 Four mammal fossil calibrations: balancing competing palaeontological and molecular considerations
Four mammal fossil calibrations: balancing competing palaeontological and molecular considerations
Article number: 18.1.5FC
https://doi.org/10.26879/490
Copyright Palaeontological Association, February 2015
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 13 May 2014. Acceptance: 14 June 2014
{flike id=777}
ABSTRACT
With the introduction of relaxed-clock molecular dating methods, the role of fossil calibration has expanded from providing a timescale, to also informing the models for molecular rate variation across the phylogeny. Here I suggest fossil calibration bounds for four mammal clades, Monotremata (platypus and echidnas), Macropodoidea (kangaroos and potoroos), Caviomorpha-Phiomorpha (South American and African hystricognath rodents), and Chiroptera (bats). In each case I consider sources of uncertainty in the fossil record and provide a molecular dating analysis to examine how the suggested calibration priors are further informed by other mammal fossil calibrations and molecular data.
Matthew J. Phillips. School of Earth, Environmental and Biological Sciences, Queensland University of Technology, 2 George Street, Brisbane 4000, Australia, mphillips.biol@gmail.com
Keywords: Caviomorpha-Phiomorpha; Chiroptera; fossil calibration; Macropodoidea; Monotremata; molecular dating
Final citation: Phillips, Matthew J. 2015. Four mammal fossil calibrations: balancing competing palaeontological and molecular considerations. Palaeontologia Electronica 18.1.5FC; 1-16. https://doi.org/10.26879/490
palaeo-electronica.org/content/fc-5
Calibrations published in the Fossil Calibration Series are accessioned into the Fossil Calibration Database (www.fossilcalibrations.org). The Database is a dynamic tool for finding up-to-date calibrations, and calibration data will be updated and annotated as interpretations change. In contrast, the Fossil Calibration papers are a permanent published record of the information on which the calibrations were originally based. Please refer to the Database for the latest data.
INTRODUCTION
The use of fossils to calibrate molecular evolutionary timescales has become increasingly complex over the past decade. Single point calibrations have been superseded by multiple sets of calibration bounds, which in turn are often modelled in Bayesian analyses as prior (e.g., uniform, normal, lognormal) distributions between minimum and maximum ages (see Ho and Phillips, 2009). In step with these changes, molecular and palaeontological workers are increasingly working together to frame calibration procedures (Ksepka et al., 2011; Parham et al., 2012) to consider the sources of error attributable to fossil records within the context of modern molecular dating methods.
Many mammalian molecular dating studies continue to rely heavily on identifying the oldest members of clades by searching reference classifications (e.g., McKenna and Bell, 1997) that were not intended as calibration sources and include many poorly known taxa. Calibration compilations such as Benton et al. (2009) have begun to overhaul this practice and reflect diverse considerations that need to be applied to justifying calibration bounds. Here I suggest calibration bounds for four widely separated mammalian crown clades that were not considered by Benton et al. (2009): Monotremata (platypus and echidnas), Macropodoidea (kangaroos and potoroos), Caviomorpha-Phiomorpha (African and South American hystricognath rodents), and Chiroptera (bats).
The Australian terrestrial mammal fossil record includes a 30 Ma hiatus from the earliest Eocene through to the latest Oligocene (Black et al., 2012). The earlier fossils, although including crown marsupials, do not clearly fit into modern orders (Beck et al., 2008), while many calibrations derived from the latest Oligocene faunas (e.g., Vombatiformes and Phalangeroidea) are expected to substantially postdate the actual divergence times (Meredith et al., 2009, Black et al., 2012). In contrast, records from the latest Oligocene to Early Miocene include numerous ‘transitional’ macropodoids morphologically close to expectations for the kangaroo-potoroo divergence (Prideaux and Warburton, 2010). Hence, Macropodoidea likely provides one of the most informative calibrations above the family level among Australian marsupials.
Sparse Australian mammal fossil records have also hampered calibration of Monotremata, with minimum age suggestions ranging from 15.92 Ma (Beck et al., 2008) to 121 Ma (Rowe et al., 2008). The platypus (Ornithorhynchidae) morphotype extends at least back to the Paleocene ( Monotrematum, Pascual et al., 1992), while fossil echidnas (Tachyglossidae) date to no older than the Middle Miocene. This disparity raises the uncertainty of whether echidnas have a very long ghost lineage or are instead derived from platypus-like ancestors (Musser, 2003). Recent analysis of both morphological and molecular data (Phillips et al., 2009) strongly supports the latter hypothesis, and illuminates the selection of calibration bounds for Monotremata.
The Caviomorpha-Phiomorpha and chiropteran bounds suggested here provide opportunities to more closely constrain calibrations among small placental mammals. The most precise calibrations, and those that are typically the most informative in molecular dating analyses, tend to have been among large-bodied clades (e.g., Whippomorpha, Perissodactyla), within which parallel slowdowns in molecular rates could bias molecular dating estimates among placental mammals more broadly (Springer et al., 2003; Waddell, 2008; Welch et al., 2008; Steiper and Seiffert, 2012). The recently published oldest record for South American rodents (Antoine et al., 2011) permits the new Caviomorpha-Phiomorpha calibration, while phylogenetic and fossil record improvements over the past decade (see Teeling et al., 2005; Eiting and Gunnell, 2009) allow re-evaluation of crown Chiroptera calibration.
The development of calibration strategies has carefully considered uncertainties associated with the fossil record and its interpretation for placing both minimum and maximum bounds on nodes (e.g., Reisz and Müller, 2004; Benton and Donoghue, 2007). More attention, however, may need to be paid to how fossil calibrations interact with each other and with the evolutionary models that underpin molecular dating (Ho and Phillips; 2009; Pyron, 2010; Warnock et al., 2012). These interactions have become more important due to recent developments in molecular dating and have implications for balancing the imperative to closely calibrate divergences with the need to avoid erroneous calibrations.
One important molecular dating development is that universal clocks have given way to ‘relaxed clock’ methods that model variation in molecular evolutionary rates across the phylogeny (see Welch and Bromham, 2005). Sets of calibration bounds now simultaneously calibrate molecular divergence and substantially inform the model for how rates of molecular evolution vary across the phylogeny. This rate variation is typically assumed to follow a specific distribution (e.g., lognormal) among branches or to be correlated among parent-daughter branches. It is likely that most molecular datasets violate these assumptions (Ho et al., 2005), and the role of fossil calibrations in correcting for these violations is generally underappreciated. In one example, Meredith et al. (2011) show that without the influence of certain calibrations, rate variation model misspecification among their mammal dataset led to some groups being inferred to be up to 50% too old and others >50% too young.
A further consideration is that multiple calibrations do not act as a consensus that would swamp any erroneous calibration information. Instead, calibration can often be more like an auction, which can be dominated by one or a few highest (and lowest) bidders, as Hallström and Janke (2010) found for their whale-cow calibration. For this reason even one set of bounds that does not include the true divergence can substantially bias date estimates at other nodes, if those nodes or their neighbours are not themselves closely bound. This provides an argument for cautious calibration. Conversely, close calibration of minimum and maximum bounds lends precision to dating estimates and is important both for containing the influence of any calibrations that are erroneous and for correcting molecular evolutionary rate models.
With a view to balancing the competing demands on calibration, there is a broad consensus that soft bounds provide a suitable solution for maximum bounds (Parham et al., 2012; Warnock et al., 2012). However, there is less agreement on the treatment of minimum bounds (Yang and Rannala, 2006; Benton et al., 2009; Lee and Skinner, 2011). Bayes theory, which underpins many recent molecular dating methods would tend to favour soft minima unless there is near-certainty that a minimum bound postdates the crown origin. The need for caution is underlined by even some strongly supported morphological clades being shown to be paraphyletic in molecular studies, such as for Microchiroptera and Ratites (see Teeling et al., 2000, 2005; Harshman et al., 2008; Phillips et al., 2010). Where defensible, however, I advocate the use of hard minimum bounds, which can more effectively contain large errors associated with other calibrations or with misspecified models of molecular rate variation (Inoue et al., 2010).
For each of the four calibrations outlined below I also consider whether uniform (flat) or non-uniform (peaked) priors available in molecular dating programs such as BEAST (Drummond and Rambaut, 2007) provide the most appropriate reflection of fossil record expectations. A molecular dating analysis is included to examine how the suggested calibration priors are further informed by other mammal fossil calibrations and molecular data.
METHODS
Parsimony Bootstrap Analyses
The arguments for or against employing particular fossil taxa to define calibration bounds for each of the clades considered here depend at least in part on previously published maximum parsimony analyses of morphological data matrices. I re-ran these as parsimony analyses with non-parametric bootstrapping for the kangaroo and rodent morphological data matrices of Kear and Pledge (2007) and Antoine et al. (2011) respectively, in order to ascertain whether their proposed phylogenies are robust to character sampling error. In each case, 500 replicates were run in PAUP* 4.0b10 (Swofford, 2002), with 20 random-addition heuristic searches per replicate, and characters ordered and unordered as originally specified.
