|
|
|
DISCUSSIONThere is abundant evidence of syndepositional dissolution of calcareous tests in certain modern environments including both terrigenous and carbonate sediments (for example, marshes: Jonasson and Patterson 1992; De Rijk and Troelstra 1999; Fatela et al. 2009; shallow water: Green et al. 1993; Murray and Alve 1999a; Berkeley et al. 2007; fjord: Alve and Nagy 1986; Murray et al. 2003; shelf seas: Murray 1989; shelf deeps: Alexandersson 1979; enclosed seas: Exon 1972; deep sea: Schröder 1988). This manifests itself in calcareous tests having an etched surface (Murray 1967; Murray and Wright 1970; Peebles and Lewis 1991; Fatela et al. 2009), breakage or loss of later chambers (Murray and Alve 1999a; Moreno et al. 2007) and a higher abundance of agglutinated tests in the dead assemblages than could be accounted for by differential production between species (Murray and Alve 1999a). Dissolution may be caused by corrosive bottom waters (Alexandersson 1979), or sediment pore waters where the metabolic decay of organic matter in the top few cm leads to lowered pH (Reaves 1986; Walter and Burton 1990), or destruction through bacterial activity (Freiwald 1995). Moreno et al. (2007) point out that foraminifera secrete magnesian calcite that is chemically heterogeneous and structurally disordered, and these factors play a role in its reactivity. They calculated the solubility index using a model based on pure calcite but noted that the index for magnesian calcite may be slightly different. In their Portuguese estuary, where the index is zero, calcareous tests are abundant, but where it is negative dissolution takes place, and calcareous tests are either low in abundance or absent altogether. However, it is relatively rare in modern environments for there to be total dissolution loss of calcareous taxa. The exceptions are marshes and the deep sea at depths greater than the lysocline. Dissolution may also take place during sediment burial through subsidence, as part of the processes of fossilisation and finally weathering of deposits exposed at outcrop following uplift. The original dead assemblages (ODAs) not only represent the accumulation of tests derived from successive live assemblages over a period of years or decades but also the results of any post-mortem modification, especially transport of tests. As the ATAs are based on ODAs they also show such features. This chain of reasoning affects their species diversity and patterns of distribution. There is also some destruction of agglutinated tests, especially those that are poorly cemented and therefore fragile (Schröder 1988; De Rijk and Troelstra 1999; Fatela et al. 2009). In our study some Miliammina fusca tests were sufficiently fragile to collapse during mounting (see also Culver et al. 1996). Leptohalysis is also fragile, and some individuals may be destroyed during sample processing (e.g., Murray et al. 2003). Similarly, agglutinated tubular foraminifera become broken during sample collection and processing; we have therefore excluded them from assemblage counts. In arctic fjords, shelf seas and deep sea, early diagenesis destroys many agglutinated tests (Barmawidjaja et al. 1992; Majewski and Zajączkowski 2007; Hald and Steinsund 1996; Wollenburg and Kuhnt 2000). According to Schröder (1988) Reophax scorpiurus is considered to have low fossilisation potential while Hormosinella distans, Eratidus foliaceus, Psammosphaera fusca, Glomospira gordialis, Ammolagena clavata, Karreriella apicularis, Nodellum membranaceum, Ammobaculites filiformis, Haplophragmoides sphaeriloculus, Cribrostomoides subglobosus and Adercotryma glomeratum show progressively greater preservation potential. However, Kuhnt et al. (2000) disagreed with Schröder's conclusions as they consider habitat preference to be equal in importance to wall structure. They point out that many Cretaceous and Palaeogene deep-sea agglutinated assemblages are formed of small infaunal taxa. Increased preservation in coastal settings where relatively high sediment accumulation rates cause rapid burial below the redox cline in the sediment seem to promote preservation of agglutinated forms as it slows down organic matter degradation (discussion in e.g., Alve 