 The enigmatic arthropod Camptophyllia
The enigmatic arthropod Camptophyllia
Article number: 15.2.15A
https://doi.org/10.26879/268
Copyright Palaeontological Association, April 2012
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 3 February 2011. Acceptance: 18 February 2012.
{flike id=218}
ABSTRACT
The enigmatic Upper Carboniferous arthropod genus Camptophyllia is known from 11 fossils, found at five Coal Measures Lagerstätten. These siderite-hosted fossils reveal only the organism's dorsal surface – its ventral and appendage morphology is entirely unknown, hampering efforts to place the genus taxonomically or phylogenetically. This study reports the application of high-resolution X-ray micro-tomography (XMT) to six Camptophyllia specimens, from four Carboniferous Lagerstätten. This XMT-based restudy has provided new morphological detail, confirming the anteriormost segment is cephalic and facilitating more informed speculation regarding the organism's mode of life. However, despite scanning all but one of the known representatives of the genus, ventral anatomy has not been resolved; it is possible this is taphonomic, resulting from a poorly sclerotized ventral region. Pending the discovery of further material, the affinities of Camptophyllia remain unclear.
Russell J. Garwood. Manchester X-ray Imaging Facility, School of Materials, The University of Manchester, Oxford Rd., Manchester M13 9PL, UK. russell.garwood@manchester.ac.uk
Mark Sutton. Department of Earth Science and Engineering, Imperial College, London SW7 2AZ, UK. m.sutton@imperial.ac.uk
KEY WORDS: Camptophyllia; Carboniferous; siderite; computed tomography; VAXML
Final citation: Garwood, Russell J. and Sutton, Mark D., 2012. The enigmatic arthropod Camptophyllia. Palaeontologia Electronica Vol. 15, Issue 2;15A,12p;
palaeo-electronica.org/content/2012-issue-2-articles/218-the-arthropod-camptophyllia
INTRODUCTION
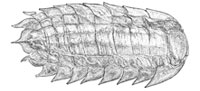
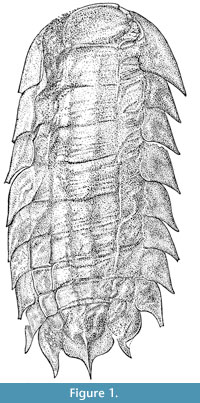 Camptophyllia is a rare and enigmatic arthropod genus found exclusively in Upper Carboniferous Coal Measures deposits of England, including the Tyne Coalfield (Crawcrook, Durham; Gill, 1924), Crock Hey (Wigan, Greater Manchester; Braznell, 2005), Coseley (Dudley, West Midlands; Rolfe, 1969), Westhoughton (Lancashire; Anderson et al., 1999) and Sparth Bottoms (Lancashire; Hansman, 1972). The multi-segmented, onisciform specimens are typically around 25 mm in length, although examples in excess of 45 mm in length do exist in the collections of the Natural History Museum, London (NHM).
Camptophyllia is a rare and enigmatic arthropod genus found exclusively in Upper Carboniferous Coal Measures deposits of England, including the Tyne Coalfield (Crawcrook, Durham; Gill, 1924), Crock Hey (Wigan, Greater Manchester; Braznell, 2005), Coseley (Dudley, West Midlands; Rolfe, 1969), Westhoughton (Lancashire; Anderson et al., 1999) and Sparth Bottoms (Lancashire; Hansman, 1972). The multi-segmented, onisciform specimens are typically around 25 mm in length, although examples in excess of 45 mm in length do exist in the collections of the Natural History Museum, London (NHM).
Only the dorsal aspect of the genus is known – the tergites are split ex-sagittally into three lobes, with a further two lateral plates forming the sides of each segment (Figure 1). The appendages – and any other ventral or cephalic details – are unknown. As such, the affinities of the genus remain unclear; a superficial similarity to the Isopoda inspired early workers to suggest peracarid affinities, while more recent suggestions have focussed on a relationship with the Arthropleuridea. All known examples are found within siderite nodules – a common mode of preservation for the era. Recent work has demonstrated the efficacy of X-ray micro-tomography in the investigation of such fossils (Selden et al., 2008; Garwood et al., 2009; Garwood and Sutton, 2010; Garwood and Dunlop, 2011). This technique reveals morphology hidden within the host concretion of the fossil (Abel et al., 2012), making it ideal for solving long-standing queries surrounding this enigmatic genus. We report here the results of an XMT-based restudy using all available Camptophyllia material, and provide also a historical background and review of all work to date on the genus.
