 Subfossil cyclostome bryozoans from Daidokutsu submarine cave, Okinawa, Japan
Subfossil cyclostome bryozoans from Daidokutsu submarine cave, Okinawa, Japan
Article number: 28.2.a29
https://doi.org/10.26879/1546
Copyright Palaeontological Association, July 2025
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 5 March 2025. Acceptance: 7 July 2025.
ABSTRACT
A sediment core (Core 19) taken in Daidokutsu cave on Ie Island, Okinawa, spans the last 7,000 years. The sampling of multiple taxa from this submarine cave has been aimed at understanding the Holocene history of biodiversity and ecological dynamics. The results have already been published for ostracods, molluscs, foraminifera and cheilostome bryozoans. The current study focuses on the cyclostome bryozoan fauna, establishing a taxonomic foundation that will contribute to an understanding of responses by the bryozoan community in this cave habitat to environmental and climate changes through the Holocene. Very little has been published on modern and Quaternary fossil cyclostomes from Japan, and nearly all publications predate the routine use of scanning electron microscopy in cyclostome taxonomy. Fifteen cyclostome species are described here from Daidokutsu. Eight of these are new species, the remaining seven were identified only to the genus level. The high proportion of new species may not only reflect the uniqueness of the Daidokutsu cyclostome fauna but also the scarcity of studies on Japanese cyclostomes and the inadequacy of descriptions and figures in older publications, which make it difficult or impossible to interpret the species they describe. Unlike cyclostome cave faunas from the Mediterranean, erect cyclostomes strongly outnumber species with encrusting colonies. In addition, the secondary homonymy of Parasmittina ligulata, used for both a new species from Daidokutsu Cave and a Western Atlantic species, is resolved by renaming the Japanese species Parasmittina vieirai nom. nov.
Paul D. Taylor. Natural History Museum, Cromwell Road, London, SW7 5BD, UK. p.taylor@nhm.ac.uk; ORCID: 0000-0002-3127-080X
Emanuela Di Martino. Dipartimento di Scienze Biologiche, Geologiche e Ambientali, Università di Catania, Corso Italia 57, 95129, Catania, Italy. Corresponding author; emanuela.dimartino@unict.it; ORCID: 0000-0002-3892-4036
Antonietta Rosso. Dipartimento di Scienze Biologiche, Geologiche e Ambientali, Università di Catania, Corso Italia 57, 95129, Catania, Italy. rosso@unict.it; ORCID: 0000-0001-5565-9513
Ruby W.T. Chiu. School of Biological Sciences and Swire Institute of Marine Science, The University of Hong Kong, Kadoorie Biological Sciences Building, Hong Kong, China. rubychiu@ymail.com; ORCID: 0000-0001-6663-9526
Kazuhiko Fujita. Department of Physics and Earth Sciences, Faculty of Science and Tropical Biosphere Research Center, University of the Ryukyus, Okinawa, 903-0213, Japan. fujitaka@sci.u-ryukyu.ac.jp; ORCID: 0000-0002-9833-007X
Akihisa Kitamura. Institute of Geosciences, Shizuoka University, Shizuoka, 422-8529, Japan. kitamura.akihisa@shizuoka.ac.jp; ORCID: 0000-0002-9928-3746
Moriaki Yasuhara. School of Biological Sciences, Area of Ecology and Biodiversity, Swire Institute of Marine Science, Institute for Climate and Carbon Neutrality, Musketeers Foundation Institute of Data Science, and State Key Laboratory of Marine Pollution, The University of Hong Kong, Kadoorie Biological Sciences Building, Hong Kong, China. yasuhara@hku.hk; ORCID: 0000-0003-0990-1764
Keywords: Cyclostomatida; Holocene; new species; taxonomy
Final citation: Taylor, Paul D., Di Martino, Emanuela, Rosso, Antonietta, Chiu, Ruby W.T., Fujita, Kazuhiko, Kitamura, Akihisa, and Yasuhara, Moriaki. 2025. Subfossil cyclostome bryozoans from Daidokutsu submarine cave, Okinawa, Japan. Palaeontologia Electronica, 28(2):a29.
https://doi.org/10.26879/1546
palaeo-electronica.org/content/2025/5573-holocene-cyclostomes-from-japan
Copyright: July 2025 Palaeontological Association.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
Biotic responses to climatic and environmental changes are being addressed through a multitaxon study of subfossils sampled from cores spanning the last 7,000 years in Daidokutsu submarine cave on Ie Island, Okinawa, Japan. Results have already been published on ostracods (Chiu et al., 2016, 2017), molluscs (Kitamura et al., 2007a, b, 2013; Yamamoto et al., 2009, 2010; Chiu et al., 2017), foraminifera (Omori et al., 2010; Chiu et al., 2017) and cheilostome bryozoans (Di Martino et al., 2025). The current paper extends the study to cyclostomes, a lesser-known order of bryozoans.
According to Bock and Gordon (2013), cyclostomes are represented by an estimated 543 species in modern oceans. This is roughly one-tenth of the diversity of cheilostomes, which are the dominant order of bryozoans living today. Like cheilostomes, cyclostomes have calcareous skeletons and locally contribute significant quantities of biogenic carbonate sediment to the seafloor. The fossil record of cyclostomes extends back to the Ordovician (Taylor, 2020), and they have colonised submarine caves since at least the Late Jurassic (Taylor and Palmer, 1994). Cyclostome zooids have relatively simple skeletons which are typically tubular, in contrast to the more complex, generally box-shaped skeletons of cheilostome zooids. As a result, the range of skeletal morphological characters available for cyclostome taxonomy is more limited, a fact recognised by bryozoologists since the nineteenth century (e.g., Waters, 1884, 1887; Gregory, 1896), and the first descriptions of species in the early bryozoan literature were considered to be inadequate even as far back as 1904 (Waters, 1904, p. 172). High levels of morphological plasticity also characterise cyclostome species (Harmelin, 1976a, b), while molecular phylogenetic studies have revealed major discordances between the existing classification of cyclostomes and phylogeny (Waeschenbach et al., 2009). These factors have contributed to cyclostomes being justifiably branded as a difficult group, which in part may account for the scarcity of modern taxonomic studies.
Knowledge of cyclostomes from Japan is particularly poor. While some studies of bryozoan faunas have included cyclostomes (Ortmann, 1890; Okada and Mawatari, 1936; Mawatari, 1952; Kataoka, 1960), very few papers have focused solely on cyclostomes. Okada (1917) reported and described 33 species of cyclostomes from the modern seas of Japan. Unfortunately, this paper, which introduces seven new species, lacks illustrations, making it particularly difficult to use for species identification. In a subsequent paper, Okada (1928) described 12 species of cyclostomes from Mutsu Bay, five new, this time accompanied by drawings and photographic illustrations. A checklist of Japanese cyclostomes (Mawatari, 1955), which included some fossils, listed 78 species from Japan. Mawatari and Mawatari (1973) described 10 species of crisiid cyclostomes around the coast of Hokkaido, two of which were new. From the same region, they later described 21 species of non-crisiid cyclostomes, including a single new species (Mawatari and Mawatari, 1974). Finally, Taylor and Grischenko (2015) described two new cyclostome species unusual for forming heavily calcified colonies in the intertidal zone of Akkeshi Bay, Hokkaido. Turning to the Quaternary fossil record, Dick et al. (2008) compiled data from the literature on Japanese Pleistocene bryozoans. These authors recorded 22 cyclostome species, representing only 6.1% of the diversity of the total bryozoan fauna. However, a subsequently discovered Pleistocene locality in the Setana Formation at Kuromatsunai in Hokkaido alone was found to contain 24 species of cyclostomes (Taylor et al., 2013). This compares with 96 species of cheilostomes from the same locality, meaning that cyclostomes make up 20% of total bryozoan diversity at Kuromatsunai.
MATERIAL AND METHODS
Material used for this study was sorted from 62 samples (Di Martino et al., 2025, table 1), each corresponding to 1 cm sections of a sediment core (Core 19) obtained from a depth of 29 m inside the Daidokutsu submarine cave (26.72°N, 127.83°E; see Yamamoto et al., 2009; Chiu et al., 2017, figure 1A, B), which is located on the east coast of Ie Island, Okinawa, Japan. Core 19 was drilled 32.5 m from the entrance of the cave (see Chiu et al., 2017, figure 1C). Encompassing sediments deposited through approximately the last 7,000 years based on radiocarbon dating (Yamamoto et al., 2009; Kitamura et al., 2013), Core 19 is 5 cm in diameter and 233 cm long. Samples were wet-sieved using a 63 μm sieve, dried, and then dry-sieved using 150 μm, 250 μm, 500 μm and 1 mm sieves. Bryozoans were picked from the >500 μm size fractions.
Imaging of uncoated specimens employed low-vacuum scanning electron microscopes operating in back-scattered mode. These instruments were a LEO VP-1455 and a JEOL IT500 at the Natural History Museum, London, UK; a Tescan Vega 2 LMU at the Department of Biological, Geological and Environmental Sciences, University of Catania, Italy; and a Hitachi TM4000plus Tabletop at the Natural History Museum, University of Oslo, Norway.
Morphometric measurements were taken from SEM images using the image processing program ImageJ (available at https://imagej.nih.gov/). Each measurement is given as the mean value plus or minus the standard deviation, followed by the observed range, with the number of specimens used and the total number of measurements both enclosed in parentheses. Please note that the measurements correspond to the illustrated specimens and occasionally to other specimens listed under each species.
Three of us (PDT, EDM and AR) were responsible for the systematic part of this paper and are to be considered the authors for all new species described.
All specimens are deposited in the collections of the Palaeontological Museum (PMC) of the Department of Biological, Geological, and Environmental Sciences, University of Catania (Italy).
SYSTEMATIC PALAEONTOLOGY
Phylum BRYOZOA Ehrenberg, 1831
Class STENOLAEMATA Borg, 1926
Order CYCLOSTOMATIDA Busk, 1852
Suborder ARTICULATA Busk, 1859
Family CRISIIDAE Johnston, 1847
Genus CRISIA Lamouroux, 1812
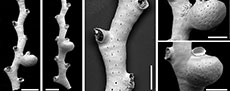
Crisia ramalhoae sp. nov. Taylor, Di Martino and Rosso
Figure 1
zoobank.org/0291833E-FC44-4617-BD0B-6E4040F58B08
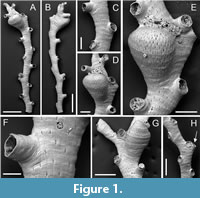 Type material. Holotype PMC. B74. 26.2.2025a, sample 19052 (Figure 1A-D, F); paratypes PMC. B74. 26.2.2025b1, sample 19056 (Figure 1E); PMC. B74. 26.2.2025b2 sample 19085 (Figure G-H); PMC. B74. 26.2.2025b3, sample 19085. Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Type material. Holotype PMC. B74. 26.2.2025a, sample 19052 (Figure 1A-D, F); paratypes PMC. B74. 26.2.2025b1, sample 19056 (Figure 1E); PMC. B74. 26.2.2025b2 sample 19085 (Figure G-H); PMC. B74. 26.2.2025b3, sample 19085. Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Etymology. Named for Lais Ramalho in recognition of her studies on bryozoans from Brazil and the Mediterranean Sea.
Diagnosis. Crisia with narrow internodes, comprising more than 10 zooids; nodes located immediately proximal of an autozooidal aperture, one or two per internode; autozooids small, apertures circular, pseudopores slit-shaped; brood chamber intervening between two autozooids along one side of internode, inverted pear-shaped, bulbous, densely pseudoporous, pseudopores slit-shaped; ooeciostome very short, located distally of brood chamber; ooeciopore transversely elliptical, about as wide as an autozooidal aperture.
