 Cells and soft tissues in fossil bone: A review of preservation mechanisms, with corrections of misconceptions
Cells and soft tissues in fossil bone: A review of preservation mechanisms, with corrections of misconceptions
Article number: 25.3.a34
https://doi.org/10.26879/1248
Copyright Society for Vertebrate Paleontology, December 2022
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 18 October 2022. Acceptance: 2 December 2022.
ABSTRACT
In the most recent three decades, there has been an outpouring of research on the preservation of cells and soft tissues within fossil bones. Cells and soft tissues that are documented to have been preserved in fossil bones include osteocytes, chondrocytes, blood vessels, nerve fibers, nerves, and the sheets of collagen in bone matrix. Recent studies identify Fenton reactions as a plausible preservation mechanism for cells and soft tissues within bones, document the chemical signatures of Fenton reactions in the cells and soft tissues of fossil bones, and indicate that such preservation occurs early in diagenesis and is facilitated by oxidizing depositional environments and by protection via external concretions and other factors. Additionally, recent advances in the study of archaeological bone have identified a suite of factors that enable a bone and its cellular and soft tissue contents to survive into the fossil record. Despite these advances, two unfortunate situations persist. One is that there is little connection between the literature on archaeological bone and the literature on fossil bone. The other is that the literature of science voices numerous misconceptions regarding the preservation of cells and soft tissues in fossil bones, many of which are rooted in young-Earth creationist (YEC) opposition to the hypothesized role of Fenton reactions. To alleviate these problems, this review corrects misconceptions and links studies of archaeological bone to studies of fossil bone, to elucidate the mechanisms by which cells and soft tissues are preserved in bones for hundreds, then thousands, then millions of years.
Philip J. Senter. Department of Biological and Forensic Sciences, Fayetteville State University, 1200 Murchison Road, Fayetteville, North Carolina 28301, U.S.A, psenter@uncfsu.edu
Keywords: fossil bone; archaeological bone; soft tissue; osteocyte; collagen; Fenton reaction
Final citation: Senter, Philip J.. 2022. Cells and soft tissues in fossil bone: A review of preservation mechanisms, with corrections of misconceptions. Palaeontologia Electronica, 25(3):a34. https://doi.org/10.26879/1248
palaeo-electronica.org/content/2022/3739-soft-tissues-in-fossil-bone
Copyright: December 2022 Society of Vertebrate Paleontology.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
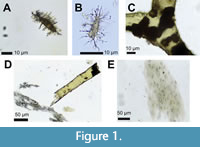 In the current century, few issues in paleontology have generated as much excitement from scientists and as much attention from anti-evolution authors as the discovery of preserved cells and soft tissues in fossil bone. In recent decades, researchers have described numerous examples of fossil bones of Mesozoic reptiles, including non-avian dinosaurs (hereafter called simply “dinosaurs” for brevity), in which internal soft tissue structures and cells are preserved. Such cells and soft tissue structures include osteocytes (bone cells) (Pawlicki, 1966, 1977, 1978, 1995; Pawlicki and Nowogrodzka-Zagóriśka, 1998; Schweitzer et al., 2005, 2007, 2013, 2016; Cadena and Schweitzer, 2012; Armitage and Anderson, 2013, 2014; Armitage, 2015, 2016; Wiemann et al., 2018; Ullmann et al., 2019; Armitage and Solliday, 2020; Cadena, 2020; Surmik et al., 2021; Schroeter et al., 2022), chondrocytes (cartilage cells) (Bailleul et al., 2020, 2021; Zheng et al., 2021), blood vessels (Pawlicki, 1966; Pawlicki and Nowogrodzka-Zagóriśka, 1998; Yao et al., 2002; Schweitzer et al., 2005, 2007, 2014, 216; Peterson et al., 2010; Armitage and Anderson, 2013; Armitage, 2015, 2016; Cleland et al., 2015; Surmik et al., 2016; Wiemann et al., 2018; Boatman et el., 2019; Ullmann et al., 2019; Armitage and Solliday, 2020; Schroeter et al., 2022), nerve fibers (Pawlicki and Nowogrodzka-Zagóriśka, 1998), nerves (Armitage and Solliday, 2020; Armitage, 2021), and the extracellular sheets of type I collagen that form the non-mineral part of bone matrix (hereafter abbreviated CBM, for collagen in bone matrix) (Schweitzer et al., 2005, 2007, 2016; Lindgren et al., 2011; Armitage and Anderson, 2013; Armitage, 2015, 2016; Bertazzo et al., 2015; Wiemann et al., 2018; Miller et al., 2019; Ullmann et al., 2019; Schroeter et al., 2022) (Figure 1). Blood cells have also been reported from the fossil bones of reptiles from the Mesozoic (Pawlicki and Nowogrodzka-Zagóriśka, 1998; Yao et al., 2002; Schweitzer et al., 2005, 2007; Armitage and Anderson, 2013; Armitage, 2015, 2016; Bertazzo et al., 2015; Plet et al., 2017) and Paleozoic (Kiseleva et al., 2019), but their identification as blood cells is dubious (Saitta et al, 2017; Korneisel et al., 2021; this paper: see the section on Misconception 10, below). Preservation of cells and soft tissues in fossil bone was unexpected at first, but it now appears to be relatively common in Cenozoic and Mesozoic fossil bone (Schweitzer et al., 2007; Huang et al., 2022). Although such preservation in Mesozoic reptiles has received the most attention, it has also been reported in Paleozoic reptiles (Kiseleva et al., 2019), Cenozoic reptiles (Cadena and Schweitzer, 2012; Cadena, 2016, 2020; Voegele et al., 2021), fossil birds (Bailleul and Zhou, 2021), fossil mammals (Schweitzer et al., 2007; Buckley and Collins, 2011; Buckley, 2015; Cadena, 2016; Barker et al., 2021; Schmidt-Schultz et al., 2021; Gatti et al., 2022), non-mammalian synapsids (Armitage, 2022a), and fossil fishes (Dutta et al., 2020).
In the current century, few issues in paleontology have generated as much excitement from scientists and as much attention from anti-evolution authors as the discovery of preserved cells and soft tissues in fossil bone. In recent decades, researchers have described numerous examples of fossil bones of Mesozoic reptiles, including non-avian dinosaurs (hereafter called simply “dinosaurs” for brevity), in which internal soft tissue structures and cells are preserved. Such cells and soft tissue structures include osteocytes (bone cells) (Pawlicki, 1966, 1977, 1978, 1995; Pawlicki and Nowogrodzka-Zagóriśka, 1998; Schweitzer et al., 2005, 2007, 2013, 2016; Cadena and Schweitzer, 2012; Armitage and Anderson, 2013, 2014; Armitage, 2015, 2016; Wiemann et al., 2018; Ullmann et al., 2019; Armitage and Solliday, 2020; Cadena, 2020; Surmik et al., 2021; Schroeter et al., 2022), chondrocytes (cartilage cells) (Bailleul et al., 2020, 2021; Zheng et al., 2021), blood vessels (Pawlicki, 1966; Pawlicki and Nowogrodzka-Zagóriśka, 1998; Yao et al., 2002; Schweitzer et al., 2005, 2007, 2014, 216; Peterson et al., 2010; Armitage and Anderson, 2013; Armitage, 2015, 2016; Cleland et al., 2015; Surmik et al., 2016; Wiemann et al., 2018; Boatman et el., 2019; Ullmann et al., 2019; Armitage and Solliday, 2020; Schroeter et al., 2022), nerve fibers (Pawlicki and Nowogrodzka-Zagóriśka, 1998), nerves (Armitage and Solliday, 2020; Armitage, 2021), and the extracellular sheets of type I collagen that form the non-mineral part of bone matrix (hereafter abbreviated CBM, for collagen in bone matrix) (Schweitzer et al., 2005, 2007, 2016; Lindgren et al., 2011; Armitage and Anderson, 2013; Armitage, 2015, 2016; Bertazzo et al., 2015; Wiemann et al., 2018; Miller et al., 2019; Ullmann et al., 2019; Schroeter et al., 2022) (Figure 1). Blood cells have also been reported from the fossil bones of reptiles from the Mesozoic (Pawlicki and Nowogrodzka-Zagóriśka, 1998; Yao et al., 2002; Schweitzer et al., 2005, 2007; Armitage and Anderson, 2013; Armitage, 2015, 2016; Bertazzo et al., 2015; Plet et al., 2017) and Paleozoic (Kiseleva et al., 2019), but their identification as blood cells is dubious (Saitta et al, 2017; Korneisel et al., 2021; this paper: see the section on Misconception 10, below). Preservation of cells and soft tissues in fossil bone was unexpected at first, but it now appears to be relatively common in Cenozoic and Mesozoic fossil bone (Schweitzer et al., 2007; Huang et al., 2022). Although such preservation in Mesozoic reptiles has received the most attention, it has also been reported in Paleozoic reptiles (Kiseleva et al., 2019), Cenozoic reptiles (Cadena and Schweitzer, 2012; Cadena, 2016, 2020; Voegele et al., 2021), fossil birds (Bailleul and Zhou, 2021), fossil mammals (Schweitzer et al., 2007; Buckley and Collins, 2011; Buckley, 2015; Cadena, 2016; Barker et al., 2021; Schmidt-Schultz et al., 2021; Gatti et al., 2022), non-mammalian synapsids (Armitage, 2022a), and fossil fishes (Dutta et al., 2020).
The three most commonly reported categories of cells and soft tissue structures in fossil bone are blood vessels, osteocytes, and CBM. A given fossil bone may contain all three, or only one or two of the three, or none. Such variability exists even among fossils representing the same slice of ancient time, and there is no apparent temporal pattern as to whether a given fossil bone will contain one, two, all three, or none of the three (Schweitzer et al., 2007; Wiemann et al., 2018; Ullmann et al., 2019). This phenomenon has prompted a series of paleontological investigations into the mechanisms of cellular and soft tissue preservation in fossil bone through geologic time and how it is that certain cells and soft tissues are preserved in some bones and not others (Schweitzer et al., 2007, 214; San Antonio et al., 2011; Surmik et al., 2016, 2021; Wiemann et al., 2018; Boatman et al., 2019; Ullmann et al., 2019, 2021, 2022).
To be preserved for millions of years after death, any tissue must first be preserved for hundreds and then thousands of years. Unfortunately, there is little connection between the literature on cellular and soft tissue preservation in bones of ages of these three orders of magnitude. That is, there is a body of literature on cells and soft tissues in bones that are hundreds of years old (e.g., medieval bones), a body literature on cells and soft tissues in bones that are thousands of years old (e.g., late Pleistocene and early Holocene bones), and a body of literature on cells and soft tissues in bones that are millions of years old (e.g., pre-Pleistocene fossil bones), with little connection between the three bodies of literature. A review that connects the three to elucidate the bigger picture would be useful. Here, I provide such a review.
To fully address the pertinent issues, a review of cellular and soft tissue preservation mechanisms in bone must include a synopsis of bone composition, Fenton chemistry, the distinction between archaeological and fossil bone, and the differences in how fossil bone is viewed between the paradigm of science and the paradigm of young-Earth creationist (YEC) ideology. The subject of Fenton chemistry is important to a review such as this, because Fenton chemistry has been identified as a crucial factor in certain forms of cellular and soft tissue preservation in fossil bone (Schweitzer et al., 2007, 2013, 2014; Surmik et al., 2016; Wiemann et al., 2018; Boatman et al., 2019). The distinction between archaeological and fossil bone is important to a review such as this, because much of the literature on cellular and soft tissue preservation in bone focuses on one category of bone or the other, and statements regarding the state of cells and soft tissue in bone of one category do not necessarily apply to the other. The view of fossil bone within the YEC paradigm is important to a review such as this, because part of the purpose of a review article is to correct misconceptions, and several misconceptions of Fenton chemistry and of cells and soft tissue in fossil bone that have been voiced in the primary literature of science are rooted in the YEC view.
SYNOPSES OF BACKGROUND INFORMATION
Bone Composition
Bone is a living tissue in which cells called osteoblasts secrete an extracellular mixture of CBM and the mineral hydroxyapatite: Ca5(PO4)3 (OH). Reactions with water in the body convert CO2 (waste from the body’s cells) into carbonate ions (CO3-2), and carbonate ions replace so many of the phosphate (PO4-2) and hydroxide (OH -) ions in the hydroxyapatite that its chemical formula must be rewritten as Ca5(PO4, CO3)3 (OH, CO3). This altered mineral is called carbonated hydroxyapatite, dahllite, or simply bone mineral (Wings, 2004; Kendall et al., 2018; Senter, 2020). The extracellular mixture of CBM and bone mineral is called bone matrix.
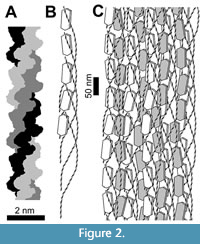 In the CBM of bone matrix, collagen molecules are arranged in rope-like units called fibrils, within which are multiple levels of helical winding. The smallest unit of collagen is the collagen molecule, also called the tropocollagen molecule, which consists of three helical protein chains that are bonded together with numerous hydrogen bonds and are tightly wound around each other to form a triple helix (Bella, 2016; Amirrah et al., 2022) (Figure 2A). These collagen molecules aggregate in staggered groups of five, in which the five collagen molecules are helically wound around each other. Such groups of five are called collagen microfibrils (Veis, 2003; Sweeney et al., 2008) (Figure 2B). The microfibrils further aggregate into collagen fibrils, each of which consists of many staggered microfibrils that are helically wound around each other (Veis, 2003; Sweeney et al., 2008; Alexander et al., 2012) (Figure 2C). Within CBM, collagen fibrils form covalent cross-links (molecular bonds between polymers) with each other (Alexander et al., 2012), as do adjacent microfibrils within fibrils (Sweeney et al., 2008) and adjacent chains within the triple helix of the collagen molecule (Light and Bailey, 1982). The cross-links confer long-term stability (Kendall et al., 2018), which is of importance in the preservation of cells and soft tissue in archaeological and fossil bone.
In the CBM of bone matrix, collagen molecules are arranged in rope-like units called fibrils, within which are multiple levels of helical winding. The smallest unit of collagen is the collagen molecule, also called the tropocollagen molecule, which consists of three helical protein chains that are bonded together with numerous hydrogen bonds and are tightly wound around each other to form a triple helix (Bella, 2016; Amirrah et al., 2022) (Figure 2A). These collagen molecules aggregate in staggered groups of five, in which the five collagen molecules are helically wound around each other. Such groups of five are called collagen microfibrils (Veis, 2003; Sweeney et al., 2008) (Figure 2B). The microfibrils further aggregate into collagen fibrils, each of which consists of many staggered microfibrils that are helically wound around each other (Veis, 2003; Sweeney et al., 2008; Alexander et al., 2012) (Figure 2C). Within CBM, collagen fibrils form covalent cross-links (molecular bonds between polymers) with each other (Alexander et al., 2012), as do adjacent microfibrils within fibrils (Sweeney et al., 2008) and adjacent chains within the triple helix of the collagen molecule (Light and Bailey, 1982). The cross-links confer long-term stability (Kendall et al., 2018), which is of importance in the preservation of cells and soft tissue in archaeological and fossil bone.
Crystallites (microscopic crystals) of bone mineral form between fibrils, between microfibrils, and in the gaps between the tips of the staggered collagen molecules within microfibrils (Veis, 2003; Alexander et al., 2012) (Figure 2). Collagen molecules form bonds with bone mineral (Veis, 2003), which probably contributes to the long-term stability of both materials (Zazzo, 2014).
 In a mature bone, two main types of bone tissue are present: compact bone (also called Haversian bone or cortical bone) and spongy bone (also called cancellous bone or medullary bone). Compact bone is the type that forms the external part of a bone (Figure 3). In compact bone, concentric circular layers of bone matrix surround vascular canals called Haversian canals, which contain blood vessels and nerves. Additional vascular canals called Volkmann’s canals, which also contain blood vessels and nerves, form crosswise links between Haversian canals (Figure 3). Some Volkmann’s canals perforate the surface of the bone and link the bone’s blood supply to that of the periosteum, the sheet of fibrous connective tissue that covers the external surface of the bone (Junqueira et al., 1998; Eurell, 2004). Spongy bone is the type of bone that occupies the interior of a bone. In spongy bone, the bone matrix is shaped into a network of struts called trabeculae (Figure 3). Occupying the spaces between the trabeculae of spongy bone are blood vessels and marrow (Junqueira et al., 1998; Eurell, 2004).
In a mature bone, two main types of bone tissue are present: compact bone (also called Haversian bone or cortical bone) and spongy bone (also called cancellous bone or medullary bone). Compact bone is the type that forms the external part of a bone (Figure 3). In compact bone, concentric circular layers of bone matrix surround vascular canals called Haversian canals, which contain blood vessels and nerves. Additional vascular canals called Volkmann’s canals, which also contain blood vessels and nerves, form crosswise links between Haversian canals (Figure 3). Some Volkmann’s canals perforate the surface of the bone and link the bone’s blood supply to that of the periosteum, the sheet of fibrous connective tissue that covers the external surface of the bone (Junqueira et al., 1998; Eurell, 2004). Spongy bone is the type of bone that occupies the interior of a bone. In spongy bone, the bone matrix is shaped into a network of struts called trabeculae (Figure 3). Occupying the spaces between the trabeculae of spongy bone are blood vessels and marrow (Junqueira et al., 1998; Eurell, 2004).
Within the matrix of both compact bone and spongy bone are osteocytes (former osteoblasts that have surrounded themselves with bone matrix) (Figure 1), each of which inhabits a space in the matrix called a lacuna. Osteocytes connect to each other and to blood vessels, using elongate extensions called dendritic processes or filipodia (sometimes spelled filopodia or filapodia). The dendritic processes inhabit tiny canals in the matrix called canaliculi (Figure 3). The vascular canals and canaliculi and lacunae form a continuous network of voids within the bone matrix. The network of canalicular and lacunar voids is called the lacuno-canalicular network (Eurell, 2004; Buenzli and Sims, 2015; Kendall et al., 2018). The combination of the lacunar-canalicular network and vascular canals could aptly be called the vascular-canalicular network (hereafter abbreviated VCN). After death, the fate of each component of bone—bone mineral, CBM, osteocytes, blood vessels, and the voids of the VCN—depends on numerous factors (see below).
Fenton Chemistry, Its Products, and Their Effects on Biomolecules
The term “Fenton chemistry” refers to the Fenton reaction and similar chemical reactions (Barbusiński, 2001). In the Fenton reaction, ferrous iron (Fe2+) reacts with hydrogen peroxide (H2O2). The products of this reaction are ferric iron (Fe3+), a hydroxide ion (OH-), and a hydroxyl radical (OH·). The Fenton reaction is:
Fe2+ + H2O2 → Fe3+ + OH- + OH·
The Fenton reaction can occur in the body of a vertebrate when red blood cells and peroxisomes are damaged. Red blood cells contain the oxygen-carrying molecule hemoglobin (Hb), part of which is heme, which contains an iron atom. If red blood cells undergo lysis (rupture), the Hb is released, and its breakdown liberates the iron (Balla et al., 2005, 2007). Peroxisomes are organelles that contain H2O2, which is used therein to oxidize various molecules. If peroxisomes are damaged, they release H2O2 (Valko et al., 2007). When liberated iron contacts H2O2, the Fenton reaction takes place (Balla et al., 2005, 2007). The Fenton reaction initiates a chain of subsequent reactions, often also called Fenton reactions (but not called “the Fenton reaction”), the products of which oxidize organic compounds (Barbusiński, 2001).
Hydroxyl radicals are both useful and highly toxic to the body. Hydroxyl radicals are involved in the activation of certain proteins, such as the tumor suppressor protein p53 (Wang et al., 2000) and proteins that regulate autophagy (destruction of dysfunctional cell components) during chemotherapy (Sumkhemthong et al., 2021). However, if left unchecked, hydroxyl radicals in excessive amounts can cause significant damage to biomolecules, and hydroxyl radicals have been implicated in a variety of diseases, such as atherosclerosis, cancer, diabetes, and some neurological disorders (Lipinski, 2011). Reactions with hydroxyl radicals can cleave polysaccharides (Duan and Kasper, 2011), cleave DNA (Dizdaroglu and Jaruga, 2012), forge cross-links between and within DNA strands (Dizdaroglu and Jaruga, 2012), forge cross-links between DNA and proteins (Dizdaroglu and Jaruga, 2012), generate conformational changes in proteins (Hawkins and Davies, 2001; Li et al., 2012; Zhang et al., 2021; Lei et al., 2022), cleave some proteins (Nyaisaba et al., 2019), forge cross-links between adjacent protein molecules (Dunlop et al., 2002; Xiong et al., 2010; Li et al., 2012; Nyaisaba et al., 2019; Zhang et al., 2021; Lei et al., 2022), convert some amino acids into other amino acids (Leeuwenburgh et al., 1998; Hawkins and Davies, 2001), convert some amino acids into other kinds of molecules (Hawkins and Davies, 2001), and cause chain reactions of lipid peroxidation in lipid molecules with fatty acid chains (McCord, 1998; Yurkova et al., 2004; Catalá, 2009). In some tissues, iron overload leads to Fenton reactions that generate a kind of cell death called ferroptosis (Yan et al., 2021; Gao et al., 2022; Liu et al., 2022). Ferroptosis is implicated in Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, dementia, liver necrosis, ischemia/reperfusion injury, and osteoporosis (Doll et al., 2017; Yan et al., 2021; Gao et al., 2022; Liu et al., 2022).
Despite the destructive potential of hydroxyl radicals toward biomolecules, a healthy body has a variety of mechanisms in vivo that prevent and halt such damage. For example, various substances in the body scavenge hydroxyl and other free radicals, thereby protecting biomolecules from them. Such scavengers include the amino acid derivative glutathione (GSH), the plasma protein albumin, antioxidant enzymes, and dietary antioxidants (He et al., 2002; Lipinski, 2011; Veira et al., 2017). Such scavengers also include type I collagen. Type I collagen is the type of collagen in CBM and in the tunica adventitia, the outer layer of connective tissue of a blood vessel (Junqueira et al., 1998). Its long, fibrous shape provides that after hydroxyl radicals have caused conformational change in the collagen by reacting with its amides, more residues containing v (C=O) are externally exposed than in most other proteins, enabling them to react with and scavenge more hydroxyl radicals (Xiao et al., 2007). The presence of type I collagen reduces the generation of hydroxyl radicals by the Fenton reaction (He et al., 2002; Xiao et al., 2007), scavenges hydroxyl radicals that the Fenton reaction generates (Xiao et al., 2007), inhibits lipid peroxidation of polyunsaturated fatty acids (PUFAs) by hydroxyl radicals (He et al., 2002), protects GSH from hydroxyl radicals (He et al., 2002; Xiao et al., 2007), and inhibits apoptosis (He et al., 2002).
Additionally, the phospholipid membranes of cells have built-in protection from the chain reactions of lipid peroxidation that hydroxyl radicals would otherwise cause. In such membranes, the double bonds in the PUFAs of phospholipid molecules scavenge hydroxyl radicals (Lipinski, 2011), thereby preventing or stopping such chain reactions. The myelin sheaths that surround nerve fibers are especially rich in plasmalogen phospholipids, which also halt such chain reactions (Luoma et al., 2015; cf. Sindelar et al., 2015). This built-in protection is important to note, in order to understand how the phospholipid membranes of cells escape complete destruction when Fenton reactions take place in the cells and soft tissues of what will later become fossil bone.
Although they damage proteins by causing conformational changes and by altering some amino acids, hydroxyl radicals also contribute to protein preservation by generating cross-links between protein molecules (Dunlop et al., 2002; Xiong et al., 2010; Li et al., 2012; Nyaisaba et al., 2019; Lei et al., 2022). The conformational changes that hydroxyl radicals generate in proteins make some proteins more susceptible to fragmentation by apoptotic enzymes (Lei et al., 2022), but they make other proteins less susceptible to fragmentation by apoptotic enzymes (Zhang et al., 2021), thus contributing to protein preservation. Either way, apoptotic fragmentation of proteins does not lead to the complete destruction of the proteins, because the cross-links contribute to preservation of the resulting fragments. In the case of collagen in CBM, such fragmentation is not expected anyway, because CBM is extracellular and is therefore not subject to attack from apoptotic enzymes within cells. In collagen, reactions with hydroxyl radicals cleave the side chains of some amino acids, separating them from the parent collagen molecule, but reactions with hydroxyl radicals do not cleave the main chain of the parent collagen molecule itself (Hawkins and Davies, 1997). Furthermore, hydroxyl radicals generate cross-links in collagen (Windhager et al., 1998), thereby contributing to its preservation. These aspects of the interaction between collagen and hydroxyl radicals are important to note, in order to fully understand how Fenton reactions may contribute to long-term preservation of CBM and of the type I collagen in the walls of blood vessels. They also demonstrate that the oft-repeated YEC assertion that Fenton reactions cannot have been involved in the preservation of fossil collagen (DeMassa and Boudreaux, 2015; Anderson, 2016a, 2018; Armitage and Solliday, 2020) is inaccurate.
As with the hydroxyl radical, ferric and ferrous iron are both useful and highly toxic to the body. Iron must be in the ferric state to be transported into cells, where it may afterward be reduced to the ferrous state as needed (Waldvogel-Abramowski et al., 2014). Ferrous iron is a component of the heme unit in metalloproteins that carry oxygen, such as Hb and myoglobin (Thomas and Lumb, 2012; Arcon et al., 2015), and ferric iron is a component of some metalloprotein enzymes (Balogh et al., 2018). Iron that transitions between ferric and ferrous states during redox reactions is a component of some biomolecules in electron transport systems, such as cytochromes and ferredoxins (Liu et al., 2014). Both ferrous and ferric irons are therefore necessary for some biological processes, but excessive amounts of free iron can cause significant damage to biomolecules. For example, Fenton reactions induced by iron liberated from heme during Hb breakdown can induce oxidation of the fatty acids, cholesterol, and apolipoprotein B-100 in low-density lipoprotein (LDL) particles, making the LDL particles cytotoxic to endothelial cells (the cells that line the inner surface of a blood vessel) and contributing to the generation of atherosclerosis (Balla et al., 2005, 2007). To prevent such problems, the protein transferrin tightly controls the delivery of iron to cells that need it (Rouault, 2003), and excess iron within cells is scavenged and sequestered by the protein ferritin (Balla et al., 2005, 2007). Endothelial cells increase their production of ferritin to scavenge excess iron when they are exposed to increased levels of Hb due to red blood cell lysis (Balla et al., 2005, 2007). These aspects of the interaction between cells and iron are important to note, in order to fully understand how Fenton reactions may contribute to long-term preservation of cells and soft tissues.
