 Sexual discrimination at work: Spinicaudatan ‘Clam Shrimp’ (Crustacea: Branchiopoda) as a model organism for the study of sexual system evolution
Sexual discrimination at work: Spinicaudatan ‘Clam Shrimp’ (Crustacea: Branchiopoda) as a model organism for the study of sexual system evolution
Article number: 15.2.20A
https://doi.org/10.26879/307
Copyright Palaeontological Association, June 2012
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 11 October 2011. Acceptance: 23 May 2012
{flike id=262}
ABSTRACT
Biological interactions are rarely preserved in the fossil record and where they do occur, are often difficult to discern. Therefore, the evolution of sexual systems over geologic time in animals has been difficult to investigate. The reproductively labile Spinicaudata ('clam shrimp') are a model clade for the study of sexual systems, containing dioecious (males and females), androdioecious (males and hermaphrodites) and self-fertilizing hermaphrodites. Herein we present a methodology in which mating systems can be inferred by the quantification of carapace shape differences attributable to sexual dimorphism in fossil specimens. We develop our methodology by comparing the carapaces of six species from two families of extant Spinicaudatans using eigenshape analyses. Sexual dimorphism was successfully quantified using morphometric techniques combined with discriminant analyses, correctly identifying males and females/hermaphrodites 92% of the time in extant taxa. Thirty-four specimens of the Jurassic clam shrimp Carapacetheria disgragaris were analyzed utilizing the methods developed with extant species. From these fossil data, we were able to detect two distinct carapace shapes and assign 100% of individuals to either shape. The mean carapace shapes of the fossil specimens fit well with the average outlines for males and females in the extant species, enabling us to calculate a sex ratio of 51:49 males:females and thereby assign the sexual system of dioecy. This study begins to successfully utilize the fossil record of the Spinicaudata to elucidate ancient sexual systems, which will likely have far reaching implications for our understanding of the evolutionary dynamics of sexual systems over geologic timescales.
T.I. Astrop. Program in Integrated Bioscience, Department of Biology, The University of Akron, Akron, Ohio, USA. tia10@zips.uakron.edu
L.E. Park. Department of Geology and Environmental Sciences, Environmental Scanning Electron Microscope Laboratory, University of Akron, Akron, Ohio 44325. lepark@uakron.edu
B. Brown. Department of Biology, The University of Akron, Akron, Ohio 44325-3908, USA. bpb8@zips.uakron.edu
S.C. Weeks. Program in Integrated Biosciences, Department of Biology, The University of Akron, Akron, Ohio 44325-3908 USA. scw@uakron.edu
Keywords: eigenshape; morphometrics; carapace; Conchostraca; Ostracod
Final citation: Astrop, T.I. , Park, L.E., Brown, B., and Weeks, S.C. 2012. Sexual discrimination at work: Spinicaudatan ‘Clam Shrimp’ (Crustacea: Branchiopoda) as a model organism for the study of sexual system evolution. Palaeontologia Electronica Vol. 15, Issue 2;20A,15p;
palaeo-electronica.org/content/2012-issue-2-articles/262-sexual-systems-in-fossils
INTRODUCTION
Within evolutionary biology, one of the most perplexing questions is: why are there so many methods of reproduction? (Williams, 1975; Bell, 1982; Barrett, 2002; Kondrashov, 1993; Charlesworth, 2006; Jarne and Auld, 2006; Vallejo-Marin et al., 2010). The more common reproductive forms include asexuality (clonal females only), hermaphroditism (all individuals are both male and female) and dioecy (separate males and females). The list of less common forms is enormous, including cyclic parthenogenesis (asexuality punctuated by periods of dioecy), sequential hermaphroditism (one sex first then switching to the other sex), gynodioecy (females and hermaphrodites) and androdioecy (males and hermaphrodites), to name a few.
One of the most frustrating aspects of studying the evolution of reproductive systems is that we have been unable, to date, to utilize information locked within the fossil record to assess breeding system evolution in deep time. While the fossil record provides us with information on an organism's living environment, as well as some aspects of its ecology, the preservation of biological interactions (sex, feeding, symbiosis) in the fossil record is an exceedingly rare phenomenon. When evidence of these interactions is present within the fossil record, they almost exclusively are restricted to examples of epibiosis (Brande, 1982; Fernandez-Leborans, 2010), predator-prey interactions (Kelley and Hansen, 2003) or plant-insect relationships (Labandeira and Sepkoski, 1993; Pott et al., 2008). Although the importance to evolutionary biology of understanding the long-term evolution of reproductive systems cannot be overstated, information concerning the mating systems of extinct taxa remain almost completely unknown (Sassaman, 1995) except for rare instances (Klimov and Sidorchuk, 2011).
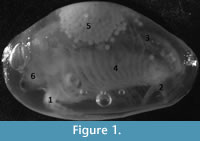 "Clam shrimp" (Spinicaudata) are a group of primitive crustaceans within the Class Branchiopoda; their fossil record is very good and extant taxa exhibit a diverse array of reproductive systems. They are identifiable as adults due to their distinctive bivalved carapace from which they derive their common name (Figure 1). Extant clam shrimp are geographically widespread, occurring on every continent except Antarctica (although there is an excellent Antarctic fossil record for these crustaceans; Tasch, 1987) with a particularly good freshwater fossil record extending back 400 Ma to the Devonian period (Novojilov, 1961; Tasch, 1969; Shen, 1978). Restricted to freshwater, the majority of modern taxa inhabit lakes, ponds and ephemeral water bodies (Weeks et al., 2009; Dumont and Negrea, 2002). Like other branchiopods, they exhibit naupliar larval stages and have a variable number of body segments (Frank, 1988).
"Clam shrimp" (Spinicaudata) are a group of primitive crustaceans within the Class Branchiopoda; their fossil record is very good and extant taxa exhibit a diverse array of reproductive systems. They are identifiable as adults due to their distinctive bivalved carapace from which they derive their common name (Figure 1). Extant clam shrimp are geographically widespread, occurring on every continent except Antarctica (although there is an excellent Antarctic fossil record for these crustaceans; Tasch, 1987) with a particularly good freshwater fossil record extending back 400 Ma to the Devonian period (Novojilov, 1961; Tasch, 1969; Shen, 1978). Restricted to freshwater, the majority of modern taxa inhabit lakes, ponds and ephemeral water bodies (Weeks et al., 2009; Dumont and Negrea, 2002). Like other branchiopods, they exhibit naupliar larval stages and have a variable number of body segments (Frank, 1988).
