 Paleoneurology of Teyumbaita sulcognathus (Diapsida: Archosauromorpha) and the sense of smell in rhynchosaurs
Paleoneurology of Teyumbaita sulcognathus (Diapsida: Archosauromorpha) and the sense of smell in rhynchosaurs
Article number: 17.1.15A
https://doi.org/10.26879/378
Copyright Society for Vertebrate Paleontology, April 2014
Plain-language and multi-lingual abstracts
PDF version
Submission: 11 February 2013. Acceptance: 12 March 2014
{flike id=705}
ABSTRACT
Rhynchosaurs were a group of archosauromorphs that dominated the guild of herbivores during the early Late Triassic. Despite the large number of specimens available, paleobiological studies are rare in the literature, especially concerning the South American species. The present study analyzes the paleoneurology of Teyumbaita sulcognathus, a Brazilian hyperodapedontine rhynchosaur, along with its nasal cavity, based on tomographic images of the specimen UFRGS-PV-0232-T. Although the endocast only reveals the morphology of the posterior half of the encephalon due to the incompletely ossified braincase, it is possible to infer the presence of great olfactory bulbs because of their impressions left on the ventral surface of the frontals. Although the snout is relatively short, the areas of the nasal cavity probably devoted to olfaction were also large and, along with the size of the olfactory bulbs, it is possible to infer that olfaction was important for the behavior and ecology of T. sulcognathus, as previously proposed for Hyperodapedon.
Marcos A.F. Sales. Departamento de Paleontologia e Estratigrafia, Instituto de Geociências, Universidade Federal do Rio Grande do Sul, Av. Bento Gonçalves, 9500, Agronomia, CEP 91501-970, Porto Alegre, Rio Grande do Sul, Brazil. marcos.paleo@yahoo.com
Cesar L. Schultz. Departamento de Paleontologia e Estratigrafia, Instituto de Geociências, Universidade Federal do Rio Grande do Sul, Av. Bento Gonçalves, 9500, Agronomia, CEP 91501-970, Porto Alegre, Rio Grande do Sul, Brazil. cesar.schultz@ufrgs.br
Keywords: paleoneurology; rhynchosaurs; cranial endocast; computed tomography; behavior; olfaction
Final citation: Sales, Marcos A.F. and Schultz , Cesar L. 2014. Paleoneurology of Teyumbaita sulcognathus (Diapsida: Archosauromorpha) and the sense of smell in rhynchosaurs. Palaeontologia Electronica Vol. 17, Issue 1;15A; 10p. https://doi.org/10.26879/378
palaeo-electronica.org/content/2014/705-olfaction-in-rhynchosaurs
INTRODUCTION
Paleoneurology is the branch of paleobiology devoted to the study of brains of extinct vertebrates (Butler and Hodos, 2005), although it usually includes other structures housed in the braincase. Therefore, it represents the interface between two broader areas, paleontology and neurology. Paleoneurology can be also defined as the study of brain evolution (Buchholtz and Seyfarth, 2001; Witmer and Ridgely, 2008; Witmer et al., 2008).
The study of brains of extinct taxa is based in the majority of cases on analyzes of cranial endocasts, which will be termed throughout this text solely as endocasts. They are naturally formed by the infilling of the cavity of braincase by sediments and their consolidation during fossil diagenesis. As these casts replicate the internal morphology of the braincases, they also provide data concerning the morphology of the encephalon that was housed within the braincases, but the amount of detail that can be recovered from these casts varies among taxa. This is explained by the fact that the brains of many animals do not entirely fill the cranial cavity and, therefore, the meninges and venous sinuses located between the brain and the braincase walls are thicker preventing the brain surface from leaving detailed impressions on the internal surface of the braincase (Hopson, 1979; Buchholtz and Seyfarth, 2001; Rogers, 1999). In this context, mammals and birds have brains whose morphologies and some other features of their surfaces can be recovered with greater precision from endocasts (Radinsky, 1969, 1971; Buchholtz and Seyfarth, 2001; Dominguez-Alonso et al., 2004; Macrini et al., 2007a, 2007b; Dong, 2008). Among fossil diapsids, pterosaurs, oviraptorosaurs and probably other small theropods are clearly examples of exceptions among non-avian archosaurs because they also have brains nearly filling the cranial cavity completely (Dominguez-Alonso et al., 2004; Osmólska, 2004; Kundrát, 2007).
Ancient brains can also be accessed indirectly by impressions left on the ventral surfaces of skull roofs and artificial endocasts (Hopson, 1979; Buchholtz and Seyfarth, 2001; Osmólska, 2004; Zelenitsky et al., 2009). Although these techniques have been applied for many decades, the employment of computed tomography gave a new impulse for paleoneurology allowing non-invasive analyzes of rare specimens (Buchholtz and Seyfarth, 2001; Witmer et al., 2008; Witmer and Ridgely, 2008). From CT images it is possible to digitally reconstruct with specialized software the endocast of many taxa, and dinosaurs are classic examples whose paleoneurology has been revised and greatly expanded recently (Rogers, 1999; Brochu, 2000; Larsson et al., 2000; Fransoza and Rowe, 2005; Knoll and Schwarz-Wings, 2009; Rogers, 2005; Sanders and Smith, 2005; Kundrát, 2007; Sampson and Witmer, 2007; Sereno et al., 2007; Witmer et al., 2008; Witmer and Ridgely, 2008; Witmer and Ridgely, 2009).
