 A revised listing of fossil mammals from the Haasgat cave system ex situ deposits (HGD), South Africa
A revised listing of fossil mammals from the Haasgat cave system ex situ deposits (HGD), South Africa
Article number: 15.3.29A
https://doi.org/10.26879/337
Copyright Palaeontological Association, November 2012
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 17 July 2012. Acceptance: 1 November 2012
{flike id=323}
ABSTRACT
Haasgat is a formerly mined cave system in the western part of the Schurveberg Mountain Range. In 1988 fossiliferous ex situ calcified sediment blocks were collected from Haasgat and mechanically processed. Limited publication on the resulting Haasgat Dumpsite faunal assemblage (HGD) described a large sample of extinct terminal Pliocene or early Pleistocene primates, including Papio angusticeps and a novel species of extinct Cercopithecoides, but an essentially modern sample of ungulates. Starting in 2010, renewed geologic and paleontologic research at Haasgat included a critical reevaluation of the published HGD assemblage and the first analysis of nearly 1,500 specimens that were never cataloged or described in the literature. This paper presents a significantly revised Haasgat faunal list, specimen counts, and a basic description of the taxonomically identifiable assemblage with an emphasis on the non-primate mammalian specimens. In contrast to the original faunal descriptions, the occurrence of several modern and fossil artiodactyls in the sample cannot be supported. The only carnivores originally described from HGD (a Chasmaporthetes nitidula maxilla and Canis mesomelas mandible), along with two primate crania and a handful of other specimens have been lost from the collections; however, analysis of the undocumented specimens yielded previously unidentified primate remains and a cf. Dinofelis sp. fourth metatarsal. In contrast to the original interpretations of Haasgat based on HGD, the revised and expanded assemblage does not necessitate the local occurrence of closed forest or swamp environs, or imply a depositional age between 1.5 and 0.5 million years.
Justin W. Adams. Department of Biomedical Sciences, Grand Valley State University, 312 Padnos Hall, Allendale, Michigan 49401 USA. adamjust@gvsu.edu
KEYWORDS: Africa; Pliocene-Pleistocene; Bovidae; Suidae; Carnivora; Rodentia
Final citation: Adams, Justin, W. 2012. A revised listing of fossil mammals from the Haasgat cave system ex situ deposits (HGD), South Africa. Palaeontologia Electronica Vol. 15, Issue 3; 29A,88p;
palaeo-electronica.org/content/2012-issue-3-articles/323-haasgat-hgd-assemblage
INTRODUCTION
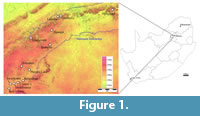 The Haasgat cave system (25°51'31"S, 27°50'9"E) formed in the Precambrian dolomitic limestone of the Malmani Subgroup (Eccles Formation) on the western slope of a narrow north-south valley in the western Schurveberg Mountain Range (Keyser and Martini, 1991; Figure 1). Lime mining of the Haasgat system during the early twentieth century obliterated the original cave entrance and most of the system. As a result the modern cave is a large east-west horizontal tunnel partially filled with miner's rubble, some remnant columns and undisturbed bands of calcified sediments along the walls and ceiling, and a massive ex situ dumpsite that extends from the modern entrance to the valley floor below (Keyser and Martini, 1991).
The Haasgat cave system (25°51'31"S, 27°50'9"E) formed in the Precambrian dolomitic limestone of the Malmani Subgroup (Eccles Formation) on the western slope of a narrow north-south valley in the western Schurveberg Mountain Range (Keyser and Martini, 1991; Figure 1). Lime mining of the Haasgat system during the early twentieth century obliterated the original cave entrance and most of the system. As a result the modern cave is a large east-west horizontal tunnel partially filled with miner's rubble, some remnant columns and undisturbed bands of calcified sediments along the walls and ceiling, and a massive ex situ dumpsite that extends from the modern entrance to the valley floor below (Keyser and Martini, 1991).
The occurrence of presumably Pliocene (5.333-2.588 m.y.a.; Gradstein et al., 2012) and/or Pleistocene (2.588-0.0117 m.y.a.; ibid.) mammal fossils in the Haasgat calcified sediments led to the collection of an unknown number of ex situ blocks for mechanical preparation at the Council for Geosciences in Pretoria in 1988 (Keyser, 1991). The resulting faunal sample (referred to here as the Haasgat Dumpsite assemblage [HGD]) was partially listed and described in a sequence of publications, with the more detailed analyses focused largely on the primate craniodental specimens (Keyser, 1991; McKee and Keyser, 1994; Plug and Keyser, 1994; von Mayer, 1999; McKee et al., 2011). The first publications on Haasgat fossils (Keyser, 1991; Keyser and Martini, 1991) noted the occurrence of three different extinct primate species: Parapapio broomi, 'Simopithecus sp.', and Cercopithecoides williamsi. Subsequent analysis (McKee and Keyser, 1994) of the papionins attributed the entire sample of 83 craniodental specimens to Papio angusticeps, which has been previously identified at Kromdraai A and B and the Cooper's deposits (Freedman, 1957; Heaton, 2006; Note P. angusticeps was included within Papio izodi by Jablonski and Frost, 2010). The HGD colobin sample originally attributed to C. williamsi has recently been reallocated to a small bodied novel species, Cercopithecoides haasgati (McKee et al., 2011), distinct from C. williamsi as reported from other South African localities with colobin samples (Makapansgat, Bolt's Farm, Swartkrans, Sterkfontein, Cooper's, and Kromdraai B; Eisenhart, 1974; McKee et al., 2011). At present the HGD samples are among the largest and most demographically diverse accumulations of these two primate taxa from a single locality in South Africa (Heaton, 2006; Jablonski and Frost, 2010).
A published description of the other HGD mammals only addressed the taxonomically identifiable Artiodactyla, Perissodactyla, and Hyracoidea specimens from the sample (Plug and Keyser, 1994). The two carnivores (Chasmaporthetes nitidula and Canis mesomelas) initially noted by Keyser (1991) were never formally described and have subsequently gone missing from the collections. The mammalian taxa listed by Plug and Keyser (1994) are broadly similar to other terminal Pliocene and Pleistocene South African faunal assemblages, but contrasts from the well-described deposits from Sterkfontein, Swartkrans, and Kromdraai in being numerically dominated by rocky landscape browsing antelope (Oreotragus major and Oreotragus oreotragus) and hyrax (Procavia transvaalensis and Procavia antiqua). Only relatively few specimens of other species were identified from the processed sample, although the list includes a range of equids (Equus capensis and Equus burchelli), giraffe (Giraffa camelopardalis), a suid (cf. Metridiochoerus sp.), two genera of tragelaphin (Tragelaphus and Taurotragus), two genera of reduncin (Kobus and Redunca), and four genera of alcelaphins (Alcelaphus, Connochaetes, Damaliscus, and Megalotragus).
Active research on the Haasgat karstic system and non-primate fossils ended after these initial publications and no further ex situ sampling or fossil processing occurred after the early 1990s. Starting in 2010 our research team reopened the Haasgat cave system as part of an ongoing interdisciplinary project addressing the geology and paleontology of South African fossil localities in the northern portion of the Cradle of Humankind UNESCO World Heritage Site. In order to establish an interpretive framework for specimens excavated from Haasgat the original HGD assemblage curated at the Council for Geosciences was evaluated. This survey of the collection revealed that only 946 of the 2,413 HGD specimens had been individually labeled, only 740 had entries in the single Council for Geosciences HGD catalog, several specimens could not be located, and many of the original taxonomic and element identifications required revisions that would affect the composition of the faunal list.
Because of the need for a more accurate baseline accounting of the fauna at Haasgat our original project goals were expanded to include a complete cataloging and reanalysis of the entire HGD sample. This publication provides an updated faunal listing and basic description of the fossil specimens in the HGD assemblage based on this reassessment, with an emphasis on describing the identifiable non-primate mammalian specimens recovered from the dumpsite. Given their paleoprimatological significance, comprehensive paleobiological description and comparative analysis of the HGD colobin and papionin craniodental specimens and indeterminate cercopithecid postcrania will be published separately.
MATERIALS AND METHODS
Before describing the methods employed in this analysis of the Haasgat HGD specimens it is important to note several known issues with the assemblage. First, as with other South African ex situ dumpsite-derived samples, the HGD faunal assemblage must be assumed to be an artificially spatially- and/or temporally-aggregated accumulation of specimens from across the cave system deposits. Unfortunately, the degree of aggregation bias in the sample cannot be gauged as neither the original publications nor the handwritten HGD catalog records information on the collection protocols, geological characteristics of the processed blocks, or the within-block association of individual specimens. Second, the HGD assemblage fossils were mechanically processed using air scribes and hand tools (Plug and Keyser, 1994). Many specimens were apparently only processed until identification could be attempted, meaning that in some cases adhering matrix (or heavy application of liquid consolidant to stabilize specimens during preparation) obscured key anatomical features or prevented metric analysis. This processing method introduced scribe marks and recent breakage to 1,080 (44.8%) of the specimens that limited specific identification and/or comparative analysis.
Third, an initial survey of the assemblage in 2010 revealed inconsistencies between: 1) the numbers of specimens listed in the Council for Geosciences HGD catalog; 2) the originally published specimen counts and descriptions; 3) both of these accountings of the assemblage and the number and identification of specimens in the collections (Plug and Keyser, 1994). As previously noted, the HGD catalog only includes entries for 740 of the 1,475 specimens listed by Keyser (1991), Keyser and Martini (1991), McKee and Keyser (1994), Plug and Keyser (1994), and McKee et al. (2011). As one example, none of the Papio and Cercopithecoides specimens assigned HGD numbers and described by McKee and Keyser (1994) and McKee et al. (2011) were entered in the sole HGD catalog maintained by the Council for Geosciences. Even with only a partial catalog, 21 taxonomically identified specimens with catalog entries could not be found in the collections (Appendix A). A further three specimens without catalog entries but known to exist could also not be located, including a reported Chasmaporthetes nitidula maxilla (Keyser, 1991, figure 7; Keyser and Martini, 1991, plate 4) and two Cercopithecoides haasgati crania (HGD 1166 and 1167; McKee et al., 2011, figure 2); although fortunately casts of the latter two specimens exist.
Lacking a complete catalog of specimens it is impossible to gauge the extent specimen loss has shaped the current composition of the collections. To illustrate the most extreme example encountered during reanalysis, Plug and Keyser (1994) reported large samples of two hyrax species, Procavia transvaalensis (number of individual specimens [NISP]: 214; minimum number of individuals [MNI]: 15) and Procavia capensis (NISP: 107; MNI: 10). In contrast, the HGD catalog only records 28 P. transvaalensis and 18 P. capensis specimens; all but two of these Procavia specimens were found in the collections. Even though analysis of the unlabeled part of the HGD sample identified additional hyrax specimens the final tally of HGD Procavia specimens (NISP: 67; Table 1) does not approach the originally published sample sizes for the genus. Unless the counts reported by Plug and Keyser (1994) represent a typographic error, the disparity between the published faunal list, catalog, and curated collections could suggest a minimum loss of 254 identifiable Procavia specimens from the HGD sample.
All processed fossil specimens from the HGD assemblage that were ultimately located or known to exist via published photographs (n=2,414; total includes casts of the missing HGD 1166 and 1167 specimens and the uncatalogued Chasmaporthetes nitidula maxilla) were sorted and coded in an electronic database for taxonomic, demographic, and taphonomic variables following the methods described in Adams (2006) and Adams (2010). All previously unlabeled specimens were assigned an HGD catalog number, marked, and incorporated into the database. Taxonomic identifications of the craniodental and postcranial specimens were made in reference to modern skeletal collections at the Ditsong National Museum of Natural History, previously described fossil specimens at the Ditsong Museum (Swartkrans, Kromdraai, Cooper's, Sterkfontein Type Site, Gondolin, and Hoogland) and the Bernard Price Institute (Makapansgat), and published diagnostic criteria (summarized in Adams, 2006). Bovid specimens that could only be confidently attributed to Family were sorted into Size Classes after Brain (1976). All measurements of specimens reported here were taken using Mitutoyo 150 mm calipers with a direct digital input, including relevant dental metrics (mesiodistal [MD] and buccolingual [BL]; taken at the level of occlusion unless otherwise noted). All statistical comparisons reported here were run using PASW Statistics 18 (SPSS, Inc., 2011).
Although I have provided authors and dates for all taxonomic names directly referenced in the results, the HGD assemblage preserves a diverse range of mammals that are also commonly represented in African Pliocene, Pleistocene, and Holocene fossil and archaeological assemblages. These two factors, along with unresolved debates on specimen attribution across localities, make comprehensive synonym citation and summaries of the geographic and temporal range of genera and species beyond the scope of this publication. Additional synonym information and data on the occurrence and ranges of mammals discussed here can be found in Wilson and Reader (2005) and Werdelin and Sanders (2010).
RESULTS
An updated catalog of the 2,414 specimens in the Haasgat HGD assemblage is provided in Appendix B. This catalogue lists both the 1,446 taxonomically identifiable mammal fossils summarized in Table 1 and the 968 taxonomically indeterminate specimens (49 craniodental, 692 postcranial, and 227 unclassifiable). The specimen entries in Appendix B have been organized to correspond to the sequence of taxonomic groups in Table 1 and as discussed in the following section.