PREVIOUS STUDY
Gill (1924) was the first to describe Camptophyllia; he erected two species – C. eltringhami and C. fallax – based upon specimens from the Phoenix Brickworks quarry, Crawcook, near Newcastle, UK. He reported a minimum of nine segments, the last modified to form a small tail. Each somite has three tergites bordered by two lateral plates ('a leaf like pleural expansion'), forming an onisciform organism with a somewhat box-like cross-section. Gill noted similarities to the Isopoda and was 'inclined to consider it as such provisionally' (Gill, 1924, p. 467). The type (and only known example) of C. eltringhami was reported to have a tail segment with lateral plates as the rest of the body and a terminal median dorsal keel drawn into a spine. Interpretation of the features was complicated, however, by the fact that the true dorsal surface was only preserved on the lateral plates of each segment; elsewhere lines representing both dorsal and ventral surfaces were reported to appear superimposed in the flattened fossil. Gill was uncertain as to whether the anterior represented a head, or merely the record of prematurely truncated, broken remains, with the visible 'head' region actually belonging to the ventral surface. C. fallax – known only from the flattened posterior five segments of a single specimen – differs in details of the tail segment, which in this species is 'without pleural expansions' and possesses a broad flange rather than keel and spine.
Van Straelen (1931) listed the genus as Eumalacostraca (incertae sedis), providing no further comments (p. 86). Brooks (1962) mentions the taxon once, saying "Van Straelen (1931, p. 86) was correct in removing them from serious consideration as a representative of any of the Malacostraca. They may be related to the Arthropleurida." Thus the genus was not formally removed from a placement in the Malacostraca, the suggestion of arthropleuridan affinities – universally accepted since – has never been critically appraised, and no supporting evidence has been proffered. Rolfe (1969) based his account on undescribed specimens from Coseley in the NHM (Hansman, 1972) and placed Camptophyllia amongst taxa doubtfully classifiable as Arthropleurida. He recorded 10 segments, 'telson' excluded, a semi-circular first somite and posited a head hidden beneath the first segment.
Hansman (1972) described an additional, poorly preserved specimen in the collections of the Sedgwick Museum, Cambridge, from Sparth Bottoms, Rochdale, Lancashire. He reported a possible fraction of the head and the first seven thoracic segments of the specimen, the rest being missing. Due to the fragmentary preservation the author did not assign this example to a species and also reported personal communication from W.D.I. Rolfe suggesting the type of C. fallax could in fact be the opisthosoma of an arachnid. Anderson et al. (1999) reported a single Camptophyllia specimen collected from the roof shales of the Wigan Four Foot coal seam, Westhoughton, Lancashire, with possible gut contents preserved. Most recently Pollard et al. (2008) suggested that the trace fossils Diplichnites triassicus and Rusophycus versans from Carboniferous lacustrine siltstones in Lancashire could have been produced by Camptophyllia. The authors justified this interpretation – despite uncertainties of affinity and mode of life of the genus – on the basis of body size, a length / width ratio of 2:1, 7–10 pairs of homopodous limbs, and a benthic habit. Further, opposed appendage tracks lie under the lateral regions of each body segment when a scaled reconstruction of Camptophyllia is placed on the tracks. This trackway matches reconstructions of arthropleurid locomotion and burrowing behaviour found in Devonian fluvial deposits, reported by Smith et al. (2003) and Morrissey and Braddy (2004).
Camptophyllia is thus currently referred to the Arthropleuridea, although little real supporting evidence exists in the literature. No ventral surface or features of the head have been reported. The number of segments is thought to be 10, and each is split into three dorsal and two lateral plates. Little work has been conducted on the excellent Camptophyllia specimens from the Coseley Lagerstätte held in the Natural History Museum, London (NHM), and many questions regarding this unusual genus remain unanswered.
METHOD
Six Camptophyllia specimens were scanned; the type specimen of C. eltringhami (Crawcook, nr Ryton-On-Tyne, Durham, Westphalian B) and the majority of (previously undescribed) examples held in the NHM (I13951, I13952, In22843, In22844 - all Coseley, Westphalian B, ca. 311 Ma in age) that were the basis of Rolfe's (1969) account (Hansman, 1972). Additionally, a fossil from the private collection of Mr. Sean Sale (CH3, Crock Hey, Westphalian A) was scanned. Further, four specimens were X-rayed prior to scanning to ascertain if any morphology was preserved within the nodule. In these cases it was clear that all information was available for visual inspection, revealed by the split in the nodule, and hence no scanning was undertaken. These include the type of C. fallax (NHM In41503, Crawcook, Durham) and another poorly preserved specimen from the Tyne Coalfields (NHM In41505), an example from the private collection of Mr. Stephen Livesley (CH304a &b, Crock Hey), and a specimen from the Sedgwick Museum (E.16925, Sparth Bottoms, Lancashire, Westphalian A, Late Carboniferous ca. 314 Ma in age). The example held in the Manchester Museum (MM) from Westhoughton was not scanned.
