 Unique fossils of caddisfly larvae from Baltic amber and in situ amber formation in aquatic ecosystems
Unique fossils of caddisfly larvae from Baltic amber and in situ amber formation in aquatic ecosystems
Article number: 26.2.a34
https://doi.org/10.26879/1278
Copyright Paleontological Society, August 2023
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 6 March 2023. Acceptance: 17 August 2023.
ABSTRACT
Amber is formed by tree resins in terrestrial habitats. Therefore, a preservation of animals living in water in amber may appear surprising. Still more and more finds of such animals were reported in recent years. The central question around these finds is, whether the animals became entrapped in the resin in their original habitat (in situ), or whether the aquatic animals have left their original habitat to become entrapped in the resin (ex situ). We report additional finds of caddisfly larvae (Trichoptera; Leptoceridae, Lepidostomatidae) in Baltic amber. Two of these still bear their cases, which should not be present for caddisfly larvae, which escaped the waterbodies and became entrapped ex situ. One of the two is preserved with additional caddisfly larvae as well as a larva of a seemingly aquatic non-biting midge (Diptera: Chironomidae) attached to its caddis case. These finds further support a possible in situ preservation of aquatic animals in amber.
Viktor Baranov. Estación Biológica de Doñana-CSIC, Avd. Americo Vespucio 26, 41092 Seville, Spain. viktor.baranov@ebd.csic.es. Corresponding author.
Jörg Hammel. Helmholtz-Zentrum Hereon, Institute of Materials Physics, Max-Planck-Straße 1, 21502 Geesthacht, Germany. joerg.hammel@hereon.de
Carsten Gröhn. University of Hamburg, Mittelweg 177, 20148 Hamburg, Germany. ambertop@t-online.de
Joachim T. Haug. Ludwig-Maximilians-Universität München, Großhaderner Str. 2, 82152 Planegg-Martinsried, Germany. jhaug@biologie.uni-muenchen.de
Key words: Trichoptera; Chironomidae; larvae; aquatic animals; amber; taphonomy
Final citation: Baranov, Viktor, Hammel, Jörg, Gröhn, Carsten, and Haug, Joachim T. 2023. Unique fossils of caddisfly larvae from Baltic amber and in situ amber formation in aquatic ecosystems. Palaeontologia Electronica, 26(2):a34. https://doi.org/10.26879/1278 palaeo-electronica.org/content/2023/3938-caddisfly-larvae-from-amber
Copyright: August 2023 Paleontological Society. This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made. creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Aquatic animals preserved in amber still represent a mystery, as amber is formed from tree resin. Numerous proposals have been put forward to explain the presence of larvae and pupae of merolimnic organisms, which spent part of the life cycle in the water, in amber (Wichard et al., 2009). One of the largest points of contention is whether the aquatic animals were preserved in situ, i.e., directly within an aquatic environment, or ex situ, i.e., within a terrestrial environment (Serrano-Sánchez et al., 2015; Schmidt and Dilcher, 2007; Schmidt et al., 2018). Most common explanations for this very mystery were: 1) migration of aquatic animals from drying-up water bodies overland; 2) entrapment of inhabitants of phytotelmata, i.e., waterpools in epiphytic plants, such as bromeliads, and 3) transfer of smaller organisms by wind to tree trunks, on which resin was flowing (Wichard et al., 2009; Schmidt et al., 2018; Xing et al., 2021). While all these explanations likely did take place at different occasions, it appears that none of them provides a single, predominant mechanism of the entrapment of aquatic organisms (Schmidt and Dilcher, 2007; Schmidt et al., 2018; Schädel et al., 2021a, b).
Actuo-palaeontological studies in wet forests of Florida showed that large flows of resin are forming on submerged parts of tree trunks (up to a volume of 200 mm x 100 mm x 30 mm; Schmidt and Dilcher, 2007). These flows stay viscous within the water for days and even weeks, entrapping microorganisms, and aquatic animals. Schmidt and Dilcher (2007), hence, showed that entrapment of large and agile aquatic organisms in amber in situ is possible. Large organisms appear to collide with resin flows under water (Schmidt and Dilcher, 2007; Xing et al., 2021). Additionally, water isolates resin from any contact with high concentrations of oxygen in the air, slowing the resin polymerization, allowing heavier (and stronger) animals to break through several upper millimeters of a polymerized crust, and be entrapped even weeks after the resin flow was first deposited (Schmidt and Dilcher, 2007). Numerous larvae of merolimnic insects have been reported from amber before, such as dragonflies (Wichard et al., 2009; Schädel et al., 2020; Haug et al., 2021), mayflies (Wichard et al., 2009), stoneflies (Wichard et al., 2009), lacewings (Haug et al., 2020), alderflies (Wichard et al., 2009; Baranov et al., 2022), and caddisflies (Wichard et al., 2009). Recent discoveries of marine plankton (Girard et al., 2008), seed shrimps (Wang et al., 2020), aquatic isopodan crustaceans (Schädel et. al., 2021a), and even an ammonite (likely transported by exogenous force) provide additional indications of abundance of aquatic organisms in amber (Yu et al., 2019).
Trichoptera (the group of caddisflies) is one of the groups of merolimnic animals of which adult representatives are very abundant in amber, yet their aquatic larvae are very rare in fossil resins, many of the records were reviewed by Wichard et al. (2009). Here we report a unique aquatic assemblage preserved in Baltic amber, preserving four aquatic caddisfly larvae, one representing an even rarer find of a larva with its case (second known occurrence), and one aquatic larva of a non-biting midge (Diptera, Chironomidae). In addition, we present some additional caddisfly larvae from Baltic amber, expanding the fossil record of the larvae of this group of merolimnic animals. The unique amber piece with several larvae further supports a widespread entrapment of aquatic organisms in situ rather than in terrestrial habitats.
