 Sorex bifidus n. sp. and the rich insectivore mammal fauna (Erinaceomorpha, Soricomorpha, Mammalia) from the Early Pleistocene of Żabia Cave in Poland
Sorex bifidus n. sp. and the rich insectivore mammal fauna (Erinaceomorpha, Soricomorpha, Mammalia) from the Early Pleistocene of Żabia Cave in Poland
Article number: 16.2.12A
https://doi.org/10.26879/376
Copyright Palaeontological Association, May 2013
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 1 February 2013. Acceptance: 4 May 2013
{flike id=428}
ABSTRACT
Thirteen species of insectivore mammals, among them the new taxon Sorex bifidus n. sp.and twelve others are described from one of the few middle Early Pleistocene localities of Poland, Żabia Cave. They are: Erinaceus sp. (Erinaceidae), Talpa minor and T. europaea (Talpidae), Paenelimnoecus pannonicus, Sorex minutus, S. runtonensis, S. (Drepanosorex) praearaneus, S. (D.) margaritodon, Asoriculus gibberodon, Neomys newtoni, Petenyia hungarica and Beremendoia fissidens (Soricidae). Measurements, systematic positions, palaeoecological requirements and illustrations are given. The four species of Sorex indicate a humide sylvan paleoenvironment. The abundance of moles is also connected with relatively humid conditions and more or less open areas with soft soils. On the other hand, S. runtonensis is probably an indicator of arid and relatively open biotope and the hedgehog, Erinaceus sp., of forest, shrubs and open areas. Neomys newtoni is connected with open water bodies. This shows a mosaic landscape – forest, open habitat patches and water bodies in the vicinity of the studied cave. The impoverishment of the shrew fauna of Europe during the Late Pliocene and Early Pleistocene caused by the extinction of the Pliocene taxa and the appearance of more numerous species of the genus Sorex are presented.
Barbara Rzebik-Kowalska. Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, Sławkowska 17, 31-016 Kraków, Poland
Keywords: new species; fossil insectivore mammals; Pleistocene
Final citation: Rzebik-Kowalska, Barbara, 2013. Sorex bifidus n. sp. and the rich insectivore mammal fauna (Erinaceomorpha, Soricomorpha, Mammalia) from the Early Pleistocene of Żabia Cave in Poland, Palaeontologia Electronica Vol. 16, Issue 2;12A; 35p. https://doi.org/10.26879/376
palaeo-electronica.org/content/2013/428-a-new-shrew-species
http://zoobank.org/6FE0DE39-CBA1-4F61-914C-7FF1ADE039C2
INTRODUCTION
Żabia (Frog) Cave (50o35'N and 19o31'E) is situated in the Częstochowa Upland in southern Poland. This extensive limestone area with numerous rock-shelters and caves is developed in Upper Jurassic limestone of Oxfordian age. Geological studies of the Żabia Cave deposits showed that they were formed in several sedimentation cycles in the Early Pleistocene, and the character of the sediments indicated several climatic cycles during their deposition (Bosák et al., 1982, Nadachowski et al., 2011).
Żabia Cave lies at an altitude of 406.3 m a.s.l. The length of its known passages is over 60 m, and the depth is about 18.5 m (about 12 m near the entrance shaft). It owes its name to the dead bodies of recent frogs and toads often found at its bottom.
The cave has been known since 1944, but only in 1979 previously inaccessible chambers filled by karst deposits with bone remains were found. Since 1980 Żabia Cave has been systematically explored by the Department of Palaeozoology and Institute of Geological Sciences of the University of Wrocław. Twenty-one layers were distinguished and most of them contained bone remains. The animal remains include slugs, amphibians (toads and frogs), reptiles (lizards and snakes), few birds, and mammals including insectivores, bats, lagomorphs, rodents, carnivores, cervids and bovids. A detailed description of cave morphology and cave deposits can be found in Stefaniak et al., 2009; the profile of the sediments in Bosák et al., 1982 and Ivanov, 1997; the first faunal list in Bosák et al., 1982 and more detailed information on fauna in Stefaniak et al., 2009 and Nadachowski et al., 2011.
METHODS
Measurements of specimens were taken according to De Jong (1988) for Erinaceidae, and according to Hutchison (1974) and Reumer (1984) for Talpidae and Soricidae, respectively. Homologous elements (e.g., right first lower molar m1 or right humerus) were used to represent the minimum number of individuals (MNI). Soricidae teeth I1 and i1 were measured along their buccal sides; all others were measured on their occlusal surfaces. Abbreviations: NS = number of specimens, L = maximum length, W = maximum width, H = maximum height and DW = width of humerus diaphysis.The specimens described are housed in the collections of the Department of Palaeozoology, Wrocław University and the Institute of Paleobiology of the Polish Academy of Sciences, Warszawa.
SYSTEMATIC PALAEONTOLOGY
Class MAMMALIA Linnaeus, 1758
Superorder INSECTIVORA sensu Novacek, 1986
Order ERINACEOMORPHA Gregory, 1910
Family ERINACEIDAE Fischer von
Waldheim, 1814
Subfamily ERINACEINAE Fischer von Waldheim, 1814
Genus ERINACEUS Linnaeus, 1758
Erinaceus sp.
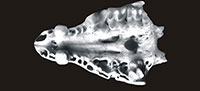
 Material. Table 1.
Material. Table 1.
Description. The size and morphology of hedgehog remains from Żabia Cave are comparable with those of the recent European hedgehogs, Erinaceus europaeus Linnaeus, 1758 and Erinaceus roumanicus Barrett-Hamilton, 1900. Descriptions are given by Holz and Niethammer (1990) in Handbuch der Säugetiere Europas.
Measurements. Table 2, Table 3, and Table 4.
Systematic position. The characters and size of remains described above indicate that they represent a fairly large hedgehog of the genus Erinaceus Linnaeus, 1758. As mentioned above it does not differ much in morphology from European Erinaceus roumanicus and E. europaeus living today in Poland. The first species inhabits most of Poland east of the Odra River, and the second species is present to the west of this river. Differences in their dentition are visible only in the morphology of the third upper incisor (I3), which has two roots, whereas I3 of E. roumanicus and E. europaeus are single-rooted. However, in Żabia Cave the one and only preserved mandible with an angular process shows similarity to the mandible of E. europaeus. In both forms the horizontal crest of the angular process divides its lingual surface into upper and lower parts and both parts (surfaces) are concave. In E. roumanicus the horizontal crest is situated in the upper border of the lingual surface of the angular process and this only surface is flat (Wolff, 1976).
The hedgehog teeth from Żabia Cave are not very large. Most tooth dimensions (Table 2 and Table 3) do not exceed the average dimensions of E. roumanicus and E. europaeus teeth collected in Poland as well as in other parts of their distributions (Holz and Niethammer, 1990). However the hedgehog from Żabia Cave was larger than Erinaceus samsonowiczi Sulimski, 1959 described from the Early Pliocene (MN15) locality of Węże 1 in Poland, Erinaceus lechei described by Kormos (1934) from Beremend in Hungary, a locality dated to the Early Pleistocene, and Erinaceus ostramosi described by Jánossy (1972) from the Early Pleistocene of Osztramos 8, also in Hungary. In addition teeth of E. samsonowiczi differ from the teeth of recent European hedgehogs as well as from the Żabia Cave hedgehog by robust cingula especially on the lingual side of M3 and strongly reduced trigonids of m3; teeth of E. lechei differ by a strong reduction of the paraconids of p4 and m3, and teeth of E. ostramosi by small lower molars m1 and m2 especially in proportion to p4 which is more elongated than in all other Erinaceus species.
Large species of fossil European hedgehogs are represented by Erinaceus praeglacialis described by Brunner (1933) from the Early Pleistocene locality of Windloch (Germany) and Erinaceus davidi Jammot, 1973 from the Middle Pleistocene of La Fage in France. According to Brunner (1933), E. praeglacialis is characterized by slightly larger size than the Recent E. europaeus, elongated metastyles of P4 and M2, and a special morphology and position of the upper P3. With more numerous material from Hundsheim (Middle Pleistocene, Austria), Rabeder (1972) called into question its large dimensions and wrote that only the position and morphology of P3 and the size of M3 discriminate between Recent and fossil species. According to this author the M3 is very large in E. praeglacialis, larger than in any other species known so far (Table 4).
A comparison (Table 4) of M3 measurements of different Erinaceus species shows that the tooth of E. praeglacialis was very large. Unfortunately it was represented only by one specimen. Moreover, Jánossy (1972) did not give the M3 dimensions of E. ostramosi and this tooth is unknown in all other species, i.e., in E. lechei, E. davidi and especially in E. praeglacialis from its type locality (Brunner, 1933). According to Jammot (1973) the teeth of E. davidi are also similar to the teeth of recent European hedgehogs but are the largest of all fossil and Recent species living in this territory. Its M3 could have been equal to or larger than the M3 of E. praeglacialis.
Unfortunately, all fossil species of the genus Erinaceus from Europe were described on the grounds of rather limited material and therefore inter-individual and geographical variation as well as their validity are not well established. In this situation it is difficult to say how many species of the genus Erinaceus have lived in the past in Europe.