 Initial reconstructions of all scanned specimens were created to assess the quality of preservation. Models were created using the custom SPIERS software suite (Sutton et al., 2012). Inverse linear thresholds of each slice were created, and a rough reconstruction was investigated. Of the scanned specimens, the two best preserved examples were chosen to be manually cleaned and are presented here. For these models the threshold data were manually cleaned for each slice to remove noise and artefacts. Structures were assigned to different colour zones, or masks, on each slice, allowing the model to be rendered as a number of isosurfaces where more than one was required. Some thin elements, which were visible in tomograms but were not picked out by the thresholding, were manually drawn by interpolating spline curves between the slices. Finished models were exported to open source ray-tracing software Blender (blender.org) for rendering. The specimens were further studied visually using conventional microscopy.
Initial reconstructions of all scanned specimens were created to assess the quality of preservation. Models were created using the custom SPIERS software suite (Sutton et al., 2012). Inverse linear thresholds of each slice were created, and a rough reconstruction was investigated. Of the scanned specimens, the two best preserved examples were chosen to be manually cleaned and are presented here. For these models the threshold data were manually cleaned for each slice to remove noise and artefacts. Structures were assigned to different colour zones, or masks, on each slice, allowing the model to be rendered as a number of isosurfaces where more than one was required. Some thin elements, which were visible in tomograms but were not picked out by the thresholding, were manually drawn by interpolating spline curves between the slices. Finished models were exported to open source ray-tracing software Blender (blender.org) for rendering. The specimens were further studied visually using conventional microscopy.
 Of the two reconstructions presented, the first, NHM I13952 (Figure 2.8 and Animation 1), is in a small siderite nodule and was scanned at the NHM on a NIKON X-Tek HMX-ST scanner, with an unfiltered tungsten reflection target, a 225 kV voltage and 190 µA current. 3142 projections were used with a 0.18 second exposure, the 2000 x 2000 (4MP) panel giving a resolution (voxel size) for this specimen of 12 µm. The same settings were used for NHM In22843 (Figure 2.9 and Animation 2), another small siderite nodule, giving a resolution of 13 µm. The rest of the scans used similar settings, the resolution of these lying between 15 and 16 µm. Both of these fossils
Of the two reconstructions presented, the first, NHM I13952 (Figure 2.8 and Animation 1), is in a small siderite nodule and was scanned at the NHM on a NIKON X-Tek HMX-ST scanner, with an unfiltered tungsten reflection target, a 225 kV voltage and 190 µA current. 3142 projections were used with a 0.18 second exposure, the 2000 x 2000 (4MP) panel giving a resolution (voxel size) for this specimen of 12 µm. The same settings were used for NHM In22843 (Figure 2.9 and Animation 2), another small siderite nodule, giving a resolution of 13 µm. The rest of the scans used similar settings, the resolution of these lying between 15 and 16 µm. Both of these fossils  are included in the Supplemental Material here as downloadable virtual models. Both are in the VAXML interchange suggested by Sutton et al.(2012; see also www.spiers-software.org). The models are zipped; when extracted and SPIERS is installed, models can be viewed by double-clicking on the .vaxml files. Low-performance systems may struggle to render the model.
are included in the Supplemental Material here as downloadable virtual models. Both are in the VAXML interchange suggested by Sutton et al.(2012; see also www.spiers-software.org). The models are zipped; when extracted and SPIERS is installed, models can be viewed by double-clicking on the .vaxml files. Low-performance systems may struggle to render the model.
SYSTEMATIC PALAEONTOLOGY
Phylum ARTHROPODA incertae sedis
Genus CAMPTOPHYLLIA Gill, 1924
Type and only species. Camptophyllia eltringhami Gill, 1924, by original designation.
1924 Camptophyllia Gill, pp. 466-471.
Localities and Ages. Upper Carboniferous Coal Measures, United Kingdom: Tyne Coalfield (Crawcrook, Durham, Westphalian B), Crock Hey (Wigan, Lancashire, Westphalian A), Coseley (Dudley, West Midlands, Westphalian B), Westhoughton (Wigan, Lancashire, Westphalian A), and Sparth Bottoms (Rochdale, Lancashire, Westphalian A).