Science vs. YEC Ideology: Methodologies, Conceptions of Geological Time, and Conceptions of Archaeological and Fossil Bone
Most of the misconceptions regarding cellular and soft tissue preservation in fossil bone that have been voiced in science journals have their origin in the anti-evolution movement. There are a variety of anti-evolution views (Scott, 2009; Kaden, 2019), but most of the authors voicing misconceptions of cellular and soft tissue preservation in fossil bone advocate one anti-evolution view in particular: the young-Earth creationist (YEC) view. It is unusual to address the YEC view in a science journal, but it is important and appropriate to do so in this case, for a number of reasons. Firstly, a paper in a science journal is the most appropriate venue to address misconceptions that have been voiced in papers in science journals. Secondly, the misconceptions in question are testable hypotheses, and it is appropriate to address testable hypotheses in a science journal. Thirdly, to address such misconceptions in such a way as to be comprehensible to the reader, it is necessary in this case to delineate how the relevant vocabulary is used differently by two opposing camps: that of science and that of the YEC movement.
It is important to note that my use of the term “science” in this review excludes the YEC discipline that is known as “creation science.” The latter discipline resembles science in that it involves publication in peer-reviewed technical journals but differs from science in that its journals require conformity with the YEC view (Senter and Mackey, 2017a). Another major difference is procedural. Within the discipline of science, one first collects observational and experimental data then draws conclusions from those data. In contrast, within the discipline of “creation science,” one begins with pre-decided conclusions, then interprets data from within the paradigm of those pre-decided conclusions (Creation Research Society, 1964; Prothero, 2017; Senter and Mackey, 2017a), often ignoring or dismissing data that contradict the pre-decided conclusions (Isaak, 2007; Niemenen et al., 2015; Prothero, 2017; Senter, 2011, 2019). Thus, from the perspective of science, so-called “creation science” is procedurally backwards. It is also foundationally unsound, because the foremost of its pre-decided conclusions—the conclusion that the text of the biblical book of Genesis is an accurate record of ancient events—is rebutted by a vast body of physical evidence (Isaak, 2007; Willoughby, 2016; Keesey, 2016; Prothero, 2017; Senter, 2011; online Table 1 of Senter, 2022a) and also by the ancient texts upon which the YEC view is ostensibly based (Senter, 2022a).
Although YEC ideology is famously opposed to certain implications of the results of scientific studies—especially macroevolution and the passage of millions of years (Ham, 2017; Kaden, 2019)—it is favorably disposed toward science in general. YEC ideology therefore encourages both science and so-called “creation science.” As a result, there are scientists who espouse the YEC view and yet conduct good science that is published in science journals, whether or not they additionally conduct “creation science” to publish in YEC journals. Some are competent microscopists and histologists who have published descriptions of cells and soft tissues in Mesozoic fossil bone in articles in science journals (Armitage and Anderson, 2013, 2014; Armitage, 2016, 2021; Armitage and Solliday, 2020). Some of the misconceptions that are addressed here are voiced in those articles.
 According to the literature of science, abundant physical evidence indicates that the Earth is billions of years old (Schmitz, 2020; Strachan et al., 2020) and that all organisms on Earth evolved from a common ancestor (Prothero, 2017). The literature of science divides ancient time into eras, which are further divided into periods, which are further divided into epochs. The current epoch is the Holocene Epoch of the Quaternary Period of the Cenozoic Era (Figure 4). The context of the Holocene Epoch is important to grasp in order to understand the distinction between archaeological and fossil bone. According to the timescale of science, the Holocene Epoch includes all of human history in addition to a stretch of prehistory that extends back approximately to the beginning of settled agriculture (Bowles et al., 2019). The Holocene Epoch was once described as having begun about 10,000 years ago (Gibbard and van Kolfschoten, 2004), but recent developments have redefined its beginning by tying it to a stratum that is dated to approximately 11,700 years before the year A.D. 2000 by the counting of annual layers in arctic ice cores (Walker et al., 2009). Radiometric dating places the beginning of the previous epoch, the Pleistocene Epoch, at 2.58 million years ago (Gibbard and Head, 2020). Together, the Pleistocene and Holocene Epochs make up the Quaternary Period, the last period of the Cenozoic Era. According to radiometric dating, the Cenozoic Era began 66 million years ago (Renne et al., 2013). The previous era was the Mesozoic Era, which was preceded by the Paleozoic Era, which in turn was preceded by a vast stretch of time called the Precambrian Supereon, which began with the formation of the Earth about 4.6 billion years ago (Strachan et al., 2020) (Figure 4).
According to the literature of science, abundant physical evidence indicates that the Earth is billions of years old (Schmitz, 2020; Strachan et al., 2020) and that all organisms on Earth evolved from a common ancestor (Prothero, 2017). The literature of science divides ancient time into eras, which are further divided into periods, which are further divided into epochs. The current epoch is the Holocene Epoch of the Quaternary Period of the Cenozoic Era (Figure 4). The context of the Holocene Epoch is important to grasp in order to understand the distinction between archaeological and fossil bone. According to the timescale of science, the Holocene Epoch includes all of human history in addition to a stretch of prehistory that extends back approximately to the beginning of settled agriculture (Bowles et al., 2019). The Holocene Epoch was once described as having begun about 10,000 years ago (Gibbard and van Kolfschoten, 2004), but recent developments have redefined its beginning by tying it to a stratum that is dated to approximately 11,700 years before the year A.D. 2000 by the counting of annual layers in arctic ice cores (Walker et al., 2009). Radiometric dating places the beginning of the previous epoch, the Pleistocene Epoch, at 2.58 million years ago (Gibbard and Head, 2020). Together, the Pleistocene and Holocene Epochs make up the Quaternary Period, the last period of the Cenozoic Era. According to radiometric dating, the Cenozoic Era began 66 million years ago (Renne et al., 2013). The previous era was the Mesozoic Era, which was preceded by the Paleozoic Era, which in turn was preceded by a vast stretch of time called the Precambrian Supereon, which began with the formation of the Earth about 4.6 billion years ago (Strachan et al., 2020) (Figure 4).
It was once customary to restrict the term “fossil” to pre-Holocene remains (Shimer, 1914), but that formerly sharp line is often blurred in the literature of science in the current century. Currently, the literature of science categorizes ancient bone into two groupings: archaeological bone and fossil (or paleontological) bone. Neither term has an official definition, but the way in which each term is used clarifies its intended meaning. In the literature of science, publications that mention both archaeological bone and fossil bone use the terms in such a way as to imply a distinction based on age, with archaeological bone the younger of the two (e.g., Trueman and Martill, 2002; Dobberstein et al., 2009; Kendall et al., 2018). Archaeology is the study of human material cultures, i.e., items that humans have made or modified, from scratched rock surfaces to pottery to buildings to civilizations. Accordingly, scientists reserve the term archaeological bone for bone that dates from historical times (times from which written records are known) and from prehistoric time spans coeval with early humans and their nearest relatives. Scientists therefore use the term “archaeological bone” for bone from the Holocene Epoch (e.g., Zazzo et al., 2012; Zazzo and Saliège, 2011) and use the term “fossil bone” for pre-Pleistocene bone (e.g., Schweitzer et al., 2007; Brumfitt et al., 2013). A terminological zone of overlap is present in the Pleistocene and the early Holocene, as bone from either time span may be called “fossil bone” (e.g., Zazzo et al., 2012; van der Plicht and Palstra, 2016; Devièse et al., 2018) or “archaeological bone” (e.g., Trueman and Martill, 2002; Dobberstein et al., 2009; Marom et al., 2013; Gatti et al., 2022) in the literature of science.
According to the YEC view, the Earth has existed only for about 6000 years (Ham, 2017; Kaden, 2019). In current YEC literature, Paleozoic, Mesozoic, and pre-Quaternary Cenozoic strata are usually considered to have been deposited by the Genesis Flood in less than one year, between 4000 and 4500 years ago, and Quaternary strata are usually considered to be post-Flood deposits (Walker, 2014; Clarey, 2020; Oard and Carter, 2021; Clarey et al., 2022; Tomkins and Clarey, 2022). Current YEC literature thus accepts the sequence of major time spans that science accepts (the geologic column), but it does not accept that those time spans were millions of years long (Figure 4).
Current YEC literature has much to say on fossil bone but little on archaeological bone. My search for the phrase “archaeological bone” through pdfs of the complete twenty-first century runs of YEC technical journals through July 2022 (Answers Research Journal, Creation Research Society Quarterly, Journal of Creation, Journal of Creation Theology and Science Series B, Occasional Papers of the Baraminological Study Group, Origins, and Proceedings of the International Conference on Creationism) found the phrase only in one passing mention (Thomas and Nelson, 2015) and one bibliographical entry (Price, 2020). Current YEC literature applies the term “fossil” to pre-Holocene remains (Oard, 2011, 2020; Institute for Creation Research, 2015; Clarey, 2015; DeMassa and Boudreaux, 2015; Thomas and Nelson, 2015; Morris, 2016; Anderson, 2017a; Tacker, 2018; Oard and Carter, 2021; Sinclair and Wood, 2021), and it applies the phrase “fossil bone” accordingly. According to YEC authors, preservation of cells and soft tissues in fossil bone is easily explained: the bones are only thousands, not millions, of years old (Oard, 2011; Institute for Creation Research, 2015; Clarey, 2015, 2020; DeMassa and Boudreaux, 2015; Anderson, 2016b, 2018; Thomas, 2015; Miller et al., 2019; Oard and Carter, 2021). As shown below (in the section on Misconception 11), that explanation is inadequate, even within the YEC paradigm.
MECHANISMS OF DECOMPOSITION AND PRESERVATION OF THE COMPONENTS OF BONE
Bone Mineral: Factors Promoting Decomposition
The physical and chemical changes that occur in sediments and fossils over time are collectively called diagenesis. The term diagenesis is also applied to such changes in bone after death. Although bone mineral is not a soft tissue, its diagenesis is relevant to that of the cells and soft tissues in bone, because it surrounds those cells and soft tissues, and their preservation depends upon its protective presence.
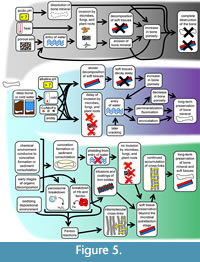 In some cases, diagenesis of bone mineral is destructive and may lead to the complete annihilation of the bone (Figure 5). Some destructive changes are wrought by living organisms. In aquatic environments, grazing fishes and invertebrates damage bone by eroding its surfaces (Sorg et al., 1997; Haglund and Sorg, 2002; Stuart and Ueland, 2017). In terrestrial environments, scavenging animals damage bone in a variety of ways (Young, 2017; Sincerbox and DiGangi, 2018). Plant roots also damage buried bone mineral, breaking it as they grow, absorbing it to obtain nutrients (Janaway, 1997), and creating holes and grooves in it (Schultz, 1997). Bone-decomposing bacteria, fungi, and algae bore tunnels through buried bone mineral (Janaway, 1997; Jans et al., 2004) and can disintegrate a bone completely (Schultz, 1997). Some bone-decomposing bacteria come from the intestine and are released as the intestinal wall decomposes. The damage that they cause tends to begin in the bones nearest the abdomen and can begin as early as 15 h after death (Jans et al., 2004). Once it has started, bioerosion by microbes and other organisms eventually results in the complete destruction of a bone within a few thousand years, preventing it from lasting long enough to be considered fossil bone, unless the bioerosion is interrupted by some physical or chemical change (Trueman and Martill, 2002). Even if such interruption occurs, fossil bone may be invaded by microbes, plants, and fungi late in its existence (Saitta et al., 2019), introducing the risk that even a bone that has lasted since the Mesozoic or Paleozoic Era may later succumb to complete destruction.
In some cases, diagenesis of bone mineral is destructive and may lead to the complete annihilation of the bone (Figure 5). Some destructive changes are wrought by living organisms. In aquatic environments, grazing fishes and invertebrates damage bone by eroding its surfaces (Sorg et al., 1997; Haglund and Sorg, 2002; Stuart and Ueland, 2017). In terrestrial environments, scavenging animals damage bone in a variety of ways (Young, 2017; Sincerbox and DiGangi, 2018). Plant roots also damage buried bone mineral, breaking it as they grow, absorbing it to obtain nutrients (Janaway, 1997), and creating holes and grooves in it (Schultz, 1997). Bone-decomposing bacteria, fungi, and algae bore tunnels through buried bone mineral (Janaway, 1997; Jans et al., 2004) and can disintegrate a bone completely (Schultz, 1997). Some bone-decomposing bacteria come from the intestine and are released as the intestinal wall decomposes. The damage that they cause tends to begin in the bones nearest the abdomen and can begin as early as 15 h after death (Jans et al., 2004). Once it has started, bioerosion by microbes and other organisms eventually results in the complete destruction of a bone within a few thousand years, preventing it from lasting long enough to be considered fossil bone, unless the bioerosion is interrupted by some physical or chemical change (Trueman and Martill, 2002). Even if such interruption occurs, fossil bone may be invaded by microbes, plants, and fungi late in its existence (Saitta et al., 2019), introducing the risk that even a bone that has lasted since the Mesozoic or Paleozoic Era may later succumb to complete destruction.
Abiotic chemical factors may also cause bone mineral to decompose. In buried bone, such decomposition is often preceded by delay of about two years, during which bone mineral survives. After that period, bone mineral dissolves in soil that is acidic (Janaway, 1997; Nord et al., 2015), contains calcium aluminum phosphate (Berna et al., 2004), or is porous and light. Porous and light soil allows greater exchange with water and free oxygen, which promote decomposition (Janaway, 1997). Decomposition of bone mineral is also facilitated by small bone size and flat bone shape (Janaway, 1997). Size and shape also influence the tendency of bones to break. Smaller sizes and more spherical shapes make bones more resistant to breakage, whereas larger sizes and less spherical shapes make bones more prone to breakage (Darwent and Lyman, 2002). In aquatic environments, water movement can cause bones to break if surf or currents batter bones against rocks or other hard surfaces (Haglund and Sorg, 2002; Stuart and Ueland, 2017).
Bone porosity also influences the decomposition of bone mineral. The more porous a portion of bone is, the more easily water flows through its VCN (Hedges and Millard, 1995). Hence, the more of its internal surface area is exposed to destructive agents that water carries, and the faster its bone mineral is dissolved by such agents (Jans et al., 2004; Turner-Walker, 2008; Kendall et al., 2018). The VCN of a bone makes it very porous to begin with, even in the compact (Haversian) bone of its exterior. The spongy bone of its interior is even more porous. Therefore, if a bone is broken so that its interior is exposed, bone mineral decomposes faster in its spongy interior than in its compact exterior (Colleary et al., 2021). Decomposition of bone mineral is also accelerated after destructive agents have increased its porosity. For example, the tunnels that bacteria and fungi bore through bone mineral constitute an increase in porosity and accelerate its decomposition (Jans et al., 2004; Kendall et al., 2018).
Another process that increases a bone’s porosity is the decomposition of CBM. When the CBM between bone mineral crystals is removed by decomposition, that removal creates more space (hence more porosity) for water and destructive agents to enter. Exposure to water also causes the remaining CBM to swell, cracking the bone, which creates new avenues for invasion of the bone mineral by destructive agents (Pfretzschner, 2016).
Bone Mineral: Factors Promoting Preservation
In buried bone, certain factors promote the preservation of bone mineral beyond the initial post-burial period of two years. Such factors include alkaline soil, the presence of sand or organic material or calcium carbonate in the soil, extreme cold, and anaerobic conditions (Janaway 1997; Nord et al., 2015; Piombino-Mascali et al., 2017; Junkins and Carter, 2017). The presence of bactericidal metals such as copper, manganese, and/or iron in the soil confers further protection (Schultz, 2001) (Figure 5). Other factors that promote bone mineral preservation are large bone size, tubular bone shape (Janaway, 1997; Berna et al., 2004), deep burial (which usually leads to exposure to colder temperatures), and burial in a coffin (Nord et al., 2015). In non-human animals, which do not bury their dead, scavenging protects bones from invasion by gut bacteria, because scavenging results in dismemberment that separates bones from the gut and from blood vessels through which gut bacteria could invade (Trueman and Martill, 2002). In addition, the framework of collagen around the bone mineral in bone matrix provides a physical barrier that protects bone mineral from water, which would otherwise slowly dissolve it (Trueman and Martill, 2002). Of course, that protection only lasts as long as the collagen does. However, collagen is a very long-lasting protein (see the section on CBM preservation, below).
Over the long term, preserved bone mineral undergoes diagenesis. Such diagenesis continues through time, so as a general rule, fossil bone mineral is more extensively altered than archaeological bone mineral (Trueman, 1999; Berna et al., 2004). A fraction of bone mineral retains its original elemental composition even in fossil bone, but that fraction decreases through time (Goodwin et al., 2007).
When osteocytes decay away, they leave behind empty voids in lacunae and canaliculi. Likewise, when blood vessels in bone decay away, they leave behind empty voids in the vascular canals that had housed them. The infilling of such voids with minerals is called permineralization. Under some conditions, bacteria accomplish permineralization. Into the voids, such bacteria may precipitate calcite (a form of CaCO3) (Carpenter, 2005) or pyrite (FeS2) (Wings, 2004). The decomposition of bone collagen, which releases sulfide ions (S-2), can be a second source of permineralization. This causes iron sulfides such as pyrite to precipitate onto the surfaces of voids and cracks (Pfretzschner, 2004). After the initial microbial phase of diagenesis has taken place, a third means of permineralization occurs: the precipitation of ions dissolved in water that percolates through the VCN. Such precipitation may deposit calcite, other carbonates, pyrite, siderite (FeCO3), kutnohorite (Ca(Mn,Mg)(CO3)2), barite (BaSO4), pyrolusite (MnO2), or other minerals in the voids (Pfretzschner, 2004; Wings, 2004; Pfretzschner and Tütken, 2011). Partial permineralization of VCN may be present in archaeological bone as young as 2000-7000 years (Duffett Carlson et al., 2022; Mandl et al., 2022). Experimentation shows that in a natural setting, artificially sliced bone cubes can undergo permineralization within a few weeks (Daniel and Chin, 2010).
Permineralization contributes to bone mineral preservation by decreasing the bone’s porosity (Kendall et al., 2018) and blocking destructive agents from entering the VCN. Permineralization may also occur in the spaces that collagen breakdown creates within the matrix (Hubert et al., 1996; Trueman et al., 2008) and that bioerosion has created before it is halted (Trueman and Martill, 2002), filling such spaces and thereby further decreasing porosity and inhibiting the entry of destructive agents (Trueman et al., 2008). Additional similar protection can be provided by encrustation, the precipitation of minerals onto the external surfaces of bones and onto the surfaces of cracks in the bone when ions in groundwater precipitate out of solution.
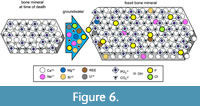 Another diagenetic change in bone mineral is recrystallization, in which bone mineral exchanges ions with groundwater as the groundwater percolates through the VCN (Trueman et al., 2008; Senter, 2020) (Figure 6). Fluorination is one such change that promotes long-term preservation. It occurs when hydroxide ions in bone mineral are exchanged for fluoride (F-) ions, converting bone mineral into francolite (Ca5 (PO4, CO3)F) and later into fluoroapatite (also spelled fluorapatite) (Ca5 (PO4)F). The fluorination of bone mineral increases the size of its crystals (Figure 6), providing the mineral with greater stability through time (Berna et al., 2004; Kocsis et al., 2010; Kendall et al., 2018) and reducing the porosity of the bone (Trueman, 1999).
Another diagenetic change in bone mineral is recrystallization, in which bone mineral exchanges ions with groundwater as the groundwater percolates through the VCN (Trueman et al., 2008; Senter, 2020) (Figure 6). Fluorination is one such change that promotes long-term preservation. It occurs when hydroxide ions in bone mineral are exchanged for fluoride (F-) ions, converting bone mineral into francolite (Ca5 (PO4, CO3)F) and later into fluoroapatite (also spelled fluorapatite) (Ca5 (PO4)F). The fluorination of bone mineral increases the size of its crystals (Figure 6), providing the mineral with greater stability through time (Berna et al., 2004; Kocsis et al., 2010; Kendall et al., 2018) and reducing the porosity of the bone (Trueman, 1999).
Recrystallization of bone mineral may also include the replacement of its calcium ions with ions of sodium (Na+), strontium (Sr+2), magnesium (Mg+2), uranium (U+4), or rare earth elements such as scandium, yttrium, lanthanum, and ytterbium (Pfretzschner, 2004; Trueman et al., 2008; Ullmann et al., 2021, 2022); the replacement of its phosphate ions with carbonate, orthosilicate (SiO4-4), or hydrogen phosphate (HPO4-2) ions, or with metal hydroxides that may subsequently become metal oxides (Pfretzschner, 2004; Trueman et al., 2008); and the replacement of its hydroxide ions with chloride (Cl-) or carbonate ions (Trueman, 1999). In carbonate-rich water, replacement of bone phosphate by carbonate is increased and can result in replacement of most of the bone phosphate with carbonate (Fernández-Jalvo et al., 2016). Recrystallization proceeds inward from the external surface of a bone, and if water has entered the bone’s medullary cavity, then recrystallization also proceeds outward from within the bone (Ullmann et al., 2022). The fluorination of fossil bone confers high stability that slows down recrystallization but does not stop it (Berna et al., 2004; Kocsis et al., 2010; Kendall et al., 2018), and recrystallization continues to occur late in the existence of a fossil bone (Kocsis et al., 2010; Piga et al., 2013; Suarez and Passey, 2014; Keenan et al., 2015).
If cracking occurs early in digenesis, it facilitates the entry of agents that are destructive to bone mineral. However, if it occurs later, after the initial microbial decomposition stage has taken place, cracking can contribute to preservation by increasing the rate and spread of permineralization, encrustation, and recrystallization (Pfretzschner and Tütken, 2011; Pokines et al., 2018). Cracking is facilitated by wet-dry cycles and freeze-thaw cycles in sufficiently moist conditions, especially on exposed bone surfaces (Pokines et al., 2018). It is also facilitated in arid environments as the bone dries out (Pfretzschner and Tütken, 2011).
Blood Vessels: Factors Promoting Decomposition
Soft tissue decomposition tends to occur in three stages: autolysis, then putrefaction, then decay. Autolysis is the self-destruction of cells that occurs shortly after death, when hypoxia and changes in pH cause breakdown of the membranes of lysosomes (Piombino-Mascali et al., 2017). Lysosomes are organelles that contain digestive enzymes that are used in vivo to break down worn out organelles and other damaged structures within cells. In autolysis, their digestive enzymes are released and break down various parts of the cell. Putrefaction occurs as anaerobic bacteria digest tissues and produce gaseous wastes that cause bloating. During subsequent decay, cells and soft tissues are consumed by abiotic factors and aerobic organisms, including insects and other arthropods (Piombino-Mascali et al., 2017; Junkins and Carter, 2017). For cells and soft tissues to be preserved, these stages of decomposition must be arrested or slowed.
Much ink has been spilled on factors promoting the decomposition of soft tissues after death (Galloway et al., 1989; Bass, 1997; Galloway, 1997; Gill-King, 1997; Janaway, 1997; Forbes et al., 2017; Piombino-Mascali et al., 2017; Junkins and Carter, 2017; Stuart and Ueland, 2017). However, such studies typically focus on soft tissues outside bone. The literature search for this study uncovered no studies outside the literature of paleontology that specifically tackled the question of whether there are factors that influence the speed of decomposition of blood vessels within bone. Outside bone, factors that increase the speed of decomposition of soft tissues include non-burial, alkaline pH, high temperature, moisture, and the presence of other decomposing organic matter (Galloway et al., 1989; Galloway, 1997; Gill-King, 1997; Janaway, 1997; Forbes et al., 2017; Piombino-Mascali et al., 2017; Junkins and Carter, 2017).
After death, the blood vessels and blood cells in bone can succumb quickly to microbial attack. As I have personally observed, the interior of a bone that remains on the surface of the ground can lose its blood vessels and their contents within a few months or years after death. Even in human bones protected by burial, blood vessels and red and white blood cells can decay completely away within 200 years (Graf, 1949), and those that survive longer usually decay completely away within a millennium, in the absence of further protective factors (Lengyel, 1968).
Blood Vessels: Factors Promoting Preservation
Factors that slow the decomposition of soft tissues outside bone include acidic pH, low temperatures, burial (especially deep burial, which exposes the body to lower temperatures), dryness, sandy sediment (which promotes bodily dessication), anaerobic conditions (which occur, for example, in deep water), and salt water (Galloway et al., 1989; Gill-King, 1997; Janaway, 1997; Forbes et al., 2017; Piombino-Mascali et al., 2017; Junkins and Carter, 2017; Stuart and Ueland, 2017). It is plausible that decomposition of cells and soft tissues within bone is slowed by the same set of factors that slow the decomposition of soft tissues outside bone, but this has yet to be demonstrated with certainty.
It is important to note that the factors that slow decomposition usually do not prevent soft tissue decomposition altogether. A body is usually skeletonized within a few months (Galloway et al., 1989; Bass, 1997; Galloway, 1997)—albeit at a reduced speed—even in a dry, cold environment, whether it is buried or not (Galloway et al., 1989; Galloway, 1997). Long-term preservation of soft tissues therefore requires shielding them from destructive factors that are present even in cold soil. Similarly, water can have a preservative effect if it is cold, salty, and anaerobic (in the deep sea, for example), but under other conditions, water is destructive to soft tissues, because it is a medium that can conduct microbes that consume soft tissues. Long-term preservation of soft tissues therefore requires shielding them from incoming water.
Preserved blood vessels in bone from arid sites in North America have been reported, but without details on the ages of the bones in question (Stout and Teitelbaum, 1976). Aridity may be a preservative factor that contributed to the preservation of blood vessels in these cases. In another case, a small artery was preserved in a Haversian canal in bone from a skeleton from the Viking age (c. 900-1200 years old) of Sweden (Graf, 1949). Little else has been written on preserved blood vessels in archaeological bone.
In fossil bone, preserved blood vessels are known in bone from fluvial (e.g., Schweitzer et al., 2007; Armitage and Anderson, 2013; Ullmann et al., 2019; Schroeter et al., 2022), estuarine (e.g., Schweitzer et al., 2005), and marine (e.g., Surmik et al., 2016; Voegele et al., 2022) depositional paleoenvironments. Because such paleoenvironments were aquatic, aridity is implausible as the initial factor promoting the preservation of the blood vessels. Consequently, some other protective factor must have been present. Protective factors other than aridity that can shield a bone from incoming water include burial in a fine-grained sediment with low permeability, rapid mineral cementation of sediment around the bone, rapid encasement in a mineral concretion, and blockage of the bone’s external pores (Peterson et al., 2010; Plet et al., 2017; Ullmann et al., 2019, 2021, 2022). Protective blockage of a bone’s external pores can occur if mineralized bacterial biofilms fill the pores soon after death (Peterson et al., 2010). With such blockage in place, microbes cannot enter the VCN and therefore cannot degrade the blood vessels and osteocytes that the VCN houses (Figure 5). Likewise, external water cannot enter the VCN, where it would otherwise cause damage by hydrolysis or by conducting other destructive factors into the bone’s interior. Encasement in a mineral concretion is often a result of initial decomposition, but it then prevents the entry of water and microbes into the VCN of a bone and thereby inhibits further decomposition. Different chemical environments produce concretions of different minerals. Organic decomposition releases bicarbonate (HCO3-), and if the bicarbonate reacts with calcium ions in the pore water of deep-sea clay or silt, the concretion that forms is calcite (CaCO3) (Yoshida et al., 2018). If the release of bicarbonate occurs in a zone of sulfate reduction and methanogenesis on the seafloor, the concretion that forms is dolomite (CaMg(CO3)2) (Muramiya et al., 2020). If the organic decomposition occurs in an anoxic or dysoxic, iron-rich sediment on the ocean floor, the concretion that forms is siderite (FeCO3) (Trzęsiok et al., 2014). If the organic decomposition occurs in a calcareous sediment in which the pore water is rich with silica (SiO2), the lowering of the pH by organic decomposition lowers the solubility of the silica, generating a silica concretion (Yoshida et al., 2021). Where iron-carrying water meets oxygenated groundwater, the concretion that forms is an iron oxide (such as hematite) or an iron oxyhydroxide (such as goethite) (Chan et al., 2007; Parry, 2011). If decomposing organic matter hosts sulfate-reducing bacteria in a freshwater or marine environment that is rich in ferrous sulfide (FeS), the bacteria produce hydrogen sulfide (H2S), which reacts with FeS to coat the decomposing organic matter with a layer of pyrite (FeS2) (Schoonen, 2004).