Systematic Palaeontology
Order PRIMATES Linnaeus, 1758
Family CERCOPITHECIDAE Gray, 1821
Subfamily COLOBINAE Jerdon, 1867
Tribe COLOBINI Blyth, 1875
Genus CERCOPITHECOIDES Mollet, 1947
Type species Cercopithecoides williamsi Mollet, 1947
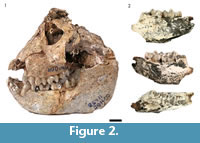 The entire HGD colobin sample of 25 specimens was originally attributed to Cercopithecoides williamsi (Keyser, 1991; McKee and Keyser, 1994), but was subsequently proposed to represent a novel smaller bodied species, Cercopithecoides haasgati (McKee et al., 2011), distinct from C. williamsi as reported the other South African localities (Makapansgat, Bolt's Farm, Swartkrans, Sterkfontein, Cooper's, and Kromdraai B; Eisenhart, 1974; von Mayer, 1999; Jablonski and Frost, 2010) (Figure 2.1). Reanalysis of the HGD colobins found five of the originally identified specimens inconsistent with attribution to the subfamily; however, eight additional colobin craniodental specimens were identified from the previously undocumented collections (Figure 2; Kegley et al., 2011). In contrast to McKee et al. (2011), Kegley (personal commun., 2012) has identified no fewer than three different colobin species present in the HGD assemblage, including C. haasgati (McKee et al., 2011), C. williamsi, and a third unassigned colobin represented by the HGD 2452 partial mandible. Metrically and morphologically, HGD 2452 approximates the large Kromdraai B specimens [KB 680/683, KB 5349 and KB 3114] and the Makapansgat Member 3 M 3018 M3 noted by Eisenhart (1974) and Leakey (1982) as resembling Paracolobus or Rhinocolobus (Figure 2.2; Kegley et al., 2011). Interestingly, two of the three C. williamsi mandibles (HGD 1175 and HGD 1180) share features with comparable specimens from Sterkfontein Member 4 (Sts 394B and Sp 21), yet differ from those recovered from Makapansgat Member 3 (M 622) and Bolt's Farm Pit 23 (BF 42B) (Kegley, personal commun., 2012).
The entire HGD colobin sample of 25 specimens was originally attributed to Cercopithecoides williamsi (Keyser, 1991; McKee and Keyser, 1994), but was subsequently proposed to represent a novel smaller bodied species, Cercopithecoides haasgati (McKee et al., 2011), distinct from C. williamsi as reported the other South African localities (Makapansgat, Bolt's Farm, Swartkrans, Sterkfontein, Cooper's, and Kromdraai B; Eisenhart, 1974; von Mayer, 1999; Jablonski and Frost, 2010) (Figure 2.1). Reanalysis of the HGD colobins found five of the originally identified specimens inconsistent with attribution to the subfamily; however, eight additional colobin craniodental specimens were identified from the previously undocumented collections (Figure 2; Kegley et al., 2011). In contrast to McKee et al. (2011), Kegley (personal commun., 2012) has identified no fewer than three different colobin species present in the HGD assemblage, including C. haasgati (McKee et al., 2011), C. williamsi, and a third unassigned colobin represented by the HGD 2452 partial mandible. Metrically and morphologically, HGD 2452 approximates the large Kromdraai B specimens [KB 680/683, KB 5349 and KB 3114] and the Makapansgat Member 3 M 3018 M3 noted by Eisenhart (1974) and Leakey (1982) as resembling Paracolobus or Rhinocolobus (Figure 2.2; Kegley et al., 2011). Interestingly, two of the three C. williamsi mandibles (HGD 1175 and HGD 1180) share features with comparable specimens from Sterkfontein Member 4 (Sts 394B and Sp 21), yet differ from those recovered from Makapansgat Member 3 (M 622) and Bolt's Farm Pit 23 (BF 42B) (Kegley, personal commun., 2012).
Subfamily CERCOPITHECINAE Gray, 1821
Tribe PAPIONINI Burnett, 1828
Genus PAPIO Statius Müller, 1773
Type species Papio papio Desmarest, 1820
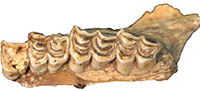
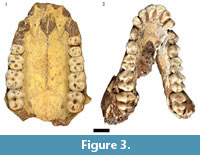 As previously noted, initial publications on the HGD fossils suggested the occurrence of two papionin species, Parapapio broomi Jones, 1937 and "Simopithecus sp." (syn. Theropithecus Geoffroy Saint-Hilaire, 1843) (Keyser, 1991; Keyser and Martini, 1991). A more detailed analysis of the 83 papionin craniodental specimens (MNI: 29; 15 male, 10 female, four indeterminate sex) classified the entire sample to the extinct species Papio angusticeps Freedman, 1957 and highlighted the similarities to specimens from Kromdraai A, B and Cooper's (McKee and Keyser, 1994). This study of the previously undocumented collections has expanded the diagnostically P. angusticeps sample to include 88 total specimens, derived from a minimum of 37 individuals (10 male, 12 female, 15 indeterminate sex) (Figure 3). A further 21 isolated Papio craniodental remains identified during this reanalysis are too incomplete to confidently attribute to P. angusticeps, but may represent additional specimens of the species.
As previously noted, initial publications on the HGD fossils suggested the occurrence of two papionin species, Parapapio broomi Jones, 1937 and "Simopithecus sp." (syn. Theropithecus Geoffroy Saint-Hilaire, 1843) (Keyser, 1991; Keyser and Martini, 1991). A more detailed analysis of the 83 papionin craniodental specimens (MNI: 29; 15 male, 10 female, four indeterminate sex) classified the entire sample to the extinct species Papio angusticeps Freedman, 1957 and highlighted the similarities to specimens from Kromdraai A, B and Cooper's (McKee and Keyser, 1994). This study of the previously undocumented collections has expanded the diagnostically P. angusticeps sample to include 88 total specimens, derived from a minimum of 37 individuals (10 male, 12 female, 15 indeterminate sex) (Figure 3). A further 21 isolated Papio craniodental remains identified during this reanalysis are too incomplete to confidently attribute to P. angusticeps, but may represent additional specimens of the species.
Cercopithecidae gen. et sp. indet.
In addition to the identifiable papionin and colobin craniodental specimens, there is a large collection of indeterminate cercopithecid craniodental (n=31) and postcranial (n=86) specimens and a single partial upper molar (HGD 2453) identifiable only as primate (but may represent Cercopithecoides).
Order ARTIODACTYLA Owen, 1848
Family BOVIDAE Gray, 1821
Tribe ALCELAPHINI de Rochebrune, 1883
The alcelaphins were the second largest taxonomic category reported in the original description of the HGD assemblage, with 230 specimens representing minimally 39 individuals (Plug and Keyser, 1994). In contrast, the original HGD catalog only records 134 specimens assigned to genera within the Tribe. This resurvey of the HGD collections only supports an alcelaphin sample size (n=132) close to that originally cataloged and not as published. Despite the superficial numerical similarity, most specimens have been classified as 'indeterminate' rather than to a more specific taxonomic level. This shift reflects a more conservative treatment of the isolated and damaged teeth necessitated by the lack of associated horn cores and overlapping dental morphologies of extant and extinct alcelaphin lineages. This approach prohibited attribution of any of the alcelaphin specimens to two species (Alcelaphus buselaphus Pallas, 1766 and Connochaetes taurinus Burchell, 1824) originally listed in the assemblage by Plug and Keyser (1994).
Genus CONNOCHAETES Lichtenstein, 1814
Type species Connochaetes gnou Zimmerman, 1780
Connochaetes gnou (Zimmerman, 1780)
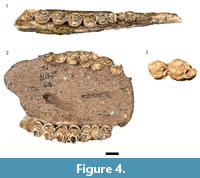 Three partial mandibles (HGD 42, 43, 85) and four isolated mandibular fourth premolars (HGD 292, 312, 378, 1259) exhibited morphological features of the corpus, premolar cusp fusion, and overall size consistent with attribution to black wildebeest (Figure 4.1). The occlusal wear of the fourth premolars indicates minimally five different fully adult individuals in the sample, with HGD 312 and 378 likely antimeres.
Three partial mandibles (HGD 42, 43, 85) and four isolated mandibular fourth premolars (HGD 292, 312, 378, 1259) exhibited morphological features of the corpus, premolar cusp fusion, and overall size consistent with attribution to black wildebeest (Figure 4.1). The occlusal wear of the fourth premolars indicates minimally five different fully adult individuals in the sample, with HGD 312 and 378 likely antimeres.
Connochaetes sp.
A small collection of isolated alcelaphin teeth (HGD 55, 95, 1254), a mandible (HGD 52), and maxilla (HGD 66) are consistent with Connochaetes in both size and morphology but were insufficiently preserved to confidently attribute to the specific level given the phylogenetic diversity of Pleistocene Connochaetes species. The morphology of the HGD 52 left mandible, originally cataloged as Connochaetes taurinus is consistent with a C. taurinus-sized alcelaphin; however, the heavily worn and damaged m1 appears extremely small relative to similarly occluded C. taurinus individuals and the specimen does overlap with larger extant C. gnou specimens. Similarly, the HGD 66 maxilla preserves large dentition with complex central cavities consistent with extant C. taurinus and fossil Connochaetes sp. aligned with the species described from Swartkrans (Vrba, 1976). These five specimens are derived from a minimum of three adult individuals.
Genus DAMALISCUS Sclater and Thomas, 1894
Type species Damaliscus dorcas Pallas, 1766
Damaliscus dorcas (Pallas, 1766)
Only one of the 19 originally cataloged Damaliscus dorcas specimens was attributable to the species. The HGD 44 is a nearly complete maxilla preserving parts of both fully erupted toothrows, with molar crowns displaying the 'squared' protocone and metaconule exhibited by modern D. dorcas specimens (Figure 4.2).
Damaliscus sp.
Two craniodental specimens from different individuals exhibit features diagnostic to the genus Damaliscus but could not be more specifically attributed. HGD 97 is a partial mandible from an immature individual preserving the lingual aspect of the corpus, p3, deciduous p4 (with the permanent p4 erupting), m1, and the mesial part of the m2. The first molar exhibits the mesiodistal 'pinching' of the protoconid and hypoconid typical of the genus, with the p3 (MD: 10.39 mm, BL: 5.37 mm) and m1 (MD: 19.67mm, BL: 10.17mm) larger than present in Swartkrans fossil specimens attributed to D. dorcas (no p3s attributed; m1 MD: mean=16.6 mm, n=6, BL: mean=8.2 mm, n=6) and as potentially representative of the extinct species Damaliscus niro (SKB 5979 p3, MD: 8.8 mm, BL: 6.5 mm; m1 MD: mean=14.1 mm, n=7; BL: mean =8.4 mm, n=7) (Vrba, 1978). Given the noted diversity of species within Damaliscus (see Gentry, 2010) the specimen can only be generically identified at present.
The HGD 1263 specimen preserves the occipital, both temporals inclusive of the petrous portions, both parietals, and parts of both frontals from a likely fully mature individual. In lateral view, the angle between the occipital and parietals is approximately 135 degrees and the small frontal portions indicate extensive sinus development that likely penetrated into the pedicles of the horncores. The breadth of the occipital and parietals makes a shared base for the horncores unlikely (excluding attribution to either Alcelaphus buselaphus or Parmularius Hopwood, 1934), and the pedicles are too anteriorly positioned for Connochaetes. The specimen is comparable to extant Damaliscus lunatus Burchell, 1824 crania in all assessable features, although the nuchal ridges on the occipital are more robust.
Genus MEGALOTRAGUS van Hoepen, 1932
Type species Megalotragus priscus Broom, 1909a
Megalotragus sp.
One isolated, occluded left mandibular third molar (HGD 311) is consistent with attribution to the extinct genus (Figure 4.3). Slight distortion along the distal and buccal margins of the crown limited direct metric comparison, but the minimum mesiodistal length (39 mm) places it within the range of Megalotragus specimens from Swartkrans Member 1, Kromdraai A, and Sterkfontein Member 4 (mean =39.7 mm, range=39.0-42.0 mm, n=7) and outside the observed range of fossil Connochaetes from those same localities (mean =33.9 mm, range=31.0-36.5 mm, n=24) (Vrba, 1976).
Alcelaphini gen. et sp. indet.
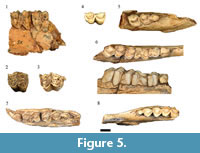 Most of the recovered alcelaphins in the HGD sample preserved insufficient morphological features to clearly attribute below the Tribal-level, but are derived from at least eight adult and two immature individuals. Of particular note within this grouping of specimens is a collection of 15 maxillary teeth (HGD 40, 53, 54, 59, 56, 61, 73, 92, 118, 145, 306, 2184, 2193, 2194, 2195) and 14 partial mandibles and mandibular teeth (HGD 4, 49, 51, 63, 90, 93, 100, 108, 113, 129, 385, 381, 425, 1253) (Figure 5). The maxillary molars in this subset exhibit protostyles, a modest basal pillar on one specimen (HGD 61), strong mesostyles with flat buccal walls of metacones, and M3s with exaggerated metastyles (Figure 5.2-5.3). The mandibular remains have an extremely mesiodistally compressed premolar row, fused p3 cusp elements, and molars exhibiting gracile goatfolds and U- to V-shaped 'pointed' protoconids and hypoconids (and a mammillary-like basal pillar on the HGD 90 m2) (Figure 5.6-5.8). Collectively these hypsodont specimens exhibit features shared with primitive alcelaphins (e.g., Damalacra Gentry, 1980; Gentry, 2000; Brink and Stynder, 2009) as well as specimens identified by Vrba (1976) from Swartkrans Member 1 Hanging Remnant as possibly representing Ovibovini (cf. Makapania sp. Wells and Cooke, 1956; SK 2693, SK 2965, SK 3005, SK 3065; Figure 5.1, 5.4-5.5). Although treated here as indeterminate alcelaphins, further analysis of this subset of specimens may lead to their attribution as Ovibovini.
