 The oldest fossil record of bandicoots (Marsupialia; Peramelemorphia) from the late Oligocene of Australia
The oldest fossil record of bandicoots (Marsupialia; Peramelemorphia) from the late Oligocene of Australia
Article number: 16.2.13A
https://doi.org/10.26879/363
Copyright Palaeontological Association, May 2013
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 6 November 2012. Acceptance: 30 January 2013
{flike id=400}
ABSTRACT
Two new late Oligocene representatives of the marsupial order Peramelemorphia (bandicoots and bilbies) from the Etadunna Formation of South Australia are described here. Bulungu muirheadae sp. nov., from Zone B (Ditjimanka Local Fauna [LF]), is represented by several dentaries and isolated upper and lower molars. Bulungu campbelli sp. nov., from Zone C (Ngapakaldi LF), is represented by a single dentary and maxilla. Together, they represent the oldest fossil bandicoots described to date. Both are small (estimated body mass of <250 grams) in comparison to most living bandicoot species and were probably insectivorous based on their dental morphology. They appear to be congeneric with Bulungu palara from Miocene local faunas of the Riversleigh World Heritage Area (WHA), Queensland and the Kutjamarpu LF (Wipajiri Formation) of South Australia. However, the Zone B peramelemorphian appears to be more plesiomorphic than B. palara in its retention of complete centrocristae on all upper molars.
K. J. Travouillon. School of Earth Sciences, University of Queensland, St. Lucia, Queensland 4072, Australia
and School of Biological, Earth and Environmental Sciences, University of New South Wales, New South Wales 2052, Australia
R.M.D. Beck. School of Biological, Earth and Environmental Sciences, University of New South Wales, New South Wales 2052, Australia
S.J. Hand. School of Biological, Earth and Environmental Sciences, University of New South Wales, New South Wales 2052, Australia
M. Archer. School of Biological, Earth and Environmental Sciences, University of New South Wales, New South Wales 2052, Australia
Keywords: Peramelemorphian; Oligocene; South Australia; bandicoot; new species; marsupials
Final citation: Travouillon, K.J., Beck, R.M.D., Hand, S.J., and Archer M. 2013. The oldest fossil record of bandicoots (Marsupialia; Peramelemorphia) from the late Oligocene of Australia, Palaeontologia Electronica Vol. 16, Issue 2; 13A 52p. https://doi.org/10.26879/363
palaeo-electronica.org/content/2013/400-late-oligocene-bandicoot
http://zoobank.org/A47937B6-444F-4BF1-B66A-0ECDE2E6BC27
INTRODUCTION
The fossil record of bandicoots and bilbies (Marsupialia: Peramelemorphia) remains relatively poorly known, with only 13 species described to date (excluding Quaternary records of extant species) from sites spanning the late Oligocene to Pleistocene. The only extinct species to have been described from the Pleistocene is Perameles sobbei from the Darling Downs, Queensland (Price, 2002; 2005). Five Pliocene peramelemorphians have been described including Perameles allinghamensis from the Bluff Downs Local Fauna (LF), Queensland (Archer and Wade, 1976), Perameles bowensis from the Bow and Wellington Caves LFs, New South Wales (Muirhead et al., 1997), cf. Peroryctes tedfordi and cf. Peroryctes sp. from the Hamilton LF, Victoria (Turnbull et al., 2003) and Ischnodon australis, from the Palankarinna LF, South Australia (Stirton, 1955). The bizarre, dentally autapomorphic Numbigilga ernielundeliusi from the Bluff Downs LF (Beck et al., 2008a) may represent a sixth Pliocene peramelemorphian species.
Ongoing research on fossil peramelemorphians from late Oligocene and early Miocene sites (Muirhead, 1994, 2000; Muirhead and Filan, 1995; Case, 2001; Schwartz, 2006; Travouillon et al., 2010, 2013; Gurovich et al., 2013), particularly relatively complete specimens from the Riversleigh World Heritage Area (WHA), has led to increasingly detailed knowledge of the evolutionary history of Peramelemorphia during this key period. Six species have been described from the Miocene, namely Yarala burchfieldi (Muirhead and Filan, 1995; Muirhead, 2000), Galadi speciosus (Travouillon et al., 2010), G. adversus, G. grandis, G. amplus (Travouillon et al., 2013) from Riversleigh LFs, and Bulungu palara (Gurovich et al., 2013) from multiple LFs at Riversleigh WHA and the Kutjamarpu LF, South Australia. Yarala kida (Schwartz, 2006) from the late Oligocene or early Miocene Kangaroo Well LF (see Metzger and Retallack, 2010) is currently the oldest described peramelemorphian. However, Woodburne et al. (1993) and Case (2001) reported a number of peramelemorphian species from the Etadunna and Wipajiri Formations of South Australia, some of which may predate the Kangaroo Well LF (see also Woodburne and Case, 1996, 144, 150-151).
Here we describe two new peramelemorphian species from two of the oldest faunal zones of the Etadunna Formation, the Ditjimanka LF (Faunal Zone B) and Ngapakaldi LF (Faunal Zone C). We assess the phylogenetic relationships of these to other peramelemorphians to shed light on the early evolutionary history of the order.
MATERIALS AND METHODS
Collecting and Processing
Specimens described here were collected from Tedford Locality (Site 2), site code RV 7230, Lake Palankarinna, Etadunna Formation, Ditjimanka LF, Zone B and Ngapakaldi Quarry, site code V 5858, Lake Ngapakaldi, Etadunna Formation, Ngapakaldi LF, Zone C, by Stirton, Tedford, Woodburne, Archer and co-workers between 1957 and 1972. All specimens collected after 1970 were recovered by screen-washing in the field (Campbell, 1976).
Anatomical Terminology
Dental terminology follows Archer (1976), Muirhead and Filan (1995), Travouillon et al. (2010) and Turnbull et al. (2003), with molar loci homology following Luckett (1993). Peramelemorphian systematics follows Muirhead (1994, 2000), Muirhead and Filan (1995) and Travouillon et al. (2010). Higher-level marsupial systematics follow the classification of Aplin and Archer (1987).
Institutional Abbreviations
QM F Queensland Museum Fossil collection, Brisbane, Australia; SAM P South Australian Museum, Palaeontology Collection, Adelaide, Australia; UCMP University of California, Museum of Paleontology, Berkeley, USA; UCR University of California, Riverside, USA.
Body Mass Estimates
The maximum length and width of each tooth on each specimen were measured (see Table 1 and Table 2). These measurements were used to estimate individual body mass of each specimen, using Myers' (2001) allometric relationships between dental measurements and body mass for predicting marsupial body mass. We used the highest ranked equation from Myers (2001) in the "all species excluding dasyuromorphians" dataset, which is the most suitable dataset for bandicoots. For example, when more than one tooth was present in a specimen, we used the highest ranked equation on the tooth row (e.g., 4LMA > 3LMA > 2LMA > 1LMA). All body mass estimates are shown in grams in Table 1 and Table 2. Average body mass for the species was calculated from these estimates.