MATERIALS AND METHODS
Material
Three pieces of amber were directly studied in for this communication. We have examined photographs of additional specimens. The most spectacular piece includes several fossil larvae preserved together in a single piece of a Baltic amber. The piece was found at the Yantarny mine, Kaliningrad (Russia), and originally belonged to the personal collection of Jonas Damzen, who acquired it from a commercial source in Yantarny, Kaliningrad District. The specimen is now deposited in the research collection of the Palaeo-Evo-Devo group (LMU) under the accession number PED1383. We are committed to the future deposition of the studied specimens in State Natural History Museum (Braunschweig), to increase its public accessibility, but since the piece is a part of the ongoing research project, it currently remains in PED collection.
Additional pieces of amber, each containing a single larva of the group Trichoptera are also from Baltic amber. One piece is from the collection of Carsten Gröhn (Gliinde) under repository number L7698 (but will be deposited in the Centrum für Naturkunde, CeNak, Hamburg University, Hamburg); the other specimen, PED1635, is in the research collection of the Palaeo-Evo-Devo group (LMU). The specimens were documented using both optical microscopy and synchrotron radiation-based X-ray computed microtomography (SR-µCT).
Images of additional specimens of caddisfly larvae preserved in Baltic amber for comparison were provided by Mr. Jonas Damzen. Description of the caddisfly larvae was initially conducted in form of a descriptive spreadsheet (see Appendix).
Age and geological provenance of Baltic amber is subject of an ongoing discussion. While most authors accept a Priabonian (Eocene) age of the amber, a growing body of evidence supports a possible younger age of Baltic amber (Baranov et al., 2019a; Kasiński et al., 2020; Sadowski et al., 2020). Identification of the material relies on the suboptimal approach of using combination of characters, as viable larval autapomorphies for the taxonomic groups involved were not available at the specimens available for this study.
Optical Imaging
The directly studied specimens were documented using a Keyence VHX-6000 digital microscope with different illumination settings. All photos used here are composite images. Stacks of images were recorded for specimens in different focus levels. Then each stack was fused into a single sharp image. In cases in which an object was too large for a single vertical stack, adjacent images were merged into larger panoramas. All processing was done automatically by the built-in software. Some specimens were also imaged using a Keyence BZ-9000 fluorescence microscope with 20× objective. Images were taken using brightfield illumination (Haug et al., 2013).
µCT Tomography
Specimens were scanned on the Imaging Beamline P05 (Lytaev et al., 2014) operated by the Helmholtz-Zentrum Hereon at the PETRA III storage ring (Deutsches Elektronen Synchrotron - DESY, Hamburg, Germany), using a photon energy of 18 keV and a sample-to-detector distance of 100 mm. Projections were recorded with a custom 20 MP CMOS imaging system with an effective pixel size of 1.28 µm (Lytaev et al., 2014). For each tomographic scan 3601 projections were recorded at equal intervals between 0 and π. Reconstruction was conducted by applying a transport of intensity phase retrieval approach and using the filtered back projection algorithm (FBP) carried out in a custom reconstruction pipeline using Matlab (Math-Works) and the Astra Toolbox (Moosmann et al., 2014; van Aarle et al., 2015, 2016). Raw projections were binned twice for further processing, resulting in an effective pixel size of the reconstructed volume (voxel) of 2.56 µm. We have reconstructed the scanned volumes using Drishti ver. 2.6.6 (Limaye, 2012). To decrease the demands for computer memory, we converted all stacks into 8-bit tiffs, downscaled all tiffs by 50% and subsequently cropped the empty space around the amber piece using Fiji ‘scale’ and ‘crop’ functions (Schindelin et al., 2012). After that we rendered the volumes in Drishti ver. 2.6.6 (Limaye, 2012). Volumes were segmented and rendered using Drishti Paint 2.6.6, by making a mesh out of the segmented-out part of the volume.
SYSTEMATIC PALAEONTOLOGY
TRICHOPTERA Kirby, 1813
LEPIDOSTOMATIDAE Ulmer, 1903
Caddisfly Morphotype 1
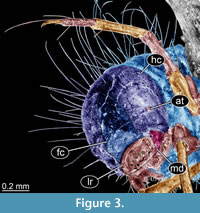
(Figure 1A-D, Figure 2A-F, Figure 3, Figure 4A-G)
Material. All specimens of this morphotype were recorded from amber piece PED 1383
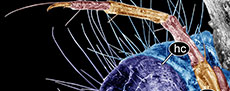
The amber piece contains five inclusions: four caddisfly larvae, all strongly resembling each other hence likely representing a single morphotype and one non-biting midge larva. This latter larva is attached to the dorsal side of the case of the largest caddisfly larva.
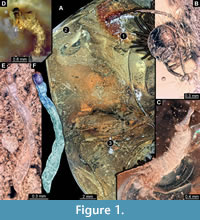 The caddisfly larvae are of different sizes. Larvae are 1.5 mm, 4.2 mm, 4.7 mm, and 15 mm long (last measurement is for the specimen in the case, approximate length). These specimens are labelled 1 through 3 (Figure 1A), while the fourth specimen (Figure 1D) is not visible on the same side of the piece of amber from which you can see all the rest of the specimens. All larvae seemingly belong to the same morphotype, probably representing several larval instars.
The caddisfly larvae are of different sizes. Larvae are 1.5 mm, 4.2 mm, 4.7 mm, and 15 mm long (last measurement is for the specimen in the case, approximate length). These specimens are labelled 1 through 3 (Figure 1A), while the fourth specimen (Figure 1D) is not visible on the same side of the piece of amber from which you can see all the rest of the specimens. All larvae seemingly belong to the same morphotype, probably representing several larval instars.