As seen above, a comparison of Erinaceus remains from Żabia Cave with other species of this genus could not unambiguously affiliate the material to any of these species. According to the morphology of its angular process, these specimens could belong to E. europaeus or its ancestor which in the Early Pleistocene was probably smaller. It could have increased in size with geological age and the roots of its I3 could have united and become single-rooted as in Recent E. europaeus. On the other hand, the remains from Żabia Cave can also represent a new species. The material is, however, too fragmentary (mostly isolated teeth) to describe another species.
In general, because hedgehogs are comparatively large mammals and are well protected by their spines when attacked by predators, they are rare in fossil material especially from owl-pellets. This is why in most cases their remains from the Pliocene and Pleistocene localities (also in Poland) are not specifically identified. The remains from Żabia Cave are also tentatively identified as Erinaceus sp.
Order SORICOMORPHA Gregory, 1910
Family TALPIDAE Fischer von Waldheim, 1814
Subfamily TALPINAE Fischer von Waldheim, 1814
Tribe TALPINI Fischer von Waldheim, 1814
Genus TALPA Linnaeus, 1758
Talpa minor Freudenberg, 1914
Material. Table 5.
Description. The teeth and humeri are small and their morphology is typical for the recent Talpa europaea Linnaeus, 1758.
Measurements. Table 6, Table 7, and Table 8.
Systematic position and distribution. The morphology of teeth and humeri identify the remains from Żabia Cave as belonging to the genus Talpa Linnaeus, 1758. From the recent European mole, T. europaea living also in Poland, they differ mostly in smaller size. This suggests that they represent Talpa minor Freudenberg, 1914 described from the early Middle Pleistocene of Hundsheim in Austria as Talpa europaea var. minor n. subsp.
Two other small fossil Talpa known from European literature, Talpa gracilis Kormos, (1930a) described from the Early Pleistocene of Somlyóberg near Püspökfürdö (today Betfia 2) in Romania and the Pliocene Talpa csarnotana described by Kretzoi (1959) from Csarnóta 2 (MN 15, Hungary) were considered as junior synonyms of T. minor, at first by Kretzoi (1938) and then by van Cleef-Roders and van den Hoek Ostende (2001).
Talpa neagui Rădulescu and Samson, 1989 described from Bereşti (MN14) and also found in Măluşteni (MN15a) (Romania) is known only from its humeri. According to the authors they are characterized by a wide diaphysis (DW = 3.00-3.50mm, n=10), wider than the diaphysis in T. minor. The authors are of the opinion that specimens cited as T. minor from the Early Pliocene Polish localities of Podlesice (MN14, Kowalski, 1956; DW = 3.20-3.50mm, n=4) and from Węże 1 [MN15, Sulimski, 1959, 1962; DW = 3.10-3.50mm, n=? (1959) and 2.40-2.60mm, n=6 (1962)] most probably belong to T. neagui. It seems, however, that the lack of dentition and a single character (width of the diaphysis) do not meet the requirements for a new species description especially since the size of the diaphysis of T. neagui overlaps the size of the diaphysis in T. minor and Talpa fossilis/T. europaea. Besides, so far the species was found only in two Pliocene localities of Romania and its presence among the Pleistocene remains of Żabia Cave is unlikely.
The presence of the recent Talpa caeca Savi, 1822 in the fossil material of Żabia Cave is also improbable. According to some authors (Kurtén, 1968, Robert, 1983), T. caeca could be derived from T. minor. According to van Cleef-Roders and van den Hoek Ostende (2001) however, there is no data supporting that T. minor was ancestral to the Recent blind mole T. caeca. Although T. minor and T. caeca are more or less of the same size, the molars of T. caeca are larger, comparable to those of T. europaea. Moreover the Recent range of T. caeca is much more to the south and west of Europe. Also Talpa levantis Thomas, 1906 living today in Bulgaria, Thrace, Anatolia (Turkey) and the adjacent Caucasus and described as T. cf. levantis from the Early Pleistocene (former MN17) of Varshets (North Bulgaria) is larger (e.g., DW = 3.50-4.40, n=26) and its morphology is different (upper molars are devoid of parastyles while parastyles are present in the Żabia Cave mole, Popov, 2004).
The presence of other small recent Talpa in Żabia Cave (Talpa occidentalis Cabrera, 1907 and Talpa stankovici Martino and Martino, 1931), endemics of Mediterranean peninsulas, is even more improbable. Rabeder (1972) suggested that all small moles described from the Pliocene and Pleistocene belong to one species which, according to priority, should be T. minor. If this is true, then T. minor appeared in Europe during the beginning of the Early Pliocene. It was very common and it has been excavated from almost all European countries (Rzebik-Kowalska, 2009). In Poland it was present practically in all fossil localities of the Pliocene and Pleistocene and it survived to the Holocene (Krucza Skała Rock Schelter, Poland, Rzebik-Kowalska, 2006, 2009).
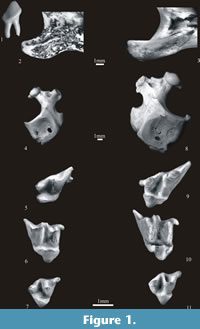
Talpa europaea Linnaeus, 1758
Figure 1. 8-11
Material. Table 9.
Description. The detailed description, history and synonymy of T. europaea and Talpa fossilis can be found in van Cleef-Roders and van den Hoek Ostende (2001) and Rzebik-Kowalska (2000b, 2006).
Measurements. Table 10, Table 11, and Table 12.
Systematic position and distribution. The large mole from Żabia Cave resembles the recent T. europaea from Poland as well as T. europaea described by van Cleef-Roders and van den Hoek Ostende (2001) from different European countries. The size and morphology of teeth and humeri are identical except for the morphology of the mesostyle of the upper molars. According to van Cleef-Roders and van den Hoek Ostende (2001) the T. europaea mesostyle is undivided and its two cusps may be discernible only in unworn specimens. Instead, in Żabia Cave the mesostyle in the unworn upper M1 is slightly or undivided, clearly two-cusped in M2 and usually two-cusped in M3.
Large Early Pleistocene moles of Europe are often determined as T. fossilis Petényi, 1864 described from the Early Pleistocene of Somlyóberg near Püspökfürdö (today Betfia 2) in Romania. However, many authors (Kretzoi, 1938, Heller, 1958, von Koenigswald, 1970, Rabeder, 1972 and others) are of the opinion that T. fossilis and Talpa praeglacialis Kormos, (1930), described from the same locality and based on the same material as well as recent T. europaea, are similar in size and morphology and should be considered as a single species.
On the other hand, Robert (1983) proposed to retain the name T. fossilis as a chronospecies. He argued that geologically older specimens of Talpa similar to T. europaea were smaller and had two-cusped mesostyles in their upper molars. During the Pleistocene they increased in size and their two-cusped mesostyles gradually became monocuspulate as in the recent T. europaea.
Van Cleef-Roders and van den Hoek Ostende (2001) disagreed with this evolutionary trend. They concluded that Robert (1983) had based tooth identification on Crochet and Michaux (1981) but did not confirm this by examining humeri and therefore these teeth could belong to T. caeca. The latter species is similar in size and the mesostyle of its upper molars is divided.
Moles from Żabia Cave are not smaller than recent T. europaea measured by van Cleef-Roders and van den Hoek Ostende (2001). However they have two-cusped mesostyles in upper M2 and M3. This character does not seem very stable because double-cusped mesostyles also occur in teeth of recent T. europaea collected in Poland. Moreover, the presence of T. caeca in Poland is unlikely.
T. europaea lives today throughout all of temperate Europe including Great Britain to the Ob and Irtysh rivers in Asia. The oldest record is known from the French locality Montoussé 5, dated to the Early Pleistocene (former MN17, Clot et al., 1976). On the other hand the oldest remains of T. fossilis come from the Late Miocene (MN13) locality Maramena in Greece (Doukas et al., 1995) and the youngest are cited from the Middle Pleistocene localities of several European countries. The decision as to which name (T. fossilis or T. europaea) is used in a particular locality seems completely arbitrary. Until a revision of the systematics of the two forms (T. fossilis or T. europaea) is completed, the larger mole from Żabia Cave is tentatively identified as T. europaea.
Family SORICIDAE Fischer von Waldheim, 1814
Subfamily ALLOSORICINAE Fejfar, 1966
Genus PAENELIMNOECUS Baudelot, 1972
Paenelimnoecus pannonicus (Kormos, 1934)
Figure 2.1-2
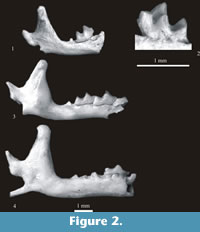 Material. Right fragment of mandible with m2 (slightly damaged) and coronoid and condyloid processes. Minimum number of individuals = 1. Layer 21 (Warsaw - No. ZPAL M. 12/4).
Material. Right fragment of mandible with m2 (slightly damaged) and coronoid and condyloid processes. Minimum number of individuals = 1. Layer 21 (Warsaw - No. ZPAL M. 12/4).
Description. The m2 is devoid of the entoconid and the entoconid crest. Instead, the entostylid takes the shape of a cusp. The buccal re-entrant valley opens at some distance above the buccal cingulum. This cingulum is wide. The horizontal ramus of the mandible is small and slender. The mental foramen is situated below the posterior alveolus of m1. The coronoid process is high and narrow. The coronoid spicule is large and placed nearly halfway between the tip of the coronoid process and the upper sigmoid notch. The condyloid process is massive. Its upper facet is triangular in shape, the lower facet is wide and slightly concave. The interarticular area is also wide. A description of detailed morphology and synonymy of this form can be found in Reumer (1984).