Among non-avian archosaurs, along with non-avian dinosaurs, crocodilyforms, aetosaurs and pterosaurs are examples of groups with endocasts figured in the literature (Hopson, 1979; Witmer et al., 2003; Kley et al., 2010). Benton (1983) showed a natural internal mould of the braincase of a basal archosauromorph, more precisely, the rhynchosaur Hyperodapedon gordoni, also reporting the occurrence of impressions of olfactory bulbs on the ventral surface of the frontals of this species. Considering the size of these impressions and the nasal cavity of Hyperodapedon, this author proposed that rhynchosaurs had a keen sense of olfaction.
While the interespecific variation and evolution of neurological features is somehow well-known in taxa like dinosaurs and extinct carnivores (Radinsky, 1969, 1971; Dong, 2008; Sereno et al., 2007; Witmer and Ridgely, 2009), the same cannot be said about rhynchosaurs. Therefore, testing any generalization for this group requires the study of other species. Thus, we present here some paleoneurological data gathered by computed tomography (CT) from Teyumbaita sulcognathus, a rhynchosaur from the Late Triassic of Brazil. We also discuss briefly its nasal cavity to provide more clues for inferences concerning the olfaction of this taxon.
MATERIAL AND METHODS
The analyzed material is the holotypic skull of Teyumbaita sulcognathus (UFRGS-PV-0232-T), housed at the Universidade Federal do Rio Grande do Sul. It was found at the Linha Facão locality (29°40'12"S; 52°43'30"W), near the boundary between the towns of Candelária and Vera Cruz, in the State of Rio Grande do Sul, Brazil, in the stratigraphic levels known as the lower parts of Caturrita Formation, which encase fossils of the Hyperodapedon Assemblage Zone (Azevedo, 1982; Schultz, 1986, Azevedo and Schultz, 1987; Langer et al., 2007; Montefeltro et al., 2010). These levels are now considered to be the upper portions of a highstand system tract named Santa Maria Sequence 2 and are probably late Carnian in age (Azevedo and Schultz, 1987; Zerfass et al., 2003; Langer et al., 2007). Teyumbaita sulcognathus was first described as belonging to the genus Scaphonyx (Azevedo, 1982; Schultz, 1986, Azevedo and Schultz, 1987), but its reallocation in the present genus was made in 2010 (Montefeltro et al., 2010). It is a derived form of rhynchosaur and is included in the subfamily Hyperodapedontinae, being probably the sister-taxon of Hyperodapedon (Montefeltro et al., 2010).
The skull is fairly complete, being well preserved and articulated, ideal for CT scanning. In fact, the only disarticulated elements are both epipterygoids, but only the right one is missing. The scanning procedure took place at the Hospital das Clínicas de Porto Alegre, Porto Alegre, Brazil, using a Phillips Brilliance 16-Slice CT Scanner and yielded 249 slices in coronal slice plan with dimensions of 512x512 pixels, each pixel measuring 0,666 mm. The other CT scan parameters are: slice thickness of 1 mm, slice increment (interslice spacing) of 1 mm, field of view of 341 mm, 140 kV and 275 mA. Data were output from the scanner in DICOM format, and then imported into InVesalius 3.0 - Beta 2 (Centro de Tecnologia da Informação Renato Archer - CTI, Brazil). This software was used to visualize the impressions of the olfactory bulb and to digitally extract a natural endocast preserved inside the braincase of Teyumbaita sulcognathus. The discussion about the nasal cavity is based on the morphology of the osseous nasal cavity and employs the anglicized version of the terms adopted by Parsons (1970).
PALEONEUROLOGY OF TEYUMBAITA SULCOGNATHUS
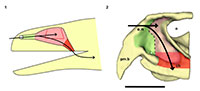
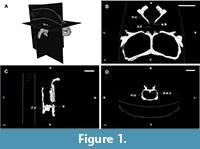 The braincase can be totally or partially ossified. In the first case, the endocast reflects the whole shape of the encephalon, and, in the second case, the preserved endocast corresponds to a varying degree of the entirety of the encephalon depending on the taxa. Rhynchosaurs fall within the second scenario and, hence, their endocasts are the internal moulds of the cavity delimited by the parietals, prootics, opistothics, supraoccipital, exooccitpitals, basioccipital and basisphenoid (Figure 1 and Figure 2; Benton, 1983; Azevedo, 1982; Montefeltro et al., 2010).