Similar parsimony bootstrap analyses were also run on a 38-taxon combined data matrix that matched the morphological data of Gunnell and Simmons (2005) with the 26-gene nuclear DNA sequences of Meredith et al. (2011) for all extant chiropteran families (and outgroups Tupaia, Cynocephalus, Erinaceus, Sus and Felis). The DNA excluded the six Eocene fossil taxa, but included an additional modern pteropodid, Nyctimene. The morphological data included greater representation within Vespertilionidae (Antrozoini, Vespertilioninae, Murininae, and Kerivoulinae, in addition to Myotinae and Miniopterinae, which were shared with the DNA data) and within Molossidae (Tomopeatinae, in addition to Molossinae, which was shared with the DNA data).
Fossil Calibration
Identification of calibration bounds here largely follows Barnett et al. (2005). The minimum bound marks the first appearance of a generally accepted member of the crown group, for which it possesses diagnostic characters. If the reference fossil is too recent for a consensus to have formed in the literature, then a matrix-based analysis that provides substantial statistical confidence was required. The maximum bound covers the time back until relatively well sampled fossil assemblages in potential geographic regions of origin that contain no putative crown group members, but contain stem members or ecological equivalents. Where applicable, numerical conversion of stratigraphic ages follows Ogg et al. (2008), with their younger and older bounds applied here to minimum and maximum calibration bounds, respectively.
Divergence Time Estimation
Mammalian molecular dates were inferred from the 26-gene, 169-taxon DNA dataset compiled by Meredith et al. (2011), using the Bayesian molecular dating program MCMCTREE (Rannala and Yang, 2007) within the PAML package (Yang, 2007). The analyses followed the methodology described by Meredith et al. (2011), employing their favoured DNA supermatrix topology and obtaining the input (.BV) files under GTR+G substitution models for each gene. MCMCTREE analysis was then run under the “independent” lognormal rates model, with every 20th of 100,000 generations sampled after discarding a burning of 20,000 generations.
Meredith et al. (2011) employed several calibrations based on reference fossil taxa that have not been subject to any formal phylogenetic analysis, matrix-based or otherwise (e.g., the tree shrew Eodendrogale and an undescribed putative Mormoopid bat). In other cases the placement of the reference taxon has been contradicted by matrix-based analyses, such as for the putative anomaluroid, Pondaungimys (see Marivaux et al., 2011) and the putative procyonid, Pseudobassaris (see Finarelli, 2008). Instead, I included the four new calibrations alongside the primary calibration set from Phillips et al. (2009) when the same clade is included (Amniota, Sauropsida, Theria, Marsupialia, Australasian marsupials, Petauroidea, Xenarthra, Primates, Carnivora, Erinaceidae-Soricidae, Paenungulata, Perissodactyla, Suina-Ruminatia, and Whippomorpha). In addition I include Benton et al.’s (2009) rodent calibration bounds (55.6-65.8 Ma) and Meredith et al.’s (2011) root height of 416-425.4 Ma for crown Osteichthyes, which Phillips et al. (2009) did not cover. In total, 20 sets of calibration bounds are employed.
The new calibration priors were bound as follows, Macropodoidea (15.97-54.65 Ma), Monotremata (15.97-113.0 Ma), Caviomorpha-Phiomorpha (40.94-56.0 Ma), and Chiroptera (45.0-58.9 Ma). All calibrations were employed as flat priors with hardbound minima and softbound (2.5% prior probability) maxima. Given the concerns for parallel rate slowdowns in large bodied, long-lived clades and in turn, potentially inflated date estimates for neighbouring and deeper clades, I ran the MCMCTREE analyses with and without calibrating Perissodactyla, Suina-Ruminatia, Whippomorpha, and Paenungulata. Median adult body mass among representatives of these taxa within the DNA dataset are 250 kg, 213 kg, 1,706 kg, and 341 kg, respectively (De Magalhães and Costa, 2009; Jones et al., 2009). The highest median adult body mass among the other calibrated clades is 8 kg for Carnivora.
CROWN MACROPODOIDEA
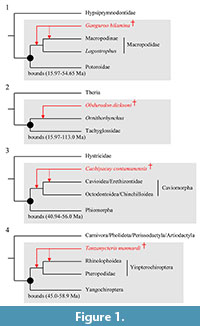 Node Calibrated. Macropodoidea, the divergence between Macropodidae (kangaroos and wallabies) and Potoroidae (potoroos and bettongs). See Figure 1.1.
Node Calibrated. Macropodoidea, the divergence between Macropodidae (kangaroos and wallabies) and Potoroidae (potoroos and bettongs). See Figure 1.1.
Fossil Taxon. Ganguroo bilamina (Cooke, 1997).
Specimens. QMF19915 (Queensland Museum), holotype of Ganguroo bilamina is a near-complete left dentary, with most of the tooth row preserved. Other partial dentaries and cheek teeth are described by Cooke (1997). Additional support for the affinities of G. bilamina is provided by QMF30845, a partial, articulated post-cranium (Kear et al., 2001) with an undescribed skull. QMF30845 follows the dental diagnosis for the holotype and conspecificity is further justified by both being recovered in the same phylogenetic position, albeit from postcranial (Kear et al., 2001) and mandibulo-dental characters (Cooke, 1997), respectively.
Phylogenetic Justification. The well-sampled, matrix-based cladistic analysis of Prideaux and Warburton (2010) provides 97% bootstrap support for grouping Ganguroo with or within Macropodidae to the exclusion of Potoroidae. Several mandibulo-dental and postcranial characters provided unambiguous synapomorphies for this relationship, including bilophodont molars and a straight acromion process on the scapula. All other cladistic analyses concur with this relationship for Ganguroo, although its precise position among basal macropodids is uncertain (see Kear and Pledge, 2007). The living macropodoid clades, Macropodidae and Potoroidae, are sister taxa in all recent molecular studies (e.g., Phillips and Pratt, 2008; Meredith et al., 2008a) and each of the morphological cladistic analyses that include G. bilamina (see above) is consistent with this relationship.
Hard Minimum Age. 15.97 Ma.
Soft Maximum Age. 54.65 Ma.
Age Justification. Ganguroo bilamina occurs in Faunal Zones B and C of the Riversleigh local faunas (northwestern Queensland). The older of these is Faunal Zone B, from which G. bilamina is known from sites such as Wayne’s Wok (including the holotype), Camel Sputum and Mike’s Menagerie. Early Miocene dates have consistently been attributed to Faunal Zone B sites by biocorrelation (Black, 1997; Travouillon et al., 2006). More recently, Black et al. (2012) indicated that U/Pb radiometric dating of speleothems now confirms this timing. However, until the new dates are published I consider the top of the Early Miocene to provide a hard minimum for Riversleigh Faunal Zone B and hence, for the crown Macropodoidea divergence.
Several putative crown macropodoids occur in Late Oligocene (~25 Ma, Woodburne et al., 1993; Megirian et al., 2010) Etadunna and Riversleigh Faunal Zone A sites and as such, an older maximum bound is required for this clade. Unfortunately, the preceding hiatus in the Australasian terrestrial mammal fossil record stretches back to the earliest Eocene (54.6 ± 0.05 Ma) Tingamarra site near Murgon, Queensland (Godthelp et al., 1992). This gap provides for a very conservative maximum bound, because the Tingamarra Fauna represents an early stage in the evolution of Australian marsupials (Beck et al., 2008), well before the evolution of the highly derived kangaroos and potoroos.
Discussion. Calibration of Macropodoidea has been heavily influenced by Woodburne et al.’s (1993) identification of both potoroids (e.g., Purtia) and macropodids (e.g., Nambaroo) in the Late Oligocene Etadunna formation. Accordingly, numerous studies have since used ~23-25 Ma to calibrate the node as a point estimate or minimum bound (e.g., Kirsch et al., 1997; Westerman et al., 2002). However, Meredith et al. (2008b) acknowledged that several morphological appraisals (Flannery, 1989; Cooke, 2006) provide substantial uncertainty for the placement of these fossils, and instead used a more conservative minimum bound of 12 Ma. This younger date is based on clearer macropodid material (see Prideaux, 2004). The oldest generally accepted potoroid, Bettongia moyesi (Flannery and Archer, 1987) further supports a divergence of at least this age, although its Middle Miocene age is controversial (Travouillon et al., 2006). Ganguroo bilamina allows a less conservative and therefore more informative minimum bound (15.97 Ma) for the potoroid-macropodid divergence. As noted by Phillips et al. (2013), Ganguroo is also a candidate for calibrating the next shallowest clade, Macropodidae, although its placement within this clade to the exclusion of lagostrophines is thus far only weakly supported (Prideaux and Tedford, 2012).