Description. Colony erect, articulated, internodes narrow, 110-168 µm wide, comprising 10-14 or more zooids, biserial, autozooids opening alternately on either side of branch, zooidal boundaries not apparent on wrinkled colony surface. Articulation bases located immediately proximal of an autozooidal aperture, one or two per internode, about the same diameter or slightly larger than an autozooidal aperture, facing frontolaterally or outwardly. Ancestrula and colony base unknown. Autozooids small, slender, frontal wall covered by slit-shaped pseudopores located in shallow grooves between strips of calcification 14-20 µm wide, pseudopores 9-12 µm long by 1-2 µm wide, centre-to-centre spacing of pseudopores in same groove 45-105 µm; apertures small, circular, 52-83 µm in diameter, facing distolaterally; peristomes short with high angle of divergence and shorter pseudopores. Gonozooids inverted pear-shaped, bulbous, intervening between two autozooids along one side of internode; brood chamber densely covered by slit-shaped pseudopores located in grooves between narrow strips of calcification 7-13 µm wide, pseudopores 7-11 µm long by 1-2 µm wide, centre-to-centre spacing of pseudopores in same groove 30-46 µm; ooeciostome very short, terminal, located distally of brood chamber sometimes off-centred relative to brood chamber axis, slightly flared; ooeciopore transversely elliptical, about as wide as an autozooidal aperture.
Measurements (µm). Zooid length 329±32, 307-397 (2, 7); aperture diameter 63±10, 52-83 (2, 10); brood chamber length 296±40, 255-350 (4, 4); brood chamber width 246±33, 217-291 (4, 4); ooeciopore length 30±6, 24-36 (3, 3); ooeciopore width 76±9, 69-86 (4, 4).
Remarks. Crisia is a speciose genus containing almost 80 extant species, very few of which have been illustrated or described comprehensively by modern standards. Ziko et al. (2012) identified six species of Crisia from the Red Sea, paying particular attention to the morphology of the pseudopores. To judge from figure 6 of their paper, none of these species have pseudopores as elongate as those in C. ramalhoae sp. nov. where they are up to ten times longer than wide (Figure 1F). Slit-shaped pseudopores characterise Crisia spissus Chae, Kil, Zágoršek and Seo, 2018, but this species from Korea has broader branches than C. ramalhoae sp. nov., and the ooeciopore is circular rather than transversely elliptical. The Brazilian species Crisia fragosa Ramalho, Muricy and Taylor, 2009 also has slit-like pseudopores but the autozooids are much larger (length 863-1212 µm compared to 307-397 in C. ramalhoae sp. nov.) and the ooeciostome is long rather than short. Another Brazilian species, C. ficulnea Buge, 1979, is distinctive among species of Crisia with slit-shaped pseudopores in lacking any trace of an ooeciostome, while the Madeiran species Crisia noronhai sp. Souto, Ramalhosa and Canning-Clode in Souto et al., 2023 has an almost circular ooeciopore contrasting with the transversely elliptical ooeciopore of C. ramalhoae sp. nov. Finally, C. guang Liu, Liu and Zágoršek, 2019, from the South Yellow Sea of China differs from the new species in having a very long and slender gonozooid.
Crisia prominens sp. nov. Taylor, Di Martino and Rosso
Figure 2
zoobank.org/5B8A3AF5-6D7A-4CC7-A5B3-B79BE4E815E6
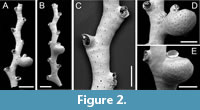 Type material. Holotype PMC. B75. 26.2.2025a, sample 19056 (Figure 2A, C-D); paratype PMC. B75. 26.2.2025b1, sample 19036 (Figure 2B, E). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Type material. Holotype PMC. B75. 26.2.2025a, sample 19056 (Figure 2A, C-D); paratype PMC. B75. 26.2.2025b1, sample 19036 (Figure 2B, E). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Etymology. Named for the prominent brood chambers.
Diagnosis. Crisia with narrow internodes, zigzagging; nodes close to base of internode proximolaterally of an autozooidal aperture; autozooids very small, apertures circular, pseudopores longitudinally elongate, sparse; gonozooids small, bulbous, vicarious, replacing an autozooid in proximal part of internode, brood chamber protruding, bean-shaped, densely pseudoporous, pseudopores longitudinally elongate, ooeciostome short, hooked, located on distal-facing top of brood chamber; ooeciopore transversely elliptical.
Description. Colony erect, articulated; internodes narrow, 67-121 µm wide, zigzagging, comprising 9 or more zooids, biserial, autozooids opening alternately on either side of branch, zooidal boundaries not apparent on colony surface. Articulation bases located proximolaterally of an autozooidal aperture, close to base of internode, facing distolaterally. Ancestrula and colony base unknown. Autozooids small, slender, frontal wall covered by sparse, longitudinally elongated pseudopores located between ill-defined strips of calcification, pseudopores sparse, 5-8 µm long by 2-3 µm wide, nearest neighbour centre-to-centre spacing 23-29 µm; apertures small, circular, 45-58 µm in diameter, facing distolaterally; peristomes short. Gonozooids small, bulbous, vicarious, replacing an autozooid in proximal part of internode; brood chamber protruding from internode, bean-shaped, about 215 µm long by 190 µm wide, densely covered by longitudinally elongate pseudopores, 5-9 µm long by 2-3 µm wide, more closely spaced than on autozooidal frontal walls, nearest neighbour centre-to-centre spacing of pseudopores 11-20 µm; ooeciostome short, hooked, located on distal-facing top of brood chamber; ooeciopore transversely elliptical, about as wide as an autozooidal aperture, 36 µm long by 58 µm wide in measured example, facing distolaterally.
Measurements (µm). Zooid length 299±21, 269-327 (2, 9); aperture diameter 49±3.5, 45-58 (2, 11).
Remarks. The most distinctive feature of Crisia prominens sp. nov. is the gonozooid with its bean-shaped, protruding brood chamber (Figure 2D, E). Brood chambers of similar morphology are found in Crisia cuneata Maplestone, 1905, as figured by Harmer (1915, pl. 8, figures 16-17) and Chae et al. (2020, figure 1E), C. circinata Waters, 1914, from Zanzibar, as well as C. tenella longinodata Rosso, 1998, from the Pleistocene of southern Italy (Rosso, 1998, plate 1, figures 9-10). However, the branches of C. prominens sp. nov. are more gracile than those of C. cuneata, with the autozooids appearing slenderer and aperture spacing about three times branch width compared to twice or less in C. cuneata. Crisia circinata has autozooids of similar length (distance between autozooidal series about 0.27 mm according to Waters (1914) vs. 0.27-0.33 mm in C. prominens sp. nov.) and gonozooids positioned low in the internodes, apertural diameter is larger than in C. bulbosa sp. nov. (0.08 mm vs. 0.05-0.06 mm). Another East African species, C. zanzibarensis Brood, 1976, also has protruding brood chambers but the gonozooids in this species are larger and the internodes seemingly longer containing 15-30 zooids compared to fewer than 10 zooids seen in C. prominens sp. nov. Crisia tenella longinodata differs in having longer and more slender internodes as well as peristomes of smaller diameter.
The autozooids of C. prominens sp. nov. are slightly smaller than those of Crisia ramalhoae sp. nov. However, apart from the contrasting morphology of the gonozooids, the most obvious difference concerns the pseudopores. These are roughly three times longer than wide in C. prominens sp. nov. (Figure 2C), compared with about 5-10 times longer in Crisia ramalhoae sp. nov. (Figure 1F) where they are more clearly slit-shaped.
Genus CRISIONA Canu and Bassler, 1927
Crisiona sp.
Figure 3
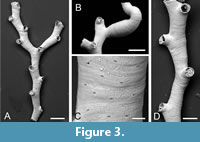 Figured material. PMC EDM-Collection J. H. B.152a.1, sample 19031 (Figure 3A); PMC EDM-Collection J. H. B.152a.2, sample 19031 (Figure 3C-D); PMC EDM-Collection J. H. B.152a.3, sample 19058 (Figure 3B). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Figured material. PMC EDM-Collection J. H. B.152a.1, sample 19031 (Figure 3A); PMC EDM-Collection J. H. B.152a.2, sample 19031 (Figure 3C-D); PMC EDM-Collection J. H. B.152a.3, sample 19058 (Figure 3B). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Description. Colony erect, seemingly not articulated; branches narrow, averaging 105 µm wide (range 72-143 µm), zigzagging mildly, biserial, autozooids opening alternately on either side of branch, zooidal boundaries not visible; bifurcations at an angle of about 80°, one of the daughter branches diverging immediately distally of an aperture. Ancestrula and colony base unknown. Autozooids slender, frontal wall relatively smooth; pseudopores small, about twice as wide as long, 2-3 µm long by 5-6 µm wide, nearest neighbour centre-to-centre spacing 17-29 µm, lacking spines or other constrictions; apertures very small, circular, facing somewhat laterally; peristomes short. Gonozooids unknown.
Measurements (µm). Zooid length 392±57, 285-461 (2, 10); aperture diameter 61±6.7, 48-73 (2, 10).
Remarks. This species is assigned tentatively to the little-known genus Crisiona , which was introduced by Canu and Bassler (1927) for crisiids with non-articulated colonies. The type species, C. baculifera Canu and Bassler, 1927, comes from Hawaii, and one other Recent species, two Cretaceous species and one Miocene species were referred by Canu and Bassler to their new genus. The type species has bifurcating branches with long tubular prolongations (?kenozooids) on the sides of the branches. One specimen of the Daidokutsu species has two questionable bifurcations near the distal end of the fragment, one on the left is short and presumably broken, and a longer one on the right is strongly curved (Figure 3B). In the absence of a gonozooid, however, we hesitate to introduce a new species. The broad pseudopores (Figure 3C) enable distinction from the two species of Crisia described above in addition to the lack of articulation nodes.
Suborder TUBULIPORINA Milne-Edwards, 1838
Family ONCOUSOECIIDAE Canu, 1918
Genus FILISPARSA d’Orbigny, 1853
Filisparsa haywardi sp. nov. Taylor, Di Martino and Rosso
Figure 4
zoobank.org/038007A9-3CA8-45E2-A3A0-EDB1AA503EE1
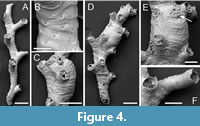 Type material. Holotype PMC. B76. 26.2.2025a, sample 19085 (Figure 4D-E); paratypes PMC. B76. 26.2.2025b.1 (Figure 4C), PMC. B76. 26.2.2025b.2, PMC. B76. 26.2.2025b.3 (Figure 4F) sample 19085; PMC. B76. 26.2.2025b.4, sample 19029 (Figure 4A-B). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Type material. Holotype PMC. B76. 26.2.2025a, sample 19085 (Figure 4D-E); paratypes PMC. B76. 26.2.2025b.1 (Figure 4C), PMC. B76. 26.2.2025b.2, PMC. B76. 26.2.2025b.3 (Figure 4F) sample 19085; PMC. B76. 26.2.2025b.4, sample 19029 (Figure 4A-B). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Etymology. Named for Peter J. Hayward in recognition of his numerous contributions to bryozoology, including a synopsis on British cyclostome bryozoans (Hayward and Ryland, 1985b).
Diagnosis. Filisparsa with small autozooids, length averaging about 400 µm, frontal wall smooth with transversely elliptical pseudopores; gonozooids longitudinally ovoidal, margins indented by autozooidal peristomes, ooeciopore subcircular, small.
Description. Colony erect, seemingly not articulated, bifurcations not observed, branches narrow, averaging 166 µm wide (range 84-320 µm), biserial, becoming quadriserial where gonozooids occur, often slightly curved, autozooids opening alternately on either side of branch, zooidal boundaries obscure. Ancestrula and colony base unknown. Autozooids small, slender, frontal wall rugose with strong transverse folds; pseudopores transversely elliptical, 4-7 µm long by 6-10 µm wide, proximal edge somewhat raised, nearest neighbour centre-to-centre spacing 20-34 µm, with a sunken iris-like constriction restricting the opening; apertures small, circular, facing distolaterally; peristomes moderately long, up to 250 µm, pseudopores similar shape and density to frontal walls. Gonozooids small, moderately bulbous; brood chamber longitudinally ovoidal, 344-415 µm long by 220-257 µm wide, edges indented by adding autozooids, densely pseudoporous, the pseudopores subcircular, 5-8 µm in diameter, nearest neighbour centre-to-centre spacing 12-19 µm; ooeciostome short, terminal; ooeciopore circular or variably elliptical, smaller than an autozooidal aperture 54-60 µm long by 42-66 µm wide.
Measurements (µm). Zooid length 402±63, 312-539 (3, 11); aperture diameter 80±9.3, 69-101 (3, 10).