Measurements. The m2 – L = 1.03 mm, W = 0.57* mm (* - slightly damaged), H of mandible below m2 = 0.89 mm, H of ascending ramus = 2. 68 mm, W of coronoid process = 0.48 mm, H of condyloid process = 1.24 mm, W of interarticular area = 0.38 mm.
Systematic position and distribution. The very small size, the m2 devoid of entoconid and entoconid crest and the triangular upper facet of the condylar process show that this mandibular fragment is from the genus Paenelimnoecus Baudelot, 1972 and its only Pliocene species Paenelimnoecus pannonicus (Kormos, 1934). The presence of P. pannonicus in the Early Pleistocene locality must be treated with caution because in Europe as well as in Asia representatives of the genus Paenelimnoecus probably did not survive the Pliocene/ Pleistocene boundary (?MN16/MN17). In Poland the youngest localities with P. pannonicus were Rębielice Królewskie 1A and 2 also dated to MN16 and Mokra Cave 1 (fauna 2) dated to the ?MN16/MN17 (Rzebik-Kowalska, 2009).
Subfamily SORICINAE Fischer von Waldheim, 1814
Tribe SORICINI Fischer von Waldheim, 1814
Genus SOREX Linnaeus, 1758
Sorex minutus Linnaeus, 1766
Figure 2.3
Material. Table 13.
Description. A description of detailed morphology and synonymy of fossil Sorex minutus can be found in Reumer (1984) and Rzebik-Kowalska (1991).
Measurements. Table 14 and Table 15.
Systematic position and distribution. The very small size and morphology assign the specimens from Żabia Cave to the Recent species Sorex minutus. They do not differ from S. minutus living today in Poland. The taxonomy of the small fossil Sorex species similar to S. minutus (Sorex subminutus Sulimski, 1962, Sorex praeminutus Heller, 1963, Sorex biharicus Terzea, 1970) was discussed by Rzebik-Kowalska (1991, 2000a) and does not need reiteration.
Today S. minutus inhabits a large area from northern Spain in Europe to Baikal Lake in Asia. It is the oldest specifically identified Sorex in Europe. Its oldest remains were found in the Early Pliocene (MN14, MN15) localities of Poland and other European countries. The Late Pliocene and Pleistocene localities also yielded abundant remains of this small shrew. The species did not change much over the long period of its existence (Rzebik-Kowalska, 1998, 2009).
Sorex runtonensis Hinton, 1911
Figure 2.4
Material. Table 16.
Description. The morphology and size of the specimens do not differ from the specimens described by Hinton (1911) and Harrison (1996) from the type locality as well as from other Sorex runtonensis described by Rzebik-Kowalska (1991, 2000a) from Poland.
Measurements. Table 17 and Table 18.
Systematic position and distribution. The intermediate size between S. minutus and Sorex araneus Linnaeus, 1758 and the morphology of upper teeth and the coronoid and condyloid processes allocate this Sorex shrew to S. runtonensis Hinton, 1911. It was described from the Middle Pleistocene locality of West Runton in England and is one of the most abundant and widespread Sorex species in the Pleistocene of Europe. In Central Europe it probably survived until the Holocene (Rzebik-Kowalska, 2006, 2009).
The origin of S. runtonensis is unknown. According to Osipova et al., (2006) it is very similar to the extant Siberian shrew Sorex tundrensis Merriam, 1900. However, a considerable spatial gap between the known ranges of Western Palearctic S. runtonensis and Eastern Palearctic S. tundrensis (boundary of its western range passes from the lower Pechora River in the north to the Western Ural Mountains and Perm Region to Kostanay Region in Kazakhstan in the south, Yudin, 1989) as well as its intraspecific variability (eight subspecies of S. tundrensis have been described from East Siberia, Yudin, 1989) precluded conspecific arrangement (Osipova et al., 2006). However, the more recent discovery of fossil S. runtonensis by Agadzhanyan (2009) in the Russian Plain visibly diminishes this gap. Further studies are needed to resolve this interesting problem.
S. tundrensis inhabits different biotopes from arctic tundra to steppe and prefers more open and arid areas than other Sorex species. It is therefore one of the most eurytopic species of this genus. Fossil S. runtonensis is very similar to recent S. tundrensis and may therefore have the same ecological requirements as S. tundrensis and may serve as an indicator of an arid and relatively open paleobiotope (Osipova et al., 2006).
Sorex bifidus n. sp.
Figure 3.1-7
http://zoobank.org/61E16B68-5216-463A-A715-750BEE1FE45D
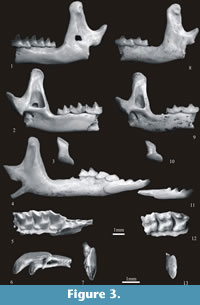 Etymology. The new species has a bifid (dichotomous) first upper incisor I1.
Etymology. The new species has a bifid (dichotomous) first upper incisor I1.
Holotype. Left mandibular fragment with m1-m3 and coronoid and condyloid processes (Warsaw - No. ZPAL M. 12/7/1). It is housed in the collection of the Institute of Paleobiology of the Polish Academy of Sciences in Warsaw.
Type locality. Żabia Cave (JZ).
Studied localities. Mokra Cave I, fauna 2 (MO1 wf2) and Żabia Cave (JZ).
Stratigraphic range. ?Late Pliocene/ Early Pleistocene boundary and middle Early Pleistocene (?MN16/?MN17 – Q1).
Material. Żabia Cave, Table 19.
Mokra Cave I, fauna 2 (MO1 wf2) - two fragments of mandibles with p4 – m2 and processes except angular process. ?Late Pliocene/ Pleistocene boundary, ?MN16/17 (Warsaw - No. ZPAL M. 13/1), minimum number of individuals = 1.
Diagnosis. Comparatively large species of Sorex with large but not bulbous (exoedaenodont) teeth, bifid I1, slender paracone on P4, large (in comparison to m1) m2, high coronoid and particularly high condyloid processes, the interarticular area of which is more or less equally wide throughout its length.
Differential diagnosis. Eleven Recent and fossil Sorex species living in Europe and western Asia are similar in size to S. bifidus n. sp.
S. araneus Linnaeus, 1758 today occupies a large area including almost all of Europe and continental Asia north of the steppe zone. It appeared at about the Pliocene/Pleistocene boundary and was common in Pleistocene and the Holocene localities of Europe.
Sorex isodon Turov, 1924 widely distributed from Scandinavia in Europe to Kamchatka, Kuril and Sakhalin Islands and Primorie in Asia, in fossil state is only known from the Late Pleistocene of the Irkutsk Region. Sorex satunini Ognev, 1922 living in the Northern Caucasus and Sorex raddei Satunin, 1895 known from northern Turkey, Armenia, Georgia and adjacent Russia have only been excavated in Treugolnaya Cave (Northern Caucasus), a locality dated to the Middle Pleistocene.
Among the extinct species of this genus Sorex (Drepanosorex) praearaneus (Kormos, 1934) described from Villany 3 in Hungary, a locality dated to the Early Pleistocene (former MN17), is known from several dozen Pleistocene localities of Europe. Sorex subaraneus Heller, 1958, described from the Middle Pleistocene of Erpfingen in Germany was also excavated in almost all European localities dated from the Early/Late Pliocene to the Late Pleistocene. Sorex macrognathus Jánossy, 1965 described from the Middle Pleistocene of Uppony 1 in Hungary was collected in Germany, Romania and Austria in localities of Middle to Late Pleistocene age. Sorex thaleri Jammot, 1989, found first in France in the Late Pleistocene from La Baume de Gigny, was also excavated in Belgium and Poland in localities of the same age. Sorex hundsheimensis Rabeder, 1972, S. doronichevi Zaitsev and Baryshnikov, 2002, and Drepanosorex rupestris Zaisev and Baryshnikov, 2002 are only known from their type localities, the first from the Middle Pleistocene of Hundsheim in Austria, the latter two from the Late Pleistocene of Treugonaya Cave in Russia.
S. bifidus n. sp. differs from S. (D.) praearaneus, a second large species of Sorex present in Żabia Cave, by not having inflated teeth, by having a narrow paracone, a small parastyle and a distinct parastylar crest in P4 [in S. (D.) praearaneus the paracone is bulbous and its large parastyle is almost devoid of a parastylar crest], by having a long m2 in comparison to m1 [its length is 82.7 – 92.1% of m1 length (n=60) while in S. (D.) praearaneus this value amounts to 74.1 – 81.9% of the m1 (n=58)], by having a wider paraconid/ metaconid valley in lower molars, especially in m1 and m2. In addition, its condyloid process is higher (the dimensions of the condyles of both species originating in Żabia Cave are excluded) and its interarticular area is of nearly the same width over its whole height [while in S. (D.) praearaneus condyle the interarticular area is narrow in its upper part and much broader in its lower part; in some specimens its width is equal to the length of the lower facet]. Its coronoid process is of the Sorex type (more or less narrow on the top and widening in the direction of the upper sigmoid notch, while in the Drepanosorex type it is more or less wide on the top and relatively narrow at the level of the upper sigmoid notch). It is therefore of nearly the same width over its whole height. The mental foramen of the new species is situated slightly more posteriorly.
S. bifidus n. sp. differs from S. subaraneus and from S. hundsheimensis by being visibly larger. Also Recent S. isodon is on average slightly smaller and above all the entoconid crests of its lower molars are in the form of cusps (not crests). This characteristic structure is unknown in other species.