Meredith et al. (2011) recently cited the Late Oligocene Bulungamaya in a return to using a 25 Ma minimum bound for crown Macropodoidea. This taxon, however, is not well suited for use as a hard minimum, because unambiguous synapomorphies linking Bulungamaya to Macropodidae derive only from dental characters, which as Flannery (1989) and Prideaux (2004) argued, may be unreliable among early kangaroos. Moreover, its placement with Macropodidae was supported in only 29% of replicates in my parsimony bootstrap analysis of Kear and Pledge’s (2007) data matrix. Even if Bulungamaya is unsuitable for defining a hard minimum, the balance of evidence appears to favour Late Oligocene crown macropodid origins. Other similar aged taxa, Purtia and Ngamaroo are morphologically very close to the stem-crown transition (Prideaux and Warburton, 2010) and the mitochondrial molecular timetree calibrated independently of Macropodidae (Nilsson et al., 2004) places this node at about 20 Ma. As an alternative to the uniform (flat) prior between minimum and maximum bounds, molecular dating programs such as BEAST (Drummond and Rambaut, 2007) can allow for higher prior probability of divergence centred between the bounds. A lognormal prior could provide a hard minimum at 15.97 Ma for Macropodoidea, with a median at 25 Ma and a soft maximum at 54.65 Ma.
The molecular dating estimates for Macropodoidea here (Table 1) bump up against the minimum hard bound, suggesting that the rate model for the nuclear data is misspecified. Removal of the macropodoid calibration shows this more clearly, with the mean estimate for Macropodoidea falling to 10.9 Ma, younger even than expected divergences within the shallower Macropodidae and Potoroidae (Prideaux, 2004). This result shows the potential importance of calibrating Macropodoidea.
CROWN MONOTREMATA
Node Calibrated. Monotremata, the divergence between Tachyglossidae (echidnas) and Ornithorhynchidae (platypus). See Figure 1.2.
Fossil Taxon. Obdurodon dicksoni (Archer et al., 1992).
Specimens. QMF20568 (Queensland Museum), holotype of Obdurodon dicksoni is a near-complete skull with left and right upper pre-molars and is sufficient for phylogenetic placement. A partial, edentulous dentary (QMF18977) and several cheek teeth (QM F18978, QMF30249, QMF30716 and QMF30717) are slightly older, providing the calibration minimum bound. The slightly older cheek teeth closely match the holotype skull for size and “insertion” and are near-identical to other dental material from the same site as the skull, thus justifying conspecificity (Archer et al., 1993; Musser and Archer, 1998).
Phylogenetic Justification. All formal and informal cladistic analyses of monotremes favour grouping Obdurodon with the modern Ornithorhynchus to the exclusion of tachyglossids (e.g., Musser, 1999; Luo et al., 2007; Rowe et al., 2008). Moreover, Phillips et al. (2009) found high statistical support for an Obdurodon - Ornithorhynchus sister-grouping, for which unambiguous synapomorphies include rostral elements (nasal, maxilla, septomaxilla) forming a board ‘bill’, a robust posterolateral maxillary process and several endocranial characters (see Macrini et al., 2006). The sister relationship between living ornithorhynchids and tachyglossids is uncontroversial in molecular and morphological studies (e.g., van Rheede et al., 2006; Luo et al., 2007; Phillips et al., 2009).
Hard Minimum Age. 15.97 Ma.
Soft Maximum Age. 113.0 Ma.
Age Justification. Obdurodon dicksoni occurs in Faunal Zones B and C of the Riversleigh local faunas (northwestern Queensland). The holotype is known from the early Middle Miocene Faunal Zone C (Ringtail Site). However, slightly older Ob. dicksoni molars and a partial dentary are known from Faunal Zone B sites (Neville’s Garden and Dirk’s Towers). Early Miocene dates have consistently been attributed to Faunal Zone B sites by biocorrelation (e.g., Black, 1997; Travouillon et al., 2006). More recently Black et al. (2012) noted that U/Pb radiometric dating of speleothems now confirms this timing. However, until the new dates are published I consider the top of the Early Miocene to provide a minimum for Riversleigh Faunal Zone B and hence, for the crown Monotremata divergence.
Potential crown monotremes are traceable at least back to the Paleocene (~61 Ma) Monotrematum sudamericanum from Argentina, which is known from several ornithorhynchid-like molars (Pascual et al., 1992) and distal femora (Forasiepi and Martinelli, 2003). Earlier (Maastrichtian) well-sampled South American faunas lack any monotremes. However, sparse Australasian fossil records provide no solid evidence for mammal faunas lacking crown monotremes until the Albian Lightning Ridge (Flannery et al., 1995) and Dinosaur Cove (Rich and Vickers-Rich, 2003) faunas. I use the base of the Albian as a soft maximum for Monotremata.
Discussion. In light of sparse fossil records and ‘platypus’ morphology being ancestral among crown monotremes (see Gregory, 1947; Musser, 2003; Phillips et al., 2009), molecular timetrees calibrated independently of Monotremata have been particularly important for estimating monotreme crown divergence. Modern relaxed-clock molecular dating estimates concur on a Tertiary divergence between the platypus and echidnas, with most estimates falling between 21-48 Ma (e.g., Janke et al., 2002; Hugall et al., 2007; Warren et al., 2008). One exception (Rowe et al., 2008) provided mean estimates of 79.5 Ma and 88.9 Ma, although their exclusion of non-mammals prevented accurate estimation of evolutionary rates on either side of the root between monotremes and therians. Rectifying this issue again resulted in mid-Tertiary estimates (Phillips et al., 2009). Hence, molecular dates for the crown monotreme divergence (including the estimates in Table 1) fall within the younger end of the 15.97-113.0 Ma bounds.
Although the Early Miocene minimum bound for crown monotreme origins is based primarily on the platypus, Obdurodon dicksoni, further support comes from the earliest tachyglossid fossil, the already somewhat derived Gulgong echidna, Megalibgwilia robusta (Dun, 1895). The age of the Gulgong deposit is 13-14 Ma, based on estimates from overlying basalt (Woodburne et al., 1985), although this has been contentious, because the fossil preservation is similar to much younger nearby Pleistocene sites (Augee et al., 2006).
It does not necessarily follow that the platypus affinity of Obdurodon dicksoni secures crown monotreme placement for the dentally similar Late Oligocene (~25 Ma) Obdurodon insignis or Paleocene Monotrematum. None of the unambiguous synapomorphic characters linking Obdurodon dicksoni with Or. anatinus from Rowe et al. (2008) are preserved in either of these older ‘platypuses’. Determining where Monotrematum and Ob. insignis fall relative to the divergence of the platypus from the edentulous echidnas will require further non-dental material.
The suggested basal Albian soft maximum bound for Monotremata is challenged by the recent proposals of Rowe et al. (2008) that the Albian Kryoryctes cadburyi (an isolated, incomplete humerus) could be a stem tachyglossid and the Aptian Teinolophos trusleri (several partial dentaries) is a stem ornithorhynchid. The former suggestion is based on gross morphology and ignores features such as a shallow ulna trochlea and an olecranon fossa, which place the specimen well outside platypuses and echidnas, for which distal humeri are substantially more specialized (Pridmore et al., 2005). Furthermore, Rowe et al.’s (2008) placement of T. trusleri depends on numerous redundant characters all based on an enlarged mandibular canal. Without this redundancy, cladistic analyses place T. trusleri outside of crown monotremes with high statistical support (Luo et al., 2007; Phillips et al., 2009).
The mid-Tertiary molecular dates for the monotreme crown divergence indicate that the 113.0 Ma maximum softbound is conservative, although necessarily so, reflecting the sparse and fragmentary nature of the monotreme fossil record. In the absence of any narrow temporal range within which stem-crown transitional monotremes appear it may be advisable to employ the monotreme bounds as a uniform prior.
CROWN CAVIOMORPHA-PHIOMORPHA
Node Calibrated. The divergence between Caviomorpha (e.g., guinea pigs) and Phiomorpha (e.g., cane rats). See Figure 1.3.
Fossil Taxon. Cachiyacuy contamanensis (Antoine et al., 2011).
Specimens. MUSM1871 (Museum of Natural History in Lima, Peru), holotype of Cachiyacuy contamanensis is a right M2 (2nd upper molar). Several additional isolated upper and lower cheek teeth also contribute to defining the phylogenetic placement of the taxon, particularly MUSM1870, MUSM1872, MUSM1873, MUSM1874, and MUSM1880. Antoine et al. (2011) argue that these teeth (collected from the same site) are conspecific on the basis of size and distinctive molar cusp and style patterns. Moreover, all of the rodent dental material published from the site supports the calibration in both phylogenetic and temporal aspects.
Phylogenetic Justification. Antoine et al. (2011) described five new caviomorph rodents from the Yahuarango Formation in Peru, Cachiyacuy contamanensis, Cachiyacuy kummeli, Canaanimys maquiensis, Eobranisamys sp., and Eoespinasp. All five species grouped with Caviomorpha in Antoine et al.’s (2011) cladistic analysis of dental characters. Extraordinary dental diversity among rodents as a whole precluded any unambiguous synapomorphies characterising Caviomorpha (including the new species), although the presence of a mesostyle on P4 and the absence of paracone-metacone compression on P4 are unambiguous caviomorph synapomorphies, among hystricognaths.
