 An acid-free method of microfossil extraction from clay-rich lithologies using the surfactant Rewoquat
An acid-free method of microfossil extraction from clay-rich lithologies using the surfactant Rewoquat
Article number: 16.3.7T
https://doi.org/10.26879/382
Copyright Paleontological Society, November 2013
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 10 March 2013. Acceptance: 29 October 2013
{flike id=530}
ABSTRACT
Marine rocks characterized by high clay content provide excellent conditions for fossil preservation, particularly for organic-walled microfossils such as retiolitid graptolites and chitinozoans. Nevertheless, the phyllosilicate minerals, which constitute the clay component, make microfossil extractions difficult. The problem results from the tendency of phyllosilicates to form aggregates in low pH values, as standard methods of microfossil extraction employ acids for rock digestion. Consequently, the use of acids for clay-rich rocks is often inefficient and time-consuming. We propose a method of rock disintegration using the surfactant Rewoquat and compare it with two commonly applied approaches: digestion in buffered acetic acid and in HCl-HF. Using examples from the Mulde Brick-clay Member from the Silurian of Gotland and the Daleje Shale from the Devonian of the Prague Basin we observed that disintegration in Rewoquat was faster (days) than digestion in acid (months), and allowed to recover calcareous in addition to organic-walled fossils. The yield and preservation was comparably good, except for conodonts, which were strongly etched after using HCl-HF. Retiolitid graptolites recovered using Rewoquat were preserved in 3D and showed a lower degree of fragmentation. The fossil content of the residue obtained using Rewoquat was higher due to dispersion of clay aggregates. For observation of delicate fossils we recommend to coat the sample with the surfactant. Application of Rewoquat can reveal the most delicate forms and growth stages, and thus provide a better insight into the ontogeny, autecology, and body size distributions of a number of fossil groups.
Emilia Jarochowska. GeoZentrum Nordbayern, Fachgruppe Paläoumwelt, Universität Erlangen-Nürnberg, Loewenichstrasse 28, 91054 Erlangen, Germany; Emilia.Jarochowska@gzn.uni-erlangen.de
Petra Tonarová. Institute of Geology at Tallinn University of Technology, Ehitajate tee 5, 19086 Tallinn, Estonia; Czech Geological Survey, Geologická 6, 152 00 Prague 5, Czech Republic; petra.tonarova@geology.cz
Axel Munnecke. GeoZentrum Nordbayern, Fachgruppe Paläoumwelt, Universität Erlangen-Nürnberg, Loewenichstrasse 28, 91054 Erlangen, Germany; Axel.Munnecke@gzn.uni-erlangen.de
Lenka Ferrová. Czech Geological Survey, Geologická 6, 152 00 Prague 5, Czech Republic; Faculty of Science, Charles University in Prague, Albertov 6, 128 43 Prague 2; ferrova@natur.cuni.cz
Jan Sklenář. Palaeontological Department, Natural History Museum, National Museum, Václavské náměstí 68, 115 79 Praha 1, Czech Republic; jan_sklenar@nm.cz
Stanislava Vodrážková. GeoZentrum Nordbayern, Fachgruppe Paläoumwelt, Universität Erlangen-Nürnberg, Loewenichstrasse 28, 91054 Erlangen, Germany; Czech Geological Survey, P.O.B. 85, 118 21 Prague 1, Czech Republic; stana.vodrazkova@seznam.cz
Keywords: fossils, calcareous; microfossils, organic-walled; microfossils, phosphatic; clay dispersion; acid digestion; three-dimensional preservation
Final citation: Jarochowska, Emilia, Tonarová, Petra, Munnecke, Axel, Ferrová, Lenka, Sklenář, Jan, and Vodrážková, Stanislava. 2013. An acid-free method of microfossil extraction from clay-rich lithologies using the surfactant Rewoquat, Palaeontologia Electronica Vol. 16, Issue 3; 7T; 16p. https://doi.org/10.26879/382
palaeo-electronica.org/content/2013/530-microfossil-extraction
INTRODUCTION
Most techniques of microfossil extraction rely on acid digestion (e.g., Upshaw et al., 1957; Schopf, 1965; Miller, 1967; Chauff, 1978; Grahn and Afzelius, 1980; Jeppsson et al., 1985, 1999; Etherington and Austin, 1993; Jeppsson and Anehus, 1995; Ford and Lee, 1997) or rock expansion through freeze-thaw (e.g., Hanna and Church, 1928; Pojeta and Balanc, 1989; Remin et al., 2012). Highly argillaceous lithologies, such as claystones and marlstones, cause significant difficulties in the application of these methods due to their very low porosity and formation of clay coating on the sample surface. Yet, fine-grained rocks are an excellent source of well-preserved fossils, and are the most typical lithology of open shelf deposits, which are of great interest to biostratigraphers due to their stratigraphic completeness. In the present work, we propose a method of fossil extraction from clay-rich lithologies using the surfactant Rewoquat.
Rewoquat W 3690 PG is a trade name for a concentrate of 75% cationic surfactant 1-methyl-2-oleyl-3-oleyl-amidoethyl-imidazolium methosulfate with 24% propylenglycol. It is widely used for cleaning fossils (Lierl, 1992; Krüger, 1994; Riegraf and Niemeyer, 1996; Babinot and Colin, 2011). Less commonly, it is also used by foraminifer specialists for whole-rock disintegration (e.g., Holbourn and Kuhnt, 1998; Nagy, 2005; Heldt et al., 2008). However, we have not encountered any reports on the use of Rewoquat for extraction of phosphatic or organic-walled microfossils, to which belong the most important groups for Lower Palaeozoic biostratigraphy, i.e., conodonts and graptolites. In this study, we present the results of comparisons made between well-established methods of acid digestion and extraction using Rewoquat, applied to two Palaeozoic argillaceous samples: the Mulde Brick-clay Member from the middle Silurian of Gotland and the Daleje shale from the Lower Devonian of the Prague Basin. We demonstrate the advantage of surfactant-based rock disintegration with special focus being put on the recovery of Palaeozoic orthostratigraphic fossils, including graptolites, chitinozoans, and conodonts.