The clam shrimp are a problematic group, taxonomically. Traditionally referred to as the 'Conchostraca' containing the orders Spinicaudata, Laevicaudata and Cyclestheridae, the group is now deemed paraphyletic with the Cyclestheridae showing affiliations with the closely related Cladocera (proposed Cladoceramorpha; Olesen, 2007). The comprehensive work on crustacean taxonomy by Martin and Davis (2001) suggests that the Conchostraca should be discarded and that the group, including the clam shrimp, Notostraca (tadpole shrimp) and Anostraca (fairy shrimp) be referred to as Phyllopoda (Phyllopoda + Cladoceramorpha being the Branchiopoda).
The Spinicaudata are of particular interest as they are diverse, geographically widespread and have an excellent fossil record, as noted above. The Order Spinicaudata comprises three extant families: the Limnadiidae, Cyzidae and Leptestheriidae. The Leptestheriidae are not as diverse or abundant as either the Cyzidae or Limnadiidae and are definitely not as well represented in the fossil record.
The Spinicaudata contain species that exhibit dioecious, androdioecious and hermaphroditic mating systems (Sassaman, 1995; Weeks et al., 2009; Brantner, 2011) spread across the three constituent families, providing an excellent opportunity to study the evolution of sexual systems over geologic time. Many authors have proposed that androdioecy is a relatively unstable system that should not persist over long periods of time (Lloyd, 1975; Charlesworth, 1984; Pannell, 1997; Wolf and Takebayashi, 2004) and is transitional between dioecy and hermaphroditism. The widespread occurrence of androdioecy within the family Limnadiidae (Weeks et al., 2006, 2009), along with a hypothesized age of the androdioecious genus Eulimnadia at between 180 (Weeks et al., 2006) – 45 mya (Tasch, 1969), would seem to be evidence to the contrary. It is also important to note that the closely related Notostraca also exhibit dioecy, androdioecy and hermaphroditism (Sassaman, 1989, 1991; Zierold et al., 2007), and that the divergence of these two groups is estimated to have occurred approximately 330 Ma (Regier et al., 2005). Thus, these branchiopod crustaceans provide an interesting set of taxa with clearly labile breeding systems (Korpolainen, 2007) and a good fossil record, which could prove exceptionally useful for the study of breeding system evolution.
A likely candidate for assessing fossil reproductive system is the sex ratio, which has been described as the 'only information concerning reproductive systems able to enter the fossil record' in fossil ostracodes (Abe, 1990). Sex ratio information has been successfully used to determine reproductive systems in extant clam shrimp (Weeks et al., 2008). Ongoing taphonomic studies also indicate no bias in preservation potential between sexes; meaning extension of this method into the fossil record should yield reliable information (Astrop and Hegna, unpublished data). Sex ratio is thus a promising trait for assessing fossil reproductive modes. Many palaeontological works have suggested that discrete variation in clam shrimp carapace shape is indeed evidence of sexual dimorphism (Table 1). The bimodal distribution of these data in many cases, and the similar number of growth rings in the two morphs, supports the idea that these two categories represent sexual dimorphism rather than variation attributable to differences in ontogenetic stages of development (Brown and Astrop, unpublished data).
The carapace of the Spinicaudata has not been subjected to a contemporary, comprehensive study and observations that have been made are often incomplete or conflicting (Martens, 1983, 1985; Rieder et al., 1984; Wang, 1989; Chen and Hudson, 1991; Olempska, 2004). This neglect has resulted in a poor understanding of a unique anatomical feature. What is known is that the carapace is constructed via multiple lamellae from successive growth stages, and that these lamellae are found in three discrete layers: a thin epicuticle on top of a thicker exocuticle and protocuticle (Martin, 1992). The carapace itself is weakly biomineralized with a chitin and calcium-phosphate complex (Stigall and Hartman, 2008), the structural details of which are poorly known (Tasch, 1969). The Spinicaudatan carapace is unique in that partial molting during ecdysis produces discrete growth lines that record the ontogenetic history of each individual. Although the growth lines represent discrete ontogenetic stages, the formation of the lines appears to be influenced by their growth environment rather than a consistent set of lines set down in a specific time sequence (Bishop, 1967). The exact mechanisms involved in the partial molting in clam shrimp are not understood but are thought to differ from the molt retention seen in some ostracodes, such as the Cytherellidae (Jones, 2003) and Eridostraca (Olempska, 2011). A dedicated contemporary investigation of the Spinicaudatan carapace is needed in order to understand the structure and function of this important diagnostic feature.
The quantification of carapace shape using contemporary morphometric techniques, as implemented in this study, builds on historical suggestions of preserved dimorphism in clam shrimp in order to diagnose both sex ratio and by extension the reproductive system. A more detailed examination of carapaces in relation to sexual dimorphism allows a new approach to fossil "Conchostracan" studies and the interpretation of mating systems in fossil species (via sex ratio comparisons and/or generic identities – see below). This new approach is of great importance for studies of breeding system evolution.
METHODS
The Morphometric 'revolution' (Rohlf and Marcus, 1993) and the subsequent development of modern, computer-based techniques (see Adams et al., 2004; Laffont et al., 2011 for reviews) has enabled the investigation of morphological variability by quantifying features traditionally assigned qualitative values based on primary homology assessments between and within taxonomic groups (de Pinna, 1991).
Eigenshape analysis (MacLeod, 1999) is a powerful contemporary technique employed in the ordination of curved outlines (Lohmann, 1983; Lohmann and Schweitzer, 1990). It has been effectively applied in previous biological, paleontological and paleoenvironmental analyses (MacLeod, 2002; Krieger et al., 2007; Astrop, 2011) and has also been successful in detecting sexual dimorphism in fossil Ostracoda (Elewa, 2003). Standard eigenshape analysis is suitable for the exploration of variation in the Spinicaudatan carapace because multiple, discrete, homologous features (i.e., landmarks in geometric morphometric analyses; Bookstein, 1986) are absent. The changes in carapace shape (associated with the maintenance of a brood chamber, development of claspers and other anatomical differences between the sexes) are not only suspected to be significant, but in light of previous applications, likely to be detected through analysis of outlines. Changes in the shape of carapace outlines are described as changes to individual margins. The location of margins and major anatomical features of a clam shrimp can be seen in Figure 1.