Overall phylogenetic signal strongly supports grouping the five new taxa with Caviomorpha; my parsimony analysis of Antoine et al.’s (2011) full data matrix provided 97% bootstrap support for this grouping. I chose Cachiyacuy contamanensis as the focal species for the calibration because it has the most completely sampled dentition. Consistent with Antoine et al. (2011), caviomorphs and phiomorphs form a clade in most recent morphological assessments (e.g., Marivaux et al., 2004; Asher et al., 2005) and importantly, their living representatives are sister taxa in all recent molecular studies (e.g., Poux et al., 2006; Meredith et al., 2011).
Hard Minimum Age. 40.94 Ma.
Soft Maximum Age. 56.0 Ma.
Age Justification. Cachiyacuy contamanensis occurs in theCTA-27 locality towards the top of the Yahuarango Formation. Antoine et al. (2011) used 40Ar/39Ar step heating to date biotite grains overlying the fossil-bearing sediments to 43.44 ± 2.5 Ma, suggesting a minimum age of 40.94 Ma for CTA-27. No taxa presently considered as putative Caviomorpha-Phiomorpha crown members are known from earlier Lutetian (Middle Eocene) mammal faunas. However, the Lutetian is particularly poorly sampled for small mammal fossils in Africa, a potential geographic origin for this clade (Gheerbrant and Rage, 2006; Marivaux et al., 2011). Hence, the absence of any hystricognaths from the better sampled Early Eocene faunas (Marivaux et al., 2011; Coster et al., 2012) provides a more appropriate soft maximum of basal Ypresian age (56.0 Ma).
Discussion. The Caviomorpha-Phiomorpha clade has not previously been calibrated as far as I am aware. This may be due in part to uncertainty surrounding the affinities of early ‘phiomorphs’ and because the previously oldest caviomorphs were putatively early Oligocene in age and already somewhat derived (Vucetich et al., 2010). The Yahuarango caviomorphs provide a more informative calibration, being late Middle Eocene in age and according to Antoine et al. (2011), represent a morphologically early stage subsequent to the divergence from African and Asian hystricognaths. The expectation of a Middle Eocene Caviomorpha-Phiomorpha divergence is also consistent with the oldest phiomorphs being at least latest Eocene (33.8 Ma) in age (Gagnon, 1987; Coster et al., 2012), and with the stem/crown transitional nature of other Eocene African ‘phiomorphs’ and Eurasian baluchimyines. Independently calibrated mean molecular dating estimates for the Phiomorpha-Caviomorpha divergence also fall in the Middle to Late Eocene (e.g., Poux et al., 2006; Huchon et al., 2007; Honeycutt 2009).
Calibration hardbounds require the researcher to decide that some level of uncertainty is sufficiently low to disregard. There are two minor sources of uncertainty for the age of the CTA-27 locality that might lead some researchers to employ the 40.94 Ma 40Ar/39Ar lower bound as a soft minimum. The first is that the next oldest South American rodent fauna is ~9 Ma younger, with rodents being absent from several faunas of intermediate age, albeit at higher latitudes (Goin et al., 1998, 2010; Bond and Deschamps, 2010). The second is that Barrancan-Mustersan (35.8-41.6 Ma) South American Land Mammal Age biochronological bounds inferred by Antoine et al. (2011) for the CTA-27 locality barely overlap with the lower bound of the 40Ar/39Ar estimate. Both concerns very likely arise from limited sampling and regionalization of the primarily dental-based South American Middle Eocene to Early Oligocene mammal record (Kay et al., 1999; Bond and Deschamps, 2010). Moreover, the newly described ~32 Ma Andean assemblage of crown caviomorphs (Bertrand et al., 2012) that are substantially more derived than the Yahuarango fossils further suggests a long South American history for these rodents.
MCMCTREE molecular dating in the present study (Table 1) is consistent with the hard minimum bound; even the lower bound of the posterior distribution for the age of Caviomorpha-Phiomorpha is several million years older than the radiometric minimum age. However, the absence of even putative caviomorphs or phiomorphs older than the CTA-27 locality makes it difficult to justify a peaked prior between the 40.94-56.0 Ma bounds, such that a uniform distribution is most appropriate.
Antoine et al. (2011) found that Cachiyacuy was sister to Cavioidea/Erethizontidae and hence, is also a candidate for calibrating the slightly younger Caviomorpha crown node. However, this grouping received only 20% parsimony bootstrap support in my analysis of Antoine et al.’s (2011) dataset. Even then crown caviomorph placement for Cachiyacuy relies on Oligocene “octodontoids” also being crown caviomorphs, which needs further testing in light of their paraphyly in Antoine et al. (2011) and deeper than expected placement in the analysis of Vucetich and Kramarz (2003).
CROWN CHIROPTERA
Node Calibrated. Chiroptera, the divergence between Yangochiroptera (e.g., pipistrelles, sheathtail bats) and Yinpterochiroptera (e.g., flying foxes, horseshoe bats). See Figure 1.4.
Fossil Taxon. Tanzanycteris mannardi (Gunnell et al., 2003).
Specimen. TNM MP-207 (Tanzanian National Museum), holotype and only specimen of Tanzanycteris mannardi is a partial skeleton including skull, mandibles, vertebral column anterior to the sacrum, shoulder girdle, partial humeri, and left radius. Teeth are unknown.
Phylogenetic Justification. Gunnell et al. (2003) identified a suite of characters that place T. mannardi within Yinpterochiroptera, specifically with Rhinolophoidea. These include extremely enlarged cochlea, broadened first rib, and a dorsally flared iliac blade (this later character is also shared with some probable stem chiropterans). In my combined parsimony analysis of morphological data from Gunnell and Simmons (2005) and DNA sequences from Meredith et al. (2011), T. mannardi groups with rhinolophoids with 58% bootstrap support, while its placement within crown Chiroptera receives 83% bootstrap support. In this analysis the enlarged cochlea and broadened 1st rib are unambiguous apomorphies for Rhinolophoidea, including T. mannardi. These characters are highly conserved among bats. The enlarged cochlea is otherwise only known from one species of mormoopid (although, without the enlarged cochlea fenestra) and a similar rib morphology is known from one other genus, Nycteris.
Hard Minimum Age. 45.0 Ma.
Soft Maximum Age. 58.9 Ma.
Age Justification. Tanzanycteris mannardi was recovered from the lacustrine Mahenge locality in north-central Tanzania. Zircon at the base of the Mahenge sequence (~1.2 m below the fossil) was 206Pb/238U dated by Harrison et al. (2001) to 45.83 ± 0.17 Ma. The authors also considered sedimentation rates, for which minimum estimates and error on the 206Pb/238U dates allow a minimum bound of 45.0 Ma for T. mannardi and the crown chiropteran divergence. This mid-Eocene age is also consistent with the Mahenge fossil fish fauna (e.g., Murray, 2000).
Several possible crown bats with putative yangochiropteran affinities occur in Early Eocene localities (Eiting and Gunnell, 2009). Bats are remarkable among mammals in that accepted crown fossil records are closely bracketed by older stem fossils from all continents except Antarctica (Ravel et al., 2011). Although no stem bats are known from prior to the Eocene, some of these records that may be used to bracket the calibration are very close to the Thanetian-Ypresian boundary and so I use the base of the Thanetian (no older than 58.9 Ma) as a soft maximum for the age of crown Chiroptera.
Discussion. Modern bats have traditionally been divided morphologically into the mainly frugivorous or nectivorous Megachiroptera (Pteropodidae, flying foxes, etc.) and the primarily insectivorous Microchiroptera (all other families). Many of the oldest fossil bats share features associated with echolocation and general body form with microchiropterans to the exclusion of megachiropterans and other mammals. This in turn has resulted in some of the earliest bats (e.g., Icaronycteris, Australonycteris) being linked phylogenetically to microchiropterans (Simmons and Geisler, 1998) and used to calibrate the chiropteran crown divergence (e.g., dos Reis et al., 2012). Analyses of multiple nuclear genes (Teeling et al., 2000, 2005) and mitochondrial genomes (Lin et al., 2002) now provide overwhelming evidence for microchiropteran paraphyly, with rhinolophoids grouping with pteropodids. The use of molecular phylogenetic scaffolds have resulted in all Early Eocene bats that have been included in matrix-based cladistic analyses falling as stem chiropterans (Teeling et al., 2005; Simmons et al., 2008), with their “microchiropteran” traits found to be plesiomorphic for bats.
The finding that incorrect placement of pteropodids distorts character covariation on the tree for inferring the placement of fossil bats (Teeling et al., 2005) is also relevant here. Hermsen and Hendricks’ (2008) molecular scaffold analysis of Gunnell and Simmons’ (2005) morphological matrix clearly favoured rhinolophoid affinities for Tanzanycteris. In contrast, their combined data analysis found this rhinolophoid placement to be equally parsimonious with exclusion of Tanzanycteris from crown Microchiroptera, but with this fossil taxon still falling within crown Chiroptera. By replacing the Teeling et al. (2005) molecular matrix with the nearly 3-fold longer Meredith et al. (2011) DNA matrix, pteropodids fall back into their expected placement and most parsimonious trees again favour rhinolophoid affinities for Tanzanycteris. Hence, Tanzanycteris might yet prove to be appropriate for calibrating the younger Yinpterochiroptera node. However, only 58% bootstrap support in the present combined analysis is reason for caution. Additionally, it would have to be shown that exclusion of pteropodids from the Tanzanycteris/Rhinolophoidea grouping is not also an artefact of the morphological homoplasy that attracts pteropodids towards the chiropteran root.