CASE STUDIES
Mulde Brick-clay Member, middle Silurian, Gotland
Sample Characteristics. We have used a sample from the middle Silurian Mulde Brick-clay Member (MBCM) of the Halla Formation. The uppermost part of MBCM is exposed in the Blåhäll 1 locality in western Gotland, Sweden (Hede, 1960; Laufeld, 1974a, 1974b; Calner et al., 2000, 2004a, 2004b). The MBCM represents an upper part of transgressive deposits of the intra- to peri-cratonic carbonate platform developed during the Early Palaeozoic on the East European Craton. They are characterized by a high content of argillaceous material and a very rich and diverse fauna (Calner et al., 2000, 2012), which includes calcareous fossils: brachiopods (e.g., Spjeldnaes, 1984; Wertel, 1995), ostracods (Martinsson, 1967), tentaculitids (Larsson, 1979), bryozoans, and a mass occurrence of trilobites (Calner et al., 2012); organic-walled fossils such as graptolites (Hede, 1942; Mierzejewski, 1988; Kozłowska-Dawidziuk, 1991; Bates et al., 2005), chitinozoans (Laufeld, 1974b), scolecodonts (Bergman, 1989); and (rare) conodonts (Jeppsson, 1983; Calner and Jeppsson, 2003).
Extraction
Two samples of 2 kg each, consisting of several boulder- and gravel-sized rock pieces, were used for comparison between acid dissolution and Rewoquat treatment.
Disintegration in Rewoquat. For Rewoquat treatment, the sample was placed in a 20 L plastic bucket filled with 4 L of Rewoquat and tightly closed. The bucket was opened every other day to gently mix the content with a gloved hand. No hard objects were used to avoid crushing of fossils. After 10 days the bucket was filled with water and slowly poured in small portions over a stack of sieves with the following mesh sizes: 63 μm, 500 μm, and 1 mm. Sieving was performed with excess water, and the remainder of the extract in the bucket was diluted each time to reduce sieve clogging. Residues from each sieve were collected and, while wet, examined for floating fragments of retiolitid rhabdosomes. These have been collected with a pipette and stored in glycerin, then washed with water before transferring them onto SEM stubs. The residues were dried overnight at 30ºC, separated in sodium polytungstate to facilitate picking of phosphatic microfossils, and examined. Calcareous fossils were additionally cleaned in an ultrasonic bath for one hour. The extraction took 10 days of Rewoquat treatment and approximately 14 h of sieving.
Digestion in acetic acid. For acid dissolution, the sample was placed in extruded mesh (90–160 mm diameter) and hung in the upper part of a 30 L plastic bucket filled with 7% acetic acid buffered using the method of Jeppsson et al. (1999). The initial pH of the acid was 3.6. The bucket was tightly closed to prevent evaporation. Undissolved sample was rubbed gently every day to remove accumulated clay cover, which would prevent dissolution. Each time when the acid reached the pH of 4.6, it was exchanged, and the residue collected at the bottom was washed out, sieved over a stack of sieves (63 μm, 500 μm and 1 mm), and dried overnight in 30ºC. The bucket was re-filled with fresh buffered acid until the sample was completely dissolved. Only the 63-500 μm fraction was collected, but sieving was performed through a stack of sieves in order to disperse the water stream. The residues were dried overnight at 30ºC and separated in sodium polytungstate. The entire dissolution took 3.5 months.
In order to quantify the difference in microfossil recovery, 3 subsamples of 10 g each were retrieved from the acid- and Rewoquat-digested residues and the number of chitinozoans and scolecodonts (including fragmented specimens) was counted. The two groups were chosen as they were yielded in sufficient amounts by both methods and thus allowed a comparison. Average counts are given in Table 1.
Results
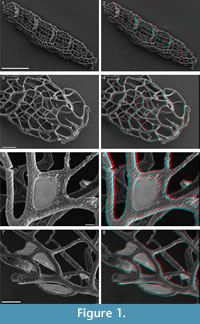 Recovered residues amounted to 202 g (10%) and 485 g (24%) for Rewoquat- and acid-extracted samples, respectively. The residue extracted with Rewoquat contained numerous rhabdosomes of the retiolitid graptolite Gothograptus nassa (Holm, 1890) preserved in three dimensions, exhibiting excellent preservation of cortical lists (Figure 1.5-8) and having partly preserved membranes (Figure 1.5-8). Recovered non-calcareous microfossils included chitinozoans (Figure 2.1-5), scolecodonts (Figure 2.6-10), two complete conodont elements (Figure 2.17-18), and one fragmented (not shown).
Recovered residues amounted to 202 g (10%) and 485 g (24%) for Rewoquat- and acid-extracted samples, respectively. The residue extracted with Rewoquat contained numerous rhabdosomes of the retiolitid graptolite Gothograptus nassa (Holm, 1890) preserved in three dimensions, exhibiting excellent preservation of cortical lists (Figure 1.5-8) and having partly preserved membranes (Figure 1.5-8). Recovered non-calcareous microfossils included chitinozoans (Figure 2.1-5), scolecodonts (Figure 2.6-10), two complete conodont elements (Figure 2.17-18), and one fragmented (not shown). 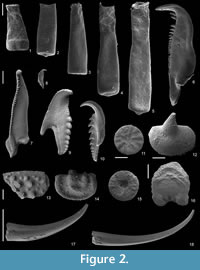 The dominant proportion of the residue was formed by calcareous micro- and macrofossils, including tentaculites (Figure 3.1-3), tabulate corals (Figure 3.4-6), brachiopods (Figure 3.10-14), ostracods (Figure 2.12-14), echinoderms (Figure 2.11, 2.15-2.16), bryozoans (Figure 3.7), and trilobites (Figure 3.8-9), many of which bear encrustations from e.g., cornulitids (Figure 3.6-7), encrusting bryozoans, and other epibionts of unclear affinity, such as Allonema sp. (Figure 3.5, 3.8), ?Condranema parvula (Condra and Elias, 1944) and Ascodictyon venustum Kiepura, 1965 (not shown).