Phylogenetic Analysis
Phylogenetic relationships within Peramelemorphia were assessed using a revised version of the matrix used by Travouillon et al. (2010), Travouillon et al. (2013), and Gurovich et al. (2013), comprising 156 qualitative morphological characters, of which 123 are dental and 33 cranial (see Appendix 1 and Appendix 2). To help resolve relationships among fossil peramelemorphians, most of which are known only from isolated dental remains, we have added 96 new dental and cranial characters relative to previous iterations of this matrix. Some characters from the original matrix were modified by adding, removing, or revising ambiguous character states, and some characters were deleted to be replaced by more comprehensive characters (see Appendix 1). Seventy-one characters represent putative morphocline/transformation series (see Appendix 1) and so were ordered in all analyses. All taxa were rescored for this study, to confirm previous scoring decisions and to correct errors present in previous versions of the matrix (e.g., we revised a number of characters scorings for Ischnodon australis). We used the same ingroup peramelemorphian species used by Travouillon et al. (2013) and Gurovich et al. (2013), except as follows. We deleted Microperoryctes ornata from the matrix because the specimens from the Australian Museum that were previously (ibid) used to score this taxon have subsequently been reassigned by museum staff to Microperoryctes longicauda. We have also scored and added all fossil bandicoots described to date to the matrix (cf. Peroryctes tedfordi, cf. Peroryctes sp., Perameles sobbei, Perameles bowensis, Perameles allinghamensis, Ischnodon australis, Yarala burchfieldi, Yarala kida, Galadi speciosus, Galadi grandis, Galadi amplus, Galadi adversus and Bulungu palara). Numbigilga ernielundeliusi has not been included, due to its highly autapomorphic dental morphology and questionable peramelemorphian affinities. In addition to the outgroup taxa used by Travouillon et al. (2013) and Gurovich et al. (2013) – namely the fossil dasyuromorphians Barinya wangala and Mutpuracinus archibaldi and the early Eocene stem-australidelphan Djarthia murgonensis, we also scored five new extant outgroup species from the family Dasyuridae: the dasyurines Dasyurus hallucatus, Dasyuroides byrnei, Phascogale tapoatafa and Antechinus stuartii,and the sminthopsine Sminthopsis macroura.
Unconstrained Maximum Parsimony Analysis
Unconstrained maximum parsimony analysis of the matrix was performed using PAUP* 4.0b10 (Swofford, 2002). Following Worthy et al. (2006) and Beck et al. (2008b), a two-stage search strategy was used with an initial search comprising 1,000 heuristic replicates, saving .10 trees per replicate, followed by a second heuristic search within these saved trees. The multiple most parsimonious trees produced were summarised using strict consensus. Bootstrap values for each node in the strict consensus were calculated using 1000 bootstrap replicates, each of which itself comprises 10 random addition replicates. Decay indices were also calculated using AutoDecay 5.0 (Eriksson, 2001) in combination with the perl script perlRat, which implements the parsimony ratchet; the default settings for perlRat (five batches of 200 replicates, randomly upweighting 25% of characters by a factor of two) were used to implement parsimony ratchet searches to calculate decay indices for all nodes in the strict consensus.
Molecular Scaffold Parsimony Analysis
For this analysis, the matrix was analysed using the same methods as for the unconstrained maximum parsimony analysis, but with a "molecular scaffold" as a "backbone" constraint. For the molecular scaffold, relationships among extant peramelemorphians were constrained to match the recent molecular phylogeny of Westerman et al. (2012); only those peramelemorphian taxa analysed by Westerman et al. (2012) were included in the molecular scaffold, and only those clades that received ?70% partitioned maximum likelihood bootstrap support and ?0.95 partitioned Bayesian posterior probability in the analyses of Westerman et al. (2012, table 2) were enforced as monophyletic. The five extant dasyurid outgroup taxa were also included in the molecular scaffold, with their relationships constrained to match those recovered by Krajewski et al. (2007, figure 5) and Westerman et al. (2008, figure 2). As in the unconstrained maximum parsimony analysis, bootstrap support values were calculated for all nodes present in the strict consensus of most parsimonious trees and using 1000 bootstrap replicates of 10 random addition sequence replicates each, but enforcing the molecular scaffold. The molecular scaffold topology is included in the supplementary information accompanying this paper (Appendix 3).
Unconstrained Bayesian Analysis
Bayesian analysis of the matrix using the Lewis (2001) "Mk" model for discrete morphological characters was implemented with MrBayes 3.2. Because the matrix included autapomorphies, the analysis assumed that variable (rather than purely parsimony-informative) characters were scored ("coding=var"). The analysis was run for 5 million generations but with otherwise default settings. The first 1.25 million generations (i.e., 25%) were discarded as burn-in. The potential scale reduction factor (PSRF) and minimum and average effective sample sizes (ESS) for both the tree length and alpha parameters were 1.0 and >5000, respectively, for the post-burn-in trees, indicating that stationarity and convergence had been achieved. The post-burn-in trees were summarised using 50% majority rule consensus, with Bayesian posterior probabilities as support values.
Molecular Scaffold Bayesian Analysis
The Bayesian analysis was repeated enforcing the same molecular scaffold as for the molecular scaffold parsimony analysis. The molecular scaffold was implemented using multiple "partial" constraints, with each constraint corresponding to a node in the molecular scaffold. All other settings were the same as for the unconstrained Bayesian analysis. The potential scale reduction factor (PSRF) and minimum and average effective sample sizes (ESS) for both the tree length and alpha parameters were 1.0 and >5000, respectively, for the post-burn-in trees, indicating that stationarity and convergence had been achieved.
SYSTEMATIC PALEONTOLOGY
Order: PERAMELEMORPHIA (Kirsch, 1968) Aplin and Archer, 1987
Superfamily: Incertae sedis
Family: Incertae sedis
Genus: BULUNGU Gurovich et al., 2013
Type Species. Bulungu palara Gurovich et al., 2013
Revised Generic Diagnosis
The species of Bulungu are small peramelemorphians (estimated body mass of ~110-210 grams) that differ from all others in the following combination of features: M3 preprotocrista terminates at the lingual base of paracone, resulting in an incomplete anterior cingulum on M3; posterior cingulum absent on M3; stylar cusp B (StB) on M3 large and oval with a crest present, but this crest does not connect to StD; StC present as a small cusp on M3 but StD1 is absent; metaconule small and never as lingually positioned as protocone (hence molars subtriangular rather than quadrilateral in occlusal outline); preentocristid anteroposteriorly-orientated on m1-4; no cuspids exist within hypoflexid region between talonid and trigonid on buccal side but a small shelf is present on m1-4; cristid obliqua contacts trigonid buccal to protoconid on m1, just lingual to protoconid on m3 and lingual to midpoint of metacristid on m4; paraconid-metaconid distance shorter than metaconid-protoconid distance in m2-3 but longer in m4; entoconid large on m4; posthypocristid oblique to tooth row axis on m2 but perpendicular on m3; talonid reduced on m4; molar crowns are distinct from roots.
The following combination of features seen in Bulungu palara may also be generically diagnostic, but is as yet unknown for other species in this genus (as they are currently known only from dental specimens): small canines; nasals extend posteriorly past anterior margin of orbit; two pairs of large palatal fenestrae present in palate posterior to incisive foramina; alisphenoid and parietal in contact on lateral wall of braincase (squamosal-frontal contact absent); sphenorbital fissure and foramen rotundum elongate and tube-like, not opening directly into endocranial cavity; primary foramen ovale very large and between petrosal and alisphenoid; bulbous alisphenoid tympanic wings cover anterolateral and medial sides of auditory recess; rostral tympanic process of petrosal an elongate ridge extending length of promontorium but without forming a hypotympanic sinus; epitympanic recess relatively shallow; an arch is formed by paroccipital and mastoid processes, through which is exposed part of the petrosal immediately posterior to fenestra cochleae; rim of postglenoid foramen formed almost exclusively by squamosal with only small contribution from petrosal; in posterior view, occiput is wider than high.
BULUNGU MUIRHEADAE, sp. nov.