The braincase can be totally or partially ossified. In the first case, the endocast reflects the whole shape of the encephalon, and, in the second case, the preserved endocast corresponds to a varying degree of the entirety of the encephalon depending on the taxa. Rhynchosaurs fall within the second scenario and, hence, their endocasts are the internal moulds of the cavity delimited by the parietals, prootics, opistothics, supraoccipital, exooccitpitals, basioccipital and basisphenoid (Figure 1 and Figure 2; Benton, 1983; Azevedo, 1982; Montefeltro et al., 2010).
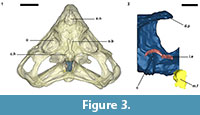
 An endocast was naturally formed and is partially preserved inside the braincase of Teyumbaita and is similar to the one of Hyperodapedon gordoni (Benton, 1983) in its overall shape (Figure 1.3 and Figure 3). It corresponds to the posterior half of the endocranial cavity and so brings information concerning only the posterior end of the encephalon, which limits the range of discussion. The contour of its anterior surface, if present, was probably destroyed during the preparation of the skull much before this study. The foramen magnum was almost empty, so its cavity was digitally filled and this procedure extended until the point where the supraoccipital stopped covering the medullary channel. This procedure also prevented the endocast from containing also the cast of the hypoglossal foramen.
An endocast was naturally formed and is partially preserved inside the braincase of Teyumbaita and is similar to the one of Hyperodapedon gordoni (Benton, 1983) in its overall shape (Figure 1.3 and Figure 3). It corresponds to the posterior half of the endocranial cavity and so brings information concerning only the posterior end of the encephalon, which limits the range of discussion. The contour of its anterior surface, if present, was probably destroyed during the preparation of the skull much before this study. The foramen magnum was almost empty, so its cavity was digitally filled and this procedure extended until the point where the supraoccipital stopped covering the medullary channel. This procedure also prevented the endocast from containing also the cast of the hypoglossal foramen.
 A dorsal protrusion or peak in the point of inflexion of the dorsal surface of the endocast is easily recognized and its extent is considerable. Some endocasts of dinosaurs also show peaks or expansions in the same location, and they were identified by Witmer et al. (2008) and Witmer and Ridgely (2009) as a dorsal venous sinus or dural expansions, which are soft structures located between the brain and the inner surface of the braincase walls. However, these structures are much less developed and pointed than in Teyumbaita sulcognathus; therefore, a dorsal venous sinus can explain only partially this peak of this rhynchosaur. As this structure projects itself parallel to the roof of the skull and seems to be absent in the endocast of Hyperodapedon gordoni (Benton, 1983), it is probably the result of a taphonomic distortion, despite the good preservation of the skull. In fact, Montefeltro et al. (2010) considered this skull to be more flat than it was when the animal was alive, consequently some degree of dorsoventral distortion is admissible to PV-0232-T. If we accept that a force compressed the skull in a dorsoventral plane, it is possible to assume that the vertical walls changed their orientation slightly, and this movement could have enlarged the space that was later filled, forming or, at least, enlarging the dorsal protrusion in the endocast.
A dorsal protrusion or peak in the point of inflexion of the dorsal surface of the endocast is easily recognized and its extent is considerable. Some endocasts of dinosaurs also show peaks or expansions in the same location, and they were identified by Witmer et al. (2008) and Witmer and Ridgely (2009) as a dorsal venous sinus or dural expansions, which are soft structures located between the brain and the inner surface of the braincase walls. However, these structures are much less developed and pointed than in Teyumbaita sulcognathus; therefore, a dorsal venous sinus can explain only partially this peak of this rhynchosaur. As this structure projects itself parallel to the roof of the skull and seems to be absent in the endocast of Hyperodapedon gordoni (Benton, 1983), it is probably the result of a taphonomic distortion, despite the good preservation of the skull. In fact, Montefeltro et al. (2010) considered this skull to be more flat than it was when the animal was alive, consequently some degree of dorsoventral distortion is admissible to PV-0232-T. If we accept that a force compressed the skull in a dorsoventral plane, it is possible to assume that the vertical walls changed their orientation slightly, and this movement could have enlarged the space that was later filled, forming or, at least, enlarging the dorsal protrusion in the endocast.
However, Benton (1983) identified in Hyperodapedon gordoni a pit in the ventral surface of the parietals and considered it to be the site of the pineal organ. Such a pit is also found in Teyumbaita sulcognathus, although it seems to be placed more posteriorly than in Benton's (1983) illustration (Figure 2). In T. sulcognathus, this pit covers the dorsal surface of the tip of the dorsal protrusion of the endocast, which may suggest that this structure is actually a cast of an anomalously enlarged pineal organ. Witmer and Ridgely (2009) found two dorsal peaks in digital endocasts of the theropod dinosaurs Majungasaurus, Allosaurus and Struthiomimus, of which the anteriormost one was identified as a cast of a pineal organ. Nevertheless, the location of the inferred pineal cast is much more anterior than the one suggested for rhynchosaurs. Moreover, the dorsal protrusion found in T. sulcognathus is located in a position more similar to the dural peaks found in those theropods. Thus, we suggest that the pit under the parietals is not the site of a pineal organ, and the dorsal peak in the endocast is in fact a taphonomically enlarged cast of a venous sinus or a dural expansion. More specimens will certainly clarify this question.
