 Nannotanyderinae: a new subfamily of Tanyderidae (Diptera)
Nannotanyderinae: a new subfamily of Tanyderidae (Diptera)
Article number: 19.3.56A
https://doi.org/10.26879/551
Copyright Palaeontological Association, December 2016
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 17 March 2015. Acceptance: 5 December 2016
{flike id=1721}
ABSTRACT
Wing venation and male genitalia characters were examined for fossil and extant representatives of family Tanyderidae. Based on morphological evidence, it is hypothesized that two separate evolutionary lineages at the subfamily level exist, namely Tanyderinae Osten-Sacken, 1879 and Nannotanyderinae subfam. nov. Differences between them are stated. New genus and species from Eocene Baltic amber is described and illustrated.
Kornelia Skibińska. Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, Sławkowska str. 17, 31-016 Kraków, Poland. yukisiak@gmail.com
Keywords: systematics, taxonomy; Baltic amber; fossil Diptera; new subfamily; new genus; new species
Final citation: Skibińska, Kornelia. 2016. Nannotanyderinae: A new subfamily of Tanyderidae (Diptera). Palaeontologia Electronica 19.3.56A: 1-16. https://doi.org/10.26879/551
palaeo-electronica.org/content/2016/1721-nannotanyderinae-new-subfamily
http://zoobank.org/A6FB4B00-8753-4F97-9711-EE24B7E69EA2
INTRODUCTION
Tanyderidae Osten-Sacken, 1879, is a family of Diptera including 38 extant species among 10 genera (Table 1). More than half of all described species in this family are distributed in the temperate zone of the northern or southern hemisphere, others are either in the subtropical or tropical zone. The tanyderid fossil fauna includes 27 species in seven genera (Table 2), all recorded from Eurasia. Only one genus, Protanyderus Handlirsch, 1909, is represented in both the fossil and extant fauna (Alexander, 1932; Krzemiński and Judd, 1997; Exner and Craig, 1976).
 The family is morphologically distinct from other groups of Diptera, both in larval and adult life stages. Adults are small (6 mm body length) to large (30 mm length) in size, with patterned wings and frail, elongate legs (Figure 1). The wings are often sexually dimorphic in size, shape or intensity of banding pattern. The wing venation differs from other Diptera in a greater proportion of plesiomorphic characters (one anal vein reaching the wing margin; five radial veins reaching the wing margin; d-cell closed) (Krzemiński and Judd, 1997). Immature stages of extant species are aquatic to semiaquatic in lotic habitats. Microhabitats include silt and gravel in stream beds, wet, sandy soil in the marginal zone of streams or the outer layers of rotting logs submerged in streams. Adult males sometimes congregate in large swarms in the evenings; spending the daylight hours among the riparian vegetation near the borders of streams (Krzemiński et al., 2013a).
The family is morphologically distinct from other groups of Diptera, both in larval and adult life stages. Adults are small (6 mm body length) to large (30 mm length) in size, with patterned wings and frail, elongate legs (Figure 1). The wings are often sexually dimorphic in size, shape or intensity of banding pattern. The wing venation differs from other Diptera in a greater proportion of plesiomorphic characters (one anal vein reaching the wing margin; five radial veins reaching the wing margin; d-cell closed) (Krzemiński and Judd, 1997). Immature stages of extant species are aquatic to semiaquatic in lotic habitats. Microhabitats include silt and gravel in stream beds, wet, sandy soil in the marginal zone of streams or the outer layers of rotting logs submerged in streams. Adult males sometimes congregate in large swarms in the evenings; spending the daylight hours among the riparian vegetation near the borders of streams (Krzemiński et al., 2013a).
Macrochile spectrum Loew, 1850, from Upper Eocene Baltic amber, was the first described species of family Tanyderidae; however, some of the earliest described tanyderids were incorrectly placed in Ptychopteridae (Loew, 1850; Alexander, 1913). In 1879, Osten-Sacken proposed Tanyderina to include three genera: Macrochile Loew, 1850, Protoplasa Osten-Sacken, 1859 and Tanyderus Philippi, 1865. Alexander (1920) recognized Tanyderina as a family and divided it into two subfamilies, Tanyderinae and Bruchomyiinae. Subsequently, Alexander (1965) determined that Bruchomyiinae actually belonged to family Psychodidae, effectively eliminating as such the subfamilial classification in Tanyderidae.
The current work is based on an examination of many fossil tanyderids as well as representatives in the extant fauna. A new genus and species from Eocene Baltic amber is described, with particular attention given to characters of the wing and male genitalia. Some fossil and extant species can be grouped taxonomically based on similarities of these structures. Considering these characters, at the subfamily level, two evolutionary lineages in Tanyderidae are apparent; therefore, a new subfamily name is proposed here. Outcrops localities of the new subfamily are presented in Figure 2.
