', 'Diversidad florística y endemismo en la Reserva de la Biósfera El Cielo', 'Diversidad y riqueza vegetal de los substratos rocosos del centro del estado de Veracruz', 'Estudio de la diversidad florística en la región de la Chinantla', 'Flora vascular del cerro El Zamorano', 'Flora y vegetación de la Sierra de San Carlos en el municipio de San Nicolás', 'La etnobiología de los recursos nutritivos en las comunidades Tzeltales en los Altos de Chiapas' (Comisión nacional para el conocimiento y uso de la biodiversidad); Sistema de Información de la vegetación Ibérica y Macaronésica (GBIF-Spain); South African National Biodiversity Institute (SANBI); South Australia Flora Observations, South Australia, Department of Environment and Natural Resources; South East Wales Biodiversity Records Centre - CCW Regional Data ; Southern Cape herbarium, South African National Biodiversity Institute; Specimen Database of Colorado Vascular Plants, University of Colorado Museum of Natural History; Staatliche Naturwissenschaftliche Sammlungen Bayerns (MB); Staatliches Museum für Naturkunde Stuttgart; Suffolk Biological Records Centre; Sukkulentensammlung Zürich, Herbaria of the University and ETH Zürich (Z+ZT); Swan Coastal Plain Survey, Department of Environment and Conservation, Western Australia; Swedish Museum of Natural History (S); Taiwan e-Learning and Digital Archives Program (TELDAP); Taiwan Forestry Research Institute (TAIF); Take a Pride in Fife Environmental Information Centre (Records for Fife from TAPIF EIC); The Danish Biodiversity Information Facility (DanBIF); The Danish Royal Veterinary and Agricultural University's Arboretum; The Deaver Herbarium (ASC); The European Genetic Resources Search Catalogue; The European Genetic Resources Search Catalogue (EURISCO); The Flora of County Waterford, The Flora of County Wexford, Heritage Trees of Ireland, Ireland’s BioBlitz 2011, Irish vascular plant data 1999-2009, Irish vascular plant data 2011, (National Biodiversity Data Centre); The Herbarium of the Université Libre de Bruxelles (ULB); The Himalayan Uplands Plant database (HUP Version 1), Global Mountain Biodiversity Assessment (GMBA); The Norwegian Biodiversity Information Centre (NBIC) (NOF); The Shimane Nature Museum of Mt. Sanbe, National Museum of Nature and Science, Japan; The System-wide Information Network for Genetic Resources (SINGER); The Vascular Plant Collection at the Botanische Staatssammlung München; Tipos de plantas vasculares, UNIBIO, IBUNAM (MEXU); Tiroler Landesmuseum Ferdinandeum; Tracheophyta collection of Biodiversity Center of Japan, National Museum of Nature and Science, Japan; Tropicos Specimen Database, Missouri Botanical Garden; UAM Botany Specimens; UCBG TAPIR Provider; UCJEPS TAPIR Provider; UK National Biodiversity Network (Thames Valley Environmental Records Centre; United States National Plant Germplasm System Collection; Universidad de Extremadura; Universidad de León; Universidad de Málaga; Universidad de Málaga (MGC-Cormof); Universidad Politécnica de Madrid; Universität Salzburg; Universitatsherbarium Gottingen; Université d'Abomey-Calavi, Faculté des Sciences Agronomiques (ABC); Université de Strasbourg herbier de nouvelle-caledonie; University and Jepson Herbaria (DiGIR provider); University of Alberta Museums (UA); University of Arizona Herbarium (ARIZ); University of British Columbia Herbarium (UBC) - Vascular Plant Collection; University of California Botanical Garden (DiGIR provider); University of California, Davis (UCD); University of Connecticut (CONN); University of Copenhagen Arboretum; University of Ghana - Ghana Herbarium, Ghana Biodiversity Information Facility (GhaBIF); University of Kansas Biodiversity Institute; University of Michigan Museum of Zoology (UMMZ); University of Oslo (ZMO); University of Washington Burke Museum (UWBM); USDA PLANTS Database; Utah State University (UTC); Utah Valley State College Herbarium (UVSC); Vascular Plant Collection - University of Washington Herbarium (WTU); Vascular Plant Specimen Database of Kanagawa Prefectural Museum of Natural History; Vascular plant specimens of Akita Prefectural Museum; Vascular plants collection of Hiratsuka City Museum; Vascular Plants Collection of National Museum of Nature and Science; Vascular Plants Collection of Sagamihara City Museum; Vascular plants database of Atugi City Museum; Vascular plants of south-central China (Harvard University Herbaria); Visual Plants (144.41.33.158) - Private collection of Helmut Dalitz and Vindas Jorge, Herbarium specimen from “BIEL,” Plants from Costa Rica, Plants from Southern Ecuador, Plants from the Kakamega Forest, Kenya; Dana Uster, Institute of Botany, University of Hohenheim.; Western Australian Herbarium (PERTH); Wildlife Institute of India (WII); Wilson Botanical Garden - Las Cruces Biological Station, Organization for Tropical Studies; Wroclaw University, Fac. Natural Sciences; Yale University Peabody Museum (PB), (Accessed through GBIF Data Portal, data.gbif.org, 2013-11-26).
APPENDIX 9. NEW EXTANT LEAF LITTER SAMPLES
Summary of physiognomic data from extant leaf litter collections in New Zealand and Australia made by the author, and predicted values for MAT according to methods of different authors.
APPENDIX 10. PODOCARPUS WIDTHS
Figure 21 and Figure 22 indicate the spread of MAT ranges for Podocarpus species in order of approximate leaf size.
 |
 |
APPENDIX 11: REEVALUTION OF SLUITER ET AL. (1995)
 The extensive GBIF database available today used in association with DIVA–GIS and its climate database allow a reevalution of Sluiter et al. (1995). These authors arrived at a MAT of c. 18°C for the Miocene of the Australian Latrobe Valley coal. The most important result is that the cooler end of some climate envelopes can be extended (Figure 23). These include the genera Macaranga, Mallotus and Ilex. Tropical data also fill the apparent 'gap' pointed out by Sluiter et al. (1995) between the climatic ranges of the Australian and New Zealand Agathis. Of interest is that although there is now greater overlap between the cool Phyllocladus and Nothofagus sg. Lophozonia and the warmer taxa, they remain cool outliers. This broadening of the climate envelopes focusses attention on genera or even species, whose presence in the Latrobe fossil record has never been rigorously demonstrated, for example Banksia integrefolia and Tasmannia. Essentially, the precision of Sluiter et al.,'s (1995) Latrobe Valley MAT estimate of 18°C can no longer be maintained.
The extensive GBIF database available today used in association with DIVA–GIS and its climate database allow a reevalution of Sluiter et al. (1995). These authors arrived at a MAT of c. 18°C for the Miocene of the Australian Latrobe Valley coal. The most important result is that the cooler end of some climate envelopes can be extended (Figure 23). These include the genera Macaranga, Mallotus and Ilex. Tropical data also fill the apparent 'gap' pointed out by Sluiter et al. (1995) between the climatic ranges of the Australian and New Zealand Agathis. Of interest is that although there is now greater overlap between the cool Phyllocladus and Nothofagus sg. Lophozonia and the warmer taxa, they remain cool outliers. This broadening of the climate envelopes focusses attention on genera or even species, whose presence in the Latrobe fossil record has never been rigorously demonstrated, for example Banksia integrefolia and Tasmannia. Essentially, the precision of Sluiter et al.,'s (1995) Latrobe Valley MAT estimate of 18°C can no longer be maintained.