Diagnosis. Onisciform arthropod with 10 somites, each dorsally split by two longitudinal axial furrows to create tri-lobed dorsal surface, and each with two lateral plates, posteriorly imbricating. First somite semi-circular, and posterior with small terminal spine or tubercle.
Camptophyllia eltringhami Gill, 1924
Figure 3, Figure 4
1924 Camptophyllia eltringhami n. sp. Gill, p.467, 1 fig.
1924 Camptophyllia fallax n. sp. Gill, p. 469, 1 fig. syn. nov.
1931 Camptophyllia eltringhami Gill; Van Straelen, p.71.
1931 Camptophyllia fallax Gill; Van Straelen, p.71.
1962 Camptophyllia eltringhami Gill; Brooks, p.269.
1962 Camptophyllia fallax Gill; Brooks, p.269.
1969 Camptophyllia Gill; Rolfe, p. R618, 1 fig.
1972 Camptophyllia sp.; Hansman, p. 315, 1 fig.
1984 Camptophyllia fallax; Selden and White, p. 43.
1998 Camptophyllia; Dunlop and Selden, p. 224.
1999 Camptophyllia eltringhami Gill; Anderson et al. p. 326, 1 fig.
2008 Camptophyllia sp. Gill; Pollard, Selden, and Watts, p. 399, 1 fig.
2010 Camptophyllia sp. Gill; Sadlok and Machalski, p. 121.
Holotype. NHM In 41504.
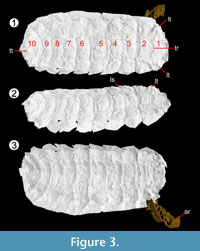 Type locality and horizon. Crawcrook, near Ryton-On-Tyne, Durham. Middle Coal Measures, Westphalian B in age, similis-pulchra Zone.
Type locality and horizon. Crawcrook, near Ryton-On-Tyne, Durham. Middle Coal Measures, Westphalian B in age, similis-pulchra Zone.
Additional material. Natural History Museum, London: NHM In41503 (= holotype of C. fallax), In41505 (Tyne Coalfield); NHM I13952, In22843, In22844, I13951, (Coseley); Sedgwick Museum, Cambridge: E.16925 (Sparth Bottoms); Private collection of Mr. Sean Sale, CH3 (Crock Hey); Private collection of Mr. Stephen Livesley, CH304a and b (Crock Hey); Manchester Museum: MM LL11153 (Westhoughton).
Distribution. UK Coal Measures, Westphalian A-B.
Diagnosis. As for genus.
Description. Onisciform arthropod, 15-45 mm long, 8-20 mm wide (NHM In 41504 a larger specimen, NHM I. 13952 the smallest; Figure 2). Both scans (Figure 3, Figure 4) and complete hand specimens (Figure 2) possess 10 segments, the segmentation most clearly demonstrated in the Coseley specimens (Figure 2.7-2.11). The segments decrease in length posteriorly; from 1.75 mm (second) to 0.88 mm (penultimate) in scanned specimen, NHM I. 13952.
The first segment is semi-circular in form (e.g., NHM I. 13952, Figure 4), with a rounded anterior and straight posterior margin. The first segment is longer than the others (NHM I. 13952: 2.22 mm), and is rarely flat; in scanned specimen NHM In 22843 it possesses lateral tubercles and a transverse ridge, while NHM I. 13952 features lateral and median tubercles. 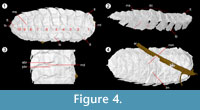 Each somite is split ex-sagittally into the diagnostic wide median axis (at greatest 3/5 the total width of the organism, well demonstrated by NHM In 22843) and two lateral paratergal folds, whose form is clearest in CH304a in which the lateral aspect is preserved (Figure 2.6). The median axis narrows posteriorly (most pronounced in NHM I. 13952) and possesses a posterior median keel resolved in the scanned specimen NHM I. 13952; the posterior five tergites possess a small median terminal spike (Figure 4; MS). This specimen demonstrates similar spikes / ornamentation at the boundary between the dorsal sclerite and lateral plates (Figure 4; LS). The lateral plates are posteriorly imbricated, as shown in the well-preserved lateral aspect of CH304a from Crock Hey (Figure 2.6) in addition to the scanned specimens (Figure 3.2, Figure 4.2). The shape of these lateral plates is also best shown in the Crock Hey example – they are posteriorly drawn into a distal point, possess a slight posterior recurvature, and in this specimen the posterior borders possess serrations in some segments.