In comparison with S. macrognathus, S. bifidus n. sp. has a high condyloid process [Condylus Index (Bx100/H, B – length of a lower facet, H – height of the condyloid process) of 22 specimens = 58.3 – 69. 4 and according to Rabeder (1992) in S. macrognathus it is over 80]. On the other hand S. thaleri has a larger p4 and a low and massive condyloid process with a long lower facet (its condylus index based on Obłazowa Cave specimens from Poland is also higher than in S. bifidus n. sp. and equals 85.14 - 102.65, n=24).
From Recent S. araneus, more or less similar in size, S. bifidus n. sp. differs by having a higher condyloid processes [H of condyloid process = 1.65 – 2.05 mm (n=39) and 2.15 – 2.57 mm (n=24), respectively] that is different in shape; from S. raddei it differ by a low and wide condyloid process, low internal temporal fossa and anteriorly placed mental foramen. Fossil species described from the Caucasus, S. doronichevi and D. rupestris, are also different: the first has a short mandible and low condyloid process, the second is much larger. Its H of the ascending ramus equals 5.90 – 6.60 mm (n=12) while in S. bifidus n. sp. this value amounts to only 4.50 – 5.39 mm (n=95). Moreover D. rupestris has slightly bulbous teeth and a very large, bicuspulate a1.
Description of holotype. The horizontal ramus of the mandible is wide and only slightly concave below the m1/m2 junction. The ascending ramus is massive; the coronoid process is also massive and Sorex-like. Its anterior edge is slightly concave and the rounded apex bends slightly towards the anterior. The coronoid spicule is pronounced and placed at about two-thirds of the height of the process. The external temporal fossa is deep and clearly delimited. It reaches below the upper sigmoid notch. The internal temporal fossa is high and triangular, shallow in its upper part. The external pterygoid fossa is shallow and a pterygoid boss is absent. The high condyloid process is placed backwards. Its upper facet is cylindrical, the lower is short, wide and slightly concave. The interarticular area is long and flat and nearly rectangular in shape. Two comparatively large and open mandibular foramina are placed below the posterior corner of the internal temporal fossa. The mental foramen is situated below the anterior root of m1.
The lower molars are of typical Sorex shape. Talonids of both m1 and m2 are wider than trigonids; entoconid crests are rather high and entostylids are present. The oblique crests bear small mesoconids. Cingula are well developed. They are broader and more flat on the lingual sides. The m2 is long in comparison with m1 (it is shorter than m1 by only about 8 – 17%). The m3 is not reduced. Its talonid is well developed, basined and provided with both a hypoconid and an entoconid. Cingula are present with the exception of the posterior cingulum.
Description of the remaining specimens. The upper I1 is massive and bifid. Its buccal posterior margin is undulate, more convex in the upper than in the lower parts. The apex and large talon are placed at a sharp angle. The buccal cingulum runs along1/2 of the lower posterior margin and then fades away. It is broad but not very protruding.
The P4 has a long buccal margin as its rather small parastyle is connected to the paracone by a high and long parastylar crest. The paracone is high and slender. The protocone and hypocone are well developed and separated by a wide valley. A broad cingulum surrounds the parastyle, the protocone/hypocone valley and the posterior margin of the tooth. The M1 has a well developed protocone. It is connected by ridges with both the paracone and metacone, but the protocone/metacone ridge (the metaloph) is not very high. As in P4 the hypocone and hypoconal flange are also well developed. A wide valley is present between the protocone and hypocone. There is a narrow cingulum on the lingual side and wider cingulum on the posterior side of the tooth. The M2 is similar, but smaller. The zygomatic process is short and, as seen from below, lies in the extension of the metastyle of M2.
The i1 is long and tricuspulate. A weak cingulum may be present along its upper buccal margin. The a1 is short, single-cusped. The cusp is situated in the middle of the tooth. Its postero-lingual basin is shallow. A wide and unprotruding cingulum is present on the buccal, lingual and posterior sides of the tooth. The p4 is two-cusped. Its postero-lingual basin is shallow but deeper than in a1. It is bordered posteriorly by a well-developed ridge. Its cingulum is similar to the cingulum in a1. Molars and mandibles do not differ from their equivalents in the holotype.
There are no differences in size and morphology between specimens from the two localities - Żabia Cave (JZ) and Mokra Cave I, fauna 2 (MO1 wf2).
Measurements. Table 20 and Table 21.
Systematic position. The presence of a posterior emargination in P4 and upper molars M1 and M2, tricuspulate i1, lower molars m1 and m2 with entoconid crests, m3 unreduced and so on (Repenning, 1967) assign the specimens described above to the genus Sorex Linnaeus, 1758. On the other hand the composition of characters presented in the diagnosis indicates that they differ in size and morphology from all large Recent and fossil Sorex species described from the Pliocene and Pleistocene of Europe and western Asia.
The upper incisors, I1, of S. bifidus and S. (D.) praearaneus are similar in morphology but do not overlap in size and therefore also isolated teeth can be separated [in S. (D.) praearaneus L of I1 = 1.58 – 2.02 mm, n=51 (Reumer, 1984, Rzebik-Kowalska, 1991) and in S. bifidus = L of I1 = 2.04 – 2. 16 mm, n=8). Unfortunately, the dimensions of the first lower incisors, i1, of both species overlap and have been only tentatively assigned to particular species based on their size – smaller lower incisors to the generally smaller S. (D.) praearaneus and larger lower incisors to the larger S. bifidus. The isolated upper molars are also similar in both species and remain difficult to differentiate.
Subgenus SOREX (DREPANOSOREX) Kretzoi, 1941
Sorex (Drepanosorex) praearaneus Kormos, 1934
Figure 3.8-13
Material. Table 22.
Description. The original description of this species is in Kormos (1934) and a detailed description, systematic account, generic status and synonymy in Reumer (1984, 1985) and Rzebik-Kowalka (1991). In general, specimens from Żabia Cave do not differ in size and morphology from specimens from the type locality and from other European populations. Some characters which separate this species from the newly described S. bifidus are mentioned in the chapter comparing the two forms (Differential diagnosis).
Measurements. Table 20 and Table 21.
Systematic position and distribution. A fissident upper incisor I1, slightly bulbous teeth, yellow tooth pigmentation, as well as anteriorly placed mental foramen indicate that the remains belong to the subgenus Sorex (Drepanosorex) Kretzoi, 1941. Comparatively small size, only slightly exoedaenodont dentition and tricuspulate lower incisor i1 with bulbous cuspules designate the material as the oldest known and the most primitive species, Sorex (Drepanosorex) praearaneus Kormos, 1934.
As mentioned above the species is known from several European countries (Rzebik-Kowalska, 1998, 2009). Its oldest remains come from the Early Pleistocene (former MN17) of Villány Kalberg in Hungary (Kormos, 1934) but it has been cited as S. (D.) cf. praearaneus from the late Early Pliocene (MN15) from Ivanovce in Slovakia (Fejfar, 1966). The youngest remains were found in the Late Pleistocene of Ukraine (Mezhzherin, 1972).
Sorex (Drepanosorex) margaritodon Kormos, 1930
Figure 4.1
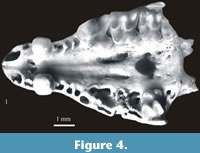 Material. The facial part of the skull with teeth - A2 and P4-M3 on the left side and A2 and M2 on the right side. Minimum number of individuals = 1. Layer 15 (Wrocław - No. ZPALUWr/JZ/II/SDm/1).
Material. The facial part of the skull with teeth - A2 and P4-M3 on the left side and A2 and M2 on the right side. Minimum number of individuals = 1. Layer 15 (Wrocław - No. ZPALUWr/JZ/II/SDm/1).
Description. The above mentioned skull is characterized by a large and massive A2 with a very distinctive cingulum, large P4 with very massive cusps, small rectangular in shape M2 (in comparison to large, more or less square M1) and small and massive M3. In occlusal view the wide zygomatic process is visible in extension of the entire buccal side of M2.
Measurements. Table 23.
Systematic position and distribution. A combination of characters such as relatively large size, exoedaenodonty and weak pigmentation of teeth classify the specimen from Żabia Cave to the subgenus Sorex (Drepanosorex) Kretzoi, 1941. Species of this subgenus that are larger than S. (D.) praearaneus include Sorex (Drepanosorex) margaritodon Kormos, 1930 (Kormos, 1930a) and Sorex (Drepanosorex) savini Hinton, 1911. The shape and size of M2, the LM1/LM2 ratio as well as the position of the M2 in relation to the zygomatic process indicate that the specimen from Żabia Cave belongs to S. (D.) margaritodon. S. (D.) savini has a larger M2 which is square and similar to M1 (L M1/L M2 ratio equals almost 1) and its zygomatic process is visible in the extension of the M2 mezostyle and metastyle only.
The presence of S. (D.) margaritodon in Poland is unexpected. So far the species was only known from the Early Pleistocene of Hungary and the Early and early Middle Pleistocene of Romania. It was never found on the opposite side of the Carpathians. It is a new element in the Polish fossil shrew fauna and Żabia Cave is the most northern locality across its range.