Figure 1–Figure 2
http://zoobank.org/F7059F8F-F295-4AD2-9084-54874CF57076
 Specific Diagnosis
Specific Diagnosis
Bulungu muirheadae differs from Bulungu palara in the following combination of features: postparacrista and premetacrista contact each other, forming a complete centrocrista on M1-3; StC large on M1 (sometimes larger than StB); StD1 larger and distinct on M1 and M2; metaconule smaller and positioned more buccally on M1-3; preparacrista connects to StB on M2, but ends at the base of StA on M3; StE is a distinct cusp on M3; anterior cingulid present on m1; cristid obliqua terminates posterior to protoconid on m2; short postentocristid present on m4, and runs anteroposteriorly; posthypocristid is oblique to the tooth row axis on m4; hypoconulid present as small cusp on m4.
Specific Etymology
The species name honours vertebrate palaeontologist Jeanette Muirhead for her contribution to our understanding of fossil bandicoot taxonomy and evolution.
 Holotype
Holotype
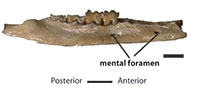
QM F10666, left dentary with m1-3 (Figure 1).
Paratypes
QM F10668, left dentary fragment with m4 (Figure 1); QM F10669, right dentary fragment with m1-2 (Figure 1); UCR 15307, LM1 (Figure 2); QM F10664, LM2 (Figure 2); and UCR 15275, RM3 (Figure 2).
Referred Material
QM F10661, Rm1; QM F10662, RM3; QM F10665, RM3; QM F10667, right dentary with m2-3 and trigonid of m4; QM F12414, Rm3; QM F12415, Lm3; QM F12416, RM1; QM F12417, LM3; QM F12418, Lm3; QM F12419, Lm1; QM F12420, RM2; UCMP 108063, left maxilla fragment with M3; UCR 15266, Rm4; UCR 15268, Rm1; UCR 15270, LM3; UCR 15279, Lm2; UCR 15285, LM3; UCR 15299, RM2; UCR 15301, Lm2; UCR 15306, Rm3; UCR 15318, Lm2; UCR 15332, RM2; UCR 16996, LM1; UCR 17002, Lm3.
Type Locality
Tedford Locality (Site 2), site code RV 7230, Lake Palankarinna, Etadunna Formation, Ditjimanka LF, Zone B.
Age and Stratigraphy
The Ditjimanka LF lies within Etadunna Faunal Zone B. Based on palaeomagnetic and drill-core data, Metzger and Retallack (2010) estimated the Etadunna Formation to span 26.1 to 23.6 m.y.a. The Ditjimanka LF is therefore late Oligocene in age.
DESCRIPTION
Dentary
Alveolar margin of the dentary increases in height from p1 to p3, decreases below m1, and then increases again from m2 to m4 (Figure 1). Depth of ramus greatest below m4. Two short subequal diastemata present: first between the alveoli for c1 and p3; second between alveoli for p1 and p2. The symphysis extends as far as posterior alveolus for p2. Two mental foramina present. Anterior of these two is below diastema between alveoli for p1 and p2; posterior foramen is below anterior alveolus for m1. Coronoid process forms an angle with body of dentary of approximately 120 degrees.
Lower Dentition
The description of p3 and m1 to m3 is based on QM F10666; that of m4 is based on QM F10668 (Figure 1). Only posterior part of p3 is preserved. The posterior cusp is low and obscured by paraconid of m1. Posterior width of p3 narrower than trigonid of m1.
Protoconid and metaconid of m1 closer together than either is to paraconid. Protoconid tallest cusp on crown, followed by entoconid, metaconid, paraconid, hypoconid, and hypoconulid. A thin anterior cingulid extends from buccal flank of paraconid to anterobuccal flank of protoconid. Paraconid anterobuccally positioned relative to metaconid. Metaconid posterolingually positioned with respect to protoconid. Talonid wider than trigonid. Entoconid is oval in shape, with short preentocristid connecting it to posterior flank of metaconid. Small cingulid-like hypoconulid, posterobuccal to entoconid, connects to hypoconid via posthypocristid. Almost linear cristid obliqua extends from hypoconid to posterobuccal flank of protoconid. Very small low ridge occurs posterobuccal to protoconid. Posterior cingulid absent.
Morphology of m2 similar to that of m1 except as follows. Paraconid more lingually positioned, almost directly anterior to metaconid. Protoconid more buccally-positioned increasing length of paracristid and metacristid. Distance between paraconid and metaconid relatively shorter. Anterior cingulid larger, extending further anterobuccally. Talonid wider with hypoconid positioned further anterobuccally, resulting in a longer posthypocristid, but shorter cristid obliqua. Angle formed by posthypocristid and cristid obliqua at hypoconid smaller. Cristid obliqua terminates more lingually, directly posterior to apex of protoconid. Entoconid is wider, almost conical, with short postentocristid running perpendicular to preentocristid. Postentocristid increases in length with wear.
Morphology of m3 similar to that of m2 except as follows. Paraconid-metaconid distance shorter. Talonid shorter, with hypoconid positioned further anteriorly, decreasing length of cristid obliqua. Entoconid shorter in length, almost completely conical, despite retaining preentocristid.
Morphology of m4 similar to that of m3 except as follows. Distance between paraconid and metaconid greater, almost equal to paraconid-protoconid distance and metaconid-protoconid distance. Talonid reduced, with hypoconid positioned posterolingual to protoconid. Hypoconulid small. Cristid obliqua longer than in m3 and terminates against trigonid posterior flank, level with midpoint between protoconid and metaconid. Angle between cristid obliqua and posthypocristid relatively wider. Entoconid small but taller than hypoconid. Well-defined preentocristid extends anteriorly from entoconid and ends against posterior flank of metaconid. A short postentocristid extends from tip of entoconid posteriorly to position just anterior to hypoconulid.
Upper Dentition
The description of M1 is based on UCR 15307 (Figure 2). It is anteroposteriorly longer than buccolingually wide. StD tallest cusp on crown. Small cusp (StD1, sensu Turnbull et al., 2003) immediately anterior to StD. Large StC very slightly smaller than StD and positioned anterior to StD1, immediately anterior to ectoflexus. A crest connects StD1 to StC through the ectoflexus and connects anteriorly to StB. Paracone directly lingual to StB; preparacrista connects these two cusps. Postparacrista longer than preparacrista and connects to premetacrista just lingual to ectoflexus. Premetacrista longer than postparacrista, but shorter than postmetacrista. Metacone directly lingual to StD. Distance between these two cusps twice as long as distance between StB and paracone. Postmetacrista ends at most posterobuccal end of tooth and connects to stylar crest, which connects StD to metastyle via small remnant of StE. Anteriorly to StD, this crest does not connect to StD1 but instead, curves lingually. StA/parastyle just anterior to StB with small crest present running anteroposteriorly from most anterior part of tooth; this small crest ends prior to StB. Small anterior cingulum occurs lingual to StA, ending at a point just anterobuccal to paracone. Preprotocrista connects protocone to anterolingual base of paracone. Postprotocrista connects to small metaconule just lingual to base of metacone and ends posterolingual to latter. No posterior cingulum.
The description of M2 is based on QM F10664 (Figure 2). Morphology of M2 similar to that of M1except as follows. Crown wider, with protocone more lingually situated. Metaconule smaller and more buccally situated. StA taller. Larger, more anteriorly and more lingually situated anterior cingulum. Paracone more posterolingually situated, increasing length of preparacrista and reducing length of postparacrista. StB much taller, almost as tall as StD and more posteriorly situated, increasing distance between it and StA. Connection between preparacrista and StB weaker, with anterior part of StB more rounded. StC highly reduced and as tall as StD1. StE reduced, almost indistinguishable along stylar crest between StD and metastyle.