Each somite is split ex-sagittally into the diagnostic wide median axis (at greatest 3/5 the total width of the organism, well demonstrated by NHM In 22843) and two lateral paratergal folds, whose form is clearest in CH304a in which the lateral aspect is preserved (Figure 2.6). The median axis narrows posteriorly (most pronounced in NHM I. 13952) and possesses a posterior median keel resolved in the scanned specimen NHM I. 13952; the posterior five tergites possess a small median terminal spike (Figure 4; MS). This specimen demonstrates similar spikes / ornamentation at the boundary between the dorsal sclerite and lateral plates (Figure 4; LS). The lateral plates are posteriorly imbricated, as shown in the well-preserved lateral aspect of CH304a from Crock Hey (Figure 2.6) in addition to the scanned specimens (Figure 3.2, Figure 4.2). The shape of these lateral plates is also best shown in the Crock Hey example – they are posteriorly drawn into a distal point, possess a slight posterior recurvature, and in this specimen the posterior borders possess serrations in some segments.
The terminal segment bears a tubercle (e.g., NHM In 22843), which is – in some specimens – elongate and almost spinous in nature (notably scanned specimen NHM I. 13952, and type NHM In 41504). The posterior margin of the terminal segment is drawn into a spike, such as that in NHM In 41504 (slightly exaggerated in the reconstruction of Gill, 1924). The fossils demonstrate an ability to both coil dorso-ventrally and curve laterally. The former is seen in a number of the fossils. I. 13952 demonstrates a degree of dorso-ventral curvature in segments 4-7 (Figure 4.2, DC). Where the organism is curved in this manner more complex boundaries between the different tergites are visible. Normally the boundary between segments is a single transverse depression, but when coiled two transverse ridges are seen – at the sclerite margins – with a small depression between these, presumably to accommodate the curvature (Figure 4.3). This pattern is also seen in NHM In 22844 between segments 4 and 7, and NHM I. 13951 between segments 2 and 5. Lateral curvature is seen in I. 13952, causing the lateral plates on the right to overlap and those on the left to be more widely spaced (Figure 4).
In the scanned specimens no sternites or ventral structures were resolved. The ventral morphology of the tergites was recovered, however, best shown in NHM I. 13952. In this view the margin between the lateral elements of the tergites is more complex. Rather than perpendicular to the long axis of the arthropod (and hence parallel to segment boundaries in the median lobe), the boundaries between segments are angled at ~50 degrees to this. These create chevron-shaped regions, but while they continue into the median axis in the form of a ridge, they do not meet in the centre (Figure 4.4, LM and MM).
Specimen NHM In 22843 appears to possess an element of sternal cuticle, which is not complete enough to discern any elements of the sternal morphology, but the thin loop of material found in the head region does give the impression of a box-like construction. Visible on a relatively small number of anterior slices (Figure 5), it suggests at the head the pleural margins are angled inwards, and a flat sternite bridges the gap between them.
 Remarks. The holotype of Camptophyllia fallax Gill, 1924, is incomplete, preserving only six posterior segments with a limited number of fragments of cuticle attached. As a result of the poor preservation the only discernible difference between C. fallax and the type species is the lack of a terminal spine in the former. Indeed the preservation of this specimen is such that Hansman (1972) reported the suspicions of W.D.I. Rolfe that it might be the opisthosoma of an arachnid. Rolfe (1969), Hansman (1972), and Pollard et al. (2008) provide no distinguishing characters – on this basis, and the variability within other Camptophyllia specimens, such as those from Coseley, we regard C. fallax as a junior synonym of C. eltringhami by page priority.
Remarks. The holotype of Camptophyllia fallax Gill, 1924, is incomplete, preserving only six posterior segments with a limited number of fragments of cuticle attached. As a result of the poor preservation the only discernible difference between C. fallax and the type species is the lack of a terminal spine in the former. Indeed the preservation of this specimen is such that Hansman (1972) reported the suspicions of W.D.I. Rolfe that it might be the opisthosoma of an arachnid. Rolfe (1969), Hansman (1972), and Pollard et al. (2008) provide no distinguishing characters – on this basis, and the variability within other Camptophyllia specimens, such as those from Coseley, we regard C. fallax as a junior synonym of C. eltringhami by page priority.
DISCUSSION
None of the scans undertaken reveal the ventral morphology of Camptophyllia, although some ventral aspects of the tergites are resolved. Previous tomographic work on fossils from Coseley (Garwood et al., 2009; Garwood and Sutton, 2010) and Crock Hey (Garwood and Dunlop, 2011) has revealed the preservation of appendages in arthropod taxa. Further, we have scanned in excess of 50 Coseley arthropods and more than 10 Crock Hey fossils; of these more than half preserved appendages. Having used either tomography or radiography to examine 10 of the 11 known fossil specimens, it seems unlikely that the absence of appendages here is purely the result of poor preservation. It also seems improbable, however, that these could all be moults – of the posited sister groups to Camptophyllia, isopods show biphasic moulting (George, 1972), and arthropleurids moulted with a suture at the posterior cephalic margin (Kraus, 2005). Both would be apparent in any exuviae, whereas these fossils appear complete and articulated. It is clear nevertheless that the body and appendages of the scanned specimens are missing; the most parsimonious explanation for these observations is that the body and limbs were less heavily sclerotized than the protective dorsal and lateral plates, and hence that their absence is taphonomic.