The anterior portion of the encephalon of Teyumbaita sulcognathus cannot be accessed in the endocast. When the frontals are the only ossified elements that surrounded the anterior half of the encephalon, it is necessary to look for impressions of the central nervous system in the ventral surface of frontals that usually have an hourglass shape (Ali et al., 2008; Zelenitsky et al., 2009). The frontals of T. sulcognathus do have a groove clearly referable to the impressions of the olfactory peduncle and the olfactory bulbs (Figure 1.2). These impressions are constrained by the cristae cranii and were already observed in Hyperodapedon gordoni by Benton (1983). As can be seen, the olfactory bulbs are connected to the brain by a developed olfactory peduncle or olfactory tract as in most tetrapods. On the other hand, pterosaurs, birds and some dinosaurs have reduced olfactory tracts or seem to lack them in a recognizable fashion (Witmer et al., 2003; Dominguez-Alonso et al., 2004; Evans, 2006; Kundrát, 2007; Witmer et al., 2008). Near the midpoint of the length of frontals, the groove bifurcates and the rising branches acquire an oval shape before reaching a large and ellipsoid depression, the last one almost entirely placed under the nasals. Benton (1983) inferred that the olfactory bulbs were located in large circular pits under the frontals of H. gordoni, so these pits must be topologically equivalent to the oval grooves found in T. sulcognathus. In Tyrannosaurus, the bifurcated portion of the groove was once interpreted as representing larges olfactory bulbs (Brochu, 2000). However, it is now interpreted as part of the olfactory region of the nasal cavity because the structure that divides the course of the groove is considered to be the mesethmoid, marking the anterior end of the olfactory bulbs (Ali et al., 2008; Witmer and Ridgely, 2009). A similar inference would be applicable to Teyumbaita due to the general topological correspondence. So, the shallow osseous structure that divides the impressions under the frontals could be the ossified portion of the nasal septum that corresponds to the avian mesethmoid. Thus, the olfactory bulbs would have occupied the space in the groove between the mesethmoid and the anterior end of the constricted portion of the hourglass, i.e., the olfactory peduncle. However, it would imply that the anterior end of the encephalon would be much more caudally positioned under the frontals in rhynchosaurs than in other diapsids. In addition, as these pits do not cross the boundaries between frontals and nasals (Figure 3.1), we prefer following Benton (1983) and supporting that the olfactory bulbs of rhynchosaurs occupied most part of the pits under the frontals, implying that the small ossified ventral keel of the frontals may not be an ossification of the nasal septum.
Finally, two of the three semicircular canals of each inner ear–the anterior and posterior ones– are recognizable within the braincase walls, but they await a full description and appreciation. However, they enable us to infer the approximate location of the cerebellum (Figure 3.2).
THE NASAL CAVITY OF TEYUMBAITA SULCOGNATHUS
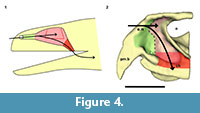 The nasal cavity can be seen as a tripartite structure in which the first and the last portion act as ducts that communicate the middle portion with the environment and the oral cavity, respectively (Figure 4). The first part is called the (nasal) vestibule which is usually a duct-like chamber that opens anteriorly to the environment through the external nares and posteriorly to the middle part called the nasal cavity proper. The middle portion is indeed a chamber in most cases and houses outpocketings known as conchae in reptiles and birds and turbinates in mammals. The third part is the nasopharyngeal duct and connects the nasal cavity proper to the oral cavity through the choanae. Only the nasal cavity proper presents olfactory epithelium and the other regions are covered by respiratory epithelium (Parsons, 1970; Evans, 2006; Schwenk, 2008).
The nasal cavity can be seen as a tripartite structure in which the first and the last portion act as ducts that communicate the middle portion with the environment and the oral cavity, respectively (Figure 4). The first part is called the (nasal) vestibule which is usually a duct-like chamber that opens anteriorly to the environment through the external nares and posteriorly to the middle part called the nasal cavity proper. The middle portion is indeed a chamber in most cases and houses outpocketings known as conchae in reptiles and birds and turbinates in mammals. The third part is the nasopharyngeal duct and connects the nasal cavity proper to the oral cavity through the choanae. Only the nasal cavity proper presents olfactory epithelium and the other regions are covered by respiratory epithelium (Parsons, 1970; Evans, 2006; Schwenk, 2008).
The description of the nasal cavity of Teyumbaita sulcognathus begins with the external nares. As in Hyperodapedon gordoni (Benton, 1983) and all rhynchosaurs, they are united in a single opening at the end of the snout, limited dorsally by the nasals and ventrolaterally by the pre-maxillae (Figure 3.1, Figure 4.2). With few exceptions among amniotes, the fleshy nostrils are located anteriorly and ventrally within the boundaries of the external nares and the same should have been true for rhynchosaurs (Witmer, 2001). Exceptions to this rule are always related to particular behavioral and physiological aspects that apparently were not present in rhynchosaurs, but even if they were, the terminal position of the external nares would make the fleshy nostrils functionally comparable to the most common condition among amniotes.