Experimental evidence shows that the breakdown of hemoglobin (Hb) may generate Fenton reactions that contribute to long-term preservation of blood vessels. In an experiment reported in 2014 (Schweitzer et al., 2014), hereafter called the Ostrich Hb Experiment, researchers extracted blood vessels from ostrich bone and soaked them in liquid for two years. The control samples were soaked in water or phosphate buffered saline, in each case with some samples oxygenated and others deoxygenated. The experimental samples were soaked in a solution of Hb, with some samples oxygenated and others deoxygenated. Within weeks, microbial action almost completely consumed the blood vessels in the control samples. After two years, some blood vessels in the control samples had escaped complete destruction but were nonetheless degraded, collapsed, and invaded by fungi. In contrast, the blood vessels in the experimental samples were still free of microbial invasion after two years. They were much more intact than the remaining vessels in the control samples, with no collapse or fungal invasion if oxygenated, and little of either if deoxygenated. Additionally, the blood vessels in the experimental samples were infused with iron oxyhydroxide (FeO(OH)) (Schweitzer et al., 2014), an iron oxide precursor (Chan et al., 2007).
In the same report, the researchers presented a hypothesis as to the mechanism by which Hb contributes to the long-term preservation of blood vessels in fossil bone, based on the following facts that were already known. When red blood cells break down, the Hb that they contain is released. When that Hb breaks down, the iron that it contains is liberated (Balla et al., 2005, 2007). When iron contacts hydrogen peroxide, the Fenton reaction occurs, generating hydroxyl radicals, which are known to generate cross-links between adjacent proteins, making them more resistant to breakdown (Dunlop et al., 2002; Xiong et al., 2010; Li et al., 2012; Zhang et al., 2021; Lei et al., 2022). Putting these facts together with their experimental results, the researchers hypothesized that in the fossil bones with preserved blood vessels, the rupture of decomposing red blood cells had released Hb, which induced Fenton reactions. When the Hb broke down, the released iron had reacted with hydrogen peroxide from decomposing cells, generating hydroxyl radicals that generated cross-links between adjacent collagen molecules in the blood vessel walls, thus making them resistant to decomposition (Schweitzer et al., 2014) (Figure 5). In subsequent studies, the spectroscopic properties of the collagen cross-links in blood vessels in Mesozoic reptile bone confirmed that Fenton chemistry and iron had been involved in the cross-linking (Surmik et al., 2016; Boatman et al., 2019). Although Fenton reactions can damage biomolecules, that damage is minimized in the presence of collagen, which scavenges hydroxyl radicals (Xiao et al., 2007) and inhibits lipid peroxidation of the PUFAs of phospholipids (He et al., 2002). The PUFAs, in turn, provide further protection by scavenging hydroxyl radicals (Lipinksi, 2011). The protection afforded by collagen and PUFAs plausibly would have allowed Fenton reactions to generate tissue-stabilizing cross-links and iron oxide precipitates without completely destroying the altered biomolecules.
As with bone mineral, preserved blood vessels undergo diagenesis after death. In fossil bone, if blood vessels are preserved, their diagenetic changes include the chemical signatures of Fenton reactions. For example, blood vessels preserved in fossil bone are usually enriched in iron (Schweitzer et al., 2014; Cadena, 2016; Lee et al., 2017; Ullmann et al., 2019), although there are exceptions (Cadena, 2020). This is due to infusion and coating with iron oxides such as hematite (alpha-Fe2O3) and the iron oxide precursor iron oxyhydroxide, the latter of which is present both in the crystalline form goethite and an amorphous form (Lindgren et al., 2011; Schweitzer et al., 2014; Surmik et al., 2016; Lee et al., 2017; Ullmann et al., 2019; Boatman et al., 2019). In some cases, the collagen that makes up part of the blood vessel wall is preserved (Pawlicki, 1966; Cadena, 2016; Boatman et al., 2019), although it is diagenetically altered in that the number of cross-links has increased (Boatman et al., 2019) (see the section on CBM preservation, below), and endothelial cells are preserved in some cases (Pawlicki and Nowogrodzka-Zagóriśka, 1998). In other cases, most of the organic matter has decayed away, leaving behind the iron oxide that had infused and coated the blood vessels and which retains the shapes of the vessels (Lindgren et al., 2011).
It is probable that the Fenton reactions that stabilize cells and soft tissues within bone occur early during diagenesis, because their occurrence appears to be tied to conditions that exist early in the postmortem history of the bone. One such condition is deposition in an oxidizing environment (such as fluvial, lacustrine, and shallow marine sediments). Evidence of Fenton reactions in fossil bone comes mainly from fossils from depositional environments that were oxidizing at the time of deposition (Wiemann et al., 2018; Ullmann et al., 2021, 2022). Another condition to which Fenton reactions appear to be tied is an initial prevention of groundwater from percolating through the VCN, which might otherwise disrupt Fenton reactions. Factors that confer such prevention include burial in a fine-grained sediment with low permeability, rapid mineral cementation of sediment around the bone, and/or rapid encasement in a mineral concretion (Plet et al., 2017; Ullmann et al., 2019, 2021, 2022). Concretions around decomposing organic matter tend to form during early diagenesis (Trzęsiok et al., 2014; Yoshida et al., 2018; Muramiya et al., 2020). In addition, Fenton reactions occurred within the two-year time frame of the Ostrich Hb Experiment (Schweitzer et al., 2014). This suite of factors suggests that when Fenton reactions stabilize cells and soft tissues within bone, it happens early in diagenesis.
In addition to Hb, ferritin is another plausible source of the iron in the iron oxides that infuse and coat the preserved blood vessels in fossil bone. The presence of superoxide radicals (O2·-) can cause ferritin to release the iron that is sequestered in its core (McCord, 1998). Ferritin is produced by osteocytes (Spanner et al., 1995; Li et al., 2018) and by the endothelial cells (Balla et al., 2005, 2007) and smooth muscle cells (Zarjou et al., 2009) of blood vessels. Its production therefore occurs in locations consistent with ferritin as the source of the iron in the iron oxides that infuse and coat blood vessels within fossil bone. It is also possible that at least some of these iron oxides derive directly from ferritin cores. The iron-sequestering cores of ferritin molecules contain the mineral ferrihydrite (a form of iron oxyhydroxide), which is known to form goethite when dissolved and hematite during dehydration (Gutiérrez et al., 2009). Intracellular and extracellular deposits of goethite and hematite, apparently from ferritin cores, form in vertebrate tissues undergoing iron overload in vivo (Gutiérrez et al., 2009).
The literature search for this review uncovered numerous reports of preserved blood vessels in fossil bone (Pawlicki, 1966; Pawlicki and Nowogrodzka-Zagóriśka, 1998; Schweitzer et al., 2005, 2007, 2014, 2016; Peterson et al., 2010; Armitage and Anderson, 2013; Armitage, 2015, 2016; Cadena, 2016; Surmik et al., 2016; Wiemann et al., 2018; Boatman et el., 2019; Kiseleva et al., 2019; Ullmann et al., 2019; Cadena et al., 2020; Armitage and Solliday, 2020; Bailleul and Zhou, 2021; Barker et al., 2021; Schroeter et al., 2022; Voegele et al., 2022) but few on preserved blood vessels in archaeological bone (Graf, 1949; Stout and Teitelbaum, 1976). The researchers reporting on archaeological bone did not test for signs of iron oxide or Fenton reactions in the blood vessels. It is possible that the dearth of reports of preserved blood vessels in archaeological bone is due to a dearth of researchers looking for blood vessels therein. However, it is also possible that the dearth of reports of preserved blood vessels in archaeological bone is not an artifact but represents a real lack of such preservation in the bones in question. This, in turn, may be because cellular and soft tissue stabilization by Fenton reactions did not occur in the studied archaeological bone. A plausible explanation for this is that most studies of archaeological bone focus on human bones, which tend to have been subjected to funerary burials. Humans usually do not bury their dead beneath river, lake, or ocean sediments, whereas such sediments tend to be the ones that entomb the fossils of non-human vertebrates. The initial conditions that bones undergo are therefore usually very different for fossil bones with preserved cells and soft tissues than they are for most of the archaeological bones that have been studied.
Blood Cells and Plasma Proteins: Factors Promoting Decomposition
The literature search for this study did not uncover studies on factors promoting the decomposition of blood cells and plasma proteins in bone. It is plausible that the same factors that promote the decomposition of blood vessels in bone also promote the decomposition of their contents.
Blood Cells and Plasma Proteins: Factors Promoting Preservation
Few examples of blood cells are reported from archaeological bone. In one case, red blood cells were preserved in the marrow cavity of a human vertebra between 400 and 700 years old, from southwestern North America (Stout and Teitelbaum, 1976). It is possible that the local aridity was an initial preservative factor that contributed to the preservation of blood cells in that case. In another case, bone marrow cells were preserved in bone from a skeleton from the Viking age of Sweden, as were red blood cells in one of its Haversian canals (Graf, 1949). In another set of cases, red and white blood cells were preserved in the marrow cavities of human bones from Kuwait that were approximately 2200 years old. Aridity cannot have been a preservative factor, because the area had become arid only since the 1950s (Maat, 1991, 1993). Anoxic conditions and a low burial temperature may have contributed to blood cell preservation in the bones from Kuwait (Maat, 1991, 1993). The author of the report further hypothesized that infusion with minerals from groundwater had contributed to the cells’ preservation, but he did not confirm the presence of minerals with chemical tests.
To date, there are no undisputed reports of blood cells in fossil bone. Several examples of putative blood cells in fossil bone have been reported (Pawlicki and Nowogrodzka-Zagóriśka, 1998; Yao et al., 2002; Schweitzer et al., 2005, 2007; Armitage and Anderson, 2013; Armitage, 2015, 2016; Kiseleva et al., 2019), but their identification as blood cells is questionable (Saitta et al., 2017; Korneisel et al., 2021; this paper: see the section on Misconception 10, below).
Proteins that are normally found in blood plasma have been identified in archaeological and fossil bone. Albumin has been identified in archaeological bone 300-4200 years old (Cattaneo et al., 1990, 1992a, b; Sawafuji et al., 2017) and in archaeological and Pleistocene bone from an array of sites with ages between 4000 and 900,000 years (Tuross, 1989; Montgelard, 1992; Wadsworth and Buckley, 2014). Although albumin is a component of blood plasma, it is also produced by osteoblasts and bone marrow cells (Ishida et al., 2004) and is incorporated into bone matrix (Owen and Triffitt, 1976), where it binds to bone mineral (Clarke, 2008). It is therefore possible that the albumin in archaeological and fossil bone is from bone matrix, rather than from blood plasma. If so, its entrapment in (and protection by) bone matrix may contribute to its long-term preservation (Wadsworth and Buckley, 2014).
Archaeological bone has also tested positive for plasma proteins involved in coagulation. Such proteins include coagulation factor X in bones as old as 6000 years, and prothrombin, fibrinogen, and coagulation factors VII and IX in bones as old as 20,000-60,000 years (Wadsworth and Buckley, 2014). In the latter cases, cold appears to have been a major factor in the survival of the proteins for > 20,000 years, because cold is conducive to cellular and soft tissue preservation, and the bones in question were from sediments beneath the cold North Sea (Wadsworth and Buckley, 2014).
Other plasma proteins from archaeological bone include immunoglobulin G in bones as old as 1490-1540 years (Cattaneo et al., 1992b) and alkaline phosphatase in bones as old as 100,000 years (Weser et al., 1996). In addition to plasma proteins, Hb has been identified in archaeological bone as young as 800-1900 years and as old as 4000-4500 years (Ascenzi et al., 1985; Smith and Wilson, 1990). The number of plasma proteins that can be recovered from archaeological and young fossil bone decreases with increasing age, as does the amount of each protein that is recovered from the bone (Ascenzi et al., 1985; Cattaneo et al., 1992a, b; Weser et al., 1996; Wadsworth and Buckley, 2014). This suggests that even after favorable conditions have allowed a plasma protein to last longer than usual, it may become subject to decay if conditions conducive to its decay subsequently arise.
Plasma proteins are expected to be present in bone canaliculi, because canaliculi conduct materials from blood plasma (Feng et al., 2006). Unlike vascular canals, canaliculi are too narrow for most bacteria to enter. Plasma proteins within canaliculi may therefore survive the microbial decomposition stage of bone decomposition, even after blood vessels have succumbed to it. Bone canaliculi are about 0.1 μm in diameter (Fritton and Weinbaum, 2009). Although bacteria as small as 0.02 μm are known (Velimirov, 2001), most bacteria are 0.2-5 μm (Levinson et al., 2020), too large to enter bone canaliculi.
Osteocytes: Factors Promoting Decomposition
Like blood vessels, osteocytes deteriorate faster than CBM and can decay completely away within 200 years (Graf, 1949). In the absence of protective factors, osteocytes that survive longer usually decay completely away within a millennium (Lengyel, 1968). Preserved osteocytes were absent in bone from a skeleton from Viking age Sweden, despite that marrow cells, red blood cells, and an artery were preserved in bone from the same individual (Graf, 1949). Bones of three Egyptian mummies 2500-3500 years old were devoid of preserved osteocytes (Graf, 1949), despite the arid environment that is expected to have discouraged microbial decomposition. Little else has been published on preserved osteocytes in archaeological bone.
Osteocytes and Chondrocytes: Factors Promoting Preservation
As mentioned above, osteocytes are protected from microbial degradation by the small size of the canaliculi, which are too narrow for most bacteria to enter, which means that most bacteria cannot reach osteocytes. Nevertheless, to be preserved, osteocytes must avoid destruction during autolysis and during the abiotic phase of bone decomposition that occurs after the initial microbial phase.
Osteocytes that are preserved in fossil bone are iron-rich (Pawlicki, 1995; Schweitzer et al., 2013, 2014; Cadena, 2016, 2020; Ullmann et al., 2019; Surmik et al., 2021). One pair of researchers suggested that preserved osteocytes in a Triceratops horn core lacked the ruffled borders that one would expect from iron nanoparticles (Armitage and Anderson, 2014), but they did not chemically test for the presence of iron, whereas researchers who did test osteocytes in Mesozoic bone for the presence of iron found it in abundance (Pawlicki, 1995; Schweitzer et al., 2013, 214; Cadena, 2016, 2020; Ullmann et al., 2019; Surmik et al., 2021). In at least one case of Mesozoic fossil bone, hematite is present in the lacunae housing the preserved osteocytes, even though their canaliculi are blocked by carbonate infills (Lee et al., 2017). In another case, the large amount of iron oxide is inconsistent with exogenous origin (Surmik et al., 2021). These cases indicate that the source of the iron is endogenous. This is consistent with the hypothesis that the iron in the preserved osteocytes and the iron oxide that surrounds them are derived from iron-associated proteins such as ferritin, which is produced by osteocytes (Spanner et al., 1995; Li et al., 2018). As with blood vessels, it is plausible that this iron contributed to the preservation of the osteocytes, via Fenton reactions.
Iron and iron oxides have also been found to coat and infuse chondrocytes and cartilage matrix in the articular cartilage of a dinosaur specimen (Zheng et al., 2021). Although the researchers who reported the discovery hypothesized that the iron percolated in from groundwater, it is also possible that the iron is endogenous and came from ferritin, which is known to be present in chondrocytes (Khan et al., 2000) and in the synovial fluid adjacent to articular cartilages (Cai et al., 2022). It is plausible that the iron in this case did not come from Hb in red blood cells, because cartilage matrix lacks blood vessels. In two Cretaceous bird fossils, preserved chondrocytes were infused with aluminum and silica, rather than iron (Bailleul and Zhou, 2021). This suggests that metals other than iron may be involved in cellular and soft tissue preservation in some cases.
Micropetrosis, the infilling of osteocyte lacunae with bone mineral during an organism’s life, has been described as fossilization in vivo (Bell et al., 2008). Uncommon in young and healthy bones, its frequency increases with old age and in cases of osteoporosis (Carpentier et al., 2012). The plasma membrane, nuclear material, and cytoskeleton of a micropetrotic osteocyte can survive for over 400 years after death. However, in all known examples of micropetrotic lacunae in fossil bones, the organic components of the osteocytes have decayed away and have been replaced by mineral bodies with the shapes of the cells (Carpentier et al., 2012). In such cases, the cells themselves cannot be said to have fossilized, because they did not survive into the fossil record. However, because micropetrosis contributes to the formation of mineral casts of osteocytes, and because a mineral cast of all or part of an organism is considered a category of fossil (Prothero, 2013), it is correct to say that micropetrosis contributes to fossilization in that it produces mineral casts of osteocytes.
CBM: Factors Promoting Decomposition
Bone collagen is vulnerable to microbial attack, and its preservation requires protection from such attack. A watery environment without special protective factors facilitates microbial action and is therefore a factor that promotes collagen decomposition. Small bone size is another such factor. In a watery environment without special protective factors, microbial action completely eliminates the CBM of small bones such as those of rodents within a few months (Pfretzschner, 2004). In larger bones, such as those of humans, microbes in watery environments consume the CBM around the periphery of a bone within a few months, whereas CBM in the interior of the bone outlasts the initial microbial phase of post-mortem bone alteration (Pfretzschner, 2004). However, in a watery environment, after the initial microbial phase of decomposition, the CBM in the interior of a large bone undergoes an abiotic decomposition phase in which water breaks down the CBM by hydrolysis (Pfretzschner, 2004). During this phase, the influx of water causes CBM to swell, generating cracks in the bone that admit more water, which keeps the process going (Pfretzschner, 2004). In some large fossil bones, CBM has decayed completely away (Saitta et al., 2019; Suarez and Kohn, 2020), and processes such as these may be responsible for its disappearance.
In environments that are not saturated with water, mineralized collagen such as CBM is protected from hydrolysis by its intimate association with bone mineral (Trueman and Martill, 2002). In such environments, microbial activity may be necessary for hydrolysis of CBM to occur. However, microbes tend to require water and tend not to gain access to the CBM without it (Trueman and Martill, 2002). Thus, in an arid environment without groundwater, CBM may survive for millennia, even in the absence of further protective factors.
However, no bone that survives long enough to enter the fossil record has completely escaped exposure to groundwater, because on a scale of millennia, aridity is temporary. Even the areas that are now occupied by major deserts were rainy earlier in the Holocene. For example, according to radiometric dates, such was the case for the area now occupied by the Gobi Desert as recently as 2000 years ago (Rosen et al., 2019), the Chihuahuan Desert 4000 years ago (Castiglia and Fawcett, 2006), the Sahara Desert 5000 years ago (Tierney et al., 2017), the Patagonian Desert 5200 years ago (Iglesias et al., 2011), the Arabian Desert 6000 years ago (Petraglia et al., 2020), the Great Basin 8000 years ago (Steponaitis et al., 2015), and the Colorado Plateau 10,000 years ago (Louderback et al., 2020). Patterns of recrystallization, in which ions in bone mineral are exchanged for ions in groundwater, demonstrate that groundwater can penetrate the VCN of buried bone (Trueman et al., 2008; Ullmann et al., 2021, 2022), which can then conduct the water to internal CBM, unless the bone is protected from the influx of water, for example by permineralization or an external mineral concretion.
Collagen breakdown is accelerated in environments that are hotter, extremely acidic, or extremely basic, and around cracks in bone (Kendall et al., 2018). It also occurs when collagen is exposed to hydroxyl ions (OH -), although the hydroxyl-mediated hydrolysis of collagen that is protected by bone mineral is slower than that of unmineralized collagen and is also slower than collagen hydrolysis by collagenase enzymes such as those that collagen-consuming microbes use (Trueman and Martill, 2002).
CBM: Factors Promoting Preservation
Collagen is the most long-lasting of all proteins (Wadsworth and Buckley, 2014). A major reason for this is that cross-links confer long-term stability upon organic molecules, and adjacent collagen molecules readily form cross-links with each other in vivo (Kendall et al., 2018; San Antonio et al., 2011), both within and between collagen fibrils (Veis, 2003; Sweeney et al., 2008; Alexander et al., 2012). This cross-linking does not stop at death. Instead, collagen continues to accumulate cross-links post-mortem, contributing to the continuing survival of collagen in bone long after death (Kendall et al., 2018). The bonds that collagen forms with bone mineral appear to contribute further long-term stability to both materials (Zazzo, 2014). The tightly packed, triple helical structure of the collagen molecule provides additional stability (Kendall et al., 2018). As a result of its stability, CBM may persist in archaeological bone for centuries after blood vessels and osteocytes have decayed away (Graf, 1949; Lengyel, 1968; Duffett Carlson et al., 2022; Mandl et al., 2022).
Further protective factors for CBM include dryness, extreme temperatures, and bactericidal metals. CBM can escape microbial decomposition in a dry environment, if it undergoes dessication before microbes can begin their attack (Trueman and Martill, 2002). Extremely cold or hot temperatures also inhibit microbial degradation of CBM, as does the presence of bactericidal metals in sediments entombing bones (Jans et al., 2004). In some cases, preserved CBM in fossil bone is enriched in iron (Ullmann et al., 2019), a bactericidal metal (Schultz, 2001). In other cases, it is not (Cadena, 2016). Iron enrichment alone is therefore not sufficient to explain the preservation of CBM in all cases.
Large bone size is another protective factor for CBM. In human skeletal remains, decomposition of CBM during the initial microbial decomposition phase may be restricted to the external cortex of a bone, due to its size (Pfretzschner, 2004). The collagen in the internal cortex may escape the initial phase of decomposition but needs protection to avoid decomposition during the following, hydrolytic phase of decomposition.
If mineralized bacterial biofilms fill the bone’s external pores soon enough after death, this can prevent water and microbes from entering the VCN. The blockage protects the internal CBM from microbial attack and from hydrolysis by groundwater (Peterson et al., 2010). Blockage of canaliculi by the precipitation of endogenous iron can have a similar protective effect (Lee et al., 2017). Such processes are effective only if the bone is not fractured post-mortem, because fracturing allows destructive agents to bypass the blockages via cracks in the bone (Peterson et al., 2010).
As with the other components of bone, CBM undergoes diagenesis after death. Collagen in fossil bone matrix includes AGEs (advanced glycation end-products) (Wiemann et al., 2018; Boatman et al., 2019) and ALEs (advanced lipoxidation end-products) (Wiemann et al., 2018). AGEs are proteins or lipids that have become covalently bonded to monosaccharides (Goldin et al., 2006). ALEs are proteins that have become covalently bonded to reactive carbonyl species, which are products of lipid peroxidation (Gianazza et al., 2019). AGEs and ALEs are resistant to microbial digestion. In the CBM of fossil bones, such modified collagen molecules have also undergone a dramatic increase in the number of cross-links, reinforcing them further against breakdown and making them hydrophobic and thus resistant to hydrolysis (Wiemann et al., 2018). Depositional environments that are oxidizing (for example, fluvial, lacustrine, and shallow marine sediments) encourage such cross-linking, whereas depositional environments that are reducing (for example, deep marine sediments) discourage it (Wiemann et al., 2018). Consequently, collagen-rich fossil bone is more characteristic of oxidizing than reducing depositional environments (Wiemann et al., 2018). However, the preservation of cells and soft tissues in fossil bone does not seem to be compromised by a microenvironment within the bone that is oxidizing at first and becomes reducing later (Ullmann et al., 2022).
The AGEs, ALEs, and increased cross-linking in fossil CBM are signs of Fenton reactions (Wiemann et al., 2018; Boatman et al., 2019). Another sign of Fenton reactions is the presence of iron oxide precipitates. Researchers have found such precipitates in numerous examples of preserved cells and soft tissue from fossil bones (Lindgren et al., 2011; Schweitzer et al., 2014; Surmik et al., 2016, 2021; Lee et al., 2017; Ullmann et al., 2019; Boatman et al., 2019; Zheng et al., 2021). Furthermore, in fossil CBM, the connection between cross-linking and oxidizing environments is consistent with Fenton chemistry as a source of the cross-linking.
Despite the protection that Fenton chemistry confers via hydroxyl radical-mediated cross-links, it is possible that some collagen breakdown may yet occur. Reactions with hydroxyl radicals cause a degree of unfolding of the main chain of the collagen molecule (Xiao et al., 2007), which could conceivably expose vulnerable parts of the molecule that were previously protected by its three-dimensional shape, leaving those parts exposed to destructive agents later. This could explain how the main chain of collagen in fossil CBM may have undergone cleavage (San Antonio et al., 2011). The intact amino acid sequences between the breaks are those that are shielded by tight packing between adjacent collagen molecules, those that are hydrophobic and therefore resistant to hydrolysis, and those that have few acidic amino acids (San Antonio et al., 2011).
Even in a degraded state, CBM is still recognizable as collagen, even in bone far older than the Holocene (Lengyel, 1968; Schweitzer et al., 2007; Wadsworth and Buckley, 2014; Schroeter et al., 2017). Despite the breaks in some of its amino acid sequences in fossil bone, the amino acid sequences that remain are recognizable as collagen, and the relative abundances of the different amino acids are typical of collagen (San Antonio et al., 2011; Lindgren et al., 2011; Bertazzo et al., 2015; Cleland et al., 2016; Surmik et al., 2016; Schroeter et al., 2017; Boatman et al., 2019; Bailleul et al., 2020). Hydroxyl radicals generate cross-links in collagen (Windhager et al., 1998), and even after other destructive agents have fragmented the collagen chain, the fragments may remain cross-linked together, which undoubtedly contributes to their preservation.
During CBM diagenesis, cross-links form not only between adjacent collagen molecules but also between collagen and incoming organic contaminants (van Klinken and Hedges, 1995). Organic contaminants in archaeological and fossil bone can come from soil or from invading algae, fungi, and other microbes (Colleary et al., 2021). Whether cross-links with organic contaminants contribute to the long-term preservation of CBM has not been established.