The dominant proportion of the residue was formed by calcareous micro- and macrofossils, including tentaculites (Figure 3.1-3), tabulate corals (Figure 3.4-6), brachiopods (Figure 3.10-14), ostracods (Figure 2.12-14), echinoderms (Figure 2.11, 2.15-2.16), bryozoans (Figure 3.7), and trilobites (Figure 3.8-9), many of which bear encrustations from e.g., cornulitids (Figure 3.6-7), encrusting bryozoans, and other epibionts of unclear affinity, such as Allonema sp. (Figure 3.5, 3.8), ?Condranema parvula (Condra and Elias, 1944) and Ascodictyon venustum Kiepura, 1965 (not shown).
The residue obtained with acid extraction consisted mainly of claystone fragments which hampered picking of fossils. Only chitinozoans and scolecodonts were obtained in large quantities (Figure 4). Calcareous fossils were absent, and retiolitid graptolites occurred only as small fragments. The average number of chitinozoans and scolecodonts in residue sample of the same weight was approximately one third of that obtained from the Rewoquat residue (38% and 31%, respectively; Table 1), mainly due to clay aggregates.
Transition layers between Zlíchov Limestone and Daleje Shale (Emsian, Lower Devonian, Barrandian)
Sample Characteristics.  We have used samples from the Pekárek Mill section, located SW of Prague in the varcava Valley of the Prague Basin. The transition from carbonaceous sedimentation (Zlíchov Limestone) to calcareous shales (Daleje Shale of the Daleje-Třebotov Formation) reflects the onset of the Daleje Event (Chlupáč and Kukal, 1986; Ferrová et al., 2012). As the transition between the Zlíchov Limestone and the Daleje Shale is gradual, the predominance of argillaceous versus carbonate sedimentation is conventionally regarded as the lower boundary of the Daleje Shale. Pekárek Mill is well known due to common occurrence of dacryoconarid tentaculites (the Daleje Shale is referred to as "tentaculite shale"), trilobites, brachiopods, and, less frequent, but characteristic goniatites (Chlupáč, 1959; Chlupáč et al., 1979; Chlupáč and Lukeš, 1999). The petrography of the shales has been studied by Petránek (1950), who recorded higher CaCO3 content linked especially to abundant faunal remains, particularly dacryoconarid tentaculites and calcified radiolarians. Detailed petrographic and chemical analyses and study of diagenetic pathways, however, have never been performed.
We have used samples from the Pekárek Mill section, located SW of Prague in the varcava Valley of the Prague Basin. The transition from carbonaceous sedimentation (Zlíchov Limestone) to calcareous shales (Daleje Shale of the Daleje-Třebotov Formation) reflects the onset of the Daleje Event (Chlupáč and Kukal, 1986; Ferrová et al., 2012). As the transition between the Zlíchov Limestone and the Daleje Shale is gradual, the predominance of argillaceous versus carbonate sedimentation is conventionally regarded as the lower boundary of the Daleje Shale. Pekárek Mill is well known due to common occurrence of dacryoconarid tentaculites (the Daleje Shale is referred to as "tentaculite shale"), trilobites, brachiopods, and, less frequent, but characteristic goniatites (Chlupáč, 1959; Chlupáč et al., 1979; Chlupáč and Lukeš, 1999). The petrography of the shales has been studied by Petránek (1950), who recorded higher CaCO3 content linked especially to abundant faunal remains, particularly dacryoconarid tentaculites and calcified radiolarians. Detailed petrographic and chemical analyses and study of diagenetic pathways, however, have never been performed.
Extraction
For this study five shale samples were chosen from the interval CH14 to CH18 sensu Chlupáč and Lukeš (1999). Two samples of about 100 g have been used from each bed, one for the Rewoquat and the other one for acid treatment.
Disintegration in Rewoquat. Each sample was soaked in Rewoquat and left in a tightly closed preserving jar for 2—3 days, after that the remaining liquid was decanted, the jar was filled with water, and the sample was left to soak for at least one more day. Sieves with mesh sizes of 50 μm, 200 μm and 1 mm were used for all five samples. Ethanol was added during the sieving process to prevent foaming and to improve the cleaning of microfossils. The residues were dried at 60°C except for the smallest fraction (50—200 μm), which was partially picked in a wet state.
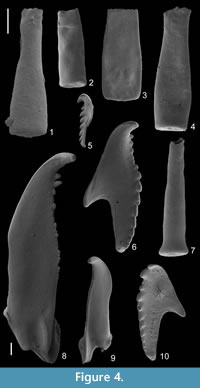 HCl-HF-HCl treatment. This technique represents a "classical" method for extracting organic microfossils (Schopf, 1965; Miller, 1967). The samples were treated with 35% HCl for three days. After dilution with water they were transferred into a plastic beaker and poured over with 40% HF (diluted to 30% by the remaining water) and left soaking until complete dissolution (for a week in this case), followed by the next dilution. In the last step, samples were boiled in concentrated HCl for a short (few minutes) time. Sieves of mesh sizes of 10 μm and 50 μm were used. The >50 μm fraction was hand-picked from a wet residue.
HCl-HF-HCl treatment. This technique represents a "classical" method for extracting organic microfossils (Schopf, 1965; Miller, 1967). The samples were treated with 35% HCl for three days. After dilution with water they were transferred into a plastic beaker and poured over with 40% HF (diluted to 30% by the remaining water) and left soaking until complete dissolution (for a week in this case), followed by the next dilution. In the last step, samples were boiled in concentrated HCl for a short (few minutes) time. Sieves of mesh sizes of 10 μm and 50 μm were used. The >50 μm fraction was hand-picked from a wet residue.
Results
Residues obtained by Rewoquat techniques consisted of abundant calcareous fossils such as dacryoconarid tentaculites (Figure 5.1-2), ostracodes (Figure 5.3-4), 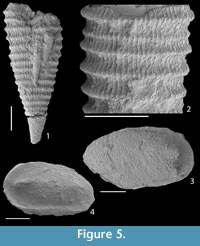 brachiopods, rugose corals, trilobites, palynomorphs including chitinozoans (Figure 6.14-17, 6.22), scolecodonts (Figure 6.18-21, 6.23), prasinophytes (Figure 6.24), and a small proportion of clay particles. Only one conodont species was recovered (Figure 6.25-26), all specimens displaying preservation of the outer hyaline tissue. Larger fossils (more than 5 mm) were often present as undeterminable scattered fragments. Some of the microfossils were covered with adherent particles (Figure 6.14-26), and therefore additional cleaning using hydrofluoric acid would be necessary.
brachiopods, rugose corals, trilobites, palynomorphs including chitinozoans (Figure 6.14-17, 6.22), scolecodonts (Figure 6.18-21, 6.23), prasinophytes (Figure 6.24), and a small proportion of clay particles. Only one conodont species was recovered (Figure 6.25-26), all specimens displaying preservation of the outer hyaline tissue. Larger fossils (more than 5 mm) were often present as undeterminable scattered fragments. Some of the microfossils were covered with adherent particles (Figure 6.14-26), and therefore additional cleaning using hydrofluoric acid would be necessary.