Eigenshape analyses (sensu MacLeod, 1999) operate via the conversion of the digitized outline of an individual specimen into equidistant, Cartesian (x-y) coordinates. The digitized co-ordinates are then transformed into a shape function as angular deviations (phi function: φ; Zahn and Roskies, 1972) from the previous step (coordinate) in order to describe the shape of the curve (sequestering size from the analysis). This description is derived from a set of empirical, orthogonal shape functions via an eigenfunction analysis of a matrix of correlations between shapes. Eigenshape 'scores' can be then used to project individual specimens into a multi-dimensional morphospace that allows the visualization of individual vectors of shape change and highlight whether particular vectors of deviation from the 'mean shape' are characteristic of a particular group.
This study utilizes three independent datasets. The first consists of four species from two genera within the family Limnadiidae: the dioecious species Limnadia badia and Limnadia stanleyana, along with the androdioecious species Eulimnadia dali, and Eulimnadia texana. Fifteen males and 15 females/hermaphrodites were selected from populations reared in the laboratory, producing a dataset of n=120. This data set was designed to investigate the strength and extent of any dimorphic 'signal' in carapace shape. A second, smaller dataset was assembled to assess the phylogenetic consistency of shape dimorphism within the group by using the Australian endemic species Limnadopsis occidentalis and a representative of the family Cyzidae, Cyzicus mexicanus.
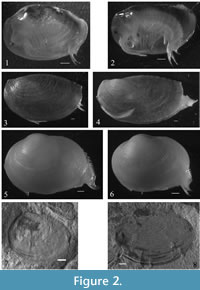 In order to apply the above protocol to extinct taxa, it was important to develop a methodology to validate the existence of 'morphotypes' within a fossil sample. Therefore a third dataset of the Lower Jurassic (Ferrar Group, Toarcian Stage), Estheriid Carapacestheria disgragaris (Tasch 1987), was assembled. Fossil specimens were originally collected from the Shackleton Glacier region, Carapace Nunatak, South Victoria Land, Antarctica (Stigall and Hartman, 2008). The sedimentary interbeds of Kirkpatrick Basalt deposits from which these fossils are derived represent high palaeolatitude lakes where algal biofilms enabled the preservation of weakly biomineralized lake flora and fauna (Stigall and Hartman, 2008) (For detailed geologic, stratigraphic and palaeontological information see Stigall and Hartman, 2008; Stigall-Rode et al., 2005; Tasch, 1969; Tasch. 1987). All specimens were imaged using a Jenoptik Progres C5 digital camera attached to a binocular microscope and desktop computer with Jenoptik Capture Pro software. The outlines of individual carapaces were digitized using tpsDig v2.10 (Rohlf, 2008) and then subjected to an eigenshape analysis. Digitized outline data were saved as .tps files which were then processed using modified versions of the Eigenshape v2.6 and Guide to models v0.7 Mathematica notebooks available via the morphotools site. The analysis interpolates and standardizes the raw Cartesian data before performing a singular value decomposition to produce eigenvalues, eigenscores and eigenshapes that describe variation of shape within the dataset. The eigenshapes produced by the analysis describe two-dimensional axes of shape change that can be used to construct morphospaces that specimens may be projected into, allowing trends in shape variation to be observed. All specimens used in this study are adult, exhibiting multiple growth lines, claspers (in males) and egg clutches in the brood chamber (in females/hermaphrodites) (Figure 2).
In order to apply the above protocol to extinct taxa, it was important to develop a methodology to validate the existence of 'morphotypes' within a fossil sample. Therefore a third dataset of the Lower Jurassic (Ferrar Group, Toarcian Stage), Estheriid Carapacestheria disgragaris (Tasch 1987), was assembled. Fossil specimens were originally collected from the Shackleton Glacier region, Carapace Nunatak, South Victoria Land, Antarctica (Stigall and Hartman, 2008). The sedimentary interbeds of Kirkpatrick Basalt deposits from which these fossils are derived represent high palaeolatitude lakes where algal biofilms enabled the preservation of weakly biomineralized lake flora and fauna (Stigall and Hartman, 2008) (For detailed geologic, stratigraphic and palaeontological information see Stigall and Hartman, 2008; Stigall-Rode et al., 2005; Tasch, 1969; Tasch. 1987). All specimens were imaged using a Jenoptik Progres C5 digital camera attached to a binocular microscope and desktop computer with Jenoptik Capture Pro software. The outlines of individual carapaces were digitized using tpsDig v2.10 (Rohlf, 2008) and then subjected to an eigenshape analysis. Digitized outline data were saved as .tps files which were then processed using modified versions of the Eigenshape v2.6 and Guide to models v0.7 Mathematica notebooks available via the morphotools site. The analysis interpolates and standardizes the raw Cartesian data before performing a singular value decomposition to produce eigenvalues, eigenscores and eigenshapes that describe variation of shape within the dataset. The eigenshapes produced by the analysis describe two-dimensional axes of shape change that can be used to construct morphospaces that specimens may be projected into, allowing trends in shape variation to be observed. All specimens used in this study are adult, exhibiting multiple growth lines, claspers (in males) and egg clutches in the brood chamber (in females/hermaphrodites) (Figure 2).
A total of 120 adult specimens were imaged (left valve only) to create the first dataset. Populations of L.badia, L.stanleyana, E.texana and E.dahli were reared in the laboratory following the protocols outlined in Weeks and Zucker (1999). Of these taxa, 15 of each sex (male/female for Limnadia, male/hermaphrodite for Eulimnadia) were processed. Preserved specimens of L. occidentals and C. mexicanus were used to create the smaller, second dataset, while 34 well preserved specimens of C. disgragaris from palaeontological collections at Ohio University and the Natural History Museum, London (Figure 2.7, 2.8) were identified to create the third, fossil dataset. All specimens were imaged facing left and outlines digitized to 500 equidistant points beginning from the junction of the anterior and dorsal margins of the carapace. A composite .tps file of outline data for all 120 specimens was constructed using the free tpsUtil v1.44 (Rohlf, 2009) software. These data were then subjected to a standard eigenshape analysis. The outlines were treated as closed curves and were mean centered and standardized to remove size, scale and rotation from the analysis (see Slice, 2005). All statistical analyses (ANOVA, Clustering, Discriminant) where performed using PAST v2.14 (Hammer et al., 2001).