Most of the recovered alcelaphins in the HGD sample preserved insufficient morphological features to clearly attribute below the Tribal-level, but are derived from at least eight adult and two immature individuals. Of particular note within this grouping of specimens is a collection of 15 maxillary teeth (HGD 40, 53, 54, 59, 56, 61, 73, 92, 118, 145, 306, 2184, 2193, 2194, 2195) and 14 partial mandibles and mandibular teeth (HGD 4, 49, 51, 63, 90, 93, 100, 108, 113, 129, 385, 381, 425, 1253) (Figure 5). The maxillary molars in this subset exhibit protostyles, a modest basal pillar on one specimen (HGD 61), strong mesostyles with flat buccal walls of metacones, and M3s with exaggerated metastyles (Figure 5.2-5.3). The mandibular remains have an extremely mesiodistally compressed premolar row, fused p3 cusp elements, and molars exhibiting gracile goatfolds and U- to V-shaped 'pointed' protoconids and hypoconids (and a mammillary-like basal pillar on the HGD 90 m2) (Figure 5.6-5.8). Collectively these hypsodont specimens exhibit features shared with primitive alcelaphins (e.g., Damalacra Gentry, 1980; Gentry, 2000; Brink and Stynder, 2009) as well as specimens identified by Vrba (1976) from Swartkrans Member 1 Hanging Remnant as possibly representing Ovibovini (cf. Makapania sp. Wells and Cooke, 1956; SK 2693, SK 2965, SK 3005, SK 3065; Figure 5.1, 5.4-5.5). Although treated here as indeterminate alcelaphins, further analysis of this subset of specimens may lead to their attribution as Ovibovini.
Tribe ANTILOPINI Gray, 1821
Genus ANTIDORCAS Sundevall, 1847
Type species Antidorcas marsupialis Zimmerman, 1780
Antidorcas bondi (Cooke and Wells, 1951)
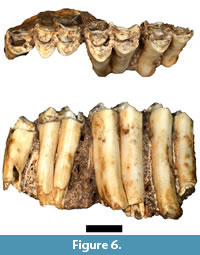 Although five Antidorcas bondi specimens were listed by Plug and Keyser (1994) only a single specimen (HGD 326) was cataloged, and no additional antilopin specimens identified during this survey could be attributed to the taxon. HGD 326 is a small portion of the left maxillary alveolus and the near complete crowns of the P4-M3 in full occlusion (Figure 6). The crowns are extremely hypsodont and are directly comparable to A. bondi specimens recovered from the Kromdraai A deposits (e.g., KA 1157; Vrba, 1976).
Although five Antidorcas bondi specimens were listed by Plug and Keyser (1994) only a single specimen (HGD 326) was cataloged, and no additional antilopin specimens identified during this survey could be attributed to the taxon. HGD 326 is a small portion of the left maxillary alveolus and the near complete crowns of the P4-M3 in full occlusion (Figure 6). The crowns are extremely hypsodont and are directly comparable to A. bondi specimens recovered from the Kromdraai A deposits (e.g., KA 1157; Vrba, 1976).
Antidorcas australis/marsupialis (Hendey and Hendey, 1968)/(Zimmerman, 1780)
A total of 28 Antidorcas marsupialis specimens were noted by Plug and Keyser (1994); however, only 10 specimens were cataloged (HGD 30, 88, 104, 120, 139, 144, 318, 330, 357, and 1079). The HGD 120 left fourth premolar could not be located, while the HGD 144 is a partial proximal phalanx that lacks diagnostic features allowing for attribution beyond Size Class. The HGD 318 left M3 was located but found to have a duplicate specimen number with an indeterminate cercopithecoid left proximal femur (and was renumbered HGD 825). Of the remaining originally cataloged specimens, most (HGD 88, 330, 357, and 1079) exhibit crown morphologies inconsistent with Antidorcas but aligned with the neotragin Oreotragus (see below), while the HGD 30 right maxillary molar is too incomplete to confidently allocate and is considered an indeterminate Antilopini/Neotragini.
Only four specimens derived from minimally two adult individuals could be confidently attributed to the Antidorcas australis/Antidorcas marsupialis lineage (probable synonyms; see Gentry, 2010), including three of the originally cataloged specimens (HGD 104 left mandible with m2 and m3; HGD 139 partial maxilla; HGD 825 left M3 [formerly HGD 318]). The HGD 422 left lower molar, originally considered Antidorcas sp., exhibits a well-defined central cavity and robust crown analogous to A. australis/A. marsupialis from Swartkrans (e.g., SK 3057, SK 4006, SK 4081) and contrasts with both Antidorcas bondi and Antidorcas recki Schwarz, 1932 comparatives.
Antidorcas sp.
While four specimens were attributed to Antidorcas sp. by Plug and Keyser (1994), a total of five specimens (HGD 328, 335, 367, 418, 422) were cataloged. After review none of the originally cataloged specimens could be retained as Antidorcas. The HGD 328 (radial carpal) and HGD 335 (lumbar vertebra) do not preserve diagnostic features that exclude other bovid genera. The HGD 367 maxillary tooth (possibly an M1) only preserves the mesial aspect of the crown and is considered here an indeterminate Antilopini/Neotragini. Both the HGD 418 (P4) and HGD 422 (m2 or m3) were identifiable as Oreotragus sp. and Antidorcas marsupialis, respectively.
Five specimens are cataloged here as representing indeterminate Antidorcas in the HGD assemblage. The HGD 36 left partial m3 is occluded but hypsodont, potentially derived from either Antidorcas recki or Antidorcas bondi. The HGD 313 and 794 partial upper molars resemble specimens attributed to both A. recki and A. marsupialis but are too brachydont to be derived from A. bondi. From the previously undocumented assemblage, the HGD 1283 right M3 is heavily occluded and may be A. recki, while the HGD 1285 ectoloph fragment is extremely hypsodont and consistent with the morphology of the HGD 326 A. bondi maxillary dentition.
Tribe HIPPOTRAGINI Retzius and Lovén, 1845
Genus HIPPOTRAGUS Sundevall, 1846
Type species Hippotragus equinus Geoffroy Saint-Hilaire, 1803
Hippotragus sp.
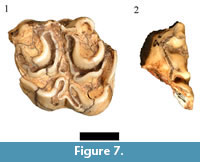 Only one of the four originally listed and cataloged Hippotragus specimens (HGD 89, right occluded M3) exhibits the boödont occlusal features consistent with Hippotragus sp. (Figure 7.1). Of the other originally cataloged Hippotragus specimens, the HGD 140 left lower molar only preserves an undiagnostic part of the mesial crown and goatfold and is reclassified here as an indeterminate Hippotragini/Reduncini, while the HGD 268 specimen is a Class III bovid distal tibia that is insufficiently diagnostic to specifically attribute. The only other cataloged Hippotragus specimen is the HGD 40 isolated right M2 listed as Hippotragus niger Harris, 1838, although this species is not included in Plug and Keyser (1994). However, the molar lacks an entostyle and exhibits cusp morphology only consistent with attribution to the Alcelaphini.
Only one of the four originally listed and cataloged Hippotragus specimens (HGD 89, right occluded M3) exhibits the boödont occlusal features consistent with Hippotragus sp. (Figure 7.1). Of the other originally cataloged Hippotragus specimens, the HGD 140 left lower molar only preserves an undiagnostic part of the mesial crown and goatfold and is reclassified here as an indeterminate Hippotragini/Reduncini, while the HGD 268 specimen is a Class III bovid distal tibia that is insufficiently diagnostic to specifically attribute. The only other cataloged Hippotragus specimen is the HGD 40 isolated right M2 listed as Hippotragus niger Harris, 1838, although this species is not included in Plug and Keyser (1994). However, the molar lacks an entostyle and exhibits cusp morphology only consistent with attribution to the Alcelaphini.
In addition to the HGD 89 molar, four additional isolated Hippotragus teeth were identified during reanalysis. The HGD 9 right M3 is a largely complete tooth that indicates the occurrence of a second fully adult individual in the assemblage. The HGD 106 left deciduous third premolar was originally cataloged as Connochaetes taurinus, however the size and shape of the crown elements (particularly the hypoconulid and paraconulid) are only consistent with attribution to Hippotragus (Figure 7.2). Of the two left p2 specimens, HGD 1261 appears to be somewhat buccolingually expanded relative to extant Hippotragus equinus specimens, but does appear to overlap with some of the largest male extant comparative specimens.
Tribe NEOTRAGINI Sclater and Thomas, 1894
Genus OREOTRAGUS Smith, 1834
Type species Oreotragus oreotragus Zimmerman, 1783
Oreotragus sp.
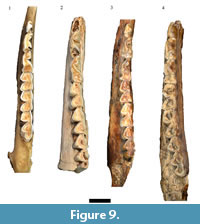
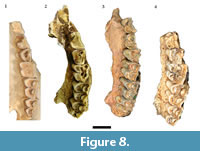 The largest single categorization of specimens (NISP: 450, MNI: 30) in the original Haasgat faunal description was to Oreotragus, with specimens (directly or via 'cf.') assigned to either extant Oreotragus oreotragus or the extinct Oreotragus major Wells, 1951 (Plug and Keyser, 1994; Figure 8 and Figure 9). Although 21 specimens were listed as O. oreotragus/O. cf. oreotragus, only 232 specimens (63 craniodental, 169 postcranial) – all assigned to O. major/O. cf. major - were cataloged. This reanalysis has been able to document a total of 158 specimens (90 craniodental, 68 postcrania) attributable to Oreotragus, representing the remains of at least 19 adult and five immature individuals. The increases to the craniodental sample largely resulted from identifying additional specimens from the previously undocumented assemblage, while the reduction in assigned postcrania arose from a more conservative approach to classifying partial elements.
The largest single categorization of specimens (NISP: 450, MNI: 30) in the original Haasgat faunal description was to Oreotragus, with specimens (directly or via 'cf.') assigned to either extant Oreotragus oreotragus or the extinct Oreotragus major Wells, 1951 (Plug and Keyser, 1994; Figure 8 and Figure 9). Although 21 specimens were listed as O. oreotragus/O. cf. oreotragus, only 232 specimens (63 craniodental, 169 postcranial) – all assigned to O. major/O. cf. major - were cataloged. This reanalysis has been able to document a total of 158 specimens (90 craniodental, 68 postcrania) attributable to Oreotragus, representing the remains of at least 19 adult and five immature individuals. The increases to the craniodental sample largely resulted from identifying additional specimens from the previously undocumented assemblage, while the reduction in assigned postcrania arose from a more conservative approach to classifying partial elements.
 Despite the large sample size the specific allocation of the HGD Oreotragus specimens is complicated. The phylogenetic status of Oreotragus relative to the other Neotragini (a probable polyphyletic group) and other extant bovids is unresolved, but the genus may represent a primitive bovid lineage that has persisted since the Miocene (Gentry, 1992; Hernández Fernández and Vrba, 2005). Unfortunately, early evidence of the genus is lacking, there is no reported occurrence of the species in eastern African fossil assemblages, and currently the oldest Oreotragus fossil specimens in the record are those from Makapansgat Member 3 (3.03-2.58 m.y.a.; Herries, 2003; Warr, 2009). These specimens were allocated to Oreotragus major, a species originally erected for a specimen recovered from a cave on the Swartkrans Farm (not the hominin-bearing site Swartkrans), an undated locality near the Makapansgat Limeworks (Wells, 1951). In contrast to extant Oreotragus oreotragus, O. major exhibits absolutely larger dentition and a relatively greater M2 mesiodistal length (Wells, 1951; Wells and Cooke, 1956). The validity of O. major was questioned by Watson and Plug (1995) who evaluated Oreotragus specimens from the South African localities, including those from the Haasgat HGD assemblage. Their analysis found no metric or morphological separation between O. major and extant O. oreotragus craniodental and postcranial remains, concluding that only one species with variable body size (O. oreotragus) existed through the Plio-Pleistocene. Following this interpretation, the presence of O. oreotragus in the Makapansgat Member 3 deposits would make it the earliest occurrence of a modern African bovid species in the fossil record (Gentry, 2010).
Despite the large sample size the specific allocation of the HGD Oreotragus specimens is complicated. The phylogenetic status of Oreotragus relative to the other Neotragini (a probable polyphyletic group) and other extant bovids is unresolved, but the genus may represent a primitive bovid lineage that has persisted since the Miocene (Gentry, 1992; Hernández Fernández and Vrba, 2005). Unfortunately, early evidence of the genus is lacking, there is no reported occurrence of the species in eastern African fossil assemblages, and currently the oldest Oreotragus fossil specimens in the record are those from Makapansgat Member 3 (3.03-2.58 m.y.a.; Herries, 2003; Warr, 2009). These specimens were allocated to Oreotragus major, a species originally erected for a specimen recovered from a cave on the Swartkrans Farm (not the hominin-bearing site Swartkrans), an undated locality near the Makapansgat Limeworks (Wells, 1951). In contrast to extant Oreotragus oreotragus, O. major exhibits absolutely larger dentition and a relatively greater M2 mesiodistal length (Wells, 1951; Wells and Cooke, 1956). The validity of O. major was questioned by Watson and Plug (1995) who evaluated Oreotragus specimens from the South African localities, including those from the Haasgat HGD assemblage. Their analysis found no metric or morphological separation between O. major and extant O. oreotragus craniodental and postcranial remains, concluding that only one species with variable body size (O. oreotragus) existed through the Plio-Pleistocene. Following this interpretation, the presence of O. oreotragus in the Makapansgat Member 3 deposits would make it the earliest occurrence of a modern African bovid species in the fossil record (Gentry, 2010).