The description of M3 is based on UCR 15275 (Figure 2). Morphology of M3 similar to that of M2 except as follows. Crown wider than long, with protocone more lingually situated. Anterior cingulum larger and more anteriorly extensive. Crest running through StA anteroposteriorly connects to anterior flank of StB. Preparacrista runs toward midpoint of StA's crest before curving anteriorly parallel to this crest, ending below StA. Angle between preparacrista and postparacrista wider. StC minute, barely visible above crest connecting StB to StD. StD1 absent. StE distinct, taller cusp. StE and metastyle more buccally situated, elongating postmetacrista. No stylar crest runs between StD and StE.
Measurements of upper and lower dentitions presented in Table 1 and Table 2. Average body mass estimated to 135.58 grams.
BULUNGU CAMPBELLI, sp. nov.
Figure 3
http://zoobank.org/15133E7C-0456-46F3-8DE0-A2CF72C6A006
 Specific Diagnosis
Specific Diagnosis
Bulungu campbelli differs from B. palara and B. muirheadae in the following combination of features: StA and StB connected by a crest on M3; StE absent on M3; postparacrista and premetacrista not connected to form a complete centrocrista on M3, but instead postparacrista ends on lingual flank of StC, and premetacrista ends on lingual flank of StD; metaconule on M3 minute and situated on lingual flank of metacone; metacristid perpendicular to tooth row on m3 only; postentocristid present; talonid slightly longer anteroposteriorly than trigonid on m3.
Specific Etymology
The species name honours vertebrate palaeontologist Colin Campbell for his significant contributions to peramelemorphian systematics and in particular the preliminary analysis in his PhD thesis (Campbell, 1976) of specimens referable to the taxa named and described here.
Holotype
SAM P13853, right dentary with m1-4 (Figure 3) and associated right maxilla fragment with M3 and partial M4 (Figure 3).
Type Locality
Ngapakaldi Quarry, site code V 5858, Lake Ngapakaldi, Etadunna Formation, Ngapakaldi LF, Zone C.
Age and Stratigraphy
The Ngapakaldi LF, (Etadunna Zone C) was regarded by Woodburne et al. (1993) to be late Oligocene in age, between 24-26 m.y.a. Based on palaeomagnetic and drill-core data, Metzger and Retallack (2010) estimated the Etadunna Formation to span from 26.1 to 23.6 m.y.a. The Ngapakaldi LF is therefore late Oligocene in age, but younger than the underlying Ditjimanka LF (Etadunna Zone B).
DESCRIPTION
Dentary
Dentary poorly preserved, missing anterior section from the anterior root of m1 (Figure 3). Posterior part of dentary also missing. Preserved base of coronoid process forms an angle of 120 degrees with the ramus. Dentary is deepest below m3.
Lower Dentition
Only molars preserved. The m1 (originally intact in the dentary but now loose) roots are preserved in the alveoli. Anterior root smaller and rounder than posterior root (Figure 3). All cusps except hypoconid damaged at tips. Protoconid tallest cusp followed (in decreasing order) by metaconid, entoconid, paraconid, hypoconid, and hypoconulid (Figure 3). Narrow anterior cingulid damaged on anterolingual side of tooth. Very little of paraconid preserved. In occlusal view, paraconid anterolingual to protoconid and anterobuccal to metaconid. Metaconid posterolingual to protoconid; hence, metacristid not perpendicular to tooth row. Hypoconid positioned posterobuccal to protoconid. Cristid obliqua departs obliquely from tip of hypoconid and terminates on posterior flank of protoconid. Hypoflexid present as a small shelf between buccal flank of protoconid and anterior flank of hypoconid. Steeply inclined posthypocristid connects hypoconid to hypoconulid. Very little of hypoconulid preserved but would have been directly posterior to entoconid. Entoconid directly posterior to metaconid. Entoconid narrow, with preentocristid visible on undamaged anterior surface; preentocristid terminates within valley separating entoconid and metaconid. Talonid wider than trigonid. No posterior cingulid.
The morphology of m2 resembles that of m1 except as follows. Anterior cingulid of m2 extends from base of paraconid, perpendicular to tooth row, then curves posterobuccally toward base of protoconid (Figure 3). Trigonid narrow, with metaconid and paraconid closer to each other than either is to protoconid. Distance between paraconid and protoconid greater than distance between metaconid and protoconid. Entoconid highly worn or damaged. Cristid obliqua terminates below centre of metacristid.
The morphology of m3 resembles that of m2 except as follows (Figure 3). Paraconid and metaconid closer together, narrowing trigonid. Metacristid perpendicular to tooth row and parallel to posthypocristid. Metaconid tallest cusp, followed by entoconid, protoconid, paraconid, hypoconulid and hypoconid. Preentocristid connects entoconid to posterior base of metaconid. Entoconid wider posteriorly than anteriorly, almost teardrop-shaped. Short crest (postentocristid) runs at an angle buccally, almost perpendicular to tooth row, and ends near base of entoconid. Trigonid and talonid wider but shorter in length than on m2.
The morphology of m4 resembles that of m3 except as follows (Figure 3). Distances between paraconid and protoconid, and metaconid and protoconid all shorter. Angle formed by trigonid blades wider. Metaconid remains tallest cusp but followed by protoconid, paraconid, entoconid, hypoconid, and hypoconulid. Talonid reduced. Hypoconid more lingually situated, almost level with middle of metacristid. Very small hypoconulid posterobuccal to entoconid. These two cusps connected by postentocristid. Cristid obliqua runs anteriorly from the posterior flank of the trigonid, just buccal to the anterior tip of preentocristid and ends posteriorly at the tip of hypoconid, joining with posthypocristid at 120 degree angle. Posthypocristid ends at posterior extremity of tooth.
Upper Dentition
The maxilla (Figure 3) is poorly preserved. Only the alveoli for M2, the M3, and the anterior tip of M4 are preserved.
M3 is wider than long (Figure 3). Preparacrista extends from paracone in a buccal direction then curves anteriorly to join StA. A stylar crest extends from tip of StA posteriorly, then curves lingually before curving again at the posterior flank of StA buccally to connect to tip of StB. This crest then continues posteriorly through a minute StC and ends prior to reaching the ectoflexus region. StD1 absent. No stylar crest connects StD to metastyle. StE absent. Postparacrista straight, obliquely orientated and ends at base of posterolingual flank of StC. Postparacrista does not join premetacrista, and hence the centrocrista is incomplete. Premetacrista ends at anterolingual base of flank of StD. Postmetacrista longest crest and runs from metacone to metastyle. Anterior cingulum is wide and runs lingually from lingual flank below StA to almost level with middle of preparacrista. Protocone posterolingual to paracone. Preprotocrista runs parallel to preparacrista from anterior flank of paracone to protocone, and forms an angle of almost 90 degrees with postprotocrista. Postprotocrista then curves posteriorly almost parallel to tooth row and joins small metaconule, before ending on lingual flank of metacone. Metaconule directly lingual to metacone. Posterior cingulum absent.
Very little of M4 is preserved (Figure 3). What can be seen resembles the anterior part of M3 but differs as follows. StA large and tall. Preparacrista runs from paracone to StA, parallel to preparacrista of M3. Stylar crest runs obliquely from StA to the broken edge of the tooth. Morphology of anterior cingulum same as in M3.
Measurements of the upper dentition of B. campbelli are presented in Table 1 and of the lower dentition in Table 2. Average body mass estimated to 112.87 grams.