RESULTS
 Standard eigenshape analysis of all 120 outlines produced 119 eigenshapes, the first four of which account for ~30% of the observed variance captured by the analysis (Figure 3).
Standard eigenshape analysis of all 120 outlines produced 119 eigenshapes, the first four of which account for ~30% of the observed variance captured by the analysis (Figure 3).
By observing the trends in shape change captured by each eigenshape axis, it is possible to describe corresponding biological change seen in the extant clam shrimp datasets. The first eigenshape axis (Figure 3) exhibits considerable change in the anterior portion of the dorsal margin which corresponds to the presence/absence of the brood chamber in egg-bearing individuals and males, respectively. This axis also captures change in angularity of the junction between the posterior and dorsal margins. The second eigenshape axis is related to the relative position of the brood chamber. Eigenshape axis three captures change in the posterior and ventral margins while eigenshape four represents the 'roundedness' of the dorsal margin.
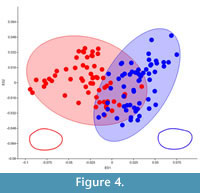 A plot of the first two eigenshapes clearly shows a divergent trend between the two sexes along either axis (Figure 4). Despite some clear overlap, the two groups are well defined when this dataset is assessed using a hierarchical clustering approach (Ward's method) (Figure 5). The distinction between the two sexes was highly significant (Table 2). The fact that the overlap on the first two eigenshape axes did not translate to higher levels of mis-scoring (Figure 5) and exceptionally high levels of distinction between the two sexes (Table 2) underscores the importance of the second two eigenshapes at further distinguishing the two sexes. A MANOVA of this dataset using the first four eigenshapes reports a significant difference between scores for both groups.
A plot of the first two eigenshapes clearly shows a divergent trend between the two sexes along either axis (Figure 4). Despite some clear overlap, the two groups are well defined when this dataset is assessed using a hierarchical clustering approach (Ward's method) (Figure 5). The distinction between the two sexes was highly significant (Table 2). The fact that the overlap on the first two eigenshape axes did not translate to higher levels of mis-scoring (Figure 5) and exceptionally high levels of distinction between the two sexes (Table 2) underscores the importance of the second two eigenshapes at further distinguishing the two sexes. A MANOVA of this dataset using the first four eigenshapes reports a significant difference between scores for both groups.
The raw eigenscores for the first four eigenshapes produced were utilized in discriminant analyses that successfully identified 96% of all specimens based on sex (Table 2). The five misclassified individuals are likely outliers in terms of erroneous shape capture, mis-identifications or possibly juveniles. Mis-classified individuals occurred in both genera.
 Carapace shape can also be used to discriminate between taxa. This has been shown in previous studies using combinations of linear measurements and area-estimations (e.g., Zierold, 2007; Hethke al., 2010) as well as landmark-based methods (Stoyan et al., 1994). However, these studies did not take into account carapace shape dimorphism. Using the eigenshape method described here, it is possible to correctly identify species for 92.3% (112 of 120 properly classified) of the measured individuals.
Carapace shape can also be used to discriminate between taxa. This has been shown in previous studies using combinations of linear measurements and area-estimations (e.g., Zierold, 2007; Hethke al., 2010) as well as landmark-based methods (Stoyan et al., 1994). However, these studies did not take into account carapace shape dimorphism. Using the eigenshape method described here, it is possible to correctly identify species for 92.3% (112 of 120 properly classified) of the measured individuals.
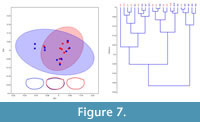
Using these same techniques to analyze smaller datasets (n= 20-25) of the phylogenetically disparate clam shrimp Cyzicus mexicanus (Figure 2.5, 2.6) and morphologically disparate Limnadopsis occidentalis (Figure 2.3, 2.4), sexes were also successfully discriminated 76% and 73% of the time, respectively (Table 2). MANOVAs performed on the first four eigenshape scores of either data sets (Table 2) revealed that the between-group difference in carapace shape is not significant for Cyzicus (p=0.151; Figure 6)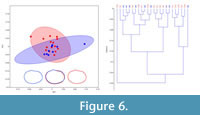 and weakly significant for Limnadopsis (p=0.0497876; Figure 7), in these samples. Although these data exhibit a less clear distinction between sexes (Figure 6 and Figure 7), than the first data set (Figures 4 and 5), it is likely that the smaller sample size and more subtle dimorphism influenced the ability of the analyses to both capture the shape of and discern the sexes present, highlighting an important consideration for future work.
and weakly significant for Limnadopsis (p=0.0497876; Figure 7), in these samples. Although these data exhibit a less clear distinction between sexes (Figure 6 and Figure 7), than the first data set (Figures 4 and 5), it is likely that the smaller sample size and more subtle dimorphism influenced the ability of the analyses to both capture the shape of and discern the sexes present, highlighting an important consideration for future work.
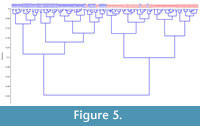
 Initial observations of C. disgragaris revealed two distinct carapace shapes: one elongate (Figure 2.8) and one more rounded (Figure 2.7). These shapes are reflected in both the outline of the carapace (Figure 8) and the growth lines preserved. Eigenshape analysis of the fossil dataset produced four major vectors of shape change that accounted for ~30% of the observed variance. Hierarchical cluster analysis of C. disgragaris using the first four eigenshapes produces two distinct groups (Figure 8, which were highly significantly different from one another (Table 2). These groups correspond to the morphotypes suspected as being different sexes. The branch lengths supporting these groups are slightly shorter than those for the extant dataset. This length difference is likely due to increased noise in the fossil dataset attributable to taphonomic sources.