The HCl-HF-HCl technique resulted in a residue composed almost solely of organic matter, consisting of acid-resistant microfossils: chitinozoans (Figure 6.1-5), scolecodonts (Figure 6.6-10), prasinophytes (Figure 6.13), and up to now undetermined palynomorphs of spore affinity). Conodonts were also recovered using this method, but the preservation was very poor, with the outer hyaline tissue entirely etched out (Figure 6.11-12). In processing of all five samples no indication of differences in mineralogical or physical properties was observed.
MINERALOGICAL COMPOSITION
 Sample preparation and measurement parameters
Sample preparation and measurement parameters
Small fragments (2 g) of matrix from each lithology, i.e., MBCM and the Daleje Shale, sample CH15b following the scheme of Chlupáč and Lukeš (1999), were ground in isopropanol using McCrone micronising mill with agate beads. Measurements of oriented samples mounted on flat plates were performed using Siemens D5000 theta-theta diffractometer with CuKα radiation, at 40 kV, 35 mA, graphite secondary monochromator, theta compensating slit, stepscan 0.02°, 3 sec counting time. Evaluation was performed using the Rietveld software "Topas".
XRD Results
The rock-forming clay mineral in the studied lithologies is illite, which consisted 35.63% of the MBCM sample and 24.76% of the Daleje Shale sample (Table 2). Both samples contained approximately 5% of clinochlore, also a phyllosilicate, and 20-23% of quartz. MBCM had a significantly smaller content of carbonate minerals (32.55% of calcite, dolomite and ankerite) than the sample representing the Daleje Shale (41.82% of calcite).
DISCUSSION
Comparison of Rewoquat- and Acid-based Extractions
The two described experiments were performed on samples representing highly clayey lithologies, from which the extraction of orthostratigraphic fossils poses a difficulty, both in terms of efficiency and time consumption. In both cases, extractions using Rewoquat yielded results after several (3-10) days, in contrast to up to 3.5 months of acid dissolution.
Organic-walled fossils (chitinozoans and scolecodonts) were obtained using both approaches, but, based on the MBCM sample, the amount of residue necessary to obtain a given number of specimens was approximately three times larger in the case of acid digestion, as the residue consisted of clay aggregates which likewise coated the fossils. The difference in recovery was pronounced with respect to retiolitid graptolites. We were able to extract long (up to 2 cm) fragments of rhabdosomes, including the proximal parts, which are most useful for taxonomic identification. On the contrary the buffered acid method yielded only small (<0.5 mm) rhabdosome fragments.
An obvious shortcoming of the acid treatment lies in the use of hazardous chemicals, especially hydrofluoric acid, whereas Rewoquat can irritate the skin and the eyes, but does not require a specialized laboratory to handle.
Extraction with Rewoquat allowed recovery of not only organic-walled, but also calcareous fossils. This is an advantage in situations where the available rock volume is limited, as it is in the case of drillcores, as well as when calcareous fossils, such as dacryoconarid tentaculites, can supplement biostratigraphic information obtained using non-calcareous groups. On the other hand, the method of whole-sample immersion with Rewoquat involves more intensive sieving in order to wash the chemical out of the residue, which incurs more human labour and a higher risk of breaking the most fragile fossils (e.g., trilobites). For such cases we recommend a cyclic procedure consisting of coating the sample surface by Rewoquat (see in the Recommendations). Argillaceous lithologies have been long recognized as the best sources of delicate fossils, such as graptolites (Barrande, 1850). Malformed and parasitized graptolite rhabdosomes, as well as their body parts which are normally not fossilized, are known virtually only from exceptionally preserved three-dimensional forms recovered from fine-grained sediments (Teller, 1998; Zalasiewicz et al., 2013). Extraction with Rewoquat from clay-rich rocks allowed us to observe a number of features important for the ontogeny and autecology, e.g., (1) the otherwise usually lost membranes between cortical lists of retiolitid graptolites, whose presence plays an important role in the reconstruction of retiolitid development (Kozłowska-Dawidziuk, 2004; Dobrowolska, 2012) (Figure 1.5-8); (2) well preserved protegula of juvenile brachiopods, the size and form of which is an indicator of the larval mode of life (planktotrophic vs. lecitotrophic, Freeman and Lundelius, 2007); (3) epibionts, which can be used as indicators of the growth habit of their hosts (Blind, 1970), and (4) shell repair marks in tentaculitoids, demonstrating the intensity of predation in these organisms with relatively poorly known ecology (Vinn, 2009, 2010).
Difficulties Arising due to a High Content of Phyllosilicates
In both discussed examples, the rock-forming clay mineral was illite, which is the dominant phyllosilicate in open shelf deposits. In acid solutions, illite displays a decrease in the apparent surface dissociation constant and forms aggregates (e.g., Jozefaciuk, 2002). Aggregation in low pH leads to coating the sample and clogging the pore space, which puts a rapid stop to further dissolution. This problem is well known to palaeontologists and archaeologists (e.g., Zhao and Pearsall, 1998; Lentfer and Boyd, 1999), and the only widespread approach is the use of dispersing agents whose action is based on replacement of cations in clay aggregates by hydrated sodium cations, which – due to their large hydrated radius – contribute to the dispersion of clay micelles (Zhao and Pearsall, 1998). These reagents include sodium hexametaphosphate (formerly referred to in the literature as Calgon, but note that the chemical sold under this trade name is not sodium hexametaphosphate anymore), sodium phosphate [NaPO3]6/Na2CO3, and Na2H2EDTA (Bates et al., 1978; Hodgkinson, 1991; Zhao and Pearsall, 1998). All these chemicals require numerous cycles of heating and sieving the released clay particles, which in marls need to be usually followed by another round of acid digestion. Grinding or shaking can improve deflocculation (Lentfer and Boyd, 1999; Coil et al., 2003), but is not applicable to brittle and delicate microfossils such as conodonts. In addition to significantly increasing the workload required for extraction, the use of sodium-based dispersing agents increases the mechanical stress applied to microfossils through multiple sieving and washing. Moreover, etching and formation of salt precipitates on the surface of microfossils have been reported in the literature for all these chemicals (Hodgkinson, 1991) and confirmed by our own (EJ's) observations. The time required for acid digestion of marlstones, even without the subsequent use of deflocculants, is measured in weeks to months, whereas in Rewoquat disintegration time is of the order of several (1-14) days depending on the degree of cementation.