RESULTS
 Unconstrained maximum parsimony analysis of our 156 craniodental character matrix recovered 8 most parsimonious trees of 889 steps; the strict consensus of these is illustrated in Figure 4.1, with bootstrap values above the branches and decay indices below. Parsimony analysis of the same matrix but enforcing a molecular scaffold as a "backbone" constraint recovered 24 most parsimonious trees of 917 steps, the strict consensus of which is given in Figure 4.2, with bootstrap values given above the branches. Unconstrained Bayesian analysis of the same matrix resulted in a post-burn-in log likelihood (harmonic mean) of -2898.10, whereas the post-burn-in log likelihood for the Bayesian analysis with the molecular scaffold enforced was -2933.45; the 50% majority rule consensuses from the unconstrained and molecular scaffold Bayesian analyses are given in Figures 5.1 and 5.2, respectively, with Bayesian posterior probabilities given at the nodes.
Unconstrained maximum parsimony analysis of our 156 craniodental character matrix recovered 8 most parsimonious trees of 889 steps; the strict consensus of these is illustrated in Figure 4.1, with bootstrap values above the branches and decay indices below. Parsimony analysis of the same matrix but enforcing a molecular scaffold as a "backbone" constraint recovered 24 most parsimonious trees of 917 steps, the strict consensus of which is given in Figure 4.2, with bootstrap values given above the branches. Unconstrained Bayesian analysis of the same matrix resulted in a post-burn-in log likelihood (harmonic mean) of -2898.10, whereas the post-burn-in log likelihood for the Bayesian analysis with the molecular scaffold enforced was -2933.45; the 50% majority rule consensuses from the unconstrained and molecular scaffold Bayesian analyses are given in Figures 5.1 and 5.2, respectively, with Bayesian posterior probabilities given at the nodes.
In all four phylogenetic analyses presented here, Bulungu campbelli and B. muirheadae lie outside crown-group Peramelemorphia. Monophyly of Bulungu is recovered in the unconstrained maximum parsimony analysis, but with weak support (bootstrap <50%; decay index +1; Figure 4.1). However, the relationships of the three Bulungu species are unresolved in the unconstrained Bayesian analysis (Figure 5.1), and B. palara and B. campbelli are recovered as closer to the crown-group than is B. muirheadae in both parsimony and Bayesian analyses when a molecular scaffold is enforced (Figure 4.2,Figure 5.2). The other Oligo-Miocene bandicoot taxa described to date, namely Yarala burchfieldi, Y. kida and the four species of Galadi also lie outside crown-group Peramelemorphia in all four analyses, in agreement with the results of previous phylogenetic analyses (Travouillon et al., 2010; 2013; Gurovich et al., 2013). Monophyly of Yarala and Galadi is also relatively strongly supported in all four analyses. However, the relative branching order of Bulungu, Yarala and Galadi varies between the analyses, with Bulungu closer to the crown-group in the unconstrained parsimony and Bayesian analyses but more distant when a molecular scaffold was enforced.
Another notable feature of our analyses is the fact that the two bandicoot taxa described by Turnbull et al. (2003) from the early Pliocene Hamilton Fauna, namely cf. Peroryctes tedfordi and cf. Peroryctes sp., consistently fall outside the crown-group. In the unconstrained maximum parsimony analysis, these taxa form a clade with Bulungu, although this is not the case in the other three analyses. Based on these results, we believe that referral of these Pliocene taxa to Peroryctes is unjustified and that they are more likely late-surviving stem-peramelemorphians; if so, all known records of Peroryctes are from New Guinea, and crown-group peramelemorphians do not appear to be present in the Hamilton LF.
 However, in contrast to the results of a previous study (Gurovich et al., 2013), analyses of our revised and expanded craniodental matrix consistently place the Pliocene Ischnodon australis in a clade with the Recent thylacomyids Macrotis lagotis and M. leucura with moderate-to-strong support, supporting the referral of Ischnodon to Thylacomyidae. The other Pliocene peramelemorphians, namely Peramelesalllinghamensis and P. bowensis, and the Pleistocene P. sobbei also consistently fall within the crown-group, and in all but the unconstrained Bayesian analysis form a clade with Recent Perameles and Isoodon species (albeit with weak support); thus, our results confirm that P. allinghamensis and P. bowensis represent the oldest known records of the family Peramelidae (Peramelinae sensu Wilson and Reeder, 2005).
However, in contrast to the results of a previous study (Gurovich et al., 2013), analyses of our revised and expanded craniodental matrix consistently place the Pliocene Ischnodon australis in a clade with the Recent thylacomyids Macrotis lagotis and M. leucura with moderate-to-strong support, supporting the referral of Ischnodon to Thylacomyidae. The other Pliocene peramelemorphians, namely Peramelesalllinghamensis and P. bowensis, and the Pleistocene P. sobbei also consistently fall within the crown-group, and in all but the unconstrained Bayesian analysis form a clade with Recent Perameles and Isoodon species (albeit with weak support); thus, our results confirm that P. allinghamensis and P. bowensis represent the oldest known records of the family Peramelidae (Peramelinae sensu Wilson and Reeder, 2005).
Our unconstrained maximum parsimony analysis shows greater congruence with current molecular phylogenies than do previous morphological analyses: monophyly of Peramelidae (i.e., Perameles + Isoodon; = Peramelinae sensu Wilson and Reeder, 2005) to the exclusion of Chaeropus and thylacomyids, and of Peroryctidae (i.e., Echymipera + Microperoryctes + Rhynchomeles + Peroryctes) is supported, although Chaeropus and Thylacomyidae are placed as successive sister-taxa to Peramelidae, rather than outside Peramelidae and Peroryctidae, as is the case in recent molecular phylogenies. By contrast, the unconstrained Bayesian analysis shows greater conflict: Peramelidae is not recovered as monophyletic to the exclusion of Chaeropus and thylacomyids, and Peroryctidae is recovered as paraphyletic. Nevertheless, the parsimony results are encouraging, and suggest that the increased taxon and character sampling in our craniodental matrix presented here has reduced the incongruence between morphological and molecular estimates of peramelemorphian phylogeny.
DISCUSSION
The origin of Peramelemorphia, like all marsupial orders in Australia, remains unclear. Recent phylogenetic analyses of higher-level marsupial relationships consistently place Peramelemorphia in a clade together with Dasyuromorphia and Notoryctemorphia (Beck, 2008, 2012; Beck et al., 2008b; Meredith et al., 2008, 2009, 2011; Nilsson et al., 2004), but the precise branching order between these three orders remains unclear. The oldest potential fossil record of Peramelemorphia may be as yet undescribed early Eocene specimens from the Tingamarra LF, near Murgon, southeastern Queensland (Black et al., 2012). Prior to the current paper, the oldest described bandicoot species were Yarala kida from the late Oligocene or early Miocene Kangaroo Well LF in the Northern Territory (Schwartz, 2006) and Y. burchfieldi, Bulungu palara, Galadi speciosus, G. adversus, G. Amplus, and G. adversus from early to late Miocene LFs of the Riversleigh WHA.
Fossil bandicoots from the late Oligocene Etadunna and early Miocene Wipajiri Formations of the Lake Eyre Basin, have been reported (Campbell, 1976; Woodburne et al., 1993; Case, 2001) but until now none has been formally described. Campbell (1976) reported two taxa: one small taxon from Zones B (Ditjimanka LF) and C (Ngapakaldi LF) and the Kutjamarpu LF (early Miocene), and one large taxon from the Kutjamarpu LF. The taxa described in this current paper and in Gurovich et al. (2013) are based on samples that include the specimens Campbell (1976) referred to the small species. With the advantage of additional specimens collected since Campbell's study, we have concluded that this material represents three distinct taxa: Bulungu muirheadae from the Ditjimanka LF (this work), B. campbelli from the Ngapakaldi LF (this work), and B. palara from the Kutjamarpu and several Riversleigh LFs (Gurovich et al., 2013). Campbell's (1976) large species remains undescribed. It appears to be unique to the Kutjamarpu LF. However, we have identified a third species from the Kutjamarpu LF that is also known from Riversleigh Faunal Zone B (early Miocene) and C (mid Miocene) LFs. Woodburne et al. (1993) and Case (2001) note but do not name other peramelemorphian specimens from the Etadunna and Wipajiri Formations. Pending formal description of these specimens, their relationships to taxa described in this work remain uncertain.