Previous workers have suggested that the first somite is not cephalic, being rather the premature termination of the trunk (Gill, 1924), or that the head is concealed beneath this first tergite (Rolfe, 1969). This study demonstrates this anterior region has lateral and (admittedly limited) sternal sclerotized elements (Figure 5), a flat and rather box-like cross-section, and no preserved 'head' beneath the carapace. The possibility that this region represents the anterior of the column of a myriapod, and that the fossils are moults which have lost the head is discounted here, as there is no clear opening in the cuticle through which the head could have been withdrawn during ecdysis. Thus, we interpret this first, semi-circular somite as the anteriormost (head) somite, lacking (preserved) cephalic appendages or obvious eyes (although the lateral tubercles described might conceivably have housed a small visual apparatus).
The variations in the nature of the inter-tergal margins noted (Figure 4.3) could be due to dorso-ventral flexure, as this complexity is only seen in the regions where the curvature is concentrated. In these areas the two ridges appear to be the termination of the tergites anterior and posterior to the depression, or gap, in between the two. An articular membrane may have been present to facilitate this flexibility, or a more complex tergal arrangement analogous to the half-rings of trilobites may have accommodated flexure.
This work has also highlighted differences between the forms of Camptophyllia found in Coseley, upon which the descriptions of Rolfe (1969) are based (Hansman, 1972), and all other examples. The Coseley specimens are universally smaller, about half the size of those from Tyne, the single example known from Sparth Bottoms, and those of Crock Hey. They are otherwise identical. If this difference in size is ontogenetic, it would be indicative of epimorphic growth. It could also result from endemism of small forms in the Coseley locality, and the age difference between this and the other deposits, however.
Affinities
The affinities of Camptophyllia remain problematic, although we are confident the genus belongs within the Arthropoda. A trilobed body is widespread within the arthropods, and without appendage information there is little to validate or invalidate any posited placements. Gill's (1924) suggestion of isopod affinities appears to have been based simply on general appearance; the low number of segments seen in Camptophyllia and the presence of pleurites (uncommon amongst the Crustacea) argue against an isopod model. We suggest here that the low segment number, unusual head, and presence of lateral plates also argue against an arthropleurid affinity. We note that epimorphic growth is present in a number of myriapod groups (Shear and Edgecombe, 2010; Vedel et al., 2010). However, this character is homoplastic within the Arthropoda (Edgecombe et al., 2000), and even if unambiguously present here, would by no means be a clear indicator of myriapod affinity. The box-like construction revealed by these scans (Figure 5) is reminiscent of the arrangement seen in the euthyacarcinoid Heterocrania rhyniensis, known from the Devonian Rhynie and Windyfield cherts (Anderson and Trewin, 2003; Hirst and Maulik, 1926). Euthycarcinoids are known from a number of Carboniferous deposits; the most pertinent to the current study are Coseley (Wilson and Almond, 2001), Bickershaw (Anderson et al., 1997), and Westhoughton (Anderson et al., 1999), but members of the order are also found in Montceau-Les-Mines, France (Racheboeuf et al., 2008), Ibbenbüren, Germany (Schultka, 1991), and Mazon Creek, USA (Schram, 1971). Their presence in these deposits suggests euthycarcinoids were relatively widespread in the Carboniferous, and this is reflected in fairly high morphological diversity during this period (Schram and Rolfe, 1982). It is clear that Camptophyllia had very different tagmosis to the Euthycarcinoidea, which have a differentiated pre- and post-abdomen, but the box-like construction and thin cuticle are closer to the euthycarcinoid arrangement than that of the arthropleurid. Pending the discovery of more informative fossils, any further speculations on the position of the genus within the Arthropoda seems ill-advised.
Functional Morphology
The organism's seemingly well-sclerotized dorsal and lateral plates are likely to be defensive in function. Furthermore, the slight flexure seen in specimen NHM In22844 and Crock Hey example CH3 suggests the creature could roll up in life as a defensive strategy. The recurvature in the pleurites is presumably linked to this ability, providing lateral protection from interlocking plates when enrolled. Further, the terminal spines on posterior segments (external on a coiled Camptophyllia) were presumably also defensive.