The air entered the nasal cavity through the fleshy nostrils and then ran along the vestibule to reach the nasal cavity proper. Although there is no nasal septum preserved, there is no reason to suppose that it was not present (Montefeltro et al., 2010), and this inference is even more plausible because the vomers support a dorsal expansion above which should have laid the septum (Figure 1.4; Benton, 1983). Because of the cartilaginous nature of other structures in the nasal capsule, it is not possible to determine exactly the specific internal details, specially the boundary between the vestibule and the nasal cavity proper. However, some attempts are possible. The vestibule is usually short and simple in most tetrapods, but there is a great diversity of forms among squamates. This diversity may be explained by the different lifestyles adopted by lizards and snakes, and the vestibules have the most complex courses and shapes in fossorial and aquatic species (Parsons, 1970). However, the position of the external nares and the abbreviated snout make it improbable that rhynchosaurs could have had nasal vestibules morphologically comparable to the extreme ones of some squamates. Rhynchosaurs seem to have lacked Jacobson's organs or have had them in a vestigial form (Sales and Schultz, in preparation). The cartilaginous capsule in the anterior portion of the nasal cavity that housed them should also have been absent or at least reduced, which implies that possibly there were no major obstacles to the airstream within the vestibule. Thus it is quite possible that the vestibules in Teyumbaita and other rhynchosaurs resembled those of terrestrial chelonians in general structure and had a relatively straight and horizontal course from the external nares to the nasal cavity proper (Figure 4.2; Parsons, 1970).
In many reptiles a ridge in the lateral wall of the cartilaginous capsule coincides with the boundary between the vestibule and the nasal cavity proper (Parsons, 1970). Such a ridge in the osseous wall of the nasal cavity was reported by Benton (1983) for Hyperodapedon gordoni and he suggested that it marked the limits between the anterior portion of the cartilaginous nasal capsule and the posterior, olfactory region. A similar ridge was found in each side of the internal surface of the nasal cavity of Teyumbaita sulcognathus, but they seem to be more posteroventrally inclined and more curved than in H. gordoni. If these ridges also coincided with the end of the vestibules, the last ones probably extended until near the anterior end of the choanae of T. sulcognathus, as in H. gordoni (Benton, 1983). These inferences would also imply that the nasal cavity proper was restricted to the posterior half of the cavity delimited by the bones of the snout (Figure 4.2).
The nasal cavity proper of Teyumbaita sulcognathus is as difficult to consider as the vestibule. The snout of T. sulcognathus is very deep and wide in cross-section, which suggests that the nasal cavity proper was also large (Figure 1.4, Figure 4.2). The size of the nasal cavity proper must be explained at least partially by the possession of large conchae. With the exception of turtles, amniotes in general have at least one concha in each nasal capsule (Parsons, 1970), and there is no reason to suppose it was absent in rhynchosaurs. The top of the nasal cavity proper must have been the large ellipsoid concavity in the ventral surface of the nasals, being posterior to the ridges in the internal surface of the snout (Figure 1.2). Dorsally its posterior end would have been just anterior to the olfactory bulbs. The olfactory epithelium is usually located posterodorsally within the nasal cavity proper and also covers the concha (Parsons, 1970). Due to the deepness and width of the snout, it seems that there was much space available for the sensory epithelium.
After the nasal cavity proper, the airstream passes through the nasopharyngeal duct to reach the oral cavity and then the upper respiratory tract. The nasopharyngeal ducts are longer in animals with secondary palate or with posteriorly displaced choanae (Parsons, 1970; Schwenk, 2008). There was no secondary palate in rhynchosaurs, and the nasal cavity proper lied dorsal to the choanae, which indicates that the ducts were extremely reduced, or absent, as in tuataras and some lizards (Figure 4.2).
Finally, the nasal capsule was probably separated from the rest of the skull by internal ridges anterior to the orbits. They coincide with the inflexion point of the dorsal ridge of the palate considered by Benton (1983) to mark the boundary between the nasal septum and the interorbital septum. Thus, we also support the same inference.
DISCUSSION
Paleobiological inferences must be based, whenever possible, in a phylogenetic framework following the Extant Phylogenetic Bracket (Witmer, 1995). However the recognition of true osteological correlates of some structures of the nasal capsule is usually not possible because many of them rely on cartilaginous supports and do not leave clear impressions on the inner surface of the snout (Parsons, 1970). Another fact that complicates the employment of the EPB is that it is difficult to reconstruct the plesiomorphic condition of Lepidosauria, one of the clades that brackets rhynchosaurs in cladograms, due to the great variety of types of nasal capsules among squamates (Parsons, 1970). Further considerations about paleoneurology of Teyumbaita sulcognathus are restricted to the preserved traits of the brain and its associated structures. In fact, as the brain of most reptiles does not entirely fill the endocranial cavity and the same should be expected for rhynchosaurs, we must be cautious about the proposed inferences.
