FUNDAMENTALS OF VISION
 The production of a neural image of the light converging upon an animal begins with the
focusing of that light onto a sheet of receptors.
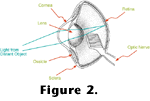
Figure 2 depicts a generalized bird eye. In terrestrial animals most of the
focusing power of the eye is at the air/cornea interface. In aquatic vertebrates the refractive index of the cornea is similar to that of the water in which it is immersed, and hence most of the focussing power is provided by the lens. In either case, light impinging upon the eye from particular directions is redirected to particular locations on the retina, the tissue lining the inside of the eye. Within the retina are photoreceptors, cells specialized for transducing relative light intensities into neurochemical signals that can be passed along to the central nervous system.
The production of a neural image of the light converging upon an animal begins with the
focusing of that light onto a sheet of receptors.
Figure 2 depicts a generalized bird eye. In terrestrial animals most of the
focusing power of the eye is at the air/cornea interface. In aquatic vertebrates the refractive index of the cornea is similar to that of the water in which it is immersed, and hence most of the focussing power is provided by the lens. In either case, light impinging upon the eye from particular directions is redirected to particular locations on the retina, the tissue lining the inside of the eye. Within the retina are photoreceptors, cells specialized for transducing relative light intensities into neurochemical signals that can be passed along to the central nervous system.
The Molecular Basis of Phototransduction
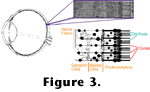 Figure 3 contains a drawing of a cross-section of a human retina showing the position and orientation of the photoreceptors.
Figure 4 diagrams the photoreceptor outer segments, where light is absorbed and transduced in vertebrates.
Figure 3 contains a drawing of a cross-section of a human retina showing the position and orientation of the photoreceptors.
Figure 4 diagrams the photoreceptor outer segments, where light is absorbed and transduced in vertebrates.
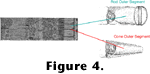 The outer segments consist of sets of disks stacked like pancakes and constructed of lipid bilayers. Embedded in the disk membranes are large numbers of photopigment molecules. Each photopigment consists of a chromophore covalently bound to a protein called an opsin. In all known visual photoreceptors the chromophore is a vitamin A derivative such as the retinal diagrammed in
Figure 5. Aside from minor differences in casting, the phototransduction processes described here are the same in all animals with eyes. When a photopigment is created, the retinal is in a configuration named 11-cis to denote the orientation of the bonds around the eleventh carbon atom
(Figure 5).
The outer segments consist of sets of disks stacked like pancakes and constructed of lipid bilayers. Embedded in the disk membranes are large numbers of photopigment molecules. Each photopigment consists of a chromophore covalently bound to a protein called an opsin. In all known visual photoreceptors the chromophore is a vitamin A derivative such as the retinal diagrammed in
Figure 5. Aside from minor differences in casting, the phototransduction processes described here are the same in all animals with eyes. When a photopigment is created, the retinal is in a configuration named 11-cis to denote the orientation of the bonds around the eleventh carbon atom
(Figure 5).
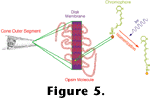 When the chromophore absorbs a photon, an electronic rearrangement may occur, which results in a rotation of the constituent atoms around the eleventh carbon bond. This isomerization of the chromophore causes a change in the shape of the opsin, and in its new configuration, the opsin behaves as an enzyme.
When the chromophore absorbs a photon, an electronic rearrangement may occur, which results in a rotation of the constituent atoms around the eleventh carbon bond. This isomerization of the chromophore causes a change in the shape of the opsin, and in its new configuration, the opsin behaves as an enzyme.
 The rate at which the reaction catalyzed by the opsin occurs sets the rate at which the photoreceptor releases neurotransmitters to other retinal neurons.