Initial observations of C. disgragaris revealed two distinct carapace shapes: one elongate (Figure 2.8) and one more rounded (Figure 2.7). These shapes are reflected in both the outline of the carapace (Figure 8) and the growth lines preserved. Eigenshape analysis of the fossil dataset produced four major vectors of shape change that accounted for ~30% of the observed variance. Hierarchical cluster analysis of C. disgragaris using the first four eigenshapes produces two distinct groups (Figure 8, which were highly significantly different from one another (Table 2). These groups correspond to the morphotypes suspected as being different sexes. The branch lengths supporting these groups are slightly shorter than those for the extant dataset. This length difference is likely due to increased noise in the fossil dataset attributable to taphonomic sources.
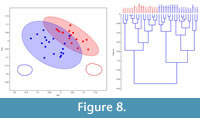 In order to test the initial observations of two discreet morphotypes within C. disgragaris, a hierarchical cluster analysis was performed (using the first four eigenshapes and applying Ward's clustering method). The hierarchical cluster seen in Figure 8 shows branch lengths based on distance, clearly illustrating the existence of two distinct groups.
In order to test the initial observations of two discreet morphotypes within C. disgragaris, a hierarchical cluster analysis was performed (using the first four eigenshapes and applying Ward's clustering method). The hierarchical cluster seen in Figure 8 shows branch lengths based on distance, clearly illustrating the existence of two distinct groups.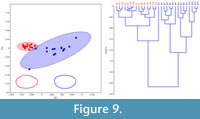 Discriminant analysis of these two groups (proposed by the hierarchical cluster analysis approach) using the first four eigenshapes, resulted in 97% success of classifying individual fossils into these two groups (Table 2).
Discriminant analysis of these two groups (proposed by the hierarchical cluster analysis approach) using the first four eigenshapes, resulted in 97% success of classifying individual fossils into these two groups (Table 2).
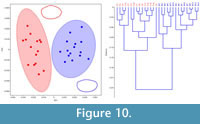
In order to test the effectiveness of the hierarchical clustering approach, extant androdioecious E. texana (Figure 9) and dioecious L. badia (Figure 10), where the sex of individuals is known a priori, were tested using the same protocol described above. Hierarchical clustering produced two-group assignments that successfully assigned sex 97 or 100% of the time, respectively. MANOVAs using the first four eigenshapes of either data set reveal that between group differences are highly significant (Table 2).
DISCUSSION
 The long-term evolutionary forces driving the diversity and maintenance of breeding systems have traditionally been addressed by theory alone (Williams, 1975; Kondrashov, 1993; Charnov, 1982; Charlesworth, 1984, 2006; Nunney, 1989; Pannell, 2009) due to the lack of detailed historical information and absence of breeding system assignments in the fossil record. This study successfully quantifies and interprets sex-specific carapace morphology for the first time in fossil Spinicaudata and allows informative biological inferences to be made from fossil taxa.
The long-term evolutionary forces driving the diversity and maintenance of breeding systems have traditionally been addressed by theory alone (Williams, 1975; Kondrashov, 1993; Charnov, 1982; Charlesworth, 1984, 2006; Nunney, 1989; Pannell, 2009) due to the lack of detailed historical information and absence of breeding system assignments in the fossil record. This study successfully quantifies and interprets sex-specific carapace morphology for the first time in fossil Spinicaudata and allows informative biological inferences to be made from fossil taxa.
We have shown that carapace shape does indeed carry a strong, sex-based signal, as had been previously suggested but not rigorously tested (Bock, 1953; Oleynikov, 1969; Tasch, 1969; Tintori and Brambilla, 1991; Gallego, 2005, 2010; Li et al., 2007, 2008, 2009a, 2009b, 2010; Zierold, 2007). Traditional attempts at utilizing morphometric measurements to quantify carapace shape via linear measurements (Oleynikov, 1969; Tintori and Brambilla, 1991; Machado et al., 1999; Jones and Chen, 2000; Babcock et al., 2002; Zierold, 2007) and, in one case, landmark-based methods (Stoyan et al. 1994), have produced mixed results that likely do not best represent the gross dimorphic carapace morphology. Although dimorphism had been previously noted in C. disgragaris by Tasch (1987), Shen (1994) deemed dimorphism "more difficult to determine" due to the distorting effects associated with fossilization despite being able to provide some distinction between proposed morphotypes (similar to those described via eigenshape analysis in this study) using linear height/length measurements of the valves. Although some degree of tectonism in the form of folding is reported in the Kirkpatrick Basalt from where specimens of C. disgragaris were collected (Stigall and Hartman, 2008), the specimens selected for this study showed no signs of warping or distortion of any kind. Ongoing taphonomic studies (Astrop and Hegna, unpublished data) have revealed that the Spinicaudatan carapace is resistant to extensive aqueous post-mortem transport. Also of import is that after deposition, remains of Spinicaudatans are surrounded by sediment and subsequently prevented from laterally expanding during compaction, as found by Briggs and Williams (1981).
The usefulness of the current approach is our ability to use these methods on both extant and fossil clam shrimp and underscores the ability of the detailed eigenshape methodology to correctly distinguish sexes in species where sex is known a priori. The observations that these methods clearly extract sexual type in extant species, the clear similarity of shape between the fossil and extant male/female (hermaphrodite) carapaces, as well as the strong signal of two morphotypes in the fossil data show these methods can be successfully used to assess individual sex in fossil clam shrimp carapaces. Using individual gender determination we can further distinguish population sex ratios in fossilized death assemblages.
The results from the extant taxa highlight that morphological dimorphism in the form of carapace shape occurs throughout the Spinicaudata. Differences in the mode of dimorphism between disparate taxa is likely the result of differing anatomical locations of the egg-clutch/brood chamber (dorsal in the Limnadiidae, lateral in the Cyzidae and Leptestheriidae), and possibly the size and position of claspers in males of all taxa. Upon application of the eigenshape method to a fossil taxon, it is clear that it is indeed possible to detect dimorphism in extinct clam shrimp and subsequently, it is reported here that the sex ratio between the two morphotypes of C. disgragarisis 51:49 male:female. Weeks et al. (2008, 2009) have noted that sex ratio can be used to assign breeding system in extant clam shrimp species: 0% male samples are self-fertilizing hermaphrodites, 20-30% males are androdioecious and 50+% males are dioecious. It is unlikely that the bimodal variance observed in both the clustering and scatter plot (Figure 8) is the result of ontogenetic variation as all specimens exhibit several growth lines, which do not occur in juveniles. It is also unlikely that the two 'morphotypes' are the result of the preservation of co-occurring species: most modern clam shrimp populations are monospecific, but where clam shrimp species do occur together they are morphologically and phylogenetically disparate (Orr and Briggs, 1999; Dumont and Negrea, 2002). Given that these two morphotypes are consistent with shape differences between the sexes observed in extant clam shrimp taxa (Figure 2), the morphotypes may be considered male and female (Figure 8; compare also with average shapes in Figure 9 and Figure 10). Thus, it is likely that C. disgragaris was a dioecious clam shrimp species.