Tribe NEOMYINI Matschie, 1909
Genus ASORICULUS Kretzoi, 1959
Asoriculus gibberodon (Petényi, 1864)
Figure 5. 1-3
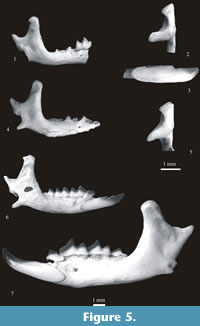 Material. Table 24.
Material. Table 24.
Description. The i1 is short and bicuspulate. The first cusp situated close to the apex is hardly visible and its part between the apex and the main (second) cusp is shortened. A buccal cingulum is present. The upper facet of the condyle is narrow and cylindrical, the lower facet is strongly elongated lingually and separated from the lower sigmoid notch by a narrow groove. The interarticular area is narrow and of nearly the same width over its whole height. The mental foramen is situated underneath the talonid of m1 (between the buccal re-entrant valley and the hypoconid of m1).
Measurements. Table 25.
Systematic position and distribution. Comparatively short i1 with shortened distal part and a narrow interarticular area of the condyle classify these specimens to the genus Asoriculus Kretzoi, 1959 and small size to Asoriculus gibberodon (Petényi, 1864). The similar in size and morphology Asoriculus castellarini (Pasa, 1947) is most probably a synonym of A. gibberodon (Reumer, 1984). On the other hand A. thenii (Malez and Rabeder, 1984) had a much larger i1. It was described from Croatia and never found outside of this country (Malez and Rabeder, 1984). The same is true in the case of the large insular species Asoriculus burgioi described by Masini and Sarà (1998) from Sicily.
The narrow interarticular area of the condyloid process is also characteristic of representatives of the genus Neomys Kaup, 1829 present in Pleistocene localities of Europe and in Żabia Cave. However the shape of the condyloid process and its interarticular area are different in Neomys species. The upper and lower facets lie closer to one another and the interarticular area is shorter and narrower in its central part than near its ends. The i1 is also different in Asoriculus species. It is comparatively shorter and especially its anterior part (between the apex and the main cusp) is shorter than in Neomys. The ratio of overall length of the i1 (A) to its anterior part (B) ranges between 2.72 – 3.38 (n= 6), whereas in Neomys it does not exceed 2.5 (2.19 – 2.43, n= 10 [calculated for Neomys fodiens (Pennant, 1771) and Neomys anomalus Cabrera, 1907, Poland, Recent].
The oldest fossil remains of A. gibberodon were collected in the Late Miocene (MN13) locality Maramena in Greece (Doukas, 2005). Throughout the Pliocene and the Early Pleistocene this species was widely distributed in Europe (from Spain to Bulgaria and Greece Rzebik-Kowalska, 2009) and it was also found in Pliocene localities of Asia Minor (Storch et al., 1998). So far, in Poland the species is known from 7 localities dated to MN14 – MN17. Its presence in Żabia Cave is probably the youngest record in Poland and possibly elsewhere in Europe.
Genus NEOMYS Kaup, 1829
Neomys newtoni Hinton, 1911
Figure 5.4-5
Material. Two fragments of mandibles, one with coronoid and condyloid processes and the second with the condyloid process, layer 17. Minimum number of individuals = 1 (Wrocław – No. ZPALUWr/JZ/II/Nn).
Description. The coronoid process is narrow and its tip is rounded. The coronoid spicule is distinct, situated in 2/3 of the height of the coronoid process. The upper facet of the condyloid process is small, the lower is wide on the buccal side and narrow on the lingual side. The interarticular area is very narrow in the middle and it widens towards the upper and lower facets (see Asoriculus). The mental foramen is situated just in front of the buccal re-entrant valley of m1. More details on the morphology of N. newtoni can be found in Hinton, 1911, and Rzebik-Kowalska, 1991.
Measurements. Table 26.
Systematic position and distribution. The morphology of the condyle described above indicates a representative of the tribe Neomyini and the genus Neomys Kaup, 1829. On the other hand the mandible fragments are similar to mandibles of the small Recent species, Neomys anomalus. However they differ by having two mandibular foramina and a straight (not buccaly recurvate) tip of the coronoid process. The size and morphology of these two specimens correspond almost exactly to the size and morphology of Neomys newtoni Hinton, 1911 described from West Runton in England (Hinton, 1911). They differ from Neomys browni Hinton, 1911 described from the Late Pleistocene of Grays Thurrock, also in England, and only known from its type locality because, according to Hinton, the species is large and only slightly smaller than the Recent N. fodiens.
The validity of Neomys intermedius Brunner, 1952 known from two localities in Germany dated to the Middle and Late Pleistocene, was questioned by Jammot (1977) because of the laconic diagnosis and the scarcity of the accessible material. Neomys hintoni Zaitsev and Baryshnikov, 2002 described from the Middle Pleistocene of Treugolnaya Cave in the Caucasus is similar in size, but differs by a deep depression situated in the buccal side of the ascending ramus between its upper and lower sigmoid notches. This depression is absent in the mandibles from Żabia Cave. The mandibles from Żabia Cave differ from the similarly sized mandibles of Asoriculus, also present in Żabia Cave, by several details mentioned on page 22.
N. newtoni is the oldest described species of this genus. In Europe it is known from many localities of several countries dated to the Early and Middle Pleistocene. So far in Poland it was collected in two localities (Zalesiaki 1A and Kozi Grzbiet), dated to the Early and Early/Middle Pleistocene (Agadzhanyan, 2009, Rzebik-Kowalska, 1998, 2000a, 2009), respectively. Its remains are always scarce in the fossil material.
Tribe BLARINELLINI Reumer, 1984
Genus PETENYIA Kormos, 1934
Petenyia hungarica Kormos, 1934
Figure 5.6
Material. Table 27.
Description. Petenyia hungarica Kormos, 1934 is very well known from the Pliocene and Pleistocene of Europe. Its original description was given by Kormos (1934) for the typical population from Villány-Kalkberg (now Villány 3). A detailed description of its morphology was also given by Reumer (1984), Rzebik-Kowalska (1989) and others, and differences between Pliocene and Early Pleistocene populations can be found e.g., in Rzebik-Kowalska (2000a).
Measurements. Table 28 and Table 29.
Systematic position and distribution. The remains listed above clearly belong to the tribe Blarinellini and the genus Petenyia Kormos, 1934. Features including a nearly straight posterior emargination of upper P4, first and second molars M1 and M2 with very weakly separated hypocones (very often the protocone and hypocone are connected and they form a continuous endoloph), short and massive mandible with a large coronoid process and a protruding coronoid spicule dividing the external temporal fossa into two parts roughly equal in size, a massive condyloid process with very broad lower condylar facet and interarticular area, mental foramen situated below the posterior root of m1, lower molars having very high entoconid crests, the m3 with reduced talonid (to a single cusp) and dark red (almost black) pigmentation of teeth permit an unambiguous assignment to P. hungarica. The older European species of Petenyia, Perenyia dubia Bachmayer and Wilson, 1970 was smaller and it did not survive later than the early Early Pliocene.
P. hungarica was very numerous and very common in European localities, almost as ubiquitous as Beremendia fissidens (Petényi, 1864). It was collected from more than 50 localities from Spain to Poland and Bulgaria (Rzebik-Kowalska, 1998, 2009). The remains of Petenyia were also very common in Asia (Rzebik-Kowalska, 2009).
Tribe BEREMENDIINI Reumer, 1984
Genus BEREMENDIA Kormos, 1934
Beremendia fissidens (Petényi, 1864)
Figure 5.7
Material. Table 30.
Description. The species was described by Petenyi in 1864 and redescribed in detail by Rzebik-Kowalska in 1976 and Reumer in 1984.
Measurements. Table 31 and Table 32.
Systematic position and distribution. Such characters as strongly fissident I1, presence of four upper antemolars of which A1 and A2 are large and about equal in size while A3 and especially A4 are much smaller, an acuspulate i1, the coronoid process short and bent strongly forwards, the internal temporal fossa small and deep, the condyloid process massive with lower facet broad and placed far anteriorly in respect to the lower sigmoid notch, the interarticular area broad and somewhat excavated indicating that the remains belong to the genus Beremendia Kormos, 1934, and their large size points to B. fissidens (Petényi, 1864).
So far two species of Beremendia are known in Europe: B. fissidens and Beremendia minor Rzebik-Kowalska, 1976. B. fissidens was described in Hungary (Beremend 1, Early Pleistocene) and is also known from almost all European countries from Spain to England, Russia, Ukraine, Greece, Croatia and Italy. It was found in dozens of localities dated from the Early Pliocene (?MN14) to the boundary of the Early/Middle Pleistocene (Poland, Kozi Grzbiet, Q2//Q3) (van den Hoek Ostende et al., 2005, Rzebik-Kowalska, 1998, 2009).
B. minor was described in Poland, in locality Rębielice Królewskie 1A (MN16) and is also known from Hungary (Osztramos 1, MN14 and Osztramos 7, MN16). It has not been found in Pleistocene localities. Both species also occur in Asia from Pliocene localities of western Siberia and Transbaikalia.