The dental morphology (tribosphenic dentition, lacking development of the metaconule) and size of Bulungu campbelli (~112.87 grams, this work) and B. muirheadae (~135.58 grams, this work) is similar to that of B. palara (~130 grams, Gurovich et al., 2013) suggesting that they were small insectivores. Gurovich et al. in press noted that B. palara, along with Yarala burchfieldi (~75 grams, Travouillon et al., 2009) and Y. kida (Schwartz et al., 2006) were smaller than any living species of bandicoot. B. muirheadae and B. campbelli add to the diversity of small bodied bandicoots, which provide further support that Oligo-Miocene Australian peramelemorphians filled ecological niches that today are mostly occupied by dasyurids (Muirhead, 1994, 2000; Muirhead and Filan, 1995; Schwartz, 2006; Travouillon et al., 2010, 2013; Gurovich et al., 2013).
Bulungu muirheadae appears to be more plesiomorphic than either B. campbelli or B. palara in its retention of complete centrocristae on all upper molars. This is supported by two of our four phylogenetic analyses, where Bulungu muirheadae branches of earlier than the other two Bulungu species (Figure 4-Figure 5). Based on their craniodental morphology all of the Oligo-Miocene fossil peramelemorphians described to date, including Bulungu campbelli and B. muirheadae from the Etadunna formation described here, appear more plesiomorphic than any extant bandicoot (Muirhead and Filan, 1995; Muirhead, 2000; Travouillon et al., 2010, 2013; Gurovich et al., 2013). Previous studies (Travouillon et al., 2010, 2013; Gurovich et al., 2013), as well as the results of the more comprehensive phylogenetic analyses presented here (Figure 4-Figure 5), also suggest that all of these taxa lie outside crown-group Peramelemorphia. Our results indicate that the oldest described crown-group bandicoots are early Pliocene in age, namely the thylacomyid Ischnodon australis and the peramelids Perameles allinghamensis and P. bowensis.
These results appear to be in conflict with dated molecular phylogenies, which suggest that the earliest divergences among crown-group peramelemorphians occurred >20 m.y.a. (Meredith et al., 2008) or even 27 m.y.a. or older, depending on the method and calibrations used (Meredith et al., 2008, 2011; Westerman et al., 2012). These relatively ancient estimated divergence dates are possible, given the relatively scanty nature of the peramelemorphian fossil record and despite recent improvements in our knowledge. Nevertheless, if the South Australian, Northern Territory and Queensland Oligocene and Miocene fossil assemblages described to date are truly representative of peramelemorphian diversity at that time, this would imply that crown-group peramelemorphians were either much rarer than their stem-relatives or were restricted to geographical areas that have yet to be sampled, such as Western Australia. However, it should be noted that additional fossil species from Riversleigh (Archer et al., 2006) and the Lake Eyre Basin (Case, 2001) remain to be described, and could potentially include crown-group taxa.
Alternatively, it is possible that the molecular divergence dates are overestimates (see Gurovich et al., 2013). The latter possibility is perhaps rendered more likely by the fact that both Meredith et al. (2008) and Westerman et al. (2012) used "cf. Peroryctes" tedfordi and "cf. Peroryctes" sp. from the early Pliocene Hamilton fauna (Turnbull et al., 2003) to calibrate the split between Peroryctes (= Peroryctinae) and Echymipera+Microperoryctes (= Echymiperinae), whereas our phylogenetic analyses consistently place "cf. Peroryctes" tedfordi and "cf. Peroryctes" sp. outside crown-group Peramelemorphia, and hence neither fossil taxon is suitable for calibrating divergences within the crown-group (see below). As we note above, according to the results of our phylogenetic analyses, the oldest known crown-group peramelemorphians are the early Pliocene Ischnodon australis and the early Pliocene peramelids Perameles allinghamensis and P. bowensis, which, we argue, are suitable for specifying minimum dates for the Thylacomyidae-(Peramelidae+Peroryctidae) and Peramelidae-Peroryctidae splits, respectively. Although beyond the scope of the current paper, it would be interesting to see what impact the use of these alternative calibrations has on molecular divergence dates.
Given the occurrence of crown-group peramelemorphian clades in the Pliocene, namely Thylacomyidae (represented by Ischnodon australis) and Peramelidae (represented by Perameles allinghamensis and P. bowensis), it seems that these more modern groups had begun to supplant the archaic stem-lineages by this time, probably following a rapid but poorly documented interval of diversification in the late Miocene. Nevertheless, some stem-peramelemorphians appear to have survived this apparent late Miocene faunal turnover event, as indicated by the presence of "cf. Peroryctes" tedfordi and "cf. Peroryctes" sp. (both of which were consistently recovered outside the crown-group in our phylogenetic analyses) in the early Pliocene Hamilton LF. Interestingly, Hocknull (2005) described two peramelemorphian species from middle Pleistocene (Hocknull et al., 2007) cave deposits at Mount Etna, central-eastern Queensland that could not be referred to any modern family. Hocknull (2005) noted similarities between these species and both "cf. Peroryctes" tedfordi and Yarala burchfieldi. It is therefore possible that stem-peramelemorphians survived in Australia until at least the middle Pleistocene, perhaps going extinct with the onset of more xeric conditions some time after 280,000 years ago (Hocknull et al., 2007). It is worth mentioning that both Hamilton LF and the middle Pleistocene cave deposits at Mount Etna have been interpreted as rainforest environments. This could potentially suggest that stem-peramelemorphians were adapted to rainforest environments and that they survived in these relictual habitats.
If the early Pliocene "cf. Peroryctes" tedfordi and "cf. Peroryctes" sp. and the two middle Pleistocene peramelemorphian species described by Hocknull (2005: 78) belong to stem-Peramelemorphia rather than to the extant family Peroryctidae, then peroryctids are currently unknown from Australia, with the sole exception of the extant Echymipera rufescens, which appears to have dispersed to Australia from New Guinea during the Plio-Pleistocene. Thus, it is possible that the early evolutionary of peroryctids was restricted to New Guinea (contra Westerman et al., 2012). However, this hypothesis must be weighed against geological evidence (Quarles van Ufford and Cloos, 2005, summarised by Westerman et al., 2012) that suggests that only small areas of land were emergent off the north coast of the Australian continent prior to ~12 m.y.a., and that these were separated from Australia by deepwater barriers. In this context, accurate estimates of divergence times within Peramelemorphia and other marsupial clades (e.g., Pseudocheiridae, Meredith et al., 2009; Phalangeridae, Raterman et al., 2006) will be key to understanding the differential roles that the Australian mainland and New Guinea played in the diversification of marsupials in the Australo-Papuan region. Given the essential role that fossils play in calibrating molecular divergence dates, a better understanding of the fossil record and phylogeny of Peramelemorphia will be crucial to this endeavour, as will improvements in molecular sampling and methods (dos Reis et al., 2012).