Analyzing the functionality and physiology of any structure of the nervous system requires the knowledge about the number of neurons and their arrangements along with the number and nature of interactions among them, i.e., the synapses. This sort of information cannot be gathered from fossils or endocasts (Hopson, 1979; Rogers, 2005) and so the discussion about paleoneurology relies on the principle of proper mass. In few words, this principle announces that the proportion (volume or mass) of a particular neural structure is proportional to the amount of information processed or function carried out by it (Witmer et al., 2003; Butler and Hodos, 2005; Witmer et al., 2008). The unpreserved portion of the encephalon precludes inferences about the brain hemispheres and visual lobes and comparisons between them and olfactory bulbs (Figure 3). In such a framework, Benton (1983) suggested that vision was good in Hyperodapedon gordoni because of the large orbits and well-developed sclerotic rings. He also proposed for that taxon a developed sense of smell due to the large size of the olfactory bulbs and nasal cavity as a whole. Rhynchosaurs in general have large orbits (Figure 2; Langer and Schultz, 2000) and the impressions of the olfactory bulbs of Teyumbaita sulcognathus are quite similar to the ones of H. gordoni.
Kundrát (2007) noticed reduced olfactory bulbs and the absence of olfactory peduncles in Conchoraptor gracilis, a condition also observed in birds. This could suggest reduced olfaction in this theropod, but the author hypothesized that the sensory epithelium could have been developed in a way to compensate for the small size of the nasal cavity and olfactory bulbs. However, this sort of inference has no support and relies only on speculation (Witmer, 1995; Benton, 2010). On the other hand we must consider that the general proportions of sensory and neural structures may vary among animals without clear implications for their sensory biology. For example, crocodiles and theropods have different olfactory ratios and the former possesses the highest ratios (Zelenitsky et al., 2009). However, tyrannosaurids are thought to have a keen sense of olfaction, maybe better developed or with a greater relative importance for behavior than in crocodiles (Rogers, 2005; Brochu, 2000; Witmer et al., 2008; Witmer and Ridgely, 2009). Although rhynchosaurs had abbreviated snouts (Langer and Schultz, 2000), the regions devoted to olfaction within the nasal cavities seem to have been very deep and there was no considerable reduction of the olfactory system during the evolution of the snout, with the retention of the olfactory tract and the bulbs being wider than the tract (Figure 1.2, Figure 3), different from what is observed in some herbivorous and omnivorous dinosaur taxa (Evans, 2006; Kundrát, 2007; Witmer et al., 2008). It may be difficult to ideally appreciate these features without mathematically and statistically comparing proportions and ratios, once that it is not possible to determine precisely the size of olfactory bulbs and cerebral hemispheres for rhynchosaurs as was performed by Zelenitsky et al. (2009). However, along with the terminal position of the external nares (Witmer, 2001), these features do suggest a great importance of olfaction in the ecology and behavior of rhynchosaurs because these are the sort of evidences of the possession of a good sense of smell expected to be found in fossils. Moreover, the reduced size of the snout of rhynchosaurs seems to be more apparent than real after Chatterjee (1974) noticed a greater development of the temporal region of the skull during the evolution of this taxon, instead of a true reduction of the snout. These assertions are in agreement with the observations here made about the olfactory system not being reduced or possibly even more developed than in other reptiles.
The inferences made for Teyumbaita sulcognathus are similar to those previously proposed for Hyperodapedon gordoni, and both taxa are included in the subfamily Hyperodapedontinae; therefore, good olfaction is possibly a feature of other hyperodapedontine rhynchosaurs. If it is also applicable to other rhynchosaurs it requires the analysis of other species. Hyperodapedontine rhynchosaurs probably had their perception of the environment based on good vision and olfaction (Benton, 1983). Moreover, olfaction might have been the only or the main chemical sense because of the reduction or absence of vomerolfaction (Sales and Schultz, in preparation) and the degree of development of the olfactory system. So, olfaction may have conveyed some social roles once played by vomerolfaction (Schwenk, 2008) and others like the recognition of offspring or location of predators in the surroundings. It may have been also useful for locating some kind of food resource and the nostrils far rostrally placed would favour the collection of any sort of odor. However, reconstructing the behavior and its physiological aspects are more difficult than making anatomical inferences, because the last ones rely on osteological correlates and preserved soft tissues, whereas the former requires other pieces of evidence (Benton, 2010). New clues are necessary to test the hypotheses proposed for the ecological role of the olfactory system of rhynchosaurs.
ACKNOWLEDGMENTS
We thank the Hospital das Clínicas de Porto Alegre for CT scanning of the specimen UFRGS-PV-0232-T. We also thank P. Rodrigues, D. Fortier and A. Liparini for their helpful comments and advices about CT scanning and 3D image softwares. M. Benton and an anonymous reviewer contributed greatly to improve this paper. Finally, we especially thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for funding this research.