Overview of the Post-Mortem Fate of Cells and Soft Tissues in Bone: Illustrative Scenarios
As shown above, the fate of a bone and its cells and soft tissues after death depends upon a complex set of factors that at first glance is a bewildering labyrinth of possibilities. Navigating that labyrinth becomes easier if we consider a simplified set of hypothetical examples, such as the following. Note that numerous other scenarios and combinations of scenarios are possible than the few that are given below.
Bone A is surrounded by soil that is acidic, contains calcium aluminum phosphate, and is porous and light. These abiotic factors cause the bone mineral of Bone A to dissolve in the soil. As the bone mineral dissolves, cracks in Bone A are exploited by bone-consuming bacteria, fungi, and plant roots. These invaders consume the bone mineral and CBM of bone matrix, exposing more of Bone A to destructive agents, which accelerates its decomposition in the vicinity of the cracks. Bone A vanishes before it can fossilize.
Bone B is surrounded by soil that is alkaline, cold, and sandy, and contains calcium carbonate. As a result, Bone B remains intact longer than Bone A. After a thousand years, its blood vessels and osteocytes have succumbed to decay, leaving behind a relatively empty VCN, but the matrix of Bone B remains intact. Over the next tens of thousands of years, as groundwater percolates through the VCN, it exchanges ions with those of the bone mineral. The exchange of hydroxide ions in bone mineral with fluoride from groundwater converts the bone mineral into francolite and later into fluoroapatite, stabilizing it and enabling it to last yet longer. Permineralization occurs as ions in groundwater precipitate as mineral crystals onto the surfaces of the VCN, filling the voids and further contributing to preservation by preventing the entry of destructive agents later. Subsequent drying (during freeze-thaw cycles, during wet-dry cycles, or as a result of the local environment becoming arid) generates cracks, which create more surfaces on which minerals precipitate, contributing further to long-term preservation. Bone B will last for millions of years after its blood vessels and their contents have vanished.
Bone C is deposited in an oxidizing environment beneath sediment at the floor of a body of water. Water and microbes invade the VCN of the bone’s cortex and consume organic matter. As they do, they increase the porosity of the bone, allowing more water to enter. The entry of water causes the collagen in the matrix of the bone’s cortex to swell, which creates cracks in the cortex that let in yet more water, accelerating the rate of collagen destruction by hydrolysis even after the initial microbial phase of decomposition has taken place. If Bone C were tiny, these processes would consume it before it had a chance to fossilize. However, Bone C is very large, and the destructive processes are restricted to its external cortex. As microbes in the VCN of the bone’s exterior precipitate minerals that plug the VCN, they block both themselves and water from entering further into the bone. Deeper inside Bone C, iron is released as the ferritin in its osteocytes and the endothelial lining of its blood vessels decomposes. The resulting Fenton reactions split numerous organic molecules and forge cross-links between their fragments, enabling those fragments to remain intact. Further Fenton reactions, generating further breakdown of organic molecules and cross-linking between their fragments, occurs as iron is released from decomposing heme that is liberated from decomposing red blood cells. The release of iron also generates a protective coating of iron oxyhydroxide over the internal and external surfaces of the blood vessels in Bone C, a coating that will later be converted into iron oxide. Over time, cross-links accumulate between collagen molecules in the CBM that was shielded from the Fenton reactions by bone mineral, increasing its stability and its resistance to hydrolysis. Millions of years later, the body of water dries up. Freeze-thaw cycles and wet-dry cycles generate cracks in the bone, allowing water into the VCN. Where the coating of iron oxides over the blood vessels is continuous, the vessels are shielded from hydrolysis. Where it is interrupted, the blood vessels succumb to decay as water and other destructive agents arrive, leaving behind an empty shell of iron oxides that maintains their shape. Some collagen also succumbs to decay by hydrolysis and other destructive agents, but such decay is prevented where cross-links are particularly abundant and where water has not entered the adjacent VCN. As groundwater seeps through some parts of the VCN, recrystallization and permineralization take place in those parts of the bone and contribute to its long-term preservation. As Bone C illustrates, different magnitudes and modes of cellular and soft tissue preservation can exist in different parts of a bone, due to differences in microenvironment and diagenetic history in different parts of the bone.
Bone D is covered in sediment at the floor of a body of water. If the pore water in the sediment is calcium-rich, a concretion of calcite forms around Bone D, shielding its VCN from the entry of destructive agents and allowing Fenton reactions to preserve its osteocytes and blood vessels as iron is released from decomposing ferritin and Hb. The same occurs if the pore water is iron-rich and a concretion of siderite forms around the bone, or if the bone is located where iron-rich water and oxygenated groundwater meet and a concretion of iron oxide forms around it. If the water is not rich in substances that facilitate concretions, then no concretion forms around Bone D. Water invades its VCN, and the organic matter in Bone D succumbs to decomposition by microbial action and hydrolysis. Over the years, the bone mineral slowly dissolves in the water, unless it is protected by sufficiently timely recrystallization and permineralization as water percolates through its VCN.
MISCONCEPTIONS ABOUT CELLULAR AND SOFT TISSUE PRESERVATION IN FOSSIL BONE
Misconceptions 1-13 below were voiced in science journals, including peer-reviewed journals in most cases (Misconceptions 2-4 and 9-13). Most of these misconceptions are rooted in the YEC view (Misconceptions 1, 3-8, and 11-13), although a few are not. Much YEC literature opposes the hypothesis that Fenton chemistry is a plausible explanation for the preservation of cells and soft tissues in fossil bone through geologic time (hereafter called the Fenton hypothesis, abbreviated FH). Most of the arguments that are presented to support such opposition are based on misconceptions of Fenton chemistry.
Misconception 1: Fenton Reactions Would Destroy More Soft Tissues Than They Preserve
Prominent among oft-repeated misconceptions of Fenton chemistry is the misconception that Fenton chemistry would destroy more soft tissue and protein than it preserves. This misconception was first voiced in YEC literature (DeMassa and Boudreaux, 2015; Anderson, 2016a, 2017a, 2018), then in a science journal (Armitage and Solliday, 2020).
Experimental data rebut this misconception. Although collagen is affected by the hydroxyl radicals that Fenton chemistry produces, it is not destroyed by them. Reactions with hydroxyl radicals alter some of the exposed functional groups of the amino acids in collagen (Xiao et al., 2007), remove the side chains of some amino acids (Hawkins and Davies, 1997), and cause a degree of unfolding of the main chain of the collagen molecule (Xiao et al., 2007). However, such reactions do not cleave the main chain of collagen (Hawkins and Davies, 1997). The collagen is therefore altered but not destroyed. Furthermore, hydroxyl radicals generate cross-links in collagen (Windhager et al., 1998), thereby contributing to its long-term preservation.
It is plausible that the unfolding of collagen’s main chain exposes vulnerable parts of the molecule that would otherwise have been protected by the three-dimensional shape of the molecule leaving those parts vulnerable to destructive agents (see the section on CBM preservation, above). However, fossil CBM is rich in cross-links (Wiemann et al., 2018; Boatman et al., 2019) that would keep adjacent collagen fragments united if the collagen were fragmented. In such a case, the CBM would be damaged but not disintegrated.
Nor is Fenton chemistry expected to destroy a cell’s plasma membrane. When hydroxyl radicals generate chain reactions of peroxidation in the phospholipids of a cell’s plasma membrane, the results of these reactions can include cleavage of phospholipids (Yurkova et al., 2004), in addition to further changes that stop the membrane from functioning properly, which may culminate in cell death (McCord, 1998; Yurkova et al., 2004; Catalá, 2009). However, a severely damaged cell membrane may be altered and functionally compromised and yet remain a continuous sheet of material that encloses and maintains the shape of the cell, just as a blanket that has become worn, abraded, and oxidized is still a coherent sheet of fabric. Furthermore, there is only so far that such chain reactions can go, because the double bonds in the PUFAs of phospholipid molecules scavenge hydroxyl radicals (Lipinski, 2011), thereby preventing or stopping such chain reactions, thus limiting damage.
The misconception that cellular and soft tissue preservation in fossils is incompatible with Fenton chemistry has also been falsified in a more direct way. Collagen cross-links produced by Fenton chemistry and iron can be distinguished by spectroscopy from collagen cross-links that formed via some other mechanism. Spectroscopy confirms that the blood vessels and CBM of Mesozoic reptile bones bear the diagnostic signs of Fenton reactions (Surmik et al., 2016; Wiemann et al., 2018; Boatman et al., 2019).
Misconception 2: Not Enough Iron
In a 2016 article on soft tissue preservation in fossil reptile bones from the Triassic Period, researchers hypothesized that the iron source for the iron oxides that coated the blood vessels within the bone was exogenous. They based that hypothesis on the assumption that “the total body iron content of various animals...may be insufficient for the precipitation of iron (hydro)oxides” (Surmik et al., 2016: p. 20). This misconception was repeated in a YEC book in which the author cited the article as a reference for the statement that “blood would probably not carry sufficient levels of iron to the tissue,” to cast doubt upon the FH (Anderson, 2017a: p. 58). The latter author made similar statements in other publications (Anderson, 2016a, Anderson 2017b).
Published data rebut this misconception. In the Ostrich Hb Experiment, iron oxyhydroxides precipitated from 20 mL of ostrich blood (electronic supplementary information of Schweitzer et al., 2014), a tiny fraction of the total blood volume of an ostrich or a multi-ton dinosaur. The Ostrich Hb Experiment therefore demonstrated that red blood cells have enough iron to precipitate iron oxyhydroxide, an iron oxide precursor. Moreover, the endogenous iron that contributes to cellular and soft tissue stabilization within bone need not all have come from blood. Another plausible source is ferritin, the iron-sequestering cores of which contain the mineral ferrihydrite, which is known to form iron oxides (Gutiérrez et al., 2009). Ferritin is produced by osteocytes (Spanner et al., 1995; Li et al., 2018) and by the endothelial cells (Balla et al., 2005, 2007) and smooth muscle cells (Zarjou et al., 2009) of blood vessels. It is therefore produced in locations that make it a plausible a source of iron involved in precipitating the iron oxides in and around cells and soft tissue within fossil bone.
Misconception 3: Controlled Laboratory Conditions
According to this misconception, the Ostrich Hb Experiment did not support the FH, because the experiment was not conducted under natural conditions but was instead conducted under controlled laboratory conditions. This misconception was first voiced in a peer-reviewed science journal (Armitage and Anderson, 2014) before it was repeated in subsequent YEC literature (DeMassa and Boudreaux, 2015; Anderson, 2017a; Armitage, 2017; Anderson, 2018) and another science journal (Armitage and Solliday, 2020). The FH opponents who voiced this misconception cited other biochemical studies that were conducted under controlled laboratory conditions, without apparent misgivings (DeMassa and Boudreaux, 2015; Anderson, 2017a; Armitage and Solliday, 2020). Therefore it appears that their objection to the drawing of conclusions based on controlled laboratory experiments applies only to experiments producing inconvenient results.
If this misconception were accurate, it would invalidate nearly all laboratory experiments in physiological biochemistry. The use of controlled laboratory conditions is necessary to test most biochemical hypotheses, because it is often the only way to ensure that a specific experimental result is due to a given factor (Lazic, 2016). When there are too many potential causative factors present in vivo for a given experiment to pinpoint one, it is necessary to conduct a controlled experiment in which the number of causative factors is limited, so that the experimenters can determine which factor caused a given result (Lazic, 2016). In the case of the Ostrich Hb Experiment, the potential causative factors were sufficiently limited to enable the determination that Hb was the source of the iron in the resulting infusion of iron oxyhydroxide.
Misconception 4: Ostrich Hb Experiment Duration
According to this misconception, the two-year duration of the Ostrich Hb Experiment was too short to support its conclusions. This misconception was first voiced subtly in a peer-reviewed science journal: “it examined tissue preservation for just a few months” (Armitage and Anderson, 2014, p. 1274). Since then, it has been more bluntly voiced in YEC literature, in which the authors assert that the two-year duration of the Ostrich Hb Experiment is insufficient to demonstrate that the preservation mechanism would continue for millions of years (DeMassa and Boudreaux, 2015; Thomas, 2015; Anderson, 2017a, 2018; Armitage, 2017).
Obviously, it is impossible for researchers to keep an experiment running for millions of years, so it is unreasonable to insist that an experiment run that long. Moreover, the short duration of the Ostrich Hb Experiment does not invalidate its main conclusions: that Hb is an iron source that can produce an infusion of the iron oxide precursor iron oxyhydroxide in cells and soft tissues, and that cells and soft tissues are then protected from microbial degradation (at least for a time). The experiment’s results unambiguously confirm these conclusions.
Misconception 5: Intact Vein Valve Cuspids
According to this misconception, the finding of intact valve cuspids (the curved flaps of tissue that make up the valves in veins) within Triceratops bones invalidates the FH, because Fenton reactions should have damaged the cuspids (Armitage and Solliday, 2020). Recent findings on the effects of Fenton reactions on venous valves rebut this misconception. Venous valves are extensions of the tunica intima, the tissue that lines the lumen (the internal cavity) of the vein. The tunica intima consists of a thin endothelium composed of a single layer of cells, plus an underlying subendothelial layer of loose connective tissue (Junqueira et al., 1998). In vivo, damage by hydroxyl radicals generated by Fenton reactions causes the tunica intima to thicken, due to changes in the organization of extracellular fibers composed of the protein elastin within the subendothelial layer, rendering the valves incompetent (Krzyściak et al., 2012). Despite being functionally compromised, incompetent valves are nonetheless intact (Lane et al., 2007). Because the changes that thicken the tunica intima in response to Fenton reactions occur in the extracellular matrix of its connective tissue, they are apparently not dependent upon conditions within living cells and would therefore be expected to occur even after death if Fenton reactions took place in the tissue of a valve. Fenton reactions within the valve of a vein after death therefore do not destroy its cusps but instead thicken them, making them more—not less—likely to be intact in a fossil bone with preserved veins.
Misconception 6: Preserved Osteocyte Dendritic Processes
According to this misconception, Fenton reactions should have fragmented the dendritic processes of osteocytes. The authors who voiced this misconception stated that dendritic processes “just outside of the vessel canals are long and unfragmented, suggesting that Fenton reactions never occurred within the lacuna-canalicular network...” (Armitage and Solliday, 2020: p. 34).
Fragmentation of dendritic processes would entail rupture of the cell membrane. However, Fenton reactions in bone cells undergoing iron overload generate ferroptosis (Gao et al., 2022; Liu et al., 2022). In cells undergoing ferroptosis, the cell membrane is damaged but not ruptured (Yan et al., 2021; Gao et al., 2022; Liu et al., 2022). Fenton reactions are therefore not expected to result in fragmentation of the dendritic processes of osteocytes.
Misconception 7: Blood Clots vs. Fenton Reactions
According to this misconception, preserved blood clots in veins in a Triceratops bone would have prevented free iron from arriving at cells and soft tissues to preserve them: “free iron was unavailable, at least in these Triceratops bones, because clotted vessels would have blocked water from flowing over lysed RBCs (red blood cells)” (Armitage and Solliday, 2020: p. 38).
Two lines of reasoning rebut this misconception. Firstly, blood clots in a vein do not prevent water from flowing over lysed red blood cells. A blood clot that forms in an artery has a core of red blood cells, surrounded by a layer of platelets, which in turn is surrounded by a mesh of fibers of the protein fibrin (Chernysh et al., 2020). The fibrin mesh is not a continuous coating but resembles a net (Chernysh et al., 2020). A blood clot in a vein lacks the platelet layer (Chernysh et al., 2020), so the red blood cells next to the external fibrin mesh are in direct contact with the surrounding water, just as a bird in a net is in contact with the surrounding air. Secondly, Hb from red blood cells is not the only plausible source of iron. Another plausible source is ferritin (see the section on Misconception 2, above).
Misconception 8: Blood Clots and Drowning
According to this misconception, a prevalence of blood clots in fossil bones of the Permian synapsid Dimetrodon and the dinosaurs Triceratops and Nanotyrannus indicates disseminated intravascular coagulation (DIC), which suggests death by drowning (Armitage and Solliday, 2020; Armitage, 2022a, b). However, DIC can be caused by many things, including trauma, cancer, cardiovascular diseases, pancreatitis, inflammatory gastrointestinal disorders, immune-mediated hemolytic anemia, heat stroke, sepsis, envenomation, and protozoal infection (Honse et al., 2013; Hampton, 2022; Stokol, 2022), which means that DIC is not diagnostic of drowning. If the singling out of drowning is what it appears to be (a subtle promotion of blood clots in fossil bones as evidence of drowning during the Genesis Flood), such promotion is misguided. In fact, blood clots are evidence against drowning. The kind of DIC that drowning causes is hyperfibrinolytic DIC. In hyperfibrinolytic DIC, clots that begin to form are immediately destroyed, so that a patient’s blood cannot clot (Schwameis et al., 2015). Furthermore, of the two references that the authors cited in favor of blood clots as evidence of drowning (Armitage and Solliday, 2020), one does not mention drowning at all (Levi and ten Cate, 1999), and the other meticulously documents that there are not blood clots in the blood vessels of drowning victims (Schwameis et al., 2015).
Misconception 9: Soft Tissues as Microbial Biofilms
Several authors have voiced the misconception that pre-Quaternary fossil bone does not have soft tissues and that putative soft tissues in fossil bone are actually bacterial biofilms (Kaye et al., 2008; Saitta et al., 2019). One group analyzed vessel-like structures from within fossil bones from the Cretaceous Period. They found that the vessel-like structures were spectroscopically more similar to modern biofilm than to modern collagen and had surface troughs that were suggestive of microbial movement through a viscous medium. They concluded that the vessel-like structures were bacterial biofilms and that putative soft tissues from other Mesozoic fossil bones were misidentified microbial biofilms (Kaye et al., 2008). A second group showed by microscopy that vessel-like structures in another set of fossil dinosaur bones from the Cretaceous Period had surface morphology that differed from that of blood vessels. They further found by spectroscopy that collagen was absent from the fossil bones. Their phylogenetic analysis of DNA recovered from the bone revealed a diverse bacterial community. They concluded that the vessel-like structures were bacterial biofilms and that putative soft tissues in other Mesozoic fossil bones were misidentified microbial biofilms (Saitta et al., 2019).
It appears that the two groups correctly identified microbial biofilms in the bones that they studied. However, the presence of biofilms and a lack of collagen in one set of fossil bones do not demonstrate the same in any other set, especially given that taphonomic conditions and their ensuing alterations can vary substantially among specimens, even among bones from the same deposit (e.g., Bertazzo et al., 2015; Cadena, 2016; Voegele et al., 2022). The findings by Kaye et al. (2008) and Saitta et al. (2019) do not rebut or disprove the enormous amount and variety of evidence of preserved soft tissues that numerous independent researchers have found with numerous independent techniques in a plethora of pre-Quaternary fossil bones. Morphological and biochemical evidence demonstrates that in such bones the soft tissues in question are not bacterial biofilms but are genuine soft tissues. Putative blood vessels in fossil bone have a lumen (internal cavity) and walls of uniform thickness, whereas biofilms have neither trait (Schweitzer et al., 2009, 2016; Cleland et al., 2015). Antibodies specific to proteins found in blood vessels bind to putative blood vessels in fossil bone, whereas they do not bind to biofilms (Cleland et al., 2015; Schweitzer et al., 2016; Boatman et al., 2019). Antibodies specific to peptidoglycan, a substance that only bacteria produce, bind to biofilms and not to the putative blood vessels in fossil bone (Schweitzer et al., 2016; Boatman et al., 2019). Antibodies specific to tubulin and actin, proteins that are found in eukaryotic but not prokaryotic cells, bind to putative osteocytes in fossil bone and not to biofilms (Schweitzer et al., 2013). Putative CBM in fossil bone is spectroscopically similar to modern collagen and is spectroscopically very different from modern biofilm (Lindgren et al., 2011; Lee et al., 2017; Tahoun et al., 2022), and antibodies specific to collagen bind to it, as confirmed by independent immunoassay results from different fossil bones (Asara et al., 2007; Schweitzer et al., 2009; Schroeter et al., 2017; Tahoun et al., 2022). Multiple lines of evidence therefore clearly indicate that these putative soft tissues in fossil bone are genuine soft tissues of bone, not biofilms.
Misconception 10: Fossilized Blood Cells
 Although blood vessels, osteocytes, and CBM have been convincingly identified in fossil bone, the same is not the case for blood cells. Putative blood cells in fossil bone include spherical structures within blood vessels from Cretaceous dinosaur bone, reported as possible red blood cells (Pawlicki and Nowogrodzka-Zagóriśka, 1998; Yao et al., 2002; Schweitzer et al., 2005, 2007; Armitage and Anderson, 2013; Armitage, 2015, 2016) (Figure 1C, Figure 7A). They also include spherical structures within Haversian canals in bone of the Permian parareptile Deltavjatia vjatkensis, reported as possible white blood cells (Kiseleva et al., 2019). Irregularly shaped structures with spiky surfaces, within blood vessels of bone from the Cretaceous dinosaur Tarbosaurus bataar, have been reported as possible red blood cells that underwent hyperosmotic stress (Pawlicki and Nowogrodzka-Zagóriśka, 1998). Concave, circular discoid structures on the surfaces of trabeculae of spongy bone from a Cretaceous theropod dinosaur and a Cretaceous ichthyosaur have been reported as possible red blood cells (Bertazzo et al., 2015; Plet et al., 2017), and ovoid structures on the surfaces of trabeculae of spongy bone from the same ichthyosaur have been reported as possible white blood cells (Plet et al., 2017). However, the identification of such structures as blood cells does not hold up to scrutiny, as shown below.
Although blood vessels, osteocytes, and CBM have been convincingly identified in fossil bone, the same is not the case for blood cells. Putative blood cells in fossil bone include spherical structures within blood vessels from Cretaceous dinosaur bone, reported as possible red blood cells (Pawlicki and Nowogrodzka-Zagóriśka, 1998; Yao et al., 2002; Schweitzer et al., 2005, 2007; Armitage and Anderson, 2013; Armitage, 2015, 2016) (Figure 1C, Figure 7A). They also include spherical structures within Haversian canals in bone of the Permian parareptile Deltavjatia vjatkensis, reported as possible white blood cells (Kiseleva et al., 2019). Irregularly shaped structures with spiky surfaces, within blood vessels of bone from the Cretaceous dinosaur Tarbosaurus bataar, have been reported as possible red blood cells that underwent hyperosmotic stress (Pawlicki and Nowogrodzka-Zagóriśka, 1998). Concave, circular discoid structures on the surfaces of trabeculae of spongy bone from a Cretaceous theropod dinosaur and a Cretaceous ichthyosaur have been reported as possible red blood cells (Bertazzo et al., 2015; Plet et al., 2017), and ovoid structures on the surfaces of trabeculae of spongy bone from the same ichthyosaur have been reported as possible white blood cells (Plet et al., 2017). However, the identification of such structures as blood cells does not hold up to scrutiny, as shown below.
The concave, circular discoid structures are rich in carbon (Bertazzo et al., 2015; Plet et al., 2017) and are spectroscopically similar to red blood cells from modern emu blood (Bertazzo et al., 2015). However, the discoid structures are only about 2 μm across (Bertazzo et al., 2015; Plet et al., 2017), which is < 20% the length of avian red blood cells (Palomeque and Planas, 1977), < 10% the length of crocodilian red blood cells (Hartman and Lessler, 1964), and < 15% the length of lizard, snake, and turtle red blood cells (Hartman and Lessler, 1964). The discoid structures are morphologically similar to folds on the surfaces of degraded sheets of collagen-rich connective tissue (Saitta et al., 2017) and morphologically dissimilar to avian and reptilian red blood cells, which are elliptical (Claver and Quaglia, 2009) (Figure 7C). The size and morphology of the concave, circular discoid structures therefore do not match those expected for avian and reptilian red blood cells. Their spectroscopic similarities to emu blood cells could be due to having originated from organic matter of some other kind (Saitta et al., 2017).
In Cretaceous dinosaur bone, the spherical structures (Figure 1C and Figure 7A) have usually been found within and not outside blood vessels, which is consistent with identification as blood cells (Schweitzer et al., 2005, 2007). They have a diameter of 5-10 μm (Pawlicki and Nowogrodzka-Zagóriśka, 1998; Yao et al., 2002; Schweitzer et al., 2005; Kaye et al., 2008; Peterson et al., 2010; Armitage and Anderson, 2013; Armitage, 2016; Kiseleva et al., 2019), which is consistent with avian blood cells (Palomeque and Planas, 1977), although it is only 34%-68% the length of the smallest red blood cells of extant reptiles (Hartman and Lessler, 1964). They are iron-rich (Pawlicki and Nowogrodzka-Zagóriśka, 1998; Peterson et al., 2010), which is suggestive of the heme in red blood cells. However, the iron content of red blood cells is only about 0.3% by weight, too small to be detectable by energy-dispersive X-ray spectroscopy (EDS) (Bertazzo et al., 2015), whereas that of the spherical structures from the blood vessels of Cretaceous dinosaurs is over 8% by weight (Pawlicki and Nowogrodzka-Zagóriśka, 1998) and is strongly detectable by EDS (Peterson et al., 2010). Furthermore, avian and reptilian red blood cells are not spherical but elliptical (Claver and Quaglia, 2009) (Figure 7C), with a length to width ratio of 1.5-2.0 in crocodilians and birds (Hartman and Lessler, 1964; Palomeque and Planas, 1977) (Figure 7). Furthermore, spectroscopic data show no evidence of heme in the spherical structures (Korneisel et al., 2021), and the discovery of similar structures within the vascular channels of petrified wood casts further doubt upon their identification as blood cells (Korneisel et al., 2021).
Some researchers have identified the spherical structures within the blood vessels of Mesozoic fossil bone as framboids (Kaye et al., 2008; Peterson et al., 2010). Framboids are spheroidal aggregations of pyrite microcrystals (Figure 7) that range in size from < 1 to 250 μm (Ohfuji and Rickard, 2005) but are usually around 4-10 μm in diameter (Ohfuji and Rickard, 2005; Vietti et al., 2015) (Figure 7B). Framboids form in sediments and water columns where an oxidizing environment and a reducing environment meet, when pyrite is so highly concentrated that supersaturation occurs, causing pyrite nucleation to outpace pyrite crystal growth (Ohfuji and Rickard, 2005). They also form in reducing microenvironments within microbial mats on decomposing bone that is surrounded by oxygenated water on the seafloor, early in diagenesis (Vietti et al., 2015). However, the spherical structures in Mesozoic fossil bone are not framboids. Although they are iron-rich (Pawlicki and Nowogrodzka-Zagóriśka, 1998; Peterson et al., 2010), their chemical composition is inconsistent with pyrite in that they have little sulfur (Schweitzer et al., 2005, 2007; Peterson et al., 2010). Some researchers have hypothesized that they are products of diagenesis of clay that had infilled the lumens of the blood vessels after burial (Korneisel et al., 2021). A test of that hypothesis and a more definite identification await further study. For now, the identity of the spheres remains a mystery. The same is the case for the identity of irregularly shaped structures with spiky surfaces from within blood vessels (putative red blood cells: Pawlicki and Nowogrodzka-Zagóriśka, 1998), and the ovoid structures on the surfaces of trabeculae of spongy bone (putative white blood cells: Plet et al., 2017). In both cases, detailed investigation into their identity has not yet occurred.