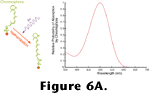
Figure 6 shows the absorption probability of a typical vertebrate photopigment as a function of wavelength and, essentially, the absorption spectrum of a photoreceptor as well, since in most cases, all of the opsin molecules within a given photoreceptor are chemically identical. Neglecting some nuances such as adaptation processes (that are quite important for visual function but are not important for the arguments presented here) the rate at which opsins are catalyzing within a photoreceptor can be determined by integrating the product of the quantal flux through the photoreceptor's outer segment and the photopigment's absorption spectrum as shown in
Figure 6B.
The rate at which the reaction catalyzed by the opsin occurs sets the rate at which the photoreceptor releases neurotransmitters to other retinal neurons.
Figure 6 shows the absorption probability of a typical vertebrate photopigment as a function of wavelength and, essentially, the absorption spectrum of a photoreceptor as well, since in most cases, all of the opsin molecules within a given photoreceptor are chemically identical. Neglecting some nuances such as adaptation processes (that are quite important for visual function but are not important for the arguments presented here) the rate at which opsins are catalyzing within a photoreceptor can be determined by integrating the product of the quantal flux through the photoreceptor's outer segment and the photopigment's absorption spectrum as shown in
Figure 6B.
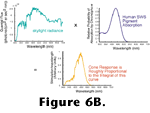 Embedded in this description is an attribute of visual function that is so essential it has been given a name - the principle of univariance. This principle states that once a photon has been absorbed by a photoreceptor, all information about the wavelength of the photon is lost. This is because the photopigment's activation is like a switch. Either the photopigment is acting as a catalyst or it is not. Isomerization of the chromophore caused by the absorption of one photon is identical to the isomerization caused by the absorption of any other photon irrespective of the photon's wavelength. Consequently, a single photoreceptor cannot convey any information about the spectral energy distribution
(read color) of the light passing through it.
Embedded in this description is an attribute of visual function that is so essential it has been given a name - the principle of univariance. This principle states that once a photon has been absorbed by a photoreceptor, all information about the wavelength of the photon is lost. This is because the photopigment's activation is like a switch. Either the photopigment is acting as a catalyst or it is not. Isomerization of the chromophore caused by the absorption of one photon is identical to the isomerization caused by the absorption of any other photon irrespective of the photon's wavelength. Consequently, a single photoreceptor cannot convey any information about the spectral energy distribution
(read color) of the light passing through it.
Color is in the Brain of the Beholder
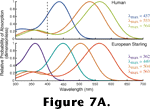 Animals extract information about the spectrum of light striking different regions of their retinas by contrasting the responses of photoreceptors containing different photopigments. Animals generate different photopigments by expressing different opsins. Opsins with different amino acid sequences may have drastically different absorption spectra. However, the shapes of the absorption spectra vary in regular ways such that almost any photopigment absorption spectrum can be adequately described by knowing only the structure of the chromophore, and the wavelength at which the photopigment's absorption peaks
(Stavenga et al. 1993;
Palacios et al. 1998). Figure 7A depicts the absorption spectra of the three types of photopigment that mediate human color vision and the four types of photopigment that mediate color vision in the European starling.
Animals extract information about the spectrum of light striking different regions of their retinas by contrasting the responses of photoreceptors containing different photopigments. Animals generate different photopigments by expressing different opsins. Opsins with different amino acid sequences may have drastically different absorption spectra. However, the shapes of the absorption spectra vary in regular ways such that almost any photopigment absorption spectrum can be adequately described by knowing only the structure of the chromophore, and the wavelength at which the photopigment's absorption peaks
(Stavenga et al. 1993;
Palacios et al. 1998). Figure 7A depicts the absorption spectra of the three types of photopigment that mediate human color vision and the four types of photopigment that mediate color vision in the European starling.