In terms of overall peramelemorphian diversity through time, although inferences based on raw taxon counts (which do not take into account geological, taphonomic, collecting or other biases) should be treated as highly tentative, taxonomic diversity appears to have been relatively low during the late Oligocene, with no more than two species known from any one site (Case, 2001). In the early Miocene, diversity seems to have significantly increased, with some Riversleigh fossil localities containing seven different species (Archer et al., 2006). In the middle Miocene, a maximum of five species have been recorded at Gag Site at Riversleigh. In the late Miocene, two species have been reported from Encore Site in Riversleigh (Myers et al., 2001) and two specimens (unclear whether these specimens represent 1 or 2 species) from Alcoota (Murray and Megirian, 1992). Whether the apparent drop in peramelemorphian diversity seen in the late Miocene is related to the inferred faunal turnover event discussed above will require more detailed study of appropriately aged sites; unfortunately, the late Miocene vertebrate fossil record is poorly known in Australia, particularly for small mammals (Archer et al., 1999).
CONCLUSIONS
Two small, insectivorous peramelemorphian marsupials, Bulungu muirheadae and B. campbelli, are described from the late Oligocene of the Etadunna Formation, one from the Ditjimanka LF and one from the Ngapakaldi LF in central Australia. These two are related to a third species, Bulungu palara, from the Miocene Kutjamarpu LF of central Australia and several LFs from Riversleigh in northwestern Queensland. The two new species are currently among the oldest formally described bandicoots. The results of our phylogenetic analysis also reveal that early Pliocene "cf. Peroryctes" tedfordi and "cf. Peroryctes" sp. from Hamilton LF belong to stem-Peramelemorphia rather than to the extant family Peroryctidae.
ACKNOWLEDGMENTS
Support for research at Riversleigh has come from the Australian Research Council (DP0453262, LP0453664, LP0989969 and LP100200486 grants to M. Archer and S.J. Hand and DE120100957 to R.M.D. Beck at the University of New South Wales); XSTRATA Community Partnership Program (North Queensland); the University of New South Wales; Phil Creaser and the CREATE Fund, the Queensland National Parks and Wildlife Service; Environment Australia; the Queensland Museum; the Riversleigh Society Inc.; Outback at Isa; Mount Isa City Council; and private supporters including K. and M. Pettit, E. Clark, M. Beavis and M. Dickson. Assistance in the field has come from many hundreds of volunteers as well as staff and postgraduate students of the University of New South Wales. Assistance in central Australia came from the University of California, Berkeley with significant input in the field from C. Campbell, M. Woodburne and B. Clemens. We thank C. Campbell for enabling us to study the bandicoots he examined during his PhD research. We thank R. Day for providing funding to the University of Queensland to create a postdoctoral position for K.J. Travouillon. We thank S. Ingleby and A. Divljan from the Australian Museum, H. Janetzki from the Queensland Museum and C. Stevenson from the Western Australian Museum for providing access to the modern bandicoot collections. We thank the UNSW Palaeosciences Lab and the UQ Palaeo Hub for their support and anonymous reviewers for helpful comments.
REFERENCES
Aplin, K.P. and Archer, M. 1987. Recent advances in marsupial systematics with a new syncretic classification, p. xv-lxxii. In Archer, M. (ed.), Possums and Opossums, Studies in Evolution. Surrey Beatty and Sons, Sydney, Australia.
Archer, M. 1976. The dasyurid dentition and its relationships to that of didelphids, thylacinids and borhyaenids. Australian Journal of Zoology, Supplementary Series 39:1-34.
Archer, M. and Wade, M. 1976. Results of the Ray E. Lemley expeditions, part 1: The Allingham Formation and a new Pliocene vertebrate fauna from northern Queensland. Memoirs of the Queensland Museum, 17:54-58.
Archer, M., Arena, D.A., Bassarova, M., Beck, R.M.D., Black, K., Boles, W.E., Brewer, P., Cooke, B.N., Crosby, K., Gillespie, A., Godthelp, H., Hand, S.J., Kear, B.P., Louys, J., Morrell, A., Muirhead, J., Roberts, K.K., Scanlon, J.D., Travouillon, K.J., and Wroe, S. 2006. Current status of species-level representation in faunas from selected fossil localities in the Riversleigh World Heritage Area, northwestern Queensland. Alcheringa Special Issue, 1: 1-17.
Archer, M., Arena, R., Bassarova, M., Black, K., Brammall, J., Cooke, B., Creaser, P., Crosby, K., Gillespie, A., Godthelp, G., Gott, M., Hand, S.J., Kear, B., Krikmann, A., Mackness, B., Muirhead, J., Musser, A., Myers, T., Pledge, N., Wang, Y., and Wroe, S. 1999. The evolutionary history and diversity of Australian mammals. Australian Mammalogy, 21:1-45.
Beck, R.M.D. 2008. A dated phylogeny of marsupials using a molecular supermatrix and multiple fossil constraints. Journal of Mammalogy, 89:175-189.
Beck, R.M.D. 2012. An 'ameridelphian' marsupial from the early Eocene of Australia supports a complex model of Southern Hemisphere marsupial biogeography. Naturwissenschaften, 99:715-29.
Beck, R.M.D., Godthelp, H., Weisbecker, V., Archer, M., and Hand, S.J. 2008b. Australia's oldest marsupial fossil and their biogeographical implications. PloS ONE, 3:e1858.
Beck, R.M.D., Archer, M., Godthelp, H., Mackness, B.S., Hand, S.J., and Muirhead, J. 2008a. A bizarre new family of Marsupialia (Incertae sedis) from the early Pliocene of northeastern Australia: implications for the phylogeny of bunodont marsupials. Journal of Paleontology, 82:749-762.
Black, K.H., Archer, M., Hand, S.J., and Godthelp, H. 2012. The rise of Australian marsupials: a synopsis of biostratigraphic, phylogenetic, palaeoecologic and palaeobiogeographic understanding. In Talent, J.A., (ed.), Earth and Life: Global Biodiversity, Extinction Intervals and Biogeographic Perturbations Through Time. Dordrecht: Springer. pp. 983-1078.
Campbell, C.R. 1976. Tertiary Dasyuridae and Peramelidae (Marsupialia) from the Tirari Desert, South Australia. Unpublished PhD Thesis, University of California, Berkeley, USA.
Case, J. A. 2001. Turnover of bandicoots in the Oligo-Miocene of South Australia. Journal of Vertebrate Paleontology, 21:39A.
dos Reis, M., Inoue, J., Hasegawa, M., Asher, R.J., Donoghue, P.C.J., and Yang, Z. 2012.
Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny. Proceedings of the Royal Society B, 279:3491-3500.
Eriksson, T. 2001. AutoDecay ver. 5.0. Bergius Foundation, Royal Swedish Academy of Sciences, Stockholm.
Gurovich, Y., Travouillon, K.J., Beck, R.M.D., Muirhead, J., and Archer, M. 2013. Biogeographical implications of a new mouse-sized fossil bandicoot (Marsupialia: Peramelemorphia) occupying a dasyurid-like ecological niche across Australia. Journal of Systematic Paleontology, DOI:10.1080/14772019.2013.776646.
Hocknull, S.A. 2005. Ecological succession during the late Cainozoic of central eastern Queensland: extinction of a diverse rainforest community. Memoirs of the Queensland Museum, 51:39-122.
Hocknull, S.A., Zhao, J.-x., Feng, Y.-x., and Webb, G.E. 2007. Responses of Quaternary rainforest vertebrates to climate change in Australia.Earth and Planetary Science Letters,264: 317-331.
Kirsch, J.A.W. 1968. Prodromus of the comparative serology of Marsupialia. Nature, 217:418-420.
Krajewski, C., Torunsky, R., Sipiorski, J.T., and Westerman, M.2007. Phylogenetic relationships of the dasyurid marsupial genus Murexia. Journal of Mammalogy, 88:696-705.
Lewis, P.O. 2001. A likelihood approach to inferring phylogeny from discrete morphological characters. Systematic Biology, 50:913-925.