Given the historical difficulties for inferring relationships among bats from morphological data, some authors may reasonably consider that a soft, rather than hard minimum bound of 45 Ma is warranted, based on the non-dental Tanzanycteris alone. Support for the minimum bound firms however, when considered in the wider fossil record context. A Tunisian rhinolophoid (dental) taxon described by Sigé (1991) adds slightly older dental evidence for Rhinolophoidea on the same continent. Chiroptera is also very likely constrained to be at least 47 Ma by Tachypteron, which is generally regarded as an emballonurid (Storch et al., 2002), placed on the opposite side of the chiropteran root to Tanzanycteris.
More material is needed for the Tunisian rhinolophoid, and Tachypteron is yet to be tested with formal matrix-based phylogenetic analyses, but both push the balance of evidence substantially in favour of Chiroptera being at least as old as the hard minimum bound suggested here. Complete mitochondrial genome molecular dating for the chiropteran crown divergence (~54 Ma, Phillips et al., 2009), independent of chiropteran calibrations further supports the hard minimum suggested here being conservative.
Bats have relatively low fossil record completeness at the genus level (Eiting and Gunnell, 2009). This may be due in part to often sparse diagnostic characters available at this taxonomic level from mandible fragments and isolated teeth, as well as the restriction of many genera to regions with low preservation potential or that are poorly sampled. At the ordinal level, however, the global bat fossil record is exceptional among mammals in its potential for providing a tight maximum bound. As flying mammals that filled new ecological space, bats first appear early in the Ypresian almost simultaneously in North America, Asia, Europe, Australia, Africa, and South America. In all cases (except for fragmentary dental material of uncertain affinities) these first appearances have been assigned stem, rather than crown placements (Simmons et al., 2008, Tabuce et al., 2009; Ravel et al., 2011; Smith et al., 2007). Hence, it is likely that crown Chiroptera originated in the Ypresian, although I use the base of the Thanetian as a more conservative soft maximum.
More primitive stem bats are likely to have been geographically restricted without strong flight and difficult to distinguish from archaic insectivores (Gunnell and Simmons, 2005), but this is not directly relevant to the question of crown Chiroptera calibration. Substantially earlier absence of crown bats is also unlikely to be explained by sampling artefacts. From their first appearance, bats occur in every subsequent sub-epoch in the fossil records of North America, Eurasia, and in at least one of the Gondwanan continents (Gunnell and Simmons, 2005).
Most molecular dates for the origin of crown bats fall inside or very close to the bounds suggested here (e.g., Nikaido et al., 2001; Jones et al., 2005; dos Reis et al., 2012). The best estimate from the present study (Table 1, excluding large-bodied clade calibrations) is 55 Ma, coincident with the initial radiation of bats in the fossil record. Several studies have dated crown Chiroptera more than 5 Ma older than the 58.9 Ma maximum suggested here. These studies typically employ multiple calibrations among large-bodied groups with far slower rates of molecular evolution and use possible stem bats (e.g., Ageina, Honrovits) as crown calibrations (e.g., Bininda-Emonds et al., 2007; Meredith et al., 2011) or place a mean prior on the age of Chiroptera that is far older than any recognised bats (e.g., 65 Ma in Teeling et al., 2005).
Even without the questionable bat calibrations, re-including the large-bodied clade calibrations in the present study and removing the maximum bound for Chiroptera increases the crown age of bats to 60.2 Ma, with only 13% of the posterior distribution younger than the maximum bound. Employing the soft maximum bound buffers the influence of the large-bodied clade calibrations and pulls the mean estimate and 65% of the posterior distribution within the chiropteran calibration bounds (Table 1). This underlines the potential importance of the chiropteran maximum bound for informing models of substitution rate variation in mammalian molecular dating studies.
CONCLUSION
In this study I have suggested minimum and maximum bounds based on fossil evidence, for the crown age of four mammal clades, Monotremata (platypus and echidnas), Macropodoidea (kangaroos and potoroos), Caviomorpha-Phiomorpha (South American and African hystricognath rodents), and Chiroptera (bats). Bayesian inference molecular dating performed on a 26 nuclear gene DNA data matrix showed close agreement among these and previously available well-corroborated calibrations, with exceptions that illustrate the importance of fossil calibrations for informing models of molecular rate variation across the phylogeny. Of particular note is the underappreciated role of maximum bounds in buffering against undercorrection for substitution rate deceleration in large-bodied, long-lived mammals.
ACKNOWLEDGEMENTS
This study has benefited substantially from the comments of R.M.D. Beck and an anonymous reviewer, a discussion with J. Alroy and helpful suggestions from E.A. Lindsay. I am also grateful to D.T. Ksepka and J.F. Parham for inviting me to the National Evolutionary Synthesis Center (NESCent) Fossil Calibrations meeting, which provided the impetus for this work.
REFERENCES
Antoine, P-O., Marivaux, L., Croft, D.A., Billet, G., Ganerød, M., Jaramillo, C., Martin, T., Orliac, M.J., Tejada, J., Altamirano, A.J., Duranthon, F., Fanjat, G., Rousse, S., and Gismondi, R.S. 2011. Middle Eocene rodents from Peruvian Amazonia reveal the pattern and timing of caviomorph origins and biogeography. Proceedings of the Royal Society of London B, 279:1319-1326.
Archer, M., Murray, P., Hand, S.J., and Godthelp, H. 1993. Reconsideration of monotreme relationships based on the skull and dentition of the Miocene Obdurodon dicksoni, p. 75-94. In Szalay, F.S., Novacek, M.J., and MacKenna, M.C. (eds.), Mammalian Phylogeny, Vol. 1. Mesozoic Differentiation, Multituberculates, Monotremes, Early Therians and Marsupials. Springer-Verlag, New York.
Archer, M., Jenkins, F.A., Hand, S.J., Murray, P., and Godthelp, H. 1992. Description of the skull and non-vestigial dentition of a Miocene platypus ( Obdurodon dicksonin. sp.) from Riversleigh, Australia, and the problem of monotreme origins, p. 15-27. In Augee, M.L. (ed.), Platypus and Echidnas. The Royal Zoological Society of New South Wales, Mosman.
Asher, R.J., Meng, J., Wible, J.R., McKenna, M.C., Rougier, G.W., Dashzeveg, D., and Novacek, M.J. 2005. Stem Lagomorpha and the antiquity of Glires. Science, 307:1091-1094.
Augee, M.L., Musser, A.M., and Gooden, B. 2006. Echidna, Extraordinary Egg-laying Mammal. CSIRO Publishing, Canberra.
Barnett, R., Barnes, I., Phillips, M.J., Martin, L.D., Harington, C.R., Leonard, J.A., and Cooper, A. 2005. Evolution of the extinct Sabretooths and the American cheetah-like cat. Current Biology, 15:R589-590.
Beck, R.M.D., Godthelp, H., Weisbecker, V., Archer, M., and Hand, S.J. 2008. Australia’s oldest marsupial fossils and their biogeographical implications. PLoS One, 3:e1858.
Benton, M.J. and Donoghue, P.C.J. 2007. Paleontological evidence to date the tree of life. Molecular Biology and Evolution, 24:889-891.
Benton, M.J., Donoghue, P.C.J., and Asher, R.J. 2009. Calibrating and constraining molecular clocks, p. 35-86. In Hedges, S.B. and Kumar, S. (eds.), The Timetree of Life. Oxford University Press, Oxford.
Bertrand, O.C., Flynn, J.J., Croft, D.A., and Wyss, A.R. 2012. Two new taxa (Caviomorpha, Rodentia) from the Early Oligocene Tinguiririca Fauna (Chile). American Museum Novitates, 3750:1-36.
Bininda-Emonds, O.R., Cardillo, M., Jones, K.E., MacPhee, R.D.E., Beck, R.M.D., Grenyer, R., Price, S.A., Vos, R.A., Gittleman, J.L., and Purvis, A. 2007. The delayed rise of present-day mammals. Nature, 446:507-512.
Black, K. 1997. Diversity and biostratigraphy of the Diprotodontoidea of Riversleigh, northwestern Queensland. Memoirs of the Queensland Museum, 41:187-192.
Black, K.H., Archer, M., Hand, S.J., and Godthelp, H. 2012. The rise of Australian marsupials: a synopsis of biostratigraphic, phylogenetic, palaeoecologic and palaeobiogeographic understanding, p. 983-1078. In Talent, J.A. (ed.), Earth and Life, International year of Planet Earth. Springer, Dordrecht, Netherlands.