Methodological Biases Arising from the Differences between Extraction Techniques
In all approaches to microfossil extraction the effects of chemical or mechanical treatment do not have the same effect on all fossils, e.g., in acid digestion smaller conodonts are selectively more vulnerable to dissolution (Jeppsson et al., 1985, 1999), and in the Glauber's salt method the plaktonic foraminifers are destroyed in a higher proportion than benthic forms (Remin et al., 2012). Therefore an accurate account of the extraction protocol and reduction in the number of individuals lost through crushing, dissolving, and etching is crucial in collecting biostratigraphic data and for their statistical handling (Jeppsson, 2005). The bias introduced through selective destruction or inefficient extraction of the smallest and most fragile forms has a number of paleoecological and biostratigraphical implications (e.g., Jeppsson, 1997, 2005; McGowan et al., 2009). For instance, different conodont elements belonging to a single species are usually not recovered with expected frequencies. This is usually attributed to taphonomic processes (Bitter and Purnell, 2005; Purnell and Donoghue, 2005), but laboratory processing leading to the loss of smaller elements can enhance this disparity, and even completely obliterate the presence of the most fragile taxa (Jeppsson, 1997).
In addition to the importance for biostratigraphic and ontogenetic studies, detection and extraction of smallest individuals is essential in reconstructing the development of body size distributions. Intermittent shifting of body size distributions towards dwarf forms (the 'Lilliput effect', Urbanek, 1993) has been discussed for a number of taxonomic groups, e.g., during major paleoecological crises (Payne, 2005; He et al., 2007; McGowan et al., 2009; Posenato, 2009; Brayard et al., 2010; Payne et al., 2011; Sigurdsen, 2012). In the Silurian Period, the tendency to micromorphism also has been observed with respect to conodonts (Slavík et al., 2010), graptolites (Urbanek, 1993), and in brachiopods (Erlfeldt, 2006; Pakhnevich, 2009), and these trends have commonly been linked to unfavourable environmental conditions. Brachiopod populations inhabiting soft-bottom, marly habitats, and, in general, deeper waters, are considered to develop smaller body sizes (Bitner, 2002; Peck and Harper, 2010). As many studies are based on the collection of brachiopod specimens from bedding planes (e.g., Wertel, 1995; Pakhnevich, 2009), the proportion of micromorphic brachiopods may be easily underestimated. In the present study, from the MBCM sample we were able to recover brachiopod shells as small as 450 μm in length, and we suggest that the employment of Rewoquat may help in improving the representation of body-size trends of brachiopods and other groups of organisms with calcareous skeletons, e.g., trilobites and echinoderms, in which micromorphism has been less recognized. Rewoquat is efficient with most argillaceous, non-cemented lithologies, which have been shown to generally preserve higher biodiversity than cemented rocks, both due to taphonomic processes in the sediment, and to methodological problems in extracting fossils from indurated rocks (Hendy, 2009; Sessa et al., 2009; Hendy, 2011; Nawrot, 2012).
RECOMMENDATIONS FOR EXTRACTIONS WITH REWOQUAT
Disintegration should be performed on a dry sample and take place in a closed container to prevent evaporation. Broad, flat containers and gentle crushing of the sample with bare hands will increase the surface contacting with the reagent. Stirring with a soft object (e.g., a gloved hand or wooden stick) can speed up disintegration. Once disintegration comes to an end, Rewoquat should be decanted, the residue diluted with water and left to soak for at least one more day in a closed container. The decanted reagent can be recovered through decantation and sieving over a 63 μm mesh; the resulting reagent will contain clay particles, but it still can be used several times to recover larger fractions. The residue can be sieved in small portions and washed with a water brush. Ethanol can be added to reduce foaming. The procedure can be adapted for extraction of a particular group of fossils, e.g., for retiolitid graptolites the sieving and water brushing steps should be reduced to avoid fragmentation of rhabdosomes, and for delicate fossils supported by matrix, such as trilobites or articulated echinoderms, we recommend to coat the sample using a brush and hermetically close it in a plastic container. Following disintegration it can be handled in the same way as described above, with the remaining large pieces washed separately. They can be studied under the microscope for fragile fossils, which remain supported by the matrix. Rock pieces larger than 200 μm should be coated with Rewoquat again and the procedure repeated. This method allows release of the rock matrix with microfossils and examine delicate calcareous structures in the same time.
Disintegration in Rewoquat can be included in a multi-step procedure, e.g., to remove the clay and increase the effective porosity of a carbonate- or silica-cemented rock sample, which can be further subjected to acid dissolution or etching.
Rewoquat is inflammable and may irritate the skin (Xi R36/38). It should be stored in air-tight containers. Gloves and goggles are necessary (also during sieving and washing), and handling of samples in Rewoquat should, if possible, take place under a fume-cupboard.
CONCLUSIONS
- Microfossil extraction in the organic surfactant Rewoquat W 3690 PG was up to 10 times faster than acid dissolution when applied to two highly clayey rock types, thanks to preventing formation of clay aggregates. In both cases, Rewoquat allowed to recover calcareous fossils which were lost in acid extraction.
- We recommend the use of Rewoquat in microfossil extraction from marls and claystones. The method does not work for firmly cemented rocks such as silicaceous shales or dolostones.
- Extraction with Rewoquat is performed by soaking the sample in the surfactant in a sealed container. Following complete disintegration the remaining Rewoquat can be recovered by decantation, the residue should be then soaked with water for one more day and then sieved with excess water.