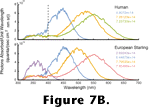 Figure 7B depicts the transformation from an incident spectrum of light to the relative responses that each of these photopigments would generate. The numbers inset in the plots are the integrals of the correspondingly-colored curves. Note that since starlings have four cone photopigments, there are four different outputs, and note that none are identical to any of the three outputs of the human cones. Because these photoreceptor outputs are all that nervous systems retain for the analysis of color, the colors we see are different from the colors seen by a starling (or indeed any other animal). It, therefore, makes little sense to talk of color in the vernacular sense as a property of objects or lights. Such nomenclature works well when the subjects of discussion are all humans because the vast majority of us have essentially the same photopigments. However, when the subjects of a visual study are animals other than humans great care must be
taken. Objects having the same hue to the experimenter may have drastically different hues to an animal and vice versa
(Bennett et al. 1994,
Fleishman et al. 1998).
Figure 7B depicts the transformation from an incident spectrum of light to the relative responses that each of these photopigments would generate. The numbers inset in the plots are the integrals of the correspondingly-colored curves. Note that since starlings have four cone photopigments, there are four different outputs, and note that none are identical to any of the three outputs of the human cones. Because these photoreceptor outputs are all that nervous systems retain for the analysis of color, the colors we see are different from the colors seen by a starling (or indeed any other animal). It, therefore, makes little sense to talk of color in the vernacular sense as a property of objects or lights. Such nomenclature works well when the subjects of discussion are all humans because the vast majority of us have essentially the same photopigments. However, when the subjects of a visual study are animals other than humans great care must be
taken. Objects having the same hue to the experimenter may have drastically different hues to an animal and vice versa
(Bennett et al. 1994,
Fleishman et al. 1998).
Visual Pigment Diversity
Absorption spectra from a large variety of animal photopigments have been determined since microspectrophotometry (a technique for measuring spectral absorption in individual photoreceptors) was developed 35 years ago
(Leibman and Entine 1964). Consequently we know that many animals not only have photopigments that differ from our own, but, like the starling, many have more than three
(Goldsmith 1990). Furthermore, recent analyses of the cloned sequences of a variety of opsin genes indicate that the MRCA of sarcopterygians and actinopterygians had four opsins mediating light-adapted vision
(Hisatomi et al. 1996,
Heath et al. 1997,
Wilkie et al. 1998,
Yokoyama et al.
1998).
 With such data we can reasonably conclude that the starling's photopigment complement has been handed down through every generation since before its ancestors became terrestrial; that is, the starling's four cone pigments are likely orthologs of the four cone pigments of the common ancestor of a starling and a goldfish.
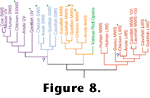
Figure 8 contains a cladogram of visual pigment genes from a subset of those that have been sequenced. Molecular biologists have been disproportionately sequencing the pigment genes of eutherian mammals. To date, at least partial sequence data have been collected for about 50 different cone pigments, and half of these come from eutherians. Consequently the groupings depicted in
Figure 8 should be viewed cautiously. However, if the groupings withstand analysis after the addition of more sequences, several fascinating conclusions may be drawn about evolution in tetrapod vision.
With such data we can reasonably conclude that the starling's photopigment complement has been handed down through every generation since before its ancestors became terrestrial; that is, the starling's four cone pigments are likely orthologs of the four cone pigments of the common ancestor of a starling and a goldfish.
Figure 8 contains a cladogram of visual pigment genes from a subset of those that have been sequenced. Molecular biologists have been disproportionately sequencing the pigment genes of eutherian mammals. To date, at least partial sequence data have been collected for about 50 different cone pigments, and half of these come from eutherians. Consequently the groupings depicted in
Figure 8 should be viewed cautiously. However, if the groupings withstand analysis after the addition of more sequences, several fascinating conclusions may be drawn about evolution in tetrapod vision.