Misconception 11: Soft Tissue Preservation as Evidence of Young Age
In a peer-reviewed article in a science journal, a group of authors expressed a misconception that the preservation of soft tissues in Mesozoic fossil bones demonstrates that the bones are only thousands of years old (Miller et al., 2019). However, if the bones are thousands of years old, then they are too old for soft tissues to have survived without some special preservation mechanism. This is because without some special preservation mechanism, soft tissues in bone decay away in less than 1000 years (Lengyel, 1968). Bones that are thousands of years old are therefore too old for young age to explain the preservation of soft tissues within them. Hence, some other explanation is required.
In their article, Miller et al. (2019) subtly advocated the YEC view that Mesozoic fossils were buried by the Genesis Flood (see the section on Misconception 13, below), which current YEC thought places at over 4300 years ago (Jones, 2016). Therefore, even within the YEC paradigm, Mesozoic fossil bones are far too old for young age to explain the preservation of soft tissues within them.
This misconception is inconsistent with the YEC view for another reason as well. According to current YEC thought, all Mesozoic and pre-Pleistocene Cenozoic strata were deposited during the single year of the Genesis Flood (Walker, 2014; Clarey, 2020; Oard and Carter, 2021; Clarey et al., 2022; Tomkins and Clarey, 2022). If that is correct, then the Mesozoic fossil bones in which soft tissues are preserved are the same age as the Mesozoic fossil bones in which soft tissues are not preserved. Therefore, even within the YEC paradigm, the preservation of soft tissues in fossil bones requires an explanation that is not based on age.
Given the empirical support for Fenton reactions as a mechanism to provide long-term stability in biomolecules (Schweitzer et al., 2014; Wiemann et al., 2018; Surmik et al., 2016; Boatman et al., 2019), and given that the short time frame involved in that explanation (Schweitzer et al., 2014; Ullmann et al., 2021, 2022) is consistent with the YEC view of the young age of the fossils in question, Fenton chemistry is a plausible explanation even within the YEC paradigm. Therefore, it would make more sense for YEC authors to embrace the FH than to oppose it.
Misconception 12: Radiocarbon in Mesozoic CBM as Evidence of Young Age
Miller et al. (2019) expressed a misconception that radiocarbon in Mesozoic CBM demonstrates that the bones are only thousands of years old. They had subjected CBM from Mesozoic bones to radiocarbon dating and found radiocarbon “dates” between 22,000 and 42,000 years (Miller et al., 2019). In that article, Miller et al. concluded that “the 65 to 150 million year ages attributed to dinosaurs are apparently erroneous” and “the 45 million years between the Late Cretaceous and Late Jurassic epochs [sic.] are also mistaken, since dinosaur fossils and coal from these strata exhibit equivalent 14C ages.”
However, the radiocarbon “dates” that the Mesozoic CBM yielded are not correct dates. Radiometric dating with isotopes other than radiocarbon (such as 238U/206Pb, 235U/207Pb, 87Rb/86Sr, 40K/40Ar, and 40Ar/39Ar) consistently shows that the sediments that entomb Mesozoic fossils are millions of years old (Schmitz, 2020). Advances in radiocarbon dating have made such dating reliable for organic substances other than collagen if the samples are less than 50,000 years old (Wood, 2015), but collagen’s tendency to form cross-links with contaminants makes it a problematic substance to date with radiocarbon (van Klinken and Hedges, 1995; Devièse et al., 2017, 2018; Spindler et al., 2021). Miller et al. (2019) used the ABA (acid-base-acid) decontamination technique on the CBM, but ABA does not circumvent the problem. Although ABA is an appropriate decontamination technique for sufficiently young archaeological CBM that is not cross-linked to its contaminants, it is not sufficient to decontaminate fossil CBM. That is because CBM continues to accumulate new cross-links with incoming organic contaminants through geologic time (van Klinken and Hedges, 1995). As a result, fossil CBM is molecularly bonded with substances containing new radiocarbon that yields a falsely young radiocarbon “date” (Senter, 2022b). The discrepancy between the bone’s correct age and the radiocarbon “date” becomes significant in bones older than 10,000 years and increases with each passing millennium (Zazzo, 2014). The curve that expresses the difference between a sample’s correct age and its radiocarbon “date” at different levels of contamination flattens between 45,000 and 50,000 years. For example, a sample with 1% contamination by new radiocarbon will yield a falsely young date of about 35,000 years, whether the sample is 50,000, 50,000,000, or 500,000,000 years old (Wood, 2015).
No decontamination technique can separate cross-linked contaminants from fossil collagen. Even decontamination techniques that are stronger than ABA, such as ultrafiltration and acid base oxidation - stepped combustion (ABOx-SC), cannot do it. As a result, even when these decontamination techniques are used, fossil collagen still yields a falsely young radiocarbon “date” (Higham et al., 2009; Higham, 2011; Marom et al., 2012, 2013; Devièse et al., 2017, 2018; Spindler et al., 2021).
One can get fossil collagen to yield a correct radiocarbon date only by using the HYP method, in which one separates the amino acid hydroxyproline (HYP) from collagen and subjects only it to radiocarbon dating (Marom et al., 2012, 2013; Nalawade-Chavan et al., 2014; Devièse et al., 2017, 2018; Spindler et al., 2021). This method works because hydroxyproline is abundant in collagen and little else, so subjecting only HYP to radiocarbon dating ensures that only collagen-specific material (and no contaminant) contributes to the radiocarbon date. If a fossil is too old to date with radiocarbon (i.e., older than about 50,000 years), then radiocarbon dating of its CBM with the HYP method yields a date of “greater than x ” (“greater than 43,000 years,” “greater than 45,000 years,” etc.) (Devièse et al., 2018; Spindler et al., 2021). Miller et al. (2019) did not use the HYP method. Their radiocarbon “dates” of between 22,000 and 42,000 years are therefore falsely young. Had they used the HYP method, the collagen would have yielded a correct radiocarbon date of “greater than x.”
To support their conclusions, Miller et al. (2019) pointed out that radiocarbon in coal and other Mesozoic materials yielded “dates” of thousands and not millions of years. Previous authors had also found that Mesozoic fossil bone mineral yielded “dates” of thousands of years (Dahmer et al., 1990; Fields et al., 1990; Helfinstine and Roth, 2007; Thomas and Nelson, 2015). However, such materials undergo recrystallization, which adds new radiocarbon via carbonate, thereby yielding a falsely young radiocarbon “date” (Senter, 2020). For a correct radiocarbon date, one must use organic substances (which do not undergo recrystallization), not minerals (Senter, 2020). The use by Miller et al. (2019) of radiocarbon “dates” of Mesozoic minerals, which cannot yield accurate radiocarbon dates, is therefore an unsound argument. Further unsound arguments from Miller et al. (2019) and their rebuttals are detailed in Appendix 1.
Misconception 13: Radiocarbon Dating of Mesozoic CBM as Evidence of the Genesis Flood.
Miller et al. (2019: p. 13) asserted that their radiocarbon “dates” of Mesozoic CBM are evidence of the Genesis Flood: “The 19th century hypothesis that sedimentary rock formations took millions of years to form is clearly contradicted by 14C ages...This leads to the possibility that extensive sedimentary formations were deposited by one or more cataclysmic events only thousands of years ago rather than millions.” Although the group did not explicitly mention the Genesis Flood, their wording is transparently a reference to it.
As has already been shown above, radiocarbon “dates” from Mesozoic samples are unreliable and therefore not evidence that all Mesozoic fossils were entombed by cataclysmic sedimentation that occurred within a geologically short time frame mere thousands of years ago. Radiometric dates from isotopes other than radiocarbon (such as 238U/206Pb, 235U/207Pb, 87Rb/86Sr, 40K/40Ar, 40Ar/39Ar) consistently demonstrate that Mesozoic sediments represent a range of ages between 262 and 66 million years (Schmitz, 2020). YEC arguments to the contrary have been rebutted elsewhere (Willoughby, 2016).
Furthermore, a worldwide Flood would unquestionably qualify as a megaflood. “Megaflood” is the term that geologists use for a flood of gargantuan magnitude. Several have occurred in the late Cenozoic, discharging over a million cubic meters of water per second during peak flow (Baker, 2010b, c). Megafloods leave a consistent geological signature: an enormous deposit or deposits of gravel (which is called conglomerate if it has lithified), often several meters in height (Blair, 2002; Moscariello et al., 2002; Russell and Knudsen, 2002; Komatsu et al., 2009; Baker, 2010a; Carling, 2013; Waitt, 2021). A worldwide megaflood would leave such a deposit worldwide. The fact that the worldwide geologic record lacks an immense stratum of gravel or conglomerate across continents demonstrates that there has never been a worldwide megaflood. Additionally, a plethora of Paleozoic, Mesozoic, and pre-Quaternary Cenozoic deposits have features that indicate subaerial deposition, deposition under calm conditions, or deposition over the course of many years. Those parts of the geologic record demonstrate that the idea that all Paleozoic, Mesozoic, and pre-Quaternary Cenozoic sediments were deposited by a worldwide megaflood within a single year, is incorrect (Senter, 2011).
Miller et al. (2019) further attempted to connect Mesozoic CBM with the Genesis Flood by stating that “the minute amounts or absence of collagen found in dinosaur bones could be at least partly attributed to their burial in megaflood deposits, with associated leaching...” (Miller et al., 2019: p. 13). However, no dinosaur fossils are known from megaflood deposits. There are a few deposits of Mesozoic gravel or conglomeratic sandstone from which dinosaur fossils are known (Jacobs et al., 1996; Gangloff et al., 1998; Van Itterbeeck et al., 2005; Mankar et al., 2019), but dinosaur fossils are, as a rule, found in sediments that indicate deposition under much calmer conditions: sandstone, claystone, mudstone, siltstone, shale, limestone, and paleosol (Dodson et al., 1980; Zhiming, 1983; Sun et al., 1985; Colbert, 1989; Cox et al., 1992; Sander, 1992; Currie et al., 1993; Eberth, 1993, 2018; Jacobs et al., 1996; Sereno et al., 1996, 2008; Blows, 1998; Gangloff et al., 1998; Rogers et al., 2000; Tang et al., 2001; Hunt et al., 2003; Leanza et al., 2004; Van Itterbeeck et al., 2004; Dingus et al., 2005; Eberth, 2005; Lucas et al., 2005; Catuneanu et al., 2006; Fürsich et al., 2007; Kirkland and Madsen, 2007; Smith et al., 2007; Dashzeveg et al., 2008; Bussert et al., 2009; Getty et al., 2010; Lyson et al., 2011; Martínez et al., 2013; Oreska et al., 2013; Tucker et al., 2013; Schoch et al., 2014; Leahey et al., 2015; Brownstein, 2018; Bell et al., 2019; Mankar et al., 2019; Yu et al., 2019; Xu et al., 2022). No dinosaur fossils are known from any megaflood conglomerate deposit. This demonstrates that the preservation of collagen in dinosaur bones has nothing to do with megaflood conditions.
Further Misconceptions from YEC Literature
Many misconceptions about cellular and soft tissue preservation in fossil bone have been voiced only in YEC literature. Of these misconceptions, most are expressions of opposition to the FH that are based on misunderstandings of the Ostrich Hb Experiment and of Fenton chemistry. These misconceptions are summarized in Appendix 2, Table 1, along with facts that rebut them.
DISCUSSION AND CONCLUSIONS
In the most recent three decades, knowledge of the mechanisms of cellular and soft tissue preservation in fossil bone has grown by leaps and bounds. Recent findings identify Fenton reactions as a plausible mechanism for the preservation of blood vessels and cells (Schweitzer et al., 2014; Surmik et al., 2016; Wiemann et al., 2018; Boatman et al., 2019), show that cells and soft tissues in fossil bones bear the chemical signatures of Fenton reactions (Surmik et al., 2016; Wiemann et al., 2018; Boatman et al., 2019), and rebut arguments against Fenton reactions as a plausible preservation mechanism (Hawkins and Davies, 1997; Windhager et al., 1998; Feng et al., 2006; Lipinski, 2011; Krzyściak et al., 2012; Schweitzer et al., 2014; Surmik et al., 2016; Wiemann et al., 2018; Boatman et al., 2019; Chernysh et al., 2020; Liu et al., 2022). Recent findings also indicate that such reactions occur early in diagenesis and are facilitated by oxidizing depositional environments and external concretions (Plet et al., 2017; Wiemann et al., 2018; Ullmann et al., 2019, 2021, 2022). Recent findings further elucidate the diagenetic changes occur in fossilized cells and soft tissues (Pawlicki, 1995; Pawlicki and Nowogrodzka-Zagóriśka, 1998; Lindgren et al., 2011; Schweitzer et al., 2013, 2014; Cadena, 2016; Surmik et al., 2016, 2021; Lee et al., 2017; Boatman et al., 2019; Ullmann et al., 2019; Cadena, 2020; Bailleul and Zhou, 2021; Zheng et al., 2021) and indicate that cross-links are important in the preservation of fossil collagen (Antonio et al., 2011; Zazzo, 2014; Kendall et al., 2018), which does not necessarily require Fenton reactions to be preserved (Cadena, 2016).
Of course, for cells and soft tissue to persist within fossil bone, the bone that encases it must first survive long enough to enter the fossil record. Recent findings in the study of archaeological bone have identified factors that contribute to its long-term survival, such as alkaline sediment, low temperature, anaerobic conditions, bactericidal metals, large bone size, tubular bone shape, fluorination, late permineralization, and lowering of the bone’s porosity (Janaway, 1997; Schultz, 2001; Berna et al., 2004; Trueman et al., 2008; Kocsis et al., 2010; Pfretschner and Tütken, 2011; Nord et al., 2015; Kendall et al., 2018; Pokines et al., 2018).
Archaeological bone often contains CBM (Graf, 1949; Lengyel, 1968; Jans et al., 2002; Wadsworth and Buckley, 2014; Kendall et al., 2018; Duffett Carlson et al., 2022; Mandl et al., 2022), but there are few reports of preserved blood vessels, osteocytes, or blood cells in archaeological bone. As hypothesized here, this may be because studies of archaeological bone tend to focus on bone from human burials, and the initial conditions that such bones undergo are different from those of fossil bones that were entombed by fluvial, lacustrine, estuarine, or marine sediments. To fill in gaps in the knowledge of cellular and soft tissue preservation in bone, it might therefore be fruitful to search for blood vessels, osteocytes, and blood cells in bones of archaeological age that did not undergo funerary burial but instead were entombed by fluvial, lacustrine, estuarine, or shallow marine sediments, especially if said bones were protected by external concretions or sediments with low permeability.
Some of the factors that preserve bone and its cells and soft tissues are products of biological decomposition. For example, organic decomposition may be involved in the formation of external concretions that shield a bone from further destructive factors (Trzęsiok et al., 2014; Yoshida et al., 2018; Muramiya et al., 2020). Likewise, the Fenton reactions that stabilize cells and soft tissues by generating cross-links are produced by the breakdown of organic molecules (Balla et al., 2005, 2007). Ironically, then, a bone and its cells and soft tissues must initially undergo a little decomposition in order to afterwards undergo a lot of preservation. A certain balance between destructive and preservative factors must occur in order to ensure the long-term preservation of a bone and its soft-tissue contents. Long-term preservation must also entail balance between factors that slow the decomposition of bone mineral and those that slow the decomposition of cells and soft tissues. Fortunately, some factors slow the decomposition of both: deep burial, low temperature, dryness, bactericidal metals, and blockage of the VCN (Galloway et al., 1989; Galloway, 1997; Gill-King, 1997; Janaway, 1997; Schultz, 2001; Jans et al., 2004; Trueman et al., 2008; Peterson et al., 2010; Nord et al., 2015; Kendall et al., 2018).
It is puzzling at first that YEC authors have expressed such vehement opposition to the FH and have instead embraced young age as the explanation of cellular and soft tissue preservation in fossil bone. It is puzzling because, as shown above, the FH is compatible both with science and with the YEC view, whereas the young age hypothesis is compatible with neither (see the section on Misconception 11, above). Likewise, YEC literature has a long history of insisting that biological degeneration occurs, while simultaneously denying the existence of vestigial organs (Senter and Mackey, 2017a), and it has a long history of insisting that adaptive and heritable biological change occurs, while simultaneously denying the existence of beneficial mutations and natural selection (Senter and Mackey, 2017b; Senter, 2017). Similarly, the very basis of the YEC paradigm is the acceptance of certain ancient texts as authority sources while simultaneously ignoring the parts of those texts that rebut the YEC view (Senter, 2022a). Some YEC authors have challenged the YEC tendency toward self-contradiction by recognizing the existence of vestigial organs, beneficial mutations, and natural selection (Senter and Mackey, 2017a, 2017b; Senter, 2017). Such about-faces, which involve replacing the denial of demonstrated facts with the acceptance of those facts, are steps in the right direction that should be encouraged. It will be interesting to see whether future YEC authors take similar steps regarding cellular and soft tissue preservation in fossil bone, by endorsing the FH.
The preservation of cells and soft tissues in fossil bone was once thought to be surprising. However, the plethora of examples of preserved cells and soft tissues in fossil bone that are now known perhaps ought to inspire a reconsideration of surprise, because it appears that such preservation is quite common in the fossil record. Now that plausible mechanisms and paleoenvironmental parameters that facilitate such preservation have been identified, perhaps it should be unsurprising to find preserved cells and soft tissues within bones entombed under conditions that satisfy those parameters.
ACKNOWLEDGMENTS
I thank the two anonymous reviewers, who provided useful input that improved this paper.
REFERENCES
Alexander, B., Daulton, T.L., Genin, G.M., Lipner, J., Pasteris, J.D., Wopenka, B., and Thomopoulos, S. 2012. The nanometre-scale physiology of bone: steric modelling and scanning transmission electron microscopy of collagen - mineral structure. Journal of the Royal Society Interface, 9:1774-1786. https://doi.org/10.1098/rsif.2011.0880
Amirrah, I.M., Lokanathan, Y., Zulkiflee, I., Razip Wee, M.F.M., Motta, A., and Fauzi, Mh.B. 2022. A comprehensive review on collagen type I development of biomaterials for tissue engineering: from biosynthesis to bioscaffold, Biomedicines, 10(2307):1-44.
https://doi.org/10.3390/biomedicines10092307
Anderson, K. 2016a. Can iron preserve tissue for millions of years? An iDINO progress report. Creation Research Society Quarterly, 53:63.
Anderson, K. 2016b. Media review: Dinosaur Blood and the Age of the Earth. Creation Research Society Quarterly, 53:73-79.
Anderson, K. 2017a. Echoes of the Jurassic. Discoveries of Dinosaur Soft Tissue. CRS Books, Chino Valley, Arizona.
Anderson, K. 2017b. iDINO update: Testing the role of iron for prolonged tissue preservation. Creation Research Society Quarterly, 54:158.
Anderson, K. 2018. Biomaterial from dinosaur fossils: Implications and challenges, part 2. Creation Matters, 23(2):1, 4-6.
Arcon, J.P., Rosi, P., Petruk, A.A., Marti, M.A., and Estrin, D.A. 2015. Molecular mechanism of myoglobin autoxidation: Insights from computer simulations. Journal of Physical Chemistry B, 8:1802-1813. https://doi.org/10.1021/jp5093948
Armitage, M.H. 2015. Soft bone material from a brow horn of a Triceratops horridus from Hell Creek Formation, Montana. Creation Research Society Quarterly, 51:248-258.
Armitage, M.H. 2016. Preservation of Triceratops horridus tissue cells from the Hell Creek Fm, MT. Microscopy Today, 24(1):18-22. https://doi.org/10.1017/s1551929515001133
Armitage, M. 2017. Utterly preserved cells are not remnants—a critique of Dinosaur Blood and the Age of the Earth. Journal of Creation, 31(1):45-50.
Armitage, M.H. 2021. First report of peripheral nerves in bone from Triceratops horridus occipital condyle. Microscopy Today, 29(2):2-7. https://doi.org/10.1017/s1551929521000468
Armitage, M.H. 2022a. UV autofluorescence microscopy of Oklahoma Permian synapsid femur (Dimetrodon Cope, 1878) reveals blood clots in vascular canals. Microscopy Today, 30(1):2-7. https://doi.org/10.1017/s1551929521001565
Armitage, M.H. 2022b. Ultraviolet autofluorescence microscopy of Nanotyrannus lancensis sections reveals blood clots in vessel canals. Microscopy Today. https://doi.org.10.1017/S1551929522001262
Armitage, M.H. and Anderson, K.L. 2013. Soft sheets of fibrillar bone from a fossil of the supraorbital horn of the dinosaur Triceratops horridus. Acta Histochemica, 115:603-608. https://doi.org/10.1016/j.acthis.2013.01.001
Armitage, M.H. and Anderson, K. 2014. Light and electron microscopic study of soft bone osteocytes from a Triceratops horridus supraorbital horn. Microscopy and Microanalysis, 20 (Supplement 3):1274-1275. https://doi.org/10.1017/s1431927614008101
Armitage M.H. and Solliday, J. 2020. UV autofluorescence microscopy of dinosaur bone reveals encapsulation of blood clots within vessel canals. Microscopy Today, 28(5):30-38. https://doi.org/10.1017/s1551929520001340
Ascenzi, A., Brunori, M., Citro, G., and Zito, R. 1985. Immunological detection of hemoglobin in bones of ancient Roman times and of Iron and Eneolithic Ages. Proceedings of the National Academy of Sciences of the United States of America, 82:7170-7172.
https://doi.org/10.1073/pnas.82.21.7170
Bailleul, A.M., Zheng, W., Horner, J.R., Hall, B.K., Holliday, C.M., and Schweitzer, M.H. 2020. Evidence of proteins, chromosomes and chemical markers of DNA in exceptionally preserved dinosaur cartilage. National Science Review, 7:815-822.
https://doi.org/10.1093/nsr/nwz206
Bailleul, A.M. and Zhou, Z. 2021. SEM analyses of fossilized chondrocytes in the extinct birds Yanornis and Confuciusornis: Insights on taphonomy and modes of preservation in the Jehol biota. Frontiers in Earth Science, 9:718588. https://doi.org/10.3389/feart.2021.718588
Balla, J., Vercellotti, G.M., Jeney, V., Yachie, A., Varga, Z., Eaton, J.W., and Balla, G. 2005. Heme, heme oxygenase and ferritin in vascular endothelial cell injury. Molecular Nutrition and Food Research, 49:1030-1043. https://doi.org/10.1002/mnfr.200500076
Balla, J., Vercellotti, G.M., Jeney, V., Yachie, A., Varga, Z., Jacob, H.S., Eaton, J.W., and Balla, G. 2007. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment, Antioxidants and Redox Signaling, 9:2119-2137. https://doi.org/10.1089/ars.2007.1787
Baker, V.R. 2010a. Channeled scablands: A megaflood landscape. pp. 21-28. In Migoń, P. (ed.), Geomorphological Landscapes of the World. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-3055-9_3
Baker, V.R. 2010b. Overview of megaflooding: Earth and Mars, p. 1-12. In Burr, D.M, Carling, P.A. and Baker, V.R. (eds.), Megaflooding on Earth and Mars. Cambridge University Press, Cambridge.
Baker, V.R. 2010c. Channeled scabland morphology. pp. 65-77. In Burr, D.M., Carling, P.A., and Baker, V.R. (eds.), Megaflooding on Earth and Mars. Cambridge University Press, Cambridge.
Balogh, E., Paragh, G., and Jeney, V. 2018. Influence of iron on bone homeostasis. Pharmaceuticals, 11(107):1-18. https://doi.org/10.3390/ph11040107
Barber, R. 1992. Bestiary MS Broadley 764. Boydell, Woodbridge.
Barbusiński, K. 2001. Fenton reaction - controversy concerning the chemistry. Ecological Chemistry and Engineering S, 16:347-358
Barker, K., Terry, D.O., and Ullmann, P.V. 2021. Recovering endogenous cells and soft tissues from fossil bones: Does the depositional environment matter? p. 225-10. In Geological Society of America (ed.), Proceedings of the GSA Connect, Portland, Oregon, 10-13 October 2021, 2021. Geological Society of America, Boulder.
https://doi.org/10.1130/abs/2021am-369460
Bass, W.M. III. 1997. Outdoor decomposition rates in Tennessee. pp. 181-186. In Haglund, W.D. and Sorg, M.H. (eds.), Forensic Taphonomy: The Postmortem Fate of Human Remains. CRC, Boca Raton. https://doi.org/10.1201/9781439821923
Bell, L.S., Kayser, M., and Jones, C. 2008. The mineralized osteocyte: A living fossil. American Journal of Physical Anthropology, 137:449-456. https://doi.org/10.1002/ajpa.20886
Bell. P.R., Fanti, F., Hart, L.J., Milan, L.A., Craven, S.J., Brougham, T., and Smith, E. 2019. Revised geology, age, and vertebrate diversity of the dinosaur-bearing Griman Creek Formation (Cenomanian), Lightning Ridge, New South Wales, Australia. Palaeogeography, Palaeoclimatology, Palaeoecology, 514:655-671.
https://doi.org/10.1016/j.palaeo.2018.11.020
Bella, J. 2016. Collagen structure: new tricks from a very old dog. Biochemical Journal, 473:1001-1025. https://doi.org/10.1042/bj20151169
Berna, F., Matthews, A., and Weiner, S. 2004. Solubilities of bone mineral from archaeological sites: the recrystallization window. Journal of Archaeological Science, 31:867-882. https://doi.org/10.1016/j.jas.2003.12.003
Bertazzo, S., Maidment, S.C.R., Kallepitis, C., Fearn, S., Stevens, M.M., and Xie, H. 2015. Fibres and cellular structures preserved in 75-million-year-old dinosaur specimens. Nature Communications, 6(7352):1-8. https://doi.org/10.1038/ncomms8352
Blair, T.C. 2002. Alluvial-fan sedimentation from a glacial-outburst flood, Lone Pine, California, and contrasts with meteorological flood deposits. pp. 113-140. In Martini, P.I., Baker, V.R., and Garzón, G. (eds.), Flood and Megaflood Processes and Deposits: Recent and Ancient Examples. Blackwell Science, Oxford. https://doi.org/10.1002/9781444304299.ch8
Blows, W.T. 1998. A review of Lower and Middle Cretaceous dinosaurs of England. New Mexico Museum of Natural History and Science Bulletin, 14:29-38.