- Rewoquat does not dissolve or etch fossils. It allows to recover all types of microfossils, e.g., conodonts, retiolitid graptolites, chitinozoans, foraminifers, ostracodes, scolecodonts, etc. However, matrix-supported fossils such as trilobites may fall apart upon rock disintegration in Rewoquat; we recommend that in such cases Rewoquat should be used to clean them on a rock bedding plane.
ACKNOWLEDGMENTS
EJ and AM acknowledge the support of the Deutsche Forschungsgemeinschaft (project no. Mu 2352/3), PT, LF and SV — of the Czech Science Foundation (project No P210/12/2018); PT was also supported by the European Union through the European Social Fund (Mobilitas grant No MJD407); JS — by the Ministry of Culture of the Czech Republic (DKRVO 2013/04, National Museum, 00023272); S.V. — by the Alexander von Humboldt Foundation, funded by the Federal Ministry for Education and Research (Germany). EJ and AM thank A. Bancroft for instructions on the Jeppsson's buffered acid method, and the colleagues at GZN Nordbayern: B. Leipner-Mata and Ch. Schulbert for help in sample preparation and SEM work, S. Krumm and M. Hertel for the XRD analyses, O. Lehnert for help with heavy liquid separations, student assistants K. Frisch and S. Pröpster for help in lab work, and C. Färber for collecting the MBCM sample in Gotland. We thank two anonymous reviewers and the PE Editor S. Gerber for their suggestions which helped us to improve the previous version of the manuscript, O. Hints for help in identification of scolecodonts, P. Lukeš — of dacryoconarid tentaculites, V. Nestor — of chitinozoans, E. Olempska — of ostracods, P. Taylor and M. Wilson — of Allonema; O. Vinn — of cornulitids and M. Zapalski — of tabulate corals. This paper is a contribution to the International Geoscience Programme (IGCP) Project 591 – The Early to Middle Paleozoic Revolution and 596 – Climate change and biodiversity patterns in the Mid-Palaeozoic.
REFERENCES
Babinot, J.-F. and Colin, J.-P. 2011. Barremian ostracods from the Serre de Bleyton (Drôme, SE France). Annalen des Naturhistorischen Museums Wien, Serie A, 113.
Barrande, J. 1850. Graptolites de Bohême. Extrait du Systême Silurien du centre de la Bohême. Self-published, Prague.
Bates, C.D., Coxon, P., and Gibbard, P.L. 1978. A new method for the preparation of clay-rich sediment samples for palynological investigation. New Phytologist, 81:459-463.
Bates, D.E.B., Kozłowska, A., and Lenz, A.C. 2005. Silurian retiolitid graptolites: Morphology and evolution. Acta Palaeontologica Polonica, 50:705-720.
Bergman, C.F. 1989. Silurian paulinitid polychaetes from Gotland. Fossils and Strata, 25:1-128.
Bitner, M.A. 2002. Size-frequency distributions of Miocene micromorphic brachiopods: interpretation tool for population dynamics. Marine Ecology, 23:19-30.
Bitter, v.P.H. and Purnell, M. 2005. An experimental investigation of post-depositional taphonomic bias in conodonts. Special Papers in Palaeontology, 73:39-56.
Blind, W. 1970. Epizoen als Anzeiger der Lebendstellung von Tentaculites sp. Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen, 136:243-261.
Brayard, A., Nützel, A., Stephen, D.A., Bylund, K.G., Jenks, J., and Bucher, H. 2010. Gastropod evidence against the Early Triassic Lilliput effect. Geology, 38:147-150.
Calner, M. and Jeppsson, L. 2003. Carbonate platform evolution and conodont stratigraphy during the middle Silurian Mulde Event, Gotland, Sweden. Geological Magazine, 140:173-203.
Calner, M., Jeppsson, L., and Eriksson, M.J. 2004a. Ytterholmen revisited – implications for the Late Wenlock stratigraphy of Gotland and coeval extinctions. GFF, 126:231-241.
Calner, M., Jeppsson, L., and Munnecke, A. 2004b. The Silurian of Gotland - Part I: Review of the stratigraphic framework, event stratigraphy, and stable carbon and oxygen isotope development. Erlanger geologische Abhandlungen - Sonderband, 5:113-131.
Calner, M., Lehnert, O., and Jeppsson, L. 2012. New chemostratigraphic data through the Mulde Event interval (Silurian, Wenlock), Gotland, Sweden. GFF, 134:65-67.
Calner, M., Sandström, O., and Mõtus, M.-A. 2000. Significance of a Halysitid-Heliolitid Mud-facies Autobiostrome from the Middle Silurian of Gotland, Sweden. PALAIOS, 15:511-523.
Chauff, K.M. 1978. Recovery of Ordovician conodonts by hydrochloric acid from phosphate nodules reworked into the Sulphur Springs Formation (Devonian) in Missouri, USA, Geological Magazine, 115:205-209.
Chlupáč, I. 1959. Facial development and biostratigraphy of Daleje Shales and Hlubočepy Limestones (Eifelian) in the Devonian of Central Bohemia. Sborník Ústředního ústavu geologického, 25:445-511.
Chlupáč, I. and Kukal, Z. 1986. Reflections of possible global Devonian events in the Barrandian area, C.S.S.R., p. 169-179. In Walliser, O. (ed.), Global Bio-Events. Lecture Notes in Earth Science. Springer-Verlag, Berlin.
Chlupáč, I. and Lukeš, P. 1999. Pragian/Zlíchovian and Zlíchovian/Dalejan boundary sections in the Lower Devonian of the Barrandian area, Czech Republic. Newsletters on Stratigraphy, 37:75-100.
Chlupáč, I., Lukeš, P., and Zikmundová, J. 1979. The Lower/Middle Devonian boundary beds in the Barrandian area, Czechoslovakia. Geologica et palaeontologica, 13:125-155.
Coil, J., Korstanje, M.A., Archer, S., and Hastorf, C.A. 2003. Laboratory goals and considerations for multiple microfossil extraction in archaeology. Journal of Archaeological Science, 30:991-1008.