Remarks. Although the type species of Filisparsa - F. neocomiensis d’Orbigny, 1853 - is of Early Cretaceous age, at least 15 Recent species have been assigned to the genus. Unfortunately, gonozooids have not been described in F. neocomiensis (Pitt and Taylor, 1990, p. 81) but in a second Early Cretaceous species, F. gasteri Pitt and Taylor, 1990, they are small and longitudinally ovate with an ooeciopore smaller than the autozooidal apertures, as in F. haywardi sp. nov. Among extant species, F. haywardi sp. nov. resembles F. rugosa Canu and Bassler, 1929 from the Philippines, which is described as having a transversely wrinkled surface. However, the Philippine species has much larger zooids, their length given as 1.12-1.84 mm (cf. 0.31-0.54 mm in the present species) and with peristomes measuring 0.28-0.30 mm in width, at least three times greater than in F. haywardi sp. nov. Canu and Bassler’s material of F. rugosa was, unfortunately, infertile. The new species shares with the East African species F. gracilis Brood, 1976 delicate colonies, but F. gracilis lacks surface wrinkles and has a larger ooeciopore with a hooked ooeciostome. The Mediterranean deep-water species ‘Filisparsa’ profunda Harmelin and d’Hondt, 1982 has larger zooids than F. haywardi sp. nov., with peristomes 175-205 µm in diameter. A similar, unnamed species of Filisparsa described by Di Martino and Taylor (2014) from the Miocene of East Kalimantan differs from F. haywardi sp. nov. in having teardrop-shaped pseudopores; unfortunately, the gonozooid is unknown in this Indonesian fossil.
Family ANNECTOCYMIDAE Hayward and Ryland, 1985a
Genus ANNECTOCYMA Hayward and Ryland, 1985a
Annectocyma sp.
Figure 5
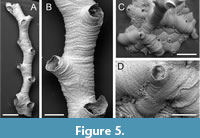 Figured material. PMC EDM-Collection J. H. B.153a.1, sample 19019 (Figure 5A-B); PMC EDM-Collection J. H. B.153a.2, sample 19112 (Figure 5C-D). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Figured material. PMC EDM-Collection J. H. B.153a.1, sample 19019 (Figure 5A-B); PMC EDM-Collection J. H. B.153a.2, sample 19112 (Figure 5C-D). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Description. Colony base encrusting, initially uniserial, rapidly giving rise to first erect branch, narrow, averaging 362 µm wide (range 289-555 µm), biserial, autozooids opening alternately on either side of branch, zooidal boundaries obscure. Ancestrula short, aperture 125 µm in diameter, protoecium 205 µm wide with pseudopores sparsely scattered over surface; three periancestrular zooids: a conventional distal bud and two adventitious lateral buds on either side of ancestrular aperture. Autozooids slender, frontal wall transversely wrinkled; pseudopores longitudinally elliptical or circular, tiny, 3-5 µm long by 2-4 µm wide, nearest neighbour centre-to-centre spacing 21-29 µm, lacking spines or other constrictions; apertures, circular, facing distolaterally; peristomes short. Gonozooids not observed.
Measurements (µm). Zooid length 963±123, 819-1124 (1, 10); aperture diameter 173±29.1, 149-215 (1, 4).
Remarks. Erect branches of this species resemble Crisiona sp. but the autozooids are considerably larger, have more rugose surfaces, and the pseudopores are pinprick-like (Figure 5B) and typically longitudinally rather than transversely elongate. Assignment to Annectocyma is based mainly on the presence of adventitious budding from the ancestrula, with zooids budded on either side of the ancestrular aperture in the Okinawan species (Figure 5D). While Annectocyma colonies are often entirely encrusting, erect branches sometimes occur and in the Daidokutsu species erect growth is initiated in very early astogeny. The discovery of gonozooids would help to test this generic placement, which for now must be somewhat tentative.
Family DIAPEROECIIDAE Canu, 1918
Genus NEVIANIPORA Borg, 1944
Nevianipora sp.
Figure 6
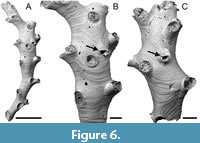 Figured material. PMC EDM-Collection J. H. B.154a.1, sample 19029 (Figure 6A-B); PMC EDM-Collection J. H. B.154a.2, sample 19056 (Figure 6C). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Figured material. PMC EDM-Collection J. H. B.154a.1, sample 19029 (Figure 6A-B); PMC EDM-Collection J. H. B.154a.2, sample 19056 (Figure 6C). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Description. Colony erect, branches narrow, about 300-700 µm wide, with a frontal and an abfrontal side, generally quadriserial with two rows of connate or closely spaced zooids on each side of branch opening alternately, zooidal boundaries very slightly grooved. Autozooids slender, frontal wall with faint transverse growth checks; pseudopores longitudinally elliptical, tiny, 4-6 µm long by 2-3 µm wide, nearest neighbour centre-to-centre spacing 16-29 µm, lacking spines or other constrictions; apertures, somewhat longitudinally elongate, facing distolaterally; peristomes short. Gonozooids narrow; brood chamber about three times longer than wide, 1268-1783 µm long by 478-552 µm wide (N = 2), modestly inflated, roof indented or penetrated by a few autozooids, densely pseudoporous, pseudopores larger than those on autozooidal frontal walls, longitudinally elongate, 4-7 µm long by 3-5 µm wide, nearest neighbour centre-to-centre spacing 14-23 µm; ooeciostome located approximately mid-length, broken in both examples, off-centred in one; ooeciopore transversely elongate, semielliptical, the distal edge almost straight, 73-97 µm long by 145-146 µm wide (N =2).
Measurements (µm). Zooid length 1011±123, 822-1186 (1, 16); zooid width 227±22.5, 213-253 (1, 3); aperture length 193±43.3, 143-278 (1, 8); aperture width 172±8.4, 151-179 (1, 10).
Remarks. Two fertile branches of this species have been imaged using SEM, both showing an elongate gonozooid with an ooeciostome situated at mid-length, corresponding to the non-terminal position typical of the genus. In Nevianipora milneana (d’Orbigny, 1842), the type species of the genus, the branches are flatter and wider, with three rows of autozooids on each side of the midline, compared with two rows in the Daidokutsu species. A couple of Chinese species of Nevianipora redescribed by Liu et al. (2019) also have broader branches with more rows of autozooids, as does N. arcuata Winston, Vieira and Woollacott, 2014 from Brazil. We defer from introducing a new species for the Okinawan species without a more detailed study of its morphology, which is hindered by the small size of the fragments found.
Genus PSILOSOLEN Canu and Bassler, 1922
Psilosolen harmelini sp. nov. Taylor,
Di Martino and Rosso
Figure 7
zoobank.org/CB514D44-E508-4FA9-96A3-A09373660B80
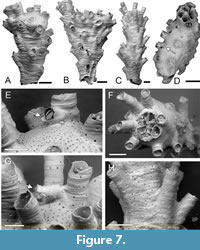 Type material. Holotype PMC. B77. 26.2.2025a, sample 19013 (Figure 7A, E); paratypes PMC. B77. 26.2.2025b.1, sample 19031 (Figure 7B, D); PMC. B77. 26.2.2025b.2, sample 19058 (Figure 7C, F, H); PMC. B77. 26.2.2025b.3, sample 19018; PMC. B77. 26.2.2025b.4, sample 19035 (Figure 7G). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Type material. Holotype PMC. B77. 26.2.2025a, sample 19013 (Figure 7A, E); paratypes PMC. B77. 26.2.2025b.1, sample 19031 (Figure 7B, D); PMC. B77. 26.2.2025b.2, sample 19058 (Figure 7C, F, H); PMC. B77. 26.2.2025b.3, sample 19018; PMC. B77. 26.2.2025b.4, sample 19035 (Figure 7G). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Etymology. Named for Jean-Georges Harmelin in recognition of his seminal research on cyclostome bryozoans.
Diagnosis. Psilosolen with narrow branches; about 8-15 autozooids opening all around branch circumference; autozooids small; pseudopores teardrop-shaped ; gonozooids terminal with brood chambers circular to elliptical in outline penetrated or indented by about 5-8 autozooidal apertures; ooeciostome adnate to a peristome, oriented subparallel to brood chamber roof; ooeciopore transversely elongate.
Description. Colony erect, branches narrow vincularian, 300-1100 µm wide, broadening distally, autozooids opening around the entire circumference in about 8-15 longitudinal rows. Autozooids slender, zooidal boundaries marked by narrow fissures, frontal wall with transverse growth checks, convex; pseudopores teardrop-shaped , pointed distally, usually longer than wide, 13-17 µm long by 11-16 µm wide, nearest neighbour centre-to-centre spacing 28-48 µm, countersunk; apertures, longitudinally elliptical, some closed by terminal diaphragms lacking pseudopores; peristomes moderately long, preserved length up to 330 µm, oriented at a shallow angle to colony surface, sparsely pseudoporous. Gonozooids located at distal ends of branches; brood chamber circular or elliptical in outline, 565 by 308 µm and 788 by 629 µm in two measured examples, roof indented or penetrated by about 5-8 autozooidal apertures, sutured, densely pseudoporous, pseudopores teardrop-shaped , 10-13 µm long by 7-9 µm wide, nearest neighbour centre-to-centre spacing 14-23 µm, lumen occluded by about half a dozen stout spines; ooeciostome adnate to a peristome, oriented subparallel to brood chamber roof; ooeciopore transversely elongate, 50-65 µm long by 81-90 µm wide.
Measurements (µm). Zooid length 443±86.5, 311-593 (2, 10); zooid width 134±12.8, 118-153 (2, 10); aperture length 102±14.3, 81-122 (2, 10); aperture width 90±7.3, 78-101 (2, 10).
Remarks. Several fragments of vincularian tubuliporines have terminal gonozooids capping the ends of branches. This trait characterises the little-known genus Psilosolen Canu and Bassler, 1922. The type species of Psilosolen, P. capitiferax Canu and Bassler, 1922 from the Pleistocene of California, was more completely illustrated by Canu and Bassler (1923) whose figures (plate 44, figures 11-21) show the ‘Entalophora’ -like branches, with fixed-walled autozooids opening all around the branch, and gonozooids with roofs penetrated by autozooidal peristomes at the terminal distal ends of a couple of branches. Additional species seem not to have been assigned to this genus, but it has clear utility for vincularian tubuliporines with terminal gonozooids. The family placement of Psilosolen is unclear but previous assignments to Cerioporidae are inappropriate, as skeletal organization in the genus is tubuliporine, not cerioporine as in Cerioporidae. In view of the erect colony and perforated gonozooids, Diaperoeciidae is most apt.
Psilosolen harmelini sp. nov. is substantially more gracile than P. capitiferax in which the branches are up to 2 mm in diameter, twice the width of those in P. harmelini sp. nov. Transverse sections of P. capitiferax reveal 50 or more zooids (compared to about 10 in P. harmelini . sp. nov., Figure 7F), and autozooidal length is 500-1350 µm (compared to 311-593 µm in P. harmelini sp. nov.).
Family TUBULIPORIDAE Johnston, 1837
Genus EXIDMONEA David, Mongereau and Pouyet, 1972
Exidmonea lineata sp. nov. Taylor, Di Martino and Rosso
Figure 8
zoobank.org/18A29343-944A-41E5-A999-B6909DEE44C8
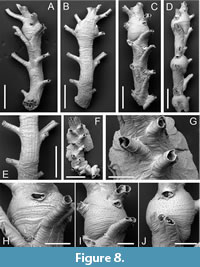 Type material. Holotype PMC. B78. 26.2.2025a, sample 19058 (Figure 8A-B, E, H); paratypes PMC. B78. 26.2.2025b.1, sample 19049 (Figure 8C, I); PMC. B78. 26.2.2025b.2, sample 19059; PMC. B78. 26.2.2025b.3, sample 19055; PMC. B78. 26.2.2025b.4, sample 19069 (Figure 8D, J); PMC. B78. 26.2.2025b.5, sample 19019 (Figure 8F-G). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Type material. Holotype PMC. B78. 26.2.2025a, sample 19058 (Figure 8A-B, E, H); paratypes PMC. B78. 26.2.2025b.1, sample 19049 (Figure 8C, I); PMC. B78. 26.2.2025b.2, sample 19059; PMC. B78. 26.2.2025b.3, sample 19055; PMC. B78. 26.2.2025b.4, sample 19069 (Figure 8D, J); PMC. B78. 26.2.2025b.5, sample 19019 (Figure 8F-G). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Diagnosis. Exidmonea with narrow branches generally having two series of initially connate autozooids either side of the midline; dorsal branch surface mildly convex, lineated with a series of longitudinal ridges; gonozooids simple, small, spanning two autozooidal series, with short, terminal ooeciostome and transversely elliptical ooeciopore typically larger than an autozooidal aperture.