Luckett, W.P. 1993. An ontogenetic assessment of dental homologies in therian mammals, p. 182-204. In Szalay, F.S., Novacek, M.J., and McKenna, M.C. (eds.), Mammal Phylogeny: Mesozoic Differentiation, Multituberculates, Monotremes, Early Eutherians and Marsupials. Springer-Verlag, New York.
Meredith, R.W., Westerman, M., and Springer, M.S. 2008. A timescale and phylogeny for "Bandicoots" (Peramelemorphia: Marsupialia) based on sequences for five nuclear genes. Molecular Phylogenetics and Evolution, 47:1-20.
Meredith, R.W., Krajewski, C., Westerman, M., and Springer, M.S. 2009. Relationships and divergence times among the orders and families of Marsupialia. Museum of Northern Arizona Bulletin, 65:383-406.
Meredith, R.W., Janecka, J.E., Gatesy, J., Ryder, O.A., Fisher, C.A., Teeling, E.C., Goodbla, A., Eizirik, E., Simao, T.L.L., Stadler, T., Rabosky, D.L., Honeycutt, R.L., Flynn, J.J., Ingram, C.M., Steiner, C., Williams, T.L., Robinson, T.J., Burk-Herrick, A., Westerman, M., Ayoub, N.A., Springer, M.S., and Murphy, W.J. 2011. Impacts of the Cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science, 334:521-524.
Metzger, C.A. and Retallack, G.J. 2010. Paleosol record of Neogene climate change in the Australian outback. Australian Journal of Earth Sciences, 57:871-885.
Muirhead, J. 1994. Systematics, evolution and palaeobiology of recent and fossil bandicoots (Peramelemorphia, Marsupialia). Unpublished PhD Thesis, University of New South Wales, Sydney, Australia.
Muirhead, J. 2000. Yaraloidea (Marsupialia, Peramelemorphia), a new superfamily of marsupial and a description and analysis of the cranium of the Miocene Yarala burchfieldi. Journal of Paleontology, 74:512-523.
Muirhead, J. and Filan, S.L. 1995. Yarala burchfieldi, a plesiomorphic bandicoot (Marsupialia, Peramelemorphia) from Oligo-Miocene deposits of Riversleigh, northwestern Queensland. Journal of Paleontology, 69:127-134.
Muirhead, J., Dawson, L., and Archer, M. 1997. Perameles bowensis, a new species of Perameles (Peramelomorphia, Marsupialia) from Pliocene faunas of Bow and Wellington caves, New South Wales. Proceedings of the Linnean Society of New South Wales, 17:163-174.
Murray, P. and Megirian, D. 1992. Continuity and contrast in middle and late Miocene vertebrate communities from Northern Territory, p. 195-218. In Murray, P.F. and Megirian, D. (eds.), Proceedings of the 1991 Conference on Australasian Vertebrate Evolution, Palaeontology and Systematics, The Beagle, Records of the Northern Territory Museum of Arts and Sciences, Alice Springs, Australia.
Myers, T.J. 2001. Prediction of marsupial body mass. Australian Journal of Zoology, 49:99-118.
Myers, T.J., Crosby, K., Archer, M., and Tyler, M. 2001. The Encore local Fauna, a late Miocene assemblage from Riversleigh, northwestern Queensland. Memoirs of the Association of Australasian Palaeontologists, 25:147-154.
Nilsson, M.A., Arnasson, U., Spencer, P.B.S., and Janke, A. 2004. Marsupial relationships and a timeline for marsupial radiation in South Gondwana. Gene, 340:189-196.
Price, G.J. 2002. Perameles sobbei, sp. nov. (Marsupialia, Peramelidae), a Pleistocene bandicoot from the Darling Downs, south-eastern Queensland. Memoirs of the Queensland Museum, 48:193-197.
Price, G. J. 2005. Fossil bandicoots (Marsupialia, Peramelidae) and environmental change during the Pleistocene on the Darling Downs, southeastern Queensland, Australia. Journal of Systematic Palaeontology, 4:347-356.
Quarles van Ufford, A. and Cloos, M. 2005. Cenozoic tectonics of New Guinea. American Association of Petroleum Geologists Bulletin, 89:119-140.
Raterman, D., Meredith, R.W., Reudas, L.A., and Springer, M.S. 2006. Phylogenetic
relationships of the cuscuses and brushtail possums (Marsupialia:
Phalangeridae) using the nuclear gene BRCA1. Australian Journal of Zoology, 54:353-361.
Schwartz, L.R.S. 2006. A new species of bandicoot from the Oligocene of Northern Australia and implications of bandicoots for correlating Australian Tertiary mammal faunas. Palaeontology, 49:991-998.
Stirton, R.A. 1955. Late Tertiary marsupials from South Australia. Records of the South Australian Museum, 11:247-268.
Swofford, D. L. 2002. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4 (updated to 10 beta). Sinauer Associates, Sunderland, Massachussets.
Travouillon, K.J., Gurovich, Y., Beck, R.M.D., and Muirhead, J. 2010. An exceptionally well-preserved short-snouted bandicoot (Marsupialia; Peramelemorphia) from Riversleigh's Oligo-Miocene deposits, northwestern Queensland, Australia. Journal of Vertebrate Paleontology, 30:1528-1546.
Travouillon, K.J., Legendre, L., Archer, M., and Hand, S.2009. Palaeoecological analyses of Riversleigh's Oligo-Miocene sites: Implications for Oligo-Miocene climate change in Australia. Palaeogeography, Palaeoclimatology, Palaeoecology, 276:24-37.
Travouillon, K.J., Gurovich, Y., Archer, M., Hand, S.J., and Muirhead, J., 2013. The genus Galadi: three new bandicoots (Marsupialia; Peramelemorphia) from Riversleigh's Miocene deposits, north-western Queensland, Australia. Journal of Vertebrate Paleontology, 33:153-168.
Turnbull, W.D., Lundelius, E.L.Jr., and Archer, M. 2003. Dasyurids, perameloids, phalangeroids, and vombatoids from the early Pliocene Hamilton fauna, Victoria, Australia. Bulletin of the American Museum of Natural History, 279:513-540.
Westerman, M., Young, J., and Krajewski, C. 2008. Molecular relationships of Pseudantechinus, Parantechinus, and Dasykaluta (Marsupialia: Dasyuridae). Australian Mammalogy, 29:201-212.
Westerman,M., Kear, B.P., Aplin, K., Meredith, R.W., Emerling, C., and Springer, M.S. 2012. Phylogenetic relationships of living and recently extinct bandicoots based on nuclear and mitochondrial DNA sequences. Molecular Phylogenetics and Evolution, 62:97-108.
Wilson, D.E. and Reeder D.M. 2005. Mammal Species of the World. A Taxonomic and Geographic Reference (third edition), Johns Hopkins University Press, Baltimore, USA.
Woodburne, M.O. and Case, J.A. 1996. Dispersal, vicariance, and the late Cretaceous to early Tertiary land mammal biogeography from South America to Australia. Journal of Mammalian Evolution, 3:121-162.
Woodburne, M.O., Macfadden, B.J., Case, J.A., Springer, M.S., Pledge, N., Power, J.D.,
Woodburne, J., and Springer, K.B. 1993. Land mammal biostratigraphy and magnetostratigraphy of the Etadunna Formation (late Oligocene) of South Australia. Journal of Vertebrate Paleontology, 13:483-515.
Worthy, T.H., Tennyson, A.J.D., Archer, M., Musser, A.M., and Hand, S.J. 2006. Miocene mammal reveals a Mesozoic ghost lineage on insular New Zealand, southwest Pacific. Proceedings of the National Academy of Sciences of the United States of America, 103:19419-19423.