Dobrowolska, K. 2012. Reconstruction of the proximal ends of retiolitid rhabdosomes (Graptolithina) from the Upper Wenlock and the Lower Ludlow. Paläontologische Zeitschrift, 87:1-17.
Erlfeldt, Å. 2006. Brachiopod faunal dynamics during the Silurian Ireviken Event, Gotland, Sweden. Examensarbeten i Geologi vid Lunds universitet, Nr. 199, 22 sid. 20 poäng. Lund University, Lund.
Etherington, R.L. and Austin, R.L. 1993. Note on the use of hydrofluoric acid for the recovery of conodonts from Ordovician cherts in the Southern Uplands of Scotland and the significance of the conodonts. Journal of Micropalaeontology, 12:194.
Ferrová, L., Frýda, J., and Lukeš, P. 2012. High-resolution tentaculite biostratigraphy and facies development across the Early Devonian Daleje Event in the Barrandian (Bohemia): implications for global Emsian stratigraphy. Bulletin of Geosciences, 87:587-624.
Ford, P.B. and Lee, D.E. 1997. Note on a new method of using hydrofluoric acid for the study of conodonts in cherts in the Torlesse terrane, New Zealand. Journal of Micropalaeontology, 16:158.
Freeman, G., and Lundelius, J.W. 2007. Variance in protegular size: a correlate of larval trophic mode in Early Palaeozoic brachiopods. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 98:233-238.
Grahn, Y. and Afzelius, B.A. 1980. Ultrastructural studies of some chitinozoan vesicles. Lethaia, 13:119-126.
Hanna, G.D. and Church, C.C. 1928. Freezing and thawing to disintegrate shales. Journal of Paleontology, 2:131.
He, W., Shi, G.R., Feng, Q., Campi, M.J., Gu, S., Bu, J., Peng, Y., and Meng, Y. 2007. Brachiopod miniaturization and its possible causes during the Permian–Triassic crisis in deep water environments, South China. Palaeogeography, Palaeoclimatology, Palaeoecology, 252:145-163.
Hede, J.E. 1942. On the correlation of the Silurian of Gotland. Meddelanden från Lunds Geologisk-Mineralogiska Institution, 101:1-25.
Hede, J.E. 1960. The Silurian of Gotland, p. 44-89. In Regnell, G. and Hede, J.E. (eds.), The Lower Paleozoic of Scania, The Silurian of Gotland. Geological Survey of Sweden, International Geological Congress, XXI Session, Norden.
Heldt, M., Bachmann, M., and Lehmann, J. 2008. Microfacies, biostratigraphy, and geochemistry of the hemipelagic Barremian–Aptian in north-central Tunisia: Influence of the OAE 1a on the southern Tethys margin. Palaeogeography, Palaeoclimatology, Palaeoecology, 261:246-260.
Hendy, A.J.W. 2009. The influence of lithification on Cenozoic marine biodiversity trends. Paleobiology, 35:51-62.
Hendy, A.J.W. 2011. Taphonomic Overprints on Phanerozoic Trends in Biodiversity: Lithification and Other Secular Megabiases, p. 19-77. In Allison, P.A. and Bottjer, D.J. (eds.), Taphonomy. Aims & Scope Topics in Geobiology Book Series. Springer Netherlands
Hodgkinson, R.L. 1991. Microfossil Processing: A Damage Report. Micropaleontology, 37:320-326.
Holbourn, A.E.L. and Kuhnt, W. 1998. Turonian-Santonian benthic foraminifer assemblages from site 959D (Côte d'Ivoire-Ghana transform margin, Equatorial Atlantic): indication of a Late Cretaceous oxygen minimum zone, p. 375-387. In Mascle, J., Lohmann, G.P., and Moullade, M. (eds.), Proceedings of the Ocean Drilling Program, Scientific Results. Ocean Drilling Program, College Station, TX.
Jeppsson, L. 1983. Silurian conodont faunas from Gotland. Fossils and Strata, 12:121-144.
Jeppsson, L. 1997. A new latest Telychian, Sheinwoodian and Early Homerian (Early Silurian) Standard Conodont Zonation. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 88:91-114.
Jeppsson, L. 2005. Biases in the recovery and interpretation of micropalaeontological data. Special Papers in Palaeontology, 73:57-71.
Jeppsson, L. and Anehus, R. 1995. A buffered formic acid technique for conodont extraction. Journal of Paleontology, 69:790-794.
Jeppsson, L., Anehus, R., and Fredholm, D. 1999. The optimal acetate buffered acetic acid technique for extracting phosphatic fossils. Journal of Paleontology, 73:964-972.
Jeppsson, L., Fredholm, D., and Mattiasson, B. 1985. Acetic acid and phosphatic fossils: a warning. Journal of Paleontology, 59:952-956.
Jozefaciuk, G. 2002. Effect of acid and alkali treatments on surface-charge properties of selected minerals. Clays and Clay Minerals, 50:647-656.
Kozłowska-Dawidziuk, A. 1991. Agastograptus from the Mulde Beds of Gotland. Acta Palaeontologica Polonica, 36:143-149.
Kozłowska-Dawidziuk, A. 2004. Evolution of retiolitid graptolites – a synopsis. Acta Palaeontologica Polonica, 49:505-518.
Krüger, F.J. 1994. Präparieren mit REWOQUAT - eine Methode für Kalkmergel-Fossilien. Arbeitskreis Paläontologie Hannover, 22:45-47.
Larsson, K. 1979. Silurian tentaculitids from Gotland and Scania. Fossils and Strata, 11:1-180.
Laufeld, S. 1974a. Reference localities or palaeontology and geology in the Silurian of Gotland. Sveriges Geologiska Undersökning, ser. C, 705:1-172.
Laufeld, S. 1974b. Silurian chitinozoa from Gotland. Fossils and Strata, 5:1-130.
Lentfer, C.J. and Boyd, W.E. 1999. An assessment of techniques for the deflocculation and removal of clays from sediments used in phytolith analysis. Journal of Archaeological Science, 26:31-44.
Lierl, H.-J. 1992. Tenside - ihre Verwendung für die Präparation geologisch-paläontologischer Objekte. Der Präparator, 38:11-17.
Martinsson, A. 1967. The succession and correlation of ostracode faunas in the Silurian of Gotland. Geologiska Föreningen i Stockholm Förhandlingar, 89:350-386.