Because B. minor is similar in morphology to B. fissidens but is smaller and was always found in localities with B. fissidens, Rofes and Cuenca-Bescós (2009) thought that the small specimens so far identified as B. minor are in reality the females of B. fissidens. However, as the authors noticed themselves, the differences in size between the sexes of Recent soricids are not striking. Moreover there are always very few specimens belonging to B. minor in comparison with the numerous (sometimes extremely numerous, see above) remains of B. fissidens whereas the number of males and females in a population is more or less similar and this should be reflected in materials originating from owl pellets. Furthermore B. minor was found only in Pliocene faunas while B. fissidens survived until the early Middle Pleistocene. It is likely that B. minor for some reason is rarely found in fossils, similarly to some other taxa, e.g. Paenelimnoecus species, Neomys species or Sorex minutissimus Zimmermann, 1780.
DISCUSSION
The remains of insectivore mammals present in Żabia Cave were very rich and their number as well as the number of individuals high (6708 and 1720, respectively). They were found in seven layers and represent 13 species belonging to two orders (Erinaceomorpha and Soricomorpha), three families (Erinaceidae, Talpidae and Soricidae) and eight genera. The most numerous were large forms, the shrew Beremendia fissidens (2112) and the mole Talpa europaea (1594). These two species also had the highest number of individuals but in the opposite order, T. europaea (451) and B. fissidens (303). The species composition, number of specimens and the minimum number of individuals in particular layers are presented in Table 33.
The taxonomic diversity varied among layers but there are no important changes among them. The most numerous (11 species) and diverse assemblage (one hedgehog, two moles, eight shrews) was collected in layers 15 and 21 in which the quantity of remains was greater than in other layers. Layers 15 and 21 represent well-marked periods of cooling and increased humidity while layers 17–19 show a distinct warming of climate.
In general, shrews (Soricidae) were more successful and diversified in warm and humid climates. The progressive climatic deterioration since the beginning of the Pleistocene resulted in the extinction or decreased ranges of numerous old Miocene and Pliocene Soricidae taxa. Many of them (e.g., species of Blarinoides Sulimski, 1959, Sulimskia Reumer, 1984, Deinsdorfia Heller, 1963) disappeared at the Villanyan/Q1 boundary and are absent from Żabia Cave deposits.
On the other hand the Recent Sorex, the most diverse genus of the Soricinae, has gradually adapted to a cooler climate and in the course of geological time has become more numerous and diversified. Being better adapted to deteriorating climatic conditions during the Pleistocene, it occupied the empty ecological niches left by extinct Pliocene forms. In Żabia Cave it was represented by five species and in younger Kozi Grzbiet (Poland), a locality dated to the Early/Middle Pleistocene (Q2/Q3), the number of species increased to seven (Rzebik-Kowalska, 2009). Of the Pliocene Sorex species only S. minutus survived to the Recent, all others appeared for the first time in Europe most probably with immigration waves from Asia during the Pleistocene.
In six Polish localities dated to the Zone Q1, 17 species of 11 genera have been found (Table 34). Many of them (Erinaceus sp., Desmana sp., Talpa minor, Paenelimnoecus pannonicus, Sorex minutus, Sorex bor Reumer, 1984, Blarinoides mariae Sulimski, 1959, Beremendia fissidens, Petenyia hungarica, Asoriculus gibberodon) survived from the Pliocene. Talpa fossilis/Talpa europaea, Sorex runtonensis, Sorex bifidus, Sorex (Drepanosorex) praearaneus, Sorex (Drepanosorex) margaritodon, Crocidura obtusa Kretzoi, 1938 and Neomys newtoni appeared as new species (Rzebik-Kowalska, 2009).
In more than 20 European localities described as belonging to Zone Q1 (e.g., in Betfia IX, X in Romania, Rzebik-Kowalska, 2000a, b, Hohensülzen in Germany, Tobien 1980, Osztramos 8 in Hungary, Reumer, 1984, Podumci 1 in Croatia, Malez and Rabeder, 1984, Mas Rambault in France, Jammot, 1977, Ilinka 2 in Russia, Agadzhanyan, 2009, Kolinany 3 in Slovakia, Horáček and Loek, 1988 and others) one genus (Galemys Kaup, 1829) and 11 species more than in Poland were found. They include Talpa episcopalis Kormos, 1930 (1930a), Desmana thermalis Kormos, 1930 (1930b), Desmana moschata (Linnaeus, 1758), Galemys kormosi (Schreuder, 1940), Crocidura cf. kornfeldi Kormos, 1934, Crocidura cf. suaveolens (Pallas, 1811), Asoriculus thenii (Malez and Rabeder, 1984) and several Sorex species such as S. minutissimus, S. praealpinus Heller, 1930, S. cf. subaraneus Heller, 1958, and S. (D.) savini Hinton, 1911.
D. thermalis, T. episcopalis, C. kornfeldi and A. thenii belong to the south European fauna and have never been collected in Poland. The remaining species appeared earlier (G. kormosi, S. subaraneus, S. praealpinus) or later (D. moschata, C. cf. suaveolens, S. minutissimus, S. (D.) savini) in Poland (Rzebik-Kowalska, 2009).
The middle Early Pleistocene assemblage of Żabia Cave is very valuable because it is one of the few sites rich in animal and insectivore remains in Poland and Europe. At the time of deposition a zone of boreal coniferous and mixed forest and steppe area came into existence in Europe as a result of cooling waves in the middle part of the Early Pleistocene. This marked the beginning of a faunal turnover caused by both the evolution and migration of species in Eurasia which continued to the end of the Early and Middle Pleistocene. Insectivore mammals and especially shrews were also affected, and the assemblage in Żabia Cave provides evidence for these processes.
These successive changes are seen very well in Early-Middle Pleistocene localities and particularly in Kozi Grzbiet in which only one species of Pliocene shrew, Beremendia fissidens, survived and the Soricidae fauna was dominated by the genus Sorex. This genus contained 70% of all shrew species in Kozi Grzbiet, while in the older Żabia Cave this value amounted to 50%, and in the Pliocene localities of Poland only 15-30%. Today in Poland Sorex species constitute 50% of the shrew assemblage.
Interesting and extraordinary is the very high number of remains (2213) and individuals (675) of moles (Talpa minor and T. europaea) found in Żabia Cave. As Recent T. europaea spend practically all their lives in underground tunnels, they are not easy to catch and the number of remains in owl-pellets (and in cave breccia) is usually not very high. The case of Żabia is different. Most probably its deep entrance shaft (ca. 12 m) acted as a trap for animals (also moles) if they accidentally fell inside.
Generally speaking, insectivore mammals do not appear to be very good indicators of the paleoenvironment. Moreover differences in the number of remains of particular forms belonging to disparate ecological groups may be connected with taphonomic factors (e.g., different predators responsible for accumulation of insectivore remains).
CONCLUSION
Some general conclusions can be drawn from the species composition of the assemblage in Żabia Cave. The four species of Sorex, indicators of a humid sylvan paleoenvironment; one species of Sorex (Sorex runtonensis) being probably a representative of an arid and relatively open biotope; the abundance of moles also connected with relatively humid conditions and more or less open area with soft soil; the presence of Erinaceus – an indicator of a mosaic landscape of forests, shrubs and open areas; the presence of Neomys newtoni – an indicator of open water bodies, show that a mosaic landscape – forests, open habitat patches and water bodies were present in the vicinity of the studied cave during its existence. The presence in Żabia Cave of opportunistic species (Petenyia hungarica and Beremendia fissidens) found throughout European Pliocene localities and representing forest and steppe environments as well as warmer and cooler climates does not alter these conclusions. The diversified mammal assemblage of Żabia Cave is unique for the reconstruction of the Early Pleistocene history of fauna in this part of Europe (Stefaniak et al., 2009, Nadachowski et al., 2011).
ACKNOWLEDGEMENTS
I am particularly indepted to Dr. K. Stefaniak and Dr. P. Socha (the Department of Palaeozoology, Wrocław University), who kindly offered me the material of insectivore mammals from Żabia Cave for study. I am grateful to Mr. A. Pereswiet-Soltan for preparing the illustrations as well as to Dr. D. Harrison and an anonymous referee for their insightful comments that improved the manuscript.
REFERENCES
Agadzhanyan, A.K. 2009. The Pliocene and Pleistocene small mammals of the Russian Plain. Trudy Paleontologicheskogo Instituta, 289, Nauka, Moskva (In Russian).
Bachmayer, F. and Wilson, R. 1970. Die Fauna der altpliozänen Höhlen- und Spalten-Füllungen bei Kohfidisch, Burgenland (Österreich). Annalen des Naturhistorischen Museums, 74:533-587.
Barrett-Hamilton, G.E.H. 1900. Note on the common hedgehog (Erinaceus europaeus, Linnaeus) and its subspecies or local variations. The Annals and Magazine of Natural History, 5:360-368.
Baudelot, S. 1972. Étude des Chiroptres, Insectivores et Rongeurs du Miocène de Sansan (Gers). Unpublished PhD Thesis, University of Toulouse, France.
Bosák, P., Głazek, J., Horáček, I., and Szynkiewicz, A. 1982. New locality of early Pleistocene vertebrates – Żabia Cave at Podlesice, Central Poland. Acta Geologica Polonica, 32:217-226.
Brunner, G. 1933. Eine präglaziale Fauna aus dem Windloch bei Sackdilling (Oberpfalz). Neues Jahrbuch für Geologie und Paläontologie. Abhandlungen B, 71:303-328.
Brunner, G. 1952. Die Markgrabenhöhle bei Pottenstein (Oberfranken). Neues Jahrbuch für Geologie und Paläontologie. Monatshefte 1952:457-471.
Cabrera, A. 1907. Three new Spanish insectivores. Annals and Magazine of Natural History, London.