Description. Body length 1.5-15 mm. Body differentiated into presumably 20 segments, ocular segment plus 19 post-ocular segments. Ocular segment and post-ocular segment 1-5 (presumably) forming a distinct capsule (head capsule).
 Head capsule longer than wide. Surface of the head capsule is smooth and glossy, light-brown. Head capsule width 0.4-1.3 mm (0.4 mm, 0.7 mm, 0.7 mm, 1.3 mm; last value measured approximately due to the position of specimen in amber). Ocular segment recognisable by its appendage derivative, clypeo-labral complex. Fronto-clypeus mostly parallel-sided, abruptly narrowing to a point at the anterior end of the coronal suture. Labrum bearing six strong setae. Post-ocular segment 1 with a short, rounded antenna. Antenna is about as long as wide. Post-ocular segment 2 (intercalary segment) without externally recognizable structures. Post-ocular segment 3 recognisable by its pair of appendages, mandibles. Mandibles massive, stout, only visible laterally. Post-ocular segment 4 recognisable by its appendage, maxilla (maxillula). Post-ocular segment 5 recognizable by its appendages, forming the labium [conjoined left and right maxillae], massive with short palps. Labium appears overall elongated, tongue-like fleshy protrusion.
Head capsule longer than wide. Surface of the head capsule is smooth and glossy, light-brown. Head capsule width 0.4-1.3 mm (0.4 mm, 0.7 mm, 0.7 mm, 1.3 mm; last value measured approximately due to the position of specimen in amber). Ocular segment recognisable by its appendage derivative, clypeo-labral complex. Fronto-clypeus mostly parallel-sided, abruptly narrowing to a point at the anterior end of the coronal suture. Labrum bearing six strong setae. Post-ocular segment 1 with a short, rounded antenna. Antenna is about as long as wide. Post-ocular segment 2 (intercalary segment) without externally recognizable structures. Post-ocular segment 3 recognisable by its pair of appendages, mandibles. Mandibles massive, stout, only visible laterally. Post-ocular segment 4 recognisable by its appendage, maxilla (maxillula). Post-ocular segment 5 recognizable by its appendages, forming the labium [conjoined left and right maxillae], massive with short palps. Labium appears overall elongated, tongue-like fleshy protrusion.
 Trunk with 11 visible units, interpreted as three thorax segments, seven abdomen units and a trunk end representing a conjoined structure of undifferentiated abdomen segments (8-11?). Trunk is brown. Thorax consists of three segments, pro-, meso-, and methathorax. Pronotum fully sclerotized, apparently without strongly sclerotized suture, i.e., transverse carina.
Trunk with 11 visible units, interpreted as three thorax segments, seven abdomen units and a trunk end representing a conjoined structure of undifferentiated abdomen segments (8-11?). Trunk is brown. Thorax consists of three segments, pro-, meso-, and methathorax. Pronotum fully sclerotized, apparently without strongly sclerotized suture, i.e., transverse carina.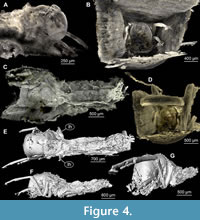 Sternum of the prothorax bears short prothoracic “horn”, a sclerotized cuticular protrusion. Front legs the longest of all three pairs of legs, with long, wavy setae on the distal end of the tibia. Mesothorax with fully sclerotized notum. Midleg shorter than front legs, with short setae on the top of the tibia. Metatorax without (visible) sclerites.
Sternum of the prothorax bears short prothoracic “horn”, a sclerotized cuticular protrusion. Front legs the longest of all three pairs of legs, with long, wavy setae on the distal end of the tibia. Mesothorax with fully sclerotized notum. Midleg shorter than front legs, with short setae on the top of the tibia. Metatorax without (visible) sclerites.
Metanotum of any of the specimens is not available for optical examination, and based of our examination of µCT scans, it is our opinion that the metanotum is probably membranous. Hind legs longer than midleg, but shorter than front legs. First unit of the abdomen with two pronounced lateral humps, but without dorsal hump. Abdominal units 2-7 without pronounced gills. Trunk end (undifferentiated abdomen units 8-11?), without any pronounced brushes of setae at their base, but with two strong setae at the base of the posterior parapods.
Case rectangular in cross section, made of flat pieces of wood (present only in largest specimen).
TRICHOPTERA Kirby, 1813
LEPTOCERIDAE Leach in Brewster, 1815
Caddisfly Morphotype 2
(Figure 5A-B)
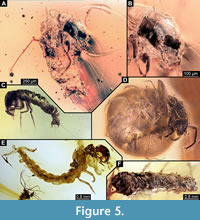 Material. Amber piece L7698. Single larva, 2.1 mm long. Larva is lacking the case (Figure 5A-B). Body length 2.1 mm. Body differentiated into presumably 20 segments, ocular segments plus 19 post-ocular segments.
Material. Amber piece L7698. Single larva, 2.1 mm long. Larva is lacking the case (Figure 5A-B). Body length 2.1 mm. Body differentiated into presumably 20 segments, ocular segments plus 19 post-ocular segments.
Ocular segment and post-ocular segment 1-5 (presumably) forming a distinct capsule (head capsule). Head capsule longer than wide, smooth and glossy, dark brown. Ocular segment recognisable by its appendage derivative, clypeo-labrum complex. Post-ocular segment 1 with a long antenna, six times longer than wide. Post-ocular segment 2 (intercalary segment) without externally recognizable structures. Post-ocular segment 3 recognisable by its pair of appendages, mandibles. Mandibles not well visible, no detail accessible. Post-ocular segment 4 recognisable by its appendage, maxilla [maxillula]. Maxilla is not well visible. Post-ocular segment 5 recognizable by its appendages, forming the labium [conjoined left and right maxillae]. Labium not well visible.