MATERIAL AND METHODS
 Specimens examined included the extant genera Peringueyomyina, Protoplasa, Mischoderus, Araucoderus and Protanyderus (all housed in the collection of Institute of Systematics and Evolution of Animals, Polish Academy of Sciences [ISEZ PAS]) and fossil genera Nannotanyderus (JG. 385/2B: Acra Collection; I-F/MP/2/1621/13: ISEZ PAS; LGA 1145: Museum für Naturkunde, Germany; LGA 2222: coll. J. Ansorge; No. 2066/2182 and No. 4270/2075: Palaeontological Institute, Russian Academy of Sciences, Moscow [PIN]); Dacochile (Bu 1262: the American Museum of Natural History, New York, USA); Macrochile (MP/2920 and MP/2921: ISEZ PAN); Podemacrochile (MP/2922: ISEZ PAS) and a new genus described below (MP/3376 and MP/3377: ISEZ PAS).
Specimens examined included the extant genera Peringueyomyina, Protoplasa, Mischoderus, Araucoderus and Protanyderus (all housed in the collection of Institute of Systematics and Evolution of Animals, Polish Academy of Sciences [ISEZ PAS]) and fossil genera Nannotanyderus (JG. 385/2B: Acra Collection; I-F/MP/2/1621/13: ISEZ PAS; LGA 1145: Museum für Naturkunde, Germany; LGA 2222: coll. J. Ansorge; No. 2066/2182 and No. 4270/2075: Palaeontological Institute, Russian Academy of Sciences, Moscow [PIN]); Dacochile (Bu 1262: the American Museum of Natural History, New York, USA); Macrochile (MP/2920 and MP/2921: ISEZ PAN); Podemacrochile (MP/2922: ISEZ PAS) and a new genus described below (MP/3376 and MP/3377: ISEZ PAS).
Three specimens (two males and one female) of a new genus described below from Eocene Baltic amber were examined. Two of them (both males) are well preserved, with many pertinent characters clearly visible. The specimens housed in the Natural History Museum in Kraków, Poland.
Specimens were examined with a Leica stereomicroscope (MZFLII) equipped with a camera lucida for line drawing and Leica digital camera (DFC 295). Drawings were also completed by tracing photographs. Vein nomenclature follows that of Byers (1989) and Krzemiński and Krzemińska (2003); morphological terminology is that presented in Cumming and Wood (2009).
SYSTEMATIC PALEONTOLOGY
Order DIPTERA Linnaeus, 1758
Infraorder PSYCHODOMORPHA Hennig, 1968
Family TANYDERIDAE Osten-Sacken, 1879
Subfamily TANYDERINAE Osten-Sacken, 1879
Type Genus. Macrochile Loew, 1850.
Type Species.Macrochile spectrum Loew, 1850.
Genera included. Fossil genera Praemacrochile; Podemacrochile; Macrochile; Similinannotanyderus and the following extant genera Araucoderus, Eutanyderus, Mischoderus, Neoderus, Nothoderus, Protanyderus (also in fossil fauna), Radinoderus and Tanyderus .
Differential diagnosis. Male genitalia small with short and wide gonopods; gonocoxite usually broad at base and about three or four times as long as wide; specimens medium or large size compared to those of a new subfamily described below; vein Sc markedly longer than half the wing length; wing with clearly visible coloration.
Description. Species rather large size with a wing length from 7-20 mm; wings with patterning; vein Sc longer than half the wing length; rostrum short; antennae usually with 16 flagellomeres; legs elongated; male genitalia short and wide, definitely shorter than 3/4 abdomen length.
NANNOTANYDERINAE subfam. nov.
zoobank.org/E5AABC67-E73E-400C-9007-E5C3B5BF992D
Type Genus.Nannotanyderus Ansorge, 1994.
Type Species.Nannotanyderus ansorgei Krzemiński, Azar and Skibińska, 2013a.
Genera included. Fossil genera Nannotanyderus; Dacochile; a new genus described below, and the extant genus Peringueyomyina.
 Differential diagnosis. The primary feature distinguishing the new subfamily is the unique external structure of the male genitalia. Gonopods elongate, its length when at rest being at least as long as one third the length of abdomen (Figure 3.1); male terminalia either unrotated or rotated 180 degrees (inverted like in all subfamilies of Psychodidae except Sycoracinae); species small compared to those of Tanyderinae, without elongated abdominal segments (except for extant species); wing with anal lobe reduced, inconspicuous, compared to prominent in Tanyderinae; wing membrane hyaline (without patterning).