Clot, J., Chaline, J., Jammot, D., Mourer-Chauviré, C., and Rage, J.C. 1976. Les poches ossiféres du Pléistocéne moyen et inférieur de Montoussé (Hautes-Pyrénées). Bulletin de la Société d'Histoire Naturelle de Toulouse, 112:146-161.
Crochet, J.-Y. and Michaux, J. 1981. Une faune de vertébrés du Pléistocéne moyen sur le Causse du Larzac: Saint-Sauver, près Nant (Aveyron). Paléobiologie Continentale, 12:131-143.
De Jong, F. 1988. Insectivora from the Upper Aragonian and the Lower Vallesian of the Daroca-Villafeliche area in the Calatayud-Teruel Basin (Spain). Scripta Geologica, special ssue, 1:253-286.
Doukas, C.S. 2005. Greece, p. 99-112. In Hoek Ostende, L.W. van den, Doukas, C.S., and Reumer, J.W.F. (eds.), The fossil record of the Eurasian Neogene insectivores (Erinaceomorpha, Soricomorpha, Mammalia). Part I, Scripta GeologicaSpecial Issue, 5.
Doukas, C.S., Hoek Ostende, L.W. van den, Theocharopoulos, C.D. and Reumer, J.W.F. 1995. The vertebrate locality Maramena (Macedonia, Greece) at the Turolian-Ruscinian boundary (Neogene). Münchner Geowissenschaftliche Abhandlungen (A) 28:43-64.
Fejfar, O. 1966. Die plio-pleistozänen Wirbeltierfaunen von Hajnáčka und Ivanovca (Slowakei), SSR. V. Allosorex stenodus n. g. n. sp. aus Ivanovce A. Neues Jahrbuch für Geologie und Paläontologie. Abhandlungen, 123:221-248.
Fischer von Waldheim, G. 191-1914. Zoognosia tabulis synopticis illustrata. Nicolai Sergeidis Vsevolozsky, Moscow.
Freudenberg, W. 1914. Die Säugetiere des älteren Quartärs von Mitteleuropa mit besonderer Berücksichtigung der Fauna von Hundsheim und Deutschaltenburg in Niederösterreich etc. Geologische und palaeontologische Abhandlungen (Neue Folge) 12:1-219.
Gregory, W.K. 1910. The orders of mammals. Bulletin of the American Museum of Natural History, 37:1-524.
Harrison, D.L. 1996. Systematic status of Kennard's shrew (Sorex kennardi Hinton, 1911, Insectivora, Soricidae): a study based on British and Polish material. Acta zoologica cracoviensia, 39:201-212.
Heller, F. 1930. Eine Forest-Bed-Fauna aus der Sackdillinger Höhle (Oberpfalz). Neues Jahrbuch für Mineralogie, Geologie und Paläontologie. Beilagebände Abteilung B, 63:247-298.
Heller, F. 1958. Eine neue altquartäre Wirbeltierfauna von Erpfingen (Schwäbische Alb). Neues Jahrbuch für Geologie und Paläontologie. Abhandlungen, 107:1-102.
Heller, F. 1963. Eine altquartäre Wirbeltierfauna des unteren Cromerium aus der nördlichen Frankenalb. Neues Jahrbuch für Geologie und Paläontologie. Abhandlungen, 118:1-20.
Hinton, M.A.C. 1911. The British fossil shrews. Geological Magazine, 8:529-539. Hoek Ostende, L.W. van den and Furió, M. 2005. Spain, p. 148-284. In Hoek Ostende, L.W. van den, Doukas, C.S., and Reumer, J.W.F. (eds.), The fossil record of the Eurasian Neogene insectivores (Erinaceomorpha, Soricomorpha, Mammalia). Part I, Scripta Geologica Special Issue 5.
Holz, H. von and Niethammer, J. 1990. Erinaceus europaeus Linnaeus, 1758-Braunbrustigel, Westigel and Erinaceus concolor Martin, 1838-Weissbrustigel, Ostigel, p. 26-64. In Niethammer, J. and Krapp, F. (eds.), Handbuch der Säugetiere Europas, AULA-Verlag ,Wiesbaden.
Horáček, I. and Loek, V. 1988. Palaeozoology and the Mid-European Quaternary past: scope of the approach and selected results. Rozpravy Československé Akademie Vĕd, 98:3-102.
Hutchison, J.H. 1974. Notes on type specimens of European Miocene Talpidae and a tentative classification of Old World Tertiary Talpidae (Insectivora: Mammalia). Geobios, 7:211-256.
Ivanov, M. 1997. Old Biharian reptiles of Żabia Cave (Poland). Acta zoologica cracoviensia, 40:249-267.
Jammot, D. 1973. Les insectivores (Mammalia) du gisement Pléistocène moyen des abimes de La Fage à Noailles (Corrèze). Nouvelles Archives du Muséum d' Histoire Naturelle de Lyon, 11:41-51.
Jammot, D. 1977. Les musaraignes (Soricidae-Insectivora) du Plio-Pléistocène d' Europe. Unpublished PhD Thesis, University of Dijon, France.
Jammot, D. 1989. Les insectivores, p. 111-120. In Campy, M., Chaline, J., and Vuillemey, M. (eds.), La Baume de Gigny (Jura). Gallia Préhistoire 27.
Jánossy, D. 1965. Nachweis einer jungmittelpleistozänen Kleinvertebratenfauna aus der Felsnische Uppony I (Nordungarn). Karszt és Barlang, 4:55-68.
Jánossy, D. 1972. Ein kleiner Hystrix aus dem Altpleistozän der Fundstelle Osztramos 8 (Nord-Ungarn). Vertebrata Hungarica, 13:163-182.
Kaup, J.J. 1829. Skizzierte Entwicklungsgeschichte und Natürliches System der Europäischen Thierwelt, p. 1-203. In Commission bei Carl Wilhelm Leske, Darmstadt und Leipzig.
Kormos, T. 1930a. Diagnosen neuer Säugetiere aus der oberpliozänen Fauna des Somlyóberger bei Püspökfürdö. Annales Musei Nationalis Hungarici, 27:237-246.
Kormos, T. 1930b. Desmana thermalis n. sp. eine neue präglaziale Bisamspitzmaus aus Ungarn. Annales Musei Nationalis Hungarici, 27:1-19.
Kormos, T. 1934. Neue Insektenfresser, Fledermäuse und Nager aus dem Oberpliozän der Villanyer Gegend. Földtani Közlöny, 64:296-321.
Kowalski, K. 1956. Insectivores, bats and rodents from the Early Pleistocene bone breccia of Podlesice near Kroczyce (Poland). Acta Palaeontologica Polonica, 1:331-394.
Kretzoi, M. 1938. Die Raubtiere von Gombaszög nebst einer bersicht der Gesamtfauna. (Ein Beitrag zur Stratigraphie des Altquartaers). Annales Historico-naturales Musei Nationalis Hungarici (1937-1938), 31:88-157.
Kretzoi, M. 1941. Weitere Beiträge zur Kenntnis der Fauna von Gombaszög. Annales Historico-Naturales Musei Nationalis Hungarici, Pars Mineralogica, Geologica et Palaeontologica, 34:105-139.
Kretzoi, M. 1959. Insectivoren, Nagetiere und Lagomorphen der jüngstpliozänen Fauna von Csarnóta im Villányer Gebirge (Südungarn). Vertebrata Hungarica, 1:237-246.
Kurtén, B. 1968. Pleistocene Mammals of Europe. Aldine Publishing, Chicago.
Linnaeus, C. 1758. Systema naturae pre regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio decima, reformata. Laurentius Salvus, Stockholm.
Linnaeus, C. 1766. Systema naturae per regna tria nature , secundum classes, ordines, genera, species cum characteribus, differentiis, synonymis, locis. 12th edition, 1 (1).
Malez, M. and Rabeder, G. 1984. Neues Fundmaterial von Kleinsäugern aus der altpleistozäne Spaltenfüllung Podumci 1 in Norddalmatien (Kroatien, Jugoslawien). Beiträge zur Paläontographie von Österreich, 11:439-510.
Martino, V. and Martino, E. 1931. A new form of Mole from Jugoslavia. Journal of Mammalogy, 12:53.
Masini, F. and Sarà, M. 1998. Asoriculus burgioi sp. nov. (Soricidae, Mammalia) from the Monte Pellegrino faunal complex (Sicily). Acta zoologica cracoviensia, 41:111-124.
Matschie, P. 1909. Mammalia, p. 9. In Brauer, M.(ed.). Die Süsswasserfauna Deutschlands. Eine Exkursionsfauna. Gustav Fischer (Pub.), Jena.
Merriam, C.H. 1900. Description of twenty-six new mammals from Alaska and British North America. Proceedings of the Washington Academy of Sciences, 2:13-30.
Mezhzherin, V.A. 1972. Shrews (Sorex, Insectivora, Mammalia) from Pleistocene deposits of the USSR, p. 117-130. In Kolosov, L.D.and Lukyanova, I.V. (eds.), Nauka, Novosibirsk, (In Russian).
Nadachowski, A., Stefaniak, K., Szynkiewicz, A., Marciszak, A., Socha, P., Schick, P., and August, C. 2011. Biostratigraphic importance of the Early Pleistocene fauna from Żabia Cave (Poland) in Central Europe. Quaternary International, 243:204-218.