Trunk with 11 visible units, interpreted as three thorax segments, seven abdomen units, and a trunk end representing a conjoined structure of undifferentiated abdomen segments (8-11?). Trunk is brown. Thorax consists of three segments, pro-, meso-, and metathorax. Prothorax with pronotum lightly coloured, sclerotized. Front legs the longest of all three pairs of legs, with long, straight setae along the entire length of leg. Mesothorax with mesonotum, membranous. Midleg shorter than front legs. Metathorax with metanotum, membranous. Hind legs longer than mid leg, but shorter than front legs. First unit of the abdomen with a pair of lateral humps and a dorsal hump.
Abdomen units 2-7 without pronounced gills. Trunk end (undifferentiated abdomen units 8-11?), with anal claw. The specimen is very reminiscent of some of the specimens available at the commercial market of amber fossils (Figure 5 C-E).
TRICHOPTERA Kirby, 1813
LEPTOCERIDAE Leach in Brewster, 1815 Kirby,
Caddisfly Morphotype 3
(Figure 5F, Figure 6A-F)
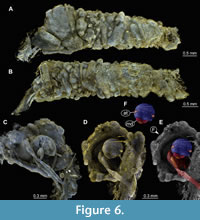 Material. Amber piece PED 1635. Caddisfly larva in its case, which is made of sand grains. Total length of the case is 4.9 mm.
Material. Amber piece PED 1635. Caddisfly larva in its case, which is made of sand grains. Total length of the case is 4.9 mm.
Body differentiated into presumably 20 segments, ocular segment plus 19 post-ocular segments. Ocular segment and post-ocular segment 1-5 (presumably) forming a distinct caspule (head capsule). Head capsule longer than wide, smooth and glossy, dark-brown. Ocular segment recognisable by its appendage derivative, clypeo-labrum complex. Post-ocular segment 1 with a long antenna, six times longer than wide. Post-ocular segment 2 (intercalary segment) without externally recognizable structures. Post-ocular segment 3 recognisable by its pair of appendages, mandibles. Mandibles not well visible. Post-ocular segment 4 recognisable by its appendage, maxilla [maxillula]. Maxilla is not well visible. Post-ocular segment 5 recognizable by its appendages, forming the labium [conjoined left and right maxillae]. Labium not well visible. Trunk with 11 visible units, interpreted as three thorax segments, seven abdomen units and a trunk end representing a conjoined structure of undifferentiated abdomen units (8-11?). Trunk is brown. Thorax consists of three segments, pro-, meso-, and methathorax. Prothorax with pronotum lightly coloured, sclerotized. Front legs the longest of all three pairs of legs, with long, straight setae along the entire length of leg.
Mesothorax with mesonotum, membranous. Midleg shorter than front legs. Metathorax with metanotum membranose. Hind legs longer than mid leg, but shorter than front legs.
DIPTERA Linnaeus, 1758
CHIRONOMIDAE Newman, 1838
CHIRONOMINAE Macquart, 1838
CHIRONOMINI Macquart, 1838
Non-biting midge larva
(Figure 1E, F, Figure 7A-D, Figure 8A-H)
Material. Amber piece PED 1383, this piece has a fly larva, attached to the case of the largest caddisfly larva (Figure 1A-3). Total length of the larva is 2.1 mm.
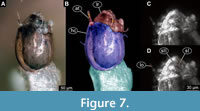 Ocular segment and post-ocular segment 1-5 (presumably) forming a distinct capsule (head capsule). Head capsule well developed, oval in dorsal view, 240 µm long, 130 µm wide (Figure 7A-D, Figure 8C-H). Surface of the head capsule is smooth. Ocular segment recognisable by its appendage derivative, clypeo-labrum complex. Clypeus (clypeal sclerite) trapezoid. Labrum narrow, crescent-shaped, with labral setae (S1 and S2), which appear to be simple.
Ocular segment and post-ocular segment 1-5 (presumably) forming a distinct capsule (head capsule). Head capsule well developed, oval in dorsal view, 240 µm long, 130 µm wide (Figure 7A-D, Figure 8C-H). Surface of the head capsule is smooth. Ocular segment recognisable by its appendage derivative, clypeo-labrum complex. Clypeus (clypeal sclerite) trapezoid. Labrum narrow, crescent-shaped, with labral setae (S1 and S2), which appear to be simple. 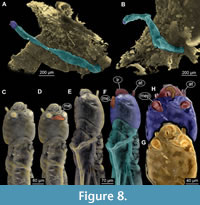 Post-ocular segment 1 with prominent antenna. Antenna well developed, apparently with five units (flagellomeres), with Lauterborn organs sitting atop the second flagellomere. Post-ocular segment 2 (intercalary segment) without externally recognizable structures. Post-ocular segment 3 recognisable by its pair of appendages, mandibles. Shape of mandibles is difficult to discern on the µCT scans, and optical observation is impossible, as the ventral side of the larva is facing the caddisfly case. Post-ocular segment 4 recognisable by its appendage, maxilla [maxillula]. Maxilla shape including shape of the palp is difficult to discern, due to the position of the larva. Post-ocular segment 5 recognizable by its appendages, forming the labium [conjoined left and right maxillae]. Only one part of the labium is visible, namely the sclerotized mentum (sensu Sæther, 1980). Mentum, seemingly trapezoid, exact morphology hard to ascertain, as is only visible on µCT scans. Submental (likely derivatives of the mentum) about 1.5 times wider than high.