Boatman, E.M., Goodwin, M.B., Holman, H.N., Fakra, S., Zheng, W., Gronsky, R., and Schweitzer, M.H. 2019. Mechanisms of soft tissue and protein preservation in Tyrannosaurus rex. Scientific Reports, 9(15678):1-12. https://doi.org/10.1038/s41598-019-51680-1
Bowles, S. and Choi, J.K. 2019. The Neolithic agricultural revolution and the origins of private property. Journal of Political Economy, 127:2186-2228. https://doi.org/10.1086/701789
Brownstein, C. 2018. The biogeography and ecology of the Cretaceous non-avian dinosaurs of Appalachia. Palaeontologia Electronica, 21.1.5A:1-56. https://doi.org/10.26879/801
Brumfitt, I.M., Chinsamy, A., and Compton, J.S. 2013. Depositional environment and bone diagenesis of the Mio/Pliocene Langebaanweg bonebed, South Africa. South African Journal of Geology, 116.2:241-258. https://doi.org/10.2113/gssajg.116.2.241
Buckley, M. 2015. Ancient collagen reveals evolutionary history of the endemic South American ‘ungulates’. Proceedings of the Royal Society B, 282:1-9. https://doi.org/10.1098/rspb.2014.2671
Buckley, M. and Collins, M.J. 2011. Collagen survival and its use for species identification in Holocene-lower Pleistocene bone fragments from British archaeological and paleontological sites. Antiqua, 1(e1):1-7. https://doi.org/10.4081/antiqua.2011.e1
Buenzli, P.R. and Sims, N.A. 2015. Quantifying the osteocyte network in the human skeleton. Bone, 75:144-150. https://doi.org/10.1016/j.bone.2015.02.016
Burgess, D. and Marshall, W. 2009. Romans in Tucson? The story of an archaeological hoax, Journal of the Southwest, 51:3-135. https://doi.org/10.1353/jsw.2009.0020
Burnett, S.E. 2019. A stegosaur carving on the ruins of Ta Prohm? Think again. Skeptical Inquirer, 43(4):45-49.
Bussert, R., Heinrich, W.-D., and Aberhan, M. 2009. The Tendaguru Formation (Late Jurassic to Early Cretaceous, southern Tanzania): definition, palaeoenvironments, and sequence stratigraphy. Fossil Record, 12:141-174. https://doi.org/10.5194/fr-12-141-2009
Cadena, E. 2016. Microscopical and elemental FESEM and Phenom ProX-SEM-EDS analysis of osteocyte- and blood vessel-like microstructures obtained from fossil vertebrates of the Eocene Messel Pit, Germany. PeerJ, 4:e1618. https://doi.org/10.7717/peerj.1618
Cadena, E.A. 2020. In situ SEM/EDS compositional characterization of osteocytes and blood vessels in fossil and extant turtles on untreated bone surfaces; different preservational pathways microns away. PeerJ, 8:e9833. https://doi.org/10.7717/peerj.9833
Cadena, E.A. and Schweitzer, M.H. 2012. Variation in osteocytes morphology vs bone type in turtle shell and their exceptional preservation from the Jurassic to the present. Bone, 51:614-620. https://doi.org/10.1016/j.bone.2012.05.002
Cai, C., Hu, W., and Chu, T. 2022. Interplay between iron overload and osteoarthritis: Clinical significance and cellular mechanisms. Frontiers in Cell and Developmental Biology, 9:817104. https://doi.org/10.3389/fcell.2021.817104
Carling, P.A. 2013. Freshwater megaflood sedimentation: What can we learn about generic processes? Earth Science Reviews, 125:87-113. https://doi.org/10.1016/j.earscirev.2013.06.002
Carpenter, K. 2005. Experimental investigation of the role of bacteria in bone fossilization. Neues Jahrbuch für Geologie und Paläontologie Monatshefte, 2:83-94. https://doi.org/10.1127/njgpm/2005/2005/83
Carpentier, V.T., Wong, J., Yeap, Y., Gan, C., Sutton-Smith, P., Badiei, A., Fazzalari, N.L., and Kuliwaba, J.S. 2012. Increased proportion of hypermineralized osteocyte lacunae in osteoporotic and osteoarthritic human trabecular bone: Implications for bone remodeling. Bone, 50:688-694. https://doi.org/10.1016/j.bone.2011.11.021
Carriveau, G.W. 1976. Thermoluminescent dating and the monsters of Acambaro. American Antiquity, 41:497-500. https://doi.org/10.2307/279018
Castiglia, P.J. and Fawcett, P.J. 2006. Large Holocene lakes and climate change in the Chihuahuan Desert. Geology, 34:113-116. https://doi.org/10.1130/g22036.1
Catalá, A. 2009. Lipid peroxidation of membrane phospholipids generates hydroxyl-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chemistry and Physics of Lipids, 157:1-11. https://doi.org/10.1016/j.chemphyslip.2008.09.004
Cattaneo, C., Gelsthorpe, K., Phillips, P., and Sokol, R.J. 1990. Blood in ancient human bone. Nature, 347:339. https://doi.org/10.1038/347339a0
Cattaneo, C., Gelsthorpe, K., Phillips, P., and Sokol, R.J. 1992a. Reliable identification of human albumin in ancient bone using ELISA and monoclonal antibodies. American Journal of Physical Anthropology, 87:365-372. https://doi.org/10.1002/ajpa.1330870311
Cattaneo, C., Gelsthorpe, K., Phillips, P., and Sokol, R.J. 1992b. Detection of blood proteins in ancient human bone using ELISA: a comparative study of the survival of IgG and albumin. International Journal of Osteoarchaeology, 2:103-107.
https://doi.org/10.1002/oa.1390020202
Catuneanu, O., Khalifa, M.A., and Wanas, H.A. 2006. Sequence stratigraphy of the Lower Cenomanian Bahariya Formation, Bahariya Oasis, Western Desert, Egypt. Sedimentary Geology, 190:121-137. https://doi.org/10.1016/j.sedgeo.2006.05.010
Chan, M.A., Ormö, J., Park, A.J., Stich, M., Souza-Egipsy, V., and Komatsu, G. 2007. Models of iron oxide concretion formation: field, numerical, and laboratory comparisons. Geofluids, 7:1-13. https://doi.org/10.1111/j.1468-8123.2007.00187.x
Chernysh, I.N., Nagaswami, C., Kosolapova, S., Peshkova, A.D., Cuker, A., Cines, D.B., Cambor, C.L., Litvinov, R.I., and Weisel, J.W. 2020. The distinctive structure and composition of arterial and venous thrombi and pulmonary emboli. Scientific Reports, 10:5112. https://doi.org/10.1038/s41598-020-59526-x
Clarey, T. 2015. Dinosaurs: Marvels of God’s Design. Master Books, Green Forest, Arkansas.
Clarey, T. 2020. Carved in Stone: Geological Evidence of the Worldwide Flood. Institute of Creation Research, Dallas.
Clarey, T.L., Werner, D.J., and Tomkins, J.P. 2022. Globally extensive Cenozoic coals indicate high post-Flood boundary. Journal of Creation, 36(1):13-15.
Clarke, B. 2008. Normal bone anatomy and physiology. Clinical Journal of the American Society of Nephrology, 3(supplement 3):S131-S139. https://doi.org/10.2215/cjn.04151206
Claver, J.A. and Quaglia, A.I.E. 2009. Comparative morphology, development, and function of blood cells in nonmammalian vertebrates. Journal of Exotic Pet Medicine, 18:89-97. https://doi.org/10.1053/j.jepm.2009.04.006
Cleland, T.P., Schroeter, E.R., Zamdborg, L., Zheng, W., Lee, J.E., Tran, J.C., Bern, M., Duncan, M.B., Lebleu, V.S., Ahlf, D.R., Thomas, P.M., Kalluri, R., Kelleher, N.L., and Schweitzer, M.H. 2015. Mass spectrometry and antibody-based characterization of blood vessels from Brachylophosaurus canadensis. Journal of Proteome Research, 14:5252-5262. https://doi.org/10.1021/acs.jproteome.5b00675
Cleland, T.P., Schroeter, E.R., Feranec, R.S., and Vashishth, D. 2016. Peptide sequences from the first Castoroides ohioensis skull and the utility of old museum collections for paleoproteomics. Proceedings of the Royal Society B, 283:20160593.
https://doi.org/10.1098/rspb.2016.0593
Colbert, E.H. 1989. The Triassic dinosaur Coelophysis. Museum of Northern Arizona Bulletin, 57:1-160.
Colleary, C., Lamadrid, H.M., O’Reilly, S.S., Dolocan. A., and Nesbitt, S.J. 2021. Molecular preservation in mammoth bone and variation based on burial environment. Scientific Reports, 11:2662. https://doi.org/10.1038/s41598-021-81849-6
Cournoyer, J.J., Pittman, J.L., Ivleva, V.B., Fallows, E., Waskell, L., Costello, C.E., and O’Connor, P.B. 2005. Deamidation: Differentiation of aspartyl from isoaspartyl products in peptides by electron capture dissociation. Protein Science, 14:452-463.
https://doi.org/10.1110/ps.041062905
Cox, B.M., Hudson, J.D., and Martill, D.M. 1992. Lithostratigraphic nomenclature of the Oxford Clay (Jurassic). Proceedings of the Geologists’ Association, 103:343-345. https://doi.org/10.1016/s0016-7878(08)80130-7
Creation Research Society. 1964 (untitled statement of purpose). Creation Research Society Annual, 1:frontispiece.
Currie, P.J. and Eberth, D.A. 1993. Palaeontology, sedimentology and palaeoecology of the Iren Dabasu Formation (Upper Cretaceous), Inner Mongolia, People’s Republic of China. Cretaceous Research, 14:127-144. https://doi.org/10.1006/cres.1993.1011
Dahmer, L., Koutznetsov, D., Ivenov, A., Hall, J., Whitmore, J., Detwiler, G., and Miller, H. 1990. Report on chemical analysis and further dating of dinosaur bones and dinosaur petroglyphs, pp. 371-374. In Walsh, R.E. (ed.), Proceedings of the Second International Conference on Creationism, Vol. 2. Creation Science Fellowship, Pittsburgh.
Daniel, J.C. and K. Chin. 2010. The role of bacterially mediated precipitation in the permineralization of bone. Palaios, 25:507-516. https://doi.org/10.2110/palo.2009.p09-120r
Darwent, C.M. and Lyman, R.L. 2002. Detecting the postburial fragmentation of carpals, tarsals, and phalanges, pp. 355-377. In Haglund, W.E. and Sorg, M.H. (eds.), Advances in Forensic Taphonomy: Method, Theory, and Archaeological Perspectives. CRC, Boca Raton. https://doi.org/10.1201/9781420058352
Dashzeveg, D., Dingus, L., Loope, D.B., Swisher, C.C. III, Dulam, T., and Sweeney, M.R. 2008. New stratigraphic subdivision, depositional environment, and age estimate for the Upper Cretaceous Djadokhta Formation, southern Ulan Nur Basin, Mongolia. American Museum Novitates, 3616:1-40. https://doi.org/10.1206/0003-0082(2005)498[0001:nssdea]2.0.co;2
DeMassa, J.M. and Boudreaux, E. 2015. Dinosaur peptide preservation and degradation. Creation Research Society Quarterly, 51:268-285.
Devièse, T., Karavanić, I., Comeskey, D., Kubiak, C., Korlević, P., Hajdinjak, M., Radović, S., Procopio, N., Buckley, M., Pääbo, S., and Higham, T. 2017. Direct dating of Neanderthal remains from the site of Vindija Cave and implications for the Middle to Upper Paleolithic transition. Proceedings of the National Academy of Sciences of the United States of America, 114:10606-10611. https://doi.org/10.1073/pnas.1709235114
Devièse, T., Stafford, T.W. Jr., Waters, M.R., Wathen, C., Comeskey, D., Becerra-Valdivia, L., and Higham, T. 2018. Increasing accuracy for the radiocarbon dating of sites occupied by the First Americans. Quaternary Science Reviews, 198:171-180.
https://doi.org/10.1016/j.quascirev.2018.08.023
Dingus, L., Loope, D.V., Dashzeveg, D., Swisher, C.C. III, Minjin, C., Novacek, M.J., and Norell, M.A. 2005. The geology of Ukhaa Tolgod (Djadokhta Formation, Upper Cretaceous, Nemegt Basin, Mongolia). American Museum Novitates, 3498:1-31. https://doi.org/10.1206/442.1
Dizdaroglu, M. and Jaruga, P. 2012. Mechanisms of free radical-induced damage to DNA, Free Radical Research, 46:382-419. https://doi.org/10.3109/10715762.2011.653969
Dobberstein, R.C., Collins, M.J., Craig, O.E., Taylor, G., Penkman, K.E.H., and Ritz-Timme, S. 2009. Archaeological collagen: Why worry about collagen diagenesis? Archaeological and Anthropological Sciences, 1:31-42. https://doi.org/10.1007/s12520-009-0002-7
Dodson, P., Behrensmeyer, A.K., Bakker, R.T., and McIntosh, J.S. 1980. Taphonomy and paleoecology of the dinosaur beds of the Jurassic Morrison Formation. Paleobiology, 6:208-232. https://doi.org/10.1017/s009483730000676x
Doll, S., Proneth, B., Tyurina, Y.Y., Panzilius, E., Kobayashi, S., Ingold, I., Irmler, M., Beckers, J., Aichler, M., Walch, A., Prokisch, H., Trümbach, D., Mao, G., Qu, F., Bayir, H., Füllekrug, J., Scheel, C., Wurst, W., Schick, J.A., Kagan, V.E., Friedmann Angeli, J.P., and Conrad, M. 2021. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nature Chemical Biology, 13:91-98. https://doi.org/10.1038/nchembio.2239
Duan, J. and Kasper, D.L. 2011. Oxidative depolymerization of polysaccharides by reactive oxygen/nitrogen species. Glycobiology, 21:401-409. https://doi.org/10.1093/glycob/cwq171
Duffett Carlson, K.S., Mandl, K., McCall, A., Brönnimann, D., Teschler-Nicola, M., Weiss-Krejci, E., and Metscher, B. 2022. 3D visualization of bioerosion in archaeological bone. Journal of Archaeological Science, 145(105646):1-11. https://doi.org/10.1016/j.jas.2022.105646
Dunlop, R.A., Rodgers, K.L., and Dean, R.T. 2002. Recent developments in the intracellular degradation of oxidized proteins. Free Radical Biology and Medicine, 33:894-906. https://doi.org/10.1016/s0891-5849(02)00958-9
Dutta, S., Kumar, S., Singh, H., Khan, M.A., Barai, A., Tewari, A., Rana, R.S., Bera, S., Sen, S., and Sahni, A. 2020. Chemical evidence of preserved collagen in 54-million-year-old fish vertebrae. Palaeontology, 63:195-202. https://doi.org/10.1111/pala.12469
Eberth, D.A. 1993. Depositional environments and facies transitions of dinosaur-bearing Upper Cretaceous redbeds at Bayan Mandahu (Inner Mongolia, People’s Republic of China). Canadian Journal of Earth Sciences, 30:2196-2213. https://doi.org/10.1139/e93-191
Eberth, D. 2005. The geology, pp. 54-82. In Currie, P.J. and Koppelhus, E.B. (eds.), Dinosaur Provincial Park: A Spectacular Ancient Ecosystem Revealed. Indiana University Press, Bloomington.
Eberth, D.A., 2018. Stratigraphy and paleoenvironmental evolution of the dinosaur-rich Baruungoyot-Nemegt succession (Upper Cretaceous), Nemegt Basin, southern Mongolia. Palaeogeography, Palaeoclimatology, Palaeoecology, 494:29-50.
https://doi.org/10.1016/j.palaeo.2017.11.018
Eurell, J.A.C. 2004. Veterinary Histology. Teton New Media, Jackson, Wyoming.
Feng, J.Q., Ward, L.M., Liu, S., Lu, Y., Xie, Y., Yuan, B., Yu, X., Rauch, F., Davis, S.I., Zhang, S., Rios, H., Drezner, M.K., Quarles, L.D., Bonewald, L.F., and White, K.E. 2006. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nature Genetics, 38:1310-1315. https://doi.org/10.1038/ng1905
Fernández-Jalvo, Y., Dolores Pesquero, M., and Tormo, L. 2016. Now a bone, then calcite. Palaeogeography, Palaeoclimatology, Palaeoecology, 444:60-70. https://doi.org/10.1016/j.palaeo.2015.12.002
Fields, W., Miller, H., Whitmore, J., Davis, D., Detwiler, G., Ditmars, J., Whitelaw, R., and Novaez, G. 1990. The Paluxy River footprints revisited, pp. 155-175. In Walsh, R.E. (ed.), Proceedings of the Second International Conference on Creationism, Vol. 2. Creation Science Fellowship, Pittsburgh.
Forbes, S.L., Perrault, K.A., and Comstock, J.L. 2017. Microscopic post-mortem changes: the chemistry of decomposition, pp. 26-38. In Schotsmans, E.M.J., Márquez-Grant, N., and Forbes, S.L. (eds.), Taphonomy of Human Remains: Forensic Analysis of the Dead and the Depositional Environment. Wiley, Oxford. https://doi.org/10.1002/9781118953358.ch2
Fritton, S.P. and Weinbaum, S. 2009. Fluid and solute transport in bone: Flow-induced mechanotransduction. Annual Review of Fluid Mechanics, 41:347-374. https://doi.org/10.1146/annurev.fluid.010908.165136
Fürsich, F.T., Sha, J., Jiang, B., and Pan, Y. 2007. High resolution palaeoecological and taphonomic analysis of Early Cretaceous lake biota, western Liaoning (NE-China). Palaeogeography, Palaeoclimatology, Palaeoecology, 253:434-457.
https://doi.org/10.1016/j.palaeo.2007.06.012
Galloway, A. 1996. The process of decomposition: A model from the Arizona-Sonoran desert, pp. 139-150. In Haglund, W.D. and Sorg, M.H. (eds.), Forensic Taphonomy: The Postmortem Fate of Human Remains. CRC, Boca Raton.
Galloway, A., Birkby, H.W., Jones, A.M., Henry, T.E., and Parks, B.O. 1989. Decay rates of human remains in an arid environment. Journal of Forensic Sciences, 34:607-616. https://doi.org/10.1520/jfs12680j
Gangloff, R.A. 1998. Arctic dinosaurs with emphasis on the Cretaceous record of Alaska and the Eurasian-North American connection. New Mexico Museum of Natural History and Science Bulletin, 14:211-220.
Gao, Z., Chen, Z., Xiong, Z., and Liu, X. 2022. Ferroptosis - a new target of osteoporosis. Experimental Gerontology, 165:111836. https://doi.org/10.1016/j.exger.2022.111836
Gatti, L., Lugli, F., Sciutto, G., Zangheri, M., Prati, S., Mirasoli, M., Silvestrini, S., Benazzi, S., Tütken, T., Douka, K., Collina, C., Boschin, F., Romandini, M., Iacumin, P., Guardigli, M., Roda, A. and Mazzeo, R. 2022. Combining elemental and immunochemical analyses to characterize diagenetic alteration patterns in ancient skeletal remains. Scientific Reports, 12:5112. https://doi.org/10.1038/s41598-022-08979-3
Getty, M.A., Loewen, M.A., Roberts, E., Titus, A.L., and Sampson, S.D. 2010. Taphonomy of horned dinosaurs (Ornithischia: Ceratopsidae) from the Late Campanian Kaiparowits Formation, Grand Staircase-Escalante National Monument, USA, pp. 478-494. In Ryan, M.J., Chinnery-Allgeier, B.J., and Eberth, D.A. (eds.), New Perspectives on Horned Dinosaurs: The Royal Tyrrell Museum Ceratopsian Symposium. Indiana University Press, Bloomington.
Gianazza, E., Brioschi, M., Martinez Fernandez, A., and Banfi, C. 2019. Lipoxidation in cardiovascular diseases. Redox Biology, 23:101119. http://doi.org/10.1016/j.redox.2019.101119
Gibbard, P.J. and Head, M.J. 2020. The Quaternary Period, pp. 1217-1255. In Gradstein, F.M., Ogg, J.G., Schmitz, M.D., and Ogg, G.M. (eds.), Geologic Time Scale 2020. Elsevier, Amsterdam.
Gibbard, P. and van Kolfschoten, T. 2004. The Pleistocene and Holocene Epochs, pp. 441-452. In Gradstein, F.M., Ogg, J.G., Schmitz, M.D., and Ogg, G.M. (eds.), Geologic Time Scale 2020. Elsevier, Amsterdam.
Gill-King, H. 1997. Chemical and ultrastructural aspects of decomposition, pp. 93-108. In Haglund, W.D. and Sorg, M.H. (eds.), Forensic Taphonomy: The Postmortem Fate of Human Remains. CRC, Boca Raton. https://doi.org/10.1201/9781439821923
Goldin, A., Beckman, J.A., Schmidt, A.M., and Creager, M.A. 2006. Advanced glycation end products. Sparking the development of diabetic vascular injury. Circulation, 114:597-605. https://doi.org/10.3390/nu14153086
Goodwin, M.D., Grant, P.G., Bench, G., and Holroyd, P.A. 2007. Elemental composition and diagenetic alteration of dinosaur bone: Distinguishing micron-scale spatial and compositional heterogeneity using PIXE. Palaeogeography, Palaeoclimatology, Palaeoecology, 253:458-476. https://doi.org/10.1016/j.palaeo.2007.06.017
Graf, W. 1949. Preserved histological structures in Egyptian mummy tissues and ancient Swedish skeletons. Cells Tissues Organs, 8:236-250.
Gutiérrez, L., Quintana, C., Patiño, C., Bueno, J., Coppin, H., Roth, M.P., and Lázaro, F.J. 2009. Iron speciation study in Hfe knockout mice tissues: Magnetic and ultrastructural characterization. Biochimica et Biophysica Acta, 1792:541-547.
Hachlili, R. 1998. Iconographic elements of Nilotic scenes on Byzantine mosaic pavements in Israel. Palestine Exploration Quarterly, 130:106-120. https://doi.org/10.1179/peq.1998.130.2.106
Haglund, W.D. and Sorg, M.H. 2002. Human remains in water environments, pp. 201-218. In Haglund, W.D. and Sorg, M.H. (eds.), Advances in Forensic Taphonomy: Method, Theory, and Archaeological Perspectives. CRC, Boca Raton.
Ham, K. 2017. Young earth creationism, pp. 17-48. In Ham, K., Ross, H., Haarsma, D.B., Meyer, S.C., and Stump J.B. (eds.). Four Views on Creation, Evolution, and Intelligent Design. Zondervan, Grand Rapids.
Hampton, K.K. 2022. Disseminated intravascular coagulation, pp. 294-297. In Sinclair, A.J., Morley, J.E., Vellas B., Cesari, M., and Munshi, M. (eds.), Pathy’s Principles and Practice of Geriatric Medicine, 6th ed. Wiley, Hoboken.
Hartman, F.A. and Lessler, M.A. 1964. Erythrocyte measurements in fishes, amphibia, and reptiles. Biological Bulletin, 126:83-88.
Hawkins, C.L. and Davies, M.J. 1997. Oxidative damage to collagen and related substrates by metal ion/hydrogen peroxide systems: random attack or site-specific damage? Biochimica et Biophysica Acta, 1360:84-96. https://doi.org/10.1016/s0925-4439(96)00069-5
Hawkins, C.L. and Davies, M.J. 2001. Generation and propagation of radical reactions on proteins, Biochimica et Biophysica Acta, 1504:196-219. https://doi.org/10.1016/S0005-2728(00)00252-8
He, Y., Chen, J., Ren, J., Wang, G., and Cai, G. 2002. Type I collagen inhibits hydroxyl radical-induced apoptosis. Journal of Biochemistry, 132:373-379. https://doi.org/10.1093/oxfordjournals.jbchem.a003232
Heck, C. and Cordonnier, R. 2018. The Grand Medieval Bestiary. Animals in Illuminated Manuscripts. Abbeville, New York.
Hedges, R.E.M. and Millard, A.R. 1995. Bones and groundwater: toward the modelling of diagenetic processes. Journal of Archaeological Science, 22:155-164. https://doi.org/10.1006/jasc.1995.0017
Helfinstine, R.F. and Roth, J.D. 2007. Texas Tracks and Artifacts. Do Texas Fossils Indicate Coexistence of Men and Dinosaurs? R & J Pub, Anoka, Minnesota.
Higham, T. 2011. European Upper and Middle Palaeolithic radiocarbon dates are often older than they look: Problems with previous dates and some remedies. Antiquity, 85:235-249. https://doi.org/10.1017/s0003598x00067570
Higham, T., Brock, F., Poresani, M., Broglio, A., Wood, R., and Douka, K. 2009. Problems with radiocarbon dating the Middle to Upper Paleolithic transition in Italy. Quaternary Science Reviews, 28:1257-1267. https://doi.org/10.1016/j.quascirev.2008.12.018
Honse, C.O., Figueiredo, F.B., de Alencar, N.X., de Fátima Madeira, M., Gremião, I.D.F., and Schubach, T.M.P. 2013. Disseminated intravascular coagulation in a dog naturally infected by Leishmania (Leishmania) chagasi from Rio de Janeiro - Brazil. BMC Veterinary Research, 9:1-5. https://doi.org/10.1186/1746-6148-9-43
Huang, T.D., Lee, Y., Chiang, C., and Reisz, R.R. 2022. The generality of preservation of organic remains in body fossils by FTIR spectra. Biopetrology, 1:48-60.
Hubert, J.F., Panish, P.T., Chure, D.J., and Prostak, K.S. 1996. Chemistry, microstructure, petrology, and diagenetic model of Jurassic dinosaur bones. Dinosaur National Monument, Utah. Journal of Sedimentary Research, 66:531-547.
https://doi.org/10.1306/d426839c-2b26-11d7-8648000102c1865d
Hunt, A.P. and Lucas, S.G. 2003. Origin and stratigraphy of historic dinosaur quarries in the Upper Cretaceous Fruitland Formation of the Fossil Forest Research Natural Area, northwestern New Mexico, pp. 383-388. In Lucas, S.G., Semken, S.C., Berglof, W., and Ulmer-Scholle, D. (eds.), New Mexico Geological Society Guidebook, 54th Field Conference, Geology of the Zuni Plateau. New Mexico Geological Society, Socorro.
https://doi.org/10.56577/ffc-54.383
Iglesias, V., Whitlock, C., Bianchi, M.M., Villarosa, G., and Outes, V. 2011. Holocene climate variability and environmental history at the Patagonian forest/steppe ecotone: Lago Mosquito (42°29.37.89”S, 71°24’14.57”W) and Laguna del Cóndor (42°20’47.22”S, 71°17’0762”W). The Holocene, 22:1297-1307. https://doi.org/10.1177/0959683611427330
Institute for Creation Research. 2015. Guide to Dinosaurs. Harvest House, Eugene, Oregon.
Isaak, M. 2007. The Counter-Creationism Handbook, University of California Press, Berkeley.
Ishida, K., Sawada, N., and Yamaguchi, M. 2004. Expression of albumin in bone tissues and osteoblastic cells: Involvement of hormonal regulation. International Journal of Molecular Medicine, 14:891-895. https://doi.org/10.3892/ijmm.14.5.891
Jacobs, L.L., Winkler, D.A., and Gomani, E.M. 1996. Cretaceous dinosaurs of Africa: examples from Cameroon and Malawi. Memoirs of the Queensland Museum, 39:595-610.