In addition, it is also important to note that due to their thin, chitinous carapace and weakly sclerotized soft integument, clam shrimp may be susceptible to pre-depositional (transport, predation, desiccation) and post-depositional (decay, compaction) processes, and as such, these sources of variation may distort carapace shape in particular ways. In order to account for these sources of variation, a dedicated taphonomic study is currently underway to allow us to characterize how such variables influence the entry of clam shrimp into the fossil record and elucidate how to recognize and account for these sources of taphonomic variation.
The successful elucidation of sex-specific carapace shape, and by extension sex ratios, in this study shows that contemporary morphometric analyses of fossil clam shrimp are capable of providing the quantitative information required to assign breeding system to extinct clam shrimp. The extraction of breeding system from fossils can now allow tests of hypotheses concerning the long-term evolution and maintenance of breeding systems in these interesting, and reproductively labile, crustaceans.
ACKNOWLEDGMENT
We thank A. Stigall, T. Hegna, J. Brantner, A. Calabrese, L. Vaz Tassi and L. Roketenetz for their helpful advice, correspondence and material, the Natural History Museum, London for access to their collections, D. Polly, our handling editor, and two anonymous reviewers for their helpful suggestions. This research was funded in part by a Denton Belk Memorial Scholarship from the Crustacean Society to T.I. Astrop. L.E. Park (Boush) was supported by the National Science Foundation as part of the author's Individual Research Plan. She is currently a Program Officer within the Earth Science Division of the Geoscience Directorate.
REFERENCES
Abe, K. 1990. What the sex ratio tells us: A case from marine ostracods, p.175-185. In Ostracoda and Global Events, Whatley, C.R. and Maybury, C. (ed.), Chapman and Hall, Cambridge.
Adams, D.C., J.F. Rohlf, and Slice, D.E. 2004. Geometric morphometrics: Ten years of progress following the 'revolution'. Italian Journal of Zoology, 71:5-16.
Astrop, T.I. 2011. Phylogeny and evolution of Mecochiridae (Decapoda: Reptantia: Glypheoidea): an integrated morphometric and cladistic approach. Journal of Crustacean Biology, 31(1):114-125.
Babcock, L.E., Isbell, J.L., Miller, M.F. and Hasiotis, S.T. 2002. New Late Paleozoic Conchostracan (Crustacea: Branchiopoda) from the shackleton glacier area, Antarctica: age and paleoenvironmental implications. Journal of Paleontology, 76(1):70-75.
Barrett, S.C.H. 2002. The evolution of plant sexual diversity. Nature Reviews Genetics, 3:274-284.
Bell, G. 1982. The Masterpiece of Nature. University of California Press, Berkeley, CA.
Bishop, J.A. 1967. Seasonal occurrence of a branchiopod crustacean, Limnadia stanleyana King (Conchostraca) in Eastern Australia. Journal of Animal Ecology, 36(1):77-95
Bock, W. 1953. American Triassic Estherids. Journal of Paleontology, 27(1):62-76.
Bookstein, F. L. 1986. Size and shape spaces for landmark data in two dimensions. Statistical Science, 1(2):181-222.
Brande, S. 1982. Epibiont analysis of the fossil interactions among a benthic infaunal bivalve, a barnacle and a drilling gastropod. Journal of Paleontology, 56(5):1230-1234.
Brantner, J. 2011.Mating system inferences in representatives from two clam shrimp families (Limnadiidae and Cyzcidae) using histological and cellular observations. Thesis: Master of Science, University of Akron.
Briggs, D.E.G. and Williams, S.H. 1981.The restoration of flattened fossils. Lethaia 14, 157-164.
Charlesworth, D. 1984. Androdioecy and the evolution of dioecy. Biological Journal of the Linnean Society, 22:333-348.
Charlesworth, D. 2006. Evolution of plant breeding systems. Current Biology, 16(17):R726-R735.
Charnov, E.L. 1982. The Theory of Sex Allocation. Princeton University Press, 363 p.
Chen, P., and Hudson, J.D. 1991.The Conchostracan fauna of the great estuarine group, Middle Jurassic, Scotland. Palaeontology, 34(3):515-545.
de Pinna, M.C.C. 1991. Concepts and tests of homology in the cladistic paradigm. Cladistics. 7: 367-394.
Dumont, H.J. and Negrea, S.V. 2002. Introduction to the class Branchiopoda. In H.J. Dumont (ed.), Backhuys Publishers, Leiden.
Elewa, A. 2003. Morphometric studies on three ostracod species of the genus Digmocythere mandelstam from the middle Eocene of Egypt. Palaeontologia Electronica, 6(2):1-11.
Fernandez-Leborans, G. 2010.Epibiosis in Crustacea: an overview. Crustaceana, Volume 83(Number 5):549-640(92).
Frank, P.W. 1988. Conchostraca. Palaeogeography, Palaeoclimatology, Palaeoecology. 62(1-4), 399-403.
Gallego, O. F. 2005. First record of the family Palaeolimnadiopseidae Defretin-le Franc, 1965 (Crustacea–Conchostraca) in the Triassic of Argentina. Journal of South American Earth Sciences, 18(2), 223-231
Gallego, O.F. 2010. A new crustacean clam shrimp (Spinicaudata: Eosestheriidae) from the Upper Triassic of Argentina and its importance for 'Conchostracan' taxonomy. Alcheringa: An Australasian Journal of Palaeontology, 34(2):179-195.