Post-ocular segment 1 with prominent antenna. Antenna well developed, apparently with five units (flagellomeres), with Lauterborn organs sitting atop the second flagellomere. Post-ocular segment 2 (intercalary segment) without externally recognizable structures. Post-ocular segment 3 recognisable by its pair of appendages, mandibles. Shape of mandibles is difficult to discern on the µCT scans, and optical observation is impossible, as the ventral side of the larva is facing the caddisfly case. Post-ocular segment 4 recognisable by its appendage, maxilla [maxillula]. Maxilla shape including shape of the palp is difficult to discern, due to the position of the larva. Post-ocular segment 5 recognizable by its appendages, forming the labium [conjoined left and right maxillae]. Only one part of the labium is visible, namely the sclerotized mentum (sensu Sæther, 1980). Mentum, seemingly trapezoid, exact morphology hard to ascertain, as is only visible on µCT scans. Submental (likely derivatives of the mentum) about 1.5 times wider than high.
Thorax and abdomen appear to be smooth and without prominent setae. Posterior parapods are not accessible neither via optical methods nor via µCT-scanning, as they are immersed within the matrix of caddisfly case.
DISCUSSION
Caddisfly Morphotype 1
Caddisfly larvae of this morphotype are all interpreted as representatives of the group Lepidostomatidae based on the following combination of characters: labrum with six strong setae, antennae about as long as wide, situated latero-dorsally on the headcapsule, mesonotum sclerotized, metanotum membranous, post-ocular segment 9 (abdomen unit 1) with a pair of lateral humps but without dorsal hump, anal claw hook-shaped, base of anal claw without prominent brush of setae, larval case distinctly rectangular in the cross-section, of the so-called panel type (figure 4 in Weaver, 1988) (Weaver, 1988, Merrit and Cummins, 1996). Further identification of the larvae is difficult as many important characters (e.g., gula, i.e., posterior ventral head sclerite) are not observable via optical means, and µCT scans do not provide sufficient resolution for these structures
It is quite remarkable that we have found four Lepidostomatidae larvae in the same piece of amber. Hoffmann (1997) has supplied a possible explanation, by noting that larvae of Lepidostomatidae tend to aggregate in large groups (up to 500 individuals), especially before the pupation (Hoffmann, 1997). This behaviour is a likely reason why we have found several larvae in the same piece of amber.
Caddisfly Morphotype 2
This caddisfly larva has antennae that are about six times as long as wide, a lightly coloured pronotum, a membranous mesonotum and metanotum, post-ocular segment 9 (abdomen unit 1) with a pair of lateral humps and a dorsal hump, anal claw hook-shaped, base of the anal claw without prominent brush of the setae. The combination of these characters strongly suggests that larva is a representative of the group Leptoceridae (Merrit and Cummins, 1996).
Caddisfly Morphotype 3
As in case of the morphotype 2 this larva has an antenna that is about six times as long as it is wide (Figure 6C). Hence, also for this morphotype the combination of these characters suggests that larva might be a representative of Leptoceridae (Merrit and Cummins, 1996).
Non-Biting Midge Larva
The non-biting midge larva is interpreted as a representative of the group Chironominae, due to the presence of a large, leaf shaped Lauterborn organ at the top of the second flagellomere of the antenna and a pair of apparent submental plates, flanking the mentum of the labium (Epler et al., 2013).
Caddisfly Larvae Preserved in Baltic Amber
The overall record of trichopteran larvae preserved in Baltic amber is rather sparse. Wichard et al. (2009) provided a report on three caddisfly larvae from this deposit with illustrations and mentioned that 15 larvae in total are known from Baltic amber.
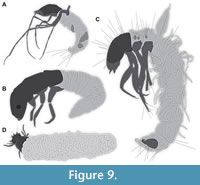 One of the three figured larvae is a representative of the group of long-horned caddisflies (Leptoceridae; Wichard et al., 2009 their figure 09.04 p. 136; interpretive drawing in Figure 9A), similar to the two specimens representing morphotype 2 and 3. Wichard et al. (2009) reported that larvae of Leptoceridae, in particular, are relatively abundant in Baltic amber, at least compared to the other types of caddisfly larvae. Numerous additional larvae of Leptoceridae are or were available on the commercial market in the past (we have secured the permissions to use the images from the sellers, but it often was impossible to pinpoint a date of the sale; Figure 5 C-E).
One of the three figured larvae is a representative of the group of long-horned caddisflies (Leptoceridae; Wichard et al., 2009 their figure 09.04 p. 136; interpretive drawing in Figure 9A), similar to the two specimens representing morphotype 2 and 3. Wichard et al. (2009) reported that larvae of Leptoceridae, in particular, are relatively abundant in Baltic amber, at least compared to the other types of caddisfly larvae. Numerous additional larvae of Leptoceridae are or were available on the commercial market in the past (we have secured the permissions to use the images from the sellers, but it often was impossible to pinpoint a date of the sale; Figure 5 C-E).
Another larval specimen reported by Wichard et al. (2009) is likely a representative of Ecnomidae (Wichard et al., 2009 their figure 09.05 p. 137; Figure 9B). Finally, Wichard et al. (2009) reported a stunningly preserved larva of the group Phrygaenidae (their figure 09.06a p. 138, figure 09.06b, p. 139 b; Figure 9C).
Gröhn (2015) refigured (his figure 3230, p. 333) the third specimen from Wichard et al. (2009). Additionally, he depicted an unusual trichopteran larva preserved with its case (his figure 7665, p. 333). This was the first instance of a larva preserved with its case. Naturally many details of the larva are not accessible in the provided image (Figure 9D).