The lack of obvious eyes (or areas where the attachment of stalked eyes would be possible) is indicative of poor visual acuity in Camptophyllia, and thus an environment in which these were unnecessary. Pollard et al. (2008) reported possible Camptophyllia traces, which were formed on siliciclastic substrate on a shallow lake floor with possible input from fluvial crevasse-splay sedimentation. Further, the authors use reports of phosphatised gut contents from Anderson et al. (1999) coupled with the traces to suggest Camptophyllia could have been a deposit feeder. This supposition is supported by the posited environments of Coseley (where most fossils originate in lacustrine deposits, Braznell, 2005), Crock Hey (a lacustrine delta complex, Braznell, 2005), and Sparth Bottoms (Pocock, 1911). As such, a murky sediment-rich environment can be envisaged, in which vision would not be useful. The flattened, onisciform appearance could also be interpreted as a 'snow-shoe' adaptation to prevent sinking into soft sediment on a lake floor and to facilitate shallow burrowing (Pollard et al., 2008).
ACKNOWLEDGMENTS
We thank C. Mellish (NHM) and M. Riley (Sedgwick) for access to material in their care, and S. Livesley and S. Sale for access to their collections. Thanks to A. Tenny for providing photographs. We thank two anonymous reviewers for helpful comments. R.G.s work was funded by a NERC Ph.D. studentship and he would like to acknowledge the assistance provided by the Manchester X-ray Imaging Facility, which was funded in part by the EPSRC (grants EP/F007906/1, EP/F001452/1 and EP/I02249X/1).
REFERENCES
Abel, Richard Leslie, Laurini, Carolina Rettondini, and Richter, Martha 2012. A palaeobiologist’s guide to ‘virtual’ micro-CT preparation. Palaeontologia Electronica Vol. 15, Issue 2;6T,17p;
palaeo-electronica.org/content/issue-2-2012-technical-articles/233-micro-ct-workflow
Anderson, L.I. and Trewin, N.H. 2003. An Early Devonian arthropod fauna from the Windyfield Cherts, Aberdeenshire, Scotland. Palaeontology, 46(3):467-509.
Anderson, L.I., Dunlop, J.A., Eagar, R.M.C., Horrocks, C.A., and Wilson, H.M. 1999. Soft-bodied fossils from the roof shales of the Wigan Four Foot coal seam, Westhoughton, Lancashire. UK. Geological Magazine, 135:321-329.
Anderson, L.I., Dunlop, J.A., Horrocks, C.A., Winkelmann, H.M., and Eagar, R.M.C. 1997. Exceptionally preserved fossils from Bickershaw, Lancashire UK (Upper Carboniferous, Westphalian A (Langsettian)). Geological Journal, 32(3):197-210.
Braznell, L.J. 2005. Exceptional preservation in the Upper Carboniferous Coseley Lagerstätte. Unpublished Ph.D. Thesis, University of Birmingham, UK.
Brooks, H.K. 1962. The Paleozoic Eumalacostraca of North America. Bulletin of American Paleontology, 44:1-338.
Dunlop, J. A., and Selden, P. A. 1998. The early history and phylogeny of the chelicerates. In Proceedings of the International Symposium on the Relationships of the Major Arthropod Groups, ed. R. A. Fortey and R. H. Thomas, 55:221–236. London: Chapman and Hall.
Edgecombe, G.D., Wilson, G.D.F., Colgan, D.J., Gray, M.R., and Cassis, G. 2000. Arthropod cladistics: combined analysis of histone H3 and U2 snRNA sequences and morphology. Cladistics, 16:155-203.
Garwood, R.J. and Dunlop, J.A. 2011. Morphology and Systematics of Anthracomartidae (Arachnida:Trigonotarbida). Palaeontology 54:145-161.
Garwood, R.J. and Sutton, M.D. 2010. X-ray micro-tomography of Carboniferous stem-Dictyoptera: new insights into early insects. Biology Letters, 6:699-702.
Garwood, R.J., Dunlop, J.A., and Sutton, M.D. 2009. High-fidelity X-ray micro-tomography reconstruction of siderite-hosted Carboniferous arachnids. Biology Letters, 5:841-844.
George, R. 1972. Biphasic moulting in Isopod Crustacea and the finding of an unusual mode of moulting in the Antarctic genus Glyptonotus. Journal of Natural History, 6:651-656.
Gill, E.L. 1924. Fossil arthropods from the Tyne Coalfield. Geological Magazine, 61:455-471.