Differential diagnosis. The primary feature distinguishing the new subfamily is the unique external structure of the male genitalia. Gonopods elongate, its length when at rest being at least as long as one third the length of abdomen (Figure 3.1); male terminalia either unrotated or rotated 180 degrees (inverted like in all subfamilies of Psychodidae except Sycoracinae); species small compared to those of Tanyderinae, without elongated abdominal segments (except for extant species); wing with anal lobe reduced, inconspicuous, compared to prominent in Tanyderinae; wing membrane hyaline (without patterning).
Description. Fossil species small in size, without elongated abdominal segments. Wings small with lengths ranging from 1.56-4 mm, only Peringueyomyina with a larger wing, approximately 10 mm in length. Wing membrane hyaline, without patterning; usually with a developed anal lobe; vein Sc short in fossil species, approximately half wing length, longer in Peringueyomyina; d-cell closed; Rs longer than R4+5. Extant species characterized by conspicuous, elongate rostrum. Antennae elongate (except in Peringueyomyina); usually with 16 articles (17 in Dacochile) with elongate, cylindrical flagellomeres. Legs elongate, each with two tibial spurs (absent in Dacochile). Male genitalia conspicuous, with strikingly elongate gonopods, their length when at rest being equal to as much as 3/4 abdomen length.
Genus NANNOTANYDERUS Ansorge, 1994
Type Species. Nannotanyderus ansorgei Krzemiński, Azar and Skibińska, 2013a.
Diagnosis. Species belonging to the genus Nannotanyderus are characterized by very small 1.56 mm body length with a wing length of 2-4 mm. Vein R2 is several times shorter than vein R2+3. Vein Sc ends at the mid length of the wing, usually before fork of Rs into R2+3 and R4+5. Anal lobe present.
Species included. Nannotanyderus oliviae; N. krzeminskii ; N. grimmenensis; N. incertus, N. kubekovensis; N. ansorgei.
Nannotanyderus oliviae Skibińska, Krzemiński and Coram, 2014
Figure 4
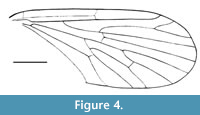 Remarks. This species is represented by two specimens preserved as wing imprints in a sedimentary rock. Wing length 2.8 mm. It is the oldest record of Tanyderidae, described from the Lower Jurassic (Sinemurian, 195 Ma; Cohen et al ., 2013) of England. Compared to other described species of Nannotanyderus, N. oliviae differs in that veins Rs, R4+5 and R4 form a nearly straight line, and vein R4+5 is shorter.
Remarks. This species is represented by two specimens preserved as wing imprints in a sedimentary rock. Wing length 2.8 mm. It is the oldest record of Tanyderidae, described from the Lower Jurassic (Sinemurian, 195 Ma; Cohen et al ., 2013) of England. Compared to other described species of Nannotanyderus, N. oliviae differs in that veins Rs, R4+5 and R4 form a nearly straight line, and vein R4+5 is shorter.
Nannotanyderus krzeminskii Ansorge, 1994
Figure 5
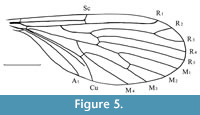 Remarks. Nannotanyderus krzeminskii was the first member of the genus described based on several isolated wings from the Lower Toarcian of Grimmen, Dobbertin and Kerhhofen, Germany. It has also been found in the Lower Toarcian of Braunschweig. The species is characterized by cross vein m-cu situated exactly in the fork of M3+4 into M3 and M4, as well as the lack of a strongly angled anal lobe.
Remarks. Nannotanyderus krzeminskii was the first member of the genus described based on several isolated wings from the Lower Toarcian of Grimmen, Dobbertin and Kerhhofen, Germany. It has also been found in the Lower Toarcian of Braunschweig. The species is characterized by cross vein m-cu situated exactly in the fork of M3+4 into M3 and M4, as well as the lack of a strongly angled anal lobe.
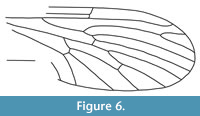
Nannotanyderus grimmenensis Ansorge and Krzemiński, 2002
Figure 6
Remarks. As for N. krzeminskii, this species is known from the Lower Toarcian of Grimmen, Germany. It is the largest species of this genus, with a wing length of approximately 4 mm. This species differs from other members of Nannotanyderus (except N. ansorgei) in that vein Sc ends apical to the fork of Rs into R4+5 and R2+3. It differs from N. ansorgei in that vein R1 ends apical to the fork of R2+3 into R 2 and R4 and in the shorter length of vein R4+5.