All caddisfly larvae in Baltic amber discussed by Wichard et al. (2009) lacked cases. We have added several additional specimens in the same condition. Wichard et al. (2009) interpreted the absence of cases as a corroboration of the assumption that these larvae were entrapped in resin on dry land, while trying to escape a drought. The few records of caddisfly larvae in amber, still with their cases, as reported by Gröhn (2015) and here, are important for understanding the mode and circumstances of aquatic organisms’ entrapment in amber (see further below). The newly reported caddisfly larvae substantially expand the records from Baltic amber. Especially the group Lepidostomatidae seems to be well represented. Modern-day larvae of the group are known as xylophages, associated with wood debris (Hoffmann, 2000). Their presence in Baltic amber might be indicative of a relatively high abundance of waterlogged wood in the water bodies in the Baltic amber forest.
Non-Biting Midge Larvae Preserved in Amber
The group of non-biting midges (Diptera: Chironomidae) is one of the most species rich and abundant one among the different merolimnic representatives of Insecta. The geological record of Chironomidae spans back 200 million years and numerous fossils of this group have been recovered (Armitage et al., 1995; Baranov et al., 2019b). Larvae of Chironomidae are very abundant in some sedimentary deposits, which formed in freshwater (Kalugina, 1974). In some fossil deposits, non-biting midges make up most fossils.
In Baltic amber, over 34% of all animal inclusions are adult non-biting midges (Wichard et al., 2009). Despite such an abundance of adults, so far only a single larva of Chironomidae has been recorded from any amber (Baranov et al., 2019b). This single larva could be identified as a terrestrial morphotype, rather than a merolimnic one (Baranov et al., 2019b). The new non-biting midge larva reported here is so far unique. Several aspects of its morphology strongly indicate an aquatic lifestyle of the larva.
Firstly, the larva appears to be a representative of the group Chironominae, and there are no known representatives of this ingroup with terrestrial larvae (Armitage et al., 1995). This actualist perspective provides a useful indication, but does not fully exclude a terrestrial lifestyle of the larva.
Secondly, the larva is attached to the external side of the case of a caddisfly larva (Figure 8A-B). Since caddisfly larvae tend to abandon their cases upon leaving water, the presence of the case indicates that caddisfly larva was in the water when entrapped (Otto, 1983). The non-biting midge larva hence provides additional support, that the amber piece contains an aquatic taphocoenosis.
In Situ vs. Ex Situ Preservation
The rarity of caddisfly larvae (and non-biting midge larvae for that matter) in Baltic amber, seems easily explained by the fact that they are merolimnic organisms, i.e., these larvae are mostly living in water. Wichard and co-authors (2009) suggested that the lack of cases on, at the time, all recorded caddisfly larvae from amber points to a desiccation-stress reaction, and should be understood as an escape reaction from drying water (Wichard et al., 2009).
The argumentation here relies on the fact that the known larvae are larvae of lineages of which larval representatives normally carry cases, but in the amber fossil record they all missed one. Hence, the idea of the Wichard et al. (2009) is that larvae have abandoned their cases to escape desiccating water bodies. In the modern fauna certain larvae of the group Integripalpia are indeed known to leave their cases behind when trying to escape from desiccating water bodies in extreme situations (Otto, 1983). Yet, larvae of other groups of caddisflies are known to retreat into the cases or drag them on when confronted with drought (Otto, 1983).
Hence, in the modern fauna, different species of caddisfly larvae have different case abandonment reactions in relation to drought (Otto, 1983). The mere absence or presence of a case cannot be used to infer if a larva was entrapped within or outside of a water body. Additionally, there are situations when caddisfly larvae abandon their cases under the water, especially in case of burial under catastrophically increased sedimentation (i.e., during flood; Dobson et al., 2000).
The fact that four larvae occur in a single piece of amber could be interpreted in different ways. In any case it indicates that a large flow of resin embedded the three larvae. The question is how the three larvae came so close together to be embedded in a single piece. This is likely related to the tendency of Lepidostomatidae to aggregate together as larvae (Hoffmann, 1997) or could have simply happened by chance.
Overall the reported additional records of aquatic larvae from Baltic amber strongly support the idea that at least some of these were trapped in resin flows within the water bodies. On the contrary there are no clear facts supporting ex situ entrapment of aquatic organisms. Additional support for an in situ entrapment is provided by numerous pieces of detritus and debris in the large amber piece, which is reminiscent of modern benthic sedimentary particles (Figure 1A).
ACKNOWLEDGMENTS
We are grateful to the handling editor D. de Vries, Executive Editor R. Bicknell, L. Pongsak, and another anonymous reviewer for their efforts in improving this manuscript. Thanks to all people providing free software. We are grateful to J. Damzen for the invaluable support and donating the specimens, which made this study possible. J.M. Starck and C. Haug, both LMU Munich, are thanked for long standing support. We are also thankful to C. Haug for kindly translating paper‘s abstract into German and to Juan Pascual Gil De Gomez for kindly translating the abstract into Spanish.
This project is supported by the Volkswagen Foundation in the frame of a Lichtenberg Professorship of J.T. Haug (J.T. Haug; V. Baranov). V. Baranov’s work is also funded by the State Agency of Innovation, within the Ramon y Cajal Program, grant number RYC2021-032144-I. Scanning of the specimen was supported by the DESY Block Allocation Group project “Scanning the past - Reconstructing the diversity in million years old fossil amber specimens using SRµCT” at PETRA III.
REFERENCES
Armitage, P.D., Pinder, L.C., and Cranston, P.S. 1995. The Chironomidae: biology and ecology of non-biting midges. Springer Science & Business Media, Dordrecht, Germany.