1996; Berkeley et al. 2007). Species DiversityOur first study showed that deep sea ATAs, like the ODAs from which they were drawn, have high diversity (Murray and Alve 1994). Our subsequent studies in a wide range of environments have confirmed that ATAs provide much information on species diversity even when only a few agglutinated individuals are recorded in the ODA. The progressive increase in species diversity of ATAs from low in marginal marine environments to high in the deep sea (Figure 2, Table 2) is comparable with that both of ODAs (Figure 2) and living assemblages (see Murray 2006, figure 8.4.). Of course, the fields differ to some degree and the range of species diversity in ODAs is greater not only than that of the ATAs but also that of the living assemblages from which they were drawn. The consequences are apparent from the statistics: the means and standard deviations of alpha and H(S) are comparable (Table 2, N.B. shelf deeps were included with shelves by Murray 2006). Modern calcareous forms have three basic wall structures: calc-agglutinated, porcelaneous, hyaline. We pose the question: do any of the calcareous groups mimic the diversity pattern of the whole assemblages? It is unlikely that this would be true of calc-agglutinated or porcelaneous forms as both are restricted to a limited range of environments, the former to normal marine shelf seas and upper bathyal, and the latter mainly to shallow water normal marine or hypersaline environments – elsewhere they are rare. Since the majority of taxa in most modern environments have hyaline walls (Murray 2006) it follows that they are highly likely to mimic the diversity of the whole assemblage. These observations are important from a practical point of view. If the non-calcareous agglutinated and hyaline components of an assemblage both individually mimic the diversity of the whole assemblage then a study carried out on just one or the other subset in fossil material should suffice to give enough diversity information to help determine the original environment. Distribution PatternsUnlike calcareous foraminifera, agglutinated forms occupy the full range of brackish/marine/hypersaline environments, from almost supratidal to the deepest ocean. However, with the exception of marshes, agglutinated foraminifera are minor elements of most modern foraminiferal assemblages. The limited literature giving details of agglutinated species ecology is summarised in the Taxonomic notes and comments on species ecology listed below. In this study, the MDS plots show the similarities and differences between the various environments. Because marshes have a distinct fauna they are readily separated from the adjacent non-vegetated flats (Figure 5). For subtidal areas, the limited overlap of the North Sea with the Channel/Celtic Sea shows these areas to be faunally distinct (Figure 6), perhaps partly due to finer sediment and lower organic flux in the Forties and Ekofisk areas of the North Sea than in the Celtic Sea. Likewise, Loch Etive has little in common with the Norwegian fjords (Lyngsdalsfjord, Oslofjord or Hardangerfjord). The deeps on the Scottish shelf and the Skagerrak are quite similar and there is a progression from the Skagerrak to Hardangerfjord to the deep sea, which partly reflects increasing water depth. Also, the Skagerrak and Hardangerfjord have finer (muddy) substrates than the deep sea (which although muddy contain sand-size planktonic foraminiferal tests). The environmental parameters we have available are not comprehensive enough to explain the faunal differences we record between the investigated areas. However, the relationship with broad environments is very clear (Figure 7, Figure 8). Overall, the data show that even if only the agglutinated components of the assemblages from these areas are considered, their composition reflects differences in environmental characteristics. The distributions of species and genera fail to reveal any clear depth-related boundaries but there is a progression in faunal change with increasing water depth from shore to deep sea (Figure 7, Figure 8, Figure 9). A similar environmental distribution of agglutinated foraminifera exists on the NW Atlantic margin (Schafer et al. 1981).