A comprehensive analysis of the Haasgat HGD Oreotragus sample relative to the larger South African Oreotragus record is beyond the scope of this publication, but has been undertaken and will be published separately. Two key results from that analysis do, however, directly impact the allocation of the Haasgat HGD Oreotragus specimens. First, statistical tests (independent sample t-test, alpha=0.05) failed to identify any statistically significant sex differences in extant Oreotragus oreotragus craniodental variables, confirming that the observed body monomorphism of the modern species (Smithers and Chimimba, 2005) extends to cranial metrics (Table 2). While some of the assessed maxillary and mandibular dimensions of specimens from Haasgat do overlap with the upper boundaries of extant Oreotragus (Table 2), the HGD specimens are significantly different in every measure from extant Oreotragus except in two morphological indices (PMRL: mandibular premolar row length/mandibular molar row length; MDMR: depth of the mandible at m2-m3/mandibular molar row length; Sponheimer et al., 1999). There are also statistical differences identified in most maxillary and mandibular measures between the two other large fossil samples (Makapansgat Member 3 and Gondolin GD 2) and modern O. oreotragus. Second, while the three substantial fossil Oreotragus samples (Haasgat HGD, Makapansgat Member 3, Gondolin GD 2) do overlap with each other in some craniodental measurements, there are statistically significant differences in the dentition and maxillary and mandibular indices between each group. The Haasgat HGD specimens are the absolute largest craniodental remains of the genus identified to date, overlapping somewhat with those from Makapansgat Member 3 (Table 2). The Makapansgat Member 3 specimens are in turn generally larger than those recovered from Gondolin GD 2, but exhibit fewer metric differences with the GD 2 sample than the Makapansgat sample does with Haasgat HGD Oreotragus.
These results contrast with those reported by Watson and Plug (1995) and raises questions about both the temporal and regional evolution of the genus during the Plio-Pleistocene and the appropriate taxonomic attribution of the Oreotragus remains from these sites. Until the results of a more comprehensive evaluation of the Haasgat HGD and South African Oreotragus record have been published, the most appropriate classification of the Haasgat HGD Oreotragus materials is to the generic level.
Tribe REDUNCINI Lydekker and Blaine, 1914
Reduncini gen. et sp. indet.
A total of 25 reduncin specimens representing minimally 9 individuals were originally allocated by Plug and Keyser (1994) to one of four species (Kobus ellipsiprymnus Ogilby, 1833, Kobus leche/Kobus cf. leche Gray, 1850, Redunca arundinum Boddaert, 1785, and Redunca fulvorufula Afzelius, 1815). Only 13 reduncin specimens were cataloged, and of those, only 12 were found during the reassessment of the assemblage. Three of these originally attributed specimens (HGD 253, 323, 324) are partial bovid Class II-III postcranial elements that preserved insufficiently diagnostic morphological features to warrant even Tribal-level attribution. There was likely an error in cataloging the HGD 317 specimen, as it is listed as a Redunca arundinum right m2 but is a cercopithecoid left proximal femur.
 The remaining eight reduncin craniodental specimens were originally cataloged as Kobus leche (HGD 39 and HGD 41), Redunca arundinum (HGD 38 and HGD 84), Redunca fulvorufula (HGD 333), and Redunca sp. (HGD 37, 321, 351); however, the specimens are damaged, incomplete, or only preserve indeterminate metric and morphological features (Figure 10). As such, identification of these specimens below the Tribal-level cannot be supported. The HGD 39 right m3 is most similar to Redunca darti Wells and Cooke, 1956 from Makapansgat Member 3 and modern Kobus in overall size (MD: 25.73 mm, BL: 10.51 mm), although the hypoconulid projects more lingually than typical in R. darti and the specimen falls outside the assessed ranges of R. darti and extant K. leche (Table 3). Similarly, the preserved left M1 (MD: 12.23 mm, BL: 15.1 mm), M2 (MD: 16.21 mm, BL: 14.2 mm) and M3 (MD: 18.6 mm, BL: 12.53 mm) on the relatively complete HGD 41 palate are large compared to extant specimens of Redunca and overlap with K. leche, but also metrically overlaps with Redunca arundinum from Elandsfontein, the largest Redunca sp. from Gondolin GD 2, and Redunca darti from Makapansgat Member 3.
The remaining eight reduncin craniodental specimens were originally cataloged as Kobus leche (HGD 39 and HGD 41), Redunca arundinum (HGD 38 and HGD 84), Redunca fulvorufula (HGD 333), and Redunca sp. (HGD 37, 321, 351); however, the specimens are damaged, incomplete, or only preserve indeterminate metric and morphological features (Figure 10). As such, identification of these specimens below the Tribal-level cannot be supported. The HGD 39 right m3 is most similar to Redunca darti Wells and Cooke, 1956 from Makapansgat Member 3 and modern Kobus in overall size (MD: 25.73 mm, BL: 10.51 mm), although the hypoconulid projects more lingually than typical in R. darti and the specimen falls outside the assessed ranges of R. darti and extant K. leche (Table 3). Similarly, the preserved left M1 (MD: 12.23 mm, BL: 15.1 mm), M2 (MD: 16.21 mm, BL: 14.2 mm) and M3 (MD: 18.6 mm, BL: 12.53 mm) on the relatively complete HGD 41 palate are large compared to extant specimens of Redunca and overlap with K. leche, but also metrically overlaps with Redunca arundinum from Elandsfontein, the largest Redunca sp. from Gondolin GD 2, and Redunca darti from Makapansgat Member 3.
The most complete of the mandibles, HGD 38, preserves a nearly intact (if crushed) left toothrow and corpus. Metrically, the measurable p2 (MD: 5.98 mm, BL: 4.52 mm), p4 (MD: 11.4 mm, BL: 7.68 mm), m1 (MD: 13.33 mm, BL: 8.81 mm), m2 (MD: 16.05 mm, BL: 8.7 mm), and m3 (MD: 20.47 mm, BL: 8.27 mm) fall within the overlapping ranges of Elandsfontein Redunca arundinum, Makapansgat Member 3 Redunca darti, Gondolin GD 2 Redunca sp., and extant Kobus leche (Table 3). Morphologically, the dentition and assessable corpus features are most similar to R. darti specimens (e.g., M879, M893, M6470, M6609), particularly in the wide separation of the p4 paraconid and metaconid (in contrast to extant Redunca and Redunca sp. from Gondolin GD 2; Adams and Conroy, 2005; Adams, 2006). Both the HGD 84 mandible, preserving the posterior portions of the corpus and the m3 (MD: 24.2 mm, BL: 9.58 mm), and the HGD 37 mandible, preserving the posterior corpus with a complete m2 (MD: 19.67 mm, BL: 9.85 mm) and partial m3, are larger than the HGD 38 individual and have teeth that lie just outside the range of the Makapansgat R. darti, Elandsfontein R. arundinum and extant K. leche samples.
The remaining reduncin specimens (HGD 321, 333, 351, 1252; the latter not previously cataloged) are isolated teeth that largely appear to be derived from individuals with a body size between modern Redunca arundinum and Kobus leche. The exception is the HGD 333 right m3, which was damaged during preparation but is from a smaller reduncin (a small R. arundinum, Redunca fulvorufula, or possibly Redunca sp. from Gondolin GD 2) than represented by the HGD 37, 38, and 84 individuals.
Tribe TRAGELAPHINI Blyth, 1863
Genus TAUROTRAGUS Wagner, 1855
Type species Taurotragus oryx Pallas, 1766
Taurotragus sp.
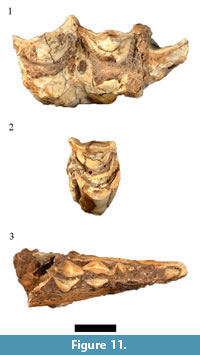 Only nine of the 13 originally listed Taurotragus oryx specimens were cataloged. Although the generic identification of four of these specimens (HGD 126, 132, 797, 799) was confirmed, the isolated and fragmentary nature of the sample prohibited specific allocation (Figure 11.1). In addition to the previously catalogued sample, a deciduous fourth premolar (HGD 315, originally attributed to Tragelaphus strepsiceros Pallas, 1766) has been reclassified here to Taurotragus sp., indicating the presence of at least two immature individuals in addition to two adults in the assemblage. The other originally cataloged T. oryx specimens (HGD 250, 257, 320, 1058) are partial Class III-IV bovid postcranial elements that cannot be confidently attributed to the species. A previously attributed single left P3 (HGD 119) only preserves part of the buccal crown and is heavily worn and is treated here as an indeterminate Tragelaphini/Hippotragini.
Only nine of the 13 originally listed Taurotragus oryx specimens were cataloged. Although the generic identification of four of these specimens (HGD 126, 132, 797, 799) was confirmed, the isolated and fragmentary nature of the sample prohibited specific allocation (Figure 11.1). In addition to the previously catalogued sample, a deciduous fourth premolar (HGD 315, originally attributed to Tragelaphus strepsiceros Pallas, 1766) has been reclassified here to Taurotragus sp., indicating the presence of at least two immature individuals in addition to two adults in the assemblage. The other originally cataloged T. oryx specimens (HGD 250, 257, 320, 1058) are partial Class III-IV bovid postcranial elements that cannot be confidently attributed to the species. A previously attributed single left P3 (HGD 119) only preserves part of the buccal crown and is heavily worn and is treated here as an indeterminate Tragelaphini/Hippotragini.
Genus TRAGELAPHUS de Blainville, 1816
Type species Tragelaphus scriptus Pallas, 1766
Tragelaphus sp.
Six of the 11 specimens listed as Tragelaphus strepsiceros by Plug and Keyser (1994) were cataloged, and five of these specimens were located; however, only one specimen (HGD 96, left probable M1) is consistent with the dentition of the extant species and lacking additional associated cranial remains is retained at the generic level (Figure 11.2). Other than the HGD 315 specimen discussed above, the HGD 98 left maxilla and HGD 113 right mandible preserve dental features only found within the Alcelaphini. The HGD 123 right mandible preserves most of the deciduous premolar dentition (Figure 11.3). While the teeth are too damaged to measure the specimen most closely resembles extant Tragelaphus angasii Gray in Angas, 1848 (as does the HGD 329 left P2).
Tragelaphini gen. et sp. indet.
An additional large tragelaphin specimen, the HGD 110 partial maxillary deciduous third premolar, may represent either Taurotragus oryx or Tragelaphus strepsiceros and is therefore maintained as an indeterminate Tragelaphini.
Family SUIDAE Gray, 1821
Subfamily SUINAE Gray, 1821
Tribe PHACOCHOERINI Gray, 1868
Phacochoerini gen. et sp. indet.
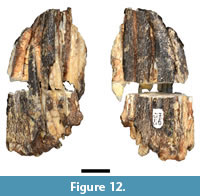 In the original description, Plug and Keyser (1994) attributed a partial suid third molar (HGD 1323; Figure 12) to the extinct genus Metridiochoerus Hopwood, 1926. The specimen was mechanically sectioned at some point prior to 2010, which eliminated ~2 mm of crown and caused significant fragmentation of the specimen. I was able to rebuild the majority of these fragments into two halves that preserve a contact point along the lingual aspect of the tooth. The degree of enamel crenulation, apical curvature, and pillar morphology (both at the occlusal plane and in the exposed section) is consistent with that of progressive phacochoerin morphology; specifically to advanced Metridiochoerus andrewsi Hopwood, 1926 mandibular third molar specimens recovered from Bolt's Farm, Gondolin GD 2, Swartkrans Members 1 and 2, and Kromdraai A (Harris and White, 1979; Cooke, 1993; Adams, 2005). Although HGD 1323 exhibits thicker enamel than typical for extant Phacochoerus aethiopicus Pallas, 1766 individuals, some Phacochoerus third molar specimens recovered from Swartkrans (e.g., SK 382, SK 4005) also have thicker enamel and exhibit a broadly similar third molar talon/id morphology. Given the preservation and indeterminate morphology it is not possible to generically attribute the specimen.
In the original description, Plug and Keyser (1994) attributed a partial suid third molar (HGD 1323; Figure 12) to the extinct genus Metridiochoerus Hopwood, 1926. The specimen was mechanically sectioned at some point prior to 2010, which eliminated ~2 mm of crown and caused significant fragmentation of the specimen. I was able to rebuild the majority of these fragments into two halves that preserve a contact point along the lingual aspect of the tooth. The degree of enamel crenulation, apical curvature, and pillar morphology (both at the occlusal plane and in the exposed section) is consistent with that of progressive phacochoerin morphology; specifically to advanced Metridiochoerus andrewsi Hopwood, 1926 mandibular third molar specimens recovered from Bolt's Farm, Gondolin GD 2, Swartkrans Members 1 and 2, and Kromdraai A (Harris and White, 1979; Cooke, 1993; Adams, 2005). Although HGD 1323 exhibits thicker enamel than typical for extant Phacochoerus aethiopicus Pallas, 1766 individuals, some Phacochoerus third molar specimens recovered from Swartkrans (e.g., SK 382, SK 4005) also have thicker enamel and exhibit a broadly similar third molar talon/id morphology. Given the preservation and indeterminate morphology it is not possible to generically attribute the specimen.
In addition to the previously described specimen, the HGD assemblage includes an unworn, partial indeterminate suid tooth crown (HGD 1324) that most closely resembles the mandibular deciduous fourth premolar of the SK 381 Metridiochoerus andrewsi specimen from the Swartkrans Member 1 deposits.
?Suidae gen. et sp. indet.
An immature proximal metaphysis/diaphysis (HGD 2309) may be a suid calcaneus given its shared morphology with the M. andrewsi calcaneus (G 126) from Gondolin GD 2 (Adams, 2006).
Order CARNIVORA Bowdich, 1821
As previously noted neither of the originally described and/or cataloged carnivore specimens (Chasmaporthetes nitidula Ewer, 1954 [not cataloged]; Canis mesomelas Schreber, 1776 [HGD 101]) recovered from the Haasgat ex situ blocks could be located in 2010. Lacking documentation for the purported C. mesomelas mandible the occurrence of species at Haasgat cannot be confirmed.
Family HYAENIDAE Gray, 1821
Hyaenidae gen. et sp. indet.