Baranov, V.A., Schädel, M., and Haug, J.T. 2019a. Fly palaeo-evo-devo: immature stages of bibionomorphan dipterans in Baltic and Bitterfeld amber. PeerJ, 7:e7843:1–49.
https://doi.org/10.7717/peerj.7843
Baranov, V., Hoffeins, C., Hoffeins, H.W. and, Haug, J.T. 2019b. More than dead males: reconstructing the ontogenetic series of terrestrial non-biting midges from the Eocene amber forest. Bulletin of Geosciences, 94(2):187–199.
https://doi.org/10.3140/bull.geosci.1739
Baranov, V., Haug, C., Fowler, M., Kaulfuss, U., Mueller, P., and Haug, J.T. 2022. Summary of the fossil record of megalopteran and megalopteran-like larvae, with a report of new specimens. Bulletin of Geosciences, 97(1):89–108.
https://doi.org/10.3140/bull.geosci.1840
Dobson, M., Poynter, K., and Cariss, H. 2000. Case abandonment as a response to burial by Potamophylax cingulatus (Trichoptera: Limnephilidae) larvae. Aquatic Insects, 22(2): 99–107.
Epler, J.H., Ekrem, T., and Cranston, P.S. 2013. The larvae of Holarctic Chironominae (Diptera: Chironomidae). p. 387–556. In Andersen, T., Cranston, P.S., Epler, J.H. (eds.), Chironomidae of the Holarctic Region. Keys and diagnoses. Entomological Society of Lund, Sweden.
Girard, V., Schmidt, A.R., Saint Martin, S., Struwe, S., Perrichot, V., Saint Martin, J.P., Grosheny, D., Breton, G., and Neraudeau, D. 2008. Evidence for marine microfossils from amber. Proceedings of the National Academy of Sciences, 105(45):17426–17429.
https://doi.org/10.1073/pnas.0804980105
Gröhn, C. 2015. Einschlüsse im baltischen Bernstein. Wachholtz Verlag, Hamburg, Germany.
Haug, C., Shannon, K.R., Nyborg, T., and Vega, F.J. 2013. Isolated mantis shrimp dactyli from the Pliocene of North Carolina and their bearing on the history of Stomatopoda. Boletín de la Sociedad Geológica Mexicana, 65:273–284. https://doi.org/10.18268/BSGM2013v65n2a9
Haug, J.T., Baranov, V., Schädel, M., Müller, P., Gröhn, C., and Haug, C. 2020. Challenges for understanding lacewings: how to deal with the incomplete data from extant and fossil larvae of Nevrorthidae? (Neuroptera). Fragmenta entomologica, 52(2):137–168.
https://doi.org/10.13133/2284-4880/472
Haug, J.T., Müller, P., and Haug, C. 2021. Fossil dragonfly-type larva with lateral abdomen protrusions and implications on the early evolution of Pterygota. IScience, 24(103162): 1–8.
https://doi.org/10.1016/j.isci.2021.103162
Hoffmann, A. 1997. To settle or not to settle? The aggregation behaviour of (Kol.)(Trichoptera: Lepidostomatidae) larvae prior to pupation. Proceedings of the 8th International Symposium on Trichoptera, Minneapolis and Lake Itasca, Minnesota, p. 496.
Hoffmann, A. 2000. The association of the stream caddisfly Lasiocephala basalis (Kol.) (Trichoptera: Lepidostomatidae) with wood. International Review of Hydrobiology: A Journal Covering all Aspects of Limnology and Marine Biology, 85(1):79–93.
https://doi.org/10.1002/(SICI)1522-2632(200003)85:1<79::AID-IROH79>3.0.CO;2-U
Kalugina, N.S. 1974. Changes in the subfamily composition of chironomids (Diptera, Chironomidae) as indicators of a possible eutrophication of bodies of water, during the Late Mesozoic. Byulleten Moskovskogo Obshchestva Ispytatelei Prirodi: Otdelenie Biologii, 79:45–56.
Kasiński, J.R., Kramarska, R., Słodkowska, B., Sivkov, V., and Piwocki, M. 2020. Paleocene and Eocene deposits on the eastern margin of the Gulf of Gdańsk (Yantarny P-1 borehole, Kaliningrad region, Russia). Geological Quarterly, 64(1):29–53.
https://doi.org/10.7306/gq.1513
Kirby, W. 1813. VI. Strepsiptera, a new order of insects proposed; and the characters of the order, with those of its genera, laid down. Transactions of the Linnean Society of London, 1:86–122.
Leach, W.E. 1815. Entomology, p. 57–172. In Brewster, D. (ed.), The Edinburgh encyclopedia, volume 9. William Blackwood, Edinburgh.
Limaye, A. 2012. Drishti: a volume exploration and presentation tool. Developments in X-ray Tomography VIII. Proceedings of the Society of Photo-Optical Instrumentation Engineers (SPIE) 8506:85060X.
https://doi.org/10.1117/12.935640
Linnaeus, C. 1758. Systema naturae per regna tria naturae, secundem classes, ordines, genera, species cum characteribus, differentis, synonymis, locis. Tomus I. Editio decima, reformata. Laurentii Salvii, Holmiae.
Lytaev, P., Hipp, A., Lottermoser, L., Herzen, J., Greving, I., Khokhriakov, I., Meyer-Loges, S., Plewka, J., Burmester, J., Caselle, M., Vogelgesang, M., Chilingaryan, S., Kopmann, A., Balzer, M., Schreyer, A., and Beckmann, F. 2014. Characterization of the CCD and CMOS cameras for grating-based phase-contrast tomography. Developments in X-Ray Tomography IX(921218): 1–11.
https://doi.org/10.1117/12.2061389
Macquart, J. 1838. Diptères exotiques nouveaux ou peu connus. Memoires de la Société (Royale) des sciences, de l'agriculture et des arts à Lille, 1:9–225.
Merritt, R.W. and Cummins, K.W. 1996. An Introduction to the Aquatic Insects of North America. Kendall Hunt, Dubuque.