Hansman, R.H. 1972. Camptophyllia from the Lower Coal Measures of Lancashire. Journal of Paleontology, 46:315-316.
Hirst, S. and Maulik, S. 1926. On some arthropod remains from the Rhynie Chert (Old Red Sandstone). Geological Magazine, 63:69-71.
Kraus, O. 2005. On the structure and biology of Arthropleura species (Atelocerata, Diplopoda; Upper Carboniferous/Lower Permian). Abhandlungen und Verhandlungen des Naturwissenschaftlichen Vereins in Hamburg, 41:5-23.
Morrissey, L.B. and Braddy, S.J. 2004.Terrestrial trace fossils from the Lower Old Red Sandstone, southwest Wales. Geological Journal, 39:315-336.
Pocock, R.I. 1911. A monograph of the terrestrial Carboniferous Arachnida of Great Britain. Monographs of the Palaeontographical Society 64:1–84.
Pollard, J.E., Selden, P.A., and Watts, S. 2008. Trace fossils of the arthropod Camptophyllia from the Westphalian (Carboniferous) rocks of Lancashire, UK and their palaeoenvironmental context. Palaeogeography, Palaeoclimatology, Palaeoecology, 270:399-406.
Racheboeuf, P.R., Vannier, J., Schram, F.R., Chabard, D., and Sotty, D. 2008. The euthycarcinoid arthropods from Montceau-les-Mines, France: functional morphology and affinities. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 99:11-25.
Rolfe, W.D.I. 1969. Camptophyllia. p.R618-R619 In Moore, R.C. (ed.), Treatise on Invertebrate Paleontology, Part R, Arthropoda 4 vol 2. Geological Society of America and University of Kansas Press, Lawrence, Boulder, Colorado and Lawrence, Kansas, 1-651.
Sadlok, G. and Machalski, M. 2010. The trace fossil Rusophycusversans from the Furongian (Upper Cambrian) of central Poland – an example of behavioural convergence amongst arthropods. Acta Geologica Polonica, 60:119-123.
Schram, F.R. 1971. A Strange arthropod from the Mazon Creek of Illinois and the Trans Permo-Triassic Merostomoidea. Fieldiana Geology, 20(6):85-102.
Schram, F.R. and Ian Rolfe, W.D. 1982. New Euthycarcinoid Arthropods from the Upper Pennsylvanian of France and Illinois. Journal of Paleontology, 56(6):1434-1450.
Schultka, S. 1991. Erster Nachweis der Gattung Euthycarcinus (Arthropoda) aus dem Oberkarbon von Ibbenbüren (Nordrhein-Westfalen, Deutschland). Paläontologische Zeitschrift, 65(3):319-332.
Selden, P.A. and White, D.E. 1984. A new Silurian arthropod from Lesmahagow, Scotland, p. 41-50. In Briggs, D.E.G. and Lane, P.D. (eds.), Trilobites and other early arthropods: papers in honour of Professor H. B. Whittington FRS. Special Papers in Palaeontology 30. Palaeontological Association, London.
Selden, P.A., Shear, W.A., and Sutton, M.D. 2008. Fossil evidence for the origin of spider spinnerets, and a proposed arachnid order. Proceedings of the National Academy of Sciences of the United States of America, 105(52):20781-20785.
Shear, W.A. and Edgecombe, G.D. 2010. The geological record and phylogeny of the Myriapoda. Arthropod Structure and Development, 39:174-90.
Smith, A., Braddy, S.J., Marriott, S.B., and Briggs, D.E.G. 2003. Arthropod trackways from the Early Devonian of South Wales: a functional analysis of producers and their behaviour. Geological Magazine, 140:63-72.
Sutton, Mark D., Garwood, Russell J., Siveter, David J., and Siveter, Derek J. 2012. SPIERS and VAXML; A software toolkit for tomographic visualisation and a format for virtual specimen interchange. Palaeontologia Electronica Vol. 15, Issue 2;4T,14p;
palaeo-electronica.org/content/94-issue-2-2012-technical-articles/226-ct-toolkits
Van Straelen, V. 1931. Crustacea. Eumalacostraca (Crustaceis Decapodis Exclusis). Fossilium Catalogus, Animalia, Berlin.
Vedel, V., Apostolou, Z., Arthur, W., Akam, M., and Brena, C. 2010. An early temperature-sensitive period for the plasticity of segment number in the centipede. Strigamiamaritima. Evolution and Development, 12:347-352.
Wilson, H.M. and Almond, J.E. 2001. New euthycarcinoids and an enigmatic arthropod from the British Coal Measures. Palaeontology, 44(1):143-156. doi: 10.1111/1475-4983.00174.