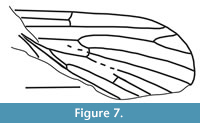
Nannotanyderus incertus Lukashevich, 2011
Figure 7
Remarks. Nannotanyderus incertus has a wing length of approximately 2 mm. Its description is based on an incomplete wing from the Upper Jurassic of Shar-Teg, Mongolia. Provisional placement of this species in Nannotanyderus was due to the small size, short vein R2, three times shorter than R2+3, and Sc as long as approximately half wing length.
Nannotanyderus kubekovensis Skibińska and Krzemiński, 2013
Figure 8
 |
 |
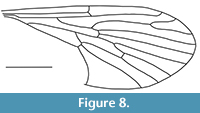 |
Remarks. The wing of N. kubekovensis is approximately 2.9 mm long. It is described from the Upper Jurassic of Karatau, Kazakhstan. Veins in the medial sector are markedly longer than other species in this genus. Moreover, vein M1 in this species is longer than the length of d-cell, and cross-vein m-cu is located behind the fork of M3+4.
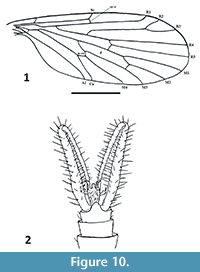
Nannotanyderus ansorgei Krzemiński, Azar and Skibińska, 2013a
Figure 9-Figure 10
Remarks. Nannotanyderus ansorgei is the smallest known tanyderid, with a wing length of 1.56 mm. It is described from Lower Cretaceous Lebanese amber and is the species for which the morphology of Nannotanyderus could first be observed in its entirety (others described prior to this were based only on wings). The male habitus of N. ansorgei is exemplary of Nannotanyderinae; thus, it was selected as type species of the subfamily. The length of the gonopod at rest in this species is approximately 3/4 the total length of the abdomen (Figure 9). In the wing, vein Sc ends apical to the fork of Rs into R4+5 and R2+3 and cross-vein r-m is placed at a level approximately 2/3 along the length of the d-cell (Figure 10.1).
 |
 |
Genus DACOCHILE Poinar and Brown, 2004
Type Species. Dacochile microsoma Poinar and Brown, 2004, monotypic genus.
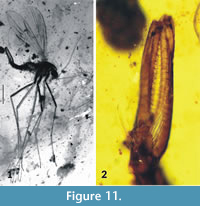
Dacochile microsoma Poinar and Brown, 2004
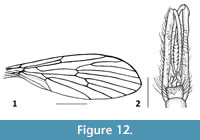
Figure 11-Figure 12
Remarks. Dacochile microsoma is described from Cretaceous Burmese amber and is among the smaller species of Nannotanyderinae; however, with a wing length of 2.8 mm, it is significantly larger than N. ansorgei. In the wing, the anal lobe is greatly reduced and vein Sc terminates at a point corresponding to one third the length of vein Rs (Figure 12.1). Woodley (2005) discussed the placement of Dacochile in Tanyderidae and stated that this genus should be transferred to Psychodidae. Despite a detailed explanation and justification for placing Dacochile in Tanyderidae, and the description of a new specimen by Poinar and Brown (2006), the taxonomic placement of this genus remained uncertain. Subsequently, Krzemiński et al. (2013a) described from Myanmar amber a male of D. microsoma showing pertinent characters that confirm its classification as a tanyderid.
 |
 |
Coramus gen. nov.
zoobank.org/DE8A2586-03C8-4275-BA57-2226027EB0A6
Etymology. The new genus name is dedicated to Dr. Robert Coram for his assistance in this research.
Differential diagnosis. Wing length 4 mm; width 1.7 mm; vein R2+3 is short, about half the length of R2; vein Sc apex at about one-third length of R2+3; vein R2 is long, about twice as long as R4+5. Coramus gen. nov. differs from Dacochile in the length of vein Sc, which in the latter ends at one-third length of Rs and in proportion of Rs to R2+3. Coramus gen. nov. differs from Nannotanyderus in having a long vein R2, about twice as long as R2+3. Male genitalia with gonopods elongate; gonocoxite cylindrical, about five times as long as wide; gonostylus bifurcate apically, with branches of bifurcation short, their length equal to half the width of the gonostylus, each with a prominent, spiniform seta inserted apically. Aedeagal complex conspicuous, aedeagus with three phallotrema.
Description. Monotypic genus. Description of genus same as for species.
Coramus gedanensis sp. nov.
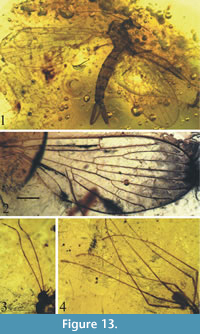
Figure 13-Figure 14
zoobank.org/81155960-C556-42C8-B299-064DCCDE537B
 Etymology. " Gedanensis " given after old name of Gdańsk -- capital of amber in Poland.