REFERENCES
Ali, F., Zelenitsky, D.K., Therrien, F., and Weishampel, D.B. 2008. Homology of the ethmoid complex of tyrannosaurids and its implications for the reconstruction of the olfactory apparatus of non-avian theropods. Journal of Vertebrate Paleontology, 28:123-133.
Azevedo, S.A.K. 1982. Scaphonyx sulcognathus (sp. nov.) um novo rincossaurídeo do Neotriássico do Rio Grande do Sul, Brasil. Unpublished MSc Thesis, Instituto de Geociências, Universidade Federal do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul, Brazil.
Azevedo, S.A.K. and Schultz, C.L. 1987. Scaphonyx sulcognathus (sp. nov.), um novo rincossaurídeo do Neotriássico do Rio Grande do Sul, Brasil, p. 99-113. In Moura, J.A., Gilson H.M.N., Campos, D.A., Beurlen, G., Macedo, A.C.M., and Brito, I.A.M. Brito (eds.), X Congresso Brasileiro de Paleontologia. Sociedade Brasileira de Paleontologia: Rio de Janeiro.
Benton, M.J. 1983. The Triassic reptile Hyperodapedon from Elgin: functional morphology and relationships. Philosophical Transactions of the Royal Society of London B, 302:605-717.
Benton, M.J. 2010. Studying function and behavior in the fossil record. PLoS ONE, 8(3):1-5.
Brochu, C.A. 2000. A digitally-rendered endocast for Tyrannosaurus rex. Journal of Vertebrate Paleontology, 20:1-6.
Buchholtz, E. A. and Seyfarth, E.-A. 2001. The study of "fossil brains": Tilly Edinger (1897-1967) and the beginnings of paleoneurology. Bioscience, 51:674-682.
Butler, A.B. and Hodos, W. 2005. Comparative Vertebrate Neuroanatomy: Evolution and Adaptation (second edition). Jon Wiley and Sons, Hoboken, New Jersey.
Chatterjee, S. 1974. A rhynchosaur from the Upper Triassic Maleri Formation of India. Philosophical Transactions of the Royal Society of London B, 267:209-261.
Dominguez-Alonso, P., Milner, A.C., Ketcham, R.A., Cookson, M.J., and Rowe, T. 2004. The avian nature of the brain and inner ear of Archaeopteryx. Nature, 430:666-669.
Dong, W. 2008. Virtual cranial endocast of the oldest giant panda (Ailuropoda microta) reveals great similarity to that of its extant relative. Naturwissenschaften, 95:1079-1083.
Evans, D.C. 2006. Nasal cavity homologies and cranial crest function in lambeosaurine dinosaurs. Paleobiology, 32:109-125.
Fransoza, J. and Rowe, T. 2005. Cranial endocast of the Cretaceous theropod dinosaur Acrocanthosaurus atokensis. Journal of Vertebrate Paleontology, 25:859-864.
Hopson, J.A. 1979. Paleoneurology, p. 39-146. In Gans, C., Northcutt, R.C., and Ulinski, P. (eds.), Biology of the Reptilia (volume 9). Academic Press, New York.
Kley, N.J., Sertich, J.J.W., Turner, A.H., Krause, D.W., O'Connor, P.M., and Georgi, J.A. 2010. Craniofacial morphology of Simosuchus clarki (Crocodyliformes: Notosuchia) from the Late Cretaceous of Madagascar. Society of Vertebrate Paleontology Memoir 8, Journal of Vertebrate Paleontology, 30 (Supplement 6):13-98.
Knoll, F. and Schwarz-Wings, D. 2009. Paleoneuroanatomy of Brachiosaurus. Annales de Paléontologie, 95:165-175.
Kundrát, M. 2007. Avian-like attributes of a virtual brain model of the oviraptorid theropod Conchoraptor gracilis. Naturwissenschaften, 94:499-504.
Langer, M.C. and Schultz, C.L. 2000. Rincossauros – herbívoros cosmopolitas do Triássico, p. 246–272. In Holz, M. and de Ros, L.F. (eds.), Paleontologia do Rio Grande do Sul. CIGO/UFRGS, Porto Alegre.
Langer, M.C., Ribeiro, A.M., Schultz, C.L., and Ferigolo, J. 2007. The continental tetrapod-bearing Triassic of South Brazil, p. 201-208. In Lucas, S.G. and Spielmann, J.A. (eds.), The Global Triassic: New Mexico Museum of Natural History and Science Bulletin. New Mexico Museum of Natural History and Science, Albuquerque, New Mexico.
Larsson, H.C.E., Sereno, P.C., and Wilson, J.A. 2000. Forebrain enlargement among nonavian theropod dinosaurs. Journal of Vertebrate Paleontology, 20:615-618.
Macrini, T.E., de Muizon, C., Cifelli, R.L., and Rowe, T. 2007a. Digital cranial endocast of Pucadelphys andinus, a Paleocene metatherian. Journal of Vertebrate Paleontology, 27:99-107.