Moosmann, J., Ershov, A., Weinhardt, V., Baumbach, T., Prasad, M.S., LaBonne, C., Xiao, X., Kashef, J., and Hoffmann, R. 2014. Time-lapse X-ray phase-contrast microtomography for in vivo imaging and analysis of morphogenesis. Nature Protocols, 9:294–304.
https://doi.org/10.1038/nprot.2014.033
Newman, E. 1834. Attempted division of British insects into natural order. Entomological Magazine, 2:379–431.
Otto, C. 1983. Behavioural and physiological adaptations to a variable habitat in two species of case-making caddisfly larvae using different food. Oikos, 41:188–194.
https://doi.org/10.2307/3544262
Sadowski, E.M., Schmidt, A.R., and Denk, T. 2020. Staminate inflorescences with in situ pollen from Eocene Baltic amber reveal high diversity in Fagaceae (oak family). Willdenowia, 50(3):405–517.
https://doi.org/10.3372/wi.50.50303
Sæther, O.A. 1980. Glossary of chironomid morphology terminology (Diptera: Chironomidae). Entomologica Scandinavica Supplement, 14:1–51.
Schädel, M., Müller, P., and Haug, J.T. 2020. Two remarkable fossil insect larvae from Burmese amber suggest the presence of a terminal filum in the direct stem lineage of dragonflies and damselflies (Odonata). Rivista Italiana di Paleontologia e Stratigrafia, 126(1):13–35.
https://doi.org/10.13130/2039-4942/12720
Schädel, M., Hörnig, M.K., Hyžný, M., and Haug, J.T. 2021a. Mass occurrence of small isopodan crustaceans in 100-million-year-old amber: an extraordinary view on behaviour of extinct organisms. PalZ, 95:429–445.
https://doi.org/10.1007/s12542-021-00564-9
Schädel, M., Hyžný, M., and Haug, J.T. 2021b. Ontogenetic development captured in amber-the first record of aquatic representatives of Isopoda in Cretaceous amber from Myanmar. Nauplius, 29:1–29.
https://doi.org/10.1590/2358-2936e2021003
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, E., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J.Y., White, D.J., Hartenstein, V., Eliceiri, K., Tomancak, P., and Cardonaet, A. 2012. Fiji: an open-source platform for biological-image analysis. Nature Methods, 9:676–682.
https://doi.org/10.1038/nmeth.2019
Schmidt, A.R. and Dilcher, D.L. 2007. Aquatic organisms as amber inclusions and examples from a modern swamp forest. Proceedings of the National Academy of Sciences, 104(42):16581–16585.
https://doi.org/10.1073/pnas.0707949104
Schmidt, A.R., Grabow, D., Beimforde, C., Perrichot, V., Rikkinen, J., Saint Martin, S., Thiel, V., and Seyfullah, L.J. 2018. Marine microorganisms as amber inclusions: insights from coastal forests of New Caledonia. Fossil Record, 21(2):213–221.
https://doi.org/10.5194/fr-21-213-2018
Serrano-Sánchez, M. de L., Hegna, T.A., Schaaf, P., Pérez, L., Centeno-García, E., and Vega, F.J. 2015. The aquatic and semiaquatic biota in Miocene amber from the Campo La Granja mine (Chiapas, Mexico): paleoenvironmental implications. Journal of South American Earth Sciences, 62:243–256.
https://doi.org/10.1016/j.jsames.2015.06.007
Ulmer, G. 1903. Über die Metamorphose der Trichopteren. Abhandlungen des Naturwissenschaftlichen Vereins in Hamburg, 18:1–154.
Van Aarle, W., Palenstijn, W.J., De Beenhouwer, J., Altantzis, T., Bals, S., Batenburg, K.J., and Sijbers, J. 2015. The ASTRA Toolbox: A platform for advanced algorithm development in electron tomography. Ultramicroscopy, 157:35–47.
https://doi.org/10.1016/j.ultramic.2015.05.002
Van Aarle, W., Palenstijn, W.J., Cant, J, Janssens, E., Bleichrodt, F., Dabravolski, A., De Beenhouwer, J., Batenburg, K.J., and Sijbers, J. 2016. Fast and flexible X-ray tomography using the ASTRA toolbox. Optics Express, 24:25129–25147.
https://doi.org/10.1364/OE.24.025129
Wang, H., Schädel, M., Sames, B., and Horne, D. J. 2020. New record of podocopid ostracods from Cretaceous amber. PeerJ, 8(e10134):1–10.
https://doi.org/10.7717/peerj.10134
Weaver, J.S. 1988. A synopsis of the North American Lepidostomatidae (Trichoptera). American Entomological Institute. Contributions of the American Entomological Institute, 24 (2):1–141.
Wichard, W., Gröhn, C., and Seredszus, F. 2009. Aquatic insects in Baltic amber: Wasserinsekten im baltischen Bernstein. Remagen, Kessel.
Xing, L., Liu, Y., McKellar, R.C., Luque, J., Li, G., Wang, Y., Yi, Q., Sun, R., Wang, E., and Audo, D. 2021. The first shrimp preserved in mid-cretaceous Kachin amber: systematics, palaeoecology, and taphonomy. Science Bulletin, 66(17):1723–1726.
https://doi.org/10.1016/j.scib.2021.05.008https://doi.org/10.1016/j.scib.2021.05.008
Yu, T., Kelly, R., Mu, L., Ross, A., Kennedy, J., Broly, P., Xia, F., Zhang, H., Wang, B., and Dilcher, D. 2019. An ammonite trapped in Burmese amber. Proceedings of the National Academy of Sciences, 116(23):11345–11350.
https://doi.org/10.1073/pnas.1821292116