Etymology. " Gedanensis " given after old name of Gdańsk -- capital of amber in Poland.
Material. Holotype No MP/3376; Paratype No MP/3377. Both from Upper Eocene Baltic amber. They are deposited in the Institute of Systematics and Evolution of Animals, PAS, Krakow, Poland.
Description. Antennae elongate, delicate, with 16 articles, slightly shorter than the whole body length (Figure 13); scapus short and cylindrical, pedicel short and ovoid, flagellomeres one and two elongate, significantly longer than remaining flagellomeres; flagellomeres 3−14 progressively shorter; flagellomeres 10−14 short and wider than all preceding flagellomeres; mouthparts shorter than the width of head (Figure 13);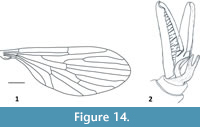 tibia with short and wide spine; palpus elongate, with apical segment longer than preceding segment; wing length 3.8 mm, width 1.7 mm; vein Sc ends almost opposite one-third length of R2+3; Rs about 1/5 longer than R2+3; R2 twice as long as R2+3; cross-vein r-m almost in a half of upper margin of d-cell length; vein m-cu situated about 1/10 length of vein M4; d-cell almost equal in length to vein M2; vein A1 strongly angled at the end; anal lobe poorly developed (Figure 14.1); terminalia well developed, with slender and elongated basistylus (0.73 mm long) and gonostylus (0.64 mm long) aedeagus with three phallotrema (Figure 13.1, Figure 14.2).
tibia with short and wide spine; palpus elongate, with apical segment longer than preceding segment; wing length 3.8 mm, width 1.7 mm; vein Sc ends almost opposite one-third length of R2+3; Rs about 1/5 longer than R2+3; R2 twice as long as R2+3; cross-vein r-m almost in a half of upper margin of d-cell length; vein m-cu situated about 1/10 length of vein M4; d-cell almost equal in length to vein M2; vein A1 strongly angled at the end; anal lobe poorly developed (Figure 14.1); terminalia well developed, with slender and elongated basistylus (0.73 mm long) and gonostylus (0.64 mm long) aedeagus with three phallotrema (Figure 13.1, Figure 14.2).
Remarks. Eocene Baltic amber is the youngest deposit in which Nannotanyderinae subfam. nov. is recorded. Wing venation and external built of hypopygium in Coramus gen. nov. is coincident for morphological scope of Nannotanyderinae subfam. nov. Coramus gen. nov. significantly differs from others genera in the wing venation (e.g., length of vein R2; ending of vein Sc). Aedegal complex is bigger than in other genera, conspicuous, aedeadus with three phallotrema. Members of Nannotanyderinae subfam. nov. were in all probability very few because despite 150 years of research, Coramus gen. nov. is the first to represent this subfamily in Baltic amber. So far two fossil genera are known Macrochile Loew, 1850 and Podemacrochile Podenas, 1997, which are classifies as subfamily Tanyderinae.
Genus PERINGUEYOMYINA Alexander, 1921
Type Species.Peringueyomyina barnardi Alexander, 1921.
Peringueyomyina barnardi Alexander, 1921
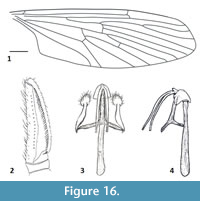
Figure 15-Figure 16
Remarks. Peringueyomyina is a monotypic genus and the only tanyderid known to occur in South Africa. With a wing length of approximately 10 mm, P. barnardi is the largest species of Nannotanyderinae. This species is unique within extant Tanyderidae in having an elongate rostrum and a hyaline wing membrane with a lesser-developed anal lobe (Figure 15). In the wing vein Sc is longer than most other species in this subfamily, ending opposite about two-thirds the length of R2+3 (Figure 16.1). As with the fossil member of Nannotanyderinae, Peringueyomyina can be characterized by elongated gonopods (Figure 15, Figure 16.2). However, in the latter, the number and arrangement of spiniform setae on the gonopods differs from any fossil species.
 |
 |
KEYS
For Keys to Subfamilies and Genera see Appendix.
DISCUSSION
Representative fossil and extant Tanyderidae were examined in this study. Differences in characters including male genitalia, wing shape and body size indicate two evolutionary lineages, in the family, treated here as subfamilies. As detailed above, the main distinguishing features between the two subfamilies are the length of wing vein Sc, shorter abdominal segments and unique structure of the male genitalia (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15, Figure 16). Nannotanyderinae subfam. nov. includes mostly fossil genera Nannotanyderus, Dacochile and Coramus gen. nov. and one extant genus, Peringueyomyina. Tanyderinae is represented by the remaining extant and fossil genera.