Etymology. In reference to the lineations on the dorsal surface of the branches.
Description. Colony erect, branches narrow, 269-492 µm wide, widest at level of gonozooids, bifurcations not observed; autozooids opening on frontolateral surfaces, usually in series of two, occasionally apertures either side of midline; dorsal branch surface slightly convex, marked by longitudinal ridges 30-82 µm apart, with pseudoporous exterior wall between, the pseudopores simple, small, 4-5 µm in diameter, nearest neighbour centre-to-centre spacing 17-26 µm. Colony base encrusting, the single observed example lacking the ancestrula and earliest autozooids, comprising six or seven autozooidal series along each side of a curved branch with a selvedge of kenozooids closed by pseudoporous calcification; some basal autozooids with emergent peristomes. Autozooids long, zooidal boundaries well defined, grooved; frontal wall with transverse growth checks, convex; pseudopores subcircular, small, 3-5 µm in diameter, nearest neighbour centre-to-centre spacing 20-33 µm, not constricted; apertures connate, rounded rectangular to subcircular, small, about 60-65 µm in diameter; peristomes long, preserved length up to 335 µm, oriented at about 60° to colony surface, with fewer pseudopores than frontal walls. Gonozooids small, located along branch midlines, sometimes closely spaced, spanning two or slightly more series of autozooids; brood chamber globular, almost circular in outline shape, 380-595 µm long by 439-506 µm wide (N = 4), densely pseudoporous, pseudopores subcircular, small, 3-5 µm in diameter, nearest neighbour centre-to-centre spacing 12-23 µm, lacking spines or other constrictions; ooeciostome adnate to a proximal side of peristome of an autozooid close to branch midline, short, oriented subparallel to brood chamber roof; ooeciopore transversely elongate, 47-71 µm long by 88-109 µm wide (N = 4).
Measurements (µm). Zooid length 551±84.9, 380-673 (3, 8); zooid width 111±10.7, 99-130 (3, 8).
Remarks. The main distinguishing feature of this new species is the presence of longitudinal ridges on the dorsal surface of the branches (Figure 8E). The ridges probably mark the basal interzooidal boundaries of the autozooids that open on the frontolateral surfaces of the branches, a hypothesis which would be testable using CT-scanning. Other species of Exidmonea generally lack such ridges, the dorsal surfaces often featuring arcuate growth lines instead. An exception is the Antarctic species E. hula (Borg, 1944), redescribed by Ostrovsky and Taylor (1996), but this species has longer gonozooids, which extend for 2-6 series compared to the single series of the globular gonozooid of E. lineata. Exidmonea watersi (Kluge, 1946) also has globular gonozooids but in this Arctic species the ooeciostome opens to the side and not along the midline of the gonozooid. There are usually three apertures instead of one or two per series. Globular gonozooids are atypical of species assigned to Exidmonea . Indeed, Gordon and Taylor (2010) were led to refer an extant species with an Exidmonea -like colony-form to the Cretaceous genus Filicisparsa (as F. albobrunnea Gordon and Taylor, 2010) on account of its simple gonozooids. The Daidokutsu species, however, lacks the pinnate colonies seen in the type species of Filicisparsa (see Girod and Martha, 2021, figure 12). An un-named species of Exidmonea from the Miocene of East Kalimantan (Di Martino and Taylor, 2014) has similar shaped gonozooids to E. lineata but there are 3-4 connate autozooids in each series (cf. 2-3 in E. lineata ) and no longitudinal striations on the dorsal surface.
Exidmonea unda sp. nov. Taylor, Di Martino and Rosso
Figure 9
zoobank.org/36205043-D1C5-4F8D-9414-562A6469A22B
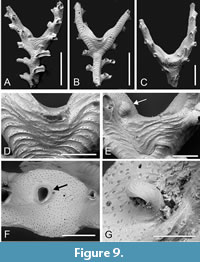 Type material. Holotype PMC. B79. 26.2.2025a, sample 19053 (Figure 9 A-B, D-F); paratype PMC. B79. 26.2.2025b.1, sample 19044 (Figure 9C, G). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Type material. Holotype PMC. B79. 26.2.2025a, sample 19053 (Figure 9 A-B, D-F); paratype PMC. B79. 26.2.2025b.1, sample 19044 (Figure 9C, G). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Etymology. From the Latin unda (used as a name in apposition) in reference to the ripple-like appearance of the ridges on the dorsal surface of the branches.
Diagnosis. Exidmonea with narrow branches having 1-2 series of initially connate autozooids either side of the midline; dorsal branch surface mildly convex, coarsely rugose with arcuate pseudoporous ridges separated by concave hollows containing few or no pseudopores; gonozooids located in branch axils, buttressed by rugose calcification, brood chamber roof flat or concave, non-rugose, densely pseudoporous, ooeciostome off-centred, hooked, ooeciopore transversely elliptical, about the same size as an autozooidal aperture.
Description. Colony erect, branches bifurcating, narrow, 167-277 µm wide; autozooids opening on frontolateral surfaces in 1-2 series either side of midline; dorsal branch surface slightly convex, coarsely rugose with arcuate, distally convex ridges 26-57 µm apart, pseudopores concentrated on ridges, sparse between ridges, small, subcircular, often teardrop-shaped, 7-9 µm long by 4-8 µm wide, nearest neighbour centre-to-centre spacing very variable, 9-35 µm. Autozooids long, zooidal boundaries grooved; frontal wall with transverse ridges, more irregular and less pronounced than on dorsal branch surfaces, convex; pseudopores teardrop-shaped, small, 4-5 µm in length by 4-7 µm in width, nearest neighbour centre-to-centre spacing 13-42 µm, partly occluded by septa-like spines; apertures connate, subcircular or rounded rectangular, diameter about 78-109 µm when peristomes are fully developed; peristomes long, up to 250 µm long, curved towards dorsal surface so that apertures face away from branch frontal, sparsely pseudoporous, bearing growth bands but not strongly ridged. Gonozooids located in axils at branch bifurcations, buttressed by a lunate expanse of ridged calcification stretching between the two daughter branches; brood chamber with flat or concave roof facing distally, lacking ridges, in the two measured examples 447 by 344 µm and 384 by 236 µm, densely pseudoporous, the pseudopores teardrop-shaped or subcircular, 5-9 µm long by 5-8 µm wide, nearest neighbour centre-to-centre spacing 11-19 µm, partly filled by spines; ooeciostome positioned eccentrically closer to one of the daughter branches, about 95 µm long, hooked inwardly, the surface lacking pseudopores but with faint growth lines; ooeciopore transversely elongate, 78 µm long by 90 µm wide in the single measured example.
Measurements (µm). Zooid length 377±84.9, 337-425 (2, 10); zooid width 100±11.2, 87-114 (1, 7).
Remarks. The buttressed, non-bulbous gonozooid located in branch axils is a distinctive feature of this new species, as are the prominent arcuate ridges on the surface of the branches on which the pseudopores are concentrated. Arcuate growth bands are known on the dorsal surfaces of branches of several species of Exidmonea and the related Idmidronea . These include Idmidronea obtecta Borg, 1944 and I. fraudulenta Ostrovsky and Taylor, 1996, both from the Antarctic, as well as Exidmonea intercalata Liu, Liu and Zágoršek, 2019 from China and Korea (Chae et al., 2022), and Exidmonea filiformis (Canu and Bassler, 1929) from the Philippines. However, the bands in these species do not form pronounced ridges, unlike E. unda sp. nov. (Figure 9B, D), and none of these species have gonozooids resembling those of E. unda sp. nov. Species erroneously identified by Canu and Bassler (1928, plate 34, figure 9) as Idmonea atlantica Forbes, 1847 [i.e., Idmidronea atlantica (Forbes in Johnston, 1847)] from the Pliocene of Panama, and also by Harmer (1915, plate 10, figure 4) from the Pacific as Tubulipora atlantica Forbes are notable in having arcuate lines of pseudopores on the dorsal surfaces of the branches. What was provisionally identified as the same species as that misidentified by Canu and Bassler (1928) was refigured by Taylor (2001), whose figure 4.11 shows the dorsal pseudopores to be large, longitudinally elongate and arranged in a single row spaced evenly along each ridge. This contrasts with the more disordered and smaller pseudopores found in E. unda sp. nov. There is some similarity between the gonozooids of this new species and those of an un-named species of Exidmonea from Singapore figured by Jain et al. (2022, figure 2A, B), particularly with respect to the location of the gonozooids at branch bifurcations and the off-centred and hooked ooeciostome. However, the gonozooid in the Singaporean species is not buttressed, nor are the branch surfaces rugose.
Exidmonea sp.
Figure 10
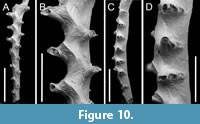 Figured material. PMC EDM-Collection J. H. B.155a.1, sample 19140 (Figure 10C-D); PMC EDM-Collection J. H. B.155a.3, sample 19140 (Figure 10A, B). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Figured material. PMC EDM-Collection J. H. B.155a.1, sample 19140 (Figure 10C-D); PMC EDM-Collection J. H. B.155a.3, sample 19140 (Figure 10A, B). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Description. Colony erect, branches narrow, 220-350 µm wide; autozooids opening on frontolateral surfaces in three series either side of midline. Autozooids long, zooidal boundaries grooved; frontal walls flat; pseudopores inconspicuous, sparse, irregularly arranged, longitudinally elliptical or lens-shaped, 6-8 µm long by 4-6 µm wide, nearest neighbour centre-to-centre spacing 18-47 µm, lumen mostly occluded; apertures connate, rounded rectangular or subcircular, decreasing in size and becoming more circular in shape from inner to outermost series; preserved peristomes short, bearing growth bands. Gonozooid medial, brood chamber not bulbous, forming a zigzag axial ridge on branch frontal, elongate, in single incomplete example exceeding 1800 µm long by about 200 µm wide, extending for more than eight autozooidal series; pseudopores 7-11 µm long by 5-8 µm wide, slightly more abundant than on autozooidal frontal walls, nearest neighbour centre-to-centre spacing 11-30 µm; ooeciostome not observed.
Measurements (µm). Zooid length 391±39.4, 340-466 (2, 11); zooid width 79±6.8, 70-90 (1, 10).
Remarks. The gonozooid in this species is very different from those of the other two species of Exidmonea from the Daidokutsu cave being extremely long. The most obvious manifestation of the gonozooid is a zigzag ridge along the medial axis of branch frontal surfaces, dividing the left and right sides of the brood chamber (Figure 10B). Unfortunately, the ooeciostome cannot be identified in the single example available, compromising species identification. Although, we are unaware of any named species of Exidmonea (or Idmidronea ) having a gonozooid with such a pronounced zigzag keel, Brood (1976, figure 15H-J) depicted a species he identified as Crisina radians (Lamarck, 1816) with a very similar gonozooid. Brood’s material from East Africa is unlike material identified as C. radians by Harmer (1915) and Borg (1941), in which the large pores in the frontal walls of the autozooids and gonozooids point to a free-walled morphology that contrasts with the fixed-walled autozooids and gonozooids with pseudoporous frontal walls evident in Brood’s material.
Family PLAGIOECIIDAE Canu, 1918
Genus PLAGIOECIA Canu, 1918
Plagioecia subcircularis sp. nov. Taylor, Di Martino and Rosso
Figure 11
zoobank.org/E8D2190D-F933-4014-9A34-BEBA0AC8E2CF
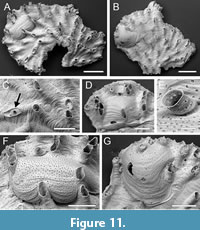 Type material. Holotype PMC. B80. 26.2.2025a, sample 19047 (Figure 11 A, C, E-F); paratypes PMC. B80. 26.2.2025b.1, sample 19058 (Figure 11B, G); PMC. B80. 26.2.2025b.2, sample 19058 (Figure 11D). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Type material. Holotype PMC. B80. 26.2.2025a, sample 19047 (Figure 11 A, C, E-F); paratypes PMC. B80. 26.2.2025b.1, sample 19058 (Figure 11B, G); PMC. B80. 26.2.2025b.2, sample 19058 (Figure 11D). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Etymology. Referring to the outline shape of the brood chambers.