Novacek, M.J. 1986. The skull of leptictid insectivorans and the higher-level classification of eutherian mammals. Bulletin of the American Museum of Natural History, 183:1-112.
Ognev, S.I. 1922. Contribution à la classification des mammifères insectivores de la Russie. Annuaire du Musée Zoologique de l'Académie des Sciences de Russie, 22:311-350.
Osipova, V.A., Rzebik-Kowalska, B., and Zaitsev, M.V. 2006. Intraspecific variability and phylogenetic relationships of the Pleistocene shrew Sorex runtonensis (Soricidae). Acta Theriologica 51:129-138.
Pallas, P.S. 1811 [1831]. Zoographia Rosso-Asiatica, sistens omnium `Animalium in extenso Imperio Rossico et adjacentibus maribus observatorum recensionem, domicillia, mores et descriptions, anatomem atque icons plurimorum. [ICZN Opinion 212 – dates of volumes: 1 & 2 :1811, 3:1814]. Petropoli, in officina Caes. Academiae scientiarum.
Pasa, A. 1947. I mammiferi di alcune antiche brecce Veronesi. Memorie del Museo Civico di Storia Naturale di Verona, 1:1-111.
Pennant, T. 1771. Synopsis of Quadrupeds. J. Monk, Chester.
Petényi, S.J. 1864. Hátrahagyott Munkái. F. Eggenberger, Budapest.
Popov, V.V. 2004. Late Plicene Erinaceidae and Talpidae (Mammalia: Insectivora) from Varshets (North Bulgaria). Acta zoologica cracoviensia, 47:61-80.
Rabeder, G. 1972. Die Insectivoren und Chiropteren (Mammalia) aus dem Altpleistozän von Hundsheim (Niederösterreich). Annalen des Naturhistorischen Museums, 76:375-474.
Rabeder, G. 1992. Die Soriciden (Insectivora, Mammalia) aus dem jüngsten Pleistozän des Nixlochs bei Losenstein-Ternberg(Oberösterreich). Mitteilung der Kommission für Quartärforschung der Österreichischen Akademie der Wisenschaften, 8:143-151.
Rădulescu, C. and Samson, P.-M. 1989. Contributions to the knowledge of the mammalian faunas from Măluşteni and Bereşti (Romania). Miscellanea Speologica Romanica, 1:303-311.
Repenning, C.A. 1967. Subfamilies and genera of the Soricidae. Geological Survey Professional Paper, Washington.
Reumer, J.W.F. 1984. Ruscinian and early Pleistocene Soricidae (Insectivora, Mammalia) from Tegelen (The Netherlands) and Hungary. Scripta Geologica, 73:1-173.
Reumer, J.W.F. 1985. The generic status and species of Drepanosorex reconsidered (Mammalia, Soricidae). Revue de Paléobiologie, 4:53-58.
Robert, C. 1983. Recherches sur les Taupes (Talpa, Insectivora) de quelques quaternares de France. PhD Thesis, University of Bordeaux I, France.
Rofes, J. and Cuenca-Bescós, G. 2009. A new genus of red-toothed shrew (Mammalia, Soricidae) from the Early Pleistocene of Gran Dolina (Atapuerca, Burgos, Spain), and a phylogenetic approach to the Eurasiatic Soricinae. Zoological Journal of the Linnean Society, 155:904-925.
Rzebik-Kowalska, B. 1976. The Neogene and Pleistocene insectivores (Mammalia) of Poland. III. Soricidae: Beremendia and Blarinoides. Acta zoologica cracoviensia, 22:359-385.
Rzebik-Kowalska, B. 1989. Pliocene and Pleistocene (Insectivora, Mammalia) of Poland. V. Soricidae: Petenyia Kormos, 1934 and Blarinella Thomas, 1911. Acta zoological cracoviensia, 31 (11):521-546.
Rzebik-Kowalska, B. 1991. Pliocene and Pleistocene Insectivora(Mammalia) of Poland. VIII. Soricidae: Sorex Linnaeus, 1758, Neomys Kaup, 1829, Macroneomys Fejfar, 1966, Paenelimnoecus Baudelot, 1972 and Soricidae indeterminata. Acta zoologica cracoviensia, 34:323-424.
Rzebik-Kowalska, B. 1998. Fossil history of shrews in Europe, p. 23-92. In Wójcik J.M. and Wolsan M. (eds.), Evolution of shrews. Mammal Research Institute Polish Academy of Sciences, Białowieża.
Rzebik-Kowalska, B. 2000a. Insectivora (Mammalia) from the Early and early Middle Pleistocene of Betfia in Romania. I. Soricidae Fischer von Waldheim, 1817. Acta zoologica cracoviensia 43:55-77.
Rzebik-Kowalska, B. 2000b. Insectivora (Mammalia) from the Early and early Middle Pleistocene of Betfia in Romania. II. Erinaceidae Bonaparte, 1838 and Talpidae Gray, 1825. Acta zoologica cracoviensia, 43:55-77.
Rzebik-Kowalska, B. 2006. Erinaceomorpha and Soricomorpha (Mammalia) from the Late Pleistocene and Holocene of Krucza Skała Rock Shelter and Komarowa Cave (Poland). Acta zoologica cracoviensia 49A:83-118.
Rzebik-Kowalska, B. 2009. Biodiversity of Polish fossil insectivores (Erinaceomorpha, Soricomorpha, Insectivora, Mammalia) compared to the European and global faunas. Institute of Systematic and Evolution of Animals, Polish Academy of Sciences, Kraków.
Satunin, K.A. 1895. Sorex raddei Satunin n. sp. und Melles taxus aranarius Satunin n. subsp. Zwai neue Säugethierarten aus dem Kaukasus und aus dem nuteren Wolggebiete. Archiv für Naturgeschichte, 1:1-109.
Savi, P. 1822. Osservazioni sopra il Mustietto, o Mustiolo, nuova specie di Topo ragno Toscano. Nuovo Giornale de Letterati, 1:60-71.
Schreuder, A. 1940. A revision of the fossil water-moles (Desmaninae). Archives Néerlandaises de Zoologie, 4:201-333.
Stefaniak, K., Nadachowski, A., Marciszak, A., Szynkiewicz, A., and Socha, P. 2009. Early Pleistocene fauna and sediments of Żabia Cave, p. 173-189. In Stefaniak, K., Tyc, A., and Socha, P. (eds.), Karst of the Częstochowa Upland and of the Eastern Sudetes, palaeoenvironments and protection. Faculty of Earth Sciences, University of Silesia Zoological Institute, University of Wrocław.
Storch, G., Qiu, Zh., and Zazhigin, V.S. 1998. Fossil history of shrews in Asia, p. 92-1. In Wójcik, J.M. and Wolsan, M. (eds.), Evolution of shrews. Mammal Research Institute Polish Academy of Sciences, Białowieża.
Sulimski, A. 1959. Pliocene insectivores from Węże. Acta Palaeontologica Polonica, 4:119-173.
Sulimski, A. 1962. Supplementary studies on the insectivores from Węże 1 (Poland). Acta Palaeontologica Polonica, 7:441-502.
Terzea, E. 1970. La faune de mammifères quaternaires de la grotte Magura de Sighiştel (Bihor, Roumania). Travaux de l'Institut de Spéologie "Émile Racovitza," 9:201-230.
Thomas, O. 1906. New Insectivores and Voles collected by Mr. A. Robert near Trebizond. The Annals and Magazine of Natural History, London, s. 7, 17:415-421.
Tobien, H. 1980. Taxonomic status of some Cenozoic Mammalian local faunas from the Mainz Basin. Mainzer Geowissenschaftliche Mitteilungen, 9:203-235.
Turov, S.S. 1924. Sur la faune des Vertebrés du litorale NE du lac Baikal. Comptes Rendus de l'Académie des Zcioences de Russie, A:109-112.
Yudin, B.S. 1989. Insectivorous mammals of Siberia. Nauka, Novosibirsk (in Russian).
van Cleef-Roders, J.T. and van den Hoek Ostende, L.W. 2001. Dental morphology of Talpa europaea and Talpa occidentalis (Mammalia: Insectivora) with a discussion of fossil Talpa in the Pleistocene of Europe. Zoologische Mededelingen, 75:51-67.
von Koenigswald, W. 1970. Mittelpleistozäne Kleinsäugerfauna aus der Spaltenfüllung Petersbuch bei Eichstätt. Mitteilungen der Bayerischen Staatssammlung für Paläontologie und Historische Geologie, 10:407-432.
von Zimmermann, E.A. W. 1778-1783. Geographische Geschichte des Menschen, und der allgemein verbreiteten vierfussigen Thiere, nebst einer Hieher gehorigen zoologischen Weltcharte. Geographische Geschichte des Menschen, und der vierfussigen Thiere. Zweiter Band. Enthalt ein vollstandiges. Verzeichniss aller bekannten Quadrupeden. Weygandschen Buchhandlung, Leipzig, 3.
Wolff, P. 1976. Unterscheidungsmerkmale am Unterkiefer von Erinaceus europaeus L. und Erinaceus concolor Martin. Annalen Naturhistorisches Museum Wien, 80:337-341.
Zaitsev, M.V. and Baryshnikov, G.F. 2002. Pleistocene Soricidae (Lipotyphla, Insectivora, Mammalia) from Treugolnaya Cave, Northern Caucasus, Russia. Acta zoologica cracoviensia 45:283-305.