The earliest record of subfamily Nannotanyderinae is Nannotanyderus oliviae from the Lower Jurassic (Sinemurian, about 190 Ma) of England (Figure 4). All Jurassic species have been described only on the basis of wing venation from isolated wing imprints in sedimentary rock. Later, the description of Nannotanyderus ansorgei from Lower Cretaceous Lebanese amber (about 130-135 Ma: Maksoud et al., 2014) enabled the first observation of an intact body of a Mesozoic tanyderid. This made it possible to examine all important morphological features, particularly the male genitalia. Coramus gen. nov. from Eocene Baltic amber (aged within the range of 38-47 Ma: Ritzkowski, 1997; Perkovsky et al., 2007) has a male genitalia with an external structure comparable to that of genera Nannotanyderus, Dacochile and Peringueyomyina. The new genus, therefore, helps to bridge the temporal gap between Lower Cretaceous and extant members of the subfamily.
Tanyderidae and Psychodidae are the only extant families currently placed in suborder Diarchineura, a group hypothesized as the most ancient lineage of Diptera (Krzemiński, 1992; Krzemiński and Krzemińska, 2003). This group also includes extinct families such as Grauvogeliidae (Lower/Middle Triassic), Kuperwoodidae (Upper Triassic), Hennigmatidae (Lower Jurassic to Lower Cretaceous) and Nadipteridae (Lower/Middle Triassic to Lower Jurassic) (Krzemiński and Krzemińska, 2003; Shcherbakov et al., 1995). Recent phylogenetic studies based primarily on molecular data have found that Trichoceridae, Tipulidae, Nymphomyiidae, Deuterophlebiidae, Ptychopteridae and Blephariceridae are also among the most ancient lineages of the order (Bertone et al., 2008; Wiegmann et al., 2011). To date no phylogenetic study of Tanyderidae is existing, and its relationships within the family remain poorly understood; however, continued study of both fossil and extant species is likely to help in reconstructing the phylogeny. This will also be helpful for clarifying relationships of Tanyderidae with other families such as Psychodidae and Blephariceridae.
ACKNOWLEDGMENTS
I wish to thank W. Krzemiński for all his assistance and for all the discussions on amber and its inclusion. I am deeply indebted to G. Curler for his helpful suggestions and improvements to this manuscript and I. Madriz for lending photos. I would like to acknowledge the reviewers for their instructive comments on elder version of this paper.
REFERENCES
Alexander, C.P. 1913. Revision of the South American dipterous insects of the family Ptychopteridae. Proceedings of the United States National Museum, 44:331-335.
Alexander, C.P. 1920. A new genus and species net- winged midge (Blephariceridae) and an undescribed species of Tanyderidae (Diptera). Arkiv for Zoologi, 13(7):1-7.
Alexander, C.P. 1921. A new genus and species of Tanyderidae ( Peringueyomyina barnardi) in the South African museum (Diptera). Annals of the South African Museum, 18:231-234.
Alexander, C.P. 1928. The Tanyderidae of Australia (Diptera) . Proceedings of the Linnean Society of New South Wales, 4:367-374.
Alexander, C.P. 1929. Diptera of Patagonia and South Chile. Part I. Crane-flies (Tipulidae, Trichoceridae, Tanyderidae). The British Museum (Natural History), London.
Alexander, C.P. 1932. The Dipterous family Tanyderidae in Japan (Insecta). Annotationes Zoologicae Japonenses, 13:273-281.
Alexander, C.P. 1965. Superfamily Psychodoidea Family Tanyderidae, p. 90. In Stone, A., Sabrosky, C.W., Wirth, W.W., Foote, R.H., and Coulson, J.R. (eds.), A Catalog of the Diptera of America North of Mexico. U.S. Government Printing Office, Washington, D.C.
Ansorge, J. 1994. Tanyderidae and Psychodidae (Insecta: Diptera) from the Lower Jurassic of northeastern Germany. Paläontologisches Zeitschrift, 68:199-209.
Ansorge, J. and Krzemiński, W. 2002. Lower Jurassic tanyderids (Diptera: Tanyderidae) from Germany. Studia Dipterologica, 9:21-29.
Bertone, M.A., Courtney, G.W., and Wiegmann, B.M. 2008. Phylogenetics and temporal diversification of the earliest true flies (Insecta: Diptera) based on multiple nuclear
genes. Systematic Entomology, 33(4):668-687.