Although the originally reported Chasmaporthetes nitidula left maxilla is currently missing, and the specific allocation cannot be confirmed, photographs of the specimen (Keyser, 1991, figure 7; Keyser and Martini, 1991, plate 4) minimally establish the occurrence of a hyaenid at the site.
Family FELIDAE Fischer von Waldheim, 1817
Genus DINOFELIS Zdansky, 1924
Type species Dinofelis abeli Zdansky, 1924
cf. Dinofelis sp.
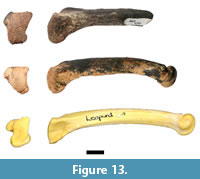 Analysis of the previously undocumented assemblage identified two carnivore postcranial elements, including a right fourth metatarsal preserving the proximal articular surface and approximately 75% of the diaphysis (HGD 950; Figure 13). Only minimal dimensions of the proximal articular surface could be measured (anteroposterior [AP]: 18.98mm, mediolateral [ML]: 14.92mm) because of abrasions to the margins of the specimen, and it is important to note that the woven appearance of the articular surface suggests that the specimen may be from an immature individual. In its preserved state HGD 950 is larger than comparative specimens of modern and fossil Panthera pardus Linnaeus, 1758 and Megantereon whitei Broom, 1937 fourth metatarsal specimens from Kromdraai B (KB 5334a and 5339c [AP: 16.70 mm, ML: 10.82 mm]) and differs in proximal articular surface morphology. It is similarly sized to Dinofelis sp. metapodials from Swartkrans and Kromdraai and fourth metatarsals from Drimolen (Dinofelis aff. piveteaui Ewer, 1955; DN 14; AP: 18.6 mm, ML: 10.8 mm) and Malapa (Dinofelis barlowi Broom, 1937; UW 88-594; ML: 13.3 mm; Kuhn et al., 2012). HGD 950 does differ from the Drimolen D. aff. piveteaui specimen in exhibiting a mediolaterally-broader proximal articular surface with a straighter anterior margin, a more gracile facet for the third metatarsal, and discrete anterior and posterior facets for the fifth metatarsal (O'Regan and Menter, 2009, figure 5D-F). Those differences aside, a distinct feature of the HGD specimen is the presence of a wide groove on the ventral diaphysis that extends from the proximal end to the break edge. This morphology has been previously noted by Werdelin and Lewis (2001) on an eastern African Dinofelis piveteaui fourth metatarsal (KNM-ER 722U). This generic identification is considered provisional, however, given the remote possibility that HGD 950 represents an immature individual of Panthera leo Linnaeus, 1758.
Analysis of the previously undocumented assemblage identified two carnivore postcranial elements, including a right fourth metatarsal preserving the proximal articular surface and approximately 75% of the diaphysis (HGD 950; Figure 13). Only minimal dimensions of the proximal articular surface could be measured (anteroposterior [AP]: 18.98mm, mediolateral [ML]: 14.92mm) because of abrasions to the margins of the specimen, and it is important to note that the woven appearance of the articular surface suggests that the specimen may be from an immature individual. In its preserved state HGD 950 is larger than comparative specimens of modern and fossil Panthera pardus Linnaeus, 1758 and Megantereon whitei Broom, 1937 fourth metatarsal specimens from Kromdraai B (KB 5334a and 5339c [AP: 16.70 mm, ML: 10.82 mm]) and differs in proximal articular surface morphology. It is similarly sized to Dinofelis sp. metapodials from Swartkrans and Kromdraai and fourth metatarsals from Drimolen (Dinofelis aff. piveteaui Ewer, 1955; DN 14; AP: 18.6 mm, ML: 10.8 mm) and Malapa (Dinofelis barlowi Broom, 1937; UW 88-594; ML: 13.3 mm; Kuhn et al., 2012). HGD 950 does differ from the Drimolen D. aff. piveteaui specimen in exhibiting a mediolaterally-broader proximal articular surface with a straighter anterior margin, a more gracile facet for the third metatarsal, and discrete anterior and posterior facets for the fifth metatarsal (O'Regan and Menter, 2009, figure 5D-F). Those differences aside, a distinct feature of the HGD specimen is the presence of a wide groove on the ventral diaphysis that extends from the proximal end to the break edge. This morphology has been previously noted by Werdelin and Lewis (2001) on an eastern African Dinofelis piveteaui fourth metatarsal (KNM-ER 722U). This generic identification is considered provisional, however, given the remote possibility that HGD 950 represents an immature individual of Panthera leo Linnaeus, 1758.
Carnivora gen. et sp. indet.
The only other definitive carnivore specimen is a partial articular surface from a distal metapodial (HGD 2333). Although it could be derived from the same individual represented by the HGD 950 cf. Dinofelis sp. specimen, it is insufficiently diagnostic to confidently attribute to the genus to the exclusion of other large-bodied felids and hyaenids.
Order PERISSODACTYLA Owen, 1848
Family EQUIDAE Gray, 1821
Genus EQUUS Linnaeus, 1758
Type species Equus caballus Linnaeus, 1758
Equus capensis (Broom, 1909b)
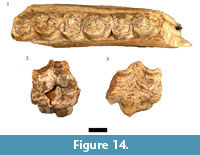 This species is represented in the HGD sample by nine craniodental and 10 postcranial specimens derived from minimally a single adult individual. The most complete Equus capensis specimen is the HGD 1105 left mandible, which preserves the occluded crowns of three of the premolars and molars (p3-m1 or p4-m2; Figure 14.1). The teeth exhibit the diagnostic features of E. capensis mandibular dentition including the absence of a protostylid, the large hypolophids, and parastylids that do not reach the lingual margin of the crown near the metaconid (Cooke, 1950; Churcher and Richardson, 1978); and the overall size of the teeth (p3/p4 MD: 29.61 mm, BL: 17.45 mm; p4/m1 MD: 28.99 mm, BL: 17.51 mm; m1/m2 BL: 16.71 mm) are comparable to that of E. capensis mandibular specimens from Swartkrans, Sterkfontein, and Kromdraai (Churcher, 1970).
This species is represented in the HGD sample by nine craniodental and 10 postcranial specimens derived from minimally a single adult individual. The most complete Equus capensis specimen is the HGD 1105 left mandible, which preserves the occluded crowns of three of the premolars and molars (p3-m1 or p4-m2; Figure 14.1). The teeth exhibit the diagnostic features of E. capensis mandibular dentition including the absence of a protostylid, the large hypolophids, and parastylids that do not reach the lingual margin of the crown near the metaconid (Cooke, 1950; Churcher and Richardson, 1978); and the overall size of the teeth (p3/p4 MD: 29.61 mm, BL: 17.45 mm; p4/m1 MD: 28.99 mm, BL: 17.51 mm; m1/m2 BL: 16.71 mm) are comparable to that of E. capensis mandibular specimens from Swartkrans, Sterkfontein, and Kromdraai (Churcher, 1970).
Equus cf. quagga (Boddaert, 1785) (sensu Klingel, in press cited by Bernor et al., 2010)
Only three maxillary specimens (HGD 1109, left P2; HGD 1091 and 1260, indeterminate premolar/molars) from minimally a single adult Equus quagga individual were identified from the HGD sample (Figure 14.2-14.3). Although only a single measurement could be confidently taken (HGD 1260 MD: 26.85mm), the specimens are similarly proportioned and morphologically indistinguishable from the extant plains zebra.
Equus sp.
In addition to the specifically identifiable equid remains a total of 51 specimens, including 13 partial teeth and 38 postcranial elements, could only be confidently attributed to the genus Equus.
Equidae gen. et sp. indet.
Two partial teeth (HGD 1265, 1269) were too damaged to be attributed to the genus, and although likely derived from either Equus capensis and/or Equus quagga individuals, they lack diagnostic features that exclude the possible contribution of a hipparionin to the HGD sample.
Order HYRACOIDEA Huxley, 1869
Family PROCAVIIDAE Thomas, 1892
Genus PROCAVIA Storr, 1780
Type species Procavia capensis Storr, 1780
Procavia antiqua (Broom, 1934)
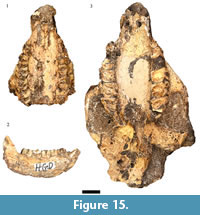 As noted above there are irreconcilable differences in the counts of hyrax specimens in the Council for Geosciences HGD catalog, the originally published HGD sample (Plug and Keyser, 1994), and as located during our recent survey (Table 1). The resurvey of the collections found 17 craniodental specimens originally attributed to Procavia capensis in the collections. Despite an attempt to subsume Procavia antiqua into P. capensis based on metric grounds (McMahon and Thackeray, 1994), later analysis identified a suite of characteristics that separate fossil P. antiqua from extant P. capensis (Schwartz, 1997). The small HGD Procavia specimens exhibit the elongated ectolophs, bunodont protocones, strong M2 mesostyles, and well-developed M3 hypocones consistent with attribution to P. antiqua (Broom, 1934; Schwartz, 1997). Within the sample of minimally eight individuals are three adults with fully erupted dentitions and an additional five immature individuals, ranging from the approximately one year old HGD 1134 right mandible (with second molars just in occlusion) to the approximately one month old HGD 1151 right mandible (preserving deciduous molars and an erupting permanent m1) (Fairall, 1980; Figure 15.1-15.2).
As noted above there are irreconcilable differences in the counts of hyrax specimens in the Council for Geosciences HGD catalog, the originally published HGD sample (Plug and Keyser, 1994), and as located during our recent survey (Table 1). The resurvey of the collections found 17 craniodental specimens originally attributed to Procavia capensis in the collections. Despite an attempt to subsume Procavia antiqua into P. capensis based on metric grounds (McMahon and Thackeray, 1994), later analysis identified a suite of characteristics that separate fossil P. antiqua from extant P. capensis (Schwartz, 1997). The small HGD Procavia specimens exhibit the elongated ectolophs, bunodont protocones, strong M2 mesostyles, and well-developed M3 hypocones consistent with attribution to P. antiqua (Broom, 1934; Schwartz, 1997). Within the sample of minimally eight individuals are three adults with fully erupted dentitions and an additional five immature individuals, ranging from the approximately one year old HGD 1134 right mandible (with second molars just in occlusion) to the approximately one month old HGD 1151 right mandible (preserving deciduous molars and an erupting permanent m1) (Fairall, 1980; Figure 15.1-15.2).
Procavia transvaalensis (Shaw, 1937)
A minimum of 13 individuals are represented in the sample of 44 specimens attributable to the extinct large bodied hyrax Procavia transvaalensis, including 11 adults with fully erupted dentitions and two immature individuals (HGD 1144: m2 partially erupted; HGD 1145: m3 partially erupted) (Figure 15.3). If the modern Procavia capensis dental eruption schedule (Fairall, 1980) is applied to these immature specimens they would represent ~10 month old and ~15 month old individuals at the time of deposition, respectively.
Procavia sp.
Six of the identifiable hyrax specimens, including three partial mandibles (HGD 675, 1159, 1357), an isolated lower molar (HGD 676), a maxilla (HGD 1201), and maxillary central incisor (HGD 1270) could not be confidently attributed to either Procavia capensis or Procavia transvaalensis because of damage or distortion and are retained at the generic level.
Order LAGOMORPHA Brandt, 1855
Family LEPORIDAE Fischer von Waldheim, 1817
Genus PRONOLAGUS Lyon, 1904
Type species Pronolagus crassicaudatus Geoffroy, 1832
Pronolagus rupestris (Smith, 1834)
Despite the name 'Haasgat' meaning 'hare hole' in Afrikaans, only three total leporid specimens were identified in the HGD deposits. Two of these specimens (HGD 827, right mandible; HGD 1113, near complete humerus) are attributable to the extant red rock hare Pronolagus rupestris.
Leporidae gen. et sp. indet.
A second mandible (HGD 1264, probable right) is a leporid and may also represent another P. rupestris individual but will require further removal of adhering matrix to diagnose.
Order RODENTIA Bowdich, 1821
Family HYSTRICIDAE Fischer de Waldheim, 1817
Genus HYSTRIX Linnaeus, 1758
Type species Hystrix cristata Linnaeus, 1758
Hystrix africaeaustralis (Peters, 1852)
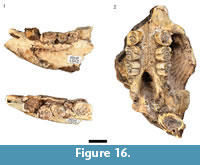 Although Hystrix africaeaustralis remains from the processed blocks were noted by Keyser (1991) and Keyser and Martini (1991), no counts or descriptions of the HGD porcupine specimens were published, and no entries were made in the HGD catalog. Other than a partial right femur (HGD 936), the identifiable H. africaeaustralis specimens located in this analysis are craniodental and include six partial crania, 12 mandibular specimens, and 19 isolated teeth from a minimum of nine individuals (Figure 16). One of the partial crania (HGD 1354) and six of the mandibles (HGD 1341, 1342, 1343, 1348, 1349, 1350) are derived from minimally five adult individuals (>24 months of age) with fully erupted and variably-occluded fourth premolars (Stage VI or higher; van Aarde, 1985). At least three subadult individuals (between six and 11 months of age; Stage III-IV) with deciduous fourth premolars and variably-erupted molars are represented by three partial crania (HGD 1351, 1352, 1353) and three mandibles (HGD 1345, 1346, 1347). A somewhat younger individual (between five and six months of age; Stage III) is represented by the HGD 1344 partial mandible that preserves a deciduous forth premolar and lightly occluded first molar.