Balticammina pseudomacrescens, Haplophragmoides wilberti, Jadammina macrescens and Trochammina inflata are cosmopolitan species restricted to marshes (Murray 2006). In tidal areas these species show distinctive patterns of distribution that are controlled mainly by elevation but also by salinity (see Patterson 1990; De Rijk 1995). The marshes around the Skagerrak, Oslofjord and Kattegat are essentially non-tidal with water level changes due primarily to barometric pressure and wind stress. The difference in elevation between the marsh front and the landward limit is often only a few tens of cm. Balticammina pseudomacrescens occurs only on the landward and higher parts of the marsh with a cover of dry leaf litter indicating infrequent flooding (Murray and Alve 1999a). These marshes are very reliable indicators of the upper limit of sea level and probably also of salinity (Alve and Murray 1999). Although Miliammina fusca has a restricted distribution in our study area being confined to marsh, intertidal and to a lesser extent in shallow fjord (Figure 7) the genus Miliammina is also common in deep shelf sea environments off Antarctica (Murray and Pudsey 2004). Based on DNA analysis and wall structure, Habura et al. (2005) conclude that Miliammina fusca is descended from a calcareous miliolid. The wall structure is considered not to be typical of agglutinated taxa. However, from an ecological perspective it behaves like an agglutinated form. Ammotium cassis is absent from Britain because of the absence of suitable non-tidal environments. Eggerella europea has not previously been reported living in the study area but recent data show it is common subtidally along the Norwegian Skagerrak coast (Alve, unpublished data). There are difficulties in distinguishing between Reophax fusiformis and R. micaceus, and this probably accounts for the disparity between the live and ATA distributions. The total (live plus dead) assemblages of Baffin Island fjords range in depth from ~215 to 708 m. They are ice covered for up to 10 months per year, and the bottom sediment is >85% silt plus clay. Textularia earlandi is dominant in fjords north of Lat. 68°N while Spiroplectammina biformis, Adercotryma glomeratum (which may really be A. wrighti as their illustrations show only three chambers) and Cuneata arctica dominate in those fjords south of Lat. 70°N (Schafer and Cole 1986). Similarly, Corner et al. (1996) found live Adercotryma glomeratum and Spiroplectammina biformis to be indicative of inner fjord at depths of 26-124 m in Tana, Norway. Hardangerfjord is of particular interest because although it reaches bathyal depths (850 m) its connection with true deep sea is across a continental shelf (<200 m) and also shallow sills (140 m) within the fjord system. Nevertheless, there are some living continental slope to deep-sea species present including Ammolagena clavata, Ammoscalaria tenuimargo, Cribrostomoides subglobosus, Haplophragmoides sphaeriloculus, Hormosinella guttifer, Hyperammina laevigata, Lagenammina arenulata and Lagenammina tubulata, and these were perhaps introduced as propagules (Alve et al. 2011). In the ATAs discussed here most are present at less than 5%, and the maximum for any species is 7%. From the estimated sea floor organic flux (2.5 gCm-2 y-1) this area is comparable with that of the continental slope. On the Newfoundland, Canada, upper slope (500 m) live Adercotryma glomeratum, Spiroplectammina biformis and Cribrostomoides jeffreysii are found in the seasonally variable, cold Labrador Current water mass while Earlandammina bullata (given as Trochamminella) are more common in mid-slope NADW and in mixed NADW and NSOW at 2000 m together with Eratidus foliaceus (given as Ammomarginulina) (Schafer and Cole 1982). According to Schmiedl et al. (1997) in the eastern South Atlantic live Lagenammina, Psammosphaera and Reophax are related to sandy sediments in areas of bottom currents but this is not obviously so in our study area. A comparison of >150 ?m stained (living) foraminifera in the axis of a Portuguese submarine canyon and those on the adjacent continental slope shows low standing crops (<30 individuals 10 cm-2) from 332-4969 m in the canyon, and higher values at 307 and 1000 m on the slope (140 and 40 individuals 10 cm-2) with just a few individuals at 4798 m. Arborescent agglutinated taxa are more abundant on the slope and almost absent from the canyon except at 4969 m. The distributions in the canyon were attributed to harsh physical factors such as high bottom currents especially in the upper and middle canyon (Garcia et al. 2007). Samples from terraces in the upper and middle canyon have larger standing crops dominated by calcareous infaunal species. The presence of fragile agglutinated taxa such as Crithionina hispida is taken as evidence of the absence of sediment disturbance by physical factors. The lower canyon faunas are more similar to those of the adjacent slope, dominated by agglutinated forms including Reophax and Lagenammina with low phytodetritus and most organic material present is refractory (Koho et al. 2007). According to Van der Zwaan et al. (1999) there is a correlation between uniserial agglutinated forms (their Reophax spp.) and Mn: remobilisation of Mn leads to reduced number of Reophax. In 1985 Jones and Charnock proposed a morphogroup model based on samples from the UK continental margin, and this model has been widely used for the interpretation of palaeoecology of ancient sediments. However, it is now known that the original model needs some revision, and the present large data set will provide an excellent opportunity to do this. Relationships between Live, Dead and Acid-treated AssemblagesWhere agglutinated foraminifera dominate living assemblages, as in some shallow water areas around the Skagerrak-Kattegat, the main species is the same in live, dead and acid-treated assemblages; for instance, dominance of Balticammina pseudomacrescens, Haplophragmoides wilberti, Miliammina fusca or Jadammina macrescens (Murray and Alve 1999b). However, in a few cases the living assemblage has a dominant species different from that of the dead and ATA, e.g., live dominant Miliammina fusca leading to Jadammina macrescens or Trochammina inflata dominated ODA or ATA. This is probably due to a bloom in the dominant living species at the time of sampling. In this same area, seven different calcareous ODAs give rise to Miliammina fusca ATAs making this the most ubiquitous shallow water species representing environments ranging from marsh edge to water depths of 6 m (limit of sampling) with a wide range of temperatures and salinities. In the North Sea many living and dead assemblages are dominated by the opportunistic species Stainforthia fusiformis. Following dissolution, these give rise to a variety of ATAs dominated by: Adercotryma wrighti (given as A. glomeratum), Cuneata arctica (given as Clavulina obscura), Eggerelloides medius or E. scaber, Morulaeplecta bulbosa, Reophax fusiformis or Leptohalysis catella (but the latter is unlikely to be well preserved due to its fragility, Alve and Murray 1995a). Elphidium excavatum ODAs give rise to Eggerelloides scaber ATAs. In the Skagerrak shelf deep Pullenia osloensis ODAs give rise to Textularia earlandi ATAs (given as T. tenuissima), Stainforthia fusiformis ODAs give rise to one or other T. earlandi, Eggerelloides medius, Haplophragmoides bradyi or Trochamminopsis quadriloba ATAs (given as T. pusillus). The English Channel and, to a lesser extent, the Celtic Sea both experience strong bottom currents. Consequently, many of the agglutinated taxa adopt a clinging or attached mode of life. Such forms comprise >70% of ATAs from the western Channel and >40% from the Western Approaches (areas of strong tidal currents) and lower values in those parts of the Celtic Sea least affected by such currents. Whereas there are three distinct inner shelf sea ODAs, they all give rise to a single Eggerelloides scaber ATA that Murray and Alve (2000a and 2000b) interpreted as indicating that the ODAs are better discriminators of subtle environmental differences than the ATAs in this instance. However, the more tranquil outer shelf sea areas each have distinctive ODAs, which are reasonably well preserved in the ATAs. In the deep-sea NE Atlantic all the ODAs are essentially calcareous, and the dominant genus is Cassidulina. In the ATAs trochamminids are abundant (>30%) in the majority of samples. The situation is similar on the Newfoundland slope (Schafer et al. 1981). Genera occurring with localised abundances >10% in the present deep sea samples include Haplophragmoides, Reophax, Trochammina, Cribrostomoides, Psammosphaera, Glomospira, Adercotryma, Portatrochammina, Eratidus, Hormosinella, Repmanina, Saccammina, Lagenammina and Cystammina (Figure 8.2). There is a broad pattern of distribution with respect to water depth. Tube fragments are most abundant on the continental slope and rise and sparse from the deeper basin. They are particularly abundant off NW Africa that may be related to nutrient-rich upwelling (Murray and Alve 1994). |
|
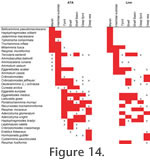 Figure 14
Figure 14