Bode, A. 1953. Die Insektenfauna des ostniedersächsischen oberen Lias. Palaeontographica A, 103:1-375.
Byers, G.W. 1989. Homologies in wing venation of primitive Diptera and Mecoptera. Proceedings of the Entomological Society of Washington, 91(4):497-501.
Cohen, K.M., Finney, S.C., Gibbard, P.L., and Fan, J.X. 2013. The ICS International Chronostratigraphic Chart. Episodes, 36(3):199-204.
Cumming, J.M. and Wood, D.M. 2009. Adult morphology and terminology, p. 9-50. In Brown, B.V., Borkent, A., Cumming, J.M., Wood, D.M., Woodley N.E., and Zumbado, M.A. (eds.), Manual of Central American Diptera. Vol.1. NRC Research Press, Ottawa.
Dong, F., Shih, C.K., and Ren, D. 2015. A new genus of Tanyderidae (Insecta: Diptera) from Myanmar amber, Upper Cretaceous. Cretaceous Research, 54:260-265.
Dong, F., Shih, C.K., Skibińska, K., Krzemiński, W., and Ren, D. 2015. New species of Tanyderidae (Diptera) from the Jiulongshan Formation of China. Alcheringa: An Australian Journal of Palaeontology, 39(4):494-507.
Exner, K. and Craig, D.A. 1976. Larvae of Alberta Tanyderidae Diptera: Nematocera. Quaestiones Entomologicae, 12:219-237.
Handlirsch, A. 1909. Zur Phylogenie und Flügelmorphologie der Ptychopteriden (Dipteren). Annalen des K. K. Naturhistorischen Hofmuseums, Wien, 23:263-272.
Hennig, W. 1968. Kritische Bemerkungen über den Bau der Flügelwurzel bei den Dipteren und die Frage nach der Monophylie der Nematocera. Stuttgarter Beiträge zur Naturkunde , 193:1-23.
Kalugina, N.S. 1988. Mesozoic Diptera: Psychodomorpha and Tipulomorpha (Tanyderidae, Eoptychopteridae, Limoniidae). Trudy Sovmestnaya Sovetsko-Mongol’skaya Paleontologicheskaya Ehkspeditsiya [New species of fossil invertebrates of Mongolia], 33:81-89. (In Russian)
Kalugina, N.S. 1992. Psychodomorphian Diptera from the Jurassic of Mongolian Altai (Diptera: Tanyderidae, Eoptychopteridae). Paleontological Journal, 26(3):110-113.
Kalugina, N.S. and Kovalev, V.G. 1985. Dvukrylienasekomye Yury Sibirii [Diptera from the Jurassic of Siberia]. Akademia Nauk, Moscow. (In Russian)
Krzemiński, W. 1992. Triassic and Lower Jurassic stage of Diptera evolution. Mitteilungen der Schweizerischen Entomologischen Gesellschaft, 65:39-59.
Krzemiński, W., Azar, D., and Skibińska, K. 2013a. Nannotanyderus ansorgei sp. n., the first member of the family Tanyderidae from Lebanese amber (Lower Cretaceous), p. 131-143. In Azar, D., Engel, M.S., Jarzembowsky, E., Krogmann, L., Nel, A., and Santiago-Blay, J. (eds.), Insect Evolution in an Amberiferous and Stone Alphabet (Proceedings of the 6 th International Congress on Fossil Insects, Arthropods and Amber). Brill, Leiden.
Krzemiński, W. and Judd, D.D. 1997. Family Tanyderidae, p. 281-289. In Papp, L. and Darvas, B. (eds.), Contributions to A Manual of Palaearctic Diptera, Vol. 2. Science Herald, Budapest.
Krzemiński, W. and Krzemińska, E. 2003. Triassic Diptera: descriptions, revisions and phylogenetic relations. Acta Zoologica Cracoviensia, 46(suppl. - Fossil Insects):153-184.
Krzemiński, W., Krzemińska, E., Kania, I., and Ross, A.J. 2013b. New Taxa of Tanyderidae (Diptera) from Eocene Baltic amber . Zootaxa, 3599(1):59-66.
Krzemiński, W. and Ren, D. 2001. Praemacrochile chinensis sp. n. from the Middle Jurassic of China (Diptera: Tanyderidae). Polish Journal of Entomology, 70:133-135.
Linnaeus, C. 1758. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis. Ed. 10. Salviae, Holmiae [= Stockholm]. (In Latin)
Loew, H. 1850. Dipterologische Beiträge. Vierter Beitrag. Die Gallmücken. [Progr]. K.Friedrich-Wilhelms-Gymnasiums Posen, (volume unknown):1-40.