Janaway, R.C. 1997. The decay of buried human remains and their associated materials, pp. 58-85. In Hunter, J., Roberts, C., and Martin, A. (eds.). Studies in Crime: An Introduction to Forensic Archaeology. Routledge, London. https://doi.org/10.4324/9780203714461-7
Jans, M.M.E., Nielsen-Marsh, C.M., Smith, C.I., Collins, M.J., and Kars, H. 2004. Characterisation of microbial attack on archaeological bone. Journal of Archaeological Science, 31:87-95. https://doi.org/10.1016/j.jas.2003.07.007
Jones, F.N. 2016. The Chronology of the Old Testament, revised color ed., Master Books, Green Forest, Arkansas.
Junkins, E.N. and Carter, D.O. 2017. Relationships between human remains, graves and the depositional environment, pp. 145-154. In Schotsmans, E.M.J., Márquez-Grant, N., and Forbes, S.L. (eds.), Taphonomy of Human Remains: Forensic Analysis of the Dead and the Depositional Environment. Wiley, Oxford. https://doi.org/10.1002/9781118953358.ch11
Junqueira, L.C., Carneiro, J., and Kelley, R.O. 1998. Basic Histology, 9th ed. Appleton and Lange, Stamford.
Kaden, T. 2019. Creationism and Anti-Creationism in the United States. A Sociology of Conflict. Springer, Cham, Switzerland.
Kaye, T.G., Gaugler, G., and Sawlowicz, Z. 2008. Dinosaurian soft tissues interpreted as bacterial biofilms. PLoS ONE, 3(7:e32808):1-7. https://doi.org/10.1371/journal.pone.0002808
Keenan, S.W., Engel, A.S., Roy, A., and Bovencamp-Langlois, G.L. 2015. Evaluating the consequences of diagenesis and fossilization on bioapatite lattice structure and composition. Chemical Geology, 413:18-27. https://doi.org/10.1016/j.chemgeo.2015.08.005
Keesey, T.M. 2016. Three histories of the human body, pp. 189-264. In Kane, J., Willoughby, E., and Keesey, T.M. (eds.), God’s Word or Human Reason? An Inside Perspective on Creationism. Inkwater, Portland.
Kendall, C., Høier Eriksen, A.M., Kontopoulos, I., Collins, M.J., and Turner-Walker, G. 2018. Diagenesis of archaeological bone and tooth. Palaeogeography, Palaeoclimatology, Palaeoecology, 491:21-37. https://doi.org/10.1016/j.palaeo.2017.11.041
Khan, I.M., Tew, S.R., Thompson, B., and Archer, C.W. 2000. Expression of ferritin, an iron-storage protein, in superficial cells of articular cartilage. Orthopaedic Research Society Transactions, 46:58.
Kirkland, J.I. and Madsen, S.K. 2007. The Lower Cretaceous Cedar Mountain Formation, eastern Utah: The view up an always interesting learning curve, pp. 1-108. In Lund, W.R. (ed.), Field Guide to Geological Excursions in Southern Utah, Geological Society of America Rocky Mountain Section 2007 Annual Meeting. Utah Geological Association, Salt Lake City.
Kiseleva, D., Shilovsky, O., Shagalov, E., Ryanskaya, A., Chervyakovskaya, M., Pankrushina, E., and Cherednichenko, N. 2019. Composition and structural features of two Permian parareptile (Deltavjatia vjatkensis, Kotelnich Site, Russia) bone fragments and their alteration during fossilization. Palaeogeography, Palaeoclimatology, Palaeoecology, 526:28-42.
https://doi.org/10.1016/j.palaeo.2019.04.015
Kocsis, L., Trueman, C.N., and Palmer, M.R. 2010. Protracted diagenetic alteration of REE contents in fossil bioapatites: direct evidence from Lu-Hf isotope systematics. Geochimica et Cosmochimica Acta, 74:6077-6092. https://doi.org/10.1016/j.gca.2010.08.007
Komatsu, G., Arzhannikov, S.G., Gillespie, A.R., Burke, R.M., Miyamoto, H., and Baker, V.R. 2009. Quaternary paleolake formation and cataclysmic flooding along the upper Yenisei River. Geomorphology, 104:143-164. https://doi.org/10.1016/j.geomorph.2008.08.009
Korneisel, D.E., Nesbitt, S.J., Werning, S., and Xiao, S. 2021. Putative fossil blood cells reinterpreted as diagenetic structures. PeerJ, 9:e12651. https://doi.org/10.7717/peerj.12651
Krzyściak, W., Kowalska, J., Kózka, M., Papież, M.A., and Kwiatek, W.M. Iron content (PIXE) in competent and incompetent veins is related to the vein wall morphology and tissue antioxidant enzymes. Bioelectrochemistry, 87:114-123.
https://doi.org/10.1016/j.bioelechem.2011.12.011
Lane, R.J., Graiche, J.A., Cuzzilla, M.L., and Coroneos, J.C. 2007. Incompetent venous valves: ultrasound imaging and exo-stent repair. Phlebolymphology, 14:105-115.
Lazic, S.E. 2016. Experimental Design for Laboratory Biologists. Maximising Information and Improving Reproducibility. Cambridge University Press, Cambridge.
Leahey, L.G., Molnar, R.E., Carpenter, K., Witmer, L.M., and Salisbury, S.W. 2015. Cranial osteology of the ankylosaurian dinosaur formerly known as Minmi sp. (Ornithischia: Thyreophora) from the Lower Cretaceous Allaru Mudstone of Richmond, Queensland, Australia. PeerJ, 3:e1475. https://doi.org/10.7717/peerj.1475
Leanza, H.A., Apesteguía, S., Novas, F.E., and de la Fuente, M.S. 2004. Cretaceous terrestrial beds from the Neuquén Basin (Argentina) and their tetrapod assemblages. Cretaceous Research, 25:61-87. https://doi.org/10.1016/j.cretres.2003.10.005
Lee, C., Chiang, C., Huang, P., Chung, C., Huang, T.D., Wang, C., Chen, C., Chang, R., Liao, C., and Reisz, R.R. 2017. Evidence of preserved collagen in an Early Jurassic sauropodomorph dinosaur revealed by synchrotron FTIR microspectroscopy. Nature Communications, 8:14220. https://doi.org/10.1038/ncomms14220
Leeuwenburgh, C., Hansen, P., Shaish, A., Holloszy, J.O., and Heinecke, J.W. 1998. Markers of protein oxidation by hydroxyl radical and reactive nitrogen species in tissues of aging rats. American Journal of Physiology, 274:R453-R461.
https://doi.org/10.1152/ajpregu.1998.274.2.r453
Lei, Y., Deng, X., Zhang, Z., Guo, X., and Zhang, J. 2022. Effects of oxidation on the physicochemical properties and degradation of mutton myofibrillar proteins. Journal of Food Science, 87: 2932-2942. https://doi.org/10.1111/1750-3841.16166
Lengyel, I. 1968. Biochemical aspects of early skeletons, pp. 271-288. In Brothwell, D.R. (ed.), The Skeletal Biology of Earlier Human Populations. Pergamon Press, Oxford.
Levi, M. and ten Cate, H. 1999. Disseminated intravascular coagulation. New England Journal of Medicine, 341:586-592.
Levinson, W., Chin-Hong, P., Joyce, E.A., Nussbaum, J., and Schwartz, B. 2020. Review of Medical Microbiology and Immunology: A Guide to Clinical Infectious Diseases, 16th ed. McGraw Hill, New York.
Li, C., Xiong, Y.L., and Chen, J. 2012. Oxidation-induced unfolding facilitates myosin cross-linking in myofibrillar protein by microbial transglutaminase. Journal of Agricultural and Food Chemistry, 60:8020-8027. https://doi.org/10.1021/jf302150h
Li, Y., Bai, B., and Zhang, Y. 2018. Expression of iron-regulators in the bone tissue of rats with and without iron overload. Biometals, 31:749-757. https://doi.org/10.1007/s10534-018-0133-3
Light, N.D. and Bailey, A.J. 1982. Covalent cross-links in collagen. Methods in Enzymology, 82:360-372. https://doi.org/10.1126/science.180.4086.561
Lindgren, J., Uvdal, P., Engdahl, A., Lee, A.H., Alwmark, C., Bergquist, K.-E., Nilsson, E., Ekström, P., Rasmussen, M., Douglas, D.A., Polcyn, M.J., and Jacobs, L.L. 2011. Microspectroscopic evidence of Cretaceous bone proteins. PLoS ONE, 6(4):e19445.
https://doi.org/10.1371/journal.pone.0019445
Lipinski, B. 2011. Hydroxyl radical and its scavengers in health and disease. Oxidative Medicine and Cellular Longevity, 2011:809696. https://doi.org/10.1155/2011/809696
Liu, J., Chakraborty, S., Hosseinzadeh, P., Yu, Y., Tian, S., Petrik, I., Bhagi, A., and Lu, Y. 2014. Metalloproteins containing cytochrome, iron-sulfur, or copper redox centers. Chemical Reviews, 114:4366-4469. https://doi.org/10.1021/cr400479b
Liu, P., Wang, W., Li, Z., Li, Y., Yu, X., Tu, J., and Zhang, Z. 2022. Ferroptosis: A new regulatory mechanism in osteoporosis. Oxidative Medicine and Cellular Longevity, 2022:2634431. https://doi.org/10.1155/2022/2634431
Louderback, L.A., Kiahtipes, C.A., Janetski, J.C. 2020. Holocene vegetation and climate change on the Colorado Plateau in southern Utah, USA. Quaternary Research, 94:31-45. https://doi.org/10.1111/j.1365-2699.2009.02161.x
Lucas, S.G., Heckert, A.B., and Tanner, L.H. 2005. Arizona’s Jurassic fossil vertebrates and the age of the Glen Canyon Group. New Mexico Museum of Natural History and Science Bulletin, 29:94-103.
Luoma, A.M., Kuo, F., Cakici, O., Crowther, M.N., Denninger, A.R., Avila, R.L., Brites, P., and Kirschner, D.A. 2015. Plasmalogen phospholipids protect intermodal myelin from oxidative damage. Free Radical Biology and Medicine, 84:296-310.
https://doi.org/10.1016/j.freeradbiomed.2015.03.012
Lyson, T.R. and Longrich, N.R. 2011. Spatial niche partitioning in dinosaurs from the latest Cretaceous (Maastrichtian) of North America. Proceedings of the Royal Society B, 278:1158-1164. https://doi.org/10.1098/rspb.2010.1444
Maat, G.J.R. 1991. Ultrastructure of normal and pathological fossilized red blood cells compared with pseudopathological biological structures. International Journal of Osteoarchaeology, 1:209-214. https://doi.org/10.1002/oa.1390010312
Maat, G.J.R. 1993. Bone preservation, decay, and its related conditions in ancient human bones form Kuwait. International Journal of Osteoarchaeology, 3:77-86. https://doi.org/10.1002/oa.1390030204
Mandl, K., Duffett Carlson, K.S., Brönnimann, D., McCall, A., Grassberger, M., Teschler-Nicola, M., Weiss-Krejci, E., and Metscher, B. 2022. Substantiating microCT for diagnosing bioerosion in archaeological bone using a new Virtual Histological Index (VHI). Archaeological and Anthropological Sciences, 14:104. https://doi.org/10.1007/s12520-022-01563-w
Mankar, R.S. and Srivastava, A.K. 2019. Revised paleogeography of the dinosaur bearing Maastrichtian Lameta Formation, central and western India: in the perspective of newly identified Salbardi-Belkher Inland Basin. Journal of Sedimentary Environments, 4:53-71. https://doi.org/10.12957/jse.2019.39318
Marom, A., McCullagh, J.S.O., Higham, T.F.G., Sinitsyn, A.A., and Hedges, R.E.M. 2012. Single amino acid radiocarbon dating of Upper Paleolithic Modern humans. Proceedings of the National Academy of Sciences of the United States of America, 109:6878-6881. https://doi.org/10.1073/pnas.1116328109
Marom, A., McCullagh, J.S.O., Higham, T.F.G., and Hedges, R.E.M. 2013. Hydroxyproline dating: Experiments on the 14C analysis of contaminated and low-collagen bones. Radiocarbon, 55:698-708. https://doi.org/10.2458/azu_js_rc.55.16301
Martínez, R.N., Apaldetti, C., Alcober, O.A., Colombi, C.E., Sereno, P.C., Fernandez, E., Santi Malnis, P., Correa, G.A., and Abelin, D. 2013. Vertebrate succession in the Ischigualasto Formation. Society of Vertebrate Paleontology Memoir, 12:10-30.
McCord, J.M. 1998. Iron, free radicals, and oxidative injury. Seminars in Hematology, 35:5-12.
Miller, H., Bennett, R., de Pontcharra, J., Giertych, M., van Oosterwyck-Gastuche, M.C., Kline, O., White, B., Owen, H., and Taylor, J. 2019. The search for solutions to mysterious anomalies in the geologic column. Geology, Earth and Marine Sciences, 1:1-15. https://doi.org/10.31038/gems.2019114
Montgelard, C. 1992. Albumin preservation in fossil bones and systematics of Malpaisomys insularis (Muridae, Rodentia) and extinct rodent of the Canary Islands. Historical Biology, 6:293-302. https://doi.org/10.1080/10292389209380437
Morris, H.M. III, 2016. Unlocking the Mysteries of Genesis. Harvest House, Eugene, Oregon.
Moscariello, A., Marchi, L., Maraga, F., and Mortara, G. 2002. Alluvial fans of the Italian Alps: Sedimentary facies and processes, pp. 141-166. In Martini, P.I., Baker, V.R., and Garzón, G. (eds.), Flood and Megaflood Processes and Deposits: Recent and Ancient Examples. Blackwell Science, Oxford. https://doi.org/10.1002/9781444304299.ch9
Muramiya, Y., Yoshida, H., Kubota, K., and Minami, M. 2020. Rapid formation of gigantic spherical dolomite concretion in marine sediments. Sedimentary Geology, 404:105664. https://doi.org/10.1016/j.sedgeo.2020.105664
Nalawade-Chavan, S., Zazula, G., Brock, F., Southon, J., MacPhee, R., and Druckenmiller, P. 2014. New single amino acid hydroxyproline radiocarbon dates for two problematic American mastodon fossils from Alaska. Quaternary Geology, 20:23-28.
https://doi.org/10.1016/j.quageo.2013.10.004
Niemenen, P., Ryökäs, E., and Mustonen, A.-M. 2015. Experiential thinking in creationism—a textual analysis. PLoS ONE, 10(4):e0118314. https://doi.org/10.1371/journal.pone.0118314
Nord, A.G., Kars, H., Ullén, I., Tronner, K., and Kars, E. 2005. Deterioration of archaeological bone - a statistical approach. Journal of Nordic Archaeological Science, 15:77-86.
Nyaisaba, B.M., Liu, X., Zhu, S., Fan, X., Sun, L., Hatab, S., Miao, W., Chen, M., and Deng, S. 2019. Effect of hydroxyl-radical on the biochemical properties and structure of myofibrillar protein from Alaska pollock (Theragra chalcogramma). LWT Food Science and Technology, 106:15-21. https://doi.org/10.1016/j.lwt.2019.02.045
Oard, M.J. 2009. Are the Ashfall site sediments and fossils post-Flood? Creation Research Society Quarterly, 46:881-91.
Oard, M.J. 2011. Dinosaur Challenges and Mysteries. Creation Book Publishers, Atlanta.
Oard, M.J. 2020. Land bridges after the Flood. Journal of Creation, 34(3):109-117.
Oard, M.J., Bates, G., and Wolfe, T. 2016. Exploring Dinosaurs with Mr. Hibb. Creation Book Publishers. Powder Springs, Georgia.
Oard, M.J. and Carter, R.W. 2021. Biblical Geology 101. Creation Book Publishers, Powder Springs, Georgia.
Ohfuji, H. and Rickard, D. 2005. Experimental synthesis of framboids—a review. Earth Science Reviews, 71:147-170. https://doi.org/10.1016/j.earscirev.2005.02.001
Oreska, M.P.J., Carrano, M.T., and Dzikiewicz, K.M. 2013. Vertebrate paleontology of the Cloverly Formation (Lower Cretaceous), I: Faunal composition, biogeographical relationships, and sampling. Journal of Vertebrate Paleontology, 33:264-292.
https://doi.org/10.1080/02724634.2012.717567
Owen, M. and Triffitt, J.T. 1976. Extravascular albumin in bone tissue. Journal of Physiology, 257:293-307. https://doi.org/10.1113/jphysiol.1976.sp011369
Palomeque, J. and Planas, J. 1977. Dimensions of the erythrocytes of birds. Ibis, 119:533-535. https://doi.org/10.1111/j.1474-919x.1977.tb02065.x
Parry, W.T. 2011. Composition, nucleation, and growth of iron oxide concretions. Sedimentary Geology, 233:53-68. https://doi.org/10.1016/j.sedgeo.2010.10.009
Pawlicki, R. 1966. Cells, collagen fibrils and vessels in dinosaur bone. Nature, 211:655-657. https://doi.org/10.1038/211655a0
Pawlicki, R. 1977. Histochemical reactions for mucopolysaccharides in the dinosaur bone studies on Epon- and methacrylate-embedded semithin sections as well as on isolated osteocytes and ground sections of bone. Acta Histochemica, 58:75-78.
https://doi.org/10.1016/s0065-1281(77)80110-4
Pawlicki, R. 1978. Morphological differentiation of the fossil dinosaur bone cells. Acta Anatomica, 100:411-418. https://doi.org/10.1159/000144925
Pawlicki, R. 1995. Histochemical demonstration of DNA in osteocytes from dinosaur bones. Folia Histochemica et Cytobiologica, 33:183-186.
Pawlicki, R. and Nowogrodzka-Zagóriśka, M. 1998. Blood vessels and red blood cells preserved in dinosaur bones. Annals of Anatomy, 180:73-77. https://doi.org/10.1016/s0940-9602(98)80140-4
Peterson, J.E., Lenczewski, M.E., and Scherer, R.P. 2010. Influence of microbial biofilms on the preservation of primary soft tissue in fossil and extant archosaurs. PLoS ONE, 5(10):e13334. https://doi.org/10.1371/journal.pone.0013334
Petraglia, M.D., Groucutt, H.S., Guagnin, M., Breeze, P.S., and Boivin, N. 2020. Human responses to climate and ecosystem change in ancient Arabia. Proceedings of the National Academy of Sciences of the United States of America, 117:8263-8270.
https://doi.org/10.1073/pnas.1920211117
Pfretzschner, H.-U. 2004. Fossilization of Haversian bone in aquatic environments. Comptes Rendus Paleovol, 3:605-616. https://doi.org/10.1016/j.crpv.2004.07.006
Pfretzschner, H.-U. and Tütken, T. 2011. Rolling bones - taphonomy of Jurassic dinosaur bones inferred from diagenetic microcracks and mineral infillings. Palaeogeography, Palaeoclimatology, Palaeoecology, 310:117-123.
https://doi.org/10.1016/j.palaeo.2011.01.026
Piga, G., Santos-Cubedo, A., Brunetti, A., Piccinini, M., Malgosa, A., Napolitano, E., and Enzo, S. 2011. A multi-technique approach by XRD, XRF, FT-IR to characterize the diagenesis of dinosaur bones from Spain. Palaeogeography, Palaeoclimatology, Palaeoecology, 310:92-107. https://doi.org/10.1016/j.palaeo.2011.05.018
Piombino-Mascali, D., Gill-Frerking, H., and Beckett, R.G. 2017. The taphonomy of natural mummies, pp. 101-119. In Schotsmans, E.M.J., Márquez-Grant, N., and Forbes, S.L. (eds.), Taphonomy of Human Remains: Forensic Analysis of the Dead and the Depositional Environment. Wiley, Oxford. https://doi.org/10.1002/9781118953358.ch8
Plet, C., Grice, K., Pagès, A., Verrall, M., Coolen, M.J.L., Ruebsam, W., Rickard, W.D.A., and Schwark, L. 2017. Palaeobiology of red and white blood cell-like structures, collagen and cholesterol in an ichthyosaur bone. Scientific Reports, 7:13776.
https://doi.org/10.1038/s41598-017-13873-4
Pokines, J.T., Faillace, K., Berger, J., Pirtle, D., Sharpe, M., Curtis, A., Lombardi, K., and Admans, J. 2018. The effects of repeated wet-dry cycles as a component of bone weathering. Journal of Archaeological Science: Reports, 17:433-441.
https://doi.org/10.1016/j.jasrep.2017.11.025
Polidoro, M. 2002. Ica Stones: Yabba-dabba-doo! Skeptical Inquirer, 26(5):24.
Price, P. 2020. The upper limits of survivability of bone material. Journal of Creation, 34(1):11-12.
Prothero, D.R. 2013. Bringing Fossils to Life: An Introduction to Paleobiology, 3rd ed. Columbia University Press, New York.
Prothero, D.R. 2017. Evolution. What the Fossils Say and Why It Matters, 2nd ed. University of California Press, Berkeley.
Renne, P.R., Deino, A.L., Hilgen, F.J., Kuiper, K.F., Mark, D.F., Mitchell, W.S. III, Morgan, L.E., Mundil, R., and Smit, J. 2013. Time scales of critical events around the Cretaceous Paleogene boundary. Science, 339:684-687. https://doi.org/10.1126/science.1230492
Robinson, A.B. and Robinson, L.R. 1991. Distribution of glutamine and asparagine residues and their near neighbors in peptides and proteins. Proceedings of the National Academy of Sciences of the United States of America, 88:8880-8884.
https://doi.org/10.1073/pnas.88.20.8880
Rogers, R.R., Hartman, J.H., and Krause, D.W. 2000. Stratigraphic analysis of Upper Cretaceous rocks in the Mahajanga Basin, northwestern Madagascar: Implications for ancient and modern faunas. Journal of Geology, 108:275-301.
https://doi.org/10.1086/321963
Rosen, A.M., Hart, T.C., Farquhar, J., Schneider, J.S., and Tadmaa, Ts. 2019. Holocene vegetation cycles, land-use, and human adaptations to desertification in the Gobi Desert of Mongolia. Vegetation History and Archaeobotany, 28:295-309.
https://doi.org/10.1007/s00334-018-0710-y
Rouault, T.A. 2003. How mammals acquire and distribute iron needed for oxygen-based metabolism. PLoS Biology, 1(3):326-328. https://doi.org/10.1371/journal.pbio.0000079
Russell, A.J. and Knudsen, O. 2002. The effects of glacial-outburst flood flow dynamics on ice-contact deposits: November 1996 Jökulhlaup, Skei ðarársandur, Iceland, pp. 67-83. In Martini, P.I., Baker, V.R., and Garzón, G. (eds.), Flood and Megaflood Processes and Deposits: Recent and Ancient Examples. Blackwell Science, Oxford.
https://doi.org/10.1002/9781444304299.ch5
Saitta, E.T., Liang, R., Lau, M.C.Y., Brown, C.M., Longrich, N.R., Kaye, T.G., Novak, B.J., Salzberg, S.L., Norell, M.A., Abbott, G.D., Dickinson, M.R., Vinther, J., Bull, I.D., Brooker, R.A., Martin, P., Donohoe, P., Knowles, T.D.J., Penkman, K.E.H., and Onstott, T. 2019. Cretaceous dinosaur bone contains recent organic material and provides an environment conducive to microbial communities. eLife, 8:e46205. https://doi.org/10.7554/eLife.46205
Saitta, E.T., Rogers, C.S., Brooker, R.A., and Vinther, J. 2017. Experimental taphonomy of keratin: A structural analysis of early taphonomic changes. Palaios, 32:647-657. https://doi.org/10.2110/palo.2017.051
San Antonio, J.D., Schweitzer, M.D., Jensen, S.T., Kalluri, R., Buckley, M., and Orgel, J.P.R.O. 2011. Dinosaur peptides suggest mechanisms of protein survival. PLoS ONE, 6(6):e20381. https://doi.org/10.1371/journal.pone.0020381
Sander, P.M. 1992. The Norian Plateosaurus Bonebeds of central Europe and their taphonomy. Palaeogeography, Palaeoclimatology, Palaeoecology, 93:255-299. https://doi.org/10.1016/0031-0182(92)90100-J
Sawafuji, R., Cappellini, E., Nagaoka, T., Fotakis, A.K., Jersie-Christensen, R.R., Olsen, J.V., Hirata, K., and Ueda, S. 2017. Proteomic profiling of archaeological human bone. Royal Society Open Science, 4:161004. https://doi.org/10.1098/rsos.161004
Schmidt-Schultz, T.H., Reich, M., and Schultz, M. 2021. Exceptionally preserved extracellular bone matrix proteins from the late Neogene proboscidean Anancus (Mammalia: Proboscidea). Paläontologische Zeitschrift, 95:757-765.
https://doi.org/10.1007/s12542-021-00566-7
Schmitz, M.D. 2020. Radioisotopic ages used in GTS2020, pp. 1285-1349. In Gradstein, F.M., Ogg, J.G., Schmitz, M.D., and Ogg, G.M. (eds.), Geologic Time Scale 2020. Elsevier, Amsterdam. https://doi.org/10.1016/b978-0-12-824360-2.00046-2
Schoch, R.R. and Seegis, D. 2014. Taphonomy, deposition and pedogenesis in the Upper Triassic dinosaur beds of Trossingen. Palaeobiodiversity and Palaeoenvironments, 94:571-593. https://doi.org/10.1007/s12549-014-0166-8
Schoonen, M.A.A. 2004. Mechanisms of sedimentary pyrite formation. Geological Society of American Special Papers, 379:117-134. https://doi.org/10.1130/0-8137-2379-5.117
Schroeter, E.R., DeHart, C.J., Cleland, T.P., Zheng, W., Thomas, P.M., Kelleher, N.L., Bern, M., and Schweitzer, M.H. 2017. Expansion for the Brachylophosaurus canadensis collagen I sequence and additional evidence of the preservation of Cretaceous protein. Journal of Proteome Research, 16:920-932.