Although Hystrix africaeaustralis remains from the processed blocks were noted by Keyser (1991) and Keyser and Martini (1991), no counts or descriptions of the HGD porcupine specimens were published, and no entries were made in the HGD catalog. Other than a partial right femur (HGD 936), the identifiable H. africaeaustralis specimens located in this analysis are craniodental and include six partial crania, 12 mandibular specimens, and 19 isolated teeth from a minimum of nine individuals (Figure 16). One of the partial crania (HGD 1354) and six of the mandibles (HGD 1341, 1342, 1343, 1348, 1349, 1350) are derived from minimally five adult individuals (>24 months of age) with fully erupted and variably-occluded fourth premolars (Stage VI or higher; van Aarde, 1985). At least three subadult individuals (between six and 11 months of age; Stage III-IV) with deciduous fourth premolars and variably-erupted molars are represented by three partial crania (HGD 1351, 1352, 1353) and three mandibles (HGD 1345, 1346, 1347). A somewhat younger individual (between five and six months of age; Stage III) is represented by the HGD 1344 partial mandible that preserves a deciduous forth premolar and lightly occluded first molar.
Species Removed from the Haasgat HGD Faunal List
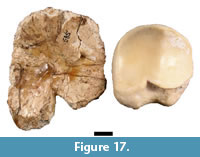 Part of a single intermediate phalanx (HGD 585) was cataloged as Giraffa camelopardalis Linnaeus, 1758 and described by Plug and Keyser (1994) as similar to that of the modern giraffe. Although the specimen was damaged during preparation and distorted when stabilized using consolidant, HGD 585 does not conform to intermediate phalanges of extant G. camelopardalis in either morphology or size (Figure 17). The gross resemblance of the HGD 585 specimen outline to that of a modern giraffe intermediate phalanx is due to preparation damage to the margins rather than shared morphology. The specimen, which is flattened cortical bone only a few millimeters thick, lacks a clearly defined articular surface on the 'proximal surface,' and the 'distal' aspect of the element does not have trabecular bone or a broken metaphyseal surface that would be expected of an intermediate phalanx.
Part of a single intermediate phalanx (HGD 585) was cataloged as Giraffa camelopardalis Linnaeus, 1758 and described by Plug and Keyser (1994) as similar to that of the modern giraffe. Although the specimen was damaged during preparation and distorted when stabilized using consolidant, HGD 585 does not conform to intermediate phalanges of extant G. camelopardalis in either morphology or size (Figure 17). The gross resemblance of the HGD 585 specimen outline to that of a modern giraffe intermediate phalanx is due to preparation damage to the margins rather than shared morphology. The specimen, which is flattened cortical bone only a few millimeters thick, lacks a clearly defined articular surface on the 'proximal surface,' and the 'distal' aspect of the element does not have trabecular bone or a broken metaphyseal surface that would be expected of an intermediate phalanx.
In the original faunal description Plug and Keyser (1994) list six individual specimens from minimally one individual as representing Sylvicapra grimmia Linnaeus, 1758 in the HGD collections. The recovery of S. grimmia in the potentially early Pleistocene Haasgat deposits was a significant finding and would represent the oldest documented occurrence of the species in the African fossil record (Gentry, 2010). Evaluation of the original catalog indicates only two of the six specimens were recorded as S. grimmia. The HGD 327 specimen, cataloged as a left maxilla preserving a complete molar row, could not be located. A specimen labeled HGD 327 was found but it is a cercopithecid distal femur. The HGD 31 right maxilla, preserving a partial M2 and complete M3, was found during our survey (Figure 18). 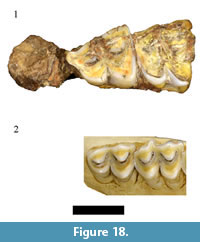 The preserved molars are visibly larger than those of extant Sylvicapra and lack the prominent rib and metacone development on the ectoloph that are diagnostic to cephalophins generally (Gentry, 2011). The specimen is, however, consistent in both molar morphology and dimensions (M3 MD: 13.13, BL: 11.03) with Oreotragus maxillae preserved in the HGD assemblage (see above; Figure 8 and Figure 9). As no additional cephalophin specimens were identified during our 2010 survey, attribution of the HGD 31 specimen to Oreotragus sp. eliminates the only evidence of the S. grimmia from the HGD sample.
The preserved molars are visibly larger than those of extant Sylvicapra and lack the prominent rib and metacone development on the ectoloph that are diagnostic to cephalophins generally (Gentry, 2011). The specimen is, however, consistent in both molar morphology and dimensions (M3 MD: 13.13, BL: 11.03) with Oreotragus maxillae preserved in the HGD assemblage (see above; Figure 8 and Figure 9). As no additional cephalophin specimens were identified during our 2010 survey, attribution of the HGD 31 specimen to Oreotragus sp. eliminates the only evidence of the S. grimmia from the HGD sample.
There is a limited record for the oribi (Ourebia ourebi Zimmerman, 1783) at African fossil localities.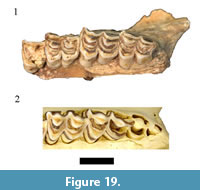 Only five specimens from Swartkrans Member 2 (Vrba, 1976) and a single possible specimen from Kanjera, Kenya (Gentry, 2010) have been reported, making the described HGD collection of 17 specimens the largest single sample in the African fossil record. Only five of the originally listed O. ourebi specimens were cataloged (HGD 24, 25, 27, 35, 36) and although all were located, none were found to exhibit morphologies consistent with attributing them to O. ourebi (Figure 19). The HGD 36 left partial m3 has a flat lingual enamel surface, small parastylid, and simple central cavities that align the specimen with Antidorcas (and most closely resembles Antidorcas recki). The dentition on the maxillae (HGD 24, 27, 35) lack the projecting ribs and styles and exhibit more angled surfaces to the protocones and metaconules than in extant O. ourebi (Figure 19). The assigned mandible (HGD 25) similarly lacks the projecting metaconid, entoconid, stylids, and persistent central cavities exhibited in oribi mandibles. In these respects, the HGD specimens are morphologically identical to other Oreotragus sp. specimens in the assemblage (see above; Figure 8 and Figure 9). With no additional neotragin specimens from the previously undocumented collections attributable to Ourebia, this reanalysis has found no support for the presence of the species within the HGD assemblage.
Only five specimens from Swartkrans Member 2 (Vrba, 1976) and a single possible specimen from Kanjera, Kenya (Gentry, 2010) have been reported, making the described HGD collection of 17 specimens the largest single sample in the African fossil record. Only five of the originally listed O. ourebi specimens were cataloged (HGD 24, 25, 27, 35, 36) and although all were located, none were found to exhibit morphologies consistent with attributing them to O. ourebi (Figure 19). The HGD 36 left partial m3 has a flat lingual enamel surface, small parastylid, and simple central cavities that align the specimen with Antidorcas (and most closely resembles Antidorcas recki). The dentition on the maxillae (HGD 24, 27, 35) lack the projecting ribs and styles and exhibit more angled surfaces to the protocones and metaconules than in extant O. ourebi (Figure 19). The assigned mandible (HGD 25) similarly lacks the projecting metaconid, entoconid, stylids, and persistent central cavities exhibited in oribi mandibles. In these respects, the HGD specimens are morphologically identical to other Oreotragus sp. specimens in the assemblage (see above; Figure 8 and Figure 9). With no additional neotragin specimens from the previously undocumented collections attributable to Ourebia, this reanalysis has found no support for the presence of the species within the HGD assemblage.
Finally, of the two specimens reported by Plug and Keyser (1994) as Raphicerus sp. Smith, 1827, only a single proximal metacarpal specimen (HGD 591) was cataloged. The specimen is a very small Class I metacarpal consistent with extant Raphicerus specimens in size, but the noted overlap in size and general morphology with some larger cephalophin metacarpals prohibited confident allocation of the specimen below the Family level.
DISCUSSION
Several factors contribute to the described differences between the previously published Haasgat fossil faunas and the revised HGD assemblage. Although interobserver differences in classification methodology undoubtedly underlie some of the variance in the faunal lists, the addition of nearly twice the number of HGD specimens in the analysis and loss of specimens likely play a more significant role. Unfortunately, other than the handful of documented specimens that are no longer in the collection, the incomplete original catalog makes it impossible to gauge the degree to which loss has shaped the current HGD assemblage. Direct categorical comparison of the previously published counts with the existing collections, however, implies the loss of hundreds of identifiable Bovidae and Hyracoidea. As a result, even though the taxonomic composition of the originally described HGD assemblage and the revised listing presented here are not radically different, the sample size for generating these two faunal listings may have been.
The changes to the HGD faunal list do impact several previously proposed paleobiological, paleoecological, and chronological interpretations of the site. The reassignment of the single cataloged and locatable specimen of Sylvicapra grimmia eliminates the earliest proposed occurrence of the species in the fossil record prior to the Late Pleistocene (Gentry, 2010). The reclassification of Ourebia ourebi and Giraffa camelopardalis specimens also removes two species that are rarely encountered in South African Pliocene or Pleistocene cave assemblages (Gentry, 2010; Harris et al., 2010). Simultaneously, the revisions to the classification of Primates, the identification of cf. Dinofelis, and inclusion of the Lagomorpha and Rodentia from the previously undescribed HGD collections has expanded the range of mammals documented from the site.
Initially the fauna identified from the HGD assemblage was used to reconstruct a heterogeneous paleoecosystem around Haasgat: proximate montane forests on rocky slopes (Cercopithecoides, Oreotragus, Procavia), nearby open woodlands and/or grasslands (Alcelaphini, Giraffa, Tragelaphus), and seasonally to permanently inundated floodplains or swampland (Kobus) (McKee and Keyser, 1994; Plug and Keyser, 1994; McKee et al., 2011). While the diverse range of browse- and graze-adapted species in the revised HGD assemblage still indicate a mixed Haasgat paleoenvironment, the modified faunal list no longer supports some of the specifically proposed paleohabitat types. The dietary and specialized locomotor adaptations of extant Oreotragus and Procavia do indicate that the topography of the Haasgat region broadly resembled the modern landscape of rocky hillsides with bush and open tree cover (Kingdon, 1982: Olds and Shoshani, 1982; Smithers and Chimimba, 2005). While reduncins generally prefer grazing in edaphic grassland or river margin habitats there is substantial variation in the diet and habitat utilization across extant Kobus and Redunca species (Jungius, 1971; Irby, 1976; Kingdon, 1982; Smithers and Chimimba, 2005), and the Tribal-level attribution of the HGD specimens advocated here would not specifically support the presence of local swamp environs.
Establishing the presence and extent of closed forest habitats at Haasgat on the occurrence of colobins is complicated. The reconstructed body size and postcranial adaptations of Cercopithecoides williamsi indicate a greater degree of terrestriality and less reliance on closed forest habitats than extant colobins (Benefit and McCrossin, 1990). Recent studies have also detected significant C4 components in the diet of some sampled C. williamsi individuals and greater within-species paleodietary diversity than occurs in other contemporaneous primate genera (Codron et al., 2005; El-Zataari et al., 2005; Fourie et al., 2008). Although these terrestrial/open habitat indicators for C. williamsi may ultimately not bear on biobehavioral interpretations of the syntopic Cercopithecoides haasgati, it does suggest it is premature to assume taxonomic uniformity in habitat requirements between fossil and extant colobins without further analysis.
Finally, the revisions to the HGD assemblage impact site biochronology. Initially, Keyser and Martini (1991) suggested that the elevation of the system relative to the modern valley floor and erosional deroofing might broadly indicate fossil deposition during the terminal Pliocene. Subsequently, McKee and Keyser (1994) suggested that the shared occurrence of Papio angusticeps at Haasgat, Kromdraai A and B (1.8-1.7 m.y.a.; Herries et al., 2009) and Cooper's deposits (1.526-1.4 m.y.a.; de Ruiter et al., 2009) implied the deposits were likely contemporaneous. In slight contrast, with a non-primate faunal list dominated by extant species Plug and Keyser (1994) concluded that the sampled HGD deposits were likely younger than 1 m.y.a., but adjusted their potential depositional age estimate (1.5-0.5 m.y.a.) to accommodate the presence of P. angusticeps.
The recovery of Equus indicates that at least some of the deposits represented in the ex situ HGD assemblage are no older than 2.33 m.y.a., given the first occurrence of the genus in Africa in the Omo Shungura lower Member G deposits (Geraads et al., 2004). Although neither of the two recovered suid teeth were specifically attributable, the morphology of the HGD 1324 specimen is analogous to phacochoerin specimens from the 2.3-1.7 m.y.a. deposits at Bolt's Farm, Gondolin GD 2, Swartkrans Members 1 and 2, and Kromdraai A (Harris and White, 1979; Cooke, 1993; Adams, 2005; Herries et al., 2006; Herries et al., 2009; Pickering et al., 2011). At maximum, the morphology of this partial tooth is very advanced compared to Metridiochoerus from Makapansgat Member 3 (3.03-2.58 m.y.a.; Harris and White, 1979; Herries, 2003; Cooke, 2005), but primitive relative to extant Phacochoerus and advanced Metridiochoerus species (Metridiochoerus modestus van Hoepen and van Hoepen, 1932 and Metridiochoerus compactus van Hoepen and van Hoepen, 1932) recovered from the <1.89 m.y.a. eastern African deposits (Harris and White, 1979; White, 1995) and Cornelia (1.07-0.99 m.y.a.; Harris and White, 1979; Brink et al., 2012).
Establishing site biochronology from the HGD primates is complicated by the uniqueness of the recovered species relative to those identified at other South African assemblages. At present, Papio angusticeps is only recognized from Haasgat and Kromdraai as most of the specimens historically attributed to P. angusticeps have since been subsumed into Papio izodi Gear, 1926 (Szalay and Delson, 1979; McKee and Keyser, 1994; Heaton, 2006; Jablonski and Frost, 2010). Although this shared occurrence may suggest similar ages for the deposits, there is as yet no defined first or last appearance date for the species. There is no chronological data on the novel colobin Cercopithecoides haasgati as it has yet to be identified from any other fossil deposit. Specimens of Cercopithecoides williamsi have been identified in eastern African deposits as early as 3.5 m.y.a. (Jablonski and Frost, 2010) and South African deposits ranging in age from Makapansgat Member 3 (3.03-2.58 m.y.a.; Herries et al., 2009) and Bolt's Farm Pit 23 (likely contemporaneous with Makapansgat Member 3; Cooke, 1993) to Swartkrans Member 2 (1.7-1.1 m.y.a.; Herries et al., 2009). As noted above, two of the C. williamsi mandibles more closely resemble those from Sterkfontein Member 4 (2.6-2.0 m.y.a.; Pickering and Kramers 2010; Herries and Shaw, 2011) than those recovered from Makapansgat Member 3 and Bolt's Farm Pit 23.