Hammer, Ø., Harper, D.A.T., and Ryan, P.D. 2001. PAST: Palaeontological statistics software package for education and data analysis. Palaeontologica Electronica 4(1):9p
Hethke, M., Fursich, F.T. and Jiang, B. 2010. Determining morphotypes of fossil clam shrimps by fourier analysis: Possible implications and limitations tested on Middle Jurassic specimens from Gansu, China. Earth Science Frontiers 17:217-218
Jarne, P. and Auld, J.R. 2006. Animals mix it up too: The distribution of self-fertilization among hermaphroditic animals. Evolution 60:1816-1824.
Jones, P.J. 2003. Ankumia van Veen, 1932 (nomen dubium): pathological moult retention in the Cytherellidae (Platycopida: Ostracoda). Journal of Micropalaeontology, 22(1):85-99.
Jones, P.J. and Chen, P. 2000. Carboniferous and Permian Leaioidea (Branchiopoda: Conchostraca) from Australia: taxonomic revision and biostratigraphic implications. Records of the Australian Museum, 52(2):223-244.
Kelley, P.H. and Hansen, T.A. 2003. Predator–Prey Interactions in the Fossil Record, p. 464. Kelley, P.H., Kowalewski, M., and Hansen, T.A. (eds.), Kluwer Academic/Plenum, New York.
Klimov, P.B. and Sidorchuk, E.A. 2011. An enigmatic lineage of mites from Baltic amber shows a unique, possibly female-controlled, mating. Biological Journal of the Linnaean Society, 102(3):661-668.
Kondrashov, A.S. 1993. Classification of hypotheses on the advantage of amphimixis. Journal of Heredity, 84:372-387.
Korpelainen, H. 2007. Labile sex expression in plants. Biological Reviews, 73(2):157-180.
Krieger, J.D., Guralnick, R.P. and Smith, D.M. 2007. Generating empirically determined continuous measures of leaf shape for paleoclimate reconstruction. Palaios, 22(2):212-219.
Labandeira, C.C. and Sepkoski, J.J. 1993. Insect diversity in the fossil record. Science, 261(5119):310-315.
Laffont, R., Firmat, C., Alibert, P., David, B., Montuire, S., and Saucède, T. 2011. Biodiversity and evolution in the light of morphometrics: From patterns to processes. Comptes Rendus Palevol, 10(2-3):133-142.
Li, G., Shen, Y., and Batten, D.J. 2007. Yanjiestheria, Yanshania and the development of the Eosestheria Conchostracan fauna of the Jehol Biota in China. Cretaceous Research, 28(2):225-234.
Li, G., Hirano, H., Kozai, T., Sakai, T., and Pan, Y. 2009a.Middle Jurassic Spinicaudatan Shizhuestheria from the Sichuan Basin and its Ontogenetic Implication. Science in China Series D: Earth Sciences, 52(12).
Li, G., Wan, X., Batten, D.J., Bengston, P., Xi, D., Wang, P. 2009b Spinicaudatans from the Upper Cretaceous Nenjiang Formation of the Songliao Basin, northeast China: taxonomy and biostratigraphy. Cretaceous Research, 30: 687–698
Li, G., Hirano, H., Batten, D.J., Wan, X., Willems, H., and Zhang, X. 2010. Biostratigraphic significance of Spinicaudatans from the Upper Cretaceous Nanxiong Group in Guangdong, South China. Cretaceous Research, 31(4):387-395.
Li, G., Wan, X., Batten, D.J., Bengtson, P., Xi, D., and Wang, P. 2009b. Spinicaudatans from the Upper Cretaceous Nenjiang Formation of the Songliao Basin, Northeast China: taxonomy and biostratigraphy. Cretaceous Research, 30(3):687-698.
Lloyd, D.G. 1975. The maintenance of gynodioecy and androdioecy in angiosperms. Genetica, 45(3):325-339.
Lohmann, G.P. 1983. Eigenshape analysis of microfossils: A general morphometric procedure for describing changes in shape. Mathematical Geology, 15(6):659-672.
Lohmann, G.P. and Schweitzer, P. N. 1990.On eigenshape analysis, p.147-166., In
Rohlf, F.J. and Bookstein, F. (eds.), Proceedings of the Michigan Morphometrics Workshop. University of Michigan Museum of Zoology, Ann Arbor.
Machado, M., Cristo, M., and Fonseca, L.C.D. 1999. Non-Cladoceran Branchiopod Crustaceans from Southwest Portugal. I. Occurrence Notes. Crustaceana, 72(6):591-602.
Macleod, N. 1999.Generalizing and extending the eigenshape method of shape space visualization and analysis. Paleobiology, 25(1):107-138.
Macleod, N. 2002. Geometric morphometrics and geological shape-classification systems. Earth-Science Reviews, 59(1-4):27-47.
Martens, T. 1983. zurTaxonomie und biostratigraphie der Conchostraca (Phyllopoda, Crustacea) des Jungpaläozoikums des DDR, Teil I. Freiberger Forschungshefte, C 382:7-105.
Martens, T. 1985. Taxonomische probleme der Conchostraca (Crustacea, Phyllopoda) unterbesonderer Berficksichtigung der Variabilitat des Carapax. Freiberger Forschungshefte, C 400:44-76.
Martin, J.W. 1992. Branchiopoda, p.25-224. In Harrison, F.W. and Humes, A.G. (eds.), Microscopic Anatomy of Invertebrates. Vol. 9, Wiley–Liss, New York.
Martin, J.W. and Davis, G.E. 2001. An updated classification of the recent Crustacea. Science Series, 39 1-124.
Novojilov, N.I. 1961. Dvustvorchaty elistonogie devona (Devonian bivalve crustaceans).Akademiâ Nauk SSSR Trudy Paleontogices kogoInstituta, 81:1-132.
Nunney, L. 1989. The Maintenance of Sex by Group Selection. Evolution, 43(2):245-257.
Olempska, E. 2011. Morphology and affinities of Eridostracina: Palaeozoic ostracods with moult retention. Hydrobiologia, 1-27.
Olesen, J. 2007. Monophyly and phylogeny of Branchiopoda, with focus on morphology and homologies of branchiopod phyllopodous limbs. Journal of Crustacean Biology, 27(2):183.