Diagnosis. Plagioecia with lobate colonies; autozooids elongate, frontal wall with subcircular pseudopores containing about a dozen radial spines, apertures small, longitudinally elongate, sometimes closed by terminal diaphragms; secondary nanozooids developed in a few autozooids; gonozooids with bulbous, subcircular brood chambers partly covering proximal zooids, densely pseudoporous; ooeciostome terminal, pseudoporous; ooeciopore transversely elliptical, smaller than autozooidal apertures.
Description. Colony encrusting, multiserial, unilamellar, lobate, thin. Ancestrula and early astogeny unknown. Autozooids elongate, frontal walls convex, crossed by growth lines; pseudopores subcircular, occasionally rounded diamond-shaped, on average slightly longer than wide, length 9-11 µm, width 7-11 µm, nearest neighbour centre-to-centre spacing 18-35 µm, with about a dozen spines restricting the opening; apertures small, usually isolated and arranged quincuncially, sometimes aligned radially or clustered into small groups and connate, longitudinally elliptical and often somewhat pointed at distal end, or rounded polygonal when connate, short mural spines visible through apertures on interior walls; preserved peristomes short; terminal diaphragms with sparse pseudopores of variable size. Secondary nanozooids budded intramurally within some autozooids and possibly a gonozooid; a short, preserved peristome emerging from the centre of a pseudoporous terminal diaphragm, with an aperture about 25 µm in diameter. Gonozooids located close to distal growing edge; brood chamber bulbous, subcircular, in the three examples scanned measuring 545 µm long by 866 µm wide, 656 mm long by 548 µm wide, and 610 µm long by 547 µm wide, proximal edge extending proximally to overlap initial frontal wall and adjacent autozooids; autozooidal peristomes indenting edges of brood chamber, one peristome enveloped by brood chamber; pseudopores similar to those of autozooids, 9-11 µm long by 8-12 µm wide, dense, nearest neighbour centre-to-centre spacing 11-25 µm, lumen occluded by radial spines; ooeciostome terminal, about 60 µm long, slightly flared, pseudoporous, the pseudopores smaller than those on the gonozooid/autozooid surface, and with longitudinal strips of calcification, not adnate to a peristome; ooeciopore transversely elliptical, 52-55 µm long by 67-68 µm wide (N = 3).
Measurements (µm). Zooid length 554±88.3, 459-750 (2, 10); zooid width 117±10.1, 97-130 (2, 10); longitudinal aperture diameter 84±16.6, 58-113 (3, 15); transverse aperture diameter 66±7.5, 56-78 (3, 15).
Remarks. The generic assignment of this new species deserves some explanation as the simple, subcircular gonozooids are unlike those of the type and most other species of Plagioecia , which have transverse brood chambers profusely penetrated by autozooidal peristomes. The occasional groups of connate peristomes seen in P. subcircularis sp. nov. (Figure 11F) are also not usually associated with Plagioecia . Two other genera were considered before opting for Plagioecia: Tubulipora and Microeciella. Tubulipora was discounted as terminal diaphragms and secondary nanozooids are absent in this genus and the brood chambers tend to have sinuous outlines. While Microeciella has gonozooids close in shape to those of P. subcircularis sp. nov., it too lacks terminal diaphragms and secondary nanozooids. In addition, the pseudopores of extant species of Microeciella do not have the radial spines seen in those of P. subcircularis sp. nov. (Taylor and Zatoń, 2008) nor have they been observed in fossil species of the genus (Zatoń and Taylor, 2009). In contrast, terminal diaphragms and secondary nanozooids (Figure 11C), both developed in P. subcircularis sp. nov., are typical of Plagioecia (Silén and Harmelin, 1974). It is, however, unusual in species of Plagioecia to have some zooids with terminal diaphragms but others with secondary nanozooids. There are some similarities with Plagioecia ambigua Osburn, 1953 from Alaska. This species also has simple gonozooids but there is no mention of secondary nanozooids, and the autozooids of P. ambigua are considerably larger with a width of 0.20 mm quoted by Osburn compared with less than 0.10-0.13 mm in P. subcircularis sp. nov. Compared with the new species, Plagioecia parva Gordon and Taylor, 2010 from New Zealand seamounts has small gonozooids but these are broader and have peristomes penetrating the brood chambers, and secondary nanozooids are not present.
Plagioecia sp.
Figure 12
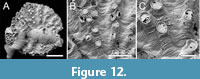 Figured material. PMC EDM-Collection J. H. B.156a.1, sample 19019 (Figure 12 A-C). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Figured material. PMC EDM-Collection J. H. B.156a.1, sample 19019 (Figure 12 A-C). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Description. Colony encrusting, multiserial, unilamellar, discoidal. Ancestrula and early astogeny obscured by a fouling colony of Annectocyma . Autozooids very variable in length, frontal walls convex, rugose, crossed by strong growth lines; pseudopores subcircular, on average slightly longer than wide, length 4-6 µm, width 3-5 µm, sparse and unevenly spaced, nearest neighbour centre-to-centre spacing 19-42 µm, without spines or other constrictions; apertures small, non-connate, roughly aligned in radial rows, closely but unevenly spaced, generally circular; preserved peristomes short; terminal diaphragms common, irregularly pseudoporous, the pseudopores mostly marginal, some pseudopores seemingly enlarged by boring. Gonozooids not observed.
Measurements (µm). Zooid length 246±76.3, 132-355 (1, 10); zooid width 89±7.3, 73-98 (1, 10); longitudinal aperture diameter 65±7.3, 50-76 (1, 10); transverse aperture diameter 62±6.5, 50-72 (1, 10).
Remarks. Only one specimen of this species has been studied using SEM. A gonozooid is not present in this colony, and its assignment to Plagioecia is based on the overall similarity of the autozooids to those of the type species, Plagioecia patina Canu, 1918 (non Tubulipora patina Lamarck, 1816; see Håkansson et al., 2024, pp. 17-18). The close spacing of the apertures and tendency for them to be aligned radially seen in the Daidokutsu species are characteristic features of P. patina (e.g., Harmelin, 1976b, plate 18, figure 6; Hayward and Ryland, 1985b, figure 33B). In P. patina , however, the pseudopores contain spines (Hayward and McKinney, 2002, figure 53F), radial rows of apertures are clearer, and the autozooids are larger than the Okinawan species; Hayward and McKinney gave a range for apertural width of 85-130 µm for P. patina compared with 50-76 µm for Plagioecia sp.
Suborder CANCELLATA Gregory, 1896
Family HORNERIDAE Smitt, 1867
Genus SPINIHORNERA Brood, 1979
Spinihornera parva sp. nov. Taylor, Di Martino and Rosso
Figure 13
zoobank.org/F447C722-F31E-483C-BADE-0B6B184DEE95
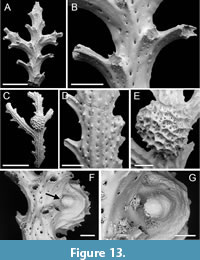 Type material. Holotype PMC. B81. 26.2.2025a, sample 19125 (Figure 13 C-G); paratype PMC. B81. 26.2.2025b.1, sample 19132 (Figure 13A-B). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Type material. Holotype PMC. B81. 26.2.2025a, sample 19125 (Figure 13 C-G); paratype PMC. B81. 26.2.2025b.1, sample 19132 (Figure 13A-B). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Etymology. The name parva alludes to the small size of the zooids in this species.
Diagnosis. Spinihornera with small autozooids, aperture diameter averaging 64 µm, arranged in pairs on either side of branch, outermost autozooids with long peristome; gonozooids with brood chamber globular in shape, coarsely reticulate; ooeciostome located at side of branch, hook-like with ooeciopore directed downwards.
Description. Colony erect, pinnate, branches narrow, 230-375 µm wide, autozooids arranged in pairs alternating on either side of branch midline, the peristome of the outer zooid oriented at about 45-60° to the branch. Branch frontal smoothly calcified, median line of autozooids forming a ridge most pronounced distally on peristome; cancelli arranged in rows on each side of ridge, longitudinally elliptical, variable in size, 7-25 µm in diameter. Branch reverse with 3-4 nervi bearing a row of short spines; ridge-like nervi spaced approximately 70 µm apart, with a row of cancelli about 15 µm in diameter between nervi. Autozooids with tiny apertures, short mural spines visible on interior walls through apertures, spacing between apertures in successive series about 300-400 µm; peristomes moderately long, terminating in about 6-8 apertural spines. Gonozooid known from a single example, small, located on branch reverse, off-centred; brood chamber bulbous, 597 µm long by 560 µm wide, cancellate, the surface a coarse reticulum of spine-bearing ridges surrounding groups of cancelli 9-30 µm in diameter; ooeciostome located at side of branch, short, strongly curved, hook-like with ooeciopore directed downwards towards branch surface.
Measurements (µm). Aperture diameter 64±6.2, 52-70 (1, 8); peristome length 125±29.8, 85-174 (1, 9).
Remarks. The type species, and hitherto only species attributed to Spinihornera is Hornera spinigera Kirkpatrick, 1888, originally described from Mauritius. Material of S. spinigera described by Brood (1979, p. 159) when he introduced the genus Spinihornera came from the Philippines, and the species has also been recorded from East Africa (Brood, 1976) and Lifou in New Caledonia (Harmer, 1915). Brood (1979, p. 159) diagnosed Spinihornera as a “Hornerid genus with pinnate branches, autozooecia arranged in lateral lines and with numerous apertural spines”. The gonozooid is mentioned by Brood as being located on the dorsal side of the branches, globular and with the ooeciostome turned towards the frontal side. These characters are all seen in S. parva sp. nov., but this new species differs from S. spinigera in having a more gracile colony. More similar in colony-form to S. parva is Hornera pinnata Canu and Bassler, 1929, which should probably be transferred to Spinihornera. However, the small diameter of the zooidal apertures in S. parva distinguishes it from S. pinnata for which Canu and Bassler (1929) cited a peristome diameter of 0.10 mm. Canu and Bassler’s species also has shorter peristomes, although this may be related to the older age of the branches.
There are clear similarities between this new species and Pseudidmonea johnsoni Di Martino and Taylor, 2014 from the Miocene of East Kalimantan, Indonesia. However, the latter differs in having a well-developed median keel on the branch frontal surface, 3-4 rows of apertures on each side of this keel, and small, circular or polygonal cancelli on the branch dorsal surface.
Family Incertae sedis
Genus MESONEA Canu and Bassler, 1920
?Mesonea sp.
Figure 14
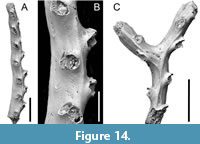 Figured material. PMC EDM-Collection J. H. B.157a.1, sample 19031 (Figure 14A-B); PMC EDM-Collection J. H. B.157a.2, sample 19029 (Figure 14C). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Figured material. PMC EDM-Collection J. H. B.157a.1, sample 19031 (Figure 14A-B); PMC EDM-Collection J. H. B.157a.2, sample 19029 (Figure 14C). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Description. Colony erect, branches bifurcating, narrow, 174-217 µm wide, autozooids arranged in pairs alternating on either side of branch midline, innermost apertures prominent, facing distolaterally, outermost apertures mostly hidden behind inner apertures in frontal view. Branch frontal smoothly calcified with a strong axial ridge and a few weaker lateral ridges; cancelli mostly arranged in a single longitudinal row along the axis of each zooid, longitudinally elliptical, 5-10 µm in diameter, spaced 26-50 µm apart, larger cancelli up to 70 µm long in bifurcation axil. Autozooids with small apertures, paired apertures connate; peristomes short, ragged. Gonozooid unknown.
Measurements (µm). Aperture diameter 98±9.2, 84-113 (2, 11); distance between apertural series 406±61.9, 352-535 (2, 8).