Walls (1942) provided the first serious attempt at mapping the evolution of vertebrate color vision. Using Walls' analysis,
Gauthier (1994) proposed that "excellent hue discrimination" is a reptilian apomorphy. Opsin sequence data suggest instead that tetrachromatic color vision was the plesiomorphic condition for sarcopterygians (and hence tetrapods). Indeed, although opsin sequence data are not yet available to establish the homology of tetrachromacy among all reptiles, at least four visual pigments are known to mediate light-adapted vision in turtles
(Bowmaker 1991) and alligators (Sillman et al. (1991) as well as the lizards (Kawamura and Yokoyama 1997) and birds (Vorobyev et al. 1998) for which sequence data do suggest homology as indicated in
Figure 8. Since the opsin genes do not necessarily cluster together on purely functional grounds the apparent homology of tetrachromacy is not likely due to convergence. That is, the nearest neighbors in the tree are not generally the genes that code for the pigments with the most similar absorption spectra, so the position of the pigment genes on the tree is substantially determined by the sequences of segments that preserve adaptively neutral phylogenetic signals rather than sequences convergently selected for particular spectral tunings. Upon finding four cone pigments in
Alligator,
Sillman et al. (1991) suggested that dinosaurs, which (like alligators) were large reptiles, had color vision. At present the Sillman et al. suggestion appears too tenuous. Since modern reptiles bracket most dinosaurian lineages we can argue that dinosaurs not only had color vision, but actually had color vision that was, in at least some ways, better than ours.
Walls (1942) suspected that mammalian color vision arose
de novo because either mammals secondarily lost their capacity for color vision during the Mesozoic Era, or their pre-Mesozoic ancestors never acquired it. In a thorough review,
Jacobs (1993) demonstrated that the most parsimonious view of more recently acquired data is that the MRCA of eutherian mammals had two opsins. This conclusion is strongly supported by the phylogenetic analyses of opsin sequence data, but another rather interesting twist is suggested by the results of these analyses.
The chicken, goldfish, and American anole, all have opsins similar in spectral sensitivity to the short wave sensitive pigment found in most orders of mammals (e.g., see the human visual pigment absorption spectra in
Figure 7A). However, instead of clustering with those opsin genes, the genes coding for the short wave sensitive pigments of
human, marmoset, squirrel monkey, talapoin monkey, and cow, as well as the UV sensitive pigments of
mouse and rat, cluster with the UV sensitive pigments of goldfish, parakeet, and
anole and the almost UV sensitive pigments of chicken, pigeon and clawed frog
(Hisatomi et al. 1996,
Yokoyama and Yokoyama 1996,
Heath et al. 1997,
Wilkie et al. 1998,
Yokoyama et al.
1998), suggesting these pigments are all orthologs. Therefore, the short-wave sensitive pigment retained by eutherian mammals was likely a UV pigment ancestrally. Prior to the discovery of UV sensitivity in rodents
(Jacobs et al.
1991), it seemed implausible that any mammals had visual sensitivity to UV light
(Goldsmith 1990). Ironically it is now a defensible position that ancestrally all eutherians were UV-sensitive. That possibility raises intriguing questions about why this particular pigment was retained whereas two others were lost, and why the pigment's absorption spectrum was shifted toward longer wavelengths for most eutherians other than a subset of murid rodents.
 The production of a neural image of the light converging upon an animal begins with the
focusing of that light onto a sheet of receptors.
Figure 2 depicts a generalized bird eye. In terrestrial animals most of the
focusing power of the eye is at the air/cornea interface. In aquatic vertebrates the refractive index of the cornea is similar to that of the water in which it is immersed, and hence most of the focussing power is provided by the lens. In either case, light impinging upon the eye from particular directions is redirected to particular locations on the retina, the tissue lining the inside of the eye. Within the retina are photoreceptors, cells specialized for transducing relative light intensities into neurochemical signals that can be passed along to the central nervous system.
The production of a neural image of the light converging upon an animal begins with the
focusing of that light onto a sheet of receptors.
Figure 2 depicts a generalized bird eye. In terrestrial animals most of the
focusing power of the eye is at the air/cornea interface. In aquatic vertebrates the refractive index of the cornea is similar to that of the water in which it is immersed, and hence most of the focussing power is provided by the lens. In either case, light impinging upon the eye from particular directions is redirected to particular locations on the retina, the tissue lining the inside of the eye. Within the retina are photoreceptors, cells specialized for transducing relative light intensities into neurochemical signals that can be passed along to the central nervous system.





 Figure 7B
Figure 7B