Macrini, T.E., Rougier, G.W., and Rowe, T. 2007b. Description of a cranial endocast from the fossil mammal Vinceleste neuquenianus (Theriiformes) and its relevance to the evolution of endocranial characters in therians. Anatomical Record, 290:875-892.
Montefeltro, F.C., Langer, M.C., and Schultz, C.L. 2010. Cranial anatomy of a new genus of hyperodapedontine rhynchosaur (Diapsida, Archosauromorpha) from the Upper Triassic of Southern Brazil. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 101:27-52.
Osmólska, H. 2004. Evidence on relation of brain to endocranial cavity in oviraptorid dinosaurs. Acta Palaeontologica Polonica, 49:321-324.
Parsons, T.S. 1970. The nose and Jacobson's organ, p. 99-191. In Gans, C. and Parsons, T.S. (eds.), Biology of the Reptilia (volume 2). Academic Press, London.
Radinsky, L. 1969. Outlines of canid and felid brain evolution. Annals of the New York Academy of Science, 167:277-288.
Radinsky, L. 1971. An example of parallelism in carnivore brain evolution. Evolution, 25:518-522.
Rogers, S.W. 1999. Allosaurus, crocodiles, and birds: evolutionary clues from spiral computed tomography of an endocast. Anatomical Record, 257:162-173.
Rogers, S.W. 2005. Reconstructing the behaviors of extinct species: an excursion into comparative paleoneurology. American Journal of Medical Genetics, 134:349-356.
Sampson, S. and Witmer, L.M. 2007. Craniofacial anatomy of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the Late Cretaceous of Madagascar. Society of Vertebrate Paleontology Memoir 8, Journal of Vertebrate Paleontology, 27(Supplement 2):32-102.
Sanders, R.K. and Smith, D.K. 2005. The endocranium of the theropod dinosaur Ceratosaurus studied with computed tomography. Acta Palaeontologica Polonica, 50:601-616.
Schultz, C.L. 1986. Osteologia parcial do pós crânio de Scaphonyx sulcognathus Azevedo 1982 (Reptilia, Diapsida, Rhynchocephalia). Unpublished MSc Thesis, Instituto de Geociências, Universidade Federal do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul, Brazil.
Schwenk, K. 2008. Comparative anatomy and physiology of chemical senses in nonavian aquatic reptiles, p. 65-81. In Thewissen, J.G.M. and Nummela, S. (eds.), Sensory Evolution on the Thresholds: Adaptations in Secondarily Aquatic Vertebrates. University of California Press, Berkeley, California.
Sereno, P.C., Wilson, J.A., Witmer, L.M., Whitlock, J.A., Maga, A., Ide, O., and Rowe, T.A. 2007. Structural extremes in a Cretaceous dinosaur. PLoS ONE, 2(11):1-9.
Witmer, L.M. 1995. The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils, p. 19-33. In Thomason, J.J. (ed.), Functional Morphology in Vertebrate Paleontology. Cambridge University Press, Cambridge.
Witmer, L.M. 2001. Nostril position in dinosaurs and other vertebrates and its signifcance for nasal function. Science, 293:850-853.
Witmer, L.M., Chatterjee, S., Fransoza, J., and Rowe, T. 2003. Neuroanatomy of flying reptiles and implications for flight, posture and behavior. Nature, 425:950-953.
Witmer, L.M. and Ridgely, R.C. 2008. Structure of the brain cavity and inner ear of the centrosaurine ceratopsid dinosaur Pachyrhinosaurus based on CT scanning and 3D visualization, p. 117-144. In Currie, P.J., Langston, W., Jr., and Tanke, D.H. (eds.), A New Horned Dinosaur from an Upper Cretaceous Bone Bed in Alberta. National Research Council of Canada Monograph Series, Ottawa.
Witmer, L.M. and Ridgely, R.C. 2009. New insights into the brain, braincase, and ear region of tyrannosaurs (Dinosauria, Theropoda), with implications for sensory organization and behavior. Anatomical Record, 292:1266-1296.
Witmer, L.M., Ridgely, R.C., Dufeau, D.L., and Semones, M.C. 2008. Using CT to peer into the past: 3D visualization of the brain and ear regions of birds, crocodiles, and nonavian dinosaurs, p. 67-87. In Edno, H. and Frey, R. (eds.), Anatomical Imaging: Towards a New Morphology. Springer-Verlag, Tokyo.
Zelenitsky, D.K., Therrien, F., and Kobayashi, Y. 2009. Olfactory acuity in theropods: palaeobiological and evolutionary implications. Proceedings of the Royal Society B, 276:667-673.
Zerfass, H., Lavina, E.L., Schultz, S.C., Garcia, A.G.V., Faccini, U.F., and Chemale, F., Jr. 2003. Sequence stratigraphy of continental Triassic strata of southernmost Brazil, a contribution to Southwestern Gondwana palaeogeography and palaeoclimate. Sedimentary Geology, 161:85-105.