Bond, M. and Deschamps, C.M. 2010. The Mustersan age at Gran Barranca: a review, p. 255-263. In Madden, R.H., Carlini, A.A., Vucetich, M.G., and Kay, R.F. (eds.), The Paleontology of Gran Barranca: Evolution and Environmental Change Through the Middle Cenozoic of Patagonia. Cambridge University Press, Cambridge.
Cooke, B.N. 1997. New Miocene bulungamayine kangaroos (Marsupialia: Potoroidae) from Riversleigh, northwestern Queensland. Memoirs of the Queensland Museum, 41:281-294.
Cooke, B.N. 2006. Kangaroos, p. 647-672. In Merrick, J.R., Archer, M., Hickey, G.M., and Lee, M.S.Y. (eds.), Evolution and Biogeography of Australian Vertebrates. Auscipub Pty Ltd, Sydney.
Coster, P., Benammi, M., Mahboubi, M., Tabuce, R., Adaci, M., Marivaux, L., Bensalah, M., Mahboubi, S., Mahboubi, A., Mebrouk, F., Maameri, C., and Jaeger, J-J. 2012. Chronology of the Eocene continental deposits of Africa: Magnetostratigraphy and biostratigraphy of the El Kohol and Glib Zegdou Formations, Algeria. Geological Society of America Bulletin, 124:1590-1606.
De Magalhães, J.P. and Costa, J. 2009. A database of vertebrate longevity records and their relation to other life-history traits. Journal of Evolutionary Biology, 22:1770-1774.
dos Reis, M., Inoue, J., Hasegawa, M., Asher, R.J., Donoghue, P.C.J., and Yang, Z. 2012. Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny. Proceedings of the Royal Society of London B, 279:3491-3500.
Drummond, A.J. and Rambaut, A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology, 7:214.
Dun, W.S. 1895. Notes on the occurrence of monotreme remains in the Pliocene of New South Wales. Records of the Geological Survey of New South Wales, 4:118-126.
Eiting, T.P. and Gunnell, G.F. 2009. Global completeness of the bat fossil record. Journal of Mammalian Evolution, 16:151-173.
Finarelli, J.A. 2008. A total evidence phylogeny of the Arctoidea (Carnivora: Mammalia): relationships among basal taxa. Journal of Mammalian Evolution, 15:231-259
Flannery, T. 1989. Phylogeny of the Macropodoidea; a study in convergence, p. 1-46. In Grigg, G., Jarman, P., and Hume, I. (eds.), Kangaroos, Wallabies and Rat-kangaroos. Surrey-Beatty and Sons, Sydney.
Flannery, T.F. and Archer, M. 1987. Bettongia moyesi, a new and plesiomorphic kangaroo (Marsupialia: Potoroidae) from Miocene sediments of northwestern Queensland, p. 759-767. In Archer, M. (ed.), Possums and Opossums: Studies in Evolution. Surrey Beatty and Sons, Sydney.
Flannery, T.F., Archer, M., Rich, T.H., and Jones, R. 1995. A new family of monotremes of the Cretaceous of Australia. Nature, 377:418-420.
Forasiepi, A.M. and Martinelli, A.G. 2003. Femur of a monotreme (Mammalia, Monotremata) from the Early Paleocene Salamanca Formation of Patagonia, Argentina. Ameghiniana, 40:625-630.
Gagnon, M. 1987. Ecological diversity and community ecology in the Fayum sequence (Egypt). Journal of HumanEvolution, 32:133-160.
Gheerbrant, E. and Rage, J-C. 2006. Paleobiogeography of Africa: how distinct from Gondwana and Laurasia? Palaeogeography, Palaeoclimatology, Palaeoecology, 241:224-246.
Godthelp, H., Archer, M., Cifelli, R., Hand, S.J., and Gilkeson, C.F. 1992. Earliest known Australian Tertiary mammal fauna. Nature, 356:514-516.
Goin, F.J., Abello, M.A., and Chornogubsky, L. 2010. Middle Tertiary marsupials from central Patagonia (early Oligocene of Gran Barranca): understanding South America’s Grande Coupure, p. 69-105. In Madden, R.H., Carlini, A.A., Vucetich, M.G., and Kay, R.F. (eds.), The Paleontology of Gran Barranca: Evolution and Environmental Change Through the Middle Cenozoic of Patagonia. Cambridge University Press, Cambridge.
Goin, F.J., Candela, A.M., López, G.M. 1998. Middle Eocene marsupials from Antofagasta de la Sierra, North Western Argentina. Geobios, 31:75-85.
Gregory, W.K. 1947. The monotremes and the Palimpsest theory. Bulletin of the American Museum of Natural History, 88:7-52.
Gunnell, G.F. and Simmons, N.B. 2005. Fossil evidence for the origin of bats. Journal of Mammalian Evolution, 12:209-246.
Gunnell, G.F., Jacobs, B.F., Herendeen, P.S., Head, J.J., Kowalski, E., Msuya, C.P., Mizambwa, F.A., Harrison, T., Habersetzer, J., and Storch, G. 2003. Oldest placental mammal from sub-saharan Africa: Eocene microbat from Tanzania - evidence for early evolution of sophisticated echolocation. Palaeontologia Electronica, 5(3):10pp, 672KB; http://palaeo-electronica.org/paleo/2002_2/africa/issue2_02.htm
Hallström, B.M. and Janke, A. 2010. Mammalian evolution may not be strictly bifurcating. Molecular Biology and Evolution, 27:2804-2816.
Harrison, T., Msuya, C.P., Murray, A.M., Fine-Jacobs, B., Báez, A.M., Mundil, R., and Ludwig, K.R. 2001. Paleontological investigations at the Eocene locality of Mahenge in north-central Tanzania, East Africa, p. 40-74. In Gunnell, G.F. (ed.), Eocene Biodiversity - Unusual Occurrences and Rarely Sampled Habitats. Kluwer Academic/Plenum Publishers, New York.
Harshman, J., Braun, E.L., Braun, M.J., Huddleston, C.J., Bowie, R.C.K., Chojnowski, J.L., Hackett, S.J., Han, K.L., Kimball, R.T., Marks, B.D., Miglia, K.J., Moore, W.S., Reddy, S., Sheldon, R.C.K., Steadman, D.W., Steppan, S.J., Witt, C.C., and Yuri, T. 2008. Phylogenomic evidence for multiple losses of flight in ratite birds. Proceedings of the National Academy of Sciences USA, 105:13462-13467.
Hermsen, E.J. and Hendricks, J.R. 2008. W(h)ither fossils? studying morphological character evolution in the age of molecular sequences. Annals of the Missouri Botanical Garden, 95:72-100.
Ho, S.Y.W. and Phillips, M.J. 2009. Accounting for calibration uncertainty in phylogenetic estimation of evolutionary divergence times. Systematic Biology, 58:367-380.
Ho, S.Y.W., Phillips, M.J., Drummond, A.J., and Cooper, A. 2005. Accuracy of rate estimation using relaxed-clock models with a critical focus on the early metazoan radiation. Molecular Biology and Evolution, 22:1355-1363.
Honeycutt, R.L. 2009. Rodents, p. 490-494. In Hedges, S.B. and Kumar. S. (eds.), The Timetree of Life. Oxford University Press, Oxford.
Huchon. D., Chevret, P., Jordan, U., Kilpatrick, C.W., Ranwez, V., Jenkins, P.D., Brosius, J., and Schmitz, J. 2007. Multiple molecular evidences for a living mammalian fossil. Proceedings of the National Academy of Sciences USA, 104:7495-7499.
Hugall, A.F., Foster, R., and Lee, M.S.Y. 2007. Calibration choice, rate smoothing, and the pattern of tetrapod diversification according to the long nuclear gene RAG-1. Systematic Biology, 56:543-563.
Inoue, J., Donoghue, P.C.J., and Yang, Z. 2010. The impact of the representation of fossil calibrations on Bayesian estimation of species divergence times. Systematic Biology, 59:74-89.
Janke, A., Magnell, O., Wieczorek, G., Westerman, M., and Arnason, U. 2002. Phylogenetic analysis of 18S rRNA and the mitochondrial genomes of the wombat, Vombatus ursinus, and the spiny anteater, Tachyglossus aculeatus : increased support for the Marsupionta hypothesis. Journal of Molecular Evolution, 54:71-80.
Jones, K.E., Bininda-Emonds, O.R., and Gittleman, J.L. 2005. Bats, clocks and rocks: diversification patterns in Chiroptera. Evolution, 59:2243-2255.
Jones, K.E., Bielby, J., Cardillo, M., Fritz, S.A., O’Dell, J., Orme, C.D.L., Safi, K., Sechrest, W., Boakes, E.H., Carbone, C., Connolly, C., Cutts, M.J., Foster, J.K., Grenyer, R., Habib, M., Plaster, C.A., Price, S.A., Rigby, E.A., Rist, J., Teacher, A., Bininda- Emonds, O.R.P., Gittleman, J.L., Mace, G.M., and Purvis, A. 2009. PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology, 90:2648-2648.