Although most of the bovid specimens could not be confidently assigned to species because of insufficient preservation or associated diagnostic elements (e.g., horn cores), the groups represented in the HGD assemblage are broadly shared with other nearby South African terminal Pliocene and Pleistocene assemblages (Vrba, 1976; Brain, 1981; Adams, 2010; Gentry, 2010). The identification of Damaliscus dorcas (with a current first appearance at Florisbad; 0.295-0.225 m.y.a.; Brink, 1987; Herries, 2011) and Connochaetes gnou (with a current first appearance at Cornelia; 1.07-0.99 m.y.a.; Brink et al., 2012) in the HGD sample would imply a mid- to late-Pleistocene age for at least some of the sampled fauna. In contrast, assessed differences between the most common bovid in the HGD assemblage, Oreotragus sp., indicate that they are not derived from extant Oreotragus oreotragus or the same populations as represented at Makapansgat Member 3 or Gondolin GD 2 (~1.8 m.y.a.; Herries et al., 2006). Even though the differences between the HGD and Makapansgat Member 3 Oreotragus specimens could be attributed to both temporal and/or geographic separation of the populations, Gondolin is located only 4 km away from Haasgat; potentially within the 15-49 hectare home range area encompassed by even a single mated pair of Oreotragus (Norton, 1981). Without evidence for geographic isolation of the HGD and Gondolin GD 2 Oreotragus breeding populations, the significant differences between samples suggest that contemporaneous deposition at ~1.8 m.y.a. is highly unlikely. While this could be taken to only imply that the HGD specimens either pre- or post-date the Gondolin GD 2 deposits, formation of the Oreotragus-bearing components of the HGD assemblage prior to 1.8 m.y.a. is more parsimonious given the shared features of the Haasgat and Makapansgat Oreotragus samples to the exclusion of extant Oreotragus oreotragus.
When integrated with the other biochronological indicators, this interpretation of the HGD Oreotragus collection suggests a depositional period of between 2.3 m.y.a. and 1.9 m.y.a. for at least some of the sampled fauna; although the recovery of extant species indicates that fauna from younger deposits is also present in the assemblage. However, as a limited ex situ sample, accurate assessment of species representation at the site, the paleoecology of the region, and the chronology of the Haasgat cave system can only be resolved once results from future in situ excavations, paleomagnetic sampling and uranium-lead analysis of the Haasgat deposits have been integrated.
ACKNOWLEDGMENTS
I wish to thank L. Marais-Botes, E. De Kock, and A. Raath of the Council for Geosciences for their assistance accessing the Haasgat fossil sample and for allowing our team to curate and reanalyze the HGD assemblage. I am indebted to S. Potze, L. Kgasi, T. Kearney, S. Badenhorst, and T. Perregil of the Ditsong National Museum of Natural History, and B. Zipfel and B. Kuhn of the Bernard Price Institute for access to the modern and Plio-Pleistocene fossil mammal collections used in this analysis. A. D.T. Kegley and J. Hemingway provided essential assistance in recalculating the NISP and MNI counts for the Cercopithecoides sample, and J. Brink provided valuable insight on the Haasgat alcelaphins. Two anonymous reviewers provided valuable comments and suggestions on the draft manuscript. Finally, I would like thank the Tetley family and entire Kalkheuvel West community for their support of our continued research at Haasgat. Research on the Haasgat site and faunal samples was funded by the National Science Foundation (NSF BCS 0962564).
REFERENCES
Adams, J. 2005. A methodology for the intraspecific assessment of heterogeneously worn hypsodont teeth using computerized tomography. Journal of Taphonomy, 3:161-172.
Adams, J. 2006. Taphonomy and paleoecology of the Gondolin Plio-Pleistocene cave site, South Africa. Unpublished Ph.D. Thesis, Washington University in St. Louis, St. Louis, Missouri, USA.
Adams, J. 2010. Taphonomy of the Gondolin GD 2 in situ deposits and its bearing on interpretations of South African Plio-Pleistocene karstic fossil assemblages. Journal of Taphonomy, 8:81-116.
Adams, J. and Conroy, G.C. 2005. Plio-Pleistocene faunal remains from the Gondolin GD 2 in situ assemblage, North West Province, South Africa, p. 243-261. In Lieberman, D., Smith, R.J., and Kelley, J. (eds.), Interpreting the past: essays on human, primate and mammal evolution in honor of David Pillbeam. Brill Academic Publishers, Boston.
Afzelius, A. 1815. De Antelopis in Genere et Speciatim Guineenfibus. Nova Acta Regiae Societatis Scientiarum Upsaliensis, 7:195-270.
Angas, G. 1848. Description of Tragelaphus Angasii Gray, with some account of its habits. Proceedings of the Zoological Society of London, 1848:89-90.
Benefit, B. and McCrossin, M. 1990. Diet, species diversity, and distribution of African fossil baboons. Kroeber Anthropological Society Papers, 71:79-93.
Bernor, R., Armour-Chelu, M., Gilbert, H., Kaiser, T., and Schulz, E. 2010. Equidae, p. 685-722. In Werdelin, L. and Sanders, W. (eds.), Cenozoic mammals of Africa. University of California Press, Berkeley.
Blyth, E. 1863. Catalogue of the Mammalia in the museum of the Asiatic Society of Bengal. The Asiatic Society, Calcutta.
Blyth, E. 1875. Catalogue of mammals and birds of Burma. Journal of the Asiatic Society of Bengal, Part II.
Boddaert, P. 1785. Elenchus animalium, volumen 1: Sistens quadrupedia huc usque nota, erorumque varietates. C.R. Hake, Rotterdam.
Bowditch, T. 1821. An analysis of the natural classifications of Mammalia for the use of students and travelers. Smith, Paris.
Brain, C. 1976. Some principles of in the interpretation of bone accumulations associated with man, p. 97-116. In Isaac, G. and McCown, E. (eds.), Human origins: Louis Leakey and the east African evidence. W.A. Benjamin, Inc., Menlo Park.
Brain, C. 1981. The hunters or the hunted? An introduction to African cave taphonomy. University of Chicago Press, Chicago.
Brandt, J. 1855. Beitrage zur nahern Kenntniss der Säugethiere Russland's. Kaiserlichen Akademie der Wissenschaften, Saint Petersburg, Mémoires Mathématiques, Physiques et Naturelles, 7:1-365.
Brink, J. 1987. The archaeozoology of Florisbad, Orange Free State. Memoirs van die Nasionale Museum Bloemfontein, 23:1-151.
Brink, J., Herries, A., Moggi-Cecchi, J., Gowlett, J., Bousman, C., Hancox, J., Grün, R., Eisenmann, V., Adams, J., and Roussouw, L. 2012. First hominin remains from a ~1.0 million year old bone bed at Cornelia-Uitzoek, Free State Province, South Africa. Journal of Human Evolution 63:527-535.
Brink, J. and Stynder, D. 2009. Morphological and trophic distinction in the dentitions of two early alcelaphine bovids from Langebaanweg (genus Damalacra). Palaeontologia africana, 44:139-193.
Broom, R. 1909a. On a large extinct species of Bubalis. Annals of the South African Museum, 7:279-280.
Broom, R. 1909b. On evidence of a large horse recently extinct in South Africa. Annals of the South African Museum, 7:281-282.
Broom, R. 1934. On the fossil remains associated with Australopithecus africanus. South African Journal of Science, 31:471-480.
Broom, R. 1937. Notices of a few more new fossil mammals from the caves of the Transvaal. Annals and Magazine of Natural History, 10:509-514.
Burchell, W. 1824. Travels in the interior of Southern Africa. Longman, Hurst, Rees, Orme, Brown and Green, London.
Burnett, G. 1828. Illustrations of the manupeda, or apes and their allies; being the arrangement of the quadrumana or anthropomorphous beasts indicated in outline. Quarterly Journal of Science, Literature, and Art, 25:300-307.
Churcher, C. 1970. The fossil Equidae from the Krugersdorp caves. Annals of the Transvaal Museum, 26:145-179.
Churcher, C. and Richardson, M. 1978. Equidae, p. 379-422. In Maglio, V. and Cooke, H. (eds.), Evolution of African Mammals. Harvard University Press, Cambridge.
Codron, D., Luyt, J., Lee-Thorp, J., Sponheimer, M., de Ruiter, D.J., and Codron, J. 2005. Utilization of savanna-based resources by Plio-Pleistocene baboons. South African Journal of Science, 101:245-248.
Cooke, H. 1950. A critical revision of the Quarternary Perissodactyla of Southern Africa. Annals of the South African Museum, 31:393-476.
Cooke, H. 1993. Undescribed suid remains from Bolt's Farm and other Transvaal cave deposits. Palaeontologia Africana, 30:7-23.
Cooke, H. 2005. Makapansgat suids and Metridiochoerus. Palaeontologia Africana, 41:131-140.
Cooke, H. and Wells, L. 1951. Fossil remains from Chelmer, near Bulawayo, Southern Rhodesia. South African Journal of Science, 47:205-209.
de Blainville, H. 1816. Su plusieurs espèces d'animaux mammifères, de l'ordre des Ruminants. Bulletin des sciences par la Société philomathique de Paris, 1816:73-82.
de Rochebrune, A. 1883. Faune de la Sénégambie. O. Doin, Paris.
de Ruiter, D., Pickering, R., Steininger, C., Kramers, J., Hancox, P., Churchill, S., Berger, L., and Backwell, L. 2009. New Australopithecus robustus fossils and associated U-Pb dates from Cooper's Cave (Gauteng, South Africa). Journal of Human Evolution 56: 497-513.
Desmarest, A. 1820. Mammalogie ou description des especes des Mammifères. Veuve Agasse, Paris.
Eisenhart, W. 1974. The fossil cercopithecoids of Makapansgat and Sterkfontein, Unpublished BA Thesis, Harvard College, Cambridge, Massachusetts, USA.
El-Zaatari, S., Grine, F., Teaford, M., and Smith, H. 2005. Molar microwear and dietary reconstructions of fossil Cercopithecoidea from the Plio-Pleistocene deposits of South Africa. Journal of Human Evolution, 49:180-205.
Ewer, R. 1954. The fossil carnivores of the Transvaal caves: the Lycyaenas of Sterkfontein and Swartkrans, together with some general considerations of the Transvaal fossil hyaenids. Proceedings of the Zoological Society London, 124:839-57.
Ewer, R. 1955. The fossil carnivores of the Transvaal caves: Machairodontinae. Proceedings of the Zoological Society London, 125:587-615.
Fairall, N. 1980. Growth and age determination in the hyrax Procavia capensis. South African Journal of Zoology, 15:16-21.
Fischer von Waldheim, J. 1817. Adversaria Zoologica, fasciculus primus. Memoires de la Societe des Naturalistes de Moscou, 5:357-446.
Fourie, N., Lee-Thorp, J., and Ackerman, R. 2008. Biogeochemical and craniometric investigation of dietary ecology, niche separation, and taxonomy of Plio-Pleistocene cercopithecoides from the Makapansgat Limeworks. American Journal of Physical Anthropology, 135:121-135.
Freedman, L. 1957. The fossil Cercopithecoidea of South Africa. Annals of the Transvaal Museum, 23:121-262.
Gear, J. 1926. A preliminary account of the baboon remains from Taungs. South African Journal of Science, 23:731-747.
Gentry, A. 1980. Fossil Bovidae (Mammalia) from Langebaanweg South Africa. Annals of the South African Museum, 79:213-337.
Gentry, A. 1992. The subfamilies and tribes of the family Bovidae. Mammal Review, 22:1-32.
Gentry, A. 2000. Caprinae and Hippotragini (Bovidae, Mammalia) in the upper Miocene, p. 65-83. In Vrba, E. and Schaller, G. (eds.), Antelopes, deer and relatives: fossil record, behavioral ecology, systematics and conservation. Yale University Press, New Haven.
Gentry, A. 2010. Bovidae, p. 741-796. In Werdelin, L. and Sanders, W. (eds.), Cenozoic Mammals of Africa. University of California Press, Berkeley.
Gentry, A. 2011. Bovidae, p. 363-465. In Harrison, T. (ed.), Paleontology and Geology of Laetoli: Human Evolution in Context. Vertebrate paleobiology and paleoanthropology series. Springer, New York.
Geoffroy Saint-Hilaire, É. 1803. Catalogue des mammifères du Muséum National d'Histoire Naturelle. Muséum National d'Histoire Naturelle, Paris.
Geoffroy Saint-Hilaire, É. 1843. Description des mammifères: nouveaux ou imparfaitement connus de la collection du Muséum d'histoire naturelle et remarques sur la classification et les caractères des mammifères. Archives du Muséum d'histoire naturelle, Paris, 2:486-592.
Geoffroy, I. 1832. A queue epaisse. L. crassicaudatus. Magasin de Zoologie, 2:cl. 1, pl. 9.
Geraads, D., Raynal, J., and Eisenmann, V. 2004. The earliest human occupation of North Africa: a reply to Sahnouni et al. (2002). Journal of Human Evolution, 46:751-761.
Gradstein, F., Ogg, J., Schmitz, M., and Ogg, G. 2012. The Geologic Time Scale 2012. Elsevier, Oxford.
Gray, J. 1821. On the natural arrangement of vertebrose animals. London Medical Repository, 15:296-310.