Remarks. The absence of gonozooids inhibits unequivocal generic identification of this species. The morphology of the colony and free-walled autozooids match species that are commonly referred to as the genus Crisina (e.g., Waters, 1914; Harmer, 1915; Borg, 1941). However, the type species of Crisina, C. normaniana d’Orbigny, 1853 from the Upper Cretaceous of Fécamp, Seine Maritime, France, has yet to be revised. Syntypes in the Muséum national d'Histoire naturelle (MNHN), Paris, registered as d’Orbigny Collection 8332, examined by one of us (PDT) comprise a mixture of specimens with free- and fixed-walled morphologies, neither seeming likely to be cancellate cyclostomes. Mesonea , with the extant type species Retepora radians Lamarck, 1816, has free-walled autozooids and seems a better fit for the Okinawan species. Although Mesonea is customarily placed in Crisinidae d’Orbigny, 1853, this assignment cannot be sustained in view of the ambiguous morphology of the type genus Crisina ; hence the uncertain family placement of the Okinawan species.
Suborder RECTANGULATA Waters, 1887
Family LICHENOPORIDAE Smitt, 1867
Genus DISPORELLA Gray, 1848
Disporella sp.
Figure 15
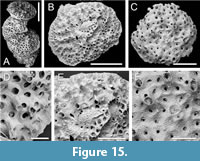 Figured material. PMC EDM-Collection J. H. B.158a.1, sample 19038 (Figure 15B, E); PMC EDM-Collection J. H. B.158a.2, sample 19019 (Figure 15A, D); PMC EDM-Collection J. H. B.158a.3, sample 19029 (Figure 15C, F). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Figured material. PMC EDM-Collection J. H. B.158a.1, sample 19038 (Figure 15B, E); PMC EDM-Collection J. H. B.158a.2, sample 19019 (Figure 15A, D); PMC EDM-Collection J. H. B.158a.3, sample 19029 (Figure 15C, F). Daidokutsu cave, Core 19, Holocene, Okinawa, Japan.
Description. Colony encrusting, discoidal, or semi-erect and formed by a vertical stack of 6 or 7 low cup-shaped subcolonies, autozooids arranged quincuncially near colony centre, becoming aligned into non-connate radial rows towards colony edge, separated by alveoli; surface calcification variably pustulose, covered by tiny spines. Autozooids with small, longitudinally elliptical apertures, in one example occluded by pustulose calcification with a small central pore; peristomes up to about 100 µm long, with spines terminal processes visible in some. Alveoli not subdivided, polygonal, variable in size, iris-like with central elliptical lumen. Possible gonozooid present in one colony, chamber exposed by surface breakage, positioned close to colony centre; ooeciostome and ooeciopore not observed.
Measurements (µm). Longitudinal aperture diameter 84±7.5, 76-98 (3, 16); transverse aperture diameter 70±5.2, 62-77 (3, 16); alveolus maximum external diameter 110±17.5, 78-137 (3, 20); alveolus minimum lumen diameter 36±14.8, 21-66 (3, 20).
Remarks. As Håkansson et al. (2024, p. 19) remarked, rectangulate cyclostomes including Disporella present major taxonomic challenges. This is because the colony surface from which most morphological characters are recorded is dynamic, changing as the colony ages, develops gonozooids and subsumes these structures beneath further calcification. In some species, compound colonies may form by adding subcolonies, either at the outer margins of the colony or vertically stacked. The degree of variability within species has yet to be fully described, making it difficult to decide whether morphological differences in fossil samples represent intraspecific variations or signify different species. Of the three specimens imaged using SEM, one is compound and semi-erect (Figure 15A), and one of the two non-compound colonies differs in having a more densely pustulose surface (Figure 15F). However, the morphometrics, notably autozooidal aperture diameter, of the three colonies are close enough to suggest that they may represent ecophenotypic or astogenetic variants of a single species. Compared to D. xianqiureni Liu et al., 2019 from China, the alveoli are much more constricted in the Okinawan species, whereas several other species of Disporella from the Indo-Pacific have connate or very closely spaced apertures arranged in radial rows, e.g., D. compta Dick et al., 2006 from Hawaii, and D. ovoidea Osburn, 1953 from Galapagos and southern California.
Class GYMNOLAEMATA Allman, 1856
Order CHEILOSTOMATIDA Busk, 1852
Superfamily SMITTINOIDEA Levinsen, 1909
Family SMITTINIDAE Levinsen, 1909
Genus PARASMITTINA Osburn, 1952
Parasmittina vieirai nom. nov.
zoobank.org/8136CF1B-1C7B-41FC-BA95-5F51AFF8C3FE
v. 2025 Parasmittina ligulata Di Martino, Rosso and Taylor in Di Martino et al., p. 56, fig. 32.
Remarks. After the publication of the study on the cheilostome bryozoans of Daidokutsu cave, Leandro Manzoni Vieira informed us that the name Parasmittina ligulata , proposed for a new species from the cave, was preoccupied. Farias et al. (2024) had established a new combination, Parasmittina ligulata ( Ridley, 1881) for Smittia trispinosa v ar. ligulata Ridley, 1881. In recognition of Vieira’s contributions to bryozoology and his courtesy in bringing this issue to our attention, we propose Parasmittina vieirai nom. nov. as a replacement name for the Japanese species.
DISCUSSION
Fifteen species of cyclostome bryozoans are recognised in Core 19 of the Daidokutsu submarine cave: Crisia ramalhoae sp. nov., Crisia prominens sp. nov., Crisiona sp., Filisparsa haywardi sp. nov., Annectocyma sp., Nevianipora sp., Psilosolen harmelini sp. nov., Exidmonea lineata sp. nov., E. unda sp. nov., Exidmonea sp., Plagioecia subcircularis sp. nov., Plagioecia sp., Spinihornera parva sp. nov., ?Mesonea sp. and Disporella sp. Open nomenclature is employed for seven of the Daidokutsu species, either because critical characters, such as gonozooids, have not been observed, or species descriptions in the literature are inadequate to permit the Daidokutsu species to be confidently matched with them. Pseudopore morphology has been seldom described or illustrated, especially in pre-SEM studies, yet these minute perforations in the frontal exterior walls are of great utility in distinguishing between species among the character-poor cyclostomes (e.g., Zatoń and Taylor, 2009).
None of the Daidokutsu cyclostomes could be identified as an existing species. In contrast, 26 of the 63 cheilostome bryozoans in the same samples were identified either as existing species or designated as cf. an existing species (Di Martino et al., 2025). In addition to the increased challenges in cyclostome taxonomy already mentioned, this discrepancy may also reflect the scarcity of modern studies on cyclostome bryozoans in Japan and elsewhere as outlined in the Introduction. Aside from a paper by Taylor and Grischenko (2015) describing two intertidal species of cyclostomes from Akkeshi Bay in northern Japan, the most recent publications on living cyclostomes in Japan were published over 50 years ago and lack SEM imagery (Mawatari and Mawatari, 1973, 1974). Perhaps coincidentally in view of the greater uncertainties in cyclostome identification, the proportion of cyclostome species (53%) considered to be new is similar to the proportion of new cheilostome species (48%) in the Daidokutsu cave.
Three of the Daidokutsu cyclostomes are classified in the suborder Articulata, nine in Tubuliporina, two in Cancellata, and one in Rectangulata. Therefore, four of the five extant suborders of cyclostomes are represented at Daidokutsu, with only Cerioporina lacking. The dominance of Tubuliporina is unsurprising. Molecular phylogenetic studies (see Waeschenbach et al., 2009; Taylor et al., 2021 and references therein) have corroborated the non-monophyletic status of Tubuliporina previously suggested by morphological analysis (Taylor and Weedon, 2000). However, it should be noted that taxon sampling in cyclostomes is still too sparse to be certain that the other suborders are monophyletic.
In terms of colony-form, three of the Daidokutsu cyclostomes are multiserial encrusters, whereas the remaining 12 species are narrow-branched erect forms, two with articulated colonies and 10 having rigid colonies. The high proportion of erect cyclostome species contrasts with some Mediterranean submarine caves. Harmelin (1997, p. 149) noted a total absence of erect bryozoans (cyclostomes and cheilostomes) on the walls of the ‘3PP’ cave near La Ciotat in France, although erect species did colonise settlement panels. In two Aegean submarine caves studied by Rosso et al. (2018), bryozoans with erect colonies were also less diverse than at Daidokutsu. Of the 14 cyclostome species listed by Rosso et al. (2018, table 1), slightly less than half (6) have characteristically erect colonies. A similar proportion is evident in Sicilian submarine caves (Rosso et al. 2013a, b).
The 15 cyclostome species recognised in the Daidokutsu submarine cave fauna can be added to the 63 cheilostome species described earlier by Di Martino et al. (2025), making a total of 78 bryozoan species. Proportionally, cyclostomes account for 19% of species diversity, which is typical for Holocene bryozoan faunas (Lidgard et al., 2021, figure 1a), including those from Mediterranean submarine caves (Harmelin, 1997; Rosso et al., 2018). A higher proportion of cyclostomes might have been predicted on the grounds that caves could provide a refuge for cyclostomes, which tend to lose competitive encounters for space with cheilostomes (McKinney, 1992) and seem on average to have lower energy intakes (McKinney, 1993), which may make them better suited to habitats with depleted food resources like caves. Regarding abundance, cyclostomes are also less common in the Daidokutsu cave where they are represented by 9912 fragments compared with 39239 cheilostome fragments. Therefore, cyclostomes comprise about 20% of total bryozoan abundance, a very similar proportion to the 19% of bryozoan species diversity in the cave.
ACKNOWLEDGEMENTS
We thank Sophie Crump and Tom Perkins for helping sorting bryozoans from the core samples during their year as volunteers at the Department of Earth Sciences of the Natural History Museum, London. EDM received support from the Leverhulme Trust Research Project ‘Origin of high tropical diversity: a test using bryozoans’ (2016-2018) (award no. RGP-2015-036 to PDT), the Young Research Talent Grant of the Research Council of Norway (2019-2025) (award no. 314499 to EDM), and the University of Catania through PIAno di inCEntivi per la RIcerca di Ateneo 2020/2022 - Linea di Intervento 3 “Starting Grant” (2024). AR was funded by the University of Catania through PIAno di inCEntivi per la RIcerca di Ateneo 2020/2022 - Linea di Intervento 2. Additional support to EDM and AR was provided by PiaCeRi 2024/2026. MY was funded by Research Grants Council of the Hong Kong Special Administrative Region, China (project codes: RFS2223-7S02, C7013-19G to MY); the Seed Funding Programme for Basic Research of the University of Hong Kong (project codes: 202111159167, 2302101483 to MY); and SKLMP Seed Collaborative Research Fund (project codes: SKLMP/SCRF/0073 to MY). We thank two anonymous reviewers for their constructive comments, which greatly contributed to improving the final version of this work. This is Catania Paleontological Research Group contribution no. 527.
REFERENCES
Allmann, G.J. 1856. A monograph of the Freshwater Polyzoa, including all the known species, both British and Foreign. The Ray Society, London.
https://doi.org/10.5962/bhl.title.9143
Bock, P.E. and Gordon, D.P. 2013. Phylum Bryozoa Ehrenberg, 1831. Zootaxa, 3703:67-74.
https://doi.org/10.11646/zootaxa.3703.1.14
Borg, F. 1926. Studies on Recent cyclostomatous Bryozoa. Zoologiska Bidrag från Uppsala, 10:181-507.
Borg, F. 1941. On the structure and relationships of Crisina (Bryozoa, Stenolaemata). Arkiv för Zoologi, 33A:1-44.
Borg, F. 1944. The stenolaematous Bryozoa Volume 3, p. 1-276. In Bock, S. (ed.), Further Zoological Results of the Swedish Antarctic Expedition 1901-1903 under the direction of Dr Otto Nordenskjöld. Norstedt & Söner, Stockholm.
Brood, K. 1976. Cyclostomatous Bryozoa from the coastal waters of East Africa. Zoologica Scripta, 5:277-300.
https://doi.org/10.1111/j.1463-6409.1976.tb00709.x
Brood, K. 1979. Spinihornera, a new bryozoan genus. Zoologica Scripta, 8:159-160.
https://doi.org/10.1111/j.1463-6409.1979.tb00627.x
Buge, E. 1979. Bryozoaires cyclostomes. Resultats Scientifiques des Campagnes de la Calypso, 11:207-252.
Busk, G. 1852. Catalogue of marine Polyzoa in the collection of the British Museum. I. Cheilostomata. Trustees of the British Museum, London:1-54.
https://doi.org/10.1242/jcs.s1-1.2.136b
Busk, G. 1859. A monograph of the fossil Polyzoa of the Crag. The Palaeontographical Society, London:1-136.
https://doi.org/10.1080/02693445.1859.12027922
Canu, F. 1918. Les ovicelles des bryozoaires cyclostomes. Études sur quelques familles nouvelles et anciennes. Bulletin de la Société Géologique de France, Série 4, 16 [for 1916]:324-335.