McGowan, A.J., Smith, A.B., and Taylor, P.D. 2009. Faunal diversity, heterogeneity and body size in the Early Triassic: testing post-extinction paradigms in the Virgin Limestone of Utah, USA. Australian Journal of Earth Sciences, 56:859-872.
Mierzejewski, P. 1988. Encrusting graptolites from the Mulde Beds of Gotland. Acta Palaeontologica Polonica, 33:261-266.
Miller, T.H. 1967. Techniques for processing and photographing chitinozoans. The University of Kansas Paleontological Contributions, 21:1-10.
Nagy, J. 2005. Delta-influenced foraminiferal facies and sequence stratigraphy of Paleocene deposits in Spitsbergen. Palaeogeography, Palaeoclimatology, Palaeoecology, 222:161-179.
Nawrot, R. 2012. Decomposing lithification bias: preservation of local diversity structure in recently cemented storm-beach carbonate sands, San Salvador Island, Bahamas. PALAIOS, 27:190-205.
Pakhnevich, A.V. 2009. Reasons of micromorphism in modern or fossil brachiopods. Paleontological Journal, 43:1458-1468.
Payne, J.L. 2005. Evolutionary dynamics of gastropod size across the end-Permian extinction and through the Triassic recovery interval. Paleobiology, 31:269-290.
Payne, J.L., Summers, M., Rego, B.L., Altiner, D., Wei, J., Yu, M., and Lehrmann, D.J. 2011. Early and Middle Triassic trends in diversity, evenness, and size of foraminifers on a carbonate platform in south China: implications for tempo and mode of biotic recovery from the end-Permian mass extinction. Paleobiology, 37:409-425.
Peck, L.S. and Harper, E.M. 2010. Variation in size of living articulated brachiopods with latitude and depth. Marine Biology, 157:2205-2213.
Petránek, J. 1950. Petrografická studie o nejmladších devonských vrstvách v Dalejském údolí u Prahy (Petrological Study on Youngest Devonian Formations in the Daleje Valley (near Prague). Rozpravy II. třídy České akademie, 60(19):1-16.
Pojeta, J.J. and Balanc, M. 1989. Freezing and thawing of fossils, p. 223-226. In Feldmann, R.M., Chapman, R.E., and Hannibal, J.T. (eds.), Paleotechniques. The Paleontological Society, Special Publication. The Paleontological Society, Knoxville.
Posenato, R. 2009. Survival patterns of macrobenthic marine assemblages during the end-Permian mass extinction in the western Tethys (Dolomites, Italy). Palaeogeography, Palaeoclimatology, Palaeoecology, 280:150-167.
Purnell, M. and Donoghue, C.J. 2005. Between death and data: biases in interpretation of the fossil record of conodonts. Special Papers in Palaeontology, 73:7-25.
Remin, Z., Dubicka, Z., Kozłowska, A., and Kuchta, B. 2012. A new method of rock disintegration and foraminiferal extraction with the use of liquid nitrogen [LN2]. Do conventional methods lead to biased paleoecological and paleoenviromental interpretations? Marine Micropaleontology, 86-87:11-14.
Riegraf, W. and Niemeyer, J. 1996. Agglutinierte Foraminiferen aus Graptolithen-Schwarzschiefern des Llanvirnium (Ordovizium) von Plettenberg im Sauerland (Nordrhein-Westfalen, NW-Deutschland). Paläontologische Zeitschrift, 70:19-36.
Schopf, J.M. 1965. A method for obtaining small acid-resistant fossils from ordinary solution residues, p. 301-304. In Kummel, B. and Raup, D.M. (eds.), Handbook of paleontological techniques. W.H. Freeman and Company, San Francisco.
Sessa, J.A., Patzkowsky, M.E., and Bralower, T.J. 2009. The impact of lithification on the diversity, size distribution, and recovery dynamics of marine invertebrate assemblages. Geology, 37:115-118.
Sigurdsen, A. 2012. Size change in brachiopods and trilobites of the Oslo Region during the Ordovician and during the Ordovician-Silurian transition. Evidence of Cope's Rule and the Lilliput Effect?, Master thesis, University of Oslo, Oslo.
Slavík, L., Kříž, J., and Carls, P. 2010. Reflection of the mid-Ludfordian Lau Event in conodont faunas of Bohemia. Bulletin of Geosciences, 85:395-414.
Spjeldnaes, N. 1984. Epifauna as a tool in autecological analysis of Silurian brachiopods. Special Papers in Palaeontology, 32:225-235.
Teller, L. 1998. Abnormalities in development of colonies in some Monograptidae. In Gutiérrez-Marco, J.C. and Rábano, I. (eds.), 6th International Conference & Field Meeting, IUGS Subcommission on Silurian Stratigraphy. Temas Geologico-Minerós ITGE, Madrid, pp. 264-265.
Upshaw, C.F., Todd, R.G., and Allen, B.D. 1957. Fluoridization of Microfossils. Journal of Paleontology, 31(4):793-795.
Urbanek, A. 1993. Biotic crises in the history of Upper Silurian graptoloids: A Palaeobiological model. Historical Biology, 7:29-50.
Vinn, O. 2009. Attempted predation on Early Paleozoic cornulitids. Palaeogeography, Palaeoclimatology, Palaeoecology, 273:87-91.
Vinn, O. 2010. Adaptive strategies in the evolution of encrusting tentaculitoid tubeworms. Palaeogeography, Palaeoclimatology, Palaeoecology, 292:211-221.
Wertel, C. 1995. Die articulaten Brachiopoden der Mulde Schichten (Wenlock) von Gotland, Schweden, Diploma thesis, Ruprecht-Karls-Universität, Heidelberg.
Zalasiewicz, J.A., Page, A., Rickards, R.B., Williams, M., Wilby, P.R., Howe, M.P.A., and Snelling, A.M. 2013. Polymorphic organization in a planktonic graptoloid (Hemichordata: Pterobranchia) colony of Late Ordovician age. Geological Magazine, 150:143-152.
Zhao, Z. and Pearsall, D.M. 1998. Experiments for improving phytolith extraction from soils. Journal of Archaeological Science, 25:587-598.