Schroeter, E.R., Ullmannn, P.V., Macauley, K., Ash, R.D., Zheng, W., Schweitzer, M.H., and Lacovara, K.J. 2022. Soft-tissue, rare earth element, and molecular analyses of Dreadnaughtus schrani, an exceptionally complete titanosaur from Argentina. MDPI Biology, 11:1158. https://doi.org/10.3390/biology11081158
Schultz, M. 1997. Microscopic investigation of excavated skeletal remains: A contribution to paleopathology and forensic medicine, pp. 201-222. In Haglund, W.D. and Sorg, M.H. (eds.), Forensic Taphonomy: The Postmortem Fate of Human Remains. CRC, Boca Raton. https://doi.org/10.1201/9781439821923
Schultz, M. 2001. Paleohistopathology of bone: A new approach to the study of ancient diseases. Yearbook of Physical Anthropology, 44:106-147. https://doi.org/10.1002/ajpa.10024
Schwameis, M., Schober, A., Schörgenhofer, C., Reinhard Sperr, W., Schöchl, H., Janata-Schwatczek, K., Istepan Kürkciyan, E., Sterz, F., and Jilma, B. 2015. Asphyxia by drowning induces massive bleeding due to hyperfibrinolytic disseminated intravascular coagulation. Critical Care Medicine, 43:2394-2402. https://doi.org/10.1097/ccm.0000000000001273
Schweitzer, M.H., Wittmeyer, J.W., Horner, J.R., and Toporski, J.K. 2005. Soft-tissue vessels and cellular preservation in Tyrannosaurus rex. Science, 307:1952-1955. https://doi.org/10.1126/science.1108397
Schweitzer, M.H., Wittmeyer, J.W., and Horner, J.R. 2007. Soft tissue and cellular preservation in vertebrate skeletal elements from the Cretaceous to the present. Proceedings of the Royal Society B, 274:183-197. https://doi.org/10.1098/rspb.2006.3705
Schweitzer, M.H., Zheng, W., Organ, C.L., Avci, R., Suo, Z., Freimark, L.M., Lebleu, V.S., Duncan, M.B., Vander Heiden, M.G., Neveu, J.M., Lane, W.S., Cottrell, J.S., Horner, J.R., Cantley, L.C., Kalluri, R., and Asara, J.M. 2009. Biomolecular characterization and protein sequences of the Campanian hadrosaur B. canadensis. Science, 324:626-631.
https://doi.org/10.1126/science.1165069
Schweitzer, M.H., Zheng, W., Cleland, T.P., and Bern, M. 2013. Molecular analyses of dinosaur osteocytes support the presence of endogenous molecules. Bone, 52:414-423. https://doi.org/10.1016/j.bone.2012.10.010
Schweitzer, M.H., Zheng, W., Cleland, T.P., Goodwin, M.B., Boatman, E., Theil, E., Marcus, M.A., and Fakra, S.C. 2014. A role for iron and oxygen chemistry in preserving soft tissues, cells and molecules from deep time. Proceedings of the Royal Society B, 281:20132741. https://doi.org/10.1098/rspb.2013.2741
Schweitzer, M.H., Moyer, A.E., and Zheng, W. 2016. Testing the hypothesis of biofilm as a source for soft tissue and cell-like structures preserved in dinosaur bone. PLoS ONE, 11(2):e0150238. https://doi.org/10.1371/journal.pone.0150238
Scott, E.D. 2009. Evolution vs. Creationism: An Introduction, 2nd edition, University of California Press, Berkeley.
Senter, P. 2011. The defeat of Flood Geology by Flood Geology: The ironic demonstration that there is no trace of the Genesis Flood in the geologic record. Reports of the National Center for Science Education, 30(3):1.1-1.14.
Senter, P. 2012. More “dinosaur” and “pterosaur” rock art that isn’t. Palaeontologia Electronica, 15.2.22A. https://doi.org/10.26879/321
Senter, P.J. 2017. The evolution of creation science, part 3: Natural selection and convergent evolution. Perspectives on Science and Christian Faith, 69:159-184.
Senter, P.J. 2019. Fire-Breathing Dinosaurs? The Hilarious History of Creationist Pseudoscience at Its Silliest. Cambridge Scholars, Newcastle upon Tyne.
Senter, P.J. 2020. Radiocarbon in dinosaur fossils: Compatibility with an age of millions of years. The American Biology Teacher, 82:72-79. https://doi.org/10.1525/abt.2020.82.2.72
Senter, P.J. 2022a. Apparent conflict between the Pentateuch and modern science: The early Church’s solution, the solution’s biblical origin, and ancient Christian approval of evidence-based reasoning. Science and Christian Belief, 34:7-28.
Senter, P.J. 2022b. Radiocarbon in dinosaur bones revisited: Problems with collagen. The American Biology Teacher, 84:336-341. https://doi.org/10.1525/abt.2022.84.6.336
Senter, P. and Cole, S.J. 2011. “Dinosaur” petroglyphs at Kachina Bridge site, Natural Bridges National Monument, southeastern Utah: not dinosaurs after all. Palaeontologia Electronica, 14(1):1-5. https://palaeo-electronica.org/2011_1/236/index.html
Senter, P.J. and Mackey, J.J. 2017a. The evolution of creation science, part 1: Vestigial structures and biological degeneration, Perspectives on Science and Christian Faith, 69:27-41.
Senter, P.J. and Mackey, J.J. 2017b. The evolution of creation science, part 2: Beneficial mutations, Perspectives on Science and Christian Faith, 69:87-497.
Sereno, P.C. and Brusatte, S.L. 2008. Basal abelisaurid and carcharodontosaurid theropods from the Lower Cretaceous Elrhaz Formation of Niger. Acta Palaeontologica Polonica, 53:15-46. https://doi.org/10.4202/app.2008.0102
Sereno, P.C., Dutheil, D.B., Iarochene, M., Larsson, H.C.E., Lyon, G.H., Magwene, P.M., Sidor, C.A., Varricchio, D.J., and Wilson, J.A. 1996. Predatory dinosaurs from the Sahara and Late Cretaceous faunal differentiation. Science, 272:986-991.
https://doi.org/10.1126/science.272.5264.986
Shimer, H.W. 1914. An Introduction to the Study of Fossils. MacMillan, New York.
Sincerbox, S.N. and DiGangi, E.A. 2018. Forensic Taphonomy and Ecology of North American Scavengers. Academic Press, London.
Sinclair, P. and Wood, T.C. 2021. Revising hominin baraminology with medoid partitioning and fuzzy analysis. Answers Research Journal, 14:451-462.
Sindelar, P.J., Guan, Z., Dallner, G., and Erstner, L. 2015. The protective role of plasmalogens in iron-induced lipid peroxidation. Free Radical Biology and Medicine, 26:318-324. https://doi.org/10.1016/s0891-5849(98)00221-4
Smith, N.D., Makovicky, P.J., Pol, D., Hammer, W.R., and Currie, P.J. 2007. The dinosaurs of the Early Jurassic Hanson Formation of the Central Transantarctic Mountains: Phylogenetic review and synthesis. USGS Open File Report 2007-1047 Short Research Paper, 3:1-5. https://doi.org/10.3133/ofr20071047srp003
Smith, P.R. and Wilson, M.T. 1990. Detection of haemoglobin in human skeletal remains by ELISA. Journal of Archaeological Science, 17:2552-268. https://doi.org/10.1016/0305-4403(90)90023-x
Sorg, M.H., Dearborn, J.H., Monahan, E.I., Ryan, H.F., Sweeney, K.G., and David, E. 1997. Forensic taphonomy in marine contexts, pp. 567-604. In Haglund, W.D. and Sorg, M.H. (eds.), Forensic Taphonomy: The Postmortem Fate of Human Remains. CRC, Boca Raton. https://doi.org/10.1201/9781439821923
Spanner, M., Weber, K., Lanske, B., Ihbe, A., Siggelkow, H., Schütze, H., and Atkinson, M.J. 1995. The iron-binding protein ferritin is expressed in cells of the osteoblastic lineage in vitro and in vivo. Bone, 17:161-165. https://doi.org/10.1016/s8756-3282(95)00176-x
Spindler, L., Comeskey, D., Chabai, V., Uthmeier, T., Buckley, M., Devièse, T., and Higham, T. 2021. Dating the last Middle Palaeolithic of the Crimean Peninsula: New hydroxyproline AMS dates from the site of Kabazi II. Journal of Human Evolution, 156:102996. https://doi.org/10.1016/j.jhevol.2021.102996
Steponaitis, E., Andrews, A., McGee, D., Quade, J., Hsieh, Y., Broecker, W.S., Shuman, B.N., Burns, S.J., and Cheng, H. 2015. Mid-Holocene drying of the U.S. Great Basin recorded in Nevada speleothems. Quaternary Science Reviews, 127:174-185.
https://doi.org/10.1016/j.quascirev.2015.04.011
Stokol, T. 2022. Disseminated intravascular coagulation. pp. 837-847. In Brooks, M.B., Harr, K.E., Seelig, D.M., Wardrop, K.J., and Weiss, D.J. (eds.), Schalm’s Veterinary Hematology, 7th ed. Wiley, Hoboken. https://doi.org/10.1002/9781119500537.ch92
Stout, S.D. and Teitelbaum, S.L. 1976. Histological analysis of undecalcified thin sections of archaeological bone. American Journal of Physical Anthropology, 44:263-270. https://doi.org/10.1002/ajpa.1330440208
Strachan, R., Murphy, B.J., Darling, J., Storey, C., and Shields, G. 2020. Precambrian (4.56-1 Ga), pp. 481-493. In Gradstein, F.M., Ogg, J.G., Schmitz, M.D., and Ogg G.M. (eds.), Geologic Time Scale 2020. Elsevier, Amsterdam.
https://doi.org/10.1016/b978-0-12-824360-2.00016-4
Stuart, B.H. and Ueland, M. 2017. Decomposition in aquatic environments, pp. 235-250. In Schotsmans, E.M.J., Márquez-Grant, N., and Forbes, S.L. (eds.), Taphonomy of Human Remains: Forensic Analysis of the Dead and the Depositional Environment. Wiley, Oxford. https://doi.org/10.1002/9781118953358
Suarez, C.A. and Kohn, M.J. 2020. Caught in the act: A case study on microscopic scale physicochemical effects of fossilization on stable isotopic composition of bone. Geochimica et Cosmochimica Acta, 268:277-295. https://doi.org/10.1016/j.gca.2019.10.008
Suarez, M.B. and Passey, B.H. 2014. Assessment of the clumped isotope composition of fossil bone carbonate as a recorder of subsurface temperatures. Geochimica et Cosmochimica Acta, 140:142-159. https://doi.org/10.1016/j.gca.2014.05.026
Sumkhemthong, S., Prompetchara, E., Chanvorachote, P., and Chaotham, C. 2021. Cisplatin-induced hydroxyl radicals mediate pro-survival autophagy in human lung cancer H460 cells. Biological Research, 54:22. https://doi.org/10.1186/s40659-021-00346-2
Sun, A., Cui, G., Li, Y., and Wu, X. 1985. A verified list of Lufeng saurischian fauna. Vertebrata PalAsiatica, 23:1-12.
Surmik, D., Boczarowski, A., Balin, K., Dulski, M., Szade, J., Kremer, B., and Pawlicki, R. 2016. Spectroscopic studies on organic matter from Triassic reptile bones, Upper Silesia, Poland. PLoS ONE, 11(3):e0151143. https://doi.org/10.1371/journal.pone.0151143
Surmik, D., Dulski, M., Kremer, B., Szade, J., and Pawlicki, R. 2021. Iron-mediated deep-time preservation of osteocytes in a Middle Triassic reptile bone. Historical Biology, 33:186-193. https://doi.org/10.1080/08912963.2019.1599884
Sweeney, S.M., Orgel, J.P., Fertala, A., McAuliffe, J.D., Turner, K.R., Di Lullo, G.A., Chen, S., Antipova, O., Perumal, S., Ala-Kokko, L., Forlino, A., Cabral, W.A., Barnes, A.M., Marini, J.C., and San Antonio, J.D. 2008. Candidate cell and matrix interaction domains on the collagen fibril, the predominant protein in vertebrates. Journal of Biological Chemistry, 28:21187-21197. https://doi.org/10.1074/jbc.m709319200
Swift, D.L. 2006. Secrets of the Ica Stones and Nazca Lines. No publisher listed.
Tacker, D. 2018. Is Genesis History? (DVD). Compass Cinema, Nashville.
Tahoun, M., Engeser, M., Namasivayam, V., Sander, P.M., and Müller, C.E. 2022. Chemistry and analysis of organic compounds in dinosaurs. MDPI Biology, 11:1-25. https://doi.org/10.3390/biology11050670
Tang, F., Luo, Z., Zhou, Z., You, H., Georgi, J.A., Tang, Z., and Wang, Z. 2001. Biostratigraphy and palaeoenvironment of the dinosaur-bearing sediments in Lower Cretaceous of Mazongshan area, Gansu Province, China. Cretaceous Research, 22:115-129. https://doi.org/10.1006/cres.2000.0242
Thomas, B. 2015. Original biomaterials in fossils. Creation Research Society Quarterly, 51:234-247.
Thomas, B. and Nelson, V. 2015. Radiocarbon in dinosaur and other fossils. Creation Research Society Quarterly, 51:299-311.
Thomas, C. and Lumb, A.B. 2012. Physiology of haemoglobin. Continuing Education in Anaesthesia, Critical Care and Pain, 12:251-256. https://doi.org/10.1093/bjaceaccp/mks025
Tierney, J.E., Pausata, F.S.R., and deMenocal, P.D. 2017. Rainfall regimes of the Green Sahara. Science Advances, 3:e1601503. https://doi.org/10.1126/sciadv.1601503
Tomkins, J.P. and Clarey, T.L. 2022. South American paleontology supports a Neogene-Quaternary (N-Q) Flood boundary. Journal of Creation, 36(1):18-21.
Trueman, C.N. 1999. Rare earth element geochemistry and taphonomy of terrestrial vertebrate assemblages. Palaios, 14:555-568. https://doi.org/10.2307/3515313
Trueman, C.N. and Martill, D.M. 2002. The long-term survival of bone: The role of bioerosion. Archaeometry, 44:371-382. https://doi.org/10.1111/1475-4754.t01-1-00070
Trueman, C.N., Palmer, M.R., Field, J., Privat, K., Ludgate, N., Chavagnac, V., Eberth, D.A., Cifelli, R., and Rogers, R.R. 2008. Comparing rates of recrystallisation and the potential for preservation of biomolecules from the distribution of trace elements in fossil bones. Comptes Rendus Paleovol, 7:145-158. https://doi.org/10.1016/j.crpv.2008.02.006
Trzęsiok, D., Krzykawski, T., Niedźwiedzki, R., Brom, K., Gorzelak, P., and Salamon, M.A. 2014. Palaeoenvironment of the Upper Cretaceous (Coniacian) concretion-bearing Lagerstätten from Poland. Palaeogeography, Palaeoclimatology, Palaeoecology, 401:154-165. https://doi.org/10.1016/j.palaeo.2014.02.030
Tucker, R.T., Roberts, E.M., Hu, Y., Kemp, A.I.S., and Salisbury, S.W. 2013. Detrital zircon age constraints for the Winton Formation, Queensland: Contextualizing Australia’s Late Cretaceous dinosaur faunas. Gondwana Research, 24:767-779.
https://doi.org/10.1016/j.gr.2012.12.009
Turner-Walker, G. 2008. The chemical and microbial degradation of bones and teeth, pp. 3-29. In Pinhasi, R. and Mays, S. (eds.), Advances in Human Palaeopathology. Wiley, Chichester. https://doi.org/10.1002/9780470724187.ch1
Tuross, N. 1989. Albumin preservation in the Taima-taima mastodon skeleton. Applied Geochemistry, 4:255-259. https://doi.org/10.1016/0883-2927(89)90026-7
Ullmann, P.V., Pandya, S.H., and Nellermoe, R. 2019. Patterns of soft tissue and cellular preservation in relation to fossil bone tissue structure and overburden depth at the Standing Rock Hadrosaur Site, Maastrichtian Hell Creek Formation, South Dakota, USA. Cretaceous Research, 99:1-13. https://doi.org/10.1016/j.cretres.2019.02.012
Ullmann, P.V., Macauley, K., Ash, R.D., Shoup, B., and Scannella, J.B. 2021. Taphonomic and diagenetic pathways to protein preservation, part II: The case of Tyrannosaurus rex specimen MOR 1125. Biology, 10:1193. https://doi.org/10.3390/biology10111193
Ullmann, P.V., Ash, R.D., and Scannella, J.B. 2022. Taphonomic and diagenetic pathways to protein preservation, part II: The case of Brachylophosaurus canadensis specimen MOR 2598. Biology, 11:1177. https://doi.org/10.3390/biology11081177
Valko, M., Leibfritz, D., Moncol, J., Cronin, M.T.D., Mazur, M., and Telser, J. 2007. Free radicals and antioxidants in normal physiological functions and human disease. International Journal of Biochemistry and Cell Biology, 39:44-84. https://doi.org/10.1016/j.biocel.2006.07.001
van der Plicht, J. and Palstra, S.W.L. 2016. Radiocarbon and mammoth bones: What’s in a date? Quaternary International, 406:246-251. https://doi.org/10.1016/j.quaint.2014.11.027
Van Itterbeeck, J., Sarasan, E., Codrea, V., Sarasan, L., and Bultynck, P. 2004. Sedimentology of the Upper Cretaceous mammal- and dinosaur-bearing sites along the Râul Mare and Barbat Rivers, Haţeg Basin, Romania. Cretaceous Research, 25:517-530. https://doi.org/10.1016/j.cretres.2004.04.004
Van Itterbeeck, J., Bolotsky, Y., Bultynck, P., and Godefroit, P. 2005. Stratigraphy, sedimentology, and palaeoecology of the dinosaur-bearing Kundur section (Zeya-Bureya Basin, Amur Region, Far Eastern Russia). Geological Magazine, 142:735-750.
https://doi.org/10.1017/s0016756805001226
van Klinken, G. and Hedges, R.E.M. 1995. Experiments on collagen-humic interactions: Speed of humic uptake, and effects of diverse chemical treatments. Journal of Archaeological Science, 22:263-270. https://doi.org/10.1006/jasc.1995.0028
Veis, A. 2003. Mineralization in organic matrix frameworks, pp. 249-289. In Dove, P.M., DeYoreo, J.J., and Weiner, S. (eds.), Reviews in Mineralogy and Geochemistry, Vol. 54. Mineralogical Society of America, Washington. https://doi.org/10.1515/9781501509346-014
Velimirov, B. 2001. Nanobacteria, ultramicrobacteria and starvation forms: A search for the smallest metabolizing bacterium. Microbes and Environments, 16:67-77. https://doi.org/10.1264/jsme2.2001.67
Vieira, S.A., Zhang, G., and Decker, G.A. 2017. Biological implications of lipid oxidation products. Journal of the American Oil Chemists’ Society, 94:339-351. https://doi.org/10.1007/s11746-017-2958-2
Vietti, L.A., Bailey, J.V., Fox, D.L., and Rogers, R.R. 2015. Rapid formation of framboidal sulfides on bone surfaces from a simulated marine carcass fall. Palaios, 30:327-334.
https://doi.org/10.2110/palo.2014.027
Voegele, K.K., Boles, Z.M., Ullmannn, P.V., Schroeter, E.R., Zheng, W., and Lacovara, K.J. 2002. Soft tissue and biomolecular preservation in vertebrate fossils in glauconitic, shallow marine sediments of the Hornerstown Formation, Edelman Fossil Park New Jersey. Biology, 11:1161. https://doi.org/10.3390/biology11081161
Wadsworth, C. and Buckley, M. 2014. Proteome degradation in fossils: investigating the longevity of protein survival in ancient bone. Rapid Communications in Mass Spectrometry, 28:605-615. https://doi.org/10.1002/rcm.6821
Waitt, R.B. 2021. Roads less travelled by—Pleistocene piracy in Washington’s northwestern Channeled Scabland, pp. 351-384. In Waitt, R.B., Thackray, G.D., and Gillespie, A.R. (eds.). Untangling the Quaternary Period—A Legacy of Stephen C. Porter. Geological Society of America, Boulder. https://doi.org/10.1130/2021.2548(18)
Waldvogel-Abramowski, S., Waeber, G., Gassner, C., Buser, A., Frey, B.M., Favrat, B., and Tissot, J.-D. 2014. Physiology of iron metabolism. Transfusion Medicine and Hemotherapy, 41:213-221. https://doi.org/10.1159/000362888
Walker, T. 2014. Perth area, Western Australia—Recessive Stage of Flood began in the mid-Cretaceous and eroded kilometres of sediment from continent. Journal of Creation, 28(1):84-90.
Walker, M., Johnsen, S., Rasmussen, S.O., Popp, T., Steffensen, J.-P., Gibbard, P., Hoek, W., Lowe, J., Andrews, J., Björck, S., Cwynar, L.C., Hughen, K., Kershaw, P., Kromer, B., Litt, T., Lowe, D.J., Nakagawa, T., Newnham, R., and Schwander, J. 2009. Formal definition and dating of the GSSP (Global Stratotype Section and Point) for the base of the Holocene using the Greenland NGRIP ice core, and selected auxiliary records. Journal of Quaternary Science, 24:3-17. https://doi.org/10.1002/jqs.1227
Wang, S., Leonard, S.S., Ye, J., Ding, M., and Shi, X. 2000. The role of hydroxyl radical as a messenger in Cr(VI)-induced p53 activation. American Journal of Physiology: Cell Physiology, 279:C868-C875. https://doi.org/10.1152/ajpcell.2000.279.3.c868
Weser, U., Kaup, Y., Etspüler, H., Kenward, N., and Hedges, R.E.M. 1996. Biochemically and immunologically active alkaline phosphatase in archaeologically important bone samples. Journal of Archaeological Science, 23:723-730. https://doi.org/10.1006/jasc.1996.0068
Wiemann, J., Fabbri, M., Yang, T., Stein, K., Sander, P.M., Norell, M.A., and Briggs, D.E.G. 2018. Fossilization transforms vertebrate hard tissue proteins into N-heterocyclic polymers. Nature Communications, 9:4741. https://doi.org/10.1038/s41467-018-07013-3
Willoughby, R. 2016. Radiometric dating and the age of the Earth, pp. 57-98. In Kane, J., Willoughby, E., and Keesey, T.M. (eds.), God’s Word or Human Reason? An Inside Perspective on Creationism. Inkwater, Portland.
Windhager, R., Nemethova, M., Mutsaers, S., Lang, S., Kotz, R., Kitzmueller, E., and Lubec, G. 1998. Evidence for the involvement of the hydroxyl radical in the pathogenesis of excessive connective tissue proliferation in patients with tumor-endoprostheses. Life Sciences, 62:1261-1269. https://doi.org/10.1016/s0024-3205(98)00056-3
Wings, O. 2004. Authigenic minerals in fossil bones from the Mesozoic of England: poor correlation with depositional environments. Palaeogeography, Palaeoclimatology, Palaeoecology, 204:15-32. https://doi.org/10.1016/s0031-0182(03)00709-0
Woetzel, D. 2012. Chronicles of Dinosauria: The History and Mystery of Dinosaurs and Man, Green Forest. Arkansas, Master Books.
Wood, R. 2015. From revolution to convention: the past, present, and future of radiocarbon dating. Journal of Archaeological Science, 56:61-72. https://doi.org/10.1016/j.jas.2015.02.019
Xiao, H., Cai, G., and Liu, M. 2007. Hydroxyl radical induced structural changes of collagen. Spectroscopy, 21:91-103. https://doi.org/10.1155/2007/496174
Xiong, Y.L., Blanchard, S.P., Ooizumi, T., and Ma, Y. 2010. Hydroxyl radical and ferryl-generating systems promote gel network formation of myofibrillar protein. Journal of Food Science, 75:C215-C221. https://doi.org/10.1111/j.1750-3841.2009.01511.x
Xu, X., Clark, J.M., Eberth, D.A., and Currie, P.J. 2022. The Shishugou fauna of the Middle-Late Jurassic transition period in the Junggar Basin of western China. Acta Geologica Sinica, 96:1115-1135. https://doi.org/10.1111/1755-6724.14996
Yan, H., Zou, T., Tuo, Q., Xu, S., Li, H., Belaidi, A.A., and Lei, P. 2021. Ferroptosis: mechanisms and links with diseases. Signal Transduction and Targeted Therapy, 6:49. https://doi.org/10.1038/s41392-020-00428-9
Yao, J., Zhang, Y., and Tang, Z. 2002. Small spheres preserved in a therizinosauroid dinosaur’s blood vessels from northeast China. Acta Scientarum Naturalium, 38:221-225.
Yoshida, H., Yamamoto, K., Minami, M., Katsuta, N., Sin-ichi, S., and Metcalfe, R. 2018. Generalized conditions of spherical carbonate concretion formation around decaying organic matter in early diagenesis. Scientific Reports, 8:6308.
https://doi.org/10.1038/s41598-018-24205-5
Yoshida, H., Kuma, R., Hasegawa, H., Katsuta, N., Sirono, S., Minami, M., Nishimoto, S., Takagi, N., Kadowaki, S., and Metcalfe, R. 2021. Syngenetic rapid growth of ellipsoidal silica concretions with bitumen cores. Scientific Reports, 11:4230.
https://doi.org/10.1038/s41598-021-83651-w
Young, A. 2017. The effects of terrestrial mammalian scavenging and avian scavenging on the body, pp. 212-229. In Schotsmans, E.M.J., Márquez-Grant, N., and Forbes, S.L. (eds.), Taphonomy of Human Remains: Forensic Analysis of the Dead and the Depositional Environment. Wiley, Oxford. https://doi.org/10.1002/9781118953358.ch16
Yu, Z., He, H., Li, G., Deng, C., Wang, H., Zhang, X., Yang, Q., Xia, X., Zhou, Z., and Zhu, R. 2019. SIMS U-Pb geochronology for the Jurassic Yanliao Biota from Bawanggou section, Qinglong (northern Hebei Province, China). International Geology Review, 63:265-275. https://doi.org/10.1080/00206814.2019.1707127
Yurkova, I., Shadyro, O., Kisel, M., Brede, O., and Arnhold, J. 2004. Radiation-influenced free-radical transformation of phospholipids: MALDI-TOF MS study. Chemistry and Physics of Lipids, 132:235-246. https://doi.org/10.1016/j.chemphyslip.2004.08.006
Zarjou, A., Jeney, V., Arosio, P., Poli, M., Antal-Szalmás, P., Agarwal, A., Balla, G., and Balla, J. 2009. Ferritin prevents calcification and osteoblastic differentiation of vascular smooth muscle cells. Journal of the American Society of Nephrology, 20:1254-1263.
https://doi.org/10.1681/asn.2008070788
Zazzo, A. 2014. Bone and enamel carbonate diagenesis: A radiocarbon perspective. Palaeogeography, Palaeoclimatology, Palaeoecology, 416:168-178. https://doi.org/10.1016/j.palaeo.2014.05.006
Zazzo, A. and Saliège, J.-F. 2011. Radiocarbon dating of biological apatite: A review. Palaeogeography, Palaeoclimatology, Palaeoecology, 310:52-61. https://doi.org/10.1016/j.palaeo.2010.12.004
Zazzo, A., Saliège, J.-F., Lebon, M., Lepetz, S., and Moreau, C. 2012. Radiocarbon dating of calcined bones: Insights from combustion experiments under natural conditions. Radiocarbon, 54:855-866. https://doi.org/10.1017/s0033822200047500
Zhang, Z., Liu, P., Deng, X., Guo, X., Mao, X., Guo, X., and Zhang, J. 2021. Effects of hydroxyl radical oxidation on myofibrillar protein and its susceptibility to μ-calpain proteolysis. LWT Food Science and Technology, 137:110453. https://doi.org/10.1016/j.lwt.2020.110453
Zheng, X., Bailleul, A.M., Li, Z., Wang, X., and Zhou, Z. 2021. Nuclear preservation in the cartilage of the Jehol dinosaur Caudipteryx. Communications Biology 4:1125. https://doi.org/10.1038/s42003-021-02627-8
Zhiming, D. 1983. The Dinosaurian Remains from Sichuan Basin, China. Science Press, Beijing.