Oleynikov, A.N. 1969. Recognition of Dimorphic Groups in Conchostraca [Branchiopoda], p.28-33 .In Westermann, G.E.G. (ed.), Sexual Dimorphism in Fossil Metazoa and Taxonomic Implications, E. Schweizerbart'sche Verlagsbuchhandlung, Stuttgart.
Orr, P.J. and Briggs, D.E. 1999. Exceptionally preserved Conchostracans and other crustaceans from the Upper Carboniferous of Ireland. Special Papers Palaeontology, 62:1-68.
Pannell, J.R. 1997. The maintenance of gynodioecy and androdioecy in a metapopulation. Evolution, 51:10-20.
Pannell, J.R. 2009. On the problems of a closed marriage: celebrating Darwin 200. Biology Letters, 5(3):332-335.
Pott, C., Labandeira, C.C., Krings, M., and Kerp, H. 2008. Fossil Insect Eggs and Ovipositional Damage on Bennettitalean Leaf Cuticles from the Carnian (Upper Triassic) of Austria. Journal of Paleontology, 82(4):778-789.
Regier, J.C., Shultz, J.W., and Kambic, R.E. 2005. Pancrustacean phylogeny: hexapods are terrestrial crustaceans and maxillopods are not monophyletic. Proceedings of the Royal Society B: Biological Sciences, 272(1561):395-401.
Rieder, N., Abaffy, P., Hauf, A., Lindel, M., and Weishaupl, H. 1984. Funktionsmorphologische Untersuchungenan den Conchostracen Leptestheria dahalacensis und Limnadia lenticularis (Crustacea, Phyllopoda, Conchostraca). Zoologische Beitrdge N.F.28:417-444.
Rohlf, F.J. 2008. tpsDig2, Image editing software, 2.12.
Rohlf, F.J. 2009. tpsUtil, File Utility Program, 1.44.
Rohlf, F.J. and Marcus, L.F. 1993. A revolution in morphometrics. Trends in Ecology and Evolution, 8:129-132.
Sassaman, C. 1989. Inbreeding and sex ratio variation in female-biased populations of a clam shrimp, Eulimnadia texana. Bulletin of Marine Science, 45:425-432.
Sassaman, C. 1991. Sex ratio variation in female-biased populations of Notostracans. Hydrobiologia, 212:169-179.
Sassaman, C. 1995. Sex determination and evolution of unisexuality in the Conchostraca. Hydrobiologia, 298(1-3):45-65.
Shen, Y. 1978. Leaiid conchostracans from the Middle Devonian of South China with notes on their origin, classification and evolution. Paper VIII. In: Papers for the International Symposium on the Devonian System, Bristol, 1978. Nanking Institute of Geology and Palaeontology, p. 9.
Shen, Y. 1994. Jurassic Conchostracans from Carapace Nunatak, southern Victoria Land, Antarctica. Antarctic Science, 6(1):105-113.
Slice, D. 2005. Modern morphometrics. Modern morphometrics in physical anthropology.
Stigall, A.L. and Hartman, J.H. 2008.A new Spinicaudatan genus (Crustacea: ?Conchostraca?) from the Late Cretaceous of Madagascar. Palaeontology, 51(5):1053-1067.
Stigall-Rode, A.L., Leslie, S.A., and Babcock, L.E. 2005. Ontogenetic change in the microstructure of Jurassic conchostracans (Crustacea, Spinicaudata, Cyzicidae) from Antarctica: Implications for conchostracan phylogeny and taxonomy. PaleoBios, 25:112-113.
Stoyan, D., Frenz, M., Goretzki, J., and Schneider, J. 1994. Tests zur form statistischen Klassifikation von Conchostraken (Crustacea, Branchiopoda) mittels Prokrustesanalyse. Freiberger Forschungsheft, C452:153-162.
Tasch, P. 1987.Fossil Conchostraca of the Southern Hemisphere and continental drift: Paleontology, biostratigraphy, and dispersal. Geological Society of America, Memoir, 165: 290.
Tintori, A. and Brambilla, E. 1991. Sexual dimorphism in a Conchostracan population from the Late Ladinian of Southern Calcareous Alps (N.Italy). Contributions from the Paleontological Museum, University of Oslo, 364:65-66.
Vallejo-Marin, M., Dorken, M.E., and Barrettz, S.C.H. 2010. The Ecological and evolutionary consequences of clonality for plant mating. Annual Review of Ecology, Evolution, and Systematics, 41:193-213.
Wang, S. 1989. The mechanics and the evolution and functional morphology of Conchostracan carapace. Science in China Series B, 32(5):631-640.
Weeks, S.C. and Zucker, N. 1999. Rates of inbreeding in the androdioecious clam shrimp Eulimnadia texana. Canadian Journal of Zoology, 77(9):1402-1408.
Weeks, S.C., Benvenuto, C., and Reed, S.K. 2006. When males and hermaphrodites coexist: a review of androdioecy in animals. Integrative and Comparative Biology, 46(4):449-464.
Weeks, S.C., Sanderson, T.F., Zofkova, M., and Knott, B. 2008. Breeding systems in the clam shrimp family Limnadiidae (Branchiopoda, Spinicaudata). Invertebrate Biology, 127(3):336-349.
Weeks, S.C., Chapman, E.G., Rogers, D.C., Senyo, D.M., and Hoeh, W.R. 2009. Evolutionary transitions among dioecy, androdioecy and hermaphroditism in limnadiid clam shrimp (Branchiopoda: Spinicaudata). Journal of Evolutionary Biology, 22(9):1781-1799.
Williams, G.C. 1975. Sex and Evolution. Princeton University Press, Princeton.
Wolf, D.E. and Takebayashi, N. 2004. Pollen limitation and the evolution of androdioecy from dioecy. The American Naturalist, 163(1):122-137.
Zahn, C.T. and Roskies, R.S. 1972. Fourier Descriptors for plane closed curves. IEEE Transactions on Computers, 21:269-281.
Zierold, T. 2007. Der carapax der muschelschaler – einwerkzeugefür die paläontologie? Veröff. Museum Für Naturkunde Chemnitz, 30:83-96. Zierold, T., Hanfling, B., and Gómez, A. 2007. Recent evolution of alternative reproductive modes in the 'living fossil' Triops cancriformis. Evolutionary Biology, 7:161-173.