Lukashevich, E.D. 2011. New Nematocerans (Insecta: Diptera) from the Late Jurassic of Mongolia. Paleontological Journal, 45(6):620-628.
Lukashevich, E.D. and Krzemiński, W. 2009. New Jurassic Tanyderidae (Diptera) from Asia with first find of larvae. Zoosymposia, 3:155-172.
Maksoud, S., Granier, B., Azar, D., Gèze, R., Paichele, J.-C., and Moreno-Bedmar, J.A. 2014. Revision of "Falaise de BLANCHE" (Lower Cretaceous) in Lebanon, with the definition of a Jezzinian Regional Stage. Carnets de Géologie, 14(18):401-427.
Osten-Sacken, C.R. 1859. New genera and species of North Ametica Tipulidae with short palpi, with an attempt at a new classification of the tribe. Proceedings of the Academy of Natural Sciences of Philadelphia, 1859:197-254.
Osten-Sacken, C.R. 1879. Die Tanyderina, eine merkwürdige Gruppe der Tipuliden. Verhandlungen der kaiserlich-koeniglichen zoologisch-botanischen Gesellschaft in Wien , 29:517-522.
Perkovsky, E.E., Rasnitsyn, A.P., Vlaskin, A.P., and Taraschuk, M.V. 2007. A comparative analysis of the Baltic and Rovno amber arthropod faunas: representative samples. African Invertebrates, 48(1):229-245.
Philippi, R.A. 1865. Aufzählung der chilenischen Dipteren. Verhandlungen der kaiserlich-koeniglichen zoologisch-botanischen Gesellschaft in Wien, 15:595-782.
Podenas, S. 1997. New Macrochile Loew, 1850 (Diptera, Tanyderidae) from the Baltic amber Mitteilungen des Geologisch Paläontologischen Instituts der Universität Hamburg , 80:173-177
Poinar, G.O. and Brown, A.E. 2004. A new genus of primitive crane flies (Diptera: Tanyderidae) in Cretaceous Burmese amber, with a summary of fossil tanyderids. Proceedings of the Entomological Society of Washington, 106(2):339-345.
Poinar, G.O. and Brown, A.E. 2006. The enigmatic Dacochile microsoma Poinar and Brown: Tanyderidae or Bruchomyiinae? Zootaxa, 1162:19-31.
Ritzkowski, S. 1997. K-Ar-Altersbestimmung der Bernsteinführenden Sedimente des Sammlandes (Paläogen, Bezirk Kaliningrad). Metalla (Sonderheft), 66:19-23.
Shcherbakov, D.E., Lukashevich, E.D., and Blagoderov, V.A. 1995. Triassic Diptera and initial radiation of the order. International Journal of Dipterological Research , 6(2):75-115.
Skibińska, K. and Krzemiński, W. 2013. Nannotanyderus kubekovensis sp. nov. (Diptera: Tanyderidae) from the Middle Jurassic of Kubekovo (Russia). Annales Zoologici, 63(3):409-412.
Skibińska, K., Krzemiński, W., and Coram, R. 2014. Discovery of the most ancient member of the family Tanyderidae (Diptera: Nematocera) from the Lower Jurassic (Sinemurian) of England. Zootaxa, 3857(1):125-130.
Soszyńska-Maj, A. and Krzemiński, W. 2013. Family Panorpodidae (Insecta, Mecoptera) from Baltic amber (Upper Eocene): new species, redescription and palaeogeographic remarks of relict scorpionflies. Zootaxa, 3636(3):489-499.
Wiegmann, B.M., Trautwein, M.D., Winkler, I.S., Barr, N.B., Kim, J.W., Lambkin, C., Bertone, M.A., Cassel, B.K., Bayless, K.M., Heimberg, A.M., Wheeler, B.M., Peterson, K.J., Pape, T., Sinclair, B.J., Skevington, J.H., Blagoderov, V., Caravas, J., Kutty, S.N., Schmidt-Ott, U., Kampmeier, G.E., Thompson, F.C., Grimaldi, D.A., Beckenbach, A.T., Courtney, G.W., Friedrich, M., Meier, R., and Yeates, D.K. 2011. Episodic radiations in the fly tree of life. Proceedings of the National Academy of Sciences (USA), 108:5690-5695.
Woodley, N.E. 2005. Dacochile microsoma Poinar & Brown, not a tanyderid but a bruchomyiine psychodid (Diptera: Psychodidae, Tanyderidae). Zootaxa, 1012:53-60.
Zhang, J.F. 2004. Nematoceran dipterans from the Jurassic of China (Insecta, Diptera: Limmoniidae, Tanyderidae). Paleontological Journal, 38(5):522-527.