Kay, R., Madden, R., Vucetich, M.G., Carlini, A., Mazzoni, M., Re, G., Heizler, M., and Sandeman, H. 1999. Revised geochronology of the Casamayoran South American Land Mammal Age: climatic and biotic implications. Proceedings of the National Academy of Sciences USA, 96:13235-13240.
Kear, B.P. and Pledge, N.S. 2007. A new fossil kangaroo from the Oligocene-Miocene Etadunna Formation of Ngama Quarry, Lake Palankarinna, South Australia. Australian Journal of Zoology, 55:331-339.
Kear, B.P., Archer, M., and Flannery, T.F. 2001. Postcranial morphology of Ganguroo bilamina, Cooke 1997 (Marsupialia: Macropodidae) from the middle Miocene of Riversleigh, northwestern Queensland. Memoirs of the Association of Australasian Palaeontologists, 25:123-138.
Kirsch, J.A.W., Lapointe, F.-J., and Springer, M.S. 1997. DNA hybridisation studies of marsupials and their implications for metatherian classification. Australian Journal of Zoology, 45:211-280.
Ksepka, D.T., Benton, M.J., Carrano, M.T., Gandolfo, M.A., Head, J.J., Hermsen, E.J., Joyce, W.G., Lamm, K.S., Patané, J.S.L., Phillips, M.J., Polly, P.D., van Tuinen, M., Ware, J.L., Warnock, R.C.M., and Parham, J.F. 2011. Synthesizing and databasing fossil calibrations: divergence dating and beyond. Biology Letters, 7:801-803.
Lee, M.S.Y. and Skinner, A. 2011. Testing fossil calibrations for vertebrate molecular trees. Zoologica Scripta, 40:538-543.
Lin, Y.H., McLenachan, P.A., Gore, A.R., Phillips, M.J., Ota, R., Hendy, M.D., and Penny, D. 2002. Four new mitochondrial genomes and the increased stability of evolutionary trees of mammals from improved taxon sampling. Molecular Biology and Evolution, 19:2060-2070.
Luo, Z-X., Chen, P., Li, G., and Chen, M. 2007. A new eutriconodont mammal and evolutionary development in early mammals. Nature, 446:288-293.
Macrini, T.E., Rowe, T., and Archer, M. 2006. Description of a cranial endocast from a fossil platypus, Obdurodon dicksoni (Monotremata, Ornithorhynchidae), and the relevance of endocranial characters to monotreme monophyly. Journal of Morphology, 267:1000-1015.
Marivaux, L., Vianey-Liaud, M., and Jaeger, J-J. 2004. High-level phylogeny of early Tertiary rodents: dental evidence. Zoological Journal of the Linnean Society, 142:105-134.
Marivaux, L., Adaci, M., Bensalah, M., Rodrigues, H.G., Hautier, L., Mahboubi, M., Mebrouk, F., Tabuce, R., and Vianey-Liaud, M. 2011. Zegdoumyidae (Rodentia, Mammalia), stem anomaluroid rodents from the Early to Middle Eocene of Algeria (Gour Lazib,Western Sahara): new dental evidence. Journal of Systematic Paleontology, 9:563-588.
McKenna, M.C. and Bell, S.K. 1997. Classification of Mammals - Above the Species Level. Columbia University Press, New York.
Megirian, D., Prideaux, G.J., Murray, P.F., and Smit, N. 2010. An Australian land mammal age biochronological scheme. Paleobiology, 36:658-671.
Meredith, R.W., Westerman, M., and Springer, M.S. 2008a. A phylogeny and timescale for the living genera of kangaroos and kin (Macropodiformes: Marsupialia) based on nuclear DNA sequences. Australian Journal of Zoology, 56:395-410.
Meredith, R.W., Westerman, M., and Springer, M.S. 2009. A phylogeny of Diprotodontia (Marsupialia) based on sequences for five nuclear genes. Molecular Phylogenetics and Evolution, 51:554-571.
Meredith, R.W., Westerman, M., Case, J.A., and Springer, M.S. 2008b. A phylogeny and timescale for marsupial evolution based on sequences for five nuclear genes. Journal of Mammalian Evolution, 15:1-36.
Meredith, R.W., Janecka, J.E., Gatesy, J., Ryder, O.A., Fisher, C.A., Teeling, E.C., Goodbla, A., Eizirik, E., Simão, T.L.L., Stadler, T., Rabosky, D.L., Honeycutt, R.L., Flynn, J.J., Ingram, C.M., Steiner, C., Williams, T.L., Robinson, T.J., Burk-Herrick, A., Westerman, M., Ayoub, N.A., Springer, M.S., and Murphy, W.J. 2011. Impacts of the Cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science, 334:521-524.
Murray, A.M. 2000. Eocene cichlid fishes from Tanzania, East Africa. Journal of Vertebrate Paleontology, 20:651-664.
Musser, A.M. 1999. Diversity and relationships of living and extinct monotremes. Australian Mammalogy, 21:8-9.
Musser, A.M. 2003. Review of the monotreme fossil record and comparison of palaeontological and molecular data. Comparative Biochemistry Physiology A: Molecular Integrative Physiology, 136:927-942.
Musser, A.M. and Archer, M. 1998. New information about the skull and dentary of the Miocene platypus Obdurodon dicksoni, and a discussion of ornithorhynchid relationships. Philosophical Transactions of the Royal Society of London B, 353:1063-1079.
Nikaido, M., Kawai, K., Cao, Y., Harada, M., Tomita, S., Okada, N., and Hasegawa, M. 2001. Maximum likelihood analysis of the complete mitochondrial genomes of eutherians and a reevaluation of the phylogeny of bats and insectivores. Journal of Molecular Evolution, 53:508-516.
Nilsson, M.A., Arnason, U., Spencer, P.B.S., and Janke, A. 2004. Marsupial relationships and a timeline for marsupial radiation in South Gondwana. Gene, 340:189-196.
Ogg, G.J., Ogg, G., and Gradstein, F.M. 2008. The Concise Geologic Time Scale. Cambridge University Press, Cambridge.
Parham, J.F., Donoghue, P.C.J., Bell, C.J., Calway, T.D., Head, J.J., Holroyd, P.A., Inoue, J., Imris, R.B., Joyce, W.G., Ksepka, D.T., Patané, J.S.L., Smith, N.D., Tarver, J.E., van Tuinen, M., Yang, Z., Angielczyk, K.D., Greenwood, J.M., Hipsley, C.A., Jacobs, L., Makovicky, P.J., Müller, J., Smith, K.T., Theodor, J.M., Warnock, R.C.M., and Benton, M.J. 2012. Best practices for justifying fossil calibrations. Systematic Biology, 61:346-359.
Pascual, R., Archer, M., Ortiz-Jaureguizar, E., Prado, J.L., Godthelp, H., and Hand, S.J. 1992. First discovery of monotremes in South America. Nature, 356:704-705.
Phillips, M.J. and Pratt, R.C. 2008. Family-level relationships among the Australasian marsupial “herbivores” (Diprotodontia : Koala, wombats, kangaroos and possums). Molecular Phylogenetics and Evolution, 46:594-605.
Phillips, M.J., Bennett, T.H., and Lee, M.S.Y. 2009. Molecules, morphology, and ecology indicate a recent, amphibious ancestry for echidnas. Proceedings of the National Academy of Sciences USA, 106:17089-17094.
Phillips, M.J., Gibb, G.C., Crimp, E.A., and Penny, D. 2010. Tinamous and moa flock together: mitochondrial genome sequence analysis reveals independent losses of flight among ratites. Systematic Biology, 59:90-107.
Phillips, M.J., Haouchar, D., Pratt, R.C., Gibb, G.C., and Bunce, M. 2013. Inferring kangaroo phylogeny from incongruent nuclear and mitochondrial genes. PLoS ONE, 8:e57745.
Poux, C., Chevret, P., Huchon, D., de Jong, W.W., and Douzery, E.J.P. 2006. Arrival and diversification of caviomorph rodents and platyrrhine primates in South America. Systematic Biology, 55:228-244.
Prideaux, G.J. 2004. Systematics and evolution of the sthenurine kangaroos. University of California Publications in Geological Sciences, 146: i-xvii, 1-623.
Prideaux, G.J. and Tedford, R.H. 2012. Tjukuru wellsi, gen. et sp. nov., a lagostrophine kangaroo (Diprotodontia, Macropodidae) from the Pliocene (Tirarian) of northern South Australia. Journal of Vertebrate Paleontology, 32:717-721.
Prideaux, G.J. and Warburton, N.M. 2010. An osteology-based appraisal of the phylogeny and evolution of kangaroos and wallabies (Macropodidae: Marsupialia). Zoological Journal of the Linnean Society, 159:954-987.
Pridmore, P.A., Rich, T.H., Vickers-Rich, P., and Gambaryan, P.P. 2005. A tachyglossid-like humerus from the Early Cretaceous of south-eastern Australia. Journal of Mammalian Evolution, 12:359-378.