Canu, F. and Bassler, R.S. 1920. North American early Tertiary Bryozoa. United States National Museum Bulletin, 106:1−879.
https://doi.org/10.5479/si.03629236.106.i
Canu, F. and Bassler, R.S. 1922. Studies on the cyclostomatous Bryozoa. Proceedings of the United States National Museum, 61:1-160.
https://doi.org/10.5479/si.00963801.61-2443.1
Canu, F. and Bassler, R.S. 1923. North American later Tertiary and Quaternary Bryozoa. United States National Museum Bulletin, 125:1-302.
https://doi.org/10.5962/bhl.title.159661
Canu, F. and Bassler, R.S. 1927. Bryozoaires des iles Hawaï. Bulletin de la Société des Sciences de Seine-et-Oise, 8:1-67.
Canu, F. and Bassler, R.S. 1928. Fossil and Recent Bryozoa of the Gulf of Mexico region. Proceedings of the United States National Museum, 72:1-199.
https://doi.org/10.5479/si.00963801.72-2710.1
Canu, F. and Bassler, R.S. 1929. Bryozoa of the Philippine region. United States National Museum Bulletin, 100:1-685.
Chae, H.S., Hyun, J.K., Zágoršek, K., and Seo, J.E. 2018. Four Korean cyclostomatous bryozoans - new additions to the Korean fauna. Journal of Species Research, 7:348-353.
https://doi.org/10.12651/JSR.2018.7.4.348
Chae, H.S., Min, B.S., Zágoršek, K., Yang, H.J., Hyun, J.K., and Seo, J.E. 2020. Crisiidae (Bryozoa: Cyclostomata) of Korea. Journal of Species Research, 9:280-287.
https://doi.org/10.12651/JSR.2020.9.3.280
Chae, H.S., Min, B.S., Yang, H.J., and Seo, J.E. 2022. Three New Records of Family Tubuliporidae (Bryozoa: Cyclostomata) from Korea. Animal Systematics, Evolution and Diversity, 38(1):20-25.
https://doi.org/10.5635/ASED.2022.38.1.042
Chiu, W.-T.R., Yasuhara, M., Iwatani, H., Kitamura, A., and Fujita, K. 2016. An enigmatic Holocene podocopid ostracod from a submarine cave, Okinawa, Japan: “living fossil” or adaptive morphotype. Journal of Systematic Palaeontology, 14:643-652.
https://doi.org/10.1080/14772019.2015.1094754
Chiu, W-T.R., Yasuhara, M., Iwatani, H., Kitamura, A., and Fujita, K. 2017. Response of subtropical submarine-cave ecosystem to Holocene cave development and Asian monsoon variability. Paleobiology, 43(3):425-434.
https://doi.org/10.1017/pab.2016.53
David, L., Mongereau, N., and Pouyet, S. 1972. Bryozoaires du Néogène du bassin du Rhône. Gisements burdigaliens de Mus (Gard). Documents des Laboratoires de Géologie de la Faculté des Sciences de Lyon, 52:1-118.
Dick, M.H., Tilbrook, K.J., and Mawatari, S.F. 2006. Diversity and taxonomy of rocky-intertidal Bryozoa on the Island of Hawaii, USA. Journal of Natural History, 40:2197-2258.
https://doi.org/10.1080/00222930601062771
Dick, M.H., Takashima, R., Kaneko, N., and Mawatari, S.F. 2008. Overview of Pleistocene bryozoans in Japan, p. 83-91. In Okada, H., Mawatari, S.F., Suzuki, N., and Gautam, P. (eds.), Proceedings of the International Symposium, The Origin and Evolution of Natural Diversity, 1-5 October 2007. Hokkaido University, Sapporo.
Di Martino, E. and Taylor, P.D. 2014. Miocene Bryozoa from East Kalimantan, Indonesia. Part I: Cyclostomata and Cheilostomata ‘Anasca’. Scripta Geologica, 146:17-126.
Di Martino, E., Rosso, A., Taylor, P.D., Chiu, R.W.T., Fujita, K., Kitamura, A., and Yasuhara, M. 2025. Unveiling the cheilostome bryozoan fauna of Daidokutsu submarine cave (Okinawa, Japan) over the last 7,000 years. Palaeontologia Electronica, 28(1):a7.
https://doi.org/10.26879/1433
Ehrenberg, C.G. 1831. Symbolae Physicae, seu Icones et descriptiones Corporum Naturalium novorum aut minus cognitorum, quae ex itineribus per Libyam, Aegyptum, Nubiam, Dongalam, Syriam, Arabiam et Habessiniam. Studio Annis 1820-1825. Berolini, Berlin.
https://doi.org/10.5962/bhl.title.107403
Farias, J., Vieira, L.M., and Almeida A.C.S. 2024. Revealing the diversity of Parasmittina Osburn, 1952 (Bryozoa, Cheilostomatida) from the Southwest Atlantic: Species complexes, non-native and new species. Plos One, 19(8):e0304347.
https://doi.org/10.1371/journal.pone.0304347
Girod, P. and Martha, S.O. 2021. Die Bryozoenforschung und der APH. Arbeitskreis Paläontologie Hannover, 49:75-87.
Gordon, D.P. and Taylor, P.D. 2010. New seamount- and ridge-associated cyclostome Bryozoa from New Zealand. Zootaxa, 2533:43-68.
https://doi.org/10.11646/zootaxa.2533.1.3
Gray, J.E. 1848. List of the specimens of British animals in the collections of the British Museum. Part 1. Centrionae or radiated animals. Trustees of the British Museum, London:91−151.
https://doi.org/10.5962/bhl.title.1582https://doi.org/10.5962/bhl.title.1582
Gregory, J.W. 1896. Catalogue of the fossil Bryozoa in the Department of Geology, British Museum (Natural History). The Jurassic Bryozoa. pp.1-239. Trustees of the British Museum, London:1-129.
https://doi.org/10.5962/bhl.title.112427
Harmelin, J.-G. 1976a. Relations entre la forme zoariale et l'habitat chez les bryozoaires cyclostomes. Consequences taxonomiques. Documents de Laboratoires de Géologie Faculté de Sciences de Lyon, HS 3, fasc. 2:369-384.
Harmelin, J.-G. 1976b. Le sous-ordre des Tubuliporina (Bryozoaires Cyclostomes) en Méditerranée: écologie et systématique. Mémoires de l'Institut océanographique, 10:1-326.
Harmelin, J.-G. 1997. Diversity of bryozoans in a Mediterranean sublittoral cave with bathyal-like conditions: role of dispersal processes and local factors. Marine Ecology Progress Series, 153:139-152.
https://doi.org/10.3354/meps153139
Harmelin, J.-G. and d’Hondt, J.-L. 1982. Bryozoaires cyclostomes bathyaux des campagnes océanographiques de l’"Atlantis II" du "Chain" et du "Knorr" 1967-1972. Bulletin du Muséum national d’Histoire naturelle, 4(A):3-16.
https://doi.org/10.5962/p.286025
Harmer, S.F. 1915. Polyzoa of the Siboga Expedition. Part 1. Entoprocta, Ctenostomata and Cyclostomata. Siboga Expedition Reports, 28A:1-180.
Hayward, P.J. and McKinney, F.K. 2002. Northern Adriatic Bryozoa from the vicinity of Rovinj, Croatia. Bulletin of the American Museum of Natural History, 270:1-139.
https://doi.org/10.1206/0003-0090(2002)270<0001:NABFTV>2.0.CO;2
Hayward, P.J. and Ryland, P.L. 1985a. Systematic notes on some British Cyclostomata (Bryozoa). Journal of Natural History, 19:1073-1078.
https://doi.org/10.1080/00222938500770671
Hayward, P.J. and Ryland, P.L. 1985b. Cyclostome Bryozoans. Synopses of the British Fauna (n.s.), 34:1-147.
https://doi.org/10.1163/9789004627598_003
Håkansson, E., Gordon, D.P. and Taylor, P.D. 2024. Bryozoa from the Maastrichtian Korojon Formation, Western Australia. Fossils and Strata, 70:1-155.
https://doi.org/10.18261/9788215072081-2024-01
Jain, S.S., Gordon, D.P., Huang, D., Kukliński, P., and Liow, L.H. 2022. Targeted collections reveal new species and records of Bryozoa and the discovery of Pterobranchia in Singapore. Raffles Bulletin of Zoology, 70:257-274.
https://doi.org/10.26107/RBZ-2022-0011https://doi.org/10.26107/RBZ-2022-0011
Johnston, G. 1837. A catalogue of the zoophytes of Berwickshire. History of the Berwickshire Naturalists’ Club, 4(1):107-108.
Johnston, G. 1847. A history of British Zoophytes. Volume 1. Van Voorst, London:1-499.
https://doi.org/10.5962/bhl.title.19627
Kataoka, J. 1960. Bryozoa from Mogami-Tai, Japan Sea. Science Reports Tôhoku University, 4:393-399.
Kirkpatrick, R. 1888. Polyzoa of Mauritius. Annals and Magazine of Natural History, 1(6):72-85.
https://doi.org/10.1080/00222938809460685
Kitamura, A., Hiramoto, M., Kase, T., Yamamoto, N., Amemiya, M., and Ohashi, S. 2007a. Changes in cavernicolous bivalve assemblages and environments within a submarine cave in the Okinawa Islands during the last 5,000 years. Paleontological Research, 11:163-182.
https://doi.org/10.2517/1342-8144(2007)11[163:CICBAA]2.0.CO;2
Kitamura, A., Yamamoto, N., Kase, T., Ohashi, S., Hiramoto, M., Fukusawa, H., Watanabe, T., Irino, T., Kojitani, H., Shimamura, M., and Kawakami, I. 2007b. Potential of submarine-cave sediments and oxygen isotope composition of cavernicolous micro-bivalve as a late Holocene paleoenvironmental record. Global and Planetary Change, 55:301-316.
https://doi.org/10.1016/j.gloplacha.2006.09.002
Kitamura, A., Kobayashi, K., Tamaki, C., Yamamoto, N., Irino, T., Miyairi, Y., and Yokoyama, Y. 2013. Evidence of recent warming in the Okinawa region, subtropical northwestern Pacific, from an oxygen isotope record of a cave-dwelling marine micro-bivalve. Paleontological Research, 17:58-68.
https://doi.org/10.2517/1342-8144-17.1.58
Kluge, G.A. 1946. Novye i maloizvestnye mshanki iz severnogo Ledovitogo okeana [New and little-known species of Bryozoa from the Arctic Ocean]. Trudy dreifuyushchei ekspeditsya Glavsevmorputi na l/s G. Sedov. 1937-40, 3:194-223.
Lamarck, J.-B.P.A. 1816. Histoire naturelle des Animaux sans Vertèbres. Vol. 2. Paris, Verdière, pp. 1-568.
https://doi.org/10.5962/bhl.title.12712
Lamouroux, J.V.F. 1812. Extrait d'un mémoire sur la classification des Polypiers coralligènes non entièrement pierreux. Nouveau Bulletin scientifique de la Société Philosophique, 3:181-188.
Levinsen, G.M.R. 1909. Morphological and systematic studies on the cheilostomatous Bryozoa. Nationale Forfatterers Forlag, Copenhagen.
https://doi.org/10.5962/bhl.title.54983
Lidgard, S., Di Martino, E., Zágoršek, K., and Liow, L.H. 2021.When fossil clades ‘compete’: local dominance, global diversification dynamics and causation. Proceedings of the Royal Society, Series B, 288(1959):1-10.
https://doi.org/10.1098/rspb.2021.1632
Liu, H., Liu, X., and Zágoršek, K. 2019. Cyclostome bryozoans from Qingdao, South Yellow Sea, China. Zootaxa, 4603:473-500.
https://doi.org/10.11646/zootaxa.4603.3.3
Maplestone, C.M. 1905. Lord Howe Island Polyzoa. Proceedings of the Royal Society of Victoria, new series, 17:386-390.
Mawatari, S. 1952. Bryozoa of Kii Peninsula. Publications of the Seto Marine Biological Laboratory, 2:261-288.
https://doi.org/10.5134/174675
Mawatari, S. 1955. A check list of known species of Japanese cyclostomatous Bryozoa. Bulletin of the Biogeographical Society of Japan, 16-19:44-50.