Gray, J. 1850. Catalogue of the specimens of Mammalia in the collection of the British Museum. Part III. Ungulata Furcipeda. British Museum, London.
Gray, J. 1868. Synopsis of the species of pigs (Suidae) in the British Museum. Proceedings of the Zoological Society of London, 1868:17-49.
Harris, W. 1838. On a new species of antelope. Proceedings of the Zoological Society of London, 1838:1-3.
Harris, J. and White, T. 1979. Evolution of the Plio-Pleistocene African Suidae. Transactions of the American Philosophical Society, 69:5-128.
Harris, J., Solounias, N., and Geraads, D. 2010. Giraffoidea, p. 797-811. In Werdelin, L. and Sanders, W. (eds.), Cenozoic mammals of Africa. University of California Press, Berkeley.
Heaton, J. 2006. Taxonomy of the Sterkfontein fossil Cercopithecinae: the Papionini of Members 2 and 4 (Gauteng, South Africa), Unpublished Ph.D. Thesis, Indiana University, Bloomington, Indiana, USA.
Hendey, Q. and Hendey, H. 1968. New Quaternary fossil sites near Swartklip, Cape Province. Annals of the South African Museum, 52:43-73.
Hernandez Fernandez, M. and Vrba, E. 2005. A complete estimate of the phylogenetic relationship in Ruminantia: a dated species-level supertree of the extant ruminants. Biological Reviews, 80:269-302.
Herries, A. 2003. Magnetostratigraphic seriation of South African hominin palaeocaves. Unpublished Ph.D. Thesis, University of Liverpool, Liverpool, UK.
Herries, A. 2011. A chronological perspective on the Acheulian and its transition to the Middle Stone Age in Southern Africa: the question of Fauresmith. International Journal of Evolutionary Biology, 9641401:25pp.
Herries, A. and Shaw, J. 2011. Palaeomagnetic analysis of the Sterkfontein palaeocave deposits; age implications for the hominin fossils and stone tool industries. Journal of Human Evolution, 60:523-539.
Herries, A., Curnoe, D., and Adams, J. 2009. A multi-disciplinary seriation of early Homo and Paranthropus bearing palaeocaves in southern Africa. Quaternary International, 202:14-28.
Herries, A., Reed, K.E., Kuykendall, K., and Latham, A. 2006. Speleology and magnetobiostratigraphic chronology of the Buffalo Cave fossil site, Makapansgat, South Africa. Quaternary Research, 66:233-245.
Hopwood, A. 1926. Some Mammalia from the Pliocene of Homa Mountain, Victoria Nyanza. Annals and Magazine of Natural History, 9:266-272.
Hopwood, A. 1934. New fossil mammals from Olduvai, Tanganyika Territory. Annals and Magazine of Natural History, 10:546-550.
Huxley, T. 1869. An Introduction to the Classification of Animals. John Churchill and Sons, London.
Irby, L. 1976. The ecology of mountain reedbuck in southern and eastern Africa, Unpublished Ph.D. Thesis, Texas A&M University, Dallas, USA.
Jablonski, N. and Frost, S. 2010. Cercopithecoidea, p. 393-428. In Werdelin, L. and Sanders, W. (eds.), Cenozoic mammals of Africa. University of California Press, Berkeley.
Jerdon, T. 1867. The mammals of India: a natural history of all the animals known to inhabit continental India. Thomas College Press, Roorkee.
Jones, T. 1937. A new fossil primate from Sterkfontein, Krugersdorp, Transvaal. South African Journal of Science, 33:709-728.
Jungius, H. 1971. The biology and behaviour of the reedbuck (Redunca arundinum Boddaert 1785) in the Kruger National Park. Mammalia Depicta. Verlag Paul Parey, Hamburg.
Kegley, A., Hemingway, J., and Adams, J. 2011. Odontometric analysis of the reanalyzed and expanded Cercopithecoides sample from the Haasgat fossil assemblage, Cradle of Humankind, South Africa. American Journal of Physical Anthropology, S144: 183.
Keyser, A.W. 1991. The palaeontology of Haasgat: a preliminary account. Palaeontologia africana, 28:29-33.
Keyser, A.W. and Martini, J. 1991. Haasgat: a new Plio-Pleistocene fossil occurrence, p. 119-129. In Heine, K. (ed.), Palaeoecology of Africa. A.A. Balkema, Rotterdam.
Kingdon, J. 1982. Bovids. East African mammals: an atlas of evolution in Africa, Part IIIC. University of Chicago, Chicago.
Kuhn, B., Werdelin, L., Hartstone-Rose, A., Lacruz, R., and Berger, L.R. 2012. Carnivoran remains from the Malapa hominin site, South Africa. PLOS One, 6:e26940.
Leakey, M. 1982. Extinct large colobins from the Plio-Pleistocene of Africa. American Journal of Physical Anthropology, 58:153-172.
Lichtenstein, M. 1814. Die Gattung Antilope. Magazin der Gesellschaft naturforschender Freunde zu Berlin, 6:147-160, 163-182.
Linnaeus, C. 1758. Systema naturae. Laurentii Salvii, Stockholm.
Lydekker, R. and Blaine, G. 1914. Catalogue of the ungulate mammals in the British Museum (Natural History). Trustees of the British Museum, London, United Kingdom 2:1-295, 3:1-283.
Lyon, M. 1904. Classification of the hares and their allies. Smithsonian Miscellaneous Collections, 44:322-443.
McKee, J. and Keyser, A.W. 1994. Craniodental remains of Papio angusticeps from the Haasgat cave site, South Africa. International Journal of Primatology, 15:823-841.
McKee, J., von Mayer, A., and Kuykendall, K. 2011. New species of Cercopithecoides from Haasgat, North West Province, South Africa. Journal of Human Evolution, 60:83-93.
McMahon, C. and Thackeray, J.F. 1994. Plio-Pleistocene Hyracoidea from Swartkrans cave, South Africa. South African Journal of Zoology, 29:40-45.
Mollett, O. 1947. Fossil mammals from the Makapan Valley, Potgietersrust. 1. Primates. South African Journal of Science, 43:295-303.
Norton, P. 1981. Activity patterns of klipspringers in two areas of the Cape Province. South African Journal of Wildlife Research, 11:126-134.
Ogilby, W. 1833. Characters of a new species of Antelope (Antilope ellipsiprymna), from the collection of Mr. Steedman. Proceedings of the Zoological Society of London, 1833:47-48.
Olds, N. and Shoshani, J. 1982. Procavia capensis. Mammalian Species, 171:1-7.
O'Regan, H. and Menter, C.G. 2009. Carnivora from the Plio-Pleistocene hominin site of Drimolen, Gauteng, South Africa. Geobios, 42:329-350.
Owen, R. 1848. Description of teeth and portions of jaws of two extinct Anthracotherioid quadrupeds (Hyopotamus vectianus and Hyop. bovinus) discovered by the Marchioness of Hastings in the Eocene deposits on the NW coast of the Isle of Wight: with an attempt to develop Cuvier's idea of the Classification of Pachyderms by the number of their toes. Quarterly Journal of the Geological Society of London, 4:103-141.
Pallas, P. 1766. Miscellanea Zoologica. P. van Cleef, Hagae.
Peters, W. 1852. Naturwissenschaftliche Reise nach Mosambique, Georg Reimer, Berlin.
Pickering, R. and Kramers, J. 2010. Re-appraisal of the stratigraphy and determination of new U-Pb dates for the Sterkfontein hominin site, South Africa. Journal of Human Evolution, 59:70-86.
Pickering, R., Kramers, J., Hancox, P., de Ruiter, D., Woodhead, J. 2011. Contemporary flowstone development links early hominin bearing cave deposits in South Africa. Earth and Planetary Science Letters, 306:23-32.
Plug, I. and Keyser, A.W. 1994. Haasgat Cave, a Pleistocene site in the central Transvaal: geomorphological, faunal and taphonomic considerations. Annals of the Transvaal Museum, 36:139-145.
Retzius, A. and Loven, S. 1845. Bericht von Retzius und Loven über Sundevall's Abhandlung, betitelt: Methodische Uebersicht der wiederkauenden Thiere. Archiv Skandinavischer Beitrage zur Naturgeschichte, Greifswald, 1:440-446.
Schreber, J. 1776. Die Säugthiere in Abbildungen nach der Natur mit Bescribungen, Teil 2. Wolfgang Walther, Erlangen.
Schwarz, E. 1932. Neue diluviale Antilopen aus Ostafrika. Zentralblatt für Mineralogie, Geologie und Paläontologie B, 1932:1-4.
Schwartz, G. 1997. Re-evaluation of the Plio-Pleistocene hyraxes (Mammalia: Procaviidae) from South Africa. Neues Jahrbuch fur Geologie und Paläontologie, Abhandlungen, 206:365-383.
Sclater, P. and Thomas, O. 1894. The Book of Antelopes. R.H. Porter, London.
Shaw, J. 1937. Evidence concerning a large fossil hyrax. Journal of Dental Research, 16:37-40.
Smith, C. 1827. Ruminantia, p. 342. In Griffith, E., Smith, C., and Pidgeon, E. (eds.), The animal kingdom arranged in conformity, with its organization by the Baron Cuvier, with additional descriptions of all the species hitherto named, and of many not before noticed. G.B. Whittaker, London.
Smith, A. 1834. An epitome of African zoology; or, a concise description of the objects of the animal kingdom inhabiting Africa, its islands and seas. South African Quarterly Journal, 2.
Smithers, R. and Chimimba, C. 2005. The Mammals of the Southern African Subregion. Cambridge University Press, Cambridge.
Sponheimer, M., Reed, K.E., and Lee-Thorp, J. 1999. Combining isotopic and ecomorphological data to refine bovid paleodietary reconstruction: a case study form the Makapansgat Limeworks hominin locality. Journal of Human Evolution, 36:705-718.
Statius Muller, P. 1773. Des Ritters C. von Linné: vollständiges Natursystem nach der zwölften Lateinischen Ausgabe und nach Anleitung des Holländischen Houttuynischen Werks, mit einer ausführlichen Erklärung Ausgefertiget von P.L.S. Müller. G.N. Raspe, Nurnberg.
Storr, G. 1780. Prodromus methodi mammalium. Litteris Reissianis, Tubingen.
Sundevall, C. 1846. Methodisk öfversigt af Idislande djuren, Linnés Pecora. Kongliga Vetenskaps Ajademiens Handlinger för år, 1844:121-210.
Sundevall, C. 1847. Methodisk öfversigt af Idislande djuren, Linnés Pecora. Kongliga Vetenskaps Ajademiens Handlinger för år, 1847:1-330.
Szalay, F.S. and Delson, E. 1979. Evolutionary History of the Primates. Academic, New York.
Thomas, O. 1892. On the species of the Hyracoidea. Proceedings of the Zoological Society of London, 1892:50-76.
Van Aarde, R. 1985. Age determination of Cape porcupines, Hystrix africaeaustralis. South African Journal of Zoology, 20:232-236.
van Hoepen, E. 1932. Voorlopige beskrywing van Vrystaatse soogdiere. Paleontologiese Navorsing van die Nasionale Museum Bloemfontein, 2:63-65.
van Hoepen, E. and van Hoepen, H. 1932. Vrystaate wilde varke. Paleontologiese Navorsing van die Nasionale Museum Bloemfontein, 2:39-62.
von Mayer, A. 1999. A reassessment of Cercopithecoides in southern Africa, Unpublished MSc Thesis, University of the Witwatersrand, Johannesburg, South Africa.
Vrba, E. 1976. The fossil Bovidae of Sterkfontein, Swartkrans and Kromdraai. Transvaal Museum Memoir, 21. Transvaal Museum, Pretoria.
Wagner, J. 1855. Eine Zusammenstellung der neuesten Entdeckungen auf dem Gebiete der Säugethierkunde., p. 394-461, Die Säugthiere in Abbildungen nach der Natur mit Beschreibungen. Supplement 5.
Warr, G. 2009. Chronology of the western Limeworks australopithecine site, Makapansgat, South Africa: magnetostratigraphy, biochronology and implications for hominin evolution, Unpublished Ph.D. Thesis, University of Liverpool, Liverpool, UK.
Watson, V. and Plug, I. 1995. Oreotragus major Wells and Oreotragus oreotragus (Zimmerman) (Mammalia: Bovidae): two species? Annals of the Transvaal Museum, 36:183-191.
Wells, L. 1951. A large fossil klipspringer from Potgietersrust. South African Journal of Science, 47:167-168.
Wells, L. and Cooke, H. 1956. Fossil Bovidae from the Limeworks Quarry, Makapansgat, Potgeitersrus. Palaeontologia Africana, 4:1-55.
Werdelin, L. and Lewis, M. 2001. A revision of the genus Dinofelis (Mammalia, Felidae). Zoological Journal of the Linnean Society, 132:147-258.
Werdelin, L. and Sanders, W.J. 2010. Cenozoic Mammals of Africa. University of California Press, Berkeley.
White, T. 1995. African omnivores: global climatic change and Plio-Pleistocene hominids and suids, p. 369-384. In Vrba, E., Denton, G., Partridge, T., and Burckle, L. (eds.), Paleoclimate and evolution, with emphasis on human origins. Yale University Press, New Haven.
Wilson, D.E. and Reeder, D.M. 2005. Mammal species of the world. A taxonomic and geographic reference. Third Edition. Johns Hopkins University Press, Baltimore.
Zdansky, O. 1924. Jungtertiäre Carnivoren Chinas. Palaeontologia Sinica serie C, 2:1-149.
Zimmerman, J. 1780. Geographische geschichte des Menschen und der vierfüssigen Thiere, Volume 2. Weygandschen Buchhandlung, Leipzig.
Zimmerman, J. 1783. Geographische geschichte des Menschen und der vierfüssigen Thiere, Volume 3. Weygandschen Buchhandlung, Leipzig.