Pyron, R.A. 2010. A likelihood method for assessing molecular divergence time estimates and the placement of fossil calibrations. Systematic Biology, 59:185-194.
Rannala, B. and Yang, Z. 2007. Inferring speciation times under an episodic molecular clock. Systematic Biology, 56:453-466.
Ravel, A., Marivaux, L., Tabuce, R., Adaci, M., Mahboubi, M., Mebrouk, F., and Bensalah, M. 2011. The oldest African bat from the early Eocene of El Kohol (Algeria). Naturwissenschaften, 98:397-405.
Reisz, R.R. and Müller, J. 2004. Molecular timescales and the fossil record: a paleontological perspective. Trends in Genetics, 20:237-241.
Rich, T.H. and Vickers-Rich, P. 2003. Diversity of Early Cretaceous mammals from Victoria, Australia. Bulletin of the American Museum of Natural History, 285:36-53.
Rowe, T., Rich, T.H., Vickers-Rich, P., Springer, M., and Woodburne, M.O. 2008. The oldest platypus and its bearing on divergence timing of the platypus and echidna clades. Proceedings of the National Academy of Sciences USA, 105:1238-1242.
Sigé, B. 1991. Rhinolophoidea et Vespertilionoidea (Chiroptera) de Chambi (Eocene inferieur de Tunisie). Aspects biostratigraphique, biogeographique et paléoécologique de l’origine des chiropteres modernes. Neues Jahrbuch für Geologie und Paläontologie, 182:355-376.
Simmons, N.B. and Geisler, J.H. 1998. Phylogenetic relationships of Icaronycteris, Archaeonycteris, Hassianycteris, and Palaeochiropteryx to extant bat lineages, with comments on the evolution of echolocation and foraging strategies in Microchiroptera. Bulletin of the American Museum of Natural History, 235:1-182.
Simmons, N.B., Seymour, K.L., Habersetzer, J., and Gunnell, G.F. 2008. Primitive Early Eocene bat from Wyoming and the evolution of flight and echolocation. Nature, 451:818-821.
Smith, T., Rana, R.S., Missiaen, P., Rose, K.D., Sahni, A., Singh, A., Singh, H., and Singh, L. 2007. High bat (Chiroptera) diversity in the Early Eocene of India. Naturwissenschaften, 94:1003-1009.
Springer, M.S., Murphy, W.J., Eizrik, E., and O’Brien, S.J. 2003. Placental mammal diversification and the Cretaceous-Tertiary boundary. Proceedings of the National Academy of Sciences USA, 100:1056-1061.
Steiper, M.E. and Seiffert, E.R. 2012. Evidence for a convergent slowdown in primate molecular rates and its implications for the timing of early primate evolution. Proceedings of the National Academy of Sciences USA, 109:6006-6011.
Storch, G., Sigé, B., and Habersetzer, J. 2002. Tachypteron franzeni n. gen., n. sp., earliest emballonurid bat from the Middle Eocene of Messel (Mammalia, Chiroptera). Paläontologische Zeitschrift, 76:189-199.
Swofford, D.L. 2002. PAUP*: Phylogenetic Analysis Using Parsimony (* and other methods), version 4.0b10. Sinauer Associates Inc., Sunderland, MA.
Tabuce, R., Antunes, M.T., and Sigé, B. 2012. A new primitive bat from the earliest Eocene of Europe. Journal of Vertebrate Paleontology, 29:627-630.
Teeling, E.C., Scally, M., Kao, D.J., Romagnoli, M.L., Springer, M.S., and Stanhope, M.J. 2000. Molecular evidence regarding the origin of echolocation and flight in bats. Nature, 403:188-192.
Teeling, E.C., Springer, M., Madsen, O., Bates, P., O’Brien, S.J., and Murphy, W.J. 2005. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science, 307:580-584.
Travouillon, K.J., Archer, M., Hand, S.J., and Godthelp, H. 2006. Multivariate analyses of Cenozoic mammalian faunas from Riversleigh, northwestern Queensland. Alcheringa, 30 S1:323-349.
van Rheede, T., Bastiaans, T., Boone, D.N., Hedges, S.B., de Jong, W.W., and Madsen, O. 2006. The platypus is in its place: Nuclear genes and indels confirm the sister group relation of monotremes and therians. Molecular Biology and Evolution, 23:587-597.
Vucetich, M.G. and Kramarz, A.G. 2003. New Miocene rodents from Patagonia *Argentina) and their bearing on the early radiation of the octodontoids (Hystricognathi). Journal of Vertebrate Paleontology, 23:435-444.
Vucetich, M.G., Vieytes, E.C., Pezez, M.E., and Carlini, A.A. 2010. The rodents from La Cantera and the early evolution of caviomorphs, p. 193-205. In Madden, R.H., Carlini, A.A., Vucetich, M.G., and Kay, R.F. (eds.), The Paleontology of Gran Barranca: Evolution and Environmental Change Through the Middle Cenozoic of Patagonia. Cambridge University Press, Cambridge.
Waddell, P.J. 2008. Fit of fossils and mammalian molecular trees: dating inconsistencies revisited. arXiv:0812.5114.
Warnock, R.C.M., Yang, Z., and Donoghue, P.C.J. 2012. Exploring uncertainty in the calibration of the molecular clock. Biology Letters, 8:156-159.
Warren, W.C., Hillier, L.W., Graves, J.A.M., Birney, E., Ponting, C.P., Grützner, F., Belov, K., Miller, W., Clarke, L., Chinwalla, A.T., Yang, S-P., Heger, A., Locke, D.P., Miethke, P., Waters, P.D., Veyrunes, F., Fulton, L., Fulton, B., Graves, T., Wallis, J., Puente, X.S., López-Otín, C., Gonzalo, R., Ordóñez, E.E.E., Lin, C., Ze, C., Deakin, J.E., Alsop, A., Thompson, K., Kirby, P., Papenfuss, A.T., Wakefield, M.J., Olender, T., Lancet, D., Huttley, G.A., Smit, A.F.A., Pask, A., Temple-Smith, P., Batzer, M.A., Walker, J.A., Konkel, M.K., Harris, R.S., Whittington, C.M., Wong, E.S.W., Gemmell, N.J., Buschiazzo, E., Vargas Jentzsch, I.M., Merkel, A., Schmitz, J., Zemann, A., Churakov, G., Kriegs, J.O., Brosius, J., Murchison, E.P., Sachidanandam, R., Smith, C., Hannon, G.J., Tsend-Ayush, E., McMillan, D., Attenborough, R., Rens, W., Ferguson-Smith, M., Lefèvre, C.M., Sharp, J.A., Nicholas, K.R., Ray, D.A., Kube, M., Reinhardt, R., Pringle, T.H., Taylor, J., Jones, R.C., Nixon, B., Dacheux, J-L., Niwa, H., Sekita, Y., Huang, X., Stark, A., Kheradpour, P., Kellis, M., Flicek, P., Chen, Y., Webber, C., Hardison, R., Nelson, J., Hallsworth-Pepin, K., Delehaunty, K., Markovic, C., Minx, P., Feng, Y., Kremitzki, C., Mitreva, M., Glasscock, J., Wylie, T., Wohldmann, P., Thiru, P., Nhan, M.N., Pohl, C.S., Smith, S.M., Hou, S., Renfree, M.B., Mardis, E.R., and Wilson, R.K. 2008. Genome analysis of the platypus reveals unique signatures of evolution. Nature, 453:175-183.
Welch, J.J. and Bromham, L. 2005. Molecular dating when rates vary. Trends in Ecology and Evolution, 20:320-327.
Welch, J.J., Bininda-Emonds, O.R., and Bromham, L.B. 2008. Correlates of substitution rate variation in mammalian protein-coding sequences. BMC Evolutionary Biology, 8:53.
Westerman, M., Burk, A., Amrine, H.M., Prideaux, G.J., Case, J.A., and Springer, M.S. 2002. Molecular evidence for the last survivor of an ancient kangaroo lineage. Journal of Mammalian Evolution, 9: 209-223.
Woodburne, M.O., Tedford, R.H., Archer, M., Turnbull, W.D., Plane, M.D., and Lundelius, E.L. 1985. Biochronology of the continental mammal record of Australia and New Guinea. Special Publication, South Australia Department of Mines and Energy, 5:347-363.
Woodburne, M.O., Macfadden, B.J., Case, J.A., Springer, M.S., Pledge, N.S., Power, J.D., Woodburne, J.M., and Springer, K.B. 1993. Land mammal biostratigraphy and magnetostratigraphy of the Etadunna Formation (late Oligocene) of South Australia. Journal of Vertebrate Paleontology, 13:483-515.
Yang, Z. 2007. PAML 4: a program package for phylogenetic analysis by maximum likelihood. Molecular Biology and Evolution, 24:1586-1591.
Yang, Z. and Rannala, B. 2006. Bayesian estimation of species divergence times under a molecular clock using multiple fossil calibrations with soft bounds. Molecular Biology and Evolution, 23:212-226.