Mawatari, S. and Mawatari, S.F. 1973. Notes on marine Bryozoa from Hokkaido. 1. Crisiidae (Cyclostomata). Journal of the Faculty of Science, Hokkaido University, Zoology, 19:95-104.
Mawatari, S. and Mawatari, S.F. 1974. Notes on the marine Bryozoa from Hokkaido II. Cyclostomata other than Crisiidae. Journal of the Faculty of Science, Hokkaido University, Zoology, 19:349-360.
McKinney, F.K. 1992. Competitive interactions between related clades: evolutionary implications of overgrowth interactions between encrusting cyclostome and cheilostome bryozoans. Marine Biology, 114:645-652.
https://doi.org/10.1007/BF00357261
McKinney, F.K. 1993. A faster-paced world?: contrasts in biovolume and process rates in cyclostome (Class Stenolaemata) and cheilostome (Class Gymnolaemata) bryozoans. Paleobiology, 19:335-351.
https://doi.org/10.1017/S0094837300000312
Milne-Edwards, H. 1838. Mémoire sur les Crisies, les Hornéres et plusieurs autres Polypes vivants ou fossiles dont l'organisation est analogue à celle des Tubulipores. Annales des Sciences naturelles, Zoologie & Biologie animale, 9(2):193-238.
Okada, Y. 1917. A report on the cyclostomatous Bryozoa of Japan. Annotationes Zoologicae Japonenses, 9:335-360.
Okada, Y. 1928. Report of the biological survey of Mutsu Bay. 8. Cyclostomatous Bryozoa of Mutsu Bay. Science Report of Tôhoku Imperial University, Ser. 4, Biology, 3:481-496.
Okada, Y. and Mawatari, S. 1936. Bryozoa fauna collected by the ‘Misago’ during the Zoological Survey around Izu Peninsula (II). Science Reports of the Tokyo Bunrika Daigaku, Section B, 3:53-73.
Omori, A., Kitamura, A., Fujita, K., Honda, K., and Yamamoto, N. 2010. Reconstruction of light conditions within a submarine cave during the past 7,000 years based on the temporal and spatial distribution of algal symbiont-bearing large benthic foraminifers. Palaeogeography, Palaeoclimatology, Palaeoecology, 292:443-452.
https://doi.org/10.1016/j.palaeo.2010.04.004
Orbigny, d’ A.1842. Voyage dans l'Amérique méridionale. P. Bertrand, Paris: pp. 6-13, pls 2, 4.
https://doi.org/10.5962/bhl.title.110540
Orbigny, d’ A. 1851-1854. Paléontologie française. Description des Mollusques et Rayonées fossils. Terrains crétacés. Tome 5, Bryozoaires. Victor Masson, Paris.
https://doi.org/10.5962/bhl.title.50510https://doi.org/10.5962/bhl.title.50510
Ortmann, A. 1890. Die Japanische Bryozoenfauna. Bericht über die von Herrn Dr. L. Döderlein in Jahre 1880-81, gemachten Sammlungen. Archiv für Naturgeschichte, 56:1-74.
Osburn, R.C. 1952. Bryozoa of the Pacific coast of America, part 2, Cheilostomata-Ascophora. Report of the Allan Hancock Pacific Expeditions, 14:271-611.
https://doi.org/10.5962/bhl.title.6542
Osburn, R.C. 1953. Bryozoa of the Pacific coast of America. Part 3, Cyclostomata, Ctenostomata, Entoprocta and Addenda. Report of the Allan Hancock Pacific Expeditions, 14:613-841.
Ostrovsky, A.N. and Taylor, P.D. 1996. Systematics of some Antarctic Idmidronea and Exidmonea (Bryozoa, Cyclostomata). Journal of Natural History, 30:1549-1575.
https://doi.org/10.1080/00222939600770881
Pitt, L.J. and Taylor, P.D. 1990. Cretaceous Bryozoa from the Faringdon Sponge Gravel (Aptian) of Oxfordshire. Bulletin of the British Museum (Natural History) (Geology), 46:61-152.
Ramalho, L.V., Muricy, G., and Taylor, P.D. 2009. Cyclostomata (Bryozoa, Stenolaemata) from Rio de Janeiro State, Brazil. Zootaxa, 2057:32-52.
https://doi.org/10.11646/zootaxa.2057.1.2
Ridley, S.O. 1881. Polyzoa, in Account of the zoological collections made during the survey of H.M. “Alert” in the Straits of Magellan and on the coast of Patagonia. Proceedings of the Zoological Society, London, 1881: 44-61.
Rosso, A. 1998. New deep-sea bryozoan species from the Pleistocene of southern Italy. Rivista Italiana di Paleontologia e Stratigrafia, 104:423-430.
Rosso, A., Sanfilippo, R., Taddei Ruggiero, E., and Di Martino, E. 2013a. Faunas and ecological groups of Serpuloidea, Bryozoa and Brachiopoda from submarine caves in Sicily (Mediterranean Sea). Bollettino della Società Paleontologica Italiana, 52(3): 167-176.
https://doi.org/10.4435/BSPI.2013.18
Rosso A., Di Martino E., Sanfilippo R., and Di Martino V. 2013b. Bryozoan communities and thanatocoenoses from submarine caves in the Plemmirio Marine Protected Area (SE Sicily), p. 251-269. In Ernst, A., Schäfer, P., and Scholz, J. (eds.), Bryozoan Studies 2010. Lecture Notes in Earth System Sciences,143. Springer, Berlin.
https://doi.org/10.1007/978-3-642-16411-8_17https://doi.org/10.1007/978-3-642-16411-8_17
Rosso, A., Gerovasileiou, V., Sanfilippo, R., and Guido, A. 2018. Bryozoan assemblages from two submarine caves in the Aegean Sea (Eastern Mediterranean). Marine Biodiversity, 49:707-726.
https://doi.org/10.1007/s12526-018-0846-0
Silén, L. and Harmelin, J.-G. 1974. Observations on living Diastoporidae (Bryozoa, Cyclostomata), with special regard to polymorphism. Acta Zoologica Stockholm, 55:81-96.
https://doi.org/10.1111/j.1463-6395.1974.tb00182.x
Smitt, F.A. 1867. Kritisk förteckning öfver Scandinaviens Hafs-Bryozoer. Öfversigt af Kongl. Vetenskaps-Akademiens Förhandlingar, Årg, 23:395-534.
Souto, J., Ramalhosa, P., Ferrario, J., Png-Gonzalez, L., Álvarez, S., Gestoso, I., Nogueira, N., and Canning-Clode, J. 2023. New species and new records of bryozoan species from fouling communities in the Madeira Archipelago (NE Atlantic). Marine Biodiversity, 53:49.
https://doi.org/10.1007/s12526-023-01355-y
Taylor, P.D. 2001. Preliminary systematics and diversity patterns of cyclostome bryozoans from the Neogene of the Central American Isthmus. Journal of Paleontology, 75:578-589.
https://doi.org/10.1666/0022-3360(2001)075<0578:PSADPO>2.0.CO;2
Taylor, P.D. 2020. Bryozoan Paleobiology. Wiley Blackwell, Oxford, 320 pp.
https://doi.org/10.1002/9781118454961
Taylor, P.D. and Grischenko, A.V. 2015. Two new species of heavily calcified bryozoans from the intertidal of Akkeshi Bay, Hokkaido, Japan. Journal of Natural History, 49:1763-1775.
https://doi.org/10.1080/00222933.2015.1006287
Taylor, P.D. and Palmer, T.J. 1994. Submarine caves in a Jurassic reef (La Rochelle, France) and the evolution of cave biotas. Naturwissenschaften, 81:357-360.
https://doi.org/10.1007/BF01136225
Taylor, P.D. and Weedon, M.J. 2000. Skeletal ultrastructure and phylogeny of cyclostome bryozoans. Zoological Journal of the Linnean Society, 128:337-399.
https://doi.org/10.1111/j.1096-3642.2000.tb01521.x
Taylor, P.D. and Zatoń, M. 2008. Taxonomy of the bryozoan genera Oncousoecia, Microeciella and Eurystrotos (Cyclostomata: Oncousoeciidae). Journal of Natural History, 42:2557-2574.
https://doi.org/10.1080/00222930802277640
Taylor, P.D., Dick, M.H., Clements, D., and Mawatari, S.F. 2013. A diverse bryozoan fauna from Pleistocene marine gravels at Kuromatsunai, Hokkaido, Japan, p. 367-383. In Ernst, A., Schäfer, P., and Scholz, J. (eds.), Bryozoan Studies 2010. Lecture Notes in Earth System Sciences ,143. Springer, Berlin.
https://doi.org/10.1007/978-3-642-16411-8_25
Taylor, P.D., Harmelin, J.-G., Waeschenbach, A., and Bouchon, C. 2021. Disporella guada sp. nov., an erect-ramose rectangulate cyclostome (Bryozoa, Stenolaemata) from the Caribbean Sea: convergent evolution in bryozoan colony morphology. European Journal of Taxonomy, 773:1-18.
https://doi.org/10.5852/ejt.2021.773.1507
Waeschenbach, A., Cox, C.C., Littlewood, D.T.J., Porter, J.S., and Taylor, P.D. 2009. First molecular estimate of cyclostome bryozoan phylogeny confirms extensive homoplasy among skeletal characters used in traditional taxonomy. Molecular Phylogenetics and Evolution, 52:241-251.
https://doi.org/10.1016/j.ympev.2009.02.002
Waters, A.W. 1884. Fossil cyclostomatous Bryozoa from Australia. Quarterly Journal of the Geological Society of London, 40:674-697.
https://doi.org/10.1144/GSL.JGS.1884.40.01-04.55
Waters, A.W. 1887. On Tertiary cyclostomatous Bryozoa from New Zealand. Quarterly Journal of the Geological Society of London, 43:337-350.
https://doi.org/10.1144/GSL.JGS.1887.043.01-04.27
Waters, A.W. 1904. Bryozoa from Franz-Josef Land, collected by the Jackson-Harmsworth Expedition, 1896-1897. Part 2, Cyclostomata, Ctenostomata, and Endoprocta. Journal of the Linnean Society of London, Zoology, 29:161-184.
https://doi.org/10.1111/j.1096-3642.1904.tb00433.x
Waters, A.W. 1914. The marine fauna of British East Africa and Zanzibar, from collections made by Cyril Crossland M.A., B. Sc., F.Z.S., in the years 1901-1902. Bryozoa - Cyclostomata, Ctenostomata, and Endoprocta. Proceedings of the Zoological Society of London, 1914:831-858.
https://doi.org/10.1111/j.1469-7998.1914.tb07065.x
Winston, J.E., Vieira, L.M., and Woollacott, R.M. 2014. Scientific Results of the Hassler Expedition. Bryozoa. No. 2. Brazil. Bulletin of the Museum of Comparative Zoology, 161(5):139-239.
https://doi.org/10.3099/MCZ14.1
Yamamoto, N., Kitamura, A., Ohmori, A., Morishima, Y., Toyofuku, T., and Ohashi, S. 2009. Long-term changes in sediment type and cavernicolous bivalve assemblages in Daidokutsu submarine cave, Okinawa Islands: evidence from a new core extending over the past 7,000 years. Coral Reefs, 28:967-976.
https://doi.org/10.1007/s00338-009-0536-2
Yamamoto, N., Kitamura, A., Irino, T., Kase, T., and Ohashi, S. 2010. Climatic and hydrologic variability in the East China Sea during the last 7,000 years based on oxygen isotope records of the submarine cavernicolous micro-bivalve Carditella iejimensis . Global and Planetary Change, 72:131-140.
https://doi.org/10.1016/j.gloplacha.2010.01.025
Zatoń, M. and Taylor, P.D. 2009. Middle Jurassic cyclostome bryozoans from the Polish Jura. Acta Palaeontologica Polonica, 54:267-288.
https://doi.org/10.4202/app.2008.0088
Ziko, A., El Safori, Y.A., El Sorogy, A., Abd El-Wahab, M., El Dera, N., and Sheata, W. 2012. Bryozoa from northern Red Sea, Egypt: 1 Crisia (Cyclostomata). Historical Biology, 24:113-119.
https://doi.org/10.1080/08912963.2011.587184

